Open Access Article
Open Access ArticleUpcycling mixed-material waste with elemental sulfur: applications to plant oil, unseparated biomass, and raw post-consumer food waste†
Bárbara G. S.
Guinati
a,
Perla Y.
Sauceda Oloño
 a,
Nawoda L.
Kapuge Dona
a,
Katelyn M.
Derr
a,
Shalini K.
Wijeyatunga
a,
Andrew G.
Tennyson
a,
Nawoda L.
Kapuge Dona
a,
Katelyn M.
Derr
a,
Shalini K.
Wijeyatunga
a,
Andrew G.
Tennyson
 *ab and
Rhett C.
Smith
*ab and
Rhett C.
Smith
 *a
*a
aDepartment of Chemistry, Clemson University, Clemson, South Carolina 29634, USA. E-mail: rhett@clemson.edu
bDepartment of Materials Science and Engineering, Clemson University, Clemson, South Carolina 29634, USA
First published on 9th May 2024
Abstract
Herein we report the preparation of high sulfur-content materials (HSMs) using food waste and elemental sulfur. Peanut hulls and peanut oil were combined with sulfur to yield HxOyS90 (x = wt% peanut hulls, y = wt% peanut oil) to enable systematic investigation of the relationship between unsaturated oil content and HSM properties. Next, post-consumer food wastes cooked in peanut oil, specifically fresh as well as desiccated French fries, were combined with elemental sulfur to afford WFFS90 and DFFS90, respectively. Differential scanning calorimetry revealed all HSMs in this study were readily remeltable and provided evidence for the presence of oligo/polysulfide chains crosslinking the food waste-derived organic matter. Impressively, the compressive strengths of HxOyS90, WFFS90, and DFFS90 were comparable or superior to that of ordinary Portland cement. Quantitative assessment of the HSMs described in this study using the environmental metrics of atom economy, E-factor, and global warming potential (GWP) yielded values ranging from 93.5% to 100% for atom economy, 0 to 0.07 for E-factor, and −0.574 to +0.608 kg CO2 eq. per kg for GWP. Moreover, the syntheses of these HSMs achieved 8 of the 12 Principles of Green Chemistry and 5 of the 17 UN Sustainable Development Goals. Collectively, the findings reported in this work provide strong evidence that HxOyS90, WFFS90, and DFFS90 will function as thermally-recyclable, mechanically-robust sustainable alternatives to concretes.
Sustainability spotlightTo address the environmental impact of waste generation, this study upcycles food waste, such as peanut hulls and post-consumer French fries, with elemental sulfur to create high sulfur-content materials (HSMs). These HSMs exhibit high compressive strengths, comparable or superior to conventional concrete, making them sustainable alternatives for construction materials. The syntheses of HSMs achieve impressive atom economy, low E-factor, and low global warming potential, aligning with UN Sustainable Development Goals 2 (Zero Hunger), 9 (Industry, Innovation and Infrastructure), 11 (Sustainable Cities and Communities), 12 (Responsible Consumption and Production), and 13 (Climate Action). Our study highlights the potential of waste-derived composites to contribute to sustainable practices and support multiple SDGs, addressing the global challenges of waste management and environmental conservation. |
Introduction
Modern human civilization generates titanic amounts of wastes, with the agriculture, construction, and petrochemical industries being among the most prolific waste generators.1,2 Wastes can be classified as avoidable or unavoidable, wherein the former can be reduced by better resource management (e.g., more sensible purchasing of foodstuffs to reduce waste caused by spoilage). Unavoidable wastes, however, are intrinsic to essential processes (e.g., inedible or indigestible byproducts from foodstuff production, elemental sulfur from petroleum refining, etc.) and cannot be mitigated without significant advances in technology (e.g., sustainable asphalts from non-petroleum sources) and/or dramatic changes in consumer behaviors (e.g., sustainable food economy based on algae, fungi, yeast, etc.). Given the scale of energy and resources expended in the agriculture, construction, and petrochemical industries, there is an urgent, ongoing need to develop valuable, productive uses for the avoidable and unavoidable wastes they produce. Despite the significant differences between these industries and their wastes, there is a powerful strategy to upcycle these wastes into valuable and useful products – conversion to high sulfur-content materials (HSMs, Scheme 1).3–13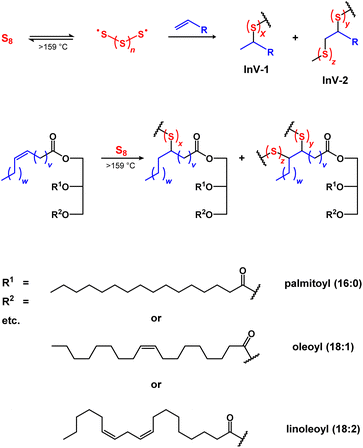 | ||
| Scheme 1 General scheme for inverse vulcanization (upper), and an example of inverse vulcanization-induced C–S bond formation in triglycerides (lower). | ||
Between 30 to 40% of the foodstuffs produced in the United States are wasted, amounting to 60 Mt of avoidable waste each year.14 Plant- and animal-derived agricultural products are highly complex, heterogeneous mixtures containing a diverse array of chemically-distinct small-molecule and polymeric species. The most common components are carbohydrates (cellulose, starch, saccharides, etc.), triglycerides (e.g., fats and oils), proteins, and polyphenols (e.g., lignin, flavonoids, etc.). Given the chemical complexity of foodstuffs and their inedible byproducts, it is difficult to use either class directly as chemical feedstocks for upcycling processes. Instead, foodstuff waste and inedible byproducts must be separated into more chemically-homogenous product streams. Although remarkable advances have been made in upcycling these wastes into valuable goods, ranging from fuels15 to plastics16 to cements17,18 to packaging goods,19 the energy and economic costs of the necessary separation processes have significantly hindered commercialization of food waste-derived products.
Petroleum refining generates 8.6 Mt of elemental sulfur each year20 as an unavoidable waste byproduct of hydrodesulfurization processes.21,22 Until recently, there were relatively few productive uses of elemental sulfur, industrial-scale synthesis of H2SO4 being the highest-volume. Inverse vulcanization, a reaction pioneered by Pyun in 2013, enabled large amounts of elemental sulfur to be converted into polysulfide chains using a relatively small amounts of organic substrates.23 In addition to petroleum-derived organic species, elemental sulfur is a versatile reagent for upcycling a remarkable array of agricultural products, such as plant oils/extracts,24–28 animal fats,29 terpenes,30 lignin derivatives,31–33 cellulose derivatives,34 starch derivatives,35,36 and unseparated lignocellulosic biomass.37,38 Notably, inverse vulcanization reactions of biologically-derived organic feedstocks represent processes that formally remove CO2 from the atmosphere.
Peanuts are an attractive agricultural product to employ in upcycling processes owing to their chemical composition and large annual crop sizes. Peanut farming in the US generates an annual output of roughly 6 Gt with >1.2 Gt deriving peanut hulls, which have low digestibility and nutritional value for human consumption.39 Peanut hulls comprise primarily cellulose and lignin 52 wt% and 35 wt%, respectively, but small amounts of peanut oil are also present (∼1%). Peanut oil is particularly useful for inverse reactions with elemental sulfur, given that peanut oil is nearly 50 wt% olein (comprising 3 monounsaturated fatty acid side-chains) and 33 wt% lanolin (comprising 3 diunsaturated fatty acid side-chains).
Previous studies elucidated strategies for preparing composites from peanut hulls and sulfur waste demonstrated that high-strength materials from peanut hulls are possible due to the presence of a small amount of olefin-containing peanut oil (∼1% by weight) in the hulls.37,38,40 The strength characteristics of these materials were investigated in the context of the interplay between filler dispersion in the network and the less favorable interactions between the hydrophilic biomass filler and the hydrophobic sulfur network. Fractionation of biomass into narrow particle size distributions was found to be advantageous in achieving uniform filler dispersion.
In the US alone, demolition and new construction projects generate more than 380 Mt of concrete waste each year, with demolition projects contributing >90% to this total.41 Concrete production generates roughly 1 kg of atmospheric CO2 per kg of concrete, therefore generating 380 Mt of concrete waste corresponds to releasing 380 Mt of CO2 into the atmosphere without contributing anything productive or valuable to human civilization. Developing a sustainable alternative to concrete, in which the synthesis thereof generated no CO2 as a gaseous byproduct, would dramatically reduce the carbon footprint of construction and demolition. Inverse vulcanization of food waste oils and other bio-derived precursors has been actively pursued in an effort to prepare more sustainable materials.42–47 Our group, for example, has previously demonstrated that HSMs with mechanical strength properties comparable or superior to concrete could be obtained via the formation of oligo/polysulfide chains that crosslink biologically-derived organic feedstocks (e.g., free fatty acids, cellulose/starch/lignin following transesterification by free fatty acids, triglycerides, etc.).36–38
We therefore hypothesized that peanut oil-containing food wastes would undergo inverse vulcanization with elemental sulfur to yield HSMs with high mechanical strength values. To establish proof of principle, we first performed inverse vulcanization using 90 wt% elemental sulfur with mixtures of isolated peanut hull waste and isolated peanut oil, ranging from 10 wt% peanut hulls and 0 wt% peanut oil to 0 wt% peanut hulls and 10 wt% peanut oil, to obtain the HSMs HxOyS90 (where x and y are wt% of hulls and oil, respectively, in the composite, Scheme 2). After demonstrating proof of principle with HxOyS90, we then performed inverse vulcanization (again using 90 wt% sulfur) on recovered food waste, in the form of uneaten French fries (23 wt% peanut oil), to yield HSMs WFFS90 and DFFS90.
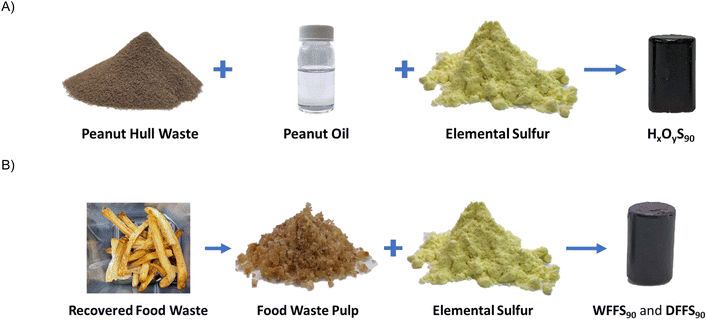 | ||
| Scheme 2 Illustration of composite synthesis from the reaction of fossil fuel waste sulfur with waste peanut hulls/peanut oil (A) or recovered post-consumer food waste (B). | ||
Herein we report the chemical and thermomechanical analyses of HxOyS90, WFFS90, and DFFS90, which revealed that each HSM contained polymeric sulfur chains and exhibited compressive strength values superior to that of conventional concrete. Collectively, our findings provide strong evidence that avoidable food waste can be combined with unavoidable sulfur waste to yield higher-value HSM products that could serve as sustainable concrete replacements and thereby dramatically reduce anthropogenic CO2 generated by human construction and demolition activities.
Results and discussion
Synthesis of peanut hull waste/peanut oil–sulfur composites
Our group has previously demonstrated that HSMs exhibiting high compressive strengths can be prepared by performing inverse vulcanization on peanut hulls with 90 wt% elemental sulfur, wherein the ∼1 wt% peanut oil found in the hulls is essential for HSM synthesis. In these composites inverse vulcanization of the olefins is accompanied by transesterification between triglycerides and hull saccharide/lignin hydroxyl groups to facilitate crosslinking between all components. In the current study, it was of interest to elucidate the dependence of HSM properties on the ratio of peanut oil to peanut hull mass. A series of composites HxOyS90 were thus prepared in which x = wt% hulls, y = wt% oil and sulfur composition was held constant at 90 wt%.The olefinic groups in peanut oil are the intended sites for covalent crosslinking by sulfur, therefore the olefin content of peanut oil was quantified via1H NMR spectroscopy, using 2,3,4,5,6-pentafluorobenzaldehyde added as an internal integration standard (Fig. S1†). Integration of the alkene region (4.5–5.5 ppm) relative to the aldehyde signal (10.3 ppm) in the standard enabled calculation of a total olefin content of 6.0 mmol g−1.
Prior work in our group has shown that HSMs derived from peanut hull particle sizes of 150–212 μm exhibit the highest compressive strength values.40 Therefore, the peanut hulls for the current study came from the same lot as our prior work and were sifted for passing between ASTM sieve numbers 100 and 70 to give the 150–212 μm hull particle sizes for use as feedstocks to prepare HxOyS90. The peanut hull powders were then combined with varying amounts of peanut oil to elucidate the relationship between HxOyS90 properties and the absolute olefin content (in mmol g−1) of the peanut hull/peanut oil reaction mixture.
Composites HxOyS90 were synthesized by combining the peanut hull powder and peanut oil with elemental sulfur, then heating this reaction mixture to 180 °C for 24 h with mechanical stirring (Scheme 2A). This process produced H0O10S90 as a brown/black solids, while H5O5S90 and H8O2S90 were brown/black solids. Composite H10O0S90 was previously reported to be a dark brown/black composite as well. Each of these HSMs were remeltable at 160 °C and could be easily cast into a variety of shapes using silicone molds (e.g., test cylinders for compressive strength analysis, Fig. 1A–C).
 | ||
| Fig. 1 Photos of (A) H0O10S90, (B) H5O5S90, (C) H8O2S90, (D) WFFS90 and (E) DFFS90 that have been shaped into cylinders for compressive strength analysis. | ||
Synthesis of food waste–sulfur composites
Unsaturated plant oils are sustainable alternatives to petrochemical olefins, however using nutritionally-valuable foodstuffs to make inedible construction materials would dramatically increase food insecurity, a significant concern for a world facing greater food scarcity and declining arable land. Using post-consumer food waste material as HSM feedstocks, however, does not increase food insecurity because these are avoidable wastes that are unsafe for human or animal consumption. We selected post-consumer uneaten French fries recovered from refuse from a fast-food restaurant known to cook French fries in peanut oil, given that their chemical compositions were similar to peanut hulls – cellulose and peanut oil, whereby the former does not react directly with elemental sulfur and the latter does. The French fries used in this work were recovered and used on the same day they had been discarded.The primary chemical constituents of the fries were water (41 wt%), peanut oil (23 wt%), and starch (33 wt%), with ∼3 wt% other materials (e.g., proteins, vitamins, etc.). We previously reported that starch, lacking olefin functionalities, cannot undergo crosslinking with elemental sulfur without prior covalent functionalization with olefins (e.g., transesterification). In this work, the necessary olefin functional groups were supplied by the peanut oil in the fries. Peanut oil extracted from the fries has an olefin content of 3.4 mmol olefin per g (1H NMR spectra are provided in Fig. S2 of the ESI†). The olefin content in the whole fries was thus 0.9 mmol g−1 for as-collected fries and 1.3 mmol g−1 for dehydrated fries (fries dried in a vacuum oven at 50 °C for 15 h).
French fry wastes were modified in one of two ways before reacting with sulfur. The as-collected (“wet”) French fries (WFF) and the desiccated French fries (DFF) were ground into a coarse paste and a dry particulate (DFF). Inverse vulcanization of WFF and DFF with 90 wt% sulfur using the same conditions as with HxOyS90 afforded the HSMs WFFS90 and DFFS90, respectively. Both WFFS90 and DFFS90 were isolated as brittle, brown/black solids. Both HSMs could be readily remelted at 160 °C and cast into a variety of shapes using silicone molds (Fig. 1D and E). Only water is produced as a side product of these processes.
Chemical and morphological analysis
The infrared spectrum of peanut oil showed peaks attributable to olefin C–H bond stretching (∼3000 cm−1) and cis-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bond bending (∼720 cm−1) vibrational modes. Consistent with the consumption of C
C–H bond bending (∼720 cm−1) vibrational modes. Consistent with the consumption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups by reaction with elemental sulfur, these peaks observed in the IR spectrum of peanut oil were absent from the spectra of all HSMs, providing strong evidence that all olefin functional groups were consumed via reaction with the polysulfide radicals generated during inverse vulcanization (ESI Fig. S3†).
C groups by reaction with elemental sulfur, these peaks observed in the IR spectrum of peanut oil were absent from the spectra of all HSMs, providing strong evidence that all olefin functional groups were consumed via reaction with the polysulfide radicals generated during inverse vulcanization (ESI Fig. S3†).
Sulfur species that are physically entrapped within an HSM, not covalently linked via S–C bonds, are classified as “dark sulfur” species.48,49 The relative quantity of dark sulfur within HSMs has been shown to influence their thermal and mechanical properties. Following a modified literature procedure, dark sulfur was extracted from each of the HSMs in this study (HxOyS90, WFFS90, and DFFS90) using ethyl acetate, followed by UV-visible spectroscopic analysis of the ethyl acetate solutions (Table 1). The relative dark sulfur content determined from its UV-vis absorbance at 275 nm was consistent across this series of HSMs, ranging from 11–14 wt%.
| Materials | T d /°C | T m /°C | T g,DSCc/°C | Sulfur rankd | Dark sulfure (wt%) |
|---|---|---|---|---|---|
| a The temperature at which the 5% mass loss was observed. b The temperature at the peak maximum of the endothermic melting. c Glass transition temperature. d Sulfur atoms per cross-link. Given as a range to reflect the possibility of one or two sulfur atom attachments to olefinic carbons as shown in Scheme 1. e Percent ethyl acetate-extractable sulfur species. f Previously reported. The composite was referred to as PS90 in the original publication. g Previously reported using a different procedure than the more updated procedure used herein for the new composites. | |||||
| H0O10S90 | 216 | 117 | −37 | 24–48 | 11 |
| H5O5S90 | 218 | 117 | −37 | 47–94 | 12 |
| H8O2S90 | 219 | 117 | −36 | 117–234 | 12 |
| H10O0S90 | 217 | 117 | NA | 129–258 | 24g |
| WFFS90 | 213 | 118 | −37 | 89.5–179 | 14 |
| DFFS90 | 217 | 116 | −37 | 108–216 | 13 |
| S8 | 229 | 118 | NA | NA | NA |
Scanning electron microscopy with elemental mapping by energy dispersive X-ray analysis (SEM and EDX) was used to analyze composites (Fig. 2). All of the composites were microscopically homogeneous materials having uniform distributions of carbon, oxygen, and sulfur. The successful homogenization of the materials is consistent with prior work on biomass–sulfur composites containing plant oils or other hydrophobic comonomers.19,34,36–38,40,50,51
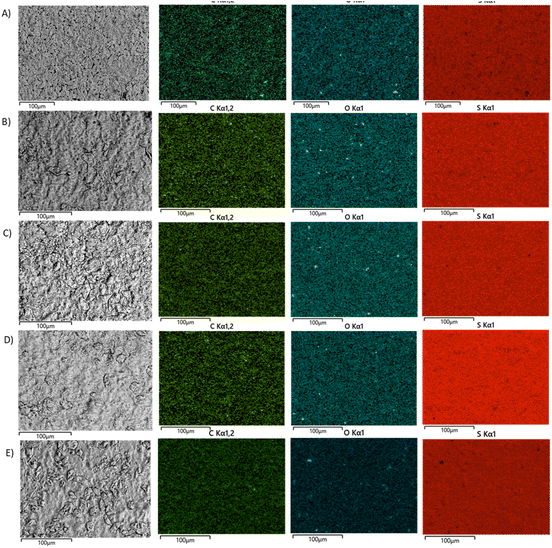 | ||
| Fig. 2 SEM and EDX images of HxOyS90 showing the EM image and the carbon, oxygen and sulfur elements mapping for (A) H0O10S90, (B) H5O5S90, (C) H8O2S90, (D) WFFS90 and (E) DFFS90. | ||
Thermal and mechanical characterization
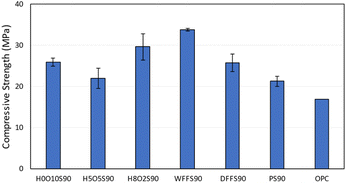
Thermogravimetric analysis (TGA, Fig. S4–S8†) and differential scanning calorimetry (DSC, Fig. S9–S13†) of all HSMs were conducted under a nitrogen atmosphere, which revealed highly-conserved decomposition (Td,5%, 5% mass loss), glass-transition (Tg), and melting (Tm) temperatures across the entire series of HxOyS90, WFFS90, and DFFS90 (Table 1). The range of Td,5% (213 to 219 °C), Tm (116 to118 °C), and Tg,DSC (−36 to −37 °C) values observed from these HSMs are in good agreement with corresponding values observed from previously reported HSMs.29,52,53 The 5% mass loss can be attributed to the sublimation of elemental sulfur out of the HSM from dark sulfur as well as that formed by rearrangement of covalently-linked sulfur species. The glass transitions observed are characteristic for polymeric sulfur chains tethered to carbon anchors.29,52,53The previously-reported HSM H10O0S90 exhibited a compressive strength of 21.3 ± 1.2 MPa, which depended strongly on the presence or absence of peanut oil. When the peanut hulls were first extracted with hexanes to remove the peanut oil prior to the inverse vulcanization reaction, the hulls separated from the sulfur during heating and uniform solids could not be shaped from the resultant mixture. In this study, the peanut oil content of peanut hulls was increased to afford H5O5S90 and H2O8S90, but no statistically-significant changes in compressive strength were observed along this series (Fig. 3, stress–strain plots are provided in ESI Fig. S18–S22†). The HSMs derived from food waste – WFFS90 and DFFS90 – exhibited compressive strength values similar to H0O10S90, H5O5S90, and H8O2S90. Impressively, the compressive strength values of all HSMs in this work were comparable or superior to that of ordinary Portland cement (OPC).
Environmental metrics, principles of green chemistry and UN sustainable development goals
The environmental impact and sustainability of chemical processes can be quantified by atom economy, E-factor, and global warming potential metrics. Atom economy is determined by the relative wt% of the atoms in the starting materials relative to the relative wt% of these atoms in the product materials. The only mass losses observed from the inverse vulcanization reactions of peanut hulls and food waste derived from evaporation of water (8 wt%, as determined by TGA analysis, Fig. S9 in the ESI†). For the HSMs HxOyS90 and WFFS90, water evaporation occurred during the heating stage of the inverse vulcanization reaction, while for DFFS90, the water content of the food waste was removed by drying in a vacuum oven at 50 °C for 15 h prior to the vulcanization step. Collectively, the syntheses of HxOyS90, WFFS90, and DFFS90 achieved impressive atom economy, ranging from 93.5% to 100%.The E-factor of a chemical process can be calculated by dividing the mass of waste generated by the mass of useful product obtained. As a benchmark, E factors for commercial bulk chemicals range from <1 to over 50.54 Because the syntheses of HxOyS90, WFFS90, and DFFS90 generated water vapor as the only waste product, the E-factors for these HSMs were impressively low (0 to 0.07).
The global warming potential of a process is an estimate of its net carbon footprint, which can be calculated using a set of assumptions about the mass inputs and energy costs of that. In the current context, global warming potentials of −0.574 to +0.608 kg CO2 eq. per kg were calculated (Table 2; calculation details are provided in the ESI†). A negative value for a process is particularly impressive, given that it indicates that process formally removes CO2 from the atmosphere.
| Materials | Atom economya (%) | E-Factorb | GWPc (kg CO2e per kg) |
|---|---|---|---|
| a Percent by mass of atoms included in the final, isolated product. b Mass of starting material not in the final isolated product divided by mass of starting material in the final isolated composite. c Carbon footprint calculated according to the assumptions outlined in the ESI. d Previously reported. The composite was referred to as PS90 in the original publication. | |||
| H0O10S90 | 100 | 0 | +0.608 |
| H5O5S90 | 99.6 | 0.004 | +0.158 |
| H8O2S90 | 99.4 | 0.006 | −0.113 |
| H10O0S90 | 99.2 | 0.008 | −0.293 |
| WFFS90 | 95.9 | 0.04 | −0.331 |
| DFFS90 | 93.5 | 0.07 | −0.574 |
Furthermore, the HSMs prepared in this work achieve most of the 12 Principles of Green Chemistry: prevention – preventing waste by upcycling food and industrial wastes into value-added products; atom economy – retaining 93.5% to 100% of all atoms in the HSM products; less hazardous chemical synthesis – using non-toxic peanut shells, peanut oil, and food wastes as carbon feedstocks, instead of petrochemicals; safer solvents and auxiliaries – performing solvent-free syntheses which generate water as the only byproduct; design for energy efficiency – preparing sustainable concrete alternatives at a significantly lower temperature than for OPC-based concretes (180 °C vs. 1400 °C, respectively); use of renewable feedstocks –utilizing unavoidable and avoidable food wastes (peanut hulls and post-consumer food waste) instead of petrochemical feedstocks; reduce derivatives –transforming feedstocks directly into HSM products in a one-pot reaction; design for degradation – literature precedent suggests that HSMs comprising biosourced feedstocks can be used as fertilizers.55,56
Furthermore, the current work also supports the broader social missions of sustainability and resilience. The United Nations Sustainable Development Goals (UNSDGs) provide a framework for assessing processes with respect to these missions. Multiple UNSDGs that will benefit directly from the HSMs described in this work: Goal #2, zero hunger – productively employing post-consumer food wastes and potentially using the HSMs as fertilizers once they have reached the end of their operational lifespans; Goal #9, industry, innovation, and infrastructure – developing new methods to create value-added materials from agricultural and industrial wastes will spur innovation in the growing green chemical industrial sector; Goal #11, sustainable cities and communities – creating sustainable alternatives to environmentally-harmful construction materials (e.g., concrete) from the waste streams of agricultural and industrial processes; Goal #12, responsible consumption and production – upcycling food wastes will promote reasonable consumption and decrease overall waste production; Goal #13, climate action– replacing high global-warming potential materials with low global-warming potential materials.
Although these simplified environmental metrics show the promise of the composite synthesis, several potential drawbacks remain to be evaluated. For example, potential release of environmentally harmful H2S or SO2 gases is always a danger when sulfur is heated with organics. While we did not detect these gases on the small laboratory scale reported herein, researchers who have conduced these types of experiments on larger scales have noted the dangers of temperature spikes that have caused toxic gas release in some cases.57 Industrial scaleup of these processes would require careful engineering controls to avoid these temperature control problems.
Collectively, the HSMs HxOyS90, WFFS90, and DFFS90 represent significant advances in multiple environmental metrics and actively promote green chemistry principles in conjunction with conscientious attention to downstream broader societal impacts. Given the generality of the syntheses of these HSMs, coupled with the diverse array of food wastes and other biomass species that comprise plant oil, lignocellulose, starch, etc., the methodology described herein will be readily adaptable to a panoply of other waste streams. The extent to which these advantages may be practically applicable will depend on the property stability of such materials over years-long exposure to environmental factors necessary for a particular target application. Samples of the materials are currently being monitored over a months to years timescale, and results of those studies will be reported when available.
Conclusions
High-sulfur content materials HxOyS90, WFFS90, and DFFS90 were prepared with high atom economy, low E-factor, and low global warming potential via upcycling of food waste (indigestible peanut hulls and uneaten post-consumer French fries) with petroleum refining waste (elemental sulfur). The only byproduct formed by the syntheses of these HSMs was water vapor released from the peanut hulls or uneaten French fries during the heating stage. The compressive strength values of these HSMs ranged from 22.0–33.8 MPa, making them viable sustainable alternatives to mineral-based bricks, cements, and concretes, the production of which requires environmentally-harmful mining practices and energy-intensive heating temperatures exceeding 1200 °C. Collectively, the composites HxOyS90, WFFS90, and DFFS90 realize most of the 12 Principles of Green Chemistry. Furthermore, the use of these HSMs as substitutes for legacy mineral-based building materials would achieve multiple United Nations Sustainability Goals. In order for these perceived beneficial properties of waste-derived composites unveiled by fundamental investigations herein to find applicability in real world contexts, issues of scalability, cost and regulatory compliance will need to be further investigated and addressed. Only in the context of these practical considerations can these ostensibly sustainable materials find appropriate application.Experimental section
Chemicals and materials
Sulfur powder (99.5%) was purchased from Alfa Aesar. Ground peanut shells were obtained from Golden Peanut and Tree Nuts (Product ES). Hexanes were purchased from Fisher Scientific, and 2,3,4,5,6-pentafluorobenzaldehyde was obtained from Acros Organics. Peanut oil was purchased from LouAna, Ventura Foods, LLC. The chemicals were used without further purification unless otherwise noted. Post-consumer French fries were made from Idaho potatoes produced in Idaho Falls, Idaho, USA in the 2023 growing season. Fries were collected at a fast-food franchise on the day of disposal by customers. All materials were used as received unless otherwise specified.General considerations
A Blendtec Total blender was used to blend French fries used in this study.UV-Vis data were collected to obtain the dark sulfur content on Agilent Technologies Cary 60 UV-Vis using Simple Reads software. The data was collected at 275 nm.
Shimadzu IR Affinity-1S instrument with an ATR attachment operating over 400–4000 cm−1 at ambient temperature was used to obtain Fourier transform infrared spectra.
The proton NMR spectra were acquired using a Bruker NEO-300 MHz spectrometer at room temperature. To process the data, SpinWorks 4.2.11 software was used.
Thermogravimetric analysis (TGA) data were recorded on a TA SDT Q600 instrument over the range 25 to 800 °C, with a heating rate of 10 °C min−1 under a N2 flow of 20 mL min−1.
Differential scanning calorimetry (DSC) data were acquired using a Mettler Toledo DSC 3 STARe System from −60 to 140 °C, with a heating rate of 10 °C min−1 under a flow of N2 (50 mL min−1). Each DSC measurement was carried out over three heat-cool cycles, and data were reported for the third cycle.
Compressional analyses were performed on a Mark-10 ES30 test stand equipped with an M3-200 force gauge (1 kN maximum force with ±1 N resolution). Compression cylinders were cast from silicone resin molds (Smooth-On Oomoo® 25 tin-cure) with diameters of ∼6 mm and heights of ∼10 mm. Samples were manually sanded to ensure uniform dimensions. Compressional analysis was performed in triplicate, and the results were averaged.
SEM and EDX were acquired on a Schottky Field Emission Scanning Electron Microscope SU5000 operating in variable pressure mode with an accelerating voltage of 15 keV.
Quantifying peanut oil olefin content
This procedure was used to quantify olefin content both for the virgin peanut oil used to prepare HxOyS90 and the peanut oil extracted from the post-consumer French fries. A solution of 2,3,4,5,6-pentaflourobenzaldehyde (PFBA) was prepared in CDCl3. First, around 25 mg of PFBA was dissolved in 0.5 mL of CDCl3. Afterward, 25 mg of the virgin peanut oil or extracted peanut oil samples were solubilized in 1.0 mL of chloroform. An aliquot of 0.1 mL of the as-prepared internal standard was added to the sample. The sample was homogenized and transferred to the NMR tube for analysis.Peanut hull preparation
The industrial peanut shell product was fractionated using the same protocol described elsewhere.36 Using ASTM standard sieves (sizes 100 and 70), particles larger than 212 μm and smaller than 150 μm were removed, and particles with dimensions of 212–150 μm were used to prepare HxOyS90.Isolation of peanut oil from fries
This procedure is based on literature precedent for removing peanut oil from biomass sources. French fries' powder (1 g) was suspended in hexane (50 mL) and stirred at room temperature for 30 min. The suspension was filtered, and the hexane was removed from the filtrate under reduced pressure. The yield of oil material was determined to be 23 wt%.French fry moisture content quantification
Post-consumer French fries were weighed and vacuum dried at 50 °C for 12 h at 4 torr and weighed after the drying process. The gravimetric difference was calculated as moisture content as 41%. The moisture content determined in this way was consistent with the mass loss below 100 °C observed in the TGA for the fries (Fig. S10 in the ESI†).French fry preparation for composite synthesis
Fries used in the preparation of WFFS90 were taken as-recovered from restaurant refuse and ground in a (Blender make/model) to give an aggregate of small fry pieces.Fries used in the preparation of DFFS90 were recovered from restaurant refuse and oven-dried at 50 °C for 12 h at 4 torr in a vacuum oven. They were then ground in a blender (Blendtec Total) to give an aggregate of small fry pieces.
French fry particle size assessment
WFF and DFF were blended using the same protocol. The obtained powder from each was weighed, and fractionated using the ASTM standard sieve (1 mm). WFF and DFF yielded 13% and 42% of particles having dimensions smaller than 1 mm, respectively.Synthesis of composites
CAUTION: heating elemental sulfur with organics can result in the formation of H2S gas. H2S is toxic, foul-smelling, and corrosive.General synthesis of HxOyS90
Elemental sulfur and peanut hulls were first combined in an Erlenmeyer flask. The flask was placed in an oil bath at 160 °C, and mechanical stirring was started when sulfur melted. The reaction temperature was then increased to 180 °C. For reactions in which peanut oil was used, the desired amount of oil was added to the mixture when the temperature was stable at 180 °C. The reactions were carried out for 24 h. The brown/black solid product was allowed to cool to ambient temperature.Quantifying dark sulfur content
To determine the dark sulfur content, the method used is based on the method reported by Hasell's group,48 which uses absorbance to correlate to the amount of dark sulfur in high sulfur content polymeric materials. In this way, a small fraction (between 6 and 7 mg) of the material was placed in a 250 mL volumetric flask containing ethyl acetate, and it was stirred for 30 min. The obtained solution was measured using a Carry-UV at 275 nm. A calibration curve was used to determine the concentration based on sample absorbance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding for this project from the National Science Foundation (CHE-2203669 to RCS) is gratefully acknowledged. We would like to thank Jake Harrell of Golden Peanut and Tree Nuts (Alpharetta, Georgia, USA) for supplying the peanut hull product used in this study.Notes and references
-
J. Inumaru, T. Hasegawa, H. Shirai, H. Nishida, N. Noda and S. Ohyama, in Advances in Power Boilers, Elsevier, 2021, pp. 1–56 Search PubMed
.
- W. Russ and R. Meyer-Pittroff, Crit. Rev. Food Sci. Nutr., 2004, 44, 57–62 CrossRef PubMed
.
- A. D. Smith, R. C. Smith and A. G. Tennyson, Sustainable Chem., 2020, 1, 209–237 CrossRef
.
- F. G. Mueller, L. S. Lisboa and J. M. Chalker, Adv. Sustainable Syst., 2023, 7, 2300010 CrossRef CAS
.
- M. J. H. Worthington, R. L. Kucera and J. M. Chalker, Green Chem., 2017, 19, 2748–2761 RSC
.
- T. Lee, P. T. Dirlam, J. T. Njardarson, R. S. Glass and J. Pyun, J. Am. Chem. Soc., 2022, 144, 5–22 CrossRef CAS PubMed
.
- Y. Zhang, R. S. Glass, K. Char and J. Pyun, Polym. Chem., 2019, 10, 4078–4105 RSC
.
- J. Bao, K. P. Martin, E. Cho, K.-S. Kang, R. S. Glass, V. Coropceanu, J.-L. Bredas, W. O. N. Parker Jr, J. T. Njardarson and J. Pyun, J. Am. Chem. Soc., 2023, 145, 12386–12397 CrossRef CAS PubMed
.
- A. Amin, N. M. Mahmoud and Y. W. Z. Nisa, Green Mater., 2020, 8, 172–180 CrossRef
.
-
J. M. Chalker, M. J. H. Worthington, N. A. Lundquist and L. J. Esdaile, in Sulfur Chemistry, ed. X. Jiang, Springer International Publishing, Cham, 2019, pp. 125–151, DOI:10.1007/978-3-030-25598-5_4
.
- J. J. Dale, V. Hanna and T. Hasell, ACS Appl. Polym. Mater., 2023, 5, 6761–6765 CrossRef CAS
.
- H. Berk, M. Kaya, M. Topcuoglu, N. Turkten, Y. Karatas and A. Cihaner, React. Funct. Polym., 2023, 187, 105581 CrossRef CAS
.
- T. Thiounn, M. S. Karunarathna, L. M. Slann, M. K. Lauer and R. C. Smith, J. Polym. Sci., 2020, 58, 2943–2950 CrossRef CAS
.
- USDA, https://www.usda.gov/foodwaste/faqs, accessed February 4, 2024.
- H. Wei, W. Liu, X. Chen, Q. Yang, J. Li and H. Chen, Fuel, 2019, 254, 115599 CrossRef CAS
.
- A. P. Shirsath and M. M. Henchion, Trends Food Sci. Technol., 2021, 118, 57–70 CrossRef CAS
.
- S. K. Wijeyatunga, K. M. Derr, C. P. Maladeniya, P. Y. Sauceda-Oloño, A. G. Tennyson and R. C. Smith, J. Polym. Sci., 2024, 62, 554–563 CrossRef CAS
.
- P. Y. Sauceda-Olono, A. C. Borbon-Almada, M. Gaxiola, A. D. Smith, A. G. Tennyson and R. C. Smith, J. Compos. Sci., 2023, 7, 248 CrossRef CAS
.
- M. K. Lauer and R. C. Smith, Compr. Rev. Food Sci. Food Saf., 2020, 19, 1–53 CrossRef PubMed
.
-
US Geological Survey, Sulfur production worldwide in 2022, by country, Statista, retrieved December 27, 2023, from https://www-statista-com.libproxy.clemson.edu/statistics/1031181/sulfur-production-globally-by-country/ Search PubMed
.
- S. Brunet, D. Mey, G. Pérot, C. Bouchy and F. Diehl, Appl. Catal., A, 2005, 278, 143–172 CrossRef CAS
.
- A. Tanimu and K. Alhooshani, Energy Fuels, 2019, 33, 2810–2838 CrossRef CAS
.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed
.
- A. E. Davis, K. B. Sayer and C. L. Jenkins, Polym. Chem., 2022, 13, 4634–4640 RSC
.
- C. Herrera, K. J. Ysinga and C. L. Jenkins, ACS Appl. Mater. Interfaces, 2019, 11, 35312–35318 CrossRef CAS PubMed
.
- C. V. Lopez, M. S. Karunarathna, M. K. Lauer, C. P. Maladeniya, T. Thiounn, E. D. Ackley and R. C. Smith, J. Polym. Sci., 2020, 58, 2259–2266 CrossRef CAS
.
- A. D. Tikoalu, N. A. Lundquist and J. M. Chalker, Adv. Sustainable Syst., 2020, 4, 1900111 CrossRef CAS
.
- C. V. Lopez, C. P. Maladeniya and R. C. Smith, Electrochem, 2020, 1, 226–259 CrossRef
.
- C. V. Lopez, A. D. Smith and R. C. Smith, RSC Adv., 2022, 12, 1535–1542 RSC
.
- C. P. Maladeniya, M. S. Karunarathna, M. K. Lauer, C. V. Lopez, T. Thiounn and R. C. Smith, Mater. Adv., 2020, 1, 1665–1674 RSC
.
- M. S. Karunarathna, M. K. Lauer, A. G. Tennyson and R. C. Smith, Polym. Chem., 2020, 11, 1621–1628 RSC
.
- A. Hoefling, D. T. Nguyen, Y. J. Lee, S.-W. Song and P. Theato, Mater. Chem. Front., 2017, 1, 1818–1822 RSC
.
- M. S. Karunarathna, M. K. Lauer and R. C. Smith, J. Mater. Chem. A, 2020, 8, 20318–20322 RSC
.
- M. K. Lauer, A. G. Tennyson and R. C. Smith, ACS Appl. Polym. Mater., 2020, 2, 3761–3765 CrossRef CAS
.
- Y. Fu, C. Yang, Y. Zheng, J. Jiang, Y. Sun, F. Chen and J. Hu, J. Mol. Liq., 2021, 328, 115420 CrossRef CAS
.
- M. K. Lauer, A. G. Tennyson and R. C. Smith, Mater. Adv., 2021, 2, 2391–2397 RSC
.
- M. K. Lauer, M. S. Karunarathna, A. G. Tennyson and R. C. Smith, Mater. Adv., 2020, 1, 2271–2278 RSC
.
- M. K. Lauer, M. S. Karunarathna, A. G. Tennyson and R. C. Smith, Mater. Adv., 2020, 1, 590–594 RSC
.
- USDA, https://downloads.usda.library.cornell.edu/usda-esmis/files/k3569432s/9306v916d/wm119139b/cropan23.pdf, accessed February 4, 2024.
- M. K. Lauer, Z. E. Sanders, A. D. Smith and R. C. Smith, Mater. Adv., 2021, 2, 7413–7422 RSC
.
- EPA, https://www.epa.gov/sites/default/files/2020-03/documents/final_cd-eol-management_2015_508.pdf, accessed February 4, 2024.
- A. Gupta, M. J. H. Worthington, H. D. Patel, M. R. Johnston, M. Puri and J. M. Chalker, ACS Sustainable Chem. Eng., 2022, 10, 9022–9028 CrossRef CAS
.
- B. Ding, C. Yuan, L. Shen, G. Xu, P. Nie and X. Zhang, Chem.–Eur. J., 2013, 19, 1013–1019 CrossRef CAS PubMed
.
- A. Hoefling, Y. J. Lee and P. Theato, Macromol. Chem. Phys., 2017, 218, 1600303–1600308 CrossRef
.
- J. A. Smith, S. J. Green, S. Petcher, D. J. Parker, B. Zhang, M. J. H. Worthington, X. Wu, C. A. Kelly, T. Baker, C. T. Gibson, J. A. Campbell, M. J. Jenkins, H. Willcock, J. M. Chalker and T. Hasell, Chem.–Eur. J., 2019, 25, 10433–10440 CrossRef CAS PubMed
.
- N. A. Lundquist, A. D. Tikoalu, M. J. H. Worthington, R. Shapter, S. J. Tonkin, F. Stojcevski, M. Mann, C. T. Gibson, J. R. Gascooke, A. Karton, L. C. Henderson, L. J. Esdaile and J. M. Chalker, Chem.–Eur. J., 2020, 26, 10035–10044 CrossRef CAS PubMed
.
- M. J. H. Worthington, R. L. Kucera, I. S. Albuquerque, A. Sibley, A. D. Slattery, J. A. Campbell, S. F. K. Alboaiji, K. A. Muller, J. Young, N. Adamson, J. R. Gascooke, D. Jampaiah, Y. M. Sabri, S. K. Bhargava, S. j. Ippolito, D. A. Lewis, J. S. Quinton, A. V. Ellis, A. Johs, G. J. L. Bernardes and J. M. Chalker, Chem.–Eur. J., 2017, 23, 16219–16230 CrossRef CAS PubMed
.
- J. J. Dale, J. Stanley, R. A. Dop, G. Chronowska-Bojczuk, A. J. Fielding, D. R. Neill and T. Hasell, Eur. Polym. J., 2023, 112198, DOI:10.1016/j.eurpolymj.2023.112198
.
- J. J. Dale, S. Petcher and T. Hasell, ACS Appl. Polym. Mater., 2022, 4, 3169–3173 CrossRef CAS
.
- M. K. Lauer, A. G. Tennyson and R. C. Smith, Mater. Adv., 2022, 3, 4186–4193 RSC
.
- M. K. Lauer, T. A. Estrada-Mendoza, C. D. McMillen, G. Chumanov, A. G. Tennyson and R. C. Smith, Adv. Sustainable Syst., 2019, 3, 1900062 CrossRef CAS
.
- M. S. Karunarathna, C. P. Maladeniya, M. K. Lauer, A. G. Tennyson and R. C. Smith, RSC Adv., 2023, 13, 3234–3240 RSC
.
- C. P. Maladeniya and R. C. Smith, J. Compos. Sci., 2021, 5, 257 CrossRef CAS
.
- T. V. T. Phan, C. Gallardo and J. Mane, Green Chem., 2015, 17, 2846–2852 RSC
.
- S. F. Valle, A. S. Giroto, R. Klaic, G. G. F. Guimaraes and C. Ribeiro, Polym. Degrad. Stab., 2019, 162, 102–105 CrossRef CAS
.
- M. Mann, J. E. Kruger, F. Andari, J. McErlean, J. R. Gascooke, J. A. Smith, M. J. H. Worthington, C. C. C. McKinley, J. A. Campbell, D. A. Lewis, T. Hasell, M. V. Perkins and J. M. Chalker, Org. Biomol. Chem., 2019, 17, 1929–1936 RSC
.
- J. J. Griebel, G. Li, R. S. Glass, K. Char and J. Pyun, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 173–177 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Proton NMR spectral data, FTIR spectra, TGA curves, analysis of char yield versus composition; DSC curves. See DOI: https://doi.org/10.1039/d4su00104d |
| This journal is © The Royal Society of Chemistry 2024 |