 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A self-assembling protein–DNA complex with an inbuilt DNA release system for quantitative immuno-PCR applications†
A. E.
Sorenson
 and
P. M.
Schaeffer
and
P. M.
Schaeffer
 *
*
Molecular and Cell Biology, College of Public Health, Medical and Veterinary Sciences, James Cook University, The Science Place, Building 142, University Drive, Douglas, QLD 4811, Australia. E-mail: patrick.schaeffer@jcu.edu.au; Tel: +61 (0)7 4781 4448
First published on 16th October 2024
Abstract
Site-specific protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA conjugation is gaining increasing importance in detection technologies such as quantitative immuno-PCR (qIPCR). Until now, DNA-binding proteins have been a relatively untapped source of protein
DNA conjugation is gaining increasing importance in detection technologies such as quantitative immuno-PCR (qIPCR). Until now, DNA-binding proteins have been a relatively untapped source of protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA conjugation systems. In Escherichia coli, the biotin protein ligase (BirA) is a biotin-dependent DNA-binding protein that offers a means to connect a protein of interest (POI) with DNA. Here, we explored BirA as a unique on–off protein
DNA conjugation systems. In Escherichia coli, the biotin protein ligase (BirA) is a biotin-dependent DNA-binding protein that offers a means to connect a protein of interest (POI) with DNA. Here, we explored BirA as a unique on–off protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA connection switch for the production of self-assembling POI
DNA connection switch for the production of self-assembling POI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA conjugates. Green fluorescent protein (GFP) is a versatile protein tag and reporter, commonly quantified by fluorescence detection. However, low GFP concentrations are challenging to detect and require more sensitive methods. A multitude of high-affinity antibodies are available for capture and detection of GFP as an affinity tag. As such, a well-characterised GFP-tagged BirA (BirA-GFP) was selected for the development and validation of an innovative qIPCR platform technology. The unique principle of this assay involves the assembly of two BirA-GFP with the bioO repressor DNA sequence in the presence of ATP and biotin. The resulting high affinity bioO
DNA conjugates. Green fluorescent protein (GFP) is a versatile protein tag and reporter, commonly quantified by fluorescence detection. However, low GFP concentrations are challenging to detect and require more sensitive methods. A multitude of high-affinity antibodies are available for capture and detection of GFP as an affinity tag. As such, a well-characterised GFP-tagged BirA (BirA-GFP) was selected for the development and validation of an innovative qIPCR platform technology. The unique principle of this assay involves the assembly of two BirA-GFP with the bioO repressor DNA sequence in the presence of ATP and biotin. The resulting high affinity bioO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BirA-GFP complex can be applied in various formats to detect the presence of anti-GFP IgG as well as GFP immobilised on a surface. Complete release of the quantifiable bioO DNA can easily be achieved by omitting ATP and biotin in the final elution step. The new BirA-based qIPCR assay enabled picomolar (≥10−12 M) detection of GFP and anti-GFP IgG as well as their affinity profiling.
BirA-GFP complex can be applied in various formats to detect the presence of anti-GFP IgG as well as GFP immobilised on a surface. Complete release of the quantifiable bioO DNA can easily be achieved by omitting ATP and biotin in the final elution step. The new BirA-based qIPCR assay enabled picomolar (≥10−12 M) detection of GFP and anti-GFP IgG as well as their affinity profiling.
Introduction
Site-selective conjugation of protein with DNA is gaining importance in protein detection and display technologies.1,2 Immuno-PCR assays are reliant on covalent or non-covalent protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA conjugation to significantly increase the sensitivity of immunoassays by several orders of magnitude.3 High-affinity DNA-binding proteins are a relatively untapped source of connecting systems, with Tus being the only example of an Escherichia coli protein that has been successfully translated into a versatile protein
DNA conjugation to significantly increase the sensitivity of immunoassays by several orders of magnitude.3 High-affinity DNA-binding proteins are a relatively untapped source of connecting systems, with Tus being the only example of an Escherichia coli protein that has been successfully translated into a versatile protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA connection system for the production of qIPCR detection devices.3–8
DNA connection system for the production of qIPCR detection devices.3–8
In E. coli, biotin protein ligase (BirA) is a biotin-dependent DNA binding protein. The primary function of BirA is to covalently attach biotin to the biotin carboxyl carrier protein (BCCP) subunit of acetyl-coA carboxylase (ACC).9–11 The biotin prosthetic group is essential for malonyl-CoA synthesis. The process involves sequential binding of biotin followed by ATP to BirA to yield the adenylated intermediate biotinyl-5′-AMP (bio-5′-AMP) with concomitant release of pyrophosphate.12 Once formed, bio-5′-AMP dissociates 1000-fold slower (half-life >30 min) from E. coli BirA than biotin.13,14 The bio-5′-AMP bound BirA is the holoenzyme (holoBirA) that catalyses the formation of an amide bond between biotin and a specific lysine residue of the BCCP subunit within the ACC complex. BirA has been successfully developed for site-specific biotinylation of proteins containing small peptide tags such as the AviTag both in vivo and in vitro.15–17 The commercial AviTag system is now routinely applied for protein biotinylation.18 However, despite the high profile of E. coli BirA in protein biotinylation, its well-characterised DNA binding property19 has not yet been exploited.
E. coli holoBirA regulates expression of bio genes and subsequently biotin synthesis through its tight binding to the biotin operator (bioO), a 40 bp inverted DNA repeat that overlaps and controls both biotin operon promoters.20–28 Binding of E. coli holoBirA to bioO yields a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 protein
1 protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA complex with nanomolar affinity.29 The affinity of E. coli BirA for bioO increases substantially when bound to biotin and even further when it is bound to bio-5′-AMP in the presence of Mg2+ ions.30 The dissociation half-life for E. coli holoBirA
DNA complex with nanomolar affinity.29 The affinity of E. coli BirA for bioO increases substantially when bound to biotin and even further when it is bound to bio-5′-AMP in the presence of Mg2+ ions.30 The dissociation half-life for E. coli holoBirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO is 400 s in a buffer containing 200 mM KCl.21E. coli BirA requires Mg2+ ions to catalyse the formation of bio-5'AMP. It is proposed that it is the formation of bio-5′-AMP that triggers the dimerization of E. coli holoBirA and increases its affinity for bioO.25 As such, E. coli BirA offers the possibility of dimerizing two proteins of interest such as GFP through a tightly-controlled association mechanism with bioO (Fig. 1).
bioO is 400 s in a buffer containing 200 mM KCl.21E. coli BirA requires Mg2+ ions to catalyse the formation of bio-5'AMP. It is proposed that it is the formation of bio-5′-AMP that triggers the dimerization of E. coli holoBirA and increases its affinity for bioO.25 As such, E. coli BirA offers the possibility of dimerizing two proteins of interest such as GFP through a tightly-controlled association mechanism with bioO (Fig. 1).
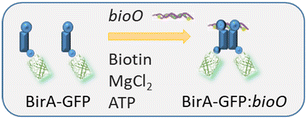 | ||
Fig. 1 Principle of E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO self-assembly. Two E. coli BirA-GFP bind to biotin and ATP and assemble with bioO into a stable protein bioO self-assembly. Two E. coli BirA-GFP bind to biotin and ATP and assemble with bioO into a stable protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA complex following formation of bio-5′-AMP. DNA complex following formation of bio-5′-AMP. | ||
GFP-tagged proteins are useful reporter molecules that can be detected with flow cytometry or imaging applications to monitor physiological processes, detect transgenic expression in vivo, and visualize protein localization.31 The autofluorescence of GFP can easily be measured at nanomolar and higher concentrations using a fluorescence plate reader. However, ultrasensitive quantification of GFP-tagged proteins is still challenging. Low expression or fluorescence can be amplified with anti-GFP antibodies and antibody conjugates, and detected using imaging, western blotting, flow cytometry and immunoassays. Quantification of picomolar concentrations of GFP-tagged proteins, expressed, secreted or circulating in complex matrices is not trivial. A TT-lock qIPCR assay has been shown to detect pM concentrations of GFP and GFP-fusion proteins.4,6,7 GFP-tagged nanobodies32 and combinations of bispecific antibodies with affinity towards GFP or comprising a GFP33–37 present new application areas for ultrasensitive GFP detection.
We have previously shown that GFP-tagged E. coli BirA (E. coli BirA-GFP) is fully functional, including its binding to bioO.18,38 Here, we evaluated E. coli BirA as a unique on–off protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA connection switch for the production and release of self-assembling protein
DNA connection switch for the production and release of self-assembling protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA conjugates. We present the first application of this system with E. coli BirA-GFP as a useful qIPCR detection device for GFP quantitation and anti-GFP antibody affinity profiling.
DNA conjugates. We present the first application of this system with E. coli BirA-GFP as a useful qIPCR detection device for GFP quantitation and anti-GFP antibody affinity profiling.
Materials and methods
Protein targets
E. coli BirA-GFP, B. pseudomallei BirA-GFP and M. tuberculosis BirA-GFP were expressed and purified as previously described.19,39,40 BirA-GFP were resuspended in BirA buffer (25 mM Tris pH 8, 100 mM NaCl, 5% v/v glycerol). Protein concentrations were determined by Bradford assay and protein purity by SDS-PAGE.Differential scanning fluorimetry of GFP-tagged proteins (DSF-GTP)
E. coli BirA-GFP (1 μM) in PBS-T (ProteOn running buffer: phosphate buffered saline, pH 7.4, 0.005% Tween 20) in the presence of various combinations of ligands (MgCl2, biotin, ATP and bioO) was incubated at room temperature (RT) for 5 min then heated in a real-time thermocycler (CFX96, Bio-Rad) from 25–90 °C, increasing in 0.5 °C increments every 30 s. Final ligand concentrations were 5 mM, 1 mM, 1 mM and 1 μM respectively for MgCl2, biotin, ATP and bioO. A series of experiments was also performed in PBS-T with 300 mM final NaCl (PBS-T (HS)). Data were analysed using Bio-Rad CFX Maestro software for transition midpoint (Tm) determination as previously described.41,42qPCR standard curve and data analyses
Solutions of 1 μM bioO specific primers (in water) were spiked with bioO template to yield final concentrations ranging from 250 pM to 50 fM. qPCR reactions contained 10 μL of the above spiked bioO solutions and 10 μL Sso Advanced SYBR Green qPCR master mix (Bio-Rad). The qPCR protocol was performed in a Bio-Rad CFX96 thermocycler with an initial incubation of 10 min at 95 °C followed by 40 cycles of 95 °C for 10 s and 60 °C for 20 s. All reactions were run in duplicate. Fluorescence threshold, window-of-linearity and PCR efficiency were determined using LinRegPCR software (Heart Failure Research Center).43 Linear regression was also performed using GraphPad Prism 7.04.
E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) bioO qIPCR for development and evaluation phase
bioO qIPCR for development and evaluation phase
Polyclonal goat anti-GFP IgG (Abcam ab6673) was adsorbed (i.e. dilutions ranging from 16.5 nM to 100 fM in PBS-T (ProteOn, Bio-Rad)) onto the surface of Nunc clear polystyrene (PS) U96 MicroWell™ plate with a MaxiSorp™ surface for solid phase immunoassays (Nunc, 449824) overnight at RT in 50 μL aliquots. Microplates were blocked for 1 h at RT with 100 μL 1% BSA in PBS-T. E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO was assembled at 1 μM at RT for 15 min in PBS-T with 1 mM biotin and 1 mM ATP (PBS-TBA). The E. coli BirA-GFP
bioO was assembled at 1 μM at RT for 15 min in PBS-T with 1 mM biotin and 1 mM ATP (PBS-TBA). The E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO complex was diluted to 2.5 nM and in PBS-TBA and 50 μL applied to the well for 30 min at RT. The diluted complex was aspirated and the microplates were washed four times with PBS-TBA (300 μL). The bioO was released from the complex with the addition of 50 μL of 1 μM bioO-specific primers for 30 min at RT (see ESI† for sequence details of the bioO template sequence and primers). Supernatant (10 μL) was combined with 10 μL Sso Advanced SYBR Green qPCR master mix (Bio-Rad) in 96-well hard-shell full-skirted PCR plates (Bio-Rad). Negative controls were performed as before without antibody in PBS-T and measured the background signal (indirect and direct non-specific bioO binding to well surface). All qPCRs were run as above in duplicate. Additional experiments were conducted as described with assembly, dilution and wash steps performed in PBS-TBA with 5 mM MgCl2 (see ESI†).
bioO complex was diluted to 2.5 nM and in PBS-TBA and 50 μL applied to the well for 30 min at RT. The diluted complex was aspirated and the microplates were washed four times with PBS-TBA (300 μL). The bioO was released from the complex with the addition of 50 μL of 1 μM bioO-specific primers for 30 min at RT (see ESI† for sequence details of the bioO template sequence and primers). Supernatant (10 μL) was combined with 10 μL Sso Advanced SYBR Green qPCR master mix (Bio-Rad) in 96-well hard-shell full-skirted PCR plates (Bio-Rad). Negative controls were performed as before without antibody in PBS-T and measured the background signal (indirect and direct non-specific bioO binding to well surface). All qPCRs were run as above in duplicate. Additional experiments were conducted as described with assembly, dilution and wash steps performed in PBS-TBA with 5 mM MgCl2 (see ESI†).
