Open Access Article
Open Access ArticleA highly efficient catalytic method for the synthesis of phosphite diesters†
Yuki
Saito
,
Soo Min
Cho
,
Luca Alessandro
Danieli
,
Akira
Matsunaga
and
Shū
Kobayashi
 *
*
Department of Chemistry, School of Science, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo, Japan. E-mail: shu_kobayashi@chem.s.u-tokyo.ac.jp
First published on 17th April 2024
Abstract
In contrast to conventional methods that rely on stoichiometric activation of phosphonylating reagents, we have developed a highly efficient catalytic method for the synthesis of phosphite diesters using a readily available phosphonylation reagent and alcohols with environmentally benign Zn(II) catalysts. Two alcohols could be introduced consecutively on the P center with release of trifluoroethanol as the sole byproduct, without any additive, under mild conditions. The products could be oxidized smoothly to access phosphate triesters. A range of alcohols, including sterically demanding and highly functionalized alcohols such as carbohydrates and nucleosides, can be applied in this reaction.
Introduction
Organophosphates are a fundamental class of compounds that are required for all life.1–9 They are also useful as biologically active molecules and functional materials. While phosphate monoesters and triesters are used as drugs,10,11 pesticides,12 flame-retardants,13 and plasticizers,14 their most important applications are as the fundamental units of biomolecules such as DNA, RNA, and oligonucleotides.15–19 In general, organophosphates are synthesized by condensation reactions between alcohols and phosphorus reagents. Although there are several reports on the use of phosphoryl chloride and its derivatives, limited functional group tolerance and decreased selectivity are common problems.20–37 On the other hand, P(III) reagents have been developed as reactive and selective phosphorous reagents38–44 and products could be converted into various P(V) compounds by consecutive reactions.45,46 The phosphoramidite method, which has been the most reliable approach, has been applied for the synthesis of complex molecules such as oligonucleotides, mainly by using solid-phase techniques (Scheme 1).47–52 Meanwhile, Baran and co-workers have undertaken extensive studies on the use of P(V) reagents and demonstrated a series of elegant syntheses of oligonucleotides (Scheme 1).53–59 The above methods always require super-stoichiometric amounts of additives and activating reagents. Although catalytic phosphorylation reactions using P(V) compounds have recently been reported,60–63 these reactions are only applicable to phosphate monoesters; catalytic selective syntheses of phosphate di- and triesters have remained unexplored.Our group reported zinc-catalyzed phosphonylation of alcohols using dimethyl phosphite 2′ as a P(III) reagent.64 The reaction proceeded under mild conditions to afford various monophosphonylated alcohols 3 with high functional group tolerance in high yields. We envisioned that the products of this reaction could react further with other alcohols under catalytic conditions for the selective synthesis of phosphite diesters 4. A combination of these two-step catalytic transformations and conventional oxidation with I2 or TBHP would offer a facile, efficient, and selective synthesis of phosphate di- and triesters (Scheme 1).65,66 Based on this hypothesis, we started the investigation; however, after extensive study, it was found that control of the selectivity was difficult because of an overreaction of product 4a (Tables S1 and S2†). To address this issue, we decided to change the leaving group to the trifluoroethoxy group. We hypothesized that the electron-deficient nature and better leaving ability of the group would improve both the reactivity and selectivity of the transesterification, and the low nucleophilicity of the resulting alcohol would suppress the side reactions.67–70 Herein, we describe a catalytic and additive-free selective synthesis of phosphite diesters that proceeds in one-pot using readily available P(III) reagents.
Results and discussion
We first investigated the selective mono-phosphonylation of cyclohexanol (1a) with readily available phosphites under Zn catalysis (Table 1). The reaction with dimethyl phosphite 2′ proceeded slowly in the presence of Zn(acac)2 catalyst at 0 °C for 1 h to afford the corresponding phosphonylated compound 3a′ in 44% yield (entry 1). On the other hand, phosphite 2, bearing a trifluoroethoxy group, showed higher reactivity to afford the target compound 3a in 92% yield under the same conditions; moreover, the target compound was formed in 58% yield without any side product when only 1 eq. of 2 was used (entries 2 and 3). This result is in sharp contrast to the previous report in which 2 eq. of 2′ was necessary to suppress the overreaction of 3′. Moreover, the reaction even proceeded with 46% yield in the absence of 5 A molecular sieves (MS) (entry 4). The effect of solvents was then examined. Halogenated solvents such as benzotrifluoride (BTF) and dichloromethane (DCM) were found to be effective (entries 5–7), and the yield could be improved to 90% by extending the reaction time to 3 h and increasing the reaction temperature to room temperature (rt) (entry 8). Interestingly, the use of sterically hindered bis(2,2,6,6-tetramethyl-3,5-heptanedionato) zinc(II) (Zn(TMHD)2) further improved the yield to 97% without the formation of side products (entry 9) and the reaction hardly proceeded in the absence of the catalyst (entry 10).| Entry | P (x eq.) | MS | Solv. | Yielda (%) |
|---|---|---|---|---|
| a Yield was determined by 1H NMR analysis using 1,3,5-trimethylbenzene as an internal standard. b The reaction was performed for 3 h at rt. c Zn(TMHD)2 was used as a catalyst. d The reaction was performed in the absence of the catalyst. | ||||
| 1 | 2′ (2 eq.) | + | BTF | 44 |
| 2 | 2 (2 eq.) | + | BTF | 92 |
| 3 | 2 (1 eq.) | + | BTF | 58 |
| 4 | 2 (1 eq.) | − | BTF | 46 |
| 5 | 2 (1 eq.) | − | Toluene | 15 |
| 6 | 2 (1 eq.) | − | THF | 36 |
| 7 | 2 (1 eq.) | − | DCM | 42 |
| 8b | 2 (1 eq.) | − | DCM | 90 |
| 9c | 2 (1 eq.) | − | DCM | 97 |
| 10d | 2 (1 eq.) | − | DCM | Trace |
Under the optimized reaction conditions (Conditions A), the substrate scope of alcohols was investigated (Scheme 2). First, a series of secondary alcohols was examined. Simple cyclic alcohols with different ring sizes, including cyclopentanol and cycloheptanol, gave the desired products 3a–c in high yields. The same holds for menthol (1d), indicating that alkyl substituents on the ring did not perturb the reaction bicyclic alcohol 1e also reacted smoothly to afford the corresponding phosphite 3e in high yield. Notably, sterically demanding alcohol 1f gave the target product 3f in excellent yield. Not only cyclic alcohols but also acyclic alcohols reacted smoothly and delivered the target products 3g–i in high to quantitative yields, irrespective of the nature of the substituents. Furthermore, a sterically demanding tertiary alcohol gave the desired compound 3j in good yield with excellent selectivity by elevating the temperature of the reaction temperature to rt.
Primary alcohols were then reacted under a completely different set of conditions because of their higher reactivity. The highly selective Zn(II) pivalate (Zn(OPiv)2) was identified as the best catalyst, and the reaction was performed at −20 °C for 12 h to give the target mono-phosphonylated compounds in excellent yields (Table S3,† Conditions B). In addition to allowing the desired phosphites to be generated with simple alkyl alcohols (1k, 1l), alkyl groups with halogen (1m), (poly)ethers (1n, 1o), and internal and terminal alkenes moieties (1p, 1q) could also be introduced to form the desired phosphites in good to excellent yields.
The phosphonylation could also be applied to a range of more complex biomolecules, starting with carbohydrates, by increasing the amount of 2 to 1.5 eq. and increasing the reaction temperature to rt to improve the reactivity (Conditions C). The use of protected ribose (1r), as well as 2′-deoxyriboses (1s–v), produced the intended phosphites in high to excellent yields irrespective of the configuration of the 1′-stereogenic center. The secondary alcohol on the 3′-position (1s, 1t) tended to be less reactive than on the primary 5′-position (3u, 3v). A protected analogue of glucose (1w) was likewise phosphonylated in high yields. The reaction system could also be applied to protected amino acids, as demonstrated by the efficient phosphonylation of a serine derivative (1x). Finally, cholesterol (1y) and estradiol (1z) gave the desired compounds in high yields. Interestingly, protection of a phenol group was not necessary and the alcohol group was selectively phosphonylated, albeit with a relatively low yield of the target compound. Most importantly, our reaction was applied to a series of nucleoside molecules simply by using a small excess of 2 at 0 °C for 19 h (Conditions D). N-Protected cytidine (1aa), adenosine (1ab), and guanosine (1ac) gave the corresponding phosphonylated compounds in excellent yields with excellent selectivity using Zn(OPiv)2 as a catalyst. Although nucleoside molecules have various Lewis basic groups on their base units, the Zn catalyst retained its activity and provided the target products selectively.
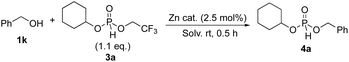
Having established the selective mono-phosphonylation of alcohols using the same molar amount of phosphonylation reagent, we next focused on the consecutive introduction of a second alcohol to phosphites 3 to synthesize phosphites diesters 4. The reaction between phosphite 3a and benzyl alcohol (1k) was selected as a model reaction, and the effects of solvents and Zn catalysts were investigated (Table 2). To our delight, the reaction proceeded smoothly with 1.1 eq. of 3a in 1 h to give the target product 4a in 71% yield with excellent selectivity (entry 1). Moreover, >95% selectivity could be achieved, whereas our previous method gave only 80% selectivity (Tables S1 and S2†). The high selectivity can be ascribed to the suppression of overreaction, which was confirmed by control experiments (see ESI†). Solvent screening revealed that aromatic solvents such as BTF and toluene were effective (entries 2–4). Several Zn catalysts were evaluated, and it was found that sterically hindered Zn(TMHD)2 showed the highest activity (entries 5–7). Finally, the desired compound was obtained in 87% yield, with trace amounts of side products, by extending the reaction time to 1.5 h (entry 8) and the reaction hardly proceeded in the absence of the catalyst (entry 9). A preliminary mechanistic study revealed an interaction between P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Lewis acid, suggesting that the phosphonylating reagents are activated for the nucleophilic addition of alcohols (Fig. S2–S5 and Table S5†). It should be noted that less Lewis basic 2 preferentially interacts with Zn(TMHD)2, which may suggest the existence of additional interaction of the CF3 moiety with the catalyst. Control experiments also revealed complete supress of the over reaction of product 3a, which is in accordance with the improved selectivity by using 2 (Scheme S1†).
O and Lewis acid, suggesting that the phosphonylating reagents are activated for the nucleophilic addition of alcohols (Fig. S2–S5 and Table S5†). It should be noted that less Lewis basic 2 preferentially interacts with Zn(TMHD)2, which may suggest the existence of additional interaction of the CF3 moiety with the catalyst. Control experiments also revealed complete supress of the over reaction of product 3a, which is in accordance with the improved selectivity by using 2 (Scheme S1†).
| Entry | Zn cat. | Solv. | Yielda (%) | Selec.a (%) |
|---|---|---|---|---|
| a Yield was determined by 1H NMR analysis using 1,2,4,5-tetramethylbenzene as an internal standard. b The reaction was performed for 1.5 h. c The reaction was performed in the absence of the catalyst. | ||||
| 1 | Zn(acac)2 | BTF | 71 | 97 |
| 2 | Zn(acac)2 | THF | 35 | 95 |
| 3 | Zn(acac)2 | DCM | 37 | 93 |
| 4 | Zn(acac)2 | Toluene | 73 | 95 |
| 5 | Zn(TMHD)2 | Toluene | 76 | 95 |
| 6 | Zn(OAc)2 | Toluene | 21 | >99 |
| 7 | Zn(OPiv)2 | Toluene | 31 | 94 |
| 8b | Zn(THHD)2 | Toluene | 87 | 92 |
| 9c | None | Toluene | N.R. | — |
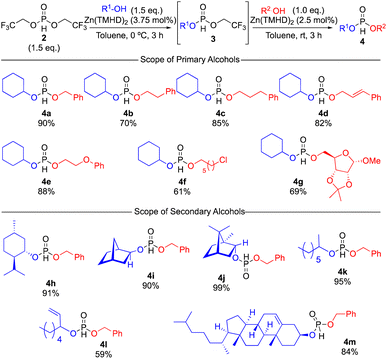
With the optimized reaction conditions for both mono-selective phosphonylation and consecutive second phosphonylation in hand, one-pot reactions to synthesize various phosphate diesters were examined (Scheme 3, see ESI† for detailed optimization). The scope of the reaction with respect to primary alcohols was examined first. The carbon chain length of primary alcohols did not affect the reactivity or selectivity significantly, and the target products 4b and 4c were obtained in good yields. Several functional groups, including alkene, ether, and alkyl chloride, were tolerated, and the corresponding phosphate diesters 4d–f were obtained in good yields. More interestingly, a protected carbohydrate could also be employed as a substrate to afford 4g in good yield. The scope of the reaction with secondary alcohols was also examined. Substituted cyclohexanol such as 1d as well as bicyclic alcohols 1e and 1f gave the target products 4h–j in high yields with excellent selectivity using benzyl alcohol 1k as the primary alcohol in the second step. Acyclic alcohol 1h also reacted smoothly to give 4k, whereas 1j gave the corresponding 4l in moderate yield. Cholesterol could also be employed in the second step of this one-pot reaction to give 4m, which was obtained in good yield. Unfortunately, tertiary alcohols were unreactive for the second step to give the corresponding phosphites in low yields.
We further challenged the catalytic synthesis of more complex phosphite diesters and phosphate triesters (Scheme 4). We first examined the synthesis of sugar phosphates using protected carbohydrates as substrates. The two-step phosphonylation of 1′,5′-protected deoxyribose with 2 and 1,2,3-protected ribose 1t gave the target product 4n in 77% yield. Subsequent oxidations with I2 in ethanol solvent gave the stable phosphate triester 5a in 63% yield over three steps (Scheme 4(a)). Similarly, two-step catalytic phosphonylation of alcohols 1ad and 1ae afforded phosphite 4o in good yield. Subsequent oxidation and deprotection gave the key intermediate of the serum albumin binder 5d (Scheme 4(b)).71 Moreover, phosphorthioate 4a-S could be synthesized with the model substrate by modifying the final oxidation step (Scheme 4(c)).72
We also investigated the catalytic synthesis of phosphate triesters 7, having two nucleoside units on the P center, which can be viewed as the smallest oligonucleotide unit (Scheme 4(d)). In the first step, a 5′-protected thymidine was reacted with 2 in the presence of 5 mol% of Zn(acac)2 to afford mono-phosphonylated compound 3af in 94% yield. The latter was then reacted with 3′-protected thymidine in the presence of 10 mol% of Zn(TMHD)2 to give phosphite diester 6a in 42% yield. The diester was oxidized to give the target compound 7a in 44% yield over three steps. This protocol was also applied to another nucleotide with a different base unit. The intermediate 3af was reacted with 2′,3′-protected cytidine under the same conditions as thymidine, and phosphite 6b was obtained in 45% yield. Again, 6b was oxidized to give phosphate triester 7b in 37% yield. These results represent the first catalytic and additive-free synthesis of phosphite diesters and its successful application to the synthesis of the smallest oligonucleotide unit.
Conclusions
In conclusion, we have developed the catalytic and additive-free synthesis of phosphite diesters. Two different alcohols were introduced on readily available phosphorylating reagent 2 in the presence of a catalytic amount of Zn complexes to afford various kinds of phosphite diesters in high selectivity. This reaction possesses high functional group tolerance including alkyne, phenol, amide, and carbamates and the products could be oxidized to phosphate triesters. Using this protocol, catalytic synthesis of nucleotides was achieved. Further investigation to reveal a detailed reaction mechanism and application to oligonucleotide synthesis is ongoing in our laboratory.Data availability
Detailed synthetic procedures, supporting experimental results, and complete characterization data for all new compounds can be found in the ESI.†Author contributions
YS and SK designed the project. YS, SMC, LAD, and AM performed and analyzed the experiments. YS and SK wrote the manuscript, SK supervised and directed the research. All authors have discussed the results and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI, Grant No. 21K14621) and the Adaptable and Seamless Technology transfer Program through Target-driven R&D (A-STEP) from the Japan Science and Technology Agency (JST) Grant No. JPMJTR22T5.Notes and references
- F. H. Westheimer, Science, 1987, 235, 1173–1178 CrossRef CAS PubMed.
- M. W. Bowler, M. J. Cliff, J. P. Waltho and G. M. Blackburn, New J. Chem., 2010, 34, 784–794 RSC.
- T. Hunter, Philos. Trans. R. Soc., B, 2012, 367, 2513–2516 CrossRef CAS PubMed.
- R. R. Siden, DNA Structure and Function Protein Synthesis, Academic Press, 1st edn, 1994 Search PubMed.
- W. Dowhan, Annu. Rev. Biochem., 1997, 66, 199–232 CrossRef CAS PubMed.
- M. J. Berridge and R. F. Irvine, Nature, 1989, 341, 197–205 CrossRef CAS PubMed.
- P. Cohen, Nat. Cell Biol., 2002, 4, E127–E130 CrossRef CAS PubMed.
- I. Smoly, N. Shemesh, M. Ziv-Ukelson, A. Ben-Zvi and E. Yeger-Lotem, PLoS Comput. Biol., 2017, 13, e1005221 CrossRef PubMed.
- J. R. Knowles, Annu. Rev. Biochem., 1980, 49, 877–919 CrossRef CAS PubMed.
- C. Meier, Synlett, 1998, 233–242 CrossRef CAS.
- M. B. Wire, M. J. Shelton and S. Studenberg, Clin. Pharmacokinet., 2006, 45, 137–168 CrossRef CAS PubMed.
- M. Tudi, H. D. Ruan, L. Wang, J. Lyu, R. Sadler, D. Connell, C. Chu and D. T. Phung, Int. J. Environ. Res. Public Health, 2021, 18, 1112 CrossRef CAS PubMed.
- S. V. Levchik and E. D. Weil, Polym. Int., 2005, 54, 981–998 CrossRef CAS.
- P. Jia, H. Xia, K. Tang and Y. Zhou, Polymers, 2018, 10, 1303 CrossRef PubMed.
- W. Brad Wan and P. P. Seth, J. Med. Chem., 2016, 59, 9645–9667 CrossRef PubMed.
- A. Khvorova and J. K. Watts, Nat. Biotechnol., 2017, 35, 238–248 CrossRef CAS PubMed.
- M. Byrne, V. Vathipadiekal, L. Apponi, N. Iwamoto, P. Kandasamy, K. Longo, F. Liu, R. Looby, L. Norwood, A. Shah, J. D. Shelke, C. Shivalila, H. Yang, Y. Yin, L. Guo, K. Bowman and C. Vargeese, Transl. Vis. Sci. Technol., 2021, 10, 23 CrossRef PubMed.
- Y. Liu, J. C. Dodart, H. Tran, S. Berkovitch, M. Braun, M. Byrne, A. F. Durbin, X. S. Hu, N. Iwamoto, H. G. Jang, P. Kandasamy, F. Liu, K. Longo, J. Ruschel, J. Shelke, H. Yang, Y. Yin, A. Donner, Z. Zhong, C. Vargeese and R. H. Brown, Nat. Commun., 2021, 12, 1–15 CrossRef PubMed.
- A. P. Guzaev, Curr. Protoc. Nucleic Acid Chem., 2013, 53, 3 Search PubMed.
- A. Deutsch and O. Fernö, Nature, 1945, 156, 604 CrossRef CAS.
- P. T. Gilham and H. G. Khorana, J. Am. Chem. Soc., 1958, 80, 6212–6222 CrossRef CAS.
- T. Sekiya, T. Takeya, E. L. Brown, R. Belagaje, R. Contreras, H. J. Fritz, M. J. Gait, R. G. Lees, M. J. Ryan, H. G. Khorana and K. E. Norris, J. Biol. Chem., 1979, 254, 5787–5800 CrossRef CAS PubMed.
- T. Sekiya, E. L. Brown, R. Belagaje, H. J. Fritz, M. J. Gait, R. G. Lees, M. J. Ryan, H. G. Khorana and K. E. Norris, J. Biol. Chem., 1979, 254, 5781–5786 CrossRef CAS PubMed.
- R. Belagaje, E. L. Brown, H. J. Fritz, R. G. Lees and H. G. Khorana, J. Biol. Chem., 1979, 254, 5765–5780 CrossRef CAS PubMed.
- S. Han and S. J. Miller, J. Am. Chem. Soc., 2013, 135, 12414–12421 CrossRef CAS PubMed.
- K. A. Coppola, J. W. Testa, E. E. Allen and B. R. Sculimbrene, Tetrahedron Lett., 2014, 55, 4203–4206 CrossRef CAS.
- J. I. Murray, R. Woscholski and A. C. Spivey, Chem. Commun., 2014, 50, 13608–13611 RSC.
- J. I. Murray, R. Woscholski and A. C. Spivey, Synlett, 2015, 985–990 CAS.
- J. Ying, Q. Gao and X. F. Wu, Chem.–Asian J., 2020, 15, 1540–1543 CrossRef CAS PubMed.
- S. Tsuneo and O. Shunji, Bull. Chem. Soc. Jpn., 1975, 48, 2084–2090 CrossRef.
- B. R. Sculimbrene and S. J. Miller, J. Am. Chem. Soc., 2001, 123, 10125–10126 CrossRef CAS PubMed.
- S. Jones and D. Selitsianos, Org. Lett., 2002, 4, 3671–3673 CrossRef CAS PubMed.
- B. R. Sculimbrene, A. J. Morgan and S. J. Miller, J. Am. Chem. Soc., 2002, 124, 11653–11656 CrossRef CAS PubMed.
- B. R. Sculimbrene, A. J. Morgan and S. J. Miller, Chem. Commun., 2003, 3, 1781–1785 RSC.
- B. R. Sculimbrene, Y. Xu and S. J. Miller, J. Am. Chem. Soc., 2004, 126, 13182–13183 CrossRef CAS PubMed.
- S. Jones, J. Northen and A. Rolfe, Chem. Commun., 2005, 30, 3832–3834 RSC.
- A. J. Morgan, S. Komiya, Y. Xu and S. J. Miller, J. Org. Chem., 2006, 71, 6923–6931 CrossRef CAS PubMed.
- R. L. Letsinger and K. K. Ogilvie, J. Am. Chem. Soc., 1969, 91, 3350–3355 CrossRef CAS.
- R. L. Letsinger, K. K. Ogilvie and P. S. Miller, J. Am. Chem. Soc., 1969, 91, 3360–3365 CrossRef CAS.
- R. L. Letsinger, J. L. Finnan, G. A. Heavner and W. B. Lunsford, J. Am. Chem. Soc., 1975, 97, 3278–3279 CrossRef CAS PubMed.
- R. L. Letsinger and W. B. Lunsford, J. Am. Chem. Soc., 1976, 98, 3655–3661 CrossRef CAS PubMed.
- S. Sigurdsson and R. Stomberg, J. Chem. Soc., Perkin Trans. 2, 2002, 2, 1682–1688 RSC.
- A. D. Dal-Maso, F. Legendre, C. Blonski and P. Hoffmann, Synth. Commun., 2008, 38, 1688–1693 CrossRef CAS.
- H. Kitamura, Y. Otake, N. Sugisawa, H. Sugisawa, T. Ida, H. Nakamura and S. Fuse, Chem. - Eur. J., 2022, 28, e202200932 CrossRef CAS PubMed.
- M. A. Maier, A. P. Guzaev and M. Manoharan, Org. Lett., 2000, 2, 1819–1822 CrossRef CAS PubMed.
- F. M. J. Tappe, V. T. Trepohl and M. Oestreich, Synthesis, 2010, 3037–3062 CAS.
- S. L. Beaucage and M. H. Caruthers, Tetrahedron Lett., 1981, 22, 1859–1862 CrossRef CAS.
- M. D. Matteucci and M. H. Caruthers, J. Am. Chem. Soc., 1981, 103, 3185–3191 CrossRef CAS.
- L. J. McBride and M. H. Caruthers, Tetrahedron Lett., 1983, 24, 245–248 CrossRef CAS.
- D. S. Sergueev and B. R. Shaw, J. Am. Chem. Soc., 1998, 120, 9417–9427 CrossRef CAS.
- S. Sigurdsson and R. Strömberg, J. Chem. Soc., Perkin Trans. 2, 2002, 2, 1682–1688 RSC.
- A. L. Featherston, Y. Kwon, M. M. Pompeo, O. D. Engl, D. K. Leahy and S. J. Miller, Science, 2021, 371, 702–707 CrossRef CAS PubMed.
- K. W. Knouse, J. N. deGruyter, M. A. Schmidt, B. Zheng, J. C. Vantourout, C. Kingston, S. E. Mercer, I. M. Mcdonald, R. E. Olson, Y. Zhu, C. Hang, J. Zhu, C. Yuan, Q. Wang, P. Park, M. D. Eastgate and P. S. Baran, Science, 2018, 361, 1234–1238 CrossRef CAS PubMed.
- D. Xu, N. Rivas-Bascón, N. M. Padial, K. W. Knouse, B. Zheng, J. C. Vantourout, M. A. Schmidt, M. D. Eastgate and P. S. Baran, J. Am. Chem. Soc., 2020, 142, 5785–5792 CrossRef CAS PubMed.
- D. T. Flood, K. W. Knouse, J. C. Vantourout, S. Kitamura, B. B. Sanchez, E. J. Sturgell, J. S. Chen, D. W. Wolan, P. S. Baran and P. E. Dawson, ACS Cent. Sci., 2020, 6, 1789–1799 CrossRef CAS PubMed.
- J. C. Vantourout, S. R. Adusumalli, K. W. Knouse, D. T. Flood, A. Ramirez, N. M. Padial, A. Istrate, K. Maziarz, J. N. Degruyter, R. R. Merchant, J. X. Qiao, M. A. Schmidt, M. J. Deery, M. D. Eastgate, P. E. Dawson, G. J. L. Bernardes and P. S. Baran, J. Am. Chem. Soc., 2020, 142, 17236–17242 CrossRef CAS PubMed.
- Y. Huang, K. W. Knouse, S. Qiu, W. Hao, N. M. Padial, J. C. Vantourout, B. Zheng, S. E. Mercer, J. Lopez-Ogalla, R. Narayan, R. E. Olson, D. G. Blackmond, M. D. Eastgate, M. A. Schmidt, I. M. McDonald and P. S. Baran, Science, 2021, 373, 1265–1270 CrossRef CAS PubMed.
- M. Ociepa, K. W. Knouse, D. He, J. C. Vantourout, D. T. Flood, N. M. Padial, J. S. Chen, B. B. Sanchez, E. J. Sturgell, B. Zheng, S. Qiu, M. A. Schmidt, M. D. Eastgate and P. S. Baran, Org. Lett., 2021, 23, 9337–9342 CrossRef CAS PubMed.
- K. W. Knouse, D. T. Flood, J. C. Vantourout, M. A. Schmidt I, M. McDonald, M. D. Eastgate and P. S. Baran, ACS Cent. Sci., 2021, 7, 1473–1485 CrossRef CAS PubMed.
- K. Domon, M. Puripat, K. Fujiyoshi, M. Hatanaka, S. A. Kawashima, K. Yamatsugu and M. Kanai, ACS Cent. Sci., 2020, 6, 283–292 CrossRef CAS PubMed.
- K. Fujiyoshi, S. A. Kawashima, K. Yamatsugu and M. Kanai, Synlett, 2021, 32, 1135–1140 CrossRef CAS.
- A. Sakakura, M. Katsukawa and K. Ishihara, Org. Lett., 2005, 7, 1999–2002 CrossRef CAS PubMed.
- A. Sakakura, M. Katsukawa and K. Ishihara, Angew. Chem., Int. Ed., 2007, 46, 1423–1426 CrossRef CAS PubMed.
- Y. Saito, S. M. Cho, L. A. Danieli and S. Kobayashi, Org. Lett., 2020, 22, 3171–3175 CrossRef CAS PubMed.
- P. J. Garegg, I. Lindh, T. Regberg, J. Stawinski, R. Stromberg and C. Henrichson, Tetrahedron Lett., 1986, 27, 4051–4054 CrossRef CAS.
- J. Dhineshkumar and K. R. Prabhu, Org. Lett., 2013, 15, 6062–6065 CrossRef CAS PubMed.
- D. E. Gibbs and C. Larsen, Synthesis, 1984, 410–413 CrossRef CAS.
- E. Kuyl-Yeheskiely, C. M. Tromp, G. A. van der Marel and J. H. van Boom, Tetrahedron Lett., 1987, 28, 4461–4464 CrossRef CAS.
- K. Tsubaki, H. Shimooka, M. Kitamura and T. Okauchi, Org. Lett., 2019, 21, 9779–9783 CrossRef CAS PubMed.
- K. Tsubaki, T. Yamanaka, T. Hisamitsu, H. Shimooka, M. Kitamura and T. Okauchi, Synthesis, 2021, 53, 3827–3835 CrossRef CAS.
- K. Zobel, M. F. T. Koehler, M. H. Beresini, L. D. Caris and D. Combs, Bioorg. Med. Chem. Lett., 2003, 13, 1512–1515 CrossRef PubMed.
- S. Sarkar and M. Kalek, Org. Lett., 2023, 25, 671–675 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc01401d |
| This journal is © The Royal Society of Chemistry 2024 |