DOI:
10.1039/D4RA04808C
(Paper)
RSC Adv., 2024,
14, 38426-38458
Versatile photocatalytic activities of indenoquinoxalines for dye reduction, single-crystal nucleation, and MNP formation with iron scrap under sunlight†
Received
2nd July 2024
, Accepted 14th August 2024
First published on 4th December 2024
Abstract
In this work, 11H-indeno[1,2-b]quinoxalin-11-one (IQ), 7-nitro-11H-indeno[1,2-b]quinoxalin-11-one (NIQ), and 7-chloro-11H-indeno[1,2-b]quinoxalin-11-one (CIQ) as indenoquinoxalines (IQPs) and 7-nitro-2′-(4-nitrophenyl)-5′,6′,7′,7a′-tetrahydrospiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-1′,1′(2′H)-dicarbonitrile (SIQPNO2) spiroheterocyclics were synthesized. These molecules photocatalytically reduced methylene blue (MB), methyl orange (MO), brilliant blue R (BBR), and Rhodamine B (RhB) in aqueous acetonitrile (aq-ACN) under sunlight (SL) for the first time. The IQPs and SIQPNO2 with a lanthanide graphene oxide template (LGT) of lanthanide sulfide nanorods (Ln2S3, Ce2S3, Tb2S3, and Ho2S3) photocatalytically reduced the dyes. IQ alone reduced MB in ∼2 min, while with LaGT, CeGT, TbGT, and HoGT in 7, 10, 11, and 13 min, respectively. NIQ and CIQ alone photocatalytically reduced MB in 18 and 32 min, while with LaGT, CeGT, TbGT, and HoGT in 18, 31, 23, and 28 min and 33, 55, 45, and 51 min, respectively. IQ with CO2 photocatalytically reduced MB and QHIn in 90 s and 17 min unlike 2 and 24 min without CO2, respectively. SIQPNO2 alone reduced MB in 190 min, while with CeGT, TbGT, HoGT, and LaGT in 242, 225, 197, and 88 min, respectively. IQ with LaGT photocatalytically reduced MB in 7 min, while SIQPNO2 with LaGT in 88 min. IQ received maximum photon (hv) producing robust redox cycles (ROCs) compared to SIQPNO2. SIQPI, SIQPII, SIQPIII, and SIQPNO2 (SIQPs) individually reduced MB in 95, 43, 54, and 190 min, while SIQPs with NIQ in 63, 35, 47, and 64 min, respectively. IQ with Fe scrap in ACN developed a single crystal in 2 weeks, while in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 5
7, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 8
3, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 aq-ACN media, the magnetic nanoparticles (MNPs) developed at normal temperature and pressure (NTP).
2 aq-ACN media, the magnetic nanoparticles (MNPs) developed at normal temperature and pressure (NTP).
Introduction
Brugnatelli in 1818, Dobereiner in 1832, Runge in 1834, Friedlander in 1906, Treibs in 1936, Chargaff in 1951, and others have synthesised and developed heterocyclic compounds with medicinal applications.1,2 Consequently, diverse heterocyclic molecules have found applications in biological fields, cosmetics, drug designing, and dye synthesis. However, to date, no scientist has delved into their potential for interfacing with solar radiation to induce electronic transitions for photocatalytic reduction (PCR) studies. Their active surfaces facilitate their use as robust photocatalysts for reducing effluents but no study has been reported yet. In this regard, our previous studies with SIQPI, SIQPII, and SIQPIII3 demonstrated novel science with a chiral carbon atom (CCA) equipartitioning eigenenergies of each constituent for generating single-valued wavefunctions on receiving hν. SIQPI, SIQPII, and SIQPIII3 explored in our previous research, demonstrating an efficacy for reducing dyes and persistent pollutants, have inspired further investigation into heterocyclic NIQ as a base unit of SIQPs in studying its energy-storing capabilities for potential applications in the PCR. Various photocatalysts are being reported to photodegrade both industrial and biological effluents, often taking a longer time. Many researchers have failed to elucidate the role of eigenenergies in analysing the photocatalytic activity, leaving a crucial aspect unexplored. Except IQPs, no heterocyclics have been reported as photocatalysts for reducing a waste dye. It has created a basis for studying IQPs in PCR, apart from SIQPs, based on eigenenergies.3 IQPs have developed ROCs by overcoming a quantum energy barrier (QEB) on lowering the band gap to photocatalytically reduce the dyes. The IQPs compared to SIQPs have investigated the role of CCA for enhancing the functionality via Förster resonance energy transfer (FRET).3 In our previously reported study, SIQPs have successfully reduced quinonoid phenolphthalein (QHIn).3 The IQPs have reduced the dyes in 1/6th time shorter than SIQPNO2. The PCR process, UV-Vis transition, quantum yield (Φ), single crystal with Fe scrap, and MNPs4 with aq-ACN indicate robust photochemical activities of IQPs having both electron-withdrawing groups (EWGs) and electron-releasing groups (ERGs). NIQ, a base matrix of SIQPs, has demonstrated a reduced dye reduction time; this inspired the synthesis of SIQPNO2, with two terminal NO2 functional group (FG), by condensing NIQ with 2-(4-nitrobenzylidene) malononitrile using L-proline in ACN. Moreover, NIQ with SIQPs has acted as a cophotocatalyst to reduce dyes. The eigenenergies of NIQ and SIQPs were mutually elevated valence bands (VBs) to conduction bands (CBs) instead of synthesizing new photocatalysts. The ERG and EWG of IQPs have simulated the eigenenergies, for example, SIQPs with NIQ have reduced MB5 in 43–190 min unlike alone NIQ in 12 min respectively. IQPs and SIQPs generate the positive and negative (h+) and (e−) holes in the same phase to photocatalytically reduce dyes in a shorter time. Moreover, their physicochemical properties have assessed a role of solvent with IQPs, SIQPNO2, SIQPNO2 + NIQ, SIQPI + NIQ, SIQPII + NIQ, and SIQPIII + NIQ for dye reduction, which are not reported yet and explored in this paper.6–12 IQPs and SIQPNO2 (ref. 13–17) are also studied with LaGT (La3+ = 4f0), CeGT (Ce3+ = 4f1), TbGT (Tb3+ = 4f8), and HoGT (Ho3+ = 4f10) respectively to photocatalytically reduce dyes followed by adsorption. IQPs + LGT and SIQPNO2 + LGT, as lanthanide organometallic interphases, have effectively reduced dyes using unpaired 4f#e configurations and never been reported yet. The IQPs and SIQPNO2 both with LGT do not mutually synchronize under hν, interact differently with each constituent and dye by elongating the PCR time. The IQ has influenced Fe rusting in different aq-ACN ratios with a novel substantial study. The electronic configurations of IQPs and SIQPs with NIQ have lowered a QEB. The IQPs and SIQPs with NIQ have reduced the dyes by maintaining in a monodispersed state. As their higher surface energies interact strongly with both the dyes and medium, such photocatalytic activities could be used as an excellent photosensitive solvent, light sensor, photoluminescence, and to monodisperse and reduce persistent pollutants. The ACN has monodispersed SIQPs and IQPs with weaker van der Waals forces without structural split while insoluble in water and unable to photocatalytically reduce the dyes along its robust split. Pure ACN nucleated a single crystal without splitting, whereas 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq ACN induced splitting, forming MNPs along with valuable products on recycling.
7 aq ACN induced splitting, forming MNPs along with valuable products on recycling.
Experimental section
Materials
Thin-layer chromatography (TLC) plate, AR-grade hexane (Sigma-Aldrich ≥99%), ethyl acetate (Sigma-Aldrich ≥99%), dichloromethane (DCM) (Sigma-Aldrich ≥99.8%), dimethylformamide (DMF) (Sigma-Aldrich ≥99.8%), acetone (Sigma-Aldrich ≥99.9%), MeOH (Sigma-Aldrich ≥99.9%), and ACN (Sigma-Aldrich ≥99.8%) were used. Para-nitro benzaldehyde, ninhydrin, malononitrile, lithium bromide (LiBr), acetic acid (CH3COOH), methanol (CH3OH), L-proline, magnetic beads, and Whatman filter paper were used as received.
Characterization methods
Structures were analyzed by 1H and 13C NMR (500 MHz, Bruker Avance Spectrometer) in CDCl3 at 500 and 125 MHz using TMS internal standard, mass spectra using an Agilent Technology 6545 Q-TOF LC-MS spectrometer at 70 eV, FT-IR spectra from 200–800 cm−1 with KBr pellets using a PerkinElmer TL8000 TG-IR interface, UV-Vis spectra from 190–1100 nm using a UV-1800 SHIMADZU (UV Spectrophotometer) in ESI mode, X-ray diffraction (XRD, powder/single), thermal gravimetric analysis (TGA) using an intercooler PerkinElmer TGA-6000 thermometer for ∼25 to 500 °C, high-resolution transmission electron microscopy (HR-TEM) using a JEOL JEM-2100 electron microscope at 200 kV operating voltage, scanning electron microscopy (SEM), stereomicroscopy, atomic force microscopy (AFM, Multimode Scanning Probe Microscope, Bruker), fluorescent spectroscopy (RF-6000 Spectro Fluorophotometer, SHIMADZU CORP), Raman spectroscopy, and elemental analysis.
Synthesis of starting material: synthesis of IQ
Scheme S1:† benzene-1,2-diamine (11 mmol, 1.1 eq., 1.1 g) and ninhydrin (10 mmol, 1.0 eq., 1.8 g) were stirred in CH3COOH (10 mL) and CH3OH (30 mL) for 30 min at RT monitored by TLC (Table S1†). A light yellowish product was filtered, washed with CH3OH, and dried in vacuum, and the structures were investigated by 1H, 13C NMR, and mass analysis (Fig. S1†).
Synthesis of NIQ
Scheme S2:† 4-nitrobenzene-1,2-diamine (11 mmol, 1.1 eq., 1.7 g) and ninhydrin (10 mmol, 1.0 eq., 1.8 g) were stirred in CH3COOH (10 mL) and CH3OH (30 mL) for 30 min at RT and monitored by TLC (Table S2†).18 A yellow product was filtered, washed with CH3OH and dried in vacuum. The structures were investigated by 1H, 13C NMR, and mass analysis (Fig. S2†).18
Synthesis of CIQ
Scheme S3:† 4-chlorobenzene-1,2-diamine (11 mmol, 1.1 eq., 1.5 g) and ninhydrin (10 mmol, 1.0 eq., 1.8 g) were stirred in CH3COOH (10 mL) and CH3OH (30 mL) for 30 min at RT monitored by TLC (Table S3†). A pale yellow product was filtered, washed with CH3OH and chilled water, and dried in vacuum, and the structures were investigated by 1H, 13C NMR, and mass analysis (Fig. S3†).
Reaction scaling of SIQPNO2 synthesis
Scheme S4:† 2-(4-nitrobenzylidene)malononitrile from 4-nitrobenzaldehyde and malononitrile in DMF with LiBr were synthesized (Table S4†). LiBr catalyzed a reaction by rearranging a dispersion phase of 4-nitrobenzaldehyde with malononitrile in DMF (Fig. S4†).
Synthesis of SIQPNO2
For Scheme S5,† 2-(4-nitrobenzylidene)malononitrile (1.0 mmol, 1.0 eq., 154.5 g) (Table S5†), was separately added to NIQ (1.0 mmol, 1.0 eq., 277 g) and L-proline (1.0 mmol, 1.0 eq., 126.5 g) in ACN (15 mL). Reacting mixtures were stirred and refluxed for 3 h monitored by TLC and visualized with UV-Vis light and iodine. Excess solvent was removed using a rota evaporator. The crude product was washed with DCM and lime water (∼3 times) to purify by column chromatography (ACME, 60–120 mesh) with 70% petroleum ether + 30% ethyl acetate (EA) eluting mixture, dried under vacuum, and analysed by 1H, 13C NMR, and mass spectroscopy (Fig. S5a and b†). IQ, NIQ, CIQ, and SIQPNO2 obtained from Schemes S1–S5† were characterised by 1H, 13C NMR spectroscopy, FT-IR spectroscopy, and TGA (Fig. 1). Scheme S6† infers SIQPI + NIQ (Fig. 2, S6a and b†), Scheme S7† infers SIQPII + NIQ (Fig. 3, S7a and b†), and Scheme S8† infers SIQPIII + NIQ (Fig. 4, S8a and b†).
 |
| | Fig. 1 FT-IR spectra for stretching and bending of (a) IQ, (b) NIQ, (c) CIQ, and (d) SIQPNO2. | |
 |
| | Fig. 2 FT-IR spectra for stretching and bending (a) SIQPI alone (b) SIQPI + NIQ. | |
 |
| | Fig. 3 FT-IR spectra for stretching and bending (a) SIQPII alone and (b) SIQPII + NIQ. | |
 |
| | Fig. 4 FT-IR spectra for stretching and bending (a) SIQPIII alone and (b) SIQPIII + NIQ. | |
Synthesis of single crystals (model: D8 QUEST, Bruker)
First, 0.001 (M) IQ with Fe scrap of 1.199 g in 5 mL of ACN in vial developed a single crystal of 95.09 mg in two weeks. The single crystal was characterized by XRD and few parameters with single-crystal XRD to obtain a contribution of Fe scrap on IQ nucleation. Robust crystals are developed by IQ with Fe than IQ alone in ACN (Fig. 5).
 |
| | Fig. 5 (i) IQ crystals alone (ii) IQ + Fe crystals in ACN at different magnitude. | |
Results and discussion
1H, 13C NMR, and FT-IR
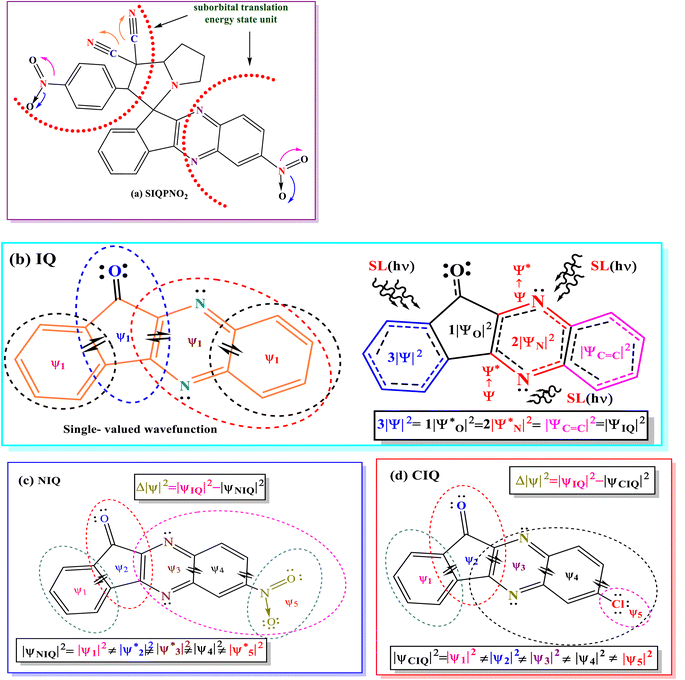
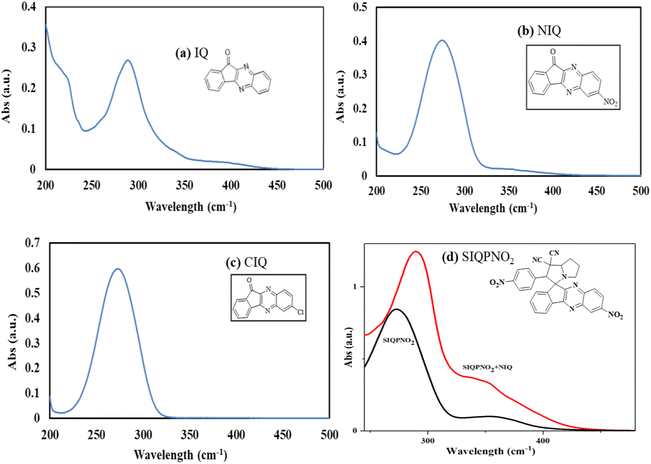
Two separate doublets in 1H NMR spectra of SIQPNO2 confirm a pyrrolizidine (pyz) ring and multiplets at δ 4.95 ppm (d, 1H, J = 5.5 Hz) pyz NCH proton. The pyz proton attached to a phenyl ring is deshielded at δ = 4.81 (d, 1H, J = 5.8) and multiplets at δ 7.49–10.16 ppm for aromatic protons (Fig. S5a†) and also confirmed by 13C NMR (Fig. S5b†). NIQ having EWG NO2 has deshielded the H atoms by attracting the density of energy state (DOS) of double bonds for robust ROCs reducing dyes in a shorter time than CIQ. IQPs with NO2 (EWG) and Cl− (ERG) have equipartitioned the DOS of π bonds from the VB to the CB to photocatalytically reduce the dyes in a shorter time. No researcher has taken a note of DOS for π bonds, which enhance a Fermi sphere to expedite photocatalyzing holes supported by the deshielding effect, noted in 1H NMR. The CCA avoids DOS intermixing of EWG and ERG by minimizing the undesired collisions influencing PCR. However, deshielding and shielding of π bond with 1H NMR for receiving hv have never been correlated with PCR. The deshielding of π bonds shortened a PCR time with photocatalysts as a novel breakthrough, it could be applied in various fields (Fig. 6).
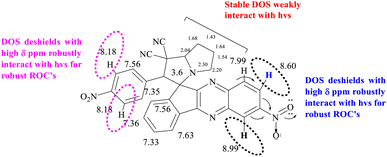 |
| | Fig. 6 NO2 deshielding effect on neighbouring H atom. | |
Moreover, the quantum energy equilibrating the DOS with nearly residual surface energy to respond under hv has never surfaced in PCR. The IQPs and SIQPs acted as rigid harmonic oscillators to synchronize the residual surface energies. Moreover, it elucidates the nature of photocatalysts acting as 1D potential energy (PE) systems with few quantum energies dented as synchronized residual energy to a single phase. The quantum resonating energies respond to SL generating ROCs as ψ1, ψ2, ψ3, ψ4, and ψ5 of IQPs and SIQPNO2 via FRET. IR stretching and bending frequencies at ∼1600–1550 and 2260 cm−1 infer –NO2 and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N in NIQ and SIQPNO2. Moreover, at 1745–1715 cm−1 infers C
N in NIQ and SIQPNO2. Moreover, at 1745–1715 cm−1 infers C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–Cl while at 850–550 cm−1 the ketonic C
O and C–Cl while at 850–550 cm−1 the ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Cl in IQPs. The strong band at 800–850 cm−1 appeared for a 1,4-disubstituted aromatic ring for SIQPNO2.
O and Cl in IQPs. The strong band at 800–850 cm−1 appeared for a 1,4-disubstituted aromatic ring for SIQPNO2.
FT-IR spectra of IQPs and SIQPs with NIQ to photocatalytically reduce dyes
Electronically, IQ stretches as the 1st order, unlike NIQ with NO2 and CIQ with Cl that slightly weaken the stretching with high intensities. The holes of IQ in a single phase, unlike NIQ and CIQ at higher intensities with EWG and ERG, shorten the PCR time. IQ broadened a peak of C–C bond from 2200 to 3500 cm−1 on aligning the charges unlike with the NO2 and Cl stretching differently. The CIQ with ERG resonates that the π conjugation increased the stretching in the same phase with sharper peaks in the 400–1200 cm−1 fingerprint region. NIQ with EWG resonates the π ring (ψ4) and NO2 (ψ5) stretching the dipoles with less intense peaks in the fingerprint region. The NIQ gains an integrated π conjugation intensifying IR transmittance. The DOS19 of NIQ linearly aligns stretching vis-à-vis conjugation. The C–C bonds are equipartitioned, leading to 6.7% min−1 PCR rate for MB. The CIQ with sharpened stretching unlike IQ with a least C–C stretching photocatalytically reduced MB in 2 min, whereas NIQ has weakened DOS due to electron–electron repulsion (EER) (Fig. 7). The π electron cloud of NIQ stretches in a fingerprint region and used with SIQPs as a cophotocatalyst via mutually monodispersion for receiving the hv. IR of SIQPI + NIQ having FP aligned with NIQ via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds has sharpened a stretching to photocatalytically reduce MB in 63 unlike 95 min with SIQPI alone (Fig. 7). The SIQPII + NIQ has also weakened stretching at 2200–400 cm−1 for CN intensifying the fingerprint region. SIQPII + NIQ with 3500–2000 cm−1 as IR active has reduced MB in 35, unlike 43 min with SIQPII alone. SIQPIII + NIQ from 1500 to 4000 cm−1 strengthened the unspent resonating sites generating a maximum stretching as IR active with a shorter PCR time than SIQPIII alone. Manifold intensities inhibited by SIQPIII + NIQ photocatalytically reduced MB in 47 min, unlike 54 with SIQPIII alone.18 The SIQPs under SL have reduced dyes in our earlier study18 and are extended now with IQPs. The wavefunctions of IQPs, SIQPs, and SIQPs + NIQ with EWG and ERG using solvation energies as a communicating FRET accessed to the dyes. The 3
C bonds has sharpened a stretching to photocatalytically reduce MB in 63 unlike 95 min with SIQPI alone (Fig. 7). The SIQPII + NIQ has also weakened stretching at 2200–400 cm−1 for CN intensifying the fingerprint region. SIQPII + NIQ with 3500–2000 cm−1 as IR active has reduced MB in 35, unlike 43 min with SIQPII alone. SIQPIII + NIQ from 1500 to 4000 cm−1 strengthened the unspent resonating sites generating a maximum stretching as IR active with a shorter PCR time than SIQPIII alone. Manifold intensities inhibited by SIQPIII + NIQ photocatalytically reduced MB in 47 min, unlike 54 with SIQPIII alone.18 The SIQPs under SL have reduced dyes in our earlier study18 and are extended now with IQPs. The wavefunctions of IQPs, SIQPs, and SIQPs + NIQ with EWG and ERG using solvation energies as a communicating FRET accessed to the dyes. The 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
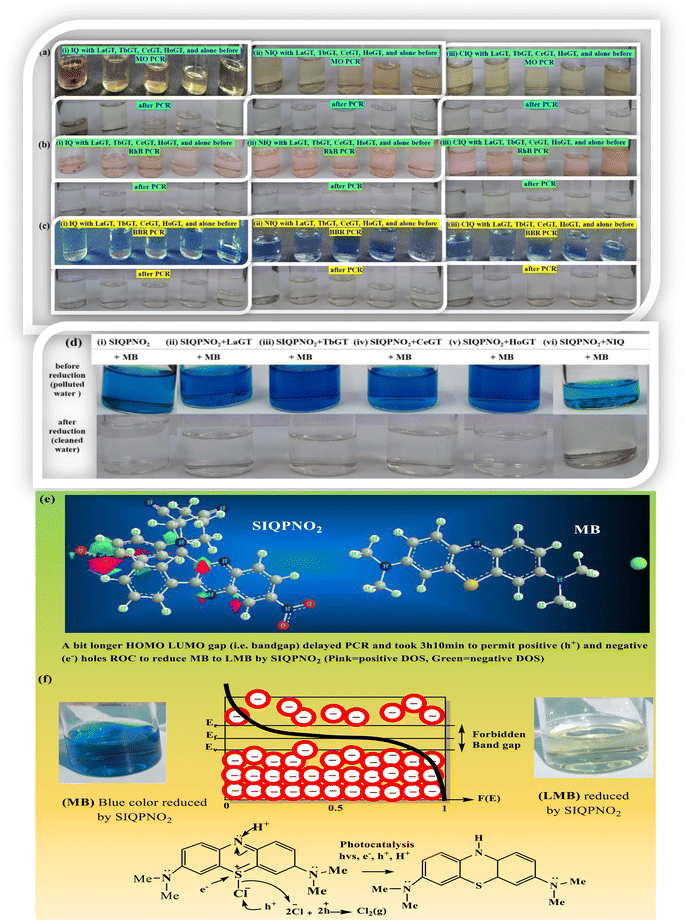
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq-ACN with π → 2π* and n → n* have quantized a photocatalytic chemistry to lay down a 2nd-generation photocatalysis. The IQPs and SIQPs with NIQ have reduced dyes in a shorter time due to single-valued wavefunctions (Fig. 7, Chart 1(a and b)). SIQPNO2 alone with enormous π → π* and n → n* has reduced MB in 2.16 times shorter period than with LGT. The 4f#e electrons of lanthanides of LGT seem to interrupt the hole generation20 (Fig. 8). The hv excites enormous π and lone pair of electrons (LPE) of SIQPNO2 that photocatalytically reduce an MB philicphobic medium in 190 min, and such studies have not been reported previously. NIQ has reduced the dyes in a shorter time with SIQPI, II, III, and SIQPNO2 than SIQPI, II, and III alone18 due to the same phase ROCs.21 IQ with π conjugation and LPE of O and N atoms have produced holes in the same phase to photocatalytically reduce in 2 min. NIQ with NO2 and CIQ with Cl have enhanced PCR time unlike IQ. IQPs with LGT have reduced dyes in longer time while the SIQPs with NIQ and LGT in shorter and longer PCR times respectively. The LaGT, CeGT, TbGT, and HoGT compared to Ln2S3, Ce2S3, Tb2S3, and Ho2S3 LSNR respectively have shortened a dye PCR time. The π → π* and 4fne → 4fne*of LGT could not compete the π → π* of IQPs, as alone LGT has reduced MB in 64 min than 2 with alone IQ, LGT with IQPs in 10–55 min (ψπ→π − ψ4fne→4fne*). IQPs + LGT with π → π* and 4fne → 4fne* have shortened the MB PCR time than our reported studies. LGT with infinite π conjugation aligns at a periphery of IQPs intensifying π conjugation via FRET for MB PCR. The π → π* and 4fne → 4fne*of LGT align with π → π* and n → n* of IQPs reducing dyes in a shorter time. The IQPs with LGT have generated IQPs + LGT. IQPs alone after MB reduction remained monodispersed resulting in a transparent nanoemulsion as thin films suitable to be used in paint industries and antireflecting coating agents.22
7 aq-ACN with π → 2π* and n → n* have quantized a photocatalytic chemistry to lay down a 2nd-generation photocatalysis. The IQPs and SIQPs with NIQ have reduced dyes in a shorter time due to single-valued wavefunctions (Fig. 7, Chart 1(a and b)). SIQPNO2 alone with enormous π → π* and n → n* has reduced MB in 2.16 times shorter period than with LGT. The 4f#e electrons of lanthanides of LGT seem to interrupt the hole generation20 (Fig. 8). The hv excites enormous π and lone pair of electrons (LPE) of SIQPNO2 that photocatalytically reduce an MB philicphobic medium in 190 min, and such studies have not been reported previously. NIQ has reduced the dyes in a shorter time with SIQPI, II, III, and SIQPNO2 than SIQPI, II, and III alone18 due to the same phase ROCs.21 IQ with π conjugation and LPE of O and N atoms have produced holes in the same phase to photocatalytically reduce in 2 min. NIQ with NO2 and CIQ with Cl have enhanced PCR time unlike IQ. IQPs with LGT have reduced dyes in longer time while the SIQPs with NIQ and LGT in shorter and longer PCR times respectively. The LaGT, CeGT, TbGT, and HoGT compared to Ln2S3, Ce2S3, Tb2S3, and Ho2S3 LSNR respectively have shortened a dye PCR time. The π → π* and 4fne → 4fne*of LGT could not compete the π → π* of IQPs, as alone LGT has reduced MB in 64 min than 2 with alone IQ, LGT with IQPs in 10–55 min (ψπ→π − ψ4fne→4fne*). IQPs + LGT with π → π* and 4fne → 4fne* have shortened the MB PCR time than our reported studies. LGT with infinite π conjugation aligns at a periphery of IQPs intensifying π conjugation via FRET for MB PCR. The π → π* and 4fne → 4fne*of LGT align with π → π* and n → n* of IQPs reducing dyes in a shorter time. The IQPs with LGT have generated IQPs + LGT. IQPs alone after MB reduction remained monodispersed resulting in a transparent nanoemulsion as thin films suitable to be used in paint industries and antireflecting coating agents.22
 |
| | Fig. 7 Functional domains of (a) SIQPNO2, (b) IQ, (c) NIQ, and (d) CIQ. | |
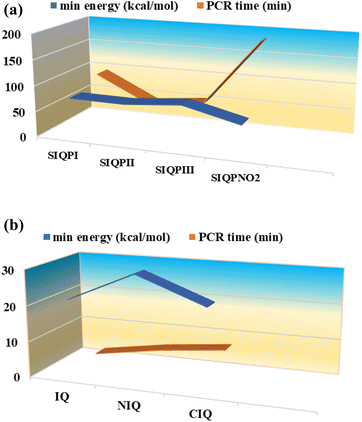 |
| | Chart 1 (a) SIQPs and (b) IQPs vs. PCR time (min) and minimum energies (kcal mol−1). | |
 |
| | Fig. 8 Photocatalysis of MO, RhB, BBR by (a) IQ, (b) NIQ, (c) CIQ each with LGT and MB PCR by (d) SIQPNO2 alone, with LGT, and NIQ, (e) molecular orbital electronic charge DOS of SIQPNO2 and MB and (f) band gap mechanism to reduce MB by SIQPNO2. | |
While LGT with IQPs that photocatalytically reduced MB settled as a black coloured nanocluster, IQPs and SIQPs alone yielded a transparent nanoemulsion (Fig. 8). The π conjugation and 4f#e of LGT with IQPs photocatalytically reduced MB in a longer time than IQPs alone on stronger inter and intra π interactions. IQPs with LGT after MB PCR did not interact with the medium as IQP + LGT have adsorbed a leuco MB (LMB) than alone IQPs (Fig. 8). Compared to 43% min−1 with IQ, NIQ and CIQ have lowered PCR MB rates by 6.7 and 2.2% min−1. NIQ photocatalytically reduced MB in 12 min while the NIQ with SIQPI, II, III and SIQPNO2 in 63, 35, 47, and 64 min respectively. The NIQ adhered with SIQPs with a higher friccohesity23 acted as cophotocatalyst with eigenenergies as SIQPI + NIQ > NIQ in 63 > 12 min to photocatalytically reduce MB respectively. Therefore, NIQ has lowered the PCR time by 34% via enormous 2n → 2n* and π → π* of >O unlike SIQPs alone. NIQ with SIQPs has reduced the PCR time from 95 to 43 min as >O with 2 LPE and π of NIQ on 2n → 2n* and π → π* synchronizing the and 4π → 4π* of 2 (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) of pyz with SIQPs along with its NO2 (Fig. 9). Alone NIQ with >O PCR in 12 min but >O FG with SIQPs via CCA reducing dye in 95 min. The NIQ with SIQPs has generated DOS by minimizing the interference of mutual DOS of pyz and free phenyl (FP) to photocatalytically reduce dyes expeditiously. SIQPs + NIQ with a higher surface energy overcome QEB to PCR pollutants in a shorter time (Fig. 9).24
N) of pyz with SIQPs along with its NO2 (Fig. 9). Alone NIQ with >O PCR in 12 min but >O FG with SIQPs via CCA reducing dye in 95 min. The NIQ with SIQPs has generated DOS by minimizing the interference of mutual DOS of pyz and free phenyl (FP) to photocatalytically reduce dyes expeditiously. SIQPs + NIQ with a higher surface energy overcome QEB to PCR pollutants in a shorter time (Fig. 9).24
 |
| | Fig. 9 UV-Vis for MB reduction using (i) SIQPNO2 and (ii) SIQPNO2 + NIQ, (a) SIQPI + NIQ, (b) SIQPII + NIQ, and (c) SIQPIII + NIQ. | |
UV-vis spectral analysis
UV-Vis maximum absorptions (λmax) for IQ, NIQ, and CIQ are at 292, 278, and 293 nm respectively, while for SIQPNO2 at 278 and 375 nm. Molar absorption coefficients (εmax) were calculated from them (Fig. 10(a–d)). Longer λmax infers less collision shortening the PCR time. The εmax values for IQ, NIQ, CIQ, and SIQPNO2 are 199, 266, 406, and 533 M−1 cm−1 with the PCR time as SIQPNO2 > CIQ > NIQ > IQ for MB respectively. IQ with the lowest εmax without ERG or EWG synchronizes eigenenergy to photocatalytically reduce MB in a shorter time. IQPs and SIQPNO2 could have synchronized the eigenenergies from one to another atom on entropic disorders (ΔS) favourably aligning the h+ and e− holes with ΔS > 0 < ΔG Gibbs free (ΔG) and activation energy (Ea) (Fig. 10 and 11).25 Enthalpy (ΔH) in the PCR process has generated entropy to reorient the photocatalysts and dyes like IQ in the same phase. The hv from solar radiation could have mildly excited 4n → 4n* and 8π → 8π* of IQ via intra-FRET mechanism (Fig. 12). The 4n → 4n* and 8π → 8π* indistinguishable energy levels of IQ have synchronized the wavefunctions fitted with time-dependent Schrödinger equation: 1.41ψIQPs =(ϕ4n* ± ϕ8π*).26 PCR normalizes as  with IQ while with NIQ and CIQ, partial orthogonal activity is depicted as eqn (1)–(3).
with IQ while with NIQ and CIQ, partial orthogonal activity is depicted as eqn (1)–(3).| |
 | (1) |
| |
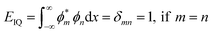 | (2) |
| |
 | (3) |
The m and n eigenenergy coefficients of wavefunctions infer a constructive interference with IQ (Fig. 12).27 While the NIQ and CIQ as |ψIQ| ± |ψNIQ/CIQ| < 1 as |%ψIQ| ± |%ψNIQ| reduced in 12 and |%ψIQ| ± |%ψCIQ| in 32 min to photocatalytically reduce MB (Fig. 12 and S9–S10†). The orientation of wavefunction (ϕ4n* ± ϕ8π*) decides PCR where IQ with synchronized ψIQ = 0.71(ϕ4n* ± ϕ8π*) PCR in 2 unlike 32 and 12 min with partially orthogonal ψNIQ = 0.71((ϕ4n* ± ϕ8π*) – (ϕ5n* ± ϕ1π*)) and ψCIQ = 0.71((ϕ4n* ± ϕ8π*) – (ϕ3n*)) for CIQ and NIQ respectively (Fig. 12). IQ generates the holes of α and β energy state coefficients from HOMO → LUMO in the same phase with ϕ4n* ± ϕ8π* differing from NIQ ϕ5n* ± ϕ1π* and CIQ (ϕ3n*) as intrinsic disintegrating eigenenergies.28 Such pioneering quantum mechanics ascertains a role of ERG for MB PCR in 32 and EWG in 12 min as phase-dependent reduction of effluents (Fig. S9 and S10†). The IQ receives the hν at the same rate at which the eigenenergies of O and 2N atoms synchronize the hole without hurdling the hv receptance, unlike CIQ and NIQ with different α and β quantum states. IQ with localised α to β states in HOMO → LUMO transition, exhibits wavefunctions ψ1 ≠ ψ1 ≠ ψ1 ≠ ψ1 ≠ ψ1 without causing undesired electron–electron collisions (EEC) or Lorentz activities, resulting in a least Ea.29 The ERG and EWG of CIQ and NIQ could have generated (ψ1 ≠ ψ2 ≠ ψ3 ≠ ψ4 ≠ ψ5) α to β quantum states, unlike (ψ1 = ψ1 = ψ1 = ψ1 = ψ1) α to β with IQ respectively. The common αO and βO energy coefficients of O atom in IQPs orientated differently to explain an extended conjugation and realigning mechanism. For example, IQ has reduced dye in 2 min, as the wavefunctions of ketonic O (ψ>CO) also receive hv at the same rate as other constituents of IQ, without any delay in localizing the activities. It elucidates the PCR mechanism as α + β simulate the wavefunctions as ψs = 0.71(ϕ4n* + ϕ8π*) for HOMO and α − β as ψs = 0.71(ϕ4n* − ϕ8π*) for LUMO. The synchronised IQ with wavefunctions ψs = c1nϕ1n + c2nϕ2n + …+ c8πϕ8π (c is coefficient of ψ and phase (ϕ)) are in single phase with least Ea.
 |
| | Fig. 10 UV-Vis absorption (a) IQ, (b) NIQ, (c) CIQ, and (d) SIQPNO2 and SIQPNO2 + NIQ. | |
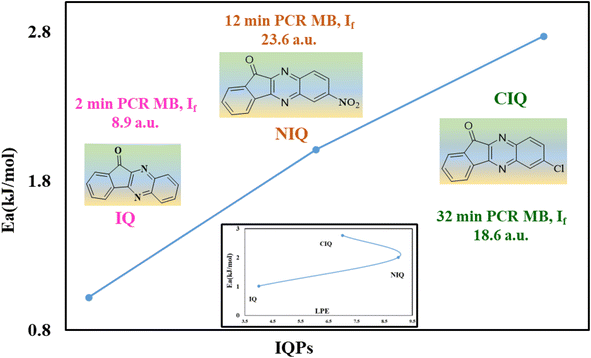 |
| | Fig. 11 Plot of Ea and LPE vs. IQPs. | |
 |
| | Fig. 12 Proposed mechanism to PCR dyes by IQPs and SIQPNO2 under SL. | |
The 4n → 4n* could have synchronized 8π → 8π*, as 2nd set of eigenenergies aligning 4n eigenfunctions + 8π eigenfunctions → 4n* eigenenergies + 8π* eigenenergies in the same phase. The PCR of MB in 2 min infers a least scattering of hv as IQ with a least Ea resonating the λ4n* + λ8π* holes transferred to MB unlike NIQ and CIQ (Fig. 12). The solvent aligning pattern around IQ and dye could have the same zetapotential around 4n of N and 8π bonds via quadrant synchronization of λ4n* + λ8π* eigenenergies with hole intensities Ir = (a4n*)2 + (a8π*)2. The λ4n* & λ8π* restricts reorientation to the spatial domain of IQ avoiding EER in PCR. With IQ, the ϕm, ϕn, ϕo…ϕi DOS w.r.t. ψ1, ψ2, ψ3, ψ4, ψ5, ψ6, andψ7 of a similarly π-conjugated electron system in the box (double bond) on receiving hv generated holes to photocatalytically reduce the dye. Their respective wavefunctions equalize the charge-deficient center of dye. IQ without EWG and ERG FG may not orient specific DOS where the hv reorient with almost similar energy and angular moments. The integrated wavefunctions  photocatalytically reduced MB in 2 min. Born Oppenheimer Approximation could be applied with balanced tentropic vibrational energies multiplied without wasting eigenenergies on a least localised electronic reorientation. Their eigenenergies could have produced the holes that differ from NIQ and CIQ. As
photocatalytically reduced MB in 2 min. Born Oppenheimer Approximation could be applied with balanced tentropic vibrational energies multiplied without wasting eigenenergies on a least localised electronic reorientation. Their eigenenergies could have produced the holes that differ from NIQ and CIQ. As 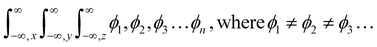 reorientation of NIQ and CIQ with different DOS does not lie in the same phase. The eigenenergy coefficients α and β from HOMO → LUMO for NIQ and CIQ having
reorientation of NIQ and CIQ with different DOS does not lie in the same phase. The eigenenergy coefficients α and β from HOMO → LUMO for NIQ and CIQ having  and
and  differ. Replacing an IQ photocatalyst with NIQ and CIQ has changed a PCR time for MB (Fig. S9 and S10†). The spontaneity for generating the hv with the IQ plane favours PCR in least time unlike with NIQ and CIQ. IQ reduced MB, MO, BBR, and RhB in 2, 5, 7, and 12 min respectively on a mutual reorientation of hv receiving electrophilic and nucleophilic sites supporting the least absorption (Fig. 12). The electrophilic and nucleophilic sites of photocatalate and photocatalyst respectively bring them together with integrated eigenenergies at a closer distance (r), where the holes reached to reduce a dye (eqn (4)).
differ. Replacing an IQ photocatalyst with NIQ and CIQ has changed a PCR time for MB (Fig. S9 and S10†). The spontaneity for generating the hv with the IQ plane favours PCR in least time unlike with NIQ and CIQ. IQ reduced MB, MO, BBR, and RhB in 2, 5, 7, and 12 min respectively on a mutual reorientation of hv receiving electrophilic and nucleophilic sites supporting the least absorption (Fig. 12). The electrophilic and nucleophilic sites of photocatalate and photocatalyst respectively bring them together with integrated eigenenergies at a closer distance (r), where the holes reached to reduce a dye (eqn (4)).
| |
 | (4) |
These 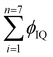 eigenenergies with a single wavefunction (ψIQ = 0.71(ϕ4n* + ϕ8π*)) photocatalytically reduce the MB in less time than MO, BBR, and RhB (Fig. 12 and eqn (5)–(8)) by synchronising as (eqn (5)) follows:
eigenenergies with a single wavefunction (ψIQ = 0.71(ϕ4n* + ϕ8π*)) photocatalytically reduce the MB in less time than MO, BBR, and RhB (Fig. 12 and eqn (5)–(8)) by synchronising as (eqn (5)) follows:
| | |
ψMB = 0.71[(ϕ2n* ± ϕ7π*) − 2(ϕ2n*)]
| (5) |
| | |
ψMO = 0.71[(ϕ2n* ± ϕ7π*) − (ϕ8n* ± ϕ3π*)]
| (6) |
| | |
ψBBR = 0.71[(ϕ2n* ± ϕ10π*) − (ϕ24n* ± ϕ13π*)]
| (7) |
and
| | |
ψRhB = 0.71[(ϕ4n* ± ϕ7π*) − (ϕ4n* ± ϕ3π*)]
| (8) |
RhB is photocatalytically reduced in >5 times compared to MB due to non-equilibrated additional
ψa of COOH, aligned differently w.r.t. wavefunction
ψo causing EER. The wavefunction
ψa of COOH generated eigenenergies in different planes unlike
ψo detaining its symmetry during hole generation as
ϕψo −
ϕψa (
eqn (8)). The IQ with differently reorientating dyes seems responsible for a high
Φ efficiency of PCR. The various eigenenergy sites of photocatalate facilitate PCR by counterbalancing the distinct electrophilic and nucleophilic electronic sites of dyes. The holes partially equilibrated photocatalysts and dyes for PCR over a longer time. BBR, with synchronised wavefunctions (
ψx) with an asymmetric electronic structure, was photocatalytically reduced in 7 min unlike MB and MO in 2 and 5 min, respectively (
Fig. 12). Symmetric π-conjugated BBR interface with the holes of IQ to mutually equilibrate their oscillations for PCR in longer time than MB and MO (
eqn (5)–(8)).
Photocatalytic degree of freedom (F) after dye PCR
Photocatalysts and dye both monodispersed in single-phase favour hν receptance while after PCR, a dye is adsorbed with photocatalyst noted as follows:| | |
F = C − P + xi (degree of freedom (F) for dye PCR)
| (9) |
The number of reacting species (C), the P phase, and xi imply quantum mechanical activities (QMA) to initiate DOS.30 The F infers heat and mechanical energy accompanied in bond breaking or making, affecting photocatalysis in a post-reaction scenario (eqn (9)). The F streamlines electrons from the VB to the CB as per the Drude and Lorentz model unlike with reacting and non-reacting systems. The F specifies generating holes assisted by medium monodispersion without undergoing any structural changes. The F for PCR was calculated for the first time where the C = 4, P = 1, and xi = 2 (hv and FRET) resulting in F = 5 at dT ≥ 0 and dP ≥ 0. F highlights a nature of photocatalyst, photocatalate, solvent (aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN), hv, and FRET. The PCR of MB with IQ releases Cl2, resulting in a single-phase solution. The F = C − P works as receiving hv and emittance is constant during PCR with C = 4 and P = 1, resulting in F = 3 photocatalyst, hv, and FRET variables. The F realigns assisting variables (AV) hv and FRET on receiving hv continuously for PCR. The IQ remained kinetically and thermodynamically stable with F = 1 due to minimum dS in quadrupolar aq
ACN), hv, and FRET. The PCR of MB with IQ releases Cl2, resulting in a single-phase solution. The F = C − P works as receiving hv and emittance is constant during PCR with C = 4 and P = 1, resulting in F = 3 photocatalyst, hv, and FRET variables. The F realigns assisting variables (AV) hv and FRET on receiving hv continuously for PCR. The IQ remained kinetically and thermodynamically stable with F = 1 due to minimum dS in quadrupolar aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN as per Prigogine. The dG = f(T,P,aq,ACN,photocatalyst (lyst),photocatalate (late)) has initiated as in eqn (10) and (11).
ACN as per Prigogine. The dG = f(T,P,aq,ACN,photocatalyst (lyst),photocatalate (late)) has initiated as in eqn (10) and (11).
| |
 | (10) |
where as

and

| | |
dG = −SdT + VdP + μlystdnlyst + μlatednlate + μaqdnaq + μACNdnACN
| (11) |
The chemical potential (
μ) for the photocatalyst and dye are
μlystd
nlyst = 0 and
μlated
nlate = 0 respectively during PCR at d
T ≥ 0 and d
P ≥ 0. Therefore,
F =
C −
P +
xi, where
xi is an electronic variable attained
via essential variables (
EV) such as robust holes, synchronization, wavefunctions, and FRET. The critical variables (
CV) at d
T and d
P are
hv and FRET, expressed as
AV by
eqn (12):
| | |
F = C − P + [EV + CV + AV]
| (12) |
Eqn (12), with
CQMA having QMA is expressed as
eqn (12a).
| | |
F = CQMA − P + [EV + CV + AV]
| (12a) |
The
μlated
nlate =
μlystd
nlyst =
CQMA stable, as
CQMA is stoichiometrically constant as =
CQMA −
P + [
EV +
CV +
AV]. On PCR, the molecularity of the dye remains the same except for the conversion of its Cl
− secondary bond into covalent bond, so Cl
− could not be ascertained as an
EV.
hv also initiated the 2Cl
− + 2h
+ → Cl
2 secondary chemical process. The 2h
+ are not generated as a fragment of IQ or solvent rather Cl
− has come out of QMAs of (h
+) holes. Cl
− neither adding nor subtracting dye alters the stoichiometric input of
CQMA in PCR. 2Cl
− + 2h
+ → Cl
2 in the PCR of dye
in situ balances the holes at quaternary N at constant
CQMA to calculate
F. The =
CQMA −
P + [
EV +
CV +
AV],
CQMA relates a dye with photocatalyst, while the e
− and h
+ balance the
EV. π → π*, n → n*, and σ → σ* of the photocatalyst with minimum Prigogine d
S streamline the holes acting as
EV. The
F =
CQMA −
P + [
EV +
CV +
AV] or =
CQMA −
P +
xi; where
xi =
EV,
CV,
AV (constant flow of
hv). The
μ of
C, at d
T and d
P for the PCR is

where
n moles of photocatalyst are constant with d
μi = 0 for generating holes without structural changes.
31–33 The d
G = 0,

as d
nlyst = 0 or
μ = 0 unlike

with
μ ≠ 0, as variables for the PCR, as
eqn (13).
| | |
dG = nlystdμlyst + nlatedμlate
| (13) |
| dG = χ = nϕlystdμCB–VB + nϕlatedμ(holesinitial)–(holesafterPCR) |
d
G < 0 infers dye PCR without degrading into n
1, n
2, n
3,…n
n molecules (
χ = PCR efficiency). On receiving
hv, water split is one of the prominent factors. n → n* and π → π* together generate
ψ(
x,
t) as the 2s
2 electron with N
+ of MO oscillate within 2s
2 → 2s
0 (
Fig. 12). N
+ connects an electron-rich π bond at the phenyl ring on the right side, while an ERG on the left side generates wavefunctions with negative charge (
ψ−) connecting
ψ+ as QMA. No electronic charge on N
+ existed that could have bypassed to the π bond on RHS while the
ψ− of ERG stabilizing
ψ+ of N
+ via FRET. The wavefunction simulating activities normalize somewhere at a margin
via an orthogonal mechanism with a differential integral. The (
ψCH3 ×
ψCH3)[
ψ+] ×
ψπN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C conjugated states of ((CH
3)
2N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
C) might have counterbalanced the DOS of N
+ (
Fig. 12). The rotational and vibrational motions might have been equipartitioned even with X
− anion like Cl
−, and the N
+ might have been stable. The MO with IQ assisted by solvent split
via CQMA reorienting its eigenenergies in one plane and MO with aq-ACN dipoles has optimized a quinonoid state. The same experiments were conducted by mixing CO
2 with IQPs for MB reduction and analysed by UV-Vis (
Fig. 13). The IQ, NIQ, and CIQ have photocatalytically reduced MB in 120 s, 12 and 32 while with CO
2 in ∼90 s, 8 and 24 min respectively (
Fig. 13 and
14). CO
2 with LPE and π bonds on getting realigned with IQ have robustly generated holes reducing MB and QHIn in ∼90 s and 10 min, acting as a cophotocatalyst. The CO
2 and IQ mutually align energies as
eqn (13a) to photocatalytically reduce MB:
| |
 | (13a) |
 |
| | Fig. 13 MB dye PCR at initial and final states under (a) UV-Vis chamber, (b) SL with IQ, (c) CO2 with IQ and (i) alone MB, MB + CO2 (ii) MB + IQ, MB + IQ + CO2 (iii) MB + NIQ, MB + NIQ + CO2 (iv) MB + CIQ, MB + CIQ + CO2 under SL. | |
 |
| | Fig. 14 Initial and final states (i) alone IQ (a) 0.1, (b) 0.01, (c) 001 M, IQ + CO2 & MB + IQ + CO2 at (a) initial, (b) 2, (c) 5 min (ii) alone NIQ (a) 0.1, (b) 0.01, (c) 001 M, NIQ + CO2 & MB + NIQ + CO2 at (a) initial, (b) 8, (c) 10 min (iii) alone CIQ (a) 0.1, (b) 0.01, (c) 001 M, CIQ + CO2 & MB + CIQ + CO2 at (a) initial, (b) 15, (c) 24 min under SL. | |
The hν interacts with the π (sp2) electrons and LPE of aligned IQPs + CO2 for VB and CB ROCs respectively and shares their eigenenergies with the π (sp2) electrons of a dye reducing to leuco state (LMB). CO2 with polar IQ has acted as a cophotocatalyst developing strong dipole–dipole interactions mutually and with aq-ACN to photocatalytically reduce a dye with higher catalytic efficiency (∼90 s) and mitigating CO2 to carbonic acid with 6n → 6n* and π → π*. The IQ, NIQ, and CIQ with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq-ACN without CO2 have produced densities (ρ) of 0.784406 (9.64), 0.794179 (6.72), and 0.802968 g cm−3 (pH = 5.84) respectively. While IQPs with CO2 have produced the ρ 0.996047 (6.95), 0.790151 (5.68), and 0.797624 g cm−3 (pH = 5.35) respectively (Chart 2). CO2 has increased the density with IQ as it split the water and activate the CO2 to form carbonic acid lowering pH from 9.64 to 6.95 (Fig. 15). The densities of alone water (0.997), ACN (0.786), and 3
7 aq-ACN without CO2 have produced densities (ρ) of 0.784406 (9.64), 0.794179 (6.72), and 0.802968 g cm−3 (pH = 5.84) respectively. While IQPs with CO2 have produced the ρ 0.996047 (6.95), 0.790151 (5.68), and 0.797624 g cm−3 (pH = 5.35) respectively (Chart 2). CO2 has increased the density with IQ as it split the water and activate the CO2 to form carbonic acid lowering pH from 9.64 to 6.95 (Fig. 15). The densities of alone water (0.997), ACN (0.786), and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq-ACN (0.568 g cm−3) at 25 °C are also lower than the IQ mixed with CO2 that have credited to the stronger dipolar–dipolar interactions. This could be helpful for monodispersion and hole generation for the PCR of MB and QHIn in ∼90 s, 17 min than without CO2 in ∼120 s, 24 min respectively (Fig. 16). The sound velocity 1507.01, 1289.83, and 1298.85 m s−1 for IQ, NIQ, and CIQ than 1510.35, 1283.44, and 1291.43 m s−1 with CO2 respectively supported the dye PCR mechanism.
7 aq-ACN (0.568 g cm−3) at 25 °C are also lower than the IQ mixed with CO2 that have credited to the stronger dipolar–dipolar interactions. This could be helpful for monodispersion and hole generation for the PCR of MB and QHIn in ∼90 s, 17 min than without CO2 in ∼120 s, 24 min respectively (Fig. 16). The sound velocity 1507.01, 1289.83, and 1298.85 m s−1 for IQ, NIQ, and CIQ than 1510.35, 1283.44, and 1291.43 m s−1 with CO2 respectively supported the dye PCR mechanism.
 |
| | Chart 2 Alone IQPs physicochemical properties (density and pH) w.r.t. with CO2 favouring dye PCR. | |
 |
| | Fig. 15 IQPs with CO2 MB PCR mechanism. | |
 |
| | Fig. 16 QHIn PCR using IQ with CO2 at t = 0, 5, and 10 min. | |
Plausible mechanism to photocatalytically reduce dyes in the presence of CO2 with IQPs
Step 1: 
Step 2: SL + 2H2O → 4H+ + 2O2− (hv split); 4H+ + 4e− → 2H2; 2O2− + 4h+ → O2
Step 3: 
IQ has acted as a moisture-sensing agent for water splitting to form carbonic acid. The carbonyl of IQ and the carboxylic group of carbonic acid aligned nearby via stronger interactions that might have orientated them in the same phase for generating robust ROC. However, NIQ and CIQ have lowered the density due to NO2 and Cl FG inducing a polarity differently by weakening the intermolecular interactions and forming carbonic acid with pH 5.68 & 5.35 respectively. The molecules like IQ lacking any FGs with symmetrically aligned π conjugated configuration could act as robust photocatalysts for the PCR of a dye than its derivatives differing by the presence of FG. This is the 1st study of PCR dyes using IQPs with CO2 to reduce dye with mitigation to carbonic acid formation (Fig. 15 and 16).
UV-vis adsorption activity of IQPs and SIQPNO2 to photocatalytically reduce dyes
The MB PCR rates were determined using eqn (14) and with IQ, the rate is as follows:| |
 | (14) |
The % MB PCR by IQ in 2 min was calculated using eqn (15):
| |
 | (15) |
where
Co is the initial % MB concentration at
t = 0 and
Ct is the reduced % MB concentration at time
t. NIQ and CIQ were photocatalytically reduced in 12 and 32 min with 80.7 and 71.1% respectively. The % MB PCR by IQ + CO
2 in 90 s was calculated using
eqn (16):
| |
 | (16) |
The reduced % MB concentration by NIQ + CO2 in 8 min is  . The % MB PCR by CIQ + CO2 in 24 min was calculated using eqn (17):
. The % MB PCR by CIQ + CO2 in 24 min was calculated using eqn (17):
| |
 | (17) |
The PCR of MB by IQ (86.0) > NIQ (80.7) > CIQ (71.1) % was calculated using eqn (14)–(16) while with CO2 using eqn (17) (Table 1). UV-Vis analysis for MB, MO, RhB, and BBR individually by IQPs and SIQPNO2, and collectively by SIQPs with LGT, infer IQPs as robust photocatalysts (Fig. S9 and S10†). The higher MB PCR with IQ in 2 min compared to 12, 32, and 180 min with NIQ, CIQ, and alone SIQPNO2 infer IQ as an upconversion nanophotocatalyst to store a higher energy (Table S6†).
Table 1 PCR of MB (18 ppm) using IQPs and SIQPNO2 (1.5 ppm) in aq-ACN under SL
| Sr. no. |
Sample |
MB reduction time (∼min) |
Rate of PCR (%) |
| aq-ACN |
Dye abs (%) |
aq-ACN |
aq-ACN (∼g min−1) |
| 1 |
IQ |
2 |
86.0 |
43.0% min−1 |
15.0 × 10–2 |
| 2 |
NIQ |
12 |
80.7 |
6.7% min−1 |
2.5 × 10–2 |
| 3 |
CIQ |
32 |
71.1 |
2.2% min−1 |
1.0 × 10–2 |
| 4 |
SIQPNO2 |
190 |
86.5 |
0.45% min−1 |
0.16 × 10–2 |
The dyes which have quaternary atoms, π-conjugated systems, LPE, extended double bonds, and fewer peripheral ERGs interfaced with those of the IQPs and SIQPNO2 via FRET to photocatalytically reduce with higher Φ. The IQ was 5 times reusable to photocatalytically reduce dyes without structural degradation, the IQPs and SIQPNO2 were reusable to reduce dyes on washing with chilled water and ethanol and dried at 100 °C for 6 h. The 0.001 M MB, MO, BBR, and RhB each was mixed with IQ for PCR, and after washing, IQ was further mixed in 0.001 M each dye. IQ after washing was used up to 5 cycles reducing 99% dyes in 2, 5, 12, 21, and 36 min respectively on repeating the same procedure for PCR efficiency. After the 5th cycle, almost 10% reduction efficiencies with IQPs and SIQPNO2 were noticed. PCR efficiencies decreased due to surface occupancy with dyes or nanoclustering. Zhu et al., studied lowering in PCR efficiency of RhB using Zn–Al–Ce–MMO after 6 runs.34 Elsayed et al. examined Na-doped ZnO NPs reducing 80% of MB after 7 runs.35 IQPs and SIQPNO2 photocatalytically reduced in 2–30 min than Zn–Al–Ce–MMO and Na-doped ZnO NPs in 60–74 min.
Fluorescence spectra analysis
Fluorescence emitted wavelengths (λem) for IQ, NIQ, CIQ, and SIQPNO2 are 453, 444, 471, and 498 nm at 295, 285, 295, and 285 nm excited wavelength (λexc) respectively (Fig. 17(a–d) and Table 2). Their fluorescence intensity (If), Stokes shift (Δλ), and Φ were calculated as follows:
| |
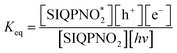 | (18) |
SIQPNO2 with milder λem than IQ infer unsynchronized transitions, detaining maximum eigenenergies (Fig. 17, eqn (17) and (18)). The higher If = 23.6 (a.u.) for NIQ at 444 nm with shorter λem synchronizes manifold intramolecular transitions through different DOS, absorbing higher energy, unlike IQ. Lowest If and Δλ values of IQ than NIQ, CIQ, and SIQPNO2 infer synchronized eigenenergies (Fig. 17). The SIQPNO2 photocatalytically reduced MB in 190 min due to the weakest FRET with highly disordered intramolecular transitions unable to overcome QEB. SIQPNO2 having two NO2 EWG at terminal positions drastically aligns electronic densities that hurdled the hole generation and could not photocatalytically reduce QHIn even after 48 h; however, the studies are continued. NO2 at phenyl of SIQPNO2 connected to pyz with maximum transitions in different energy-storing domains instead of emittance had enhanced the PCR time. The CIQ streamlines an electron flow, generating the electron-deficient sites, unlike NIQ. The If value of EWG and ERG activities has correlated the PCR time with reference to a least If 8.9 (a.u.) with IQ than NIQ, CIQ, and SIQPNO2 having 23.6, 18.6, and 1085.2 (a.u.) respectively (Fig. 17). Both NIQ and CIQ have intensified energy holding capacities with a shorter PCR time. The If = 1085.2 (a.u.) for SIQPNO2 PCR in 190 min unlike 167 (a.u.) for SIQPI in 95 min in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 1
6 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of time respectively infers intramolecular electronic transitions (Fig. S11†).18 NO2 of SIQPNO2 inhibits a PCR rate, unlike IQ exhibits the least If and shortest PCR time (Table 2). The Φ value for IQ, NIQ, CIQ, and SIQPNO2 calculated at λexc 295, 285, 295 & 285 nm has determined E = 6.734 × 10−19 J (IQ & CIQ) and 6.970 × 10−19 J (NIQ & SIQPNO2) respectively. The number of hν absorbed (na) was calculated using eqn (19) as follows:
2 ratio of time respectively infers intramolecular electronic transitions (Fig. S11†).18 NO2 of SIQPNO2 inhibits a PCR rate, unlike IQ exhibits the least If and shortest PCR time (Table 2). The Φ value for IQ, NIQ, CIQ, and SIQPNO2 calculated at λexc 295, 285, 295 & 285 nm has determined E = 6.734 × 10−19 J (IQ & CIQ) and 6.970 × 10−19 J (NIQ & SIQPNO2) respectively. The number of hν absorbed (na) was calculated using eqn (19) as follows:| |
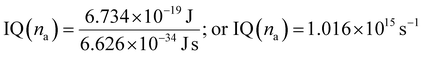 | (19) |
 |
| | Fig. 17 Fluorescence of (a) IQ, (b) NIQ, (c) CIQ, (d) SIQPNO2, and (e) Φ vs. PCR time graph. | |
Table 2 UV-Vis & fluorescence study of IQPs and SIQPNO2 in aq-ACN (1.5 × 10−3 M) at λexc = 295, 285, 295 nm (IQPs) & 285 nm (SIQPNO2), Δλ = (λem − λexc) nm with specific εmax (M−1 cm−1)
| Molecules |
λmax |
Abs |
εmax |
λem |
Δλ |
If |
Φ |
| IQ |
292 |
0.2 |
199 |
453 |
158 |
8.9 |
65.1 |
| NIQ |
278 |
0.4 |
266 |
444 |
159 |
23.6 |
64.1 |
| CIQ |
293 |
0.6 |
406 |
471 |
176 |
18.6 |
62.6 |
| SIQPNO2 |
278 |
0.8 |
533 |
496 |
285 |
1085.2 |
57.4 |
The na values for NIQ, CIQ, and SIQPNO2 are 1.052 × 1015, 1.016 × 1015, and 1.052 × 1015 s−1 at λem of IQ, NIQ, CIQ, and SIQPNO2 at 453, 444, 471, and 496 nm with E = 4.385 × 10−19, 4.474 × 10−19, 4.217 × 10−19, and 4.005 × 10−19 J respectively (Table 2). The number of hνs emitted (ne) by IQ is calculated using eqn (20):
| |
 | (20) |
The ne values for NIQ, CIQ, and SIQPNO2 are 0.662 × 1015 s−1, 0.675 × 1015, 0.636 × 1015, and 0.604 × 1015 s−1. The Φ, ratio of ne and na was obtained with IQ using eqn (21):
| |
 | (21) |
The Φ value for NIQ, CIQ, and SIQPNO2 are 64.1, 62.6, and 57.4% respectively, while 65.1% with IQ seem to minimize EER (Fig. 17 and Table 2). IQPs with negligible If than SIQPNO2 infer disordered π → π* and n → n*. Aq-ACN affects λmax and εmax as bathochromic π → π* and hypsochromic shifts n → n* of FG.36 IQPs and SIQPNO2 both with bathochromic shifts (Δλ = λ285 and λ295 < λem), at shorter λem, and If have widened applications vis-à-vis Φ, λem, and If for the PCR of a dye (Table 2 and Fig. 17). PCR studies using IQPs, IQPs + LGT, SIQPNO2, SIQPNO2 + LGT, SIQPNO2 + NIQ, and SIQPs + NIQ have reduced MB, MO, BBR, and RhB and were studied with fluorescence (Fig. S11–S24†).
Raman analysis
IQPs and SIQPs photosensitive molecules reengineer the overall eigenenergies to electronically align with single-valued wavefunctions, expressed as a deformed DOS. The Raman intensity (IR) elucidates the optimized molecular oscillations differentiating the ERG and EWG activities expressed as multiple D and G bands despite synergistic structure minimized IR on stabilizing dipoles (Fig. 18 and eqn (22)).| |
 | (22) |
 |
| | Fig. 18 Raman spectra of (a) IQ, NIQ, CIQ, and (b) SIQPNO2. | |
The 1st order of Raman shift (SR) vs. IR with IQ inferred transition energy states generating dipoles at a similar rate. The ΔG = ΔH − TΔS with ΔG = 0 infers oscillated dipoles with a laser within similar dimensions on regaining ΔH. Else any bonded atom pair would have been opted out of stabilization with different eigenenergy (Eψ) as in eqn (23):
| |
 | (23) |
T The
IR vs. SR in 1st order infers uniform PE exchanging into
 via
via symmetric
ψi(
x,
t) oscillations at distance
x in time
t as per Heisenberg.
37–39 The absence of D & G bands infers Δ
G = Δ
H −
TΔ
S, at Δ
G = 0, Δ
H =
TΔ
S, or
and
 via
via 
. The

could twist a bond with Δ
H on oscillation. The IQ as a harmonic oscillator minimized undesired collisions reducing MB in ∼2 min with uniform
SR. The partially equilibrated two centers of NIQ respond differently to
hν rather than generating holes to photocatalytically reduce dyes. EWG influences an electron cloud without deformation, and on passing a laser to IQPs, few electrons are easily excited while forbidding the other transitions due to an integrated framework. On acquiring a laser, a new energy-storing alignment within IQPs acts as stocks for reducing the dyes in a shorter PCR time. Moreover, a laser with SIQPNO
2 develops holes, detaining energy as antistokes photocatalytically reduce MB in 190 min. The CIQ generates the weaker antistokes among IQPs due to energy equipartitioning with weaker electronic activities. The SIQPNO
2 with larger Fermi sphere could not crossover QEB as electrons remain in the VB, unlike alone IQPs. SIQPNO
2 has increased the VB-to-CB volume, elongating the PCR time as the electrons are unable to generate atomic oscillations, unlike NIQ or CIQ, which show vibrant antistokes. The CIQ, IQ, and NIQ are vibrant, moderate, and weakest antistokes with minimum shifts respectively (
Table 3). With IQ, the
SR increases linearly along
IR, its higher values reach almost all the electrons to exponentially oscillate in single phase. On lowering an energy of a source, the Raman active stabilized dipoles reduced a dye. Almost all uniform structures with single and double bonds including N with equal charge develop holes at the same rate. The IQ uniformly oscillates the terminal rings (
ψ1 =
ψ1 =
ψ1 =
ψ1 =
ψ1) with a linear slope for MB PCR. NIQ with NO
2 on passing
hνs reduces MB in 12 min. However, CIQ with a weaker ERG Cl photocatalytically reduced MB in 32 min due to non-equilibrate electronic states by engaging
hν in different
IR (
Fig. 18 and
19).
Table 3 Raman slope for IQPs at different Raman shift (SR) vs. intensity IR (a.u.) at specific frequency
| Sr. no. |
SR (cm−1) |
IR (CIQ) |
IR (IQ) |
IR (NIQ) |
| 1 |
91.8142 |
7236 |
3830 |
2662 |
| 2 |
797.248 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 836 836 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 604 |
4326 |
| 3 |
1223.86 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 018 018 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 042 |
5168 |
 |
| | Fig. 19 Raman shift (SR) vs. intensity (SR) for IQPs. | |
The CIQ with intramolecular activities mildly generate the holes for PCR in a longer time unlike IQ to crossover QEB PCR dye in 2 min. SIQPNO2 with vectorized DOS throughout its functional domains via CCA photocatalytically reduce a dye in 180 min as its NIQ delocalized π ring with intensive DOS acting as the 1st oscillating center vis-à-vis differently bonded NO2 FG. The electron–electron cloud reorientations via NO2 attached with FP and CN with pyz ring generating the different DOS. The hν could have been received initially by the units developing uniform electronic shifts as no excess photonic energy is left out to reduce dye. NIQ with SIQPI generating intrinsic oscillations analyzed with Raman spectra infers no deformation as D and G bands are absent with non-uniform FRET. Single FRET with IQ infers Raman inactive while with NIQ attains an unequal distribution with a linear curve deforming a shift. The NIQ with SIQPI has synchronized its three domains with many secondary linkages (Fig. 20) without producing a single deformability pattern.
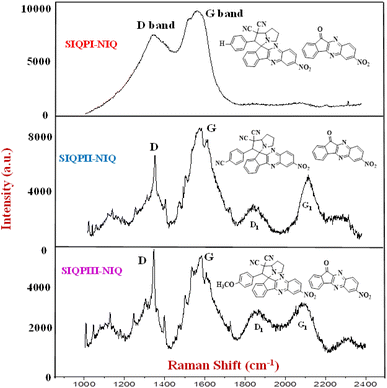 |
| | Fig. 20 Raman spectra for SIQPI + NIQ, SIQPII + NIQ, and SIQPIII + NIQ. | |
As its individual FG has developed a specific peak for reducing MB in 63 min, unlike 95 min with SIQPI alone. NIQ with EWG oscillates without deformation missing D and G bands. SIQPI having FP, pyz, and NIQ units mixed with NIQ in 1/1 ratio generated the charged centers on lowering a permittivity shortening the PCR time to 63 min unlike 95 min with SIQPI alone. The D and G bands at 1300 and 1700 cm−1 infer mild deformed SIQPI and NIQ as Rayleigh scattering40 producing a linear line with mild rate as Raman inactive (Fig. 20). The uniform activity as antistokes scattering avoided undesired collisions during the PCR. The impulse of electrons from one domain to another than of NIQ, pyz, and FP have been prevented by CCA due to trifurcation. Less broad D band than G dominated with Raman active NIQ matrix shortened the PCR time than SIQPI + NIQ on equipartitioning as G > D bands (Fig. 20) with eigenenergies Eψ. Aq-ACN SIQPI has reduced 18 ppm MB in 95 min, rate 0.18 ppm min−1 unlike 63 min, rate 0.28 ppm min−1 with SIQPI + NIQ due to a single phase. The NIQ avoids the EEC by equilibrating the oscillations of FP and pyz in continuity via CCA. SIQPI alone has produced the least scintillations as a linear line with zero slope without eigenenergies (Fig. 20). Wt loss of alone SIQPI at 110.55 °C (Fig. S25†)18 unlike SIQPI + NIQ at higher temperature 280–290 °C generating the sharper multiple peaks and has shortened a PCR time.
The NIQ in a same plane as SIQPI having excess LPE and π conjugation with prominent resonating energy |ψ|2 have shortened the PCR time. SIQPI + NIQ with D and G bands infer aligned dipoles at a shorter distance than bandgap with electronically deformed surfaces and bands. The aligned charges synchronized as Gerade symmetry or G band (Fig. 20). Broader D and G bands of SIQPII + NIQ took a shorter PCR time. The laser might have excited the electrons of EWG CN expressed as multifurcated D & G bands with the same energy of SIQPII with NIQ (Fig. 20). However, their DOS interacts differently with CN, pyz electron clouds of phenyl via CCA, and NIQ with laser generating multiple holes of wavefunctions (ψi). These resonate with the different energies as wavefunctions in the same phase as IR = a2, where a is the amplitude, and IR is the resultant phonon frequency.41 The multifurcated peaks broadened at Raman shift 1300 cm−1, 600 a.u. and 1500 cm−1, 5000 a.u. along with 1600 cm−1 small bands (Fig. 20). The 1000–1300 cm−1 aligns with a vibrating Raman shift of the residual domain of SIQPII + NIQ. The sharper D band infers CN of FP and π bonds of CN of pyz developing a broader frequency, unlike SIQPII18 that might have not fully shared their electron to overcome manifold QEB. Resonating energy overcomes QEB in closely placed scintillating intensities expressed as fringes. The bands at 1900 cm−1 at 2100 and 2100 cm−1 at 4100 a.u. infer C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NO2 without mutually resonating energies separately (Fig. 20). The 2N of NIQ along delocalization could have generated the 2nd set of deformation for D and G bands. Raman inactive SIQPI18 as a weak semiconductor could not align holes but with NIQ disrupted the electronic configuration (Fig. S26(a)†).18 The DOS of SIQPI + NIQ is more active than synchronized SIQPI alone. The laser could not affect SIQPI unlike splitting into D and G bands with NIQ at 1348 cm−1 with 6000 a.u. and 1581 cm−1 with 10
O and NO2 without mutually resonating energies separately (Fig. 20). The 2N of NIQ along delocalization could have generated the 2nd set of deformation for D and G bands. Raman inactive SIQPI18 as a weak semiconductor could not align holes but with NIQ disrupted the electronic configuration (Fig. S26(a)†).18 The DOS of SIQPI + NIQ is more active than synchronized SIQPI alone. The laser could not affect SIQPI unlike splitting into D and G bands with NIQ at 1348 cm−1 with 6000 a.u. and 1581 cm−1 with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 a.u. intermixing (Fig. 20). The bands are broadened by NO2 at NIQ and NO2 at FP on symmetric DOS as a new state of oscillations. The NO2 of NIQ has partially attracted the electron clouds of NO2 of FP and pyz. The electron-withdrawing activities of NO2 of FP and NO2 of NIQ sharpened the bands at 1550 and 1650 cm−1 (Fig. 20). The sharper G band infers a bonding of NO2 at FP as SIQPI is missing NO2 at FP as Raman inactive and IR active (Fig S26(a)†). The 2NO2 of SIQPNO2 as Raman active and FT-IR inactive broadened a band at 1900 cm−1 and sharpened at 2000 bifurcating at 2100 cm−1 of NIQ and pyz across CCA. The NIQ with SIQPNO2 might have sharpened IR. SIQPIII alone18 unlike with SIQPIII + NIQ produces a straight line by mutually inducing the sharper oscillations lowering QEB. Raman spectra have a sharper split at ∼1300 cm−1 with a lower intensity due to terminal NO2 of NIQ. The OMe of SIQPIII could develop coulombic interactions with NO2 of NIQ on sharper Raman splits with high intensity compensating the stretching and sharp oscillations. Moreover, various electron-rich and deficient sites have weakened the G band while sharpened the D band for the PCR of MB in 47 min unlike 54 min with SIQPIII alone (Fig. 20, S26(a)† and Table 4).
000 a.u. intermixing (Fig. 20). The bands are broadened by NO2 at NIQ and NO2 at FP on symmetric DOS as a new state of oscillations. The NO2 of NIQ has partially attracted the electron clouds of NO2 of FP and pyz. The electron-withdrawing activities of NO2 of FP and NO2 of NIQ sharpened the bands at 1550 and 1650 cm−1 (Fig. 20). The sharper G band infers a bonding of NO2 at FP as SIQPI is missing NO2 at FP as Raman inactive and IR active (Fig S26(a)†). The 2NO2 of SIQPNO2 as Raman active and FT-IR inactive broadened a band at 1900 cm−1 and sharpened at 2000 bifurcating at 2100 cm−1 of NIQ and pyz across CCA. The NIQ with SIQPNO2 might have sharpened IR. SIQPIII alone18 unlike with SIQPIII + NIQ produces a straight line by mutually inducing the sharper oscillations lowering QEB. Raman spectra have a sharper split at ∼1300 cm−1 with a lower intensity due to terminal NO2 of NIQ. The OMe of SIQPIII could develop coulombic interactions with NO2 of NIQ on sharper Raman splits with high intensity compensating the stretching and sharp oscillations. Moreover, various electron-rich and deficient sites have weakened the G band while sharpened the D band for the PCR of MB in 47 min unlike 54 min with SIQPIII alone (Fig. 20, S26(a)† and Table 4).
Table 4 Raman D & G with Ea levels for IQPs, SIQPs with NIQ, and SIQPNO2
| Compounds |
E1 (J) ×10−31 |
E2 (J) ×10−31 |
E3 (J) ×10−30 |
E4 (J) ×10−30 |
E5 (J) ×10−30 |
| IQ |
1st order (no T.S) |
| NIQ |
1st order |
| CIQ |
5.2 |
7.29 |
— |
— |
— |
| SIQPI + NIQ |
8.55 |
1.06 |
1.57 |
— |
— |
| SIQPII + NIQ |
8.88 |
1.06 |
1.24 |
1.42 |
— |
| SIQPIII + NIQ |
8.84 |
1.05 |
1.22 |
1.40 |
— |
| SIQPNO2 |
8.78 |
1.06 |
1.25 |
1.39 |
1.54 |
AFM analysis
IQP and SIQP photosensitive molecules realign their eigenenergies that dimensionalize a topography expressed as surface-quantized oscillations. This responds to a sharp silicon cantilever tip to scan the interatomic repulsive forces to elucidate the surface activities to receive hν accordingly. The surface profiles of SIQPNO2 did not strongly interact with dye due to its weakly equilibrated electronic clouds and took 190 min to reduce MB (Fig. 21). However, SIQPII with NIQ has further reduced to 35 min than 63, 47, and 64 with SIQPI + NIQ, SIQPIII + NIQ, and SIQPNO2 + NIQ respectively. The SIQPIII3,18 with OMe induces EER with the double bond of the phenyl ring exhibiting a nonlinear topography via LPE conjugation, unlike a wavefunction of synchronized topography to capture a maximum hν with the intensified surface energies. The wavefunctions of H3C– (3σ bonds) with –O– in the conjugated ring intensify the DOS of –OCH3 and the phenyl ring in response to the EWG exposing the CCA to different eigenenergies of SIQPIII. The position and nature of FG reorient the surfaces to respond the hν as the NO2 at FP w.r.t. NIQ in opposite directions have confined their respective terminals (Fig. 21(i)). Such reorientation of eigenenergies generates charge-deficient spaces nearby CCA that intensify eigenfunctions around each NO2 triggering in situ coulombic interactions partially supported with low IR (Fig. 21). This weakly captures the hν and generates the holes and SIQPNO2 could not overcome QEB and smoothen a topography, resulting in MB PCR in 190 min (Fig. 21(i)). Raman and FT-IR both have confirmed synchronized surface residual charges. Surface topography directly influences the efficiency of hν absorption, generation of holes, FRET, and reaction kinetics. The less pronounced surface fringes or peripheries, ranging from −22.0 to 32.6 nm, −10.8 to 11.4 nm, and −9.9 to 9.6 nm, infer a lower efficiency in ROC generation and delayed MB PCR to 190 min (Fig. 21(ii)). The larger peripheries provide active reaction sites for the photocatalyst and photocatalate for PCR. SIQPNO2 at nanometre scale, such as nanoparticles or nanosheets, inherently have small surface areas per unit volume, limiting the availability of photocatalate to reach in a vicinity of photocatalyst or to ROC. Meanwhile, the generation of holes and their utilization in reduction get affected as an ordered morphology to facilitate hole generation and alignment to photocatalytically reduce dye and vice versa. However, SIQPNO2 with a less ordered topography does not facilitate robust hole generation and alignment to reduce a dye, unlike IQPs. SIQPNO2 with the bulky molecular configuration (pyz, NIQ, & –C6H5NO2) has delayed PCR compared to single-configured IQPs. The two NO2 and two CN EWG with SIQPNO2 desynchronize alignment or charge distribution weakens the PCR activity. The less pronounced surface morphology of SIQPNO2 does not facilitate efficient SL absorption, robust ROC generation, or high quantum efficiency for PCR activities, and requires more PCR time than IQPs. These IQPs could further be enhanced by functionalizing them with N(CH3)2, NH2, CONH2, and COOH groups, enabling them to effectively trap renewable energy for dye PCR in a shorter time (Scheme S9†).18
 |
| | Fig. 21 (i) AFM topography and z axis images of SIQPNO2 at different dimensions. (ii) Morphology of SIQPI. | |
XRD of IQPs and SIQPNO2 analysis
XRD analysis determined the lattice plane of the crystal by measuring the atomic placement in terms of peak intensities and d-spacing for both IQPs and SIQPNO2 (Fig. 22). Each integrated constitutional unit, despite having covalent bonds within the IQ framework, has developed sharper peaks in the lattice plane. EWG of NIQ and ERG of CIQ realign Bravais lattice planes of different peaks unlike IQ without FG having sharper peaks (Fig. 22). IQ has produced symmetric charge distribution via extended conjugation with electron-rich and -deficient sites. No sharp peak was generated at 2θ < 10° except a sharper lattice at 2θ ∼ 11° due to the symmetric plane and bifurcated further by two planes in a single framework (Fig. 22). IQ with oscillations in one plane receives hνs without EEC unlike IQ with a maximum of d spacing as its NO2 withdraws e− from the delocalized ring influencing structural lattice. Therefore, NIQ with localized and integrated planes has generated lattices at lower 2θ ∼ 5°. Though the Cl of CIQ could have not overcome its electronic resistance resulting in a loose electronic bridging at 10° < 2θ > 45°. That could have aligned CIQ to a nanocrystalline, unlike crystalline IQ and NIQ. The EWG and ERG with NIQ and CIQ have generated sharper lattices at 2θ ∼ 5–10° unlike IQ. The other planes of NIQ and CIQ having π rings are not much affected as their internuclear radii (r nm) between negative (q1−) and positive (q1+) charges (q1+q2−/4πεr2) at slightly higher r, nm generate the similar planes at 2θ = 10–30°. The terminal rings of IQPs lie in one plane with sharper intensities. The FG NO2 and Cl− have disrupted electronic clouds producing their individual lattices. The CIQ increases DOS towards ring-enriching wavefunctions ψ1 and ψ3 with ψ2 having a π system with closely packed lattices (Fig. 22). The wavefunctions ψ2 infer multiple lattices with CIQ. However, SIQPNO2 with defused electronic cloud and least split in plane did not respond to hνs. SIQPNO2 acted as a phosphorescent material by generating the secondary transitions due to unsynchronized electronic configurations. SIQPNO2 with major and minor broad diffraction XRD peaks for two NO2 at 2θ ∼31° and 41° due to an amorphous framework unlike IQPs (Fig. 22). High-intensity peaks at 2θ ∼ 5° infer nanostructure with SIQPNO2.| |
 | (24) |
D (particle size), with k = constant (0.9), λ = 0.154 nm, 2θ = angle, β = full width of half maximum of a peak42,43 (eqn (24)). SIQPNO2 at 2θ ∼8° has larger d spacing and 2θ from ∼10–70° with a linear curve. Its 2NO2 at 2θ ∼ 27–32° generated a broader intensity on diffusing its electronic planes responding haphazardly to a laser (Fig. 22). All the planes respond to laser haphazardly without intensifying XRD peaks. NO2, pyz, and NIQ seem to integrate SIQPNO2 PE in a single plane indistinguishable towards 2θ. The unique states of PE seem to be integrated by CCA of SIQPNO2 that generated exceptional If. This energy-storing mechanism of SIQPNO2 could advance the science and technology of organic optoelectronic devices.
 |
| | Fig. 22 XRD spectra of SIQPNO2 and IQPs. | |
Effect of NIQ on SIQPs based on XRD and elemental analysis
NIQ as a cophotocatalyst has tuned a Fermi energy gap44 of SIQPs by generating sharper peaks (Fig. 23). The CCA prohibits the eigenenergies of FP, pyz, and NIQ from mutually mixing, thus, a cophotocatalyst distinctly sharpens the respective electronic planes. NIQ intensifies the DOS of SIQPI with higher scintillations, resembling a single crystal (Fig. 23). NIQ synchronized the charges of SIQPs to sharpen the planes via FRET shortening the PCR time. The new eigenenergies receive more hν for generating the holes with least Ea as the 1st-order reaction (Fig. 23, 24 and Table 5).3,45 The scintillation counts of SIQPs are counterbalanced with NIQ optimizing their overall structural sites (Fig. 23 and 24) with an integrated structure. However, SIQPNO2 alone could not sharpen an XRD pattern unlike SIQPNO2 + NIQ (Fig. 24 and Table S7†). Major deformation occurs when hν passed to SIQPNO2 with DOS of FP, pyz, and NIQ else it would have obtained a straight line due to structural stability. The SIQPNO2 might have caused a mild permittivity to photocatalytically reduce MB in 190 min, unlike SIQPs with NIQ in 64 min (Tables 5 and 6). The DOS in NIQ could have been equilibrated w.r.t. each interacting site.
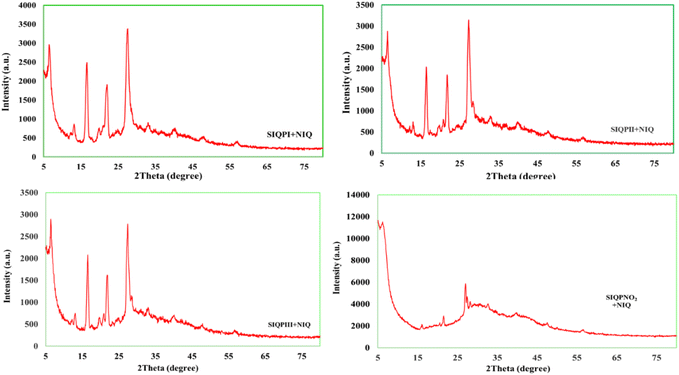 |
| | Fig. 23 XRD spectra of SIQPI + NIQ, SIQPII + NIQ, SIQPIII + NIQ, and SIQPNO2 + NIQ. | |
 |
| | Fig. 24 Dye reducing mechanism of (a) SIQPNO2 and (b) role of added (+NIQ) unit on NIQ of SIQPNO2 in dye PCR. | |
Table 5 Comparative PCR study of MB, MO, BBR, and RhB using IQPs and SIQPNO2
| Compounds |
MB (PCR min) |
MO (PCR min) |
BBR (PCR min) |
RhB (PCR min) |
| IQ |
2 |
5 |
7 |
12 |
| NIQ |
12 |
18 |
14 |
19 |
| CIQ |
32 |
27 |
28 |
38 |
| SIQPNO2 |
190 |
195 |
78 |
392 |
Table 6 Comparative MB PCR (min) study of alone SIQPNO2, SIQPNO2+NIQ, SIQPI + NIQ, SIQPII + NIQ, and SIQPIII + NIQ
| |
SIQPNO2 |
SIQPNO2 + NIQ |
SIQPI + NIQ |
SIQPII + NIQ |
SIQPIII + NIQ |
| PCR (min) |
190 |
64 |
63 |
35 |
47 |
HR-TEM analysis
The HR-TEM image of IQPs and SIQPNO2 on transmitting the electrons through a sample has produced the image with high-resolution 2D plane geometry (Fig. 25). SIQPNO2 compared to IQPs indicates its unoptimized structure requiring 190 min to align for the PCR of the effluents of wastewater like MB (Fig. 25). The NIQ, CIQ, and SIQPNO2 extended Schrodinger PE well due to the bonding of –NO2, –Cl, and –NO2 with asymmetric wavefunctions, unlike IQ. The smart quantum mechanics of IQ unlike others has acted as an ideal PE box of the Schrodinger equation performing PCR in 2 min. The HR-TEM image infers a linear pattern of IQ in one plane, expeditiously capturing the hνs with the same wavefunctions (Fig. 25).
