What's in a word? Student beliefs and understanding about green chemistry†
Laura B.
Armstrong
 *a,
Lauren M.
Irie
*a,
Lauren M.
Irie
 b,
Kelly
Chou
b,
Kelly
Chou
 b,
Mariana
Rivas
b,
Mariana
Rivas
 b,
Michelle C.
Douskey
b,
Michelle C.
Douskey
 b and
Anne M.
Baranger
b and
Anne M.
Baranger
 *ab
*ab
aUniversity of California Berkeley, Graduate Group in Science and Mathematics Education, Berkeley, CA 94720, USA. E-mail: armstronglaura@berkeley.edu; abaranger@berkeley.edu
bDepartment of Chemistry, Berkeley, CA 94720, USA
First published on 19th September 2023
Abstract
For the past decade, the College of Chemistry at UC Berkeley has iteratively redesigned general chemistry laboratory courses to introduce students to green chemistry concepts, while simultaneously using green chemistry as a relevant context to learn chemistry. To investigate the effectiveness of this curriculum we developed approaches to investigate student understanding of green chemistry. We adapted a constructivist educational framework to iteratively design fixed and free response items appropriate for large enrollment courses that probe student knowledge of green chemistry concepts and practices. Two free response items were designed to probe students’ ability to define green chemistry and make green chemistry decisions in the context of a case study. A set of fixed response items were designed to probe particular aspects of green chemistry knowledge that were included in the course. Together, we used these items to characterize (1) changes in student understanding of green chemistry and (2) how prior “green” knowledge impacts student learning of new green chemistry principles in the general chemistry laboratory course. Analysis of student responses indicated that, on average, students demonstrated increased green chemistry understanding after completing this green chemistry aligned laboratory course. Students were able to integrate more normative green chemistry principles in their answers and began to indicate awareness of complex interconnected systems. Because the items focused on assessing student knowledge of green chemistry, rather than their self-assessment of knowledge, they provided valuable insight regarding students’ prior green chemistry knowledge that will be used to develop future versions of the curriculum.
Introduction
As educators it is critical that we prepare the next generation of scientists and engineers to create systems that are more efficient, safe, equitable, and sustainable than ever before. Science, technology, engineering, and math (STEM) fields play an integral role in developing and implementing many of the changes needed for a truly sustainable world – from solar panels to carbon capture to prevention instead of remediation. Our students deserve an education that allows them to not only enter science as it is right now, but to have the vision, ethics, and skills to imagine and create the field needed for their future.Developed by and for chemists, green chemistry is a relatively new framework and set of principles that provides chemistry (and science at large) with a new way of framing and practicing chemistry as it places the safety of both people and the environment at the center chemistry research and application (Anastas, 2011). Just a few decades ago, chemicals were largely designed without evaluating environmental and health impacts (Iles, 2011) and, while chemical regulations have improved in the years since, the chemical industry remains one of the largest sources of pollution and environmental hazards (Woodhouse and Breyman, 2005; Epicoco et al., 2014). Green chemists envisioned a new framework for chemistry that would initiate a relationship between chemistry and the environment and society at large (Bodner, 2015) and their work has moved the field towards developing and using renewable feedstocks (Woodhouse and Breyman, 2005; Epicoco et al., 2014), creating chemical processes that are less energy-intensive, and finding and promoting safer alternatives to widely used hazardous materials (Epicoco et al., 2014).
Mirroring the growth of green chemistry over the past decades, interest in and development of green chemistry and sustainability curricula, instruction, and courses has steadily increased (Andraos and Dicks, 2012; Haack and Hutchison, 2016). Green chemistry has been used to enhance student learning of chemistry, provide students with an ethical framework for doing and learning chemistry, and improve the safety and cost of instructional laboratories by using safer, green chemistry aligned compounds in student experiments (Haack and Hutchison, 2016). Green chemistry can enhance student thinking and chemistry abilities (Andraos and Dicks, 2012) as it allows students to participate in more authentic problem solving and inquiry. Green chemistry projects and problems often have a variety of options that need to be evaluated with no one correct answer that instructors can offer students; often, there is a range of appropriate green chemistry solutions or the answer is not even known by the chemistry community (Andraos and Dicks, 2012). Authentic green chemistry questions and research require optimizations and tradeoffs (Kitchens et al., 2006; DeHaan, 2009) and this comparative analysis leads to deeper student analyses and richer discussions (Andraos and Dicks, 2012). Bringing green chemistry into the classroom offers improved laboratory safety and unique learning opportunities for students, both of which strengthen chemistry education.
Green chemistry courses and curricula also provide students with valuable learning opportunities that allow students to see how chemistry can help solve some of the grand challenges of sustainability, which in turn brings relevance and meaning to the chemistry classroom (Burmeister et al., 2012; Bodner, 2015). Key to solving these grand challenges is considering the interconnections between chemistry and the larger environmental and planetary systems along with societal systems. Systems thinking provides an approach for systematically understanding and interpreting these interconnections that are foundational for green chemistry and can suggest how these connections then lead to systems-level phenomena (Mahaffy et al., 2019a, 2019b; Orgill et al., 2019; Mahaffy and Elgersma, 2022). There is growing interest in preparing chemistry students to apply systems thinking to solve problems in green chemistry and sustainability.
Green chemistry education at UC Berkeley
For more than a decade, the Berkeley Chemistry Education Research Group has integrated green chemistry into UC Berkeley's undergraduate chemistry education. We developed over 30 new green chemistry experiments (Table 1) for the general chemistry laboratories and iteratively created corresponding green chemistry curricula (Buckley et al., 2013; Purcell et al., 2016; Armstrong et al., 2019).| Module (# weeks) | General chemistry principles | Green chemistry principles |
|---|---|---|
| Designing a model airbag (1) | Stoichiometry, molar mass, balanced chemical equation | Atom economy, less hazardous chemical syntheses |
| How the nose knows (1) | Functional groups, physical properties, formal charges, bond-line notation, VSEPR | Designing safer chemicals, renewable feedstocks |
| Polymers: cross-linking, toy design (2) | Cross-linking reactions, intermolecular interactions, bonding, mass ratios in mixtures | Safer chemistry, safer solvents, renewable feedstocks, atom economy, waste prevention |
| Biofuels synthesis and combustion (3) | Transesterification, calorimetry, solubility, extraction, Ccal and Hcomb | Designing safer chemicals, renewable feedstocks, catalysis, safer solvent, atom economy, safer chemistry, energy efficiency, waste prevention |
| Acids in the environment (3) | Solubility equilibria, acid/base titrations, equilibrium, Le Châtelier's Principle, buffers | Real-time analysis for pollution prevention, less hazardous chemical syntheses, inherently safer chemistry |
| Extraction of curcumin and spectroscopic analysis (1) | Transmission, absorbance, extraction, calibration curves, linearity of data | Safer solvent, energy efficiency, renewable feedstocks |
In 2018, we iteratively designed a new green chemistry curriculum – General Chemistry Green Curriculum (GC2) – to accompany these green chemistry experiments. GC2 used a constructivist learning science framework – knowledge integration (Linn and Eylon, 2006, 2011b) – to introduce students to the 12 Principles of Green Chemistry (Anastas and Warner, 1998) and systems thinking (Constable et al., 2019) into the general chemistry laboratory. We developed approximately 50 new prelab and postlab green chemistry questions (with a focus on providing students opportunities to engage in evaluation or application of green chemistry principles) using the KI framework (Linn and Eylon, 2006) and created new green chemistry introductory content for the lab manual and experiment introductions. Our goal for this curriculum was to equip students with the chemical ideas, principles, and practices inherent to green chemistry and ultimately support them in using green chemistry principles and practices to make greener chemical decisions. We purposefully focused this curriculum redesign within the non-chemistry majors’ course since this introduced the largest number of students to green chemistry and showcased how green chemistry applies to a wide range disciplines and experiences outside of chemistry.
The development and implementation of GC2 presented us with a unique opportunity to document and evaluate students’ green chemistry understanding. While green chemistry has gained a strong foothold within the chemistry education community, assessment of green curricula and courses and the resulting student outcomes is in a more nascent stage of development. It is important to document the outcomes, both expected and unexpected, from green chemistry courses to ensure the goals of the curricula are being met. Assessment not only informs and improves the development of new curricula (e.g., Marteel-Parrish, 2014; Andraos and Dicks, 2015; Garner et al., 2015; Paluri et al., 2015), but also provides support for sustained support of these courses.
Over the past decade, there has been an increased focus on assessing green chemistry outcomes. Green chemistry assessments areas have ranged from student knowledge (Mandler et al., 2012; Gron et al., 2013; Karpudewan et al., 2012, 2015a, 2015b, 2016; Guron et al., 2016; Shamuganathan and Karpudewan, 2017); to attitudes, motivation, and values (Karpudewan et al., 2012, 2015a, 2015b; Mandler et al., 2012; Guron et al., 2016; Shamuganathan and Karpudewan, 2017); to laboratory skills (Gron et al., 2013); to systems thinking (Talanquer, 2019; Reyes et al., 2023; Reynders et al., 2023). Most of these studies rely on surveys or questionnaires (Armstrong et al., 2018) that utilize Likert and sometimes free response items (Gron et al., 2013; Aubrecht et al., 2015; Purcell et al., 2016). However, this type of assessment requires respondents to self-assess their knowledge or skills, allowing the researcher to capture only what students believe they know about a given topic. Self-assessment of knowledge is often not a reliable measure of cognitive learning (Davis et al., 2006; von Blottnitz et al., 2015) and instead provides a measure of affective components (Sitzmann et al., 2010). Some researchers (e.g., Galgano et al., 2012; Gron et al., 2013; Marteel-Parrish, 2014; Andraos and Dicks, 2015) have used student course work to explore student outcomes, while others have used interviews and focus groups to provide a more holistic picture of student understanding (Mandler et al., 2012; Karpudewan et al., 2015a, 2015b; Shamuganathan and Karpudewan, 2017), but these are time intensive to conduct and analyze and thus, can often only be used with a small number of students.
Since much of the reported green chemistry assessments rely on students’ self-assessment of their understanding or takes place in much smaller enrollment classes (Armstrong et al., 2018), we focused on developing alternative modes of assessment that could be used to assess green chemistry student learning for large enrollment courses. These considerations led us to develop and use a hybrid assessment model to investigate the evolution of students’ green chemistry understanding as they complete our large enrollment green chemistry aligned course. Namely, we developed both fixed and free response green chemistry items to explore the extent to which students understand and apply green chemistry concepts and practices and document changes in student demonstrated understanding and use of green chemistry after completing GC2. We developed coding rubrics to capture the breadth and depth of students answers to green chemistry items and their development towards understanding the complex, interconnected systems that influence green chemistry decisions. Ultimately, we aim for the data from these assessments to inform future instruction and curricular content and design and provide a holistic framework for more effective teaching and assessment.
Theoretical framework
Constructivism is a theory of learning based on the idea that people are not “blank slates” that simply absorb information from others, but rather actively construct their own understanding of the world through the interplay of their prior knowledge, observations and experiences, and reflection (Bodner, 1986; Phillips, 1995; Honebein, 1996; Hyslop-Margison and Strobel, 2007; Bada and Olusegun, 2015). Constructivism posits that learning occurs when meaningful connections are made between prior and new knowledge and that these connections are mediated by both cognitive and affective considerations (Bodner, 1986; Phillips, 1995; Bada and Olusegun, 2015).Green chemistry education and assessment often inherently align with the pedagogical goals of constructivist learning environments such as embedding learning in realistic contexts and providing opportunities to evaluate alternative solutions from multiple perspectives (Honebein, 1996). In contrast with the static, segmented style of teaching chemical concepts featured in more traditional chemistry curricula, green chemistry topics are intrinsically situated within a greater system. This creates opportunities for nuanced discussion, where there is often no single correct answer and students’ prior knowledge and experiences can and should provide rich and valuable contributions. Indeed, while the term “green chemistry” may be new to students, many green chemistry concepts may be familiar from product marketing, books, documentaries, personal interests, prior courses, etc. Thus, it is especially important to make explicit this knowledge at the beginning of course or session so that instruction or curriculum can be tailored to help students add and distinguish between new ideas and their prior knowledge base (Linn and Eylon, 2011a). It is also important to continually assess if normative connections form between prior and new knowledge and, ultimately, if students exit a course having a more complete understanding of the desired green chemistry learning outcomes.
We used a constructive framework – Knowledge Integration (Linn, 2006; Linn and Eylon, 2006, 2011a) – to design our new general chemistry green curriculum (GC2), which made it the natural choice to also use for the generation and refinement of green chemistry assessment items. Knowledge integration (KI) is an ideal framework for green chemistry education and assessment as it values the prior knowledge and experiences that students bring to the course, while also providing opportunities for students to add, distinguish, and reflect on their prior ideas and new knowledge (Linn and Eylon, 2006). We used KI to design both the formative and summative green chemistry assessments integrated into the course curriculum as well as the paired pre/post research items; we designed a set of items that (1) allowed students to demonstrate any prior green-aligned knowledge or beliefs at the start of the course and (2) identified changes in student understanding after completing a green chemistry focused laboratory course.
Research questions
In this work we focus on the assessment of students’ demonstrated understanding of green chemistry during and after a one semester green chemistry laboratory course at UC Berkeley. Our research question focuses on:To what extent are students able to define green chemistry principles and make green chemistry decisions? In what ways do student understandings change after completing the General Chemistry Green Chemistry (GC2) laboratory course?
This work contributes to the body of literature on green chemistry assessment by exploring the complexity of students’ understanding of green chemistry and how different facets of student knowledge can be observed depending on item design and context. Additionally, assessment of student responses provides the opportunity to design iterative improvements to a curriculum. With such assessments in place, the goal is to create a feedback cycle for instructors and students such that we can identify areas of improvement needed in the curriculum and relay this information to the students so that they can learn from their previous work.
Methods
Research context
The same laboratory instructor taught and ran the course for both semesters of this study and 30 graduate teaching assistants (TAs) taught the individual laboratory sections each semester. TAs attended weekly meetings with the course instructor where they reviewed the upcoming laboratory experiment and grading rubric and discussed the structure for the TA prelab lecture. TAs were also provided with detailed grading rubrics and instructor notes with suggests for green chemistry topics to introduce or review during prelab lectures. The Fall 2018 semester was used to pilot the implementation of both the new curriculum (GC2) and assessment items. Full data collection occurred during the Fall 2019 semester once the curriculum and item design was finalized (Table 2).
| Fall 2018 | Fall 2019 | |
|---|---|---|
| (Pilot) | (Full data collection) | |
| Instructor | Laboratory instructor | |
| Graduate teaching assistants (TAs) | 30 TAs | 30 TAs |
| TA Training/support | Weekly TA meeting with laboratory instruction, grading rubrics for new prelab and postlab green chemistry question, and in-lab observation prompts, TA notes for green chemistry introductory content | Weekly TA meeting with laboratory instruction, grading rubrics for revised prelab and postlab green chemistry questions and in-lab observation prompts, additional TA notes with suggestions for green chemistry topics to introduce or review during prelab lectures |
| Data collection | Full data collection and analysis for one (1) free response green chemistry item; pilot of one (1) additional free response item | Full data collection and analysis for two (2) free response and three (3) fixed response green chemistry items |
| Semester | Number of students in course | Number of students who consented |
|---|---|---|
| a The total number of students in the study exceeded the total number of students in the course due to attrition during the semester. The total number of enrolled students is calculated at the end of the semester while the number of students in the study comes from both the pretest and posttest surveys. | ||
| Fall 2018 | 1086 | 1010 |
| Fall 2019 | 1031 | 1040a |
The students in this study encompassed a wide range of intended majors including, but not limited to, life sciences, bioengineering, nutrition science, public health, environmental science, and civil engineering. The majority (73% for Fall 2018 and 68% for Fall 2019) of students had at least one parent with a four-year degree and nearly 50% have a parent with a graduate degree. Nearly every student had taken at least one semester of chemistry prior to their entry into the university with, on average, having completed three prior semesters of chemistry. About half of the students had completed two semesters and ∼40% had completed four or more semesters of chemistry before entering Chem 1AL. More than half of the students had taken honors chemistry (60%) and/or AP chemistry (48%). As is typical, the courses had more female (65%) than male (35%) students. Most students were of Asian descent (∼57%) with White (∼26%), Latinx (∼12%) and African American (∼1%) students comprising the remainder of the class. More detailed demographic information can be found in the ESI.†
Description and administration of items
Students completed the in-class green chemistry items at the beginning and end of the Fall 2018 and 2019 semesters. Students were given 10 minutes to complete these two items during the first and last laboratory sections of the semester. Standardized instructions were given to each graduate teaching assistant for the administration of the quiz in their laboratory section (ESI†). Students were advised that these items would be graded based only on effort and that if they did not know how to answer an item, they should state “I don’t know but my best guess is….” Students were also asked to not discuss these items with other students in the course so students in later laboratory sections would not try to prepare for the items. Nearly every student enrolled in the course completed these items; additionally, the in-class administration allowed for student responses to be collected without access to search engines or other outside resources.
Students completed these multiple choice items at the beginning and end of the Fall 2019 semesters through an online Qualtrics survey. The pretest survey was administered during the first two weeks of the semester and the posttest survey was administered during the last two weeks of the semester. The link to the survey was distributed through a personalized announcement on the course learning management system. The course instructor also announced the survey during her lectures. Two reminder announcements were posted for each survey – one several days before the due date and one the day the survey was due. The respondents had between 7–10 days to complete the survey. All respondents received course bonus points for completing the online survey. If students did not want to complete the survey but still wanted to receive the course bonus points, they could instead write a one-page essay on a recent green chemistry innovation.
Analysis of items
Respondents who did not consent to participate in the research study were dropped from the dataset. Respondents who did not complete both quizzes (pretest and posttest) were also dropped from the dataset leaving 636 respondents for the Fall 2018 semester and 615 respondents for the Fall 2019 semester.| Category | Unsupported response | Supported response |
|---|---|---|
| Renewability | “The best way would be via cinnamon tree barks as it is a renewable method.” | “Cinnamon trees can be planted making them less scarce than fossil fuels which cannot be regenerated.” |
| Harmful byproducts | “I would choose the method based on…. whether it produces toxic byproducts” | “Look at the toxicity of the byproducts….check CO2 emissions for each method.” |
| Less waste/waste disposal | “I’d see if there are a lot of waste or byproducts by each method.” | “I think the best method to use for making cinnamaldehyde would be the method that creates less trash and pollution to begin with this is important because it is easier to create less trash in the first place, then clean it up after.” |
While coding through the initial 20% responses, several new coding categories emerged. Amount of Material included responses that discussed the amount of material involved for either of the two methods without tying the physical amount to other categories, such as hazards, waste, renewability, yield, or any of the other categories. This category captured students who understood that the amount of material used or produced (as byproducts) was important when considering green chemistry but did not articulate why it was important. Categories such as Yield and Atom Economy were also added as many students included these topics in their responses but did so without explanation or evidence to support their claims. A final category was also added to capture the responses that implied or assumed that benzaldehyde (an intermediary for one method) was a toxic or harmful substance.
Finally, a coding category – Sustainable Systems – was added that was distinct from but aligned with the 12 Principles of Green Chemistry. The original coding scheme for Two Methods Choice was based on the 12 Principles and used a similar categorization method as the Green Chemistry Definition coding scheme. However, we quickly observed that many responses focused on specific aspects of sustainability or environmentalism that fell outside the traditional 12 Principles language. Concerns about the environment had been a common but vague theme when students defined green chemistry. In contrast, in the Two Methods Choice responses, environmental concerns were much more detailed and contextualized within the actual scenario. For example, a response might focus on the potential harm that removing tree bark from a tree might cause – “is the tree still able to survive and thrive” after this process? Other examples included land use and potential deforestation for the tree bark method. For example, Stephanie said that she would “look at how each method produces the substance in terms of the amount of land…required to produce the molecule (i.e., if it diverts land from agriculture, requires deforestation to clear land for production…)”. These responses demonstrated a holistic and systems thinking approach to green chemistry, focusing on topics beyond the immediate laboratory and/or about the extent and magnitude of choices made in the laboratory. This broad category encompassed topics such as life cycle analysis, ethical considerations with respect to environmental issues, habitat/ecosystem impact, human health and safety, and practices utilized in extraction/production of raw materials.
Using this new coding scheme, two researchers independently coded an additional set of student responses and discussed their results to achieve complete agreement. The inclusion and exclusion criteria were revised based on these results. This process was repeated until no additional changes to the codebook (ESI†) were produced. The remaining student responses were divided and independently coded by two researchers. Any unexpected responses were discussed by both researchers until consensus was reached. If consensus could not be reached, a third researcher was brought in to break ties.
Breadth and depth scores were created to capture (1) the number of green chemistry components mentioned (total breadth score) and (2) the number of times a response provided a justification for including a green chemistry component (total depth score). The breadth score was simply the sum of all green chemistry components (renewability, hazardous byproducts, hazardous reactants, reducing waste, economics, yield, atom economy, amount of material) present in a response regardless of whether they were supported or unsupported. The depth category was a sum of only the supported coding categories (supported renewability, supported hazardous byproducts, supported hazardous reactants, supported reducing waste, supported economics, supported Sustainable Systems) present in a response. This summative score was designed to capture if students provided a justification for including a particular normative green chemistry component.
| Category | Definition |
|---|---|
| Full correct | Respondent selects all correct choices and no incorrect choices |
| Incomplete correct | Respondent selects only correct choices but not all of the correct choices; no incorrect choices are selected |
| Partially correct | Respondent selects one (or more) correct choice(s) but also selects one incorrect choice |
| Incorrect | Respondent selects more than one incorrect choices |
| I don’t know | Respondent selects the “I don’t know” choice |
All analyses were completed using StataSE 14.2 and Python 3.9.
Results
One of the main goals of this work was to create assessment items that allowed us to investigate student understanding of green chemistry (and related concepts) and ability to apply green chemistry principles and practices to novel scenarios. We developed both free and fixed response items to explore the depth and breadth of student green chemistry knowledge. The free response items were intentionally designed to give students the opportunity to demonstrate any and all of their knowledge about green chemistry, which would help reveal their prior “green” knowledge and beliefs at the start of the course and illustrate in what ways normative green chemistry ideas were integrated into their understanding after completing GC2. The fixed response items, while designed to probe targeted green chemistry concepts or principles, also helped measure the knowledge and beliefs that students brought into the course and how these shifted after completing GC2.Green chemistry definition
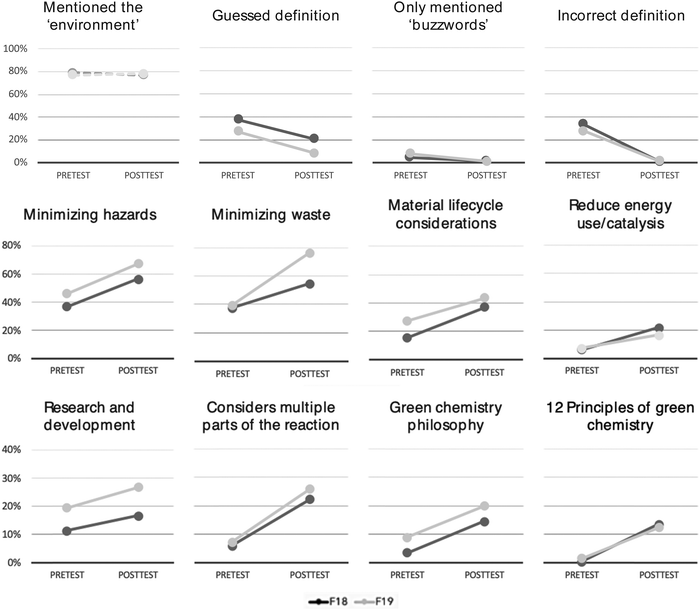
An open-ended Green Chemistry Definition item (Table 4) was used to explore how students conceptualized green chemistry as a discipline and/or as a framework for practicing chemistry. This item had been developed several years prior to the current study and had been used in a previous research (Armstrong et al., 2019). The open-ended structure of this item gave students the opportunity to demonstrate any and all of their knowledge about green chemistry and did not necessitate prior chemistry knowledge. This was important since students entered Chem 1AL with a wide range of prior chemistry experience and may or may not have ever heard of green chemistry prior to this course.Similar trends were seen across both semesters with, on average, a decrease in guessing, incorrect responses, and buzzword-only responses by the end of the semester (Fig. 1). Mentions of environmentally aligned terms did not change appreciably between the pre and posttest for either semester with approximately 78% of responses containing these phrases. An exact McNemar's test determined that there was not a statistically significant difference in the proportion of students who were coded into this category pre- and post-course (NFall19 = 615, pFall19 = 0.551; NFall18 = 636, pFall18 = 0.387). This stasis was expected since there is nothing inherently wrong with mentioning the environment when defining green chemistry. In fact, many popular definitions of green chemistry contain references to the environment including one such definition by the “father of green chemistry” Paul Anastas (2011): “Green chemistry requires looking across systems and across life cycles to design products and processes that are benign to both people and the environment.” What is important and interesting is how students connect and integrate these environmental terms with other normative green chemistry ideas and practices.
In contrast to students’ pre-course responses of which approximately 40% were incorrect or only mentioned buzzwords, student's post-course responses mentioned at least one specific component of green chemistry 82% of the time for both semesters. Minimizing hazards and waste were the two most common specific components included, followed by material lifecycle and energy use/catalysis (Fig. 1). Exact McNemar's tests showed that there was a statistically significant difference in the proportion of students who were coded into each category pre- and post-course (NFall19 = 615, pFall19 < 0.001; NFall18 = 636, pFall18 < 0.001).
“[Green chemistry is] [c]hemistry that takes into consideration the environmental impacts of any products or process included in experiments. Green Chemistry aims to create an understanding of the importance of the world's natural resources in a field that is normally seen as dealing with artificial substances/processes.”
After completing the course, Mei's view of green chemistry still included the core idea that green chemistry should “create a healthier world” but specifically linked normative green chemistry ideas to this framing, giving it clear specificity and scope. Mei took a holistic view of green chemistry arguing that it applies in both industrial and research settings and tied waste prevention, renewable resources, energy efficiency, and reducing chemical hazards to the idea of true sustainability:
“[Green chemistry means] [e]nsuring that our chemical processes, whether industrial, experimental, or otherwise are part of a sustainable cycle. This means reducing and eliminating waste, using renewable sources and limiting the production of harmful byproducts. These are only a few green chemistry concepts that aim to create a healthier world that will last for generations to come. Energy efficiency also plays a role in green chemistry.”
Students not only talked about common principles of green chemistry but also demonstrated increased holistic or systems thinking (Constable et al., 2019; Dicks et al., 2019; Flynn et al., 2019; Hutchison, 2019), as they considered the broader categories shown in the bottom row of Fig. 1. Overall, at the start of the course, very few students understood that green chemistry strives to create new technologies, methods, and other innovations (research and development) but by the end of the course 17%/27% (Fall 2018/2019) of students were able make this connection. Similarly, at the start of the course for both semesters less than 8% of student responses acknowledged that green chemistry targets all aspects of a chemical process (reactants, reaction, products/byproducts), but by the end of the semester 22%/26% (Fall 2018/2019) of student responses considered multiple components of the reaction. Additionally, after completing Chem 1AL, 14%/20% (Fall 2018/2019) of students discussed how green chemistry is a philosophy for all chemistry – not just a niche topic – demonstrating a more nuanced understanding of green chemistry as a metadiscipline (Woodhouse and Breyman, 2005; Linthorst, 2009; Epicoco et al., 2014). Finally, at the beginning of the course for both semesters, less than 1% of students explicitly mentioned the 12 Principles of Green Chemistry (Anastas and Warner, 1998) in their definition nit after completing the course, 14%/12% (Fall 2018/2019) of responses did explicitly mention this framework in their definition of green chemistry and 82% of responses implicitly mentioned one or more of these principles for both semesters (which is defined by the presence of one or more of the categories in Fig. 1).
One student, Kristen, included many holistic components in her definition of green chemistry at the end of the Fall 2019 semester. She first made explicit the many dimensions that green chemistry must include (the environment, human health, economics) and that these decisions occur along the entire lifecycle of a chemical process. She also brought specific green chemistry principles that target both the reaction process and resulting byproducts and recognized that every decision made within chemistry should be done with attention to the entire system it impacts:
“Green chemistry means acknowledging the environmental, human, and economic consequences of any decisions made before, during, or after conducting chemistry. It means striving to create the most efficient reactions and mitigating byproducts, striving for renewable, reusable compounds to be used in experimental, industrial, and everyday practice, limiting toxicity to human health, and many other concepts, all united by this prospect of being cognizant of the effects of our decision in chemistry.”
It is important to note that no one student's definition was expected to include all the coding categories for this item as the coding scheme was developed to capture the breadth of categories that could be included in a definition. Most pretest student definitions used green-aligned terms or phrases, but with minimal demonstrated understanding of those words/phrases. Students tended to integrate only one specific component of green chemistry into their pretest definition and did not demonstrate a sophisticated understanding of systems thinking. In contrast, posttest definitions still had many green-aligned terms (e.g., buzzwords), but with the addition of green chemistry principles, examples, and explanations. Students also showed an increased holistic green chemistry perspective with detailed inclusion of many of the 12 Principles. Overall, after completing the course, most students agreed that green chemistry is aligned with reducing hazards and waste. They see green chemistry as a framework for doing chemistry and a minority of students can also recognize the complex, innovative, and interconnected nature of green chemistry.
Two methods choice
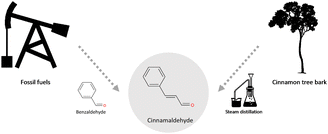
The Two Methods Choice item was developed and piloted over the Fall 2018 and Spring 2019 semesters and finalized for the Fall 2019 semester. This item was designed to complement the Green Chemistry Definition item as it was a broad scenario that would let students showcase their knowledge of green chemistry. However, in contrast to the previous definition item, Two Methods Choice did not simply ask for students to define a term but rather make a green chemistry decision and rationalize that decision (Table 3). While any green chemistry principle could have been used to support their choice, this item did naturally focus on the ideas of renewability and energy usage, which had only been infrequently mentioned when students defined green chemistry (Fig. 1).There was no one “right” answer for this item (as is often the case with green chemistry decisions) and instead it was an opportunity for students to demonstrate how they would approach using green chemistry principles to make a decision. However, most students agreed that the method that used cinnamon tree bark as a starting material was the greener method with 92% of students choosing this method on the pretest which increase to 95% on the posttest. Only 6% of students chose the method that used fossil fuels as a precursor on the pretest which dropped to 3% on the posttest. The remaining 2% of students did not indicate which method they believed was greener.
This item also showed that students prioritized different topics/ideas from the previous Green Chemistry Definition item. In this new context, student responses showed that they understood that renewable feedstocks were one of the most relevant green chemistry dimensions for justifying either method choice. For example, Gregory made an explicit comparison based on renewability between tree bark and fossil fuels. He also explicitly connected the idea of renewability to timescales stating that the timescale for fossil fuel regeneration was not a feasible option:
“Making pumpkin spice from cinnamon tree bark is preferable from making it from fossil fuels because trees are a renewable resource, while fossil fuels are a limited resource. We can always grow more cinnamon for more production of pumpkin spice, but we’d need to wait billions of years for more fossil fuels to be created.”
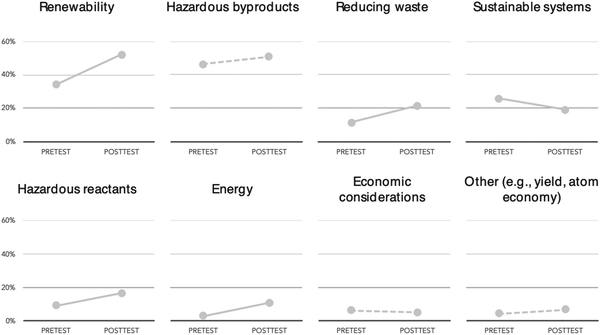
As with Gregory's response most student responses focused on how cinnamon tree bark was a potentially renewable feedstock for cinnamaldehyde especially in comparison to the fossil fuel precursor method. Overall, renewability considerations were the most prominent posttest category and showed the most growth from pretest to posttest; at the beginning of the semester 34% of students mentioned renewability considerations in their response, which increased to 52% of students by the end of the semester (Fig. 3).
While most categories increased or stayed constant from pretest to posttest, one category – Sustainable Systems – decreased significantly from pretest to posttest (N = 615, p = 0.003). Sustainable Systems included responses that focused on how the decision would have an impact beyond the immediate laboratory (e.g., ethical considerations about environmental issues, habitat/ecosystem impact, human health and safety, and practices utilized in extraction/production of raw materials). The decrease for Sustainable Systems was expected as it was hypothesized that posttest responses would shift away from broad (and often colloquial) environmental ideas towards more normative green chemistry principles as students had just completed a course focused on those formalized ideas and practices. Indeed, the number of supported responses (Table 6) for Sustainable Systems (i.e., those that provided a justification this idea) remained constant from pre to post-course at 4% while the number of unsupported responses (Table 6) for Sustainable Systems decreased from 21% to 15%. Additionally, students who had initially included Sustainable Systems ideas in their pretest response but no longer included it in their posttest response tended to make larger gains in the number of normative green chemistry categories included in their posttest response compared to students who either never had included Sustainable Systems in their pretest response or still included it in their posttest response.
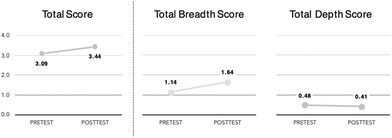
On average, the post-course breadth score was higher than pre-course scores, but the depth score was, on average, lower after the course (Fig. 4). A Wilcoxon signed-rank test indicated that the median breadth post-course ranks were statistically significantly higher than the median pre-course ranks for both semesters (Z = 9.13, p < 0.001). However, the median depth post-course ranks were statistically significantly lower than the median pre-course ranks (Z = −2.31, p = 0.02). This indicates that students included more green chemistry concepts/ideas (greater breadth) in their posttest responses but did not include additional explanations/justifications (similar depth) for using these green chemistry components to rationalize their method choice. While 89% of the post-course responses mentioned at least one specific green chemistry component (and 32% mentioned two, and 19% mentioned three or more components) only 34% of responses provide any justification for including one or more of those components. Most students tended to list the factors they consider important for making a green chemistry choice but did not provide evidence for the inclusion of those factors. For example, Brooke's response below mentions many green chemistry principles – often by name (reducing harmful byproducts, energy efficiency, renewable feedstocks, waste prevention, inherently safer chemistry) – but does not explain how or why these are important to know and/or simply asserts that these principles are better for her chosen method:
Steam distillation:
• Doesn’t produce greenhouse gases as a result of fossilization
• More energy efficient → requires less energy (i.e., heat, power) to obtain chemical
• Renewable feedstock – chemical compound obtained from cinnamon tree bark (nature) rather than drilling down to find it.
• Waste prevention – may not produce as much byproduct as fossil fuels would
• Inherent safe chemical process – the extraction process on cinnamon tree bark is safer than fossils.
Interestingly, it appears that a shift occurred from pretest to posttest where responses began to favor breadth of response over depth of response. Indeed, only 30% of students applied more than one green chemistry component for their pre-course response choice. On the posttest however, students had a wider range of green chemistry principles and practices to draw from to support their method choice; 50% of students now used two or more green chemistry components to support their method choice and 20% used three or more.
Select all green chemistry concepts
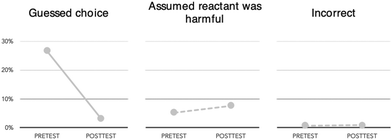
The three select all multiple choice items were designed to probe specific green chemistry ideas observed while coding the Green Chemistry Definition item during the Fall 2018 semester. These three items targeted (1) atom economy, which is a specific green chemistry metric that provides a measure for how efficient a reaction is at the molecular level, (2) lethal dose, 50% or LD50, a widely used measure for the acute toxicity of a compound, and (3) natural versus renewable processes. Many student responses to the Green Chemistry Definition item had focused on “hazards/toxicity”, “efficiency”, and/or “renewability” without clearly defining what those terms meant in the context of green chemistry. Additionally, student responses (both to the Green Chemistry Definition item and to course laboratory manual questions) had occasionally appeared to conflate renewability with natural products/process. Thus, these three fixed response items were used to probe specific concepts already observed in student responses.The select all items illustrated differences in student confidence in their prior knowledge and the degree to which that knowledge shifted towards more normative understanding after completing the GC2 laboratory course (Fig. 5). Students were largely successful on the first item, which focused on atom economy, even before experiencing most of GC2. Most students, both pre- and post-course, were either completely correct or incompletely correct (selected one of the two correct choices with no incorrect choices) on this item. The positive performance on the pretest (including the low frequency of students saying they did not know how to respond to this item) was not surprising since atom economy is the first green chemistry principle introduced to students during the course. While the pretest survey was administered during the first week of the course students would still have attended their first lab lecture and started preparing for their first experiment during this time. Indeed, several students mentioned having heard of green chemistry (or related terms) during their first lab lecture or from reading the introduction to their lab manual.
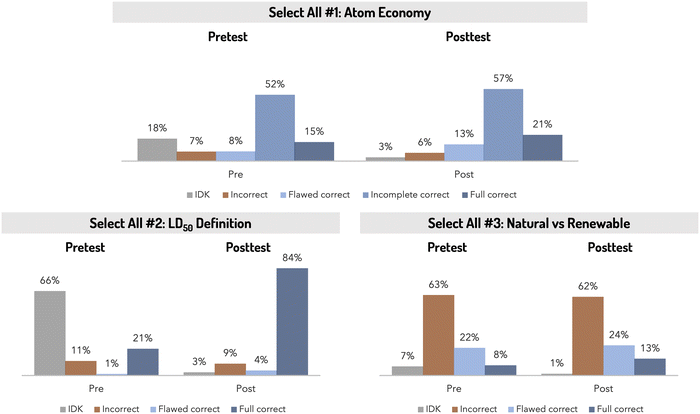 | ||
| Fig. 5 Frequency distributions for all “select all” multiple choice items (N = 508). Full item prompts can be found in Table 5. | ||
Students showed considerable gains from pretest to posttest in their ability to answer the second item, which focused on defining LD50. This concept was not covered at the beginning of the course but was introduced and then reinforced later in the semester. This concept was one that students could clearly recall: 66% stated that they did not know the answer on the pre-test, while 84% answered the item correctly on the posttest. In contrast, most students incorrectly answered the third item, which focused the differences between natural versus renewable processes, both on the pretest and the posttest. Unlike the first Atom Economy item that focused on a concept students were introduced to during the first week of the course, renewability (and especially the comparison to “naturalness”) was not introduced until after the pre-course survey had been completed. And, unlike the second LD50 item that focused on a concept students learned about through the course and subsequently correctly answered on the posttest, this third item had no appreciable shift in correctness from the pretest to the posttest indicating that the course curriculum was not able to appreciably shift student understanding or belief around natural and renewable processes, despite this topic being addressed during a three-week biofuels laboratory module midway through the course.
Discussion
The development of the general chemistry green curriculum (GC2) provided an opportunity to explore student understanding of green chemistry within the context of a large enrollment, non-chemistry major, general chemistry laboratory course. The goal of this work was to document the green chemistry ideas and beliefs students brought into the classroom as well as changes in their understanding of green chemistry after completing this course. The items developed for this study allowed students to demonstrate their understanding of green chemistry and related concepts and apply green chemistry principles to novel scenarios while making green chemistry decisions. The design and use of both free and fixed responses items enabled data to be collected from most students in the course while also allowing for meaningful and timely analysis for the hundreds (and in some cases thousands) of responses to each item by a small team of researchers.Students gain knowledge about green chemistry
Overall, our research showed that students demonstrated increased green chemistry understanding after completing the GC2 laboratory course. At the start of the semester, students defined green chemistry (Green Chemistry Definition item) using colloquial “green” terms (such as sustainability, eco-friendliness, efficiency) and included incorrect statements or assumptions; these definitions, on average, only mentioned one specific component of green chemistry and did not demonstrate an understanding of systems thinking. After completing GC2, students built upon their starting definitions and gained confidence in their understanding of green chemistry. Post-course definitions still had many green-aligned terms but integrated normative green chemistry principles, examples, and explanations and began to include explanations of how chemical reactions and processes operate as and within complex interconnected chemical, ecological, and societal systems.Students’ abilities to make choices between two generalized processes from a green chemistry perspective (Two Methods Choice item) also meaningfully changed after completing the course. Most students (nearly 90%) were able to correctly identify one or more of the main factors for deciding between the two methods. As with their definition of green chemistry, students were able to include more normative green chemistry ideas in their justification for choosing one method over the other after completing the course. However, student responses decreased in depth at the end of the course (Fig. 4). While students provided more criteria for making a green decision they did not justify how or why those criteria applied to the given problem. There are several potential explanations for this shift. First, it is possible that students had enough familiarity to know these green terms applied to the scenario but not enough robust understanding (either of the terms themselves or in the norms for justifying a decision) to fully explain the specific connections or usefulness that these criteria had for the scenario. Second, by the posttest, students may have assumed that there was a shared understanding of what these terms and principles meant within the class and thus they did not think it was necessary to define or explicitly explain why or how they were important or relevant. Finally, students only had 10 minutes to complete both this item and the Green Chemistry Definition item for both the pre and posttest. Since students, on average, included more components of green chemistry in their post-course response this may have led to decreased time for students to fully explain their increased number of green chemistry ideas and principles.
Overall students moved away from more colloquial ‘green’ and environmental language and towards more normative green chemistry ideas. In their definitions, students indicated that green chemistry is a framework for doing chemistry, and a minority of students can also recognize the complex, innovative, and interconnected nature of green chemistry. However, the decrease in Sustainable Systems (a holistic coding category that captured impacts beyond the immediate laboratory) on the posttest paired with the increase green chemistry ideas and principles introduced in the course (Fig. 4) indicated that it was difficult for students to balance adding or integrating specific green chemistry concepts with more holistic understandings of habitat/ecosystem impact, human health and safety, and environmental ethics. While it was encouraging that students were able to provide multiple relevant green chemistry ideas, they often did not include accompanying justifications or explanations for how or why those ideas apply to a given scenario. These results suggest that students need additional support and opportunities to connect their more general or holistic understandings to applicable scenarios, questions, and problems and more practice managing the complexities and tradeoffs of green chemistry systems. This outcome aligns with related assessments of student understanding of systems thinking in introductory chemistry courses in which students struggle to connect and apply theoretical understandings to practice in the real world (Talanquer, 2019; Reyes et al., 2023; Reynders et al., 2023).
Student prior knowledge of green chemistry
In alignment with constructivist theory (Honebein, 1996; Linn and Eylon, 2006, 2011a), all of the green chemistry items showed that students entered the course with “green” ideas and beliefs and in some cases sophisticated green chemistry knowledge, which was reinforced and built upon during this course. At first glance, it may appear that student understanding of green chemistry was naïve or superficial at the start of the semester. This is certainly true in some cases (and regardless, in most cases, there was still room for substantial growth), but probing specific terms using fixed response items allowed students to demonstrate latent knowledge at both the beginning and end of the course. For example, very few student responses to the free response items (Green Chemistry Definition or Two Methods Choice) mentioned the concept of atom economy at the start of the course and yet most students could partially or fully define this idea when directly asked via the fixed response select all item. Similarly, students were able to correctly apply green chemistry principles to novel green scenario even at the beginning of the semester (Fig. 3 and 4).Student responses, while aligned with the 12 Principles, also showed that students conceptualized green chemistry as more than the 12 Principles. Students brought up more holistic themes that revealed their ideas outside of the 12 Principles (ethics, values, tradeoffs, philosophy, environmentalism). A significant minority of students saw green chemistry as a new philosophy for conducting chemistry; green chemistry was not simply a list of principles to check off but rather a way of doing chemistry that considered the short- and long-term impacts that a chemical reaction or process could have across multiple dimensions (Fig. 1). Students believed that green chemistry valued and prioritized human and environmental outcomes and often used those outcomes to explain or justify the importance of many of the formalized principles of green chemistry. The emergence of Sustainable Systems category in the Two Methods Choice item demonstrated that students brought prior knowledge or experiences that aligned with green chemistry values but were not explicitly stated in those principles. These items, particularly the Two Methods Choice, allowed students to construct responses based not only on what they learned from the curriculum, but also from their own personal values and prior knowledge.
However, students’ ideas around environmentalism were mainly focused on a general or abstract idea of the “environment” and not tied to any specific outcomes or other dimensions (e.g., worker health and safety, environmental justice) indicating that instructional guidance around extensions or applications of the 12 Principles is needed. Indeed, many educators believe that green chemistry curricula need to extend beyond the 12 Principles of Green Chemistry to include societal factors (Burmeister et al., 2012) with courses grounding these societal impacts within a local geographic area or introducing students to green chemistry through case studies (Karpudewan et al., 2015a). Others advocate for the application of green chemistry (or related ideas) to social justice problems and the development of humanistic approaches to chemistry (Burmeister et al., 2012; Sjostrom and Talanquer, 2014; Sjostrom et al., 2016).
Students’ reliance on prior awareness of “naturalness” and its relationship to greenness was also seen in initial definitions of green chemistry. Melissa's initial definition of green chemistry showed that, to her, green chemistry was aligned with environmental concerns and natural products or processes:
“I don’t know but my best guess is using natural compounds to make chemicals and other compounds that are useful to us. “Green” usually has the connotation of environmentally friendly or natural, so that is why I assumed it has to do with natural compounds.”
Eric's initial definition of green chemistry showed a similar tendency towards conflating green chemistry with reducing environmental harm and naturalness while also conflating the idea of renewability and naturalness:
“I don’t know, but my best guess is that green chemistry heavily emphasizes procedures and method which try to minimize harmful effects on the environment…They want the chemistry to be natural so biofuel as opposed to regular diesel fuel…”
For a concept like natural or renewable products, students entered the class with some knowledge or familiarity about this topic as evidenced by the majority of students who felt confident providing a (non-normative) answer to the Natural vs. Renewable select all item (Fig. 5) as well as using naturalness to define green chemistry at the beginning of the semester. The course curriculum (GC2) was unable to make a meaningful impact on students’ understanding of the complicated differences between these natural and renewable products and processes as students remained attached to their previous ideas about these two topics even after completing the course. However, it appeared that students were able to easily integrate a low prior knowledge concept like LD50 into their existing conceptions of chemical safety or toxicity. Most students had not heard of this idea prior to the course so there was very little prior understanding or beliefs to shift or align with this new metric.
It was expected that students would be less successful on the Natural vs. Renewable item as understanding the differences between natural and renewable products is much more complex than learning a definition for LD50. However, it was unexpected that so many students would be so confident in their answers – especially before any direct instruction on green chemistry. These prior beliefs may make the integration of new normative ideas difficult. This is consistent with previous findings that students maintain misconceptions that limit their conceptual understanding of fundamental chemistry concepts (Bergquist and Heikkinen, 1990; Bodner, 1991). Instructors teaching green chemistry should consider that learning green chemistry terms and ideas may differ depending on how much prior exposure students have to those terms and ideas. A concept like LD50 or atom economy that has not become commonly used outside of green chemistry communities will be much easier to introduce to students than concepts like natural products or renewability. More targeted teaching of these high prior knowledge topics and more explicit surfacing of this prior knowledge (Linn, 2006; Linn and Eylon, 2011a, 2011b) is needed to aid in the learning of new, and often contradictory, information around these complex ideas that have made their way into everyday conversations and meaning.
Limitations
This research was conducted at UC Berkeley during the Fall 2018 and Fall 2019 semesters. Berkeley (both the city and the university) has the perception of being a liberal city and aligned with green policies, which potentially could lead to a liberal, green skewed incoming student population for Chem 1AL (or subsequently influence student beliefs, attitudes, and knowledge around green topics). Additionally, the knowledge that students brought into the class was necessarily influenced by events that occurred in their communities, states, and countries. The discourse around topics such as green chemistry (and the related topics of sustainability, climate change, renewable energy, etc.) is constantly changing and can vary by news source and political administration. What students knew and believed during Fall 2018 and Fall 2019 might be very different than what students believe and know several years later. Student understanding of green chemistry may have been impacted by learning about green chemistry or related ideas from other sources (e.g., they may have been taking other courses or been part of clubs that touched on green chemistry topics or ideas, exploring the topics on their own, or just hearing about related ideas from friends, family, or media).Finally, further work should be done to establish the reliability and validity of these items. While a construct map was created and used to design the items, we recognize that these five items did not and could not examine all aspects of green chemistry and our knowledge of student understanding is limited to how they chose to answer these items. Additionally, through this analysis, we have come to believe that these items may measure two different constructs: green chemistry understanding and green beliefs. Finally, it was difficult to obtain high internal consistency since we only had five unique items and, since we believe that these items are measuring different constructs, we do not expect high internal consistency. Therefore, we are not proposing that this set of items is a valid measure of a single dimension of green chemistry. Rather it is a set of items that allowed us to explore the breadth and depth of student understanding and beliefs around green chemistry. More work is needed to refine the current items and develop more items to more reliably and accurately measure these constructs.
Conclusions and implications
Our research describes the iterative development of a set of items to probe students’ demonstrated understanding of green chemistry. We focused on the specific topics, ideas, and beliefs student held prior to direct green chemistry instruction and how they developed and integrated new normative green chemistry ideas and practices following a one semester green chemistry laboratory course at UC Berkeley. Our assessment approach differs from most available in the literature by providing students with opportunities to demonstrate their knowledge of and ideas about green chemistry, rather than asking students to self-assess. The Green Chemistry Definition item probed students’ prioritization of the components to include and explanations of green chemistry. The Two Methods Choice item asked students to weigh different factors in making a green-aligned decision. Finally, the select all items probed specific aspects of student green chemistry knowledge. Together these items provided a snapshot of students’ green chemistry knowledge which can be used to improve and iterate pedagogy and curriculum. Both free response items could be applied in many different courses to reveal students’ current knowledge and beliefs, while the fixed response items could be modified to be responsive to other curricula needs or student populations. These assessment items could be applied in both small and large enrollment courses.The GC2 laboratory course was able to support students to learn green chemistry even though students entered the course with different levels of green chemistry understanding. Students’ abilities to define green chemistry, make decisions from a green chemistry perspective, identify and define green chemistry concepts, and apply green chemistry principles to novel scenarios all showed meaningful changes from the beginning to the end of the course. While students came into the course with varying awareness and experience with green chemistry, students, on average, were able to make gains in understanding green chemistry, regardless of their prior experience with chemistry or green chemistry. Not surprisingly, students need more support to connect their specific knowledge of green chemistry to the wider systems impacted by this chemistry. Finally, more work is needed to explore how students engage in core green chemistry practices as the current items only serve as a measure of “pen and paper” green chemistry understanding or application of green chemistry principles to theoretical choices.
One of the underlying themes for both the design of items and interpretation of results was the many ways that students’ prior knowledge mediated their integration of new ideas into their existing mental schema. The green chemistry curriculum effectively introduced new concepts to students, such as LD50 and atom economy, but was unable to shift existing beliefs about natural and renewable products. As posited by constructivist learning theory, students were not a “blank slates” but came into the course with knowledge of green-aligned ideas and practices (Bodner, 1986; Phillips, 1995; Honebein, 1996; Hyslop-Margison and Strobel, 2007; Bada and Olusegun, 2015). From this research, more work needs to be done to actively uncover student prior knowledge to show both instructors and students what knowledge students hold and how new ideas taught in the course support or contradict that prior understanding. The course structure needs to allow for repeated and targeted lessons that provide both normative ideas and opportunities to compare and contrast those new ideas to prior beliefs (Linn, 2006).
Additionally, these results indicated that a more nuanced understanding of both student knowledge and beliefs of specific green chemistry ideas is needed to effectively teach and assess green chemistry. Terms like “natural” or “renewable” have such varied and, in some cases, interconnected everyday meanings for both societies and individuals (e.g., the idea of conserving natural resources versus the safety and use of natural products versus determining if those natural products come from renewable sources at the necessary scale). These terms are frequently connected to choices individuals must make as consumers in part due to the proliferation of eco-labels (Brécard, 2017; Ecolabel Index|Who's Deciding What's Green? 2021) and greenwashing (Delmas and Burbano, 2011). Instructors can focus on explicitly asking students to compare the terms “natural” and “renewable and conveying nuance by making sure that not all the natural materials presented are inherently safe or the greener alternative. Additional research is needed to determine the ways in which both cognitive and affective components contribute to learning and integrating new knowledge, especially for concepts that had or will have a direct connection to students’ immediate choices and ethics.
Finally, it is important to continue developing green chemistry curricula and assessments that target populations outside of organic chemistry students, i.e., general chemistry and high school students. General chemistry and high school classes are critical points of entry and intervention to build normative green chemistry understanding (especially for important but complex topics like natural and renewable products) since most secondary and post-secondary students will never take organic chemistry. At Berkeley, many non-chemistry majors will only take one semester of general chemistry meaning that Chem 1AL is the one opportunity for their green beliefs to be challenged or changed through formal green chemistry instruction. If green chemistry is truly a framework for chemistry, then it should be present in all chemistry education to allow the greatest number of students the opportunity to hear and learn about green chemistry concepts and practices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the Dow foundation and Hayden McNeil Publishing for providing funding for this project. We would also like to thank Zeyi Zhou, Kailey Sun-Marcus, and our Chemistry Education group members for always providing valuable feedback on our green chemistry education project. We would like to thank the Instructional Support Facility staff for assistance with experiments. Finally, we would like to thank the many student participants for sharing their valuable time and making this research possible.References
- Anastas P. T. (2011), Twenty Years of Green Chemistry, Chem. Eng. News, 26(89), 62–65.
- Anastas P. T. and Warner J. C., (1998), Principles of green chemistry, Green chemistry: Theory and practice, vol. 29, pp. 14821–14842.
- Andraos J. and Dicks A. P., (2012), Green chemistry teaching in higher education: a review of effective practices, Chem. Educ. Res. Pract., 13(2), 69–79 10.1039/C1RP90065J.
- Andraos J. and Dicks A. P., (2015), The State of Green Chemistry Instruction at Canadian Universities, in Zuin V. and Mammino L. (ed.), Worldwide Trends in Green Chemistry Education, Royal Society of Chemistry, pp. 179–212 10.1039/9781782621942-00179.
- Armstrong L. B., Rivas M. C., Douskey M. C. and Baranger A. M., (2018), Teaching students the complexity of green chemistry and assessing growth in attitudes and understanding, Curr. Opin. Green Sustainable Chem., 13, 61–67 DOI:10.1016/j.cogsc.2018.04.003.
- Armstrong L. B., Rivas M. C., Zhou Z., Irie L. M., Kerstiens G. A., Robak M. T., Douskey M. C. and Baranger A. M., (2019), Developing a Green Chemistry Focused General Chemistry Laboratory Curriculum: What Do Students Understand and Value about Green Chemistry? J. Chem. Educ., 96(11), 2410–2419 DOI:10.1021/acs.jchemed.9b00277.
- Aubrecht K. B., Padwa L., Shen X. and Bazargan G., (2015), Development and Implementation of a Series of Laboratory Field Trips for Advanced High School Students to Connect Chemistry to Sustainability, J. Chem. Educ., 92(4), 631–637 DOI:10.1021/ed500630f.
- Bada D. and Olusegun S., (2015), Constructivism Learning Theory: A Paradigm for Teaching and Learning, IOSR Journal of Research Method in Education, 5(6), 66–70.
- Bergquist W. and Heikkinen H., (1990), Student ideas regarding chemical equilibrium: what written test answers do not reveal, J. Chem. Educ., 67(12), 1000 DOI:10.1021/ed067p1000.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63(10), 873 DOI:10.1021/ed063p873.
- Bodner G. M., (1991), I have found you an argument: the conceptual knowledge of beginning chemistry graduate students. J. Chem. Educ., 68(5), 385 DOI:10.1021/ed068p385.
- Bodner G. M., (2015), Understanding the Change Toward a Greener Chemistry by Those Who Do Chemistry and Those Who Teach Chemistry, Relevant Chemistry Education, Rotterdam: SensePublishers, pp. 263–284 DOI:10.1007/978-94-6300-175-5_14.
- Brécard D., (2017), Consumer misperception of eco-labels, green market structure and welfare, J. Regulatory Economics, 51(3), 340–364 DOI:10.1007/s11149-017-9328-8.
- Buckley H. L., Beck A. R., Mulvihill M. J. and Douskey M. C., (2013), Fitting It All In: Adapting a Green Chemistry Extraction Experiment for Inclusion in an Undergraduate Analytical Laboratory, J. Chem. Educ., 90(6), 771–774 DOI:10.1021/ed300510s.
- Burmeister M., Rauch F. and Eilks I., (2012), Education for Sustainable Development (ESD) and chemistry education, Chem. Educ. Res. Pract., 13(2), 59–68 10.1039/C1RP90060A.
- Constable D. J. C., Jiménez-González C. and Matlin S. A., (2019), Navigating Complexity Using Systems Thinking in Chemistry, with Implications for Chemistry Education, J. Chem. Educ., 96(12), 2689–2699 DOI:10.1021/acs.jchemed.9b00368.
- Davis D. A., Mazmanian P. E., Fordis M., Harrison R. V., Thorpe K. E. and Perrier L., (2006), Accuracy of Physician Self-assessment Compared With Observed Measures of Competence: A Systematic Review, JAMA, 296(9), 1094–1102 DOI:10.1001/jama.296.9.1094.
- DeHaan R. L., (2009), Teaching Creativity and Inventive Problem Solving in Science, Cell Biol. Educ., 8(3), 172–181 DOI:10.1187/cbe.08-12-0081.
- Delmas M. A. and Burbano V. C., (2011), The Drivers of Greenwashing, California Management Rev., 54(1), 64–87 DOI:10.1525/cmr.2011.54.1.64.
- Dickson-Spillmann M., Siegrist M. and Keller C., (2011), Attitudes toward chemicals are associated with preference for natural food, Food Quality Preference, 22(1), 149–156 DOI:10.1016/j.foodqual.2010.09.001.
- Ecolabel Index | Who's deciding what's green? (2021), https://www.ecolabelindex.com/.
- Epicoco M., Oltra V. and Saint Jean M., (2014), Knowledge dynamics and sources of eco-innovation: Mapping the Green Chemistry community, Technol. Forecasting Soc. Change, 81(C), 388–402 DOI:10.1016/j.techfore.2013.03.006.
- Galgano P. D., Loffredo C., Sato B. M., Reichardt C. and El Seoud O. A., (2012), Introducing education for sustainable development in the undergraduate laboratory: Quantitative analysis of bioethanol fuel and its blends with gasoline by using solvatochromic dyes, Chem. Educ. Res. Pract., 13(2), 147–153 10.1039/C1RP90061G.
- Garner N., Huwer J., Siol A., Hempelmann R. and Eilks I., (2015), On the Development of Non-formal Learning Environments for Secondary School Students Focusing on Sustainability and Green Chemistry, in Zuin V. and Mammino L. (ed.), Worldwide Trends in Green Chemistry Education, Royal Society of Chemistry, pp. 76–92 10.1039/9781782621942-00076.
- Gron L. U., Bradley S. B., McKenzie J. R., Shinn S. E. and Teague M. W., (2013), How to Recognize Success and Failure: Practical Assessment of an Evolving, First-Semester Laboratory Program Using Simple, Outcome-Based Tools, J. Chem. Educ., 90(6), 694–699 DOI:10.1021/ed200523w.
- Guron M., Paul J. J. and Roeder M. H., (2016), Incorporating Sustainability and Life Cycle Assessment into First-Year Inorganic Chemistry Major Laboratories, J. Chem. Educ., 93(4), 639–644 DOI:10.1021/acs.jchemed.5b00281.
- Haack J. A. and Hutchison J. E., (2016), Green Chemistry Education: 25 Years of Progress and 25 Years Ahead, ACS Sustainable Chem. Eng., 4(11), 5889–5896 DOI:10.1021/acssuschemeng.6b02069.
- Honebein P. C., (1996), Seven Goals for the Design of Constructivist Learning Environments, in Wilson B. G. (ed.), Constructivist Learning Environments: Case Studies in Instructional Design, Englewood Cliffs: Educational Technology Publications, pp. 11–24.
- Hyslop-Margison E. J. and Strobel J., (2007), Constructivism and education: misunderstandings and pedagogical implications, Teach. Educ., 43(1), 72–86 DOI:10.1080/08878730701728945.
- Iles A., (2011), Greening chemistry: emerging epistemic political tensions in California and the United States, Public Underst. Sci., 22(4), 460–478 DOI:10.1177/0963662511404306.
- Karpudewan M., Ismail Z. and Roth W.-M., (2012), Ensuring sustainability of tomorrow through green chemistry integrated with sustainable development concepts (SDCs), Chem. Educ. Res. Pract., 13(2), 120–127 10.1039/C1RP90066H.
- Karpudewan M., Roth W.-M. and Ismail Z., (2015a), Education in Green Chemistry: Incorporating Green Chemistry into Chemistry Teaching Methods Courses at the Universiti Sains Malaysia, Worldwide Trends in Green Chemistry Education, Royal Society of Chemistry, pp. 248–265 10.1039/9781782621942-00248.
- Karpudewan M., Roth W.-M. and Ismail Z., (2015b), The Effects of “Green Chemistry” on Secondary School Students’ Understanding and Motivation, The Asia-Pacific Educ. Res., 24(1), 35–43 DOI:10.1007/s40299-013-0156-z.
- Karpudewan M., Michael Roth W. and Sinniah D., (2016), The role of green chemistry activities in fostering secondary school students’ understanding of acid–base concepts and argumentation skills, Chem. Educ. Res. Pract., 17(4), 893–901 10.1039/C6RP00079G.
- Kitchens C., Charney R., Naistat D., Farrugia J., Clarens A., O’Neil A., Lisowski C. and Braun B., (2006), Completing Our Education. Green Chemistry in the Curriculum, J. Chem. Educ., 83(8), 1126 DOI:10.1021/ed083p1126.
- Linn M. C., (2006), Inquiry Learning: Teaching and Assessing Knowledge Integration in Science, Science, 313(5790), 1049–1050 DOI:10.1126/science.1131408.
- Linn M. C. and Eylon B. S., (2006), Science education: Integrating views of learning and instruction, Handbook of educational psychology DOI:10.4324/9780203874790.ch22.
- Linn M. C. and Eylon B.-S., (2011a), Experimentation and Knowledge Integration, Science Learning and Instruction, Routledge DOI:10.4324/9780203806524.
- Linn M. C. and Eylon B.-S., (2011b), Science Learning and Instruction: Taking Advantage of Technology to Promote Knowledge Integration, 1st edn, Routledge DOI:10.4324/9780203806524.
- Linthorst J. A., (2009), An overview: Origins and development of green chemistry, Found. Chem., 12(1), 55–68 DOI:10.1007/s10698-009-9079-4.
- Machado A. A. S. C., (2015), Holistic Green Chemistry Metrics for Use in Teaching Laboratories, in Zuin V. G. and Mammino L. (ed.), Worldwide Trends in Green Chemistry Education, Royal Society of Chemistry, pp. 111–137.
- Mahaffy P. G. and Elgersma A. K., (2022), Systems thinking, the molecular basis of sustainability and the planetary boundaries framework: complementary core competencies for chemistry education, Curr. Opin. Green Sustainable Chem., 37, 100663 DOI:10.1016/j.cogsc.2022.100663.
- Mahaffy P. G., Matlin S. A., Holme T. A. and MacKellar J., (2019a), Systems thinking for education about the molecular basis of sustainability, Nat. Sustainability, 2(5), 362–370 DOI:10.1038/s41893-019-0285-3.
- Mahaffy P. G., Matlin S. A., Whalen J. M. and Holme T. A., (2019b), Integrating the Molecular Basis of Sustainability into General Chemistry through Systems Thinking, J. Chem. Educ., 96(12), 2730–2741 DOI:10.1021/acs.jchemed.9b00390.
- Mandler D., Mamlok-Naaman R., Blonder R., Yayon M. and Hofstein A., (2012), High-school chemistry teaching through environmentally oriented curricula, Chem. Educ. Res. Pract., 13(2), 80–92 10.1039/C1RP90071D.
- Marteel-Parrish A. E., (2014), Teaching Green and Sustainable Chemistry: A Revised One-Semester Course Based on Inspirations and Challenges, J. Chem. Educ., 91(7), 1084–1086 DOI:10.1021/ed400393b.
- Moscato E. M. and Machin J. E., (2018), Mother natural: motivations and associations for consuming natural foods, Appetite, 121, 18–28 DOI:10.1016/j.appet.2017.10.031.
- Orgill M., York S. and MacKellar J., (2019), Introduction to Systems Thinking for the Chemistry Education Community, J. Chem. Educ., 96(12), 2720–2729 DOI:10.1021/acs.jchemed.9b00169.
- Paluri S. L. A., Edwards M. L., Lam N. H., Williams E. M., Meyerhoefer A. and Pavel Sizemore I. E., (2015), Introducing “Green” and “Nongreen” Aspects of Noble Metal Nanoparticle Synthesis: An Inquiry-Based Laboratory Experiment for Chemistry and Engineering Students, J. Chem. Educ., 92(2), 350–354 DOI:10.1021/ed5004806.
- Phillips D. C., (1995), The Good, the Bad, and the Ugly: The Many Faces of Constructivism, Educ. Res., 24(7), 5–12 DOI:10.3102/0013189X024007005.
- Purcell S. C., Pande P., Lin Y., Rivera E. J., Latisha P. U., Smallwood L. M., Kerstiens G. A., Armstrong L. B., Robak M. T., Baranger A. M. and Douskey M. C., (2016), Extraction and Antibacterial Properties of Thyme Leaf Extracts: Authentic Practice of Green Chemistry, J. Chem. Educ., 93(8), 1422–1427 DOI:10.1021/acs.jchemed.5b00891.
- Reyes K. M. D., Bruce K. and Shetranjiwalla S., (2023), Green Chemistry, Life Cycle Assessment, and Systems Thinking: An Integrated Comparative-Complementary Chemical Decision-Making Approach, J. Chem. Educ., 100(1), 209–220 DOI:10.1021/acs.jchemed.2c00647.
- Reynders M., Pilcher L. A. and Potgieter M., (2023), Teaching and Assessing Systems Thinking in First-Year Chemistry, J. Chem. Educ., 100(3), 1357–1365 DOI:10.1021/acs.jchemed.2c00891.
- Román S., Sánchez-Siles L. M. and Siegrist M., (2017), The importance of food naturalness for consumers: results of a systematic review, Trends Food Sci. Technol., 67, 44–57 DOI:10.1016/j.tifs.2017.06.010.
- Shamuganathan S. and Karpudewan M., (2017), Science writing heuristics embedded in green chemistry: a tool to nurture environmental literacy among pre-university students, Chem. Educ. Res. Pract., 18(2), 386–396 10.1039/C7RP00013H.
- Sitzmann T., Ely K., Brown K. G. and Bauer K. N., (2010), Self-Assessment of Knowledge: A Cognitive Learning or Affective Measure? Acad. Manag. Learn. Educ., 9(2), 169–191.
- Sjostrom J. and Talanquer V., (2014), Humanizing Chemistry Education: From Simple Contextualization to Multifaceted Problematization, J. Chem. Educ., 91(8), 1125–1131 DOI:10.1021/ed5000718.
- Sjostrom J., Eilks I. and Zuin V. G., (2016), Towards Eco-reflexive Science Education A Critical Reflection About Educational Implications of Green Chemistry, Sci. Educ., 25(3–4), 321–341 DOI:10.1007/s11191-016-9818-6.
- Talanquer V., (2019), Some Insights into Assessing Chemical Systems Thinking, J. Chem. Educ., 96(12), 2918–2925 DOI:10.1021/acs.jchemed.9b00218.
- von Blottnitz H., Case J. M. and Fraser D. M., (2015), Sustainable development at the core of undergraduate engineering curriculum reform: a new introductory course in chemical engineering, J. Cleaner Prod., 106, 300–307 DOI:10.1016/j.jclepro.2015.01.063.
- Wilson M., (2005), Constructing measures: An item response modeling approach, Lawrence Erlbaum Associates DOI:10.4324/9781410611697.
- Woodhouse E. J. and Breyman S., (2005), Green Chemistry as Social Movement? Sci., Technol. Human Values, 30(2), 199–222 DOI:10.1177/0162243904271726.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2rp00270a |
| This journal is © The Royal Society of Chemistry 2024 |