Open Access Article
Open Access ArticleMultiphoton tandem photoredox catalysis of [Ir(dFCF3ppy)2(dtbbpy)]+ facilitating radical acylation reactions†
Zhicong
Lin‡
,
Qian
Zhou‡
,
Yan
Liu
,
Chenli
Chen
,
Jialong
Jie
 * and
Hongmei
Su
* and
Hongmei
Su

College of Chemistry, Beijing Normal University, Beijing 100875, China. E-mail: jialong@bnu.edu.cn
First published on 27th June 2024
Abstract
Photoredox catalytic radical acylation reactions, utilizing [Ir(dFCF3ppy)2(dtbbpy)]+ (IrIII) as the photocatalyst and α-keto acids as the starting substrates, have recently emerged as an attractive strategy for preparing ketone derivatives. While there is consensus on the importance of detailed mechanistic insights to maximize the formation of desired products, efforts focused on uncovering the underlying elementary mechanisms of IrIII photocatalytic radical acylation reactions are still lacking. Herein, using time-resolved spectroscopy, we observed the efficient quenching of the triplet state, 3IrIII*, via electron transfer from α-keto acids, resulting in the generatation of the reduced IrII. Subsequently, IrII rapidly transforms into a stable IrH+ species through protonation, with α-keto acid acting as a proton donor. Upon absorbing additional photon(s), IrH+ is expected to transform into IrH3, involving further hydrogenation/protonation. Emission and Fourier transform infrared (FTIR) spectroscopy, together with global analysis, identify the character of IrH3/3IrH3* and corroborate its contribution to representative radical acylation reactions (decarboxylative 1,4-addition of α-keto acids with Michael acceptors, decarboxylative coupling of α-keto acids with aryl halides, and decarboxylative cyclization of 2-alkenylarylisocyanides with α-keto acids), where IrH3/3IrH3* serves as the key species to trigger the second photoredox cycle. These results elucidate the existence and generality of the tandem photoredox catalysis mechanism for IrIII photocatalytic radical acylation reactions, providing advanced insights into the mechanism of IrIII-based photoredox processes and potentially expanding their application in the design and development of new synthetic methodologies.
Introduction
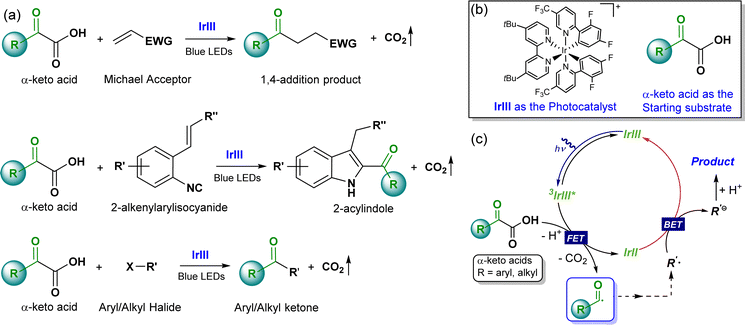
The radical acylation reaction is a highly efficient synthetic route for preparing ketone derivatives, which constitute one of the most common structural units in various organic molecules, including drugs, natural bioactive products, and pesticides.1 Compared to acyl chloride, anhydrides, and other acyl-transfer reagents, the environmentally friendly nature of the coproduct formed in decarboxylation, generating acyl radicals (only CO2), accredits α-keto acid as an ideal acylating agent.1,2 Consequently, a significant number of important radical acylation reactions utilizing α-keto acids as key starting materials have been described.2 However, reactions triggered by traditional methods generally necessitate excess oxidants and/or elevated temperatures. Instead, the photoredox catalytic conversion of α-keto acids into acyl radicals, allowing for the facile generation of a series of carbonyl products under mild conditions, recently emerged as a sustainable and attractive strategy.3 Using such rapidly burgeoning photoredox catalytic methods, impressive breakthroughs have been achieved in the development of novel radical acylation reactions over the past decade, e.g. decarboxylative 1,4-addition of α-keto acids with Michael acceptors, decarboxylative coupling of α-keto acids with aryl halides, and decarboxylative cyclization of 2-alkenylarylisocyanides with α-keto acids (Scheme 1a).4–16 For these radical acylation reactions, [Ir(dFCF3ppy)2(dtbbpy)]+ (Scheme 1b, IrIII) has proven to be one of the most effective photocatalysts,5–7,9,11–13 likely owing to its outstanding visible-absorption optical properties, efficient electron transfer capabilities, and superior photostability.17In principal, photocatalysts are primarily selected based on their ground and excited state properties, but their activities are also intrinsically tied to the nature of the transformed intermediates involved in the catalytic cycle. Catalyst reactivity often requires an inherent instability, and thus these intermediates represent a mechanistic turning point that either facilitates product formation or leads to side-reactions. In this regard, alongside the progress towards expanding the scope of photoredox catalytic reactions, there is consensus that efforts on uncovering underlying elementary mechanisms are essential to maximize the formation of desired products.18–21 Nevertheless, to date, reports providing detailed mechanistic insights into the fate of IrIII in photocatalytic radical acylation systems are still lacking. Generally, these IrIII photocatalytic radical acylation reactions are proposed as a single-photon cycle, mainly comprising one photon absorption and two single electron transfers (forward and back electron transfer, FET and BET) (Scheme 1c). Specifically, the reaction proceeds with IrIII absorbing visible light to generate the catalytically active excited state IrIII*, which is then reduced via electron transfer from α-keto acids, generating the reduced IrIII (IrII) and acyl radical. This IrII species is proposed as the key intermediate to directly reduce the further transformed species of the acyl radical, thereby completing the cycle to regenerate the catalyst.
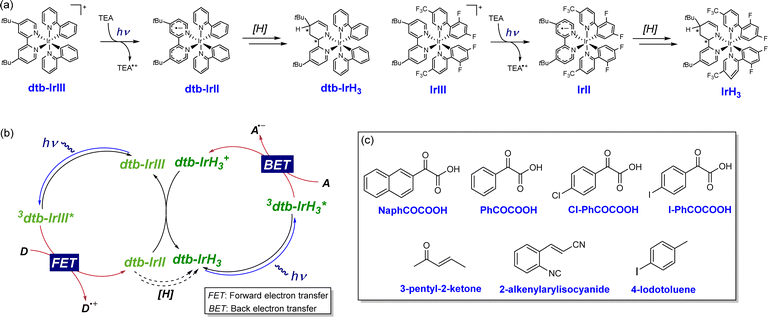
Distinguished from the proposed single-photon cycle involving IrIII, IrIII*, and IrII, the fate of heteroleptic photocatalysts [Ir(C^N)2(N^N)]+ (where C^N is a cyclometalating ligand and N^N is an ancillary diimine ligand), extensively used in photoredox catalysis, has recently been reinterpreted under typical photoredox conditions.22,23 In 2019, P. S. Francis et al. reported the immediate product of photoinduced electron transfer between [Ir(ppy)2(dtbbpy)]+ (dtb-IrIII) and a tertiary amine [e.g., TEA or DIPEA] would transforms into a new, partially reduced Ir complex, [Ir(ppy)2(H3-dtbbpy)]0 (dtb-IrH3), as confirmed by nuclear magnetic resonance, mass spectrometry, and deuterium labeling experiments (Scheme 2a).22 Upon further irradiation, dtb-IrH3 undergoes electron transfer or proton-coupled electron transfer with a representative acceptor (N-(diphenylmethylene)-1-phenylmethanamine). Turnover of this new photocatalytic cycle occurs concurrently with the reformation of dtb-IrIII (Scheme 2b). This tandem photoredox catalysis mechanism, where two distinct yet interconnected photoredox cycles involving dtb-IrIII and dtb-IrH3, has been shown to be able to facilitate numerous synthetic reactions, including reductive dehalogenation of aryl halides, carbonylative amidation of aryl and alkylhalides, reductive activation and hydrofunctionalization of olefins, and carbonylative hydroacylation of styrenes with alkyl halides.22–26 Interestingly, this particular chemical transformation is later found to be pervasive for heteroleptic [Ir(C^N)2(N^N)]+ complexes with a 2,2′-bipyridine-type ancillary ligand, including [Ir(dFCF3ppy)2(dtbbpy)]+ (Scheme 2a),23 suggesting that this tandem photoredox catalysis mechanism may also be suitable for IrIII-based photoredox processes. Spurred by these recent reports, we thus suspect that instead of a single-photon cycle, such a tandem photoredox catalysis mechanism may unknowingly contribute to these IrIII photocatalytic radical acylation reactions.
In this context, we performed joint time-resolved and steady-state spectral investigations on the fate of IrIII in typical photocatalytic radical acylation systems. In the case of IrIII photocatalytic decarboxylative 1,4-addition of α-keto acids with Michael acceptors, it was observed that the decay of the triplet excited state of IrIII (3IrIII*) can be efficiently quenched by α-keto acids (107–109 M−1 s−1). The emergence of the observed IrII intermediate generated from the reaction of 3IrIII* + α-keto acids provides direct evidence for the electron transfer quenching mechanism. Furthermore, it was found that through protonation, IrII undergoes further transformation into IrH+, rather than directly reducing substrate radicals. Surprisingly, IrH+ remains remarkably stable at ∼ ms timescale, even in the presence of Michael acceptors, suggesting that this Ir species may not be the key intermediate responsible for back electron transfer. Intriguingly, by emission and Fourier transform infrared (FTIR) spectroscopy, together with global analysis, the co-contribution of IrIII/3IrIII* and IrH3/3IrH3* to the entire photocatalytic reaction is clearly evidenced. Above data reveal that the chemical transformation of IrIII into IrH3, most possibly viaIrH+ intermediate and an extra photon cycle involving IrH3/3IrH3* as the key triggering species, indeed exists within IrIII photocatalytic decarboxylative 1,4-addition of α-keto acids with Michael acceptors. Similar observations were obtained for IrIII photocatalytic decarboxylative coupling of α-keto acids with aryl halides and IrIII photocatalytic decarboxylative cyclization of 2-alkenylarylisocyanides with α-keto acids. These results unequivocally demonstrate the generality of tandem photoredox catalysis mechanism for IrIII photocatalytic radical acylation reactions, thereby recontextualizing the role of photocatalysts and their possible side-reactions, and laying the foundation toward a greater understanding of single and multiphoton photoredox catalysis in radical acylation reactions.
Results and discussion
Quenching studies of 3IrIII* by α-keto acids
To initiate our study, we first examined IrIII photocatalytic decarboxylative 1,4-addition of α-keto acids with Michael acceptors, a representative photocatalytic radical acylation reaction. As illustrated in Fig. 1a, the absorption spectrum of IrIII photocatalyst spans from the UV to the visible region and is characterized by two absorption bands at 380 nm and 410 nm (ε ≈ (1–10) × 103 M−1 cm−1), along with a longer wavelength tail (ε < 1 × 103 M−1 cm−1). Absorptions around 380 and 410 nm are ascribed to spin-allowed ligand to ligand charge transfer (1LLCT) transitions and spin-allowed metal to ligand charge transfer (1MLCT) transitions, respectively. The tail at longer wavelengths (∼460 nm) is attributed to spin-forbidden singlet-to-triplet (3MLCT) transitions.17,27 In comparison, the substrates (Scheme 2c) used in this study, including α-keto acids (NaphCOCOOH, PhCOCOOH, I-PhCOCOOH, and Cl-PhCOCOOH), and Michael acceptor (3-pentyl-2-ketone) all exhibit absorption below 410 nm (Fig. S1–S3 and S5†). The spectra of the mixed solution of IrIII + substrates simply represent the summation of these two absorption spectra, suggesting no interaction between IrIII and the substrates. In the subsequent steady-state and time-resolved spectral investigations, a 430 nm laser was employed to selectively excite the photocatalyst IrIII.First, Stern–Volmer (SV) quenching experiments were conducted to evaluate the reaction between IrIII* and α-keto acid substrates. As shown in Fig. 1b, the steady-state emission spectrum of IrIII exhibits a structured shape peaking at ∼470 nm. In the presence of α-keto acids (NaphCOCOOH), the emission intensity of IrIII is noticeably reduced, exhibiting a linear concentration dependence in the Stern–Volmer plot. The strong spin–orbit coupling effect (ξIr = 3909 M −1 cm−1), facilitating rapid intersystem crossing from singlet to triplet states (<100 fs), attributes the steady-state emission of IrIII to the phosphorescence from its triplet state (3IrIII*), rather than the fluorescence from its singlet state (1IrIII*).28–30 Consequently, it is the electronically excited state 3IrIII* that has the catalytic activity and reacts with NaphCOCOOH.
Meanwhile, for the time-resolved emission spectra of IrIII, an initially observed structured spectrum, identical to the steady-state emission spectrum, is noted (Fig. 1c). The decay of this emission state, following in a mono-exponential behavior, is found to be sensitive to oxygen (Fig. 1d), further supporting its assignment as 3IrIII*. Under N2-saturated condition, the decay of 3IrIII* is significantly accelerated in the presence of excess NaphCOCOOH (Fig. 1e). Linear fitting of the measured pseudo-first-order reaction rate constants versus NaphCOCOOH concentration enables direct determination of the quenching efficiency of 3IrIII* by NaphCOCOOH (5.0 × 109 M−1 s−1) (Fig. 1f). Similarly, Stern–Volmer (SV) quenching experiments of 3IrIII* by the other representative α-keto acids (PhCOCOOH, I-PhCOCOOH, and Cl-PhCOCOOH), are characterized by both steady-state and time-resolved emission spectra (Fig. S1–S3†). These findings suggest that for IrIII photocatalytic decarboxylation 1,4-addition of α-keto acids with Michael acceptors, the entire cycle should be initiated by the quenching reaction of 3IrIII* + α-keto acids substrates.
Second, the possibility of proposed electron transfer catalysis mechanism, is further assessed by calculating the standard free energy change (ΔG0) using the Rehm–Weller equation. Cyclic voltammogram experiments were conducted to determine the oxidation potentials of these representative α-keto acids (Fig. S4†). The reduction potential of 3IrIII* (1.21 V) is taken from reported work.17,31 Based on these data, the calculated ΔG0 is found to be negative for all representative α-keto acids, indicating the electron transfer quenching is thermodynamically favored (Table S1†).
Moreover, minimal quenching of photocatalyst phosphorescence was observed in the presence of 3-pentyl-2-ketone, a kind of olefin Michael acceptor (Fig. S5†). The oxidation potentials of olefins typically exceed the reduction potential of 3IrIII* (E(3IrIII*/IrII) = 1.21 V vs. SCE), rendering electron transfer quenching thermodynamically unfavorable.32 It is noteworthy that the reported triplet energy values of common olefins are generally lower than that of IrIII (ET = 60.1 kcal mol−1), making energy transfer quenching of 3IrIII* by olefin become possible.33,34 However, considering the relative smaller quenching rate constant and the absence of acyl radical generation via this pathway, energy transfer catalysis can be excluded as the primary mechanism for IrIII photocatalytic decarboxylative 1,4-addition of α-keto acids with Michael acceptors.
Monitoring IrII generation via electron transfer from α-keto acids to 3IrIII*
To initiate our study, The single-electron transfer from α-keto acids to 3IrIII* results in the formation of the reduced IrII species and a corresponding carboxyl radical species. It was presumed that this open-shell dicarbonyl intermediate would promptly extrude CO2 to deliver the crucial acyl radical species,4,6 which is further supported by our DFT calculations that predicted a barrier-free reaction potential for this process (Fig. S6†). Nanosecond time-resolved UV-vis absorption spectroscopy was employed to characterize these species and determine their subsequent reaction rate constants. Upon 430 nm laser excitation of IrIII under N2-saturated condition, as shown in Fig. 2a, the phosphorescence emission (negative bands around 470 nm) and excited-state absorption (positive bands around 370 and 430 nm) are observed initially. The decay at 370, 430, and 450 nm all exhibit mono-exponential kinetics (y = A0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−k/t) with a consistent lifetime of 2.3 μs, matching the value obtained from the time-resolved emission data. This lifetime corresponds to the decay process of 3IrIII* to its ground state.
e−k/t) with a consistent lifetime of 2.3 μs, matching the value obtained from the time-resolved emission data. This lifetime corresponds to the decay process of 3IrIII* to its ground state.
For IrIII in the presence of NaphCOCOOH (Fig. 2c), the transient absorption spectra are obviously different from IrIII alone. The efficient reaction with NaphCOCOOH, notably accelerates the decay of 3IrIII*, accompanied by the emergence of a new spectral shape within 1 μs, characterized by two resolved absorption bands around 490 and 525 nm, which are distinct from those of 3IrIII*. This new spectral shape is attributed to the spectrum of IrII, previously characterized by both time-resolved spectroscopy and spectroelectrochemistry.19,20 The appearance of IrII resulting from the reaction of 3IrIII* + NaphCOCOOH provides direct evidence for the electron transfer quenching mechanism.
 | (1) |
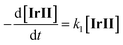 | (2) |
According to the proposed single photon cycle for IrIII photocatalytic 1,4-addition of acyl radical with Michael acceptors, IrII serves as the pivotal intermediate, marking the mechanistic turning point that affords to reduce either the acyl radical (a side-reaction) or its further transformed radical, enolate radical (the desired reaction), thereby completing the cycle to regenerate the catalyst. In this context, the decay of IrII is expected to follow a second-order reaction behavior (eqn (1)). However, contrary to expectations, the bands corresponding to IrII unexpectedly exhibit mono-exponential decay within 15 μs (eqn (2)). Furthermore, this decay behavior remains unaffected even in the presence of excess Michael acceptors (3-pentyl-2-ketone) (Fig. S7†). These results kinetically reveal that IrII is involved in a first-order decay reaction (eqn (2)), ruling out the possibility of IrII serving as the critical intermediate responsible for electron return.
The decay fate of IrII: formation of a new Ir species
As time proceeds to 15 μs, the decay of IrII is almost complete and an unexpectedly new spectral shape of a maximum below 450 nm, together with a long, featureless tail extending to 700 nm, is observed. Based on the following factors: (1) carboxyl radical species would rapidly extrude CO2 to yield critical acyl radical species; (2) such spectral evolution is unaffected with or without extra excess Michael acceptor 3-pentyl-2-ketone; (3) it has been reported that this type of acyl radical primarily absorbs below 400 nm;35 (4) similar transient spectral results are also obtained for the rest representative α-keto acids (PhCOCOOH, I-PhCOCOOH, and Cl-PhCOCOOH) (Fig. S8†), we thus conclude that the transient spectrum at 5 μs originates from a new Ir species, rather than a species related to substrate. Consequently, the spectral evolution within 15 μs likely correspond to IrII transforming into this new Ir intermediate, following a first-order reaction behavior. This particular chemical evolution of IrII into this new Ir species should be pervasive in IrIII photocatalytic radical acylation reactions.To further explore the role of this newly Ir species, we examined its kinetic at longer timescale. As depicted in Fig. S9,† our kinetics analysis reveals that this Ir species is quite long-lived and remains stable at ∼ ms timescale, even in the presence of excess Michael acceptor, 3-pentyl-2-ketone. The quite long-lived nature suggests that this Ir species may not serve as the key intermediate responsible for back electron transfer to substrate radicals. Consequently, it appears that a single photon process alone may not adequately describe the entire photocatalytic cycle of the 1,4-addition of acyl radicals with Michael acceptors. Based on the aforementioned results, we propose an alternative mechanism: instead of a single photon catalytic cycle, the tandem photoredox catalysis mechanism involving IrH3/IrH3* likely plays a predominant role in the IrIII photocatalytic radical acylation reaction.
Identifying IrH3/IrH3* as key species contributing to representative IrIII photocatalytic radical acylation reactions
The proposed tandem photoredox catalysis mechanism for IrIII photocatalytic 1,4-addition of acyl radical with Michael acceptors can be substantiated through the following additional experiments. In previous investigations of dtb-IrIII photocatalytic systems, the involvement of this tandem photoredox catalysis mechanism has been corroborated by the steady-state emission spectra of dtb-IrIII + TEA + imine, wherein the characters of both dtb-IrIII/3dtb-IrIII* and dtb-IrH3/3dtb-IrH3* were observed.22 Analogously, it is anticipated that IrH3/3IrH3* would also contribute to the emission of IrIII + α-keto acids + Michael acceptors, potentially altering the profile of the emission spectra if IrH3/3IrH3* plays a substantial role. To validate the existence and participation of IrH3/3IrH3*, steady-state emission spectroscopy and global analysis were conducted for IrIII + α-keto acids + Michael acceptors.Initially, steady-state emission spectral experiments were performed for IrIII + TEA to isolate the pure emission profile of IrH3/3IrH3*.23 As depicted in Fig. 3a–c, the emission spectra of IrIII + TEA exhibit significant variations under continuous illumination. Specifically, with increasing illumination time, a gradual decrease in the IrIII emission band at 470 nm is observed, accompanied by the emergence of a new intense emission profile peaking at 580 nm. According to earlier studies, this longer-wavelength emission profile originates from 3IrH3* species, indicating the chemical transformation of IrIII into IrH3.23 Subsequently, analogous emission experiments and species analysis were conducted for IrIII + NaphCOCOOH + 3-pentyl-2-ketone, as illustrated in Fig. 3d–f. Intriguingly, while the quenching evolution of the 3IrIII* emission profile after the continuous illumination is also noted, the eventual stable emission displays a broad spectrum spanning from 450 nm to 700 nm, characterized by a peak around 500 nm and a shoulder at longer wavelengths. Importantly, global analysis reveals that the final emission profile can be satisfactorily simulated by the weighted sum of the IrIII emission profile and the IrH3 emission profile (Fig. 3e). This progressive spectral evolution thus signifies the partial chemical transformation of IrIII into IrH3. Similar results are observed when varying the substrate of α-keto acids (Fig. S10†), providing crucial evidence for the co-contribution of both IrIII/3IrIII* and IrH3/3IrH3* to the photocatalytic reactions of IrIII + α-keto acids + Michael acceptors.
By comparing the chemical structures of IrIII and IrH3, it is evident that the conversion of these two species necessitates the transfer of a total of 4 electrons and 3 protons. Previous studies have revealed a nonlinear relationship between the maximum rate of formation of analogous dtb-IrH3 from dtb-IrIII and light intensity, suggesting the involvement of more than one photon in this specific transformation.22 Here, we conducted additional experiments to further demonstrate the correlation between the maximum rate of 1,4-addition product formation and irradiation intensity. As depicted in Figs. 4a and b, the IR spectrum of IrIII + NaphCOCOOH + 3-pentyl-2-ketone displays a broad absorption range spanning from 1420 to 1700 cm−1, characterized by three characteristic bands at ∼1475 cm−1, 1610 cm−1, and 1660 cm−1. This IR spectrum is a composite of the individual IR spectra of IrIII, NaphCOCOOH, and 3-pentyl-2-ketone. As the irradiation time progressed, a gradual decrease in the IR intensity around 1610 cm−1 was observed, accompanied by the emergence of two new absorption bands centered at 1643 cm−1 and 1672 cm−1 (Fig. 4b and c). The diminishing band around 1610 cm−1 reflects to the consumption of NaphCOCOOH, whereas the appearance of these two new absorption bands corresponds to the formation of the final 1,4-addition product (Table S2†). Consequently, the maximum formation of the final product over time under the given irradiation intensity can be obtained, which exhibits a nonlinear relationship between the maximum product formation efficiency and irradiation power (Fig. 4d). These findings clearly demonstrate the involvement of more than one photon in efficient final product generation, which cannot be explained solely through a traditional one-photon catalytic cycle. Instead, it provides extra evidence to support that the multiphoton tandem photoredox catalysis mechanism utilizing both IrIII/3IrIII* and IrH3/3IrH3* as the triggering species, governs IrIII photocatalytic decarboxylative 1,4-addition of α-keto acids with Michael acceptors.
Most interestingly, analogous steady-state emission spectral data are obtained for both IrIII photocatalytic decarboxylative coupling of α-keto acids with 4-iodotoluenes (the representative aryl halide substrate) and IrIII photocatalytic decarboxylative cyclization of 2-alkenylarylisocyanides with α-keto acids (Fig. S11†). These findings suggest the applicability of tandem photoredox catalysis mechanisms to these two IrIII photocatalytic radical acylation reactions as well, thereby providing mechanistic evidence for the generality of the tandem photoredox catalysis mechanism in IrIII photocatalytic radical acylation reactions.
Elucidating the intermediate role of this new Ir species within IrII transforming into IrH3
While more than one photon is required for this specific conversion of IrIII into IrH3, our TA experiments reveal that the formation of the new Ir species, generated from the decay of IrII, requires only one photon. Building upon these observations, we infer that this newly Ir species captured by our TA experiments, likely serves as the pivotal intermediate linking the decay of IrII and the formation of IrH3. Additionally, it is anticipated that extra photon(s) are necessary to facilitate the transformation of this new Ir intermediate into the final IrH3 state.After addressing this aspect, we delve into the characterization of this novel Ir intermediate. In general, when a species acquires an electron, its electron cloud density intensifies, thereby leading to a more alkaline nature and a greater capacity to accept protons under neutral condition. This gives us a hint that the novel Ir intermediate may correspond to the protonation product of IrII, (IrH+), with protonation being the primary process accounting for the observed decay of IrII. This is further supported by the control experiments, which clearly show that the conversion of IrII into this novel Ir intermediate is prohibited at a higher pH (Fig. S12a†). Indeed, according to previous reports on dtb-IrIII/IrIII + TEA, TEA as reductive quenchers capable of proton transfer are essential for the formation of final dtb-IrH3/IrH3.22,23 Similarly, in the reactions of IrIII + substrate α-keto acids, the additional role of reductive quenchers α-keto acids as proton donors, is taken into consideration. This is substantiated by the observation of decay kinetic of IrII exhibiting clear dependence on the concentration of α-keto acids (Fig. S12b†). Therefore, the substrate α-keto acids function not only as electron donors, initiating the conversion of 3IrIII* to IrII, but also proton donors to promote the subsequent transformation of IrII into IrH+. Upon absorbing additional photon(s), IrH+ is expected to transform into the final IrH3, involving further hydrogenation/protonation steps.
The reactions of IrIII + α-keto acids system and dtb-IrIII + TEA system, are both governed by a multiphoton tandem photoredox catalysis mechanism. This mechanism involves the conversion of IrIII/dtb-IrIII into IrH3/dtb-IrIII, where α-keto acids/TEA serve as dual electron and proton/hydrogen donors. Based on these findings, it is plausible to conclude that in IrIII photoredox catalytic reactions, whenever electron and proton/hydrogen donors are present, a multiphoton tandem photoredox catalysis process involving the transformation of IrIII into IrH3, as opposed to a single-photon cycle, should be taken into consideration.
Tandem photoredox catalysis mechanism for IrIII photocatalytic radical acylation reactions
On the basis of the accumulated evidence, we deduce that the tandem photoredox catalysis mechanism, comprising two interconnected photoredox cycles is responsible for representative IrIII photocatalytic radical acylation reactions. To illustrate, take IrIII photocatalytic decarboxylative 1,4-addition of α-keto acids with Michael acceptors, as an example. Initially, photoinduced electron transfer (PET) (Scheme 3, steps (i) and (ii)) yields IrII and acylation radicals, alongside the by-product CO2. The acyl radicals can be rapidly trapped by excess Michael acceptors to generate enolate radicals. Reducible enolate radicals may then react with IrII (E(IrIII/IrII) = −1.37 V vs. SCE)17 (step (iii)) to complete a conventional photoredox cycle, thereby hindering the formation of IrH3 (step (iv)). While our DFT calculations of the standard free energy change suggest IrII returning an electron to the enolate radical is thermodynamically favorable (Fig. S13 and Table S3†), our kinetic analysis clearly demonstrates that IrII is predominantly quenched by α-keto acids, possibly resulting in the formation of IrH+ through protonation. Subsequently, additional photon(s) are required to drive IrH+ to get more electrons and protons, finally generating IrH3. | ||
| Scheme 3 Tandem photoredox catalysis mechanism for IrIII photocatalytic radical acylation reactions. The directly observed species are marked in green. | ||
The absorption of light by IrH3 (step (v)) leads to the formation of the excited triplet, 3IrH3*, which has a reduction potential (E(IrH3+/3IrH3*) = –1.16 V vs. SCE),23 comparable with that of IrII (E(IrIII/IrII) = –1.37 V vs. SCE).173IrH3* is anticipated to undergo oxidation by enolate radicals through a back-electron transfer process, ultimately yielding the final 1,4-addition product after protonation and generation of IrH3+, with IrH3+ likely comprising Ir in the +4-oxidation state. Our DFT calculations on the standard free energy change further support this back-electron transfer process from 3IrH3* to enolate radical (Fig. S13 and Table S3†).
Similarly, the feasibility of IrH+ or IrH3 as species capable of reducing enolate radicals is thermodynamically ruled out based on the positive standard free energy change, as predicted by our DFT calculations (Fig. S13 and Table S3†). In the presence of substrates, the formation of a small amount of IrH3 early in the reaction initiates an effective photoredox cycle (steps (v–vii)), continuously generating IrH3+. The reported tandem photoredox catalysis mechanism employing analogous dtb-IrIII as the photocatalyst suggests this kind of oxidized intermediate IrH3+ (E(IrH3+/IrH3) = 1.44 V vs. SCE) may react favorably with IrII (E(IrIII/IrII) = −1.37 V vs. SCE) (step (vii)),22,23 to regenerate both IrH3 and IrIII through a single electron transfer, which is further supported by our DFT calculations on the standard free energy change of this process (Fig. S13 and Table S3†). Besides, due to the rapid decay of IrII into IrH+, we propose it may be also plausible that IrH3+ reacts with the succeeding intermediates of IrII, to facilitate a continuous flow of electrons between the two photoredox cycles.
Conclusions
Utilizing a combination of time-resolved UV-vis spectroscopy in combination with steady-state emission spectroscopy and global analysis, we have delved into the underlying mechanisms of representative IrIII photocatalytic radical reactions, including decarboxylative 1,4-addition of α-keto acids with Michael acceptors, decarboxylative coupling of α-keto acids with aryl halides, and decarboxylative cyclization of 2-alkenylarylisocyanides with α-keto acids. For IrIII photocatalytic decarboxylative 1,4-addition of α-keto acids with Michael acceptors, our direct observation of critical reactive species (3IrIII*, IrII, IrH+, IrH3/3IrH3*) has unveiled a series of successive photochemical events driving these photocatalytic radical acylation reactions. Initially, the photoexcitation of IrIII triggers the first photoredox cycle, populating 3IrIII*via rapid intersystem crossing (ISC) of 1IrIII* (<100 fs). Then, 3IrIII* oxidizes α-keto acids, yielding acyl radical and IrII, along with the by-product CO2. The formed IrII is further quenched by α-keto acids to IrH+, which may absorb additional photon(s) to generate IrH3. Serving as the coexisting photosensitizer, IrH3 absorbs new photon to give rise to the critical species, 3IrH3*, thereby sequentially triggering the second photoredox cycle. 3IrH3* facilitates the electron return to enolate radicals, generating IrH3+ and delivering 1,4-addition product after protonation. Eventually, IrH3+ may then react with subsequent intermediates of IrII, regenerating the two photoredox cycle-triggering species, IrIII and IrH3.The generality of this mechanism is further demonstrated through the observation of similar phenomena in both IrIII photocatalytic decarboxylative coupling of α-keto acids with aryl halides and IrIII photocatalytic decarboxylative cyclization of 2-alkenylarylisocyanides with α-keto acids. This constitutes the first known example of dual oxidative and reductive photoredox cycles for IrIII operating in tandem. Traditionally, IrIII photocatalytic radical acylation reactions have been considered to occur purely through a single-photon cycle. However, the combined evidence obtained in this study reveals the existence of two distinct yet interconnected photoredox cycles, which are responsible for these efficient radical acylation reactions. Most importantly, our findings suggest that if both electron and proton/hydrogen donors are present in IrIII photoredox catalytic reactions, a multiphoton tandem photoredox catalysis process involving the transformation of IrIII into IrH3 should be considered. Hence, this work extends the understanding of IrIII-based photoredox processes, and further investigation into their application in the design and development of new synthetic methodology is strongly encouraged.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Author contributions
Zhicong Lin performed steady-state UV/vis absorption spectra, steady-state emission spectra, and laser flash photolysis experiments. Qian Zhou performed fourier transform infrared spectra measurements and the theoretical calculations, and drew all the diagrams. Yan Liu and Chenli Chen assisted in the measurements of the above experiments and diagrams. Jialong Jie conceived the project, supervised the research, and wrote the manuscript, with input from all the authors. Hongmei Su provided guidance for the analysis of the results and the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2022YFA1505400), the National Natural Science Foundation of China (No. 21933005, 21727803, 22003005 and 22273007), and the Fundamental Research Funds for the Central Universities (No. 2233300007).Notes and references
- C. Chatgilialoglu, D. Crich, M. Komatsu and I. Ryu, Chem. Rev., 1999, 99, 1991–2070 CrossRef CAS PubMed.
- F. Penteado, E. F. Lopes, D. Alves, G. Perin, R. G. Jacob and E. J. Lenardão, Chem. Rev., 2019, 119, 7113–7278 CrossRef CAS PubMed.
- A. Banerjee, Z. Lei and M.-Y. Ngai, Synthesis, 2019, 51, 303–333 CrossRef CAS PubMed.
- J. Liu, Q. Liu, H. Yi, C. Qin, R. Bai, X. Qi, Y. Lan and A. Lei, Angew. Chem., Int. Ed., 2014, 53, 502–506 CrossRef CAS PubMed.
- W.-M. Cheng, R. Shang, H.-Z. Yu and Y. Fu, Chem.–Eur. J., 2015, 21, 13191–13195 CrossRef CAS PubMed.
- L. Chu, J. M. Lipshultz and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2015, 54, 7929–7933 CrossRef CAS PubMed.
- G.-Z. Wang, R. Shang, W.-M. Cheng and Y. Fu, Org. Lett., 2015, 17, 4830–4833 CrossRef CAS PubMed.
- C. Zhou, P. Li, X. Zhu and L. Wang, Org. Lett., 2015, 17, 6198–6201 CrossRef CAS PubMed.
- L. Gu, C. Jin, J. Liu, H. Zhang, M. Yuan and G. Li, Green Chem., 2016, 18, 1201–1205 RSC.
- N. Xu, P. Li, Z. Xie and L. Wang, Chem.–Eur. J., 2016, 22, 2236–2242 CrossRef CAS PubMed.
- Q.-F. Bai, C. Jin, J.-Y. He and G. Feng, Org. Lett., 2018, 20, 2172–2175 CrossRef CAS PubMed.
- Y. Lv, P. Bao, H. Yue, J.-S. Li and W. Wei, Green Chem., 2019, 21, 6051–6055 RSC.
- X. Zhang, P. Zhu, R. Zhang, X. Li and T. Yao, J. Org. Chem., 2020, 85, 9503–9513 CrossRef CAS PubMed.
- D.-L. Zhu, Q. Wu, D. J. Young, H. Wang, Z.-G. Ren and H.-X. Li, Org. Lett., 2020, 22, 6832–6837 CrossRef CAS.
- B. Yang, S.-J. Li, Y. Wang, Y. Lan and S. Zhu, Nat. Commun., 2021, 12, 5257 CrossRef CAS PubMed.
- H.-L. Zhu, F.-L. Zeng, X.-L. Chen, K. Sun, H.-C. Li, X.-Y. Yuan, L.-B. Qu and B. Yu, Org. Lett., 2021, 23, 2976–2980 CrossRef CAS PubMed.
- M. S. Lowry, J. I. Goldsmith, J. D. Slinker, R. Rohl, R. A. Pascal, G. G. Malliaras and S. Bernhard, Chem. Mater., 2005, 17, 5712–5719 CrossRef CAS.
- S. Ruccolo, Y. Qin, C. Schnedermann and D. G. Nocera, J. Am. Chem. Soc., 2018, 140, 14926–14937 CrossRef CAS PubMed.
- R. Sun, Y. Qin, S. Ruccolo, C. Schnedermann, C. Costentin and D. G. Nocera, J. Am. Chem. Soc., 2019, 141, 89–93 CrossRef CAS.
- Y. Qin, R. Sun, N. P. Gianoulis and D. G. Nocera, J. Am. Chem. Soc., 2021, 143, 2005–2015 CrossRef CAS PubMed.
- N. A. Till, S. Oh, D. W. C. MacMillan and M. J. Bird, J. Am. Chem. Soc., 2021, 143, 9332–9337 CrossRef CAS PubMed.
- T. U. Connell, C. L. Fraser, M. L. Czyz, Z. M. Smith, D. J. Hayne, E. H. Doeven, J. Agugiaro, D. J. D. Wilson, J. L. Adcock, A. D. Scully, D. E. Gómez, N. W. Barnett, A. Polyzos and P. S. Francis, J. Am. Chem. Soc., 2019, 141, 17646–17658 CrossRef CAS PubMed.
- J. C. Bawden, P. S. Francis, S. DiLuzio, D. J. Hayne, E. H. Doeven, J. Truong, R. Alexander, L. C. Henderson, D. E. Gómez, M. Massi, B. I. Armstrong, F. A. Draper, S. Bernhard and T. U. Connell, J. Am. Chem. Soc., 2022, 144, 11189–11202 CrossRef CAS PubMed.
- J. A. Forni, N. Micic, T. U. Connell, G. Weragoda and A. Polyzos, Angew. Chem., Int. Ed., 2020, 132, 18805–18813 CrossRef.
- M. L. Czyz, M. S. Taylor, T. H. Horngren and A. Polyzos, ACS Catal., 2021, 11, 5472–5480 CrossRef CAS.
- J. A. Forni, V. H. Gandhi and A. Polyzos, ACS Catal., 2022, 12, 10018–10027 CrossRef CAS.
- S.-H. Wu, J.-W. Ling, S.-H. Lai, M.-J. Huang, C. H. Cheng and I. C. Chen, J. Phys. Chem. A, 2010, 114, 10339–10344 CrossRef CAS PubMed.
- L. Flamigni, A. Barbieri, C. Sabatini, B. Ventura and F. Barigelletti, in Photochemistry and Photophysics of Coordination Compounds II, ed. V. Balzani and S. Campagna, Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, pp. 143–203, DOI:10.1007/128_2007_131.
- G. J. Hedley, A. Ruseckas and I. D. W. Samuel, J. Phys. Chem. A, 2010, 114, 8961–8968 CrossRef CAS PubMed.
- S. Tschierlei, A. Neubauer, N. Rockstroh, M. Karnahl, P. Schwarzbach, H. Junge, M. Beller and S. Lochbrunner, Phys. Chem. Chem. Phys., 2016, 18, 10682–10687 RSC.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
- D. A. Nicewicz and D. S. Hamilton, Synlett, 2014, 25, 1191–1196 CrossRef PubMed.
- T. Ni, R. A. Caldwell and L. Melton, J. Am. Chem. Soc., 1989, 111, 457–464 CrossRef CAS.
- A. Singh, K. Teegardin, M. Kelly, K. S. Prasad, S. Krishnan and J. D. Weaver, J. Organomet. Chem., 2015, 776, 51–59 CrossRef CAS.
- Y. P. Tsentalovich and H. Fischer, J. Chem. Soc., Perkin Trans. 2, 1994, 729–733, 10.1039/P29940000729.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and Methods, additional experimental results and additional calculational results. See DOI: https://doi.org/10.1039/d4sc03183k |
| ‡ Z. L. and Q. Z. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |