Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Corrosion inhibition performance of benzimidazole derivatives for protection of carbon steel in hydrochloric acid solution†
N. Timoudan a,
Arej S. Al-Gorair*b,
L. El Foujjicd,
I. Waradef,
Z. Safi
a,
Arej S. Al-Gorair*b,
L. El Foujjicd,
I. Waradef,
Z. Safi g,
B. Dikicih,
F. Benhibaai,
A. El Kacem Qaissj,
R. Bouhfidj,
F. Bentissk,
Salih S. Al-Juaidl,
Metwally Abdallah
g,
B. Dikicih,
F. Benhibaai,
A. El Kacem Qaissj,
R. Bouhfidj,
F. Bentissk,
Salih S. Al-Juaidl,
Metwally Abdallah *mn and
A. Zarrouk
*mn and
A. Zarrouk *af
*af
aLaboratory of Materials, Nanotechnology and Environment, Faculty of Sciences, Mohammed V University in Rabat, P. O. Box. 1014, Rabat, Morocco. E-mail: azarrouk@gmail.com
bChem. Depart., College of Science., Princess Nourah bint Abdulrahman Univ., Riyadh, Saudi Arabia. E-mail: asalgorir@pnu.edu.sa
cMoroccan Foundation of Advanced Science Innovation and Research MAScIR, Composites and Nanocomposites Center, Rabat Design Center, Madinat Al Irfane, Rabat, Morocco
dLaboratpire de Chimie Oganique Heterocyclique, Faculté des Sciences, Université Mohammed V de Rabat, Morocco
eDepartment of Chemistry, An-Najah National University, P. O. Box 7, Nablus, Palestine
fResearch Centre, Manchester Salt & Catalysis, Unit C, 88-90 Chorlton Rd, Manchester, M15 4AN, UK
gAl Azhar University – Gaza, Chemistry Department, Faculty of Science, P. O. Box 1277, Gaza, Palestine
hAtaturk University, Department of Mechanical Engineering, 25240 Erzurum, Turkey
iHigher Institute of Nursing Professions and Health Techniques of Agadir Annex Guelmim, Morocco
jMohammed VI Polytechnic University, Lot 660 Hay Moulay Rachid, Ben Guerir, 43150, Morocco
kLaboratory of Catalysis and Corrosion of Materials (LCCM), Faculty of Sciences, Chouaib Doukkali University, P. O. Box 20, M-24000 El Jadida, Morocco
lChem. Depart., Faculty of Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
mChem. Depart., Faculty of Science, Umm Al-Qura University, Makkah, Saudi Arabia. E-mail: metwally555@yahoo.com
nChem. Depart., Faculty of Science, Benha University, Benha, Egypt
First published on 23rd September 2024
Abstract
This paper presents a comprehensive study on the corrosion inhibition properties of new organic compounds, (1H-benzimidazol-2-yl)methanethiol (LF1) and 1-dodecyl-2-((dodecylthio)methyl)-1H-benzimidazole (LF2), have been examined for inhibiting of Carbon-Steel (C.S) in 1.0 M HCl. Numerous methods, such as potentiodynamic polarization, electrochemical impedance spectroscopy, scanning electron microscopy (SEM) and Energy Dispersive X-ray (EDX) analysis, atomic force microscopy (AFM), contact angle measurements, UV-visible spectroscopy, and theoretical calculations, were used to evaluate the effectiveness in preventing corrosion. The two benzimidazoles (LF1 and LF2)' inhibitory efficacy rose as their concentration increased, peaking at 88.2% and 95.4% respectively. The polarization graphs show a mixed behavior, with anodic predominance for LF1 and cathodic predominance for LF2. Thermodynamic investigations showed that the values of ΔGads were −40.0 kJ mol−1 for LF1 and −43.1 kJ mol−1 for LF2, highlighting their strong adsorption onto the metal surface. Their adsorption process was in line with the Langmuir isotherm. Density Functional Theory (DFT) and Molecular Dynamics (MD) modeling have been utilized to examine and clarify the relationship between the inhibitor and carbon steel (C.S).
1. Introduction
Carbon steel is often utilized as a construction material in various industrial applications due to its cost-effectiveness, favorable physicochemical properties, and widespread availability. As indicated by the references, this encompasses tasks such as constructing pipelines for the transportation of gas and oil, acid descaling, and acid pickling.1,2On the other hand, if the same material has to be cleaned and descaled using a strong acidic solution, especially 1.0 M HCl, it can be readily damaged, resulting in economic and human calamities. Because of this, this phenomenon is a critical issue that has scholars' attention. In order to reduce the corrosive assault on metallic surfaces, corrosion inhibitors, which are frequently used at low concentrations in acidic situations, are essential for protecting metals. Organic compounds having polar functionalities comprising nitrogen, sulfur, and/or oxygen in the conjugated system have been found to have effective inhibitory capabilities among alternative corrosion inhibitors.3,4 Research indicates that compounds containing N, S, and O-azoles, pyrrole, quinoline, and their derivatives, for example-have significant anti-corrosion properties.5,6
The characteristics of the corrosive media, the inhibitor's chemical structure, and the metal surface all affect adsorption.7 The interaction between the investigated metal and organic molecules has been characterized using two different mechanisms: chemical adsorption and physisorption. The kind and concentration of the medium, along with its temperature, are the main factors that influence the choice of appropriate inhibitors. Numerous writers have investigated how benzimidazole and its derivatives-especially N-heterocyclic compounds-affect carbon steel corrosion in acidic environments.8 Numerous researchers frequently employ benzimidazole bases as corrosion inhibitors. The benzimidazole derivatives demonstrated outstanding steel inhibition effectiveness in acidic solution, according to recent investigations.9–13
Benzimidazole and its many derivatives have attracted a great deal of interest in the field of metal corrosion protection. Several studies have demonstrated that these compounds are excellent inhibitors, particularly in acidic environments.14–17 Previous research has examined benzimidazole and its derivatives as corrosion inhibitors for carbon steel in a 1 M hydrochloric acid solution. These studies revealed that all three benzimidazole derivatives exhibited satisfactory corrosion inhibition efficacy.14 One study combined experimental and quantum chemical approaches to evaluate the inhibition performance of certain benzimidazole derivatives on mild steel corrosion in HCl, highlighting their effectiveness as corrosion inhibitors.
Benzimidazole and its multiple derivatives have also been studied in biochemical and pharmacological contexts.18 Literature searches have confirmed that benzimidazole molecules are considered a promising class of bioactive heterocyclic compounds, renowned for their efficacy against various strains of microorganisms. Benzimidazole derivatives exhibit a wide range of activities, including antimicrobial,19 antiparasitic,20 anti-HIV,21 and anticancer.22
The current study assesses the inhibitory efficacy of two synthetic benzimidazole compounds, (1H-benzimidazol-2-yl)methanethiol (LF1) and 1-dodecyl-2-[(dodecylsulfanyl)methyl]-1H-1,3-benzimidazole (LF2), against corrosion of C.S in 1.0 M HCl solution. The inhibition efficiency of these organic compounds was successively evaluated using potentiodynamic polarization curves, electrochemical impedance spectroscopy, isotherm calculations, SEM-EDX, AFM, contact angle, and UV-visible analysis, the efficiency of these organic compounds' inhibition was successively evaluated. DFT computations and molecular dynamics simulations (MDS) were used to determine the relationship between the investigated derivatives' molecular structures and their corrosion inhibition characteristics. Also proposed and covered was the adsorption mechanism. Thermodynamic and adsorption characteristics were also computed. Table 1 displays the molecular structures of the LF1 and LF2 under investigation.
2. Experimental
2.1. Materials
The chemical composition (in weight percentage) of the carbon steel (C.S) specimens used in this investigation is as follows: C-0.3700%, Si-0.2300%, Mn-0.6800%, S-0.0160%, Cr-0.0770%, Ti-0.0110%, Ni-0.0590%, Co-0.0090%, Cu-0.1600%, and iron (Fe) as the remaining content. SiC emery grade (180–1200) papers were used to polish these samples. After that, they were cleaned with distilled water, degreased with acetone, and dried with hot air. Distilled water was used to dilute concentrated acid (37.0%), which had a density of d = 1.18, to create the aggressive solution.2.2. Synthesis of LF1 and LF2
The general method for the synthesis of 2-substituted benzimidazoles was based on the Phillips procedure.23 The condensation of commercially available o-phenylenediamine (0.02 mol) and thioglycolic acid (0.03 mol), was conducted in the presence of boiling 4 N hydrochloric acid (20 mL) under reflux for 2 h, as shown in Scheme 1. The reaction mixture was cooled and neutralized using a sodium hydroxide solution. The crude product was washed with cold distilled water, dissolved in boiling water for recrystallization, and finally filtered and dried to obtain the desired LF1 product with a darkish aspect. Yield: 60%; 1H-NMR (600 MHz, DMSO-d6) δ (ppm), 7.56–7.18 (m, 4H), 4.22 (s, 2H). 13C-NMR (151 MHz, DMSO) δ (ppm) 36.2 CH2 (C8), 115.4 CH C(3/6), 122.3 CH C(1/2), 139.3 C(4/5), 151.1 C(7). LF2 was synthesized by direct alkylation reaction following the same procedure described in our previous work.24 LF1 (0.02 mol) was alkylated using 1-bromododecane (0.03 mol), anhydrous K2CO3 (0.04 mol) and tetra-n-butylammonium bromide TBAB as a catalyst in DMF as shown in Scheme 1. The mixture was stirred at room temperature for 48 h. The reaction progress and completion are monitored by thin-layer chromatography. After removing the salts by filtration, the DMF was removed using a rotary evaporator under reduced pressure. The obtained residue was dissolved in dichloromethane and filtered, and the solvent was removed under reduced pressure. The mixture is chromatographed on a silica gel column (eluent: ethyl acetate/cyclohexane (10/90%)). The obtained product LF2 is in a liquid form. Yield: 26%; 1H NMR (600 MHz, CDCl3) δ 7.99–7.08 (m, 4H), 4.22 (t, 2H), 3.97 (s, 2H), 2.58 (t, 2H), 1.88 (m, 2H), 1.56 (m, 36H), 1.40–1.18 (m, 2H), 0.90 (t, 6H). 13C NMR (151 MHz, CDCl3) δ (ppm) 150.8 C C(7), 142.3 C C(4), 135.5 1C C(5), 122.4, 121.8, 119.5, 109.5 4C C(1, 2, 3 and 6), 44.1, 44.05, 32.8, 31.9, 31.9, 31.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.3, 29.2, 29.1, 28.8, 28.7, 28.2, 28.1, 27.0 21C CH2, 22.7 2C CH2, 14.2 2C, CH3.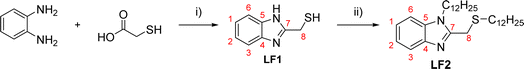 | ||
| Scheme 1 General procedure for the synthesis of LF1 and LF2. Reagents and conditions: (i) 4 N-HCl, 2 h, reflux; (ii) C12H25Br, K2CO3, TBAB, DMF, RT. | ||
2.3. Electrochemical studies
The VoltaLab potentiostat, which was attached to a typical triple-electrode cell, was used for electrochemical studies. A specimen made of carbon steel with a chosen surface area of 1.0 cm2 served as the working electrode in this configuration. With platinum wire acting as the counter electrode with the same surface area as the working electrode, the reference electrode was a saturated calomel electrode (SCE). Every experiment was finished with fewer than 30 minutes of immersion time to provide a consistent open circuit potential.
 | (1) |
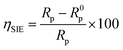 | (2) |
We used the results of the blank in the absence and presence of HCl without inhibitor for the effect of concentration and temperature, since we worked under similar conditions in a work already published by our team.23
2.4. Morphology investigations SEM
The SEM technique was utilized to examine the surfaces. A corrosive solution of molar HCl, with and without an optimal concentration of 0.001 M of inhibitors LF1 and LF2 individually, was employed. The samples were exposed to these harsh environments separately for 24 hours, then delicately removed, rinsed with purified water, dried, and subjected to surface morphological analysis using SEM. The JEOL-JSM-IT-100 model was employed for examining surface morphology. We have used the results of the blank in the absence and presence of HCl without inhibitor since we have worked under similar conditions in a work already published by our team.232.5. AFM analysis
The examination of the deposited film morphology was conducted at T = 303 K through observation using Atomic Force Microscopy (AFM) using Hitachi 5100N. The AFM studies are carried out utilizing an imaging techniques system by immersing the electrode in 1.0 M HCl in the absence and presence of 0.001 M of the two investigated compounds for 24 h.We have used the results of the blank in the absence and presence of HCl without inhibitor since we have worked under similar conditions in a work already published by our team.23
2.6. Contact angle
Based on Biolin Scientific's Attension/Theta model, contact angles were determined using the sessile water drop technique. One minute after the liquid was placed on the coated surface, water contact angles (WCA) depending on the profile of the drop attained after equilibrium were determined. Contact angle measurements were employed to analyze the changes in C.S's hydrophobicity when exposed to an acidic environment with two inhibitors, LF1 and LF2. These assessments were conducted after immersing C.S in the acid solution for 24 hours, both before and after exposure to an optimal inhibitor concentration (0.001 M) of LF1 and LF2. We have used the results of the blank in the absence and presence of HCl without inhibitor since we have worked under similar conditions in a work already published by our team.232.7. UV-visible spectrum analysis
UV-vis spectroscopy offers a better understanding of the interactions between steel and the inhibitors examined. Using an optimum concentration of 0.001 M benzimidazole derivatives at a temperature of 303 K for 24 hours, the electrolytic solution was subjected to analysis by this method before and after immersion of the C.S samples in a 1.0 M HCl solution. With a spectral range of 0 nm/400 nm, UV-vis spectrophotometer (Jasco type spectrophotometer (series V-730) with a stray light of 0.02% for exceptional absorbance linearity up to 3 abs) was used to record the spectra.2.8. Computational chemical details
Structure, electron distribution, molecule adsorption on metal and oxide surfaces, and studied inhibitory mechanisms are all subjects of investigation. The quantum chemical technique using DFT to examine the effectiveness of inhibitors in relation to their molecular characteristics.24,25 This study used the DFT/(B3LYP) calculations with base 6-311+G(d,p) performed using Gaussian 09 methods to optimize the benzimidazole derivatives completely. These are looking for molecular descriptors like LUMO (EL) and HOMO (EH) energies. Based on these values, we can calculate energy gap (ΔE = EL − EH), absolute electronegativity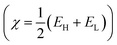 , global hardness
, global hardness  , softness
, softness  , electrophilicity
, electrophilicity  , dipole moment (μ), ionization energy (I = −EH), electron affinity (A = −EL), and number of electrons transferred from the inhibitor to the metal Fe(110)
, dipole moment (μ), ionization energy (I = −EH), electron affinity (A = −EL), and number of electrons transferred from the inhibitor to the metal Fe(110)  with Φ is work function, he energy change during the electronic back-donation process
with Φ is work function, he energy change during the electronic back-donation process  .26–28
.26–28
In addition, the vertical ionization potential (VIP) and vertical electron affinity (VEA) are calculated as:26
| IPv = E0+ − E0 and EAv = E0− − E0 | (3) |
2.9. MD simulation details
Molecular dynamics (MD) simulations have become increasingly popular in studying corrosion inhibition in recent years.29,30 This method helps researchers to understand how inhibitor molecules interact and are adsorbed onto supporting surfaces.31 In this study, we focused on a system consisting of 9Cl−, 9H3O+, and 481H2O molecules, and successfully built a monomer of the selected molecule during each simulation.We used the Forcite technique in Materials Studio 8 software to run the molecular dynamics simulations, with cell characteristics of 30 Å vacuums, periodic boundary conditions, a unit cell size of (27.30 × 27.30 × 40.13 Å3), and a (11 × 11) unit cell. Prior to the simulation, the LF1 and LF2 structures were pre-optimized using the GGA (Generalized Gradient Approximation) & DNP (Dual Numerical Polarization) functions.
The COMPASS force field was used to run the simulation, with a time interval of 1.0 fs and a duration of 2000 ps. We used the NVT ensemble with an Andersen thermostat to run all simulations at a temperature of 303 K conditions, (27.30 × 27.30 × 40.13 Å3) unit cell size, and (11 × 11) unit cell. The LF1 and LF2 structures are pre-optimized through the use of the GGA (Generalized Gradient Approximation) & DNP (Dual Numerical Polarization) functions. The simulation was run using the COMPASS force field, with a time interval of 1.0 fs and a duration of 2000 ps. The NVT ensemble with an Andersen thermostat was used to run all simulations at a temperature of 303 K.32
3. Results and discussions
3.1. Potentiodynamic polarization measurements
Fig. 1 displays potentiodynamic polarization plots for carbon steel at 303 K in 1.0 M HCl solution, both with and without different LF1 and LF2 concentrations.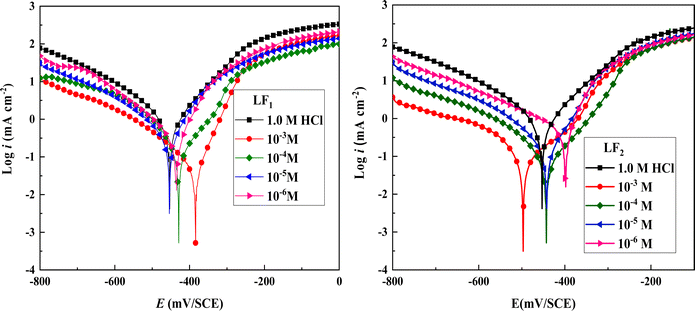 | ||
| Fig. 1 Potentiodynamic polarization graphs of C.S at 303 K, with and without different LF1 & LF2 concentrations, submerged in 1.0 M HCl solution. | ||
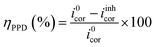
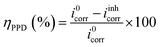
Eqn (4)33 was used in the Tafel extrapolation method to determine the corrosion inhibition efficiency (nPPD) because every reaction exhibits Tafel behavior. The corrosion current density obtained in the absence of corrosion inhibitors i0corr and the corrosion current density observed in the presence of corrosion inhibitors iinhcorr are the two variables in this equation. Table 2 displays the electrochemical corrosion parameters that were determined.
 | (4) |
| Solution | Conc. | −Ecorr/SCE (mV) | icorr (μA cm−2) | βa (mV dec−1) | −βc (mV dec−1) | ηPPD (%) |
|---|---|---|---|---|---|---|
| HCl | 1 M | 456.3 ± 6.0 | 1104.1 ± 5.0 | 112.8 | 155.4 | — |
| LF1 | 10−3 | 385.7 ± 3.2 | 130.7 ± 1.2 | 66.4 | 183.1 | 88.1 |
| 10−4 | 427.6 ± 6.2 | 234.0 ± 2.5 | 104.8 | 115.6 | 78.8 | |
| 10−5 | 454.2 ± 5.2 | 330.7 ± 3.5 | 82.6 | 125.1 | 70.0 | |
| 10−6 | 437.0 ± 7.2 | 407.5 ± 5.1 | 75.7 | 139.5 | 63.1 | |
| LF2 | 10−3 | 497.9 ± 4.1 | 50.4 ± 0.3 | 89.1 | 61.7 | 95.4 |
| 10−4 | 441.7 ± 3.2 | 85.5 ± 3.2 | 79.4 | 109.9 | 92.2 | |
| 10−5 | 441.9 ± 3.2 | 214.1 ± 2.4 | 70.6 | 125.3 | 80.6 | |
| 10−6 | 400.0 ± 3.2 | 300.3 ± 3.1 | 43.2 | 99.7 | 72.8 |
The data presented in Table 2 reveal that the introduction of LF1 and LF2 inhibitors into the corrosive medium results in a decrease in both anodic and cathodic current densities.34 This implies that both anodic metal attack and cathodic hydrogen evolution reactions are reduced following the addition of LF1 and LF2 inhibitors to the acid solution. The corrosion-inhibiting effect becomes increasingly pronounced with increasing inhibitor concentration,35 from 1 × 10−6 M to 1 × 10−3 M. Fig. 1 highlights the fact that the cathodic current–potential curves are almost parallel, suggesting that the addition of the inhibitors LF1 and LF2 to the 1.0 M HCl solution does not alter the mechanism of the hydrogen evaluation reaction. The reduction of H+ ions on the surface of carbon steel occurs mainly via a charge transfer mechanism.36–38 However, the βc value changes with the benzimidazole addition, indicating that both inhibitors influence the rate at which hydrogen is evolute, which suggests that benzimidazoles powerfully inhibit the corrosion process of C.S, and its ability as a corrosion inhibitor is enhanced as its concentration is increased. The suppression of the cathodic process can be due to the formation of a protective film of benzimidazoles at the metal/solution interface.36,39,40
It can be seen, that compared to the blank solution, the value of βa is markedly changed in the presence of benzimidazoles, which suggests that benzimidazoles can affect the kinetics of the anodic process. Given their several pairs of free electrons, nitrogen, and sulfur atoms in the benzimidazole molecules participate in the formation of the iron–benzimidazole complex. This interaction between iron and the benzimidazole molecule alters the mechanism of iron dissolution.36,41,42
As depicted in Table 2, introducing LF1 into the acidic solution results in a shift of the corrosion potential towards more positive values. This suggests that the inhibitor functions primarily as a mixed-type corrosion inhibitor, with a predominantly anodic effect,43 according to the polarization measurement data. Conversely, the addition of LF2 to the acidic solution shifts the corrosion potential towards more negative values. LF2 exhibits mixed-type corrosion inhibition, with a predominant cathodic action.44–46
3.2. Electrochemical impedance spectroscopy study
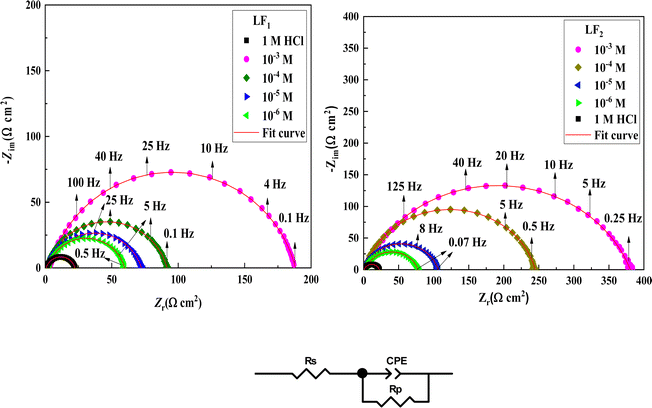
Electrochemical impedance spectroscopy tests were conducted to learn more about the electrochemical processes occurring across the electrode/solution interface and to examine these properties in the presence and absence of compounds containing benzimidazole derivatives (1 × 10−3 M, 1 × 10−4 M, 1 × 10−5 M, and 1 × 10−6 M), which are present in the 1.0 M HCl solution. Thanks for sharing the information. We'll take a look at the Nyquist charts in Fig. 2 to better understand the systems.Nyquist plots and Bode curves obtained for compounds in the LF2-1.0 M HCl and C.S-LF1 systems at open-circuit-potential are displayed in Fig. 2 and 3. Table 3 provides the corresponding fitted parameters. It is clear from the acquired spectra that a capacitive loop was present. This loop's diameter increased significantly upon the addition of chemicals LF1 and LF2, peaking at a concentration of 10−3 M for each molecule. Furthermore, as reported in the context of frequency dispersion, these plots show intact semicircles.47 Rp pointed out that it is found that the resistance to polarization rises with the use of inhibitors. An increase in the inhibitors' effectiveness is indicated by the semicircle's size growing in proportion to the LF1 and LF2 chemical concentrations. These findings suggest that a charge transfer mechanism controls the corrosion of C.S in hydrochloric solution, and that adsorption of LF1 and LF2 compounds to the C.S surface prevents corrosion.
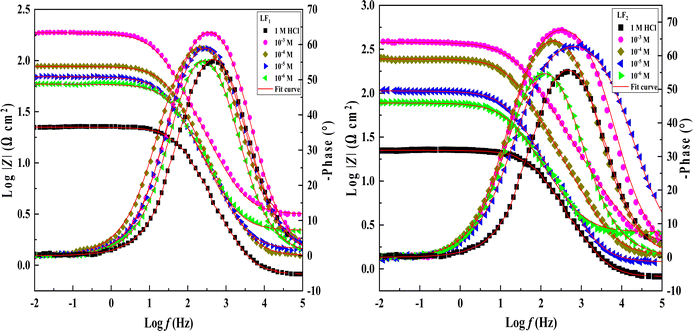 | ||
| Fig. 3 Bode plots & phase angle spectral representations of Bode for the impedance spectroscopy system in a 1.0 M HCl solution, both with and without different LF1 & LF2 concentrations at 303 K. | ||
| Medium | Conc. (M) | Rs (Ω cm2) | Rp (Ω cm2) | 106 × Q (μF sn−1 cm−2) | n | Cdl (μF cm−2) | χ2 | ηSIE (%) |
|---|---|---|---|---|---|---|---|---|
| HCl | 1 M | 0.83 ± 0.01 | 21.57 ± 0.56 | 293.9 ± 2.35 | 0.845 ± 0.003 | 116.2 | 0.002 | — |
| LF1 | 10−3 | 3.94 ± 0.03 | 184.9 ± 1.54 | 83.2 ± 0.90 | 0.862 ± 0.005 | 42.62 | 0.004 | 88.3 |
| 10−4 | 1.25 ± 0.01 | 90.4 ± 0.83 | 125.0 ± 1.32 | 0.857 ± 0.006 | 59.17 | 0.005 | 76.2 | |
| 10−5 | 1.55 ± 0.01 | 70.9 ± 0.61 | 163.0 ± 1.51 | 0.851 ± 0.004 | 74.64 | 0.005 | 69.6 | |
| 10−6 | 2.35 ± 0.02 | 57.4 ± 0.49 | 175.0 ± 1.83 | 0.849 ± 0.001 | 77.2 | 0.004 | 62.5 | |
| LF2 | 10−3 | 1.70 ± 0.02 | 378.7 ± 2.77 | 29.2 ± 0.26 | 0.851 ± 0.002 | 13.26 | 0.004 | 94.3 |
| 10−4 | 1.53 ± 0.01 | 243.5 ± 2.09 | 59.9 ± 0.56 | 0.861 ± 0.005 | 30.26 | 0.005 | 91.1 | |
| 10−5 | 1.01 ± 0.01 | 106.0 ± 0.96 | 165.0 ± 1.58 | 0.843 ± 0.001 | 77.66 | 0.007 | 79.7 | |
| 10−6 | 1.28 ± 0.01 | 76.5 ± 0.58 | 230.5 ± 2.34 | 0.831 ± 0.002 | 101.4 | 0.006 | 71.9 |
An analogous electrochemical circuit with a solution resistor (Rs), a resistance of polarization (Rp), and a constant phase element (CPE) is applied to model impedance behavior frequently. Table 3 presents the computed values of the pertinent electrochemical parameters, which are Rs, Rp, and CPEdl. Furthermore, the correctness of the simulated data was evaluated using chi-square; small chi-square values (10−3) (Table 3) show that the simulated and experimental data agree closely.
Impedance spectrum depression is commonly attributed to frequency dispersion. This frequency dispersion is due to inhomogeneities in the electrode surface (formation of corrosion products, roughness, presence of impurities, variations in the thickness or composition of a film or coating on the metal surface, or inhibitor adsorption), which induce a change in the electrode's active surface. These surface inhomogeneities are accounted for by a constant-phase element CPE (Q, n), via the coefficient n (between 0 and 1). The impedance of such an element is given by the following equation:48
| ZCPE = Q−1(iω)−1 | (5) |
| Cdl = (Q × Rp1−n)1/n | (6) |
 | (7) |
Table 3 shows the parameters obtained from the Electrochemical Impedance Spectroscopy studies. It was observed that while Cdl values show an inverse relationship, Rp increases proportionally with inhibitor concentration. This phenomenon is probably due to the adsorption of inhibitor compounds, which increased the surface coverage of the C.S. The increase in the thickness of the double layer, caused by these organic compounds replacing the water molecules at the C.S interface, is linked to the decrease in Cdl. Due to the irregularity of the metal surface induced by the development of porous layers, the deviation of Cdl from perfect capacitive behaviour can be associated with the slight divergence of the n value from unity.49,50
The chi-squared (χ2) was utilized to appraise the precision of the fitting outcomes, the small chi-squared values (Table 3) acquired for all the outcomes show that the fitted results have a great concurrence with the experimental findings. For LF2 at the optimal concentration (10−3 M), the maximum value of Rp (378.7 Ω cm2) and the minimum value of Cdl (13.26 (μF sn−1) cm−2) were discovered. This finding demonstrates that LF2 exhibits stronger inhibitory performance than LF1. For LF1, the obtained value of ηSIE is 88.3%. The inhibitory efficacy of the compound rises with the addition of a second benzimidazole segment, reaching a value of 94.3% for LF2. The interaction between the many N, S, and π-electron active sites and the open orbitals of the iron atoms explains this enhancement. Furthermore, LF2's total contact area is greater than LF1's, leading to a more noticeable interaction between the steel surface.51
The acquired results (refer to Table 2) are in good agreement with the potentiodynamic polarization measurements.
3.3. Adsorption isotherm
Various types of isotherm models were applied to better understand how the LF1 and LF2 studies interact with the C.S surface (Fig. S1 in the ESI†). The correlation coefficient (R2) was found to be the main parameter fitted to the experimental data. Surface coverage rates (θ) were determined using the following formula for different quantities:
 | (8) |
The Cinh/θ vs. Cinh plot gives a straight line with a slope near the unit (Fig. S1†), and also, a linear correlation coefficient near 1, which shows the adsorption of LF1 and LF2 obeys Langmuir isotherm that expressed according to eqn (9):51,52
| Cinh × θ−1 = K−1 + Cinh | (9) |
| ΔGads = −RT × ln(1 × 55.5 × K) | (10) |
The Langmuir isotherm adsorption parameters (Table 4) have a regression coefficient R2 of 1 for LF1 & for 1 LF2. It is generally acknowledged that a result above −20 kJ mol−1 shows physical adsorption and that chemical adsorption occurs below −40 kJ mol−1. The interaction between inhibitors and metals is thought to be mixed adsorption, comprising both chemical and physical adsorption if the value falls between these two criteria.
| R2 | Slope | K (L mol−1) | ΔGads (kJ mol−1) | |
|---|---|---|---|---|
| LF1 | 0.999 | 1.12 | 1.4261 × 105 | −40.0 |
| LF2 | 0.999 | 1.05 | 4.9907 × 105 | −43.1 |
ΔGads calculated for LF1 and LF2 at the temperature 303 K, show that the shaping of adsorbed film on the surface of the C.S is mostly the product of the inhibitory mechanism's reinforcement of the chemical adsorption process.54 Although the ΔGads values strongly indicate that chemisorption is the primary adsorption mode, it is not possible to completely discount the influence of van der Waals interactions (physical adsorption). These interactions might be significant during the initial adsorption stages or contribute to the adsorbed layer's overall stability. Consequently, the adsorption process on the C.S surface is considered a combination of chemisorption and physisorption, with chemisorption being the predominant interaction based on the estimated thermodynamic outcomes.
3.4. Effect of temperature
The temperature of the corrosive environment influences the interaction between the C.S surfaces and the inhibitors, as well as the corrosion rate of C.S in an acid solution. To examine this effect, polarization analyses were carried out on C.S in a 1.0 M HCl solution, in the presence and absence of inhibitors at a concentration of 10−3 M, over a temperature range from 303 to 333 K. The results are shown in Table 5 and Fig. 4.| Solution | T (K) | −Ecorr (mVSCE) | icorr (μA cm−2) | βa (mV dec−1) | −βc (mV dec−1) | ηpp (%) |
|---|---|---|---|---|---|---|
| 1.0 M HCl | 303 | 456.3 ± 6.0 | 1104.1 ± 5.0 | 112.8 | 155.4 | — |
| 313 | 423.5 ± 9.0 | 1477.4 ± 8.0 | 91.3 | 131.3 | — | |
| 323 | 436.3 ± 7.0 | 2254.0 ± 10.0 | 91.4 | 117.8 | — | |
| 333 | 433.3 ± 5.0 | 3944.9 ± 12.0 | 103.9 | 134.6 | — | |
| LF1 | 303 | 385.7 ± 3.2 | 130.7 ± 1.2 | 66.4 | 183.1 | 88.1 |
| 313 | 432.6 ± 6.2 | 292.9 ± 3.0 | 71.4 | 137.7 | 80.1 | |
| 323 | 420.9 ± 5.3 | 516.1 ± 5.1 | 53.1 | 146.7 | 77.1 | |
| 333 | 407.9 ± 6.0 | 1258.7 ± 10.9 | 49.2 | 209.1 | 68.1 | |
| LF2 | 303 | 497.9 ± 4.1 | 50.4 ± 0.3 | 89.1 | 61.7 | 95.4 |
| 313 | 490.9 ± 7.5 | 146.2 ± 1.5 | 85.8 | 134.0 | 90.1 | |
| 323 | 499.7 ± 1.7 | 365.1 ± 3.7 | 71.6 | 117.7 | 83.8 | |
| 333 | 451.01 ± 5.8 | 1138.2 ± 12.0 | 84.9 | 116.6 | 71.1 |
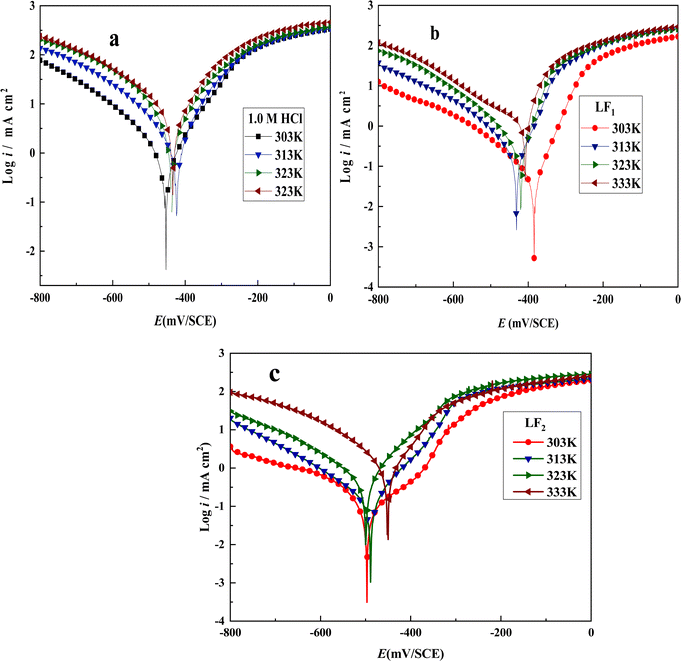 | ||
| Fig. 4 Tafel plots showing C.S in 1.0 M HCl solution with and without inhibitors at 10−3 M concentrations at varying temperatures: (a) without inhibitor, (b) with LF1, and (c) with LF2. | ||
As corrosion current density rises in both inhibited and uninhibited solutions, both compounds function as temperature-sensitive inhibitors, as shown by the results in Fig. 4 and Table 5. Moreover, as the temperature of the corrosive solutions rises, the inhibitory efficacy of both compounds falls. Therefore, inhibitory performance is decreased at higher temperatures. This observation is most likely the result of inhibitor compounds' declining adsorption capacity as temperature rises.
Eqn (11) and (12) were used to compute thermodynamic activation characteristics, such as activation energies (Ea), enthalpies (ΔHa), and activation entropies (ΔSa), which were then combined and displayed in Table 6.
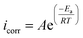 | (11) |
 | (12) |
 to (T−1) (Fig. S2†). Upon seeing the point where this line intersects the
to (T−1) (Fig. S2†). Upon seeing the point where this line intersects the 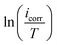 axis, we get ΔSa, which has a slope of −ΔHa × R−1.
axis, we get ΔSa, which has a slope of −ΔHa × R−1.
| R2 | Ea (kJ mol−1) | ΔHa (kJ mol−1) | ΔSa (J mol−1 K−1) | |
|---|---|---|---|---|
| 1.0 M HCl | 0.9670 | 35.40 | 32.77 | −79.2 |
| LF1 | 0.9860 | 61.50 | 59.00 | −9.88 |
| LF2 | 0.9960 | 85.90 | 83.30 | 62.50 |
The inhibition process was accelerated by the inhibitors under investigation, which raised the energy barrier (Ea) of the inhibition solution in comparison to the blank. This can be explained by these molecules gradually adhering to the C.S surface to form a protective layer. The positive indication of enthalpy (ΔHa) indicates that the C.S dissolving process is endothermic (ΔHa).55 The activation entropy ΔSa increases following the addition of LF1 and LF2, suggesting an increase in molecular disorder in the system.56,57
3.5. Surface characterizations
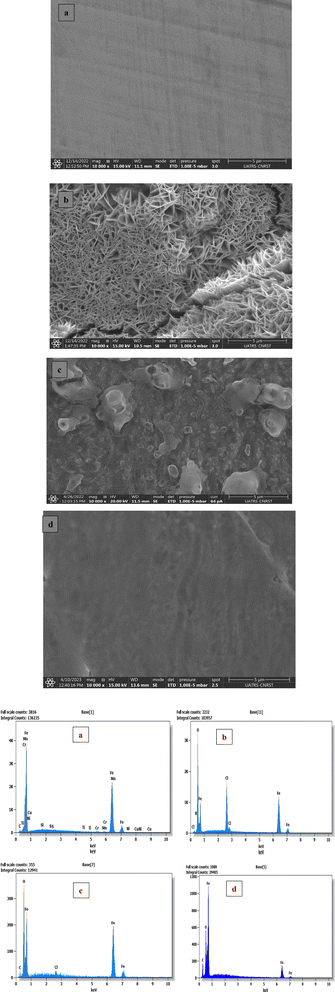 | ||
| Fig. 5 SEM/EDX of C.S only (a), with 1.0 M HCl (b), and with 1 × 10−3 M of LF1 (c) & LF2 (d) after 24 h of immersion. | ||
Severe damage to the sample's surface has been caused by rapid corrosion that was observed in a 1.0 M HCl acid solution without LF1 or LF2, as shown in the C.S picture (Fig. 5b). However, the surface appears to be covered with plate-like patterns (Fig. 5c and d) when LF1 or LF2 is present at a concentration of 1 × 10−3 M, indicating the existence of organic molecules (Fig. 5c and d). This observation suggests that the prevention of corrosion occurs when inhibiting molecules produce a deposit that creates a layer on the C.S surface.
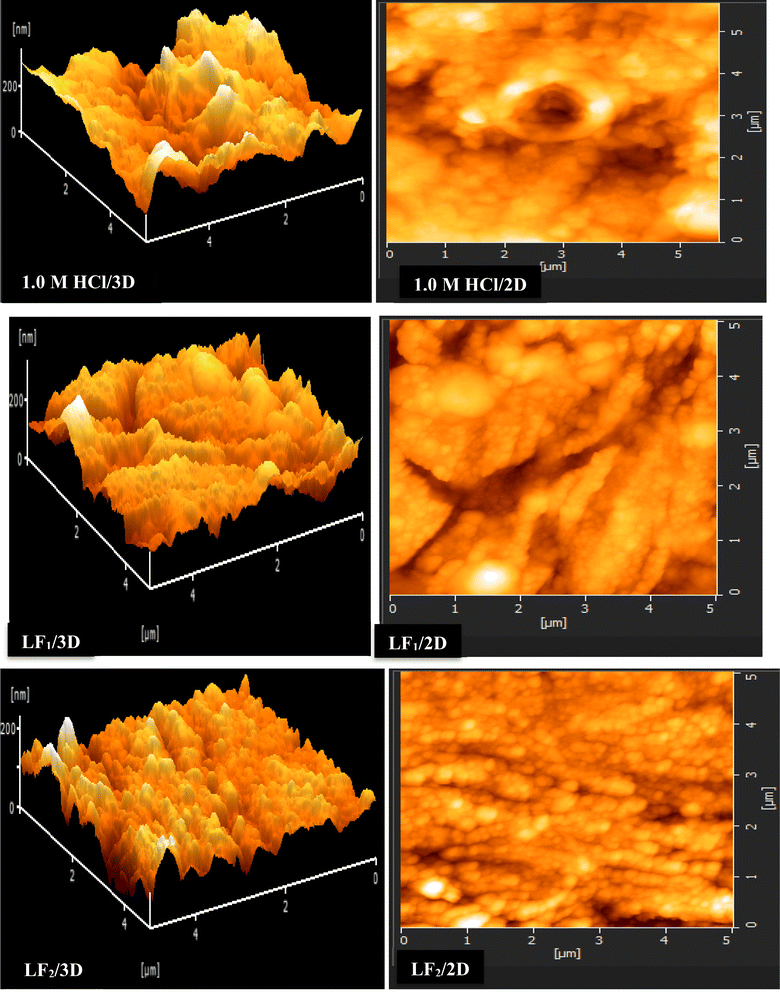 | ||
| Fig. 6 AFM images (2D and 3D) were captured for C.S specimens both in the non-existent and in the existence of 1 × 10−3 M concentrations of LF1 & LF2. | ||
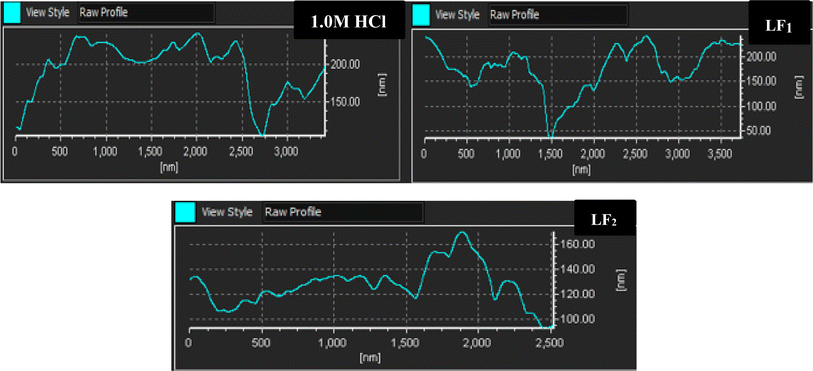 | ||
| Fig. 7 Displays the relative-height profiles extracted from 3D/2D AFM images at a temperature of 303 K. | ||
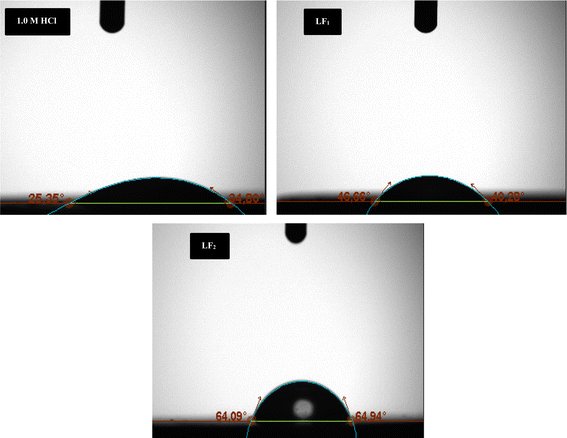
The addition of LF1 and LF2 produced a C.S surface that was noticeably uniformly smooth and deepened the corrosion grooves significantly. These findings imply that these organic substances are useful in reducing the rate at which steel samples corrode in 1.0 M HCl.
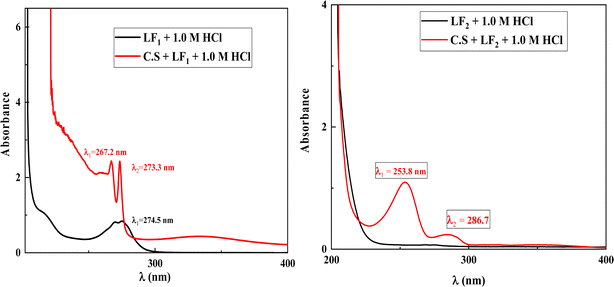
The UV-visible (UV-visible) spectra in the present investigation indicate a change in wavelength (λ) for the corrosive solution of 1.0 M HCl containing only the inhibitors LF1 and LF2 in comparison to the solution of 1.0 M HCl containing both inhibitors in the presence of C.S (Fig. 9). This demonstrates how the complexation reaction helps the two inhibitors, LF1 and LF2, decrease the solubility of ferric ions Fe2+ in the corrosive solution.6,60
3.6. DFT and MD simulations
Fig. 11 displays the optimized structures, HOMOs, LUMOs, energy gap diagrams, and molecular electrostatic potential maps (ESP) for the inhibitor compounds that are being studied. Upon inspection, it is evident that the HOMO distribution primarily occurs over the benzimidazoles moiety of the inhibitors, whereas the LUMO distribution is limited to the electron-deficient regions of the benzimidazoles moiety. This indicates that these centers are primarily involved in electron donation and acceptance during the metal–inhibitor interactions.
 | ||
| Fig. 11 The optimized geometrical structures (a), LUMO's (b), HOMO's (c), ESP maps (d) & the energy gaps of the investigated species (LF1, and LF2). | ||
Table 7 provides a summary of the global reactivity properties of the inhibitor that was studied. Higher EHOMO values indicate a higher tendency to donate electrons to electron-deficient sites, while lower values indicate a greater ability to accept electrons by the inhibitor.61,62 As shown, the EHOMO increases in the order of LF2 > LF1, indicating that the LF2 inhibitor is more likely to contribute electrons to the virtual 3d orbital of the steel. The ELUMO decreases in the order of LF1 < LF2, which means that the LF1 inhibitor molecule has the highest capacity to receive electrons from the steel surface. The smaller the energy gap, the higher the adsorption efficiency. The results show that the Egap trend is as follows: LF2 (4.801 eV) < LF1 (5.148 eV), indicating that the LF2 inhibitor is the most reactive species and is better able to adhere to the steel surface compared to the LF1 inhibitor. Similar trends can also be observed for the other global chemical reactivity parameters (see Table 7).
| LF1 | LF2 | |
|---|---|---|
| E0 (Ha) | −817.90697 | −1761.52369 |
| E0− (Ha) | −817.99001 | −1761.60258 |
| E0+ (Ha) | −817.64291 | −1761.27386 |
| EH (eV) | −7.269 | −6.813 |
| EL (eV) | −2.121 | −2.012 |
| ΔE (eV) | 5.148 | 4.801 |
| ΔE1 (eV) | 5.781 | 5.890 |
| ΔE2 (eV) | 7.118 | 6.662 |
| IPv (eV) | 7.185 | 6.798 |
| EAv (eV) | 2.260 | 2.147 |
| Fg (eV) | 4.926 | 4.652 |
| χ (eV) | 4.723 | 4.472 |
| η (eV) | 2.463 | 2.326 |
| S (eV) | 0.406 | 0.430 |
| ω (eV) | 4.528 | 4.300 |
| ΔEsteel/inh (eV) | −1.553 | −1.398 |
| ΔN110 | 0.020 | 0.075 |
| ΔEb-d (eV) | −0.616 | −0.581 |
Another important factor is the ΔN110, showing the transfer of electrons throughout the adsorption process, either from the inhibitor (positive values) to the metal surface or the other way around (negative values) when the metal surface or inhibitors are brought into close proximity from a low/electronegativity system to a high-electronegativity system until all chemical potentials are equal. If ΔN < 3.6, the summarized results in Table 7 show that the values of the (ΔN110) are positive and <3.6, illustrating that the inhibition efficiency of the investigated inhibitors is improved, and they have their ability to donate electrons to the metal surface is increased as in Cherinka et al. work.63 Our results show that the trend of the investigated inhibitors is as follows: LF2 (0.0747) > LF1 (0.020). These results show the importance of lengthening the carbon chain in increasing ΔN110 and, consequently, increasing the fraction of electron transfer from the inhibitor molecule to the metal surface.
The interaction affecting the way inhibitors and the metal surface interact can be treated in view of the energy gaps between the metal and inhibitors as calculated in the following expressions:61,62,64
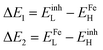 | (13) |
| fk+ = q(N + 1) − q(N) (for nucleophilic attacks) | (14) |
| fk− = q(N) − q(N − 1) (for electrophilic attacks) | (15) |
| Δfk = fk+ − fk− | (16) |
The inhibitors under investigation contain several active centers, which is advantageous based on their chemical structures. The inhibitor centers' ability to provide electrons to the steel surface rises with the amount of partially negative active centers.75 The three-dimensional isosurfaces of the Fukui functions (fk−, fk+, and Δfk) are shown in the ESI (Fig. S3†). Like the surfaces for HOMOs and LUMOs (Fig. 11), these isosurfaces confirm the findings about the active centers of the species under study. A center is generally thought to be vulnerable to nucleophilic attack if its Δfk is greater than zero and it fk+ is the highest. On the other hand, the situation is the opposite for electrophilic attack centers, where Δfk is negative and fk− is the maximum.76 The main conclusions of the fk+, fk− and Δfk condensed Fukui indices for the inhibitors under study are presented in Table 8. A full dataset can be seen in ESI Tables S1 and S2.†
| Center | fk+ | Center | fk− | Center | Δfk |
|---|---|---|---|---|---|
| LF1 neutral | |||||
| S18 | 0.1401 | C4 | 0.1306 | C4 | −0.0586 |
| C7 | 0.0898 | N12 | 0.091 | C1 | −0.0531 |
| C3 | 0.0853 | C1 | 0.0886 | C2 | −0.0325 |
| C6 | 0.0825 | C6 | 0.0868 | S18 | 0.0921 |
| N13 | 0.072 | C7 | 0.0825 | C15 | 0.0173 |
| N12 | 0.0657 | C3 | 0.0759 | C3 | 0.0093 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| LF1-protonated | |||||
| S18 | 0.1451 | S18 | 0.331 | S18 | −0.1859 |
| C7 | 0.127 | C4 | 0.08 | C1 | −0.037 |
| C3 | 0.0683 | C1 | 0.0621 | C2 | −0.0267 |
| C6 | 0.0679 | C5 | 0.0593 | C7 | 0.0894 |
| N13 | 0.0642 | C2 | 0.0519 | N13 | 0.0406 |
| N12 | 0.0634 | C7 | 0.0375 | C3 | 0.0343 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| LF2-protonated | |||||
| C7 | 0.1178 | S18 | 0.4558 | S18 | −0.3646 |
| S18 | 0.0912 | C19 | 0.0375 | C19 | −0.0308 |
| C3 | 0.0759 | C15 | 0.0329 | C15 | −0.0066 |
| C6 | 0.0722 | C4 | 0.0304 | C7 | 0.1113 |
| C5 | 0.0612 | C5 | 0.0212 | C3 | 0.0601 |
| N13 | 0.0584 | C1 | 0.0206 | C6 | 0.0564 |
Table 8 makes clear that for protonated species, S18 & C7 are the highly reactive centers that are more suited for nucleophilic assaults, whereas S18 & C4 are more vulnerable to electrophilic ones. Precisely, for the protonated form of LF1, the centers that may be favored for electrophilic attacks and nucleophilic attacks are S18 & C7, respectively. For the protonated form of LF2, the centers that may be favored for electrophilic attacks are S18 & C19. Whereas, for the same inhibitor, the sites that may be favored for the nucleophilic attacks are C7 & C3.
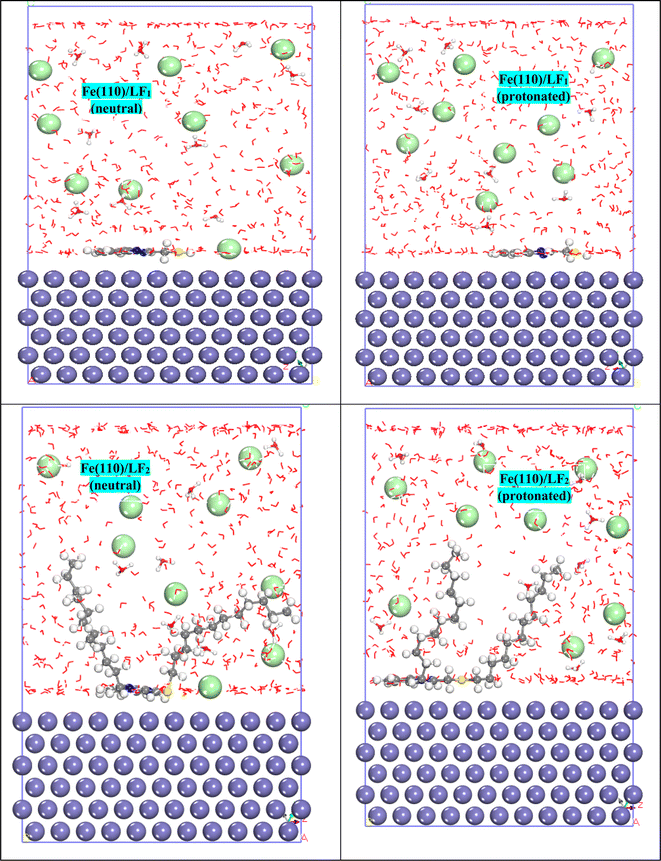 | ||
| Fig. 12 Adsorption configurations feature the most stable forms for the systems Fe(110)/LF1 (neutral), Fe(110)/LF2 (neutral), Fe(110)/LF1 (protonated), and Fe(110)/LF2 (protonated). | ||
The degree of interaction is well represented by energy parameters such as interaction and binding energies, with a more negative value of Einteraction indicating a better interaction, while a more positive Ebinding value means better adsorption. These both descriptors are computed based on the following two formulas:78,79
| Einteraction = Etotal − Einhibitor − (Esurface+solution) | (17) |
| Ebinding = −Einteraction | (18) |
In Table 9, a greater negative value for Einteraction indicates a higher level of protective adsorption due to increased interaction between the inhibitor and steel surface.80 As noted in Table 9, the Fe(110)/LF2 (neutral) system bears a more negative value for Einteraction (−1184.209 kJ mol−1) and a more positive value for Ebinding (1184.209 kJ mol−1), showing that the neutral molecule LF2 interacts more strongly with the first Fe(110) layer. This result and all the data contained in Table 9 confirm the results generated experimentally.
| Systems | Fe(110)/LF1 (neutral) | Fe(110)/LF2 (neutral) | Fe(110)/LF1 (protonated) | Fe(110)/LF2 (protonated) |
|---|---|---|---|---|
| Einteraction | −1138.360 | −1184.209 | −1144.982 | −1180.681 |
| Ebinding | 1138.360 | 1184.209 | 1144.982 | 1180.681 |
This method's primary goal is to use the radial distribution function (RDF) to assess the nature of the bonding and adsorption with the neutral and protonated atoms in the inhibitor's initial iron layer. Estimating the interatomic distance between the atoms and heteroatoms of the forms under study and Fe(110) is a particularly important use of this method.81 Prior studies have verified that chemical adsorption is more probable for bond lengths shorter than 3.5 Å, whereas physical adsorption is suggested by longer link lengths. All bond lengths have values smaller than 3.5 Å, as seen by the initial peaks in Fig. 13, indicating chemical adsorption.
3.7. Corrosion inhibition mechanism
The protective mechanism of these compounds on the C.S can be explained through the inhibition process exerted by the inhibitor over the metal surface. The main modes of adsorption of an organic molecule onto a metal surface include chemisorption, physisorption, or a combination of the two. Many parameters, such as the concentration of the inhibitor, the presence of active sites, the charge of the molecules, and the characteristics of the metal surface, influence the adsorption processes of the inhibitor.The benzimidazole compounds studied in this research have functional groups containing S, N heteroatoms and phenyl rings, which can act as adsorption sites for these inhibitors. Fig. 14 shows a potential inhibition process to provide a more detailed explanation of the corrosion inhibition mechanism of C.S in 1.0 M HCl solution.
Previous studies have established that C.S develops a positive surface charge when exposed to an acidic HCl solution. The Cl− ions present in the solution promote the formation of a negatively charged surface on the initially positively charged C.S, facilitating the adsorption of cations from the solution. Due to the presence of an unshared pair of electrons on the nitrogen atoms, benzimidazole molecules can be protonated in an acidic solution. Following an electrostatic interaction, or physisorption, the protonated molecules (LF1H+ and LF2H+) can be adsorbed onto the surface of the C.S, as shown in Fig. 14.
However, the presence of two alkyl chains (C12H25) in LF2 provides a larger surface area for adsorption onto the steel surface, this increased surface area facilitates stronger interactions between the LF2 molecules and the steel surface, leading to improved inhibition efficiency. Also, the longer alkyl chains in LF2 often contribute to increased hydrophobicity of the LF2 molecules, which enhanced hydrophobic interactions between the LF2 molecules, and the steel surface can improve the stability of the protective film formed and reduce the penetration of Cl−.82
The increased negative charge on the C.S surface is transferred from the vacant π* orbital of the inhibitor molecules to the d-orbital of Fe, leading to stronger inhibitor adsorption on the steel surface. Additionally, there is a possibility that the d-orbitals on the C.S surface can donate electrons back to the aromatic cycles. Consequently, the steel surface eventually forms an adsorbed layer of the inhibitor, which acts as a protective barrier between the metal and the corrosive medium, preventing metal corrosion.83
4. Conclusion
The effectiveness of two new benzimidazole compounds as corrosion inhibitors for C.S in a 1.0 M HCl environment was evaluated through computational and experimental studies. The following are the main conclusions:• The results showed that in a 1.0 M HCl solution, LF1 and LF2 efficiently serve as corrosion inhibitors for carbon steel substrates. With increasing inhibitor concentration, both chemicals' inhibition efficiency rise in the following order: LF1 < LF2.
• The results obtained from potentiodynamic polarization data suggest that the investigated compounds are mixed type inhibitors.
• The electrochemical impedance spectroscopy plots revealed the adsorption of both imidazole molecules, which is confirmed by the rise of the polarization resistance and the reduction of the values of the double layer capacitance.
• SEM/EDX/AFM/contact angle analysis confirms the creation of a protective film on the C.S-surface.
• The research in the UV-visible clearly showed the creation of complexes.
• The acquired result through DFT/MDs simulation strongly corroborated the findings from the laboratory experiments.
Data availability
The data supporting this article have been included within the manuscript and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors express their gratitude for Princess Nourah bint Abdulrahman University Researchers for their support project number (PNURSP2022R94). Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.References
- A. Mohagheghi and R. Arefinia, Corrosion inhibition of carbon steel by dipotassium hydrogen phosphate in alkaline solutions with low chloride contamination, Constr. Build. Mater., 2018, 187, 760–772 CrossRef CAS.
- A. A. Nkuna, E. D. Akpan, I. B. Obot, C. Verma, E. E. Ebenso and L. C. Murulana, Impact of selected ionic liquids on corrosion protection of mild steel in acidic medium: Experimental and computational studies, J. Mol. Liq., 2020, 314, 113609 CrossRef CAS.
- N. Vaidya, A. K. Bhatia and S. Dewangan, Organic corrosion inhibitors, in Computational Modelling and Simulations for Designing of Corrosion Inhibitors, Elsevier, 2023, pp. 33–53 Search PubMed.
- M. G. A. Saleh, M. Alfakeer, R. N. Felaly, M. S. Al-Sharif, S. S. Al-Juaid, K. A. Soliman, M. A. Hegazy, S. Nooh, M. Abdallah and S. A. El Wanees, Retardation of the C-Steel Destruction in Hydrochloric Acid Media Utilizing an Effective Schiff Base Inhibitor. Experimental and Theoretical Computations, ACS Omega, 2024, 9(27), 29666–29681 CrossRef CAS PubMed.
- A. El Defrawy, M. Abdallah and J. Al-Fahemi, Electrochemical and Theoretical Investigation for Some Pyrazolone Derivatives as Inhibitors for the Corrosion of C-Steel in 0.5 M Hydrochloric Acid, J. Mol. Liq., 2019, 288, 110994 CrossRef CAS.
- M. Abdallah, K. A. Soliman, M. Alshareef, A. S. Al-Gorair, H. Hawsawi, H. M. Altass, S. S. Al-Juaid and M. S. Motawea, Investigation of the anticorrosion and adsorption properties of two polymer compounds on the corrosion of SABIC iron in 1 M HCl solution by practical and computational approaches, RSC Adv., 2022, 12, 20122–20137 RSC.
- H. Zarrok, R. Saddik, H. Oudda, B. Hammouti, A. El Midaoui, A. Zarrouk, N. Benchat and M. Ebn Touhami, 5-(2-Chlorobenzyl)-2,6-Dimethylpyridazin-3-One: An efficient Inhibitor of C38 Steel Corrosion in Hydrochloric Acid, Der Pharma Chem., 2011, 3(5), 272–282 CAS.
- W. Li, Q. He, C. Pei and B. Hou, Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media, Electrochim. Acta, 2007, 52(22), 6386–6394 CrossRef CAS.
- M. Marinescu, Recent advances in the use of benzimidazoles as corrosion inhibitors, BMC Chem., 2019, 13, 1–21 CrossRef PubMed.
- Z. Cao, Y. Tang, H. Cang, J. Xu, G. Lu and W. Jing, Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies, Corros. Sci., 2014, 83, 292–298 CrossRef CAS.
- Y. Tang, F. Zhang, S. Hu, Z. Cao, Z. Wu and W. Jing, Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: Gravimetric, electrochemical, SEM and XPS studies, Corros. Sci., 2013, 74, 271–282 CrossRef CAS.
- Y. Abboud, A. Abourriche, T. Saffaj, M. Berrada, M. Charrouf, A. Bennamara, A. Cherqaoui and D. Takky, The inhibition of mild steel corrosion in acidic medium by 2,2′-bis(benzimidazole), Appl. Surf. Sci., 2006, 252, 8178–8184 CrossRef CAS.
- A. Ghanbari, M. M. Attar and M. Mahdavian, Corrosion inhibition performance of three imidazole derivatives on mild steel in 1 M phosphoric acid, Mater. Chem. Phys., 2010, 124, 1205–1209 CrossRef CAS.
- S. Zafari, A. A. Sarabi and B. Movassagh, Experimental and theoretical evaluation of two benzimidazole derivatives for steel corrosion protection in HCl, Asia-Pac. J. Chem. Eng., 2019, 14(5), e2349 CrossRef CAS.
- A. Salcı, H. Yüksel and R. Solmaz, Experimental studies on the corrosion inhibition performance of 2-(2-aminophenyl) benzimidazole for mild steel protection in HCl solution, J. Taiwan Inst. Chem. Eng., 2022, 134, 104349 CrossRef.
- M. Ouakki, M. Galai and M. Cherkaoui, Imidazole derivatives as efficient and potential class of corrosion inhibitors for metals and alloys in aqueous electrolytes: A review, J. Mol. Liq., 2022, 345, 117815 CrossRef CAS.
- M. Abdallah, I. Zaafarany, K. S. Khairou and M. Sobhi, Inhibition of carbon steel corrosion by iron(III) and imidazole in sulfuric acid, Int. J. Electrochem. Sci., 2012, 7(2), 1564–1579 CrossRef CAS.
- K. F. Ansari and C. Lal, Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives, Eur. J. Med. Chem., 2009, 44(10), 4028–4033 CrossRef CAS PubMed.
- A. H. El-masry, H. H. Fahmy and S. H. Ali Abdelwahed, Synthesis and antimicrobial activity of some new benzimidazole derivatives, Molecules, 2000, 5(12), 1429–1438 CrossRef CAS.
- G. Navarrete-Vázquez, R. Cedillo, A. Hernández-Campos, L. Yépez, F. Hernández-Luis, J. Valdez and R. Castillo, Synthesis and antiparasitic activity of 2-(trifluoromethyl) benzimidazole derivatives, Bioorg. Med. Chem. Lett., 2001, 11(2), 187–190 CrossRef PubMed.
- G. Yadav, S. Ganguly, S. Murugesan and A. Dev, Synthesis, anti-HIV, antimicrobial evaluation and structure activity relationship studies of some novel benzimidazole derivatives, Anti-Infect. Agents, 2015, 13(1), 65–77 CrossRef CAS.
- S. Ersan, A. Titi, N. Noyanalpan and E. Yeşilada, Studies on analgesic and anti-inflammatory activities of 1-dialkylaminomethyl-2-(p-substituted phenyl)-5-substituted benzimidazole derivatives, Arzneim.-Forsch., 1997, 47(7), 834–836 CAS.
- N. Timoudan, A. Titi, M. El Faydy, F. Benhiba, R. Touzani, I. Warad, A. Bellaouchou, A. Alsulmi, B. Dikici, F. Bentiss and A. Zarrouk, Investigation of the mechanisms and adsorption of a new pyrazole derivative against corrosion of carbon steel in hydrochloric acid solution: Experimental methods and theoretical calculations, Colloids Surf., A, 2024, 682, 132771 CrossRef CAS.
- C. Verma, I. B. Obot, I. Bahadur, E. S. M. Sherif and E. E. Ebenso, Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: Gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies, Appl. Surf. Sci., 2018, 457, 134–149 CrossRef CAS.
- L. Toukal, S. Keraghel, F. Benghanem and A. Ourari, Electrochemical, thermodynamic and quantum chemical studies of synthesized benzimidazole derivative as an eco-friendly corrosion inhibitor for XC52 steel in hydrochloric acid, Int. J. Electrochem. Sci., 2018, 13(1), 951–974 CrossRef CAS.
- O. Dagdag, R. Hsissou, A. El Harfi, L. El Gana, Z. Safi, L. Guo, C. Verma, E. E. Ebenso and M. El Gouri, Development and anti-corrosion performance of polymeric epoxy resin and their zinc phosphate composite on 15CDV6 steel in 3wt% NaCl: experimental and computational studies, Journal of Bio- and Tribo-Corrosion, 2020, 6, 1–9 CrossRef.
- K. Chkirate, K. Azgaou, H. Elmsellem, B. El Ibrahimi, N. K. Sebbar, M. Benmessaoud and E. M. Essassi, Corrosion inhibition potential of 2-[(5-methylpyrazol-3-yl) methyl] benzimidazole against carbon steel corrosion in 1 M HCl solution: Combining experimental and theoretical studies, J. Mol. Liq., 2021, 321, 114750 CrossRef CAS.
- Y. Boughoues, M. Benamira, L. Messaadia and N. Ribouh, Adsorption and corrosion inhibition performance of some environmental friendly organic inhibitors for mild steel in HCl solution via experimental and theoretical study, Colloids Surf., A, 2020, 593, 124610 CrossRef CAS.
- M. Damej, R. Hsissou, A. Berisha, K. Azgaou, M. Sadiku, M. Benmessaoud and N. Labjar, New epoxy resin as a corrosion inhibitor for the protection of carbon steel C38 in 1M HCl. experimental and theoretical studies (DFT, MC, and MD), J. Mol. Struct., 2022, 1254, 132425 CrossRef CAS.
- L. Ahmed, N. Bulut, O. Kaygılı and R. Omer, Quantum chemical study of some basic organic compounds as the corrosion inhibitors, Journal of Physical Chemistry and Functional Materials, 2023, 6(1), 34–42 CrossRef.
- M. Sikine, H. Elmsellem, Y. K. Rodi, Y. Kadmi, M. Belghiti, H. Steli and B. Hammouti, Experimental, Monte Carlo simulation and quantum chemical analysis of 1, 5-di (prop-2-ynyl)-benzodiazepine-2, 4-dione as new corrosion inhibitor for mild steel in 1 M hydrochloric acid solution, J. Mater. Environ. Sci., 2017, 8, 116–133 CAS.
- A. Rahimi, M. Abdouss, A. Farhadian, L. Guo and J. Neshati, Development of a novel thermally stable inhibitor based on furfuryl alcohol for mild steel corrosion in a 15% HCl medium for acidizing application, Ind. Eng. Chem. Res., 2021, 60(30), 11030–11044 CrossRef CAS.
- G. Laadam, M. El Faydy, F. Benhiba, A. Titi, H. Amegroud, A. S. Al-Gorair, H. Hawsawi, R. Touzani, I. Warad, A. Bellaouchou, A. Guenbour, M. Abdallah and A. Zarrouk, Outstanding anti-corrosion performance of two pyrazole derivatives On carbon steel in acidic medium: Experimental and quantum-chemical examinations, J. Mol. Liq., 2023, 375, 121268 CrossRef CAS.
- M. Farsak, H. Keleş and M. Keleş, A new corrosion inhibitor for protection of low carbon steel in HCl solution, Corros. Sci., 2015, 98, 223–232 CrossRef CAS.
- H. Keleş and M. Keleş, Electrochemical investigation of a schiff base synthesized by cinnamaldehyde as corrosion inhibitor on mild steel in acidic medium, Res. Chem. Intermed., 2014, 40, 193–209 CrossRef.
- Z. Rouifi, M. Rbaa, F. Benhiba, T. Laabaissi, H. Oudda, B. Lakhrissi, A. Guenbour, I. Warad and A. Zarrouk, Preparation and anti-corrosion activity of novel 8-hydroxyquinoline derivative for carbon steel corrosion in HCl molar: Computational and experimental analyses, J. Mol. Liq., 2020, 307, 112923 CrossRef CAS.
- I. Ahamad, R. Prasad and M. A. Quraishi, Experimental and theoretical investigations of adsorption of fexofenadine at mild steel/hydrochloric acid interface as corrosion inhibitor, J. Solid State Electrochem., 2010, 14, 2095–2105 CrossRef CAS.
- X. Wang, H. Yang and F. Wang, An investigation of benzimidazole derivative as corrosion inhibitor for mild steel in different concentration HCl solutions, Corros. Sci., 2011, 53(1), 113–121 CrossRef CAS.
- D. Ben Hmamou, R. Salghi, A. Zarrouk, H. Zarrok, S. S. Al-Deyab, O. Benali and B. Hammouti, The Inhibited effect of Phenolphthalein towards the corrosion of C38 Steel in Hydrochloric Acid, Int. J. Electrochem. Sci., 2012, 7, 8988–9003 CrossRef CAS.
- A. Dutta, S. K. Saha, U. Adhikari, P. Banerjee and D. Sukul, Effect of substitution on corrosion inhibition properties of 2-(substituted phenyl) benzimidazole derivatives on mild steel in 1 M HCl solution: a combined experimental and theoretical approach, Corros. Sci., 2017, 123, 256–266 CrossRef CAS.
- X. Zheng, S. Zhang, M. Gong and W. Li, Experimental and theoretical study on the corrosion inhibition of mild steel by 1-octyl-3-methylimidazolium L-prolinate in sulfuric acid solution, Ind. Eng. Chem. Res., 2014, 53(42), 16349–16358 CrossRef CAS.
- S. Hari Kumar and S. Karthikeyan, Torsemide and furosemide as green inhibitors for the corrosion of mild steel in hydrochloric acid medium, Ind. Eng. Chem. Res., 2013, 52(22), 7457–7469 CrossRef CAS.
- E. Ech-chihbi, M. Adardour, W. Ettahiri, R. Salim, M. Ouakki, M. Galai and M. Taleb, Surface interactions and improved corrosion resistance of mild steel by addition of new triazolyl-benzimidazolone derivatives in acidic environment, J. Mol. Liq., 2023, 387, 122652 CrossRef CAS.
- A. Zarrouk, B. Hammouti, R. Touzani, S. S. Al-Deyab, M. Zertoubi, A. Dafali and S. Elkadiri, Comparative Study of New Quinoxaline Derivatives Towards Corrosion of Copper in Nitric Acid, Int. J. Electrochem. Sci., 2011, 6, 4939–4952 CrossRef CAS.
- S. Kertit, F. Chaouket, A. Srhiri and M. Keddam, Corrosion inhibition of amorphous FeBSiC alloy in 1 m HCl by 3-amino-1, 2, 4-triazole, J. Appl. Electrochem., 1994, 24(11), 1139–1145 CrossRef CAS.
- M. Bouanis, M. Tourabi, A. Nyassi, A. Zarrouk, C. Jama and F. Bentiss, Corrosion inhibition performance of 2, 5-bis (4-dimethylaminophenyl)-1, 3, 4-oxadiazole for carbon steel in HCl solution: Gravimetric, electrochemical and XPS studies, Appl. Surf. Sci., 2016, 389, 952–966 CrossRef CAS.
- R. Baskar, D. Kesavan, M. Gopiraman and K. Subramanian, Corrosion inhibition of mild steel in 1.0 M hydrochloric acid medium by new photo-cross-linkable polymers, Prog. Org. Coat., 2014, 77(4), 836–844 CrossRef CAS.
- M. El Faydy, F. Benhiba, B. Lakhrissi, M. Ebn Touhami, I. Warad, F. Bentiss and A. Zarrouk, The inhibitive impact of both kinds of 5-isothiocyanatomethyl-8- hydroxyquinoline derivatives on the corrosion of carbon steel in acidic electrolyte, J. Mol. Liq., 2019, 295, 111629 CrossRef CAS.
- B. Mernari, H. El Attari, M. Traisnel, F. Bentiss and M. Lagrenee, Inhibiting effects of 3, 5-bis (n-pyridyl)-4-amino-1, 2, 4-triazoles on the corrosion for mild steel in 1 M HCl medium, Corros. Sci., 1998, 40(2–3), 391–399 CrossRef CAS.
- G. A. Zhang, X. M. Hou, B. S. Hou and H. F. Liu, Benzimidazole derivatives as novel inhibitors for the corrosion of mild steel in acidic solution: Experimental and theoretical studies, J. Mol. Liq., 2019, 278, 413–427 CrossRef CAS.
- Y. Tang, F. Zhang, S. Hu, Z. Cao, Z. Wu and W. Jing, Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: Gravimetric, electrochemical, SEM and XPS studies, Corros. Sci., 2013, 74, 271–282 CrossRef CAS.
- J. Aljourani, K. Raeissi and M. A. Golozar, Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution, Corros. Sci., 2009, 51(8), 1836–1843 CrossRef CAS.
- A. Zarrouk, H. Zarrok, R. Salghi, R. Touir, B. Hammouti, N. Benchat, L. L. Afrine, H. Hannache, M. El Hezzat and M. Bouachrine, Electrochemical impedance spectroscopy weight loss and quantum chemical study of new pyridazine derivative as inhibitor corrosion of copper in nitric acid, J. Chem. Pharm. Res., 2013, 5(12), 1482–1491 Search PubMed.
- E. Machnikova, K. H. Whitmire and N. Hackerman, Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives, Electrochim. Acta, 2008, 53(20), 6024–6032 CrossRef CAS.
- A. Ghazoui, R. Saddik, N. Benchat, B. Hammouti, M. Guenbour, A. Zarrouk and M. Ramdani, The Role of 3-Amino-2-Phenylimidazo[1,2-a]Pyridine as Corrosion Inhibitor for C38 Steel in 1M HCl, Der Pharma Chem., 2012, 4(1), 352–364 Search PubMed.
- S. El Arrouji, K. Karrouchi, A. Berisha, K. Ismaily Alaoui, I. Warad, Z. Rais, S. Radi, M. Taleb, M. Ansar and A. Zarrouk, New pyrazole derivatives as effective corrosion inhibitors on steelelectrolyte interface in 1 M HCl: Electrochemical, surface morphological (SEM) and computational analysis, Colloids Surf., A, 2020, 604, 125325 CrossRef CAS.
- R. N. Felaly, A. S. Al-Gorair, N. Fouad, S. S. Al-Juaid, D. F. Norhan Saadan, A. Y. El-Etre, E. M. Mabrouk and M. Abdallah, Enhanced corrosion protection of copper alloy in 2.0 M HNO3solution using expired solo sept, slim-lax and well derm drugs, Int. J. Electrochem. Sci., 2024, 19, 100657 CrossRef CAS.
- M. Galai, K. Dahmani, O. Kharbouch, M. Rbaa, N. Alzeqri, L. Guo and I. Warad, Surface analysis and interface properties of a newly synthesized quinoline-derivative corrosion inhibitor for mild steel in acid pickling bath: Mechanistic exploration through electrochemical, XPS, AFM, contact angle, SEM/EDS, and computational studies, J. Phys. Chem. Solids, 2024, 184, 111681 CrossRef CAS.
- W. Li, Z. Zhang, Y. Zhai, L. Ruan, W. Zhang and L. Wu, Electrochemical and computational studies of proline and captopril as corrosion inhibitors on carbon steel in a phase change material solution, Int. J. Electrochem. Sci., 2020, 15(1), 722–739 CrossRef CAS.
- M. Abdallah, T. Al-Habal, M. Alfakeer, R. El-Sayed and R. S. Abdel Hameed, Insight into the corrosion inhibition and adsorption behavior of synthetic pyrazole surfactants as an efficient inhibitor for carbon steel in 1mol/L HCl solutions, Int. J. Corros. Scale Inhib., 2023, 12(4), 2024–2045 Search PubMed.
- N. El-Aouni, R. Hsissou, Z. Safi, S. About, F. Benhiba, J. El Azzaoui and M. Rafik, Performance of two new epoxy resins as potential corrosion inhibitors for carbon steel in 1MHCl medium: Combining experimental and Computational approaches, Colloids Surf., A, 2021, 626, 127066 CrossRef CAS.
- O. Dagdag, R. Hsissou, A. El Harfi, L. El Gana, Z. Safi, L. Guo and M. El Gouri, Development and Anti-corrosion Performance of Polymeric Epoxy Resin and their Zinc Phosphate Composite on 15CDV6 Steel in 3wt% NaCl: experimental and Computational Studies, Journal of Bio- and Tribo-Corrosion, 2020, 6(4), 1–9 CrossRef.
- B. Cherinka, B. H. Andrews, J. Sánchez-Gallego, J. Brownstein, M. Argudo-Fernández, M. Blanton and R. Yan, Marvin: A tool kit for streamlined access and visualization of the SDSS-IV MaNGA data set, Astron. J., 2019, 158(2), 74 CrossRef.
- I. Lukovits, E. Kalman and F. Zucchi, Corrosion inhibitors—correlation between electronic structure and efficiency, Corrosion, 2001, 57(1), 3–8 CrossRef CAS.
- K. Jrajri,K, M. El Faydy, M. Alfakeer, S. S. Al-Juaid, Z. Safi, I. Warad, F. Benhiba, D. R. Bazanov, N. A. Lozinskaya, M. Abdallah and A. Zarrouk, Correlation between molecular structures and performance of corrosion inhibition of carbon steel by some imidazole analogs in HCl 1M: Integrating practical and theoretical aspects, Colloids Surf., A, 2024, 700, 1346 CrossRef.
- K. Adel, S. E. Hachani, I. Selatnia, N. Nebbache and S. Makhloufi, Correlating the inhibitory action of novel benzimidazole derivatives on mild steel corrosion with DFT-based reactivity descriptors and MD simulations, J. Indian Chem. Soc., 2022, 99(7), 100497 CrossRef CAS.
- R. Hsissou, F. Benhiba, M. El Aboubi, S. About, Z. Benzekri, Z. Safi and M. Rafik, Synthesis and performance of two ecofriendly epoxy resins as a highly efficient corrosion inhibition for carbon steel in 1M HCl solution: DFT, RDF, FFV and MD approaches, Chem. Phys. Lett., 2022, 139995 CrossRef CAS.
- D. Lide, Handbook of Chemistry and Physics, CRC Press, 88th edn, 2007, ch. 22, vol. 24, p. 154 Search PubMed.
- W. Yang and W. J. Mortier, The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines, J. Am. Chem. Soc., 1986, 108(19), 5708–5711 CrossRef CAS PubMed.
- K. Fukui, Role of frontier orbitals in chemical reactions, Science, 1982, 218, 747–754 CrossRef CAS PubMed.
- T. Lu and F. Chen, Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33(5), 580–592 CrossRef CAS PubMed.
- F. De Proft, J. M. Martin and P. Geerlings, Calculation of molecular electrostatic potentials and Fukui functions using density functional methods, Chem. Phys. Lett., 1996, 256(4–5), 400–408 CrossRef CAS.
- P. Geerlings, P. W. Ayers, A. Toro-Labbé, P. K. Chattaraj and F. Proft, The Woodward–Hoffmann rules reinterpreted by conceptual density functional theory, Acc. Chem. Res., 2012, 45(5), 683–695 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Local softness and chemical reactivity in the molecules CO, SCN− and H2CO, J. Mol. Struct.: THEOCHEM, 1988, 163, 305–313 CrossRef.
- M. M. Abdelsalam, M. A. Bedair, A. M. Hassan, B. H. Heakal, A. Younis, Z. I. Elbialy and S. A. Fareed, Green synthesis, electrochemical, and DFT studies on the corrosion inhibition of steel by some novel triazole Schiff base derivatives in hydrochloric acid solution, Arabian J. Chem., 2022, 15(1), 103491 CrossRef CAS.
- H. M. Abd El-Lateef, K. Shalabi, A. R. Sayed, S. M. Gomha and E. M. Bakir, The novel polythiadiazole polymer and its composite with α-Al (OH)3 as inhibitors for steel alloy corrosion in molar H2SO4: Experimental and computational evaluations, J. Ind. Eng. Chem., 2022, 105, 238–250 CrossRef CAS.
- M. Goyal, H. Vashisht, A. Kumar, S. Kumar, I. Bahadur, F. Benhiba and A. Zarrouk, Isopentyltriphenylphosphonium bromideionic liquid as a newly effective corrosion inhibitor on metal-electrolyte interface in acidic medium: Experimental, surface morphological (SEM-EDX & AFM) and computational analysis, J. Mol. Liq., 2020, 316, 113838 CrossRef CAS.
- Y. Gong, Z. Wang, F. Gao, S. Zhang and H. Li, Synthesis of new benzotriazole derivatives containing carbon chains as the corrosion inhibitors for copper in sodium chloride solution, Ind. Eng. Chem. Res., 2015, 54(49), 12242–12253 CrossRef CAS.
- Sheetal, A. K. Singh, M. Singh, S. Thakur, B. Pani, J. Singh, S. Zamindar and P. Banerjee, Understanding of remarkable corrosion combating action of N-(benzo[d]thiazol-2-yl)-1-(2-substituted phenyl) methanimines: Electrochemical, surface and computational approach, Inorg. Chem. Commun., 2024, 159, 111736 CrossRef CAS.
- J. Saranya, F. Benhiba, N. Anusuya, R. Subbiah, A. Zarrouk and S. Chitra, Experimental and computational approaches on the pyran derivatives for acid corrosion, Colloids Surf., A, 2020, 603, 125231 CrossRef CAS.
- A. Thoume, A. Elmakssoudi, D. Benmessaoud, N. Benzbiria, F. Benhiba, M. Dakir, M. Zahouily, A. Zarrouk, M. Azzi and M. Zertoubi, Amino acid structure analog as a corrosion inhibitor of carbon steel in 0.5 M H2SO4: Electrochemical, synergistic effect and theoretical studies, Chem. Data Collect., 2020, 30, 100586 CrossRef CAS.
- A. Zomorodian, R. Bagonyi and A. Al-Tabbaa, The efficiency of eco-friendly corrosion inhibitors in protecting steel reinforcement, J. Build. Eng., 2021, 38, 102171 CrossRef.
- L. Huang, Q. Zhao, H. J. Li, J. Y. Wang, X. Y. Wang and Y. C. Wu, Investigation of adsorption and corrosion inhibition property of Hyperoside as a novel corrosion inhibitor for Q235 steel in HaCl medium, J. Mol. Liq., 2022, 364, 120009 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05070c |
| This journal is © The Royal Society of Chemistry 2024 |