Open Access Article
Open Access ArticleA novel hydrophobic carborane-hybrid microporous material for reversed C2H6 adsorption and efficient C2H4/C2H6 separation under humid conditions†
Lingyao
Wang‡
a,
Shuangshuang
Wu‡
a,
Jianbo
Hu‡
b,
Yunjia
Jiang
a,
Jiahao
Li
a,
Yongqi
Hu
a,
Yan
Han
a,
Teng
Ben
 c,
Banglin
Chen
c,
Banglin
Chen
 ad and
Yuanbin
Zhang
ad and
Yuanbin
Zhang
 *a
*a
aKey Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, 321004, China. E-mail: ybzhang@zjnu.edu.cn
bZhejiang Lab, Hangzhou, 311100, P. R. China
cInstitute of Advanced Fluorine-Containing Materials, Zhejiang Normal University, Jinhua, 321004, China
dFujian Provincial Key Laboratory of Polymer Materials, College of Chemistry & Materials Science, Fujian Normal University, Fuzhou, 350007, P. R. China
First published on 12th March 2024
Abstract
Since ethylene (C2H4) is important feedstock in the chemical industry, developing economical and energy-efficient adsorption separation techniques based on ethane (C2H6)-selective adsorbents to replace the energy-intensive cryogenic distillation is highly demanded, which however remains a daunting challenge. While previous anionic boron cluster hybrid microporous materials display C2H4-selective features, we herein reported that the incorporation of a neutral para-carborane backbone and aliphatic 1,4-diazabicyclo[2.2.2]octane (DABCO) enables the reversed adsorption of C2H6 over C2H4. The generated carborane-hybrid microporous material ZNU-10 (ZNU = Zhejiang Normal University) is highly stable in humid air and maintains good C2H6/C2H4 separation performance under high humidity. Gas loaded single crystal structure and density-functional theory (DFT) calculations revealed that the weakly polarized carborane and DABCO within ZNU-10 induce more specific C–Hδ+⋯Hδ−–B dihydrogen bonds and other van der Waals interactions with C2H6, while the suitable pore space allows the high C2H6 uptake. Approximately 14.5 L kg−1 of polymer grade C2H4 can be produced from simulated C2H6/C2H4 (v/v 10/90) mixtures under ambient conditions in a single step, comparable to those of many popular materials.
Introduction
Polymer-grade ethylene (C2H4 > 99.9%) is significant feedstock to produce resins and plastics.1 During the production, ethane (C2H6) is the major impurity and needs to be removed to improve the C2H4 quality. The current state-of-the-art technique to separate C2H4/C2H6 depends on cryogenic distillation at high pressure and low temperature.2 The separation process is highly energy-intensive due to the similarity of the boiling points of C2H4 (−88.6 °C) and C2H6 (−103.7 °C) and requires very large distillation towers with 120 to 180 trays and high reflux ratios.3 Therefore, there is a need to develop novel technologies for efficient C2H4/C2H6 separation.4Recently, physisorptive separation using porous solid adsorbents has attracted increasing attention due to its environmental friendliness and reduced energy consumption.5 Porous coordination polymers (PCPs) or metal–organic frameworks (MOFs), as a new class of crystalline porous materials with a high surface area, structural tailorability and controllable properties, have been developed for the separation of C2H4/C2H6 hydrocarbon mixtures.6 Among them, most MOF materials, especially those decorated with polar functional groups or unsaturated metal sites, exhibit the preferential adsorption order of C2H4 > C2H6 based on their difference in physicochemical properties including molecular size, polarity and quadrupole moment (Table S2†).7 However, C2H4-selective MOFs are not the optimal selection for the adsorptive separation of C2H4/C2H6 mixtures. The concentration of C2H4 within the cracked gas mixtures is often over 10 times higher than that of C2H6, thus using C2H4-selective adsorbents for C2H4/C2H6 separation entails more adsorbent consumption and larger space occupation than using C2H6-selective adsorbents.8 Besides, the utilization of C2H4-selective adsorbents usually needs over four adsorption–desorption cycles to attain polymer-grade C2H4 due to the retention of C2H6 amidst adsorbent particles.9 In sharp contrast, using C2H6-selective adsorbents facilitates the direct acquisition of high-purity C2H4 through a single adsorption step, streamlining the separation process and effecting approximately a 40% reduction in energy consumption.10 Hence, the development of C2H6-selective adsorbents is highly imperative for industrial refinement of C2H4. In previous reports, reducing the polarity of pore surfaces in porous materials is promising to afford reversed adsorption of C2H6 over C2H4.11 This phenomenon can be regarded as “like adsorbs like” when compared to the “like dissolves like” principle. However, the construction of C2H6-selective MOFs is still a difficult problem at present.
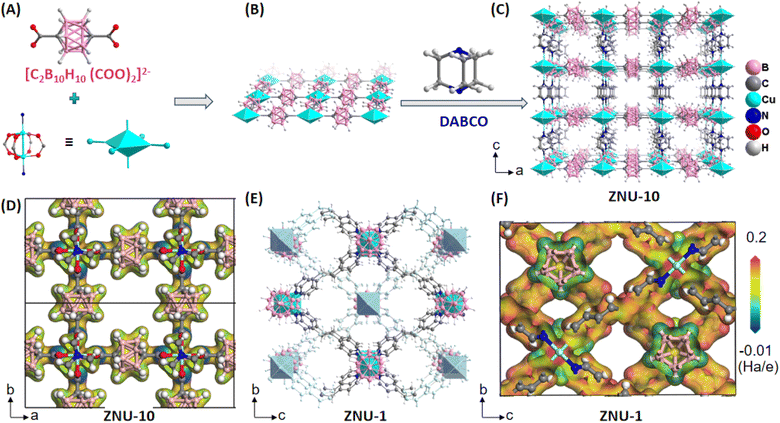
With the above consideration in mind, we envision that three dimensional neutral para-carborane (p-C2B10H12) as a derivative of the [B12H12]2− anion (Scheme 1) can serve as a weakly polarized functional group in MOFs to selectively bind C2H6 over C2H4. Although a plenty of dodecaborate [B12H12]2− anion pillared MOFs have been developed in our group for gas separation with efficient C2H2/CO2 and C2H2/C2H4 splitting performance,12 carborane-based MOFs have been rarely reported,13 and no records of light hydrocarbon separation with carborane hybrid MOFs can be traced. On the other hand, carborane hybrid MOFs seem to display higher water stability when compared to benzene-based MOF analogues, which is a benefit for real applications with moisture.13a,d
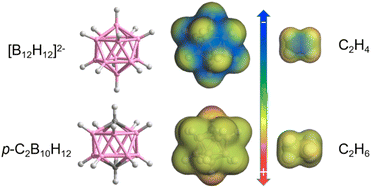 | ||
| Scheme 1 Comparison of the surface electrostatic potential of boron clusters (dodecaborate dianion and p-carborane) and C2 hydrocarbon gases (C2H6/C2H4). | ||
Herein, we reported a novel microporous MOF termed ZNU-10 (ZNU = Zhejiang Normal University) with cluster para-carboranyl dicarboxylic acid (1,12-p-C2B10H10-(COOH)2) and 1,4-diazobicyclo[2.2.2]octane (DABCO) as the backbones. A pillar-layered framework with pcu topology and saturated metal sites is generated when assembled with classical paddle-wheel dicopper(II) SBUs. As a result, these pores with uniform electrostatic potential not only help to reverse the adsorption sequence of C2H6 over C2H4, but also contribute to improving the water stability of the framework due to the hydrophobic surface of the pore channel. Despite its higher affinity towards C2H6, the C2H6 adsorption heat (26.4 kJ mol−1) in ZNU-10 is quite low, allowing its facile regeneration. In situ gas loaded single crystal structures and density-functional theory (DFT) calculations were applied to reveal the adsorption mechanism. In the poorly polarized channels, C2H6 molecules are stacked more closely and feature stronger van der Waals interactions and C–Hδ+⋯Hδ−–B dihydrogen bonding to the framework due to the larger molecular size and polarizability. Experimental breakthrough studies confirmed that one-step acquisition of high-purity C2H4 from C2H4/C2H6 mixtures can be realized with a high production of ca. 14.5 L kg−1.
Results and discussion
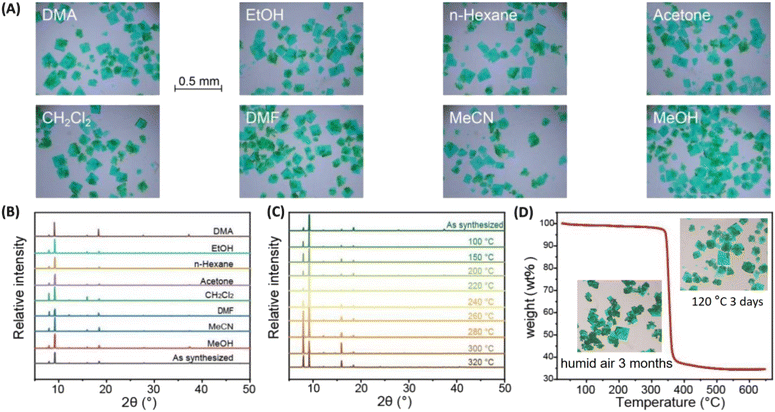
Greenish-blue single crystals of ZNU-10 {Cu(1,12-p-C2B10H10-(COO)2)(DABCO)0.5} were produced by a solvothermal reaction of Cu(NO3)2·3H2O, p-C2B10H10-(COOH)2 and DABCO in a mixed DMF/MeOH/H2O (v/v/v = 2/2/1) solution (Fig. S1†). Single crystal X-ray diffraction (SCXRD) analysis showed that Cu2(COO)4 paddle wheel secondary building units (SBUs) are formed between two Cu2+ and four carboxylate ligands (Fig. 1A). Each SBU paddle wheel is connected by p-carborane ligands to form a two-dimensional (2D) square grid layer (Fig. 1B). The layers are further pillared by the organic DABCO ligands to generate a 3D framework with pcu topology and saturated metal sites (Fig. 1C). The analysis of the surface electrostatic potential of ZNU-10 indicates that ZNU-10 features a uniform and poorly polarized pore surface (Fig. 1D). This is because ZNU-10 consists of a neutral carborane backbone and aliphatic organic building blocks. When compared, ZNU-1 {CuB12H12(dpe)2}12a constructed from −2 charged [B12H12]2− backbones and aromatic ligands displays similar 1D channels but significantly different electrostatic potential on the pore surface. Notable charge separation can be observed on the isosurface of ZNU-1, namely the anionic boron cluster surface displays highly negative electrostatic potential while the organic linker surface displays very positive electrostatic potential (Fig. 1F). As we know, ZNU-1 is one of the best MOFs to selectively capture C2H2 from various mixtures.12a,b Due to the completely different electrostatic potential distribution, ZNU-10 is yet supposed to be a C2H6 selective adsorbent for one-step C2H4 acquisition from C2H4/C2H6 mixtures.Before gas adsorption experiments, we investigated the chemical and thermal stability of ZNU-10 to optimize the activation conditions. We find that ZNU-10 is highly stable in many organic solvents including DMA, EtOH, n-hexane, acetone, CH2Cl2, DMF, MeCN, MeOH and so on, identified by a microphotograph (Fig. 2A) and powder X-ray diffraction (PXRD) (Fig. 2B). Temperature varied PXRD and thermogravimetric analysis (TGA) indicated that ZNU-10 is stable below 320 °C (Fig. 2C and D). Such thermal stability is superior to that of all the boron cluster anion hybrid MOFs and most Cu(II) based MOFs (Fig. S10 and S11†).12 According to the above results, the as-synthesized ZNU-10 was soaked in MeOH for solvent-exchange and activated under 120 °C to fully remove all the guest molecules. Besides, we find that ZNU-10 is highly stable in humid air. The as-synthesized single crystals retained the crystallinity and quality over 3 months under an ambient humid atmosphere or heating at 120 °C for 3 days under vacuum (Fig. 3D, inset), identified by single crystal X-ray analysis.
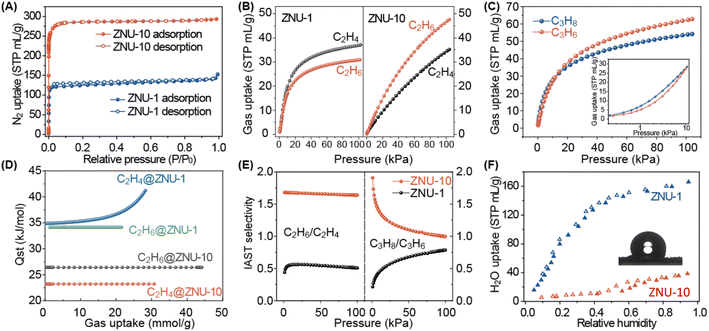
The permanent porosity of ZNU-10 was established by N2 gas adsorption experiments at 77 K. The adsorption isotherm corresponds to the representative type I adsorption isotherm, indicative of its inherent microporous characteristics (Fig. 3A). At P/P0 = 0.95, the N2 adsorption capacity of ZNU-10 was 293 STP cm3 g−1 (13.1 mmol g−1), and the Brunauer–Emmett–Teller (BET) surface area and micropore volume are calculated to be 1171 m2 g−1 and 0.45 cm3 g−1, respectively. The calculated pore size is centered at 8.59 Å (Fig. S13†), which is very close to the pore size of 7.7–10 Å from the single crystal structure evaluation (Fig. S6 and S7†). When compared, ZNU-1 only displays a BET surface area of 532 m2 g−1 and a pore volume of 0.20 cm3 g−1 (Fig. S14†). Therefore, the porosity of ZNU-10 (Fig. S4 and S5†) is significantly increased when compared to that of ZNU-1.
Single component C2H6 and C2H4 adsorption measurements at 298 K were conducted for ZNU-10 and ZNU-1. At 1.0 bar, the C2H6 and C2H4 uptakes on ZNU-10 were 47.6 and 35.1 STP cm3 g−1 (2.13 and 1.57 mmol g−1), respectively (Fig. 3B, right). This uptake trend is opposite to that of ZNU-1 (30.9 and 37.1 STP cm3 g−1 for C2H6 and C2H4) (Fig. 3B, left). With the temperature decreasing to 278 K, the C2H6 and C2H4 uptakes on ZNU-10 were improved to 67.9 and 55.1 cm3 g−1 (Fig. S17 and S18†). The reversed adsorption of C3H8 and C3H6 is also observed in the low pressure region for ZNU-10 (Fig. 3C), which is quite rare in MOFs.14
In order to quantitatively compare the adsorption affinity between the framework and gas molecules, we calculated the adsorption heats (Qst) of ZNU-10 and ZNU-1 using the Clausius–Clapeyron equation after fitting the isotherms to Langmuir equations with excellent accuracy (Tables S3 and S4†). The Qst values of C2H6 and C2H4 for ZNU-10 were 26.4 and 23.2 kJ mol−1, respectively; while those for ZNU-1 were 34.1 and 34.9 kJ mol−1, respectively (Fig. 3D). These Qst values are consistent with the slope of the adsorption isotherms in the low pressure region. Notably, the C2H6 adsorption heat on ZNU-10 is much lower than those in most C2H6-selective materials such as Fe2(O2)(dobdc) (67 kJ mol−1),15 MAF-49 (60 kJ mol−1),16 UiO-NDC (58 kJ mol−1),17 NKMOF-8-Br (40.8 kJ mol−1)18 and FJI-H11-Me (38.9 kJ mol−1),19 and even lower than that of C2H6-disfavored ZNU-1. This extremely low adsorption heat allows the facile regeneration of ZNU-10 for recyclable use.
The separation selectivity of C2H6/C2H4 is further calculated based on ideal adsorption solution theory (IAST). The C2H6/C2H4 selectivity on ZNU-10 is 1.68∼1.64 in the range of 1–100 kPa (Fig. 3E), which is approximately 3.2 times that of ZNU-1 (S = 0.51 at 100 kPa). This C2H6/C2H4 selectivity is also higher than those of many C2H6 selective MOFs such as UiO-NDC (1.35),17 UPC-612 (1.4),20 TJT-100 (1.2),21 LIFM-63 (1.56),22 JNU-2 (1.6)23 and Azole-Th-1 (1.46).24 Besides, the C2H6/C2H4 selectivity on ZNU-10 remains consistent regardless of the C2H6/C2H4 ratio (Fig. S22†). The equimolar C3H8/C3H6 selectivity for ZNU-10 and ZNU-1 from 1 to 100 kPa is calculated to be 1.91–1.00 and 0.21–0.78, respectively, further indicating the opposite affinity of ZNU-10 and ZNU-1 towards olefins and paraffins.
To further explore the pore difference of ZNU-10 and ZNU-1, we performed a water vapor adsorption test. The results showed that at a relative humidity of 0.9, the water vapor adsorption capacity of ZNU-10 was only 35.6 mL g−1, much lower than that of ZNU-1 (ca. 162 mL g−1) under the same conditions (Fig. 3F). These results showed that ZNU-10 is more hydrophobic and has high potential to remove the C2H6 impurity from C2H4/C2H6 mixtures under humid environmental conditions. The water stability is also confirmed by soaking the ZNU-10 crystals in water. Initially, ZNU-10 floated on the surface of water due to its low density (0.933 cm3 g−1) and hydrophobicity. After some time, ZNU-10 crystals sunk to the bottom of water and became blue powders slowly. Nonetheless, the PXRD pattern of the water-soaked ZNU-10 is still consistent with that of the as-synthesized one (Fig. S25†). We also measured the water contact angle of ZNU-10, and the large water contact angle of >114° also indicated the hydrophobic nature of ZNU-10 (Fig. S26†).
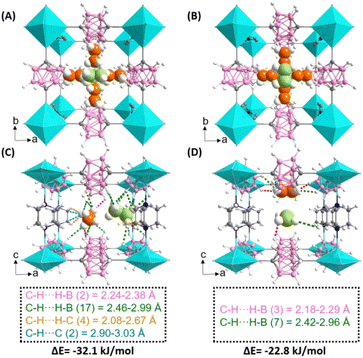
Due to the high stability of ZNU-10 single crystals, the in situ single crystal structure of ZNU-10 with the loading of C2H6 or C2H4 was studied. We found that on average 0.7 C2H6 and 0.55 C2H4 molecules are adsorbed by a carborane unit (Fig. 4A and B), which corresponds to ca. 45 and 35 STP cm3 g−1 of C2H6 and C2H4, respectively. These uptake values are close to the gas uptake from the adsorption isotherms at 1.0 bar. For both gases, two different binding sites are observed. The first site is located close to the window consisting of two carboranes and two DABCO (Fig. S9A and B†). There are four such sites in a single unit cell. The second site is located close to the window consisting of four carboranes (Fig. S9C and D†). There are two such sites in every unit cell. Therefore, six gas molecules can be adsorbed in a unit cell at maximum. However, the single crystal structure analysis indicated that the occupancy of gas molecules at each position is less than one. This could be explained by the low adsorption heats of C2H6 and C2H4 in ZNU-10 and the high symmetry of the framework. Due to the disorder, the contact distance between the gas molecules and the framework is not precise. Nonetheless, more close van der Waals interactions and C–Hδ+⋯Hδ−–B dihydrogen bonds are observed for C2H6 molecules. For example, the closest C–Hδ+⋯Hδ−–B distance between C2H6 and ZNU-10 is 2.10 Å, while that for C2H4 is 2.32 Å.
To gain more insights into the gas adsorption behavior, modeling studies using DFT calculations were further performed. The calculated average bonding energy between ZNU-10 and two gases located at different sites based on the in situ single crystal structures is −32.1 and −22.8 kJ mol−1 for C2H6 and C2H4, respectively (Fig. 4C and D), consistent with the experimental Qst trend. In site I, C2H6 interacts with two carborane backbones by multiple C–H⋯H–B interactions. The contact between C2H6 and DABCO is also observed, with H⋯H distances of 2.08–2.67 Å and H⋯C distances of 2.90–3.03 Å. In site II, there only exist weak C–H⋯H–B interactions with a distance of >2.4 Å. When compared, C2H4 displays slightly shorter contact distances (2.18 and 2.21 Å in site I and 2.29 in site II) but decreased binding energy due to the fact that the overall interactions are fewer. This is consistent with the fact that C2H4 has small molecular size and thus the confinement in the nanospace of ZNU-10 will be weaker.
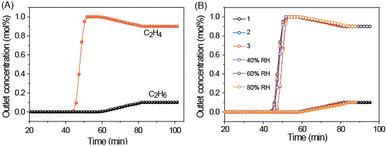
To evaluate the feasibility of ZNU-10 for practical C2H4 purification from binary C2H4/C2H6 mixtures, lab-scale breakthrough experiments were conducted for the C2H4/C2H6 (90/10) mixture to simulate the real separation conditions (Fig. S22†). Fig. 5A shows that high-purity C2H4 (>99.9%) was obtained between 44 and 58 min. The polymer grade C2H4 (>99.9%) productivity is estimated to be as high as 14.5 L kg−1 (647 mmol kg−1), superior to those of NKU-301 (9.5 L kg−1),14 Zr-TCA (6.51 L kg−1),25 MOF-801 (5.73 L kg−1),26 UiO-66 (0.46 L kg−1)25 and many other popular materials under the same conditions.11 Besides, ZNU-10 displays high recyclability and resistance to humidity. The breakthrough time is almost the same for multiple cycles or under highly humid conditions (Fig. 5B). Besides, ZNU-10 can be readily generated under Ar purge at room temperature within 1 h (Fig. S24†), which is consistent with the low adsorption heat of C2H6 and C2H4 in ZNU-10. The regeneration can also be achieved by desorption under vacuum for 1 h. In brief, the combination of high productivity of pure C2H4, good recycling performance and facile regeneration conditions of the material makes ZNU-10 as a potential adsorbent for practical C2H4 purification.
Conclusions
In conclusion, we have reported a novel hydrophobic carborane hybrid metal–organic framework ZNU-10 with reversed C2H6 adsorption properties for efficient capture of C2H6 from C2H4/C2H6 mixtures to produce high-purity C2H4 in a single step. ZNU-10 displays distinct electrostatic potential distribution in the pore surface compared to the analogue cluster boron anion pillared ZNU-1. The difference leads to reversed C2H4/C2H6 hydrocarbon adsorption orders in ZNU-10 and ZNU-1. Notably, ZNU-10 exhibits good chemical/thermal stability with resistance towards water adsorption due to its hydrophobic pore surface. The excellent C2H4/C2H6 separation performance is fully illustrated by breakthrough experiments with good recyclability and capacity retention under high humidity. In general, our work demonstrates the versatility and importance of boron cluster functionalities, which can be tuned to achieve desirable gas adsorption properties. Considering the large library of boron cluster compounds, this work will open a new avenue in boron cluster chemistry for light hydrocarbon separation applications.Data availability
All the data supporting this article have been included in the main text and the ESI.†Author contributions
L. W.: investigation, structural determination and analysis, funding, and supervision; S. W.: synthesis, characterization, and adsorption experiments; J. H.: DFT calculation; Y. J.: investigation and discussion; J. Li: adsorption experiments; Y. H.: breakthrough experiments; Y. H.: breakthrough experiments; T. B.: supervision; B. C.: supervision and funding; Y. Z.: concept, supervision, draft and funding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 22205207, 22378369 and 22008209), Major project of Natural Science Foundation of Zhejiang Province (LD24B060001) and Jinhua Industrial Key Project (No. 2021-1-088).References
- X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M. J. Zaworotko and B. Chen, Science, 2016, 353, 141–144 CrossRef CAS
.
-
(a) D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed
; (b) K. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q. Y. Zhang and M. J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS
.
- Q. Ding, Z. Zhang, C. Yu, P. Zhang, J. Wang, X. Cui, C. H. He, S. Deng and H. Xing, Sci. Adv., 2020, 6, eaaz4322 CrossRef CAS
.
- D. Lv, P. Zhou, J. Xu, S. Tu, F. Xu, J. Yan, H. Xi, W. Yuan, Q. Fu, X. Chen and Q. Xia, Chem. Eng. J., 2022, 431, 133208 CrossRef CAS
.
-
(a) Z. Zhang, L. Li, J.-X. Wang, H.-M. Wen, R. Krishna, H. Wu, W. Zhou, Z.-N. Chen, B. Li, G. Qian and B. Chen, J. Am. Chem. Soc., 2020, 142, 633–640 CrossRef
; (b) H. Yang, Y. Wang, R. Krishna, X. Jia, Y. Wang, A. N. Hong, C. Dang, H. E. Castillo, X. H. Bu and P. Y. Feng, J. Am. Chem. Soc., 2020, 142, 2222–2227 CrossRef CAS
; (c) S.-M. Wang, X.-T. Mu, H.-R. Liu, S.-T. Zheng and Q.-Y. Yang, Angew. Chem., Int. Ed., 2022, 61, e202207066 CrossRef CAS PubMed
; (d) Y. Jiang, L. Wang, T. Yan, J. Hu, W. Sun, R. Krishna, D. Wang, Z. Gu, D. Liu, X. Cui, H. Xing and Y. Zhang, Chem. Sci., 2023, 14, 298–309 RSC
; (e) Y. Jiang, J. Hu, L. Wang, W. Sun, N. Xu, R. Krishna, S. Duttwyler, X. Cui, H. Xing and Y. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202200947 CrossRef CAS
; (f) Y. Gu, J.-J. Zheng, K. Otake, M. Shivanna, S. Sakaki, H. Yoshino, M. Ohba, S. Kawaguchi, Y. Wang, F. Li and S. Kitagawa, Angew. Chem., Int. Ed., 2021, 60, 11688–11694 CrossRef CAS
; (g) Y. Jiang, W. Yang, Y. Zhang, L. Wang and B. Chen, J. Mater. Chem. A, 2024, 12, 5563–5580 RSC
; (h) Y. Zhang, W. Sun, B. Luan, J. Li, D. Luo, Y. Jiang, L. Wang and B. Chen, Angew. Chem., Int. Ed., 2023, 62, e202309925 CrossRef CAS PubMed
.
-
(a) M. Ding, R. W. Flaig, H. L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC
; (b) K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin and M. Eddaoudi, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC
; (c) L. Yang, S. Qian, X. Wang, X. Cui, B. Chen and H. Xing, Chem. Soc. Rev., 2020, 49, 5359–5406 RSC
; (d) L. Yang, L. Yan, W. Niu, Y. Feng, Q. Fu, S. Zhang, Y. Zhang, L. Li, X. Gu, P. Dai, D. Liu, Q. Zhang and X. Zhao, Angew. Chem., Int. Ed., 2022, 61, e202204046 CrossRef CAS PubMed
; (e) L. Wang, N. Xu, Y. Hu, W. Sun, R. Krishna, J. Li, Y. Jiang, S. Duttwyler and Y. Zhang, Nano Res., 2023, 16, 3536–3541 CrossRef CAS
; (f) Z. Bao, J. Wang, Z. Zhang, H. Xing, Q. Yang, Y. Yang, H. Wu, R. Krishna, W. Zhou and B. Chen, Angew. Chem., Int. Ed., 2018, 57, 16020–16025 CrossRef CAS PubMed
; (g) Y. Jiang, Y. Hu, B. Luan, L. Wang, R. Krishna, H. Ni, X. Hu and Y. Zhang, Nat. Commun., 2023, 14, 401 CrossRef PubMed
.
-
(a) E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed
; (b) S. J. Geier, J. A. Mason, E. D. Bloch, W. L. Queen, M. R. Hudson, C. M. Brown and J. R. Long, Chem. Sci., 2013, 4, 2054–2061 RSC
; (c) S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schroder, Nat. Chem., 2015, 7, 121–129 CrossRef CAS
; (d) S. Tu, D. Lin, J. Huang, L. Yu, Z. Liu, Z. Li and Q. Xia, Microporous Mesoporous Mater., 2023, 354, 112532 CrossRef CAS
; (e) R.-B. Lin, L. Li, H.-L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS PubMed
.
-
(a) D. Lv, R. Shi, Y. Chen, Y. Wu, H. Wu, H. Xi, Q. Xia and Z. Li, ACS Appl. Mater. Interfaces, 2018, 10, 8366–8373 CrossRef CAS PubMed
; (b) Y. Wang, S. Yuan, Z. Hu, T. Kundu, J. Zhang, S. B. Peh, Y. Cheng, J. Dong, D. Yuan, H.-C. Zhou and D. Zhao, ACS Sustain. Chem. Eng., 2019, 7, 7118–7126 CrossRef CAS
.
-
(a) X.-W. Gu, J. Pei, K. Shao, H.-M. Wen, B. Li and G. Qian, ACS Appl. Mater. Interfaces, 2021, 13, 18792–18799 CrossRef CAS PubMed
; (b) R.-B. Lin, H. Wu, L. Li, X.-L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS
.
- Y. P. Li, Y. N. Zhao, S. N. Li, D. Q. Yuan, Y. C. Jiang, X. Bu, M. C. Hu and Q. G. Zhai, Adv. Sci., 2021, 8, 2003141 CrossRef CAS
.
-
(a) G.-D. Wang, K. Rajamani, Y.-Z. Li, W.-J. Shi, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202213015 CrossRef CAS PubMed
; (b) G.-D. Wang, J. Chen, Y.-Z. Li, L. Hou, Y.-Y. Wang and Z. Zhu, Chem. Eng. J., 2022, 433, 133786 CrossRef CAS
; (c) Y. Ye, Y. Xie, Y. Shi, L. Gong, J. Phipps, A. M. Al-Enizi, A. Nafady, B. Chen and S. Ma, Angew. Chem., Int. Ed., 2023, 62, e202302564 CrossRef CAS PubMed
; (d) S.-Q. Yang, F.-Z. Sun, P. Liu, L. Li, R. Krishna, Y.-H. Zhang, Q. Li, L. Zhou and T.-L. Hu, ACS Appl. Mater. Interfaces, 2020, 13, 962969 Search PubMed
; (e) X. Zhang, J. X. Wang, L. Li, J. Pei, R. Krishna, H. Wu, W. Zhou, G. Qian, B. Chen and B. Li, Angew. Chem., Int. Ed., 2021, 133, 10392–10398 CrossRef
.
-
(a) L. Wang, W. Sun, Y. Zhang, N. Xu, R. Krishna, J. Hu, Y. Jiang, Y. He and H. Xing, Angew. Chem., Int. Ed., 2021, 60, 22865–22870 CrossRef CAS PubMed
; (b) W. Sun, J. Hu, S. Duttwyler, L. Wang, R. Krishna and Y. Zhang, Sep. Purif. Technol., 2022, 283, 120220 CrossRef CAS
; (c) Y. Zhang, J. Hu, R. Krishna, L. Wang, L. Yang, X. Cui, S. Duttwyler and H. Xing, Angew. Chem., Int. Ed., 2020, 59, 17664–17669 CrossRef CAS PubMed
; (d) Y. Zhang, L. Yang, L. Wang, X. Cui and H. Xing, Angew. Chem., Int. Ed., 2019, 58, 8145–8150 CrossRef CAS PubMed
; (e) Y. Zhang, L. Yang, L. Wang, X. Cui and H. Xing, J. Mater. Chem. A, 2019, 7, 27560–27566 RSC
; (f) Y. Zhang, L. Wang, J. Hu, X. Cui and H. Xing, CrystEngComm, 2020, 22, 2649–2655 RSC
; (g) W. Sun, Y. Jin, Y. Wu, W. Lou, Y. Yuan, S. Duttwyler, L. Wang and Y. Zhang, Inorg. Chem. Front., 2022, 9, 5140–5147 RSC
; (h) L. Wang, W. Sun, S. Duttwyler and Y. Zhang, J. Solid State Chem., 2021, 299, 1221167 Search PubMed
.
-
(a) L. Gan, A. Chidambaram, P. G. Fonquernie, M. E. Light, D. Choquesillo-Lazarte, H. Huang, E. Solano, J. Fraile, C. Viñas, F. Teixidor, J. A. R. Navarro, K. C. Stylianou and J. G. Plansa, J. Am. Chem. Soc., 2020, 142, 8299–8311 CrossRef CAS PubMed
; (b) L. K. Macreadie, K. B. Idrees, C. S. Smoljan and O. K. Farha, Angew. Chem., Int. Ed., 2023, 62, e202304094 CrossRef CAS PubMed
; (c) K. B. Idrees, K. O. Kirlikovali, C. Setter, H. Xie, H. Brand, B. Lal, F. Sha, C. S. Smoljan, X. Wang, T. Islamoglu, L. K. Macreadie and O. K. Farha, J. Am. Chem. Soc., 2023, 145, 23433–23441 CrossRef CAS PubMed
; (d) Z. Li, D. Choquesillo-Lazarte, J. Fraile, C. Viñas, F. Teixidor and J. G. Planas, Dalton Trans., 2022, 51, 1137 RSC
.
-
(a) X. Lian, P.-X. Liu, Y.-C. Yuan, J.-J. Pang, L. Li, S.-S. Liu, B. Yue, Y.-H. Zhang, L. Li, J. Xu and X.-H. Bu, Adv. Funct. Mater., 2024, 34, 2312150 CrossRef CAS
; (b) P. Zhang, L. Yang, X. Liu, J. Wang, X. Suo, L. Chen, X. Cui and H. Xing, Nat. Commun., 2022, 13, 4928 CrossRef CAS PubMed
.
- L. Li, L. Guo, D. Olson, S. Xian, Z. Zhang, Q. Yang, K. Wu, Y. Yang, Z. Bao, Q. Ren and J. Li, Science, 2022, 377, 335–339 CrossRef CAS PubMed
.
- P.-Q. Liao, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2015, 6, 8697 CrossRef PubMed
.
- J. Pires, J. Fernandes, K. Dedecker, J. R. B. Gomes, G. Pérez-Sánchez, F. Nouar, C. Serre and M. L. Pinto, ACS Appl. Mater. Interfaces, 2019, 11, 27410–27421 CrossRef CAS PubMed
.
- S. Geng, E. Lin, X. Li, W. Liu, T. Wang, Z. Wang, D. Sensharma, S. Darwish, Y. H. Andaloussi, T. Pham, P. Cheng, M. J. Zaworotko, Y. Chen and Z. Zhang, J. Am. Chem. Soc., 2021, 143, 8654–8660 CrossRef CAS
.
- Z. Di, C. Liu, J. Pang, S. Zou, Z. Ji, F. Hu, C. Chen, D. Yuan, M. Hong and M. Wu, Angew. Chem., Int. Ed., 2022, 61, e202210343 CrossRef CAS PubMed
.
- Y. Wang, C. Hao, W. Fan, M. Fu, X. Wang, Z. Wang, L. Zhu, Y. Li, X. Lu, F. Dai, Z. Kang, R. Wang, W. Guo, S. Hu and D. Sun, Angew. Chem., Int. Ed., 2021, 60, 11350–11358 CrossRef CAS
.
- H.-G. Hao, Y.-F. Zhao, D.-M. Chen, J.-M. Yu, K. Tan, S. Ma, Y. Chabal, Z.-M. Zhang, J.-M. Dou, Z.-H. Xiao, G. Day, H.-C. Zhou and T.-B. Lu, Angew. Chem., Int. Ed., 2018, 57, 16067–16071 CrossRef CAS PubMed
.
- C.-X. Chen, Z.-W. Wei, T. Pham, P. C. Lan, L. Zhang, K. A. Forrest, S. Chen, A. M. Al-Enizi, A. Nafady, C.-Y. Su and S. Ma, Angew. Chem., Int. Ed., 2021, 60, 9680–9685 CrossRef CAS PubMed
.
- H. Zeng, X. J. Xie, M. Xie, Y. L. Huang, D. Luo, T. Wang, Y. Zhao, W. Lu and D. Li, J. Am. Chem. Soc., 2019, 141, 20390–20396 CrossRef CAS PubMed
.
- Z. Xu, X. Xiong, J. Xiong, R. Krishna, L. Li, Y. Fan, F. Luo and B. Chen, Nat. Commun., 2020, 11, 3163 CrossRef CAS PubMed
.
- Y. Liu, H. Xiong, J. Chen, S. Chen, Z. Zhou, Z. Zeng, S. Deng and J. Wang, Chin. J. Chem. Eng., 2023, 59, 9–15 CrossRef
.
- F. Xie, J. Liu, W. Graham, S. Ullah, E. M. C. Morales, K. Tan, T. Thonhauser, H. Wang and J. Li, Chem. Eng. J., 2023, 473, 145096 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2287884, 2287885, 2287887 and 2287888. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc00424h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |