Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Children's emergent mechanistic reasoning in chemistry: a case study about early primary students’ reasoning about the phenomenon of thermal expansion of air
Astrid
Berg
 * and
Magnus
Hultén
* and
Magnus
Hultén

Department of Behavioural Science and Learning, University of Linköping, Sweden. E-mail: astrid.berg@liu.se
First published on 13th September 2023
Abstract
The importance of introducing students to mechanistic reasoning (MR) early in their schooling is emphasised in research. The goal of this case study was to contribute with knowledge on how early primary students’ (9–10 year-olds) MR in chemistry is expressed and developed in a classroom practice framed by model-based inquiry. The study focuses on the first lesson in a sequence of six that was developed as part of a design study. The teaching was designed to ensure student agency and create conditions for the students to develop, test, and evaluate simple particle models in interaction with observations cooperatively and under teacher guidance. During the lesson, students were encouraged to express their tentative explanatory models in drawing and writing, and to act as molecules to dramatize the expansion of air. A mechanistic reasoning framework based on the characterisation of system components (entities, properties, activities, organisation) was developed and used to analyse children's mechanistic reasoning. The framework included multimodal analysis of communication (speech, gestures, writing, drawing, bodily motion) and evaluation of student reasoning based on e.g., the presence of gaps in terms of explanatory black boxes or missing pieces. The results show that: (1) In model-based inquiry, young children can navigate across different representational levels in their reasoning and engage in MR; (2) children's black-boxing can be seen as an indication of epistemic work in the process of model-based inquiry; and (3) asking students to engage in multiple modes of representations support the development of student MR in model-based inquiry.
Introduction
Mechanistic reasoning (MR) focuses on the underlying, and often invisible, mechanisms that cause a phenomenon. It is characterised by hypothetical models of the mechanism being formed and then tested, evaluated, and revised against observations of the phenomenon. In this way, explanations are produced for how and why the phenomenon arises and changes (Machamer et al., 2000, Russ et al., 2008).Although early primary curricula typically focus on descriptions rather than explanations of scientific phenomena, research from the last two decades shows that from a young age, students have the ability to think in terms of mechanisms (e.g., Metz, 1991; Hammer, 2004; Buchanan and Sobel, 2011; Samarapungavan et al., 2017; Kelemen, 2019; Betz and Keil, 2021). Moreover, children seem to recall mechanistic explanations more easily than non-mechanistic ones (Betz and Keil, 2021; Kurkul et al., 2021), and when taught mechanistic explanations become more interested in science (Haeusler and Donovan, 2020). Consequently, researchers emphasise the importance of introducing students to MR early in their schooling (e.g., Russ et al., 2008; Windschitl et al., 2008; Bolger et al., 2012; Samarapungavan et al., 2017; Kelemen, 2019; Moreira et al., 2019; Betz and Keil, 2021; Kurkul et al., 2021). Also, important to note is that research has shown that children are able to form a conceptual framework about atoms and molecules at an early age (9 year-olds in Haeusler and Donovan, 2020). Samarapungavan et al. (2017) make an argument for introducing simple particle models in early years, proposing that “children's macroscopic and (sub-)microscopic concepts of matter should be viewed as co-developing rather than sequential” (p. 992).
However, we still need more knowledge on how young children's MR is expressed and develops in actual classroom practices, as part of students observing phenomena and, guided by the teacher, collaboratively develop explanatory models of these (see, e.g., Russ et al., 2008; 2009; Windschitl et al., 2008). We refer to such practice as model-based inquiry (see Windschitl et al., 2008). In response to this need, a design study was performed in which a teaching sequence of six 60 minute lessons was developed, where children, guided by a teacher, were to explore and explain their observations of a chemical phenomenon by developing a simple particle model (Hultén et al., 2020). The sequence was developed in cooperation between educational researchers and teachers. In this study, we analyse data that was collected during the first lesson to gain more knowledge of young children's first acquaintance with this type of reasoning in chemistry. The study was guided by the following research question:
1. What characterises children's emergent mechanistic reasoning in a classroom practice framed by model-based inquiry?
Students’ mechanistic reasoning in chemistry
Most previous studies on students’ MR in chemistry have focused on university students (see Becker et al., 2016; Caspari et al., 2018; Caspari and Graulich, 2019; Crandell et al., 2019; Keiner and Graulich, 2020). There are a few studies on upper secondary education (Moreira et al., 2019), lower secondary education (De Andrade et al., 2022), and primary education, grade 6 students (Krist et al., 2019) and grade 3 students (Samarapungavan et al., 2017). This means that although there is a recent and growing body of research on students’ MR, research on children as young as those in focus in this study (grade 3) is still scarce.Another characteristic of previous studies on students’ MR in chemistry is that most of them do not study how students' MR develops in a classroom practice. Rather, previous studies analyse students’ reasoning in test-like situations, thus testing their existing knowledge (e.g., Caspari et al., 2018; Crandell et al., 2019). Hence, this means that most studies are not studying students’ MR as it evolves in a knowledge-building process.
At upper primary level, Krist et al. (2019) studied 1837 sixth-grade students’ answers to tasks where they were required to explain phenomena in chemistry and biology, respectively. The aim of their study was to develop a framework for identifying and characterising students’ MR in science in general. The framework resulted in three epistemic heuristics: considering the level below the target phenomenon; differentiating between factors at the lower level; and linking lower-level interactions and behaviours to the target phenomenon. Even though Krist et al. (2019) give examples of students’ MR in chemistry, analysed through the lens of their framework, they only offer snapshots of what students’ MR in chemistry might look like. An example of highly complex student responses explaining chemical phenomena (the making of chocolate covered strawberries) is given. Student G writes:
The molecules cooled. When the molecules were hot they slid past each other and could take the shape of their container. But when they cooled, the molecules got together and started to vibrate. The liquid chocolate got hard and it cooled into a solid. (Krist et al., 2019, p. 187–188. Bold and cursive in original, indicating aspects of their coding).
In the above written explanation from Student G, the student identifies the change in temperature as a causal factor, identifies the process as a phase change and links the behaviours of the chocolate molecules to the change in temperature. It should be stressed that these students were three grade levels above the students in this study.
Regarding primary students, Samarapungavan et al. (2017) studied how students in grade 3 used simple particle models to describe and explain a variety of material phenomena. They developed what they call Modelling in the Primary Grades (MPG) lessons, a similar instruction design as the one used in the present study. Samarapungavan et al.'s (2017) focus was not to analyse MR, but their results give some insights of relevance for the present study. Also, their study used interviews to inquire into students’ explanations of material phenomena, and thus did not directly analyse students’ talk in classroom activities. During the fifteen 45 to 60 minute lessons, Samarapungavan et al. (2017, p. 1016) found that most of the children learned “to construct simple particle models that account for the behaviours and properties of matter in varied states or phases, and to explain phase changes such as melting and freezing”. The results in their study focus on quantified presentations of students' models of matter. However, one example of a more advanced explanation is given in their coding and analysis section, where the student Robbie says:
“The ice melts to water ‘cause it's hot.” He said, “Ice is solid. Water's liquid. Droplets of water move about and go all over when they get hot. You can’t see each one. They are moving faster. They are coming down and spreading. They break up and move around.” (Samarapungavan et al., 2017, p. 1006)
The above example shows that Robbie expresses an example of the category Microscopic Particles code. Thus, clearly students in their study, after having had fifteen 45 to 60 minute lessons, were able to conceptualise changes of properties of particles at a submicroscopic level and linked these to changes at the macroscopic level.
To summarise, research on primary students’ MR is scarce, and results regarding students’ MR are only presented at a general level, lacking detail in how young students approach the task of describing and explaining material phenomena. Also, the two examples given above are not examples of students learning about mechanisms in classroom practices, but students recollecting knowledge they have been taught in an interview (Robbie) or in a written task (Student G).
Prior research on MR stresses the importance of considering representational (or modal) aspects both in analysis of student MR and in teaching MR. Moreira et al. (2019) and Keiner and Graulich (2020) include a comparison between student MR as expressed in two different modes: writing and drawings. Bechtel and Abrahamsen (2005) emphasise that scientists use physical models and images when the limit for the mental animation of a mechanism is reached. They also point out that scientists prefer images of mechanisms, because they invite simulations of activities in the system in ways that words cannot. Becker et al.'s (2016) interview study with chemistry students is a telling example: although most students could only describe limited details of the mechanism (electrical attraction occurs between neutral atoms), their representations in the form of drawings were more sophisticated than their explanations in words (spoken or written). Recently, De Andrade et al. (2022) have explored drawing as a tool to promote MR. Their study follows two grade 9 students who attended a three-day workshop on chemical changes led by two of the authors. Data were drawn from the first 90 minutes of the workshop, where the two students worked together to represent a chemical phenomenon at the submicroscopic level via a drawing. The authors conclude that drawing, in terms of allowing direct perception of the entities involved, helped students to imagine and reason about aspects of the properties and organisation of entities. Also Sjøberg et al. (2023) show how drawings play an important role in undergraduate biology students' model-based reasoning process. For example, by displaying spatial relationships the drawing enabled the students to represent how the molecular process led to the observable phenomenon.
Several studies show that students spontaneously use gestures to visualise dynamic and organisational aspects of mechanisms, thus suggesting that gestures are important sensemaking and communicative resources for students MR (Russ et al., 2008; Kang and Tversky, 2016; Mathayas et al., 2019; de Andrade et al., 2022; Sjøberg et al., 2023). Although gestures have similar affordances as drawing in displaying organisational aspects, gestures can be used in a more dynamic fashion. This explains the key role of gestures for exploring e.g., molecular interactions (Mathayas et al., 2019; Sjøberg et al., 2023). Gestures are also well known for connecting students to their learning environment. Roth and Lawless (2002) describe that when students construct verbal expressions of abstract concepts and are near available artefacts such as experimental equipment, they enact representational gestures of the abstract against this perceptual ground. Consequently, this makes certain aspects become salient and, thus, not needed to be put into words. This is similar to the findings of Berg et al. (2019) although the students in this case enacted gestures against 2D-representations of the experimental equipment. Also, drawings of the submicro level may support mechanistic reasoning in a similar manner. The students in de Andrade et al. (2022) combined talk with gestures on their drawings to convey their ideas, and the authors conclude that this “made their conversation highly contextual and fluent with minimal verbal resources “thus helping the students to express more ideas (p. 221).
Given this review, there are to our knowledge no studies of how MR unfolds in classroom practice, either at the primary or secondary level, or at the university level.
Methods
Participants and empirical data
This qualitative study is part of a larger project financed by the Swedish Institute for Educational Research (Grant number 2016/148). The aim of the project is to explore benefits and challenges with the use of digital tools, animation and more traditional experiments in science teaching, more specifically in relation to the particulate nature of matter in early primary chemistry education (see Hultén et al., 2020).The empirical material for this study comes from a series of six lesson designed to create conditions for students to cooperatively, and with guidance from the teacher, develop, test, and evaluate simple particle models to explain observations of a phenomenon (cf.Samarapungavan et al., 2017; also see Talanquer, 2018a). The participants in this study were one teacher and her 11 students in grade3 in a school situated in a small town in Sweden. The language of instruction was Swedish. The teacher was an experienced primary teacher (30 years in the profession) who had served at the school in question for seven years. The students were between nine and ten years old. The overall planning, as well as the ongoing revision, of the lessons was made in co-operation between the teacher and the researchers. The teacher alone carried out the teaching.
In Sweden, science is taught from grade 1. The national curriculum at the time of the study specifies teaching at the phenomenal level for grades 1–3, “Basic properties of air and how they can be observed” (Skolverket, 2018, p. 190 – for 1–3), while introducing a particulate model of nature for grade 4–6: “Simple particle model to describe and explain the structure, recycling and indestructibility of matter. Movements of particles as an explanation for transitions between solids, liquids and gases” (Skolverket, 2018, p. 190 – för 4–6). And more advanced particle models for grades 7–9: “Particle models to describe and explain the properties of phases, phase transitions and distribution processes for matter in air, water and the ground” (Skolverket, 2018, p. 191 – for 7–9).
The lessons were video- and audio recorded to capture students' verbal and nonverbal interactions with each other and with the teacher, as well as with different resources (Derry et al., 2010). In the classroom, students were placed in three groups of three to four students each, and video cameras and dictaphones followed each group. One of these cameras also followed discussions and activities conducted as a class. The physical representations (e.g., texts and physical models) created by the students were documented photographically.
The data for the present study comes from the first of the six lessons. The reason for focusing on the first lesson is that it provides an interesting and – we believe – relevant example of how a simple particle model can be introduced to children aged 9 to 10. While the other five lessons also were centred around relevant teaching activities for promoting explanations of a phenomena using a particulate model of matter, they do not display a straightforward and systematic account of how this type of teaching could be designed as a couple of lessons were more explorative in nature, which makes it harder to follow the development of the students reasoning (see Hultén et al., 2020). Adding to this difficulty is that the constellations in the student groups changed over time, and that most students were absent at least one of the lessons. Also, in studying the six lessons, we found that the student reasoning showed remarkable development already during the first lesson, thus giving a vivid account of how much could be done already with little teaching time at disposal.
From the first lesson we chose to specifically focus on one of the three groups, consisting of the students George, Gordon, and Greta. This group was chosen because it was the most verbally active during group- and whole-class discussions, and thus provided us with the most information about their reasoning. According to the teacher, these students were not among the high achievers in science – rather the opposite. In addition, their overall achievements at the end of the six lessons did not stand out but were representative of the class. As this is a qualitative study, aiming at exploring children's emerging mechanistic reasoning in young children, a detailed qualitative analysis was deemed appropriate. As Guba and Lincoln (1994) argues, qualitative studies can provide important contextual information often lost in quantitative studies and can give important insights into human behaviour. Even though larger quantitative studies will be needed to assess what is possible on a larger scale, the concrete examples analysed and discussed in this study can provide insights on the individual level necessary for further developing both analytical frameworks and necessary instructional strategies needed for large scale studies. By focusing on a low-achieving group we believe that the results may show what is feasible for more students than the three students in this case study.
Ethical considerations
Ethical approval from the Swedish Ethical Review Authority was not required in the context of the project. No sensitive personal data was collected. However, the ethical guidelines of the Swedish Research Council (SRC) guided the planning, implementation, and reporting of the project (Swedish Research Council, 2017). The children and their parents were provided with an information letter about the project and a letter of consent, according to the school's established guidelines. The information letter included a description of the project (its purpose), how the data would be collected (video and voice) and used by the researchers, how it would be stored (security cabinet), and who would have access to it (only members of the research group). The letter also included information about how the results of the project would be reported (scientific papers). In the letter, the participants were also informed that their participation was voluntary and that they could withdraw their participation at any time. Finally, the letter also provided information about how to get in contact with the project manager (telephone and email). Teachers were provided with oral information as well as an information letter and a letter of consent. The content of the information letter was similar to that described above. Letters of consent were collected according to the school guidelines. All participants surveyed agreed to participation. Two researchers were present in the classroom. They took a passive role and did not participate in the teaching as such. All participants have been assigned new names, to ensure their anonymity.Outline of the lesson
Four researchers, the principal of the school, and the teacher collaborated to design and implement a series of six lessons focusing on the phenomenon of air expansion (for more details about the collaboration, see Hultén et al., 2020). The teacher and the research group continuously talked about the experiences gained during the lessons, including how students could be challenged in their reasoning by the teacher through asking challenging follow-up questions that force students to progress in their reasoning. During the lessons, conduction and observation of simple experiments were interspersed with conversations in small groups and as a whole class, and various forms of text and image creation with the aim of describing and formulating tentative explanations of the phenomenon at the submicroscopic level. Students used computer tablets to document the experiments using the camera function. The students were also instructed to express their tentative explanatory models in drawings and writing, and to cooperatively create a stop-motion animation that explained the observed phenomenon. The students used paper, pencils, and crayons. They also had access to clay, beads, and small building blocks that could also be integrated into the texts. The students themselves chose how they would design their models.The overall instruction design was based on the principles of learning activity (LA) (Davydov, 2008; Eriksson and Jansson, 2017). The main principle in LA is that students are invited to participate in collective activities centred around theoretical knowledge content where they experience a need for additional knowledge. In practice, this means that the teacher challenges the ideas that the students present as explanations for the phenomenon, rather than correcting or evaluating them in terms of right and wrong. In other words, students are positioned as epistemic agents (Eriksson and Lindberg, 2016; Eriksson et al., 2021). The structure of the lesson series had the teacher guide the students to cooperatively develop a simple particle model. This took place through direct interaction with the observations where the teacher encouraged the students to try different explanations for the phenomenon. The teacher propelled the process forwards by challenging and questioning the students' explanations, as well as by pitting them against each other, all based on the implicit question: “What kind of process at the submicroscopic level could reasonably explain what we observe?” This way of enabling students to develop agency is described as especially important in practices focusing on MR for the very youngest students (Russ et al. 2009). Importantly, a teaching practice where students collaboratively develop a theoretical model in interaction with observations also mimics science's own knowledge-building practice (cf.Duschl and Grandy, 2008; Schwarz et al., 2017; Windschitl et al., 2012).
This lesson included several different teaching activities, beginning with students, in groups of three, producing an experiment with a glass bottle and a balloon. Since the bottle needs to be cold, the students had been instructed to put the bottles in a freezer the day before the lesson. The freezer was situated in a room next to the classroom. At the start of the lesson, the groups were asked to retrieve one bottle each from the freezer. They were then instructed to put a balloon over the bottle's neck, wait and observe what happened to the balloon while also documenting their observations (video and/or photographs). The instruction to document the evolving phenomenon aimed to reinforce and preserve the experience (Roth and Lawless, 2002). The balloon would begin to grow as the bottle became warmer. It was clear that the children were fascinated by the experiment, and that some also knew what was going to happen, but that they did not understand what was happening. The observed phenomenon might be considered what Cheng et al. (2020) characterise as a “puzzling phenomenon”. Using an experiment to stage a puzzling phenomenon can serve as a way of getting students engaged in really trying to understand how said phenomenon can arise.
After observing what happened to the bottle and the balloon at room temperature, students conducted a new experiment, where they poured hot water on the glass bottle and observed the balloon again. While doing this, the concept of a molecule was introduced by one of the students in discussion with the teacher, who in turn took the opportunity to introduce and discuss the concept with the whole class. To visualise and let the students experience with their senses the fact that the higher the speed of the molecules, the more space they take up, the teacher then directed a “molecule dance” where the students acted as molecules. The lesson ended with students creating individual representations (drawings accompanied by text) of their tentative explanations of what they had observed.
Analytical framework
Several frameworks have been developed for analysing students' MR in chemistry education. These frameworks vary in terms of character and purpose. We will use a type of framework that Dood and Watts (2022) call philosophy of science frameworks, as they depart from philosophical work on the concept of mechanism in science. Fundamental to these frameworks is Russ et al.'s (2008) adaptation of Machamer et al.'s (2000) work on mechanisms in science. In these frameworks, a mechanistic explanation will be based on the identification of relevant entities (E) of the system, their properties (P), the activity (A) in which they are engaged, and how they are spatiotemporally organised (O) during activities. The activities and the organisation of the entities are causally responsible for the properties and behaviours of the system (Machamer et al., 2000; Russ et al., 2008). Importantly, “the mechanistic piece of a causal mechanistic explanation must have defined underlying objects or entities that are at least one scalar level below the phenomenon of interest” (Crandell et al., 2019, p. 214).Among the philosophy of science frameworks, the ones developed by Moreira et al. (2019) and Keiner and Graulich (2020) are especially interesting. Both these studies refine and adapt Russ et al.'s (2008) framework for analysis of MR in chemistry specifically. A comparative overview of the frameworks of Russ et al. (2008), Moreira et al. (2019) and Keiner and Graulich (2020) is presented in Table 1.
| MR framework | Discernible steps in the framework | |||
|---|---|---|---|---|
| Identify core mechanistic features | Characterise students’ connections and transitions among the features on one or several representational levels | Build explanatory approaches or reasoning diagrams | Evaluate explanatory approaches/reasoning diagrams | |
| X: the framework includes this step in the analysis.—: the framework does not include this step in the analysis. | ||||
| Russ et al. (2008) | X (nine categories) | — | — | X |
| Moreira et al. (2019) | X (Part A of the analysis) | — | X (first step of Part B of the analysis) | X (second step of Part B of the analysis) |
| Keiner and Graulich (2020) | X (step I) | X (step II) | X (step III) | – (discussed, but not included in the framework – potential step IV) |
In this study we will take our starting point in Keiner and Graulich's (2020) three-step procedure. Step I is dedicated to identifying the features of a mechanism present in the students’ explanations. In line with Moreira et al. (2019), they identify four “core mechanistic features” (p. 471): entities, properties, activities, and organisation. Step II of Keiner and Graulich's (2020) analysis concerns the characterisation of students’ connections and transitions among the features on one or several representational levels. In step III, they build what they call explanatory approaches, in which they visualise the identified features, connections, and transitions. We have made three modifications of Keiner and Graluich's framework as described below.
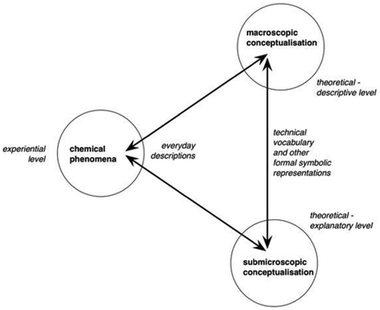 | ||
| Fig. 1 Taber's (2013) reconceptualized model of the chemistry triplet. | ||
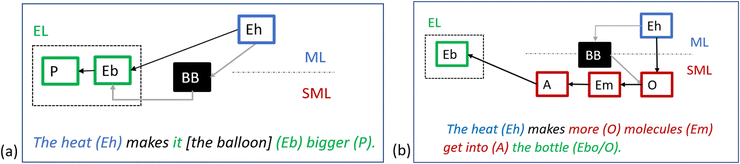
Regarding the first modification, Keiner and Graulich's (2020) use of Johnstone's (1991) chemistry triplet means that they do not separate the two possible foci for observational descriptions. Taber (2013) describes these foci as both observational descriptions of the direct experience of the phenomenon using everyday language (experiential level; e.g., the water disappears), and conceptualisation of these in relation to a macroscopic framework of theoretical concepts (macroscopic level; e.g., the water evaporates). Taber's (2013) revised model will allow us to follow children's reasoning from the direct experience of the phenomenon—the experiential level—via conceptualisation at the macroscopic level all the way to the submicroscopic level.
The second modification concerns the application of a multimodal analysis of communication (Selander, 2008; Kress, 2010). The basis of multimodal theory is that all communication is multimodal, i.e., uses a combination of different modes in communicating meaning, where examples of modes are “image, writing, layout, music, gesture, speech, moving image, soundtrack and 3D objects” (Kress, 2010, p. 79). Exactly how to define a mode may vary according to social and cultural context and the purpose of the study. For example, regarding writing, we may discern different typologies used as one separate mode of writing, and when it comes to drawings, these always contain a lot of culturally developed conventions such as use of special signs, colours, etc. For this study, we will distinguish between the following modes: speech, writing, gestures, drawings and bodily motion. As regards gestures, we focus on and will distinguish between deictic and iconic gestures, where deictic “are used in concrete or abstract pointing” and iconic “bear perceptual similarities to concrete entities, processes, or events” (Sjøberg et al., 2023, p. 9). Even though these different modes will be analysed separately, the interplay between modes is of interest, i.e., how different modes interact, complement each other, and change in children's MR over the course of the lesson (cf.Sjøberg et al., 2023). Also, modes often appear in ensembles, with different modal affordances (the potentialities and constraints of different modes in relation to communication in a specific context), and multimodal orchestration (how different modes are combined in communication in order to make meaning) (Kress, 2010. Also see Danielsson et al., 2023).
As regards the third and final modification – the evaluation of student reasoning (a fourth step in the framework) – neither of the frameworks in Table 1 treats this fourth step in an exhaustive manner. From the literature review we find that the following aspects are also important to consider in order to evaluate student reasoning:
The different types of features identified by the students: Moreira et al. (2019) conclude that reasoning in terms of activities and organisation requires higher abstraction, while it is easier for students to identify and reason about entities and their properties. This compares to Keiner and Graulich (2020) who showed that entity was the most frequently used feature in the students' reasoning, while reference to the activities, as well as properties, of entities was rare. However, Russ et al.'s (2008) rate property as a more scientifically sophisticated feature than activity.
The representational level at which the identified features of the mechanism are conceptualised: Describing the phenomenon at the experiential level is probably not as challenging as conceptualising it at the submicroscopic or macroscopic level (cf.Russ et al., 2008). In contrast to the experiential level, the macroscopic level signals conceptualisation of what is observed (Taber, 2013); i.e., “seeing” something as something specifically related to a framework of macroscopic theoretical concepts (Berg et al., 2019).
Transition between representational levels: Mechanistic explanations of chemical phenomena include a description of the mechanism at the submicroscopic level and how it gives rise to the observations at the experiential level. Keiner and Graulich (2020) describe the ability to transition between the macro- and submicro levels as “a core competency to progress towards causal mechanistic reasoning” (p. 471) (cf.Krist et al., 2019). A mechanistic explanation of a chemical phenomenon involves and demands transitions between the submicroscopic and macroscopic levels.
Causality: Mechanistic explanations are inherently causal (Russ et al., 2008). However, explanations at the macro level, although not mechanistic, may also be causal; that is, detailing why something happens (but not how). Hence, students’ reasoning may vary on a range from non-causal (such as a purely descriptive reasoning – thus non-mechanistic) to causal to mechanistic. Causality can be expressed through various causal linking words such as ‘leads to’, ‘because of’ (see Craver and Darden, 2013).
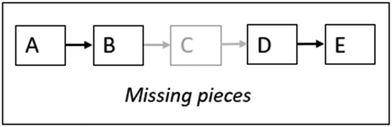
Completeness: Part of characterising MR is determining how complete the reasoning is; are there gaps in terms of mechanistic features that are missing in the chain of reasoning or is it complete (Keiner and Graulich, 2020)?
Such gaps may vary in nature. Keiner and Graulich (2020) describe the gaps they identified in students’ reasoning as “missing pieces” (p. 475) resulting in the lack of causal connections between features. For example, they found that the students described an activity (e.g. ‘B’ in Fig. 2) without mentioning the resulting property (e.g. ‘C’, Fig. 2), or they inferred the property of an entity (e.g. ‘D’ in Fig. 2) without expressing the activity (e.g. ‘C’ in Fig. 2) that caused the change in property. This reasoning pattern may be described as the act of “chaining forward” or “backward” (Russ et al., 2008, p. 510) is put to a halt.
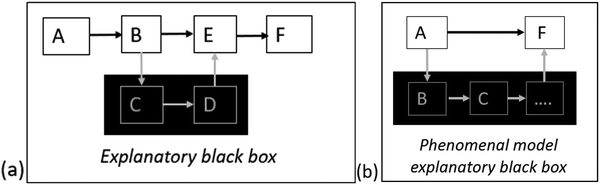
Another kind of gap in mechanistic explanations are explanatory black boxes. In these cases, the processes that underlie an established and mentioned causal link within the explanation is unknown to the person constructing the explanation. Thus, what is missing is a description as to how exactly the causal relationship occurs (see Fig. 3a). In other words, the mechanistic details are hidden in a “black box”. These kinds of incomplete mechanistic explanations that include one or several black boxes are referred to as mechanistic sketches (Craver and Darden, 2013). As opposed to explanatory black boxes within an explanation, phenomenal models hide the whole mechanism of a phenomenon in a black box (e.g. when air is heated it expands); they are complete black boxes (Craver and Darden, 2013) (see Fig. 3b). Such explanations may be regarded as simple causal (cf.Moreira et al., 2019).
The existence of black boxes is an indicator of explanatory depth – “the more black boxes there are, the less the depth of the explanation” (Haskel-Ittah, 2023, p. 8). Thus, evaluating a certain explanation in terms of its limits and mechanistic level means recognising the number of black boxes. However, as it's always possible to increase the level of detail in a model, all models would potentially include black boxes. Thus, we have to make decisions about where we accept the explanation in a certain situation to bottom out (at a sufficient level of detail), something which in turn may depend on e.g., what aspects of a phenomenon it aims to explain (Craver, 2006). Whether pieces of information in an explanation are explanatorily relevant to the phenomenon, or not, is in other words “an objective fact about the world as any other” (Craver, 2006, p. 360). As stressed by e.g., Craver and Kaplan (2020), incomplete mechanistic explanations are not always improved by adding more details.
To summarise the evaluative and fourth step in an MR analysis, we are interested the different types of features identified by the students; at which representational level they are identified; whether transitions between levels are expressed; whether and how causal connections are expressed (and at which level); and lastly, the completeness of their MR (identifying gaps as missing pieces and as explanatory black boxes).
Procedures
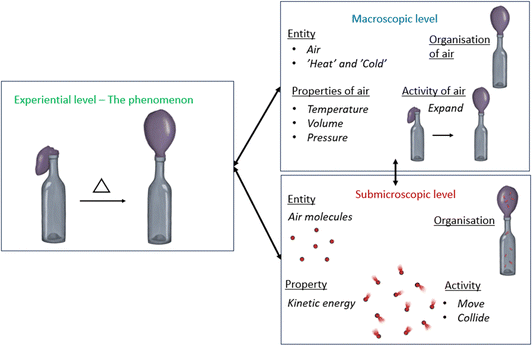
In the following paragraphs, we describe and exemplify how we apply our four-step version of Keiner and Graulich's (2020) three-step framework to the target phenomenon in focus in our study, i.e., thermal expansion of air.Step I: Identifying the activated features of a mechanism: entities (E), properties (P), activities (A), and spatiotemporal organisation (O). A mechanism explains how these features interact at the submicroscopic level and produce the phenomenon observed at a scalar level above. Hence, and as concluded by Keiner and Graulich (2020), “each mechanistic feature can be conceptualised at the respective representational level” (p. 471). Thus, the analysis of our data includes a coding of which features were activated in the students’ reasoning at the macroscopic and submicroscopic levels respectively, as well as identification of descriptions of the target phenomenon at the experiential level. Importantly, though, in chemistry, a property observed at the macro/experiential level (e.g., air pressure) emerges from the collective and continuous interaction among particles (i.e., the particle system) and not of the individual particle. In other words, the behaviour or property the particles collectively give rise to is not a property of the particles themselves (Newman, 2013; Tümay, 2016).
(1) The entities (E) of the system are the actors of the mechanism, “the things that play roles in producing the phenomenon” (Russ et al., 2008 p. 14). We consider the phenomenon being thermal expansion of air and, thus, limit the system boundary to include the entities air molecules and ‘the heat’ and ‘the cold’ (see further down) (see Fig. 4). This means that we do not consider the artefacts bottle and balloon, or the water poured on the bottle, as entities of the mechanism but rather as entities of the phenomenon at the experiential level. Forming a closed, and partly stretchable ‘container’, they enable the students to experience the phenomenon as a change in the property ‘size’ of the balloon. Further, although the system boundary could be considered as including e.g., the glass particles of the bottle, the polymers of the balloon and the air molecules surrounding the bottle + balloon, it would not be meaningful considering the specific context of the study. The students had not been introduced to the idea of matter as composed of particles prior to the studied lesson (although they may have met it elsewhere) and the content focused during the lesson neither considered the existence of particles other than air molecules nor the air (molecules) surrounding the bottle and balloon. On some occasions, both the teacher and the students referred to the air particle system and its colligative properties (the space it occupies). In these cases, we choose to consider the particle system itself as an entity of the mechanism at the submicro level.
 | ||
| Fig. 4 Example of aspects of mechanistic features for the phenomenon of thermal air expansion in an enclosed container made up of a glass bottle and a balloon. | ||
Finally, the air molecules are particles of the ‘substance’ air. Air can be conceptualised at the macro level and, thus, be regarded as an entity (E) of the mechanism at the macroscopic level. At the experiential level, air may be indirectly experienced and described as, e.g., an inflated balloon (see Fig. 4).
The students' reasoning included descriptions of the non-material factor heat energy as an entity; “the heat heats” and “the cold goes up”. In the analysis, we consider the expressions “(the) heat” and “(the) cold” to be identifications of entities of the mechanism at the macro-level (E). Conceptualisation of “heat” and “cold” at the submicroscopic level would have involved descriptions of the activities and organisation of particles (the transfer of vibrational particle motion)—a content that was not explicitly included in the lesson. Thus, the children's descriptions of heat energy (and temperature, see properties below) are expected to bottom-out at the macro level. In other words, we don't consider the submicro level process an unpacked black box.
We wish to emphasise that we cannot know what the submicro-words the children express really mean to them. In other words, we cannot know if the use of e.g., the word “molecule” implies that they have a submicroscopic perspective on matter. However, given that the study rests on sociocultural theory, we choose to interpret submicro-level words as representing a submicro-perspective in the normative sense.
(2) Entities are characterised by their properties (P). The kinetic energy of the air molecules is a submicroscopic property (P). It is in turn responsible for the macro-level properties temperature, air pressure and volume. At the experiential level, air pressure may be experienced in terms of changes in the property size (P) of the balloon (i.e., as “hanging down”, “bigger”). The property temperature is implicitly mentioned in the students' reasoning when they describe the bottle as “cold” (P) and the water poured on the bottle as “hot” (P) (although cold and hot, strictly speaking, is not a measure of temperature). In this case, we consider “hot” and “cold” as properties of the entities of the phenomenon (experiential level).
(3) Activities (A) are something that the entities ‘do’. The submicro entity air molecule is engaged in the activity ‘moves’ (A) and the macro level entity heat (E) in the activity “heats” (A).
(4) Organisation (O) is “the spatial and temporal location of entities during a determined activity and its connection to the properties or behaviour of the system” (Keiner and Graulich, 2020 p. 471). At the submicro level, organisation (O) includes information about the spatial-temporal location of air molecules. Since the bottle and the balloon make up the spaces in which the sub-micro entity air molecule, and the macro-entity air, are located they designate spatial organisation (O) for these. Hence, we choose to consider “the balloon” in, e.g., the phrase “the air goes up into the balloon” to be an identification of both an entity and of spatial organisation (E/O).
Step II: Identifying students’ connections and transitions among the features on one or several representational levels. When Greta in Episode 5 proposes that “we [the molecules] go up”, she connects the submicroscopic entity molecule (E) to an activity (A: go) and organisation (O: up). Looking at the whole statement from Greta in Episode 5, we see an example of transition from submicro level to the target phenomenon at the experiential level: “We [the molecules] go up because we take up so much space [submicroscopic level] and then it gets bigger and bigger in the balloon [experiential level]”.
Step III: Constructing explanatory approaches: we transferred the connections and transitions among features from Step II into a visual representation, as exemplified in Fig. 5. The explanatory approach visualises at which level of representation the mechanistic features in the students' reasoning are expressed (macro–submicro) as well as features of the phenomenon at the experiential level. It also visualises connections and transitions among the features at different or the same representational levels.
Step IV: In the evaluative step of an MR analysis, we are interested in the different types of features identified by the students; at which representational level they are identified; whether transitions between levels are expressed; how causal connections are expressed (and at which level); and lastly, the completeness of their MR (gaps in terms of black boxes or missing pieces).
The students' reasoning was analysed from the perspective of completeness by focusing on the following two questions: (a) Are there gaps in terms of missing pieces within the explanation (Keiner and Graulich, 2020) i.e., a lack of chaining backwards or forwards (see Fig. 2)? and (b) Are there explanatory black boxes within the explanation? Guided by Craver and Darden (2013) explanatory black boxes were identified by identifying the use of linking words. Craver and Darden (2013) describe that a causal relationship hiding an explanatory black box are characterised linking words such as “X affects Y”, “when X happens then Y”, or “X leads to Y”. This may be compared with linking words such as “X binds to Y”. These describe a clear interaction – indicates an activity rather than a (correlative) relationship – between X and Y. Identification of black boxes in students' mechanistic reasoning is, in other words, possible by attending to the use of linking words. Table 2. shows the three types of linking words used by the students that we consider indicating a black-boxed rather than mechanistic causal connection.
| Linking words that signals black boxing | Examples from the students reasoning |
|---|---|
| X becomes Y | “The water evaporates upwards so that it becomes air”. |
| X makes Y | “The heat makes the balloon bigger”. |
| When X then Y | “When you pour hot water more molecules get into the bottle”. |
When Gordon proposes that “the heat makes the balloon bigger”, he makes a causal connection between the entity heat and the phenomenon. However, there is no information on how the connection occurs. Along the same line of reasoning, Greta's suggestion that “water evaporates up so that it becomes air” is also black boxed. As opposed to Gordon, Greta's reasoning is non-canonical and there is no canonical mechanism hidden in the black box. However, our analysis focuses on children's MR regardless of scientific correctness (cf.Russ et al., 2008; 2009) and Greta may, or may not, have an idea about the missing mechanism. Black boxes are represented as black squares labelled “BB” (see Fig. 5).
Scientists are not always interested in, or cannot always provide, complete descriptions of mechanisms. Thus, within the scientific research practice, what's inside the black box is unknown to the person constructing the explanation (Craver and Darden, 2013). In terms of students’ mechanistic reasoning, we believe that what is inside the explanatory black box (but not mentioned) may, or may not be, known to the student. Further, when the mentioned causal link is non-canonical (for example, a student in our study describes that “water evaporates to air”), it is not meaningful to talk in terms of what is, or is not, ‘known’ – there is no canonical answer. Rather, the student may possibly have an idea – or not – about what is hidden in the black box or may not even discern the chain of reasoning as black-boxed.
To facilitate the presentation of the teaching and learning process during lesson 1, the lesson was chronologically divided into several discernible teaching sequences/activities in the classroom/group, which we refer to as “episodes” (cf.Sjøberg et al., 2023). These episodes ranged from one minute to several minutes. The main purpose of this was to be able to present the significant steps more clearly being taken in student MR, to link these to different teaching activities in the classroom and thus discuss implications for practice. The dialogue has been translated into English by the authors, and checked by a proofreader, after which clarifications and slight changes were made to the transcripts.
Results
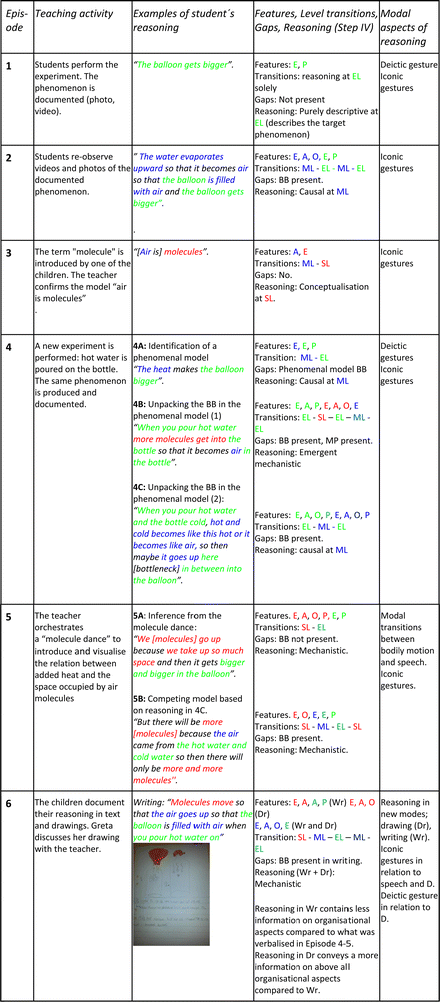
As described in the Method section, the purpose of the lesson was to get students to reflect on air as a phenomenon at the macroscopic and submicroscopic level, and to help them begin to see the macroscopic-level properties of air as arising due to processes at the submicroscopic level. The lesson included several activities corresponding to the six episodes. First, the students performed the experiment with the cold bottle and the balloon in groups of three, to produce and observe the target phenomenon: thermal expansion of air (Episode 1). Second, students tried to explain the observation (Episode 2). Third, the concept of the molecule was introduced (Episode 3). Fourth, a new experiment was performed and observed: pouring hot water on the glass bottle (Episode 4). Fifth, the teacher orchestrated a “molecule dance” in which the students acted out being air molecules (Episode 5). Lastly, the students represented their tentative explanatory models of the observed phenomenon in drawing and writing (Episode 6). Following an analysis of these six episodes, the results from each episode have been summarised in Table 3.Episode 1: Producing and describing the phenomenon at the experiential level
Once the teacher has introduced the teaching unit and the tasks of the first lesson, the students are instructed to start by taking one bottle per group out of the freezer, bringing it to their table, and then putting the balloon over the bottleneck (Table 3). The students in the group that we followed carry out the instructions and then comments on what happens:George: Look at this! Look, look! [deictic gesture: points at the balloon] Greta: It has gotten fatter. It just gets bigger and bigger… Look, it gets bigger. George: [laughs]
[…] Gordon: It stands straight up. Dung [sound; moves hand upwards]. It's just dung [sound; repeats gesture].
The students here describe their observation of the target phenomenon. In doing so, they identify the entity balloon (Eb) at the experiential level and describe how its property size (P) changes: “[It] gets bigger”. Also, they identify its spatial organisation (O): “It stands straight up” (Fig. 6). Greta documents the whole process with five pictures.
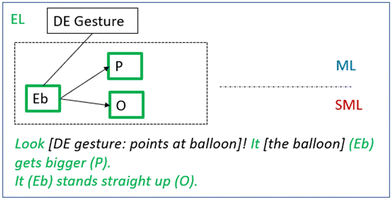 | ||
| Fig. 6 The students’ descriptions of the phenomenon at the experiential level including the entity balloon (Eb) (deictic gesture (DE)) and changes in its property (P) and organisation (O). | ||
Episode 2: Emergence of causal reasoning at the macroscopic level when re-observing the experiment
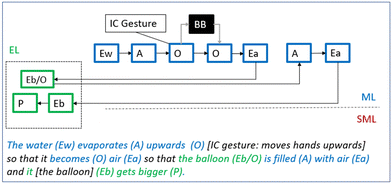
Just after the initial observation of the phenomenon, George, Greta and Gordon looks through the pictures they have taken. Gordon then notes that the balloon seems to have got even bigger and exclaims that he “knows what it is”, whereupon Greta reasons at the macroscopic level, using words and gestures:Greta: It evapo… [Gordon: …rates] rates upwards [iconic gesture: move hands upwards] and turns to air. Gordon: [nods] George: What if there are clouds in the balloon? Greta: We’ll write it like this: “The water evaporates upwards so that it becomes air so that the balloon is filled with air and it gets bigger”. That's how it works.
Greta identifies the macroscopic entity water (Ew) and its activity and correlated change in organisation – “water evaporates (A) upwards (O)”. Her talk is accompanied by an iconic gesture as she moves both her hands upwards (Fig. 7). The students talk about the macroscopic activity of evaporation (A) can be understood by the fact that condensation was formed on the outside of the glass bottle when it was taken out of the freezer. The students recognise the condensation and touch it.
 | ||
| Fig. 7 While explaining that “the water evaporates upwards” Greta makes an iconic (IC) gesture with her hands to represent the process of water evaporating upwards. | ||
Greta also identifies the macro-entity air (Ea) and connects it to the evaporating water: “The water evaporates so that it becomes air”. The linking words Greta uses indicates that the described causal connection is black-boxed: water is somehow transformed into air. Finally, Greta causally connects the formation of air to the phenomenon thus making transitions to the experiential level: “so that the balloon is filled with air, and it gets bigger”. Greta's reasoning seems to be inspired by knowledge about the water cycle, and George's comment—“What if there are clouds in the balloon?”—indicates that he interprets Greta's explanation as a description of the same.
A comparison with Episode 1 shows a development from a description of the phenomenon at the experiential level (Fig. 6) to an explanation of the phenomenon at the macro level (Fig. 8). As shown in Fig. 8, Greta's reasoning is rich in macro-level features (E, A, O) and includes several transitions between the macroscopic and experiential level.
Finally, the students call on the teacher to present their explanation. Instead of confirming or correcting their idea, she challenges them: “Did you have water in the bottle?”. In this way, she helps them to discern that the observation of condensation is not a relevant feature. Greta, Gordon, and George then revise their explanation and replace the entity water (Ew) with cold (Ec). Together they describe that “all cold goes up in the balloon”, and Greta adds that “air is formed so that it goes up”. Hence, they stick with air as a critical entity.
Episode 3: Introducing the submicroscopic level (“molecule”)
While still talking to the group, the teacher focuses the fact that the bottle is empty (no water) and that the task is to contemplate what air is:Teacher: And we’re going to think about what air is. We’ve agreed that…
Gordon: Oxygen.
Teacher: …there's air in it [the bottle]. It's oxygen. What else is it?
George: Ehm it's like, what's it called, like this [iconic gesture: moves both hands up and down]. Plup.
Greta: Those weird plups that exist.
Teacher: Yes, what are they called?
Greta: Molocules (sic!).
Teacher: Good. That's right. Molecules. Air is lots of molecules. You knew that [looks at Greta]. That's good. Now you’re going to think a little about how they work.
George: I’m better at animals.
In the conversation, George and Greta introduce the term “molecule” through the substitute word “plup” (cf.Rundgren et al., 2012). While searching for the word, George simultaneously moves his hands up and down several times, suggesting that he represents molecules as something in motion. The teacher confirms the students' idea and summarises it in relation to air: “Air is lots of molecules”.
Episode 4: A new experiment supports the emergence of mechanistic reasoning
Episode 4 revolves around a new experiment with the bottle and balloon. The students' reasoning in relation to the observations of the experiment has three distinct foci as described under the sub-episodes Episode 4A, B and C.Teacher: But there isn’t any more air in it?
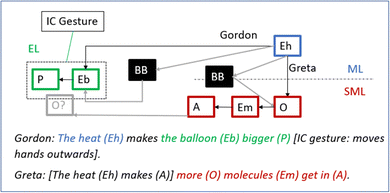
Gordon: No, maybe… the heat makes it bigger and bigger [iconic gesture: move hands outwards from each other].
With her question, the teacher relates the observed phenomenon to a puzzling fact: the balloon gets bigger although there is not more air in the balloon. Seemingly, this helps Gordon identify the macro- entity “heat” (Eh) and discern the causal relationship between added heat and the expansion of the balloon: “The heat makes it bigger”. He reinforces his verbal description by simultaneously moving his hands outwards from each other to represent a growing balloon. Importantly, George's inference. lacks a description of a mechanism and thus has the characteristics of a phenomenal model – a completely black-boxed relationship between heat (input) and the phenomenon (output) (Fig. 9).
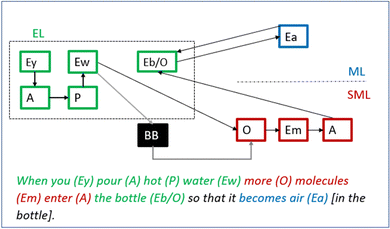
When the teacher asks her to further explain her thoughts, Greta expands her reasoning to include more features and transitions between levels (see Fig. 10): “When you pour the hot water […] more molecules get into the bottle so that it becomes air”. Notably, she exchanges the entity “heat” for the experiential entity “water” having the property “hot”. She also transitions from the submicroscopic- to the macroscopic level as she relates the entering [air] molecules to the formation of air in the bottle. Finally, her reasoning now includes the missing piece from her prior reasoning – the molecules get into the bottle (Ebo, experiential level).
In a next step, the teacher asks Greta to unpack the black box in her reasoning: “How did they [the molecules] get into the bottle?”. Greta hesitantly suggests “Through the hot water, or?”. This model is challenged by the teacher – “did the hot water pour into the bottle?” Greta says “no” i.e., agrees to that her models lack obvious observational support.
George: But the heat does, like this, it heats so maybe the hot air goes up into the balloon.
George, implicitly, suggests that the heat “heats” the air. Next, he suggests that the heating of air causes changes in its organisation; “so maybe the hot air goes up”. The underlying mechanism is, however, not described and the relationship is thus black-boxed. As with Greta, the teacher asks George to develop his idea, that is, unpack the black box:
Teacher: Can you explain your reasoning, how does it rise?
George [stands halfway to get closer to the bottle]: Because you got, pour the hot water…[iconic gesture: draws fingers along the part of the bottle where the water was poured]
Teacher: Hmm [approvingly].
George: And the bottle is cold.
Teacher: Hmm [approvingly].
George: So hot and cold becomes like this hot (sic!), or it becomes like air [sits down again]. So maybe it goes up into the balloon [rests his head in his hand].
Gordon: Maybe it goes in between here [Greta: yes] through the neck [deictic gesture: points at bottleneck]?
Gordon and Greta: Hmm [approvingly].
Teacher: Okay.
Encouraged by the teacher, George here develops a more extended reasoning. It's rich in features at the macro- and experiential level and attempts to explain the process of heating and of formation of air (see Fig. 11). He describes the pouring of “hot water” on the “cold bottle” and then reasons in terms of heat and cold as entities: “hot (Eh) and cold (Ec) becomes like this hot (sic!)”. However, next he adds, “or, it becomes like air” thus suggesting that heat and cold are somehow transformed to (hot) air in the bottle (an idea that comes back in Episode 5). Thus, like Greta, George's reasoning is based on the idea that there is no air in the bottle from the beginning. Notably, the formulation – ‘X becomes Y’ – indicates a black-boxed relationship. Finally, George suggests that the air goes up into the balloon and Gordon, then, suggests that the air “goes up here in between” while simultaneously pointing at the bottleneck. Hence, the bottle and deictic gesture enables the students to communicate a more detailed representation of the organisational change, as compared to George's “goes up in the balloon”. However, a mechanism describing how hot air rises is still missing. Thus, his reasoning includes two explanatory black boxes (see Fig. 11).
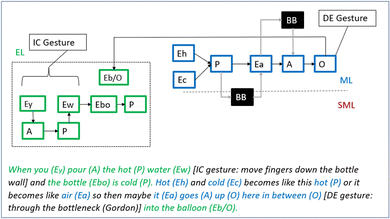 | ||
| Fig. 11 George's reasoning after the teacher asked him to develop it. The part about the air going “up here in between”, accompanied by a deictic gesture, was added by Gordon. | ||
Later, the teacher returns to George's reasoning about hot air going up whereupon he explains that “when a smith dips a warm iron object in water air comes up so I guess that should happen with the bottles, too”. George thus reveals that he used his experiences from observing a smith in work to construct the explanation (Fig. 11) of the growing balloon.
To conclude, the observation of the second experiment (pouring hot water on the bottle) was a game changer in that the students were now able to discern “heat” as an entity and causally relate it to the expanding balloon. Obviously, this inspired both Greta and George to spontaneously try to unpack the phenomenal black box. A common point in their reasoning is that they try to solve the expansion of the balloon by increasing the amount of molecules/air in the bottle. This, however, demands black boxing the entrance (Greta) and production (George) of molecules/air.
After Episode 4C, the teacher addresses the whole class and asks Greta to explain (to them) what air is. Greta answers “molecules” [–] “small plups” while simultaneously making an iconic gesture; she holds her hands at eye level, pinches forefinger and thumb together until a very small space between the fingers remains. The teacher then asks the class if “they [molecules] are alone” whereupon George says that “No, there are lots of them, there are a million, even more”. The discussion continues for several minutes and revolves around what molecules are.
Episode 5: The molecule dance supports development of mechanistic reasoning
Following the discussion about molecules the teacher arranges a ‘molecule dance’ during which she orchestrates an ensemble of speech and bodily motion. Episode 5 revolves around this representational activity. The students' reasoning has two distinct foci as described under the sub-episodes Episode 5A and 5B.The dance becomes a way of introducing and visualising a model for how heat affects the property speed of the individual molecule, and how this in turn affects the organisation of the molecules in terms of how much space they – as a system – occupy (the aggregate level). The sequence of aspects highlighted by the combination of bodily motion and the teacher's verbal comments and questions enables the students to experience and elaborate on a mechanism at the submicro level. The students state that they themselves—i.e., the molecules as a group—take up more space when they move than when standing still, as well as when they move faster (two steps instead of one step). When the teacher asks the students if they fit in the bottle (box 2 in Fig. 12), Greta infers that “that's why it goes up”. When the dance is finished the teacher asks the students: “what happens to the molecules in the bottle” (box 3). Gordon suggests that “some go up maybe” and then Greta develops:
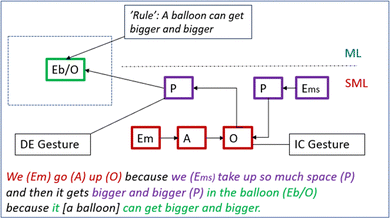
Greta: We go up [iconic gesture: move hands upwards] because we take up so much space and then it gets bigger and bigger [iconic gesture: measures with her hands] in the balloon because it can get bigger and bigger.
Greta thus relates the molecule dance to their prior reasoning (air goes up, Episode 4C) and uses it as a model to mechanistically reason about why the molecules “go up”. Greta uses the word “we” twice. A reasonable interpretation is that the first “we” refers to “we” as individual molecules, and the second to “we” as a system of molecules (entity Em and Ems, respectively in Fig. 14). She creates a causal relationship between a property change of the molecular system (Ems)—“we take up so much space”—and the activity and organisation of molecules (Em)—“we go up”—and then links these changes at the submicro level to the phenomenon – “it gets bigger in the balloon” (see Fig. 14).
What stands out in Greta's description is her continuous use of gestures to emphasise speech, of which the first visualises the upward movement of molecules. The second gesture (see Fig. 13) may be interpreted as a growing balloon (i.e., the phenomenon). However, the accompanying speech describes that the expansion goes on “in the balloon”. This indicates that the gesture (also) represents how the space occupied by the molecular system increases i.e., what was enacted during the molecule dance. In other words, Greta's iconic gesture together with her verbal description seem to form a representation of what simultaneously goes on at the experiential (the phenomenon) and submicro level. Importantly, Greta uses the rule-like statement ‘balloons can get bigger’ (see Fig. 14) to explain how an increase in volume of the molecular system may be possible within a closed container (balloons are stretchable).
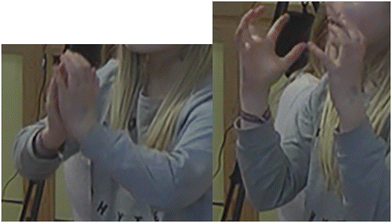 | ||
| Fig. 13 Greta employs an iconic gesture; she uses her hands to visualise how it gets “bigger and bigger in the balloon because it can get bigger and bigger”. | ||
 | ||
| Fig. 14 Greta's reasoning in conjunction with the molecule dance. The purple boxes refer to the aggregate level of the submicroscopic level (the molecular system). | ||
After Greta has presented her model (Fig. 14) the teacher challenges Greta's reasoning: “But you [molecules] didn’t get bigger?”. This suggests that the teacher wants to make sure that “it” in Greta's description “it gets bigger (P) in the balloon” refers to the space occupied by the molecular system and not the individual molecules. Since an increase in molecular size is a hypothesis that could explain why the balloon gets bigger, this is an essential question. However, Greta denies this and describes that “No, but we move away”. A reasonable interpretation of this is that she tries to clarify that what gets bigger is the space occupied by moving molecules in the balloon, not the molecules as such.
To conclude, the introduction of the molecule dance leads to a more qualified MR as compared to the students' reasoning during Episode 4 (Fig. 10 and 11vs.Fig. 14); it is more complete – there are no apparent black boxes in Greta's reasoning – and it describes a mechanism at the submicro level which then is related to the phenomenon. However, it's unclear whether Greta's model accounts for the idea presented by Gordon—some of the molecules go up—or if it presumes that all molecules go up.
Gordon: But you go outwards and outwards [iconic gesture: moves his right hand forwards], because then you touch the bottle [iconic gesture: moves his arms outwards and inwards].
Presumably, Gordon's reasoning is based on a comparison with the molecules “standing still” in the cold bottle (Box 1 in Fig. 12); they stay where they are, they do not take up more space. When describing how the molecules are “going” towards the sides of the bottle and eventually”touch” it, Gordon simultaneously moves his arms in- and outwards to represent. Importantly, this gesture adds information to what was conveyed in speech – the air molecules not only touch the bottle wall but bounces back. The teacher then points at the bottleneck and makes explicit that “it's open here” i.e., that the space-demanding molecular system is able to expand into the balloon.
Episode 6: Drawing afford explication of organisational aspects
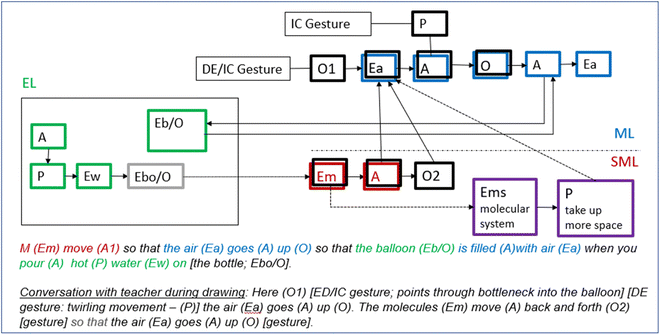
After the molecule dance, the students are instructed to create individual texts describing “what happens inside [the bottle and the balloon]”. The teacher leaves it open to the students whether they want to draw, write, or use clay. Fig. 15 shows the multimodal texts created by the students in our group. George chose to use clay together with a sketch of the bottle, while Greta and Gordon made drawings.Greta starts by drawing the contours of a bottle and three molecules presumably moving up (indicated by wavy lines) through the bottleneck. She then deliberates with the teacher on how to proceed:
Greta: Miss [says the teacher's name], I have been thinking. Here the air goes up [deictic gesture: points to her drawing of the bottle and simultaneously employs an iconic gesture: makes a twirling movement with her finger moving up through the bottleneck]. Shall I draw that these molecules move back and forth [iconic gesture: moves her arms inwards and outwards] so that the air goes up [iconic gesture: brings her arms together and upwards].
Teacher: That sounds like a good suggestion. I think you will figure out how to describe it well.
Greta's interaction with the teacher illustrates how the task of making a drawing pushes Greta to verbally consider and make decisions about the entity molecule in terms of aspects of activity, organisation, and property. Greta expresses her ideas with speech and gestures. First, she describes that the air goes up “here” while moving her finger up through the bottleneck of the drawn bottle, thus integrating a deictic and iconic gesture. Moving her finger with a twirling gesture, Greta at the same also represents how the air moves (property of movement) thus adding information to that contained in speech. Next, she describes the activity and organisation of molecules and relates this to the organisational change of air: “the molecules move back and forth so that the air goes up”. She reinforces speech with iconic gestures (raises arms up, moves arms inwards/outwards). When Greta, later, shows the teacher her ready-made drawing, she says that: “I’ve drawn it so that you can see that they [the molecules] stand completely still”, while simultaneously bringing her palms together under her chin, drawing her shoulders up towards her ears and then ‘freezes’. To conclude, Greta spontaneously employs gestures throughout her reasoning with the teacher to reinforce and complement information in her talk about aspects of activity, property, and organisation. Importantly, the drawing enables Greta to make deictic reference to specific locations of activities. Finally, it's interesting to note that Greta's reasoning carries obvious features of the molecule dance; molecules moving “back and forth” mirrors the instructions given by the teacher.
Greta’s writing and drawing – Scene 2 in Greta's writing and drawing (Fig. 15) concerns the bottle and balloon at the end of the lesson when the bottle had cooled, and the balloon had shrunk. This was just briefly commented upon, and we focus primarily on scene 1. Here, her reasoning in writing resembles a model that the group briefly reasoned about at the end of Episode 5 (was not detailed); molecules that “move” cause air to “go up”. She then connects this to the phenomenon—“the balloon is filled with air”—and finally adds the event that produced the unfolding mechanism—hot water poured on the bottle.
As for Greta's drawing in scene 1, it contains aspects that relate to her initial interaction with the teacher (above). The property of molecular movement is represented as wavy lines (mirrors the twirling with her finger) and these lines are drawn within a restricted area (mirrors “back and forth”). Importantly, these aspects are represented in writing as simply “M [molecules] move”. Thus, the drawing conveys a much more explicit description. In fact, Greta's writing adds nothing relevant to the drawing apart from labelling the entities “air” and “molecules”. Whether the air is the same as, or distinct from, the molecules is not made clear in either the image or the writing. Interpretation is complicated because Greta has not drawn what it looks like in the balloon at the submicroscopic level.
There are several aspects of features that are expressed in drawing but not in writing. These may be traced back to the molecule dance. This includes the organisation of molecules in terms of them moving within an area restricted to the upper part of the bottle (scene 1; the length of the wavy lines indicates that the molecules move within that area only), the organisation of the non-moving molecules (scene 2, represented as absence of wavy lines) as a cluster in the middle of the bottle, and the number of molecules (three). Importantly, it is not until Greta makes her drawing that these notions are expressed and thus possible for the teacher to discern. Further, since the molecules in scene 1 are represented as occupying a greater area than those in scene 2, scenes 1 and 2 together tell the story that the volume of the molecular system changes with temperature. Finally, the molecular properties of shape and size, and the organisational aspect number of molecules, are also expressed in Greta's drawing. It's interesting to note how they mirror the emphasis on conservation of size, and number of molecules during Episode 5; the molecules have the same shape and size and the number of molecules is conserved between scene 1 and 2.
Gordon and George: Like Greta, both Gordon and George represent molecular motion through wavy lines. However, Gordon's and George's drawings differ from Greta's in a decisive way: the molecules are evenly spread throughout the whole bottle. However, Gordon draws the molecules in the cold bottle similarly to Greta; i.e., clustered together in the middle of the bottle. As for George, it is not clear since he has drawn only one bottle. Finally, and similarly to Greta, neither George nor Gordon has drawn what it looks like inside the balloon (Fig. 16).
 | ||
| Fig. 16 Greta's reasoning in terms of elements of her writing (red/blue/green boxes), drawing (purple boxes) and conversation with the teacher during the drawing activity (black boxes). | ||
Summary and discussion
The purpose of this case study was to analyse early primary students' (grade 3, aged 9 to 10) mechanistic reasoning in chemistry as it evolves in classroom practice. The study focuses on the first lesson in a sequence of six that was developed as part of a design study. The teaching was designed to create conditions for students to develop, test, and evaluate simple particle models in interaction with observations cooperatively and under teacher guidance (model-based inquiry). Our research question is: What characterises children's emergent mechanistic reasoning in a classroom practice framed by model-based inquiry?When analysing students' mechanistic reasoning we used a modified version of Keiner and Graulich's (2020) framework, using Taber's (2013) revised chemistry triplet, a fourth evaluative step, and the inclusion of multimodal aspects of MR (Kress, 2010). We believe that the modified framework allowed for more nuances in the development of children's MR to be captured in the analysis, as compared to the original framework. First, by using Taber's (2013) revised model instead of Johnstone's (1991) chemistry triplet, we could follow children's reasoning from the direct experience of the phenomenon—the experiential level—via conceptualisation at the macroscopic level all the way to the submicroscopic level. Second, regarding the evaluation of student reasoning—the fourth step in the framework—neither of the frameworks in Table 1 treated this in an exhaustive manner. Based on a review of the literature, we pointed to four different aspects to be included in such an analysis: the different types of features identified by the students; at which representational level they are identified; whether transitions between levels are expressed; whether and how causal connections are expressed (and at which level); and lastly, the completeness of their MR (identifying gaps as missing pieces and as explanatory black boxes). We will elaborate on our findings in relation to black boxes below, as black boxes have not been discussed in the literature on student MR before. Lastly, our frameworkmade helped us see that the children used several modalities other than speech, and that these—and the interplay between these—played a key role in student reasoning. This will also be further discussed below.
The results shows that: (1) In model-based inquiry, young children can navigate across different representational levels in their reasoning and engage in MR; (2) children's black-boxing can be seen as an indication of epistemic work in the process of model-based inquiry; and (3) asking students to engage in multiple modes of representations support the development of student MR in model-based inquiry. In the following, we will discuss each of these points and their implications for teaching and learning young children MR.
In model-based inquiry, young children can navigate across different representational levels in their reasoning and spontaneously engage in MR
This study supports recent research showing that a simple particle model can be productively taught in the early primary years (Haeusler and Donovan, 2020; Samarapungavan et al., 2017). It also shows that when children are active participants in a guided model-based inquiry process, they can develop and use such a model to suggest and assess tentative explanatory models in interaction with observations and navigate across different representational levels in their reasoning, i.e., develop MR (cf.Samarapungavan et al., 2017). The students in our study displayed all three of the epistemic heuristics that Krist et al. (2019) found in their extensive data on sixth graders’ MR: considering the level below the target phenomenon; differentiating between factors at the lower level; and linking lower-level interactions and behaviours to the target phenomenon. In line with, e.g., Russ et al. (2008), we believe that for primary teachers to take advantage and support the development of this ability of children, striving towards promoting students’ agency and influence over what counts as scientific knowledge is of overall importance (see also Eriksson et al., 2021).This study does not reveal any obvious signs of the students having difficulties discerning the emergent nature of the submicro–macroscopic relationship, something that Newman (2013) describes as a prerequisite for building MR. Rather, the students show signs of an understanding that the expanding air at the macroscopic level is somehow arising from changes in molecular activity and organisation. Importantly, the teacher never talked with the students about, e.g., what a model or explanation means, what the submicroscopic or macro level is, and how these levels relate to each other. Hence, we suggest that this specific classroom practice, in terms of the students suggesting and assessing tentative explanatory models in interaction with observations and inquiry-oriented teaching activities (most notably experiments), supports the development of an implicit understanding of the submicro–macro relationship. This epistemic practice contrasts with a more traditional one, where submicro models are presented as facts (Talanquer, 2018a; 2018b) and the students’ task becomes to memorise and then reproduce the content rather than use it as tools to explain the observable (Windschitl et al., 2008). A particularly interesting finding of this study is that the children showed a genuine commitment to a theoretical content and, throughout the lesson, spontaneously searched for explanations to make sense of their observations.
Gaps as black-boxing – an indication of epistemic work in the process of model-based inquiry
We noticed several instances of explanatory black boxes, including a phenomenal model, in the students' reasoning. Notably, the nature of these black boxes varies. At the initial stage of the inquiry process, before the molecule dance, the students' reasoning reflects an intuitive search for a causal explanation of the growing balloon. This search is based on the idea that the amount of air in the bottle/balloon increases, and results in a specific type of black boxing present in the students' reasoning in Episodes 2–4. Here, they suggest that “water” and “hot and cold” respectively “become air” (Fig. 8 and 10) without describing the underlying mechanism. These explanatory models are non-canonical and may be characterised as transmutations, i.e., a “forbidden” transformation in chemistry in which one entity transmutes to another entity (see Andersson, 1990). This is the first type of explanatory black boxing present in our data. To construct these explanations the students probably drew on their existing knowledge about the water cycle (Episode 2), and their out of school experience of, for example, observing a smith at work (Episode 4). Lacking appropriate knowledge, they select pieces from these resources to fit their causal explanatory model: the amount of air increases. We wish to emphasise that we can’t know that the students assume that water ‘transmutes’ to air. It may be that they do not differentiate between different types of substances i.e., all transparent liquids may be called water and all gas-like entities may be called air.A second type of black boxing is constructed by Gordon when he, from observing the effect of pouring hot water on the bottle, infers that “heat makes the balloon bigger” (Episode 4, Fig. 5). In this case, the whole mechanism of the phenomenon is hidden in a black box and the account has the character of a phenomenal model. This compares with what Bolger et al. (2012) characterise as a “rule-based explanation”. Importantly, the students were not able to make this empirical inference until hot water was poured on the bottle. This illustrates how multiple representations of the same phenomenon may support patterns seeking and thus identification of phenomenal models.
Third, drawing on Gordon's phenomenal model, Greta then tries to unpack the black box and proposes that “the heat makes more [air] molecules get into the bottle” (Fig. 9). Like Gordon, she uses the linking-word “makes” thus attributing (a not detailed) agency to the entity heat. This means that she doesn't describe how the molecules get in. Moreover, her black-boxed mechanistic account is built on a non-canonical idea: air molecules can move through glass. Although non-canonical, this indicates students' commitment to search for mechanisms (Krist et al., 2019). Unlike the students’ prior non-canonical ideas – an entity transmutes to another entity (water to air) – Greta's idea has no obvious linkage to other knowledge domains (water cycle) or everyday experience (e.g., observing a smith at work).
A fourth and final kind of black boxing is present in George's proposal that warm air rises when heated (Fig. 11). This idea has the character of what De Andrade et al. (2022) describe as an “intuitive rule” learned at school, in this case presumably in the context of the water cycle. This fact and rule-like statement is typically amplified in educational visualisations of the water cycle with an arrow pointing in one direction: from sea level to clouds. Presumably, the ambition is to simplify the science of the underlying mechanism and shortcut to facts. However, this rule is not transferable to the context of the observed phenomenon and may thus stand in the way for the students to consider air molecules as moving randomly.
The findings related to black boxes are in line with prior studies showing that (1) students draw on previous knowledge to construct explanations of an observed phenomenon (De Andrade et al., 2022), and (2) when lacking knowledge about how things work, they invent non-canonical “factors” to construct a causal account for the phenomenon (Krist et al., 2019; De Andrade et al., 2022).
At the same time the findings related to black boxes contribute to the emerging picture that (young) students can engage in MR even when they lack (appropriate) knowledge about how things work (Russ et al., 2008; Manz, 2012; Andrade et al., 2022). Notably, black boxes were present in the initial inquiry phase during the lesson but faded out as the students got comfortable with using the idea that air is molecules in motion, and testing and revising this idea against observations in an iterative manner. The students' reasoning process during Episode 4–5 also mirrors the epistemic practice of science in that: (1) Gordon first identifies a phenomenal model and then the students cooperatively and gradually try to unpack the black box in the model, and (2) this unpacking reveals new black boxes (cf.Craver and Darden, 2013). Based on this, we argue that black boxes in students MR reflect their epistemic work in the process of inquiry and thus are important to consider. We thus suggest that black boxes should be included in frameworks aiming at characterising students MR during inquiry. In line with Haskel-Ittah (2023) we also suggest that identification of black boxes in students' MR should be part of the process of guiding student's MR. In other words, teachers should attend to and highlight black boxes in students’ reasoning to help them recognise them as gaps and to consider whether they need to be unpacked or not. If so, the teacher could introduce them as something acting as “placeholders indicating where investigators might most productively focus their efforts” (Craver and Darden, 2013, p. 31); i.e., mirror the way black boxes are used in scientific practice. Importantly, Haskel-Ittah (2023) refers to several studies showing that helping students to identify black boxes in their explanations is critical for learning. In addition, the author also argues that if students learn to identify black boxes in scientific explanations this will help them to see through “the illusion of explanatory depth” (p. 6) in their own explanations as well as develop an understanding about the uncertainty of science. In the present study, the teacher implicitly highlights black boxes in the students' reasoning on a few occasions. In Episode 4, she challenges Greta's idea that the number of molecules in the bottle will increase: “How did they get in there, it's closed?” as well as George's idea that hot air rises: “how does it rise?”.
Finally, we wish to clarify that our identification of black boxes serves to illustrate how the concept may be used to characterise students’ MR. We cannot claim that our identification is (or can be) correct, since it rests on our subjective beliefs about what is a reasonable level of detail in the specific classroom of this study.
Asking students to engage in multiple modes of representations support the development of student MR in model-based inquiry
The analysis shows that the children draw on several modes when expressing their reasoning.As for gestures, the students used iconic gestures to primarily represent dynamic processes, most often at the submicro level, in terms of activities (entities in motion), organisational changes (predominantly direction of movement) and property changes (space occupied by the molecular system, balloon size). This finding, explained by the dynamic affordance of hand gestures, is in line with prior research (e.g.Bolger et al., 2012; Mathayas, 2019; De Andrade et al., 2022; Sjøberg et al., 2023).
Students also used iconic gestures to clarify the meaning of speech. When Gordon describes that molecules are “small” he moves the thumb and index finger towards each other. This gesture not only emphasises “small” but also serves to illustrate ‘how small’ i.e., to relate the measure ‘small’ to something. Also Greta, when she ‘freezes’ her body while describing that the molecules “stand still”, clarifies the meaning of speech; they are not just standing still, they are totally motionless. On a few occasions the students even made iconic gestures that convey ideas beyond what is expressed in speech alone: “plups” [speech] [moves hands up and down] – i.e., gesture adds that “plups” (i.e. molecules) are ‘something’ in motion (Episode 3); “[when in motion molecules] touch the bottle” [speech] [moves hand back- and forth] – i.e., gesture adds that molecules not only touch but collide with the bottle wall and thus bounces back (Episode 5); and “air goes up” [speech] [twirling gesture with finger moving upwards] – i.e. gesture adds that air moves in a twirling way (Episode 6). Thus, these gestures represent critical mechanistic aspects. This implies that teachers need to pay attention to students' gestures – they provide silent evidence of their reasoning – and to publicly highlight their meaning to support the collective epistemic work.
Although the students' gestures were predominantly iconic, we identified a few instances of deictic references. The most interesting examples are when the students point to areas of the physical and drawn bottle (Episode 4 and 6, respectively) to highlight a critical organisational aspect – the air goes up through the bottleneck. This implicates that immediate access to the experimental artefacts may support MR in that affords communication about e.g. organisational aspects (cf.Roth and Lawless, 2002; Berg et al., 2019).
The analysis of the students’ drawings (Episode 6) illustrates how drawing affords, above all, clarification of organisational aspects. One reason for this is that drawing something always requires making explicit decisions about the structure of the things to be drawn, such as their size, shape and location in space (Kress, 2010). However, as regarding spatial organisation, the affordance of drawing is, in this study, a result of the students' choice to integrate the experiential (bottle and balloon) and submicro level in the same drawing; it's the drawn contours of the bottle that enable representation of specific location. Thus, we suggest that encouraging students to make hybrid representations may support communication of spatial aspects of a mechanism (cf.Berg et al., 2019) as well as afford discernment of the relation between the phenomenon and the mechanism at the submicro level (cf.Berg et al., 2019; Sjøberg et al., 2023).
Drawing has a communicative affordance also in relation to the guiding work of the teacher; they are a window into students' ideas, thus allowing the teacher to identify signs of understanding or misinterpretations. The conservation of molecules and the activity and spatial organisation of the same are two such examples. Greta's way of drawing non-moving molecules concentrated in a cluster in the middle of the cold bottle reveals how she has (mis)interpreted aspects of the molecule dance (see below).
The multimodal experience of the molecule dance became a game-changer for the students in that it provided a tool for them to develop an explanatory model at the submicro level. However, the molecule dance also illustrates the two-sided nature of representational activities in the classroom (cf.Kress, 2010). As all models it has limitations which, if not addressed, may lead to non-canonical ideas. One limitation of the molecule dance is that the students can only move in the horizontal direction. Also, depending on the way the dance was staged, it doesn't account for the random motion of molecules. Another aspect is the modelling of the (three) students as standing still and very close to each other. This evidently contributed to the non-canonical ideas visible in Greta's and Gordon's drawings: three molecules organised in a cluster in the middle of the bottle. One way to meet the challenges of representational work such as the molecule dance is to initiate meta-conversations. These can focus e.g. the question: What are we showing, and not showing, when we do this? This forces the students to examine and describe the limitations of the represented model and, thus, support collective meaning-making.
Other important modalities are the video and photo documentation of the experiment. Revisiting the experiment in these modalities supported conceptualisation at the macroscopic level of the observation. One reason may be that these modalities enable the students to see the phenomenon in a new way, i.e., that they amplify aspects that were not discerned during the initial observation. The videos and photos can – just as drawings – be seen as new representations of the phenomena. To make new representations, and reflect on these, are stressed as necessary steps in multimodal theories of learning (Selander, 2008).
In relation to the multimodal concept of orchestration, it's interesting to note the different ways Greta uses the material and tools at hand to represent temporal changes of activity and organisation. In drawing she uses (and omits) wavy lines, arrows, and a sequential approach (scene 1 and 2). During her communication with the teacher, Greta is able to represent the dynamics more explicitly using spoken words and, above all, gestures on the drawing. Hence, the interactions between drawing, gestures, and speech enables Greta to physically animate and communicate dynamic aspects of the mechanism. This orchestration of a multimodal animated reasoning during representational work confirms previous findings on the importance of drawings and gestures to support students' MR (e.g., De Andrade et al., 2022; Sjøberg et al., 2023).
We can conclude that: (a) different modalities interact and support communication and development of student reasoning and (b) different modes provide the teacher with different types of evidence of students’ (mechanistic) reasoning, which can be used to clarify misunderstandings or inconsistencies. Hence, we advise teachers to organise for students to engage in constructing representations in multiple modes. However, based on the findings that the students' verbal representations are at times vague, we suggest that teachers provide students with (1) representational resources to use during tentative reasoning-in-action (cf.Andrade et al., 2022) (i.e., not only to document prior reasoning); and (2) a space for publicly sharing, and further elaborating on, these representations (e.g. the whiteboard). This should not only afford the individual student's reasoning, but also the communication of ideas and thus the collective epistemic work in the classroom. Further, publicly shared representations of ideas can work as a “collective memory” of the students' reasoning (cf.Eriksson et al., 2021, p. 44).
Limitations and further research
The conclusions we draw rests on some inherent limitations of our study. It is a small case study, focusing in detail on one lesson and three children and their MR as it unfolds during this lesson. Further, it was at times challenging to interpret the students' representations and we cannot ensure that these are always correct and mirror the students' intended meaning. There is also a possibility of subjectivity in the coding process, although we placed particular emphasis on analysing the data with an unbiased mind, and continually re-evaluated our inferences to control for pre-existing assumptions. To conclude, more research needs to be done to verify both the results of this study and the analytical framework used. It would also be of interest to further investigate how different modalities may better be used and orchestrated in developing student MR. In relation to this, other selection criteria than the ones used in this study – i.e., choosing to analyse the group that was the most verbally active – should be considered, such as choosing to analyse the least verbal students (cf.Wilmes and Siry, 2021).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financed by the Swedish Institute for Educational Research (Grant number 2016/148). We want to thank the anonymous teacher and her students who willingly took part of the study. We also want to thank the anonymous reviewers for insightful comments that contributed to the quality of the final version of the manuscript.References
- Andersson B., (1990), Pupils’ conceptions of matter and its transformations (age 12–16), Studies Sci. Educ., 18(1), 53–85.
- Bechtel W. and Abrahamsen A., (2005), Explanation: a mechanist alternative, Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences, 36(2), 421–441.
- Becker N., Noyes K. and Cooper M., (2016), Characterising Students’ Mechanistic Reasoning about London Dispersion Forces, J. Chem. Educ., 93(10), 1713–1724.
- Berg A., Orraryd D., Pettersson A. J. and Hultén M., (2019), Representational challenges in animated chemistry: self-generated animations as a means to encourage students’ reflections on sub-micro processes in laboratory exercises, Chem. Educ. Res. Pract., 20(4), 710–737.
- Betz N. and Keil F., (2021), Mechanistic Learning Goals Enhance Elementary Student Understanding and Enjoyment of Heart Lessons, Proc. Annual Meeting Cognitive Sci. Soc., 43, 2031–2037.
- Bolger M. S., Kobiela M., Weinberg P. J. and Lehrer R., (2012), Children's Mechanistic Reasoning, Cognition Instruction, 30(2), 170–206.
- Buchanan D. W. and Sobel D. M., (2011), Mechanism-based causal reasoning in young children, Child Dev., 82(6), 2053–2066.
- Caspari I. and Graulich N., (2019), Scaffolding the structure of organic chemistry students’ multivariate comparative mechanistic reasoning, Int. J. Phys. Chem. Educ., 11(2), 31–43.
- Caspari I., Kranz D. and Graulich N., (2018), Resolving the complexity of organic chemistry students' reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19(4), 1117–1141.
- Cheng M. M., Danielsson K. and Lin A. M., (2020), Resolving puzzling phenomena by the simple particle model: examining thematic patterns of multimodal learning and teaching, Learning: Res. Practice, 6(1), 70–87.
- Crandell O. M., Kouyoumdjian H., Underwood S. M. and Cooper M. M., (2019), Reasoning about reactions in organic chemistry: starting it in general chemistry, J. Chem. Educ., 96(2), 213–226.
- Craver C. F., (2006), When mechanistic models explain, Synthese, 153(3), 355–376.
- Craver C. F. and Darden L., (2013), In search of mechanisms: Discoveries across the life sciences, University of Chicago Press.
- Craver C. F. and Kaplan D. M., (2020), Are more details better? On the norms of completeness for mechanistic explanations, British J. Philosophy Sci., 71(1), 287–319.
- Danielsson K., Jeppsson F., Nestlog E. B. and Tang K. S., (2023), Representations of science content in a primary classroom: combining long and short timescales for multimodal analysis, Sci. Educ. DOI:10.1002/sce.21814.
- Davydov V. V., (2008), Problems of developmental instruction: A theoretical and experimental psychological study, Nova Science Publishers, Inc.
- De Andrade V., Shwartz Y., Freire S. and Baptista M., (2022), Students' mechanistic reasoning in practice: enabling functions of drawing, gestures and talk, Sci. Educ., 106(1), 199–225.
- Derry S. J., Pea R. D., Barron B., Engle R. A., Erickson F., Goldman R., Sherin B. L., (2010), Conducting video research in the learning sciences: guidance on selection, analysis, technology, and ethics, J. Learn. Sci., 19(1), 3–53.
- Dood A. J. and Watts F. M., (2022), Mechanistic reasoning in organic chemistry: a scoping review of how students describe and explain mechanisms in the chemistry education research literature, J. Chem. Educ., 99(8), 2864–2876.
- Duschl R. and Grandy R., (2008), Teaching scientific inquiry: Recommendations for research and implementation, Sense Publishers.
- Eriksson I. and Jansson A., (2017), Designing algebraic tasks for 7 year-old students – a pilot project inspired by Davydov's learning activity, Int. J. Math. Teach. Learn., 18(2), 257–272.
- Eriksson I. and Lindberg V., (2016), Enriching ‘learning activity’ with ‘epistemic practices’ – enhancing students’ epistemic agency and authority, Nordic J. Studies Educ. Policy, 2016(1), 32432.
- Eriksson I., Fred J., Nordin A. K., Nyman M. and Wettergren S., (2021), Tasks, tools, and mediated actions–promoting collective theoretical work on algebraic expressions, Nordic Studies Math. Educ., 26(3–4), 29–52.
- Guba E. G. and Lincoln Y. S., (1994), Competing paradigms in qualitative research, in N. K. Denzin and Y. S. Lincoln (ed.), Handbook of qualitative research, Thousand Oaks, pp. 105–117.
- Haeusler C. and Donovan J., (2020), Challenging the science curriculum paradigm: teaching primary children atomic-molecular theory, Res. Sci. Educ., 50(1), 23–52.
- Hammer D., (2004), The variability of student reasoning, lectures 1–3, in E. Redish and M. Vinventini (ed.), Proceedings of the Enrico Fermi Summer School in Physics, Course CLVI, Italian Physical Society, pp. 279–340.
- Haskel-Ittah M., (2023), Explanatory black boxes and mechanistic reasoning, J. Res. Sci. Teach., 60(4), 915–933.
- Hultén M., Berg A., Danielsson K. and Eriksson I., (2020), Animerad kemi: Elever i grundskolans tidiga år forklarar kemiska samband [Animated chemistry; Students in early primary school explain chemical connections]. The Swedish National Centre for Science and Technology Education – NATDID.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assisted Learn., 7(2), 75–83.
- Kang S. and Tversky B., (2016), From hands to minds: gestures promote understanding, Cognitive Research: Principles and Implications, 1(1), 1–15.
- Keiner L. and Graulich N., (2020), Transitions between representational levels: characterization of organic chemistry students’ mechanistic features when reasoning about laboratory work-up procedures, Chem. Educ. Res. Pract., 21(1), 469–482.
- Kelemen D., (2019), The magic of mechanism: Explanation-based instruction on counterintuitive concepts in early childhood, Perspectives Psychological Sci., 14(4), 510–522.
- Kress G. R., (2010), Multimodality: A Social Semiotic Approach to Contemporary Communication, Routledge Taylor & Francis Group.
- Krist C., Schwarz C. V. and Reiser B. J., (2019), Identifying essential epistemic heuristics for guiding mechanistic reasoning in science learning, J. Learn. Sci., 28(2), 160–205.
- Kurkul K. E., Castine E., Leech K. and Corriveau K. H., (2021), How does a switch work? The relation between adult mechanistic language and children's learning. J. Appl. Dev. Psychol., 72, 101221.
- Machamer P., Darden L. and Craver C. F., (2000), Thinking about mechanisms, Philosophy Sci., 67(1), 1–25.
- Mathayas N., Brown D. E., Wallon R. C. and Lindgren R., (2019), Representational gesturing as an epistemic tool for the development of mechanistic explanatory models, Sci. Educ., 103(4), 1047–1079.
- Manz E., (2012), Understanding the co-development of modelling practice and ecological knowledge, Sci. Educ., 96(6), 1071–1105.
- Metz K. E., (1991), Development of explanation: incremental and fundamental change in children's physics knowledge, J. Res. Sci. Teach., 28(9), 785–797.
- Moreira P., Marzabal A. and Talanquer V., (2019), Using a mechanistic framework to characterise chemistry students' reasoning in written explanations, Chem. Educ. Res. Pract., 20(1), 120–131.
- Newman M., (2013), Emergence, supervenience, and introductory chemical education, Sci. Educ., 22(7), 1655–1667.
- Roth W.-M. and Lawless D., (2002), Scientific investigations, metaphorical gestures, and the emergence of abstract scientific concepts, Learn. Instruction, 12(3), 285–304.
- Rundgren C. J., Hirsch R., Chang Rundgren S. N. and Tibell L. A., (2012), Students’ Communicative Resources in Relation to Their Conceptual Understanding—The Role of Non-Conventionalized Expressions in Making Sense of Visualisations of Protein Function, Res. Sci. Educ., 42(5), 891–913.
- Russ R. S., Scherr R. E., Hammer D. and Mikeska J., (2008), Recognizing mechanistic reasoning in student scientific inquiry: a framework for discourse analysis developed from philosophy of science, Sci. Educ., 92(3), 499–525.
- Russ R. S., Coffey J. E., Hammer D. and Hutchison P., (2009), Making classroom assessment more accountable to scientific reasoning: a case for attending to mechanistic thinking, Sci. Educ., 93(5), 875–891.
- Samarapungavan A., Bryan L. and Wills J., (2017), Second graders’ emerging particle models of matter in the context of learning through model-based inquiry, J. Res. Sci. Teach., 54(8), 988–1023.
- Schwarz C. V., Passmore C. and Reiser B. J., (2017), Helping students make sense of the world using next generation science and engineering practices, NSTA Press.
- Selander S., (2008), Designs for learning: a theoretical perspective, Des. Learn., 1(1), 10–22.
- Sjøberg M., Furberg A. and Knain E., (2023), Undergraduate biology students' model-based reasoning in the laboratory: exploring the role of drawings, talk, and gestures, Sci. Educ., 107(1), 124–148.
- Skolverket [National Agency for Education], (2018), Curriculum for the compulsory school, preschool class and school-age educare 2011 (revised 2018). https://www.skolverket.se/download/18.31c292d516e7445866a218f/1576654682907/pdf3984.pdf.
- Swedish Research Council, (2017), Good research practice. https://www.vr.se/english/analysis/reports/our-reports/2017-08-31-good-research-practice.html.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Practice, 14(2), 156–168.
- Talanquer V., (2018a), Exploring mechanistic reasoning in chemistry, in J. Yeo, T. W. Teo and K. S. Tang (ed.), Science education research and practice in Asia-Pacific and beyond, Springer, pp. 39–52.
- Talanquer V., (2018b), Importance of understanding fundamental chemical mechanisms, J. Chem. Educ., 95(11), 1905–1911.
- Tümay H., (2016), Reconsidering learning difficulties and misconceptions in chemistry: emergence in chemistry and its implications for chemical education, Chem. Educ. Res. Pract., 17(2), 229–245.
- Wilmes S. E. and Siry C., (2021), Multimodal Interaction Analysis: a Powerful Tool for Examining Plurilingual Students’ Engagement in Science Practices: Proposed Contribution to RISE Special Issue: Analysing Science Classroom Discourse, Res. Sci. Educ., 51(1), 71–91.
- Windschitl M., Thompson J. and Braaten M., (2008), Beyond the scientific method: Model-based inquiry as a new paradigm of preference for school science investigations, Sci. Educ., 92(5), 941–967.
- Windschitl M., Thompson J., Braaten M. and Stroupe D., (2012), Proposing a core set of instructional practices and tools for teachers of science, Sci. Educ., 96(5), 878–903.
| This journal is © The Royal Society of Chemistry 2024 |