Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Binding of carbon dioxide and acetylene to free carboxylic acid sites in a metal–organic framework†
Christopher
Marsh
 a,
Xue
Han
ab,
Zhenzhong
Lu
a,
Ivan
da Silva
a,
Xue
Han
ab,
Zhenzhong
Lu
a,
Ivan
da Silva
 c,
Yongqiang
Cheng
d,
Luke L.
Daemen
d,
Sarah J.
Day
e,
Stephen P.
Thompson
e,
Anibal J.
Ramirez-Cuesta
c,
Yongqiang
Cheng
d,
Luke L.
Daemen
d,
Sarah J.
Day
e,
Stephen P.
Thompson
e,
Anibal J.
Ramirez-Cuesta
 d,
Sihai
Yang
d,
Sihai
Yang
 *af and
Martin
Schröder
*af and
Martin
Schröder
 *a
*a
aDepartment of Chemistry, University of Manchester, Manchester, M13 9PL, UK. E-mail: Sihai.Yang@manchester.ac.uk; M.Schroder@manchester.ac.uk
bCollege of Chemistry Beijing Normal University, Beijing 100875, China
cISIS Neutron and Muon Source, Rutherford Appleton Laboratory, Oxford, OX11 0QX, UK
dNeutron Scattering Division, Neutron Sciences Directorate, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
eDiamond Light Source, Harwell Science Campus, Oxford, OX11 0DE, UK
fBeijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: Sihai.Yang@pku.edu.cn
First published on 10th April 2024
Abstract
The functionalisation of organic linkers in metal–organic frameworks (MOFs) to improve gas uptake is well-documented. Although the positive role of free carboxylic acid sites in MOFs for binding gas molecules has been proposed in computational studies, relatively little experimental evidence has been reported in support of this. Primarily this is because of the inherent synthetic difficulty to prepare MOF materials bearing free, accessible –COOH moieties which would normally bind to metal ions within the framework structure. Here, we describe the direct binding of CO2 and C2H2 molecules to the free –COOH sites within the pores of MFM-303(Al). MFM-303(Al) exhibits highly selective adsorption of CO2 and C2H2 with a high selectivity for C2H2 over C2H4. In situ synchrotron X-ray diffraction and inelastic neutron scattering, coupled with modelling, highlight the cooperative interactions of adsorbed CO2 and C2H2 molecules with free –COOH and –OH sites within MFM-303(Al), thus rationalising the observed high selectivity for gas separation.
Porous metal–organic framework (MOF) materials have received much interest in the search for sorbent materials for a variety of gases.1–3 A commonly employed strategy to obtain MOFs with desirable affinity for target gases is to introduce functional groups to the linker, which subsequently line the pore interior and provide binding sites for gas molecules. State-of-the-art studies primarily focus on groups such as halide,4 amide,5,6 hydroxyl7,8 and amine9 groups since they can be incorporated within the protocol for MOF synthesis and the resultant materials often retain their framework structure with the functional group(s) protruding into the pores. Carboxylic acid groups (–COOH), incorporating both lone pairs of electrons and acidic protons, possess high propensity for gases such as CO2, as has been suggested by computational studies.10–12 However, due to the preference of carboxylate groups to bind to metal centres, there are only a few reports of porous MOFs that are functionalised with free carboxylic acid sites.13–20 To date, the molecular details of interactions of carboxylic acid moieties in MOFs with gas molecules have been poorly explored by experiment. Here we report the direct visualisation of binding of CO2 and C2H2 molecules to the free carboxylic acid sites in MFM-303(Al) by a combination of in situ synchrotron X-ray powder diffraction (SXPD), inelastic neutron scattering (INS) and density functional theory (DFT) calculations. These crystallographic and dynamic experiments confirm unambiguously the important role of –COOH and –OH sites in assembling a series of cooperative supramolecular interactions with adsorbed CO2 and C2H2 molecules, leading to excellent adsorption properties.
MFM-303(Al) was prepared by hydrothermal reaction of biphenyl-3,3′,5,5′-tetracarboxylic acid (H4L) with aluminium chloride in acidified water (pH < 2).21 The material, [Al(OH)(H2L)], is composed of octahedrally-coordinated Al(III) centres, with two bridging hydroxyl groups in trans positions, and each ligand has two –COOH groups that are not bound to Al(III) centres and remain protonated. This is promoted by the use of low pH conditions during the synthesis, with the potential of the carboxylic group interacting with guest molecules within the pores via hydrogen bonding. The structure of the as-synthesised material thus reveals intramolecular hydrogen bonding between neighbouring carboxylic acid groups [O⋯O = 2.637(14) Å], which helps to stabilise the overall structure. This confirms the approach to overcome the inherent preference of carboxylic acid groups to bind to metal centres via the use of strongly acidic conditions combined with multitopic ligands that can support hydrogen bonding. Along the crystallographic c axis the widest aperture is ∼8 Å, and the –COOH groups taking van der Waals radii into account are separated by ca 2.3 Å (Fig. 1). The phase purity of bulk samples of MFM-303(Al) was confirmed by PXRD (Fig. S1†). Desolvated MFM-303(Al) shows a surface area of 724 m2 g−1 (Fig. S4†).
CO2 isotherms were recorded over a wide temperature range (273–333 K) and showed adsorption–desorption hysteresis, which increased with increasing temperature (Fig. 2a). This is in contrast to many previously reported MOFs, in which hysteresis loops are enhanced at low temperature,22,23 and implies that MFM-303(Al) has a small degree of temperature/guest-dependant framework flexibility. The isosteric heat (Qst) and entropy (ΔS) of adsorption of CO2 were calculated using the van't Hoff equation (Fig. 2b). The value of Qst decreases from 37 to 33 kJ mol−1 with CO2 loading up to 0.5 mmol g−1 and then increases to 36 kJ mol−1 between 0.5 and 2.5 mmol g−1. This indicates that the strong binding sites are occupied early on initial uptake of CO2 and then notable CO2⋯CO2 interactions are generated with increasing surface coverage. The value of ΔS decreases continuously with increasing loading, indicating an increase in local order of the system, again consistent with the formation of CO2⋯CO2 interactions at higher loadings.
Pores with restricted geometry or with narrow apertures have been shown to have high potential in the field of molecular separation, particularly where the components of mixtures have similar molecular properties, such as sizes and volatilities.24 Isotherms for C2 hydrocarbons were recorded on MFM-303(Al) at 273–333 K. Hysteresis loops are present for all the C2 hydrocarbons and become more prominent at higher temperature (Fig. 2c and d). For C2H2, an uptake of 2.65 mmol g−1 is observed at 293 K and 1 bar, which decreases to 2.24 mmol g−1 at 333 K. The hysteresis loop becomes prominent at temperatures above 313 K. For C2H4, a lower uptake of 1.75 mmol g−1 was measured at 293 K and 1 bar, decreasing to 1.19 mmol g−1 at 333 K. The hysteresis loop is much more pronounced than for C2H2, being apparent for measurements at all temperatures. Meanwhile, an uptake for C2H6 of 1.45 mmol g−1 at 293 K and 1 bar was observed, reducing to 0.30 mmol g−1 at 333 K. The values for Qst for C2H2, C2H4 and C2H6 were measured to be 38–42, 34–32 and 30–24 kJ mol−1, respectively, the order of Qst values being consistent with the observed uptake capacities and inversely related to the magnitude of the observed hysteresis. This suggests that the adsorbates with the largest molecular size do not enter the framework as readily and once inside become trapped in the pore, which results in a hysteresis loop and a lower value of Qst but higher ΔS (Fig. S8†). Adsorption of CH4 shows broad hysteretic loops and much lower uptakes (Fig. S5†).
The presence of hysteresis loops in the isotherms suggests that there is a possible structural change from the activated to the guest-loaded material. The magnitude of the hysteresis loop is inversely proportional to the observed values of Qst with more strongly interacting adsorbates having a narrower loop. This suggests that the structural change can be modulated by the choice of guest, and molecules with stronger interactions can more readily overcome the energy barrier to re-organisation.25 Also, for the C2 hydrocarbons, the uptake capacities and values of Qst are inversely proportional to the kinetic diameter of the adsorbate (Table S3†). The hysteresis loops are dependent on temperature, becoming wider at higher temperature, consistent with other MOFs that feature guest-dependent flexibility;26 this is in contrast to capillary condensation in rigid porous structures.27 Within MFM-303(Al), the intramolecular hydrogen bonding can reorientate from that in the activated empty structure in order to optimise interaction of the –COOH moieties with guest molecules. The presence of flexibility in sorbent materials is often beneficial to gas separations, and materials can breathe and/or undergo a gate-opening response dependent on the size and shape of the molecule. The pressure under which structural transformation occurs is unique for each adsorbent, and thus excellent separation performance and selective uptake of target molecules can be achieved.28,29
The adsorption selectivity of MFM-303(Al) for equimolar binary mixtures was estimated using ideal adsorbed solution theory (IAST) based upon single-component isotherms.30,31 Interestingly, MFM-303(Al) exhibits an excellent IAST selectivity of 15.0 for C2H2 over C2H4 at 1 bar and 293 K (Fig. S11†). This compares well with other MOFs in the literature. For example, at 298 K, MOF-74(Fe) possesses a selectivity of 2.08 (ref. 32) whilst UTSA-100 achieves 10.72 (ref. 33) and NbU-1 reaches 5.9.34 The excellent selectivity is corroborated by comparison to other reported MOFs containing acidic –OH and –COOH groups, such as Ni-gallate35 and NTU-72 (ref. 36) which show exceptional selectivities of 43.7 and 56 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 mixtures, though in these examples their potential for other mixtures of hydrocarbons was not fully explored. A high selectivity for CO2/CH4 is also observed for MFM-303(Al) (56.1 at 293 K and 1 bar), which again compares well with other MOFs (Table S2†). Thus, the restricted pores of MFM-303(Al) lead to enhanced selectivity for C2H2 and CO2. Dynamic breakthrough separation of an equimolar mixture of C2H2/C2H4 and C2H4/C2H6 was undertaken at 333 K (Fig. 2e and f). Clear separation of components was observed in both cases with selective retention of C2H2 and C2H4 demonstrating the potential of MFM-303(Al) as a sorbent material for molecular separation.
99 mixtures, though in these examples their potential for other mixtures of hydrocarbons was not fully explored. A high selectivity for CO2/CH4 is also observed for MFM-303(Al) (56.1 at 293 K and 1 bar), which again compares well with other MOFs (Table S2†). Thus, the restricted pores of MFM-303(Al) lead to enhanced selectivity for C2H2 and CO2. Dynamic breakthrough separation of an equimolar mixture of C2H2/C2H4 and C2H4/C2H6 was undertaken at 333 K (Fig. 2e and f). Clear separation of components was observed in both cases with selective retention of C2H2 and C2H4 demonstrating the potential of MFM-303(Al) as a sorbent material for molecular separation.
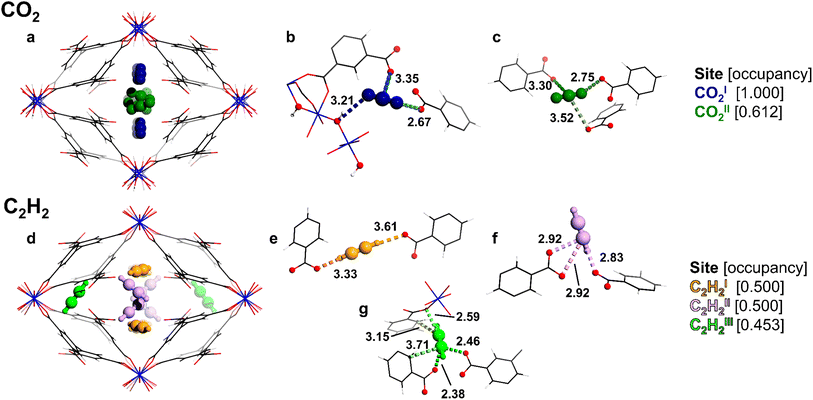
Two unique binding domains (I and II) for CO2 have been determined using Rietveld refinement of SXPD data for CO2-loaded MFM-303(Al) (Fig. 3a–c). Notable interactions are observed between CO2 molecules and the free –COOH and bridging –OH sites in the pore. CO2I (occupancy of 1.0) is stabilised by moderate hydrogen bonding interactions to the bridging hydroxyl group [μ2-OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C=O = 3.21(1) Å] and the free –COOH [O
C=O = 3.21(1) Å] and the free –COOH [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯O = 2.67(1) Å]. COII2 [occupancy of 0.61(1)] shows similar distances to neighbouring –COOH groups [O
O⋯O = 2.67(1) Å]. COII2 [occupancy of 0.61(1)] shows similar distances to neighbouring –COOH groups [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯O = 2.75(1) Å]. Furthermore, adsorbed CO2 molecules in the pore form the typical “T” shape arrangement [O
O⋯O = 2.75(1) Å]. Furthermore, adsorbed CO2 molecules in the pore form the typical “T” shape arrangement [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CI
CI![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯CO2II = 2.60(1) Å], suggesting that intermolecular dipole interaction also contributes to the packing of CO2 in the pore, consistent with the values for Qst.
O⋯CO2II = 2.60(1) Å], suggesting that intermolecular dipole interaction also contributes to the packing of CO2 in the pore, consistent with the values for Qst.
In contrast, three binding sites (I–III) were determined for adsorbed C2H2 molecules in MFM-303(Al) (Fig. 3d–g). C2H2I [occupancy of 0.50(1)] is located towards the centre of the pore and forms strong hydrogen bonds between adjacent –COOH sites [H–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯O = 3.61(5), 3.33(5) Å]. C2H2II [occupancy of 0.50(1)] is oriented differently, but also interacts with –COOH groups [H–C
C–H⋯O = 3.61(5), 3.33(5) Å]. C2H2II [occupancy of 0.50(1)] is oriented differently, but also interacts with –COOH groups [H–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯O = 2.83(4), 3.42(4) Å], suggesting moderate hydrogen-bonding interactions. Finally, C2H2III [occupancy of 0.45(1)] is in the small pocket at the corner of the pore and is stabilised primarily via confinement effects due to the restricted pore geometry, with contacts to coordinated carboxylate groups [H–C
C–H⋯O = 2.83(4), 3.42(4) Å], suggesting moderate hydrogen-bonding interactions. Finally, C2H2III [occupancy of 0.45(1)] is in the small pocket at the corner of the pore and is stabilised primarily via confinement effects due to the restricted pore geometry, with contacts to coordinated carboxylate groups [H–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯O = 2.38(6), 2.46(7) Å] and the phenyl ring [H–C
C–H⋯O = 2.38(6), 2.46(7) Å] and the phenyl ring [H–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯Cphenyl = 3.15(5), 3.72(7) Å]. Additionally, strong interactions are found between C2H2III and nearby carboxylate groups with H–C
C–H⋯Cphenyl = 3.15(5), 3.72(7) Å]. Additionally, strong interactions are found between C2H2III and nearby carboxylate groups with H–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯O distances of 2.59(4) Å. Thus, the free carboxylic acid sites, along with the hydroxyl group, play a crucial role in binding to these guest molecules. Interestingly, the present study represents the first example of observation of binding of CO2 and C2H2 molecules at free –COOH groups within MOFs.
C–H⋯O distances of 2.59(4) Å. Thus, the free carboxylic acid sites, along with the hydroxyl group, play a crucial role in binding to these guest molecules. Interestingly, the present study represents the first example of observation of binding of CO2 and C2H2 molecules at free –COOH groups within MOFs.
In situ INS measurements were conducted for MFM-303(Al) as a function of loading of CO2 and of C2H2 (Fig. 4). The difference spectra were obtained by subtracting the INS spectrum of bare MFM-303(Al) from the spectra of gas-loaded samples. DFT calculations of the INS spectra of both the bare and gas-loaded systems show excellent agreement with experimental data, allowing the full assignment of the vibrational modes. The difference spectrum of the CO2-loaded sample showed several changes. Difference peaks I and II are both combinations of vibrational modes, most significantly the out-of-plane wagging of –OH in the –COOH group, along with aromatic C–H asymmetric bending and C–H out-of-plane bending modes. Peak III is identified as a blueshift of the mode at 959–979 cm−1, assigned to the bridging –OH group rocking parallel and in the plane of the [AlO4(OH)2] chain. Peak IV is assigned to a combination of vibrational modes, most significantly the O–H of COOH in-plane wagging and C–O–H deformation, along with a contribution from the aromatic C–H in-plane wagging. For C2H2, five notable changes are observed in the difference spectrum. Peak I centred at 667 cm−1 arises from a combination of modes: the bridging –OH along-chain wagging, C2H2 bending and aromatic C–H out-of-plane wagging. Peak II is also assigned to a combination of modes, the antisymmetric C–H binding in C2H2, and the in-plane wagging of the –OH group of the free carboxylic acid group. Peak III at 850 cm−1 arises from the O–H of the free COOH in-plane wagging mode, whilst peaks IV and V arise from a combination of the aromatic C–H out-of-plane wagging and the free carboxylic acid group O–H in-plane wagging, respectively. These observations on the host–guest binding dynamics are entirely consistent with the crystallographic models determined by SXPD data for CO2- and C2H2-loaded MFM-303(Al) and further confirm the positive impact of free –COOH sites on gas adsorption in MOFs.
MFM-303(Al), bearing free, accessible –COOH groups and incorporating narrow pores, shows selective uptake of CO2 and C2H2. This selectivity is a result of multiple factors: firstly, the larger value of Qst for C2H2 than C2H4 shows that MFM-303(Al) has a stronger binding preference, with a lower ΔS indicating more ordered pores when loaded with C2H2. The strong interaction of MFM-303(Al) with CO2 and C2H2 was revealed through in situ SXPD and INS/DFT studies with adsorbed C2H2 and CO2 being well-ordered within the structure via interaction with the free carboxylic acid and hydroxyl groups, whilst also being confined within the pores. In comparison, C2H4 shows an overall lower uptake and a weaker interaction with the MOF, related also to its larger kinetic diameter. Interestingly, adsorption of small gas molecules in MFM-303(Al) shows hysteresis, which increases with increasing temperature and molecular size of the gas molecules.
MFM-303(Al) illustrates the importance of the free –COOH group in binding with CO2 and C2H2 molecules, and demonstrates that there is much potential in developing MOFs containing free carboxylic acid sites for selective gas separation. More broadly, the incorporation of functional groups on the interior of MOF materials provides favourable binding interactions with target guest molecules, and is a key strategy for improving selective uptake. This can be combined with optimisation of the pore geometry for the target gas molecules, enabling them to pack efficiently within the pores, thus increasing the overall uptake and selectivity. There is a careful balance to be struck, as efficient packing due to restricted pore geometry and volume may lead to an overall lower uptake when compared with materials with larger pore volumes. Thus, it is important to consider if the intended application is molecular separation (with greater emphasis on selectivity) or gas storage (with greater emphasis on total uptake/packing density). This work also emphasises the power of in situ SXPD and INS studies to reveal and understand the nature of host–guest interaction. This deserves further attention in studies of other porous solids showing exceptional adsorption selectivity.
Data availability
Additional details of experimental methods and procedures, crystallographic and thermodynamic data, isotherms and their fitting and analysis, and of DFT calculations are given in the ESI† for this manuscript.Author contributions
CM: synthesis of materials, and their characterisation and analysis. XH, ZL: gas adsorption studies and studies at national facilities. YC, LLD, AJR-C: inelastic neutron scattering experiments, analysis and modelling. IdS: structural analysis and refinement. SJD, SPT: synchrotron X-ray diffraction studies. SY, MS: design, development and supervision of project. Preparation of manuscript with all authors.Conflicts of interest
The authors declare no competing interest.Acknowledgements
We thank EPSRC (EP/I011870), the Royal Society, University of Manchester, BNLMS and Peking University for funding. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No. 742401, NANOCHEM). We are grateful to Diamond Light Source for access to Beamline I11. This research used resources at the Spallation Neutron Source, a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory. Computing resources for neutron data analysis were made available through the VirtuES and the ICE-MAN projects, funded by Laboratory Directed Research and Development program and Compute and Data Environment for Science (CADES) at ORNL.References
- R.-B. Lin, S. Xiang, H. Xing, W. Zhou and B. Chen, Coord. Chem. Rev., 2019, 378, 87–103 CrossRef CAS.
- J. Li, P. M. Bhatt, J. Li, M. Eddaoudi and Y. Liu, Adv. Mater., 2020, 32, 2002563 CrossRef CAS PubMed.
- E. Binaeian, E.-S. M. El-Sayed, M. Khanpour Matikolaei and D. Yuan, Coord. Chem. Rev., 2021, 430, 213738 CrossRef CAS.
- Y. Wang, C. Tan, Z. Sun, Z. Xue, Q. Zhu, C. Shen, Y. Wen, S. Hu, Y. Wang, T. Sheng and X. Wu, Chem.–Eur. J., 2014, 20, 1341–1348 CrossRef CAS PubMed.
- Y.-Q. Chen, Y.-K. Qu, G.-R. Li, Z.-Z. Zhuang, Z. Chang, T.-L. Hu, J. Xu and X.-H. Bu, Inorg. Chem., 2014, 53, 8842–8844 CrossRef CAS PubMed.
- Z. Lu, J. Bai, C. Hang, F. Meng, W. Liu, Y. Pan and X. You, Chem.–Eur. J., 2016, 22, 6277–6285 CrossRef CAS PubMed.
- M. Savage, I. da Silva, M. Johnson, J. H. Carter, R. Newby, M. Suyetin, E. Besley, P. Manuel, S. Rudić, A. N. Fitch, C. Murray, W. I. F. David, S. Yang and M. Schröder, J. Am. Chem. Soc., 2016, 138, 9119–9127 CrossRef CAS PubMed.
- X. Han, H. G. W. Godfrey, L. Briggs, A. J. Davies, Y. Cheng, L. L. Daemen, A. M. Sheveleva, F. Tuna, E. J. L. McInnes, J. Sun, C. Drathen, M. W. George, A. J. Ramirez-Cuesta, K. M. Thomas, S. Yang and M. Schröder, Nat. Mater., 2018, 17, 691–696 CrossRef CAS PubMed.
- R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, P. G. Boyd, S. Alavi and T. K. Woo, Science, 2010, 330, 650–653 CrossRef CAS PubMed.
- A. Torrisi, R. G. Bell and C. Mellot-Draznieks, Cryst. Growth Des., 2010, 10, 2839–2841 CrossRef CAS.
- Q. Yang, A. D. Wiersum, P. L. Llewellyn, V. Guillerm, C. Serre and G. Maurin, Chem. Commun., 2011, 47, 9603–9605 RSC.
- A. Torrisi, C. Mellot-Draznieks and R. G. Bell, J. Chem. Phys., 2010, 132, 044705 CrossRef PubMed.
- W. Yang, A. J. Davies, X. Lin, M. Suyetin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, J. E. Parker, C. C. Tang, M. W. George, P. Hubberstey, S. Kitagawa, H. Sakamoto, E. Bichoutskaia, N. R. Champness, S. Yang and M. Schröder, Chem. Sci., 2012, 3, 2993 RSC.
- M. B. Lalonde, R. B. Getman, J. Y. Lee, J. M. Roberts, A. A. Sarjeant, K. A. Scheidt, P. A. Georgiev, J. P. Embs, J. Eckert, O. K. Farha, R. Q. Snurr and J. T. Hupp, CrystEngComm, 2013, 15, 9408–9414 RSC.
- Q. Yang, S. Vaesen, F. Ragon, A. D. Wiersum, D. Wu, A. Lago, T. Devic, C. Martineau, F. Taulelle, P. L. Llewellyn, H. Jobic, C. Zhong, C. Serre, G. De Weireld and G. Maurin, Angew. Chem., Int. Ed., 2013, 52, 10316–10320 CrossRef CAS PubMed.
- D. H. Hong and M. P. Suh, Chem.–Eur. J., 2014, 20, 426–434 CrossRef CAS PubMed.
- Q. Zhang, J. Yu, J. Cai, R. Song, Y. Cui, Y. Yang, B. Chen and G. Qian, Chem. Commun., 2014, 50, 14455–14458 RSC.
- O. Karagiaridi, N. A. Vermeulen, R. C. Klet, T. C. Wang, P. Z. Moghadam, S. S. Al-Juaid, J. F. Stoddart, J. T. Hupp and O. K. Farha, Inorg. Chem., 2015, 54, 1785–1790 CrossRef CAS PubMed.
- X. Liu, Z. Xiao, J. Xu, W. Xu, P. Sang, L. Zhao, H. Zhu, D. Sun and W. Guo, J. Mater. Chem. A, 2016, 4, 13844–13851 RSC.
- P. Rought, C. Marsh, S. Pili, I. P. Silverwood, V. G. Sakai, M. Li, M. S. Brown, S. P. Argent, I. Vitorica-Yrezabal, G. Whitehead, M. R. Warren, S. Yang and M. Schröder, Chem. Sci., 2019, 10, 1492–1499 RSC.
- C. Marsh, X. Han, J. Li, Z. Lu, S. P. Argent, I. da Silva, Y. Cheng, L. L. Daemen, A. J. Ramirez-Cuesta, S. P. Thompson, A. J. Blake, S. Yang and M. Schröder, J. Am. Chem. Soc., 2021, 143, 6586–6592 CrossRef CAS PubMed.
- X. Lv, L. Li, S. Tang, C. Wang and X. Zhao, Chem. Commun., 2014, 50, 6886 RSC.
- S. Gao, C. G. Morris, Z. Lu, Y. Yan, H. G. W. Godfrey, C. Murray, C. C. Tang, K. M. Thomas, S. Yang and M. Schröder, Chem. Mater., 2016, 28, 2331–2340 CrossRef CAS.
- H. Wang, Y. Liu and J. Li, Adv. Mater., 2020, 32, 2002603 CrossRef CAS PubMed.
- H. Fu and J. Zhang, Chem.–Eur. J., 2015, 21, 5700–5703 CrossRef CAS PubMed.
- S. Rahman, A. Arami-Niya, X. Yang, G. Xiao, G. Li and E. F. May, Commun. Chem., 2020, 3, 186 CrossRef CAS PubMed.
- K. Morishige and N. Tateishi, J. Chem. Phys., 2003, 119, 2301–2306 CrossRef CAS.
- H. Zeng, M. Xie, Y. Huang, Y. Zhao, X. Xie, J. Bai, M. Wan, R. Krishna, W. Lu and D. Li, Angew. Chem., Int. Ed., 2019, 58, 8515–8519 CrossRef CAS PubMed.
- P. Hu, J. Hu, M. Zhu, C. Xiong, R. Krishna, D. Zhao and H. Ji, Angew. Chem., Int. Ed., 2023, 62, e202305944 CrossRef CAS PubMed.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121–127 CrossRef CAS.
- N. F. Cessford, N. A. Seaton and T. Düren, Ind. Eng. Chem. Res., 2012, 51, 4911–4921 CrossRef CAS.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed.
- T.-L. Hu, H. Wang, B. Li, R. Krishna, H. Wu, W. Zhou, Y. Zhao, Y. Han, X. Wang, W. Zhu, Z. Yao, S. Xiang and B. Chen, Nat. Commun., 2015, 6, 7328 CrossRef CAS PubMed.
- J. Li, L. Jiang, S. Chen, A. Kirchon, B. Li, Y. Li and H.-C. Zhou, J. Am. Chem. Soc., 2019, 141, 3807–3811 CrossRef CAS PubMed.
- J. Wang, L. Li, L. Guo, Y. Zhao, D. Xie, Z. Zhang, Q. Yang, Y. Yang, Z. Bao and Q. Ren, Chem.–Eur. J., 2019, 25, 15516–15524 CrossRef CAS PubMed.
- Y. Duan, Y. Huang, C. Wang, Q. Wang, K. Ge, Z. Lu, H. Wang, J. Duan, J. Bai and W. Jin, Chem. Sci., 2023, 14, 4605–4611 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2144629 and 2144630. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc00101j |
| This journal is © The Royal Society of Chemistry 2024 |