Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
3-Sulfopropyl acrylate potassium-based polyelectrolyte hydrogels: sterilizable synthetic material for biomedical application
Johanna Romischke a,
Thomas Eicknerb,
Niels Grabow
a,
Thomas Eicknerb,
Niels Grabow bc,
Udo Kragl
bc,
Udo Kragl ac and
Stefan Oschatz
ac and
Stefan Oschatz *b
*b
aUniversity of Rostock, Institute of Chemistry, Department of Industrial and Analytical Chemistry, Albert-Einstein-Str. 3A, Rostock 18059, Germany
bRostock University Medical Center, Institute for Biomedical Engineering, Friedrich-Barnewitz-Str. 4, 18119 Rostock, Germany. E-mail: stefan.oschatz@uni-rostock.de
cDepartment Life, Light & Matter (LLM), University of Rostock, Rostock, Germany
First published on 11th September 2024
Abstract
Hydrogels are extensively used in the biomedical field due to their highly valued properties, biocompatibility and antimicrobial activity and resistance to rheological stress. However, determining an efficient sterilization protocol that does not compromise the functional properties of hydrogels is one of the challenges researchers face when developing a material for a medical application. In this work, conventional sterilization methods (steam-, radiation- and gas sterilization) were investigated regarding the influence on the degree of swelling, mechanical performance and chemical effects on the poly 3-sulfopropyl acrylate potassium (pAESO3) hydrogel, which is a promising representative for biomedical engineering applications. In summary, no significant changes in the gel properties were observed after sterilization, showing the potential of the selected hydrogel for biomedical applications.
Introduction
Alongside several other abilities, such as high biocompatibility, resistance to rheological stresses, antimicrobial activity and self-healing capabilities, the high swelling ability is one of the valued properties of hydrogels. This makes this class of materials remarkably versatile for biomedical applications. In particular, hydrogels based on polymerized ionic liquids have been shown to be promising for lubricating coatings or wound dressings, and as drug delivery systems, which was shown before for polymerized acrylate-based sulfonates.1–3 Although previous studies on biocompatibility and antibacterial properties suggest further applications in the biomedical field, sterilizability is a mandatory parameter for further clinical uses. Sterilization processes are much more aggressive than simple disinfection. In the literature, the sterilization of sensitive materials is described as one of the most difficult tasks to complete, due to the effects of high temperature or radiation on their structure. Possible consequences could be the hydrolysis and degradation of the polymer during the process.4,5 There are many examples in the literature that show how strongly the sterilization method can influence the properties of gels.6–8 This often leads to a reduction in water absorption capacity or changes in mechanical properties.9,10 As sterilization aims to completely destroy microorganisms and pathogens, the treatment is usually chemically aggressive and can therefore have a negative impact on the material to be sterilized.In previous studies, poly-(3-sulfopropyl acrylate potassium) crosslinked with PEGDA (pAESO3) has shown good mechanical resilience with high compressibility paired with non-excessive swelling behaviour, good cell adhesion and biocompatibility, making it promising in the context of biomedical applications, e.g. as a drug delivery system for ion exchange controlled release.2,3 In this context, there is great interest in investigating how common sterilization protocols affect the properties of this particular material, as sterilisability is an important process in the use of medical materials. A few studies on hydrogels based on acrylates/bisacrylates have already been published in the literature, which have achieved similar results or have to struggle with some challenges, such as a decrease in water absorption capacity or changes in the elasticity mode.8,11 In order to assess the extent to which pAESO3 hydrogels are resistant to sterilization, three relevant sterilization methods in the field of biomedical device manufacturing, autoclaving, ethylene oxide (ETO) gassing and gamma irradiation, were tested on pAESO3. The influence on the swelling properties as well as on the mechanical conditions and the composition in general was investigated.
In particular autoclaving is frequently used in research laboratories and for medical instruments. It is a fast, inexpensive and widely available method. Another major advantage of autoclaving is that there are no toxic residues to be expected as in the case with e.g., ETO sterilization. However, autoclaving is associated with the disadvantage that, in the case of hydrogels, the saturated water vapor atmosphere can lead to uncontrolled water uptake. In addition, steam and heat can drastically alter the mechanical and chemical properties of many biomaterials. Including synthetic ones, in particular when it comes to materials degradable or susceptible to hydrolysis.12 Overall, autoclaving has a high penetrability but takes long time to implement.13,14
ETO, as the second relevant sterilization method, places high demands on laboratory equipment in terms of safety and is time consuming. Moreover, ETO sterilization requires gas-permeable packaging. Therefore, the hydrogel samples must be dried prior to sterilization process in order to avoid changes in the material due to uncontrolled water loss over the transport period or sterilization process and to ensure comparability. Gamma irradiation is particularly practical as the devices and products to be sterilized can be stored in various containers, e.g., glass bottles, as the radiation treatment penetrates a range of materials. Especially for hydrogels, this enables the sterilization of already hydrated or swollen samples in sealed bottles or beakers, avoiding the need for additional treatments or drying and rehydrating steps. However, the radiation energy, usually in a range of several kGy, can damage the polymer-based material. With gamma irradiation, sterilization without toxic residues can be assumed with a high penetrating power. Unfortunately this method often affects the physiochemical and structural properties of the material.15
Other sterilization techniques are also of high relevance in everyday medical practice e.g., disinfection using EtOH, plasma-based sterilization and electron beam sterilization. However, we have not opted for these methods due to the fact that EtOH must diffuse completely into the hydrogel for complete sterilization. This leads to the need for complete removal when it comes to medical application. Plasma and ion beam-based sterilization mainly affect the surface of the hydrogel, whereas microbes are able to survive inside the gel bulk.
Materials and methods
Chemicals
Poly(ethyleneglycol)diacrylate (PEGDA, Mn = 575; SigmaAldrich), N,N,N′,N′-tetra-methylethylenediamine (TMEDA; ≥99.5%; VWR International), ammonium persulfate (APS; 98%; Acros Organics), 3-sulfopropylacrylate potassium salt (AESO3; 98%; SigmaAldrich) were used as received.For 1 L of PBS 8.00 g NaCl, 0.20 g KCl, 1.44 g Na2HPO4·2H2O and 0.12 g KH2PO4 are dissolved accordingly in ultrapure water and the pH of 7.4 is adjusted with 0.1 M HCl.
Hydrogel synthesis
The hydrogel was synthesized by radical polymerization as previously described.2,3 3-Sulfopropylacrylate potassium (AESO3) and PEGDA (Mn = 575, 2 mol%) were dissolved in ultrapure water (AESO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEGDA = 49
PEGDA = 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Subsequently, ammonium persulfate (APS) solution (0.1 mol%) was added and the reaction mixture was degassed for 15 min. After degassing, tetramethylethylene diamine (TMEDA, 1.9 mol% of the total monomer concentration, TMEDA
1). Subsequently, ammonium persulfate (APS) solution (0.1 mol%) was added and the reaction mixture was degassed for 15 min. After degassing, tetramethylethylene diamine (TMEDA, 1.9 mol% of the total monomer concentration, TMEDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APS = 5
APS = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as catalyst was added and the mixture was filled into was filled into syringes (10 mL) and rested for 24 h at 22 ± 2 °C.
1) as catalyst was added and the mixture was filled into was filled into syringes (10 mL) and rested for 24 h at 22 ± 2 °C.
Gamma sterilization
Gamma sterilization was performed by an external contractor (BBF Sterilizations Service GmbH, Kernen, Germany). Samples as obtained after synthesis without further treatment were placed in screw cap glass vials and exposed to a radiation dose ≥25 Gy, using a Co-60 radiation source.Ethylene oxide sterilization
ETO sterilization was performed by an external contractor (Medicoplast International GmbH, Illingen, Germany). Prior to sterilization, samples were dried at room temperature on air for 48 h and placed in a gas-permeable well plate, which was sealed in standard sterilization bags. According to the contractors information, samples were preconditioned for 16.75 h at 35–45 °C and 45–80% relative humidity. Following, samples were exposed to ETO gas at 44.5 °C and 191 mbar with 18 kg ETO mass. Finally, desorption took place for 3 days at 35–45 °C.Autoclaving sterilization
Autoclaving was carried out in a Tuttnauer 2540 ELV autoclave (Tuttnauer, Breda, Netherlands). Samples were placed in glass vials, which were loosely covered using aluminum foil. The samples were heated to 120 °C in 20 min in the autoclaving chamber, and the temperature was maintained for 20 min. After autoclaving, the samples were cooled to ambient conditions.Mechanical testing
Uniaxial compression testing was performed under ambient conditions using a ZwickiLine ZN 2.5 testing machine (Zwick/Roell, Ulm, Germany) equipped with a 1 kN load cell and two compression plates at a crosshead speed of v = 2 mm min−1 with n = 5. Force was measured as a function of compression. The compressive modulus (E) was determined in the linear elastic region between 0.05 to 0.25% compression by linear regression. From the collected data, the compression at break (εB) and ultimate compression strength (σm) were extracted. For the rehydration of the specimens for mechanical testing of autoclaving and ETO influence, specimens were rehydrated by placing the samples on a hydrated foam mat for several hours until adequately soft and weighed afterwards to determine the missing deionized water (DW) volume. Subsequently, the missing DW volume was added using a micropipette directly on the specimen. Cyclic mechanical testing was performed using the same set-up, applying up to 20% compression over n = 10 cycles. Parameters used were a preload of σ = 0.025 MPa, a crosshead speed for loading v = 5 mm min−1 and a crosshead speed for unloading of v = 10 mm min−1. Unloading was performed until σ = 0.025 MPa, after which the next cycle started.Gravimetric swelling experiments
Water uptake of pAESO3 hydrogel before and after sterilization was studied gravimetrically using PBS-buffer at 36 ± 1 °C as a function of time in a strainer. The weights of the swollen gels were determined at different intervals, after dripping through the strainer and dabbing the samples off, until the equilibrium swelling was attained. The degree of swelling (qt) was calculated according to the following equation:
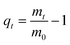 | (1) |
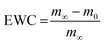 | (2) |
The kinetics of the polymer swelling was investigated in more detail an according to the calculations of one of our previous studies.16 In short, the process can be described as a second order process:
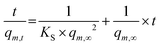 | (3) |
From the obtained slope and the y-intercepts of the linear regression of the experimental data, theoretical values of the initial swelling rates and the swelling rate constants KS were calculated.
Fourier transform infrared spectroscopy-attenuated total reflectance
Data were collected in the range of![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 400 cm−1 to 4000 cm−1 with a resolution of
= 400 cm−1 to 4000 cm−1 with a resolution of ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 4 cm−1 averaged over 32 scans in reflection mode using a Bruker Alpha T Nicolet 380 FT-IR with a Smart Orbit ATR-Unit. Atmospheric compensation has been performed. All spectra were subsequently baseline corrected and finally normalized with OPUS vector-normalization-function for better visualization.
= 4 cm−1 averaged over 32 scans in reflection mode using a Bruker Alpha T Nicolet 380 FT-IR with a Smart Orbit ATR-Unit. Atmospheric compensation has been performed. All spectra were subsequently baseline corrected and finally normalized with OPUS vector-normalization-function for better visualization.
DSC experiments
Thermal analysis was carried out using a DSC 1 Stare system (Mettler Toledo, Greifensee, Switzerland). Conventional calibration was performed using highly pure indium and zinc. Samples were heated from T = −10 °C to 310 °C with a heating rate q = 10 K min−1. Samples were dried prior to DSC analysis and sample weights were in the range of 3–10 mg.Statistical analysis
All data are given as mean ± standard deviation. For statistical analysis of swelling rate constant KS, initial swelling rate and mechanical parameters E, εB and σB, two tailed t-tests of the means versus unsterilised reference have been performed. For statistical analysis, GraphPad Prism software (V5.04, GraphPad Software Inc., Boston, MA, USA) has been used. Significances are given at a significance level of P < 0.05 and marked with an asterisk.Results and discussion
Synthesis of the hydrogels
Within this work, a hydrogel containing sulfonate groups was used. This system has shown good mechanical resilience with high compressibility paired with non-excessive swelling behaviour and good cell adhesion and biocompatibility properties.2 Furthermore, the sulfonate function enables ion exchange controlled drug release properties, which are of high interest in the field of the development of innovative drug delivery systems and medical device coatings. In brief, the hydrogel used in this study was synthesized by free radical polymerization starting from the monomer AESO3, using PEGDA (Mn = 575) as crosslinker and APS/TMEDA as initiator system (Fig. 1). PEGDA was chosen as crosslinker as it allows for in vivo degradation, which is highly beneficial as the implanted system vanishes from the body, enabling reduced long-term side effects from the foreign body reaction.17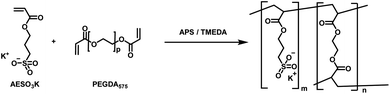 | ||
| Fig. 1 Synthesis of AESO3-PEGDA575-hydrogels with APS and TMEDA initiator system for radical polymerization. | ||
Handling of the hydrogels according to sterilization method
In contrast to gamma sterilization, where samples may be stored and treated in sealed vessels, the samples for ETO sterilization and autoclaving were dried prior to sterilization and rehydrated after sterilization before further testing. This is due to the fact that ETO sterilization requires gas permeable packaging to allow for the penetration of ETO gas during the sterilization, but leads to uncontrolled water evaporation during the whole process chain. For autoclaving, the process is carried out under a saturated steam atmosphere, which may lead to uncontrolled water uptake. To avoid uncontrolled swelling of the samples, the hydrogel specimens ns were dried before and after the sterilization process. For the mechanical testing, to restore the initial swelling state, the samples were rehydrated by adding the weight difference of deionized water to restore the initial swelling state. Notably, direct exposure to water, e.g., by adding the volume difference using a pipette, caused the samples to burst. We observed very quick swelling directly at the point of contact with the water, causing internal stress in the sample, leading to cracks. To avoid this, the samples were first slowly rehydrated by using a hydro foam mat and then, if necessary, additional water was added to achieve the desired degree of swelling.Degree of swelling characterization
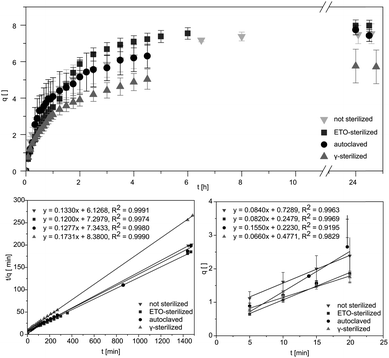
Studying the swelling behaviour is a feasible way to quickly detected material defects and serves to provide a fast characterization of the material. Data for equilibrium swelling of the synthesized hydrogels at 36 ± 1 °C are shown in Fig. 2.Investigation of swelling behaviour was performed in phosphate buffered saline (PBS). Untreated pAESO3 gel showed a degree of swelling up to q = 7.5 ± 0.5 within 390 min. Similar results were received for autoclaved hydrogels (Table 1). After ETO sterilization the samples showed a slightly faster swelling, but achieved a similar degree of swelling in equilibrium. From our data it became obvious that only gamma sterilization had the effect that the treated hydrogels showed reduced swelling up to q = 5.7 ± 0.9 within 270 min. It has been shown in the literature that the mechanical properties and swelling of hydrogels can be controlled by the use of different types of crosslinking molecules and by controlling the crosslinking density.18 It can therefore be assumed that the rather aggressive sterilization with high-energy gamma radiation leads to further linkages between monomer and crosslinker and thus increases the crosslinker density. As a result, less water can be absorbed and the degree of swelling is decreased. However, as the samples do not have to be dried for gamma sterilization, this method allows a more feasible handling of the hydrogels. Similar results were obtained by Kanjickal et al. when they sterilized their PEG hydrogels with ETO gassing, gamma radiation and H2O2. Sterilization with the first two methods mentioned led to a loss of swelling of the samples. They also suggest that this is due to the formation of free radicals, which can lead to further cross-linking in the polymer network. While sterilization with H2O2 led to the breaking up of the crosslinks and a higher degree of swelling.7 Eljarrat-Bienstock et al. even observed this effect after the autoclaving of their hydrogel sponges based on hydroxyethyl methacrylate (HEMA) crosslinked with ethylene glycol dimethylacrylate (EGDMA) samples. Here, the samples demonstrated a 10–12% decrease in their water absorption.11
| Sample | qm,∞ | Initial swelling rate [min−1] | KS [10−1] | EWC |
|---|---|---|---|---|
| Not sterilized | 7.5 ± 0.5 | 0.084 ± 0.02 | 1.33 ± 0.06 | 0.879 ± 0.007 |
| Autoclaved | 7.4 ± 0.3 | 0.115 ± 0.037 | 1.28 ± 0.05 | 0.881 ± 0.004 |
| ETO | 7.9 ± 0.3 | 0.082 ± 0.002 | 1.20 ± 0.05 | 0.888 ± 0.005 |
| Gamma | 5.7 ± 0.9 | 0.066 ± 0.004 | 1.73 ± 0.26 | 0.850 ± 0.023 |
From the obtained slope and the y-intercepts of the linear regression of the experimental data, theoretical values of the initial swelling rates and the swelling rate constants KS could be calculated (Fig. 2). The swelling rate describes the absorption of water of the hydrogel and the swelling process. It depends on the surface area to volume ratio and is also affected by the initial swelling status of the hydrogel as well as the structure of the gel. Based on thermodynamic theories the focus is more on initial states and equilibrium end-states.19 However, focused control of hydrogel swelling rates based on hydrogel structure has potential for application in both drug delivery and biomechanical actuation.19 The initial swelling rate describes the initially linear increase in the swelling curve within the first 20 minutes. By means of these parameters, conclusions can be drawn to evaluate the effects of the sterilization processes on the material at a molecular level, as cross-linking or degradation would directly influence the swelling behaviour of the hydrogel.
Additionally, the swelling rate constant was identified and the equilibrium water content (EWC) was calculated using eqn (2), representing water that was absorbed from the hydrogels in the equilibrium state, as summarized in Table 1. The EWC represents water that was absorbed from the hydrogels in the equilibrium state. It is calculated by the ratio of the mass of the gel in equilibrium subtracted by the dry mass to the mass of the gel in equilibrium.
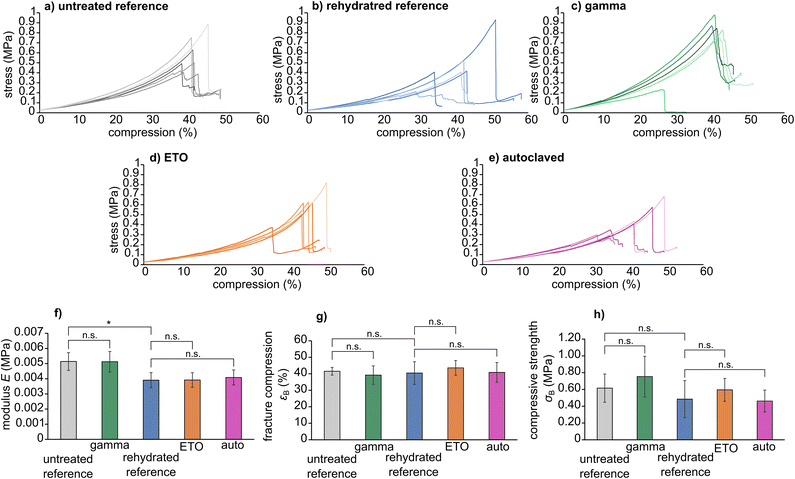
Mechanical characterization
The stress–compression curves of the sterilized samples are shown in Fig. 3. In particular, the modulus E, fracture compression εB and compressive strength σB are viable parameters for determining the influence of the sterilization method on the molecular integrity of the hydrogel, leading for example to chain breaks or crosslinking. For the presented specimens, statistical analysis showed that rehydration leads to a slight decrease in compression modulus E compared to the untreated reference (ERef = 0.0051 ± 0.0005 MPa versus Erehydrated = 0.00039 ± 0.0005 MPa with P = 0.0078). However, no significant differences were found between the respective reference groups and the sterilized samples (untreated reference versus gamma sterilized samples, and rehydrated reference versus ETO sterilized samples and samples sterilized by autoclaving). Thus, we conclude that the three sterilization methods do not have a major effect on the material's mechanical properties. The mechanical parameters extracted from compression curves are given in Table 2.| Reference | Gamma | Rehydrated | ETO | Autoclave | |
|---|---|---|---|---|---|
| E (MPa) | 0.0051 ± 0.0005 | 0.0051 ± 0.0007 | 0.0039 ± 0.0005 | 0.0039 ± 0.0005 | 0.0041 ± 0.0005 |
| εB (%) | 41.5 ± 2.3 | 39.2 ± 5.6 | 40.4 ± 6.9 | 43.6 ± 4.5 | 40.8 ± 6.0 |
| σB (MPa) | 0.61 ± 0.17 | 0.75 ± 0.24 | 0.48 ± 0.22 | 0.59 ± 0.14 | 0.46 ± 0.13 |
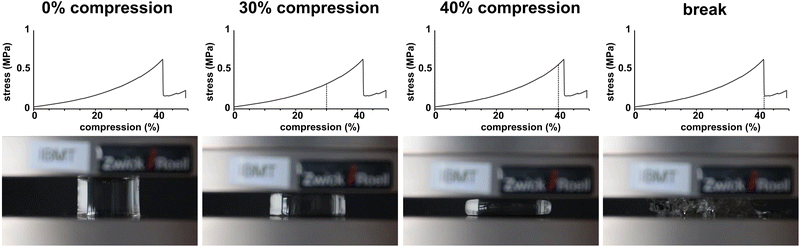
All samples showed similar compression behaviour as shown exemplarily for the untreated reference in Fig. 4 for 0%, 30%, 40% compression and directly at εB. Overall, the materials deform elastically until spontaneously fracture. Where the samples did not show partial breaking during compression in the video capture, some samples, however, showed the formation of cracks visible as steps in the stress–compression curves (Fig. 3). Still, even with these small cracks, the samples were mechanically highly resilient. As this was comparable for all tests, irrespective whether sterilized or not, only one set of images is provided.
Compared to other hydrogel materials reported in the literature, these results are of great significance. Gelatine methacryloyl hydrogels have already shown a significantly smaller compressive modulus after autoclaving.8 This behaviour was also observed after radiation treatment. Shi et al. also discovered a correlation between the mechanical changes and the dose of radiation applied to their polyvinyl alcohol-based gels. The compressive strength and the compressive modulus increase with increasing radiation dose, but a decrease is observed at doses higher than 100 kGy.20
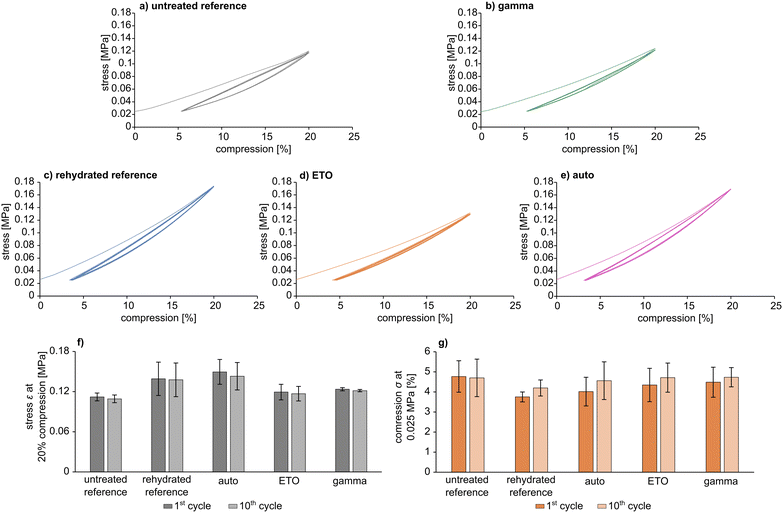
Cyclic tests (Fig. 5) showed comparable behaviour to the uniaxial mechanical test under load for all samples (Fig. 3). In particular, pAESO3 hydrogel did not show any creep behaviour after 10 cycles with 20% compression, even after sterilization. However, specimens that exhibited partial fracture, which was also observable in the uniaxial test and can be recognised as peaks in the stress–compression curves, were not included in the material evaluation, although the material still responded to the cyclic test. Differences in ε and σ were observed between the individual samples, which can presumably be attributed to intrinsic material differences. Nevertheless, the values of stress ε at 20% compression and compression σ at 0.025 MPa were in a comparable range for all cycles, and it was observed that sterilization did not affect the mechanical strength.
Fourier transform infrared spectroscopy
The results of the FT-IR measurements are shown in Fig. 6.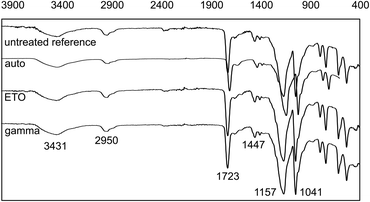 | ||
| Fig. 6 FT-IR-spectra of dried AESO3-PEGDA-hydrogels without and after sterilization. Wavenumbers (cm−1) for relevant bands are shown. | ||
The characteristic bands with comparable intensity can be clearly seen. Differences in the FT-IR were not observed. Potential influences on the chemical structure of the hydrogels due to sterilization are, in particular, additional crosslinking of unreacted PEGDA or the polymer network itself through chain scission and recombination reactions. As no observable changes in the IR spectra, such as additional signals or altered signal intensities, were detected, we conclude based on FT-IR analysis that the selected sterilization methods have no pronounced influence on the chemical composition of the material.
The previously assumed fundamental changes in the gel structure due to gamma irradiation cannot be confirmed by the IR measurements either. It can therefore be assumed on the basis of this additional analysis method that the type of sterilization has no influence on the basic composition of the gel samples. An overview of the assignment of the signals to the respective functional groups can be found in Table 3.
| Identification | Wavenumber (cm−1) | Chemical group |
|---|---|---|
| 1 | 3431 | O–H from the intermolecular and intramolecular hydrogen bonds |
| 2 | 2950 | C–H from alkyl groups |
| 3 | 1723 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| 4 | 1447 | CH2 |
| 5 | 1157 & 1041 | C–O–C |
| 6 | 880–700 | C–H (di- or trisubstituted) |
Thermal analysis
DSC analysis of the pAESO3 hydrogel showed no characteristic melting or crystallisation processes that could be identified by peaks in the endothermic or exothermic range in the heating curve (Fig. 7).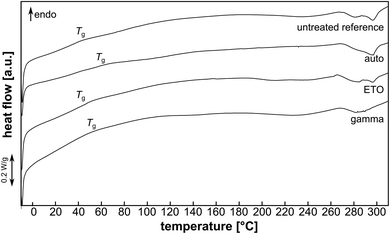 | ||
| Fig. 7 Exemplary heating curves of pAESO3-PEGDA575 hydrogel without and after sterilization. Position of glass transition (Tg) is indicated. | ||
However, when heated to approx. 270 °C, decomposition was observed, which became violent and rapid and vulcanisation of the hydrogel was observed when heated to 370 °C (not shown). The glass transition temperature Tg was comparable for the pAESO3 hydrogel before and after ETO sterilization (Table 4). Gamma sterilization and autoclaving led to a slight increase in Tg.
| Reference | Auto | ETO | Gamma | |
|---|---|---|---|---|
| Tg [°C] | 47.5 ± 2.5 | 59.6 ± 2.6 | 45.9 ± 1.2 | 52.0 ± 3.2 |
This could be due to reduced chain mobility after hydrolysis (auto) or chain scission (gamma) of the PEDGA crosslinker, but this minor degradation did not show to affect mechanical or swelling behaviour (Fig. 2, 3 & 5).22,23
Conclusions
Within this work, we showed that pAESO3 hydrogels are applicable for clinical relevant sterilization methods. Influence of sterilization on hydrogel properties was investigated with regard to mechanical characteristics and swelling behaviour. Our finding was that for ETO sterilization and autoclaving no significant influence on the hydrogel properties were found. However, a lower degree of swelling was detected for gamma-sterilization. The lower water uptake compared to the other sterilized samples may be caused from additional crosslinking of the polymer network by the energy rich radiation. To what extent the hydrogel polymer was affected, however, was below the detection limit of IR spectroscopy. As far as the mechanical properties are concerned, no changes in compressibility were detectable in the gamma-sterilized samples either. Moreover, sterilization did not show to affect the materials mechanical properties concerning cyclic mechanical load. Thermal analysis revealed a slight degradation caused by autoclaving, but this did not appear to have a strong negative impact on the general material properties, particularly the mechanical resilience. In summary, pAESO3 hydrogels are well suited for being sterilised by common sterilization methods.Data availability
Data for this article are available at Zenodo at DOI: https://doi.org/10.5281/zenodo.13384842.Author contributions
Conceptualization: J. R. & S. O.; data curation: J. R.; formal analysis: J. R. & S. O.; funding acquisition: N. G. & U. K; investigation: J. R. & S. O.; methodology: J. R. & S. O.; project administration: T. E.; resources: N. G. & U. K.; supervision: N. G. & U. K.; validation: all; visualization: S. O.; writing—original draft preparation: J. R. & S. O.; writing—review and editing: all. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was funded by Federal Ministry of Education and Research (BMBF) within RESPONSE “Partnership for Innovation in Implant Technology”, grant numbers 03ZZ0910B and 03ZZ0933L. The work and assistance of Daniela Koper, Richard Tröltzsch and Tom Kunde are gratefully acknowledged.References
- N. Nikfarjam, M. Ghomi, T. Agarwal, M. Hassanpour, E. Sharifi, D. Khorsandi, M. Ali Khan, F. Rossi, A. Rossetti, E. Nazarzadeh Zare, N. Rabiee, D. Afshar, M. Vosough, T. Kumar Maiti, V. Mattoli, E. Lichtfouse, F. R. Tay and P. Makvandi, Adv. Funct. Mater., 2021, 31, 2104148 CrossRef CAS.
- J. Claus, A. Brietzke, C. Lehnert, S. Oschatz, N. Grabow and U. Kragl, PLoS One, 2020, 15, e0231421 CrossRef CAS PubMed.
- J. Claus, T. Eickner, N. Grabow, U. Kragl and S. Oschatz, Macromol. Biosci., 2020, 20, e2000152 CrossRef PubMed.
- A. Bernhardt, M. Wehrl, B. Paul, T. Hochmuth, M. Schumacher, K. Schütz and M. Gelinsky, PLoS One, 2015, 10, e0129205 CrossRef PubMed.
- D. Kanjickal, S. Lopina, M. M. Evancho-Chapman, S. Schmidt, J. J. Inbaraj, T. B. Cardon and G. A. Lorigan, J. Biomed. Mater. Res., Part A, 2009, 88, 409–418 CrossRef PubMed.
- S. S. Karajanagi, R. Yoganathan, R. Mammucari, H. Park, J. Cox, S. M. Zeitels, R. Langer and N. R. Foster, Biotechnol. Bioeng., 2011, 108, 1716–1725 CrossRef CAS PubMed.
- D. Kanjickal, S. Lopina, M. M. Evancho-Chapman, S. Schmidt and D. Donovan, J. Biomed. Mater. Res., Part A, 2008, 87, 608–617 CrossRef PubMed.
- M. Rizwan, S. W. Chan, P. A. Comeau, T. L. Willett and E. K. F. Yim, Biomed. Mater., 2020, 15, 65017 CrossRef CAS PubMed.
- N. Haridas and M. J. Rosemary, Polym. Degrad. Stab., 2019, 163, 220–227 CrossRef CAS.
- E. Tichý, A. Murányi and J. Pšenková, Adv. Polym. Technol., 2016, 35, 198–207 CrossRef.
- E. Eljarrat-Binstock, A. Bentolila, N. Kumar, H. Harel and A. J. Domb, Polym. Adv. Technol., 2007, 18, 720–730 CrossRef CAS.
- G. Kubyshkina, B. Zupančič, M. Štukelj, D. Grošelj, L. Marion and I. Emri, J. Biomater. Nanobiotechnol., 2011, 02, 361–368 CrossRef CAS.
- Z. Dai, J. Ronholm, Y. Tian, B. Sethi and X. Cao, J. Tissue Eng., 2016, 7, 2041731416648810 Search PubMed.
- R. Galante, T. J. A. Pinto, R. Colaço and A. P. Serro, J. Biomed. Mater. Res., Part B, 2018, 106, 2472–2492 CrossRef CAS PubMed.
- C. S. A. Bento, M. C. Gaspar, P. Coimbra, H. C. de Sousa and M. E M Braga, Int. J. Pharm., 2023, 634, 122671 CrossRef PubMed.
- A. Jastram, T. Lindner, C. Luebbert, G. Sadowski and U. Kragl, Polymers, 2021, 13, 1834 CrossRef CAS PubMed.
- M. B. Browning, S. N. Cereceres, P. T. Luong and E. M. Cosgriff-Hernandez, J. Biomed. Mater. Res., Part A, 2014, 102, 4244–4251 CAS.
- K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- H. Omidian, S. A. Hashemi, P. G. Sammes and I. Meldrum, Polymer, 1998, 39, 6697–6704 CrossRef CAS.
- Y. Shi, D. Xiong and J. Zhang, Tribol. Int., 2014, 78, 60–67 CrossRef CAS.
- H. S. Mansur, C. M. Sadahira, A. N. Souza and A. A. Mansur, Mater. Sci. Eng., C, 2008, 28, 539–548 CrossRef CAS.
- G. Burke, V. Barron, T. Geever, L. Geever, D. M. Devine and C. L. Higginbotham, J. Mech. Behav. Biomed. Mater., 2019, 99, 1–10 CrossRef CAS PubMed.
- T. Şener Raman, M. Kuehnert, O. Daikos, T. Scherzer, C. Krömmelbein, S. G. Mayr, B. Abel and A. Schulze, Front. Chem., 2022, 10, 1094981 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |