Open Access Article
Open Access ArticleThe unprecedented strong paratropic ring current of a bis-PdII complex of 5,10,23-trimesityl [28]heptaphyrin(1.1.0.0.1.0.0)†
Yang
Liu
,
Ling
Xu
,
Xiaorong
Jin
,
Bangshao
Yin
,
Yutao
Rao
,
Mingbo
Zhou
,
Jianxin
Song
 * and
Atsuhiro
Osuka
* and
Atsuhiro
Osuka
Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China), Key Laboratory of the Assembly and Application of Organic Functional Molecules of Hunan Province, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China. E-mail: jxsong@hunnu.edu.cn
First published on 7th June 2024
Abstract
Acid-catalyzed Friedel–Crafts-type cyclization of tetrapyrrolic BF2 complex 1 and α,α′-dibromotripyrrin 2 gave 5,10,23-trimesityl [28]heptaphyrin(1.1.0.0.1.0.0) BF2 complex 3BF2 as a stable and moderate antiaromatic macrocycle. Demetalation of 3BF2 with methanesulfonic acid followed by treatment with HCl gave free-base salt 3HCl that holds a chloride anion at the core. This salt displays a planar structure with an inverted pyrrole and a stronger paratropic ring current. Metalation of neutral free-base 3 with PdCl2 gave bis-PdII complex 3Pd2 as a stable antiaromatic molecule. The 1H NMR spectrum of 3Pd2 displays signals due to pyrrolic β-protons in the range of −1.06 ∼ −1.90 ppm, indicating the unprecedented strong paratropic ring current.
Introduction
Stable antiaromatic molecules1 are a promising organic structural motif because of their facile redox reactivities, three-dimensional aromaticity,2 characteristically fast excited-state decays and small HOMO–LUMO gaps,3 and excellent conductivities in the solid-state.4 In the last two decades, expanded porphyrins have been demonstrated to be a nice platform to realize stable antiaromatic molecules owing to their electronically and sterically adaptable properties.5 How to strengthen the antiaromatic character of expanded porphyrins while retaining chemical stability is an interesting issue. A paratropic ring current and a theoretical NICS value are the representative indices to evaluate the strength of antiaromaticity.Representative examples of stable antiaromatic porphyrinoids exhibiting strong paratropic ring currents are shown in Scheme 1, which include meso-aryl [28]hexaphyrin(1.1.1.1.1.1) bis-AuIII complex A,6 5,14-dimesityl [16]tetraphyrin(1.0.1.0) NiII complex (NiII norcorrole) B,7 5,10,23-trimesityl [24]hexaphyrin(1.1.0.0.1.0) bis-PdII complex C8 and 5,10,19-trimesityl [20]pentaphyrin(1.1.0.1.0) ([20]smaragdyrin) D.9 Signals due to the outer β-protons are observed as a pair of doublets at 3.16 and 3.10 ppm and at 1.60 and 1.45 ppm for A and B, respectively. Correspondingly, for C and D, these signals appear in the ranges of 2.49 to 0.70 ppm and 3.88 to 2.75 ppm. The calculated NICS (nucleus-independent chemical shift) values (at the B3LYP/6-31G(d) level) within the macrocycle of A, B, C and D are largely positive (Scheme 1). Among expanded porphyrins, heptaphyrins have been poorly studied so far as compared with hexaphyrins and octaphyrins despite their interesting and characteristic reactivities and properties.10,11 In addition, the synthetic access to heptaphyrins has been rather limited. In the one-pot synthesis of meso-pentafluorophenyl-substituted expanded porphyrins by modified Lindsey condensation at high concentration,12 the yield of heptaphyrin was poor (ca. 4–5%) and its isolation needed tedious repeated separations. Later, the same heptaphyrin was prepared in a better yield (39%) by designed acid-catalyzed condensation of tripyrrane dicarbinol and tetrapyrrane.11b
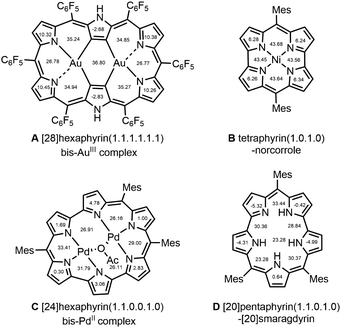 | ||
| Scheme 1 Examples and NICS(0) values of stable antiaromatic expanded porphyrins, A, B, C and D; the shown NICS(0) values are determined at the geometric or areal center. | ||
Results and discussion
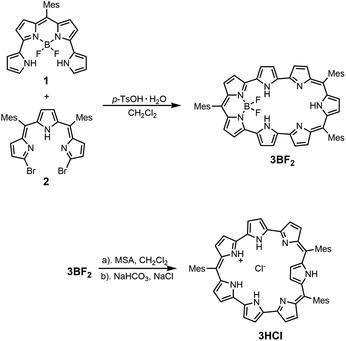
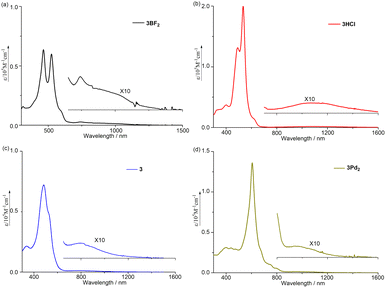
So far, most of the expanded porphyrins have been synthesized by acid-catalyzed condensation reactions of oligopyrrolic molecules and aldehydes or their equivalents.5 Recently, we have developed a new synthetic method for expanded porphyrins on the basis of acid-catalyzed Friedel–Crafts-type cyclization.13 In this work, we employed a similar cyclization of tetrapyrrolic BF2 complex 1 (ref. 14) and α,α′-dibromotripyrrin 2 (ref. 15) for the synthesis of 5,10,23-trimesityl [28]heptaphyrin(1.1.0.0.1. 0.0) 3BF2. Namely, a solution of equimolar amounts of 1 and 2 in CH2Cl2 was heated in the presence of p-toluenesulfonic acid at 50 °C for 8 h. After the usual work up, separation through an Al2O3 column gave 3BF2 in 23% yield. The parent ion peak of 3BF2 was observed at m/z = 893.4298, calcd for (C58H50BF2N7)+ = 893.4233 ([M]+) by high resolution MALDI-TOF mass spectrometry.The structure of 3BF2 has been revealed by X-ray analysis.16 Two different structures (3BF2A and 3BF2B) were found in the crystal (Fig. 1a–d). The two structures are similar, showing that the tetrapyrrolic and the tripyrrolic parts are planar but these two units are bent with an angle of ca. 50°. On the basis of Cα–N–Cα′ angles of the pyrroles, the pyrroles C, E, and G have been denoted as an aminic pyrrole and the pyrroles D and F have been denoted as an iminic pyrrole. The 1H NMR spectrum of 3BF2 in CDCl3 shows three pairs of doublets in the range of 5.42–4.98 ppm and a singlet at 4.79 ppm due to the pyrrolic β-protons, indicating a moderate paratropic ring current arising from its 28π-electronic circuit. Signals due to the inner NH protons are not observed at room temperature in CDCl3 but are observed at 18.18 ppm (2H) and at 17.73 ppm (1H) at 213 K, in line with the assigned structure. This phenomenon is common in expanded porphyrins, possibly due to rapid NH proton exchange between neighboring amino and imine pyrroles, or between amino pyrroles and solvent molecules like water. The absorption spectrum of 3BF2 shows two prominent bands at 464 and 524 nm along with a broad tail at 1200 nm, which are typical of antiaromatic porphyrinoids (Fig. 4).17
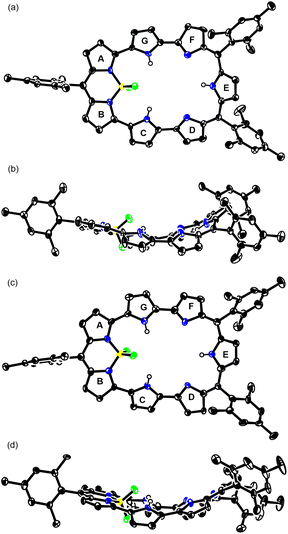 | ||
| Fig. 1 Single-crystal X-ray structures of the (a) top view and (b) side view of 3BF2A, and (c) top view and (d) side view of 3BF2B. The thermal ellipsoids are at 50% probability. | ||
In the next step, a solution of 3BF2 in CH2Cl2 was refluxed in the presence of methanesulfonic acid overnight. After the usual work up, the organic extract was treated with an aqueous NaHCO3 solution and then brine. From this residue, 3HCl was obtained in 87% yield as red solids (Scheme 2). The parent ion peak was observed at m/z = 846.4279, calcd for (C58H52N7)+ = 846.4279 ([M–Cl]+). The structure of 3HCl has been revealed by X-ray analysis to be roughly C2v symmetric and considerably planar with a mean-plane-deviation (MPD) of 0.358 Å (Fig. 2).16 On the basis of the Cα–N–Cα angles (Fig. S25†), the pyrroles A, C, D, E, and F have been denoted as aminic and the pyrroles B and G have been denoted as iminic. Interestingly, pyrrole A is inverted and the Cl anion is held by hydrogen bonding interaction with the four pyrrolic NH bonds possibly, as well as the two β-pyrrolic CH protons. Similar anion binding has been extensively studied by Sessler et al., but the involvement of β-pyrrolic CH protons is rare.18 The 1H NMR spectrum of 3HCl shows signals at 25.42 and 24.89 ppm due to the inner NH protons, a signal at 4.76 ppm due to the outer NH proton, a signal at 16.79 ppm due to the inner pyrrolic β-proton, and twelve signals in the range of 4.47–3.55 ppm due to the outer pyrrolic β-protons, indicating a stronger paratropic ring current in the nonsymmetric structure. It is considered that 3HCl takes a nonsymmetric tautomeric structure in CDCl3 solution. The absorption spectrum of 3HCl shows a split strong band at 396 and 491 nm with a shoulder at ∼617 nm and a very weak and broad tail at 1600 nm (Fig. 4).
 | ||
| Fig. 2 Single-crystal X-ray structures of the (a) top view and (b) side view of 3HCl. The thermal ellipsoids are at 50% probability. | ||
Treatment of 3HCl with a K2CO3 solution gave free base heptaphyrin 3 (Scheme 3). The 1H NMR spectrum of 3 in DMSO-d6 shows two isomers in a ratio of ca. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. These isomers were not separable. The major isomer is non-symmetric, displaying four peaks due to the pyrrolic β-protons at 9.95, 10.11, 10.76 and 10.97 ppm, indicating the presence of two inverted pyrroles. Signals due to the outer pyrrolic β-protons of the major isomer are in range of 5.02–6.08 ppm with two inner NH protons at 19.08 and 19.25 ppm. The minor isomer is symmetric with one inverted pyrrole on the symmetry axis, showing six peaks due to outer pyrrolic β-protons in the range of 5.9–4.9 ppm, one inner pyrrolic β-proton at 10.96 ppm, two peaks due to inner NH protons at 17.43 (1H) and 15.46 (2H) ppm and one peak due to an outside NH proton at 7.05 ppm. The solubility of 3 in CDCl3 is poor and the 1H NMR spectrum of 3 in CDCl3 is also complicated, showing four doublets due to the pyrrolic β-protons in the range of 11.2–9.5 ppm, suggesting the presence of a minor isomer having two inverted pyrroles. Signals due to the outer pyrrolic β-protons of major and minor isomers are observed in the range of 5.2–6.2 ppm.
1. These isomers were not separable. The major isomer is non-symmetric, displaying four peaks due to the pyrrolic β-protons at 9.95, 10.11, 10.76 and 10.97 ppm, indicating the presence of two inverted pyrroles. Signals due to the outer pyrrolic β-protons of the major isomer are in range of 5.02–6.08 ppm with two inner NH protons at 19.08 and 19.25 ppm. The minor isomer is symmetric with one inverted pyrrole on the symmetry axis, showing six peaks due to outer pyrrolic β-protons in the range of 5.9–4.9 ppm, one inner pyrrolic β-proton at 10.96 ppm, two peaks due to inner NH protons at 17.43 (1H) and 15.46 (2H) ppm and one peak due to an outside NH proton at 7.05 ppm. The solubility of 3 in CDCl3 is poor and the 1H NMR spectrum of 3 in CDCl3 is also complicated, showing four doublets due to the pyrrolic β-protons in the range of 11.2–9.5 ppm, suggesting the presence of a minor isomer having two inverted pyrroles. Signals due to the outer pyrrolic β-protons of major and minor isomers are observed in the range of 5.2–6.2 ppm.
