Calcium hydride with aluminium for thermochemical energy storage applications†
Lucie
Desage‡
 ,
Terry D.
Humphries‡
,
Terry D.
Humphries‡
 *,
Mark
Paskevicius
*,
Mark
Paskevicius
 and
Craig. E.
Buckley
and
Craig. E.
Buckley

Physics and Astronomy, Institute for Energy Transition, Curtin University, GPO Box U1987, Perth, WA 6845, Australia. E-mail: terry_humphries81@hotmail.com
First published on 27th November 2023
Abstract
Thermochemical energy storage has the potential to unlock large-scale storage of renewable energy sources by integrating with power production facilities. Metal hydrides have high thermochemical energy storage densities through reversible hydrogenation. Particularly, calcium hydride presents remarkable properties to integrate with high-temperature systems. The addition of aluminium to calcium hydride enables lower operating temperatures below 700 °C. The CaH2–2Al system reacts through a two-step reaction mechanism, which was verified via in situ powder diffraction analysis. The thermodynamics of dehydrogenation have been determined for both dehydrogenation steps with step 1 having a ΔHdes = 79 ± 3 kJ mol−1 and ΔSdes = 113 ± 4 J mol−1 K−1, while step 2 has a ΔHdes = 99 ± 4 kJ mol−1 and ΔSdes = 128 ± 5 J mol−1 K−1. The reaction kinetics for both steps were determined using the Kissinger method from DSC-TGA data to be 138 ± 12 kJ mol−1 and 98 ± 8 kJ mol−1 for step 1 and 2, respectively. Reversible hydrogenation over step 2, for 66 cycles at 670 °C under 20 bar of H2, determined the sorption capacity to be stable at 91% of the theoretical maximum of 1.1 wt% H2. A materials-based cost analysis evaluates the system at 9.2 US$ per kW hth, with an energy density of 1031 kJ kg−1.
Introduction
The global energy landscape must rapidly transition from fossil fuels to alternative systems to reduce the emission of greenhouse gases.1 Energy storage is key to ensure the decarbonisation of energy production while keeping up with energy demand. The diversification of energy storage systems is necessary to enable the efficient utilisation of renewable resources. For example, thermal energy storage (TES) is appropriate to integrate concentrated solar power (CSP) plants.2 Particularly, recent TES studies show that thermochemical energy storage (TCES) materials have the potential to be implemented into thermochemical batteries (TCB) providing energy storage solutions for power production facilities.3Metal hydrides have been reported to be suitable for diverse applications including fuel cells, hydrogen storage, and solid state batteries but they also show exceptional properties for TCES, with volumetric energy storage densities up to 2423 kW hth m−3 and operating temperatures ranging from 200 to 1000 °C.4–6 A recent paper by Adams et al. highlighted the advantages of metal hydrides for TCES, and addressed the challenges related to their implementation in large-scale systems in terms of reaction modelling, reactor material selection and design.7 Hardy et al. modelled a high-temperature TCES system operating with a metal hydride pair for solar energy storage; of which the outcomes confirmed that appropriate operating conditions and sizing are crucial to ensure the viability of the system and avoid failure due to thermal ratcheting.8 Feng et al. proposed an optimal reactor design methodology with heat transfer fluid flowing through a regular helical tube placed in the reactor bed powder to optimise energy storage using a metal hydride.9 Experimental TCB prototypes using MgH2 or Mg2FeH6 have been investigated at medium temperatures below 500 °C.10–14 For example, Poupin et al. built a prototype using 900 g of magnesium iron hydride and a coil heat exchanger flowing supercritical water; the cycling showed promising results at ∼450 °C with an energy storage capacity reaching 87% of the theoretical maximum and a heat extraction efficiency of 80%.12 Recently, the CaH2–MgH2–ZrCl4 material developed by Singh et al. showed reversible hydrogenation below 400 °C with improved kinetics, which could be used for medium temperature TCES.15 Although there have been some excellent advances in this field, the development of metal hydrides that operate at ≈700 °C is required to enable efficient heat-to-power engines to be used at their optimal operating temperature.16
The understanding of the intrinsic physical properties of metal hydrides is crucial to evaluate their potential to integrate TCES system.17 Recently, Balakrishnan et al. updated the thermodynamics and kinetics of pure calcium hydride at high temperature in the molten and solid state.18 Calcium hydride is an appealing material because of its high energy storage density (5131 kJ kg−1), but the decomposition temperature of above 900 °C and the sluggish reaction kinetics hinder the utilisation of pure CaH2 for technological applications.18 In the past decades, several studies looked at the thermodynamic destabilisation of CaH2 with additives to reduce the operating temperature and enhance the reaction kinetics.19 The CaH2–11Zn system was determined to operate at 1 bar of H2 and ∼600 °C, where cyclic reversibility of the first reaction step over 10 cycles resulted in the loss of 0.02 wt% sorption of H2.20 The addition of Si to CaH2 was shown to cause decomposition via multistep reactions forming CaxSiy compounds with reversibility at 700–800 °C and hydrogen partial pressure of 1–5 bar, which overall, showed potential for TCES applications.21 Sofianos et al. destabilised CaH2 with calcium halides.22 The resulting operating temperature (650 °C) and pressure (1–10 bar) are suitable for TCES for CSP; however, the particle sintering and slow reaction kinetics are important issues compromising the viability of these systems for application.22 Similarly, the addition of Al2O3 to CaH2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) showed sintering resulting in poor cycling capacity, but reducing the initial amount of additives may promote the sorption stability of this system operating at 636 °C and 1 bar of H2.23
1 molar ratio) showed sintering resulting in poor cycling capacity, but reducing the initial amount of additives may promote the sorption stability of this system operating at 636 °C and 1 bar of H2.23
In 1981, Veleckis investigated Ca–Al alloys and reported the formation of CaAl4 and CaAl2 in a two-step reaction process (eqn (1) and (2)), along with the conclusion that the system has potential as a high temperature hydrogen storage medium due to its reversible hydrogenation capabilities at 450–650 °C.24 An absorption enthalpy of −83.1 kJ mol−1 H2 was also determined for the reaction of CaAl2 with H2, giving a theoretical energy density of 865 kJ kg−1 CaAl2. More recently, Ward et al. studied the reversible hydrogenation of the CaAl2 system at 600 °C and 25 bar H2 over 100 cycles.25 In this case cycling was carried out following eqn (3), which has a theoretical H2 capacity of 2.1 wt%. The cycling experiment only showed a slight capacity decrease of 3% from 1.9 to 1.85 wt% H2. The thermal conductivity of CaAl2 was also measured and increases from 6.8 W m−1 K−1 at 80 °C to 20.3 W m−1 K−1 at 600 °C.25
| CaH2 + 4Al ⇌ CaAl4 + H2 | (1) |
| CaH2 + CaAl4 ⇌ 2CaAl2 + H2 | (2) |
| CaH2 + 2Al ⇌ CaAl2 + H2 | (3) |
Previous studies have shown the CaH2–Al system to be a promising TCES material due to its cyclability and reasonable cost of $14.9 USD per kW hth.20,24,25 Further work is required to determine the physical attributes of this material and determine whether cycling is achievable at higher temperatures (>650 °C). This study will determine the thermodynamics and activation energies for both steps of hydrogen desorption of the CaH2–2Al system, while also ascertaining the exact decomposition pathway using X-ray diffraction techniques. Overall, this allows the determination of the operational conditions for cycling and a direct cost evaluation to be undertaken.
Experimental section
Sample preparation
All manipulations of chemicals were undertaken in an argon atmosphere using an Mbraun Unilab glovebox to prevent air exposure and to minimise oxygen (O2 < 1 ppm) and water (H2O < 1 ppm) contamination. CaH2 + 2Al samples were prepared by ball milling (BM) CaH2 (Sigma-Aldrich, >95%) and Al (Alfa Aesar, −100 + 325 mesh, >99.5% metals basis) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio at room temperature in a planetary ball mill (Fritsch Pulverisette 6, Germany). The powders were milled at 400 rpm for 6 h (15 min milling with 5 min break, bidirectional) with a ball-to-powder mass ratio of 30
2 ratio at room temperature in a planetary ball mill (Fritsch Pulverisette 6, Germany). The powders were milled at 400 rpm for 6 h (15 min milling with 5 min break, bidirectional) with a ball-to-powder mass ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using 316 stainless steel vial (80 mL) and balls (10 mm in diameter) under an Ar atmosphere.
1 using 316 stainless steel vial (80 mL) and balls (10 mm in diameter) under an Ar atmosphere.
Powder X-ray diffraction
In-house powder X-ray diffraction (XRD) was performed using a Bruker D8 Discovery diffractometer (Co Kα radiation) utilising XRD sample holders covered with a poly(methylmethacrylate) (PMMA) airtight dome to prevent oxygen/moisture contamination during data collection. The PMMA airtight dome results in a broad hump in XRD patterns centred at ∼23° 2θ. Data were acquired over a 2θ range of 10–90°, with a step size of 0.03° and count time of 1.8 s per step. Synchrotron powder X-ray diffraction (SR-XRD) was performed at the Australian Synchrotron in Melbourne, Australia.26 Powder samples were loaded in quartz capillaries (outer diameter 0.7 mm, wall thickness 0.01 mm) while inside a glove box, and mounted using Swagelok tube fittings to a vacuum manifold and measured under dynamic vacuum. One-dimensional SR-XRD patterns (monochromatic X-rays with λ = 0.825040(5) Å) were continuously collected using a Mythen microstrip detector with an exposure time of 30 s at two different detector positions in order to cover the entire 2θ range (3–80°).27 The capillary was continuously oscillated through 100° angle during data collection to improve the powder averaging and ensure even heating. The capillary caused a broad amorphous hump between 2θ = 10 and 15° that is visible throughout the in situ experiment. Diffraction patterns were quantitatively analysed with the Rietveld method using TOPAS (Bruker-AXS).28Thermal analysis
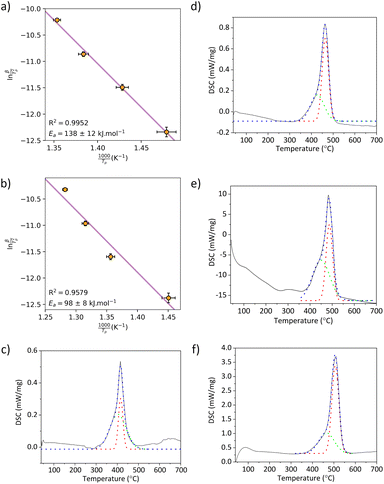
The hydrogen sorption properties of the CaH2–2Al mixture were measured with a computer controlled Sieverts/volumetric apparatus previously described elsewhere.29 Pressure Composition Isotherms (PCI) were collected between 520 and 591 °C on a sample weighing 1.7085 g in a system volume of 78.07 cm3. Each equilibrium data point was conducted with an initial wait time of 2 h followed by a check to ensure an increment of <0.008 wt% H2 was not exceeded over the following 2 h, ensuring hydrogen desorption/absorption had ceased. Subsequent equilibrium data points were then collected after a 3 bar pressure decrement (reference volume pressure) was applied. Sample temperature and pressure were recorded every 30 s using a K-type thermocouple (uncertainty of ±1.5 °C) inserted directly into the sample and a digital pressure transducer (Rosemount 3051S) with a 0.035% span accuracy (200 bar span). Powder samples were loaded into a SiC sample cell (Saint-Gobain) to prevent hydrogen permeability at high temperature.30 The sample cell was then placed under ∼25 bar H2 back pressure to inhibit decomposition and heated at a ramp rate of 10 °C min−1 to the desired temperature before each PCI was initiated. Hydrogen cycling experiments were carried out using the same experimental setup as for the PCI experiments. In this case, the ball milled powder (0.4376 g) was heated to 670 °C under vacuum for the purpose of commencing the experiment in a desorbed state. Absorption was then undertaken at 20 bar for 2.3 h, while desorption was carried out in vacuo for 1 h. A total of 66 cycles were measured.Simultaneous Differential Scanning Calorimetry (DSC) was performed with a Netzsch STA 449 F3 Jupiter analyser equipped with a Pt furnace. Samples were measured under an argon flow of 40 mL min−1 using Pt/Rh crucibles with Al2O3 liners containing approximately 15 mg of sample, loaded under argon and sealed with lids possessing a pin-hole to allow for gas release. The furnace containing the sample was evacuated prior to being placed under an argon flow to remove traces of air. The analysis was conducted by heating from 40–800 °C at 2, 5, 10 and 20 °C min−1. The temperature and sensitivity of the DSC was calibrated using In, Bi, Al, Ag and Au reference materials, resulting in a temperature accuracy of ±0.2 °C, while the balance has an accuracy of ±20 μg. The DSC signal data was processed using a Gaussian deconvolution fitting model available in the OriginPro software. The Kissinger method was applied to determine the activation energy Ea for hydrogen desorption using the peak temperature Tp of the DSC signal at the different heating rates β.31 Equation (eqn (4)) was used to generate the Kissinger plot:32
 | (4) |
The activation energy was calculated by multiplying the slope of the resulting trendline by the universal gas constant R (8.314 J K−1 mol−1).33
Results and discussion
SR-XRD analysis of the milled powder (Fig. 1a) indicated that only CaH2 (45.7(1) wt%) and Al (54.3(1) wt%) were present in the sample with no oxide contamination. This ensures that no reaction occurred during milling and the mixture is in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 CaH2