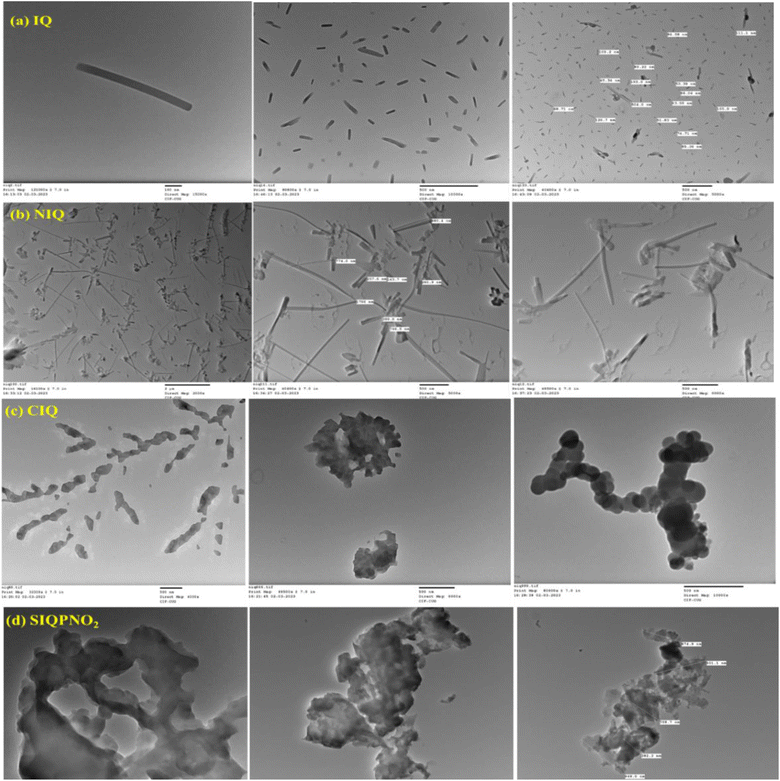 |
| | Fig. 25 HR-TEM images of (a) IQ, (b) NIQ, (c) CIQ, and (d) SIQPNO2. | |
TGA
TGA on gradually raising the temperature exciting electrons of π and σ bonds caused weight loss as unstable components lost weight plotted against temperature (KT) at specific frequency (ν) with E = kKT, E = hν or  or kKT = hν for
or kKT = hν for  , where k is the Boltzmann distribution constant, KT is the Kelvin T constant, h is the Planck constant, and E is the energy. The ν value of weight loss at KT elucidates the thermal stability of the molecules as onset weight loss. IQ lost weight as a single sharper peak at 290 °C with a rate of weight loss 350 μg min−1 with ψ1 (Fig. 26). While the NIQ and CIQ both have developed by destructively lowering a Φ where the IQ has acted as an excellent photocatalyst. NIQ has weight loss rate 425 μg min−1 at ∼350 °C due to asymmetric oscillations caused by NO2. The NIQ slightly resists the electronic activities of NO2 disrupting an overall crystallinity by delocalization. The CIQ destabilizes a crystallinity with rate of weight loss 325 μg min−1 at ∼290 °C with different FRET and hybridized |ψ2| (Fig. 26 and S25†). Two energies equilibrated as a straight line of weight loss from 200 to 300 °C with slope = 0. However, a few endothermic sharp changes appeared at 120 °C, followed by exothermic changes at 125 °C, attributed to the presence of benzene ring. It is accompanied by a slightly endothermic shoulder at 120 °C, due to O matching with the N cavity. After 300–800 °C, the closely placed oscillating peak with wavefunctions of each unit withdraw the intense changes. NIQ with SIQPI has transformed sharply into a multi-peak curve on sharing the electrons of NIQ. The molecule broadens a peak due to fused electron clouds. The CCA could have synchronised the SIQPI interactions with NIQ on broadening the peaks due to weaker FRET. The resulting electron clouds are differently reoriented as 1st order instead of 2nd for SIQPI with NIQ. The SIQPI with NIQ as 1st order TGA at a higher temperature on mutual activation of electron clouds at 20 μg min−1 at ∼300 °C than 400 μg min−1 at ∼230 °C with SIQPI alone (Fig S25†). Therefore, NIQ has enhanced the thermal stability of SIQPI by shortening a PCR time due to robust mutual resonating activity. NIQ with SIQPs expeditiously developed a FRET due to moderate electrostatic interactions lowering the weight loss without transition except for a single-crystal lattice as a novel eutectic mechanism. The SIQPs with anodic NIQ have shortened MB as cathodic PCR by 1.5, 1.2, and 1.1 times compared to SIQPs alone (Table 6). NIQ with ERG/OMe of SIQPIII has disintegrated the electron cloud of the resulting framework.
, where k is the Boltzmann distribution constant, KT is the Kelvin T constant, h is the Planck constant, and E is the energy. The ν value of weight loss at KT elucidates the thermal stability of the molecules as onset weight loss. IQ lost weight as a single sharper peak at 290 °C with a rate of weight loss 350 μg min−1 with ψ1 (Fig. 26). While the NIQ and CIQ both have developed by destructively lowering a Φ where the IQ has acted as an excellent photocatalyst. NIQ has weight loss rate 425 μg min−1 at ∼350 °C due to asymmetric oscillations caused by NO2. The NIQ slightly resists the electronic activities of NO2 disrupting an overall crystallinity by delocalization. The CIQ destabilizes a crystallinity with rate of weight loss 325 μg min−1 at ∼290 °C with different FRET and hybridized |ψ2| (Fig. 26 and S25†). Two energies equilibrated as a straight line of weight loss from 200 to 300 °C with slope = 0. However, a few endothermic sharp changes appeared at 120 °C, followed by exothermic changes at 125 °C, attributed to the presence of benzene ring. It is accompanied by a slightly endothermic shoulder at 120 °C, due to O matching with the N cavity. After 300–800 °C, the closely placed oscillating peak with wavefunctions of each unit withdraw the intense changes. NIQ with SIQPI has transformed sharply into a multi-peak curve on sharing the electrons of NIQ. The molecule broadens a peak due to fused electron clouds. The CCA could have synchronised the SIQPI interactions with NIQ on broadening the peaks due to weaker FRET. The resulting electron clouds are differently reoriented as 1st order instead of 2nd for SIQPI with NIQ. The SIQPI with NIQ as 1st order TGA at a higher temperature on mutual activation of electron clouds at 20 μg min−1 at ∼300 °C than 400 μg min−1 at ∼230 °C with SIQPI alone (Fig S25†). Therefore, NIQ has enhanced the thermal stability of SIQPI by shortening a PCR time due to robust mutual resonating activity. NIQ with SIQPs expeditiously developed a FRET due to moderate electrostatic interactions lowering the weight loss without transition except for a single-crystal lattice as a novel eutectic mechanism. The SIQPs with anodic NIQ have shortened MB as cathodic PCR by 1.5, 1.2, and 1.1 times compared to SIQPs alone (Table 6). NIQ with ERG/OMe of SIQPIII has disintegrated the electron cloud of the resulting framework.
 |
| | Fig. 26 TGA of (a) IQ, (b) NIQ, (c) CIQ, and (d) SIQPNO2. | |
SEM, stereomicroscopy, elemental analysis, single-crystal XRD, FT-IR spectroscopy, and UV-vis spectroscopy
IQ, a sharper nanorod unlike others with EWG and ERG has gained least organized edged morphology (Fig. 27). A polar solvent, ACN, has monodispersed IQ to align the crystals. The CIQ with Cl− and >O coulombic linkages developed a linear crystal analyzed using a stereomicroscope (Fig. 28). Its crystallization could be catalyzed with a suitable solvent. However, IQ with Fe scrap in ACN has nucleated a single crystal growing as sharp-edged nanorods along the walls of the vial while 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aq
1 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN has rusted out (Fig. 29 and 30).
ACN has rusted out (Fig. 29 and 30).
 |
| | Fig. 27 SEM images of (a) IQ, (b) NIQ, (d) CIQ, and (c) SIQPNO2. | |
 |
| | Fig. 28 Stereomicroscopic images of CIQ at different resolutions and dimension in ACN. | |
 |
| | Fig. 29 IQ + Fe in 100% ACN, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7 2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5 3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3 5, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and 2 7, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 of aq 8 of aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN initial, after 5 days, and after a week. ACN initial, after 5 days, and after a week. | |
 |
| | Fig. 30 Stereomicroscopic images of (a) IQ + Fe in ACN, forming crystals (b) IQ + Fe in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq 7 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN forming crystals, (c) IQ + Fe 7 ACN forming crystals, (c) IQ + Fe 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 aq 3 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN forming MNPs, and (d) elucidates π-conjugation potential of IQ to oxidised Fe2+ and Fe3+ (and dyes) that nucleate the IQ in continuity to grow robust crystals. ACN forming MNPs, and (d) elucidates π-conjugation potential of IQ to oxidised Fe2+ and Fe3+ (and dyes) that nucleate the IQ in continuity to grow robust crystals. | |
Single-crystal XRD. IQ with 1.199 g Fe scrap of 2 cm length and 0.1 cm width in 5 mL ACN, placed in a closed vial for 2 weeks, has nucleated a single crystal of orthorhombic, space group (P) = 1, a = 3.86, b = 11.65, c = 23.71 Å and α = β = γ = 90° with 1066 (Å)3 (Fig. 30(a–d)). The crystal has mosaicity at 0.66–0.68° with 0.80 Å. The dipoles of water and ACN aligned along IQ to grow shaper single crystals in solution and along the wall unlike within 3 weeks in EtOH and CH3OH separately reported elsewhere.46 π → π* and n → n* of IQ with Fe split water with least Ea for nucleation with can, unlike with EtOH and CH3OH, has elongated the nucleation time to 3 weeks due to higher solubility. The Fe scrap with IQ has nucleated sharper lattices in a shorter time, by replacing EtOH and CH3OH by ACN.46 However, the IQ in ACN with ionic interactions is linked with the Fe ion of scrap and ACN. However, IQ alone in ACN without scrap with least density has increased the vapour pressure by developing longer spikes than those of IQ with Fe in ACN remaining in the solution. The spikes and nanorods were developed along the wall due to vapor pressure. ACN has catalyzed IQ to form crystals with Fe linearly and oriented towards vapor sticking to walls on adhesion. IQ with Fe has formed crystals linearly and oriented towards vapor sticking to walls with adhesion in the ACN solvent. The spikes and nanorods were developed along the wall and aligned crystals. IQ grew sharp crystals and could act as smart electronics in emerging areas such as fingerprint and optoelectronics. Fe scrap with a larger surface area has weakened dipolar interactions of solvents for carrying IQ with Fe along the walls generating a metallic pressure. The Fe2+ and Fe3+ ions out of the Fe scrap could have aligned IQ linearly at surfaces to crystallize along the wall, interacting with oppositely charged sites of IQ as active in situ ROCs. The adequate hν has sharpened a redox process of IQ with Fe catalyzing the nucleation and photoluminescence (Fig. 30 and 31). However, the sharper IR stretching indicates that FeO, Fe2O3, and Fe3O4 and hydroxides have developed with IQ + Fe in aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN, analysed by FT-IR spectroscopy, TGA, XRD, UV-Vis spectroscopy, and EDX (Fig. S26(b) and S27–S30†). Moreover, Fe with IQ has formed MNPs in 5
ACN, analysed by FT-IR spectroscopy, TGA, XRD, UV-Vis spectroscopy, and EDX (Fig. S26(b) and S27–S30†). Moreover, Fe with IQ has formed MNPs in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 aq
5 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN characterized by XRD and SEM (Fig. 32–34).
ACN characterized by XRD and SEM (Fig. 32–34).
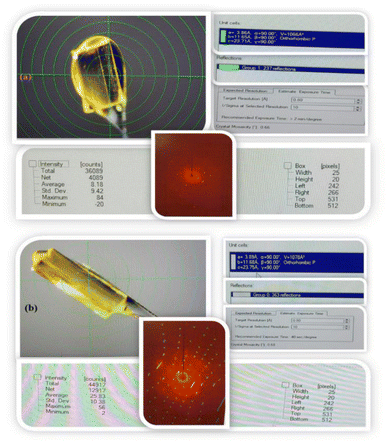 |
| | Fig. 31 IQ developed a sharp single crystal with Fe in (a) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq 7 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN and (b) 100% ACN. ACN and (b) 100% ACN. | |
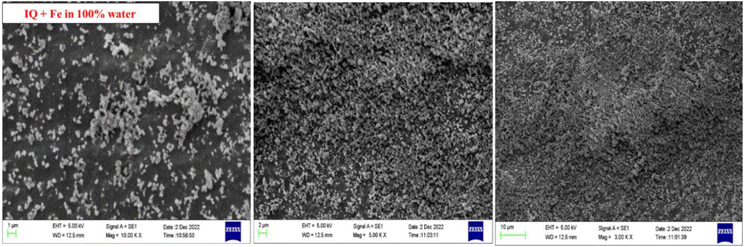 |
| | Fig. 32 SEM images of IQ + Fe in water. | |
 |
| | Fig. 33 SEM images of IQ + Fe in (a) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and (b) 3 8 and (b) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 solvent ratios of aq 7 solvent ratios of aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN. ACN. | |
 |
| | Fig. 34 SEM images of IQ + Fe in (a) 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and (b) 8 3 and (b) 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 aq 2 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN solvent. ACN solvent. | |
IQ with Fe scrap for versatile recycling: single crystals, MNPs, and for recovery of HIn from QHIn
IQ oxidizes the Fe scrap to develop the MNPs without co- or post-precipitation that eliminated a washing during synthesis (Table 7). The conventional methods trigger uncontrolled molecular and ionic activities during MNP synthesis along with other complications.47 Therefore, the MNPs required washing with excess amounts of solvents and the infrastructure was then deleted with our recycling Fe scrap by the IQ photocatalyst method (Table S8†). The MNP synthesis conventionally47 with FeSO4 and FeCl3 using excess NaOH require purification while the smart IQ photocatalyst has selectively responded to Fe scrap. However, the IQ with Fe has developed a single crystal in ACN unlike the MNPs in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 of aq
5 of aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN. The IQ catalyses the Fe scrap to generate the eigenenergies for developing MNPs without using salts as their removal complicates the process. Moreover, it has simplified and advanced the synthesis of MNPs by using renewable resources (SL) not reported yet. The XRD planes shown in Fig. 35 confirm the crystalline MNP formation with diffraction peaks at the (220), (311), (400), (511), and (440) lattice planes. The characteristic peak 311 at 2θ = 37° confirm a magnetite phase within the MNPs matching with the standard XRD data of magnetite48 (Fig. 35).
ACN. The IQ catalyses the Fe scrap to generate the eigenenergies for developing MNPs without using salts as their removal complicates the process. Moreover, it has simplified and advanced the synthesis of MNPs by using renewable resources (SL) not reported yet. The XRD planes shown in Fig. 35 confirm the crystalline MNP formation with diffraction peaks at the (220), (311), (400), (511), and (440) lattice planes. The characteristic peak 311 at 2θ = 37° confirm a magnetite phase within the MNPs matching with the standard XRD data of magnetite48 (Fig. 35).
Table 7 Cutting edge for MNPs synthesis over conventional methods
| Sr no. |
Conventional methods |
Ours methods |
| 1 |
Excess NaOH is used |
No |
| 2 |
Heating |
Under SL |
| 3 |
Multiple washings |
No |
| 4 |
Multistep with manifolds infrastructure |
 |
| 5 |
Multistep process |
Single step process |
| 6 |
Specific pH |
In situ |
| 7 |
Specific temperature |
Under SL at NTP |
| 8 |
Trouble in getting desired particle size & shape |
Not needed |
| 9 |
Manifolds centrifuge |
Not needed |
| 10 |
Autoclaving |
— |
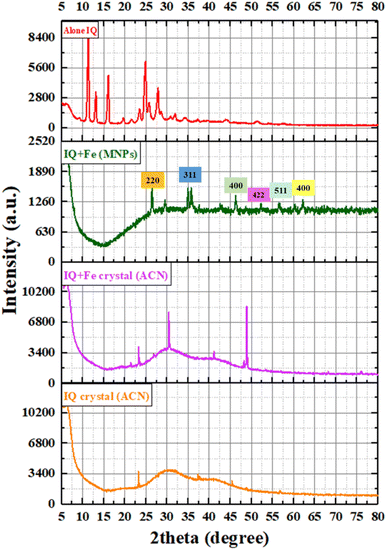 |
| | Fig. 35 XRD characterisation curve for IQ crystal, IQ + Fe crystal, MNPs, and alone IQ. | |
Moreover, IQ with Fe scrap has reduced QHIn to HIn in nanoseconds while without Fe scrap in 17 min. The IQ catalyses the Fe oxidation photocatalytically at NTP that reduced QHIn. Since inception, the HIn dye is used as an indicator in volumetric acid and base titrations and is drained out causing enormous pollution. Till date there are no remedial measures for degrading the persistent pollutant like QHIn, but IQ with Fe scrap has degraded that could further be extended to other pollutants and transition metals such as V, Cr, Mn, and Co scrap. The electrons released by Fe catalyse the reduction of QHIn by IQ, and similarly, they could reduce other persistent pollutants. Till date, no photocatalyst is reported that can advance the 1st and 2nd oxidations like Fe2+ and Fe3+ carried out by IQ to photocatalytically reduce a dye along with developing the MNPs. The same has been supported on FeSO4 hydrolysis in 5 min with IQ unlike 7 min in aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN under SL. The crystalline water was engaged by ACN via H dipolar interactions that cause the release of crystalline water from FeSO4, whereas hydrolysis without IQ takes time; however, IQ has reduced the Fe and formed metal hydroxide with release of H2 gas. However, CuSO4·5H2O with IQ took 6 h to decolourise blue copper to colourless and has formed homogeneous thin nano films at the glass vial walls under SL.
ACN under SL. The crystalline water was engaged by ACN via H dipolar interactions that cause the release of crystalline water from FeSO4, whereas hydrolysis without IQ takes time; however, IQ has reduced the Fe and formed metal hydroxide with release of H2 gas. However, CuSO4·5H2O with IQ took 6 h to decolourise blue copper to colourless and has formed homogeneous thin nano films at the glass vial walls under SL.
The 1st oxidation state of copper is highly stable due to its stable configuration; therefore, IQ dominantly split water and reduced copper. Since, IQ has developed dipolar–dipolar interactions with the medium and copper, forming a nano thin film at walls of the vials (Fig. 36). However, the solvent system catalyses the mechanism of nano monolayer thin film formation with IQ and fitted as per the Freundlich and Langmuir isotherm theory unlike metal remains at the bottom surface. In the same sequence, the starch iodide paper used with the solvent (aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN) alone, FeSO4, IQ, and IQ + FeSO4 with MB drop strain (Fig. 37). IQ + FeSO4 effectively reduced MB as the IQ and Fe have a strong interaction that has photocatalyzed MB with the highest rate as compared to solvent alone, FeSO4 or IQ alone. This process can be applied for various metal extraction processes with IQ. Hence, IQ with its active configuration has undergone a robust transition that has split water (step 2) to oxygen and –OH.49 The Fe with IQ has further catalysed a process as Fe → Fe2+ + 2e− that reduces O2 + 2e− → O2− and O2− transfers its electron to water received from HO− (step 3) to generate the holes by IQ on receiving the hν.
ACN) alone, FeSO4, IQ, and IQ + FeSO4 with MB drop strain (Fig. 37). IQ + FeSO4 effectively reduced MB as the IQ and Fe have a strong interaction that has photocatalyzed MB with the highest rate as compared to solvent alone, FeSO4 or IQ alone. This process can be applied for various metal extraction processes with IQ. Hence, IQ with its active configuration has undergone a robust transition that has split water (step 2) to oxygen and –OH.49 The Fe with IQ has further catalysed a process as Fe → Fe2+ + 2e− that reduces O2 + 2e− → O2− and O2− transfers its electron to water received from HO− (step 3) to generate the holes by IQ on receiving the hν.
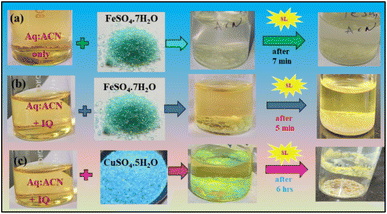 |
| | Fig. 36 (a) Alone aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN solvent study with FeSO4·7H2O (b) IQ in aq ACN solvent study with FeSO4·7H2O (b) IQ in aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN with FeSO4, and (c) IQ in aq ACN with FeSO4, and (c) IQ in aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN with CuSO4·5H2O formed nano thin film under SL. ACN with CuSO4·5H2O formed nano thin film under SL. | |
 |
| | Fig. 37 Starch-iodide paper detect the absence of oxidizing agents. (a) MB + FeSO4 on starch paper. (b) MB + IQ on starch paper. (c) MB + IQ + FeSO4 on starch paper. (d) Only MB on starch paper. | |
Mechanism
Step 1: SL (hv) + IQ → IQ* → IQ* ≈ h+ + e−;
Step 2: H2O → H+ + HO− (autosplit); HO− → O2− + H+;
Step 3: SL (hv) + H2O → 2H+ + HO−; 2O2− + 4h+ → O2;
Step 4: Fe2+ + 2HO− → Fe(OH)2 → 2FeO + 2H2O; Fe3+ + 3HO− → Fe(OH)3 → Fe2O3 + 3H2O.
The same experiment was continued in degassed and distilled water where a degassed water has mildly rusted due to less dissolved O2 (Fig. 38, S31, Tables 7 and S8†). The IQ with Fe scrap has expeditiously developed an anti-rusting sharp lattice in ACN reducing QHIn in seconds (Fig. 39). The IQPs and SIQPs having N atom, LPE, and π conjugated rings enhanced dye PCR. The intense yellow colour of IQ in aq-ACN with scrap has transition as follows: Fe → Fe2+ + 2e− and Fe → Fe3+ + 3e−. The Fe2+ & Fe3+ both have initiated the π catanionic interactions interfacing IQ with aligned wavefunctions of MNPs (Fig. 40). The Fe converted dissolved oxygen to O2− producing (HO−) Fe2O3 as a major product (step 3) in situ, with different heat capacities. The Fe2+ formed O2− in situ developing the oxides at the surfaces. The O2− sticks to Fe2+ surfaces reducing water to (HO−) in a two-step process.
 |
| | Fig. 38 Effect in degassed and distilled water on rusting with Fe scrap. | |
 |
| | Fig. 39 Images of (i) IQ + Fe in aq, (ii) aq IQ only, (iii) IQ + Fe in degassed water, (iv) IQ in ACN, (v) IQ + Fe in ACN (a) initial and after (b) 1, (c) 2, (d) 3, (e) 5, (f) 7, (g) 10, (h) 15, and (i) 17 min. | |
 |
| | Fig. 40 Recycling mechanism of Fe scrap with IQ to develop MNPs in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 aq 5 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN. ACN. | |
The 1st step is Fe → Fe2+ and the 2nd forms a product on reducing the negative charges indirectly. The Fe2+ and Fe3+ both reduce a dissolved oxygen to O2− evolving the bubbles to surfaces as water approaches Fe2+/Fe3+ (Fig. S32†). This has reduced to HO− on reacting with Fe2+/Fe3+ anodic reactions. The Fe2+/Fe3+ reacts to O2−/HO− forming hydroxides with variable chemical rates. The IQ with Fe has reduced QHIn to HIn in seconds. The electrons of IQ via ROCs have formed crystals with metal (step 5).50
Mechanism
Step 1: SL (hv) + IQ + Fe (scrap) → IQ* → (IQ* ≈ h+ + e−);
Step 2: IQ* + Fe (scrap) → Fe2+ + Fe3+; NaOH (hv) → Na+ + HO−;
Step 3: H2O (hv) → H+ + HO−; HO− → O2− + H+; 2H+ + 2e− → H2↑;
Step 4: Fe2+ + 2HO− → Fe(OH)2; Fe3+ + 3HO− → Fe(OH)3
Step 5:
This mechanism elucidates the –OH formation as O atoms with partially negative charges engage Na+ as Fe2+ and Fe3+ align along O− of the carboxyl group in QHIn (step 5). The resulting solution has yielded a white-colored HIn precipitate confirming with aq NaOH that had restored its pink color (Fig. S33†).
The recovery of dyes from effluents has enhanced a novelty of IQ. While IQ with ACN has developed a single crystal as no oxygen was dissolved unlike the ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 2
9, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 5
8, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 8
3, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of aq
2 of aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN to oxidize Fe to MNPs (Chart S1†). The dipolar ACN has trapped hv and detained SL partially forming metal oxides. Furthermore, aq-ACN has dissolved oxygen, further increasing with water %, leading to rusting. However, distilled and degassed water resulted in least rusting, depicting the oxidizing role of dissolved oxygen. ACN without dissolved oxygen acted as an anti-rusting agent in conjugation with IQ (Table S9†). The O2− may affect the dipoles of ACN to catalyse oxygen ionization. The Fe reacted with dissolved O2 where a subsequent interaction for rusting depends on increasing or decreasing the ratio of water with ACN. However, iodine with −0.54 V reduction potential could realign a delocalized IQ vis-à-vis Fe oxidation by substituting the presence of O2− with quantized activities. The Fe metal framework has more affinity to bind I2 than starch detecting the presence of Fe ions in IQ organometallic frameworks. It was found that 0.001 M IQ has degraded a 0.5 mL waste mobil oil with 1/50 ratio in 15 days with 3
ACN to oxidize Fe to MNPs (Chart S1†). The dipolar ACN has trapped hv and detained SL partially forming metal oxides. Furthermore, aq-ACN has dissolved oxygen, further increasing with water %, leading to rusting. However, distilled and degassed water resulted in least rusting, depicting the oxidizing role of dissolved oxygen. ACN without dissolved oxygen acted as an anti-rusting agent in conjugation with IQ (Table S9†). The O2− may affect the dipoles of ACN to catalyse oxygen ionization. The Fe reacted with dissolved O2 where a subsequent interaction for rusting depends on increasing or decreasing the ratio of water with ACN. However, iodine with −0.54 V reduction potential could realign a delocalized IQ vis-à-vis Fe oxidation by substituting the presence of O2− with quantized activities. The Fe metal framework has more affinity to bind I2 than starch detecting the presence of Fe ions in IQ organometallic frameworks. It was found that 0.001 M IQ has degraded a 0.5 mL waste mobil oil with 1/50 ratio in 15 days with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq
7 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN of 0 mJ m−2 interfacial energies (Fig. S34 and Table S9†).
ACN of 0 mJ m−2 interfacial energies (Fig. S34 and Table S9†).
The IQ with robust FRET has disintegrated a waste mobil oil into fragments of shorter alkyl chains splitting waste hydrocarbons. The SIQPs in an alkaline medium reduced QHIn to HIn while IQ reduced mobil oil that was dispersed in an eluting solvent. It was monitored by TLC and stained with iodine and visualized under UV-Vis light. However, hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA eluting solvent has widened IQ dispersion and degraded mobil oil salting out quasi-static adhesion on TLC. The mobil oil has developed a velocity gradient in situ with different retention factor (Rf) values. The eluting solvent hexane
EA eluting solvent has widened IQ dispersion and degraded mobil oil salting out quasi-static adhesion on TLC. The mobil oil has developed a velocity gradient in situ with different retention factor (Rf) values. The eluting solvent hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA has comparatively covered a maximum Rf value (Fig. S35†). Moreover, IQ breaks a longer C–C alkyl chain of oil into an alkene of shorter carbon chain with H2 gas via a free radical disintegration mechanism. The IQ as reference upconversion nanomaterial and quantum mechanical sensor disintegrated the saturated hydrocarbons.51,52 IQ produced single-valued wavefunction of integrated π conjugated DOS as a quantum dot including antifungal activities by inhibiting fungal growth. The ACN with hydrophobicity has tuned interaction with host on increasing water concentration that might have blocked its reacting sites (Fig. S35†). The IQ could not interact and resulted in fungal activities. The explorative synergistic effects with other antifungal agents are being pursued in laboratory.53 Moreover, IQ has effectively detached a dye from textile dyed fabric and the experiments are pursued in various aqueous waste solvent systems (Fig. S36†).
EA has comparatively covered a maximum Rf value (Fig. S35†). Moreover, IQ breaks a longer C–C alkyl chain of oil into an alkene of shorter carbon chain with H2 gas via a free radical disintegration mechanism. The IQ as reference upconversion nanomaterial and quantum mechanical sensor disintegrated the saturated hydrocarbons.51,52 IQ produced single-valued wavefunction of integrated π conjugated DOS as a quantum dot including antifungal activities by inhibiting fungal growth. The ACN with hydrophobicity has tuned interaction with host on increasing water concentration that might have blocked its reacting sites (Fig. S35†). The IQ could not interact and resulted in fungal activities. The explorative synergistic effects with other antifungal agents are being pursued in laboratory.53 Moreover, IQ has effectively detached a dye from textile dyed fabric and the experiments are pursued in various aqueous waste solvent systems (Fig. S36†).
Conclusion
SIQPNO2 has been synthesised with 85–90% and IQPs with 80–89% yields in ACN. The SIQPNO2 and IQP photocatalysts photocatalytically reduced the pollutant MB, MO, BBR, and RhB dyes under SL in 2 to 87 min. The ERG of CIQ lowered PCR Φ unlike EWG of NIQ causing the different reorientations. IQ with waste Fe has developed robust MNPs in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 aq
5 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN, recovered HIn, and developed a single crystal. SIQPNO2 with two peripheral EWG NO2 photocatalytically reduced MB in 190 than 2–32 min with IQPs. SIQPNO2 alone has reduced MB in 2.16 times shorter period than with LaGT, acting as anti-PCR due to 4f#e. The rate of reaction, minimum energy, Ea, Φ, and photocatalytic activities were calculated from UV-Vis and fluorescence. Fe scrap in ACN had catalysed IQ to nucleate a single crystal as nanorods on recycling a waste metal. IQ with 100% ACN acted as an anti-rusting agent while with water has developed MNPs. The IQPs and SIQPNO2 have reduced MB, MO, BBR, and RhB in a shorter time than with LaGT, TbGT, HoGT, and CeGT, as antireduction agents. IQPs and SIQPNO2 could be extended to adsorb several toxic heavy metals from industrial effluents. These photocatalysts have reduced and physisorbed the industrial waste dyes, pollutants, and inks and were not photodegraded during the PCR process even after 5th run. Reducing dyes with IQPs and SIQPNO2 is a sustainable separation with high Φ and could be substituted with EWG (SO4) and ERG (NH2) constitutional units for PCR. The studies are being pursued with other dyes such as naphthalene derivatives, persistent pollutants, and industrial wastes (used industrial wastes such as ink and oil). IQPs may quench ozone preventing holes and ROS in a free radical scavenging process.
ACN, recovered HIn, and developed a single crystal. SIQPNO2 with two peripheral EWG NO2 photocatalytically reduced MB in 190 than 2–32 min with IQPs. SIQPNO2 alone has reduced MB in 2.16 times shorter period than with LaGT, acting as anti-PCR due to 4f#e. The rate of reaction, minimum energy, Ea, Φ, and photocatalytic activities were calculated from UV-Vis and fluorescence. Fe scrap in ACN had catalysed IQ to nucleate a single crystal as nanorods on recycling a waste metal. IQ with 100% ACN acted as an anti-rusting agent while with water has developed MNPs. The IQPs and SIQPNO2 have reduced MB, MO, BBR, and RhB in a shorter time than with LaGT, TbGT, HoGT, and CeGT, as antireduction agents. IQPs and SIQPNO2 could be extended to adsorb several toxic heavy metals from industrial effluents. These photocatalysts have reduced and physisorbed the industrial waste dyes, pollutants, and inks and were not photodegraded during the PCR process even after 5th run. Reducing dyes with IQPs and SIQPNO2 is a sustainable separation with high Φ and could be substituted with EWG (SO4) and ERG (NH2) constitutional units for PCR. The studies are being pursued with other dyes such as naphthalene derivatives, persistent pollutants, and industrial wastes (used industrial wastes such as ink and oil). IQPs may quench ozone preventing holes and ROS in a free radical scavenging process.
Data availability
The following files are available free of charge i.e., spectral data of 1H, 13C NMR, FT-IR, XRD (powder), LC-MS, UV-Vis, EDX, and TGA spectra of all compounds (ESI†).
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
The authors are thankful to the Central University of Gujarat, India for infrastructural support, UGC DAE CSIR Indore for Raman spectroscopy, and XRD single crystal for IIT Gandhinagar.
Notes and references
- N. Shahrestani, F. Salahi, N. Tavakoli, K. Jadidi, M. Hamzehloueian and B. Notash, Asymmetric synthesis approach of enantiomerically pure spiro-indenoquinoxaline pyrrolidines and spiro-indenoquinoxaline pyrrolizidines, Tetrahedron:Asymmetry, 2015, 26(20), 1117–1129, DOI:10.1016/j.tetasy.2015.08.013.
- N. Kumar, C. Lal, B. Singh and A. K. Patel, Synthesis and Biological Activities of Some Novel Spiro Heterocyclic Pyrrolizidine Derivatives of 11H-indeno[1,2-b]quinoxaline through 1,3-Dipolar Cycloaddition, Asian J. Chem., 2020, 32(5), 1255–1258, DOI:10.14233/ajchem.2020.22630.
- R. Kumari and M. Singh, Spiroheterocyclic Photocatalyst for Reducing QHIn-Persistent Pollutants, Dyes, and Transition-Metal Ions Cocatalyzed with Electrolytes, ACS Omega, 2022, 7(44), 40203–40229, DOI:10.1021/acsomega.2c05103.
- S. R. Pandya and M. Singh, Dispersion and optical activities of newly synthesized magnetic nanoparticles with organic acids and dendrimers in DMSO studied with UV/vis spectrophotometry, J. Mol. Liq., 2015, 211, 146–156, DOI:10.1016/J.MOLLIQ.2015.06.068.
- S. Dev and M. Singh, Metallic sulfide nanoparticles anchored graphene oxide: Synthesis, characterization and reduction of methylene blue to leuco methylene blue in aqueous mixtures, J. Phys. Chem. Solids, 2020, 139, 109335, DOI:10.1016/j.jpcs.2020.109335.
- J. Liu and Y. Lu, FRET Study of a Trifluorophore-Labeled DNAzyme, J. Am. Chem. Soc., 2002, 124(51), 15208–15216, DOI:10.1021/ja027647z.
- A. G. Naikwade, M. B. Jagadale, D. P. Kale, A. D. Gophane, K. M. Garadkar and G. S. Rashinkar, Photocatalytic Degradation of Methyl Orange by Magnetically Retrievable Supported Ionic Liquid Phase Photocatalyst, ACS Omega, 2020, 5(1), 131–144, DOI:10.1021/acsomega.9b02040.
- A. S. Yusuff, L. Taofeek Popoola and E. I. Aderibigbe, Solar photocatalytic degradation of organic pollutants in textile industry wastewater by ZnO/pumice composite photocatalyst, J. Environ. Chem. Eng., 2020, 8(4), 103907, DOI:10.1016/J.JECE.2020.103907.
- L.-L. Tan, W.-J. Ong, S.-P. Chai and A. Rahman Mohamed, Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide, Nanoscale Res. Lett., 2013, 8, 465, DOI:10.1186/1556-276X-8-465.
- A. Salama, A. Mohamed, N. M. Aboamera, T. A. Osman and A. Khattab, Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation, Appl. Nanosci., 2018, 8, 155–161, DOI:10.1007/s13204-018-0660-9.
- S. Cailotto, et al., Carbon dots as photocatalysts for organic synthesis: Metal-free methylene-oxygen-bond photocleavage, Green Chem., 2020, 22(4), 1145–1149, 10.1039/C9GC03811F.
- Y. Huang, Y.-X. Huang, J. Sun and C.-G. Yan, A [3+2] cycloaddition reaction for the synthesis of spiro[indoline-3,3′-pyrrolidines] and evaluation of cytotoxicity towards cancer cells, New J. Chem., 2019, 43(23), 8903–8910, 10.1039/C9NJ00994A.
- A. L. Jadhav and G. D. Yadav, Clean synthesis of benzylidenemalononitrile by Knoevenagel condensation of benzaldehyde and malononitrile: effect of combustion fuel on activity and selectivity of Ti-hydrotalcite and Zn-hydrotalcite catalysts, J. Chem. Sci., 2019, 131(8), 79, DOI:10.1007/s12039-019-1641-6.
- M. Singh and R. Kumari, Correction to ‘Photocatalytic Reduction of Fluorescent Dyes in Sunlight by Newly Synthesized Spiroindenoquinoxaline Pyrrolizidines’, ACS Omega, 2020, 5(36), 23201–23218, DOI:10.1021/acsomega.0c05835.
- A. Suresh, T. V. Baiju, T. Kumar and I. N. N. Namboothiri, Synthesis of Spiro- and Fused Heterocycles via (4+4) Annulation of Sulfonylphthalide with o-Hydroxystyrenyl Derivatives, J. Org. Chem., 2019, 84(6), 3158–3168, DOI:10.1021/acs.joc.8b03039.
- K. Kumar, R. P. Dave, S. Dev and M. Singh, Study of molar properties of GO after doping with transition metals for photodegradation of fluorescent dyes, RSC Adv., 2022, 12(46), 29734–29756, 10.1039/d2ra04230d.
- G. A. Eldeken, F. A. El-Samahy, E. M. Zayed, F. H. Osman and G. E. H. Elgemeie, Synthesis, Biological Activities and Molecular Docking analysis
of a Novel Series of 11H-Indeno[1,2-b]quinoxalin-11-one Derivatives, J. Mol. Struct., 2022, 1261, 132929, DOI:10.1016/J.MOLSTRUC.2022.132929.
- R. Kumari and M. Singh, Photocatalytic reduction of fluorescent dyes in sunlight by newly synthesized spiroindenoquinoxaline pyrrolizidines, ACS Omega, 2020, 5(36), 23201–23218, DOI:10.1021/acsomega.0c02976.
- C. T. Campbell, The Degree of Rate Control: A Powerful Tool for Catalysis Research, ACS Catal., 2017, 7(4), 2770–2779, DOI:10.1021/acscatal.7b00115.
- Q. Yusen and E. J. Schelter, Lanthanide Photocatalysis, Acc. Chem. Res., 2018, 51(11), 2926–2936, DOI:10.1021/acs.accounts.8b00336.
- J. Lavergne and W. Junge, Proton release during the redox cycle of the water oxidase, Photosynth. Res., 1993, 38, 279–296, DOI:10.1007/BF00046752.
- D. Bouhafs, A. Moussi, A. Chikouche and J. M. Ruiz, Design and simulation of antireflection coating systems for optoelectronic devices: Application to silicon solar cells, Sol. Energy Mater. Sol. Cells, 1998, 52(1–2), 79–93, DOI:10.1016/S0927-0248(97)00273-0.
- A. K. Jangid, P. Malik and M. Singh, Mineral acid monitored physicochemical studies of oil-in-water nanoemulsions, J. Mol. Liq., 2018, 259, 439–452, DOI:10.1016/j.molliq.2018.03.005.
- J. Kou, C. Lu, J. Wang, Y. Chen, Z. Xu and R. S. Varma, Selectivity Enhancement in Heterogeneous Photocatalytic Transformations, Chem. Rev., 2017, 117(3), 1445–1514, DOI:10.1021/acs.chemrev.6b00396.
- L. A. Kurfman, T. T. Odbadrakh and G. C. Shields, Calculating Reliable Gibbs Free Energies for Formation of Gas-Phase Clusters that Are Critical for Atmospheric Chemistry: (H2SO4)3, J. Phys. Chem. A, 2021, 125(15), 3169–3176, DOI:10.1021/acs.jpca.1c00872.
- H. De Raedt, Product formula algorithms for solving the time dependent Schrödinger equation, Comput. Phys. Rep., 1987, 7(1), 1–72, DOI:10.1016/0167-7977(87)90002-5.
- M. Bawin and A. Burnel, Single-valuedness of wavefunctions from global gauge invariance in two-dimensional quantum mechanics, J. Phys. A: Math. Gen., 1985, 18(11), 212, DOI:10.1088/0305-4470/18/11/033.
- G. Ch. Mellau, Rovibrational eigenenergy structure of the [H,C,N] molecular system, J. Chem. Phys., 2011, 134, 194302, DOI:10.1063/1.3590026.
- U. Hillmann, W. Schimmack, P. Jacob and K. Bunzl, In situ γ-spectrometry several years after deposition of radiocesium, Radiat. Environ. Biophys., 1996, 35, 297–303, DOI:10.1007/s004110050043.
- J. M. Cortina, J. P. Green, K. R. Keeler and R. J. Vandenberg, Degrees of Freedom in SEM: Are We Testing the Models That We Claim to Test?, Organ. Res. Methods, 2016, 20(3), 350–378, DOI:10.1177/1094428116676345.
- L.-Q. Chen, Chemical potential and Gibbs free energy II: Q&A, MRS Bull., 2022, 47(8), 753–758, DOI:10.1557/s43577-022-00405-3.
- P. Dais, Impact of Gibbs’ and Duhem's approaches to thermodynamics on the development of chemical thermodynamics, Arch. Hydrobiol. Suppl., 2021, 75(2), 175–248, DOI:10.1007/s00407-020-00259-8.
- G. G. Láng, Basic interfacial thermodynamics and related mathematical background, ChemTexts, 2015, 1, 16, DOI:10.1007/s40828-015-0015-z.
- J. Zhu, Z. Zhu, H. Zhang and S. Küppers, Enhanced photocatalytic activity of Cedoped Zn-Al multi-metal oxide composites derived from layered double hydroxide precursors, J. Colloid Interface Sci., 2016, 481, 144–157, DOI:10.1016/j.jcis.2016.07.051.
- M. H. Elsayed, T. M. Elmorsi, A. M. Abuelela, A. E. Hassan, A. Z. Alhakemy, M. F. Bakr and H. H. Chou, Direct sunlight-active Na-doped ZnO photocatalyst for the mineralization of organic pollutants at different pH mediums, J. Taiwan Inst. Chem. Eng., 2020, 115, 187–197, DOI:10.1016/j.jtice.2020.10.018.
- J. M. Lee, S. B. Yuk, J. W. Namgoong and J. P. Kim, Mechanofluorochromism of Triphenylamine-BODIPY: Effect of twisted intramolecular charge transfer and restriction in rotation
on fluorescence, Dyes Pigm., 2021, 185(Part A), 108864, DOI:10.1016/j.dyepig.2020.108864.
- E. J. Sie, Observation of Exciton Redshift-Blueshift Crossover in Monolayer WS2, Nano Lett., 2017, 17, 4210–4216, DOI:10.1021/acs.nanolett.7b01034.
- S. Calati, Q. Li, X. Zhu and G. Stahler, Ultrafast evolution of the complex dielectric function of monolayer WS2 after photoexcitation, Phys. Chem. Chem. Phys., 2021, 23, 22640–22646, 10.1039/D1CP03437E.
- R. Salzwedel, A. Knorr, D. Hoeing, H. Lange and M. Selig, Theory of radial oscillations in metal nanoparticles driven by optically induced electron density gradients, J. Chem. Phys., 2023, 158(6), 064107, DOI:10.1063/5.0139629.
- M. Chandra, S. S. Indi and P. K. Das, Depolarized Hyper-Rayleigh Scattering from Copper Nanoparticles, J. Phys. Chem. C, 2007, 111(28), 10652–10656, DOI:10.1021/jp071847l.
- K. Thakur and B. Kandasubramanian, Graphene and Graphene Oxide-Based Composites for Removal of Organic Pollutants: A Review, J. Chem. Eng. Data, 2019, 64(3), 833–867, DOI:10.1021/acs.jced.8b01057.
- J. M. Garcia, L. F. Heald, R. E. Shaffer and S. G. Sayres, Oscillation in Excited State Lifetimes with Size of Sub-nanometer Neutral (TiO2)n Clusters Observed with Ultrafast Pump–Probe Spectroscopy, J. Phys. Chem. Lett., 2021, 12(16), 4098–4103, DOI:10.1021/acs.jpclett.1c00840.
- M. Hosni, Y. Kusumawati, S. Farhat, N. Jouini and Th. Pauporté, Effects of Oxide Nanoparticle Size and Shape on Electronic Structure, Charge Transport, and Recombination in Dye-Sensitized Solar Cell Photoelectrodes, J. Phys. Chem. C, 2014, 118(30), 16791–16798, DOI:10.1021/jp412772b.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582, DOI:10.1016/j.surfrep.2008.10.001.
- Z. A. Piskulich, O. O. Mesele and W. H. Thompson, Activation Energies and Beyond, J. Phys. Chem. A, 2019, 123(33), 7185–7194, DOI:10.1021/acs.jpca.9b03967.
- N. Elangovan and S. Sowrirajan, Synthesis, single crystal (XRD), Hirshfeld surface analysis, computational study (DFT) and molecular docking studies of (E)-4-((2-hydroxy-3,5-diiodobenzylidene)amino)-N-(pyrimidine)-2-yl) benzenesulfonamide, Heliyon, 2021, 7(8), e07724, DOI:10.1016/j.heliyon.2021.e07724.
- P. Biehl, M. von der Lühe, S. Dutz and F. H. Schacher, Synthesis, Characterization, and Applications of Magnetic Nanoparticles Featuring Polyzwitterionic Coatings, Polymers, 2018, 10, 91, DOI:10.3390/polym10010091.
- J. Sun, S. Zhou, P. Hou, Y. Yang, J. Weng, X. Li and M. Li, Synthesis and characterization of biocompatible Fe3O4 nanoparticles, J. Biomed. Mater. Res., 2006, 80A, 333–341, DOI:10.1002/jbm.a.30909.
- S. K. Sahoo, et al., Photocatalytic Water Splitting Reaction Catalyzed by Ion-Exchanged Salts of Potassium Poly(heptazine imide) 2D Materials, J. Phys. Chem. C, 2021, 125(25), 13749–13758, DOI:10.1021/acs.jpcc.1c03947.
- C. T. Campbell, The Degree of Rate Control: A Powerful Tool for Catalysis Research, ACS Catalysis, 2017, 7(4), 2770–2779, DOI:10.1021/acscatal.7b00115.
- A. Hemmati, H. Emadi and S. Reza Nabavi, Green Synthesis of Sulfur- and Nitrogen-Doped Carbon Quantum Dots for Determination of L-DOPA Using Fluorescence Spectroscopy and a Smartphone-Based Fluorimeter, ACS Omega, 2023, 8(23), 20987–20999, DOI:10.1021/acsomega.3c01795.
- T. Naghdi, S. Faham, T. Mahmoudi, N. Pourreza, R. Ghavami and H. Golmohammadi, Phytochemicals toward Green (Bio)sensing, ACS Sens., 2020, 5(12), 3770–3805, DOI:10.1021/acssensors.0c02101.
- D. Makawana and M. Singh, A new dendrimer series: synthesis, free radical scavenging, and protein binding studies, RSC Adv., 2020, 10, 21914–21932, 10.1039/D0RA04102E.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article and
Man Singh
and
Man Singh *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 5
7, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 8
3, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 aq-ACN media, the magnetic nanoparticles (MNPs) developed at normal temperature and pressure (NTP).
2 aq-ACN media, the magnetic nanoparticles (MNPs) developed at normal temperature and pressure (NTP).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq ACN induced splitting, forming MNPs along with valuable products on recycling.
7 aq ACN induced splitting, forming MNPs along with valuable products on recycling.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N in NIQ and SIQPNO2. Moreover, at 1745–1715 cm−1 infers C
N in NIQ and SIQPNO2. Moreover, at 1745–1715 cm−1 infers C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–Cl while at 850–550 cm−1 the ketonic C
O and C–Cl while at 850–550 cm−1 the ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Cl in IQPs. The strong band at 800–850 cm−1 appeared for a 1,4-disubstituted aromatic ring for SIQPNO2.
O and Cl in IQPs. The strong band at 800–850 cm−1 appeared for a 1,4-disubstituted aromatic ring for SIQPNO2.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds has sharpened a stretching to photocatalytically reduce MB in 63 unlike 95 min with SIQPI alone (Fig. 7). The SIQPII + NIQ has also weakened stretching at 2200–400 cm−1 for CN intensifying the fingerprint region. SIQPII + NIQ with 3500–2000 cm−1 as IR active has reduced MB in 35, unlike 43 min with SIQPII alone. SIQPIII + NIQ from 1500 to 4000 cm−1 strengthened the unspent resonating sites generating a maximum stretching as IR active with a shorter PCR time than SIQPIII alone. Manifold intensities inhibited by SIQPIII + NIQ photocatalytically reduced MB in 47 min, unlike 54 with SIQPIII alone.18 The SIQPs under SL have reduced dyes in our earlier study18 and are extended now with IQPs. The wavefunctions of IQPs, SIQPs, and SIQPs + NIQ with EWG and ERG using solvation energies as a communicating FRET accessed to the dyes. The 3
C bonds has sharpened a stretching to photocatalytically reduce MB in 63 unlike 95 min with SIQPI alone (Fig. 7). The SIQPII + NIQ has also weakened stretching at 2200–400 cm−1 for CN intensifying the fingerprint region. SIQPII + NIQ with 3500–2000 cm−1 as IR active has reduced MB in 35, unlike 43 min with SIQPII alone. SIQPIII + NIQ from 1500 to 4000 cm−1 strengthened the unspent resonating sites generating a maximum stretching as IR active with a shorter PCR time than SIQPIII alone. Manifold intensities inhibited by SIQPIII + NIQ photocatalytically reduced MB in 47 min, unlike 54 with SIQPIII alone.18 The SIQPs under SL have reduced dyes in our earlier study18 and are extended now with IQPs. The wavefunctions of IQPs, SIQPs, and SIQPs + NIQ with EWG and ERG using solvation energies as a communicating FRET accessed to the dyes. The 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq-ACN with π → 2π* and n → n* have quantized a photocatalytic chemistry to lay down a 2nd-generation photocatalysis. The IQPs and SIQPs with NIQ have reduced dyes in a shorter time due to single-valued wavefunctions (Fig. 7, Chart 1(a and b)). SIQPNO2 alone with enormous π → π* and n → n* has reduced MB in 2.16 times shorter period than with LGT. The 4f#e electrons of lanthanides of LGT seem to interrupt the hole generation20 (Fig. 8). The hv excites enormous π and lone pair of electrons (LPE) of SIQPNO2 that photocatalytically reduce an MB philicphobic medium in 190 min, and such studies have not been reported previously. NIQ has reduced the dyes in a shorter time with SIQPI, II, III, and SIQPNO2 than SIQPI, II, and III alone18 due to the same phase ROCs.21 IQ with π conjugation and LPE of O and N atoms have produced holes in the same phase to photocatalytically reduce in 2 min. NIQ with NO2 and CIQ with Cl have enhanced PCR time unlike IQ. IQPs with LGT have reduced dyes in longer time while the SIQPs with NIQ and LGT in shorter and longer PCR times respectively. The LaGT, CeGT, TbGT, and HoGT compared to Ln2S3, Ce2S3, Tb2S3, and Ho2S3 LSNR respectively have shortened a dye PCR time. The π → π* and 4fne → 4fne*of LGT could not compete the π → π* of IQPs, as alone LGT has reduced MB in 64 min than 2 with alone IQ, LGT with IQPs in 10–55 min (ψπ→π − ψ4fne→4fne*). IQPs + LGT with π → π* and 4fne → 4fne* have shortened the MB PCR time than our reported studies. LGT with infinite π conjugation aligns at a periphery of IQPs intensifying π conjugation via FRET for MB PCR. The π → π* and 4fne → 4fne*of LGT align with π → π* and n → n* of IQPs reducing dyes in a shorter time. The IQPs with LGT have generated IQPs + LGT. IQPs alone after MB reduction remained monodispersed resulting in a transparent nanoemulsion as thin films suitable to be used in paint industries and antireflecting coating agents.22
7 aq-ACN with π → 2π* and n → n* have quantized a photocatalytic chemistry to lay down a 2nd-generation photocatalysis. The IQPs and SIQPs with NIQ have reduced dyes in a shorter time due to single-valued wavefunctions (Fig. 7, Chart 1(a and b)). SIQPNO2 alone with enormous π → π* and n → n* has reduced MB in 2.16 times shorter period than with LGT. The 4f#e electrons of lanthanides of LGT seem to interrupt the hole generation20 (Fig. 8). The hv excites enormous π and lone pair of electrons (LPE) of SIQPNO2 that photocatalytically reduce an MB philicphobic medium in 190 min, and such studies have not been reported previously. NIQ has reduced the dyes in a shorter time with SIQPI, II, III, and SIQPNO2 than SIQPI, II, and III alone18 due to the same phase ROCs.21 IQ with π conjugation and LPE of O and N atoms have produced holes in the same phase to photocatalytically reduce in 2 min. NIQ with NO2 and CIQ with Cl have enhanced PCR time unlike IQ. IQPs with LGT have reduced dyes in longer time while the SIQPs with NIQ and LGT in shorter and longer PCR times respectively. The LaGT, CeGT, TbGT, and HoGT compared to Ln2S3, Ce2S3, Tb2S3, and Ho2S3 LSNR respectively have shortened a dye PCR time. The π → π* and 4fne → 4fne*of LGT could not compete the π → π* of IQPs, as alone LGT has reduced MB in 64 min than 2 with alone IQ, LGT with IQPs in 10–55 min (ψπ→π − ψ4fne→4fne*). IQPs + LGT with π → π* and 4fne → 4fne* have shortened the MB PCR time than our reported studies. LGT with infinite π conjugation aligns at a periphery of IQPs intensifying π conjugation via FRET for MB PCR. The π → π* and 4fne → 4fne*of LGT align with π → π* and n → n* of IQPs reducing dyes in a shorter time. The IQPs with LGT have generated IQPs + LGT. IQPs alone after MB reduction remained monodispersed resulting in a transparent nanoemulsion as thin films suitable to be used in paint industries and antireflecting coating agents.22
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) of pyz with SIQPs along with its NO2 (Fig. 9). Alone NIQ with >O PCR in 12 min but >O FG with SIQPs via CCA reducing dye in 95 min. The NIQ with SIQPs has generated DOS by minimizing the interference of mutual DOS of pyz and free phenyl (FP) to photocatalytically reduce dyes expeditiously. SIQPs + NIQ with a higher surface energy overcome QEB to PCR pollutants in a shorter time (Fig. 9).24
N) of pyz with SIQPs along with its NO2 (Fig. 9). Alone NIQ with >O PCR in 12 min but >O FG with SIQPs via CCA reducing dye in 95 min. The NIQ with SIQPs has generated DOS by minimizing the interference of mutual DOS of pyz and free phenyl (FP) to photocatalytically reduce dyes expeditiously. SIQPs + NIQ with a higher surface energy overcome QEB to PCR pollutants in a shorter time (Fig. 9).24
 with IQ while with NIQ and CIQ, partial orthogonal activity is depicted as eqn (1)–(3).
with IQ while with NIQ and CIQ, partial orthogonal activity is depicted as eqn (1)–(3).
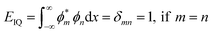

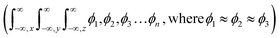 photocatalytically reduced MB in 2 min. Born Oppenheimer Approximation could be applied with balanced tentropic vibrational energies multiplied without wasting eigenenergies on a least localised electronic reorientation. Their eigenenergies could have produced the holes that differ from NIQ and CIQ. As
photocatalytically reduced MB in 2 min. Born Oppenheimer Approximation could be applied with balanced tentropic vibrational energies multiplied without wasting eigenenergies on a least localised electronic reorientation. Their eigenenergies could have produced the holes that differ from NIQ and CIQ. As 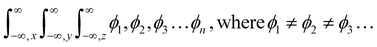 reorientation of NIQ and CIQ with different DOS does not lie in the same phase. The eigenenergy coefficients α and β from HOMO → LUMO for NIQ and CIQ having
reorientation of NIQ and CIQ with different DOS does not lie in the same phase. The eigenenergy coefficients α and β from HOMO → LUMO for NIQ and CIQ having  and
and  differ. Replacing an IQ photocatalyst with NIQ and CIQ has changed a PCR time for MB (Fig. S9 and S10†). The spontaneity for generating the hv with the IQ plane favours PCR in least time unlike with NIQ and CIQ. IQ reduced MB, MO, BBR, and RhB in 2, 5, 7, and 12 min respectively on a mutual reorientation of hv receiving electrophilic and nucleophilic sites supporting the least absorption (Fig. 12). The electrophilic and nucleophilic sites of photocatalate and photocatalyst respectively bring them together with integrated eigenenergies at a closer distance (r), where the holes reached to reduce a dye (eqn (4)).
differ. Replacing an IQ photocatalyst with NIQ and CIQ has changed a PCR time for MB (Fig. S9 and S10†). The spontaneity for generating the hv with the IQ plane favours PCR in least time unlike with NIQ and CIQ. IQ reduced MB, MO, BBR, and RhB in 2, 5, 7, and 12 min respectively on a mutual reorientation of hv receiving electrophilic and nucleophilic sites supporting the least absorption (Fig. 12). The electrophilic and nucleophilic sites of photocatalate and photocatalyst respectively bring them together with integrated eigenenergies at a closer distance (r), where the holes reached to reduce a dye (eqn (4)).
 eigenenergies with a single wavefunction (ψIQ = 0.71(ϕ4n* + ϕ8π*)) photocatalytically reduce the MB in less time than MO, BBR, and RhB (Fig. 12 and eqn (5)–(8)) by synchronising as (eqn (5)) follows:
eigenenergies with a single wavefunction (ψIQ = 0.71(ϕ4n* + ϕ8π*)) photocatalytically reduce the MB in less time than MO, BBR, and RhB (Fig. 12 and eqn (5)–(8)) by synchronising as (eqn (5)) follows:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN), hv, and FRET. The PCR of MB with IQ releases Cl2, resulting in a single-phase solution. The F = C − P works as receiving hv and emittance is constant during PCR with C = 4 and P = 1, resulting in F = 3 photocatalyst, hv, and FRET variables. The F realigns assisting variables (AV) hv and FRET on receiving hv continuously for PCR. The IQ remained kinetically and thermodynamically stable with F = 1 due to minimum dS in quadrupolar aq
ACN), hv, and FRET. The PCR of MB with IQ releases Cl2, resulting in a single-phase solution. The F = C − P works as receiving hv and emittance is constant during PCR with C = 4 and P = 1, resulting in F = 3 photocatalyst, hv, and FRET variables. The F realigns assisting variables (AV) hv and FRET on receiving hv continuously for PCR. The IQ remained kinetically and thermodynamically stable with F = 1 due to minimum dS in quadrupolar aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN as per Prigogine. The dG = f(T,P,aq,ACN,photocatalyst (lyst),photocatalate (late)) has initiated as in eqn (10) and (11).
ACN as per Prigogine. The dG = f(T,P,aq,ACN,photocatalyst (lyst),photocatalate (late)) has initiated as in eqn (10) and (11).
 and
and 
 where n moles of photocatalyst are constant with dμi = 0 for generating holes without structural changes.31–33 The dG = 0,
where n moles of photocatalyst are constant with dμi = 0 for generating holes without structural changes.31–33 The dG = 0,  as dnlyst = 0 or μ = 0 unlike
as dnlyst = 0 or μ = 0 unlike  with μ ≠ 0, as variables for the PCR, as eqn (13).
with μ ≠ 0, as variables for the PCR, as eqn (13).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C conjugated states of ((CH3)2N
C conjugated states of ((CH3)2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) might have counterbalanced the DOS of N+ (Fig. 12). The rotational and vibrational motions might have been equipartitioned even with X− anion like Cl−, and the N+ might have been stable. The MO with IQ assisted by solvent split via CQMA reorienting its eigenenergies in one plane and MO with aq-ACN dipoles has optimized a quinonoid state. The same experiments were conducted by mixing CO2 with IQPs for MB reduction and analysed by UV-Vis (Fig. 13). The IQ, NIQ, and CIQ have photocatalytically reduced MB in 120 s, 12 and 32 while with CO2 in ∼90 s, 8 and 24 min respectively (Fig. 13 and 14). CO2 with LPE and π bonds on getting realigned with IQ have robustly generated holes reducing MB and QHIn in ∼90 s and 10 min, acting as a cophotocatalyst. The CO2 and IQ mutually align energies as eqn (13a) to photocatalytically reduce MB:
C) might have counterbalanced the DOS of N+ (Fig. 12). The rotational and vibrational motions might have been equipartitioned even with X− anion like Cl−, and the N+ might have been stable. The MO with IQ assisted by solvent split via CQMA reorienting its eigenenergies in one plane and MO with aq-ACN dipoles has optimized a quinonoid state. The same experiments were conducted by mixing CO2 with IQPs for MB reduction and analysed by UV-Vis (Fig. 13). The IQ, NIQ, and CIQ have photocatalytically reduced MB in 120 s, 12 and 32 while with CO2 in ∼90 s, 8 and 24 min respectively (Fig. 13 and 14). CO2 with LPE and π bonds on getting realigned with IQ have robustly generated holes reducing MB and QHIn in ∼90 s and 10 min, acting as a cophotocatalyst. The CO2 and IQ mutually align energies as eqn (13a) to photocatalytically reduce MB:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq-ACN without CO2 have produced densities (ρ) of 0.784406 (9.64), 0.794179 (6.72), and 0.802968 g cm−3 (pH = 5.84) respectively. While IQPs with CO2 have produced the ρ 0.996047 (6.95), 0.790151 (5.68), and 0.797624 g cm−3 (pH = 5.35) respectively (Chart 2). CO2 has increased the density with IQ as it split the water and activate the CO2 to form carbonic acid lowering pH from 9.64 to 6.95 (Fig. 15). The densities of alone water (0.997), ACN (0.786), and 3
7 aq-ACN without CO2 have produced densities (ρ) of 0.784406 (9.64), 0.794179 (6.72), and 0.802968 g cm−3 (pH = 5.84) respectively. While IQPs with CO2 have produced the ρ 0.996047 (6.95), 0.790151 (5.68), and 0.797624 g cm−3 (pH = 5.35) respectively (Chart 2). CO2 has increased the density with IQ as it split the water and activate the CO2 to form carbonic acid lowering pH from 9.64 to 6.95 (Fig. 15). The densities of alone water (0.997), ACN (0.786), and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq-ACN (0.568 g cm−3) at 25 °C are also lower than the IQ mixed with CO2 that have credited to the stronger dipolar–dipolar interactions. This could be helpful for monodispersion and hole generation for the PCR of MB and QHIn in ∼90 s, 17 min than without CO2 in ∼120 s, 24 min respectively (Fig. 16). The sound velocity 1507.01, 1289.83, and 1298.85 m s−1 for IQ, NIQ, and CIQ than 1510.35, 1283.44, and 1291.43 m s−1 with CO2 respectively supported the dye PCR mechanism.
7 aq-ACN (0.568 g cm−3) at 25 °C are also lower than the IQ mixed with CO2 that have credited to the stronger dipolar–dipolar interactions. This could be helpful for monodispersion and hole generation for the PCR of MB and QHIn in ∼90 s, 17 min than without CO2 in ∼120 s, 24 min respectively (Fig. 16). The sound velocity 1507.01, 1289.83, and 1298.85 m s−1 for IQ, NIQ, and CIQ than 1510.35, 1283.44, and 1291.43 m s−1 with CO2 respectively supported the dye PCR mechanism.



 . The % MB PCR by CIQ + CO2 in 24 min was calculated using eqn (17):
. The % MB PCR by CIQ + CO2 in 24 min was calculated using eqn (17):
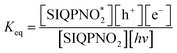
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 1
6 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of time respectively infers intramolecular electronic transitions (Fig. S11†).18 NO2 of SIQPNO2 inhibits a PCR rate, unlike IQ exhibits the least If and shortest PCR time (Table 2). The Φ value for IQ, NIQ, CIQ, and SIQPNO2 calculated at λexc 295, 285, 295 & 285 nm has determined E = 6.734 × 10−19 J (IQ & CIQ) and 6.970 × 10−19 J (NIQ & SIQPNO2) respectively. The number of hν absorbed (na) was calculated using eqn (19) as follows:
2 ratio of time respectively infers intramolecular electronic transitions (Fig. S11†).18 NO2 of SIQPNO2 inhibits a PCR rate, unlike IQ exhibits the least If and shortest PCR time (Table 2). The Φ value for IQ, NIQ, CIQ, and SIQPNO2 calculated at λexc 295, 285, 295 & 285 nm has determined E = 6.734 × 10−19 J (IQ & CIQ) and 6.970 × 10−19 J (NIQ & SIQPNO2) respectively. The number of hν absorbed (na) was calculated using eqn (19) as follows: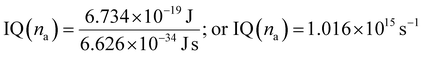


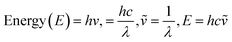

 via symmetric ψi(x,t) oscillations at distance x in time t as per Heisenberg.37–39 The absence of D & G bands infers ΔG = ΔH −TΔS, at ΔG = 0, ΔH = TΔS, or
via symmetric ψi(x,t) oscillations at distance x in time t as per Heisenberg.37–39 The absence of D & G bands infers ΔG = ΔH −TΔS, at ΔG = 0, ΔH = TΔS, or 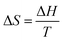 and
and 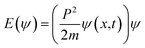 via
via  . The
. The  could twist a bond with ΔH on oscillation. The IQ as a harmonic oscillator minimized undesired collisions reducing MB in ∼2 min with uniform SR. The partially equilibrated two centers of NIQ respond differently to hν rather than generating holes to photocatalytically reduce dyes. EWG influences an electron cloud without deformation, and on passing a laser to IQPs, few electrons are easily excited while forbidding the other transitions due to an integrated framework. On acquiring a laser, a new energy-storing alignment within IQPs acts as stocks for reducing the dyes in a shorter PCR time. Moreover, a laser with SIQPNO2 develops holes, detaining energy as antistokes photocatalytically reduce MB in 190 min. The CIQ generates the weaker antistokes among IQPs due to energy equipartitioning with weaker electronic activities. The SIQPNO2 with larger Fermi sphere could not crossover QEB as electrons remain in the VB, unlike alone IQPs. SIQPNO2 has increased the VB-to-CB volume, elongating the PCR time as the electrons are unable to generate atomic oscillations, unlike NIQ or CIQ, which show vibrant antistokes. The CIQ, IQ, and NIQ are vibrant, moderate, and weakest antistokes with minimum shifts respectively (Table 3). With IQ, the SR increases linearly along IR, its higher values reach almost all the electrons to exponentially oscillate in single phase. On lowering an energy of a source, the Raman active stabilized dipoles reduced a dye. Almost all uniform structures with single and double bonds including N with equal charge develop holes at the same rate. The IQ uniformly oscillates the terminal rings (ψ1 = ψ1 = ψ1 = ψ1 = ψ1) with a linear slope for MB PCR. NIQ with NO2 on passing hνs reduces MB in 12 min. However, CIQ with a weaker ERG Cl photocatalytically reduced MB in 32 min due to non-equilibrate electronic states by engaging hν in different IR (Fig. 18 and 19).
could twist a bond with ΔH on oscillation. The IQ as a harmonic oscillator minimized undesired collisions reducing MB in ∼2 min with uniform SR. The partially equilibrated two centers of NIQ respond differently to hν rather than generating holes to photocatalytically reduce dyes. EWG influences an electron cloud without deformation, and on passing a laser to IQPs, few electrons are easily excited while forbidding the other transitions due to an integrated framework. On acquiring a laser, a new energy-storing alignment within IQPs acts as stocks for reducing the dyes in a shorter PCR time. Moreover, a laser with SIQPNO2 develops holes, detaining energy as antistokes photocatalytically reduce MB in 190 min. The CIQ generates the weaker antistokes among IQPs due to energy equipartitioning with weaker electronic activities. The SIQPNO2 with larger Fermi sphere could not crossover QEB as electrons remain in the VB, unlike alone IQPs. SIQPNO2 has increased the VB-to-CB volume, elongating the PCR time as the electrons are unable to generate atomic oscillations, unlike NIQ or CIQ, which show vibrant antistokes. The CIQ, IQ, and NIQ are vibrant, moderate, and weakest antistokes with minimum shifts respectively (Table 3). With IQ, the SR increases linearly along IR, its higher values reach almost all the electrons to exponentially oscillate in single phase. On lowering an energy of a source, the Raman active stabilized dipoles reduced a dye. Almost all uniform structures with single and double bonds including N with equal charge develop holes at the same rate. The IQ uniformly oscillates the terminal rings (ψ1 = ψ1 = ψ1 = ψ1 = ψ1) with a linear slope for MB PCR. NIQ with NO2 on passing hνs reduces MB in 12 min. However, CIQ with a weaker ERG Cl photocatalytically reduced MB in 32 min due to non-equilibrate electronic states by engaging hν in different IR (Fig. 18 and 19).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 836
836![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604
604![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 018
018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042
042![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NO2 without mutually resonating energies separately (Fig. 20). The 2N of NIQ along delocalization could have generated the 2nd set of deformation for D and G bands. Raman inactive SIQPI18 as a weak semiconductor could not align holes but with NIQ disrupted the electronic configuration (Fig. S26(a)†).18 The DOS of SIQPI + NIQ is more active than synchronized SIQPI alone. The laser could not affect SIQPI unlike splitting into D and G bands with NIQ at 1348 cm−1 with 6000 a.u. and 1581 cm−1 with 10
O and NO2 without mutually resonating energies separately (Fig. 20). The 2N of NIQ along delocalization could have generated the 2nd set of deformation for D and G bands. Raman inactive SIQPI18 as a weak semiconductor could not align holes but with NIQ disrupted the electronic configuration (Fig. S26(a)†).18 The DOS of SIQPI + NIQ is more active than synchronized SIQPI alone. The laser could not affect SIQPI unlike splitting into D and G bands with NIQ at 1348 cm−1 with 6000 a.u. and 1581 cm−1 with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 a.u. intermixing (Fig. 20). The bands are broadened by NO2 at NIQ and NO2 at FP on symmetric DOS as a new state of oscillations. The NO2 of NIQ has partially attracted the electron clouds of NO2 of FP and pyz. The electron-withdrawing activities of NO2 of FP and NO2 of NIQ sharpened the bands at 1550 and 1650 cm−1 (Fig. 20). The sharper G band infers a bonding of NO2 at FP as SIQPI is missing NO2 at FP as Raman inactive and IR active (Fig S26(a)†). The 2NO2 of SIQPNO2 as Raman active and FT-IR inactive broadened a band at 1900 cm−1 and sharpened at 2000 bifurcating at 2100 cm−1 of NIQ and pyz across CCA. The NIQ with SIQPNO2 might have sharpened IR. SIQPIII alone18 unlike with SIQPIII + NIQ produces a straight line by mutually inducing the sharper oscillations lowering QEB. Raman spectra have a sharper split at ∼1300 cm−1 with a lower intensity due to terminal NO2 of NIQ. The OMe of SIQPIII could develop coulombic interactions with NO2 of NIQ on sharper Raman splits with high intensity compensating the stretching and sharp oscillations. Moreover, various electron-rich and deficient sites have weakened the G band while sharpened the D band for the PCR of MB in 47 min unlike 54 min with SIQPIII alone (Fig. 20, S26(a)† and Table 4).
000 a.u. intermixing (Fig. 20). The bands are broadened by NO2 at NIQ and NO2 at FP on symmetric DOS as a new state of oscillations. The NO2 of NIQ has partially attracted the electron clouds of NO2 of FP and pyz. The electron-withdrawing activities of NO2 of FP and NO2 of NIQ sharpened the bands at 1550 and 1650 cm−1 (Fig. 20). The sharper G band infers a bonding of NO2 at FP as SIQPI is missing NO2 at FP as Raman inactive and IR active (Fig S26(a)†). The 2NO2 of SIQPNO2 as Raman active and FT-IR inactive broadened a band at 1900 cm−1 and sharpened at 2000 bifurcating at 2100 cm−1 of NIQ and pyz across CCA. The NIQ with SIQPNO2 might have sharpened IR. SIQPIII alone18 unlike with SIQPIII + NIQ produces a straight line by mutually inducing the sharper oscillations lowering QEB. Raman spectra have a sharper split at ∼1300 cm−1 with a lower intensity due to terminal NO2 of NIQ. The OMe of SIQPIII could develop coulombic interactions with NO2 of NIQ on sharper Raman splits with high intensity compensating the stretching and sharp oscillations. Moreover, various electron-rich and deficient sites have weakened the G band while sharpened the D band for the PCR of MB in 47 min unlike 54 min with SIQPIII alone (Fig. 20, S26(a)† and Table 4).


 or kKT = hν for
or kKT = hν for  , where k is the Boltzmann distribution constant, KT is the Kelvin T constant, h is the Planck constant, and E is the energy. The ν value of weight loss at KT elucidates the thermal stability of the molecules as onset weight loss. IQ lost weight as a single sharper peak at 290 °C with a rate of weight loss 350 μg min−1 with ψ1 (Fig. 26). While the NIQ and CIQ both have developed by destructively lowering a Φ where the IQ has acted as an excellent photocatalyst. NIQ has weight loss rate 425 μg min−1 at ∼350 °C due to asymmetric oscillations caused by NO2. The NIQ slightly resists the electronic activities of NO2 disrupting an overall crystallinity by delocalization. The CIQ destabilizes a crystallinity with rate of weight loss 325 μg min−1 at ∼290 °C with different FRET and hybridized |ψ2| (Fig. 26 and S25†). Two energies equilibrated as a straight line of weight loss from 200 to 300 °C with slope = 0. However, a few endothermic sharp changes appeared at 120 °C, followed by exothermic changes at 125 °C, attributed to the presence of benzene ring. It is accompanied by a slightly endothermic shoulder at 120 °C, due to O matching with the N cavity. After 300–800 °C, the closely placed oscillating peak with wavefunctions of each unit withdraw the intense changes. NIQ with SIQPI has transformed sharply into a multi-peak curve on sharing the electrons of NIQ. The molecule broadens a peak due to fused electron clouds. The CCA could have synchronised the SIQPI interactions with NIQ on broadening the peaks due to weaker FRET. The resulting electron clouds are differently reoriented as 1st order instead of 2nd for SIQPI with NIQ. The SIQPI with NIQ as 1st order TGA at a higher temperature on mutual activation of electron clouds at 20 μg min−1 at ∼300 °C than 400 μg min−1 at ∼230 °C with SIQPI alone (Fig S25†). Therefore, NIQ has enhanced the thermal stability of SIQPI by shortening a PCR time due to robust mutual resonating activity. NIQ with SIQPs expeditiously developed a FRET due to moderate electrostatic interactions lowering the weight loss without transition except for a single-crystal lattice as a novel eutectic mechanism. The SIQPs with anodic NIQ have shortened MB as cathodic PCR by 1.5, 1.2, and 1.1 times compared to SIQPs alone (Table 6). NIQ with ERG/OMe of SIQPIII has disintegrated the electron cloud of the resulting framework.
, where k is the Boltzmann distribution constant, KT is the Kelvin T constant, h is the Planck constant, and E is the energy. The ν value of weight loss at KT elucidates the thermal stability of the molecules as onset weight loss. IQ lost weight as a single sharper peak at 290 °C with a rate of weight loss 350 μg min−1 with ψ1 (Fig. 26). While the NIQ and CIQ both have developed by destructively lowering a Φ where the IQ has acted as an excellent photocatalyst. NIQ has weight loss rate 425 μg min−1 at ∼350 °C due to asymmetric oscillations caused by NO2. The NIQ slightly resists the electronic activities of NO2 disrupting an overall crystallinity by delocalization. The CIQ destabilizes a crystallinity with rate of weight loss 325 μg min−1 at ∼290 °C with different FRET and hybridized |ψ2| (Fig. 26 and S25†). Two energies equilibrated as a straight line of weight loss from 200 to 300 °C with slope = 0. However, a few endothermic sharp changes appeared at 120 °C, followed by exothermic changes at 125 °C, attributed to the presence of benzene ring. It is accompanied by a slightly endothermic shoulder at 120 °C, due to O matching with the N cavity. After 300–800 °C, the closely placed oscillating peak with wavefunctions of each unit withdraw the intense changes. NIQ with SIQPI has transformed sharply into a multi-peak curve on sharing the electrons of NIQ. The molecule broadens a peak due to fused electron clouds. The CCA could have synchronised the SIQPI interactions with NIQ on broadening the peaks due to weaker FRET. The resulting electron clouds are differently reoriented as 1st order instead of 2nd for SIQPI with NIQ. The SIQPI with NIQ as 1st order TGA at a higher temperature on mutual activation of electron clouds at 20 μg min−1 at ∼300 °C than 400 μg min−1 at ∼230 °C with SIQPI alone (Fig S25†). Therefore, NIQ has enhanced the thermal stability of SIQPI by shortening a PCR time due to robust mutual resonating activity. NIQ with SIQPs expeditiously developed a FRET due to moderate electrostatic interactions lowering the weight loss without transition except for a single-crystal lattice as a novel eutectic mechanism. The SIQPs with anodic NIQ have shortened MB as cathodic PCR by 1.5, 1.2, and 1.1 times compared to SIQPs alone (Table 6). NIQ with ERG/OMe of SIQPIII has disintegrated the electron cloud of the resulting framework.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aq
1 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN has rusted out (Fig. 29 and 30).
ACN has rusted out (Fig. 29 and 30).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5
3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3
5, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and 2
7, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 of aq
8 of aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN initial, after 5 days, and after a week.
ACN initial, after 5 days, and after a week.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN, analysed by FT-IR spectroscopy, TGA, XRD, UV-Vis spectroscopy, and EDX (Fig. S26(b) and S27–S30†). Moreover, Fe with IQ has formed MNPs in 5
ACN, analysed by FT-IR spectroscopy, TGA, XRD, UV-Vis spectroscopy, and EDX (Fig. S26(b) and S27–S30†). Moreover, Fe with IQ has formed MNPs in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 aq
5 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN characterized by XRD and SEM (Fig. 32–34).
ACN characterized by XRD and SEM (Fig. 32–34).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 of aq
5 of aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN. The IQ catalyses the Fe scrap to generate the eigenenergies for developing MNPs without using salts as their removal complicates the process. Moreover, it has simplified and advanced the synthesis of MNPs by using renewable resources (SL) not reported yet. The XRD planes shown in Fig. 35 confirm the crystalline MNP formation with diffraction peaks at the (220), (311), (400), (511), and (440) lattice planes. The characteristic peak 311 at 2θ = 37° confirm a magnetite phase within the MNPs matching with the standard XRD data of magnetite48 (Fig. 35).
ACN. The IQ catalyses the Fe scrap to generate the eigenenergies for developing MNPs without using salts as their removal complicates the process. Moreover, it has simplified and advanced the synthesis of MNPs by using renewable resources (SL) not reported yet. The XRD planes shown in Fig. 35 confirm the crystalline MNP formation with diffraction peaks at the (220), (311), (400), (511), and (440) lattice planes. The characteristic peak 311 at 2θ = 37° confirm a magnetite phase within the MNPs matching with the standard XRD data of magnetite48 (Fig. 35).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN under SL. The crystalline water was engaged by ACN via H dipolar interactions that cause the release of crystalline water from FeSO4, whereas hydrolysis without IQ takes time; however, IQ has reduced the Fe and formed metal hydroxide with release of H2 gas. However, CuSO4·5H2O with IQ took 6 h to decolourise blue copper to colourless and has formed homogeneous thin nano films at the glass vial walls under SL.
ACN under SL. The crystalline water was engaged by ACN via H dipolar interactions that cause the release of crystalline water from FeSO4, whereas hydrolysis without IQ takes time; however, IQ has reduced the Fe and formed metal hydroxide with release of H2 gas. However, CuSO4·5H2O with IQ took 6 h to decolourise blue copper to colourless and has formed homogeneous thin nano films at the glass vial walls under SL.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN) alone, FeSO4, IQ, and IQ + FeSO4 with MB drop strain (Fig. 37). IQ + FeSO4 effectively reduced MB as the IQ and Fe have a strong interaction that has photocatalyzed MB with the highest rate as compared to solvent alone, FeSO4 or IQ alone. This process can be applied for various metal extraction processes with IQ. Hence, IQ with its active configuration has undergone a robust transition that has split water (step 2) to oxygen and –OH.49 The Fe with IQ has further catalysed a process as Fe → Fe2+ + 2e− that reduces O2 + 2e− → O2− and O2− transfers its electron to water received from HO− (step 3) to generate the holes by IQ on receiving the hν.
ACN) alone, FeSO4, IQ, and IQ + FeSO4 with MB drop strain (Fig. 37). IQ + FeSO4 effectively reduced MB as the IQ and Fe have a strong interaction that has photocatalyzed MB with the highest rate as compared to solvent alone, FeSO4 or IQ alone. This process can be applied for various metal extraction processes with IQ. Hence, IQ with its active configuration has undergone a robust transition that has split water (step 2) to oxygen and –OH.49 The Fe with IQ has further catalysed a process as Fe → Fe2+ + 2e− that reduces O2 + 2e− → O2− and O2− transfers its electron to water received from HO− (step 3) to generate the holes by IQ on receiving the hν.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN solvent study with FeSO4·7H2O (b) IQ in aq
ACN solvent study with FeSO4·7H2O (b) IQ in aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN with FeSO4, and (c) IQ in aq
ACN with FeSO4, and (c) IQ in aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN with CuSO4·5H2O formed nano thin film under SL.
ACN with CuSO4·5H2O formed nano thin film under SL.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 2
9, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 5
8, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 8
3, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of aq
2 of aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN to oxidize Fe to MNPs (Chart S1†). The dipolar ACN has trapped hv and detained SL partially forming metal oxides. Furthermore, aq-ACN has dissolved oxygen, further increasing with water %, leading to rusting. However, distilled and degassed water resulted in least rusting, depicting the oxidizing role of dissolved oxygen. ACN without dissolved oxygen acted as an anti-rusting agent in conjugation with IQ (Table S9†). The O2− may affect the dipoles of ACN to catalyse oxygen ionization. The Fe reacted with dissolved O2 where a subsequent interaction for rusting depends on increasing or decreasing the ratio of water with ACN. However, iodine with −0.54 V reduction potential could realign a delocalized IQ vis-à-vis Fe oxidation by substituting the presence of O2− with quantized activities. The Fe metal framework has more affinity to bind I2 than starch detecting the presence of Fe ions in IQ organometallic frameworks. It was found that 0.001 M IQ has degraded a 0.5 mL waste mobil oil with 1/50 ratio in 15 days with 3
ACN to oxidize Fe to MNPs (Chart S1†). The dipolar ACN has trapped hv and detained SL partially forming metal oxides. Furthermore, aq-ACN has dissolved oxygen, further increasing with water %, leading to rusting. However, distilled and degassed water resulted in least rusting, depicting the oxidizing role of dissolved oxygen. ACN without dissolved oxygen acted as an anti-rusting agent in conjugation with IQ (Table S9†). The O2− may affect the dipoles of ACN to catalyse oxygen ionization. The Fe reacted with dissolved O2 where a subsequent interaction for rusting depends on increasing or decreasing the ratio of water with ACN. However, iodine with −0.54 V reduction potential could realign a delocalized IQ vis-à-vis Fe oxidation by substituting the presence of O2− with quantized activities. The Fe metal framework has more affinity to bind I2 than starch detecting the presence of Fe ions in IQ organometallic frameworks. It was found that 0.001 M IQ has degraded a 0.5 mL waste mobil oil with 1/50 ratio in 15 days with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 aq
7 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN of 0 mJ m−2 interfacial energies (Fig. S34 and Table S9†).
ACN of 0 mJ m−2 interfacial energies (Fig. S34 and Table S9†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA eluting solvent has widened IQ dispersion and degraded mobil oil salting out quasi-static adhesion on TLC. The mobil oil has developed a velocity gradient in situ with different retention factor (Rf) values. The eluting solvent hexane
EA eluting solvent has widened IQ dispersion and degraded mobil oil salting out quasi-static adhesion on TLC. The mobil oil has developed a velocity gradient in situ with different retention factor (Rf) values. The eluting solvent hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA has comparatively covered a maximum Rf value (Fig. S35†). Moreover, IQ breaks a longer C–C alkyl chain of oil into an alkene of shorter carbon chain with H2 gas via a free radical disintegration mechanism. The IQ as reference upconversion nanomaterial and quantum mechanical sensor disintegrated the saturated hydrocarbons.51,52 IQ produced single-valued wavefunction of integrated π conjugated DOS as a quantum dot including antifungal activities by inhibiting fungal growth. The ACN with hydrophobicity has tuned interaction with host on increasing water concentration that might have blocked its reacting sites (Fig. S35†). The IQ could not interact and resulted in fungal activities. The explorative synergistic effects with other antifungal agents are being pursued in laboratory.53 Moreover, IQ has effectively detached a dye from textile dyed fabric and the experiments are pursued in various aqueous waste solvent systems (Fig. S36†).
EA has comparatively covered a maximum Rf value (Fig. S35†). Moreover, IQ breaks a longer C–C alkyl chain of oil into an alkene of shorter carbon chain with H2 gas via a free radical disintegration mechanism. The IQ as reference upconversion nanomaterial and quantum mechanical sensor disintegrated the saturated hydrocarbons.51,52 IQ produced single-valued wavefunction of integrated π conjugated DOS as a quantum dot including antifungal activities by inhibiting fungal growth. The ACN with hydrophobicity has tuned interaction with host on increasing water concentration that might have blocked its reacting sites (Fig. S35†). The IQ could not interact and resulted in fungal activities. The explorative synergistic effects with other antifungal agents are being pursued in laboratory.53 Moreover, IQ has effectively detached a dye from textile dyed fabric and the experiments are pursued in various aqueous waste solvent systems (Fig. S36†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 aq
5 aq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN, recovered HIn, and developed a single crystal. SIQPNO2 with two peripheral EWG NO2 photocatalytically reduced MB in 190 than 2–32 min with IQPs. SIQPNO2 alone has reduced MB in 2.16 times shorter period than with LaGT, acting as anti-PCR due to 4f#e. The rate of reaction, minimum energy, Ea, Φ, and photocatalytic activities were calculated from UV-Vis and fluorescence. Fe scrap in ACN had catalysed IQ to nucleate a single crystal as nanorods on recycling a waste metal. IQ with 100% ACN acted as an anti-rusting agent while with water has developed MNPs. The IQPs and SIQPNO2 have reduced MB, MO, BBR, and RhB in a shorter time than with LaGT, TbGT, HoGT, and CeGT, as antireduction agents. IQPs and SIQPNO2 could be extended to adsorb several toxic heavy metals from industrial effluents. These photocatalysts have reduced and physisorbed the industrial waste dyes, pollutants, and inks and were not photodegraded during the PCR process even after 5th run. Reducing dyes with IQPs and SIQPNO2 is a sustainable separation with high Φ and could be substituted with EWG (SO4) and ERG (NH2) constitutional units for PCR. The studies are being pursued with other dyes such as naphthalene derivatives, persistent pollutants, and industrial wastes (used industrial wastes such as ink and oil). IQPs may quench ozone preventing holes and ROS in a free radical scavenging process.
ACN, recovered HIn, and developed a single crystal. SIQPNO2 with two peripheral EWG NO2 photocatalytically reduced MB in 190 than 2–32 min with IQPs. SIQPNO2 alone has reduced MB in 2.16 times shorter period than with LaGT, acting as anti-PCR due to 4f#e. The rate of reaction, minimum energy, Ea, Φ, and photocatalytic activities were calculated from UV-Vis and fluorescence. Fe scrap in ACN had catalysed IQ to nucleate a single crystal as nanorods on recycling a waste metal. IQ with 100% ACN acted as an anti-rusting agent while with water has developed MNPs. The IQPs and SIQPNO2 have reduced MB, MO, BBR, and RhB in a shorter time than with LaGT, TbGT, HoGT, and CeGT, as antireduction agents. IQPs and SIQPNO2 could be extended to adsorb several toxic heavy metals from industrial effluents. These photocatalysts have reduced and physisorbed the industrial waste dyes, pollutants, and inks and were not photodegraded during the PCR process even after 5th run. Reducing dyes with IQPs and SIQPNO2 is a sustainable separation with high Φ and could be substituted with EWG (SO4) and ERG (NH2) constitutional units for PCR. The studies are being pursued with other dyes such as naphthalene derivatives, persistent pollutants, and industrial wastes (used industrial wastes such as ink and oil). IQPs may quench ozone preventing holes and ROS in a free radical scavenging process.








































