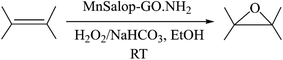Open Access Article
Open Access ArticleManganese salophen covalently anchored to amino-functionalized graphene oxide as an efficient heterogeneous catalyst for selective epoxidation†
Robabeh Hajian * and
Narjes Sadat Mousavi
* and
Narjes Sadat Mousavi
Department of Chemistry, Yazd University, Yazd 89195-741, Iran. E-mail: rhajian@yazd.ac.ir; Fax: +98-353-8210644; Tel: +98-353-31232822
First published on 5th December 2024
Abstract
Epoxidation of olefins catalyzed by manganese(III) salophen (MnSalop) immobilized on graphene oxide (GO) modified with 3-aminopropyltrimethoxysilane (GO·NH2) has been reported. Characterization of the solid catalyst by FTIR, DR UV-Vis, FESEM, XRD, elemental scanning mappings, TGA/DTG, BET measurements, and ICP analysis aided in understanding the catalyst morphology. It confirmed that there was no significant demetallation or chemical change in MnSalop-GO·NH2. The heterogeneous catalyst (MnSalop-GO·NH2) showed high efficiency in the oxidation of different olefins with H2O2 as a green oxygen donor agent assisted by NaHCO3 as co-catalyst at room temperature. The alkenes were oxidized to their corresponding epoxides with 88–100% selectivity and turnover frequency (TOF) values ranging from 40.7 to 162.8 h−1 in the presence of MnSalop-GO·NH2 under mild conditions. When supported on GO, MnSalop-GO·NH2 afforded epoxide yields comparable to those of the corresponding homogeneous analog. The prepared catalyst was selective for most olefins, with a high conversion. In addition, it could be reused four times without any remarkable loss in catalytic performance.
1. Introduction
Catalytic olefin epoxidation is a valuable reaction for the synthesis of organic materials, as epoxides serve as chemical intermediates in the production of a wide range of commercial products, including epoxy paints, agrochemicals, and pharmaceutical products.1,2 Consequently, there is a need to develop highly efficient catalysts for epoxidation reactions. Schiff base ligands have been the subject of intensive investigation because of their applications in various areas, including biological activities and coordination chemistry.3 The biomimetic catalyst models of enzymatic monooxygenase and metal-Schiff base complexes have successfully oxidized olefins and other substances.4,5 Numerous single oxygen donors, such as sodium periodate, alkyl hydroperoxide, hydrogen hydroperoxide, and iodosylbenzene, influence various oxidation processes.6–9 Several catalytic systems have been previously reported for this purpose.10 Metalloporphyrin and metal salen/salophen complexes have received notable attention because of their active redox systems, high catalytic activity, and selectivity.11–15 Metal salophen successfully catalyzes the oxidation of various organic compounds.16–18 Salophen, one of the most popular classes of tetradentate ligands, can be synthesized by condensing two equivalents of salicylaldehyde with one equivalent of 1,2-phenylenediamine derivative. Among various transition metals, manganese is particularly noteworthy and well-suited for alkene epoxidation due to its low toxicity and its presence in metalloenzymes.19–23 Manganese salophen is a valuable and powerful catalyst for epoxidation.24–26 Despite the high activity obtained with homogeneous catalysts, the aggregation of units through π–π interactions, difficulty in reusing the catalyst from the reaction medium, and self-destruction are several drawbacks of these systems.27,28 One way to overcome these drawbacks is to immobilize the homogeneous catalyst on solid supports through encapsulation in a porous structure, covalent grafting, copolymerization, and buildup of the catalyst structure to produce polymers.29–34 Graphene oxide (GO) compounds have been widely used in academic and industrial applications.35,36 The oxygen-containing functional groups in two-dimensional graphene oxide structures, such as hydroxyl, epoxy, and carboxyl, lead to unique properties, including a high specific surface area, remarkable chemical stability, and intrinsic low mass.37 Owing to the widespread flexibility in the design of composites, GOs can be tailored for various applications, including solar cells, catalysis, sensors, gas storage, and supports.36,38–40 The functionalization of GO can enhance its capability to support the immobilization of various homogeneous compounds, such as metallic Schiff bases.41,42 However, the covalent attachment of the catalyst on the surface of supports (GO) decreases the leaching of the catalyst during the reaction. Zarnegaryan et al. prepared a copper(II) Schiff base covalently anchored onto GO for the epoxidation of olefins.43 Rayati et al. reported a Cu-Schiff base complex grafted onto a graphene oxide nanocomposite as an efficient and reusable catalyst for alkene oxidation.44 Masteri-Farahani et al. functionalized graphene oxide and graphene oxide-magnetite nanocomposite with molybdenum-bidentate Schiff base complex for epoxidation of various alkenes.45 Hassan et al. prepared a vanadium(IV) Schiff base amine-tagged graphene oxide composite to oxidize olefins.46 Rezazadeh et al. synthesized Schiff base complexes of Mo(VI) immobilized on functionalized graphene oxide nanosheets for catalytic epoxidation of alkenes.47 Rasoul Pour et al. immobilized the macrocyclic Schiff base copper complex on GO nanosheets and investigated its catalytic activity for olefins epoxidation.48 As a part of our ongoing interest in developing heterogeneous catalytic systems for potential use in oxidative catalysts,11,49,50 we have previously reported that CoFe2O3/Graphene Oxide can be appropriately used for supporting the Brominated Mn(III) Salophen.51In this study, GO modified with 3-aminopropyltrimethoxysilane was prepared. Then, manganese(III) salophen was covalently attached to graphene oxide-bound amine (MnSalop-GO·NH2). The immobilized manganese Schiff base was tested as a catalyst for the epoxidation of various alkenes in the presence of H2O2/NaHCO3 in organic media. The catalyst recovery ability was also discussed (Scheme 1).
2. Experimental
2.1. Materials
All chemical materials were purchased from Fluka and Merck Chemical Company and used without further treatment. The chemical purities of all olefins were tested by gas chromatography and were confirmed to be higher than 98%. Manganese(III) salophen was prepared and characterized according to literature procedures.2.2. Instrumental
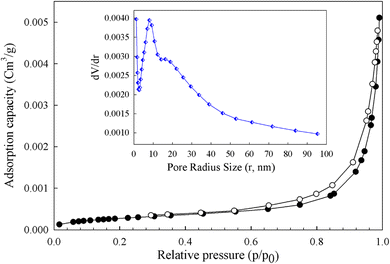
FTIR spectra of the catalyst were recorded on Bruker FT-IR Equinox-55 instrument spectrophotometer, using pellets of the materials diluted with KBr. DR UV-Vis spectra were obtained using a V-670 spectrometer. FESEM of the samples were performed using a TE-SCAN MIRA (Czech) instrument. The pattern of X-ray diffraction (XRD) of compounds was taken on a D8 Advanced Bruker diffractometer using Cu-Kα radiation. The thermogravimetric and differential analysis (TG-DTG) was performed on STD Q600 in an N2 atmosphere from 25 to 800 °C. Mn loading was performed using an ICP-Spectrociros CCD instrument. Adsorption–desorption isotherms were recorded on a BELSORB-mini II surface area analyzer at 77 K. The Brunauer–Emmett–Teller (BET) equation was calculated for specific surface area, and the Barrett–Joyner–Halenda (BJH) method was used for volume and pore diameter. The chemical products were analyzed by gas chromatography (Agilent 7890N, HP-5 19091 J-413 capillary column) with a flame ionization detector and n-decane as the internal standard using N2 as the carrier gas.2.3. Catalyst preparation
2.4. Catalytic test
In a typical experiment, alkene (0.5 mmol) in 4 mL solvent, MnSalop-GO·NH2 catalyst (100 mg), hydrogen peroxide as a green oxidant (5 mmol; 30%), and sodium bicarbonate (0.5 mmol) were mixed and stirred at ambient conditions for the appropriate time. The progress of the reaction was followed by GC equipped with an FID detector and an HP-5 column (n-decane as the internal standard). After completion of the reaction, the MnSalop-GO·NH2 catalyst was separated by centrifugation, washed with EtOH, dried carefully, and recycled for the next run.3. Results and discussion
3.1. Characterization of MnSalop-GO·NH2
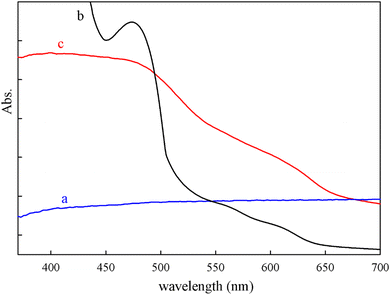
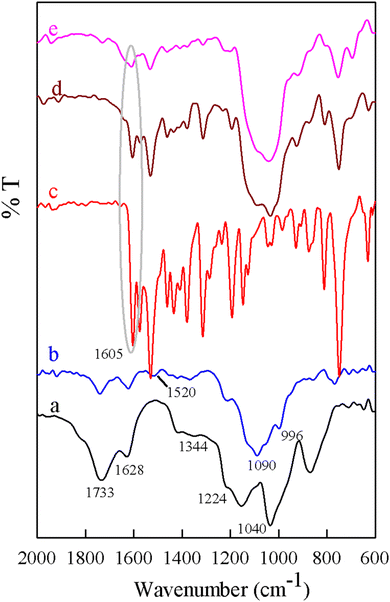
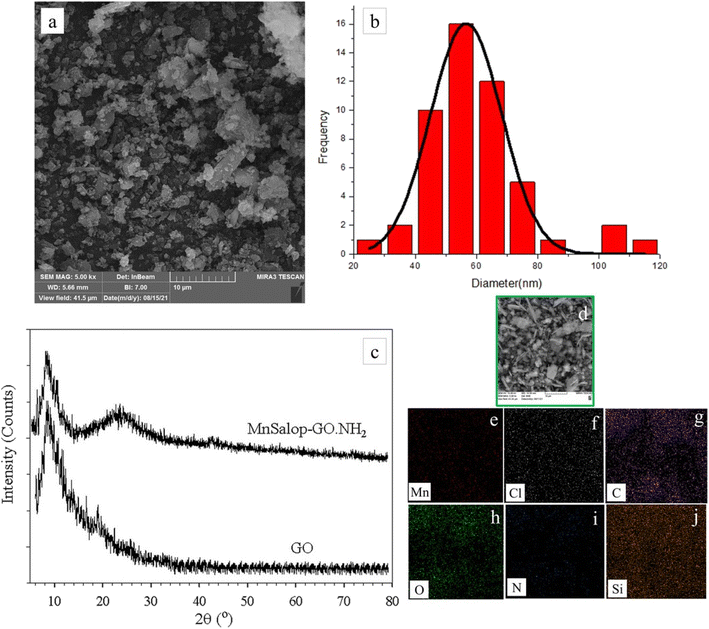
As described in Scheme 2, GO was prepared by oxidative exfoliation of graphite in the first step. GO was treated with 3-aminopropyltrimethoxysilane (APTMS) in refluxing toluene for 24 h. On the other hand, APTMS can be grafted onto GO for its –COOH, –OH, and epoxy groups to form Si–O linkages. Subsequently, Mn(III) salophen can interact with GO·NH2 through covalent bonds.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carboxyl; 1733 cm−1), C–OH (hydroxyl groups; 1224 cm−1), and C
O (carboxyl; 1733 cm−1), C–OH (hydroxyl groups; 1224 cm−1), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (aromatic ring; 1628 cm−1) can be observed in the IR spectrum of pure GO (Fig. 2a).53 The strong bands at 1090 cm−1 and 996 cm−1 correspond to the stretching vibrations of Si–O–Si and Si–O–C, respectively, indicating the silylanization of GO through chemical bonding. Two bonds around 3443 and 1520 cm−1 were attributed to the stretching and bending modes of N–H of amine-tagged graphene oxide (Fig. 2b).46 A characteristic band at 1605 cm−1 appeared in the FT-IR spectrum of MnSalop, which corresponds to the stretching of the azomethine group (C
C (aromatic ring; 1628 cm−1) can be observed in the IR spectrum of pure GO (Fig. 2a).53 The strong bands at 1090 cm−1 and 996 cm−1 correspond to the stretching vibrations of Si–O–Si and Si–O–C, respectively, indicating the silylanization of GO through chemical bonding. Two bonds around 3443 and 1520 cm−1 were attributed to the stretching and bending modes of N–H of amine-tagged graphene oxide (Fig. 2b).46 A characteristic band at 1605 cm−1 appeared in the FT-IR spectrum of MnSalop, which corresponds to the stretching of the azomethine group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of the manganese Schiff base complex (Fig. 2c).55 In the FTIR spectrum of MnSalop-GO·NH2, the peak at 1603 cm−1 was attributed to the imine group (C
N) of the manganese Schiff base complex (Fig. 2c).55 In the FTIR spectrum of MnSalop-GO·NH2, the peak at 1603 cm−1 was attributed to the imine group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) stretching in the manganese salophen molecule. Additionally, the stretching vibration bands of the silanol groups at 1092 and 1030 cm−1 and the band at 1530 cm−1 reveal amine groups. These results confirm the coordination of MnSalop to the amine-modified GO nanosheets (Fig. 2d).
N) stretching in the manganese salophen molecule. Additionally, the stretching vibration bands of the silanol groups at 1092 and 1030 cm−1 and the band at 1530 cm−1 reveal amine groups. These results confirm the coordination of MnSalop to the amine-modified GO nanosheets (Fig. 2d).
3.2. Catalytic performance
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of H2O2 to NaHCO3 (Fig. 6d). Thus, sodium bicarbonate is an essential co-catalyst of the oxidation reaction.
1 molar ratio of H2O2 to NaHCO3 (Fig. 6d). Thus, sodium bicarbonate is an essential co-catalyst of the oxidation reaction.
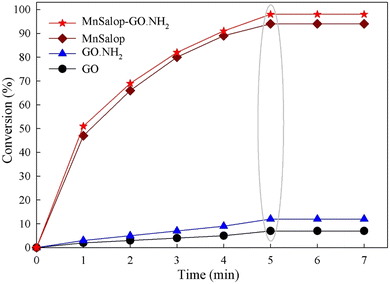
The time courses of the control components of this heterogeneous system, including GO, GO·NH2, MnSalop, and MnSalop-GO·NH2 were also investigated under the same reaction conditions. The supporting materials (GO and GO·NH2) exhibited low catalytic performances. The homogeneous catalyst (MnSalop) afforded a conversion of 94% after 5 min. The immobilization of MnSalop on the modified GO increased the conversion percentage (98% after 5 min) in the epoxidation reaction because of the isolation of the active sites of the GO solid (Fig. 7).
| Substrate | Product | Conversionb (%) | Epoxide selectivity (%) | TOF (h−1) | |
|---|---|---|---|---|---|
a Reaction conditions: the molar ratios for catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) olefin olefin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaHCO3 are (2 NaHCO3 are (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27), EtOH (4 mL).b GC yield based on the starting olefin. 27), EtOH (4 mL).b GC yield based on the starting olefin. |
|||||
| 1 |  |
 |
98 | 100 | 159.5 |
| 2 |  |
 |
93 | 88 | 151.4 |
| 3 |  |
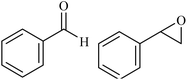 |
88 | 91 | 143.3 |
| 4 |  |
 |
92 | 94 | 149.8 |
| 5 | 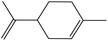 |
 |
74 | (86% exo), (14% endo) | 120.5 |
| 6 |  |
 |
100 | 90 | 162.8 |
| 7 |  |
 |
36 | 100 | 58.6 |
| 8 |  |
 |
25 | 100 | 40.7 |
To demonstrate the effectiveness of the heterogeneous catalyst, the TOF (h−1) of this work (MnSalop-GO·NH2) was compared with our previously reported system, CFeGO@MnSalBr51 (Table S1†). A more detailed analysis of the results reveals that MnSalop-GO·NH2 exhibits higher catalytic efficiency than the CFeGO@MnSalBr catalyst. This can be attributed to the reduced mobility of CFeGO@MnSalBr due to the presence of the magnetite core.
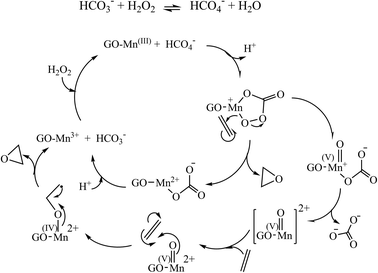
Based on Krishnan's ideas and other reports, a possible mechanism is proposed for the epoxidation of alkenes with H2O2/NaHCO3 using a manganese complex. In this mechanism, HCO4− is a more active and nucleophilic oxidant than H2O2, which can coordinate with manganese metal and form an intermediate manganese-acyl peroxymonocarbonate complex or a high-valent Mn![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate. The active atomic oxygen can be transferred from the molecule's intermediate to the C
O intermediate. The active atomic oxygen can be transferred from the molecule's intermediate to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to form the epoxide (Scheme 3).57,58
C bond to form the epoxide (Scheme 3).57,58
3.3. The reusability of catalyst
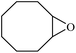
The heterogenization of different catalysts is of great importance from an economical point of view and for environmentally friendly applications. Hence, the durability and reproducibility of MnSalop-GO·NH2 as a heterogeneous catalyst in cis-cyclooctene epoxidation was studied. After completion of the reaction, the prepared catalyst was isolated by easy filtration from the reaction mixture, washed twice with ethanol, and then dried carefully before use in the subsequent run. After the fourth run, epoxidation conversion decreased from 98 to 77% (Fig. 8). In addition, atomic absorption spectroscopy was used to measure the amount of MnSalop leached. The results demonstrated that after the first two runs, no considerable Mn was detected (0.75% and 0.41%, respectively). The FT-IR spectra of MnSalop-GO·NH2 were compared before and after the fourth run and showed the same patterns (Fig. 2e), which emphasizes that this catalyst not only has good durability but also high reproducibility, instilling confidence in its application. | ||
Fig. 8 Recycling data for MnSalop-GO·NH2 as catalyst in the epoxidation of cyclooctene with H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaHCO3. NaHCO3. | ||
The heterogenization of different catalysts is of great importance from an economical point of view and for environmentally friendly applications. Hence, the durability and reproducibility of MnSalop-GO·NH2 as a heterogeneous catalyst in cis-cyclooctene epoxidation was studied. After completion of the reaction, the prepared catalyst was isolated by easy filtration from the reaction mixture, washed twice with ethanol, and dried carefully before use in the subsequent run. After the fourth run, epoxidation conversion decreased from 98% to 77% (Fig. 8). Also, atomic absorption spectroscopy was used to measure the amount of MnSalop leached. The results demonstrated that no considerable Mn was detected after the first two runs (0.75% and 0.41%). The FT-IR spectra of MnSalop-GO·NH2 was compared before and after the fourth run and showed the same patterns (Fig. 2e), emphasizing that this catalyst has good durability and high reproducibility, instilling confidence in its application.
4. Conclusion
In this study, amine-functionalized graphene oxide was synthesized and used to support the immobilization of manganese(III) salophen chloride (MnSalop). The catalytic activity of the heterogeneous catalyst MnSalop-GO·NH2 was evaluated in the epoxidation of various olefins using H2O2 as an eco-friendly oxidant and NaHCO3 as co-catalyst in EtOH. This catalytic system offers several advantages, including mild reaction conditions, high selectivity, and acceptable reusability.Data availability
All relevant data are within the manuscript and its additional files. The data are available from the corresponding author on reasonable request.Conflicts of interest
There is no conflict of interest in the article.Acknowledgements
We are thankful to the Yazd University Research Council for the partial support of this research.References
- C. Boulechfar, H. Ferkous, A. Delimi, A. Djedouani, A. Kahlouche, A. Boublia, A. S. Darwish, T. Lemaoui, R. Verma and Y. Benguerba, Inorg. Chem. Commun., 2023, 150, 110451–114062 CrossRef CAS.
- Y. Meng, F. Taddeo, A. F. Aguilera, X. Cai, V. Russo, P. Tolvanen and S. Leveneur, Catalysts, 2021, 11, 765–840 CrossRef CAS.
- A. M. Abu-Dief and I. M. Mohamed, Beni-Suef Univ. J. Basic Appl. Sci., 2015, 4, 119–133 Search PubMed.
- H. Kargar, A. A. Ardakani, M. N. Tahir, M. Ashfaq and K. S. Munawar, J. Mol. Struct., 2021, 1233, 130112–130124 CrossRef CAS.
- A. Nishinaga and H. Tomita, J. Mol. Catal., 1980, 7, 179–199 CrossRef CAS.
- B. Bahramian, V. Mirkhani, S. Tangestaninejad and M. Moghadam, J. Mol. Catal. A: Chem., 2006, 244, 139–145 CrossRef CAS.
- N. H. Lee, J.-C. Byun and T.-H. Oh, Bull. Korean Chem. Soc., 2005, 26, 454–456 CrossRef CAS.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork and N. Rasouli, Catal. Commun., 2008, 9, 2171–2174 CrossRef CAS.
- M. Sedighipoor, A. H. Kianfar, W. A. K. Mahmood and M. H. Azarian, Inorg. Chim. Acta, 2017, 457, 116–121 CrossRef CAS.
- A. Nodzewska, A. Wadolowska and M. Watkinson, Coord. Chem. Rev., 2019, 382, 181–216 CrossRef CAS.
- R. Hajian, S. Tangestaninejad, M. Moghadam, V. Mirkhani and I. Mohammadpoor-Baltork, J. Iran. Chem. Soc., 2016, 13, 1061–1067 CrossRef CAS.
- O. Kocyigit and E. Guler, J. Inclusion Phenom. Macrocyclic Chem., 2010, 67, 29–37 CrossRef CAS.
- S. Tangestaninejad, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork and R. Hajian, Inorg. Chem. Commun., 2010, 13, 1501–1503 CrossRef CAS.
- Ş. Uysal and Z. E. Koç, J. Mol. Struct., 2018, 1165, 14–22 CrossRef.
- A. Pappalardo, F. P. Ballistreri, R. M. Toscano, M. A. Chiacchio, L. Legnani, G. Grazioso, L. Veltri and G. Trusso Sfrazzetto, Catalysts, 2021, 11, 465–474 CrossRef CAS.
- C. W. Anson, S. Ghosh, S. Hammes-Schiffer and S. S. Stahl, J. Am. Chem. Soc., 2016, 138, 4186–4193 CrossRef CAS PubMed.
- M. A. Asraf, C. I. Ezugwu, C. Zakaria and F. Verpoort, Photochem. Photobiol. Sci., 2019, 18, 2782–2791 CrossRef CAS PubMed.
- M. Salavati-Niasari, A. Badiei and K. Saberyan, Chem. Eng. J., 2011, 173, 651–658 CrossRef CAS.
- N. Sarkar, P. K. Bhaumik and S. Chattopadhyay, Polyhedron, 2016, 115, 37–46 CrossRef CAS.
- M. Maiti, D. Sadhukhan, S. Thakurta, E. Zangrando, G. Pilet, A. Bauzá, A. Frontera, B. Dede and S. Mitra, Polyhedron, 2014, 75, 40–49 CrossRef CAS.
- P. Kar, M. G. Drew and A. Ghosh, Inorg. Chim. Acta, 2013, 405, 349–355 CrossRef CAS.
- R. Egekenze, Y. Gultneh and R. Butcher, Polyhedron, 2018, 144, 198–209 CrossRef CAS.
- G. De Faveri, G. Ilyashenko and M. Watkinson, Chem. Soc. Rev., 2011, 40, 1722–1760 RSC.
- M. A. Fardjahromi, M. Moghadam, S. Tangestaninejad, V. Mirkhani and I. Mohammadpoor-Baltork, RSC Adv., 2016, 6, 20128–20134 RSC.
- S. Y. Liu and D. G. Nocera, Tetrahedron Lett., 2006, 47, 1923–1926 CrossRef CAS.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad, B. Bahramian and A. Mallekpoor-Shalamzari, Appl. Catal., A, 2007, 321, 49–57 CrossRef CAS.
- G. Consiglio, I. P. Oliveri, S. Cacciola, G. Maccarrone, S. Failla and S. Di Bella, Dalton Trans., 2020, 49, 5121–5133 RSC.
- P. Kumar Mudi, A. Das, N. Mahata and B. Biswas, J. Mol. Liq., 2021, 340, 117193–117203 CrossRef CAS.
- M. Asadniaye Fardjahromi, M. Moghadam, S. Tangestaninejad, V. Mirkhani and I. Mohammadpoor-Baltork, J. Iran. Chem. Soc., 2017, 14, 1317–1323 CrossRef CAS.
- M. Balas, S. Beaudoin, A. Proust, F. Launay and R. Villanneau, Eur. J. Inorg. Chem., 2021, 2021, 1581–1591 CrossRef CAS.
- V. Mahdavi, M. Mardani and M. Malekhosseini, Catal. Commun., 2008, 9, 2201–2204 CrossRef CAS.
- J. Tong, Y. Zhang, Z. Li and C. Xia, J. Mol. Catal. Chem., 2006, 249, 47–52 CrossRef CAS.
- D. Zhu, Y. Chen, Y. Zhu, C.-Y. Liu, Q. Yan, X. Wu, K. Ling, X. Zhang, P. M. Ajayan, T. P. Senftle and R. Verduzco, Macromolecules, 2024, 57, 1038–1049 CrossRef CAS.
- Y. Zhu, Y. Liu, Q. Ai, G. Gao, L. Yuan, Q. Fang, X. Tian, X. Zhang, E. Egap, P. M. Ajayan and J. Lou, ACS Mater. Lett., 2022, 4, 464–471 CrossRef CAS.
- C. Chung, Y.-K. Kim, D. Shin, S.-R. Ryoo, B. H. Hong and D.-H. Min, Acc. Chem. Res., 2013, 46, 2211–2224 CrossRef CAS PubMed.
- A. N. Ghulam, O. A. Dos Santos, L. Hazeem, B. Pizzorno Backx, M. Bououdina and S. Bellucci, J. Funct. Biomater., 2022, 13, 77–105 CrossRef CAS PubMed.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS PubMed.
- K. Jiao, X. Wang, Y. Wang and Y. Chen, J. Mater. Chem. C, 2014, 2, 7715–7721 RSC.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed.
- P. W. Sayyad, S. S. Khan, N. N. Ingle, G. A. Bodkhe, T. Al-Gahouari, M. M. Mahadik, S. M. Shirsat and M. D. Shirsat, Appl. Phys. A, 2020, 126, 1–8 CrossRef.
- H. Su, S. Wu, Z. Li, Q. Huo, J. Guan and Q. Kan, Appl. Organomet. Chem., 2015, 29, 462–467 CrossRef CAS.
- G. Bian, P. Jiang, F. Wang, Y. Shen, K. Jiang, L. Liu and W. Zhang, New J. Chem., 2018, 42, 85–90 RSC.
- A. Zarnegaryan, Z. Pahlevanneshan, M. Moghadam, S. Tangestaninejad, V. Mirkhani and I. Mohammdpoor-Baltork, J. Iran. Chem. Soc., 2019, 16, 747–756 CrossRef CAS.
- S. Rayati, E. Khodaei, S. Shokoohi, M. Jafarian, B. Elmi and A. Wojtczak, Inorg. Chim. Acta, 2017, 466, 520–528 CrossRef CAS.
- M. Masteri-Farahani and M. Ghahremani, J. Phys. Chem. Solids, 2019, 130, 6–12 CrossRef CAS.
- H. M. A. Hassan, M. A. Betiha, E. A. El-Sharkawy, R. F. M. Elshaarawy, N. B. El-Assy, A. A. Essawy, A. M. Tolba and A. M. Rabie, Colloids Surf. A Physicochem. Eng. Asp., 2020, 591, 124520–124531 CrossRef CAS.
- B. Rezazadeh, A. R. Pourali, A. Banaei and H. Behniafar, J. Coord. Chem., 2019, 72, 3401–3416 CrossRef CAS.
- S. R. Pour, A. Abdolmaleki and M. Dinari, J. Mater. Sci., 2019, 54, 2885–2896 CrossRef CAS.
- R. Hajian and E. Bahrami, Catal. Lett., 2021, 152, 2445–2456 CrossRef.
- R. Hajian and A. Ehsanikhah, Chem. Phys. Lett., 2018, 691, 146–154 CrossRef CAS.
- R. Hajian, Inorg. Chem. Res., 2021, 6, 1–9 Search PubMed.
- D. Chen and A. Martell, Inorg. Chem., 1987, 26, 1026–1030 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- H. Su, Z. Li, Q. Huo, J. Guan and Q. Kan, RSC Adv., 2014, 4, 9990–9996 RSC.
- S. Tangestaninejad, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork and M. S. Saeedi, Appl. Catal., A, 2010, 381, 233–241 CrossRef CAS.
- S. Choudhary, H. P. Mungse and O. P. Khatri, J. Mater. Chem., 2012, 22, 21032–21039 RSC.
- B. S. Lane, M. Vogt, V. J. DeRose and K. Burgess, J. Am. Chem. Soc., 2002, 124, 11946–11954 CrossRef CAS PubMed.
- K. K. Krishnan, A. M. Thomas, K. S. Sindhu and G. Anilkumar, Tetrahedron, 2016, 72, 1–16 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05280c |
| This journal is © The Royal Society of Chemistry 2024 |