Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydroaminomethylation of methyl 10-undecenoate with integrated catalyst recycling via a thermomorphic multiphase system for the continuous production of renewable amines†
Anna
Kampwerth
 ,
Tim B.
Riemer
,
Tim B.
Riemer
 ,
Jonathan
Pöttker-Menke
,
Nadine
Oppenberg
,
Arno M.
Windisch
,
Jonathan
Pöttker-Menke
,
Nadine
Oppenberg
,
Arno M.
Windisch
 ,
Dieter
Vogt
,
Dieter
Vogt
 and
Thomas
Seidensticker
and
Thomas
Seidensticker
 *
*
TU Dortmund University, Department for Biochemical and Chemical Engineering, Laboratory of Industrial Chemistry, Emil-Figge-Straße 66, 44227 Dortmund, Germany. E-mail: Thomas.seidensticker@tu-dortmund.de; Web: https://tc.bci.tu-dortmund.de/ Tel: +49 231 7552310
First published on 29th April 2024
Abstract
A thermomorphic multiphase system (TMS) consisting of methanol and n-dodecane was successfully applied to the hydroaminomethylation (HAM) of the renewable methyl 10-undecenoate. By using different amine substrates, a variety of α,ω-bifunctional products with potential use as intermediates for the polymer industry were obtained with high yields of up to 96%. The full potential of our TMS was shown in a miniplant, with over 90 h of continuous operation showing a stable selectivity towards the desired amine product of 80%. Combining the TMS with an OSN membrane for continuous separation of the by-product water from the reaction system was a key factor for the excellent results. The water concentration in the recycle could be kept below 4% so that only 3% of the undesired aldol condensate was obtained after 90 hours. The low catalyst leaching via the TMS and the OSN membrane is particularly remarkable. The total loss of rhodium over the 90 hours continuous operation is 2.5% of the initial amount used, and for the ligand SulfoXantphos only 0.4% of the initial amount. The loss of rhodium via the TMS and the OSN is 6 ppm per produced desired amine product.
Sustainability spotlightThe industrial production of amines is mainly realised using heterogeneous catalysts under extreme reaction conditions in energy-intensive processes. Homogeneous catalysis, however, is highly active and selective, reducing energy consumption and waste. It offers another great, yet not-reachable, potential in the conversion of renewables, which are usually more functionalised than fossil raw materials and cannot be converted using classic methods. However, recycling the active catalyst complex is challenging. Our approach, the homogeneously catalysed hydroaminomethylation with an integrated catalyst recycling for the continuous production of renewable α,ω-bifunctional products with potential use as intermediates for the polymer industry, we want to pave the way to the sustainable production of amines. The work aligns with the UN sustainable development goal: responsible consumption and production (SDG12),and industry, innovation, and infrastructure (SDG9). |
Introduction
The raw materials used today for intermediate and value-added chemical products are mostly fossil raw materials whose consumption further promotes climate change. In 2022, for example, only 13% of the raw materials used in the chemical industry in Germany came from renewable resources.1 With a clever choice of substrates from renewables, nature's natural synthesis capacity can be utilized, significantly improving the sustainability of the entire process. Fatty acids and their derivatives are particularly interesting as starting materials in the chemical industry, as they contain both a carboxyl function and a long carbon chain, which often is unsaturated, hence C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds are present. These unsaturated compounds are promising for further functionalization, opening up a wide range of products and intermediates based on oleochemicals. The reviews by Biermann et al.2,3 from 2000 and 2021 provide an insight into the great potential of fatty acids and their derivatives. Their importance is also reflected in the fact that their industrial use has increased from 31 to 51 million tonnes per year in the last 10 years.3 Castor oil, which plant (Ricinus communis) is known for its fast growth and easy cultivation in dry and nutrient-poor regions,4 is of particular interest to the chemical industry, as reflected by the numerous reviews of the last years5–7 and the growing annual production.8 Castor oil is so valuable because it has a high content of ricinoleic acid (>85%), which, due to its structure, offers a wide range of applications for the chemical industry, like the production of sebacic (decanedioic) acid and methyl 10-undecenoate (1). The latter is accessible via transesterification of castor oil followed by its pyrolysis at 500 °C producing n-heptanal as a co-product.51 is already industrially used to produce ω-aminoundecanoic acid, which has been used as a monomer by Arkema for 70 years to produce Rilsan, the commercial name of polyamide 11 (PA11).9,10 Polyamides are used in all kinds of technologies, like aerospace, automotive, textiles, and electronics. The importance of PA12, which is additionally used for 3D printing and medical applications, is evident as Evonik has increased its production capacity by more than 50% in 2021.11
C double bonds are present. These unsaturated compounds are promising for further functionalization, opening up a wide range of products and intermediates based on oleochemicals. The reviews by Biermann et al.2,3 from 2000 and 2021 provide an insight into the great potential of fatty acids and their derivatives. Their importance is also reflected in the fact that their industrial use has increased from 31 to 51 million tonnes per year in the last 10 years.3 Castor oil, which plant (Ricinus communis) is known for its fast growth and easy cultivation in dry and nutrient-poor regions,4 is of particular interest to the chemical industry, as reflected by the numerous reviews of the last years5–7 and the growing annual production.8 Castor oil is so valuable because it has a high content of ricinoleic acid (>85%), which, due to its structure, offers a wide range of applications for the chemical industry, like the production of sebacic (decanedioic) acid and methyl 10-undecenoate (1). The latter is accessible via transesterification of castor oil followed by its pyrolysis at 500 °C producing n-heptanal as a co-product.51 is already industrially used to produce ω-aminoundecanoic acid, which has been used as a monomer by Arkema for 70 years to produce Rilsan, the commercial name of polyamide 11 (PA11).9,10 Polyamides are used in all kinds of technologies, like aerospace, automotive, textiles, and electronics. The importance of PA12, which is additionally used for 3D printing and medical applications, is evident as Evonik has increased its production capacity by more than 50% in 2021.11
Methyl 10-undecenoate (1) could also be utilized for the production of the monomer for PA12, 12-aminolauric acid, through highly selective functionalization using homogeneous catalysis. One possible approach is hydroaminomethylation (HAM), which offers an elegant way for producing α,ω bifunctional C12-compounds, with a carboxyl group on one end and an amine function on the other, directly from 1 in one step, as shown in Fig. 1.
 | ||
| Fig. 1 Hydroamination of methyl 10-undecenoate as a possible route for the production of polyamide 12. | ||
Hydroaminomethylation (HAM) is a tandem catalytic reaction, combining hydroformylation and reductive amination with only water as co-product. In this reaction, amines are produced directly from alkenes, whereby the carbon chain is increased by one carbon. The expensive transition metal rhodium is usually used as a homogeneous catalyst and must therefore be recycled from both economic and ecological perspectives. Recyclability is extremely important for a sustainable process, as it ensures that the products are not contaminated with heavy metals, and higher productivity can be achieved by reusing the catalyst. Many concepts have already been developed in which the homogeneous catalyst is immobilized on a solid or in a liquid phase. The liquid–liquid multiphase systems have already been widely applied, including in industry, such as in the Ruhrchemie/Rhône-Poulenc process. The disadvantage of liquid–liquid two-phase systems is the mass transfer limitation between the catalyst in one phase and the reactants in the other phase. Due to this problem, a phase mediator is often needed. For example, co-solvents, such as n-butanol,12 or phase transfer agents and surfactants like cyclodextrins13–15 or hexadecyltrimethylammonium chloride (CTAC)16 were already investigated in the HAM. Another approach to avoid these mass transfer limitations are so-called thermomorphic multiphase systems (TMS).17 These TMS, which were first presented by Bergbreiter et al. 1998,18 typically consist of two commercially available organic solvents, which exploit a temperature-dependent miscibility gap with the products. This allows a homogeneous reaction mixture at reaction conditions and easy separation by decantation after the reaction upon cooling. A general scheme of TMS and a ternary diagram of the two solvents (usually one polar and one non-polar) with the potential reactants is shown in Fig. 2.
In 2019, such a TMS was already developed by our working group for catalyst recycling in the Rh-catalyzed HAM of 1-decene with diethylamine.19 A combination of methanol and n-dodecane in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as a solvent system and a homogeneous Rhodium/SulfoXantphos catalyst was used. With this system, an almost stable process operation over 60 h with an average yield of the desired amine of 61% was achieved. Since water is a by-product of HAM, it must be removed from the process, which was achieved with an organic solvent nanofiltration (OSN) membrane.20 The combined rhodium leaching through TMS and OSN was only 11 mg kg−1 produced product instead of 48 mg kg−1 in the process without the OSN membrane. In comparison, the lowest reported leaching of rhodium in a TMS was achieved in the reductive amination of undecanal with diethylamine by our working group.21 There, only 0.6 mg of rhodium per kg of product produced was leached via the TMS and OSN membrane.
1 ratio as a solvent system and a homogeneous Rhodium/SulfoXantphos catalyst was used. With this system, an almost stable process operation over 60 h with an average yield of the desired amine of 61% was achieved. Since water is a by-product of HAM, it must be removed from the process, which was achieved with an organic solvent nanofiltration (OSN) membrane.20 The combined rhodium leaching through TMS and OSN was only 11 mg kg−1 produced product instead of 48 mg kg−1 in the process without the OSN membrane. In comparison, the lowest reported leaching of rhodium in a TMS was achieved in the reductive amination of undecanal with diethylamine by our working group.21 There, only 0.6 mg of rhodium per kg of product produced was leached via the TMS and OSN membrane.
In the HAM with the TMS, however, only 1-decene was investigated, which only has a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. Bifunctional molecules such as 1 and its derivatives can exhibit a phase-mediating effect, which could be challenging for TMS, especially during separation after the reaction. The product is prone to end up in the polar phase together with the catalyst, or the catalyst could be leached into the non-polar phase. Although no HAM of 1 in a TMS has been investigated so far, the methoxycarbonylation (hydroesterification) of 1 has already been investigated in a TMS consisting of methanol and n-dodecane by our working group in 2016.22 Here, a ratio of methanol to n-dodecane of 1
C double bond. Bifunctional molecules such as 1 and its derivatives can exhibit a phase-mediating effect, which could be challenging for TMS, especially during separation after the reaction. The product is prone to end up in the polar phase together with the catalyst, or the catalyst could be leached into the non-polar phase. Although no HAM of 1 in a TMS has been investigated so far, the methoxycarbonylation (hydroesterification) of 1 has already been investigated in a TMS consisting of methanol and n-dodecane by our working group in 2016.22 Here, a ratio of methanol to n-dodecane of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used for the recycling experiments. This was a compromise between overall activity, catalyst loss, and product extraction. With more methanol, the activity was higher, but only 9% of the formed product was extracted into the non-polar phase. With more n-dodecane, the activity was lower with a yield of only 22%. In addition, only 3 recycling runs with steadily decreasing yields were possible when no catalyst compound was replenished after each run. Only by replenishing methane sulfonic acid, 8 recycling runs were possible with slowly decreasing yields per run. These challenges could also occur in the HAM with 1 due to the bifunctionality of the substrate and in particular the product formed.
1 was used for the recycling experiments. This was a compromise between overall activity, catalyst loss, and product extraction. With more methanol, the activity was higher, but only 9% of the formed product was extracted into the non-polar phase. With more n-dodecane, the activity was lower with a yield of only 22%. In addition, only 3 recycling runs with steadily decreasing yields were possible when no catalyst compound was replenished after each run. Only by replenishing methane sulfonic acid, 8 recycling runs were possible with slowly decreasing yields per run. These challenges could also occur in the HAM with 1 due to the bifunctionality of the substrate and in particular the product formed.
In this work, the TMS consisting of methanol and n-dodecane is applied to the HAM of the renewable methyl 10-undecenoate (1) to generate α,ω bifunctional products with potential use as intermediates for the polymer industry. This involves testing the extensibility of the TMS as a recycling concept for homogeneous catalysts to bifunctional substrates and products. In addition, different amines are to be used, producing a wide range of bifunctional products, and investigating the influence of the different amines on phase separation, as well as on catalyst leaching into the non-polar product phase. To demonstrate the capability of our TMS, this reaction system will also be operated continuously in a miniplant with constant water removal.
Results and discussion
Hydroaminomethylation (HAM) is a complex tandem reaction where multiple reaction steps take place. The reaction network is shown in Fig. 3, describing the HAM with all side reactions. The HAM involves three reaction steps: starting from the alkene (1), hydroformylation to the aldehyde (2) takes place first. Both the linear (l-)aldehyde (l-2) and the branched (b-)aldehyde (b-2) can be formed. This is followed by reductive amination of the aldehydes, which consists of a condensation of the l-2 with the amine substrate (9) to form the l-enamine (l-3) and subsequent reduction of the l-enamine to form the desired l-amine (l-4). The b-aldehyde (b-2) can also react to form the b-amine (b-4) by reductive amination via (b-3). Various side reactions can also occur. 1 can be converted to the alkane (5) by hydrogenation and to the iso-alkene (6) by isomerization. Hydrogenation of 2 leads to the formation of the alcohol (7). In addition, aldol condensates (8) can be formed by the reaction of an aldehyde (2) and an enamine (3) with the elimination of the amine substrate.As the starting point for the investigation, we chose the reaction conditions of the rhodium-catalyzed hydroaminomethylation of 1-decene with diethylamine in the thermomorphic multiphase system consisting of methanol and n-dodecane, previously reported by our group.19 To gain an initial understanding of the effect of the bifunctional function of 1 and the HAM products on the TMS, the reaction conditions were adopted exactly from this previous work. The detailed reaction setup for the batch experiments is shown in Fig. 4. In this initial reaction of the HAM of 1 with diethylamine (9a), a conversion of 96% and an excellent yield of l-4a of 89% was achieved, resulting in a high selectivity of 93%. The leaching for both rhodium and the phosphorus ligand, SulfoXantphos, into the organic product phase, with less than 1%, was also very promising. However, only just under 40% of the product l-4a was extracted in the non-polar n-dodecane phase. The majority of the amine products are present in the polar methanol phase. This could lead to problems in subsequent catalyst recycling. For example, the methanol phase could be further enriched with products after each recycling, which might lead to a reduced polarity difference between the two phases and thus increase the leaching of the catalyst. It is also possible that the products have an inhibiting effect on the catalyst system, reducing the activity after each recycling. Overall, the applicability of the TMS consisting of methanol and n-dodecane to the HAM of 1 with 9a to form the bifunctional product l-4a was successfully demonstrated in this initial reaction.
To prove the general applicability of this TMS, different primary and secondary alkylamines, cyclic and heterocyclic amines as well as amino acids (9a–o) were used in the hydroaminomethylation of 1 to produce various bifunctional amine compounds (Table 1). The amine substrates also have a phase-mediating effect, which can influence the subsequent separation and, thus, the recyclability of the catalyst system. In addition, the HAM itself is also influenced by the nucleophilicity of the amine used.
a Conditions: 48.4 mmol methyl 10-undecenoate (1) (10 w%), 1 eq. amine substrate (9a–g, i–o) (0.5 eq. for 9h), 0.1 mol% Rh(acac)(COD), SulfoXantphos, Rh/P: 2/7, solvent: 86 g methanol/n-dodecane (w/w) 1/1, CO/H2: 1/2, p:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 bar, cont. gassing, T: 125 °C, 700 rpm, TSeparation: 10 °C, tSeparation: 15 min. Yield (Y) and selectivity (S) determined by GC-FID with di-n-butyl ether as internal standard. For not-determined yields, n.d. is indicated. aDimethylammonium-dimethyl carbamate (DimCarb) used as a dimethylamine precursor. bobtained as a white solid after reaction, Y indicated as isolated yield. cQualitatively determined by GC-MS after derivatization using trimethylsulfonium hydroxide (TMSH). 36 bar, cont. gassing, T: 125 °C, 700 rpm, TSeparation: 10 °C, tSeparation: 15 min. Yield (Y) and selectivity (S) determined by GC-FID with di-n-butyl ether as internal standard. For not-determined yields, n.d. is indicated. aDimethylammonium-dimethyl carbamate (DimCarb) used as a dimethylamine precursor. bobtained as a white solid after reaction, Y indicated as isolated yield. cQualitatively determined by GC-MS after derivatization using trimethylsulfonium hydroxide (TMSH).
|
|---|

|
In addition to the HAM with diethylamine (9a) investigated in the initial reaction, with which a yield of l-4a of 89% and selectivity of 93% was achieved, the HAM of 1 was also successfully carried out with other linear dialkyl amines in the methanol/n-dodecane TMS. With dimethylammonium-dimethyl carbamate as precursor for dimethylamine (9b), a yield of 84% to l-4b, with di-n-propylamine (9c) a yield of 94% to l-4c and with di-n-butylamine (9d), 94% to l-4d was reached. With 9c the highest selectivity to l-4 was achieved with 98%. However, no HAM was observed with the branched di-iso-propylamine (9e). The desired linear amines were also obtained with the cyclic amines pyrrolidine (9f) and piperidine (9g), with yields of 91% l-4f and 96% l-4g, respectively. In addition, the bis-HAM of 1 with piperazine (9h) could also be carried out in the TMS. In 2016, this bis-HAM was first investigated by our group.23,24 Both amine groups of 9h react with one methyl 10-undecenoate (1) in the HAM to form the bis-HAM product l-4h. In this TMS, it precipitated as a white solid. By further purification of the solid, an isolated yield of 65% was obtained. With morpholine (9i), a heterocyclic amine was successfully tested in the TMS with a yield of 79% to l-4i. Imidazoline (9j), an aromatic amine, could not be converted in the HAM. Furthermore, amines with a carboxyl group, the amino acids, were also used in the HAM of 1 in the TMS consisting of methanol and n-dodecane. With sarcosine (9k), only traces of the desired product l-4k were determined by GC-MS after derivatization using trimethylsulfonium hydroxide. The HAM of 1 with valine (9l) was successful in the TMS. The product l-4l precipitated as a white solid from the reaction mixture, and after washing with methanol, an isolated yield of 57% was obtained. With proline (9m), no amine products l-4m were detected, only the hydrogenation product 5 and different isomers (6) of methyl 10-undecenoate. The HAM with 9m combined with the simultaneous esterification of 9m has already been investigated, but with methyl oleate as the substrate and a different catalyst system.25 The desired esterified amine product was obtained with a yield of up to 59%. With the two linear primary amines, n-butylamine (9n) and ethylamine (9o), the corresponding product amines l-4n and l-4o were obtained with 48% and 60% yield, respectively.
Overall, the HAM of 1 was successfully carried out in the TMS with a wide variety of amines, resulting in the production of various bifunctional products (l-4a-o). However, since not only the reaction itself is to be investigated here, but above all the applicability of the TMS consisting of methanol and n-dodecane to a broader range of substrates, the separation after the reaction is of particular importance. On the one hand, the product must be extracted into the non-polar n-dodecane phase in order to remove it from the reaction mixture and, on the other hand, the catalyst system must remain immobilized in the methanol phase and must not leach into the non-polar phase.
Fig. 5 shows the product distribution of l-4a between the polar methanol phase and the non-polar n-dodecane phase. However, only the reactions in which the desired product l-4 was formed are displayed.
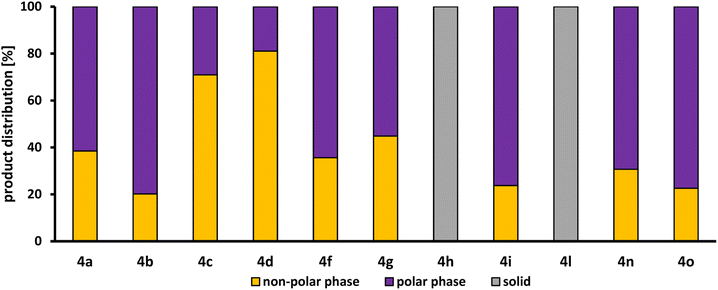 | ||
| Fig. 5 Product distribution of the desired amine product l-4 between the polar methanol phase and the non-polar n-dodecane phase determined by GC-FID (for details, see ESI†). | ||
Both the products of 1 with piperazine (h) and with valine (l) precipitated as a white solid. Only traces of the respective products could be observed in the reaction solution, which is why no product distribution between polar and non-polar phase can be determined here. Only in the HAM reactions, in which di-n-propylamine (9c) and di-n-butylamine (9d) were used as substrates, more than 50% of the product l-4 produced could be found in the n-dodecane phase (71% for l-4c and 81% for l-4d). With the other linear dialkylamines, diethylamine (9a) and dimethylamine (9b), only about 38% and 20% were extracted in the n-dodecane phase. This difference is due to the different lengths of the carbon chain of the alkyl residues. The longer the chain, the lower the polarity of the amine product formed, leading to a better solubility in n-dodecane. This tendency of product distribution can also be observed for the primary alkylamines, n-butylamine (9n) and ethylamine (9o), where 31% and 23% of the respective products l-4n and l-4o present in the n-dodecane phase, as well as for the cyclic amines, pyrrolidine (9f) and piperidine (9g), where 31% and 23% of the respective products l-4f and l-4g are present in the n-dodecane phase. 9g and morpholine (9i) are both a six membered-ring. Due to the oxygen atom in the morpholine, it possesses dipole properties, which is why it is more polar than 9g and has a lower solubility in n-dodecane. Therefore, the corresponding product l-4i is only present in the n-dodecane phase to 24%. All in all, the product distribution is clearly related to the polar character of the amine used and, thus, also to the carbon chain length of the substituents. However, even if less than 45% is extracted into the n-dodecane phase for most products l-4, the product may still be separated sufficiently for a continuous process: the polar phase will be saturated with the product, thereby increasing product extraction in ongoing production.
Since not the entire product is extracted into the n-dodecane phase, this means on the contrary that a lot of the product is present in the methanol phase, in which the catalyst is also immobilized. The products l-4 can, therefore, have a major influence on catalyst leaching to the dodecan phase. Fig. 6 shows the leaching of rhodium and the phosphorus ligand SulfoXantphos into the non-polar n-dodecane for each HAM listed in Table 1.
 | ||
| Fig. 6 Leaching results for rhodium (Rh) and SulfoXantphos (SX). Leaching determined by ICP-OES and calculated as the amount lost with respect to the initial amount of rhodium and phosphorous ligand (For details, see ESI†). | ||
The leaching of rhodium into the non-polar phase for all used amines is under 1% of the initial amount. For SulfoXantphos, it is under 0.13% except for the HAM with 9e and 9o, in which up to 0.6% of the initially used ligand is leached. These leaching results indicate that the amount of product l-4 present in the methanol phase does not have a detrimental influence on the immobilization of the catalyst system. These leaching results are promising for subsequent catalyst recycling.
