 Open Access Article
Open Access ArticleOrdered assembly of two different metal clusters with the same topological connectivity in one single coordination network†
Jian-Wei
Cao‡
 ,
Tao
Zhang‡
,
Tao
Zhang‡
 ,
Juan
Chen
,
Jin-Bo
Wang
,
Yu
Wang
and
Kai-Jie
Chen
,
Juan
Chen
,
Jin-Bo
Wang
,
Yu
Wang
and
Kai-Jie
Chen
 *
*
Key Laboratory of Special Functional and Smart Polymer Materials of Ministry of Industry and Information Technology, Xi'an Key Laboratory of Functional Organic Porous Materials, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, China. E-mail: ckjiscon@nwpu.edu.cn
First published on 25th June 2024
Abstract
The introduction of heterogeneous components within one single coordination network leads to the multifunctionality of the final material. However, it is hard to precisely control the local distribution of these different components in such a coordination network, especially for different components with identical topological connectivity. In this study, we successfully achieved the ordered assembly of [Mn3(μ3-O)] nodes and [Mn6(μ3-O)2(CH3COO)3] nodes within one pacs coordination network. The resulting new structure (NPU-6) with heterogeneous metal nodes simultaneously inherits the advantages of both parent networks (good thermal stability and high pore volume). The significant effect of the reaction concentration of competing ligand CH3COO− on the mixed assembly of these two nodes in NPU-6 is revealed by a series of control experiments. This method is anticipated to offer a valuable reference for orderly assembling heterogeneous components in coordination networks.
Introduction
Metal–organic frameworks (MOFs),1 also known as porous coordination polymers (PCPs)2 and metal–organic materials (MOMs),3 can be systematically designed by targeting the structure through reticular chemistry and crystal engineering strategies. Currently there are over 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hits in the Cambridge Structural Database (CSD) MOF subset,4 which have been widely used in the field of gas storage/separation, sensing, energy storage, etc.1,5–14 Most MOFs are composed of a single type of ligand and a single type of metallic secondary building unit (SBU),15–18 which however limit their structural diversity and functional versatility. By introducing multiple components within the parent network,19,20 the resulting structurally heterogeneous MOFs, the so-called multivariate MOFs (MTV-MOFs), reported by Yaghi et al.21,22 or solid solution reported by Kitagawa et al.,23–25 can largely enhance the structural diversity and functional versatility of MOF contents.26–29 Although as many as ten kinds of metal ions30 and eight kinds of organic ligands22 can be incorporated within one MOF crystal, the local distribution of heterogeneous contents is hard to control and recognize.31,32 In most cases, these components are arranged randomly, or as short/long duplicates or insertions,33 and the long-range ordered arrangement of heterogeneous components within one single coordination network has been still a great challenge yet.34,35 Things will become much more difficult if these heterogeneous components are topologically identical.
000 hits in the Cambridge Structural Database (CSD) MOF subset,4 which have been widely used in the field of gas storage/separation, sensing, energy storage, etc.1,5–14 Most MOFs are composed of a single type of ligand and a single type of metallic secondary building unit (SBU),15–18 which however limit their structural diversity and functional versatility. By introducing multiple components within the parent network,19,20 the resulting structurally heterogeneous MOFs, the so-called multivariate MOFs (MTV-MOFs), reported by Yaghi et al.21,22 or solid solution reported by Kitagawa et al.,23–25 can largely enhance the structural diversity and functional versatility of MOF contents.26–29 Although as many as ten kinds of metal ions30 and eight kinds of organic ligands22 can be incorporated within one MOF crystal, the local distribution of heterogeneous contents is hard to control and recognize.31,32 In most cases, these components are arranged randomly, or as short/long duplicates or insertions,33 and the long-range ordered arrangement of heterogeneous components within one single coordination network has been still a great challenge yet.34,35 Things will become much more difficult if these heterogeneous components are topologically identical.
We realize that introducing heterogeneities that are largely chemically distinguishable would be a practical way, not only because the distinguishable natures make heterogeneities recognizable but also because self-recognition and self-assembly of heterogeneities would make the ordered arrangement of multiple components possible, even when these heterogeneous components share the same topological connectivity. In 2015, Bu36 and Chen37 demonstrated the construction of a pacs (partitioned acs) network using a 9-connected [M3(μ3-O)] triangle trimer. In 2021, our group found that a brand-new double-decker topologically 9-connected [Mn6(μ3-O)2(CH3COO)3] hexamer can also be incorporated within a pacs network (NPU-1).38 And together with our following study,39 we can reason that the incorporating of CH3COO− would be the key for the formation of [Mn6(μ3-O)2(CH3COO)3]. It is possible to realize the mixed assembly of such two clusters with largely different sizes and geometries in one network if we precisely control the concentration of CH3COO− and further control the producing rate of these clusters in the reaction media.
Based on the above vision, we selected a trinuclear [Mn3(μ3-O)] cluster (CPM-153)40 and a hexanuclear [Mn6(μ3-O)2(CH3COO)3] cluster (NPU-1) of model metal clusters for assembly exploration. We successfully realized the assembly of two chemically different but topologically identical components, [Mn3(μ3-O)] and [Mn6(μ3-O)2(CH3COO)3] clusters, into a single pacs network [{Mn3(μ3-O)}{Mn6(μ3-O)2(CH3COO)3}(BDC)6(TPT)3], denoted as NPU-6; BDC = benzene-1,4-dicarboxylate; TPT = 2,4,6-tri(4-pyridinyl)-1,3,5-triazine, by controlling the concentration of CH3COO−. Single-crystal X-ray diffraction unambiguously determined the ordered arranging of ABAB packing of Mn3 and Mn6 cluster layers (Scheme 1).
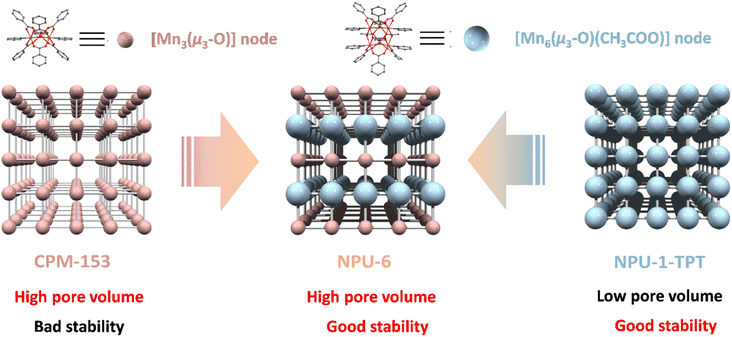 | ||
| Scheme 1 The ordered assembly of two metal clusters with different chemical nature but the same topological connectivity in one single coordination network to enhance both pore volume and stability. | ||
In addition, by inheriting the high pore volume from CPM-153 and good thermal stability from NPU-1-TPT, NPU-6 displayed the highest experimental pore volume among these three coordination networks. Consequently, NPU-6 demonstrated the best C2H6/C2H4 separation performance among these three coordination networks.
Results
Crystallographic structure of NPU-6
The yellowish hexagonal NPU-6 single crystal was harvested following a solvothermal reaction of manganese acetate tetrahydrate, TPT, and dicarboxylate ligand H2BDC in N,N′-dimethylacetamide (DMA) at 388 K for 3 days. Single-crystal X-ray diffraction data revealed that NPU-6 crystallizes in a non-centrosymmetric hexagonal space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2. In this compound, two kinds of metal clusters were observed, the classical trinuclear [Mn3(μ3-O)]6+ cluster and the rare hexanuclear [Mn6(μ3-O)2(CH3COO)3]6+ cluster (Fig. 1a). The charge neutrality of the network indicates the mixed valence state of Mn ions in these clusters, consistent with previous findings and supported by X-ray photoelectron spectroscopy (XPS) analysis (Fig. S2†).38 Two crystallographically different manganese ions in the asymmetric unit exhibit the same octahedral geometry. Each metal ion coordinated with one μ3-O atom, one N atom from the TPT ligand, and four O atoms from four carboxylates in a monodentate fashion. Nevertheless, for Mn1 of the [Mn3(μ3-O)] cluster, all four carboxylates come from four dicarboxylate ligands; meanwhile for Mn2 of the [Mn6(μ3-O)2(CH3COO)3] cluster, two carboxylates come from two bridging acetate anions, and others from two dicarboxylate ligands. The trinuclear [Mn3(μ3-O)]6+ is constructed from three Mn1 ions bridged by one μ3-O and six carboxylate groups from the BDC2− ligand, which bares the syn-syn-μ2-η1:η1 mode (Fig. S4†), leaving the axial coordination site occupied by the nitrogen atom of the TPT ligand. The distinctive hexanuclear [Mn6(μ3-O)2(CH3COO)3]6+, which was used to build NPU-1/2/3 in our recent work,38 is cooperating within NPU-6. This hexanuclear node is constructed from six Mn2 ions, which can be regarded as a face-to-face stack of two trinuclear Mn3 clusters bridged by three acetate anions with the μ4-η2:η2 mode (Fig. S4†). Each type of cluster is interconnected with three same ones in the ab plane by triangular TPT ligands but with six different ones along the c axis by BDC2− ligands, further packing as an ABAB mode with an interlayer distance of 0.93 nm (Fig. S5†). Interestingly, the Mn6 layer contains double-walled TPT ligands because of the connectivity nature of the Mn6 cluster, while the Mn3 layer only has single TPT ligand bridging, which would lead to the hyperfine spitting of the pore structures which we will discuss later. Topologically, considering both metal clusters as 9-connected nodes and the TPT ligand as a 3-connected node, the network of NPU-6 can be described by the point symbol of {421·615}{43} with the topology type of pacs (partitioned acs).41,42 Particularly, such a co-existence of highly connected metal nodes is very rare43–46 and represents the first example of mixed 9-connected nodes so far. Calculated from the single crystal data based on Platon analysis,47NPU-6 afforded a 3D open network with a porosity of 54.1% and a pore volume of 0.592 cm3 g−1, lying between the values of CPM-153 (59.1% and 0.707 cm3 g−1) and NPU-1-TPT (50.6% and 0.514 cm3 g−1).
m2. In this compound, two kinds of metal clusters were observed, the classical trinuclear [Mn3(μ3-O)]6+ cluster and the rare hexanuclear [Mn6(μ3-O)2(CH3COO)3]6+ cluster (Fig. 1a). The charge neutrality of the network indicates the mixed valence state of Mn ions in these clusters, consistent with previous findings and supported by X-ray photoelectron spectroscopy (XPS) analysis (Fig. S2†).38 Two crystallographically different manganese ions in the asymmetric unit exhibit the same octahedral geometry. Each metal ion coordinated with one μ3-O atom, one N atom from the TPT ligand, and four O atoms from four carboxylates in a monodentate fashion. Nevertheless, for Mn1 of the [Mn3(μ3-O)] cluster, all four carboxylates come from four dicarboxylate ligands; meanwhile for Mn2 of the [Mn6(μ3-O)2(CH3COO)3] cluster, two carboxylates come from two bridging acetate anions, and others from two dicarboxylate ligands. The trinuclear [Mn3(μ3-O)]6+ is constructed from three Mn1 ions bridged by one μ3-O and six carboxylate groups from the BDC2− ligand, which bares the syn-syn-μ2-η1:η1 mode (Fig. S4†), leaving the axial coordination site occupied by the nitrogen atom of the TPT ligand. The distinctive hexanuclear [Mn6(μ3-O)2(CH3COO)3]6+, which was used to build NPU-1/2/3 in our recent work,38 is cooperating within NPU-6. This hexanuclear node is constructed from six Mn2 ions, which can be regarded as a face-to-face stack of two trinuclear Mn3 clusters bridged by three acetate anions with the μ4-η2:η2 mode (Fig. S4†). Each type of cluster is interconnected with three same ones in the ab plane by triangular TPT ligands but with six different ones along the c axis by BDC2− ligands, further packing as an ABAB mode with an interlayer distance of 0.93 nm (Fig. S5†). Interestingly, the Mn6 layer contains double-walled TPT ligands because of the connectivity nature of the Mn6 cluster, while the Mn3 layer only has single TPT ligand bridging, which would lead to the hyperfine spitting of the pore structures which we will discuss later. Topologically, considering both metal clusters as 9-connected nodes and the TPT ligand as a 3-connected node, the network of NPU-6 can be described by the point symbol of {421·615}{43} with the topology type of pacs (partitioned acs).41,42 Particularly, such a co-existence of highly connected metal nodes is very rare43–46 and represents the first example of mixed 9-connected nodes so far. Calculated from the single crystal data based on Platon analysis,47NPU-6 afforded a 3D open network with a porosity of 54.1% and a pore volume of 0.592 cm3 g−1, lying between the values of CPM-153 (59.1% and 0.707 cm3 g−1) and NPU-1-TPT (50.6% and 0.514 cm3 g−1).
The mixed metal cluster assembly results in more complex pore structures, with three types of hierarchical polyhedral cages. For comparison, the same types of cages of CPM-153 based on the Mn3 cluster39,40 and NPU-1-TPT based on the Mn6 cluster are included in Fig. 1a and c. All these structures display two types of cages: cage A with a trigonal antiprism shape and cage B with a trigonal bipyramidal shape. Cage A is surrounded by six metal clusters, six BDC2− ligands and two TPT ligands to form a bicapped trigonal antiprism shaped cage. Because of the larger size of the Mn6 cluster than the Mn3 cluster, cage A in NPU-6 holds medium dimensions (2.49 × 1.46 nm for NPU-6; the height was defined by the distances between the C atoms in carboxylate groups and the width was defined by the N atoms coordinated to the metal ions, regardless of the van der Waals radius) between those of CPM-153 (2.42 × 1.13 nm, Fig. 1a) and NPU-1-TPT (2.5 × 1.79 nm, Fig. 1c). Cage B is made of five metal clusters and six BDC2− ligands to afford a trigonal bipyramidal shaped cage. As shown in Fig. 1b, the mixing metal cluster assembly nature in NPU-6 separates cage B into two different cages (B1 and B2). Cage B1 is constructed by two Mn3 clusters in the axial direction (2.22 nm) and three Mn6 clusters in the equatorial plane (2.49 nm). Cage B2 is made of two Mn6 clusters in the axial direction (2.56 nm) and three Mn3 clusters in the equatorial plane (2.49 nm). The size of cage B in CPM-153 is still the lowest one with the dimensions of 2.42 × 1.90 nm. In contrast, cage B in NPU-1-TPT has the largest size with the dimensions of 2.50 × 2.88 nm. In NPU-6, cage A arranges along the c axis and separates each other by bridging tridentate pyridyl ligands. Each cage A interconnects with three B1 cages and three B2 cages by sharing the triangle window formed by BDC2− and TPT ligands. Each cage B1 and cage B2 interconnect with six A cages, affording the overall three-dimensional network (Fig. 1b).
Possible self-assembly mechanism for NPU-6
The unique –A–B–A–B– laminar assembly mode of the tri- and hexa-nuclear clusters in NPU-6 also led us to consider possible reaction mechanisms. The main difference between trinuclear [Mn3(μ3-O)] clusters and hexanuclear [Mn6(μ3-O)2(CH3COO)3] clusters is that Mn ions are bridged by CH3COO− in Mn6, which was not needed in the Mn3 cluster. So, the relative concentrations of CH3COO− may be the key to the equilibrium shifting from the formation of trinuclear clusters to hexanuclear clusters. With the current scientific and technological means, it is extremely difficult to observe the self-assembly process of molecules at the microscopic scale. On this basis, we propose a reaction mechanism for CH3COO− concentration induced assembly (as shown in Fig. 2b). In our proposal, when there is no CH3COO− in the reaction system and the product is pure trinuclear based CMP-153; when the concentration of CH3COO− increases, the hexanuclear cluster begins to form but the formation rate is lower than that of the trinuclear cluster, which leads to the product containing trimer based CPM-153 and mixed-cluster based NPU-6; when the addition of CH3COO− is further increased, both clusters form at comparable rates, and here we can harvest mixed-cluster based NPU-6 as a pure product; when the concentration of CH3COO− is further increased, a mixture of hexamer based NPU-1-TPT and mixed-cluster based NPU-6 can be obtained owing to the higher formation rate of hexanuclear midbodies than trinuclear midbodies, and finally we can obtain the hexamer based NPU-1-TPT as a pure product with an excess amount of CH3COO−. In order to further verify our proposed mechanism and establish the correlation between the content of CH3COO− and the result of reaction assembly, a series of experiments were conducted. Firstly, we used manganese acetate tetrahydrate as the metal source and fixed the amount and proportion of metal salts and ligands. When no additional CH3COOH was added, the extremely low CH3COO− concentration severely limited the reaction kinetics of the Mn6 cluster, so only CPM-153 with the Mn3 node was generated in the system (Fig. 2a and c state I). When an additional 0.1 mL acetic acid is added, the PXRD of the product showed a strong CPM-153 peak at 8.37° and a weak NPU-6 peak at 7.59° (Fig. 2a state II), indicating that a large amount of CPM-153 and a small amount of NPU-6 were generated. It is speculated that ν(Mn3) in this reaction system is much higher than ν(Mn6). During the growth of CPM-153 with the Mn3 cluster as the node, a large number of Mn ions are consumed, which further leads to the reduction of ν(Mn3). When the kinetic rate of the two metal clusters is the same, alternating self-assembly occurs to generate NPU-6 (Fig. 2c, state II). As shown in Fig. 2a, when the acetic acid amount increased to 0.2 mL, the concentration of CH3COOH was sufficient to support the dynamic alternating fluctuation of ν(Mn3) and ν(Mn6), resulting in the alternating growth of the two metal clusters (as shown in Fig. 2a and c state III), and only the diffraction peak of NPU-6 appeared in the reaction product. When the additional amount of acetic acid is further increased to 0.3–0.7 mL (Fig. 2a), the situation is opposite to 0.1 mL of CH3COOH. At this time, ν(Mn6) is much higher than ν(Mn3), and NPU-1-TPT with hexanuclear Mn6 as the node is first generated. In this process, a large number of acetic acid ions are consumed, while ν(Mn6) gradually decreases to the same as ν(Mn3), so that alternate assembly of the two metal clusters occurs in the next stage (Fig. 2c, state IV). When acetic acid is sufficient (0.8 mL), as shown in Fig. 2a, the PXRD pattern of the reaction products only shows the characteristics of NPU-1-TPT. This is because sufficient CH3COO− makes ν(Mn6) much greater than ν(Mn3) until the Mn ions are completely consumed, resulting in the formation of only NPU-1-TPT with the Mn6 cluster as the node in the reaction system (Fig. 2c, state V). In summary, as shown in Fig. 2, with the increase of CH3COOH in the reaction system, the reaction product has 5 different states and follows the change rule of [CPM-153]–[CPM-153 & NPU-6]–[NPU-6]–[NPU-6 & NPU-1-TPT]–[NPU-1-TPT]. In addition, to eliminate the influence of CH3COO− of metal salts, we used Mn (NO3)2·4H2O as the manganese source to conduct the same experiment, and the PXRD data of the reaction products showed the same trend (Fig. S6†), which further confirmed our proposal. Furthermore, we found that substituting acetic acid with formic acid had the same effect on the reaction, i.e., with the addition of formic acid, the reaction results showed a similar trend (Fig. S7†). Although there is no HBF4-related component in the single crystal structure, we also designed experiments to explore the effect of HBF4 on the reaction system. The introduction of HBF4 can promote the dissolution of the reactants and improve the quality of the crystalline product to a certain extent, but it will not affect the structure of the product (Fig. S7†). These controlled experiments demonstrated that CH3COOH is the key to the selective nucleation of Mn ions, which provides a reference for the design and synthesis of high nuclear coordination structures in the future.Functional characterization of NPU-6
It is necessary to evaluate its versatility after realizing the directional and ordered assembly of trinuclear and hexanuclear manganese clusters. In a previous study, we found that CPM-153 with the traditional Mn3 cluster has a large theoretical pore capacity but is limited by its poor stability. The expanded NPU-1-TPT with Mn6 clusters has higher stability due to the higher connection number, which prompts us to investigate the stability and adsorption performance of NPU-6 with hybrid nodes. As shown in Fig. 3a, the powder X-ray diffraction at variable temperature shows that the diffraction peak of CPM-153 has changed significantly at 90 °C, and the appearance of new diffraction peaks at 9.5° and 14.2° indicates the partial collapse and decomposition of the network. Meanwhile, the powder X-ray diffraction of NPU-1-TPT (Fig. 3b) with the hexanuclear cluster has no significant change at 90 °C, and the decrease of the main peak at 280 °C indicates the decomposition of the material. More interestingly, the PXRD patterns show that NPU-6 (Fig. 3c) with mixed clusters can also be well maintained until 280 °C.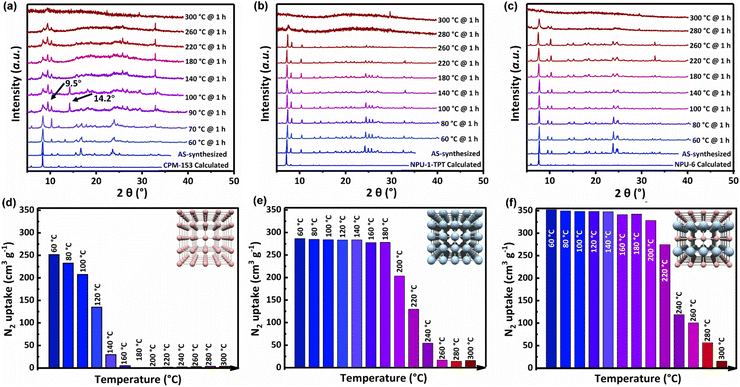 | ||
| Fig. 3 Stability and porosity tests. PXRD patterns and N2 uptake data of CPM-153 ((a) and (d)), NPU-1-TPT ((b) and (e)), and NPU-6 ((c) and (f)) samples after treatment with different temperatures. | ||
For porous materials, the characterization of porosity can provide more detailed support for their stability research. Therefore, their N2 adsorption isotherms at 77 K were used to characterize the porosity of the three networks after being treated at different temperatures. To activate the porous material at a lower temperature, the material was soaked with methylene chloride (CH2Cl2), and thermogravimetric analysis (TGA) of the as-synthesized and CH2Cl2-exchanged samples revealed that the DMA solvent in the pores was completely exchanged by CH2Cl2 (Fig. S8†). As shown in Fig. S9–S11,† the three materials showed high porosity after 60 °C treatment under high vacuum, with a reversible type I adsorption isotherm. CPM-153 with the trinuclear Mn3 cluster showed a relatively low actual N2 capacity at 77 K and 100 kPa (255.5 cm3 g−1) due to the partial collapse of the network after activation. NPU-1-TPT with the Mn6 cluster can be maintained after activation, which results in a moderate N2 uptake at 77 K and 100 kPa (285.5 cm3 g−1). Interestingly, NPU-6 with mixed clusters showed the highest N2 adsorption capacity under the same conditions (354.1 cm3 g−1), as the density of the network was effectively reduced by the introduction of Mn3 clusters. Horvath–Kawazoe (pore geometry: sphere) based pore size distribution analyses were conducted upon the above N2 sorption isotherms. As shown in Fig. S12,†CPM-153, NPU-1-TPT and NPU-6 have a similar pore-size distribution of 0.65–0.90 nm, which is consistent with the aperture obtained from the single crystal data. Assuming that liquid N2 fills the pores at 77 K and 100 kPa, the pore volume calculated from N2 absorption at 100 kPa is 0.399 for CPM-153 with the Mn3 cluster, 0.447 for NPU-1-TPT with the Mn6 cluster, and 0.552 cm3 g−1 for the mixed cluster NPU-6, which means that NPU-6 inherits the high theoretical porosity from CPM-153. With the increase in processing temperature, the decrease of the measured adsorption capacity of CPM-153 indicates the rapid collapse of the network, and the porosity of the material almost disappeared when it reached 160 °C (the detailed isotherm is shown in Fig. S13†). Interestingly, the measured N2 adsorption capacity of NPU-1-TPT with the Mn6 cluster and NPU-6 with the mixed cluster is almost unchanged even when heated to 180 °C (Fig. 3e, f, S14 and S15†), which means that the introduction of the Mn6 cluster makes NPU-6 inherit the good thermal stability of NPU-1-TPT. Further, humidity stability experiments (Fig. S17†) show that CPM-153 with the Mn3 cluster collapses rapidly at a relative humidity (R.H.) of 70%, while NPU-1-TPT with the Mn6 cluster and NPU-6 with the mixed cluster still maintain the complete crystalline state and porosity after 7 days, which indicates that the introduction of the Mn6 cluster significantly improves the humidity stability of the network. Therefore, after realizing the directional assembly of tri- and hexa-nuclear clusters, NPU-6 not only has the same stability as NPU-1-TPT, but also obtains the highest pore volume, achieving the effect of “1 + 1 > 2”.
The high porosity of NPU-6 prompts us to investigate its C2 adsorption behaviour. As we expected, the adsorption capacity of CPM-153, NPU-1-TPT and NPU-6 for C2H6 and C2H4 increased sequentially (the detailed isotherm is shown in Fig. S18–S20†). As shown in Fig. 4a, at 298 K and 100 kPa, the adsorption capacity of C2H6 for NPU-6 was up to 134.0 cm3 g−1 (120.1 for C2H4), while those of NPU-1-TPT and CPM-153 were 99.8 cm3 g−1 (91.5 cm3 g−1 for C2H4) and 85.2 cm3 g−1(79.9 cm3 g−1 for C2H4), respectively. The C2H6 uptake of all three materials is greater than that of C2H4, implying that they have the potential to purify C2H4 from the C2H6/C2H4 mixture in one step, which prompted us to calculate their IAST (Ideal Adsorbed Solution Theory)48 selectivity by fitting their adsorption isotherms at 298 K into the Langmuir–Freundlich equation49 (detailed fitting curves are given in Fig. S21–S23†). As shown in Fig. 4b, NPU-1-TPT and CPM-153 have comparable C2H6/C2H4 selectivity, while NPU-6 has the highest selectivity of 1.8. In order to investigate the effect of the simultaneous introduction of two metal clusters on the interaction mechanism of the network with C2H6 and C2H4, we used molecular simulations to confirm the interaction sites of the materials with C2H6 and C2H4. As shown in Fig. S24,† the three materials have similar pore characteristics, and C2H4 and C2H6 are preferentially located on the triangular windows composed of two BDC2− and one TPT ligand. For NPU-6, C2H6 with a larger size preferentially adsorbs in the middle of the channel compared to the other two materials, and C2H6 interacts with eight weak contact sites through four C–H⋯C (3.41–3.67 Å), two C–H⋯N (2.90 and 3.07 Å) and two C–H⋯O (3.39 and 3.39 Å). Meanwhile in NPU-1-TPT and CPM-153, C2H6 tends to bias towards one side of the channel, obtaining seven weak contact points through three C–H⋯C (3.22–3.74 Å for CPM-153; 3.7–3.82 Å for NPU-1-TPT), two C–H⋯N (3.15 and 3.25 Å for CPM-153; 3.14 and 3.27 Å for NPU-1-TPT) and two C–H⋯O (3.32 and 3.37 Å for CPM-153; 3.69 and 3.71 Å for NPU-1-TPT). Due to its smaller molecular size, C2H4 tends to act on one side of the channel, forming six weak forces with three C–H⋯C (3.26–4.05 Å), two C–H⋯N (2.95 and 3.17 Å), and two C–H⋯O (3.52 and 3.54 Å) in NPU-6, and it is a similar case for NPU-1-TPT and CPM-153. We reason that C2H4 and C2H6 are adsorbed in these three materials with minor differences confirmed by the similar binding energy of C2H4 (ECPM-153 = 24.69 kJ mol−1, ENPU-1-TPT = 25.61 kJ mol−1 and ENPU-6 = 24.78 kJ mol−1) and C2H6 (ECPM-153 = 30.73 kJ mol−1, ENPU-1-TPT = 31.72 mol−1 and ENPU-6 = 30.91 kJ mol−1) and the practical separation differences are attributed to the different effective pore volumes of these three materials. To further simulate the realistic separation properties, a single adsorption bed model was built to simulate breakthrough experiments. For these three materials, all the simulating parameters were set the same except for the parameters from isotherms. As shown in Fig. 4d, the first detections of C2H4 were found at times of 19.8, 34.0 and 48.7 min for CPM-153, NPU-1-TPT and NPU-6, respectively, in good agreement with the experimental pore volume of CPM-153 < NPU-1-TPT < NPU-6. Then, the first observations of 0.5% C2H6 in the outlet gas were at 21.3, 35.3 and 53.2 min, respectively. Thus, the retention times for the production of polymer-grade C2H4 (>99.5%) were 1.5, 1.3 and 4.5 min, in the same order of IAST selectivities of NPU-1-TPT < CPM-153 < NPU-6. In a word, by taking advantage of the high pore volume of trimer-based CPM-153 and the good stability of hexamer-based NPU-1-TPT, NPU-6 displayed the highest capacity as well as the highest selectivity among these three materials in C2H4/C2H6 separations. Further, as shown in Fig. 4c, the experimental breakthrough curve verified the actual ability of NPU-6 to produce polymer-grade C2H4 from a C2H4/C2H6 mixture in one step. In order to investigate the separation behaviour of NPU-6 for a ternary C2H2/C2H4/C2H6 mixture (Fig. S25†), we carried out a breakthrough experiment based on a C2H2/C2H4/C2H6 ternary gas mixture. As shown in Fig. S20 and S25,†NPU-6 demonstrates the selective adsorption of C2H2 and C2H6 over C2H4 and further achieves one-step C2H4 purification from the ternary gas mixture, but with only limited performance due to the subtle difference in the adsorption capacity of C2H4 and C2H2 by NPU-6 at the relatively low-pressure stage. Further, the good cycling stability and regeneration performance were proved by cycling adsorption–desorption experiments (Fig. S26†), in which the C2H6 capacity of NPU-6 did not show any attenuation within five cycles.
Conclusions
In conclusion, we utilized the large chemical difference (size and geometry) of two metal clusters to realize the ordered assembly of such two metal clusters with the same topological connectivity in one single coordination network, through the precise concentration control of the bridging CH3COO− ligand in the reaction system. The resulting mixed cluster-assembled coordination network NPU-6 demonstrates superior stability and high pore volume, and further effective C2H6/C2H4 separation performance. Subsequent in situ characterization experiments for understanding the assembly mechanism of these two metal clusters are under way.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for NPU-1-TPT and NPU-6 have been deposited at CCDC under 2347593 and 2347594.Author contributions
K.-J. C. designed the project. J.-W. C. conducted all the experimental work regarding the synthesis, gas adsorption characterisation and breakthrough experiments. T. Z. performed single crystal structure analysis and breakthrough simulation. J.-W. C., T. Z., Y. W., J. C. and K.-J. C. discussed the formation mechanism. T. Z. performed the GCMC calculation. J.-W. C., Y. W. and J.-B. W. performed the collection and analysis of PXRD data. J.-W. C., T. Z., J. C. and K.-J. C. wrote the manuscript, and all authors contributed to the revision of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the financial support from the National Natural Science Foundation of China (22071195 and 22101231), Youth Innovation Team of Shaanxi Universities, China Postdoctoral Science Foundation (No. 2022M712585), Natural Science Basic Research Plan in Shaanxi Province of China (2023-YBGY-425 and 2019JQ627), Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (CX2022071) and Open Project Program of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry (No. 2024-28). We would like to thank the Analytical & Testing Center of Northwestern Polytechnical University.Notes and references
- H. Furukawa, K.-E. Cordova, M. O Keeffe and O.-M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- J.-J. Perry Iv, J.-A. Perman and M.-J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC.
- D.-J. O'Hearn, A. Bajpai and M.-J. Zaworotko, Small, 2021, 2006351 CrossRef.
- A. Cadiau, K. Adil, P.-M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140 CrossRef CAS.
- J.-W. Cao, S. Mukherjee, T. Pham, Y. Wang, T. Wang, T. Zhang, X. Jiang, H.-J. Tang, K.-A. Forrest, B. Space, M.-J. Zaworotko and K.-J. Chen, Nat. Commun., 2021, 12, 6507 CrossRef CAS PubMed.
- J.-W. Cao, T. Zhang, Y. Wang and K.-J. Chen, ACS Appl. Mater. Interfaces, 2024, 16, 10260–10266 CrossRef CAS PubMed.
- P. Miao, T. Zhang, T. Wang, J. Chen, T. Gao, Y. Wang, J. Kong and K.-J. Chen, Chin. J. Chem., 2022, 40, 467–474 CrossRef CAS.
- X. Liu, J. Li, N. Li, B. Li and X.-H. Bu, Chin. J. Chem., 2021, 39, 440–462 CrossRef CAS.
- K.-J. Chen, D.-G. Madden, S. Mukherjee, T. Pham, K.-A. Forrest, A. Kumar, B. Space, J. Kong, Q.-Y. Zhang and M.-J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS PubMed.
- X. Cui, K.-J. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M.-J. Zaworotko and B. Chen, Science, 2016, 353, 141–144 CrossRef CAS PubMed.
- S. Zhou, O. Shekhah, A. Ramírez, P. Lyu, E.-A. Hamad, J. Jia, J. Li, P.-M. Bhatt, Z. Huang, H. Jiang, T. Jin, G. Maurin, J. Gascon and M. Eddaoudi, Nature, 2022, 606, 706–712 CrossRef CAS.
- Y.-Y. Jia, J.-C. Yin, N. Li, Y.-H. Zhang, R. Feng, Z.-Q. Yao and X.-H. Bu, Chin. J. Chem., 2022, 40, 589–596 CrossRef CAS.
- H.-L. Zhou, J. Bai, X.-Y. Tian, Z.-W. Mo and X.-M. Chen, Chin. J. Chem., 2021, 39, 2718–2724 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O'Keeffe and O.-M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- S.-S.-Y. Chui, S.-M.-F. Lo, J.-P.-H. Charmant, A.-G. Orpen and I.-D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS.
- J.-H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K.-P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- G. Ferey, C.-M. Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef CAS.
- H. Furukawa, U. Müller and O.-M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 3417–3430 CrossRef CAS PubMed.
- A.-D. Burrows, CrystEngComm, 2011, 13, 3623–3642 RSC.
- X. Kong, H. Deng, F. Yan, J. Kim, J.-A. Swisher, B. Smit, O. M. Yaghi and J. A. Reimer, Science, 2013, 341, 882–885 CrossRef CAS PubMed.
- H. Deng, C. J. Doonan, H. Furukawa, R.-B. Ferreira, J. Towne, C.-B. Knobler, B. Wang and O.-M. Yaghi, Science, 2010, 327, 846–850 CrossRef CAS.
- M. Inukai, T. Fukushima, Y. Hijikata, N. Ogiwara, S. Horike and S. Kitagawa, J. Am. Chem. Soc., 2015, 137, 12183–12186 CrossRef CAS PubMed.
- S. Horike, Y. Inubushi, T. Hori, T. Fukushima and S. Kitagawa, Chem. Sci., 2012, 3, 116–120 RSC.
- T. Fukushima, S. Horike, Y. Inubushi, K. Nakagawa, Y. Kubota, M. Takata and S. Kitagawa, Angew. Chem., Int. Ed., 2010, 49, 4820–4824 CrossRef CAS.
- T. Li, Y. Liu, T. Wang, Y. Wu, Y. He, R. Yang and S. Zheng, Microporous Mesoporous Mater., 2018, 272, 101–108 CrossRef CAS.
- Z. Dong, Y. Sun, J. Chu, X. Zhang and H. Deng, J. Am. Chem. Soc., 2017, 139, 14209–14216 CrossRef CAS.
- A.-M. Fracaroli, P. Siman, D.-A. Nagib, M. Suzuki, H. Furukawa, F.-D. Toste and O.-M. Yaghi, J. Am. Chem. Soc., 2016, 138, 8352–8355 CrossRef CAS PubMed.
- L.-M. Aguirre-Díaz, F. Gándara, M. Iglesias, N. Snejko, E. Gutiérrez-Puebla and M.-Á. Monge, J. Am. Chem. Soc., 2015, 137, 6132–6135 CrossRef.
- L.-J. Wang, H. Deng, H. Furukawa, F. Gándara, K.-E. Cordova, D. Peri and O.-M. Yaghi, Inorg. Chem., 2014, 53, 5881–5883 CrossRef CAS PubMed.
- Q. Liu, H. Cong and H. Deng, J. Am. Chem. Soc., 2016, 138, 13822–13825 CrossRef CAS.
- F. Nouar, T. Devic, H. Chevreau, N. Guillou, E. Gibson, G. Clet, M. Daturi, A. Vimont, J.-M. Grenèche, M. I. Breeze, R. I. Walton, P. L. Llewellyn and C. Serre, Chem. Commun., 2012, 48, 10237–10239 RSC.
- Z. Ji, T. Li and O.-M. Yaghi, Science, 2020, 369, 674–680 CrossRef CAS PubMed.
- L. Gagliardi and O.-M. Yaghi, Chem. Mater., 2023, 35, 5711–5712 CrossRef CAS.
- S.-J. Lee and S.-G. Telfer, Angew. Chem., Int. Ed., 2023, 62, e2023063 Search PubMed.
- X. Zhao, X. Bu, Q.-G. Zhai, H. Tran and P. Feng, J. Am. Chem. Soc., 2015, 137, 1396–1399 CrossRef CAS.
- Y.-S. Wei, M. Zhang, P.-Q. Liao, R.-B. Lin, T.-Y. Li, G. Shao, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2015, 6, 8348 CrossRef CAS.
- B. Zhu, J.-W. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K.-A. Forrest, M.-J. Zaworotko and K.-J. Chen, J. Am. Chem. Soc., 2021, 143, 1485–1492 CrossRef CAS.
- T. Zhang, J.-W. Cao, Y.-F. Dai, H. Feng, S.-Y. Zhang, J. Chen, T. Wang, Y. Wang and K.-J. Chen, Cryst. Growth Des., 2022, 22, 3594–3600 CrossRef CAS.
- Q.-G. Zhai, X. Bu, C. Mao, X. Zhao, L. Daemen, Y. Cheng, A. J. Ramirez-Cuesta and P. Feng, Nat. Commun., 2016, 7, 13645 CrossRef CAS PubMed.
- Q.-G. Zhai, X. Bu, X. Zhao, D.-S. Li and P. Feng, Acc. Chem. Res., 2017, 50(2), 407–417 CrossRef CAS PubMed.
- V. A. Blatov, Struct. Chem., 2012, 23, 955–963 CrossRef CAS.
- F. Nouar, J. F. Eubank, T. Bousquet, L. Wojtas, M. J. Zaworotko and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 1833–1835 CrossRef CAS PubMed.
- A. Schoedel, W. Boyette, L. Wojtas, M. Eddaoudi and M.-J. Zaworotko, J. Am. Chem. Soc., 2013, 135, 14016–14019 CrossRef CAS PubMed.
- D. Alezi, A. M. P. Peedikakkal, Ł. J. Weseliński, V. Guillerm, Y. Belmabkhout, A. J. Cairns, Z. Chen, Ł. Wojtas and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 5421–5430 CrossRef CAS PubMed.
- B. Tu, Q. Pang, E. Ning, W. Yan, Y. Qi, D. Wu and Q. Li, J. Am. Chem. Soc., 2015, 137, 13456–13459 CrossRef CAS PubMed.
- A.-L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- A.-L. Myers and J.-M. Prausnitz, AIChE J., 1965, 11, 121–127 CrossRef CAS.
- A. Dabrowski, J. Oscik, W. Rudzinski and M. Jaroniec, J. Colloid Interface Sci., 1979, 69, 287–300 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2347593 and 2347594. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02550d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |