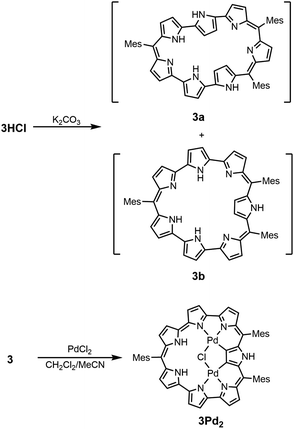
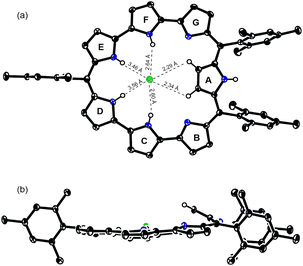
Finally, the reaction of 3 with PdCl2 in a mixture of CH2Cl2 and acetonitrile at room temperature for 4 h gave bis-Pd complex 3Pd2 in 19% yield (Scheme 3). The parent ion peak was observed at m/z = 1091.1711, calcd for (C58H48ClN7Pd2)+ = 1091.1745 ([M + H]+). The structure of 3Pd2 has been revealed by X-ray analysis to be C2v symmetric and very planar with a small MPD of 0.05 Å (Fig. 3).16 Similarly to 3HCl, only pyrrole A is inverted and the remaining pyrroles are pointing inward. The two Pd metals are bound to the two pyrrolic nitrogen atoms (the pyrroles B and G and the pyrroles C and F), the β-proton of the pyrrole A, and the Cl− anion with bond lengths of 1.979 Å, 2.192 Å, 1.958 Å, and 2.323 Å, respectively. The Cl− anion is shared by the two Pd metals and interact with the NH protons of the pyrroles D and E by hydrogen bonding. In the 1H NMR spectrum of 3Pd2, a signal due to the inner NH protons is observed at a very low field (42.93 ppm), signals due to the outer pyrrolic β-protons are observed at a high field (−1.06 ∼ −1.90 ppm), and a signal due to the outer NH proton is observed at −6.17 ppm, indicating a very strong paratropic ring current. While many antiaromatic porphyrinoids have been reported so far, the observation of pyrrolic β-protons at negative chemical shifts is unprecedented. The absorption spectrum of 3Pd2 shows a band at 606 nm with a weak and broad tail at 1200 nm (Fig. 4).
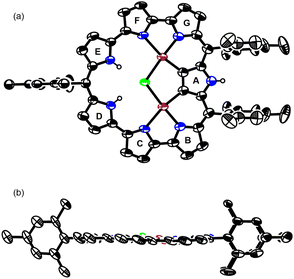 | ||
| Fig. 3 Single-crystal X-ray structures of the (a) top view and (b) side view of 3Pd2. The thermal ellipsoids are at 50% probability. | ||
The electrochemical properties were investigated by cyclic voltammetry and differential pulse voltammetry (Table 1). The electrochemical HOM–LUMO gaps of 3BF2 and 3 are certainly small (1.25 eV and 1.30 eV), but those of 3HCl and 3Pd2 are distinctly smaller, being 0.74 and 0.72 eV, respectively.
| Sample | E red.3 (V) | E red.2 (V) | E red.1 (V) | E ox.1 (V) | E ox.2 (V) | E ox.3 (V) | ΔEHLb |
|---|---|---|---|---|---|---|---|
| a Measured in anhydrous CH2Cl2 with 0.1 M nBu4NPF6 in a glovebox versus ferrocene/ferrocenium ion couple; working electrode, glassy carbon; counter electrode, Pt wire; reference electrode, Ag/0.01 M AgNO3. b ΔEHL = e(Eox.1 − Ered.1) [eV]. | |||||||
| 3BF2 | — | −1.37 | −1.15 | 0.10 | 0.42 | — | 1.25 |
| 3HCl | −1.57 | −1.23 | −0.78 | −0.04 | 0.39 | — | 0.74 |
| 3 | −1.76 | −1.45 | −1.16 | −0.14 | 0.15 | 0.34 | 1.30 |
| 3Pd2 | −1.79 | −1.33 | −0.95 | −0.23 | 0.25 | — | 0.72 |
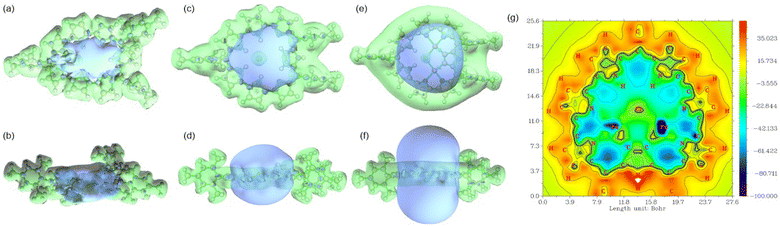
To evaluate the antiaromaticity of 3BF2, 3HCl, and 3Pd2, NICS values and ICSS (iso-chemical shielding surface)19 were calculated. These results are displayed in Fig. S49–S53 (ESI†).20 The NICS values calculated in the inner area of 3BF2 fall within the range of 9–15 ppm, suggesting its relatively weak antiaromatic character. In contrast, the NICS values for 3HCl were found to be in the range of 15–24 ppm, indicating enhanced antiaromaticity, potentially attributed to its more planar structure compared to 3BF2. Notably, for 3Pd2, the corresponding NICS values are significantly larger, ranging from 30 to 65 ppm, consistent with the observed strong paratropic ring current. As shown in Fig. 5, the deshielding isosurface ranges indicated by blue increase in the order of 3BF2 < 3HCl < 3Pd2 (Fig. 4a–f). Additionally, the ICSSZZ(1) plot for 3Pd2 was calculated to reach near −100 ppm in the inner area,21 indicating that 3Pd2 is strongly antiaromatic. It is conceivable that strong paratropic ring current is stemming from the planar, rigid, large and symmetric electronic π-network of 3Pd2.
The benchmark for aromaticity is often determined by the maximum difference in chemical shifts (Δδ) between the peripheral CH or NH moieties and the interior CH or NH moieties. Similarly, we can also use Δδ to evaluate the strength of paratropic ring current. For 3BF2, the value of ΔδCH–NH is 13.43 ppm. For 3HCl, the values of ΔδCH–NH, ΔδNH–NH and ΔδCH–CH are 21.87 ppm, 20.66 ppm and 13.24 ppm, respectively. For 3a (major isomer of 3), the value of ΔδCH–NH is 14.23 ppm while the values of ΔδCH–NH and ΔδNH–NH are 12.48 ppm and 10.38 ppm for 3b (minor isomer of 3). For 3Pd2, the values of ΔδCH–NH and ΔδNH–NH are 43.83 ppm and 49.10 ppm. This huge Δδ has never been seen in other porphyrinoids, which demonstrates the unprecedented strong paratropic ring current of 3Pd2.
To get further insights into the origin of the strong paratropic ring current of 3Pd2, we also calculated the NICS values of related structures with only atoms changing or replacement based on the optimized geometry structure of 3Pd2 (Fig. S54 and S57†). The results show that the effects of the replacement of Cl by Br, I or OH on the paratropic ring current of 3Pd2 are negligible and replacement of the meso-Mes group with hydrogen, the NICS values at those points become even larger, suggesting that the Mes group may have a negative impact on the strength of its paratropic ring current. However, upon removal of the coordinated Pd2Cl group, the paratropic ring current is noticeably weakened, as indicated by a decrease in NICS values from ca. 60 ppm to ca. 40 ppm at specific points within the macrocycle. Additionally, substituting PdII with NiII or CuII demonstrates that both NiII and CuII complexes exhibit strong paratropic ring currents, which are comparable to those observed in 3Pd2. These findings suggest that other transition metal cations may produce a similar effect, provided the complex's geometry remains essentially unaltered (Fig. S55 and S56†). Therefore, the coordination of the metal to the porphyrinoid may increase the electron density on the macrocycle, possibly due to backbonding interactions, thereby enhancing the paratropic ring current in antiaromatic porphyrinoids.
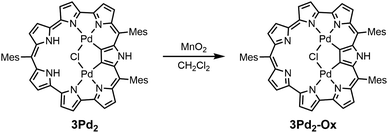
As a complement to the study of the chemical properties of heptaphyrin(1.1.0.0.1.0.0), we also investigated the reduction reaction of 3BF2 and the oxidation reaction of 3Pd2. Treating 3BF2 with NaBH4 does not produce any reducing product while 3BF2 can be reduced with Me10Fc in the presence of a large amount of HCl, similar to the case of the annulated rosarin/octaphyrin reported by Sessler and coworkers.22,23 The reduction product may be aromatic as judged by the absorption spectrum (Fig. S59†) but is too unstable to be isolated from the reaction mixture. The oxidation of 3Pd2 with MnO2 produces an aromatic counterpart 3Pd2-Ox in 52% yield (Scheme 4), and it is spontaneously reduced in solvent (Fig. S62†). The parent ion peak of 3Pd2-Ox can be observed at m/z = 1088.1606, calcd for (C58H46ClN7Pd2)+ = 1088.1668 ([M + H]+). The 1H NMR spectrum of 3Pd2-Ox in CDCl3 shows four sharp peaks and two broad peaks in the range of 11.25–9.64 ppm due to the pyrrolic β-protons, indicating a clear diatropic ring current arising from its 26π-electronic circuit. Broad peaks at 11.10 ppm and 9.94 ppm can be due to the β-protons and those in the range of −2.5 to −3.5 ppm may be ascribed to a tautomer of 3Pd2-Ox (Fig. S61†).
Conclusions
In summary, [28]heptaphyrin(1.1.0.0.1.0.0) BF2 complex 3BF2 was synthesized by acid-catalyzed Friedel–Crafts-type cyclization of tetrapyrrolic BF2 complex 1 with α,α′-dibromotripyrrin 2, and was converted to its HCl salt 3HCl and bis-Pd complex 3Pd2. While these heptaphyrins are all antiaromatic, 3Pd2 displays an unprecedentedly strong paratropic ring current. Additionally, we conducted research on the reduction of 3BF2 and the oxidation of 3Pd2. The unprecedentedly strong paratropic ring current can be attributed to its planar, rigid, large, and symmetric π-network, as well as possible backbonding interactions that augment the electron density on the macrocycle brought about by PdII coordination. Along this direction, new synthetic trials to explore even stronger antiaromatic molecules are in progress in our laboratory.Data availability
All experimental data and detailed experimental procedures are available in the ESI.†Author contributions
J. Song conducted the project. Y. Liu performed most of the synthesis and characterization, and partial DFT calculations. L. Xu carried out partial crystal analysis and partial DFT calculations. X. Jin contributed to partial synthesis. B. Yin conducted partial NMR tests. Y. Rao and M. Zhou assisted with partial crystal analysis. Y. Liu, L. Xu, Y. Rao, J. Song and A. Osuka prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant No. 22071052, 21772036, 22271091, and 22201072), Science and Technology Planning Project of Hunan Province (2018TP1017), and Science and Technology Innovation Program of Hunan Province (2021RC4059).Notes and references
- (a) R. Breslow, Acc. Chem. Res., 1973, 6, 393 CrossRef CAS; (b) H. Hopf, Angew. Chem., Int. Ed., 2013, 52, 12224 CrossRef CAS PubMed; (c) S. Nobuse, H. Miyoshi, A. Shimizu, I. Hisaki, K. Fukda, M. Nakano and Y. Tobe, Angew. Chem., Int. Ed., 2015, 54, 2090 CrossRef PubMed.
- (a) R. Nozawa, H. Tanaka, W.-Y. Cha, Y. Hong, I. Hisaki, S. Shimizu, J.-Y. Shin, T. Kowalczyk, S. Irle, D. Kim and H. Shinokubo, Nat. Commun., 2016, 7, 13620 CrossRef CAS PubMed; (b) R. Nozawa, J. Kim, J. Oh, A. Lamping, Y. Wang, S. Shimizu, I. Hisaki, T. Kowalczyk, H. Fliegl, D. Kim and H. Shinokubo, Nat. Commun., 2019, 10, 3576 CrossRef PubMed.
- J.-Y. Shin, K. S. Kim, M.-C. Yoon, J. M. Kim, Z. S. Yoon, A. Osuka and D. Kim, Chem. Soc. Rev., 2010, 39, 2733 RSC.
- J. Liu, J. Ma, K. Zhang, P. Ravat, P. Machata, S. Avdoshenko, F. Hennersdorf, H. Komber, W. Pisula, J. J. Weigand, A. A. Popov, R. Beerger, K. Müllen and X. Feng, J. Am. Chem. Soc., 2017, 139, 7513 CrossRef CAS PubMed.
- (a) J. L. Sessler and D. Seidel, Angew. Chem., Int. Ed., 2003, 42, 5134 CrossRef CAS PubMed; (b) S. Saito and A. Osuka, Angew. Chem., Int. Ed., 2011, 50, 4342 CrossRef CAS PubMed; (c) M. Stepien, N. Sprutta and L. Latos-Grażyński, Angew. Chem., Int. Ed., 2011, 50, 4288 CrossRef CAS PubMed; (d) T. Tanaka and A. Osuka, Chem. Rev., 2017, 117, 2584 CrossRef CAS PubMed; (e) B. Szyszko, M. J. Bialek, E. Pacholska-Dudziak and L. Latos-Grażyński, Chem. Rev., 2017, 117, 2839 CrossRef CAS PubMed; (f) M. Stępień, E. Gońka, M. Żyła and N. Sprutta, Chem. Rev., 2017, 117, 3479 CrossRef PubMed; (g) A. Borissov, Y. Kumar Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Chem. Rev., 2022, 122, 565 CrossRef CAS PubMed.
- S. Mori and A. Osuka, J. Am. Chem. Soc., 2005, 127, 8030 CrossRef CAS PubMed.
- T. Ito, Y. Hayashi, S. Shimizu, J.-Y. Shin, N. Kobayashi and H. Shinokubo, Angew. Chem., Int. Ed., 2012, 51, 8542 CrossRef CAS PubMed.
- Y. Liu, L. Xu, Y. Rao, B. Yin, M. Zhou, J. Song and A. Osuka, Org. Lett., 2023, 25, 8121 CrossRef CAS PubMed.
- D. X. Y. Liu, Y. Rao, G. Kim, M. Zhou, D. Yu, L. Xu, B. Yin, S. Liu, T. Tanaka, N. Aratani, A. Osuka, Q. Liu, D. Kim and J. Song, J. Am. Chem. Soc., 2018, 140, 16553 CrossRef PubMed.
- (a) S. Hiroto, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2006, 128, 6568 CrossRef CAS PubMed; (b) S. Saito and A. Osuka, Chem.−Eur. J., 2006, 12, 9095 CrossRef CAS PubMed; (c) S. Saito, K. S. Kim, Z. S. Yoon, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2007, 46, 5591 CrossRef CAS PubMed; (d) S. Saito, J.-Y. Shin, J. M. Lim, K. S. Kim, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2008, 47, 9657 CrossRef CAS PubMed; (e) S. Saito, K. Furukawa and A. Osuka, Angew. Chem., Int. Ed., 2009, 48, 8086 CrossRef CAS PubMed; (f) R. Sakamoto, S. Saito, S. Shimizu, Y. Inokuma, N. Aratani and A. Osuka, Chem. Lett., 2010, 39, 439 CrossRef CAS; (g) T. Yoneda, S. Saito, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2011, 50, 3475 CrossRef CAS PubMed; (h) M.-C. Yoon, J.-Y. Shin, J. M. Lim, S. Saito, T. Yoneda, A. Osuka and D. Kim, Chem.−Eur. J., 2011, 17, 6707 CrossRef CAS PubMed; (i) T. Higashino, B. S. Lee, J. M. Lim, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2012, 51, 13105 CrossRef CAS PubMed; (j) W.-Y. Cha, T. Yoneda, S. Lee, J. Mi. Lim, A. Osuka and D. Kim, Chem. Commun., 2014, 50, 548 RSC; (k) J. O. Kim, Y. Hong, T. Kim, W.-Y. Cha, T. Yoneda, T. Soya, A. Osuka and D. Kim, J. Phys. Chem. Lett., 2018, 9, 4527 CrossRef CAS PubMed.
- (a) J. L. Sessler, D. Seidel and V. Lynch, J. Am. Chem. Soc., 1999, 121, 11257 CrossRef CAS; (b) G. Anguera, B. Kauffmann, J. L. Borrell, S. Borros and D. Sanchez-Garcia, Org. Lett., 2015, 17, 2194 CrossRef CAS PubMed; (c) T. Kohler, D. Seidel, J. L. Sessler, Z. Ou, K. M. Kadish and J. L. Sessler, J. Am. Chem. Soc., 2003, 125, 6872 CrossRef PubMed; (d) V. R. G. Anand, S. K. Pushpan, A. Srinivasan, S. J. Narayanan, B. Srideri and T. K. Chandrashekar, Org. Lett., 2000, 2, 3829 CrossRef CAS PubMed; (e) V. G. Anand, S. K. Pushpan, S. Venkaratraman, A. Dey, T. K. Chadrashekar, R. Roy, B. S. Joshi, S. Deepa and G. N. Sastry, J. Org. Chem., 2002, 67, 6309 CrossRef CAS PubMed; (f) C. Bucher, D. Seidel, V. Lynch and J. L. Sessler, Chem. Commun., 2002, 328 RSC.
- J.-Y. Shin, H. Furuta, K. Yoza, S. Igarashi and A. Osuka, J. Am. Chem. Soc., 2001, 123, 7190 CrossRef CAS PubMed.
- W. Deng, Y. Liu, D. Shimizu, T. Tanaka, A. Nakai, Y. Rao, L. Xu, M. Zhou, A. Osuka and J. Song, Chem.−Eur. J., 2023, 29, e202203484 CrossRef CAS PubMed.
- T. Jiang, P. Zhang, C. Yu, J. Yin, L. Jiao, E. Dai, J. Wang, Y. Wei, X. Mu and E. Hao, Org. Lett., 2014, 16, 1952 CrossRef CAS PubMed.
- M. Umetani, J. Kim, T. Tanaka, D. Kim and A. Osuka, Chem. Commun., 2019, 55, 10547 RSC.
- Deposition numbers 2330167 for 3BF2, 2330168 for 3HCl, and 2330169 and for 3Pd2 contain the supplementary crystallographic data for this paper.†.
- J.-Y. Shin, K. S. Kim, M.-C. Yoon, J. M. Kim, Z. S. Yoon, A. Osuka and D. Kim, Chem. Soc. Rev., 2010, 39, 2751 RSC.
- J. L. Sessler, M. Cyr, H. Furuta, V. Kral, T. Mody, T. Morishima, M. Shionoya and S. Weghorn, Pure Appl. Chem., 1993, 65, 393 CrossRef CAS.
- S. Kloda and E. Kleinpeter, J. Chem. Soc., Perkin Trans. 2, 2001, 1893 Search PubMed.
- T. Lu and F.-W. Chen, J. Comput. Chem., 2012, 33, 580 CrossRef CAS PubMed.
- Z. Liu, T. Lu and Q. Chen, Carbon, 2020, 165, 468 CrossRef CAS.
- M. Ishida, S.-J. Kim, C. Preihs, K. Ohkubo, J. M. Lim, B. S. Lee, J. S. Park, V. M. Lynch, V. V. Roznyatovskiy, T. Sarma, P. K Panda, C.-H. Lee, S. Fukuzumi, D. Kim and J. L. Sessler, Nat. Chem., 2013, 5, 15 CrossRef CAS PubMed.
- T. Sarma, G. Kim, S. Sen, W.-Y. Cha, Z. Duan, M. D. Moore, V. M. Lynch, Z. Zhang, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2018, 140(38), 12111 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthetic procedures and characterization for all new compounds, NMR spectra, high resolution mass spectra, UV/vis spectra, and CV spectra. CCDC 2330167 for 3BF2, 2330168 for 3HCl and 2330169 for 3Pd2. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc01909a |
| This journal is © The Royal Society of Chemistry 2024 |