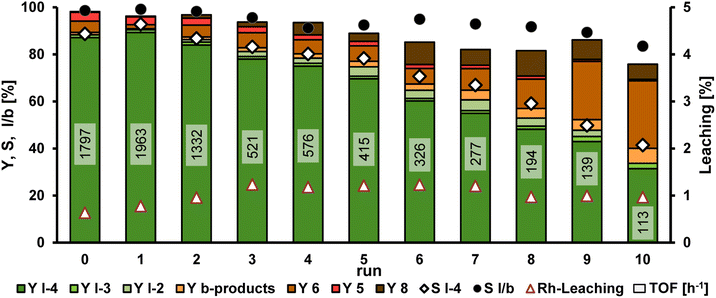
To verify whether recycling of the catalyst in this TMS is possible in the presence of bifunctional products, the next step is to carry out catalyst recycling for the HAM of 1 with 9a (Fig. 7). Detailed information on the setup for the recycling experiments is presented in the ESI.† Diethylamine (9a) was chosen as the model amine, as this allows the recycling to be compared with the previously published recycling of the HAM with 1-decene in this TMS.19 Since the catalyst phase cannot be analyzed during the recycling experiment, the yields and selectivities per recycling run shown in Fig. 7 only refer to the removed n-dodecane phase.
The first run of the recycling experiment of the HAM of 1 with 9a yielded comparable results to the previous reaction (see Table 1). It can be concluded that the yield obtained by analyzing the n-dodecane phase is a good approximation of the total yield. After a slight increase of the yield of the desired amine product l-4a in the first recycling run, the yield decreases steadily from run 2 with 84% until only 31% l-4a is obtained in run 10. The turnover frequency (TOF), indicating the activity of the catalyst system, is reduced to more than half in run 3. In this case, the TOF is determined by the pressure drop in the gas reservoir during the reaction. The obtained pressure curve can be used as an indication of how much substrate 1 has already been converted at which time. While decreasing selectivity towards l-4a, the number of by-products increases significantly. In particular, the formation of the isomeres (6) of methyl 10-undecenoate increases considerably from about 6% in run 2–6 to 29% in the last run. The yield of aldol condensates (8) also increased from run 2, with the yield varying between 6 and 11% in the last runs. Since both aldol condensation and the first step of reductive amination are condensation reactions in which water is formed as a by-product, water is accumulated in the polar methanol phase during the recycling. The calculated amount of accumulated water after the respective runs is shown in Table 2. A total of 7.3 g of water was produced over the entire recycling experiments, which corresponds to 6.8% by weight in the reaction mixture. As the condensation reaction of the aldehyde l-2a with the amine substrate to form the enamine l-3a is a reversible reaction, the accumulated amount of water shifts the equilibrium in favor of the aldehyde over the course of the recycling experiment. On the other hand, aldol condensation is not an equilibrium reaction (at least under the here present conditions). Therefore, the accumulation of water may be a reason for the decreasing yield of the desired amine l-4a and the increasing yield of aldol condensate 8 from run 4, where 3.8 wt% of water is already present in the reaction mixture. In the previous work on the HAM of 1-decene with diethylamine in TMS consisting of methanol and n-dodecane, it was also shown that the formation of by-products, especially aldol condensates, increases with increasing water content in the reaction mixture.19 In addition to the calculated amounts of accumulated water, the leaching results for rhodium and Sulfoxantphos are also given in Table 2. The leaching of rhodium is already higher from the first recycling run compared to the initial run with 0.64%, and increases to 1.23% by run 3. In total, 11.37% of the initially used amount of rhodium leaches from the catalyst phase into the n-dodecane phase over all recycling experiments. The leaching of the phosphorus ligand is also in a similar range with 12.18% lost with respect to the initial amount of ligand. The reduced concentration of the catalyst system due to leaching can also have a negative effect on the selectivity of the reaction and the TOF. However, the TOF has already dropped significantly in run 3, which suggests that the decreasing reaction performance is not only due to leaching of the catalyst system.
| Run | Water accumulationa [wt%] | Leaching into the n-dodecane phase | |||
|---|---|---|---|---|---|
| Rhb [ppm] | SXb [ppm] | Rhc [%] | SXc [%] | ||
| a Calculated water accumulation within the different recycling runs. b Measured concentration of rhodium or phosphorus by inductively coupled plasma-optical emission spectrometry (ICP-OES). c Calculated leaching as the amount lost with respect to the initial amount of rhodium or phosphorus ligand. | |||||
| 0 | 0.8 | 1 | 2 | 0.64 | 0.83 |
| 1 | 1.6 | 1 | 2 | 0.78 | 0.99 |
| 2 | 2.4 | 1 | 3 | 0.96 | 1.52 |
| 3 | 3.1 | 1 | 1 | 1.24 | 0.44 |
| 4 | 3.8 | 1 | 1 | 1.18 | 0.83 |
| 5 | 4.4 | 1 | 2 | 1.21 | 1.14 |
| 6 | 5.0 | 1 | 4 | 1.23 | 1.91 |
| 7 | 5.6 | 1 | 1 | 1.20 | 0.47 |
| 8 | 6.1 | 1 | 2 | 0.97 | 1.15 |
| 9 | 6.5 | 1 | 2 | 1.00 | 0.97 |
| 10 | 6.8 | 1 | 4 | 0.97 | 1.94 |
| Total | 11.37 | 12.18 | |||
The pressure drop in the gas reservoir allows the course of the reaction to be easily observed over time. The reaction time needed is increasing with each recycling run. The pressure curves of runs 0, 4, 6 and 8 can be found in the ESI.† It is evident that the time required to observe an initial pressure loss in the gas reservoir increases with each run. In the initial run, gas was consumed directly from the beginning, whereas in Run 8 the pressure only decreased 45 minutes after the start of the reaction. This indicates that the catalyst system was not in its active form at the start of the reaction. One possible reason for the inactive catalyst form could be the change in gas atmosphere during the separation. The separation of the two phases after the reaction is carried out under an argon atmosphere in a separating funnel made of glass. Changing the gas atmosphere can also change the active catalyst species. Hydroformylation normally requires an active rhodium hydride species. Due to the absence of hydrogen and/or carbon monoxide, the catalyst system could now be present in an inactive form, which then has to be reactivated. However, isomerization of methyl 10-undecenoate (1) can still occur during this time, as both hydrogen and carbon monoxide are not required for this. This may, therefore, be a possible explanation for the increasing amount of isomers (6).
Overall, the recycling of the catalyst system in the TMS consisting of methanol and n-dodecane for the HAM of 1 with 9a was achieved for the first time with 10 runs and a total turnover number (TTON) of almost 8.000.
In the next step, the reaction system is tested in a continuous setup. The miniplant used for continuous experiments is equipped with an organic solvent nanofiltration (OSN) membrane to get rid of the water produced by the condensation reactions. Furthermore, with this miniplant, it is possible to perform the separation without changing the gas atmosphere, which could prevent the possible alteration of the catalyst system. The flow scheme of the miniplant is shown in Fig. 8.
 | ||
| Fig. 8 Flow scheme of the miniplant for the continuous operated HAM of methyl 10-undecenoate (1) with diethylamine (9a) in the TMS consisting of methanol and n-dodecane. | ||
The miniplant consists of a continuous stirred-tank reactor (CSTR, B1), a decanter (B2), and a buffer tank (B3) connected with a membrane (M1). The substrates 1 and 9a, as well as n-dodecane, are fed to the CSTR. Hydrogen and carbon monoxide are fed via two mass flow controllers. The monophasic reaction mixture at the higher temperature, indicated in green, flows from CSTR B1 to the decanter B2via a riser tube. Here, the reaction mixture is cooled to 5 °C, forming two phases. The n-dodecane phase, indicated as yellow, leaves B2 and is depressurized and stored in a product container. The methanol phase (blue), in which the catalyst is immobilized, flows from B2 to B3. Here, the water is removed via an OSN membrane (NanoPro™ S-3012). Methanol has a similar polar character as water, and, therefore, partially leaves the process via the membrane and thus is replenished by a continuous methanol makeup stream fed to B3. This membrane setup has already successfully removed water to prevent accumulation and ensures the retention of the valuable catalyst.20,21 The recycle stream from B3 is pumped back to the CSTR and supplemented with the substrates and n-dodecane to ensure the desired residence time in B1.
The results of the continuously operated hydroaminomethylation of methyl 10-undecenoate (1) with diethylamine (9a) in the thermomorphic multiphase system consisting of methanol and n-dodecane are given in Fig. 9.
In the miniplant, continuous operation was achieved for over 90 hours with an average yield of l-4a of about 70%. After 33 h, the first stable operating point was reached with a yield of l-4a of 74%, which corresponds to a selectivity of 80%. Here, the main by-products identified were the hydrogenation product 5 and the isomerization product 6. To improve the space-time yield, the residence time in the reactor was then reduced from 4 to 2 hours. A stable operation point was reached again after about 25 h. By halving the residence time, the yield of l-4a was reduced by only 7% to 67%. Although the selectivity almost remained constant at 79%, it should be noted that the yields of the undesired by-products 5 and 6 were significantly reduced, whereas the yields of the intermediate products l-2a and l-3a increased compared to the results with a residence time of 4 h. In the end, the ratio of carbon monoxide to hydrogen was changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The change aimed to reduce the formation of hydrogenation product 5 in order to increase the selectivity to the desired amine product l-4a. Once again, a stable operating point was achieved. The yield of 5 was reduced from 4% to 2%. However, the selectivity towards l-4a only slightly increased back to 80%. This minor change is due to the increasing formation of aldol condensate 8. The yield of 8 has been increasing slowly and continuously since the start of the experiment and is at 3.3% after 90 hours of operation. Overall, stable operation of the HAM of 1 with 9a was demonstrated in the investigated TMS consisting of methanol and n-dodecane over 90 hours, with no evidence of a loss of catalytic activity. The ratio of linear to branched (l/b) products also remained constant at 98% over the entire operation, except for the first 30 hours.
1. The change aimed to reduce the formation of hydrogenation product 5 in order to increase the selectivity to the desired amine product l-4a. Once again, a stable operating point was achieved. The yield of 5 was reduced from 4% to 2%. However, the selectivity towards l-4a only slightly increased back to 80%. This minor change is due to the increasing formation of aldol condensate 8. The yield of 8 has been increasing slowly and continuously since the start of the experiment and is at 3.3% after 90 hours of operation. Overall, stable operation of the HAM of 1 with 9a was demonstrated in the investigated TMS consisting of methanol and n-dodecane over 90 hours, with no evidence of a loss of catalytic activity. The ratio of linear to branched (l/b) products also remained constant at 98% over the entire operation, except for the first 30 hours.
Fig. 10 shows the water concentration in the recycle and the permeate streams of the miniplant as well as the membrane rejection of the catalyst system over time. In addition, the water concentration in the recycle without the membrane was calculated from the water theoretically produced in the reaction. More information about membrane performance, like the membrane flux and the rejection of the products, can be found in the ESI.†
After the first stable operating point was reached, the pump that circulates the catalyst phase from the buffer tank B3 to the membrane was switched on. So, the membrane only began to run after approx. 35 h. With the help of the membrane, the water concentration can be kept below 4% for the entire operation. Without the membrane, a concentration above 5% would have been reached after the 90 h. The membrane rejection of rhodium and the ligand SulfoXantphos is over 99% during the entire operating time. In total, only 0.3% of the initially used amount of rhodium and SulfoXantphos was washed out of the process via the membrane (Table 3). The OSN was, therefore, able to successfully remove the water from the process without losing the valuable catalyst system via the membrane.
| Spezies | Initial mass [mg] | Loss totala [%] | Loss total [mg] | Loss per houra [%/h] | Loss totala [%] | Loss per product [mg kg−1] | Loss per product [mg kg−1] | |
|---|---|---|---|---|---|---|---|---|
| a Percentage losses refer to the initial mass of Rh and SX in the miniplant. | ||||||||
| Rh | 176 | 2.43 | TMS | 3.80 | 0.024 | 2.2 | 5.3 | 6.0 |
| OSN | 0.49 | 0.005 | 0.3 | 0.7 | ||||
| SX | 4687 | 0.39 | TMS | 6.37 | 0.001 | 0.1 | 9.0 | 25.6 |
| OSN | 11.85 | 0.005 | 0.3 | 16.7 | ||||
Of course, the membrane is not the only way in which the expensive catalyst system can be removed from the process. During the separation of the methanol and n-dodecane phases in the decanter, the catalyst can also leach into the n-dodecane phase and thus leave the process. The amount of catalyst lost with the organic product phase and the membrane is listed in Table 3. The ligand SulfoXantphos only leached to 0.1% of the initial used amount into the non-polar n-dodecane phase. The total loss of rhodium via the TMS is 2.2% of the initially used amount. This means that only 0.024% of the catalyst was leached per hour. Overall, the loss of rhodium via the TMS and the OSN is 6 ppm per produced l-4a (mleached Rh/mproducedl-4a). In comparison, a rhodium loss of 11 ppm per produced product was achieved in the previously published HAM of 1-decene with 9a in the miniplant setup with an OSN membrane and a loss of 48 ppm without the OSN membrane. Thus, the leaching could be reduced by a factor of 2 for the HAM with 1 in this miniplant with an OSN membrane for water removal.
Conclusion
In this work, the TMS consisting of methanol and n-dodecane was successfully applied to the hydroaminomethylation (HAM) of the renewable methyl 10-undecenoate (1). By using different amine substrates, a variety of α,ω-bifunctional products with potential use as intermediates for the polymer industry could be obtained with high yields of up to 96%. The extensibility of the TMS as a recycling concept for homogeneous catalysts was tested for these bifunctional substrates and products. First, the influence of the various produced amines on the phase separation and on the leaching of the catalyst into the non-polar product phase was investigated. Interestingly, no significant influence on the leaching of the catalyst system was observed, although a large quantity of the amine products produced remain in the methanolic catalyst phase after separation. Based on these promising results, an initial recycling was then carried out in a batch setup. In this first recycling of the HAM of 1 with diethylamine (9a) a total turnover number (TON) of almost 8.000 was achieved within 10 runs. To demonstrate the full potential of our TMS, the reaction system was finally operated continuously in a miniplant. Continuous operation over 90 h with a stable selectivity towards the desired amine product l-4a of 80% was successfully achieved. By using an OSN membrane, the water concentration in the recycle could be kept below 4%, so that only 3% aldol condensate was obtained after 90 hours. The low catalyst leaching via the TMS and the OSN membrane is particularly remarkable. The total loss of rhodium over the 90 hours continuous operation is 2.5% of the initial amount used, corresponding to 0.029% per hour. For the ligand SulfoXantphos it is only 0.4% of the initial amount. Overall, the loss of rhodium via the TMS and the OSN is 6 ppm per produced amine product l-4a. Thus, the TMS consisting of methanol and n-dodecane as a recycling strategy in homogeneous catalyzed HAM proved to be flexible in the use of a wide variety of bifunctional products and achieved in combination with an OSN mebrane to separate the water produced during the reaction excellent results in the first miniplant experiment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Gefördert durch die Deutsche Forschungsgemeinschaft (DFG)–TRR 63 “Integrierte chemische Prozesse in flüssigen Mehrphasensystemen”(Teilprojekt A11) – 56091768 – Funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)–TRR 63 “Integrated Chemical Processes in Liqiud Mulitphase Systems” (subprojects A11) – 56091768. We would also like to thank the German Federal Ministry of Food and Agriculture (Bundesministerium für Ernährung und Landwirtschaft), represented by the FNR (Fachagentur Nachwachsende Rohstoffe) for the financial support of the junior research group ‘Renewlysis’ (project number 2219NR355).References
- Daten und Fakten - Energiestatistik, Verband der Chemischen Industrie e.V., 2023 Search PubMed.
- U. Biermann, W. Friedt, S. Lang, W. Lühs, G. Machmüller, J. O. Metzger, M. R. g Klaas, H. J. Schäfer and M. P. Schneider, New Syntheses with Oils and Fats as Renewable Raw Materials for the Chemical Industry, Angew. Chem., Int. Ed. Engl., 2000, 39, 2206–2224 CrossRef CAS PubMed.
- U. Biermann, U. T. Bornscheuer, I. Feussner, M. A. R. Meier and J. O. Metzger, Fatty Acids and their Derivatives as Renewable Platform Molecules for the Chemical Industry, Angew. Chem., Int. Ed. Engl., 2021, 60, 20144–20165 CrossRef CAS PubMed.
- K. Bauddh, K. Singh, B. Singh and R. P. Singh, Ricinus communis: A robust plant for bio-energy and phytoremediation of toxic metals from contaminated soil, Ecol. Eng., 2015, 84, 640–652 CrossRef.
- D. S. Ogunniyi, Castor oil: a vital industrial raw material, Bioresour. Technol., 2006, 97, 1086–1091 CrossRef CAS PubMed.
- H. Mutlu and M. A. R. Meier, Castor oil as a renewable resource for the chemical industry, Eur. J. Lipid Sci. Technol., 2010, 112, 10–30 CrossRef CAS.
- V. R. Patel, G. G. Dumancas, L. C. Kasi Viswanath, R. Maples and B. J. J. Subong, Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production, Lipid Insights, 2016, 9, 1–12 CrossRef PubMed.
- The Food and Agriculture Organization of the United Nations (FAO), FAOSTAT. Crops and livestock products, 2022 Search PubMed.
- M. van der Steen and C. V. Stevens, Undecylenic acid: a valuable and physiologically active renewable building block from castor oil, ChemSusChem, 2009, 2, 692–713 CrossRef CAS PubMed.
- K. Weissermel, Industrial Organic Chemistry, VCH, Weinheim, New York, 1997 Search PubMed.
- Evonik Industries AG, Meilenstein in Marl: Ministerpräsident Armin Laschet weiht weltgrößte Polyamid-12-Anlage ein, 2021, https://corporate.evonik.com/de/presse/pressemitteilungen/corporate/meilenstein-in-marl-ministerpraesident-armin-laschet-weiht-weltgroesste-polyamid-12-anlage-ein-160600.html Search PubMed.
- T. A. Faßbach, F. O. Sommer and A. J. Vorholt, Hydroaminomethylation in Aqueous Solvent Systems - An Efficient Pathway to Highly Functionalized Amines, Adv. Synth. Catal., 2018, 360, 1473–1482 CrossRef.
- K. U. Künnemann, D. Weber, C. Becquet, S. Tilloy, E. Monflier, T. Seidensticker and D. Vogt, Aqueous Biphasic Hydroaminomethylation Enabled by Methylated Cyclodextrins: Sensitivity Analysis for Transfer into a Continuous Process, ACS Sustainable Chem. Eng., 2021, 9, 273–283 CrossRef.
- T. Roth, R. Evertz, N. Kopplin, S. Tilloy, E. Monflier, D. Vogt and T. Seidensticker, Continuous production of amines directly from alkenes via cyclodextrin-mediated hydroaminomethylation using only water as the solvent, Green Chem., 2023, 25, 3680–3691 RSC.
- S. A. Jagtap, S. P. Gowalkar, E. Monflier, A. Ponchel and B. M. Bhanage, Rhodium catalyzed selective hydroaminomethylation of biorenewable eugenol under aqueous biphasic condition, Mol. Catal., 2018, 452, 108–116 CrossRef CAS.
- A. Behr and A. Wintzer, Hydroaminomethylation of the Renewable Limonene with Ammonia in an Aqueous Biphasic Solvent System, Chem. Eng. Technol., 2015, 38, 2299–2304 CrossRef CAS.
- J. Bianga, K. U. Künnemann, T. Gaide, A. J. Vorholt, T. Seidensticker, J. M. Dreimann and D. Vogt, Thermomorphic Multiphase Systems: Switchable Solvent Mixtures for the Recovery of Homogeneous Catalysts in Batch and Flow Processes, Chem.–Eur. J., 2019, 25, 11586–11608 CrossRef CAS PubMed.
- D. E. Bergbreiter, Y.-S. Liu and P. L. Osburn, Thermomorphic Rhodium(I) and Palladium(0) Catalysts, J. Am. Chem. Soc., 1998, 120, 4250–4251 CrossRef CAS.
- J. Bianga, K. U. Künnemann, L. Goclik, L. Schurm, D. Vogt and T. Seidensticker, Tandem Catalytic Amine Synthesis from Alkenes in Continuous Flow Enabled by Integrated Catalyst Recycling, ACS Catal., 2020, 10, 6463–6472 CrossRef CAS.
- S. Schlüter, K. U. Künnemann, M. Freis, T. Roth, D. Vogt, J. M. Dreimann and M. Skiborowski, Continuous co-product separation by organic solvent nanofiltration for the hydroaminomethylation in a thermomorphic multiphase system, Chem. Eng. J., 2021, 409, 128219 CrossRef.
- T. B. Riemer, P. Lapac, D. Vogt and T. Seidensticker, Stable and Continuous Production of Amines via Reductive Amination in a Green Switchable Solvent System with Efficient Water Removal, ACS Sustainable Chem. Eng., 2023, 11, 12959–12966 CrossRef CAS.
- T. Gaide, A. Behr, A. Arns, F. Benski and A. J. Vorholt, Hydroesterification of methyl 10-undecenoate in thermomorphic multicomponent solvent systems—Process development for the synthesis of sustainable polymer precursors, Chem. Eng. Process., 2016, 99, 197–204 CrossRef CAS.
- T. Seidensticker, H. Busch, C. Diederichs, J. J. von Dincklage and A. J. Vorholt, From Oleo Chemicals to Polymer: Bis -hydroaminomethylation as a Tool for the Preparation of a Synthetic Polymer from Renewables, ChemCatChem, 2016, 8, 2890–2893 CrossRef CAS.
- T. Seidensticker, J. M. Vosberg, K. A. Ostrowski and A. J. Vorholt, Rhodium-Catalyzed Bis- Hydroaminomethylation of Linear Aliphatic Alkenes with Piperazine, Adv. Synth. Catal., 2016, 358, 610–621 CrossRef CAS.
- A. Behr, T. Seidensticker and A. J. Vorholt, Diester monomers from methyl oleate and proline via tandem hydroaminomethylation-esterification sequence with homogeneous catalyst recycling using TMS-technique, Eur. J. Lipid Sci. Technol., 2014, 116, 477–485 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00109e |
| This journal is © The Royal Society of Chemistry 2024 |