Open Access Article
Open Access ArticleSynthesis of NiII porphyrin—NiII 5,15-diazaporphyrin hybrid tapes†
Lina
Wang
a,
Zian
Liao
a,
Peng
Lin
a,
Yingying
Jia
a,
Le
Liu
a,
Ling
Xu
a,
Mingbo
Zhou
a,
Bangshao
Yin
a,
Yutao
Rao
a,
Akito
Nakai
b,
Takayuki
Tanaka
b,
Daiki
Shimizu
b,
Atsuhiro
Osuka
a and
Jianxin
Song
 *a
*a
aKey Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China), Key Laboratory of the Assembly and Application of Organic Functional Molecules of Hunan Province, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China. E-mail: jxsong@hunnu.edu.cn
bDepartment of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan
First published on 24th May 2024
Abstract
NiII porphyrin (P) and NiII 5,15-diazaporphyrin (DAP) hybrid tapes were synthesized by Suzuki–Miyaura cross-coupling reactions of meso- or β-borylated P with β-brominated DAP followed by intramolecular oxidative fusion reactions. Meso-β doubly linked hybrid tapes were synthesized by oxidation of singly linked precursors with DDQ-FeCl3. Synthesis of triply linked hybrid tapes was achieved by oxidation with DDQ-FeCl3-AgOTf with suppression of peripheral β-chlorination. In these tapes, DAP segments were present as a 20π-electronic unit, but their local antiaromatic contribution was trivial. Remarkably, these hybrid tapes were stable and exhibited extremely enhanced absorption bands in the NIR region and multiple reversible redox waves. A pentameric hybrid tape showed a remarkably sharp and red-shifted band at 1168 nm with ε = 5.75 × 105 M−1 cm−1. Singly linked P-DAP dyads were oxidized with DDQ-FeCl3 to give stable radicals, which were oxidized further to afford dimeric hybrid tapes possessing a nitrogen atom at the peripheral-side meso-position.
Introduction
Highly conjugated molecules have attracted considerable interest in light of their applications in diverse materials chemistry.1 As representative molecules, meso–meso, β-β, β-β triply linked ZnII-porphyrin tapes were developed by oxidative conversion of meso–meso singly linked ZnII-porphyrin oligomers.2,3 This conversion caused an orthogonal-to-coplanar structural change to realize remarkable red-shifts of absorption, which eventually reached the infrared region.2 While a range of potential applications of porphyrin tapes have been demonstrated,3 they became increasingly unstable upon elongation of the array owing to the steady lift-up of the HOMO level, which is a serious drawback.2a We hypothesized that nitrogen doping at the meso-positions of a porphyrin tape may stabilize the HOMO and induce novel optical and electronic properties.4 Furthermore, nitrogen-doped porphyrin tapes could be appealing as a unique and discrete N-doped graphene model.5 5,15-Diazaporphyrins have been explored by Matano et al. and Shinokubo et al.,4,6–8 but porphyrin-diazaporphyrin hybrid tapes have not been reported before.Results and discussion
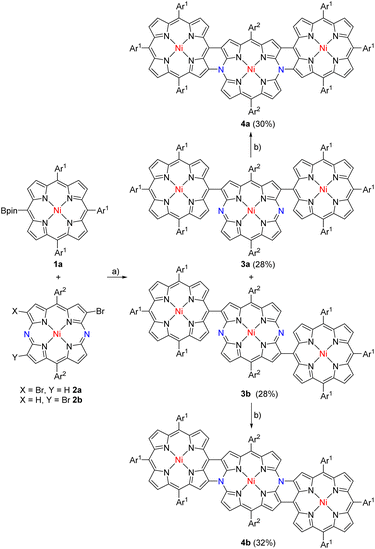
In this paper, we report the synthesis of nitrogen-doped porphyrin tapes consisting of NiII-porphyrin (P) and NiII-5,15-diazaporphyrin (DAP). Synthetic routes were concise, involving Suzuki–Miyaura coupling and a subsequent intramolecular oxidative fusion reaction. As the first trial, isomeric triads 3a and 3b were prepared in 28% yields, respectively, by the cross-coupling reactions of 1a with 2 (ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
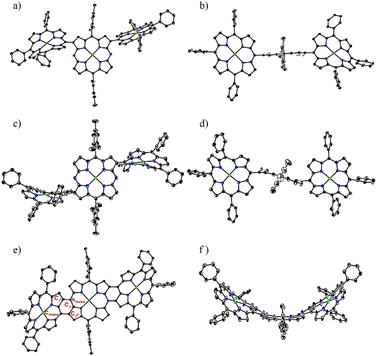
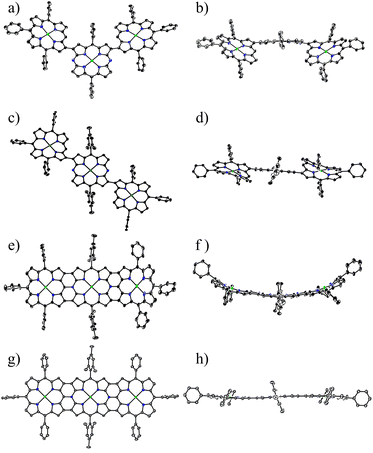
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2a and 2b) followed by chromatographic separation (Scheme 1). Triads 3a and 3b were been to be syn- and anti-isomers, respectively, by X-ray analysis (Fig. 1). In both triads, the DAP segment showed a planar structure but P segments showed less planar structures. The dihedral angles between DAP and P were 74.6° and 53.8° in 3a and 65.3° in 3b, respectively.
1 mixture of 2a and 2b) followed by chromatographic separation (Scheme 1). Triads 3a and 3b were been to be syn- and anti-isomers, respectively, by X-ray analysis (Fig. 1). In both triads, the DAP segment showed a planar structure but P segments showed less planar structures. The dihedral angles between DAP and P were 74.6° and 53.8° in 3a and 65.3° in 3b, respectively.
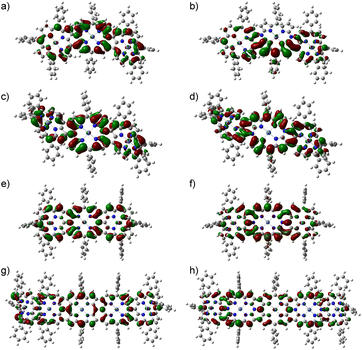
We attempted the synthesis of meso, β doubly linked porphyrin tapes from 3a and 3b. Through many attempts, we found that hybrid tapes 4a and 4b were obtained in 30% and 32% yields by oxidation of 3a and 3b, respectively, with DDQ and FeCl3 (Scheme 1). Hybrid tapes 4a and 4b showed parent-ion peaks at m/z = 2459.1427 (calcd for [C160H168N14Ni3]+ = 2459.1631) and at m/z = 2459.1584 (calcd for [C160H168N14Ni3]+ = 2459.1631), respectively. X-ray diffraction revealed the anti-structure of 4b to be considerably bent (Fig. 1). Importantly, 4b had a neutral 20π-DAP unit with average Nmeso–Cα, Nmeso–Cβ, and Cmeso– bond lengths of 1.38, 1.41, and 1.44 Å, respectively, embedded in a bent-tape structure. Observed Nmeso–Cα and Nmeso–Cβ bond distances were distinctly shorter than the normal N–C bond length (1.47 Å). In line with the structures, a singlet set and two sets of signals due to Ar2 protons were observed for 4b and 4a, respectively. In addition, singlet signals due to the β proton at the DAP unit were observed at 9.04 and 9.01 ppm in 4a and 4b, respectively. These high chemical shifts were contrary to what was expected for a paratropic ring current arising from the 20π-DAP unit judging from low chemical shifts (4.6–3.3 ppm) of antiaromatic 20π-DAP monomers.4,8 Instead, DFT calculations indicated that the molecular orbitals of 4a and 4b were well spread over entire tapes (Fig. 2a–d), and the local antiaromaticity of DAP units did not remain in 4a or 4b based on NICS(0) values therein (Fig. S6, S2†).
bond lengths of 1.38, 1.41, and 1.44 Å, respectively, embedded in a bent-tape structure. Observed Nmeso–Cα and Nmeso–Cβ bond distances were distinctly shorter than the normal N–C bond length (1.47 Å). In line with the structures, a singlet set and two sets of signals due to Ar2 protons were observed for 4b and 4a, respectively. In addition, singlet signals due to the β proton at the DAP unit were observed at 9.04 and 9.01 ppm in 4a and 4b, respectively. These high chemical shifts were contrary to what was expected for a paratropic ring current arising from the 20π-DAP unit judging from low chemical shifts (4.6–3.3 ppm) of antiaromatic 20π-DAP monomers.4,8 Instead, DFT calculations indicated that the molecular orbitals of 4a and 4b were well spread over entire tapes (Fig. 2a–d), and the local antiaromaticity of DAP units did not remain in 4a or 4b based on NICS(0) values therein (Fig. S6, S2†).
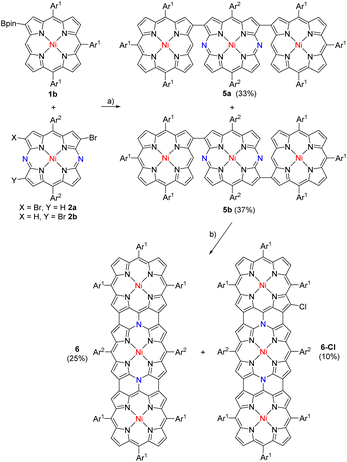
Triply linked hybrid tape 6 was synthesized via triads 5a and 5b, which were prepared in 33% and 37% yields, respectively, by the cross-coupling reactions of 1b with 2 followed by chromatographic separation (Scheme 2). Syn- and anti-structures were confirmed for 5a and 5b, respectively, by X-ray analysis (Fig. 3). In both cases, the DAP segment showed a planar structure, and P units showed less planar structures. The dihedral angles between DAP and P were 45.1° and 45.2° in 5a and 25.7° in 5b, respectively.
In the next step, oxidation of a mixture of 5a and 5b with DDQ and FeCl3 under similar conditions led to the formation of 6 (25%) along with β-chlorinated hybrid tapes 6-Cl (10%) (Scheme 2). Meanwhile, we found that addition of an excess amount of AgOTf could improve the yield of 6 by suppressing the formation of β-chlorinated tapes. Namely, a solution of 5a and/or 5b in CH2Cl2 was stirred at room temperature for 1 h in the presence of DDQ (10 equiv.), FeCl3 (10 equiv.), and AgOTf (100 equiv.), and subsequent chromatographic separation gave 6 in 45% yield. The parent-ion peak of 6 was detected at m/z = 2455.1405 (calcd for [C160H164N14Ni3]+ = 2455.1318 ([M]+)). The structure of 6 was confirmed by X-ray diffraction. In the crystal of 6, two structures were found: a bent structure (Fig. 3e and f) and planar structure (Fig. 3g and h). Here, the center was a formal 20π-DAP carrying two neutral meso-nitrogen atoms with average Nmeso–Cα, Nmeso–Cmeso, and Cβ–Cβ bond lengths of 1.381, 1.425, and 1.428 Å, and of 1.373, 1.417, and 1.410 Å, respectively. The 1H NMR spectrum indicated one pair of doublets due to edge β-protons at 8.81 and 8.76 ppm and two singlet signals due to inner β-protons at 9.14 and 8.13 ppm being much lower as compared with the trimeric ZnII porphyrin tape.9 These data also excluded a possible paratropic ring current of 20π-DAP. DFT calculations indicated that the molecular orbital of 6 was well delocalized over the whole tape (Fig. 2e and f).
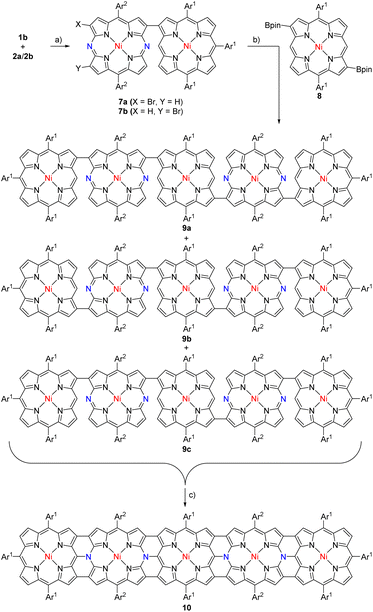
We also attempted the synthesis of a pentameric hybrid tape 10 (Scheme 3). Brominated dyad 7 (ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 7a and 7b) was prepared by the cross-coupling reaction of 1b with 2 in 51% yield. This brominated mixture was coupled with diborylated NiII porphyrin 8 to afford a mixture of pentameric precursors (9a, 9b, and 9c) in 61% yield. The parent-ion peak was detected at m/z = 3804.6985 (calcd for (C244H249N24Ni5)+ = 3804.6984 ([M + H]+)). Oxidation of pentameric precursors with DDQ, FeCl3, and AgOTf produced pentameric hybrid tape 10 in 16% yield. The parent-ion peak of 10 was detected at m/z = 3791.5733 (calcd for [C244H236N24Ni5)+ = 3791.5955 ([M]+)). The 1H NMR spectrum showed one pair of doublets at 8.82 and 8.76 ppm and four singlets at 9.17, 9.05, 8.20, and 8.00 ppm. These data indicated the trivial contribution of a paratropic ring current of 20π-DAP but a global diatropic ring current. DFT calculations indicated a delocalized nature of HOMO and LUMO+1 over the entire tape (Fig. 2g and h).
1 mixture of 7a and 7b) was prepared by the cross-coupling reaction of 1b with 2 in 51% yield. This brominated mixture was coupled with diborylated NiII porphyrin 8 to afford a mixture of pentameric precursors (9a, 9b, and 9c) in 61% yield. The parent-ion peak was detected at m/z = 3804.6985 (calcd for (C244H249N24Ni5)+ = 3804.6984 ([M + H]+)). Oxidation of pentameric precursors with DDQ, FeCl3, and AgOTf produced pentameric hybrid tape 10 in 16% yield. The parent-ion peak of 10 was detected at m/z = 3791.5733 (calcd for [C244H236N24Ni5)+ = 3791.5955 ([M]+)). The 1H NMR spectrum showed one pair of doublets at 8.82 and 8.76 ppm and four singlets at 9.17, 9.05, 8.20, and 8.00 ppm. These data indicated the trivial contribution of a paratropic ring current of 20π-DAP but a global diatropic ring current. DFT calculations indicated a delocalized nature of HOMO and LUMO+1 over the entire tape (Fig. 2g and h).
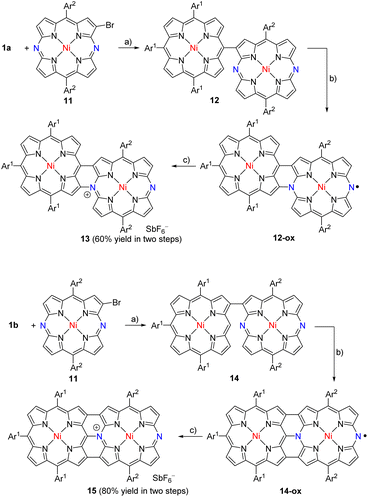
Finally, we examined the synthesis of dimeric hybrid tapes which had N-unsubstituted DAP at the periphery. Meso-β linked P—DAP dyad 12 was prepared by the cross-coupling of 1a (ref. 10) with 11 (ref. 11) in 71% yield (Scheme 4). Oxidation of 12 with DDQ and FeCl3 in a mixture of CH2Cl2 and nitromethane at room temperature for 1 h gave a stable product, 12-ox, which exhibited the parent-ion peak at 1531.6771 (calcd for C98H99N10Ni2, m/z = 1531.6756 ([M]+)), which was 2H less than that of 12. The 1H NMR spectrum of 12-ox was extremely broad, suggesting its radical character. In fact, 12-ox in degassed toluene exhibited an EPR signal at g = 1.9995 (Fig. S1–S23†). The signal did not have hyperfine coupling (hfc) features, indicating small hfc constants due to effective spin delocalization over the π-system. It is important to note that 12-ox is stable under ambient conditions. Further oxidation of 12-ox with tris(4-bromophenyl)aminium hexafluoroantimonate gave the SbF6− salt 13 in 60% yield in two steps. The fused dimer 13 showed the parent-ion peak at 1531.6747 (calcd for C98H99N10Ni2, m/z = 1531.6756 ([M-SbF6]+)). Different from the trimeric and pentameric hybrid tapes, the DAP unit had an 18π-electronic system. The 1H NMR spectrum of 13 showed peaks in the low-field region due to the diamagnetic current. On the basis of these data, we thought that 12-ox was a neutral radical (Scheme 4), but we failed to obtain its single crystals.
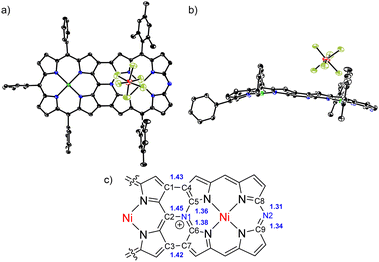
Similarly, β-β linked P—DAP dyad 14 was prepared by the cross-coupling of 1b with 11 in 75% yield. It was oxidized with DDQ and FeCl3 to yield 14-ox as an intermediate, which exhibited the parent-ion peak at 1532.6794 (calcd for C98H100N10Ni2, m/z = 1532.6834 ([M + H]+)), which was 2H less than that of 14. Radical 14-ox in toluene showed an EPR signal similar to 12-ox at g = 1.9985 without hfc splitting. Radical 14-ox was also stable. Further oxidation of 14-ox with TBPA·SbF6 gave hybrid tape 15 in a two-step yield of 80%. The parent-ion peak was observed at m/z = 1531.6747 (calcd for C98H99N10Ni2, m/z = 1531.6756 ([M-SbF6]+)). The 1H NMR spectrum of 15 exhibited broad (but characteristically high-field-shifted) signals at 6.74 and 6.70 ppm due to the β-protons at the meso-nitrogen side. The structure of 15 was confirmed by X-ray analysis to be a triply linked P—DAP hybrid bearing a SbF6− counter anion (Fig. 4). In 15, the P moiety took a domed form but the DAP moiety was almost planar. The bond lengths of C(1)–C(4), C(2)–N(1) and C(3)–C(7) bonds were 1.432(8), 1.449(7), and 1.422(8) Å, respectively. Importantly, the bond lengths of C(5)–N(1) (1.360(7) Å) and C(6)–N(1) bonds (1.375(7) Å) were distinctly shorter than the C(2)–N(1) bond, revealing conjugation in the DAP unit through a positively charged quaternary nitrogen atom.
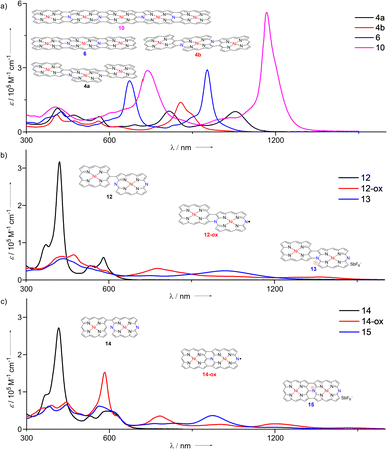
Fig. 5a shows the UV/Vis absorption spectra of 4a, 4b, 6, and 10 in CH2Cl2. The absorption spectra of 4a and 4b are different, showing absorption bands at 412, 473, 565, 814, and 1054 nm and at 409, 473, 560, 624, and 857 nm, respectively, which reflected different syn- and anti-molecular structures. Curiously, the spectral features of 4a and 4b were quite different from those of corresponding ZnII porphyrin tapes.12 The observed red-shifted band at 1054 nm for 4a was remarkable because the anti-isomer of the meso-β doubly linked ZnII porphyrin tape showed an intensified band as compared with the syn-isomer.13 Triply-linked hybrid tape 6 displayed three major absorption bands at 427, 672, and 953 nm. The molar extinction coefficient of the Q band (λ = 953 nm) was 2.90 × 105 M−1 cm−1, which is significantly larger than that of the original ZnII porphyrin trimer tape (λmax = 1406 nm, ε = 1.48 × 105 M−1 cm−1).9 It is interesting to note that 4a showed a more red-shifted absorption band than that of 6.
Dimeric tape 13 displayed a very broad absorption spectrum with weak peaks at 436, 748, and 1020 nm (Fig. 5b). 12-ox showed a very broad absorption spectrum with weak peaks at 316, 430, 472, 543, 776, and 1357 nm, which are characteristic of porphyrinoid radical species.1315 displayed a broad absorption spectrum with peaks at 381, 444, 563, 766, 834, and 974 nm (Fig. 5c). Similarly, the absorption spectrum of 14-ox shows bands at 390, 444, 583, 783, 1000, and 1200 nm with a low tail reaching to ca. 1600 nm.
Electrochemical properties were examined by cyclic voltammetry and differential pulse voltammetry (Table 1). Dimer 14 exhibited an irreversible first oxidation potential at 0.53 V and a reversible first reduction potential at −1.26 V, which corresponded to that of Ni-Por (0.58 V) and that of Ni-DAP (−1.32 V), respectively. These results revealed that first oxidation would occur at the porphyrin site, and first reduction would occur at the DAP site. Radical 14-ox showed reversible redox waves at −0.24, 0.80, and −0.59 V, and 12-ox displayed reversible redox waves at −0.32, 0.74, and −0.68 V. Fairly reversible redox waves and a narrow HOMO–LUMO gap of 14-ox (0.35 eV) and 12-ox (0.36 eV) are characteristics of porphyrinoid radicals.14 The first reduction and oxidation potentials of cation species 15 (−0.22 and 0.80 V) should correspond to the first and second oxidation potentials of radical 14-ox (−0.24 and 0.80 V), and this observation could also be found between 12-ox and 13.15
| Cpd. | E ox.5 (V) | E ox.4 (V) | E ox.3 (V) | E ox.2 (V) | E ox.1 (V) | E red.1 (V) | E red.2 (V) | E red.3 (V) | ΔEHLc (eV) |
|---|---|---|---|---|---|---|---|---|---|
| a Potentials [V] vs. ferrocene/ferrocenium ion. Scan rate = 0.05 V s−1; glassy carbon working electrode and Pt wire counter electrode; supporting electrolyte = 0.1 M nBu4NPF6 in benzonitrile; Ag/AgNO3 reference electrode. b Irreversible peaks. c Electrochemical HOMO–LUMO gaps (ΔEHL = e (Eox.1 − Ered.1) [eV]). | |||||||||
| 4a | 1.03 | 0.89 | 0.71 | 0.20 | −0.24 | −1.40 | −1.78 | −1.98 | 1.16 |
| 4b | 1.30b | 0.75 | 0.63 | 0.19 | −0.23 | −1.54 | −1.63 | −2.06 | 1.31 |
| 6 | 1.20 | 0.86 | 0.75 | 0.25 | −0.16 | −1.36 | −1.58 | −1.99 | 1.20 |
| 10 | 1.00 | 0.62 | −0.42 | −2.26 | −2.42 | 1.04 | |||
| 13 | 0.75 | −0.28 | −0.67 | 1.03 | |||||
| 15 | 0.80 | −0.24 | −0.57 | 1.04 | |||||
As a rare case, doubly linked hybrid tapes 4a and 4b showed eight reversible waves at 1.03, 0.89, 0.71, 0.20, −0.24, −1.40, −1.78, and −1.98 V, and at 1.30, 0.75, 0.63, 0.19, −0.23, −1.54, −1.63, and −2.06 V, respectively, suggesting their potential as electron reservoirs. Trimeric hybrid tape 6 showed nine reversible waves at 1.20, 0.86, 0.75, 0.25, −0.16, −1.36, −1.58, −1.99, and −2.24 V. Pentameric hybrid tape 10 displayed weak reversible waves at 0.62, probably due to its poor solubility. The electrochemical HOMO–LUMO gaps increased in the order of 10 (1.04 eV) < 4a (1.16 eV) < 6 (1.20 eV) < 4b (1.31 eV), which was in agreement with their optical properties. The cationic tapes 13 and 15 showed small HOMO–LUMO gaps, which were comparable with that of 10.
Conclusions
In summary, we have developed a concise protocol to synthesize P–DAP hybrid tapes by Suzuki–Miyaura cross-coupling reactions and subsequent suitable oxidations. In hybrid tapes with DAP, the DAP units were 20π-electronic systems, but their local antiaromatic effects were quite minor. Dimeric tapes were synthesized as cationic species carrying an 18π-electronic DAP unit. These hybrid tapes were stable and exhibited intriguing properties, such as nine reversible redox waves for 6 and extremely enhanced Q-like absorption bands at 1168 nm with ε = 5.75 × 105 M−1 cm−1 for 10. Exploration of more extended porphyrin–DAP hybrid nanotapes is underway in our laboratory.Data availability
All experimental data and detailed experimental procedures, are available in the ESI.†Author contributions
J. Song designed and conducted the project. L. Wang, Z. Liao, P. Lin, and Y. Jia performed the synthesis and characterization, and measured optical and electrochemical properties. L. Xu, and Y. Rao performed X-ray diffraction. A. Nakai, T. Tanaka, and D. Shimizu performed EPR experiments and analysis. L. Xu, Y. Rao, and T. Tanaka performed DFT calculations. L. Xu, M. Zhou, B. Yin, A. Nakai, Y. Rao, T. Tanaka, J. Song, and A. Osuka prepared the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22071052, 21772036, 21602058, 21702057), Science and Technology Planning Project of Hunan Province (2018TP1017), Science and Technology Innovation Program of Hunan Province (2021RC4059), and Hunan Provincial Innovation Foundation for Postgraduates (CX20220510).Notes and references
- (a) M. Stępień, E. Gońka, M. Żyła and N. Sprutta, Chem. Rev., 2017, 117, 3479 CrossRef; (b) A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Chem. Rev., 2022, 122, 565 CrossRef CAS PubMed.
- (a) A. Osuka and A. Tsuda, Science, 2001, 293, 79 CrossRef PubMed; (b) D. Kim and A. Osuka, J. Phys. Chem., 2003, 107, 8791 CrossRef CAS; (c) D. Kim and A. Osuka, Acc. Chem. Res., 2004, 37, 735 CrossRef CAS.
- (a) Y. Nakamura, N. Aratani, H. Shinokubo, A. Takagi, T. Kawai, T. Matsumoto, Z. S. Yoon, D. Y. Kim, T. K. Ahn, D. Kim, A. Muranaka, N. Kobayashi and A. Osuka, J. Am. Chem. Soc., 2006, 128, 4119 CrossRef CAS PubMed; (b) H. Mori, T. Tanaka and A. Osuka, J. Mater. Chem., 2013, 1, 2500 CAS; (c) T. Tanaka and A. Osuka, Chem.–Eur. J., 2018, 24, 17188 CrossRef CAS PubMed; (d) D. Bonifazi, M. Scholl, F. Song, L. Echegoyen, G. Accorsi, N. Armaroli and F. Diederich, Angew. Chem., Int. Ed., 2003, 42, 4966 CrossRef CAS PubMed; (e) D. Bonifazi, H. Spillamnn, A. Kiebele, M. de Wild, P. Seiler, F. Cheng, H.-J. Güntherodt, T. Jung and F. Diederich, Angew. Chem., Int. Ed., 2004, 43, 4759 CrossRef CAS; (f) T. Sakurai, K. Shi, H. Sato, K. Tashiro, A. Osuka, A. Saeki, S. Seki, S. Tagawa, S. Sasaki, H. Masunaga, K. Osaka, M. Takata and T. Aida, J. Am. Chem. Soc., 2008, 130, 13812 CrossRef CAS PubMed; (g) J.-R. Deng, M. T. González, H. Zhu, H. L. Anderson and E. Leary, J. Am. Chem. Soc., 2024, 146, 3651 CrossRef CAS; (h) Z. Chen, J.-R. Deng, S. Hou, X. Bian, J. L. Swett, Q. Wu, J. Baugh, L. Bogani, G. A. D. Briggs, J. A. Mol, C. J. Lambert, H. L. Anderson and J. O. Thomas, J. Am. Chem. Soc., 2023, 145, 15265 CrossRef CAS PubMed; (i) V. V. Diev, K. Hanson, J. D. Zimmerman, S. R. Forrest and M. E. Thompson, Angew. Chem., Int. Ed., 2010, 49, 5523 CrossRef CAS PubMed; (j) T. Sakurai, K. Tashiro, Y. Honsho, A. Saeki, S. Seki, A. Osuka, A. Muranaka, M. Uchiyama, J. Kim, S. Ha, K. Kato, M. Takata and T. Aida, J. Am. Chem. Soc., 2011, 133, 6537 CrossRef CAS PubMed.
- Y. Matano, Chem. Rev., 2017, 117, 3138 CrossRef CAS PubMed.
- (a) X.-Y. Wang, M. Richter, Y. He, J. Björk, A. Riss, R. Rajesh, M. Garnica, F. Hennersdorf, J. J. Weigand, A. Narita, R. Berger, X. Feng, W. Auwärter, J. V. Barth, C.-A. Palma and K. Müllen, Nat. Commun., 2017, 8, 1948 CrossRef PubMed; (b) U. Hahn, E. Maisonhaute and J.-F. Nierengarten, Angew. Chem., Int. Ed., 2018, 57, 10635 CrossRef CAS PubMed; (c) X. Guo, Z. Yuan, Y. Zhu, Z. Li, R. Huang, Z. Xia, W. Zhang, Y. Li and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 16966 CrossRef CAS.
- (a) Y. Matano, T. Shibano, H. Nakano and H. Imahori, Chem.–Eur. J., 2012, 18, 6208 CrossRef CAS PubMed; (b) Y. Matano, T. Shibano, H. Nakano, Y. Kimura and H. Imahori, Inorg. Chem., 2012, 51, 12879 CrossRef CAS PubMed.
- (a) Y. Matano, D. Fujii, T. Shibano, K. Furukawa, T. Higashino, H. Nakano and H. Imahori, Chem.–Eur. J., 2014, 20, 3342 CrossRef CAS PubMed; (b) S. Omomo, Y. Maruyama, K. Furukawa, T. Furuyama, H. Nakano, N. Kobayashi and Y. Matano, Chem.–Eur. J., 2015, 21, 2003 CrossRef CAS PubMed; (c) M. Kawamata, T. Sugai, M. Minoura, Y. Maruyama, K. Furukawa, C. Holstrom, V. N. Nemykin, H. Nakano and Y. Matano, Chem.–Asian J., 2017, 12, 816 CrossRef CAS PubMed; (d) F. Abou-Chahine, D. Fujii, H. Imahori, H. Nakano, N. V. Tkachenko, Y. Matano and H. Lemmetyinen, J. Phys. Chem. B, 2015, 119, 7328 CrossRef CAS PubMed.
- T. Sakurai, Y. Hiraoka, H. Tanaka, Y. Miyake, N. Fukui and H. Shinokubo, Angew. Chem., Int. Ed., 2023, 62, e202300437 CrossRef CAS PubMed.
- T. Tanaka, B. S. Lee, N. Aratani, M.-C. Yoon, D. Kim and A. Osuka, Chem.–Eur. J., 2011, 17, 14400 CrossRef CAS PubMed.
- H. Hata, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2005, 127, 8264 CrossRef CAS PubMed.
- Y. Matano, D. Fujii, T. Shibano, K. Furukawa, T. Higashino, H. Nakano and H. Imahori, Chem.–Eur. J., 2014, 20, 3342 CrossRef CAS PubMed.
- T. Ikeda, N. Aratani, S. Easwaaramoorthi, D. Kim and A. Osuka, Org. Lett., 2009, 11, 3080 CrossRef CAS PubMed.
- (a) Y. Hisamune, K. Nishimura, K. Isakari, M. Ishida, S. Mori, S. Karasawa, T. Kato, S. Lee, D. Kim and H. Furuta, Angew. Chem., Int. Ed., 2015, 54, 7323 CrossRef CAS PubMed; (b) P. Schweyen, K. Brandhorst, R. Wicht, B. Wolfram and M. Bröring, Angew. Chem., Int. Ed., 2015, 54, 8213 CrossRef CAS PubMed; (c) Y. Tanaka, T. Yoneda, K. Furukawa, T. Koide, H. Mori, T. Tanaka, H. Shinokubo and A. Osuka, Angew. Chem., Int. Ed., 2015, 54, 10908 CrossRef CAS PubMed; (d) T. Yoshida, W. Zhou, T. Furuyama, D. B. Leznoff and N. Kobayashi, J. Am. Chem. Soc., 2015, 137, 9258 CrossRef CAS PubMed; (e) D. Shimizu, J. Oh, K. Furukawa, D. Kim and A. Osuka, J. Am. Chem. Soc., 2015, 137, 15584 CrossRef CAS PubMed.
- (a) T. Koide, K. Furukawa, H. Shinokubo, J.-Y. Shin, K. S. Kim, D. Kim and A. Osuka, J. Am. Chem. Soc., 2010, 132, 7246 CrossRef CAS PubMed; (b) D. Shimizu, J. Oh, K. Furukawa, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2015, 54, 6613 CrossRef CAS PubMed; (c) D. Shimizu and A. Osuka, Chem. Sci., 2018, 9, 1408 RSC.
- (a) D. Shimizu, K. Fujimoto and A. Osuka, Angew. Chem., Int. Ed., 2018, 57, 9434 CrossRef CAS PubMed; (b) K. Fujimoto, D. Shimizu and A. Osuka, Chem.–Eur. J., 2019, 25, 521 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2310178–2310184 and 2336241. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc01450b |
| This journal is © The Royal Society of Chemistry 2024 |