Open Access Article
Open Access ArticleA photoelectrocatalytic system as a reaction platform for selective radical–radical coupling†
Sunghwan
Won
a,
Dongmin
Park
 b,
Yousung
Jung
b,
Yousung
Jung
 *c,
Hyunwoo
Kim
*c,
Hyunwoo
Kim
 *de and
Taek Dong
Chung
*de and
Taek Dong
Chung
 *af
*af
aDepartment of Chemistry, Seoul National University, Seoul 08826, Republic of Korea. E-mail: tdchung@snu.ac.kr
bDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea
cDepartment of Chemical and Biological Engineering, Institute of Chemical Processes, Seoul National University, Seoul 08826, Republic of Korea. E-mail: yousung.jung@snu.ac.kr
dDepartment of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang 37679, Republic of Korea. E-mail: khw7373@postech.ac.kr
eInstitute for Convergence Research and Education in Advanced Technology (I–CREATE), Yonsei University, Seoul 03722, Republic of Korea
fAdvanced Institutes of Convergence Technology, Suwon-Si, Gyeonggi-do 16229, Republic of Korea
First published on 23rd September 2024
Abstract
The selection of electrode material is a critical factor that determines the selectivity of electrochemical organic reactions. However, the fundamental principles governing this relationship are still largely unexplored. Herein, we demonstrate a photoelectrocatalytic (PEC) system as a promising reaction platform for the selective radical–radical coupling reaction owing to the inherent charge-transfer properties of photoelectrocatalysis. As a model reaction, the radical trifluoromethylation of arenes is shown on hematite photoanodes without employing molecular catalysts. The PEC platform exhibited superior mono- to bis-trifluoromethylated product selectivity compared to conventional electrochemical methods utilizing conducting anodes. Electrochemical and density functional theory (DFT) computational studies revealed that controlling the kinetics of anodic oxidation of aromatic substrates is essential for increasing reaction selectivity. Only the PEC configuration could generate sufficiently high-energy charge carriers with controlled kinetics due to the generation of photovoltage and charge-carrier recombination, which are characteristic features of semiconductor photoelectrodes. This study opens a novel approach towards selective electrochemical organic reactions through understanding the intrinsic physicochemical properties of semiconducting materials.
Introduction
The development of chemoselective synthetic methodology is of utmost importance in organic chemistry. Selectivity is particularly essential for the synthesis of complex organic molecules, as it allows for precise control over multiple reactive sites leading to the efficient and selective construction of desired target molecules. In recent decades, radical-mediated organic transformations have emerged as attractive synthetic strategies enabling the straightforward generation of complex target molecules and overcoming conventional closed-shell reactivity.1–3 Recent advances by integrating radical intermediates with well-defined homogeneous redox-active catalyst systems4 or semiconducting particles as photocatalysts5,6 have been made to achieve the desired chemoselectivity. In particular, heterogeneous electrochemical systems merged with homogeneous transition-metal catalysts pave a new way for the synthesis of target organic molecules. This method takes advantage of the intrinsic strength of electrochemical systems, which enables facile regeneration of heterogeneous catalysts and allows for straightforward scalability, especially when combined with flow systems.7–9 Typically, the outcomes of the reaction, such as reaction yield, product selectivity, and faradaic efficiency, are closely linked to the choice of electrode materials during operation, although the underlying principles governing this relationship are still largely unexplored.10However, achieving chemoselectivity in radical organic reactions at a heterogeneous interface without the use of homogeneous catalysts remains challenging. The issue lies in the formation of radical intermediates within the electric double layer (EDL) region, a confined space (ca. 1 nm) at the electrode surface, in contrast to homogeneous catalysis. Since highly reactive radical species are produced in this tight space, it is vital to control the radical production kinetics to regulate the local radical concentration and achieve chemoselectivity.11,12 This is particularly crucial for radical–radical cross-coupling reactions, where balancing the generation kinetics of the two different radical species involved in the reaction is critical for increasing selectivity toward the cross-coupled product.13–15 Yet, in a typical electrochemical system, matching the radical generation current of the two reactants having different redox potentials is challenging.
On the other hand, the photoelectrocatalytic (PEC) system can be a promising platform for organic reactions involving radical intermediates since the number of charge carriers can be controlled by modulating the illumination intensity. Recently, the use of semiconductor photoelectrodes for the transformation of organic molecules has garnered intensive research interest, owing to its energy-harvesting capability and sustainable features.9,16–24 Despite the extensive use of photoelectrodes as tools for electron transfer reactions, the impact of the semiconductor itself on the reaction outcome, such as reaction efficiency and product selectivity, remains underexplored.25 Although regioselective arene C–H amination under the PEC system was previously reported,21 it is important to note that the selectivity observed in this case was due to the hydrogen bonding network between the reaction intermediate and the solvent, rather than originating from the photoanode itself.
Herein, we describe the inherent advantages of photoelectrodes in radical–radical coupling reactions by demonstrating a mono-selective radical trifluoromethylation of arenes under the photoelectrocatalytic system, which obviates the need for a molecular catalyst (Fig. 1). In recent years, the generation of trifluoromethyl (CF3) radicals by means of single electron transfer (SET) has been shown to be a productive synthetic strategy to access a wide range of trifluoromethylated organic compounds.26–31 The electrochemical radical trifluoromethylation also has been highlighted in several precedent reports, particularly due to the ability to control the rate of radical formation in the electrochemical method.32 Recent electrosynthetic techniques such as alternating current electrolysis33 and electrophotocatalysis34 were also successfully demonstrated.
 | ||
| Fig. 1 Photoelectrocatalytic trifluoromethylation of arenes. Potential values with respect to Ag/Ag+. | ||
Most of the precedent literature regarding arene radical trifluoromethylation showed modest to low efficiency with electron-rich substrates.35 Electrochemical trifluoromethylation has recently been developed for highly electron-rich substrates, but the method was not effective for moderately electron-rich and neutral substrates.36 More importantly, although some prior studies noted the formation of a comparable amount of bis-trifluoromethylated byproducts, limited attention has been devoted to enhancing the selectivity towards mono-substituted products.33,34,37
In this study, the newly developed PEC system exhibits good yields and high mono- to bis-trifluoromethylation selectivity, particularly for electron-rich arene substrates compared to electrochemical methods using conducting electrodes such as carbon or fluorine-doped tin oxide (FTO) anodes. Photogenerated holes were capable of oxidizing both arene substrates and the CF3 radical source, eventually leading to the formation of mono-substituted products through the radical–radical coupling pathway. The exceptional selectivity of the PEC configuration originates from the charge-transfer characteristics of the photoanode itself, such as charge-carrier recombination and the generation of photovoltage.
Results and discussion
Selective mono-trifluoromethylation on the PEC system
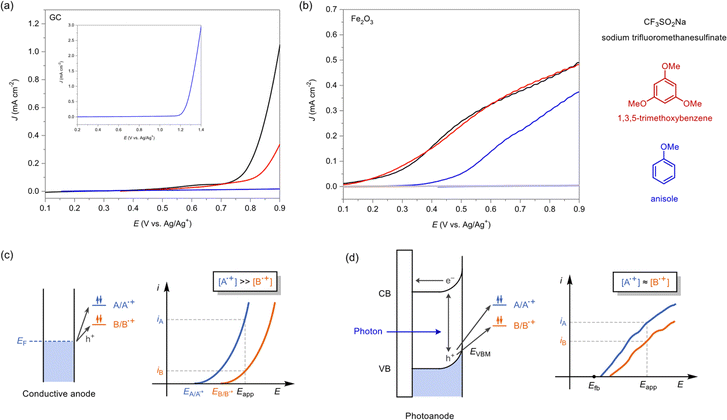
We have first examined whether radical trifluoromethylation is a suitable example for demonstrating potential advantages of photoanodes in radical–radical coupling reactions by voltammetric studies. Hematite (α-Fe2O3) is chosen as a photoanode material due to its fairly positive valence-band maximum (EVBM) and its stable operation in a wide range of organic solvents.21,38Linear sweep voltammograms (LSVs) of sodium trifluoromethanesulfinate (CF3SO2Na), a precursor of a CF3 radical, and aromatic substrates (1,3,5-trimethoxybenzene and anisole) were obtained at a glassy carbon (GC) electrode and a hematite photoanode (Fig. 2). CF3SO2Na is chosen as a CF3 radical source because it readily generates CF3 radicals via 1-electron oxidation and has a relatively low oxidation potential.32,39 At a GC electrode, each compound exhibits distinct onset potentials and current slopes, resulting in significantly different current densities at a certain overpotential (Fig. 2a). This is because current–voltage behavior follows Butler–Volmer kinetics under electrolysis conditions, which typically involve high substrate concentrations and forced convection (Fig. 2c). Thus, balancing the radical generation currents of the CF3 radical precursor and the arene substrates is challenging at the conducting anode.
In stark contrast, LSVs obtained at a hematite photoanode under illumination display relatively similar current densities to each other (Fig. 2b). The onset potentials were shifted to more negative values compared to those obtained at a GC electrode, indicating generation of photovoltage at the photoanode.40 Notably, anisole was directly oxidized by the photoanode at the given potential window, which was not the case with the carbon anode, illustrating the greater oxidation power of hematite due to its sufficiently positive EVBM (Fig. 2d and S5†). Also, the growth of photocurrents is less rapid compared to the electrochemical system due to charge-carrier recombination.41 These electrochemical data indicate that a hematite photoanode is more adequate for a radical–radical coupling reaction owing to its ability to “balance” the oxidation current of the two reaction partners (CF3SO2Na and an aromatic substrate) and sufficient oxidation power for generating aromatic radical cation.
We commenced our investigation by choosing 1,3,5-trimethoxybenzene 1a as our model substrate, hematite as the photoanode and platinum plate as the cathode (Fig. 3). The working electrode potential (EWE) was set at 0.8 V, where both the CF3 radical precursor and 1a could undergo photo-oxidation, and no dark current flowed (Fig. 2). After optimization, we observed that the application of both blue light (456 nm) and electric bias was necessary to yield the desired mono-trifluoromethylated product 1b selectively over bis-trifluoromethylated product 1c (Fig. 3, entries 1–3). This is because electron–hole pairs are generated through photo-excitation of electrons in the valence band, followed by separation of two charge carriers by electric field within the space-charge region induced by the applied potential. The optimal conditions employed 2.0 equivalent of CF3SO2Na as the radical precursor and LiClO4 as the electrolyte under dimethyl sulfoxide (DMSO) and water as the co-solvent. Since the concentration of CF3SO2Na affects the radical generation rate match and hence the mono/bis-selectivity, both the radical generation rate and the total amount of radicals should be optimized by adjusting the concentration of the radical precursors (Fig. S9†).
 | ||
| Fig. 3 Reaction parameter optimization. Yields determined by 19F NMR using CF3CO2H as an internal standard. | ||
The solvent systems other than DMSO/H2O such as acetonitrile (CH3CN) were not effective (entry 4). Both reaction yield and product selectivity decreased in the absence of water (entry 5). Furthermore, water was found to be the most efficient proton source compared to other protic co-solvents such as methanol and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) (entries 6–7). The choice of electrolyte was also crucial in both reactivity and mono/bis-selectivity (entry 8). When the reaction was carried out under ambient conditions, the mono/bis-selectivity was significantly dropped compared to the standard conditions, while the overall yield was slightly increased (entry 9).
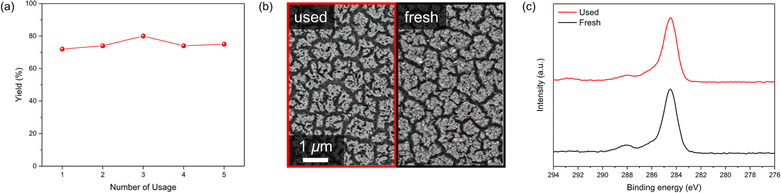
The durability of the electrode is also a crucial factor in electrochemical organic reactions. Often, the electrodes experience surface passivation which is mainly caused by the deposition of polymeric films produced by undefined side reactions of reaction intermediates.10,42,43 The catalytic activity of a hematite photoanode was retained during five iterative uses (Fig. 4a), exhibiting excellent robustness and catalyst-regeneration capability. The surface analysis by using scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD) further revealed that the morphology, chemical composition, and the band structure of the electrode surface were identical to those of a freshly-prepared photoanode (Fig. 4b, c and S6†). It is worth mentioning that the carbon-containing polymeric film was not observed, which could potentially lead to passivation of the photoelectrode. The robustness of the hematite surface also highlights its advantage in radical reactions.
Next, we employed our optimized reaction conditions to arene substrates with a range of different electronic properties (Fig. 5). For the substrates with electron-rich or neutral substituents, moderately high mono- to bis-trifluoromethylation selectivity was observed when using a hematite photoanode. Late-stage functionalization of pharmaceuticals containing electron-rich aromatic rings, such as Boc-protected melatonin (8) and gemfibrozil methyl ester (9), was successfully demonstrated, highlighting the practical utility and versatility of the PEC method. It was notable to see that 1,4-dicyanobenzene showed low reactivity, which cannot be photo-oxidized by the photoanode since its oxidation peak potential is more positive than the EVBM of hematite, ca. 1.7–1.8 V (Fig. S5†).38
For comparative purposes, the reaction was carried out using a carbon-felt anode in a two-electrode configuration, which is one of the most adopted configurations in electrochemical organic synthesis. The cell voltage was chosen within a range where the initial current is closely matched to that of the PEC system (see page S7 in the ESI† for the detailed procedure). Typical cell voltage ranged from 2.6 to 3.2 V, which corresponds to a working electrode potential of 0.6–0.8 V vs. Ag/Ag+ (Table S1†). The semiconductor electrode exhibited notably greater mono/bis-selectivity in contrast to the conducting anode, except in case where 1,4-dicyanobenzene was employed as the substrate (Fig. 5). Moreover, even though the mono/bis-selectivity slightly decreases with extended reaction time, the selectivity in the PEC system still outperforms the electrochemical analog at all reaction times (Fig. S10†).
These results suggest that a semiconductor electrode can selectively produce mono-substituted products when both reactants undergo electrochemical oxidation at the photoanode. This can be attributed to the relatively positive EVBM of hematite and the photovoltage generated within the PEC system, allowing the efficient oxidation of substrates ranging from electron-rich to neutral aromatic compounds, as well as the CF3 radical source.
DFT computational studies for possible reaction pathways
To explain the superior chemoselectivity of the PEC system, we deliberated the possible reaction pathways of the trifluoromethylation of the aromatic compounds (Fig. 6). The CF3 radical source should first be oxidized to generate a CF3 radical and consequently, the trifluoromethylated products. The reaction pathways thus bifurcate depending on whether the aromatic substrate is electrochemically oxidized or not (Fig. 6a). The formation of the radical cation intermediate 1a˙+ by photo-oxidation at a hematite photoanode is confirmed by a radical trapping experiment (Fig. S11†). However, the oxidation of 1a hardly occurs with the electrochemical system at the operating potential (i.e. 0.6–0.8 V; Fig. 2a).When the substrate 1a is oxidized at the photoanode surface (Fig. 6a-A), the corresponding radical cation 1a˙+ couples with a CF3 radical, generating the cationic intermediate [1a-CF3]+. Deprotonation of [1a-CF3]+ gives the mono-substituted product 1b. Thus, the kinetics of the radical–radical coupling pathway will also depend on the oxidation potential of the substrate. On the other hand, the reaction can also be initiated by attack of a CF3 radical on the aromatic substrate (Fig. 6a-B). This radical-attack leads to the formation of the radical intermediate [1a-CF3]˙, followed by the radical-polar crossover at the electrode surface. Rearomatization of the cationic intermediate [1a-CF3]+ gives the product 1b.
We anticipate that the radical–radical coupling pathway is dominant on the photoanode since hematite can oxidize the aromatic substrates. On the other hand, the radical-attack pathway is likely to occur when utilizing a carbon anode, since the aromatic compounds were hardly oxidized at the applied potential range, even though the system exhibited a comparable current level (ca. 2 mA) to the PEC configuration (Table S1†). The ratio of activation barriers between the mono-substitution step and the bis-trifluoromethylation step will determine the selectivity of the mono-substituted compound 1b over the bis-trifluoromethylated byproduct 1c (the ratio between ΔG‡(1a,coup) and ΔG‡(1b,coup) for the radical–radical coupling, ΔG‡(1a,att) and ΔG‡(1b,att) for the radical-attack pathway. See Fig. 6 for the abbreviations).
Density functional theory (DFT) calculations were performed to obtain kinetic insights of each step (Fig. 6b, see page S12 in the ESI† for the computational details). The mono-substitution step of the radical–radical coupling pathway occurs without a free-energy barrier since the coupling of two reactive radical species is highly favorable. On the other hand, the activation barrier of the second substitution step is found to be ΔG‡(2,coup) = 6.98 kcal mol−1, indicating that the second coupling step is kinetically less favorable than the first one. Also, because the mono-trifluoromethylated compound 1b exhibits more positive oxidation potential compared to the substrate 1a, generating the radical cation 1b˙+ is kinetically slower than 1a˙+ (Fig. S7†). Consequently, the radical–radical coupling pathway will facilitate kinetically selective formation of the mono-trifluoromethylated product.
On the other hand, relatively similar activation barriers of ΔG‡(1a,att) = 13.70 kcal mol−1 and ΔG‡(1b,att) = 14.47 kcal mol−1 were obtained for the radical-attack pathway. This result implies that achieving mono/bis-selectivity is less probable under mild overpotentials insufficient to oxidize the aromatic compounds. It is noteworthy that a CF3 radical is less likely to attack 1a or 1b instead of 1a˙+ when the oxidation potential of a hole is sufficiently high, since ΔG‡(1a,att) and ΔG‡(1b,att) are much greater than ΔG‡(1a,coup). The energy profile demonstrated in Fig. 6 represents the result at 1.8 V. However, the activation barriers of the radical-attack pathway at 0.8 V were identical to those obtained at 1.8 V, as the change in electrode potential only affects the free energy of the electron-transfer steps (Fig. S14†).
Thus, the present computational study suggests that sufficiently positive valence band position of hematite facilitates direct photoelectrochemical oxidation of the aromatic compounds, eventually leading to a selective production of mono-substituted product through radical–radical coupling. This explanation is consistent with the experimental result that both the PEC system and the carbon anode-based electrochemical setup exhibited identical reaction result with the highly electron-deficient substrate that cannot be directly oxidized by a hematite photoanode (Fig. 5).
Inherent advantages of the PEC configuration in selective radical reactions
Given that direct oxidation of the substrate may enhance the selectivity of the mono-substituted product, a series of electrochemical systems utilizing different electrode materials was employed for the trifluoromethylation of 1a to explore whether sufficient overpotential can improve the selectivity of electrochemical configurations (Fig. 7).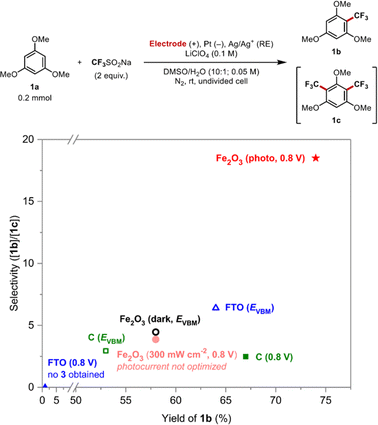 | ||
| Fig. 7 Reaction yield and selectivity depending on electrode material and applied voltage. EVBM corresponds to the valence band edge of hematite, 1.8 V vs. Ag/Ag+. | ||
First, the reaction was conducted with a hematite electrode in the dark at 1.8 V, which approximately corresponds to the EVBM, to dictate the direct electrochemical oxidation of the substrate without light irradiation (black open circle). Both the yield and mono/bis-selectivity were reduced, implying that the PEC reaction condition (red star) outperforms its purely electrochemical counterpart. It is known that electrochemical oxidation is not possible on the hematite surface in the absence of light due to its nature as an n-type semiconducting material.44 Consequently, the oxidation of organic molecules is expected to take place at the pinholes of the underlying conductive substrate, FTO.45,46 Comparable yields and product ratios to the dark condition were observed when employing a bare FTO electrode as the anode (blue open triangle), suggesting that the hematite layer does not exhibit catalytic activity under dark conditions. Hence, the reaction selectivity of the PEC system should be explained by the charge-transfer characteristics under illumination.
Conversely, no reaction took place at FTO at 0.8 V (blue filled triangle), which corresponds to the operating potential of the PEC system, as it is a relatively inert electrode material that demands a greater overpotential to facilitate the identical electron transfer reaction (Fig. S8†). The reaction was also conducted using a carbon anode at 0.8 V within a three-electrode system. Although the initial current level was almost identical to that of the PEC system, the inferior mono/bis-selectivity was observed (green filled square), consistent with the computational result.
When a potential of 1.8 V was applied to a carbon anode for the direct electrochemical oxidation of the substrate, it resulted in a slight increase in product selectivity but a decrease in the overall yield (green open square). The decrease in overall reaction yield can be attributed to the significant increase in current resulting from the application of high voltage. Owing to the elevated current density, a considerable amount of radical intermediates is generated in close proximity to the electrode surface, rendering the radical reaction uncontrollable and ultimately leading to a reduction in overall yield of the cross-coupled product.11,12 A similar reactivity pattern was observed with the semiconductor photoanode. When the initial current was increased by the strong irradiation (ca. 300 mW cm−2) while maintaining a constant overpotential, the product yield was deteriorated from 74% to 58% (pale red, filled circle). The elevated current density also deteriorated the product selectivity from the standard PEC reaction conditions, possibly because excessive charge carriers caused the oxidation of the mono-substituted product 1b, thereby facilitating the second trifluoromethylation step.
A similar trend was observed when driving PEC trifluoromethylation of 1a and anisole (6a) at 0.4 V (Fig. S12†). At this potential, 1a still oxidizes, but 6a barely does (Fig. 2b). Therefore, almost identical result was obtained for 1a. On the other hand, the selectivity of mono-trifluoromethylated product of 6a is decreased at 0.4 V. Since 6a is not oxidized at 0.4 V and only CF3 radicals can be generated, the reaction does not proceed through radical–radical coupling, leading to decreased selectivity for mono-substituted products.
Therefore, we have concluded that the reaction yield and product selectivity should be compromised at an electrochemical system utilizing a conducting electrode. At a low overpotential, the radical–radical coupling reaction cannot occur, resulting in relatively unselective behavior. At a high overpotential, even though reaction selectivity slightly increases due to radical–radical coupling, overall yield decreases because of high current density and generation of excess radical species. Since current and overpotential are coupled at a conducting electrode and cannot be independently optimized, additional strategies such as surface modification with electron-transfer mediators and/or incorporation of homogeneous catalysts are needed to enhance the selectivity of electrochemical systems.47
On the other hand, overpotential and photocurrent density can be independently tuned when using photoelectrodes because the number of photogenerated charge carriers is not only a function of overpotential but also depends on the illumination intensity. This characteristic feature of semiconductor photoelectrodes enables independent optimization of photocurrents under constant overpotential, while securing sufficient redox power by carefully selecting semiconducting material with a suitable band-edge alignment.
Numerous applications can stem from the intrinsic physicochemical properties of semiconductor photoelectrodes highlighted in this study. For instance, introducing various surface defects can help regulate the spatial distribution of ‘hole-emitting’ sites, where radical intermediates are generated.48 Since the allocation of catalytic sites significantly affects the efficiency of radical coupling reactions,49 appropriate surface treatment will maximize the benefits of PEC systems in these reactions.
Several characteristic features of a photoelectrode that have not been experimentally examined in this research may also affect the selectivity of reactions. For example, the presence of surface states is a distinctive feature of photoelectrodes compared to conducting electrodes.46,50,51 However, their role in the transformation of organic molecules has not been thoroughly investigated to date. The presence of native surface states may result in photogenerated holes having different oxidation potentials at the electrode surface, just before their transfer into the solution phase (Fig. S13†). This, in turn, could reduce the difference between the effective overpotential for each oxidation reaction (η1 and η2 in Fig. S13†), consequently decreasing the disparity in the two oxidation currents.
In summary, we have explored the impact of distinct charge-transfer characteristics of a semiconductor photoelectrode on the selectivity of radical reaction by demonstrating PEC trifluoromethylation of aromatic compounds as a model reaction. Since a PEC system is capable of controlled release of highly reactive minority charge carriers as well as balancing the radical generation currents between the two radical precursors, a selective radical–radical coupling reaction was successively achieved without help of a transition-metal-based homogeneous catalyst. Considering that the choice of electrode material significantly influences reaction outcomes, studying the impact of the charge-transfer characteristics of photoelectrodes on the reaction result will facilitate the development of more efficient PEC organic synthesis methods, as well as understanding mechanistic features.
Conclusions
In this study, to link the physical aspects of semiconductor photoelectrodes and their charge-transfer properties to reaction outcomes, we have demonstrated the radical trifluoromethylation of aromatic substrates at a hematite photoanode without employing a homogeneous catalyst. The PEC system outperformed conventional electrochemical systems utilizing conducting anodes in terms of product selectivity and reaction yield. By combining the photoelectrochemical analysis with the DFT computational studies, the outstanding selectivity of the PEC configuration is attributed to the following three characteristic charge-transfer behaviors of the hematite photoanode. First, the sufficiently positive valence band edge of the hematite enabled direct oxidation of the aromatic substrate, eventually leading to a radical–radical coupling pathway that enhances the mono- to bis-trifluoromethylation selectivity. Second, the overall photocurrent could be optimized under a constant overpotential by regulating the illumination intensity, preventing excessive generation of radical species while maintaining the oxidation power for generating radical intermediates. Third, the photocurrents for radical generation from each precursor could be well-balanced on the photoanode surface due to photocurrent generation and charge-carrier recombination. This work shows that understanding the inherent nature of semiconductor photoelectrodes can facilitate the development of more efficient and selective radical reactions.Experimental
Preparation of the photoanode
Hematite photoelectrodes were synthesized according to the previously reported procedure.52,53 Briefly, cleaned fluorine-doped tin oxide (FTO, Solaronix, Switzerland) glass was soaked in a precursor solution containing 0.15 M FeCl3 and 1.0 M NaNO3, and enclosed with stainless steel autoclave. The temperature of the system was raised to 95 °C and kept for 4 hours to form β-FeOOH film on the FTO substrate. After cooled at 4 °C for 45 minutes, the electrodes were sonicated for 3 minutes to remove poorly adsorbed particles. The substrates were then annealed at 550 °C for 2 hours to convert the as-deposited β-FeOOH film into hematite (α-Fe2O3) nanowire. Then, the hematite electrodes were additionally annealed at 800 °C for 20 minutes to improve their PEC activity.General procedure for photoelectrocatalytic trifluoromethylation of arenes
Electrolysis experiments were conducted using an MPG-2 multi-potentiostat (Biologic). Kessil PR160 blue LED (λ = 456 nm) is used as a light source. An oven-dried, 10 mL two-neck glass tube was equipped with a magnetic stir bar, a rubber septum, a threaded Teflon cap fitted with electrical feed-throughs, a hematite photoanode (1.0 × 1.0 cm2, connected to the electrical feedthrough via a 9 cm in length, 2 mm in diameter graphite rod), a platinum mesh cathode (0.5 × 1.0 cm2), and an Ag/Ag+ reference electrode. To this reaction vessel, LiClO4 (42.5 mg, 0.4 mmol), an arene substrate (0.2 mmol, 1.0 equiv.), and CF3SO2Na (62.4 mg, 0.4 mmol, 2 equiv.) were added. The cell was sealed and purged with a nitrogen-filled balloon for 15 minutes. Before initiating the photoelectrocatalytic reaction, a cyclic voltammogram was obtained in the dark to determine the potential range where dark current does not flow. Typical potential-range limit was 0.6–0.8 V, where the initial current was approximately 2 mA. The photoelectrochemical reaction was conducted under illumination with a blue LED (distance ∼5 cm) at room temperature for 14–18 hours. The reaction mixture was then filtered by silica or Celite and concentrated under reduced pressure. The residue was subjected to flash column chromatography on silica gel (eluted with hexane/ethyl acetate) to yield the desired product.Procedures for voltammetric studies
(Photo)electrochemical experiments were conducted using a Gamry Reference 600 potentiostat (Gamry Instruments). A glassy carbon (GC) rod electrode (d = 4 mm) or an FTO glass was used as a working electrode. A platinum wire was used as a counter electrode. Ag/Ag+ reference electrode was used as a reference electrode. For an FTO glass, a homemade Teflon electrochemical cell was used, and electrode area was defined by a super-viton P6 O-ring (inner diameter = 5.8 mm; Anyseal, Korea). Scan rate is 50 mV s−1, unless otherwise noted.Computational details
Density functional theory (DFT) calculations were conducted using Q-Chem 4.4 program54 to obtain optimized structures and energies of each reactant, product, transition state, and intermediate species. The range-separated ωB97X-D3 hybrid functional55 with the 6-31G* basis set56 was used for geometry optimizations and vibrational frequency analysis. Solvation effects were considered using the polarizable continuum model (PCM) as implemented in Q-Chem,57 with a dielectric constant of 46.7, corresponding to that of DMSO. To obtain the Gibbs free energy, thermal corrections were applied to the single point electron energy obtained on optimized structures with the same functional but with the larger def2-TZVP basis set.58To account for the potential effects, the computational hydrogen electrode method was applied as in the previously reported case.59 In the computational hydrogen electrode method, the chemical potential of the electron is referenced to the standard hydrogen electrode (SHE) and the applied potential relative to the SHE.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
S. Won: conceptualization, validation, investigation, methodology, writing – original draft. D. Park: formal analysis. Y. Jung: resources, software, supervision, writing – review & editing. H. Kim: conceptualization, validation, resources, supervision, funding acquisition, writing – original draft. T. D. Chung: conceptualization, validation, resources, supervision, funding acquisition, writing – original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A5A1030054). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1A2C3004327). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1C1C1004605). S. W. acknowledges Dr Su Yong Go for his assistance and advice on the product purification. S. W. also thanks Mr Jinyeong Park, Mr Donghwi Na, Ms Yun Seung Nam, Dr Ji Yong Kim, Mr Sung Il Kim, and Dr Jeonguk Kweon for their technical support and fruitful discussion.Notes and references
- F. Parsaee, M. C. Senarathna, P. B. Kannangara, S. N. Alexander, P. D. E. Arche and E. R. Welin, Nat. Rev. Chem, 2021, 5, 486–499 CrossRef CAS PubMed.
- M. Yan, J. C. Lo, J. T. Edwards and P. S. Baran, J. Am. Chem. Soc., 2016, 138, 12692–12714 CrossRef CAS PubMed.
- N. Zhang, S. R. Samanta, B. M. Rosen and V. Percec, Chem. Rev., 2014, 114, 5848–5958 CrossRef CAS.
- K. T. Mahmudov and A. J. L. Pombeiro, Chem.–Eur. J., 2023, 29, e202203861 CrossRef CAS.
- H. Kisch, Acc. Chem. Res., 2017, 50, 1002–1010 CrossRef CAS PubMed.
- G. Horner, P. Johne, R. Kunneth, G. Twardzik, H. Roth, T. Clark and H. Kisch, Chem.–Eur. J., 1999, 5, 208–217 CrossRef CAS.
- T. Noël, Y. Cao and G. Laudadio, Acc. Chem. Res., 2019, 52, 2858–2869 CrossRef.
- L. Chen, L. M. Barton, J. C. Vantourout, Y. Xu, C. Chu, E. C. Johnson, J. J. Sabatini and P. S. Baran, Org. Process Res. Dev., 2021, 25, 2639–2645 CrossRef CAS.
- Y. Chen, Y. He, Y. Gao, J. Xue, W. Qu, J. Xuan and Y. Mo, Science, 2024, 384, 670–676 CrossRef CAS PubMed.
- D. M. Heard and A. J. J. Lennox, Angew. Chem., Int. Ed., 2020, 59, 18866–18884 CrossRef CAS PubMed.
- H. Huang, X. Song, C. Yu, Q. Wei, L. Ni, X. Han, H. Huang, Y. Han and J. Qui, Angew. Chem., Int. Ed., 2023, 62, e202216321 CrossRef CAS.
- A. Wuttig and F. D. Toste, Nat. Sci., 2021, 1, e20210036 CrossRef CAS.
- R. F. C. Claridge and H. Fischer, J. Phys. Chem., 1983, 87, 1960–1967 CrossRef CAS.
- S. Wang, S. Tang and A. Lei, Sci. Bull., 2018, 63, 1006–1009 CrossRef CAS.
- Y. Mo, Z. Lu, G. Rughoobur, P. Patil, N. Gershenfeld, A. I. Akinwande, S. L. Buchwald and K. F. Jensen, Science, 2020, 368, 1352–1357 CrossRef CAS PubMed.
- H. G. Cha and K. S. Choi, Nat. Chem., 2015, 7, 328–333 CrossRef CAS PubMed.
- T. Li, T. Kasahara, J. He, K. E. Dettelbach, G. M. Sammis and C. P. Berlinguette, Nat. Commun., 2017, 8, 6–10 CrossRef PubMed.
- Z. Li, L. Luo, M. Li, W. Chen, Y. Liu, J. Yang, S. M. Xu, H. Zhou, L. Ma, M. Xu, X. Kong and H. Duan, Nat. Commun., 2021, 12, 1–13 CrossRef PubMed.
- Y. Zhao, C. Deng, D. Tang, L. Ding, Y. Zhang, H. Sheng, H. Ji, W. Song, W. Ma, C. Chen and J. Zhao, Nat. Catal., 2021, 4, 684–691 CrossRef CAS.
- H. Tateno, S. Iguchi, Y. Miseki and K. Sayama, Angew. Chem., Int. Ed., 2018, 57, 11238–11241 CrossRef CAS PubMed.
- L. Zhang, L. Liardet, J. Luo, D. Ren, M. Grätzel and X. Hu, Nat. Catal., 2019, 2, 366–373 CrossRef CAS PubMed.
- X. Liu, Z. Chen, S. Xu, G. Liu, Y. Zhu, X. Yu, L. Sun and F. Li, J. Am. Chem. Soc., 2022, 144, 19770–19777 CrossRef CAS PubMed.
- L. Wu, D. Tang, J. Xue, S. Liu, J. Wang, H. Ji, C. Chen, Y. Zhang and J. Zhao, Angew. Chem., Int. Ed., 2022, e202214580 CAS.
- J. Wang, L. Zuo, Z. Guo, C. Yang, Y. Jiang, X. Huang, L. Wu and Z. Tang, Angew. Chem., Int. Ed., 2023, e202315478 CAS.
- R. Beranek, Angew. Chem., Int. Ed., 2019, 58, 16724–16729 CrossRef CAS PubMed.
- Y. Ji, T. Brueckl, R. D. Baxter, Y. Fujiwara, I. B. Seiple, S. Su, D. G. Blackmond and P. S. Baran, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 14411–14415 CrossRef CAS.
- X. Lu, R. Kawazu, J. Song, Y. Yoshigoe, T. Torigoe and Y. Kuninobu, Org. Lett., 2021, 23, 4327–4331 CrossRef CAS.
- Y. Ye, S. H. Lee and M. S. Sanford, Org. Lett., 2011, 13, 5464–5467 CrossRef CAS PubMed.
- K. Natte, R. V. Jagadeesh, L. He, J. Rabeah, J. Chen, C. Taeschler, S. Ellinger, F. Zaragoza, H. Neumann, A. Brückner and M. Beller, Angew. Chem., Int. Ed., 2016, 55, 2782–2786 CrossRef CAS PubMed.
- E. A. Meucci, S. N. Nguyen, N. M. Camasso, E. Chong, A. Ariafard, A. J. Canty and M. S. Sanford, J. Am. Chem. Soc., 2019, 141, 12872–12879 CrossRef CAS PubMed.
- A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950–8958 CrossRef CAS PubMed.
- A. G. O-Brien, A. Maruyama, Y. Inokuma, M. Fujita, P. S. Baran and D. G. Blackmond, Angew. Chem., Int. Ed., 2014, 53, 11868–11871 CrossRef CAS PubMed.
- S. Rodrigo, C. Um, J. C. Mixdorf, D. Gunasekera, H. M. Nguyen and L. Luo, Org. Lett., 2020, 22, 6719–6723 CrossRef CAS PubMed.
- Y. Qiu, A. Scheremetjew, L. H. Finger and L. Ackermann, Chem. - Eur. J., 2020, 26, 3241–3246 CrossRef CAS.
- G. Y. Dou, Y. Y. Jiang, K. Xu and C. C. Zeng, Org. Chem. Front., 2019, 6, 2392–2397 RSC.
- Y. Deng, F. Lu, S. You, T. Xia, Y. Zheng, C. Lu, G. Yang, Z. Chen, M. Gao and A. Lei, Chin. J. Chem., 2019, 37, 817–820 CrossRef CAS.
- J. Struwe and L. Ackermann, Faraday Discuss., 2023, 247, 79 RSC.
- K. Sivula, F. Le Formal and M. Grätzel, ChemSusChem, 2011, 4, 432–449 CrossRef CAS PubMed.
- C. Zhang, Adv. Synth. Catal., 2014, 356, 2895–2906 CrossRef CAS.
- M. T. Mayer, Curr. Opin. Electrochem., 2017, 2, 104–110 CrossRef CAS.
- S. Corby, R. R. Rao, L. Steier and J. R. Durrant, Nat. Rev. Mater., 2021, 6, 1136–1155 CrossRef CAS.
- M. Ferreira, H. Varela, R. M. Torresi and G. Tremiliosi-Filho, Electrochim. Acta, 2006, 52, 434–442 CrossRef CAS.
- M. Gattrell and D. W. Kirk, J. Electrochem. Soc., 1993, 140, 903–911 CrossRef CAS.
- D. Eisenberg, H. S. Ahn and A. J. Bard, J. Am. Chem. Soc., 2014, 136, 14011–14014 CrossRef CAS PubMed.
- P. Shadabipour and T. W. Hamann, Chem. Commun., 2020, 56, 2570–2573 RSC.
- D. Seo, S. Won, J. T. Kim and T. D. Chung, Anal. Chem., 2022, 94, 2063–2071 CrossRef CAS.
- R. Francke and R. D. Little, Chem. Soc. Rev., 2014, 43, 2492–2521 RSC.
- R. Chen, F. Fan and C. Li, Angew. Chem., Int. Ed., 2022, 61, e202117567 CrossRef CAS PubMed.
- J. Yu, P. Zhang, L. Li, K. Li, G. Zhang, T. Wang, Z. Zhao and J. Gong, Nat. Commun., 2022, 13, 7909 CrossRef CAS PubMed.
- L. M. Peter, Chem. Rev., 1990, 90, 753–769 CrossRef CAS.
- L. M. Peter, K. G. U. Wijayantha and A. A. Tahir, Faraday Discuss., 2012, 155, 309–322 RSC.
- Y. Ling, G. Wang, D. A. Wheeler, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 2119–2125 CrossRef CAS.
- L. Vayssieres, N. Beermann, S. E. Lindquist and A. Hagfeldt, Chem. Mater., 2001, 13, 233–235 CrossRef CAS.
- Y. Shao, Z. Gan, E. Epifanovsky, A. T. B. Gilbert, M. Wormit, J. Kussmann, A. W. Lange, A. Behn, J. Deng, X. Feng, D. Ghosh, M. Goldey, P. R. Horn, L. D. Jacobson, I. Kaliman, R. Z. Khaliullin, T. Kus, A. Landau, J. Liu, E. I. Proynov, Y. M. Rhee, R. M. Richard, M. A. Rohrdanz, R. P. Steele, E. J. Sundstrom, H. L. Woodcock, P. M. Zimmerman, D. Zuev, B. Albrecht, E. Alguire, B. Austin, G. J. O. Beran, Y. A. Bernard, E. Berquist, K. Brandhorst, K. B. Bravaya, S. T. Brown, D. Casanova, C. M. Chang, Y. Chen, S. H. Chien, K. D. Closser, D. L. Crittenden, M. Diedenhofen, R. A. Distasio, H. Do, A. D. Dutoi, R. G. Edgar, S. Fatehi, L. Fusti-Molnar, A. Ghysels, A. Golubeva-Zadorozhnaya, J. Gomes, M. W. D. Hanson-Heine, P. H. P. Harbach, A. W. Hauser, E. G. Hohenstein, Z. C. Holden, T. C. Jagau, H. Ji, B. Kaduk, K. Khistyaev, J. Kim, J. Kim, R. A. King, P. Klunzinger, D. Kosenkov, T. Kowalczyk, C. M. Krauter, K. U. Lao, A. D. Laurent, K. V. Lawler, S. V. Levchenko, C. Y. Lin, F. Liu, E. Livshits, R. C. Lochan, A. Luenser, P. Manohar, S. F. Manzer, S. P. Mao, N. Mardirossian, A. V. Marenich, S. A. Maurer, N. J. Mayhall, E. Neuscamman, C. M. Oana, R. Olivares-Amaya, D. P. Oneill, J. A. Parkhill, T. M. Perrine, R. Peverati, A. Prociuk, D. R. Rehn, E. Rosta, N. J. Russ, S. M. Sharada, S. Sharma, D. W. Small, A. Sodt, T. Stein, D. Stück, Y. C. Su, A. J. W. Thom, T. Tsuchimochi, V. Vanovschi, L. Vogt, O. Vydrov, T. Wang, M. A. Watson, J. Wenzel, A. White, C. F. Williams, J. Yang, S. Yeganeh, S. R. Yost, Z. Q. You, I. Y. Zhang, X. Zhang, Y. Zhao, B. R. Brooks, G. K. L. Chan, D. M. Chipman, C. J. Cramer, W. A. Goddard, M. S. Gordon, W. J. Hehre, A. Klamt, H. F. Schaefer, M. W. Schmidt, C. D. Sherrill, D. G. Truhlar, A. Warshel, X. Xu, A. Aspuru-Guzik, R. Baer, A. T. Bell, N. A. Besley, J. Da Chai, A. Dreuw, B. D. Dunietz, T. R. Furlani, S. R. Gwaltney, C. P. Hsu, Y. Jung, J. Kong, D. S. Lambrecht, W. Liang, C. Ochsenfeld, V. A. Rassolov, L. V. Slipchenko, J. E. Subotnik, T. Van Voorhis, J. M. Herbert, A. I. Krylov, P. M. W. Gill and M. Head-Gordon, Mol. Phys., 2015, 113, 184–215 CrossRef CAS.
- Y. S. Lin, G. De Li, S. P. Mao and J. Da Chai, J. Chem. Theory Comput., 2013, 9, 263–272 CrossRef CAS.
- P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- A. W. Lange and J. M. Herbert, J. Phys. Chem. Lett., 2010, 1, 556–561 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- Y. Huang, R. J. Nielsen, W. A. Goddard and M. P. Soriaga, J. Am. Chem. Soc., 2015, 137, 6692–6698 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc04570j |
| This journal is © The Royal Society of Chemistry 2024 |