E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) bioO qIPCR dissociation rates
bioO qIPCR dissociation rates
Polyclonal goat anti-GFP IgG (Abcam ab6673) was adsorbed at 10 nM in PBS (ProteOn, Bio-Rad) onto the surface of Nunc clear polystyrene (PS) U96 MicroWell™ plate with a MaxiSorp™ surface for solid phase immunoassays (Nunc, 449824) overnight at RT in 100 μL aliquots. Microplates were blocked for 1 h at RT with 200 μL 1% BSA in PBS-T with shaking at 200 RPM. E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO was assembled as described in PBS-TBA and in parallel with PBS-TBA with 5 mM MgCl2 (PBS-TBA + MgCl2). Complexes were diluted to 2.5 nM and in PBS-TBA or PBS-TBA + MgCl2 and 150 μL applied to the well for 30 min at RT with shaking at 200 RPM. Aspiration and wash steps were performed as described previously with PBS-TBA or PBS-TBA + MgCl2. The bioO was released from the complex with the addition of 150 μL of 1 μM bioO-specific primers for 45 min at RT, with 10 μL aliquots of supernatant taken at 0, 2, 4, 8, 13, 30 and 45 min. qPCR were performed in duplicate as described previously.
bioO was assembled as described in PBS-TBA and in parallel with PBS-TBA with 5 mM MgCl2 (PBS-TBA + MgCl2). Complexes were diluted to 2.5 nM and in PBS-TBA or PBS-TBA + MgCl2 and 150 μL applied to the well for 30 min at RT with shaking at 200 RPM. Aspiration and wash steps were performed as described previously with PBS-TBA or PBS-TBA + MgCl2. The bioO was released from the complex with the addition of 150 μL of 1 μM bioO-specific primers for 45 min at RT, with 10 μL aliquots of supernatant taken at 0, 2, 4, 8, 13, 30 and 45 min. qPCR were performed in duplicate as described previously.
E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) bioO bridging qIPCR
bioO bridging qIPCR
GFP was adsorbed (dilutions ranging from 1 μM to 1 pM in PBS-T (ProteOn, Bio-Rad)) onto the surface of Nunc clear polystyrene (PS) U96 MicroWell™ MaxiSorp™ plate (Nunc, 449824) in 50 μL aliquots for 2 h at RT with shaking at 200 RPM. Microplates were blocked for 30 min at RT with 100 μL 1% BSA in PBS-T with shaking. E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO was assembled and diluted to 2.5 nM in PBS-TBA. Polyclonal goat anti-GFP IgG (Abcam ab6673) and HRP-conjugated protein G were added at 1 μg mL−1 to the diluted complex and 50 μL of the mixture applied to the wells for 30 min at RT with shaking. Aspiration and wash steps were performed as described previously. The bioO was released from the complex with the addition of 50 μL of 1 μM bioO-specific primers for 30 min at RT. qPCR were performed using 10 μL aliquots as described previously. TMB solution (20 μL, Sigma-Aldrich) was added to the remaining 40 μL in the wells. The reaction was developed for 15 min at RT, stopped with H2SO4 (5 μL 2 M) and read at 450 nm. The dilution of the TMB reagent yields a corresponding reduction in signal strength.
bioO was assembled and diluted to 2.5 nM in PBS-TBA. Polyclonal goat anti-GFP IgG (Abcam ab6673) and HRP-conjugated protein G were added at 1 μg mL−1 to the diluted complex and 50 μL of the mixture applied to the wells for 30 min at RT with shaking. Aspiration and wash steps were performed as described previously. The bioO was released from the complex with the addition of 50 μL of 1 μM bioO-specific primers for 30 min at RT. qPCR were performed using 10 μL aliquots as described previously. TMB solution (20 μL, Sigma-Aldrich) was added to the remaining 40 μL in the wells. The reaction was developed for 15 min at RT, stopped with H2SO4 (5 μL 2 M) and read at 450 nm. The dilution of the TMB reagent yields a corresponding reduction in signal strength.
E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) bioO qIPCR for antibody profiling
bioO qIPCR for antibody profiling
Polyclonal chicken anti-GFP (Aves, AB_2307313), polyclonal biotinylated goat anti-GFP (Abcam, ab6658) and monoclonal mouse anti-6x His (Abcam, ab18184) were adsorbed onto the surface of Nunc clear polystyrene (PS) U96 MicroWell™ MaxiSorp™ plate (Nunc, 449824) at 2 μg mL−1 in 50 mM phosphate buffer, pH 7.8 in 50 μL aliquots overnight at 4 °C. Microplates were washed and blocked as described previously. E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO was assembled and serially diluted (2.5 nM to 0.156 nM) and applied in 50 μL aliquots for 1 h at RT. Aspiration, wash, and dissociation steps were performed as described previously. qPCR was performed with BioRad iTaq Universal SYBR green supermix with an initial incubation of 2 min at 95 °C followed by 40 cycles of 95 °C for 10 s and 60 °C for 20 s. qPCR were performed in duplicate.
bioO was assembled and serially diluted (2.5 nM to 0.156 nM) and applied in 50 μL aliquots for 1 h at RT. Aspiration, wash, and dissociation steps were performed as described previously. qPCR was performed with BioRad iTaq Universal SYBR green supermix with an initial incubation of 2 min at 95 °C followed by 40 cycles of 95 °C for 10 s and 60 °C for 20 s. qPCR were performed in duplicate.
Results and discussion
Stability of E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) bioO in the presence and absence of MgCl2
bioO in the presence and absence of MgCl2
The pH and salt effects on E. coli BirA activity, dimerization and bioO binding have previously been examined.44–46 We were particularly interested in the stability of the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO complex, bound to both biotin and ATP in the absence of Mg2+ ions, which has never been investigated. We hypothesized that in the absence of Mg2+ ions, the binding of ATP and biotin to E. coli BirA would increase its affinity for bioO compared to the biotin-bound form without freezing the complex through formation of bio-5′-AMP. Here, the DSF-GTP technology41,42,47 that had previously been validated for E. coli, Burkholderia pseudomallei and Mycobacterium tuberculosis BirA-GFP19,39,40,48 provided us with an ideal assay to examine the effect of Mg2+ ions on the E. coli BirA-GFP and its various complexes in different buffer conditions.
bioO complex, bound to both biotin and ATP in the absence of Mg2+ ions, which has never been investigated. We hypothesized that in the absence of Mg2+ ions, the binding of ATP and biotin to E. coli BirA would increase its affinity for bioO compared to the biotin-bound form without freezing the complex through formation of bio-5′-AMP. Here, the DSF-GTP technology41,42,47 that had previously been validated for E. coli, Burkholderia pseudomallei and Mycobacterium tuberculosis BirA-GFP19,39,40,48 provided us with an ideal assay to examine the effect of Mg2+ ions on the E. coli BirA-GFP and its various complexes in different buffer conditions.
First, we compared the effect of biotin (1 mM), ATP (1 mM) and MgCl2 (5 mM), and combinations thereof on the transition midpoint (Tm) values of E. coli BirA-GFP (1 μM) in PBS-T, then in PBS-T high salt (HS) (Fig. 2, S1A and B†). The increase in Tm value for E. coli BirA-GFP when both ATP and biotin are present reflects formation of significant stabilizing interactions upon binding of ATP to the biotin-bound species. MgCl2 only increases the Tm of E. coli BirA-GFP when in presence of both biotin and ATP which is likely due to the formation of the holoenzyme and slow dissociation of bio-5′-AMP.
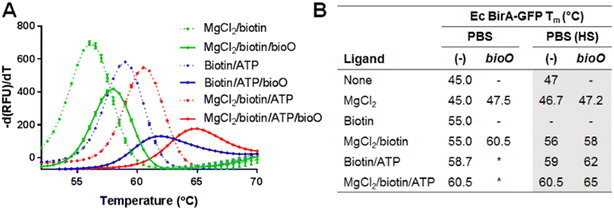 | ||
| Fig. 2 Ligand effects on E. coli BirA-GFP melt-curves in PBS-T and PBS-T (HS). A) E. coli BirA-GFP (1 μM) was incubated for 10 min at RT with combinations of biotin (1 mM), ATP (1 mM) and MgCl2 (5 mM) in PBS-T (HS) prior to analysis by DSF-GTP melt-curve protocol (35–90 °C at 0.5 °C/30 s). B) E. coli (Ec) BirA-GFP Tm with combinations of biotin, ATP, MgCl2 and bioO (1 μM). Tm were analyzed (see Fig. S1† for full data). Data represent averages and SD of three melt-curves. (−): not tested. (*): could not be determined. | ||
Next, we compared the binding of E. coli BirA-GFP to bioO (Fig. 2 and S1C–F†) with combinations of biotin, ATP, MgCl2 as well as bioO (1 μM) in PBS-T and PBS-T (HS). The presence of bioO significantly increased the Tm of all tested forms of E. coli BirA, i.e. biotin-bound, biotin/ATP-bound and holoBirA, suggesting the formation of discrete protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA complexes for these species in PBS-T. Previous EMSA and DNA foot-printing data support these findings.19,30 Stark differences in Tm between these E. coli BirA-GFP
DNA complexes for these species in PBS-T. Previous EMSA and DNA foot-printing data support these findings.19,30 Stark differences in Tm between these E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO complexes are evident. The data for E. coli BirA and bioO is consistent with a low affinity protein
bioO complexes are evident. The data for E. coli BirA and bioO is consistent with a low affinity protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA complex. The difference in Tm between biotin-bound E. coli BirA-GFP and biotin-bound E. coli BirA-GFP
DNA complex. The difference in Tm between biotin-bound E. coli BirA-GFP and biotin-bound E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO indicates a significant increase in bioO binding affinity.
bioO indicates a significant increase in bioO binding affinity.
The Tm values for both biotin/ATP-bound E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO and holoBirA-GFP
bioO and holoBirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO could not be determined in PBS-T due to the extreme thermal stabilization effects of bioO. Here, the PBS-T (HS) conditions were essential to weaken the DNA binding affinity of E. coli BirA-GFP sufficiently to measure and compare the Tm values of all bioO complexes (Fig. 2). The data clearly show that the stability of the biotin/ATP-bound E. coli BirA-GFP for bioO is intermediate between the biotin-bound and holoenzyme species, providing support to our working hypothesis.
bioO could not be determined in PBS-T due to the extreme thermal stabilization effects of bioO. Here, the PBS-T (HS) conditions were essential to weaken the DNA binding affinity of E. coli BirA-GFP sufficiently to measure and compare the Tm values of all bioO complexes (Fig. 2). The data clearly show that the stability of the biotin/ATP-bound E. coli BirA-GFP for bioO is intermediate between the biotin-bound and holoenzyme species, providing support to our working hypothesis.
Development and evaluation of the E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) bioO qIPCR
bioO qIPCR
Further to our encouraging DSF-GTP data, we hypothesized that the biotin/ATP-bound E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO complex formed in the absence of Mg2+ ions, despite being highly stable, could have the advantage of dissociating rapidly upon removal of ATP and biotin, offering a useful DNA release trigger mechanism for qIPCR detection and other display applications. In this scenario, ATP and biotin can simply be omitted in the final qIPCR elution step to promote rapid bioO DNA template dissociation.
bioO complex formed in the absence of Mg2+ ions, despite being highly stable, could have the advantage of dissociating rapidly upon removal of ATP and biotin, offering a useful DNA release trigger mechanism for qIPCR detection and other display applications. In this scenario, ATP and biotin can simply be omitted in the final qIPCR elution step to promote rapid bioO DNA template dissociation.
In our proof-of-concept qIPCR setup (Fig. 3A), anti-GFP IgG in solutions ranging from 10−13 to 10−8 M were physisorbed onto the wells of a 96-well plate. After the blocking and wash steps, 50 μL of E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO (2.5 nM in PBS-TBA) were added for 30 min at RT. Following four wash steps with PBS-TBA, bioO was released from the complex in a 50 μL aqueous primer mix solution (i.e. no ATP nor biotin) for 30 min, of which 10 μL were used for qPCR analysis.
bioO (2.5 nM in PBS-TBA) were added for 30 min at RT. Following four wash steps with PBS-TBA, bioO was released from the complex in a 50 μL aqueous primer mix solution (i.e. no ATP nor biotin) for 30 min, of which 10 μL were used for qPCR analysis.
 | ||
Fig. 3 qIPCR detection of preadsorbed polyclonal anti-GFP IgG with E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO. A) Schematic representation of the qIPCR platform principle involving binding of the E. coli BirA-GFP bioO. A) Schematic representation of the qIPCR platform principle involving binding of the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO to preadsorbed anti-GFP IgG (association) followed by release of bioO (dissociation) and qPCR detection. B) qPCR standard curve for bioO (slope = −5.064). All replicates are shown. See ESI† for sequence details of the bioO template sequence and primers. C) Background control qIPCR were performed by omitting IgG with biotin/ATP-bound E. coli BirA-GFP bioO to preadsorbed anti-GFP IgG (association) followed by release of bioO (dissociation) and qPCR detection. B) qPCR standard curve for bioO (slope = −5.064). All replicates are shown. See ESI† for sequence details of the bioO template sequence and primers. C) Background control qIPCR were performed by omitting IgG with biotin/ATP-bound E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO in PBS-T (PBS-TBA − MgCl2, black diamonds). All replicates are shown. D) Comparison of qIPCR detection of preadsorbed polyclonal anti-GFP IgG with biotin/ATP-bound E. coli BirA-GFP bioO in PBS-T (PBS-TBA − MgCl2, black diamonds). All replicates are shown. D) Comparison of qIPCR detection of preadsorbed polyclonal anti-GFP IgG with biotin/ATP-bound E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO (PBS-TBA − MgCl2, black diamonds and E. coli holoBirA-GFP bioO (PBS-TBA − MgCl2, black diamonds and E. coli holoBirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO (PBS-TBA + MgCl2, red diamonds). Concentrations of IgG (αGFP) are as indicated. All replicates are shown. The slope is steeper in the holoenzyme conditions (PBS-TBA + MgCl2) resulting in a loss of detection performance. It is important to note that buffer compositions are different and data are thus not directly comparable as background values are differently affected. All replicates are shown. E) qIPCR bioO dissociation time-course experiment for complexes formed in the presence or absence of MgCl2 was set at the highest concentration of αGFP IgG. Averages of two datapoints and standard deviations are shown. The dissociation half-life for bioO in the absence of MgCl2 (black diamonds) is 234.7 s calculated with a PCR efficiency of 1.559 (LinRegPCR). bioO (PBS-TBA + MgCl2, red diamonds). Concentrations of IgG (αGFP) are as indicated. All replicates are shown. The slope is steeper in the holoenzyme conditions (PBS-TBA + MgCl2) resulting in a loss of detection performance. It is important to note that buffer compositions are different and data are thus not directly comparable as background values are differently affected. All replicates are shown. E) qIPCR bioO dissociation time-course experiment for complexes formed in the presence or absence of MgCl2 was set at the highest concentration of αGFP IgG. Averages of two datapoints and standard deviations are shown. The dissociation half-life for bioO in the absence of MgCl2 (black diamonds) is 234.7 s calculated with a PCR efficiency of 1.559 (LinRegPCR). | ||
A qPCR standard curve spanning over a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log dynamic range with a R2 correlation coefficient of 0.998 was obtained with our selected bioO qPCR DNA template and primers (Fig. 3B). A series of background control qIPCR were set up without IgG (Fig. 3C), as well as by replacing E. coli BirA-GFP with either B. pseudomallei or M. tuberculosis BirA-GFP which do not bind bioO (Fig. S2B–D†). At a BirA-GFP
log dynamic range with a R2 correlation coefficient of 0.998 was obtained with our selected bioO qPCR DNA template and primers (Fig. 3B). A series of background control qIPCR were set up without IgG (Fig. 3C), as well as by replacing E. coli BirA-GFP with either B. pseudomallei or M. tuberculosis BirA-GFP which do not bind bioO (Fig. S2B–D†). At a BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO detection device concentration of 2.5 nM, Cq values ∼32 were obtained for the background control reactions which is equivalent to ∼10−14 M bioO (Cq value ∼32) as derived from the standard curve (cf.Fig. 3B and C). The qIPCR background Cq values are due to the concentration-dependent non-specific adsorption and dissociation of the bioO DNA (directly and indirectly) to and from a well's surface. Thus, background qIPCR Cq values represent the absolute limit of detection of the qIPCR which are mirrored by the M. tuberculosis and B. pseudomallei BirA-GFP data (Fig. S2†).
bioO detection device concentration of 2.5 nM, Cq values ∼32 were obtained for the background control reactions which is equivalent to ∼10−14 M bioO (Cq value ∼32) as derived from the standard curve (cf.Fig. 3B and C). The qIPCR background Cq values are due to the concentration-dependent non-specific adsorption and dissociation of the bioO DNA (directly and indirectly) to and from a well's surface. Thus, background qIPCR Cq values represent the absolute limit of detection of the qIPCR which are mirrored by the M. tuberculosis and B. pseudomallei BirA-GFP data (Fig. S2†).
Our proof-of-concept qIPCR assay could detect bioO molecules dissociating from wells that were coated with a ≥10−12 M anti-GFP IgG suspension (Fig. 3D). The binding affinity and kinetics of the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO detection device are unknown in the PBS-TBA conditions and cannot be examined using traditional methods such as SPR due to the changes in binding and elution conditions as well as differences in surfaces. Hence, we performed the same qIPCR assay in the presence of MgCl2 to evaluate the E. coli holoBirA-GFP
bioO detection device are unknown in the PBS-TBA conditions and cannot be examined using traditional methods such as SPR due to the changes in binding and elution conditions as well as differences in surfaces. Hence, we performed the same qIPCR assay in the presence of MgCl2 to evaluate the E. coli holoBirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO complex as a detection device. In these conditions, the qIPCR was less sensitive with a detection limit for anti-GFP IgG >10−11 M (Fig. 3Dcf. red diamonds). To shed further light onto these findings, we performed a time-course experiment with complexes formed in the presence or absence of MgCl2 aiming at comparing the dissociation kinetics of bioO from E. coli BirA-GFP after the qIPCR wash steps (Fig. 3E). The data clearly show that when the device is prepared without MgCl2, significantly more bioO molecules bind to the wells and they also dissociate faster after the wash steps. As such, the stability of the protein
bioO complex as a detection device. In these conditions, the qIPCR was less sensitive with a detection limit for anti-GFP IgG >10−11 M (Fig. 3Dcf. red diamonds). To shed further light onto these findings, we performed a time-course experiment with complexes formed in the presence or absence of MgCl2 aiming at comparing the dissociation kinetics of bioO from E. coli BirA-GFP after the qIPCR wash steps (Fig. 3E). The data clearly show that when the device is prepared without MgCl2, significantly more bioO molecules bind to the wells and they also dissociate faster after the wash steps. As such, the stability of the protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA connection is not the only essential attribute of our ultrasensitive qIPCR detection device. Here, increased binding and fast release of bioO DNA are both desirable to improve the analytical detection performance of our E. coli BirA
DNA connection is not the only essential attribute of our ultrasensitive qIPCR detection device. Here, increased binding and fast release of bioO DNA are both desirable to improve the analytical detection performance of our E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO qIPCR assay. Alternative DNA release mechanisms triggered by either BCCP or the AviTag peptide in conjunction with high affinity mutants49 like BirAG154D could be envisaged in qIPCR applications, albeit with obvious drawbacks, e.g. increased complexity and cost.
bioO qIPCR assay. Alternative DNA release mechanisms triggered by either BCCP or the AviTag peptide in conjunction with high affinity mutants49 like BirAG154D could be envisaged in qIPCR applications, albeit with obvious drawbacks, e.g. increased complexity and cost.
Bridging immunoassay format
Sensitive bridging immunoassays are useful for the detection of anti-drug antibodies50,51 taking advantage of their dimeric structure, and qIPCR techniques have demonstrated superior sensitivity.52,53 Here, we evaluated the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO in a bridging qIPCR format (Fig. 4A). We compared the performance of the E. coli BirA-GFP
bioO in a bridging qIPCR format (Fig. 4A). We compared the performance of the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO with a commercial protein G-peroxidase conjugate in the same wells, in identical conditions (Fig. 4B, cf. blue diamonds). A plateau is reached with a 10−7 M GFP suspension for well coating with either detection method (Fig. 4B). However, the detection range is much narrower with the protein G-peroxidase. Overall, the analytical sensitivity and dynamic range of the E. coli BirA-GFP
bioO with a commercial protein G-peroxidase conjugate in the same wells, in identical conditions (Fig. 4B, cf. blue diamonds). A plateau is reached with a 10−7 M GFP suspension for well coating with either detection method (Fig. 4B). However, the detection range is much narrower with the protein G-peroxidase. Overall, the analytical sensitivity and dynamic range of the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO device is impressively high compared to the peroxidase conjugate (∼10
bioO device is impressively high compared to the peroxidase conjugate (∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fold more sensitive, cf.Fig. 4B).
000 fold more sensitive, cf.Fig. 4B).
Antibody and surface profiling
SPR is the gold standard method for antibody affinity and concentration determination. However, it cannot be applied to antibody samples containing other protein contaminants and does not mirror the surface interactions occurring in an ELISA format. Here we showcase an important application of the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO detection device in antibody and capture surface profiling. The bioO release system is particularly useful in profiling surfaces coated with high affinity binders with extremely slow dissociation rates. We performed a cross comparison of a selection of polyclonal anti-GFP antibodies (chicken and biotinylated goat) as well as a mouse monoclonal anti-6His antibody (N-terminal tag of E. coli BirA-GFP).
bioO detection device in antibody and capture surface profiling. The bioO release system is particularly useful in profiling surfaces coated with high affinity binders with extremely slow dissociation rates. We performed a cross comparison of a selection of polyclonal anti-GFP antibodies (chicken and biotinylated goat) as well as a mouse monoclonal anti-6His antibody (N-terminal tag of E. coli BirA-GFP).
The BioRad iTaq Universal SYBR green supermix was selected to bring the background Cq value ≥40 at a device concentration of 1 nM and steepen the slope of Cq as a function of antibody concentration. This qPCR mix reduced the PCR efficiency to 1.376 and is better suited to distinguish small changes in bioO concentration. All antibodies were coated at a concentration of 2 μg ml−1. The wells were probed with the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO device at 2.500, 1.250, 0.625, 0.313 and 0.156 nM (Fig. 5).
bioO device at 2.500, 1.250, 0.625, 0.313 and 0.156 nM (Fig. 5).
The anti-6His antibody bound significantly less E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO than the polyclonal anti-GFP antibodies. Interestingly, while the chicken anti-GFP captured more E. coli BirA-GFP
bioO than the polyclonal anti-GFP antibodies. Interestingly, while the chicken anti-GFP captured more E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO at lower concentrations and plateaued early, the biotinylated goat anti-GFP increased steadily binding the highest number of detection device. Here, we demonstrate that the E. coli BirA-GFP
bioO at lower concentrations and plateaued early, the biotinylated goat anti-GFP increased steadily binding the highest number of detection device. Here, we demonstrate that the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO can be applied to compare different antibodies and the relative binding capacity of a surface. Our bioO and primer combination design and qPCR mix yielding a lower PCR efficiency enables reproducible and more precise determination of intermediate protein concentrations. However, this comes at a cost of reducing the dynamic range and sensitivity of the E. coli BirA-GFP
bioO can be applied to compare different antibodies and the relative binding capacity of a surface. Our bioO and primer combination design and qPCR mix yielding a lower PCR efficiency enables reproducible and more precise determination of intermediate protein concentrations. However, this comes at a cost of reducing the dynamic range and sensitivity of the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO detection device.
bioO detection device.
Conclusions and perspective
Despite its relatively small size, E. coli BirA is an exceptionally well-behaved protein offering a variety of functionalities, binding, and catalytic activities. The increased thermostability of E. coli BirA-GFP in the presence of ATP, biotin, MgCl2, and bioO offers benefits such as extended binding steps and storage at room temperature. There is no doubt that this high-profile protein will be given a special place in the protein chemist's toolbox with its new use as an on–off protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA connection switch. Here, we validated the first application of E. coli BirA as a connector between a GFP and a DNA template in various qIPCR assay formats with excellent picomolar detection performance. Our proof-of-concept data should spark the development of further qIPCR-based applications for the detection of protein
DNA connection switch. Here, we validated the first application of E. coli BirA as a connector between a GFP and a DNA template in various qIPCR assay formats with excellent picomolar detection performance. Our proof-of-concept data should spark the development of further qIPCR-based applications for the detection of protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein or protein
protein or protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand interactions. Protein and DNA display technologies requiring reversible high-density DNA or protein arrays, especially in concert with non-dissociating physisorbed or chemisorbed high-affinity IgG, could benefit from the E. coli BirA on–off protein
ligand interactions. Protein and DNA display technologies requiring reversible high-density DNA or protein arrays, especially in concert with non-dissociating physisorbed or chemisorbed high-affinity IgG, could benefit from the E. coli BirA on–off protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA connection switch. We also envisage that droplet digital PCR could be used as an alternative absolute quantitative readout method.54
DNA connection switch. We also envisage that droplet digital PCR could be used as an alternative absolute quantitative readout method.54
In the last two decades, the flexibility, streamlining and multiplexing power of qPCR for both DNA and protein quantification have created opportunities for the development of innovative bioassays. If combined with other systems such as the Tus![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ter-lock,6 the unique specificity of E. coli BirA
Ter-lock,6 the unique specificity of E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO could offer an avenue for multiplexing or polyplexing qIPCR assays. Interestingly, despite the significantly weaker complex stability30 and faster dissociation kinetics21 of E. coli BirA
bioO could offer an avenue for multiplexing or polyplexing qIPCR assays. Interestingly, despite the significantly weaker complex stability30 and faster dissociation kinetics21 of E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO compared to Tus
bioO compared to Tus![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ter-lock,6,55 both qIPCR technologies perform similarly. Our data suggest that the E. coli BirA
Ter-lock,6,55 both qIPCR technologies perform similarly. Our data suggest that the E. coli BirA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO complex is stable in our qIPCR conditions and that the bioO DNA release system is efficient. At a very low antibody concentration, dimerization of the E. coli BirA-GFP on bioO could be advantageous. Indeed, based on the crystal structure of the holoenzyme dimer,26 it can reasonably be argued that the two paratopes of an anti-GFP antibody could simultaneously bind to the two GFP in the E. coli BirA-GFP
bioO complex is stable in our qIPCR conditions and that the bioO DNA release system is efficient. At a very low antibody concentration, dimerization of the E. coli BirA-GFP on bioO could be advantageous. Indeed, based on the crystal structure of the holoenzyme dimer,26 it can reasonably be argued that the two paratopes of an anti-GFP antibody could simultaneously bind to the two GFP in the E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO detection device, resulting in improved analytical sensitivity due to increased avidity and slower dissociation of the protein complex. This could be particularly valuable for ultrahigh affinity antibody profiling.
bioO detection device, resulting in improved analytical sensitivity due to increased avidity and slower dissociation of the protein complex. This could be particularly valuable for ultrahigh affinity antibody profiling.
E. coli BirA-GFP can be expressed in much higher yields and soluble form than Tus-GFP.6,42 Other examples of E. coli BirA fusion proteins such as CBD-BirA-His have been produced in high yields for protein biotinylation,44 further supporting the flexibility of E. coli BirA for functional fusion protein production. Finally, multiple strategies are available to produce bispecific antibodies56 offering opportunities to couple a diagnostic antibody with an anti-GFP antibody for ultrasensitive detection with E. coli BirA-GFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bioO. In fact, GFP-tagged nanobodies32 and several combinations of bispecific antibodies with affinity towards GFP or comprising a GFP have already been engineered33–37 that could easily be implemented in the qIPCR assay formats presented in this work.
bioO. In fact, GFP-tagged nanobodies32 and several combinations of bispecific antibodies with affinity towards GFP or comprising a GFP have already been engineered33–37 that could easily be implemented in the qIPCR assay formats presented in this work.
Data availability
Data supporting this article have been included as part of the ESI.† Raw data are available upon request from the authors.Conflicts of interest
There are no conflicts to declare.Notes and references
- J. B. Trads, T. Torring and K. V. Gothelf, Acc. Chem. Res., 2017, 50, 1367–1374 CrossRef CAS PubMed.
- S. Fredriksson, M. Gullberg, J. Jarvius, C. Olsson, K. Pietras, S. M. Gustafsdottir, A. Ostman and U. Landegren, Nat. Biotechnol., 2002, 20, 473–477 CrossRef CAS PubMed.
- L. Chang, J. Li and L. Wang, Anal. Chim. Acta, 2016, 910, 12–24 CrossRef CAS PubMed.
- S. P. Askin and P. M. Schaeffer, Analyst, 2012, 137, 5193–5196 RSC.
- D. B. Dahdah, I. Morin, M. J. Moreau, N. E. Dixon and P. M. Schaeffer, Chem. Commun., 2009, 3050–3052 RSC.
- I. Morin, N. E. Dixon and P. M. Schaeffer, Mol. BioSyst., 2010, 6, 1173–1175 RSC.
- I. Morin, S. P. Askin and P. M. Schaeffer, Analyst, 2011, 136, 4815–4821 RSC.
- T. Sano, C. L. Smith and C. R. Cantor, Science, 1992, 258, 120–122 CrossRef CAS PubMed.
- N. R. Pendini, M. Y. Yap, S. W. Polyak, N. P. Cowieson, A. Abell, G. W. Booker, J. C. Wallace, J. A. Wilce and M. C. Wilce, Protein Sci., 2013, 22, 762–773 CrossRef CAS PubMed.
- B. P. Duckworth, K. M. Nelson and C. C. Aldrich, Curr. Top. Med. Chem., 2012, 12, 766–796 CrossRef CAS PubMed.
- T. P. Soares da Costa, W. Tieu, M. Y. Yap, N. R. Pendini, S. W. Polyak, D. Sejer Pedersen, R. Morona, J. D. Turnidge, J. C. Wallace, M. C. Wilce, G. W. Booker and A. D. Abell, J. Biol. Chem., 2012, 287, 17823–17832 CrossRef CAS PubMed.
- O. Prakash and M. A. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 5592–5595 CrossRef CAS PubMed.
- Y. Xu and D. Beckett, Biochemistry, 1994, 33, 7354–7360 CrossRef CAS PubMed.
- Y. Xu, C. R. Johnson and D. Beckett, Biochemistry, 1996, 35, 5509–5517 CrossRef CAS PubMed.
- D. Beckett, E. Kovaleva and P. J. Schatz, Protein Sci., 1999, 8, 921–929 CrossRef CAS PubMed.
- M. Fairhead and M. Howarth, Methods Mol. Biol., 2015, 1266, 171–184 CrossRef CAS PubMed.
- M. G. Cull and P. J. Schatz, Methods Enzymol., 2000, 326, 430–440 CAS.
- A. E. Sorenson, S. P. Askin and P. M. Schaeffer, Anal. Methods, 2015, 7, 2087–2092 RSC.
- S. P. Askin, T. E. H. Bond and P. M. Schaeffer, Anal. Methods, 2016, 8, 418–424 RSC.
- P. R. Adikaram and D. Beckett, J. Mol. Biol., 2012, 419, 223–233 CrossRef CAS PubMed.
- E. D. Streaker and D. Beckett, J. Mol. Biol., 2003, 325, 937–948 CrossRef CAS PubMed.
- E. D. Streaker and D. Beckett, Protein Sci., 2006, 15, 1928–1935 CrossRef CAS PubMed.
- E. D. Streaker and D. Beckett, Biochemistry, 2006, 45, 6417–6425 CrossRef CAS PubMed.
- L. H. Weaver, K. Kwon, D. Beckett and B. W. Matthews, Protein Sci., 2001, 10, 2618–2622 CrossRef CAS PubMed.
- L. H. Weaver, K. Kwon, D. Beckett and B. W. Matthews, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 6045–6050 CrossRef CAS PubMed.
- Z. A. Wood, L. H. Weaver, P. H. Brown, D. Beckett and B. W. Matthews, J. Mol. Biol., 2006, 357, 509–523 CrossRef CAS PubMed.
- H. Zhao and D. Beckett, J. Mol. Biol., 2008, 380, 223–236 CrossRef CAS PubMed.
- J. Abbott and D. Beckett, Biochemistry, 1993, 32, 9649–9656 CrossRef CAS PubMed.
- E. D. Streaker and D. Beckett, Biochemistry, 1998, 37, 3210–3219 CrossRef CAS PubMed.
- E. D. Streaker, A. Gupta and D. Beckett, Biochemistry, 2002, 41, 14263–14271 CrossRef CAS PubMed.
- R. Y. Tsien, Annu. Rev. Biochem., 1998, 67, 509–544 CrossRef CAS PubMed.
- F. Jin, C. Shen, Y. Wang, M. Q. Wang, M. X. Sun and M. Hattori, Commun. Biol., 2021, 4, 366 CrossRef CAS PubMed.
- K. Tawa, M. Umetsu, T. Hattori and I. Kumagai, Anal. Chem., 2011, 83, 5944–5948 CrossRef CAS PubMed.
- S. Stamova, A. Feldmann, M. Cartellieri, C. Arndt, S. Koristka, F. Apel, R. Wehner, M. Schmitz, M. Bornhauser, M. von Bonin, G. Ehninger, H. Bartsch and M. Bachmann, Anal. Biochem., 2012, 423, 261–268 CrossRef CAS PubMed.
- B. Jedlitzke, Z. Yilmaz, W. Dorner and H. D. Mootz, Angew. Chem., Int. Ed., 2020, 59, 1506–1510 CrossRef CAS PubMed.
- S. Hemmi, R. Asano, K. Kimura, M. Umetsu, T. Nakanishi, I. Kumagai and K. Makabe, Biochem. Biophys. Res. Commun., 2020, 523, 72–77 CrossRef CAS PubMed.
- A. Alvarez-Cienfuegos, N. Nunez-Prado, M. Compte, A. M. Cuesta, A. Blanco-Toribio, S. L. Harwood, M. Villate, N. Merino, J. Bonet, R. Navarro, C. Munoz-Briones, K. M. Sorensen, K. Molgaard, B. Oliva, L. Sanz, F. J. Blanco and L. Alvarez-Vallina, Sci. Rep., 2016, 6, 28643 CrossRef CAS PubMed.
- A. E. Sorenson and P. M. Schaeffer, Methods Mol. Biol., 2020, 2089, 159–166 CrossRef CAS PubMed.
- T. E. H. Bond, A. E. Sorenson and P. M. Schaeffer, Microbiol. Res., 2017, 205, 35–39 CrossRef CAS PubMed.
- T. E. H. Bond, A. E. Sorenson and P. M. Schaeffer, Microbiol. Res., 2017, 199, 40–48 CrossRef CAS PubMed.
- M. J. J. Moreau, I. Morin, S. P. Askin, A. Cooper, N. J. Moreland, S. G. Vasudevan and P. M. Schaeffer, RSC Adv., 2012, 2, 11892–11900 RSC.
- M. J. Moreau and P. M. Schaeffer, Mol. BioSyst., 2013, 9, 3146–3154 RSC.
- J. M. Ruijter, C. Ramakers, W. M. Hoogaars, Y. Karlen, O. Bakker, M. J. van den Hoff and A. F. Moorman, Nucleic Acids Res., 2009, 37, e45 CrossRef CAS PubMed.
- S. C. Wu, J. C. Yeung, P. M. Hwang and S. L. Wong, Protein Expression Purif., 2002, 24, 357–365 CrossRef CAS PubMed.
- H. Zhao, S. Naganathan and D. Beckett, J. Mol. Biol., 2009, 389, 336–348 CrossRef CAS PubMed.
- H. Zhao, E. Streaker, W. Pan and D. Beckett, Biochemistry, 2007, 46, 13667–13676 CrossRef CAS PubMed.
- A. E. Sorenson and P. M. Schaeffer, Methods Mol. Biol., 2020, 2089, 69–85 CrossRef CAS PubMed.
- S. Askin, T. E. H. Bond, A. E. Sorenson, M. J. J. Moreau, H. Antony, R. A. Davis and P. M. Schaeffer, Chem. Commun., 2018, 54, 1738–1741 RSC.
- C. He, G. Custer, J. Wang, S. Matysiak and D. Beckett, Biochemistry, 2018, 57, 1119–1129 CrossRef CAS PubMed.
- S. Song, L. Yang, W. L. Trepicchio and T. Wyant, J. Immunol. Res., 2016, 2016, 3072586 Search PubMed.
- N. Nath, R. Flemming, B. Godat and M. Urh, J. Immunol. Methods, 2017, 450, 17–26 CrossRef CAS PubMed.
- M. Spengler, M. Adler, A. Jonas and C. M. Niemeyer, Biochem. Biophys. Res. Commun., 2009, 387, 278–282 CrossRef CAS PubMed.
- D. Jani, E. Savino and J. Goyal, Bioanalysis, 2015, 7, 285–294 CrossRef CAS PubMed.
- H. Schroder, M. Grosche, M. Adler, M. Spengler and C. M. Niemeyer, Biochem. Biophys. Res. Commun., 2017, 488, 311–315 CrossRef PubMed.
- M. J. Moreau and P. M. Schaeffer, Mol. BioSyst., 2012, 8, 2783–2791 RSC.
- U. Brinkmann and R. E. Kontermann, mAbs, 2017, 9, 182–212 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sd00225c |
| This journal is © The Royal Society of Chemistry 2024 |