1.9 CaH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al. From these stoichiometries, a total practical H2 capacity of 2.02 wt% for this prepared material is feasible compared to the theoretical capacity of 2.1 wt% H2. During the in situ SR-XRD analysis, the sample was heated under dynamic vacuum from room temperature to 750 °C (Fig. 1b and c). No crystallographic changes occurred in the sample until ∼355 °C when the Bragg reflections of CaH2 and Al began to diminish and CaAl4 was observed. The quantity of CaAl4 reached a maximum at 535 °C with 60 wt% being present in the sample, along with 22.5 wt% CaH2 and 8 wt% Al remaining. The residual 9.5 wt% was CaAl2, which was first observed at ∼480 °C. Elemental Al was no longer observed after 560 °C, while all CaH2 was depleted by 610 °C. The Al was consumed at a greater rate than CaH2 to facilitate the formation of the Al-rich CaAl4 phase. The CaAl4 was completely consumed by 700 °C leaving only CaAl2. During the decomposition process, Ca or CaO were not detected. Therefore, the reaction pathway can be expressed as:
Al. From these stoichiometries, a total practical H2 capacity of 2.02 wt% for this prepared material is feasible compared to the theoretical capacity of 2.1 wt% H2. During the in situ SR-XRD analysis, the sample was heated under dynamic vacuum from room temperature to 750 °C (Fig. 1b and c). No crystallographic changes occurred in the sample until ∼355 °C when the Bragg reflections of CaH2 and Al began to diminish and CaAl4 was observed. The quantity of CaAl4 reached a maximum at 535 °C with 60 wt% being present in the sample, along with 22.5 wt% CaH2 and 8 wt% Al remaining. The residual 9.5 wt% was CaAl2, which was first observed at ∼480 °C. Elemental Al was no longer observed after 560 °C, while all CaH2 was depleted by 610 °C. The Al was consumed at a greater rate than CaH2 to facilitate the formation of the Al-rich CaAl4 phase. The CaAl4 was completely consumed by 700 °C leaving only CaAl2. During the decomposition process, Ca or CaO were not detected. Therefore, the reaction pathway can be expressed as:| CaH2 + 2Al ⇌ ½CaAl4 + ½CaH2 + ½H2 | (5) |
| ½CaAl4 + ½CaH2 ⇌ CaAl2 + ½H2 | (6) |
The kinetics of the hydrogen desorption reaction were studied by DSC at different heating rates in order to produce a Kissinger plot for each reaction step (Fig. 2a and b) and determine the activation energy (Ea) of the reactions. The DSC profile under flowing argon doesn't show two resolved peaks expected for this system under all ramp rates. Instead, the first decomposition step is denoted by a broad event, indicative of slow kinetics, which is convoluted with the second, well defined event. This convolution is corroborated by the in situ SR-XRD data (Fig. 1b) where the formation of CaAl4 and CaAl2 occur within overlapping temperature regions. Deconvolution of the data (Fig. 2c–e) shows peak maxima for the first step at 403, 427, 449 and 466 °C for ramp rates of 2, 5, 10 and 20 °C, respectively. This provides an Ea of 138 ± 12 kJ mol−1 H2. The peak maxima for the second step are 416, 464, 487 and 507 °C for ramp rates of 5, 10 and 20 °C, respectively. This provides Ea of 98 ± 8 kJ mol−1 H2. These values are of the same order of magnitude considering the uncertainties of the measurements, which suggests similar kinetics for hydrogen desorption for both steps. In addition, these Ea values are consistent compared to other hydrides that exhibit rapid kinetics. The second step of decomposition for SrH2 + 2Al, which is analogous to this system, was determined to be 156 ± 10 kJ mol−1 H2,34 and also comparable to other metal hydrides such as MgH2 (161 ± 15 kJ mol−1 H2),35 and NaMgH3 (160 ± 9 kJ mol−1 H2).36 Fast reaction kinetics are required for TES applications and hydrogen storage applications. In terms of TCES applications, fast absorption of hydrogen permits heat generation at rapid rates implying that electricity production is not hindered by the material physical properties. Conversely, a thermochemical battery can also charge quickly by rapid absorption of heat during hydrogen release.
The thermodynamics for both steps of the decomposition reaction were evaluated by PCI analysis over five temperatures between 520 and 591 °C (Fig. 3a). The combined H2 capacity for both steps is 2.1 wt% H2 and corresponds to the theoretical amount of 2.1 wt% H2, with step 1 and 2 having 1.1 wt% and 1 wt%, respectively. This also corroborates the 2.02 wt% H2 determined by SR-XRD on the pristine sample. The next noticeable point is that each of the hydrogen release steps has a sloping equilibrium plateau. Ideally, a TCES material should have a flat plateau at its equilibrium pressure, both for absorption and desorption, with little hysteresis. This sloping plateau has been noted in various metal hydride systems such as NaHxF1−x,37 Mg(HxF1−x)2,38 NaMgH2F39 and 2CaH2 + Si,21 and is attributed to the formation of solid solutions of varying compositions within the material. Ca and Al can form alloy systems.40 Particularly, at 7.7% Ca solubility in Al an Al–CaAl4 eutectic forms at 616 °C (above the temperatures used in this study),40 while CaAl2 is known to form CaAl2–xMx solid solutions, where M can be Cu, Mg, Ni and Zn.41 Unfortunately, it is unclear which solid solutions formed in this study, due to there being no clear evidence from the SR-XRD experiments.
 | ||
| Fig. 3 (a) Pressure composition isotherms (desorption) of CaH2 + 2Al (eqn (1)) between 520 and 591 °C, (b) van't Hoff plot for step 1 (eqn (2)) and (c) van't Hoff plot for step 2 (eqn (3)). | ||
Van't Hoff plots were constructed for both steps of the reaction and are illustrated in Fig. 3b and c. Step 1 has a ΔHdes = 79 ± 3 kJ mol−1 H2 and ΔSdes = 113 ± 4 J K−1 mol−1 H2 with a R2 factor of 0.9998, which equates to a Tdes(1bar) = 426 ± 22 °C. Step 2 has a ΔHdes = 99 ± 4 kJ mol−1 H2 and ΔSdes = 128 ± 5 J K−1 mol−1 H2 with a R2 factor of 0.9995, which equates to a Tdes(1bar) = 500 ± 28 °C. These thermodynamics show that the addition of Al to CaH2 has destabilised the hydrogen release from CaH2, causing a decrease in the ΔHdes, ΔSdes, and operating temperature compared to pure CaH2. Balakrishnan et al. recently determined the decomposition thermodynamics of molten CaH2 to be ΔHdes = 216 ± 10 kJ mol−1 H2 and ΔSdes = 177 ± 9 J K−1 mol−1 H2, giving Tdes(1bar) of 947 ± 65 °C.18 This is 524 °C higher than the first step of decomposition of CaH2 + 2Al and 447 °C higher than the second step.
Since the thermodynamics of the CaH2 + 2Al system were determined by PCI analysis, the operating temperatures can be calculated for any operating temperature and pressure, and these are illustrated in Fig. 4a. The ideal scenario for this system to achieve technological application as a storage material for a thermochemical battery is to operate at temperatures above 600 °C at a pressure as low as possible to reduce safety hazards and minimise engineering costs. A temperature of 670 °C was chosen for the cycling experiment, which has a theoretical equilibrium pressure of 15.1 bar, but due to the sloping plateau observed by PCI a working pressure of 20 bar was chosen to ensure full H2 absorption. Decomposition was undertaken in vacuo for 1 h. This allows complete cycling over the 2nd step at a theoretical hydrogen capacity of 1 wt% without operating over the 1st reaction step to maintain a single-step reaction pathway. This is complementary to the previous study by Ward et al. who cycled over both steps but at a temperature of 600 °C and 23 bar H2 (see Fig. 4a).25 Lower operating pressure and higher operating temperatures ensure better operating conditions for thermal battery applications utilising a Stirling engine and also integration in to CSP. As such, this study aimed to investigate the performance of this system at higher temperatures and at lower or similar pressures. Sixty-six sorption cycles were undertaken with fast kinetics observed and hydrogen absorption reaching 91% capacity in 2 h (Fig. 4b and c). The cycling capacity appears to decrease over cycles following a linear trend (Fig. 4b), which suggests that the capacity will be nil after 821 cycles. However, it is likely that the decreasing trend won't be linear over a large number of cycles and will converge towards an asymptote, as it is observed with most TCES materials.
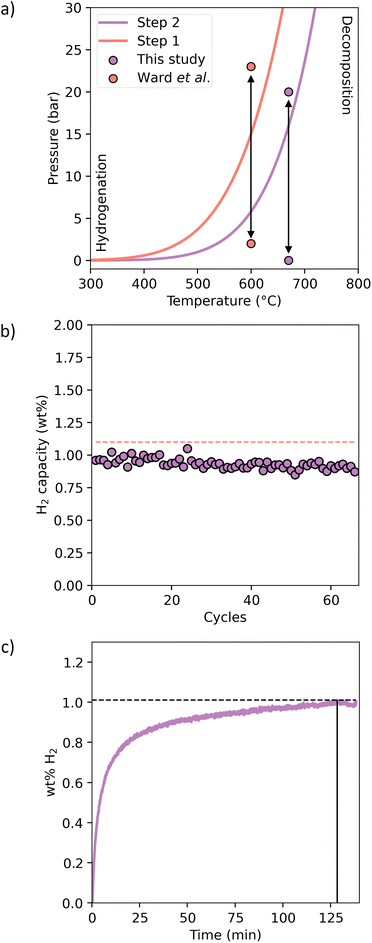 | ||
| Fig. 4 (a) Ca–Al–H2 equilibrium diagram deduced from the determined enthalpy and entropy for both reaction steps showing the cycling parameters of this study compared to a previous study (ref. 25). (b) Hydrogen sorption capacity over 66 cycles relative to the theoretical maximum sorption capacity. (c) Hydrogen absorption kinetics on cycle 1. | ||
Cycling was finalised after an absorption step and allowed to cool under 20 bar H2. The material retrieved from the reactor had agglomerated into a solid ingot. This likely attributed to the reduction in cycling capacity as H2 is restricted from reacting with the sample in a short timescale. This could be reduced by the addition of a particle refinement material such as TiB2, which has been shown to aid cyclic reversibility.36 Unfortunately, the ingot was impossible to powderise and so adequate XRD analysis and subsequent quantitative Rietveld refinement analysis was impossible. XRD analysis was performed after PCI analysis (Fig. S1†), where the same sample was employed throughout the experiments, and therefore cycled at least five times. The sample was left in a desorbed state and phase identification of the material by XRD determined only CaAl2 to be present with no other impurities such as oxides.
A materials-based cost analysis of the system was undertaken to determine the ultimate feasibility of CaH2–2Al as a TCES material (Table 1). This was calculated for systems using just the first reaction step (eqn (1)), just the second step (eqn (2)) and both reactions (eqn (3)). Assuming a cost of $2.57, $2.68 and $3 USD per kg for Ca, Al and H2, respectively, a cost of $2.6 USD per kg was determined for the starting raw material without processing costs.42 If the first step was to be used, where ΔHdes = 79 kJ mol−1 H2, an energy density of 822 kJ kg−1 is determined and therefore leads to a materials cost of $11.6 USD per kW hth.
| TES material | ΔH (kJ mol−1 H2) | ΔH (kJ kg−1) | Material cost (US$ per tonne) | Material cost (US$ per kW hth) | T (°C) | H2P (bar) | Mass requiredb (tonnes) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Note that values were calculated in an assumption of 100% conversion of reactants. b To generate 1 TJ of energy. | ||||||||
| CaH2 + 2Al (1st step) | 79 | 822 | 2641 | 11.6 | 426 | 1 | 2810 | This work |
| CaH2 + 2Al (2nd step) | 99 | 1031 | 2637 | 9.2 | 500 | 1 | 2360 | This work |
| CaH2 + 2Al (both steps) | 178 | 1853 | 2641 | 5.1 | 500 | 1 | 1310 | This work |
| CaH2 | 216 | 5131 | 2591 | 1.8 | 947 | 1 | 254 | 18 |
| SrH2 | 183 | 2041 | 5443 | 9.6 | 1070 | 1 | 322 | 34 |
| SrH2 + 2Al | 132 | 919 | 4375 | 17.1 | 846 | 1 | 742 | 34 |
| CaH2 + 11Zn | 131 | 172 | 2649 | 55.4 | 597 | 1 | 8920 | 43 |
| 3CaH2 + 2Al2O3 | 100 | 909 | 2460 | 9.7 | 636 | 1 | 1650 | 43 |
Molten salt (40NaNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60KNO3) 60KNO3) |
39 | 413 | 630 | 5.8 | 565 | — | 5250 | 44 |
Employing just the second step of the reaction leads to a higher ΔHdes = 99 kJ mol−1 H2, which leads to a higher energy density of 1031 kJ kg−1 and therefore a lower material cost of $9.2 USD per kW hth. Allowing the TES material to operate over both steps may be advantageous as the ΔHdes is 178 kJ mol−1 H2, which provides a material cost of $5.1 USD per kW hth. As such, there are advantages in operating the CaH2–2Al system over both the steps from an energy density and materials cost perspective. However, operating over both steps requires a larger gas pressure swing in the system, which may negatively impact the engineering cost of the system by requiring larger hydrogen gas storage tanks and/or reducing the efficiency of gas compression/expansion.
Compared to the other high temperature metal hydrides in Table 1, the CaH2–2Al system operates at a lower temperature (500 °C at 1 bar H2) than others, for example, CaH2–11Zn which operates at 597 °C. This is also below the maximum operating temperature for molten nitrate salts (565 °C) but is similar in cost to molten salts ∼5–6 US$ per kW hth. Conversely, due to the significantly higher energy density possessed by CaH2–2Al, only 25% of the total mass is required, which may save on storage space, despite the requirement for H2 gas storage facilities. Overall, CaH2–2Al has the potential to operate at elevated temperatures, despite the requirement for higher pressures, and is cost competitive compared to other high temperature metal hydrides. In addition, there is little chance of materials to sublime and condense (and ultimately segregate) compared to materials such as NaHxF1−x or Mg(HxF1−x)2.37,38
Conclusions
A stoichiometric mixture of CaH2 and 2Al was prepared by ball milling and its physical properties analysed to determine its feasibility as a high temperature TCES material for TCB applications. The initial decomposition reaction was determined by synchrotron radiation powder X-ray diffraction, DSC and PCI to be a two-step reaction with the onset of CaAl4 formation occurring at ∼350 °C and CaAl2 formation starting at ∼480 °C, with the two reactions being highly convoluted under vacuum or flowing argon conditions. The kinetics of both reactions were determined using the Kissinger method on the DSC data, deeming that both activation energies are of the same order of magnitude at 138 ± 12 kJ mol−1 and 98 ± 8 kJ mol−1, respectively. Determination of the thermodynamics by PCI analysis between 520 and 591 °C established that step one has a ΔHdes = 79 ± 3 kJ mol−1 H2 and ΔSdes = 113 ± 4 J K−1 mol−1 H2 giving Tdes(1bar) = 426 ± 22 °C. Step 2 has a ΔHdes = 99 ± 4 kJ mol−1 H2 and ΔSdes = 128 ± 5 J K−1 mol−1 H2, Tdes(1bar) = 500 ± 28 °C. These results allow for a determination of the operating conditions required for a sample to undergo hydrogen cycling for solely the second step. As such, a sample was cycled 66 times at 670 °C with a capacity loss of 9%. Overall, this study has shown that the CaH2–2Al system is a potential thermal energy storage material that can be used to store energy produced by renewable energy sources or high temperature waste heat.Author contributions
All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors CEB, MP, and TDH acknowledge the financial support the Department of Industry Science and Resources for the 2019 Global Innovation Linkage (GIL73589) grant and the Australian Research Council (ARC) for Discovery Project (DP200102301). The synchrotron powder diffraction data was collected on the Powder Diffraction beamline at the Australian Synchrotron, part of ANSTO. LD acknowledges the support of the Future Energy Exports CRC (https://www.fenex.org.au) whose activities are funded by the Australian Government's Cooperative Research Centre Program. This is FEnEx CRC Document 2023/22.RP2.0121-PHD-FNX-011.References
- IPCC, Strengthening and Implementing the Global Response, in Global Warming of 1.5 °C: IPCC Special Report on Impacts of Global Warming of 1.5 °C above Pre-industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, Cambridge University Press, Cambridge, 2022, ch. 4, pp. 313–444, DOI:10.1017/9781009157940.006.
- A. Palacios, C. Barreneche, M. E. Navarro and Y. Ding, Renewable Energy, 2020, 156, 1244–1265 CrossRef CAS.
- J. Sunku Prasad, P. Muthukumar, F. Desai, D. N. Basu and M. M. Rahman, Appl. Energy, 2019, 254, 113733 CrossRef CAS.
- C. Corgnale, B. Hardy, T. Motyka, R. Zidan, J. Teprovich and B. Peters, Renewable Sustainable Energy Rev., 2014, 38, 821–833 CrossRef CAS.
- M. S. Salman, Q. Lai, X. Luo, C. Pratthana, N. Rambhujun, M. Costalin, T. Wang, P. Sapkota, W. Liu, A. Grahame, J. Tupe and K.-F. Aguey-Zinsou, J. Alloys Compd., 2022, 920, 165936 CrossRef CAS.
- X. Qu, Y. Li, P. Li, Q. Wan and F. Zhai, Front. Mater. Sci., 2015, 9, 317–331 CrossRef.
- M. Adams, C. E. Buckley, M. Busch, R. Bunzel, M. Felderhoff, T. W. Heo, T. D. Humphries, T. R. Jensen, J. Klug, K. H. Klug, K. T. Møller, M. Paskevicius, S. Peil, K. Peinecke, D. A. Sheppard, A. D. Stuart, R. Urbanczyk, F. Wang, G. S. Walker, B. C. Wood, D. Weiss and D. M. Grant, Prog. Energy, 2022, 4, 032008 CrossRef.
- B. J. Hardy, C. Corgnale and S. N. Gamble, Sustainability, 2021, 13, 12117 CrossRef CAS.
- P. Feng, Y. Liu, I. Ayub, Z. Wu, F. Yang and Z. Zhang, Energy Convers. Manage., 2018, 174, 239–247 CrossRef CAS.
- D. Dong, T. D. Humphries, D. A. Sheppard, B. Stansby, M. Paskevicius, M. V. Sofianos, A. L. Chaudhary, M. Dornheim and C. E. Buckley, Sustainable Energy Fuels, 2017, 1, 1820–1829 RSC.
- M. Paskevicius, D. A. Sheppard, K. Williamson and C. E. Buckley, Energy, 2015, 88, 469–477 CrossRef CAS.
- L. Poupin, T. D. Humphries, M. Paskevicius and C. E. Buckley, Int. J. Hydrogen Energy, 2021, 46, 38755–38767 CrossRef CAS.
- R. Urbanczyk, K. Peinecke, S. Peil and M. Felderhoff, Int. J. Hydrogen Energy, 2017, 42, 13818–13826 CrossRef CAS.
- Z. Z. Fang, C. Zhou, P. Fan, K. S. Udell, R. C. Bowman, J. J. Vajo, J. J. Purewal and B. Kekelia, J. Alloys Compd., 2015, 645, S184–S189 CrossRef CAS.
- P. K. Singh, A. Singh, V. Kain, Y. Kojima, H. Miyaoka and S. Kumar, Int. J. Hydrogen Energy, 2021, 46, 34362–34368 CrossRef CAS.
- G. Lundholm, The Experimental V4X Stirling Engine - A Pioneering Development Report number: NEI-SE-497OSTI ID: 20475297, Lund Inst. of Tech., Dept. of Heat and Power Engineering, Sweden, 2003 Search PubMed.
- K. Manickam, P. Mistry, G. Walker, D. Grant, C. E. Buckley, T. D. Humphries, M. Paskevicius, T. Jensen, R. Albert, K. Peinecke and M. Felderhoff, Int. J. Hydrogen Energy, 2019, 44, 7738–7745 CrossRef CAS.
- S. Balakrishnan, T. D. Humphries, M. Paskevicius and C. E. Buckley, Int. J. Hydrogen Energy, 2023, 48(78), 30479–30488 CrossRef CAS.
- J. J. Vajo and G. L. Olson, Scr. Mater., 2007, 56, 829–834 CrossRef CAS.
- S. Balakrishnan, M. V. Sofianos, T. D. Humphries, M. Paskevicius and C. E. Buckley, Phys. Chem. Chem. Phys., 2020, 22, 25780–25788 RSC.
- A. C. M. Griffond, M. V. Sofianos, D. A. Sheppard, T. D. Humphries, A.-L. Sargent, M. Dornheim, K.-F. Aguey-Zinsou and C. E. Buckley, J. Alloys Compd., 2021, 858, 158229 CrossRef CAS.
- M. V. Sofianos, S. Randall, M. Paskevicius, K.-F. Aguey-Zinsou, M. R. Rowles, T. D. Humphries and C. E. Buckley, J. Alloys Compd., 2020, 819, 153340 CrossRef CAS.
- S. Balakrishnan, M. V. Sofianos, M. Paskevicius, M. R. Rowles and C. E. Buckley, J. Phys. Chem. C, 2020, 124, 17512–17519 CrossRef CAS.
- E. Veleckis, J. Less-Common Met., 1981, 80, 241–255 CrossRef CAS.
- P. A. Ward, J. A. Teprovich, Y. Liu, J. He and R. Zidan, J. Alloys Compd., 2018, 735, 2611–2615 CrossRef CAS.
- K. S. Wallwork, B. J. Kennedy and D. Wang, AIP Conf. Proc., 2007, 879, 879 CrossRef CAS.
- B. Schmitt, C. Bronnimann, E. F. Eikenberry, F. Gozzo, C. Hormann, R. Horisberger and B. Patterson, Nucl. Instrum. Methods Phys. Res., Sect. A, 2003, 501, 267–272 CrossRef CAS.
- R. A. Young and R. A. Young, The Rietveld Method, Oxford University Press, 1995 Search PubMed.
- M. Paskevicius, D. A. Sheppard and C. E. Buckley, J. Am. Chem. Soc., 2010, 132, 5077–5083 CrossRef CAS PubMed.
- R. A. Causey, J. D. Fowler, C. Ravanbakht, T. S. Elleman and K. Verghese, J. Am. Ceram. Soc., 1978, 61, 221–225 CrossRef CAS.
- S. Vyazovkin, Molecules, 2020, 25, 2813 CrossRef CAS.
- H. E. Kissinger, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- T. Renner, Quantities, Units and Symbols in Physical Chemistry, The Royal Society of Chemistry, 2007 Search PubMed.
- T. D. Humphries, M. Paskevicius, A. Alamri and C. E. Buckley, J. Alloys Compd., 2022, 894, 162404 CrossRef CAS.
- J. F. Fernández and C. R. Sánchez, J. Alloys Compd., 2003, 356–357, 348–352 CrossRef.
- L. Poupin, T. D. Humphries, M. Paskevicius and C. E. Buckley, Sustainable Energy Fuels, 2019, 3, 985–995 RSC.
- T. D. Humphries, D. A. Sheppard, M. R. Rowles, M. V. Sofianos and C. E. Buckley, J. Mater. Chem. A, 2016, 4, 12170–12178 RSC.
- M. S. Tortoza, T. D. Humphries, D. A. Sheppard, M. Paskevicius, M. R. Rowles, M. V. Sofianos, K. F. Aguey-Zinsou and C. E. Buckley, Phys. Chem. Chem. Phys., 2018, 20, 2274–2283 RSC.
- D. A. Sheppard, C. Corgnale, B. Hardy, T. Motyka, R. Zidan, M. Paskevicius and C. E. Buckley, RSC Adv., 2014, 4, 26552–26562 RSC.
- S. S. S. Kumari, R. M. Pillai and B. C. Pai, Int. Mater. Rev., 2005, 50, 216–238 CrossRef CAS.
- S. Amerioun, S. I. Simak and U. Häussermann, Inorg. Chem., 2003, 42, 1467–1474 CrossRef CAS PubMed.
- ISE Metal Quotes, https://ise-metal-quotes.com/, accessed Jan 22, 2023 Search PubMed.
- S. Balakrishnan, M. V. Sofianos, T. D. Humphries, M. Paskevicius and C. E. Buckley, Phys. Chem. Chem. Phys., 2020, 22, 25780–25788 RSC.
- Q. A. Zhang, Y. Nakamura, K. Oikawa, T. Kamiyama and E. Akiba, Inorg. Chem., 2002, 41, 6547–6549 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se01122d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |