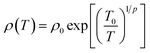Open Access Article
Open Access ArticleRetracted Article: Agile soft template array fabrication of one-dimensional (1D) polyaniline nanocomposite fibers for hydrogen storage
Ameena Parveena,
S. Manjunathab,
M. Madesh Kumarc and
Aashis S. Roy *de
*de
aDepartment of Physics, Government Degree College, Yadgir-585201, Karnataka, India
bDepartment of Physics, SSA Government First Grade College, Ballari-583101, India
cDepartment of Physics, REVA University, Bangalore-560064, Karnataka, India
dDepartment of Chemistry, S. S. Tegnoor Degree College, Gubbi Colony-585104, Karnataka, India. E-mail: aashisroy@gmail.com; Tel: +91-9108809031
eDepartment of Industrial Chemistry, Addis Ababa Science and Technology University, Addis Ababa 500013, Ethiopia
First published on 13th August 2024
Abstract
Polyaniline-Zn/V2O5 nanocomposites were prepared in the presence of toluene-4-sulfonic acid monohydrate as an anionic surfactant via an in situ oxidation polymerization method. The structural study of the nanocomposites was carried out using FTIR and XRD analysis, and their surface morphology was characterized through SEM analysis. The BET surface area of a 3 wt% nanocomposite was 386 m2 g−1, which is higher compared to that of PANI. The Kelvin two probe method was used to study DC conductivity, and it was found that the conductivity increases with increasing temperature. Among all the PANI nanocomposites, 3 wt% PANI-Zn/V2O5 shows a high conductivity of 13.8 S cm−1. Cyclic voltammetry results show the characteristic oxidation–reduction peaks at 0.93 V and 0.24 V for polyaniline and its nanocomposites, respectively. Hydrogen absorption studies were carried out using volumetric sorption measurement technique. At room temperature, it was found that the hydrogen adsorption capacity of polyaniline fibres is about 4.5 wt%, and its absorption capacity increases two-fold upon increasing the temperature up to 60 °C. Conversely, the 3 wt% PANI-Zn/V2O5 nanocomposite showed a high absorption capacity of 6.6 wt% compared with other compositions, which is may be due to the presence of nitrogen (N) molecules in polyaniline and its particular porous fiber architecture.
1. Introduction
The unique benefits of nanostructured polymer materials in green hydrogen energy conversion and hydrogen storage technologies have been significantly improved. Various structures of polyaniline can be prepared using different synthesis methods, such as in situ oxidative polymerization, electrochemical, and interfacial methods. B. Senthilkumar et al.1 reported on hydrogen adsorption on pure polyaniline of various structures. They determined that the hydrogen adsorption capacities of polyanilines with irregular granular, spherical and fibrous structures are 1.56 wt%, 3.3 wt% and 5.3 wt%, respectively. Hydrogen energy has become increasingly important in recent years owing to its potential to replace fossil fuels. Hydrogen is abundant in the universe and can be used to generate electricity and power vehicles. It can be generated from renewable sources, such as water streams and biomass, making it a clean and sustainable energy source.2 Hydrogen is nontoxic, environmentally friendly, efficient, cost-effective, and readily available, making it a possible alternative to fossil fuels. This makes hydrogen a much better option for reducing air pollution than fossil fuels, which release carbon dioxide and other pollutants into the atmosphere.3The storage medium needs to be reversible and able to hold its energy even after being charged with hydrogen several times. Making materials that can store and release gas at standard pressure and temperature is therefore essential. Metal hydrides and their complexes have been the material of choice for hydrogen storage for most of the past 10 years. Metal hydrides, particularly magnesium hydrides, can contain 6.5 H atoms per cm3 compared to liquid hydrogen's density of only 4.2 H atoms per cm3. This advantage makes it possible to maintain a safe vessel while attaining significant hydrogen gravimetric absorption. Most of the materials in this class are metal hydride complexes of lithium, aluminum, beryllium, magnesium, and sodium because of their low atomic weight. Hydrogen atoms dissociate on the metal surface, absorb into the substance, and then form chemical bonds within the lattice complex's interstitial sites. The “bottleneck” of this material is the activation energy required to break the interstitial bonds and liberate the hydrogen atoms from the material.4
The importance of lightweight metal oxide nanomaterials, which are directly related to a wide range of alternative technological solutions, is particularly crucial. There are difficult technical problems in the use of hydrogen as petroleum energy for automobiles via the development of efficient hydrogen storage systems.5–7 The adsorption of hydrogen on the vanadium pentoxide (010) surface is a selected reaction step in the nucleophilic oxidation of hydrocarbons for V2O5 cluster models, as per the ab initio Hartree–Fock method. Three different oxygen adsorption sites are considered: the vanadyl site, the bridging oxygen site between two surface vanadyl groups (V2+, V3+), and the bridging oxygen site between two surface vanadium centres. This suggests that for the vanadyl sites, hydrogen binds to the surface oxygen with a fairly strong bond, leading to the formation of stable surface groups that are bound to the surface vanadium atoms.8 The proton is localized and forms a hydride bond with vanadium. The electrons are also localized, creating V2+ and V3+ paramagnetic centres. The exchange interaction between these two paramagnetic species is the origin of chemosorption. In contrast, hydrogen approaching the bridging oxygen sites forms an even stronger surface O–H bond, but that particular surface oxygen becomes mobile so that the surface OH species can desorb with only a small or no activation barrier, leaving behind an oxygen vacancy.9,10 Storing H2 in nanostructured polymer fibers by means of a physisorption mechanism is an easy-to-control process that allows reversible H2 absorption and release, as well as quick adsorption/desorption kinetics.11–14
The most fascinating materials are conducting polymers because of their easy synthesis, environmental stability, low cost, and tunable electrical properties. Owing to these properties, they can be used in suitable technological applications, such as energy storage, battery, super capacitor and sensor applications.15–17 However, there are problems with the crystallinity of the polyaniline and mechanical robustness. To overcome these constraints, many researchers have recently focused on creating hybrid organic–inorganic nanocomposite systems consisting of nanostructured metal oxides and conducting polymer fibers.18–20 Here, we have prepared a polyaniline fiber shape at ice temperature with the ability to modify its electrical and mechanical properties to produce desired synergistic effects. PANI has also been demonstrated to be a well-known material with prospective applications in sensors, supercapacitors, corrosion, solar cells, and light-emitting diode devices.21 Recently, one-dimensional hybrid heterojunction-based nanocomposites have been investigated for use in hydrogen storage applications, owing to their high aspect ratios and high surface areas. Polyaniline can be prepared in one-dimensional fiber nanostructures using soft templates or template-less methods.22–24 The controlled modified polyaniline surface enhances the physical and chemical absorptions, owing to the rich structural modification chemistry, anisotropic geometry of the nanofiber structures, high surface area, smaller infiltration depth for marked molecules, and room-temperature operation.25
Therefore, we have made an attempt to prepare polyaniline nanofibers doped with porous Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V2O5 nanocomposites with different weight percentages. The prepared nanocomposites were characterized by Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD) pattern analysis, and their surface morphologies were studied by employing scanning electron microscopy (SEM). The DC conductivity was measured using the Kelvin two-probe method, and the activation energy was calculated. A CH Instruments 660D electrochemical work station was used for electrochemical impedance experiments, and a picoamp (pA) booster was used to identify the current and voltage. Volumetric isothermal analyses were carried out using Hy-Energy PCTPro 2000 sorption equipment.
V2O5 nanocomposites with different weight percentages. The prepared nanocomposites were characterized by Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD) pattern analysis, and their surface morphologies were studied by employing scanning electron microscopy (SEM). The DC conductivity was measured using the Kelvin two-probe method, and the activation energy was calculated. A CH Instruments 660D electrochemical work station was used for electrochemical impedance experiments, and a picoamp (pA) booster was used to identify the current and voltage. Volumetric isothermal analyses were carried out using Hy-Energy PCTPro 2000 sorption equipment.
2. Materials and methods
All chemicals were used in analytical grade for the preparation of Zn/V2O5 nanoparticles and polyaniline. The following products were purchased from Sigma-Aldrich Pvt Ltd, India and used exactly as is: toluene-4-sulfonic acid monohydrate (C7H8O3S·xH2O) with mol. wt. 172.20 and 98.6% purity; zinc acetate anhydride (Zn(CH3COO)2·(H2O)) with 99% purity; vanadium acetate (V(C2H3O2)5) with 99.99% purity; orthophosphoric acid (H3PO4) with 99.99% purity; hydrogen peroxide (H2O2) with 99.9% purity; hydrogen peroxide (H2O2) solution with mol. wt. 34.01, concentrated hydrochloric acid with mol. wt. 36.46; ammonium persulfate ((NH4)2S2O8) with 99.9% purity; and acetone with 99.8% purity.2.1 Synthesis of porous Zn/V2O5 nanoparticles
Using a round-bottom flask, 4.5 g of zinc acetate anhydride (Zn(CH3COO)2·(H2O)), 100 ml of ethanol, 100 ml of vanadium acetate (V(C2H3O2)5), and 200 ml of ethanol solvent were used to dissolve 4.5 g of sodium hydroxide (NaOH) to create Zn/V2O5 nanoparticles using the sol–gel technique. First, we combined the zinc acetate and vanadium acetate solutions in a 500 ml beaker at an equimolar ratio, while constantly stirring for 20 minutes. Subsequently, a sodium hydroxide solution was gradually added to the previously mentioned solution, and then hydrogen peroxide (H2O2) was added while stirring continuously at room temperature for three hours. The pH was maintained between 5.6 and 5.9. Less than 8.0% ethanol was used to modify the pH, such that the white colloidal solution could settle for five hours. After transferring the white colloidal solution into centrifuge tubes, the mixture was centrifuged for 15 minutes at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The liquid supernatant was disposed, and the white residue was repeatedly cleaned with distilled water. The residue was collected, dried for 60 minutes at 60 °C, and then annealed in a temperature-controlled muffle furnace for two hours at 450 °C. The annealed Zn/V2O5 (ZVO) nanoparticle residue was then crushed into tiny particles for use in the fabrication of nanocomposite materials.26–28
000 rpm. The liquid supernatant was disposed, and the white residue was repeatedly cleaned with distilled water. The residue was collected, dried for 60 minutes at 60 °C, and then annealed in a temperature-controlled muffle furnace for two hours at 450 °C. The annealed Zn/V2O5 (ZVO) nanoparticle residue was then crushed into tiny particles for use in the fabrication of nanocomposite materials.26–28
2.2 Synthesis of the polyaniline nanofiber
0.1 M of aniline aqueous solution in 100 ml of distilled water was mixed with 100 ml of 1 M HCl and 1 M toluene-4-sulfonic acid monohydrate (PTSA) solution to form an aniline hydrochloro-toluene-4-sulfonic acid solution. The mixture was soaked in ice pores by submersion in an ice bath. Then, at ice temperature, 0.25 M of 100 ml ammonium persulfate (APS) was added gradually with constant stirring. Following the addition of all of the APS, the reaction mixture was placed in a deep freezer under ice temperature to react slowly for 48 hours without stirring, resulting in the formation of a greenish-spongy precipitate. After filtering the precipitate, it was repeatedly washed with distilled water to get rid of any salt deposits, followed by 0.1 M HCl to eliminate all of the unreacted monomers. Then, the precipitate was washed with acetone to remove the water from the nanocomposite, and dried under a dynamic vacuum drier for 24 hours at 60 °C to achieve a constant weight.29,302.3 Synthesis of the polyaniline-Zn/V2O5 nanocomposite fibers
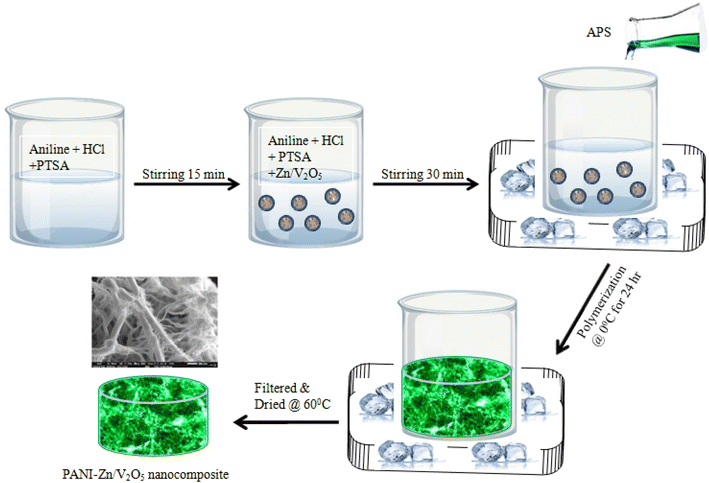
The preparation of the Zn/V2O5-doped polyaniline nanocomposites followed an identical procedure to that of the polyaniline synthesis. The aniline hydrochloro-toluene-4-sulfonic acid solution was combined with varying weight percentages (1, 2, 3, and 4 wt%) of Zn/V2O5 nanoparticles during a 30 minute stirring period in this synthesis procedure. Then, at ice temperature, 0.25 M of 100 ml ammonium persulfate (APS) was added slowly with continuous stirring. Then, the reaction mixtures were put in the deep freezer at ice temperature and allowed to react slowly for 48 hours without being agitated, resulting in the formation of a greenish-spongy precipitate once all of the APS was added. After filtering the precipitate and repeatedly washing it with distilled water to eliminate any salt build up, 0.1 M HCl was added to remove any remaining unreacted monomers. After rinsing with acetone to eliminate any remaining water from the nanocomposite, the precipitate was dried under dynamic vacuum drier for 48 hours at 60 °C to achieve a constant weight.31,32 Schematic diagram 1 shows how the PANI-Zn/V2O5 nanocomposite was prepared (Fig. 1).3. Characterization
Polyaniline and PANI-Zn/V2O5 nanocomposite were characterized via Fourier transform infrared (FTIR) spectra recorded on a PerkinElmer spectrophotometer (model 1600) within the wave number range of 400–4000 cm−1. After combining the produced PANI samples with KBr in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio to create a uniform palette, the mixture was compressed using a hydraulic press to create a plate with a diameter of 10 mm. The surface morphologies of PANI and PANI-Zn/V2O5 nanocomposites were examined by scanning electron microscopy (SEM) with gold-coated particles via sputtering. Electrochemical studies were conducted using the CH Instruments 660D-electrochemical workstation; the current and voltage were detected using a picoamp booster. Three electrodes were used in the electrochemical investigations: a platinum counter electrode, a saturated calomel electrode (SCE) as the reference electrode, and polyaniline and its nanocomposite mixed with carbon black and polyvinylidene fluoride in a 8
5 ratio to create a uniform palette, the mixture was compressed using a hydraulic press to create a plate with a diameter of 10 mm. The surface morphologies of PANI and PANI-Zn/V2O5 nanocomposites were examined by scanning electron microscopy (SEM) with gold-coated particles via sputtering. Electrochemical studies were conducted using the CH Instruments 660D-electrochemical workstation; the current and voltage were detected using a picoamp booster. Three electrodes were used in the electrochemical investigations: a platinum counter electrode, a saturated calomel electrode (SCE) as the reference electrode, and polyaniline and its nanocomposite mixed with carbon black and polyvinylidene fluoride in a 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in N-methyl-2-pyrrolidone were used to prepare a uniform paste coated over the nickel plate to create the working electrodes.
1 ratio in N-methyl-2-pyrrolidone were used to prepare a uniform paste coated over the nickel plate to create the working electrodes.
4. Results and discussion
4.1 Fourier transform infrared (FTIR) spectroscopy
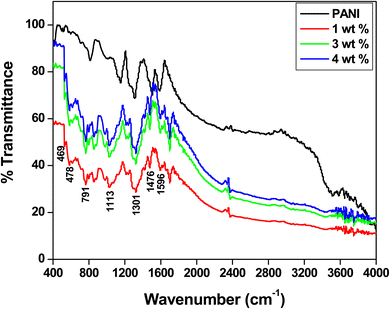
Fourier transform infrared (FTIR) spectroscopy was used to analyse the structural properties of the synthesised PANI and PANI-Zn/V2O5 nanocomposites, as shown in Fig. 2. The C–N and C–S stretching vibrations are located at 602 cm−1 and 687 cm−1, respectively, for the PANI. The C–H bending vibrations are found at 842 cm−1, the para di-substituted ring stretching vibrations at 842 cm−1, the C–N in-plane stretching vibrations at 1113 cm−1, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching band of the aromatic ring at 1301 cm−1, the benzenoid ring stretching vibrations at 1476 cm−1, the quinoid ring vibrations at 1596 cm−1, and the O–H stretching vibrations of water molecules.33–35 The Zn/V2O5 nanoparticle-doped nanocomposites show a peak at 469 cm−1, which corresponds to the Zn–O in-plane bending, 478 cm−1 is due to the vibrational mode of Zn–V–O symmetrical twisted in-plane bending, 1013 cm−1, which is related to the in-plane O–V stretching vibration, and 3456 cm−1, which is related to the O–H stretching mode of the hydroxyl group. The nanocomposites exhibit a shift of the peaks due of the loss in mass and strong chemical interaction between the nanoparticles and polyaniline, generating a red shift in the spectra that indicates the presence of Zn/V2O5 in polyaniline nanocomposites.36
N stretching band of the aromatic ring at 1301 cm−1, the benzenoid ring stretching vibrations at 1476 cm−1, the quinoid ring vibrations at 1596 cm−1, and the O–H stretching vibrations of water molecules.33–35 The Zn/V2O5 nanoparticle-doped nanocomposites show a peak at 469 cm−1, which corresponds to the Zn–O in-plane bending, 478 cm−1 is due to the vibrational mode of Zn–V–O symmetrical twisted in-plane bending, 1013 cm−1, which is related to the in-plane O–V stretching vibration, and 3456 cm−1, which is related to the O–H stretching mode of the hydroxyl group. The nanocomposites exhibit a shift of the peaks due of the loss in mass and strong chemical interaction between the nanoparticles and polyaniline, generating a red shift in the spectra that indicates the presence of Zn/V2O5 in polyaniline nanocomposites.36
4.2 X-rays diffraction study
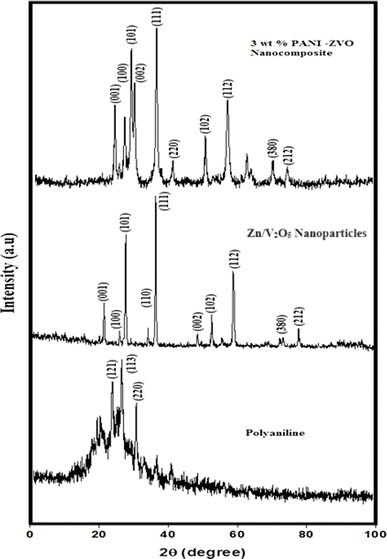
X-ray diffraction (XRD) was used to analyse the structure of the produced metal oxide and nanocomposites, as illustrated in Fig. 3. Due to the semicrystalline nature, it was discovered that the PANI exhibits the characteristic diffraction peak values at the (121), (113), and (220) planes, which correlate to 25.4 Å, 26.5 Å, and 30.4 Å, respectively (JCPDS no. 053-1890).37 The modified sol–gel method was used to prepare the porous Zn/V2O5 nanoparticles. The observed diffraction peaks were observed at the (001), 9110), (101), (110), (111), (002), (102), (11), (380), and (212) planes. These correspond to 2θ values of 21.1 Å, 25.4 Å, 25.6 Å, 36.8 Å, 39.2 Å, 45.1 Å, 47.3 Å, 69.8 Å, 72.4 Å, and 72.6 Å, indicating the orthorhombic α-phase with an interlayer spacing of 0.425 nm (JCPDS card no. 041-1426). The Debye Scherrer equation, D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, is used to calculate the crystallite size, and the result is 32 nm. Here, D stands for the crystalline size of the metal oxide nanoparticles, K is the Scherrer constant, λ is the wavelength of 1.54, and β is the full width at half maximum (FWHM) of the crystallographic peaks.38 Similar hkl peaks of the Zn/V2O5 nanoparticles in polyaniline nanocomposites can be observed at the (001), (100), (101), (002), (111), (2200, (102), (112), (380) and (212) planes, corresponding to the same 2θ values, which suggests the presence of the same crystal structure without distortion when doped in polyaniline. The observed superposition of polyaniline semicrystalline peaks in 3 weight percent of PANI-Zn/V2O5 nanocomposites suggests that the cause might be the nanoparticles embedded in the polymer matrix.
θ, is used to calculate the crystallite size, and the result is 32 nm. Here, D stands for the crystalline size of the metal oxide nanoparticles, K is the Scherrer constant, λ is the wavelength of 1.54, and β is the full width at half maximum (FWHM) of the crystallographic peaks.38 Similar hkl peaks of the Zn/V2O5 nanoparticles in polyaniline nanocomposites can be observed at the (001), (100), (101), (002), (111), (2200, (102), (112), (380) and (212) planes, corresponding to the same 2θ values, which suggests the presence of the same crystal structure without distortion when doped in polyaniline. The observed superposition of polyaniline semicrystalline peaks in 3 weight percent of PANI-Zn/V2O5 nanocomposites suggests that the cause might be the nanoparticles embedded in the polymer matrix.
4.3 Scanning electron microscopy
Polyaniline and its nanocomposites were prepared by in situ polymerization technique in the presence of an anionic surfactant. The prepared PANI, Zn/V2O5 and PANI-Zn/V2O5 nanocomposites were characterized via SEM to understand the desirable manipulation of the surface morphology that would be suitable for hydrogen application. Fig. 4(a) shows the pristine polyaniline prepared in ice in the presence of toluene-4-sulfonic acid monohydrate surfactant, which helps to form the one-dimensional polyaniline (1D PANI) fibers.36 It was observed that the prepared polyaniline is around 200 nm in length, forming a well-ordered network among each other without any agglomeration in the ice template. Nanocomposites were prepared by doping porous Zn/V2O5 nanoparticles synthesized by sol–gel method. The metal oxide formed a flower-like spherical and small fiber-like structure, and exhibited very high porosity with the size of 23 nm, as shown in Fig. 4(b). It was observed that the Zn/V2O5 nanoparticles were homogeneously distributed and completely embedded in the polyaniline fibers. In 1 wt%, 2 wt% and 4 wt%, the cluster of small broken fibers (Fig. 4(c), (d) and (f)) causes a low surface area and less adsorption sites on the PANI nanocomposites surface.39 However, it was observed that the 3 wt% of PANI-Zn/V2O5 nanocomposite shows the proper fiber form (Fig. 4(e)), which is suitable for hydrogen adsorption application.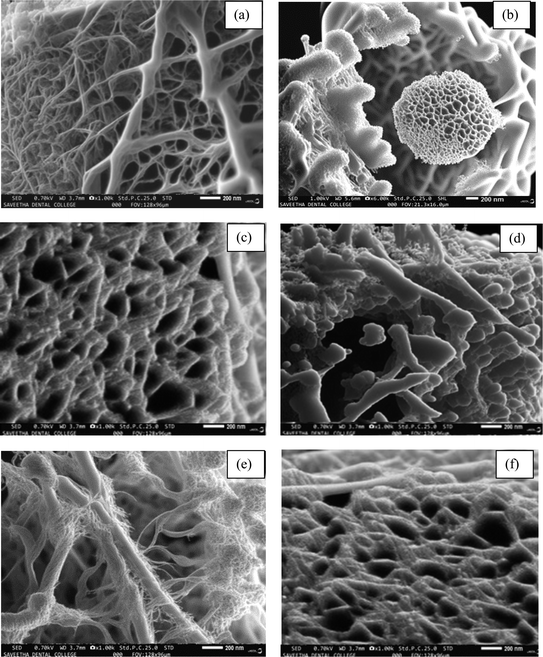 | ||
| Fig. 4 SEM image of (a) PANI, (b) porous Zn/V2O5 nanoparticles, and (c–f) 1 wt%, 2 wt%, 3 wt% and 4 wt% of PANI-Zn/V2O5 nanocomposites. | ||
4.4 Surface studies
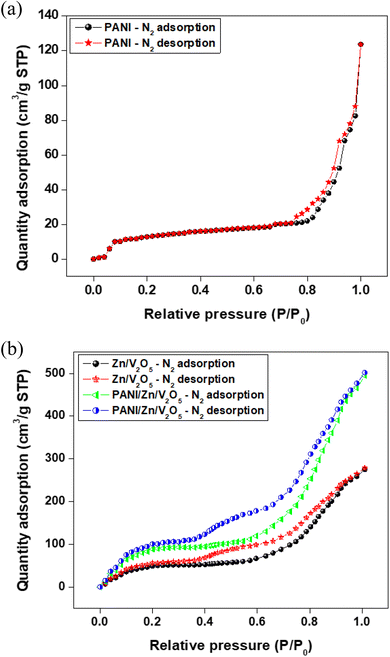 | ||
| Fig. 5 Surface area analysis using Brunauer–Emmett–Teller (BET) analysis: N2 adsorption–desorption isotherm of (a) PANI, Zn/V2O5 and (b) 3 wt% of the PANI-Zn/V2O5 nanocomposite. | ||
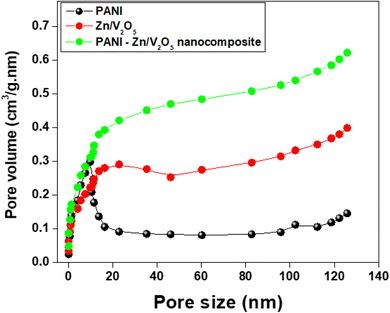 | ||
| Fig. 6 BJH pore size & volume analysis-Brunauer–Emmett–Teller (BET) of PANI, Zn/V2O5 and 3 wt% of PANI-Zn/V2O5 nanocomposite. | ||
The Barrett–Joyner–Halenda (BJH) method is employed to determine the distribution of pore size in the polyaniline nanocomposite, as shown in Fig. 6. It is observed that the average pore size of the PANI, Zn/V2O5 and 3 wt% of PANI-Zn/V2O5 nanocomposite was found to be 1.93 nm, 3.86 nm and 1.89 nm, respectively.43 It is important to note that the nanocomposites have a mesoporous structure after doping nanoparticles, and the pore diameter increases as the specific surface area decreases. The mesoporous structure and abundant gaps in the nanocomposite have advantages for achieving the desired hydrogen storage capacity of the nanocomposite.44
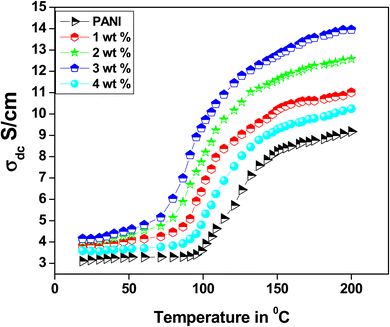
5. DC conductivity
The temperature-dependent DC conductivities of polyaniline and PANI-Zn/V2O5 nanocomposites fibers are shown in Fig. 7. The results obtained from the temperature-dependent conductivity of polyaniline and its nanocomposite fiber shows the increase of conductivity with an increase in temperature from 25 °C to 200 °C. This may be due to the linear enhancement in the hopping of polarons and bipolarons from the valence band to the conduction band.45 The increase in DC conductivity due to the increase in temperature because of the reduction in activation energy (Ea) is related to a shift in the Fermi level of doped Zn/V2O5 nanocomposites, as shown in Table 1. The temperature reliance of the conductivity of the PANI nanocomposites demonstrates a distinctive semiconductor nature, and it can be articulated by the one-dimensional variable range hopping (1D-VRH) model predicted by Mott and Davis, and is as follows;where T0 is the characteristic hopping temperature, ρ is the exponential factor, and the pre-exponential factor ‘ρ0’ is because of the lower temperature ‘T’. According to Mott and Davis, the significant change in the DC conductivity shows that the conduction transpires largely in extended polymer chain states.39 A smaller value of σo denotes a large variety of localised states, and indicates that the conduction transpired via the hopping of charge carriers from the valence bands to the conduction bands. The values of σo in the polyaniline fibre configuration are of the order of 13.8 S cm−1 for 3 wt% of PANI-Zn/V2O5 nanocomposites, amplifying conduction due to the hopping of charge carriers. This is because of the wide range of localised states available within the nanocomposites. We may infer from the results that the increase in the nanocomposite conductivity is due to the hopping process.46–48 The conduction mechanism can also be explained by the formation of polarons and bipolarons. The transport characteristics of polyaniline nanocomposites are significantly affected by the charge injection caused by polarons and bipolarons. Due to the strong lattice interaction, these self-localized defects are linked to various polyaniline backbone deformations and quantum states that are located in the energy gap.49
| Sl no. | Samples | Slope value (B) | Energy (J) | Activation energy (Ea) in eV |
|---|---|---|---|---|
| 1 | PANI | 0.42457 | 3529.291 | −2.202 |
| 2 | 1 wt% | 0.49131 | 4084.751 | −2.549 |
| 3 | 2 wt% | 0.56573 | 4703.229 | −2.935 |
| 4 | 3 wt% | 0.59763 | 4968.695 | −3.101 |
| 5 | 4 wt% | 0.47091 | 3896.186 | −2.431 |
Three stages of conductivity are visible in the polyaniline nanocomposites' conductivity as a function of temperature. The first step's nearly constant temperature range of 25 to 100 degrees celsius could be caused by the polarons and bipolarons not having enough energy to shift from one low energy state to a higher one. In the second step, the mobility of the electron charge system causes a progressive increase in conductivity between 100 and 150 °C. In the third step, an exponential increase in conductivity is observed between 150 and 200 °C. This could be because of the usual behaviour of polyaniline semiconductors, which is the hopping of polarons and bipolarons from the lower energy state to the excited state. The highest conductivity of a 3 wt% of the polyaniline-Zn/V2O5 nanocomposite is reported to be 13.8 S cm−1.50–54
6. Cyclic voltammetry
A functioning electrode was created using a polyaniline-coated polyester sheet. As a conducting substance, polyaniline and its nanocomposites were used, while polystyrene (PS) was used as a coating material. Unaccompanied polyaniline cannot generate a covering with adequate mechanical properties. As a result, polystyrene was coated as a support material in the fabrication of a covering comprising conductive polyaniline. The coating solution is produced and deposition is performed as follows. 1 g polyaniline and 1.27 g camphorsulfonic acid (C10H16O4S) (CSA) were dissolved in 20 cm3 of chloroform. After that, 1.00 g of polystyrene was added to the solution. The coating solution was created by magnetically swirling the solution for 24 hours. A polyaniline and camphorsulfonic acid chloroform solution was prepared, and a polystyrene solution was added to it. A polymer coating solution was made from the resultant solution. Using the aniline monomeric unit to calculate polyaniline, camphorsulfonic acid and polyaniline is combining to form a PANI–CSA salt in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The polyaniline and CSA salt was readily soluble in chloroform at this ratio. This blend was sufficiently conductive; thus, the experiment's PANI
1 molar ratio. The polyaniline and CSA salt was readily soluble in chloroform at this ratio. This blend was sufficiently conductive; thus, the experiment's PANI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS weight ratio was set at 1
PS weight ratio was set at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
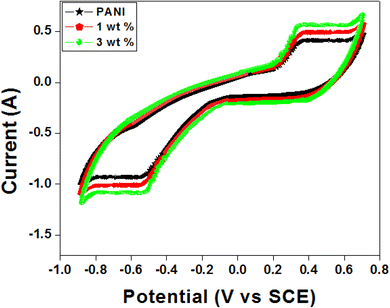
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.55–58 The electric double layer capacitance (EPLC) properties are shown by the cyclic voltammetry (CV) curves of PANI and PANI-Zn/V2O5 nanocomposite in Fig. 8, which have a rectangular shape. On the other hand, the PANI CV curve displays the typical CV feature of PANI, which is ascribed to the reduction-oxidation peaks of 0.93 V and 0.24 V. The two peaks are assigned to PANI's redox conversion from a semiconducting to a conducting state and pernigraniline.59
1.55–58 The electric double layer capacitance (EPLC) properties are shown by the cyclic voltammetry (CV) curves of PANI and PANI-Zn/V2O5 nanocomposite in Fig. 8, which have a rectangular shape. On the other hand, the PANI CV curve displays the typical CV feature of PANI, which is ascribed to the reduction-oxidation peaks of 0.93 V and 0.24 V. The two peaks are assigned to PANI's redox conversion from a semiconducting to a conducting state and pernigraniline.59
7. Hydrogen sorption measurements of the PANI nanofiber
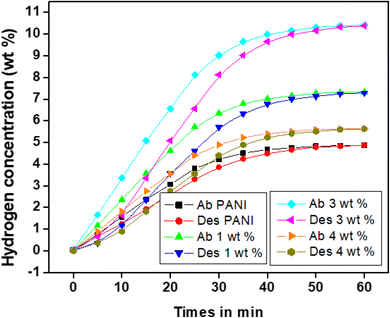
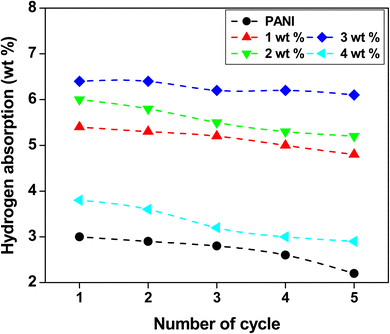
Volumetric hydrogen sorption experiments are critical for PANI fiber reactions when storing hydrogen. Experiments on hydrogen absorption were carried out at 60 °C in a high-pressure (H2 80 bar) reservoir. The volumetric isothermal analyses were performed on the Hy-Energy PCTPro 2000 sorption apparatus. This device is fully automated and has an integrated 170 bar PID-pressure regulator controlled to meet Sievert's specifications. The HyDataV2.1 Lab-View program was used to inspect subroutines software for purging hydrogen, leak testing, and PCT.A Schlenk flask with a rubberized cap was filled with the anionic surfactant of toluene-4-sulfonic acid monohydrate (PTSA)-based polyaniline and PANI-Zn/V2O5 nanocomposite fibres. The flask was then dried under vacuum at 70 °C for 60 minutes. Before being transferred to a high-pressure hydrogen reactor in a glove box with inert nitrogen gas, polyaniline and its nanocomposites were thoroughly dried.60–63 To assess the sorption performance, a volumetric setup with high hydrogen pressure was connected to the reactor and it was completely enclosed. Fig. 9 illustrates how pressure-applied hydrogen absorption changes at room temperature. Research has shown that when the pressure increases, so does the hydrogen adsorption capability.64 The hydrogen adsorption capacity of the polyaniline fibre is around 4.5 weight percent at ambient temperature, but it doubles to approximately 10.8 weight percent at 60 degrees celsius. The nitrogen (N) molecules in polyaniline are the hydrogen bond donor, and have a large number of hydrogen bond receptor sites in their molecular structure. The 3 wt% of PANI-Zn/V2O5 nanocomposites exhibit a high absorption capacity of 6.6% compared to other compositions of nanocomposites.65 It has been discovered that when pressure is released from 9.4 MPa to 1 MPa and the temperature is raised from room temperature to 60 °C, the hydrogen desorption rises. The increasing temperature enhances the kinetic energy of the molecules, thereby facilitating more effective interactions between absorbent materials and target substances. This phenomenon is particularly evident in gas absorption processes, where the elevated thermal conditions lead to greater molecular agitation, resulting in heightened adsorption rates. Consequently, the increased energy levels promote a more efficient transfer of species across phases.66 It is also crucial to remember that the desorption process is extremely sluggish at room temperature, which could be because the polyaniline backbone molecules have strong N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H bonds. At room temperature, polyaniline and PANI-Zn/V2O5 nanocomposites exhibit hydrogen desorption at rates of 78% to 86% for 3 weight percent, respectively.67
H bonds. At room temperature, polyaniline and PANI-Zn/V2O5 nanocomposites exhibit hydrogen desorption at rates of 78% to 86% for 3 weight percent, respectively.67
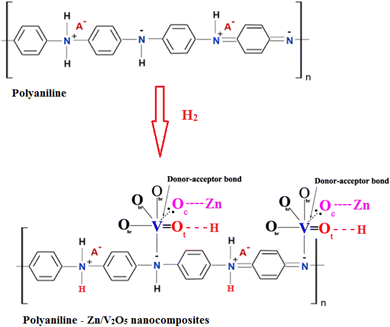
Hydrogen can adsorb two ways, as shown in Fig. 10. First, an enhanced electronic transition that interacts with the hydrogen atoms is produced by the two receptors in conductive groups on the polyaniline backbone that increase in relation to the quinoid diimine units (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).57 The H–H bond breaks through the heterolytic path to produce a hydride, bonding to the Zn2+, Vi2+ and Vi3+ sites, and a proton bonds to one oxygen to generate the (H+–H) species in the next phase, the physisorption of H2 molecules (
N).57 The H–H bond breaks through the heterolytic path to produce a hydride, bonding to the Zn2+, Vi2+ and Vi3+ sites, and a proton bonds to one oxygen to generate the (H+–H) species in the next phase, the physisorption of H2 molecules (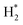 species).
species).
Later, the H+ on the VO2+ + 2e− + 4H+ → VO3+ + 2H2O site is shifted to the neighboring oxygen atom, resulting in homogenous splitting and the configuration of 2O–H hydroxyl groups is labeled (H+–H+). As seen in Fig. 8, this phase is followed by the transport of two electrons to surface vanadium oxygen sites that become reduced.68–70
Fig. 11 illustrates the first hydrogen uptake of polyaniline and PANI-Zn/V2O5 nanocomposite fibres, which are prepared via in situ polymerization in an ice bath over a period and formed at room temperature. It is discovered that the absorption of the polyaniline nanocomposite fiber reaches saturation in less than 60 minutes, meaning that a temperature increase of 60 °C results in an increase of 88% of the true uptake capacity to 10.8 weight percent. However, the hydrogen gas desorption takes a longer time of 147 minutes, causing the strong N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H bonding of the polyaniline backbone benzenoid molecules and the hygroscopic nature of the Zn/V2O5 nanoparticles.71 To determine the hydrogen sorption efficiency, a room-temperature pressure–composition–temperature (PCT) investigation was conducted on the polyaniline fiber.72 After 80 minutes, an effective hydride of around 6 bar is formed by a separate variable pressure. With a linear zone of 4.5 weight percent, it reduces PANI's hydrogen uptake exponentially up to 90%. At room temperature, the total desorption capacity of 3 weight percent Zn/V2O5-doped PANI nanocomposites decreased from 10.8% to 6.6%.
H bonding of the polyaniline backbone benzenoid molecules and the hygroscopic nature of the Zn/V2O5 nanoparticles.71 To determine the hydrogen sorption efficiency, a room-temperature pressure–composition–temperature (PCT) investigation was conducted on the polyaniline fiber.72 After 80 minutes, an effective hydride of around 6 bar is formed by a separate variable pressure. With a linear zone of 4.5 weight percent, it reduces PANI's hydrogen uptake exponentially up to 90%. At room temperature, the total desorption capacity of 3 weight percent Zn/V2O5-doped PANI nanocomposites decreased from 10.8% to 6.6%.
8. Conclusion
Polyaniline-Zn/V2O5 nanocomposites were prepared by in situ oxidation polymerization method in the presence of an anionic surfactant. The prepared nanocomposites were subjected for structural analysis by FTIR and XRD studies. An FTIR spectrum confirms the characteristic peaks of the benzenoid ring stretching vibration, quinoid ring and metal oxide peaks. The XRD pattern confirms the orthorhombic structure with the α-phase of Zn//V2O5, which has an interlayer spacing of 0.425 nm. The SEM surface morphology confirms the formation of polyaniline nanofibers, and also revealed that doping above 3 wt% interfered with the formation of the polyaniline fibre structure. The specific surface area of PANI, Zn/V2O5 and 3 wt% of the PANI-Zn/V2O5 nanocomposite is 62.53 m2 g−1, 89 m2 g−1 and 386 m2 g−1, respectively. The DC conductivity confirms that the conductivity increases with an increase in temperature. Among all nanocomposites, 3 wt% of PANI-Zn/V2O5 shows a high conductivity of 13.8 S cm−1. Cyclic voltammetry studies were performed for polyaniline and its nanocomposites. The characteristic peaks of oxidation–reduction is observed at 0.93 V and 0.24 V. Hydrogen absorption studies were carried out by volumetric sorption measurement technique. It was confirmed that at room temperature, the pristine polyaniline fibre has a hydrogen adsorption capacity of about 4.5 wt%. However, at 60 °C, its capacity doubles to around 10.8 wt%. In comparison, 3 wt% of PANI-Zn/V2O5 nanocomposite shows a high absorption capacity of 6.6 wt% compared with other nanocomposite compositions, which is due to the particular structural design and shape. This indicates the porous fiber in nature, and the nitrogen (N) molecules present in the benzenoid and quinoid rings in polyaniline. Hydrogen desorption of PANI and PANI-Zn/V2O5 nanocomposites increases with the decrease of pressure from 9.4 MPa to 1 MPa by an increase in temperature from ambient temperature to 60 °C. It was also confirmed that the hydrogen absorption–desorption capacity was stable up to 96% for 3 wt% Zn/V2O5-doped PANI nanocomposites at room temperature.Data availability
Data will be available on request to author.Author contributions
Ameena Parveen: data creation, conceptualization, methodology, writing – original draft and editing, Aashish Roy: methodology, writing – original draft and editing, S. Manjunatha: conceptualization and methodology, M. Madesh Kumar: methodology and data analysis, Ameena Parveen & Aashis S. Roy: final drafting and editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to thank the PolyTech Research Laboratory, and are grateful for partial financial support from VGST during revision of the manuscript vide ref. no. KSTEPS/VGST/KFIST-L1/GRD-1151/2022-23/672; Dated: 05.02.2024. M. Madesh Kumar acknowledge the REVA University management for funding and support for this work through a seed money grant.References
- B. Senthilkumar, P. Ashok and A. Shanmugavani, 8 - Polyaniline-based nanocomposites for hydrogen storage, in Woodhead Publishing Series in Composites Science and Engineering, Polymer-Based Nanocomposites for Energy and Environmental Applications, ed. M. Jawaid and M. M. Khan, Woodhead Publishing, 2018, pp. 219–238 Search PubMed.
- N. Mahato, H. Jang, A. Dhyani and S. Cho, Recent Progress in Conducting Polymers for Hydrogen Storage and Fuel Cell Applications, Polymers, 2020, 12(11), 2480 CrossRef CAS.
- Y. Sun, D. Liu, W. Liu, H. Liu, J. Zhao, P. Chen, Q. Wang, X. Wang and Y. Zou, Fabrication of porous polyaniline/MWCNTs coated Co9S8 composite for electrochemical hydrogen storage application, J. Phys. Chem. Solids, 2021, 157, 110235 CrossRef CAS.
- B. Sakintuna, F. Lamari-Darkrim and M. Hirscher, Metal hydride materials for solid hydrogen storage: A review, Int. J. Hydrogen Energy, 2007, 32, 1121–1140 CrossRef CAS.
- N. F. Attia, S. M. Lee, H. J. Kim and K. E. Geckeler, Nanoporous polypyrrole: Preparation and hydrogen storage properties, Int. J. Energy Res., 2014, 38, 466–476 CrossRef CAS.
- Y. Sun, D. Liu, W. Liu, H. Liu, P. Chen, J. Zhao, Y. Cheng, Q. Wang and X. Wang, Preparation of porous polyaniline/RGO composite and its application in improving the electrochemical properties of Co9S8 hydrogen storage alloy, Int. J. Hydrogen Energy, 2021, 46(80), 40239–40250 CrossRef CAS.
- N. F. Attia and K. E. Geckeler, Polyaniline as a Material for Hydrogen Storage Applications, Macromol. Rapid Commun., 2013, 34, 1043–1055 CrossRef CAS.
- D. Tinet, M. H. Legay, L. Gatineau and J. J. Fripiat, Properties of the vanadium pentoxide hydrogen bronzes (H2xV2O5), J. Phys. Chem., 1986, 90(5), 948–952 CrossRef CAS.
- M. Witko and K. Hermann, Hydrogen adsorption and OH desorption at vanadium pentoxide surfaces: ab initio cluster model studies, J. Mol. Catal., 1993, 81(2), 279–292 CrossRef CAS.
- H. Wu, J. Du, F. Cai, F. Xu, W. Wei, J. Guo and Z. Lan, Catalytic effects of V and V2O5 on hydrogen storage property of Mg17Al12 alloy, Int. J. Hydrogen Energy, 2018, 43(31), 14578–14583 CrossRef CAS.
- G. Illing, K. Hellgardt, R. J. Wakeman and A. Jungbauer, Preparation and characterization of polyaniline based membranes for gas separation, J. Membr. Sci., 2001, 184, 69–78 CrossRef CAS.
- X. Gao, Z. Zhong, L. Huang, Y. Mao, H. Wang, J. Liu, L. Ouyang, L. Zhang, M. Han, X. Ma and M. Zhu, The role of transition metal doping in enhancing hydrogen storage capacity in porous carbon materials, Nano Energy, 2023, 118, 109038 CrossRef CAS.
- J. Germain, J. Frechet and F. Svec, Nanoporous, hypercrosslinked polypyrroles: Effect of crosslinking moiety on pore size and selective gas adsorption, Chem. Commun., 2009, 12, 1526–1528 RSC.
- Z. Ding, Y. Ma, D. Peng, L. Zhang, Y. Zhao, Y. Li and S. Han, Effects of the hierarchical pyrolysis polyaniline on reversible hydrogen storage of LiBH4, Progress in Natural Science: Materials International, 2018, 28(4), 529–533 CrossRef CAS.
- S. Virji, R. B. Kaner and B. H. Weiller, Hydrogen sensors based on conductivity changes in polyaniline nanofibers, J. Phys. Chem. B, 2006, 110, 22266–22270 CrossRef CAS.
- Y. Chen, H. Zhu and Y. Liu, Preparation of activated rectangular polyaniline-based carbon tubes and their application in hydrogen adsorption, Int. J. Hydrogen Energy, 2011, 36, 11738–11745 CrossRef CAS.
- A. Rahy, T. Rguig, S. J. Cho, C. E. Bunker and D. J. Yang, Polar solvent soluble and hydrogen absorbing PANI nanofibres, Synth. Met., 2011, 161, 280–284 CrossRef CAS.
- A. R. Phani, R. M. T. De Britto, S. Srinivasan and L. Stefanakos, Polyaniline Nanofibers Obtained by Electrospin Process for Hydrogen Storage Applications, Int. J. Environ. Res. Dev., 2014, 4, 375–386 Search PubMed.
- S. S. Srinivasan, R. Ratnadurai, M. U. Niemann, A. R. Phani, D. Y. Goswami and E. K. Stefanakos, Reversible hydrogen storage in electrospun polyaniline fibers, Int. J. Hydrogen Energy, 2010, 35, 225–230 CrossRef CAS.
- W. Shen, S. Han, Y. Li, S. Yang and Q. Miao, Effect of electroplating polyaniline on electrochemical kinetics of La–Mg–Ni-based hydrogen storage alloy, Appl. Surf. Sci., 2012, 258(17), 6316–6320 CrossRef CAS.
- M. U. Jurczyk, A. Kumar, S. Srinivasan and E. Stefanakos, Polyaniline-based nanocomposite materials for hydrogen storage, Int. J. Hydrogen Energy, 2007, 32, 1010–1015 CrossRef CAS.
- B. H. Kim, W. G. Hong, S. M. Lee, Y. J. Yun, H. Y. Yu, S.-Y. Oh, C. H. Kim, Y. Y. Kim and H. J. Kim, Enhancement of hydrogen storage capacity in polyanilinevanadium pentoxide nanocomposites, Int. J. Hydrogen Energy, 2010, 35, 1300–1304 CrossRef CAS.
- J. Germain, J. M. J. Frèchet and F. Svec, Hypercrosslinked polyanilines with nanoporous structure and high surface area: Potential adsorbents for hydrogen storage, J. Mater. Chem. A, 2007, 17, 4989–4997 RSC.
- Y. Chen, X. Cao, H. Zhu and Y. Liu, Preparation of a porous carbon from ferrocene-loaded polyaniline and its use in hydrogen adsorption, Int. J. Hydrogen Energy, 2012, 37, 7629–7637 CrossRef CAS.
- M. M. Li, C. C. Wang, Y. T. Zhou and C. C. Yang, Clarifying the polyaniline effect on superior electrochemical performances of hydrogen storage alloys, Electrochim. Acta, 2021, 365, 137336 CrossRef CAS.
- M. U. Niemann, S. S. Srinivasan, A. R. Phani, A. Kumar, D. Y. Goswami and E. K. Stefanakos, Room temperature reversible hydrogen storage in polyaniline (PANI) nanofibers, J. Nanosci. Nanotechnol., 2009, 9(8), 4561–4565 CrossRef CAS.
- M. Patel, S. Mishra and R. Verma, Synthesis of ZnO and CuO nanoparticles via Sol gel method and its characterization by using various technique, Discover Materials, 2022, 2, 1–7 CrossRef.
- J. N. Hasnidawani, H. N. Azlina, H. Norita, N. N. Bonnia, S. Ratim and E. S. Ali, Synthesis of ZnO Nanostructures Using Sol-Gel Method, Procedia Chem., 2016, 19, 211–216 CrossRef CAS.
- P. Y. Gorobtsov, T. L. Simonenko, N. P. Simonenko, E. P. Simonenko and N. T. Kuznetsov, Preparation of V2O5 Thin Film by Sol–Gel Technique and Pen Plotter Printing, Colloids Interfaces, 2023, 7(1), 20 CrossRef CAS.
- A. S. Roy, Antistatic and Dielectric properties of one-dimensional Al2+:Nd2O3 nanowire doped polyaniline nanocomposites for electronic application, Sens. Actuators, A, 2018, 280, 1–7 CrossRef CAS.
- R. D. Balikile, A. S. Roy, A. Parveen, G. Ramgopal and N. Badi, Hybrid Nickel Ferrite Nanotubes Doped Polyaniline Nanocomposite and Its Dielectric Properties, J. Electron. Mater., 2020, 49, 833–841 CrossRef CAS.
- R. D. Balikile, A. S. Roy, S. C. Nagaraju and G. Ramgopal, Conductivity properties of hollow ZnFe2O4 Nanofibers doped Polyaniline Nanocomposites, J. Mater. Sci.: Mater. Electron., 2017, 28, 7368–7375 CrossRef CAS.
- J. N. Ansari, S. Khasim, A. Parveen, O. A. Al-Hartomy, Z. Khattari, N. Badi and A. S. Roy, Synthesis, Characterization, Dielectric and Rectification Properties of PANI/Nd2O3:Al2O3 Nanocomposites, Polym. Adv. Technol., 2016, 27, 1064 CrossRef CAS.
- J. Zheng, C.-G. Wang, H. Zhou, E. Ye, J. Xu, Z. Li and X. J. Loh, Current Research Trends and Perspectives on Solid-State Nanomaterials in Hydrogen Storage, Research, 2021, 23, 3750689 Search PubMed.
- U. Eberle, G. Arnold and R. von Helmholt, Hydrogen storage in metal-hydrogen systems and their derivatives, J. Power Sources, 2006, 154, 456–460 CrossRef CAS.
- R. K. Joshi, S. Krishnan, M. Yoshimura and A. Kumar, Pd nanoparticles and thin films for room temperature hydrogen sensor, Nanoscale Res. Lett., 2009, 4(10), 1191–1196 CrossRef CAS PubMed.
- H. A. Al-Aoh, N. Badi, A. S. Roy, A. M. Alsharari, S. Abd El Wanees, A. Albaqami and A. Ignatiev, Preparation of Anionic Surfactant-Based One-Dimensional Nanostructured Polyaniline Fibers for Hydrogen Storage Applications, Polymers, 2023, 15(7), 1658 CrossRef CAS PubMed.
- F. Liu, Z. Chen and G. Fang, V2O5 Nanospheres with Mixed Vanadium Valences as High Electrochemically Active Aqueous Zinc-Ion Battery Cathode, Nano-Micro Lett., 2019, 11, 25–35 CrossRef CAS PubMed.
- P. Nagaraju, Y. Vijayakumar, M. V. Ramana Reddy and U. P. Deshpande, Effect of vanadium pentoxide concentration in ZnO/V2O5 nanostructured composite thin films for toluene detection, RSC Adv., 2019, 9, 16515 RSC.
- C. Sandaruwan, H. M. P. C. K. Herath and T. S. E. F. Karunarathne, Polyaniline/palladium nanohybrids for moisture and hydrogen detection, Chem. Cent. J., 2018, 12, 93 CrossRef CAS PubMed.
- S. Nawaz, Y. Khan, S. A. M. Abdelmohsen, S. Khalid, E. M. Björk, M. A. Rasheed and M. Siddiq, Polyaniline inside the pores of high surface area mesoporous silicon as composite electrode material for supercapacitors, RSC Adv., 2022, 12, 17228–17236 RSC.
- R. Rahman, P. Rani, S. Ghosh, A. Midya, A. Pathak and T. K. Nath, Engineering Hierarchical Self-Assembled PANI-/1T@2H MoS2 Nanostructure toward Ultrahigh Performance Supercapacitor Electrodes, ACS Appl. Energy Mater., 2023, 6(21), 11012–11029 CrossRef CAS.
- H. Tan, D. Xiao, R. Navik and Y. Zhao, Facile Fabrication of Polyaniline/Pristine Graphene–Bacterial Cellulose Composites as High-Performance Electrodes for Constructing Flexible All-Solid-State Supercapacitors, ACS Omega, 2021, 6(17), 11427–11435 CrossRef CAS PubMed.
- B. Liu, X. Zhang, D. Tian, Q. Li, M. Zhong, S. Chen, C. Hu and H. Ji, In Situ Growth of Oriented Polyaniline Nanorod Arrays on the Graphite Flake for High-Performance Supercapacitors, ACS Omega, 2020, 5(50), 32395–32402 CrossRef CAS.
- U. Akhter, M. Miran, M. Susan, M. Mollah and M. Rahman, Preparation and characterization of polyaniline-silica composite material, Bangladesh J. Sci. Ind. Res., 2012, 47(3), 249–256 CrossRef.
- M. Rahim, S. Yaseen and R. Ullah, Electrochemical supercapacitor based on polyaniline/bismuth-doped zinc oxide (PANI/Bi–ZnO) composite for efficient energy storage, J. Phys. Chem. Solids, 2023, 182, 111610 CrossRef CAS.
- A. Parveen and A. S. Roy, Enhancement of microwave absorption of polyaniline-PbTiO3 composites prepared by using sodium dodecyl benzene sulfonoic acid (SDBSA), J. Mater. Res., 2013, 28, 840–847 CrossRef CAS.
- A. S. Roy, H. Shruti Gopalkrishna and A. Parveen, Synthesis, Characterization, Ac Conductivity and Diode Properties of Polyaniline-CaTiO3 Composite, Polym. Adv. Technol., 2014, 25, 130–135 CrossRef CAS.
- B. Getiren, H. Altınışık, F. Soysal, Z. Çıplak and N. Yıldız, N-doped reduced graphene oxide/MnO2/co-doped polyaniline ternary nanocomposites for electrochemical energy storage applications, J. Electroanal. Chem., 2023, 932, 117243 CrossRef CAS.
- D. Tian, H. Cheng, Q. Li, C. Song, D. Wu, X. Zhao, S. Hu, S. Chen and C. Hu, The ordered polyaniline nanowires wrapped on the polypyrrole nanotubes as electrode materials for electrochemical energy storage, Electrochim. Acta, 2021, 398, 139328 CrossRef.
- K. Ran, X. Hou, Y. Huang, W. Ye, J. Wen, Y. Zhang, S. Wang, K. Cao, W. Wang, W. Xue and R. Zhao, Preparation of polyaniline intercalated V2O5 composites and study of their self-charging mechanism, Colloids Surf., A, 2023, 671, 131703 CrossRef CAS.
- A. R. Dhanya, P. Haridoss and R. Sundara, EDA/PANI derived FeNxC with Fe-Nx active sites as room temperature hydrogen storage material, J. Alloys Compd., 2024, 970, 172596 CrossRef.
- Y. Chen, H. Zhu and Y. Liu, Preparation of activated rectangular polyaniline-based carbon tubes and their application in hydrogen adsorption, Int. J. Hydrogen Energy, 2011, 36(18), 11738–11745 CrossRef CAS.
- M. Turgeman, G. Bergman, A. Nimkar, B. Gavriel, E. Ballas, F. Malchik, M. D. Levi and D. Sharon, Unique Mechanisms of Ion Storage in Polyaniline Electrodes for Pseudocapacitive Energy Storage Devices Unraveled by EQCM-D Analysis, ACS Appl. Mater. Interfaces, 2022, 14(41), 47066–47074 CrossRef CAS.
- J. Yun, I. Echols, P. Flouda, S. Wang, A. Easley, X. Zhao, Z. Tan, E. Prehn, G. Zi, M. Radovic, M. J. Green and J. L. Lutkenhaus, Layer-by-Layer Assembly of Polyaniline Nanofibers and MXene Thin-Film Electrodes for Electrochemical Energy Storage, ACS Appl. Mater. Interfaces, 2019, 11(51), 47929–47938 CrossRef CAS.
- W. Xinming, W. Qiguan and Z. Wenzhi, et al., Enhanced electrochemical performance of hydrogen-bonded graphene/polyaniline for electrochromo-supercapacitor, J. Mater. Sci., 2016, 51, 7731–7741 CrossRef.
- G. Mashao, K. D. Modibane and S. B. Mdluli, et al., Polyaniline-Cobalt Benzimidazolate Zeolitic Metal-Organic Framework Composite Material for Electrochemical Hydrogen Gas Sensing, Electrocatalysis, 2019, 10, 406–419 CrossRef CAS.
- J. Hu, F. Yang and C. Lai, et al., Researches on a conductive polyaniline-acetylene black composite to suppress the hydrogen evolution reaction in lead-acid batteries, J. Solid State Electrochem., 2022, 26, 1153–1161 CrossRef CAS.
- L. Li, Y. Zhang and H. Lu, et al., Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage, Nat. Commun., 2020, 11, 62 CrossRef CAS PubMed.
- A. Arslan and E. Hur, Electrochemical storage properties of polyaniline-, poly(N-methylaniline)-, and poly(N-ethylaniline)-coated pencil graphite electrodes, Chem. Pap., 2014, 68, 504–515 CAS.
- K. Y. Yasoda, M. S. Kumar and S. K. Batabyal, Polyaniline decorated manganese oxide nanoflakes coated graphene oxide as a hybrid-supercapacitor for high performance energy storage application, Ionics, 2020, 26, 2493–2500 CrossRef CAS.
- H. D. Kyomuhimbo and U. Feleni, Catalytic and Energy Storage Applications of Metal/Polyaniline Nanocomposites: A Critical Review, J. Electron. Mater., 2022, 51, 5568–5585 CrossRef CAS.
- S. Wang, M. Gao, K. Xian, Z. Li, Y. Shen, Z. Yao, Y. Liu and H. Pan, LiBH4 Nanoconfined in Porous Hollow Carbon Nanospheres with High Loading, Low Dehydrogenation Temperature, Superior Kinetics, and Favorable Reversibility, ACS Appl. Mater. Interfaces, 2020, 3(4), 3928–3938 CAS.
- S. Wang, M. H. Wu, Y. Y. Zhu, Z. L. Li, Y. X. Yang, Y. Z. Li, H. F. Liu and M. X. Gao, Reactive destabilization and bidirectional catalyzation for reversible hydrogen storage of LiBH4 by novel waxberry-like nano-additive assembled from ultrafine Fe3O4 particles, J. Mater. Sci. Technol., 2024, 173, 63–71 CrossRef CAS.
- M. Wu, M. Gao, S. Qu, Y. Liu, W. Sun, C. Liang, X. Zhang, Z. Li, Y. Yang and H. Pan, LiBH4 hydrogen storage system with low dehydrogenation temperature and favorable reversibility promoted by metallocene additives, J. Energy Storage, 2023, 72, 108679 CrossRef.
- E. Boateng, J. van der Zalm and A. Chen, Design and Electrochemical Study of Three-Dimensional Expanded Graphite and Reduced Graphene Oxide Nanocomposites Decorated with Pd Nanoparticles for Hydrogen Storage, J. Phys. Chem. C, 2021, 125(42), 22970–22981 CrossRef CAS.
- Z. Li, M. Gao, J. Gu, K. Xian, Z. Yao, C. Shang, Y. Liu, Z. Guo and H. Pan, In Situ Introduction of Li3BO3 and NbH Leads to Superior Cyclic Stability and Kinetics of a LiBH4-Based Hydrogen Storage System, ACS Appl. Mater. Interfaces, 2020, 12(1), 893–903 CrossRef CAS PubMed.
- Y. Du, N. Li, T.-L. Zhang, Q.-P. Feng, Q. Du, X.-H. Wu and G.-W. Huang, Reduced Graphene Oxide Coating with Anticorrosion and Electrochemical Property-Enhancing Effects Applied in Hydrogen Storage System, ACS Appl. Mater. Interfaces, 2017, 9(34), 28980–28989 CrossRef CAS.
- I. Hussain, C. Lamiel, M. S. Javed, M. Ahmad, S. Sahoo, X. Chen, N. Qin, S. Iqbal, S. Gu, Y. Li, C. Chatzichristodoulou and K. Zhang, MXene-based heterostructures: Current trend and development in electrochemical energy storage devices, Prog. Energy Combust. Sci., 2323, 97, 101097 CrossRef.
- C. K. Maity, S. De, K. Verma, M. Moniruzzaman and S. Sahoo, Nanocellulose: A versatile nanostructure for energy storage applications, Ind. Crops Prod., 2023, 204, 117218 CrossRef CAS.
- H. Liu, W. Liu, Y. Sun, P. Chen, J. Zhao, X. Guo and Z. Su, Preparation and electrochemical hydrogen storage properties of Ti49Zr26Ni25 alloy covered with porous polyaniline, Int. J. Hydrogen Energy, 2020, 45(20), 11675–11685 CrossRef CAS.
- Y. Chen, H. Zhu and Y. Liu, Preparation of activated rectangular polyaniline-based carbon tubes and their application in hydrogen adsorption, Int. J. Hydrogen Energy, 2011, 36(18), 11738–11745 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |