Open Access Article
Open Access ArticleEffects of mixed enzymolysis alone or combined with acetylation or carboxymethylation on the role of jujube kernel fibre as a biosorbent for wastewater treatment
Nan Qin*,
Yunfei Li,
Lan Zhang,
Lili Guo,
Wenfang Zhang,
Guanwen Li and
Jun Bai
College of Medicine and Food Engineering, Shanxi University of Chinese Medicine, Taiyuan, 030619, China. E-mail: qinnan_qn@sxtcm.edu.cn; Tel: +86-13753120097
First published on 22nd July 2024
Abstract
Jujube kernel fibre (JKF) could serve as a renewable, abundant, low-cost, and environmentally friendly adsorbent for wastewater if its adsorption capacities are improved. However, data on the modification of JKF, especially on the combination of biological and chemical modifications, are scarce. Therefore, for the first time, we studied the effect of mixed enzymolysis alone or combined with acetylation or carboxymethylation on the structure and adsorption capacities of JKF. After these modifications, the microstructure of JKF became more porous, and its soluble fibre and extractable polyphenol contents, surface area and adsorption capacities for nitrite, copper, and lead ions were all significantly improved (P < 0.05). Meanwhile, mixed enzymatic hydrolysis and acetylation treated JKF showed the highest surface hydrophobicity (43.57) and oil-adsorption ability (4.47 g g−1), while mixed enzymatic hydrolysis and carboxymethylation treated JKF exhibited the highest water adsorption ability (10.66 g g−1), water expansion ability (8.50 mL g−1), and lead and copper ion chelating abilities. Additionally, mixed enzymatic hydrolyzed JKF had the highest nitrite-ion-adsorption ability (10.57 μmol g−1). It can be concluded that mixed enzymolysis combined with carboxymethylation is an optimal way to increase the hydration properties and heavy-metal-adsorption capacity of JKF, while mixed enzymolysis combined with acetylation is an effective approach to enhance the oil-adsorption capacity of JKF.
1 Introduction
Water pollution is one of the biggest problems threatening human survival and security. Pathogenic microorganisms, parasites, heavy metals, grease, detergent and phytonutrients (such as nitrogen, phosphorus and sulfur) in contaminated water can deteriorate water quality, reduce or eliminate crop yields, contaminate food and cause and spread various diseases.1 Industrial wastewater, gas and residues, pesticide and veterinary drug residues, and non-compliant packaging materials are the main sources of heavy metal pollution in water.2 Most heavy metals, such as lead(II) (Pb2+), cadmium (Cd+) and mercury (Hg+) ions, are hazardous because they accumulate in living organisms and cause fatal diseases,1 while excessive essential heavy metals in water, such as copper, iron, and manganese ions, can cause serious damage to the liver, kidney, and other organs of humans.3 Moreover, excessive phytonutrients, such as nitrite, phosphorus, and sulfur, in water can cause eutrophication, water-quality degradation, bloom, and even the death of fish and other organisms.4 Additionally, oil and sugar in domestic and industrial sewage can breed a large number of pathogenic bacteria and microorganisms, thus depleting the oxygen in water and gradually resulting in the death of animals and plants in the water.1,5 Therefore, technologies, such as adsorption, reverse osmosis, and ion exchange, that can remove heavy metals, phytonutrients, and other toxic substances from wastewater have attracted increasing attention. Among these, the use of natural biopolymers, especially fibre, starch, and chitosan, as adsorbents to treat wastewater is popular for their high availability, low cost, and renewability.4,6Compared to active carbon and starch, adsorbents derived from plant fibres have remarkable advantages, including availability, low cost, reasonable strength and stiffness, and low density.5 In recent decades, the adsorption properties of heavy metals, phytonutrients, oil and other toxic substances in wastewater on various agro-based waste fibres, such as rice bran, coconut shell, millet bran, and sugarcane bagasse, have been studied.6–8 Although insoluble fibre also plays a role in the wastewater treatment ability of fibre adsorbents, a large number of studies have shown that the wastewater treatment ability of fibre is mainly dependent on surface microstructure, soluble fibre content, hydrophobicity and hydrophilicity.1,5 However, many natural fibre adsorbents show relatively poor adsorption capacities for toxic and harmful substances in wastewater due to their low hydration properties, small specific surface area, and few functional groups.4 Thus, various physical, biological, and chemical modifications have been explored to improve the adsorption capacity of fibres.6,9 Among these methods, enzyme hydrolysis is a biological modification for increasing the hydration and adsorption properties of fibres, with the advantages of environmental friendliness, low-energy consumption, selectivity, and mild operation.10–12 Cellulase and lignin enzyme hydrolysis can cause a rupture of the glycoside bonds of fibres, exposing more hydrophilic groups and increasing the contact area of fibres with adsorbed substances.7,13 Moreover, hydroxypropylation, acetylation, and carboxymethylation can induce the introduction of hydroxypropyl, ester and carboxymethyl groups into fibres, respectively. These introduced groups can improve the polarity of fibres, thereby improving their specific area, hydration properties, and adsorption capacities.14–16 However, the composite influence of these biological and chemical modifications on the adsorption capacity of fibres is small.
Red jujube (Ziziphus jujuba Mill.) is one of the main fruits in the north of China, with a cultivation history of around 4000 years.17 Jujube kernel, a potential dietary fibre resource, is rich in fibre, of which cellulose and lignin account for around 49 and 24 g 100 g−1, respectively.18 Every year, approximately 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of jujube kernels are produced in China, but only a small part is used as medicine for nourishing the liver, and calming the heart and mind, while the majority are discarded directly, causing serious environmental pollution problems.19,20 Although jujube kernel fibre (JKF) has a relatively high water sorption ability, its soluble fibre content (6.32 g 100 g−1) is low and its sorption abilities for oil and heavy metal ions are poor, which is not conducive for its applications as a wastewater adsorbent.21 Moreover, alkaline treatment has been shown to be effective at reducing the cellulose content of date-pit fibres,22 albeit there is scant data referring to the modifications of JKF, especially the synergistic effects of physical, chemical, and biological modifications for wastewater treatment. Thus, to improve the wastewater treatment capacity of JKF, mixed enzymatic hydrolysis (cellulase, hemicellulase, and laccase hydrolysis), mixed enzymatic hydrolysis assisted with acetylation or carboxymethylation were used to modify JKF. Changes in the structure, hydration properties, and the ability of purifying wastewater by these composite modification methods were investigated with the purpose of expanding the usage of JKF as a low-cost, biodegradable and renewable wastewater adsorbent.
000 tons of jujube kernels are produced in China, but only a small part is used as medicine for nourishing the liver, and calming the heart and mind, while the majority are discarded directly, causing serious environmental pollution problems.19,20 Although jujube kernel fibre (JKF) has a relatively high water sorption ability, its soluble fibre content (6.32 g 100 g−1) is low and its sorption abilities for oil and heavy metal ions are poor, which is not conducive for its applications as a wastewater adsorbent.21 Moreover, alkaline treatment has been shown to be effective at reducing the cellulose content of date-pit fibres,22 albeit there is scant data referring to the modifications of JKF, especially the synergistic effects of physical, chemical, and biological modifications for wastewater treatment. Thus, to improve the wastewater treatment capacity of JKF, mixed enzymatic hydrolysis (cellulase, hemicellulase, and laccase hydrolysis), mixed enzymatic hydrolysis assisted with acetylation or carboxymethylation were used to modify JKF. Changes in the structure, hydration properties, and the ability of purifying wastewater by these composite modification methods were investigated with the purpose of expanding the usage of JKF as a low-cost, biodegradable and renewable wastewater adsorbent.
2 Experimental
2.1. Materials
Jujube kernels were purchased from Zaohua Red Jujube Processing Factory, Taigu, China. Laccase (75 U g−1, where one unit corresponds to the amount of enzymes that can convert 1 μmol catechol per hour at pH 5.0 and 25 °C), hemicellulase (from Aspergillus niger, 2.0 × 105 U g−1, where one unit can liberate 1.0 μmol of D-galactose from hemicellulose per hour at pH 5.5 at 45 °C), cellulase (from Trichoderma Vride G, 3.0 × 105 U g−1, where one unit corresponds to the amount of enzymes that can convert 1 μmol substrate to produce glucose per hour at pH 4.8 and 50 °C), amyloglucosidase (Aspergillus niger, 1.0 × 105 U g−1, where one unit corresponds to the amount of enzymes that can convert 1 μmol soluble starch per minute at pH 4.6 and 40 °C), and α-amylase (Bacillus licheniformis, 1.0 × 105 U g−1, where one unit corresponds to the amount of enzymes that can convert 1 μmol starch to produce glucose per hour at pH 6.9 and 25 °C) were all purchased from Peisun Enzyme Company (Nanning, China). Monochloroacetic acid, isopropenyl acrylate, and other analytical reagents were obtained from Maoyuan Chemical Reagent Company (Tianjin, China).2.2. JKF preparation
Jujube kernel was dried at 45 °C for 12 h, and then ground and sieved using a XL-30C grinder (Xulang Machinery, Guangzhou, China) and a sieve (aperture: 150 μm) (Yushang Sieve Instrument, Shaoxing, China) in sequence. Next, 80 g of the ground powder, phosphate buffer (100 μmol L−1, 1 L), and α-amylase (2.4 g) were added and mixed in a triangular glass bottle.15 The mixture in the bottle was shaken with an HEZ-004C shaker (Shangxu Instrument Factory, Shangyu, China) at 225 rpm and 90 °C, and the pH value of the mixture was maintained at 5.0 through adjusting the pH with NaOH (100 mmol L−1) or HCl (100 mmol L−1) every 30 min. Then, 90 min later, the mixture was adjusted to pH 7.0 and papain (1.2 g) was added. The mixture in the bottle was continuously shaken at 225 rpm (50 °C, 120 min). Afterward the pH value of the mixture was lowered to 4.0, and then glucoamylase (1.2 g) was added. The bottle was shaken in the HEZ-004C shaker at 225 rpm and 60 °C for 120 min, and then heated in boiling water for 10 min. Finally, the reaction mixture was filtered with a filter paper (Whatman 1004-047), and the residue on the paper was dried in a GHZ-43B blast drying oven (45 °C, 6 h). The dried residue was ground and passed through a sieve (aperture: 150 μm), and JKF was obtained.2.3. Mixed enzymatic hydrolysis of JKF
In a glass bottle, JKF (20 g), 400 mL of buffer (pH 5.0, 0.1 mol L−1), 0.15 g of cellulase, and 0.05 g of hemicellulase were mixed. The suspension in the bottle was shaken in the EZH-004C shaker (48 °C and 225 rpm) for 2 h. NaOH (0.1 mol L−1) was used for adjusting the pH value of the suspension to 6.5 ± 0.1 and the reaction temperature was adjusted to 35.0 °C, and then laccase (25 U g−1 JKF) was added. The reaction suspension was shaken at 225 rpm for 2 h, and then heated in boiling water for 10 min. Afterward, the suspension was filtered with filter paper (Whatman1004-047), and the residue on the paper was dried in the GHZ-43B blast drying oven (52 °C, 6 h). The dried residue was ground and passed through a sieve (aperture: 150 μm), and the mixed enzymatic hydrolyzed jujube kernel fibre (JKF-E) was obtained.2.4. Acetylation of JKF-E
Here, 20 g of JKF-E was placed in a triangular flask, and dimethyl sulfoxide (270 mL) was slowly added to the inside of the flask.15 The suspension in the bottle was shaken at 70 °C in the HEZ-004C shaker with a stirring speed of 45 rpm. Then, 3 h later, the temperature was lowered to 40 °C, and then sodium carbonate (0.3 g) and 7.4 mol L−1 isopropenyl acetate (16 mL) were added. The mixture was continuously shaken at 45 rpm and 40 °C in the oscillator for 70 h, and then filtered with filter paper (Whatman1004-047). Isopropanol (60 mL) was used to purify the residue on the filter paper in triplicate. Then the residue was dried at 48 °C for 7 h and the jujube kernel fibres modified by mixed enzymatic hydrolysis and acetylation (JKF-EA) was obtained.2.5. Carboxymethylation of JKF-E
The carboxymethylation of OPKEF-GE was performed by slight modification of the procedure of Zhang et al.23 First, 20 g of OPKEF-GE was alkalized using 70 mmol L−1 of NaOH (dissolved in 85% ethanol, v/v) at 35 °C for 70 min. Afterward, chloroacetic acid (3.5 mmol L−1, dissolved in 85% ethanol solution) was added. The reaction mixture was shaken in the EZH-004C shaker at a temperature of 35 °C and shaking rate of 115 rpm. Then, 75 min later, the reaction mixture was slowly mixed with 45 mL of NaOH solution (3.4 mol L−1), and then continuously shaken at 53 °C and 115 rpm. Then, 4 h later, the mixture was slowly neutralized with acetic acid (8.75 mol L−1), and then centrifuged at 4100×g for 0.5 h. The obtained precipitate was purified through washing with anhydrous ethanol (15 mL each time), and dried at 50 °C for 7 h. JKF treated by mixed enzymatic hydrolysis and carboxymethylation (JKF-EC) was finally obtained. The substitution degree was defined as the increment in carboxymethyl group content of JKF-E after carboxymethylation.242.6. Determination of the chemical constituent
The methods AOAC.955.04, AOAC.92.05, AOAC.924.05, and AOAC.920.39 were used to determine the contents of protein, fat, ash, and moisture of the jujube kernel fibres, respectively.25 The cellulose, lignin, and hemicellulose contents were measured according to the method of Zhu et al.9 Moreover, the AOAC.991.43 method was employed to determine the insoluble and total fibre contents, and the soluble fibre content is the difference between the total fibre content and the insoluble fibre content.25 Moreover, phenolic content determination was conducted with the Folin–Ciocalteu method.262.7. Determination of the particle size
JKFs were dispersed in distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 g mL−1) and then analysed with a WNINER 336B nanometre size analysis system (Xinna Particle Instrument Factory, Chengdu, China).15 The particle size was represented by the Sauter mean diameter (D3,2, μm) and the refractive index was 1.33. Moreover, the specific surface area (m2 kg−1) of the samples was read.
25 g mL−1) and then analysed with a WNINER 336B nanometre size analysis system (Xinna Particle Instrument Factory, Chengdu, China).15 The particle size was represented by the Sauter mean diameter (D3,2, μm) and the refractive index was 1.33. Moreover, the specific surface area (m2 kg−1) of the samples was read.
2.8. Changes in the structure of JKF after modification
2.9. Water absorption and expansion capacities
In a graduated centrifugal tube, dried JKFs were dispersed in distilled water with a mass to volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. The dispersion was placed at 25 °C for 230 min, and then centrifuged at 1500 g for 25 min.27 The upper water was removed, and the wet JFKs were weighed. The water absorption capacity (WAC) was calculated as follows:
15. The dispersion was placed at 25 °C for 230 min, and then centrifuged at 1500 g for 25 min.27 The upper water was removed, and the wet JFKs were weighed. The water absorption capacity (WAC) was calculated as follows:| WAC (g g−1) = (Ww − Wd)/Wd | (1) |
Moreover, 3 g of dry JKFs was placed in a glass cylinder with a plug and the volume was read (Vd, mL). Next, 54 mL distilled water was added and gently mixed for 3 min, and then the cylinder was placed at 25 °C for 20 h. Afterward the volume of the wet JKFs was read (Vw, mL), and the water expansion ability (WEA) was calculated as follows:
| WEA (mL g−1) = (Vw − Vd)/Wd | (2) |
2.10. Surface hydrophobicity
As per the method of He et al.,28 JKF and the modified JKFs (JKF-E, JKF-EA, and JKF-EC) were dispersed in 0.1 mol L−1 of phosphate buffer (pH 7.4) for formulating solutions of different concentrations (20–500 μg mL−1). Next, 2.5 mL of the JKFs solution was reacted with 25 μL of 1-anilino-8-naphthalenesulfonate (10 mmol mL−1) at 25 °C for 2 min. Then the fluorescence intensity of the reaction solution was investigated using a DOLOBF93 spectrophotometer (Lengguang Technology and Science Co. Ltd, Shanghai, China) with an emission wavelength of 470 nm and an excitation wavelength of 390 nm. The regression curve between the fluorescent intensity of the JKFs' solution and their concentration was drawn, and the initial slope of the curve was defined as the surface hydrophobicity.2.11. Adsorption properties
| OAA (g g−1) = (W2 − W1 − W3)/W1 | (3) |
| ACLI (mg g−1) = (C0 − CS) × 220/W | (4) |
| ACCI (mg g−1) = (Co − Cf) × 125/W | (5) |
2.12. Statistics analysis
The mean and standard deviation for all the data were obtained by duplicating all the experiments at least three times. Statistical differences were analysed by analysis of the variance (ANOVA) and Duncan test with the SPSS Mas program (SPSS Inc., Chicago, America). The threshold for significant difference (p < 0.05) was 95%.3 Results and discussion
3.1. Effects of different modifications on the chemical components of the JKFs
The carboxymethylated degree of JKF-EC was 4.73% ± 0.22%, suggesting that a part of the hydrogen bonds in JKF had been replaced by carboxymethyl groups. Compared with hydrogen bonds, carboxymethyl groups have higher hydrophilicity, which could probably improve the water solubility of JKF. The acetylated degree of JKF-EA was 1.87 ± 0.13%, indicating that a part of the hydrogen bonds in JKF had been replaced by acyl ester bonds, which was conducive to the hydrophobicity of DFs.31 Moreover, the composite modification methods used in this study (mixed enzymatic hydrolysis, enzymatic hydrolysis assisted with acetylation or carboxymethylation) had no obvious impact on the fat, protein, or moisture contents (p > 0.05), because the difference between JKF, JKF-E, JKF-EA, and JKF-EC was not significant (p > 0.05, Table 1). By contrast, these modification methods all showed remarkably enhanced soluble fibre content of JKF (p < 0.05). Cellulase and laccase can cause a degradation of the glycosidic bonds, which can release more hydrophilic groups and increase the soluble fibre content of JKE.27| Constituent | Jujube kernel | JKF | JKF-E | JKF-EC | JKF-EA |
|---|---|---|---|---|---|
| a Different small letters (a–e) in the same line indicate significant difference (p < 0.05). | |||||
| Moisture (g 100 g−1) | 5.25 ± 0.47a | 6.38 ± 0.11a | 5.37 ± 0.09a | 4.29 ± 0.20a | 5.12 ± 0.15a |
| Fat (g 100 g−1) | 2.74 ± 0.25a | 1.93 ± 0.27b | 1.24 ± 0.05b | 0.97 ± 0.05b | 1.01 ± 0.06b |
| Ash (g 100 g−1) | 0.81 ± 0.11b | 0.95 ± 0.04b | 1.51 ± 0.06a | 1.28 ± 0.09a | 1.41 ± 0.15a |
| Protein (g 100 g−1) | 0.87 ± 0.09a | 0.38 ± 0.06a | 0.45 ± 0.03a | 0.39 ± 0.03a | 0.62 ± 0.06a |
| Total fiber (g 100 g−1) | 87.36 ± 2.74b | 89.76 ± 2.56a | 91.36 ± 2.69a | 92.19 ± 4.52a | 90.64 ± 3.73a |
| Insoluble fiber (g 100 g−1) | 81.87 ± 1.65a | 83.44 ± 3.43a | 77.72 ± 3.48b | 73.87 ± 1.19a | 76.64 ± 2.57b |
| Soluble fiber (g 100 g−1) | 5.49 ± 0.24c | 6.32 ± 0.33c | 13.64 ± 1.42b | 18.32 ± 0.82a | 14.00 ± 1.22b |
| Extractable polyphenols (mg g−1) | 3.46 ± 0.22d | 5.82 ± 0.37c | 12.65 ± 0.16a | 9.56 ± 0.37b | 10.07 ± 0.45b |
| Cellulose (g 100 g−1) | 40.83 ± 1.64a | 41.82 ± 1.43a | 34.27 ± 1.25b | 35.45 ± 0.08b | 31.91 ± 1.02b |
| Hemicellulose (g 100 g−1) | 24.25 ± 0.39a | 24.67 ± 0.78a | 17.58 ± 0.50b | 17.49 ± 0.32b | 18.05 ± 0.67b |
| Lignin (g 100 g−1) | 24.37 ± 3.14a | 26.17 ± 3.27a | 14.34 ± 0.78b | 9.43 ± 0.37c | 8.39 ± 0.47c |
Moreover, the soluble fibre content of JKF-EC (18.32 ± 0.82 g 100 g−1) was much higher than that of JKF-E (p < 0.05), indicating that carboxymethylation significantly enhanced the effects of the mixed enzymatic hydrolysis to improve the polarity of JKF. The introduced carboxymethyl group had a relatively high polarity.16 In comparison, the soluble content of JKE-SGEA was not different from that of JKE-SGE (p > 0.05). Previous studies showed that acyl ester bonds could increase the hydrophobicity of fibres,15,16,32 therefore acetylation reduced the effect of mixed enzymatic hydrolysis on the polarity of JKF.
In addition, the cellulose, hemicellulose, and lignin contents of JKF were all decreased after the three modifications (p < 0.05), corresponding to their lower insoluble fibre content (Table 1). Cellulase, hemicellulase, and laccase can cause a degradation of cellulose, hemicellulose, and lignin,33 resulting in a decreased insoluble fibre content and increased soluble fibre content. Furthermore, the soluble fibre contents of JKF, JKF-EC, and JKF-EA (13.64–18.32 g 100 g−1) were much higher than those of rice bran fibre (3.03 g 100 g−1),7 coconut mesocarp fibre (4.01 g 100 g−1),27 soybean hulls fibre (1.76 g 100 g−1),9 and sugarcane bagasse fibre (3.12 g 100 g−1),6 highlighting their relatively high hydration properties.
Apart from that, the extractable polyphenol content of JKF was 3.46 ± 0.22 mg g−1, which was in accordance with the result of Ji et al.21 The extractable polyphenol contents of JKF-E, JKF-EA, and JKF-EC were all higher than that of JKF (p < 0.05), probably due to laccase hydrolysis. Laccase causes a degradation of lignin (polyphenol polymers) and thus significantly increases the extractable phenols.12
3.2. Effects of different modifications on particle size of JKF
The results in Table 2 show that mixed enzymatic hydrolysis, and mixed enzymatic hydrolysis combined with acetylation or carboxymethylation increased the surface area of the JKF and reduced its particle size (p < 0.05). Mixed enzymatic hydrolysis can cause the breakdown of the fibre cell well and degradation of the polysaccharide chains,11 resulting in a reduction of JKF's particle size. The surface area of JKF-EA (146.37 ± 3.72 m2 kg−1) was higher than that of the other JKFs, and its particle size (63.77 ± 1.53 μm) was lower than that of the other JKFs (p < 0.05). An increase in surface area can improve the contact chance between JKF and oil or heavy metal ions, leading to an improvement of its sorption capacity.30| Properties | JKF | JKF-E | JKF-EC | JKF-EA |
|---|---|---|---|---|
| a Different small letters (a–d) in the same line indicate significant difference (p < 0.05). | ||||
| D3,2 (μm) | 132.94 ± 3.62a | 73.35 ± 4.27c | 87.99 ± 3.39b | 63.77 ± 1.53d |
| Surface area (m2 kg−1) | 51.46 ± 1.28d | 129.22 ± 5.68b | 114.95 ± 1.73c | 146.37 ± 3.72a |
| Water-absorption capacity (g g−1) | 4.46 ± 0.30c | 7.73 ± 0.28b | 10.66 ± 0.45a | 6.47 ± 0.49b |
| Water-expansion ability (mL g−1) | 4.20 ± 0.17c | 6.42 ± 0.16b | 8.50 ± 0.20a | 5.95 ± 0.20b |
| Viscosity (cp) | 9.83 ± 0.04c | 14.34 ± 0.12b | 17.93 ± 0.84a | 16.71 ± 0.22a |
| Surface hydrophobicity | 36.50 ± 3.44b | 28.65 ± 2.59c | 22.42 ± 1.62d | 43.57 ± 3.36a |
| Oil-adsorption ability (g g−1) | 1.56 ± 0.21c | 1.32 ± 0.07c | 2.62 ± 0.12b | 4.47 ± 0.34a |
| Adsorbing capacity of nitrite ions (μmol g−1) | 3.37 ± 0.17c | 10.57 ± 0.34a | 9.69 ± 0.32a | 7.24 ± 0.33b |
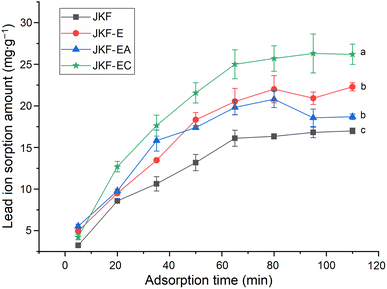
| Equilibrium sorption amount of Pb2+ (mg g−1) | 12.43 ± 0.35d | 17.22 ± 1.52c | 29.85 ± 1.64a | 25.61 ± 1.44b |
| Equilibrium sorption amount of Cu2+ (mg g−1) | 11.44 ± 1.08d | 15.97 ± 0.98c | 23.47 ± 1.33b | 19.69 ± 1.99a |
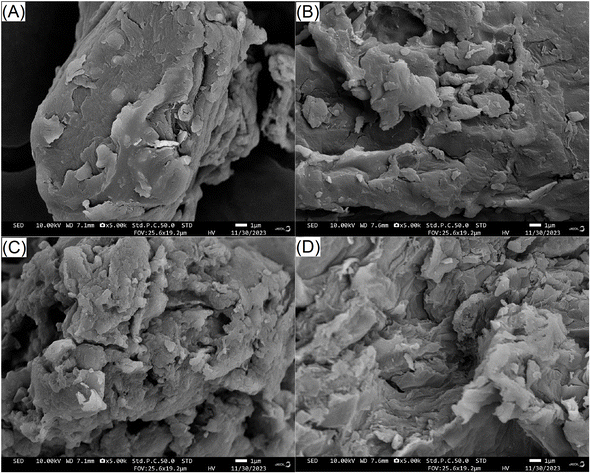
3.3. Effects of different modifications on the structure of JKF
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively) in the spectrum of JKF separately appeared at 1762, 1037, and 1508 cm−1 in the spectrum of JKE-SGEA,6 demonstrating that acyl ester groups had been introduced into the JKF molecules after acetylation. Additionally, a new peak appeared compared with the spectrum of JKF, with the spectrum of JKF-EC showing this new peak at around 964 cm−1, which was attributed to the introduction of the carboxymethyl group after carboxymethylation.2 Previous studies have shown that both acetylation and carboxymethylation could influence the structure of the fibres by changing their chemical bonds.23,24
O, respectively) in the spectrum of JKF separately appeared at 1762, 1037, and 1508 cm−1 in the spectrum of JKE-SGEA,6 demonstrating that acyl ester groups had been introduced into the JKF molecules after acetylation. Additionally, a new peak appeared compared with the spectrum of JKF, with the spectrum of JKF-EC showing this new peak at around 964 cm−1, which was attributed to the introduction of the carboxymethyl group after carboxymethylation.2 Previous studies have shown that both acetylation and carboxymethylation could influence the structure of the fibres by changing their chemical bonds.23,24
3.4. Water adsorption and expansion capacities
High WAC and WEA mean fibres have a larger expansion volume in wastewater, which is helpful for the adsorption of heavy metal ions, oil, sugar, and nitrite ions on the fibres.36 The results in Table 2 show that mixed enzymatic hydrolysis, and mixed enzymatic hydrolysis combined with carboxymethylation or acetylation all showed enhanced WACs and WEAs compared to JKF (p < 0.05), which could be mainly attributed to the improvement of the soluble fibre content and the changes in the surface area and microstructure of JKF after these modifications. Their higher soluble fibre contents (Table 1) mean that JKF-E, JKF-EA, and JKF-EC have a stronger affinity with water molecules than JKF. Moreover, the larger surface area and more porous microstructures (Fig. 1B, C and Table 2) can ensure that JKF-E, JKF-EA, and JKF-EC have more contact chance and stronger interactions with water molecules.11JKF-EC exhibited the highest WAC (10.66 g g−1) and the largest expansion volume (8.50 mL g−1) among these JKFs, corresponding to its highest soluble fibre content (18.32 ± 0.82 g 100 g−1), evidencing that mixed enzymatic hydrolysis combined with carboxymethylation was more effective for improving the water adsorption and expansion capacities of JKF than mixed enzymatic hydrolysis alone. The main reason for this was that after carboxymethylation, the introduced carboxymethyl groups can significantly increase the polarity of JKF, and enhance the steric hindrance between its polysaccharide chains, both of which play an important role in the WAC and WEA of the fibres.1 Moreover, JKF-EA also had a higher WAC and WEA than JKF (p < 0.05), but these values were not significantly different from those of JKF-E (p > 0.05), probably due to the introduced acyl ester groups having a relatively high hydrophobicity rather than hydrophilicity.14 A higher WEA is helpful for JKF to expand its structure, and contact and adsorb pollutants in effluents.
3.5. Surface hydrophobicity
Surface hydrophobicity is positively correlated with the chemical adsorption of hydrophobic harmful substances in wastewater on fibres.1 The results in Table 2 show that the surface hydrophobicities of JKF-E and JKF-EC were both lower than that of JKF (p < 0.05), highlighting that mixed enzymolysis and mixed enzymolysis combined with carboxymethylation both reduced the hydrophobicity of JKF. Mixed enzymatic hydrolysis causes degradation of the glycosidic bonds of JKF and releases more hydrophilic groups,10,37 and the introduced phosphate groups increases the hydrophilicity of JKF,15 which all contribute to the lower surface hydrophobicities of JKF-E and JKF-EC. However, a lower surface hydrophobicity is not conducive to the treatment of oil wastewater. In contrast, the surface hydrophobicity of JKF-EA was significantly higher than that of JKF (p < 0.05), because the introduced acyl ester group after acetylation have a relatively high hydrophobicity.29 Previous studies have found that acetylation improved the surface hydrophobicity of banana and oil palm empty fruit bunch fibres, too.14,383.6. Adsorption capacity
4 Conclusions
Mixed enzymatic hydrolysis alone or combined with acetylation or carboxymethylation increased the surface area and made the microstructure of JKF more porous, and improved its soluble fibre content, and water adsorption and expansion capacities. Mixed enzymatic hydrolysis combined with acetylation remarkably enhanced the surface hydrophobicity and oil-adsorption ability of JKF; while mixed enzymatic hydrolysis combined with carboxymethylation significantly increased the solubility and hydration properties of JKF, and improved its chelating abilities toward glucose, copper, and lead ions. Moreover, mixed enzymatic hydrolyzed JKF showed the highest nitrite ions-adsorption ability. Thus, JKF-EC, JKF-EA, and JKF all exhibited potential as wastewater adsorbents. However, the adsorption kinetics and mechanism of these modified JKFs on pollutants in wastewater, and the chemical oxygen demand of the effluents after the treatment of JKFs at the optimum conditions need further study.Data availability
The data supporting this article have been included as part of the manuscript.Author contributions
Nan Qin: conceptualization, writing – original draft, funding acquisition. Yunfei Li: investigation, methodology, writing – original draft. Lan Zhang: investigation, methodology. Lili Guo: validation, writing—review and editing; Wenfang Zhang: investigation, methodology. Guanwen Li: validation, writing—review and editing. Jun Bai: methodology, validation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Shanxi University of Chinese Medicine Science and Technology Innovation Team (2022TD2008), and the special fund for Science and Technology Innovation Teams of Shanxi Province (202304051001043).References
- F. Haq, S. Mehmood, M. Haroon, M. Kiran, K. Waseem, T. Aziz and A. Farid, Role of starch based materials as a bio-sorbents for the removal of dyes and heavy metals from wastewater, J. Polym. Environ., 2022, 30(5), 1730–1748, DOI:10.1007/s10924-021-02337-6.
- A. Akinterinwa, E. Oladele, A. Adebayo, E. Gurgur, O. O. Iyanu and O. Ajayi, Cross-linked-substituted (esterified/etherified) starch derivatives as aqueous heavy metal ion adsorbent: A review, Water Sci. Technol., 2020, 82(1), 1–26, DOI:10.2166/wst.2020.332.
- R. T. Prabha and T. H. Udayashankara, Adsorption of copper metal ions from aqueous solution using rice husk and groundnut shell, Int. J. Sci. Res., 2014, 8, 705–709, DOI:10.1080/19443994.2014.914446.
- N. A. Abd El-Ghany, M. H. A. Elella, H. M. Abdallah, M. S. Mostafa and M. Samy, Recent advances in various starch formulation for wastewater purification via adsorption technique: A review, J. Polym. Environ., 2023, 31(7), 2792–2825, DOI:10.1007/s10924-023-02798-x.
- S. R. Kim, J. Y. Park and E. Y. Park, Effect of ethanol, phytic acid and citric acid treatment on the physicochemical and heavy metal adsorption properties of corn starch, Food Chem., 2024, 431, 137167, DOI:10.1016/j.foodchem.2023.137167.
- D. I. L. Gil-López, J. A. Lois-Correa, M. E. Sánchez-Pardo, M. A. Domínguez-Crespo, A. M. Torres-Huerta, A. E. Rodríguez-Salazar and V. N. Orta-Guzmán, Production of dietary fibers from sugarcane bagasse and sugarcane tops using microwave-assisted alkaline treatments, Ind. Crops Prod., 2019, 135, 159–169, DOI:10.1016/j.indcrop.2019.04.042.
- D. Zadeike, R. Vaitkeviciene, R. Degutyte, J. Bendoraitiene, Z. Rukuiziene and D. Cernauskas, et al., A comparative study on the structural and functional properties of water-soluble and alkali-soluble dietary fibers from rice bran after hot-water, ultrasound, hydrolysis by cellulase, and combined pre-treatments, Int. J. Food Sci. Technol., 2021, 57(2), 15480, DOI:10.1111/ijfs.15480.
- Z. Raji, A. Karim, A. Karam and S. Khalloufi, A review on the heavy metal adsorption capacity of dietary fibers derived from agro-based wastes: Opportunities and challenges for practical applications in the food industry, Trends Food Sci. Technol., 2023, 137, 74–91, DOI:10.1016/j.tifs.2023.05.004.
- L. Zhu, B. Yu, H. Chen, J. Yu, H. Yan, Y. Luo, J. He, Z. Huang, P. Zheng, X. Mao, J. Luo and D. Chen, Comparisons of the micronization, steam explosion, and gamma irradiation treatment on chemical composition, structure, physicochemical properties, and in vitro digestibility of dietary fiber from soybean hulls, Food Chem., 2022, 366, 130618, DOI:10.1016/j.foodchem.2021.130618.
- H. Zohaib, I. Muhammad, A. M. Haseeb and K. M. Kamran, Ultrasound-Assisted Modification of Insoluble Dietary Fiber from Chia (Salvia hispanica L.) Seeds, J. Food Qual., 2021, 5035299, DOI:10.1155/2021/5035299.
- Y. Liu, S. Yi, T. Ye, Y. Leng, M. A. Hossen, D. E. Sameen and W. Qin, Effects of superfine-grinding and homogenization on physicochemical properties of okara dietary fibers for 3D printing cookies, Ultrason. Sonochem., 2021, 77, 105693, DOI:10.1016/j.ultsonch.2021.105693.
- S. Zhang, Z. Dong, J. Shi, C. Yang, Y. Fang, G. Chen, H. Chen and C. Tian, Enzymatic hydrolysis of corn stover lignin by laccase, lignin peroxidase, and manganese peroxidase, Bioresour. Technol., 2022, 361, 127699, DOI:10.1016/j.biortech.2022.127699.
- J. Y. Si, C. R. Yang, Y. Chen, J. H. Xie, S. L. Tian, Y. A. Cheng, X. B. Hu and Q. Yu, Structural properties and adsorption capacities of Mesona chinensis Benth residues dietary fiber prepared by cellulase treatment assisted by Aspergillus niger or Trichoderma reesei, Food Chem., 2023, 407, 135149, DOI:10.1016/j.foodchem.2022.135149.
- K. Rani, T. Gomathi, K. Vijayalakshmi, M. Saranya and P. N. Sudha, Banana fiber Cellulose Nano Crystals grafted with butyl acrylate for heavy metal lead (II) removal, Int. J. Biol. Macromol., 2019, 131, 461–472, DOI:10.1016/j.ijbiomac.2019.03.064.
- Y. Zheng, B. Xu, P. Shi, H. Tian, Y. Li, X. Wang, S. Wu and P. Liang, The influences of acetylation, hydroxypropylation, enzymatic hydrolysis and crosslinking on improved adsorption capacities and in vitro hypoglycemic properties of millet bran dietary fibre, Food Chem., 2022, 368, 130883, DOI:10.1016/j.foodchem.2021.130883.
- P. Kanwar, R. B. Yadav and B. S. Yadav, Cross-linking, carboxymethylation and hydroxypropylation treatment to sorghum dietary fiber: Effect on physicochemical, micro structural and thermal properties, Int. J. Biol. Macromol., 2023, 233, 123638, DOI:10.1016/j.ijbiomac.2023.123638.
- W. Cai, F. Tang, Z. Guo, Q. Zhang, X. Zhao, M. Ning and C. Shan, Effects of pretreatment methods and leaching methods on jujube wine quality detected by electronic senses and HS–SPME–GC–MS, Food Chem., 2020, 330, 127330, DOI:10.1016/j.foodchem.2020.127330.
- P. Agrawal, T. Singh, D. Pathak and H. Chopra, An updated review of Ziziphus jujube: Major focus on its phytochemicals and pharmacological properties, Pharmacol. Res. – Mod. Chin. Med., 2023, 8, 100297, DOI:10.1016/j.prmcm.2023.100297.
- L. Bai, H. Zhang, Q. Liu, Y. Zhao, X. Cui, S. Guo, L. Zhang, C. T. Ho and N. Bai, Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujube, Food Funct., 2016, 7, 2870–2877, 10.1039/C6FO00613B.
- A. K. Rashwan, N. Karim, M. R. I. Shishir, T. Bao, Y. Lu and W. Chen, Jujube fruit: A potential nutritious fruit for the development of functional food products, J. Funct. Foods, 2020, 75, 104205, DOI:10.1016/j.jff.2020.104205.
- X. Ji, C. Hou, Y. Yan, M. Shi and Y. Liu, Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit, Int. J. Biol. Macromol., 2020, 149, 1008–1018, DOI:10.1016/j.ijbiomac.2020.02.018.
- M. Al-Khalili, N. Al-Habsi, A. Al-Alawi, L. Al-Subhi, M. T. Z. Myint, M. Al-Abri, M. I. Waly, S. Al-Harthi, A. Al-Mamun and M. S. Rahman, Structural characteristics of alkaline treated fibers from date-pits: Residual and precipitated fibers at different pH, Bioact. Carbohydr. Diet. Fibre, 2021, 25, 100251, DOI:10.1016/j.bcdf.2020.100251.
- M. Y. Zhang, A. M. Liao, K. Thakur, J. H. Huang, J. G. Zhang and Z. J. Wei, Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution, Food Chem., 2019, 297, 124983, DOI:10.1016/j.foodchem.2019.124983.
- Y. Zheng, J. Li, X. Wang, M. Guo, C. Cheng and Y. Zhang, Effects of three biological combined with chemical methods on the microstructure, physicochemical properties and antioxidant activity of millet bran dietary fibre, Food Chem., 2023, 411, 135503, DOI:10.1016/j.foodchem.2023.135503.
- AOAC, Official Methods of Analysis, Association of Official Analytical Chemists, Washington, DC, 2020 Search PubMed.
- V. V. Cecilia, G. V. R. María, A. R. Carolina, L. C. Jorge, G.-G. Teresa, A. Roberto, O. P. García, J. L. Rosado, C. M. López-Sabater, A. I. Castellote, H. M. A. Monteayor and K. d. l. T. Carbot, Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin-Ciocalteu method, Food Chem., 2015, 176, 480–486, DOI:10.1016/j.foodchem.2014.12.050.
- Y. J. Zheng and Y. Li, Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution, Food Chem., 2018, 257, 135–142, DOI:10.1016/j.foodchem.2018.03.012.
- K. He, Q. Li, Y. Li, B. Li and S. Liu, Water-insoluble dietary fibers from bamboo shoot used as plant food particles for the stabilization of O/W Pickering emulsion, Food Chem., 2020, 310, 125925, DOI:10.1016/j.foodchem.2019.125925.
- R. Asadpour, S. Yavari, H. Kamyab, V. Ashokkumar, S. Chelliapan and A. Yuzir, Study of oil sorption behaviour of esterified oil palm empty fruit bunch (OPEFB) fibre and its kinetics and isotherm studies, Environ. Technol. Innovation, 2021, 22, 101397, DOI:10.1016/j.eti.2021.101397.
- M. Hua, J. Lu, D. Qu, C. Liu, L. Zhang, S. Li, J. Chen and Y. Sun, Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient, Food Chem., 2019, 286, 522–529, DOI:10.1016/j.foodchem.2019.01.114.
- K. Gao, T. Liu, Q. Zhang, Y. Wang, X. Song, X. Luo, R. Ruan, L. Deng, X. Cui and Y. Liu, Stabilization of emulsions prepared by ball milling and cellulase treated pomelo peel insoluble dietary fiber: Integrity of porous fiber structure dominates the stability, Food Chem., 2024, 440, 138189, DOI:10.1016/j.foodchem.2023.138189.
- R. Nasseri, R. Ngunjiri, C. Moresoli, A. Yu, Z. Yuan and C. Xu, Poly(lactic acid)/acetylated starch blends: Effect of starch acetylation on the material properties, Carbohydr. Polym., 2020, 229, 115453, DOI:10.1016/j.carbpol.2019.115453.
- M. Ma and T. Mu, Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure, Carbohydr. Polym., 2016, 136, 87–94, DOI:10.1016/j.carbpol.2015.09.030.
- X. Qin, C. Yang, J. Si, Y. Chen, J. Xie, J. Tang, X. Dong, Y. Cheng, X. Hu and Q. Yu, Fortified yogurt with high-quality dietary fiber prepared from the by-products of grapefruit by superfine grinding combined with fermentation treatment, LWT–Food Sci. Technol., 2023, 188, 115396, DOI:10.1016/j.lwt.2023.115396.
- B. J. Hazarika and N. Sit, Effect of dual modification with hydroxypropylation and cross-linking on physicochemical properties of taro starch, Carbohydr. Polym., 2016, 140(20), 269–278, DOI:10.1016/j.carbpol.2015.12.055.
- E. Miehle, M. Hass, S. Bader-Mittermaier and P. Eisner, The role of hydration properties of soluble dietary fibers on glucose diffusion, Food Hydrocolloids, 2022, 131, 107822, DOI:10.1016/j.foodhyd.2022.107822.
- E. Backes, C. G. Kato, R. C. G. Corrêa, R. F. P. M. Moreira, R. A. Peralta, L. Barros, I. G. F. R. Ferreira, G. M. Zanin, A. Bracht and R. M. Peralta, Laccases in food processing: Current status, bottlenecks and perspectives, Trends Food Sci. Technol., 2021, 115, 445–460, DOI:10.1016/j.tifs.2021.06.052.
- Y. J. Zheng, Y. Li and H. L. Tian, Effects of carboxymethylation, acidic treatment, hydroxypropylation and heating combined with enzymatic hydrolysis on structural and physicochemical properties of palm kernel expeller dietary fiber, LWT–Food Sci. Technol., 2020, 133, 109909, DOI:10.1016/j.lwt.2020.109909.
- X. Xia, F. Li, H. Ran, J. Zhao, X. Lei, L. Lei, J. Wen, G. Xiao, K. Zeng and J. Ming, Effect of jujube kernel powder addition on moisture absorption performance, color stability, texture properties and agglomeration characteristics of jujube powder, LWT–Food Sci. Technol., 2023, 174, 114452, DOI:10.1016/j.lwt.2023.114452.
- J. Q. Sang, L. Li, J. Wen, H. C. Liu, J. J. Wu, Y. S. Yu, Y. J. Xu, Q. Gu, M. Fu and X. Lin, Chemical composition, structural and functional properties of insoluble dietary fiber obtained from the Shatian pomelo peel sponge layer using different modification methods, LWT–Food Sci. Technol., 2022, 165, 113737, DOI:10.1016/j.lwt.2022.113737.
- A. Campos, A. R. Sena Neto, V. B. Rodrigues, B. R. Luchesi, J. M. Mattoso and J. M. Marconcini, Effect of raw and chemically treated oil palm mesocarp fibers on thermoplastic cassava starch properties, Ind. Crops Prod., 2018, 124, 149–154, DOI:10.1016/j.indcrop.2018.07.075.
- F. A. Borges, L. M. Costa, C. R. T. Tarley, G. de Fatima Lima Martins and E. C. Figueiredo, Lead determination in commercial juice samples by direct magnetic sorbent sampling flame atomic absorption spectrometry (DMSS-FAAS), Food Chem., 2023, 413, 135676, DOI:10.1016/j.foodchem.2023.135676.
| This journal is © The Royal Society of Chemistry 2024 |