Open Access Article
Open Access ArticleA CRISPR-amplified label-free electrochemical aptasensor for the sensitive detection of HbA1c†
Jianfeng
Ma
,
Youwei
Zheng
,
Yaoyao
Xie
,
Dan
Zhu
 ,
Lianhui
Wang
,
Lianhui
Wang
 * and
Shao
Su
* and
Shao
Su
 *
*
State Key Laboratory of Organic Electronics and Information Displays & Jiangsu Key Laboratory of Smart Biomaterials and Theranostic Technology, Institute of Advanced Materials (IAM), Nanjing University of Posts and Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamlhwang@njupt.edu.cn; iamssu@njupt.edu.cn
First published on 16th July 2024
Abstract
Glycated hemoglobin (HbA1c) is a pivotal biomarker for the monitoring and early diagnosis of diabetes. The CRISPR-Cas system has fascinating application prospects in the next generation of biosensors due to its high specificity, efficiency, flexibility, and customization. Herein, a label-free electrochemical aptasensor was designed for the detection of HbA1c by combining the specific recognition ability of aptamers with the signal amplification effect of the CRISPR-Cas12a system. In the presence of HbA1c, the cis–trans cleavage ability of Cas12a protein was activated, causing the pre-formed probe DNA to be heavily cleaved and the electrochemical signal to increase. With CRISPR-assisted signal amplification, the developed electrochemical aptasensor can detect as low as 0.84 ng mL−1 HbA1c. Moreover, this aptasensor can detect 10 ng mL−1 HbA1c in 50% human serum due to its high selectivity, reproducibility, and long-term stability, which is lower than its physiological level in human blood samples. All results proved that the proposed aptasensor has a promising application in the early diagnosis and long-term monitoring of diabetes.
Introduction
Diabetes mellitus has gradually become a threat to public health, which brings a heavy economic burden to society and families.1–4 According to the Global Burden of Disease Study 2021, the number of diabetes patients is estimated to reach 1.31 billion worldwide by 2050.5 It is well-known that careful blood glucose management plays a vital role in reducing long-term complications of diabetes.6,7 Therefore, rapid and accurate early diagnosis of diabetes attracts more and more scientists' attention. Compared to classical blood glucose, glycated hemoglobin (HbA1c) has been recognized as a crucial biomarker for the diagnosis of diabetes because it reflects the average glucose level over a 2- to 3-month period.8–10 Moreover, the HbA1c concentration is not affected by diets, insulin levels and emotions. In general, the HbA1c and the HbA1c level (HbA1c%) in normal individuals are approximately 3–13 mg mL−1 and 4–6%, respectively.11,12 Importantly, the American Diabetes Association (ADA) and the World Health Organization (WHO) have set a diagnostic threshold of HbA1c% ≥ 6.5% for the diagnosis of diabetes since 2009. Due to the rapid increase of diabetes, it is urgent to develop high-performance methods for HbA1c detection.Compared with antibodies, aptamers are considered as ideal recognized units for the capture and detection of HbA1c due to their high chemical stability, ease of large-scale synthesis, and high binding affinity.13 Among all methods for HbA1c detection, the use of electrochemical aptasensors has been proved as an efficient method due to their easy preparation, high selectivity and fast detection process.14–16 To further improve the performance of electrochemical aptasensors for HbA1c detection, different signal amplification strategies have been introduced into the construction of electrochemical aptasensors. For example, E. Omidinia et al. utilized hierarchical gold architecture structure-decorated reduced graphene oxide (rGO) as an electrode modifier to amplify the electrochemical signal. The large surface area and high conductivity of these nanocomposites led to a low detection limit (1 nM) for HbA1c detection.17 Similarly, Yang et al. used silver-doped hollow carbon spheres (Ag@HCS) as signal amplification probes to construct an electrochemical aptasensor for HbA1c detection. Due to the large specific surface area and superior electrical conductivity of Ag@HCS, the designed aptasensor can detect as low as 0.35 μg mL−1 HbA1c.18
Recently, clustered regularly interspaced short palindromic repeat (CRISPR) technologies have been considered as powerful tools for developing next-generation biosensors for biological molecule detection due to their high specificity, efficiency, flexibility, and customization.19–24 Similar to duplex-specific nuclease (DSN) and DNAzyme, the cleavage activity of the CRISPR-Cas system can be easily regulated.25,26 Moreover, the CRISPR-Cas system easily couples with aptamers to construct high-performance aptasensors.27–29 Nowadays, CRISPR-based aptasensors are employed to analyze proteins,27 small molecules,28 and metal ions29 with satisfactory results. For example, Wang et al. established a fluorescence biosensor based on rolling circle amplification (RCA) and the CRISPR-Cas12a system for HbA1c detection. With the assistance of RCA, the CRISPR-based biosensor can detect 0.129 pg mL−1 HbA1c with high selectivity and fast detection time.30 Inspired by the above studies, a label-free electrochemical aptasensor was designed for the sensitive detection of HbA1c by coupling the specific recognition ability of aptamers and the signal amplification effect of the CRISPR-Cas12a system. It should be noted that the combination of the cis- and trans-cleavage of the CRISPR-Cas12a system could obviously improve the analytical performance of the designed electrochemical aptasensor. In the presence of HbA1c, the cleavage activity of the CRISPR-Cas12a system was activated, leading to an outstanding increase in the electrochemical signal. By monitoring the changes of the electrochemical signal, the designed aptasensor can qualitatively and quantitatively analyze both HbA1c and HbA1c%.
Materials and methods
Materials and reagents
All DNA oligomers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China), and are listed in Table S1.† All chemicals and reagents are also recorded in the ESI.†Preparation of the recognition probe
A double-stranded DNA (dsDNA) structure composed of the aptamer and its complementary strand served as a recognition probe for recognizing HbA1c. At first, the aptamer and its complementary strand was incubated in phosphate buffer (PB) at 95 °C for 5 min. Then, the product was gradually cooled to room temperature at a rate of 2 °C min−1. Finally, the designed probe was stored in a refrigerator for sensing application.Fabrication of the electrochemical aptasensor
Firstly, the designed recognition probe was incubated with 10 mM tris(2-carboxyethyl)phosphine (TCEP) in the dark for 1 h to reduce the disulfide bonds. Subsequently, a gold electrode was pre-treated according to a classical cleaning procedure.31 After drying, the probe was assembled on the cleaned gold electrode surface via gold–sulfur (Au–S) bonding followed by incubation for 16 h at room temperature, which was defined as probe/Au. After washing and drying, 10 mM MCH was used to block the nonspecific remaining sites of probe/Au for 1 h at room temperature. Then, 8 μL different concentrations of HbA1c were incubated onto the electrode surface for 1 h at 37 °C. For the CRISPR-based signal amplification, the Cas12a–crRNA complex was incubated onto the electrode surface for 20 min at 37 °C to cleave the assembled dsDNA and single-stranded DNA (ssDNA).Electrochemical impedance spectroscopy (EIS) and square wave voltammetry (SWV) measurements were used to characterize the preparation process and the analytical performance of this aptasensor. In this work, [Fe(CN)6]3−/4− was used as a redox probe to monitor the detection process. It should be noted that the difference in current signals (ΔI = IHbA1c − Ino HbA1c) was used to evaluate the effect of signal amplification and the analytical performance of the electrochemical aptasensor. All electrochemical measurements were repeated at least 3 times.
Results and discussion
Design principle of the electrochemical aptasensor
As illustrated in Scheme 1, a label-free electrochemical aptasensor was designed for highly sensitive detection of HbA1c. At first, the HbA1c aptamer (Apt) and the complementary strand (cDNA) were pre-assembled into a dsDNA, which was used as a recognition probe to recognize and capture HbA1c. Expectedly, a slight increase in the electrochemical signal was observed after the recognition of HbA1c. The added HbA1c preferentially bound with Apt and promoted the dissociation of the dsDNA structure. As a result, the product of Apt-HbA1c was released from the electrode surface, resulting in the dsDNA assembled on the electrode surface becoming ssDNA (only cDNA immobilized on the electrode surface). At this point, the electron transfer between the electrode surface and [Fe(CN)6]3−/4− was facilitated, leading to an increase in the electrochemical signal. By further introducing the CRISPR-Cas12a system, an amplified electrochemical signal was obtained. In this work, the designed Cas12a–crRNA complex could specifically recognize the retained cDNA. Meanwhile, the cis- and trans-cleavage activity of Cas12a protein was activated. Therefore, both the assembled dsDNA and the retained ssDNA were cleaved, further facilitating the electron transfer between [Fe(CN)6]3−/4− and the electrode surface to amplify the electrochemical signal.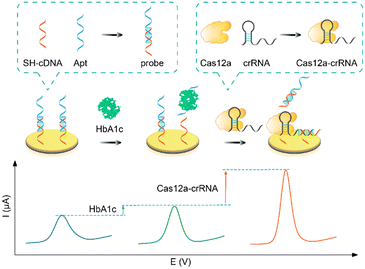 | ||
| Scheme 1 Schematic illustration of a CRISPR-amplified electrochemical aptasensor for HbA1c detection. | ||
Feasibility of the electrochemical aptasensor for HbA1c detection
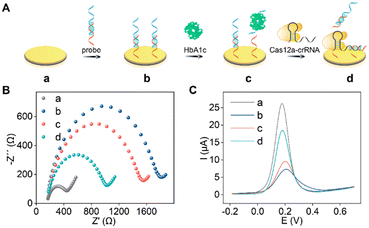
The step-by-step assembly process of the electrochemical aptasensor is demonstrated in Fig. 1A. The construction process was characterized by electrochemical impedance spectroscopy (EIS) and square wave voltammetry (SWV). As displayed in Fig. 1B, the impedance value (Ret) of probe/Au (curve b, 1612 Ω) was larger than that of the bare gold electrode (curve a, 209 Ω), proving the successful immobilization of the probe on the electrode surface. As expected, the Ret of this electrochemical aptasensor significantly decreased with the addition of HbA1c (curve c, 1325 Ω). The reason was ascribed to the dissociation of dsDNA into ssDNA. When the CRISPR-Cas12a system was further introduced on the sensing surface, the Ret of the aptasensor further decreased (Ret = 848 Ω, curve d). The specific binding of cDNA and crRNA activated the cis- and trans-cleavage activity of Cas12a, leading to the cleavage of the assembled dsDNA and retained ssDNA. Therefore, the electron transfer between [Fe(CN)6]3−/4− and the electrode surface became more convenient, resulting in a smaller impedance value. In addition, the feasibility of the electrochemical aptasensor for HbA1c detection was further validated by SWV. As shown in Fig. 1C, the bare Au electrode exhibits the highest oxidation peak current (26.03 μA, curve a). Obviously, the oxidation peak current of HbA1c/probe/Au (8.69 μA, curve c) is higher than that of probe/Au (6.31 μA, curve b). The reason is attributed to Apt in the probe which specifically recognizes HbA1c and disassociates from the electrode surface, facilitating electron transfer between the electrode and [Fe(CN)6]3−/4−. After introduction of the CRISPR-Cas12a system, the oxidation peak current of the Cas12a-crRNA/HbA1c/probe/Au further increased to 17.9 μA (curve d), which is attributed to the successful activation of the cis–trans cleavage activity of Cas12a. As a result, a large number of cDNAs and probes were cleaved. If the CRISPR-Cas12a system is introduced into probe/Au directly, the oxidation peak current shows almost no change (Fig. S1†), indicating that the CRISPR-based signal amplification is target-triggered. The above experimental results showed that the designed electrochemical aptasensor can effectively recognize and analyze HbA1c.Evaluation of CRISPR-based signal amplification
As shown in Fig. 2, ΔI was used to evaluate the effect of the CRISPR-based signal amplification strategy. Obviously, the ΔI of the CRISPR-amplified aptasensor (Fig. 2B) is about 11.66 μA for 1 μg mL−1 HbA1c detection, which is about 4.4-fold that of no CRISPR-assisted aptasensor (CRISPR-free, Fig. 2A). The experimental results proved that CRISPR-based signal amplification can greatly enhance the analysis performance of the aptasensor. | ||
| Fig. 2 Schematics and electrochemical signal change of the (A) CRISPR-free aptasensor and (B) CRISPR-amplified aptasensor for HbA1c detection in 0.5 mM [Fe(CN)6]3−/4− solution containing 0.1 M KCl. | ||
The analytical performance of the electrochemical aptasensor
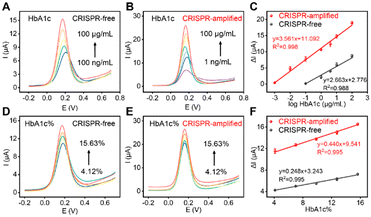
The critical experimental parameters of the electrochemical aptasensor were optimized to improve the analytical performance, and are recorded in Fig. S2.† Under the optimal conditions, the analytical performance of the CRISPR-free aptasensor and CRISPR-amplified aptasensor for HbA1c detection was tested, respectively. As shown in Fig. 3A and B, the currents of the CRISPR-free and CRISPR-amplified aptasensors increased with increasing concentrations of HbA1c, respectively. From the SWV curves of the CRISPR-free and CRISPR-amplified aptasensors, two linear relationships between the current change and the logarithm of HbA1c concentrations were obtained. The corresponding linear equations are y = 2.663x + 2.776 (R2 = 0.988) and y = 3.561x + 11.092 (R2 = 0.998), respectively (Fig. 3C). According to the equation, the detection limit of the CRISPR-amplified aptasensor was estimated to be 0.84 ng mL−1, which is lower than some published works (Table S2†).32–35 Compared with the CRISPR-free aptasensor, it can be seen that the CRISPR-amplified electrochemical aptasensor has a wider detection range (1 ng mL−1–100 μg mL−1vs. 100 ng mL−1–100 μg mL−1) and a lower limit of detection (0.84 ng mL−1vs. 98 ng mL−1) for HbA1c detection.The designed aptasensor was also used to analyze HbA1c%. Fig. 3D and E show that the electrochemical signals of the CRISPR-free and CRISPR-amplified aptasensors increased with increasing HbA1c% in the range of 4.12–15.63%, respectively. Correspondingly, two linear relationships between the ΔI and HbA1c% were obtained for the CRISPR-free and CRISPR-amplified aptasensors with equations of y = 0.248x + 3.243 (R2 = 0.995) and y = 0.440x + 9.541 (R2 = 0.995), respectively (Fig. 3F). The detection limit of the CRISPR-amplified aptasensor for HbA1c% detection was estimated to be 0.01%, which is lower than that of the CRISPR-free aptasensor (0.98%). All experimental results suggested that our developed CRISPR-amplified label-free electrochemical aptasensor has a promising application in the early diagnosis of diabetes.
Reproducibility, selectivity and stability of the CRISPR-amplified aptasensor
The reproducibility was evaluated by using ten independent CRISPR-amplified aptasensors for 100 μg mL−1 HbA1c detection under the same conditions. The relative standard deviation (RSD) of the original current and ΔI was calculated to be 4.33% (Fig. 4A) and 3.26% (Fig. 4B), respectively, suggesting that this aptasensor has excellent reproducibility. Then, the selectivity of this electrochemical aptasensor was tested by selecting common biomolecules in the blood as interfering substances, including total hemoglobin (tHb), uric acid (UA), ascorbic acid (AA), bovine serum albumin (BSA), and glucose. As shown in Fig. 4C, no obvious electrochemical signal change of this CRISPR-amplified aptasensor was observed for UA, AA, BSA and glucose detection. Moreover, the ΔI of the CRISPR-amplified aptasensor for HbA1c detection was approximately 3 times higher than that for tHb, indicating that the aptasensor could effectively distinguish HbA1c and tHb. All results proved that the designed aptasensor can detect HbA1c with high selectivity. The long-term storage stability of this aptasensor was tested by placing the aptasensor in a refrigerator for different days. Fig. 4D shows no obvious decrease in ΔI after 10 days of storage, proving that this aptasensor has excellent storage stability.Practical application of the CRISPR-amplified aptasensor
The practical application of this proposed aptasensor was evaluated by detecting various known concentrations of HbA1c (0.01, 0.1, 1 and 10 μg mL−1) in 10%, 20% and 50% human serum. As shown in Fig. 5A–C, this aptasensor can accurately detect HbA1c in the complex samples with acceptable relative standard deviation (RSD, Tables S3–S5†). Although ΔI gradually decreased with the increase of human serum concentration, the proposed aptasensor could still detect 0.01 μg mL−1 HbA1c in 50% human serum (Fig. 5D and S3†), indicating that this aptasensor had a charming practical application in the early diagnosis and long-term monitoring of diabetes.Conclusions
In this study, a CRISPR-amplified label-free electrochemical aptasensor was developed for the sensitive detection of HbA1c. Coupling the specific recognition ability of aptamers with the signal amplification effect of the CRISPR-Cas12a system, the designed electrochemical aptasensor exhibits excellent sensitivity, reproducibility, selectivity, stability and anti-interference ability, which can be employed to analyze HbA1c in human serum samples. This electrochemical aptasensor is simple, sensitive, and low-cost, proving that it has good prospects in the early diagnosis and long-term monitoring of diabetes.Author contributions
Jianfeng Ma: conceptualization, formal analysis, data curation, investigation, writing – original draft preparation. Youwei Zheng: methodology, validation, data curation, formal analysis. Yaoyao Xie: validation, data curation. Dan Zhu: methodology, formal analysis, visualization. Lianhui Wang: supervision, funding acquisition, project administration. Shao Su: writing – review and editing, supervision, funding acquisition, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Leading-edge Technology Program of Jiangsu Natural Science Foundation (BK20212012), the “Belt and Road” Innovation Cooperation Project of Jiangsu (BZ2022011), and the Natural Science Foundation of the Jiangsu Higher Education Institutions (22KJA150003).Notes and references
- L. Guariguata, D. R. Whiting, I. Hambleton, J. Beagley, U. Linnenkamp and J. E. Shaw, Diabetes Res. Clin. Pract., 2014, 103, 137–149 CrossRef CAS PubMed.
- A. J. M. Boulton, A. I. Vinik, J. C. Arezzo, V. Bril, E. L. Feldman, R. Freeman, R. A. Malik, R. E. Maser, J. M. Sosenko and D. Ziegler, Diabetes Care, 2005, 28, 956–962 CrossRef.
- J. M. Forbes and M. E. Cooper, Physiol. Rev., 2013, 93, 137–188 CrossRef CAS.
- A. Girach, D. Manner and M. Porta, Int. J. Clin. Pract., 2006, 60, 1471–1483 CrossRef CAS.
- K. L. Ong, et. al. , Lancet, 2023, 402, 203–234 CrossRef.
- N. A. ElSayed, G. Aleppo, V. R. Aroda, R. R. Bannuru, F. M. Brown, D. Bruemmer, B. S. Collins, J. L. Gaglia, M. E. Hilliard, D. Isaacs, E. L. Johnson, S. Kahan, K. Khunti, J. Leon, S. K. Lyons, M. L. Perry, P. Prahalad, R. E. Pratley, J. J. Seley, R. C. Stanton, R. A. Gabbay and A. American Diabetes, Diabetes Care, 2022, 46, S19–S40 CrossRef.
- A. American Diabetes, Diabetes Care, 2016, 40, S11–S24 CrossRef PubMed.
- S. Yazdanpanah, M. Rabiee, M. Tahriri, M. Abdolrahim, A. Rajab, H. E. Jazayeri and L. Tayebi, Crit. Rev. Clin. Lab. Sci., 2017, 54, 219–232 CrossRef CAS PubMed.
- I. M. Mostafa, H. Liu, S. Hanif, M. R. H. S. Gilani, Y. Guan and G. Xu, Anal. Chem., 2022, 94, 6853–6859 CrossRef CAS PubMed.
- Z. Zhan, Y. Li, Y. Zhao, H. Zhang, Z. Wang, B. Fu and W. J. Li, Biosensors, 2022, 12, 221 CrossRef CAS PubMed.
- A. American Diabetes, Diabetes Care, 2007, 30, S42–S47 CrossRef.
- J. Yadav, A. Rani, V. Singh and B. M. Murari, Biomedical Signal Processing and Control, 2015, 18, 214–227 CrossRef.
- H.-M. Meng, H. Liu, H. Kuai, R. Peng, L. Mo and X.-B. Zhang, Chem. Soc. Rev., 2016, 45, 2583–2602 RSC.
- S. H. Ang, M. Thevarajah, Y. Alias and S. M. Khor, Clin. Chim. Acta, 2015, 439, 202–211 CrossRef CAS PubMed.
- C. Apiwat, P. Luksirikul, P. Kankla, P. Pongprayoon, K. Treerattrakoon, K. Paiboonsukwong, S. Fucharoen, T. Dharakul and D. Japrung, Biosens. Bioelectron., 2016, 82, 140–145 CrossRef CAS PubMed.
- L. Zhou, G. Figueroa-Miranda, S. Chen, M. Neis, Z. Hu, R. Zhu, Y. Li, M. Prömpers, A. Offenhäusser and D. Mayer, Sens. Actuators, B, 2023, 386, 133730 CrossRef CAS.
- S. Y. Shajaripour Jaberi, A. Ghaffarinejad and E. Omidinia, Anal. Chim. Acta, 2019, 1078, 42–52 CrossRef CAS.
- Y. Yang, H. Dong, H. Yin, J. Gu, Y. Zhang, M. Xu, X. Wang and Y. Zhou, Bioelectrochemistry, 2023, 152, 108450 CrossRef CAS PubMed.
- M. M. Kaminski, O. O. Abudayyeh, J. S. Gootenberg, F. Zhang and J. J. Collins, Nat. Biomed. Eng., 2021, 5, 643–656 CrossRef CAS PubMed.
- Z. Weng, Z. You, J. Yang, N. Mohammad, M. Lin, Q. Wei, X. Gao and Y. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202214987 CrossRef CAS PubMed.
- P. Fozouni, S. Son, M. Díaz de León Derby, G. J. Knott, C. N. Gray, M. V. D'Ambrosio, C. Zhao, N. A. Switz, G. R. Kumar, S. I. Stephens, D. Boehm, C.-L. Tsou, J. Shu, A. Bhuiya, M. Armstrong, A. R. Harris, P.-Y. Chen, J. M. Osterloh, A. Meyer-Franke, B. Joehnk, K. Walcott, A. Sil, C. Langelier, K. S. Pollard, E. D. Crawford, A. S. Puschnik, M. Phelps, A. Kistler, J. L. DeRisi, J. A. Doudna, D. A. Fletcher and M. Ott, Cell, 2021, 184, 323–333 CrossRef CAS PubMed.
- M. Broto, M. M. Kaminski, C. Adrianus, N. Kim, R. Greensmith, S. Dissanayake-Perera, A. J. Schubert, X. Tan, H. Kim, A. S. Dighe, J. J. Collins and M. M. Stevens, Nat. Nanotechnol., 2022, 17, 1120–1126 CrossRef CAS PubMed.
- J. S. Chen, E. Ma, L. B. Harrington, M. Da Costa, X. Tian, J. M. Palefsky and J. A. Doudna, Science, 2018, 360, 436–439 CrossRef CAS PubMed.
- C. M. Ackerman, C. Myhrvold, S. G. Thakku, C. A. Freije, H. C. Metsky, D. K. Yang, S. H. Ye, C. K. Boehm, T.-S. F. Kosoko-Thoroddsen, J. Kehe, T. G. Nguyen, A. Carter, A. Kulesa, J. R. Barnes, V. G. Dugan, D. T. Hung, P. C. Blainey and P. C. Sabeti, Nature, 2020, 582, 277–282 CrossRef CAS PubMed.
- H.-L. Shuai, K.-J. Huang, Y.-X. Chen, L.-X. Fang and M.-P. Jia, Biosens. Bioelectron., 2017, 89, 989–997 CrossRef CAS PubMed.
- J. Xu, Y. Liu, Y. Li, Y. Liu and K.-J. Huang, Anal. Chem., 2023, 95, 13305–13312 CrossRef CAS PubMed.
- Y. Dai, R. A. Somoza, L. Wang, J. F. Welter, Y. Li, A. I. Caplan and C. C. Liu, Angew. Chem., Int. Ed., 2019, 58, 17399–17405 CrossRef CAS PubMed.
- M. Liang, Z. Li, W. Wang, J. Liu, L. Liu, G. Zhu, L. Karthik, M. Wang, K.-F. Wang, Z. Wang, J. Yu, Y. Shuai, J. Yu, L. Zhang, Z. Yang, C. Li, Q. Zhang, T. Shi, L. Zhou, F. Xie, H. Dai, X. Liu, J. Zhang, G. Liu, Y. Zhuo, B. Zhang, C. Liu, S. Li, X. Xia, Y. Tong, Y. Liu, G. Alterovitz, G.-Y. Tan and L.-X. Zhang, Nat. Commun., 2019, 10, 3672 CrossRef.
- Y. Xiong, J. Zhang, Z. Yang, Q. Mou, Y. Ma, Y. Xiong and Y. Lu, J. Am. Chem. Soc., 2020, 142, 207–213 CrossRef CAS PubMed.
- W. Wang, L. Geng, Y. Zhang, W. Shen, M. Bi, T. Gong, C. Liu, Z. Hu, C. Guo and T. Sun, Microchem. J., 2024, 200, 110370 CrossRef CAS.
- J. Zhang, S. Song, L. Wang, D. Pan and C. Fan, Nat. Protoc., 2007, 2, 2888–2895 CrossRef CAS PubMed.
- M. D. Mozammal Hossain, J.-M. Moon, N. G. Gurudatt, D.-S. Park, C. S. Choi and Y.-B. Shim, Biosens. Bioelectron., 2019, 142, 111515 CrossRef CAS PubMed.
- I. Pandey and J. D. Tiwari, Sens. Actuators, B, 2019, 285, 470–478 CrossRef CAS.
- P. Zhang, Y. Zhang, X. Xiong, Y. Lu and N. Jia, Sens. Actuators, B, 2020, 321, 128626 CrossRef CAS.
- A. Anand, C.-Y. Chen, T.-H. Chen, Y.-C. Liu, S.-Y. Sheu and Y.-T. Chen, Nano Today, 2021, 41, 101294 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sd00193a |
| This journal is © The Royal Society of Chemistry 2024 |