Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Protection coatings for corrosion control of mild steel using phenolic polymeric deep eutectic solvents†
Jon
Lopez de Lacalle
a,
Daniela
Minudri
 *a,
Matias L.
Picchio
a,
Antonela
Gallastegui
a,
Daniele
Mantione
*a,
Matias L.
Picchio
a,
Antonela
Gallastegui
a,
Daniele
Mantione
 ab,
Maria
Forsyth
bc and
David
Mecerreyes
ab,
Maria
Forsyth
bc and
David
Mecerreyes
 *ab
*ab
aPOLYMAT, University of the Basque Country UPV/EHU, Avenida Tolosa 72, 20018 Donostia-San Sebastián, Spain. E-mail: dminudri@gmail.com; david.mecerreyes@ehu.es
bIKERBASQUE, Basque Foundation for Science, Bilbao, 48011, Spain
cInstitute for Frontier Materials, Deakin University, Burwood, VIC 3125, Australia
First published on 7th May 2024
Abstract
The mitigation of corrosion, especially in coastal regions, stands as a significant social concern necessitating the exploration of environmentally friendly materials. This study introduces phenol-based ionic polymers as both coatings and solution corrosion inhibitors for mild steel. Three methacrylated phenolic deep eutectic monomers (DEMs) bearing catechol and gallol motifs were synthetized for this purpose, taking advantage of polyphenol chemistry. Both the polyphenol DEMs and the UV-photopolymerized ionic polymer coatings formed coordination complexes with iron ions on the surface of the mild steel, resulting in a distinctive colour evolution, transitioning from a non-coloured film to dark purple, characterized by optical imaging and UV-vis absorption analysis. The resulting interfacial complex layer demonstrated exceptional corrosion inhibition efficiency for tannic acid and protocatechuic acid based polymers, achieving efficiencies of 99.84% and 99.54% respectively. Further explanation of this phenomenon involves the investigation of phenolic deep eutectic monomers in solution as model compounds, revealing a purple hue in the solution arising from the complexation between the polyphenol chemistry and the iron ions leached from the steel surface.
Sustainability spotlightThe goal of reducing corrosion of different metallic materials has a negative impact from the social, economic and environmental points of view. Looking for sustainable corrosion inhibitors has turned into a key point, avoiding chromate-based components, lately related to pollution and toxicity. We focus on the development of new green ionic polymeric coatings based on deep eutectic solvents in order to stop any damage to metals. In this context, these polymeric materials are inexpensive, non-toxic and plant based, with fast synthesis and polymerization processes at moderate temperatures and without the need of volatile organic solvents, produced by UV curing. The obtained coatings demonstrated their anticorrosive properties in mild steel linked to their polyphenolic nature. Related to the UN goals, some of them are emphasized in our work:SDG 7 (Affordable and Clean Energy) → use of low temperatures and UV curing. SDG 9 (Industry, Innovation and Infrastructure) → avoid damage of buildings. SDG 13 (Climate Action) → avoid the use of volatile organic compounds (VOCs). SDG14 (Life Below Water) → avoid corrosion below water in the marine environment. |
Introduction
Corrosion is a natural process wherein a metal undergoes deterioration due to an (electro)chemical reaction at its surface with the environment.1 It causes important economic losses and environmental impact, and therefore different corrosion inhibitors have been searched in the last few decades. Traditional anti-corrosive materials include inorganic additives and polymer coatings. However, some of these compounds successfully used in the last few decades have been ruled out because of the associated pollution and toxicity of Cr(VI). In recent years, organic compounds have emerged as non-toxic and versatile corrosion-inhibiting alternatives.2,3 Among them, ionic compounds such as ionic liquids (ILs) and polymeric ionic liquids (PILs) are being successfully investigated as potential organic inhibitors and polymeric protective coatings.4,5Deep eutectic solvents (DESs) represent a new class of ionic solvents that have attracted substantial attention due to their environmentally sustainable characteristics, becoming potential alternatives to aforementioned materials. They are eutectic mixtures of a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD), interacting via hydrogen bonding resulting in negative deviations from thermodynamic ideality.6,7 Their green character and the easiness of incorporating biobased molecules make DESs attractive to be used in the field of corrosion inhibitors of metals.8 Among the big family of DESs, those based on choline chloride as a quaternary ammonium salt and different HBDs have been broadly investigated.9,10 Their properties as corrosion protectors have been ascribed to many key factors. Primarily, it includes the absence of oxidizing species as well as the high viscosity and low electrical conductivity.11
Polyphenols are a wide family of biobased molecules known for their anti-inflammatory, anticancer, and antibacterial properties, as well as their complexation ability with different metal ions.12 Interestingly, some studies have indicated the potential application of polyphenols as corrosion inhibitors. For instance, Kassim et al. demonstrated a positive correlation between higher phenolic content in extracted natural plants and improved corrosion efficiency against mild steel.13 In this trend, tannic acid–based materials have been demonstrated to be excellent green corrosion inhibitors for different metals and alloys.14,15 The favorable properties of polyphenols are associated with their antioxidant properties and metal-complexation ability.15,16
Introducing a polymerizable structure into DESs offers numerous advantages, showing unique properties such as ionicity, low volatility, high thermal stability, and tunable polarity, enabling their use in various chemical processes.6,7,17 Thus, polymeric deep eutectic solvents (polyDESs) offer promising opportunities to develop new innovative, sustainable, and versatile materials.18–20 Furthermore, their polymeric nature facilitates the creation of films, coatings, or membranes that can be tailored to exhibit specific properties such as self-healing capability.21,22 For instance, pioneering studies by Mota-Morales et al. proposed using polyDESs in frontal polymerizations23 and dry ionic conductors for wearable sensors.24 In another example, M. Isik et al. showed the versatility of polyDES chemistry and their applications as sorbents for CO2 capture.25 Beyond this, polyDESs have also been investigated as electrolytes,26,27 as carriers for drug delivery systems,28 and in coating applications.29
Our group recently reported the development of DESs based on polyphenols, which showed potential as (bio)adhesives. We also demonstrated that this chemical platform could be used to develop multifunctional monomers and the corresponding polyDESs.30 The resulting (meth)acrylic deep eutectic monomers (DEMs) could be easily photopolymerized, presenting good adhesion, excellent mechanical properties, and antibacterial activity. In this context, this letter aims to investigate the combination of the anti-corrosive properties of polyphenols and DESs into a polymeric protective coating. For this purpose, we selected three phenolic deep eutectic monomers (DEMs) and evaluated their coatings obtained by photopolymerization directly on the surface of mild steel. Interestingly, it was observed that the phenolic polyDES layer complexed at the steel surface, generating a colour transformation into a dark purplish film. An insight into this complex formation, colour evolution, and the anti-corrosive properties of these innovative polyDES coatings is presented.
Experimental section
Materials
[2-(Methacryloyloxy) ethyl] trimethylammonium chloride solution (M1, 75 wt% in water), 2-hydroxy-2-methylpropiophenone (Darocur 1173, 97%), tannic acid (TA, ACS reagent) and 3,4-dihydroxyhydrocinnamic acid (HCA, 98%) were purchased from Sigma-Aldrich. 3,4-dihydroxybenzoic acid (PCA, 97%) was supplied by Alfa Aesar. All chemicals were used without further purification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 for TA-M1 and 1
20 for TA-M1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for PCA-M1 and HCA-M1 under stirring at 70 °C, for 1 hour. After obtaining a homogeneous viscous coloured liquid, the mixture was freeze-dried for two days for water elimination. Different HBD
2 for PCA-M1 and HCA-M1 under stirring at 70 °C, for 1 hour. After obtaining a homogeneous viscous coloured liquid, the mixture was freeze-dried for two days for water elimination. Different HBD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
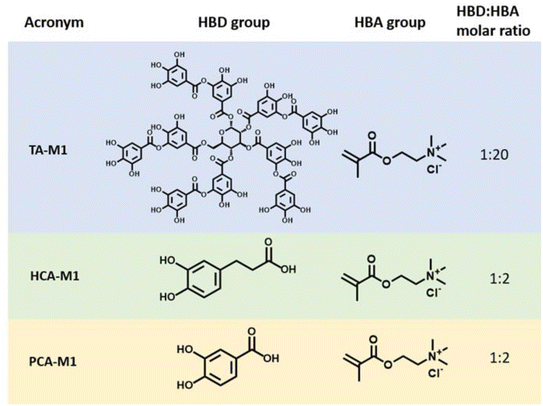
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBA molar ratios were evaluated, but only those mixtures that remained in a liquid state at room temperature after freeze-drying are reported in this work as deep eutectic monomers. The obtained DEMs are later presented in Fig. 1.
HBA molar ratios were evaluated, but only those mixtures that remained in a liquid state at room temperature after freeze-drying are reported in this work as deep eutectic monomers. The obtained DEMs are later presented in Fig. 1.
Corrosion test
An acrylic UV-curable coating, composed of DEM and 2-hydroxy-2-methylpropiophenone (5% wt), was applied onto the steel surface using a doctor blade technique. The precursor coating was kept at room temperature for 24 hours until the coating colour transformation from transparent to dark blue or violet occurs. Subsequently, the coating was irradiated for over 120 seconds using a UVC-5 (DYMAX) UV Curing Convenyor System with a 400 mW cm−2 lamp intensity. This first step is illustrated in Fig. 2. After this, a second acrylic layer based on dipropylene glycol diacrylate and 2-hydroxy-2-methylpropiophenone (5% wt.) was applied above the previous coating, also, by a doctor blade technique and also, irradiated for 120 seconds. The two step polymer coating formation scheme is shown in the ESI (Fig. S1†).
 | ||
| Fig. 2 Illustration of the Doctor Blade technique for coating preparation and representation of the complexation of the phenolic DEMs and polyDES with iron ions from mild steel. | ||
As a pristine reference coating, a single-layer coating was used. In that case, the acrylic layer based on dipropylene glycol diacrylate and 2-hydroxy-2-methylpropiophenone (5% wt) was applied onto the steel surface using a doctor blade technique, without any previous deep eutectic monomer coating.
Thicknesses of the applied double layer coatings are measured with a calliper, before and after each doctor blade technique is applied. First, the thickness of the mild steel is calculated, then, after irradiating the previously complexated DEM coating, the thickness is measured again, and finally, after the addition and polymerization of the second acrylic layer it is measured again, in order to get the exact thickness of each polymer layer. At least three different points around the coatings surfaces are selected to measure the coating thickness, taking into account possible irregularities or small defects. The overall polymeric coatings present an approximate thickness of 100 μm.
where I0corr and Icorr are the corrosion current densities without and with inhibitor coatings, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times accordingly.
000 times accordingly.
Results and discussion
Polyphenol-based methacrylic DEMs were prepared using the simple heating/solvent drying method commonly used in DES chemistry. Thus, the monomeric HBA [2-(methacryloyloxy)ethyl]trimethylammonium chloride (M1) was mixed with three phenolic derivatives acting as HBDs, namely tannic acid (TA), protocatechuic acid (PCA) and hydrocaffeic acid (HCA) in the corresponding molar ratios. These polyphenols were selected as representative examples due to the differences in their chemical structure and the variety of phenolic moieties such as gallols and catechols. The HBD/HBA mixtures were heated at 70 °C while stirring until a clear solution was obtained. The chemical structures and the corresponding acronyms of the DEMs studied in this work are illustrated in Fig. 1. The thermal characterization and spectroscopic studies, such as FTIR or 1H-NMR, are included in the ESI (Fig. S2–S7†).30The polyDES coatings were prepared on top of the mild steel by UV photopolymerization of the previously reported DEM monomers. Fig. 2 illustrates the experimental scheme for the preparation of the polymer layers. First, the three different DEMs were deposited on top of mild steel by a doctor blade technique (step 1–2). Surprisingly, minutes after the application of the DEM coating, the applied film suffers a colour transformation from non-colour to weak blue/violet. After 24 hours of exposure, all the applied coating turned completely dark (step 3), and the DEM was photopolymerized by applying UV light (step 4). Since the polyDES coatings were water soluble, a second hydrophobic polyacrylate top coating layer was applied for additional protection.
As mentioned, the colour evolution observed for each polyphenol based coating is illustrated in Fig. 3. The starting point just after applying the doctor blade technique is a transparent homogeneous coating. However, for the tannic based coating as soon as it is applied to the surface, it starts to get darker in different zones, as observed in the first image (0 minutes). Tannic acid was also the fastest to suffer a homogeneous darkening effect, turning to soft purple colour, after 15 minutes in contact with the mild steel, probably due to the more gallol and catechol moieties this molecule has in its structure. In contrast, after 15 minutes, the complexation and therefore the colour transformation are only visible in single points for HCA, and even nowhere for the PCA coating. After 8 hours, the tannic based coating undergoes a homogeneous darkening, whereas the HCA-M1 and PCA-M1 coatings become blue and violet respectively, and they exhibit some defects and irregularities, potentially linked to the worse compatibility and adhesiveness of these DEMs to the mild steel, as observed in both images, where the darkness of the coating is not uniform among all the surface. Finally, after 24 hours, coatings are completely dark, showing a slightly different black purplish colour, and the HCA based layer has slightly bluish tone. Even so, the TA-M1 coating presents uniform consistency and better adhesive contact to the mild steel surface, probably related to the higher viscosity of the applied DEM, while the HCA based coating has some irregularities, where the coating is slightly detached what could conclude in worst anticorrosive properties of that material. Further information about the thicknesses of the applied polymer coatings is added to the ESI (Table S1†).
Next, electrochemical characterization of the phenolic polyDES-coated steel was performed by Electrochemical Impedance Spectroscopy (EIS). A potentiodynamic polarization test was used to evaluate the application of the synthesized materials as protective coatings. Fig. 4 shows the EIS results of the phenolic polyDES coatings in NaCl solution after 24 hours. The impedance values for PCA-M1 and TA-M1 are higher than for the reference hydrophobic acrylate layer, with values of 109.2 Ω cm2 and 107.5 Ω cm2, respectively, indicating that the complexation is beneficial to the anti-corrosive effect of the phenolic polyDES coatings. However, the HCA-M1 exhibits a lower impedance modulus value after 24 hours, 105.4 Ω cm2. This behaviour is probably due to the surface defects caused by the complexation process. As mentioned before, the complexed surface layer of HCA-M1 exhibits some imperfection and poor compatibility which is the main reason for the non-protective electrochemical behaviour. Nyquist plots are presented for HCA-M1 in Fig. S8† as well as the fitting results are summarized in Table S1 and Fig. S9† with the corresponding circuit model.
 | ||
| Fig. 4 EIS results for the phenolic poly(DES) coatings in NaCl solution after 24 hours of immersion. | ||
These results agree with the potentiostatic polarization curve measurements, which are plotted in Fig. 5a. The corrosion parameters obtained for the coatings are summarized in Table 1. Values of the Tafel slopes demonstrated effective cathodic protection by TA and PCA based protective layers, as evidenced by the reduction in the Tafel slope, indicative of a lower cathodic reaction velocity. Both coatings also present higher inhibition efficiencies than the reference, with values above 99%. In contrast, it was concluded that the HCA-based coating did not work as a corrosion inhibitor. Corrosion inhibition seems to be deeply affected due to the imperfections the coatings has, probably because of water penetration into the mild steel. Fig. 5b shows the areas of the coatings immersed after CPP measurement, in which part of the formed complex is removed for HCA, related to the non-protective behaviour of this material, because of the removal of the polyDES coating, which acts as a corrosion inhibitor.
| Sample | E (V) | I (μA) | β a (mV dec−1) | β c (mV dec−1) | % ƞ |
|---|---|---|---|---|---|
| Blank | −0.70 | 4.3 | — | ||
| TA-M1 | −0.58 | 0.007 | 47.4 | 37.1 | 99.84 |
| HCA-M1 | −0.53 | 4.4 | 52.4 | 46.7 | — |
| PCA-M1 | −0.51 | 0.02 | 46.9 | 30.6 | 99.54 |
To further understand the anticorrosive nature of phenolic DEMs and the consequent colour evolution, different aqueous solutions were prepared at a concentration of 0.03 M DEM with 0.01 M aqueous NaCl as solvent. NaCl 0.01 M without the presence of the phenolic DEM was used as a reference. The steel electrode immersion in these solutions revealed a strong evolution of the solution colour with time. It was observed visually and characterized by UV-vis absorption, as shown in Fig. 6. The solution's UV-vis absorption spectra were measured daily for three days after immersing the electrode as shown in Fig. 6a, where the spectra measured at day 3 are plotted for all DEMs. For all the solutions, a wide band can be observed between 280 and 320 nanometres, which is attributed to the characteristic π–π* band in polyphenols.31 The inset shows that these bands are red-shifted by the strong interaction between the polyphenols and the metal cation Fe3+ and the consequent complex formation.32 Thus, TA-M1, PCA-M1, and HCA-M1 exhibit a broad absorption band with maximum peaks at 606, 601, and 742 nm, respectively. From a chemical point of view, they can be attributed to ligand–metal charge transfer (LMCT) due to the complex formation between Fe3+ and hydroxyl groups in polyphenols, which is responsible for the colour evolution observed in Fig. 6b.33 At the same time, the pH of the immersed solutions was measured every day (Table S2†), showing an acidic character which may be due to the release of protons after complexation with the iron. It was previously reported that the number of ligands surrounding the coordination centre depends on the pH value. Iron is generally bound by three polyphenols per metal ion at slightly acidic pH, resulting in dark blue-violet Fe complexes, as highlighted in Fig. 6c.34 These results indicate a complex formation associated with a dissolution of iron cations from the mild steel towards the polyphenols in solution.
Furthermore, the potential protective capacity of DEMs in solution on the stainless steel surface was further investigated. Fig. 7 shows the surfaces of the mild steels immersed in DEM solutions for seven days. As in the previous case, 0.01 M NaCl aqueous solution was employed as a control system, where it is observed that the steel experienced severe degradation as eye-catching corrosion products partially appeared on the surface after only one day of the experiment, while the steel surface is fully damaged by corrosion after one week. However, when the phenolic DEMs are present in the system, the corrosion is significantly inhibited by a single purple layer formed on the metal surface corresponding to the complexation between the iron ions and polyphenol, which drastically delays the steel degradation. It is also observed that in the case of TA-M1, iron complexation is faster than PCA-M1 or HCA-M1. After one immersion day in TA-M1, the steel surface was totally covered with a purple layer. This faster complexation is probably due to the more gallol groups of the tannic acid structure, which enable faster interaction with iron ions. According to the surface evolution, after one week there is an irregular coloured purplish layer on top of the steel, which acts as a corrosion inhibitor. These effects are obvious in the case of PCA-M1 and TA-M1 after 7 days.
Fig. S10† shows an amplified optical image of the steel surface immersed for one week. Additional electrochemical characterization is included in the ESI.† The corrosion of AS1020 steel in the presence of aqueous electrolytes with and without phenolic DEM additives was evaluated using electrochemical impedance spectroscopy and potentiodynamic polarization techniques. Fig. S11 and Table S3† show the Bode plots and corrosion efficiency values for the steel samples tested in NaCl and DEM solutions. The data prove that adding 10 mM DEM solution and forming the polyphenol–Fe complex result in an anti-corrosive coating on the steel surface. The corrosion inhibition efficiencies show that these results agree with what is highlighted in optical images.
Conclusions
In conclusion, this article introduces a novel sustainable corrosion inhibitor for mild steel both in solution and their corresponding polymeric coatings, grounded on polyphenolic and deep eutectic solvent chemistry. UV-photopolymerization was carried out to obtain the aforementioned ionic polymers on top of mild steel, yielding a surprising colour darkening within minutes. Electrochemical measurements underscore the exceptional protective properties of polyphenol-based polymeric coatings, particularly those derived from tannic acid and protocatechuic acid, thereby highlighting their potential as eco-friendly alternatives for corrosion inhibitors.Furthermore, immersion tests conducted in the presence of mild steel, utilizing dissolved polyphenol monomers, reinforces the idea of applying this material as a corrosion inhibitor. Remarkably, the solution underwent a colour transformation from non-colour to purple upon adding the steel electrode, indicative of the formation of the metal–ligand complex between the dissolved polyphenol and the leached iron ions. Electrochemical results summarize the promise of biobased DESs as green materials in addressing corrosion challenges, while aligning with environmentally conscious practices.
Author contributions
The authors' contributions are as follows: Jon Lopez de Lacalle conceptualized, planned, and carried out the experiments. Daniela Minudri, Antonela Gallastegui, Matias Luis Picchio and Daniele Mantione contributed to the analysis and interpretation of results. David Mecerreyes and Maria Forsyth supervised and critically reviewed the research and manuscript. All the authors provided valuable feedback and helped to shape the project, analysis, and manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from EU (IONBIKE 2.0 MSCA-SE), Eusko Jaurlaritza (GV-IT1525-22) and MINECO AEI (PID2020-119026GB-I00) is gratefully acknowledged. D. M. thanks Ayuda RYC2021-031668-I.Notes and references
- S. Harsimran, K. Santosh and K. Rakesh, Overview of corrosion and its control: a critical review, Proceedings on Engineering Sciences, 2021, 13–24, DOI:10.24874/PES03.01.002.
- A. Kadhim, A. A. Al-Amiery, R. Alazawi, M. K. S. Al-Ghezi and R. H. Abass, Corrosion Inhibitors. A Review, Int. J. Corros. Scale Inhib., 2021, 10(1), 54–67, DOI:10.17675/2305-6894-2021-10-1-3.
- C. Verma, E. E. Ebenso, M. A. Quraishi and C. M. Hussain, Recent Developments in Sustainable Corrosion Inhibitors: Design, Performance and Industrial Scale Applications, Mater. Adv., 2021, 3806–3850, 10.1039/d0ma00681e.
- D. Minudri, A. Somers, N. Casado, M. Forsyth and D. Mecerreyes, Poly(Ionic Liquid)s Having Coumarate Counter-Anions as Corrosion Inhibitors in Acrylic UV Coatings, RSC Appl. Polym., 2023, 1, 55, 10.1039/d3lp00017f.
- C. Verma, E. E. Ebenso and M. A. Quraishi, Ionic Liquids as Green and Sustainable Corrosion Inhibitors for Metals and Alloys: An Overview, J. Mol. Liq., 2017, 403–414, DOI:10.1016/j.molliq.2017.02.111.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Deep Eutectic Solvents: A Review of Fundamentals and Applications, Chem. Rev., 2021, 1232–1285, DOI:10.1021/acs.chemrev.0c00385.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev., 2014, 11060–11082, DOI:10.1021/cr300162p.
- E. I. Ahmed, K. S. Ryder and A. P. Abbott, Corrosion of Iron, Nickel and Aluminium in Deep Eutectic Solvents, Electrochim. Acta, 2021, 397, 139284, DOI:10.1016/j.electacta.2021.139284.
- M. Bučko and J. B. Bajat, A Review of the Electrochemical Corrosion of Metals in Choline Chloride Based Deep Eutectic Solvents, J. Electrochem. Sci. Eng., 2022, 237–252, DOI:10.5599/jese.1135.
- A. Mitar, M. Panić, J. Prlić Kardum, J. Halambek, A. Sander, K. Zagajski Kučan, I. Radojčić Redovniković and K. Radošević, Physicochemical Properties, Cytotoxicity, and Antioxidative Activity of Natural Deep Eutectic Solvents Containing Organic Acid, Chem. Biochem. Eng. Q., 2019, 33(1), 1–18, DOI:10.15255/CABEQ.2018.1454.
- A. A. Kityk, Y. D. Rublova, A. Kelm, V. V. Malyshev, N. G. Bannyk and I. Flis-Kabulska, Kinetics and Mechanism of Corrosion of Mild Steel in New Types of Ionic Liquids, J. Electroanal. Chem., 2018, 823, 234–244, DOI:10.1016/j.jelechem.2018.06.018.
- N. R. Perron and J. L. Brumaghim, A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding, Cell Biochem. Biophys., 2009, 53(2), 75–100, DOI:10.1007/s12013-009-9043-x.
- M. Jain Kassim and T. Kang Wei, Plants Polyphenols: an Alternative Source for Green Corrosion Inhibitor, The Proceedings of 2nd Annual International Conference Syiah Kuala University 2012 & 8th IMT-GT Uninet Biosciences Conference, 2012 Search PubMed.
- E. Kusmierek and E. Chrzescijanska, Tannic Acid as Corrosion Inhibitor for Metals and Alloys, Mater. Corros., 2015, 66(2), 169–174, DOI:10.1002/maco.201307277.
- W. Xu, E. H. Han and Z. Wang, Effect of Tannic Acid on Corrosion Behavior of Carbon Steel in NaCl Solution, J. Mater. Sci. Technol., 2019, 35(1), 64–75, DOI:10.1016/j.jmst.2018.09.001.
- B. Qian, M. Michailidis, M. Bilton, T. Hobson, Z. Zheng and D. Shchukin, Tannic Complexes Coated Nanocontainers for Controlled Release of Corrosion Inhibitors in Self-Healing Coatings, Electrochim. Acta, 2019, 297, 1035–1041, DOI:10.1016/j.electacta.2018.12.062.
- T. El Achkar, H. Greige-Gerges and S. Fourmentin, Basics and Properties of Deep Eutectic Solvents: A Review, Environ. Chem. Lett., 2021, 3397–3408, DOI:10.1007/s10311-021-01225-8.
- L. C. Tomé and D. Mecerreyes, Emerging Ionic Soft Materials Based on Deep Eutectic Solvents, J. Phys. Chem. B, 2020, 8465–8478, DOI:10.1021/acs.jpcb.0c04769.
- L. C. Tomé, L. Porcarelli, J. E. Bara, M. Forsyth and D. Mecerreyes, Emerging Iongel Materials towards Applications in Energy and Bioelectronics, Mater. Horiz., 2021, 3239–3265, 10.1039/d1mh01263k.
- M. Isik, F. Ruiperez, H. Sardon, A. Gonzalez, S. Zulfiqar and D. Mecerreyes, Innovative Poly(Ionic Liquid)s by the Polymerization of Deep Eutectic Monomers, Macromol. Rapid Commun., 2016, 37(14), 1135–1142, DOI:10.1002/marc.201600026.
- M. Jablonský, A. Škulcová and J. Šima, Use of Deep Eutectic Solvents in Polymer Chemistry–a Review, Molecules, 2019, 24, 3978, DOI:10.3390/molecules24213978.
- D. Carriazo, M. C. Serrano, M. C. Gutiérrez, M. L. Ferrer and F. del Monte, Deep-Eutectic Solvents Playing Multiple Roles in the Synthesis of Polymers and Related Materials, Chem. Soc. Rev., 2012, 41(14), 4996–5014, 10.1039/c2cs15353j.
- K. F. Fazende, M. Phachansitthi, J. D. Mota-Morales and J. A. Pojman, Frontal Polymerization of Deep Eutectic Solvents Composed of Acrylic and Methacrylic Acids, J. Polym. Sci., Part A: Polym. Chem., 2017, 55(24), 4046–4050, DOI:10.1002/pola.28873.
- J. D. Mota-Morales, R. J. Sánchez-Leija, A. Carranza, J. A. Pojman, F. del Monte and G. Luna-Bárcenas, Free-Radical Polymerizations of and in Deep Eutectic Solvents: Green Synthesis of Functional Materials, Prog. Polym. Sci., 2018, 139–153, DOI:10.1016/j.progpolymsci.2017.09.005.
- M. Isik, S. Zulfiqar, F. Edhaim, F. Ruiperez, A. Rothenberger and D. Mecerreyes, Sustainable Poly(Ionic Liquids) for CO2 Capture Based on Deep Eutectic Monomers, ACS Sustain. Chem. Eng., 2016, 4(12), 7200–7208, DOI:10.1021/acssuschemeng.6b02137.
- M. H. Dokoohaki, A. R. Zolghadr and A. Klein, Highly Efficient Dye-Sensitized Solar Cells Based on Electrolyte Solutions Containing Choline Chloride/Ethylene Glycol Deep Eutectic Solvent: Electrolyte Optimization, Ind. Eng. Chem. Res., 2022, 61(31), 11464–11473, DOI:10.1021/acs.iecr.2c01324.
- L. Millia, V. Dall'Asta, C. Ferrara, V. Berbenni, E. Quartarone, F. M. Perna, V. Capriati and P. Mustarelli, Bio-Inspired Choline Chloride-Based Deep Eutectic Solvents as Electrolytes for Lithium-Ion Batteries, Solid State Ionics, 2018, 323, 44–48, DOI:10.1016/j.ssi.2018.05.016.
- I. B. Qader and K. Prasad, Recent Developments on Ionic Liquids and Deep Eutectic Solvents for Drug Delivery Applications, Pharm. Res., 2022, 2367–2377, DOI:10.1007/s11095-022-03315-w.
- M. L. Picchio, D. Minudri, D. Mantione, M. Criado-Gonzalez, G. Guzmán-González, R. Schmarsow, A. J. Müller, L. C. Tomé, R. J. Minari and D. Mecerreyes, Natural Deep Eutectic Solvents Based on Choline Chloride and Phenolic Compounds as Efficient Bioadhesives and Corrosion Protectors, ACS Sustain. Chem. Eng., 2022, 10(25), 8135–8142, DOI:10.1021/acssuschemeng.2c01976.
- J. L. de Lacalle, A. Gallastegui, J. L. Olmedo-Martínez, M. Moya, N. Lopez-Larrea, M. L. Picchio and D. Mecerreyes, Multifunctional Ionic Polymers from Deep Eutectic Monomers Based on Polyphenols, ACS Macro Lett., 2023, 12(2), 125–132, DOI:10.1021/acsmacrolett.2c00657.
- G. Świderski, A. Z. Wilczewska, R. Świsłocka, M. Kalinowska and W. Lewandowski, Spectroscopic (IR, Raman, UV–Vis) Study and Thermal Analysis of 3d-Metal Complexes with 4-Imidazolecarboxylic Acid, J. Therm. Anal. Calorim., 2018, 134(1), 513–525, DOI:10.1007/s10973-018-7524-0.
- A. Espina, M. V. Cañamares, Z. Jurašeková and S. Sanchez-Cortes, Analysis of Iron Complexes of Tannic Acid and Other Related Polyphenols as Revealed by Spectroscopic Techniques: Implications in the Identification and Characterization of Iron Gall Inks in Historical Manuscripts, ACS Omega, 2022, 7(32), 27937–27949, DOI:10.1021/acsomega.2c01679.
- J. I. Ballesteros, H. J. R. Caleja-Ballesteros and M. C. Villena, Digital Image-Based Method for Iron Detection Using Green Tea (Camellia Sinensis) Extract as Natural Colorimetric Reagent, Microchem. J., 2021, 160, 105652, DOI:10.1016/j.microc.2020.105652.
- N. R. Perron and J. L. Brumaghim, A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding, Cell Biochem. Biophys., 2009, 53(2), 75–100, DOI:10.1007/s12013-009-9043-x.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00117f |
| This journal is © The Royal Society of Chemistry 2024 |