 Open Access Article
Open Access ArticleSynthesis of star-shaped poly(lactide)s, poly(valerolactone)s and poly(caprolactone)s via ROP catalyzed by N-donor tin(II) cations and comparison of their wetting properties with linear analogues†
Miroslav Novák *a,
Yaraslava Milasheuskayab,
Michael Srbb,
Štěpán Podzimek
*a,
Yaraslava Milasheuskayab,
Michael Srbb,
Štěpán Podzimek a,
Marek Bouškac and
Roman Jambor
a,
Marek Bouškac and
Roman Jambor b
b
aInstitute of Chemistry and Technology of Macromolecular Materials, Faculty of Chemical Technology, University of Pardubice, Studentská 573, 53210 Pardubice, Czech Republic. E-mail: miroslav.novak@upce.cz
bDepartment of General and Inorganic Chemistry, Faculty of Chemical Technology, University of Pardubice, Studentská 573, 53210 Pardubice, Czech Republic
cDepartment of Graphic Arts and Photophysics, Faculty of Chemical Technology, University of Pardubice, Studentská 573, 53210 Pardubice, Czech Republic
First published on 24th July 2024
Abstract
In this study, we report the use of N-coordinated tin(II) cations [L1→Sn(H2O)][OTf]2·THF (1) and [L1→SnCl][SnCl3] (2) (L1 = 1,2-(C5H4N-2-CH = N)2CH2CH2) as efficient ROP catalysts, which, in combination with benzyl alcohol, afford well-defined linear poly(ε-caprolactone) (PCL) and poly(δ-valerolactones) (PVL) via an activated monomer mechanism (AMM). Thanks to the versatility of complexes 1 and 2 as catalysts, star-shaped PCL, PVL and PLA were also prepared using three-, four-, five- and six-functional alcohols. The number of arms was determined by SEC-MALS-Visco analysis. Spin-coated thin layers of linear and selected six-armed polymers were further studied in terms of their wettability to water. Attention was focused on the influence of the composition and structure of the polymers. Finally, to increase the hydrophobic properties of the studied polymers, stannaboroxines L2(Ph)Sn[(OB-(C6H4-4-CF3))2O] and L2(Ph)Sn[(OB-(C6H4-3,5-CF3)2)2O] (L2 = C6H3-2,6-(Me2NCH2)2) were applied.
Introduction
In past decades, linear aliphatic polyesters, such as poly(lactide) (PLA) or poly(ε-caprolactone) (PCL), have received great attention due to their biodegradable and biocompatible properties.1 For this reason, they are nowadays a very suitable alternative to classic petroleum-based plastics and, in particular, poly(lactides) have found application in many areas such as building and packaging materials, electronic components, pharmacy and medicine.2 Besides these applications, biodegradable polyesters are considered as relatively hydrophobic materials and for this reason they have found an attractive application in the preparation of non-fluorinated hydrophobic materials, i.e. water-repellent, self-cleaning materials or membranes in oil-water separation, etc.3 The classification of the hydrophobic properties of materials is based on the measurement of the contact angle of water (WCA, θ), for example by the sessile drop method. The final wetting properties depend not only on the type of polymer and thus their surface energy, but also on the topography of the studied substrate, which is associated with the fabrication method. In the case of PCL, the most hydrophobic materials were prepared by electrospinning, when a series of nanofibers, meshes, scaffold, sheets and tissues was obtained.3b–h These materials showed WCAs in the range of 113–136°.3b–h The electrospinning method has also become an excellent way to manufacture hydrophobic and superhydrophobic PLA-based materials. It has been reported that PLA nanofibers prepared in this way show WCA = 152° and the application of these fibers on a cellulosic cotton substrate provides a very effective membrane for water-oil separation with an efficiency of 99.16%.3m A similar results on hydrophobic electrospun-made PLA membranes and fibers was demonstrated by Zhang and Opaprakasit, who reported materials with WCAs of 133° and 110°, respectively.3n,o On the other hand, the electrospinning process is more complex and not all PLA-based materials show a hydrophobic character. Recently, PLA fibers with a flat and smooth surface have been produced, resulting in a WCA of 86.7°.3k Besides the electrospinning process, a simple approach based on the solvent casting method of dioxane solution of PLA were implemented to obtain superhydrophobic PLA surfaces.3i While the film obtained only from dioxane solution exhibited WCA = 66.6°, the precipitation of PLA from dioxane-water or dioxane-ethanol solutions in the combination with the gelation in air led to a WCA higher than 150°.3iThe hydrophobic properties of biodegradable polymers could be influenced not only by their surface energy and topography, but also by the structure of the polymer. In particular, star-shaped polymers have become the object of great interest due to their unique structure and topological properties, which cannot be achieved in the case of linear polymers.4 These polymers are composed of several linear arms radiating from central core, which causes a high proportion of chain ends and a higher concentration of functional groups than in their linear analogues of the same molar mass.
Two possible polymerization techniques are applicable to produce star-shaped polyesters. The first one is based on polycondensation of dicarboxylic acids with polyalcohols, which, however, yield polymers with Mn < 4700 g mol−1.5 On the other, an incorporation of catalysts based on p-toluenesulfonic acid6 or triphenylphosphonium trifluoromethanesulfonate7 led to the isolation PLAs with Mn up to 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. The second and more widespread method is the ring-opening polymerization (ROP) of cyclic esters operating by the coordination insertion or activated monomer mechanism.8 This method is popular mainly due to the excellent control, robustness, versatility, and simple reaction setup. Besides the monomer, the key components of the ROP are initiator and catalyst.
000 g mol−1. The second and more widespread method is the ring-opening polymerization (ROP) of cyclic esters operating by the coordination insertion or activated monomer mechanism.8 This method is popular mainly due to the excellent control, robustness, versatility, and simple reaction setup. Besides the monomer, the key components of the ROP are initiator and catalyst.
Almost all reports used Sn(Oct)2 to catalysed the formation star-shaped polyesters.9 Sn(Oct)2, operating via coordination-insertion mechanism, is a very efficient catalyst and it has low toxicity as reported by the Food and Drug Administration (FDA, USA) for biomedical applications.10 For the synthesis of star-shaped polymer, the polymerization of L-LA in the bulk with Sn(Oct)2 as catalyst was usually carried out at 130 °C.11 Further, other tin compounds as spirocyclic tin initiators based on tin-substituted polyethylene ethoxylate,12 cyclic stannoxane,13 tin acetylacetonate,14 tetraphenyltin15 were have also been used with success. Besides tin compounds, other catalysts such as calcium hydride,16 potassium hexamethyldisilazide17 and bismuth(III) acetate18 were also employed. Further, more biologically friendly aluminium salen and salan,19,20 zinc amino-, thio-phenolate or zinc amido-oxazolinate21 and diiminate complex22 extended the range of metal mediators for the synthesis of star-shaped polyesters. In addition, Lewis acid catalysts based on tin and aluminum allowed control of polymerization and provided polymer stars with variable tactics.23
Recently, we reported the utilization of N-coordinated tin(II) cations [L1→Sn(H2O)][OTf]2·THF (1) and [L1→SnCl][SnCl3] (2) (L1 = 1,2-(C5H4N-2-CH = N)2CH2CH2) as the examples of Lewis acidic tin(II) catalysts in the ROP of L-LA (see Scheme 1).24 It has been demonstrated that polymerization operates via activated monomer mechanism and the kinetic studies indicated a pseudo-first order reaction, and thus a good control over the polymerization.
Therefore, we set out the study to prove that 1 and 2 are the universal catalysts for ROP of other monomers, δ-valerolactone (δ-VL) and ε-caprolactone (ε-CL), to prepare linear biodegradable polyesters.
In addition, the formation of activated monomer also opens the possibility to use different polyalcohols instead of benzylalcohol for the synthesis of star-shaped polymers. Thus, the application of 1 and 2 in the synthesis of star-shaped polyesters poly(L-lactide)s, poly(δ-valerolactone)s and poly(ε-caprolactone)s using glycerol, trimethylolpropane, triethanolamine, pentaerythritol, xylitol, myo-inositol, D-sorbitol and dipentaerythritol as different polyalcohols is another goal of this study. These experiments provided three-, four-, five- and six-armed stars. The linear and star-shaped polyesters were characterized by SEC-MALS-Visco analysis.
Further, since the linear PLA showed good water-repellent properties, we tested selected linear and star-shaped polyesters as hydrophobic materials. Due to the good solubility and film-forming properties, we prepared thin layers of selected linear and star-shaped polyesters by spin-coating method. The spin-coating method led to the preparation of uniform thin-layers, in which it was possible to study the hydrophobic properties only based on their composition and structure. Finally, the effect of stannaboroxines L2(Ph)Sn[(OB-(C6H4-4-CF3))2O] and L2(Ph)Sn[(OB-(C6H4-3,5-CF3)2)2O] (L2 = C6H3-2,6-(Me2NCH2)2)25a on the hydrophobic properties of thin layers of polyesters is also discussed. Both compounds with six-membered SnB2O3 central core were selected as properly soluble and good film-forming compounds,25a that structurally resemble polymer N-Boroxine-PDMS with six-membered B3O3 central core. This polymer in the combination with SiO2 nanoparticles was recently published as hydrophobic material with WCA = 160.8°.26
Results and discussion
Synthesis and characterization of linear polyesters PVL and PCL
Recently, compounds 1 and 2 proved to be effective catalysts in ROP of L-LA. The computational study confirmed that ROP operates via activated monomer mechanism. The kinetic studies indicated a pseudo-first order reaction with a similar polymerization rate (k = 5.44 × 10−2 min−1 for 1 and k = 4.93 × 10−2 min−1 for 2), and thus a good control over the polymerization. Complexes 1 and 2 are also universal in the ROP of other cyclic esters, which was demonstrated by the preparation of linear PVL and PCL (see Scheme 2).All polymerization tests were performed in bulk at 145 °C. δ-VL and ε-CL was purified by the distillation over CaH2 to avoid data fluctuations due to the variation of impurities in the technical grade monomers. The polymerization reactions were carried out at molar ratios of [catalyst]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [monomer] = 1
[monomer] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1
50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 1
100, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 1
200 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500. In all reactions, benzyl alcohol (BzOH) was added as an initiator in a molar ratio of 1
500. In all reactions, benzyl alcohol (BzOH) was added as an initiator in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 compared to the catalyst. The conversion a kinetics of the polymerization experiments was monitored by the 1H NMR spectroscopy and showed a similar process as in the case of PLA. The data thus demonstrated not only the same process, but also the same activated monomer mechanism. All isolated polymers were further characterized by the combined SEC-MALS-Visco analysis with the aim to determine the number-average molar mass (Mn) and dispersity (Ð). Results on polymerization tests are summarized in Table 1.
1 compared to the catalyst. The conversion a kinetics of the polymerization experiments was monitored by the 1H NMR spectroscopy and showed a similar process as in the case of PLA. The data thus demonstrated not only the same process, but also the same activated monomer mechanism. All isolated polymers were further characterized by the combined SEC-MALS-Visco analysis with the aim to determine the number-average molar mass (Mn) and dispersity (Ð). Results on polymerization tests are summarized in Table 1.
| Entry | Catalyst | [cat]:[BzOH]:[δ-VL] | Conva [%] | Mn,thb [g mol−1] | Mn,SECc [g mol−1] | Ð |
|---|---|---|---|---|---|---|
| a Measured by the 1H NMR spectroscopy.b Calculated Mn of PVL (g mol−1): [δ-VL]:[cat]·conv·M(δ-VL) + M(BzOH); calculated Mn of PCL (g mol−1): [ε-CL]:[cat]·conv·M(ε-CL) + M(BzOH).c Experimental Mn values were determined by SEC-MALS-VISCO analysis in THF solution. | ||||||
| 1 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
99 | 5100 | 4000 | 1.55 |
| 2 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
99 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5900 | 1.55 |
| 3 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
98 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.60 |
| 4 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
65 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.46 |
| 5 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
98 | 5000 | 4100 | 1.51 |
| 6 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
99 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7200 | 1.53 |
| 7 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
96 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.59 |
| 8 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
68 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.48 |
| Entry | Catalyst | [cat]:[BzOH]:[ε-CL] | Conva [%] | Mn,thb [g mol−1] | Mn,SECc [g mol−1] | Ð |
|---|---|---|---|---|---|---|
| 9 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
97 | 5600 | 5700 | 1.45 |
| 10 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
98 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
8000 | 1.55 |
| 11 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
99 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.58 |
| 12 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
78 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.46 |
| 13 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
98 | 5700 | 5900 | 1.42 |
| 14 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
98 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
9700 | 1.47 |
| 15 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
99 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.53 |
| 16 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
69 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.46 |
As in the case of polymerization of L-LA,24 the different charge of the tin atom in 1 and 2 does not have significant influence on the polymerization rate of δ-VL a ε-CL, since both catalysts 1 and 2 are very active and almost complete conversion of monomers (for molar ratio [catalyst]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [monomer] = 1
[monomer] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1
50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and 1
100 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) was occurred after 1 hour. However, for a molar ratio of 1
200) was occurred after 1 hour. However, for a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, the catalytic activity of 1 and 2 decreases, when after 1 hour the conversion was in the range of 65–78%. Furthermore, the isolated PVLs showed macromolecular parameters with only small deviations independently of the catalyst used. The determined Mn,SEC should correspond to Mn,th within the controlled living ROP, which is given by the equation Mn,th = [monomer]
500, the catalytic activity of 1 and 2 decreases, when after 1 hour the conversion was in the range of 65–78%. Furthermore, the isolated PVLs showed macromolecular parameters with only small deviations independently of the catalyst used. The determined Mn,SEC should correspond to Mn,th within the controlled living ROP, which is given by the equation Mn,th = [monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cat] × M(monomer) × conversion + M(alcohol). In our case, this condition is met, for both catalysts 1 and 2, especially by the molar ratio [cat]
[cat] × M(monomer) × conversion + M(alcohol). In our case, this condition is met, for both catalysts 1 and 2, especially by the molar ratio [cat]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [monomer] = 1
[monomer] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (and for PCL also 1
50 (and for PCL also 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and 1
100 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). The prepared PVLs and PCL (in the molar ratio of 1
200). The prepared PVLs and PCL (in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 1
100, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 1
200 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 for PVL and 1
500 for PVL and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 for PCL) show lower Mn,SEC than the theoretical ones, which can be caused by the traces of water in monomer and deactivation of catalyst (especially in a higher loading of monomer) resulting in the inhibition of the polymerization. So, 1 and 2 produces PVLs with Mn in the range of 4000–11
500 for PCL) show lower Mn,SEC than the theoretical ones, which can be caused by the traces of water in monomer and deactivation of catalyst (especially in a higher loading of monomer) resulting in the inhibition of the polymerization. So, 1 and 2 produces PVLs with Mn in the range of 4000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 for 1 and 4100–16
600 g mol−1 for 1 and 4100–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 for 2 and 5700–24
500 g mol−1 for 2 and 5700–24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 for 1 and 5900–16
700 g mol−1 for 1 and 5900–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 for 2 in the case of PCL. The dispersity Ð (1.46–1.60 for PVLs and 1.42–1.58 for PCLs) demonstrates a relatively uniform nature of polymers.
500 g mol−1 for 2 in the case of PCL. The dispersity Ð (1.46–1.60 for PVLs and 1.42–1.58 for PCLs) demonstrates a relatively uniform nature of polymers.
Based on these data, it can be concluded that the ROP of δ-VL and ε-CL catalysed by complexes 1 and 2 is relatively well controlled and the character of polymerization is very similar to L-LA polymerization catalysed by same complexes.24
Synthesis and characterization of star-shaped polyesters PLA, PVL and PCL
Compounds 1 and 2 showed the ability to polymerize L-LA, δ-VL and ε-CL via activated monomer mechanism providing linear biodegradable polyesters PLA, PVL and PCL. This mechanism opens the possibility of exchanging BzOH for polyalcohols, such as glycerol, trimethylolpropane, triethanolamine, pentaerythritol, xylitol, myo-inositol, D-sorbitol and dipentaerythritol as initiators. Therefore, 1 and 2 were tested in ROP of L-LA, δ-VL and ε-CL with these polyalcohols serving as the core in well-defined star-shaped polyesters (see Scheme 3).The experimental set up was the same as in the case of the synthesis of linear polyesters. The polymerization reactions were carried out in a molar ratio of [catalyst]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [initiator]
[initiator]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [monomer] = 1
[monomer] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/n
1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (where n = number of hydroxyl groups in the initiator). The conversion of the polymerization experiments was monitored by the 1H NMR spectroscopy. All isolated polymers were further characterized by the combined SEC-MALS-Visco analysis with the aim to determine the number-average molar mass (Mn,SEC) and dispersity (Ð). These two macromolecular parameters further served as criteria for the classification of complexes 1 and 2 as suitable catalysts for well-controlled ROP. Thus, a promising catalyst should produce polymers with Ð close to 1, indicating a uniform character of polymer. Furthermore, as already mentioned, Mn,SEC should ideally be equal to the theoretical value of Mn (Mn,th) within well-controlled ROP. Results of the polymerization tests are summarized in Tables 2–4.
100 (where n = number of hydroxyl groups in the initiator). The conversion of the polymerization experiments was monitored by the 1H NMR spectroscopy. All isolated polymers were further characterized by the combined SEC-MALS-Visco analysis with the aim to determine the number-average molar mass (Mn,SEC) and dispersity (Ð). These two macromolecular parameters further served as criteria for the classification of complexes 1 and 2 as suitable catalysts for well-controlled ROP. Thus, a promising catalyst should produce polymers with Ð close to 1, indicating a uniform character of polymer. Furthermore, as already mentioned, Mn,SEC should ideally be equal to the theoretical value of Mn (Mn,th) within well-controlled ROP. Results of the polymerization tests are summarized in Tables 2–4.
| Entry | Catalyst | Initiator | Conva [%] | Mn,thb [g mol−1] | Mn,SECc [g mol−1] | Ð | fd |
|---|---|---|---|---|---|---|---|
| a Measured by the 1H NMR spectroscopy.b Calculated Mn of PLA (g mol−1): [L-LA]:[cat]·conv·M(L-LA) + M(initiator).c Experimental Mn values were determined by SEC-MALS-VISCO analysis in THF solution.d Numbers in parentheses indicate the theoretical number of arms. | |||||||
| 1 | 1 | Glycerol | 96 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
6000 | 1.27 | 2.3 (3) |
| 2 | 1 | Trimethylolpropane | 85 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
5700 | 1.16 | 2.5 (3) |
| 3 | 1 | Triethanolamine | 96 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
6300 | 1.19 | 2.6 (3) |
| 4 | 1 | Pentaerythritol | 96 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
9000 | 1.70 | 3.1 (4) |
| 5 | 1 | Xylitol | 97 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7700 | 1.91 | 3.7 (5) |
| 6 | 1 | myo-Inositol | 88 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.51 | 2.7 (6) |
| 7 | 1 | D-Sorbitol | 96 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
8200 | 1.80 | 4.1 (6) |
| 8 (ref. 24) | 1 | Dipentaerythritol | 95 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
8500 | 1.53 | 6.1 (6) |
| 9 | 2 | Glycerol | 95 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
6800 | 1.09 | 2.6 (3) |
| 10 | 2 | Trimethylolpropane | 89 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
9600 | 1.22 | 2.7 (3) |
| 11 | 2 | Triethanolamine | 94 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.14 | 3.8 (3) |
| 12 | 2 | Pentaerythritol | 97 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.45 | 3.6 (4) |
| 13 | 2 | Xylitol | 97 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.32 | 4.4 (5) |
| 14 | 2 | myo-Inositol | 85 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
8500 | 1.22 | 4.1 (6) |
| 15 | 2 | D-Sorbitol | 94 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.57 | 5.2 (6) |
| 16 (ref. 24) | 2 | Dipentaerythritol | 96 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.15 | 7.2 (6) |
| 17 | Sn(Oct)2 | Dipentaerythritol | 99 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.54 | 3.9 (6) |
| Entry | Catalyst | Initiator | Conva [%] | Mn,thb [g mol−1] | Mn,SECc [g mol−1] | Ð | fd |
|---|---|---|---|---|---|---|---|
| a Measured by the 1H NMR spectroscopy.b Calculated Mn of PVL (g mol−1): [δ-VL]:[cat]·conv·M(δ-VL) + M(initiator).c Experimental Mn values were determined by SEC-MALS-VISCO analysis in THF solution.d Numbers in parentheses indicate the theoretical number of arms. | |||||||
| 1 | 1 | Glycerol | 93 | 9300 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.13 | 3.3 (3) |
| 2 | 1 | Trimethylolpropane | 90 | 9000 | 9300 | 1.15 | 3.1 (3) |
| 3 | 1 | Triethanolamine | 92 | 9200 | 9800 | 1.12 | 3.2 (3) |
| 4 | 1 | Pentaerythritol | 97 | 9700 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.14 | 3.4 (4) |
| 5 | 1 | Xylitol | 94 | 9400 | 9000 | 1.17 | 3.2 (5) |
| 6 | 1 | myo-Inositol | 90 | 9000 | 3800 | 1.15 | 2.9 (6) |
| 7 | 1 | D-Sorbitol | 91 | 9100 | 7600 | 1.17 | 3.4 (6) |
| 8 | 1 | Dipentaerythritol | 92 | 9200 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.19 | 4.2 (6) |
| 9 | 2 | Glycerol | 93 | 9300 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.96 | 7.3 (3) |
| 10 | 2 | Trimethylolpropane | 90 | 9000 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.77 | 8.0 (3) |
| 11 | 2 | Triethanolamine | 91 | 9100 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.87 | 7.2 (3) |
| 12 | 2 | Pentaerythritol | 96 | 9600 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.72 | 7.8 (4) |
| 13 | 2 | Xylitol | 95 | 9500 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.00 | 7.4 (5) |
| 14 | 2 | myo-Inositol | 87 | 8700 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.89 | 8.4 (6) |
| 15 | 2 | D-Sorbitol | 95 | 9500 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.13 | 8.1 (6) |
| 16 | 2 | Dipentaerythritol | 90 | 9000 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.26 | 12 (6) |
| 17 | Sn(Oct)2 | Dipentaerythritol | 93 | 9600 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.78 | 3.5 (6) |
| Entry | Catalyst | Initiator | Conva [%] | Mn,thb [g mol−1] | Mn,SECc [g mol−1] | Ð | fd |
|---|---|---|---|---|---|---|---|
| a Measured by the 1H NMR spectroscopy.b Calculated Mn of PCL (g mol−1): [ε-CL]:[cat]·conv·M(ε-CL) + M(initiator).c Experimental Mn values were determined by SEC-MALS-VISCO analysis in THF solution.d Numbers in parentheses indicate the theoretical number of arms. | |||||||
| 1 | 1 | Glycerol | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.43 | 2.5 (3) |
| 2 | 1 | Trimethylolpropane | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.68 | 2.8 (3) |
| 3 | 1 | Triethanolamine | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
8800 | 1.34 | 2.4 (3) |
| 4 | 1 | Pentaerythritol | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
9500 | 1.73 | 2.7 (4) |
| 5 | 1 | Xylitol | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
8600 | 1.57 | 2.8 (5) |
| 6 | 1 | myo-Inositol | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.52 | 2.3 (6) |
| 7 | 1 | D-Sorbitol | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.70 | 3.2 (6) |
| 8 | 1 | Dipentaerythritol | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
6200 | 1.90 | 3.2 (6) |
| 9 | 2 | Glycerol | 99 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
8300 | 1.63 | 7.1 (3) |
| 10 | 2 | Trimethylolpropane | 65 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.38 | 4.5 (3) |
| 11 | 2 | Triethanolamine | 95 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
8900 | 1.36 | 2.5 (3) |
| 12 | 2 | Pentaerythritol | 99 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
9900 | 1.72 | 9.5 (4) |
| 13 | 2 | Xylitol | 97 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
9700 | 3.18 | 14 (5) |
| 14 | 2 | myo-Inositol | 67 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.53 | 2.3 (6) |
| 15 | 2 | D-Sorbitol | 98 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.72 | 13 (6) |
| 16 | 2 | Dipentaerythritol | 97 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.45 | 9.8 (6) |
| 17 | Sn(Oct)2 | Dipentaerythritol | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.68 | 3.7 (6) |
For L-LA, the polymerization tests did not show any significant effect of the used catalyst on the dispersity Ð, since it is statistical across the obtained PLAs (range of 1.09–1.91) but shows a relatively uniform nature of polymers. The lowest dispersity Ð was achieved using catalytic system of 2 and glycerol (1.09, Table 2, entry 9). On the other, the complex 1 in the combination with xylitol produced polymer with the highest Ð (1.91, Table 2, entry 5). A certain influence of the catalyst is evident in the case of number-average molar mass, when the experimental Mn values (Mn,SEC) for PLAs produced using catalyst 1 generally reach approximately half of the theoretical ones (Mn,th). On the other hand, when catalyst 2 is used, the agreement of these two parameters is observed, especially when triethanolamine, xylitol and D-sorbitol are used as initiators (Table 2, entries 11, 13 and 15). These results thus suggest better control by using of 2 as catalyst producing well defined uniform star-shaped polyesters of PLAs.
In contrast, the polymerization experiments of δ-VL indicated strong influence of catalyst on dispersity Ð of isolated star-shaped PVLs. It is evident, that complex 1 produce polymers with very low dispersity Ð, which is in a very narrow range of 1.12–1.19 (Table 3, entries 1–8). Moreover, Mn,SEC values correlate very well with Mn,th, except for the case involving myo-inositol (Table 3, entry 6). In contrast, poor control over polymerization was achieved when complex 2 was used as catalyst. The isolated star-shaped PVLs revealed dispersity Ð of range 1.72–2.26 (Table 3, entries 9–16) and Mn,SEC values are almost twice as large as Mn,th. These results thus suggest better control by using of 1 as catalyst producing well defined uniform star-shaped of PVLs.
Similar results were also obtained in the polymerization experiments of ε-CL. In comparison to the ROP of L-LA and δ-VL, the polymerization of ε-CL is less controlled, but complex 1 produce star-shaped polyesters with narrower dispersity Ð ranging from 1.34 to 1.90 (Table 4, entries 1–8) and Mn,SEC values correlate with Mn,th, except for the dipentaerythritol (Table 4, entry 8). Poor control over polymerization was achieved when complex 2 was used as catalyst (dispersity Ð ranging from 1.36 to 3.18, Table 4, entries 9–16). These results thus again suggest better control by using of 1 as catalyst producing well defined uniform star-shaped polyesters of PCLs.
The different ability of complexes 1 and 2 to control the polymerization of L-LA, δ-VL and ε-CL, and thus the dispersity Ð, can be explained based on the strength of the interaction between the catalyst (as Lewis acid) and the monomer (as Lewis base) in the activated monomer. This is a prerequisite to produce well-defined polymers. In the previous work, we used theoretical calculations to determine the NPA atomic charge q of tin(II) atom in 1 and 2, which are 1.42e (for 1) and 1.21 (for 2) indicating 1 as more Lewis acidic.24 Unfortunately, to the best of our knowledge, there is no study in the literature comparing the basicity of L-LA, d-VL and e-CL. Information about this is only for δ-VL and ε-CL, which exhibit similar pKb (14.3 for δ-VL and 14.7 for ε-CL).27 However, it can be assumed that the presence of a Me group with a +I effect in the L-LA structure increases the nucleophilic character of the carbonyl oxygen, and thus the Lewis basicity. The Lewis basicity of the studied monomers can thus be estimated in order L-LA > δ-VL ≈ ε-CL. From this point of view, δ-VL and ε-CL can form a strong interaction only with more Lewis acidic complex 1, leading to well-defined star-shaped PVLs and PCLs. In contrast, L-LA can also interact strongly with complex 2, which, despite the assumption of a weaker interaction than in the case of complex 1, produces PLAs with a lower distribution.
The incorporation of polyalcohols into the polymer chain was confirmed by 1H NMR spectroscopy. A representative sample was chosen for this study, namely PCL containing trimethylolpropane as the core (Table 4, entry 2). In the 1H NMR spectrum of this polymer, the major signals with δ = 1.28, 1.55, 2.21 and 3.96 ppm corresponding to CH2 protons of the main chain of ε-caprolactone unit were found. Besides these signals, the 1H NMR spectrum revealed small peaks with δ = 0.80 and 3.89 ppm, which were assigned to the trimetholpropane core. In addition, the presence of trimethylolpropane in the prepared PCL was also detected by TG-GCMS, when the chromatogram of the studied PCL showed a peak with a retention time of tR = 3.6 min and Mw = 134.
Although these studies confirm the incorporation of polyalcohols into the structure of the prepared polymers, the branching of the polymer chain cannot be clearly determined from these data. The combined SEC-MALS-viscosity method allows not only the determination of absolute molar mass distribution, but also the estimation of the degree of branching. The detection and quantification of branching is based on the fact that a branched macromolecule has smaller size than corresponding linear molecule of identical chemical composition and molar mass.28 For small macromolecules the branching cannot be characterized on the basis of root mean square radius (radius of gyration) as this quantity cannot be determined for molecules with radii below about 10 nm, which roughly corresponds to molar mass of 105 g mol−1. This limit applies to majority of molecules in the prepared samples. Instead, branching characterization based on the intrinsic viscosity can be used:29
 | (1) |
 | (2) |
| PLA [η] = 0.025 × M0.755 |
| PCL [η] = 0.042 × M0.692 |
| PVL [η] = 0.051 × M0.659 |
The constants are valid for THF and 25 °C. Using the above equations, one can calculate the intrinsic viscosity of a hypothetical linear polymer that would have the same weight-average molar mass (Mw) as that of branched polymer (see Fig. 2). The branching ratio g′ is obtained by simple division of the experimental weight-average intrinsic viscosity ([η]w) divided by the value calculated for the linear polymer (see Fig. 2). The estimation of an average number of arms per molecules is performed using the eqn (2). As eqn (2) does not allow the explicit expression for f, one can simply calculate g′ for various f (with an increment 0.1) in Excel, and then to match the g′ calculated for a given sample with f. The obtained values of f are listed in Tables 2–4.
 | ||
| Fig. 2 Example of Mark–Houwink plots of linear and star shaped polymers. Top left: PVL, linear vs. star shaped with theoretically four arms (pentaerythritol as core, Table 3, entry 4) catalysed by 1; left bottom: g′–versus–M plot for the star shaped PVL; top right: PLA, linear vs. star shaped with theoretically four arms (pentaerythritol as core, Table 2, entry 12) catalysed by 2; left bottom: g′–versus–M plot for the star shaped PLA. | ||
In the case of star-shaped PLAs, the factor f ranges from 2.3 to 5.2 and thus the experimental number of arms is always slightly lower than the theoretical ones. As discussed, complex 2 showed better control over the polymerization of the star shaped polymers. This is also demonstrated by the values of the factor f ranging from 2.6 to 5.2. Therefore, the values correlate well with the theoretical ones, except the star shaped polymer based on myo-inositol as a core (Table 2, entry 14). As already mentioned, polymerization tests of δ-VL producing star-shaped PVLs showed better control when using catalyst 1. This fact is also reflected in factor f ranging from 2.9 to 4.2. The values thus fit well especially in the case of 3- and 4-armed star-shaped PVLs (Table 3, entries 1–4), while 5- and 6-armed PVLs revealed lower branching. The biggest discrepancy can be found again for myo-inositol as a core (Table 3, entry 6). Similar results can be found for star-shaped PCLs, where better control was achieved by catalyst 1. The factor f ranges from 2.3 to 3.2. The values again fit well for 3- and 4-armed star-shaped PCLs (Table 4, entries 1–4), while 5- and 6-armed PCLs revealed lower branching. The biggest discrepancy can be found again for myo-inositol as a core (Table 4, entry 6).
Finally, the catalytic activity of complexes 1 and 2 was compared with commercial Sn(Oct)2. Dipentaerythritol was selected as representative initiator. The experimental set up was the same as in the case of complexes 1 and 2. The macromolecular parameters Mn,SEC, Ð and f are summarized in Tables 2–4 (for PLA, see Table 2 entry 17; for PVL, see Table 3, entry 17 and for PCL, see Table 4, entry 17). For all polymers Mn,SEC is higher than Mn,th, the deviation is more pronounced than for their analogues prepared using 2 (for PLA and PCL) and 1 (for PVL). The dispersity Ð is also higher (1.54 vs. 1.15 for PLA, 1.78 vs. 1.19 for PVL and 1.68 vs. 1.45). Additionally, Sn(Oct)2 does not provide polymers with such a degree of branching compared to 2 and 1 (3.9 vs. 7.2 for PLA, 3.5 vs. 4.2 for PVL and 3.7 vs. 9.8 for PCL). By comparing these factors, it can be clearly stated that complex 2 (for PLA and PCL) and complex 1 (for PVL) control ROP much more effectively than commercial Sn(Oct)2.
Comparison of wettability of linear and star-shaped polyesters
As it has been stated linear PLA and PCL based materials are considered as hydrophobic materials.3 Thus, the comparison of the wettability of linear and star-shaped PLA, PCL and PVL prepared in this study was another goal. Since the literature clearly demonstrates dependence of the WCAs on the fabrication method, we took advantage of the good solubility of the synthesized polymers and we prepared thin layers of above mentioned polymers by an spin-coating method to avoid any influence on the method of fabrication. This method may provide thin layers with uniform surfaces and allow us to study the hydrophobicity of the polymers in terms of composition, structure, and concentration.To investigate the influence of WCA on the polymer composition, linear polyesters with similar Mn prepared by ROP catalysed by 1 or 2 were selected. Thus the linear PLA (PLA-L) with Mn = 7800 g mol−1 and Ð = 1.25,24 linear PVL (PVL-L) with Mn = 7200 g mol−1 and Ð = 1.53 (see Table 1, entry 6) and linear PCL (PCL-L) with Mn = 8000 g mol−1, Ð = 1.55 (see Table 1, entry 10) were studied further. To see the effect of the polymer structure on WCA, star-shaped polyesters with similar Mn derived from dipentaerythritol as polymers with the highest number of arms prepared by ROP catalysed by 1 or 2 were selected. Thus the star-shaped PLA (PLA-DPE) with Mn = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1, Ð = 1.15 and f = 7.2 (see Table 2, entry 16),24 star-shaped PVL (PVL-DPE) with Mn = 11
200 g mol−1, Ð = 1.15 and f = 7.2 (see Table 2, entry 16),24 star-shaped PVL (PVL-DPE) with Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1, Ð = 1.19 and f = 4.2 (see Table 3, entry 8) and star-shaped PCL (PCL-DPE) with Mn = 12
700 g mol−1, Ð = 1.19 and f = 4.2 (see Table 3, entry 8) and star-shaped PCL (PCL-DPE) with Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, Ð = 1.45 and f = 9.8 (Table 4, entry 16) were studied further. We tested THF solutions of selected polymers at concentrations 0.5, 1.0 and 2.0% (w/w) for the fabrication of the thin layers. A silicon wafer was used as a substrate to may analysed these thin layers based on atomic force microscopy (AFM), scanning electron microscopy (SEM) and variable angle spectroscopic ellipsometry (VASE) data. The thin layers were fabricated by the coating on the silicon wafer with approximately 0.3 ml of the THF solutions of polymers followed by the spinning at 4000 rpm. Then, the thin layers were dried under vacuum. The substrates modified in this way were subsequently used for the measurement of WCA by the sessile drop method. The obtained results of these measurements are summarized in Fig. 3 and Table S1 in ESI.†
800 g mol−1, Ð = 1.45 and f = 9.8 (Table 4, entry 16) were studied further. We tested THF solutions of selected polymers at concentrations 0.5, 1.0 and 2.0% (w/w) for the fabrication of the thin layers. A silicon wafer was used as a substrate to may analysed these thin layers based on atomic force microscopy (AFM), scanning electron microscopy (SEM) and variable angle spectroscopic ellipsometry (VASE) data. The thin layers were fabricated by the coating on the silicon wafer with approximately 0.3 ml of the THF solutions of polymers followed by the spinning at 4000 rpm. Then, the thin layers were dried under vacuum. The substrates modified in this way were subsequently used for the measurement of WCA by the sessile drop method. The obtained results of these measurements are summarized in Fig. 3 and Table S1 in ESI.†
 | ||
| Fig. 3 (A) A graphical representation of WCAs of tested linear and star-shaped polymers. (B) Screens of water droplet. | ||
The measured data show that WCA depends on polymer composition, polymer structure and concentration. In general, the most hydrophobic materials were obtained from 2% (w/w) solutions. The highest WCA values were found for 2% (w/w) PLA-L (97.2°), 2% (w/w) PCL-L (101.4°) and 2% (w/w) PCL-DPE (103.5°) and fall in the region of hydrophobic behaviour. Therefore, the influence of the polymer composition on WCA is evident, since hydrophobic properties are mainly exhibited by both PCLs, while both polymers based on PVLs are hydrophilic (PVL-L 74.6°, PVL-DPE 76.0°). The influence of the polymer structure on WCA can be also discussed. While the WCAs of polymers based on PVL and PCL are almost the same for both linear and star-shaped analogues, pronounced effect is observed for PLA polymers. The star-shaped PLA-DPE (2% (w/w)) has a hydrophilic character with WCA not exceeding 79.9°, while linear PLA-L (2% (w/w)) revealed hydrophobic behaviour with WCA at 97.2°. These results again proved that the values of WCA of polymer materials strongly depend on the method of the preparation and the WCA of spin-coated thin layers of PLA, PCL or PVL cannot compete with PLA nanofibers prepared by the electrospinning method, where WCA exceeding 150° were obtained.3m
As mentioned, the uniformity of thin layers was also studied. Surfaces were characterized based on atomic force microscopy (AFM), scanning electron microscopy (SEM) and variable angle spectroscopic ellipsometry (VASE) data. The SEM and AFM data showed that thin layers of hydrophobic materials have the surface with small cracks and corrugations (Fig. 4) with root mean square (RMS) roughness values (determined by AFM) in the narrow range of 14.42–20.36 nm. The thicknesses of these thin layers (determined by VASE) showed also narrow values of 155–242 nm (Table 5).
| Thin layer | |||
|---|---|---|---|
| Sample name | WCA [°] | Thickness [nm] | RMS [nm] |
| 2% (w/w) PLA-L | 97 (±1) | 155 | 20.36 |
| 2% (w/w) PCL-L | 101 (±1) | 222 | 16.59 |
| 0.5% (w/w) PVL-L | 75 (±2) | 52 | Macro defects |
| 2% (w/w) PLA-DPE | 80 (±1) | 154 | Macro defects |
| 2% (w/w) PCL-DPE | 104 (±3) | 242 | 14.42 |
| 2% (w/w) PVL-DPE | 76 (±3) | 44 | 6.31 |
In contrast, the VASE data revealed that thin layers of hydrophilic materials have smaller thicknesses ranging from 44 to 154 nm (Table 5), and the SEM and AFM data suggested that the surfaces of two samples contain macro defects and therefore AFM scans could not be measured (see Table 5).
To increase the hydrophobic properties of the studied polymers, we further focused our attention on their combination with another component. In the past decade, we have been concerned with the synthesis of N → M coordinated galla- or stannaboroxines with the general formula L2Ga(OB-Ar)2O and L2(Ph)Sn(OB-Ar)2O containing MB2O3 central ring (L2 = C6H3-2,6-(Me2NCH2)2, Ar = substituted aryl).25 The great advantage of this type of compounds is easy preparation, solubility in organic solvents and great variability of differently substituted aryls bound to the boron atoms. In addition, it has recently been found that gallaboroxine LGa(OB-Ar)2O, where Ar is C6H4-4-CH = O, exhibits very good film-forming properties, which allowed the fabrication of transparent thin films with properties comparable to amorphous oxide glasses containing B2O3 and Ga2O3 (ref. 31) (for example refractive index n = 1.44 or optical band gap Eg = 3.95 eV).25a This fact evoked us to use heteroboroxines as additives to the studied polymers to improve the uniformity of surfaces and increase the WCAs of these hydrophilic materials. Two stannaboroxines L2(Ph)Sn[(OB-(C6H4-4-CF3))2O] (SnBO-1) and L2(Ph)Sn[(OB-(C6H4-3,5-CF3)2)2O] (SnBO-2) (Fig. 5),25b were selected due to their solubility. The presence of fluorine atoms in the structures of SnBO-1 and SnBO-2 could have been a promising aspect to increase the WCA. In addition, both compounds contain six-membered SnB2O3 central core and they structurally resemble polymer N-Boroxine-PDMS with six-membered B3O3 central core.26
Therefore, THF solutions of PVL-L (0.5% w/w), PLA-DPE (2% w/w), and PVL-DPE (2% w/w) were enriched by SnBO-1 and SnBO-2 to get two ratios of polymer/SnBO at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Thus, new formulations were obtained for the study of hydrophobic properties.
1, respectively. Thus, new formulations were obtained for the study of hydrophobic properties.
The thin layers from these THF solutions of new SnBO based formulations were fabricated by similar way (spin coating on the silicon wafer) and dried under vacuum. The substrates modified in this way were subsequently used for the measurement of WCA by the sessile drop method. The obtained results of these measurements are summarized in Fig. 6 and Table S2 in ESI.†
 | ||
| Fig. 6 (A) A graphical representation of the WCA of tested formulations containing SnBO-1 and SnBO-2. (B) Screens of water droplet. | ||
Based on these data, it was found that the addition of SnBO-2 with the ratio of polymer/SnBO-2 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 significantly increased the WCAs of thin layers of PVL-L, PLA-DPE and PVL-DPE. Their hydrophilic character was changed to the hydrophobic one with the WCAs 100.3° (PVL-L), 96.5° (PLA-DPE) and 95.0° (PVL-DPE). Especially for PVL-L, the increase of WCA (25.7°) is the most striking. Of course, SnBO-1 and SnBO-2 were also added to PLA-L, PCL-L and PCL-DPE and new formulations were fabricated in the same way. However, such a significant improvement in hydrophobic properties was not observed in these cases (Table S2 and Fig. S1, ESI†).
1 significantly increased the WCAs of thin layers of PVL-L, PLA-DPE and PVL-DPE. Their hydrophilic character was changed to the hydrophobic one with the WCAs 100.3° (PVL-L), 96.5° (PLA-DPE) and 95.0° (PVL-DPE). Especially for PVL-L, the increase of WCA (25.7°) is the most striking. Of course, SnBO-1 and SnBO-2 were also added to PLA-L, PCL-L and PCL-DPE and new formulations were fabricated in the same way. However, such a significant improvement in hydrophobic properties was not observed in these cases (Table S2 and Fig. S1, ESI†).
The uniformity of these thin layers with highest WCAs (Polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnBO-2 = 1
SnBO-2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w)) was again studied. Surfaces were characterized based on AFM, SEM and VASE. The SEM and AFM data showed smooth surface without cracks and corrugations (see Fig. 7). Root mean square (RMS) roughness values determined by AFM were typically found to be lower than ∼2.35 nm (see Table 6). The thicknesses of all samples were determined by VASE showing values of 89–522 nm (Table 6). From these data, it is evident that the hydrophobicity is not influenced by the thickness of layers, which varies between 89 and 522 nm with almost the same WCAs (95–100°). In addition, the presence of oxygen, boron, fluorine and tin atoms in the spin coated thin films was confirmed by SEM EDX.
1 (w/w)) was again studied. Surfaces were characterized based on AFM, SEM and VASE. The SEM and AFM data showed smooth surface without cracks and corrugations (see Fig. 7). Root mean square (RMS) roughness values determined by AFM were typically found to be lower than ∼2.35 nm (see Table 6). The thicknesses of all samples were determined by VASE showing values of 89–522 nm (Table 6). From these data, it is evident that the hydrophobicity is not influenced by the thickness of layers, which varies between 89 and 522 nm with almost the same WCAs (95–100°). In addition, the presence of oxygen, boron, fluorine and tin atoms in the spin coated thin films was confirmed by SEM EDX.
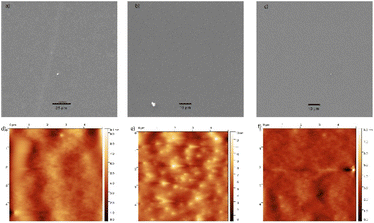 | ||
Fig. 7 SEM image and AFM scan of PVL-L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnBO-2 = 1 SnBO-2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) (a, c), PLA-DPE 1 (w/w) (a, c), PLA-DPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnBO-2 = 1 SnBO-2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) (b, e) and PVL-DPE 1 (w/w) (b, e) and PVL-DPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnBO-2 = 1 SnBO-2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) (c, f). 1 (w/w) (c, f). | ||
| Thin layer | ||||
|---|---|---|---|---|
| Sample name | WCA [°] | The increase of WCA [°] | Thickness [nm] | RMS [nm] |
0.5% (w/w) PVL-L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnBO-2 1 SnBO-2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 (±1) | 25.7 | 465 | 0.94 |
2% (w/w) PLA-DPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnBO-2 1 SnBO-2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 (±2) | 15.7 | 522 | 1.18 |
2% (w/w) PVL-DPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnBO-2 1 SnBO-2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95.0 (±1) | 19 | 89 | 0.66 |
Therefore, it is evident, that the stannaboroxines improved the uniformity of surfaces of thin layers of polymers and increased the WCAs of these hydrophilic materials and formed the hydrophobic materials.
Conclusions
Following the catalytic activity of N-coordinated tin(II) cations [L1→Sn(H2O)][OTf]2·THF (1) and [L1→SnCl][SnCl3] (2) in ROP producing well-defined linear PLAs via activated monomer mechanism, here, we determined these complexes as effective and universal also for the polymerization of ε-CL and δ-VL. The polymerization of these monomers yielded well-defined linear PCL and PVL. Moreover, the formation of activated monomer opened the possibility to use different polyalcohols for the synthesis of well-defined star-shaped analogues. Thus, the application of 1 and 2 in the synthesis of star-shaped polyesters PLAs, PVLs and PCLs led to the preparation of a series of polymers with different arm numbers. While well-defined star-shaped PLAs were obtained using complex 2, complex 1 provided well-defined star-shaped PVLs and PCLs.The preparation of well-defined linear and star-shaped PLAs, PCLs and PVLs allowed us to compare their wettability in terms of composition and polymer structure. For this study, thin layers of the linear and six-armed polymers derived from dipentaerythritol were fabricated by spin-coating method to avoid any influence on the method of fabrication. Results based on WCA measurements showed that the wetting properties of PCLs and PVLs depend only on the composition, when PCLs showed hydrophobic character (∼102°), while PVLs hydrophilic (∼75°). For PLAs, the hydrophobicity was found for linear polymer (∼97°), while star-shaped analogue (∼80°) showed the hydrophilicity. To improve the uniformity of surfaces and increase the WCAs, two stannaboroxines L2(Ph)Sn[(OB-(C6H4-4-CF3))2O] and L2(Ph)Sn[(OB-(C6H4-3,5-CF3)2)2O] were applied. The addition of stannaboroxines improved the uniformity of thin films and moreover transferred hydrophilic polyesters to hydrophobic, which was the most striking for linear PVL (ΔWCA ∼26°). This fact suggested the use of stannaboroxines and other heteroboroxines can be promising approach for the creating of hydrophobic materials.
Experimental part
General consideration
All moisture and air sensitive reactions were carried out under an argon atmosphere using standard Schlenk tube techniques. All solvents were dried using Pure Solv–Innovative Technology equipment. L-lactide, δ-valerolactone and ε-caprolactone were purchased from Sigma-Aldrich and were purified by the recrystallization from toluene or distillation over CaH2 before use. Compounds 1, 2, SnBO-1 and SnBO-2 were prepared according to the literature.24,25b The 1H NMR spectra were recorded on a Bruker 500 NMR spectrometer at 298 K. The 1H NMR spectra were referenced internally to the residual protio-solvent. The molar mass distributions and Mark–Houwink plots were determined by size exclusion chromatography (SEC) coupled with a multi-angle light scattering (MALS) detector DAWN NEON, an RI detector Optilab NEON and an online viscometer ViscoStar NEON (all detectors from Wyatt Technology). The SEC system consisted of a 1200 Series isocratic pump and an autosampler with two Mixed-C 300 × 7.5 mm 5 μm columns (all Agilent Technologies) using tetrahydrofuran (THF) as the mobile phase at a flow rate of 1 ml min−1. Samples of linear and star-branched polymers were prepared as solutions in THF at the concentrations of ≈ 6 mg ml−1, left to dissolve for at least 12 h, filtered with 0.45 μm filters and injected in the amount of 100 μL. The specific refractive index increment (dn/dc) was determined from the RI detector response and injected mass assuming 100% mass recovery. The average values obtained from four or five measurements of different linear samples are as follows:| PLA dn/dc = 0.046 ± 0.001 ml g−1 |
| PCL dn/dc = 0.072 ± 0.001 ml g−1 |
| PVL dn/dc = 0.070 ± 0.001 ml g−1 |
The values are valid for THF, the wavelength 690 nm and temperature 25 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/n
1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (where n = number of hydroxyl groups in the initiator) were weighted into a Schlenk tube and homogenized. The polymerization mixture was then placed into an oven preheated to 145 °C. After a desired time, the reaction mixture was cooled to room temperature and subjected to the 1H NMR analysis. The monomer conversion was determined by the calculation of the integration of the monomer vs. polymer characteristic resonances in the 1H NMR (CDCl3, 500 MHz) spectrum. The polymer was purified by dissolving the crude samples in CHCl3 and precipitating into cold methanol (100 ml). The obtained polymers were dried to a constant weight, and the dry polymer samples were analyzed by the SEC-MALS-Visco.
100 (where n = number of hydroxyl groups in the initiator) were weighted into a Schlenk tube and homogenized. The polymerization mixture was then placed into an oven preheated to 145 °C. After a desired time, the reaction mixture was cooled to room temperature and subjected to the 1H NMR analysis. The monomer conversion was determined by the calculation of the integration of the monomer vs. polymer characteristic resonances in the 1H NMR (CDCl3, 500 MHz) spectrum. The polymer was purified by dissolving the crude samples in CHCl3 and precipitating into cold methanol (100 ml). The obtained polymers were dried to a constant weight, and the dry polymer samples were analyzed by the SEC-MALS-Visco.The prepared solutions were deposited on silicon wafer substrates of 15 × 15 × 0.3 mm and the substrates were rotated using a Spin-Master spin coater at 4000 rpm for 60 s. After that, thin layers were dried under vacuum at 80 °C. The formulations containing the stannaboroxines SnBO-1 and SnBO-2 were prepared by adding these compounds to appropriate THF polymer solution in weight ratios of polymer:SnBO-1(-2) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For example, for formulation of 2% (w/w) solution of polymer with SnBO-2 in ratio 1
1. For example, for formulation of 2% (w/w) solution of polymer with SnBO-2 in ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w): 0.2 g of polymer and 0.2 g of SnBO-2 was dissolved in 9.80 g of THF. The thin films were prepared in the same manner as the polymer samples themselves.
1 (w/w): 0.2 g of polymer and 0.2 g of SnBO-2 was dissolved in 9.80 g of THF. The thin films were prepared in the same manner as the polymer samples themselves.
Data availability
All data generated or analyzed during this study are included in this published article and its ESI files.† For more details, please contact authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Czech Science Foundation (no. 23-06548S) for the financial support.Notes and references
- (a) E. Chiellini and R. Solaro, Adv. Mater., 1996, 8, 305 CrossRef CAS; (b) S. Mecking, Angew. Chem., Int. Ed., 2004, 43, 1078 CrossRef CAS PubMed; (c) L. S. Nair and C. T. Laurencin, Prog. Polym. Sci., 2007, 32, 762–798 CrossRef CAS; (d) B. Laycock, M. Nikolić, J. M. Colwell, E. Gauthier, P. Halley, S. Bottle and G. George, Prog. Polym. Sci., 2017, 71, 144 CrossRef CAS; (e) R. H. Platel, L. M. Hodgson and C. K. Williams, Polym. Rev., 2008, 48, 11 CrossRef CAS; (f) G. Barouti, K. Jarnouen, S. Cammas-Marion, P. Loyer and S. M. Gaillaume, Polym. Chem., 2015, 6, 5414 RSC; (g) X. Zhang, M. Fevre, G. O. Jones and R. M. Waymouth, Chem. Rev., 2018, 18, 839 CrossRef PubMed; (h) M. Suzuki, Y. Tachibana, K. Oba, R. Takizawa and K.-I. Kasuya, Polym. Degrad. Stab., 2018, 149, 1 CrossRef CAS.
- (a) L. Fambri and C. Migiliaresi, Crystallization and thermal properties, in Poly(lactic Acid): Synthesis, Structures, Properties, Processing and Applications, ed. R. Auras, L. T. Lim, S. E. M. Selke and H. Tsuji, John Wiley & Sons Inc, New York, 2010, pp. 113–124 Search PubMed; (b) M. K. Nampoothiri, N. R. Nair and R. P. John, Bioresour. Technol., 2010, 101, 8493 CrossRef PubMed; (c) M. Jamshidian, E. A. Tehrany, M. Imran, M. Jacquot and S. Desobry, Compr. Rev. Food Sci. Food Saf., 2010, 9, 552 CrossRef CAS PubMed; (d) A. Jahandideh and K. Muthukumarappan, Eur. Polym. J., 2017, 87, 360 CrossRef CAS; (e) E. S. Kim, B. C. Kim and S. H. Kim, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 939 CrossRef CAS; (f) M. B. Khajeheian and A. Rosling, J. Appl. Polym. Sci., 2016, 13, 42231/1–8 Search PubMed.
- (a) A. Cipitria, A. Skelton, T. R. Dargaville, P. D. Dalton and D. W. Hutmacher, J. Mater. Chem., 2011, 21, 9419 RSC; (b) A. Martins, E. D. Pinho, S. Faria, I. Pashkuleva, A. P. Marques, R. L. Reis and N. M. Neves, Small, 2009, 5, 1195 CrossRef CAS PubMed; (c) F. Yang, J. G. C. Wolke and J. A. Jansen, Chem. Eng. J., 2008, 137, 154 CrossRef CAS; (d) E. V. Melnik, S. N. Shkarina, S. I. Ivlev, V. Weinhardt, T. Baumbach, M. V. Chaikina, M. A. Surmeneva and R. A. Surmenev, Polym. Degrad. Stab., 2019, 167, 21 CrossRef CAS; (e) Š. Zupančič, L. Preem, J. Kristl, M. Putrinš, T. Tenson, P. Kocbek and K. Kogermann, Eur. J. Pharm. Sci., 2018, 122, 347 CrossRef PubMed; (f) H. Jeon and G. Kim, J. Mater. Chem. B, 2014, 2, 171 RSC; (g) S. Surucu, K. Masur, H. Turkoglu Sasmazel, T. Von Woedtke and K. D. Weltmann, Appl. Surf. Sci., 2016, 385, 400 CrossRef CAS; (h) N. E. Zander, J. A. Orlicki, A. M. Rawlett and T. P. Beebe, ACS Appl. Mater. Interfaces, 2012, 4, 2074 CrossRef CAS PubMed; (i) J. Shi, N. M. Alves and J. F. Mano, Bioinspiration Biomimetics, 2008, 3, 034003 CrossRef PubMed; (j) E. H. Tümer, H. Y. Erbil and N. Akdogan, Langmuir, 2022, 38, 10052 CrossRef PubMed; (k) X.-Q. Cheng, W. Liu, Ch. Zhang, X.-Ch. Chen, S.-Q. Duan and H. Fu, J. Appl. Polym. Sci., 2022, 139, e52621 CrossRef CAS; (l) J. Kingman and M. K. Dymond, Chem. Data Collect., 2022, 40, 100884 CrossRef CAS; (m) P. M. Gorea and B. Kandasubramanian, J. Mater. Chem. A, 2018, 6, 7457 RSC; (n) D. Zhang, X.-Z. Jin, T. Huang, N. Zhang, X.-D. Qi, J.-H. Yang, Z.-W. Zhou and Y. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 5073 CrossRef CAS PubMed; (o) Ch. Eang and P. Opaprakasit, J. Polym. Environ., 2020, 28, 1484 CrossRef CAS; (p) W. Li, Q. Yu, H. Yao, Y. Zhu, P. D. Topham, K. Yue, L. Ren and L. Wang, Acta Biomater., 2019, 92, 60 CrossRef CAS PubMed; (q) F. Wang, K. Liu, Y. Xi and Z. Li, Polymer, 2022, 249, 124858 CrossRef CAS; (r) G. Zhang, P. Wang, X. Zhang, Ch. Xiang and L. Li, Eur. Polym. J., 2019, 116, 386 CrossRef CAS.
- (a) W. Zhang, T. Ji, S. Lyon, M. Mehta, Y. Zheng, X. Deng, A. Liu, A. Shagan, B. Mizrahi and D. S. Kohane, ACS Appl. Mater. Interfaces, 2020, 12, 17314 CrossRef CAS PubMed; (b) Y. Deng, S. J. Liu, B. M. Zhao, P. Wang, Q. L. Fan, W. Huang and L. H. Wang, J. Lumin., 2011, 131, 2166 CrossRef CAS; (c) L. Teng, X. Xu, W. Nie, Y. Zhou, L. Song and P. Chen, J. Polym. Res., 2015, 22, 83 CrossRef; (d) I. Yu, T. Ebrahimi, S. G. Hatzikiriakos and P. Mehrkhodavandi, Dalton Trans., 2015, 44, 14248 RSC; (e) B. S. Lele and J. C. Leroux, Polymer, 2002, 43, 5595 CrossRef CAS.
- S. H. Luo, Q. F. Wang, J. F. Xiong and Z. Y. Wang, J. Polym. Res., 2019, 19, 9962/1–9 Search PubMed.
- (a) S. H. Kim and Y. H. Kim, Macromol. Symp., 1999, 144, 277 CrossRef CAS; (b) H. Tsuji and M. Suzuki, Macromol. Chem. Phys., 2014, 215, 1879 CrossRef CAS; (c) Y. Lin, A. Zhang and L. Wang, J. Appl. Polym. Sci., 2012, 124, 4496 CrossRef CAS.
- A. Abiko, Y. Sy and M. Iguchi, Polymer, 2012, 53, 3842 CrossRef CAS.
- Handbook of Ring-Opening Polymerization, ed. P. Dubois, O. Coulembier and J.-M. Raquez, Wiley-VCH, Weinheim, 2009 Search PubMed.
- (a) A. Michalski, M. Brzezinski, G. Lapienis and T. Biela, Prog. Polym. Sci., 2019, 89, 159 CrossRef CAS; (b) J. M. Ren, T. G. McKenzie, Q. Fu, E. H. H. Wong, J. Xu, Z. An, S. Shanmugam, T. P. Davis, C. Boyer and G.-G. Qiao, Chem. Rev., 2016, 116, 6743 CrossRef CAS.
- K. Karidi, P. Pladis and C. Kiparissides, Macromol. Symp., 2013, 333, 206 CrossRef CAS.
- (a) M. Trollsas, B. Atthoff, H. Claesson and J. L. Hedrick, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 1174 CrossRef CAS; (b) C. M. Dong, K. Y. Qiu, Z. W. Gu and X. D. Feng, J. Polym. Sci., Part A: Polym. Chem., 2001, 40, 409 CrossRef; (c) C. Gottschalk, F. Wolf and H. Frey, Macromol. Chem. Phys., 2007, 208, 1657 CrossRef CAS; (d) L. Wang and C. M. Dong, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2226 CrossRef CAS.
- (a) A. Finne and A. C. Albertsson, Biomacromolecules, 2002, 3, 684 CrossRef CAS; (b) K. Odelius, A. Finne and A. C. Albertsson, J. Polym. Sci., Part A: Polym. Chem., 2005, 44, 596 CrossRef; (c) J. Geschwind, S. Rathi, C. Tonhauser, M. Schoemer, S. L. Hsu and E. B. Coughlin, Macromol. Chem. Phys., 2013, 214, 1434 CrossRef CAS; (d) K. Odelius and A. C. Albertsson, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1249 CrossRef CAS; (e) H. R. Kricheldorf, Biomacromolecules, 2002, 3, 691 CrossRef CAS PubMed; (f) H. R. Kricheldorf and B. Fechner, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 1047 CrossRef CAS.
- A. Finne and A. C. Albertsson, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 1296 CrossRef CAS.
- C. A. P. Joziasse, H. Veenstra, M. D. C. Topp, D. W. Grijpma and A. J. Pennings, Polymer, 1998, 39, 467 CrossRef CAS.
- S. H. Kim, Y. K. Han, Y. H. Kim and S. I. Hong, Makromol. Chem., 1992, 193, 1623 CrossRef CAS.
- K. A. George, F. Schue, T. V. Chirila and E. Wentrup-Byrne, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4736 CrossRef CAS.
- (a) Y. Lemmouchi, M. C. Perry, A. J. Amass, K. Chakraborty and E. Schacht, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3966 CrossRef CAS; (b) Y. Lemmouchi, M. C. Perry, A. J. Amass, K. Chakraborty and E. Schacht, J. Polym. Sci., Part A: Polym. Chem., 2008, 6, 5363 CrossRef.
- (a) H. R. Kricheldorf, H. Hachmann-Thiessen and G. Schwarz, Biomacromolecules, 2004, 5, 492 CrossRef CAS PubMed; (b) S. Malberg, D. Basalp, A. Finne-Wistrand and A. C. Albertsson, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1214 CrossRef CAS.
- M. P. Shaver and D. J. A. Cameron, Biomacromolecules, 2010, 11, 3673 CrossRef CAS PubMed.
- M. J. Stanford and A. P. Dove, Macromolecules, 2009, 42, 141 CrossRef CAS.
- (a) C. Hiemstra, Z. Zhong, L. Li, P. J. Dijkstra and J. Feijen, Biomacromolecules, 2006, 7, 2790 CrossRef CAS PubMed; (b) C. Hiemstra, W. Zhou, Z. Zhong, M. Wouters and J. Feijen, J. Am. Chem. Soc., 2007, 129, 9918 CrossRef CAS PubMed; (c) R. Omar, M. Shaik, Ch. Griggs, J. D. Jensen, R. Boyd, N. Oncel, D. C. Webster and G. Du, Eur. Polym. J., 2021, 160, 110756 CrossRef CAS.
- W. Guerin, M. Helou, J. F. Carpentier, M. Slawinski, J. M. Brusson and S. M. Guillaume, Polym. Chem., 2013, 4, 1095 RSC.
- M. P. Shaver and D. J. A. Cameron, Biomacromolecules, 2010, 11, 3673 CrossRef CAS PubMed.
- M. Novák, J. Turek, Y. Milasheuskaya, Z. Růžičková, Š. Podzimek and R. Jambor, Dalton Trans., 2021, 50, 16039 RSC.
- (a) Y. Milasheuskaya, J. Schwarz, L. Dostál, Z. Růžičková, M. Bouška, Z. Olmrová Zmrhalová, T. Syrový and R. Jambor, Dalton Trans., 2021, 50, 18164 RSC; (b) M. Kořenková, B. Mairychová, A. Růžička, R. Jambor and L. Dostál, Dalton Trans., 2014, 43, 7096 RSC.
- X. Li, B. Li, Y. Li and J. Sun, Chem. Eng. J., 2021, 404, 126504 CrossRef CAS.
- D. J. Darensbourg and W.-Ch. Chung, Polyhedron, 2013, 58, 139 CrossRef CAS.
- B. H. Zimm and W. H. Stockmayer, J. Chem. Phys., 1949, 17, 1301 CrossRef CAS.
- B. H. Zimm and R. W. Kilb, J. Polym. Sci., 1959, 37, 19 CrossRef CAS.
- (a) J. Douglas, J. Roovers and K. Freed, Macromolecules, 1990, 23, 4168 CrossRef CAS; (b) Note that the f calculated this way represent an average value for the entire sample in which different molecules can differ in the number of arms..
- (a) H. Kimura and A. Miyazaki, Mater. Res. Bull., 2001, 36, 1847 CrossRef CAS; (b) A. Prabhakar Reddy, M. Chandrashekhar Reddy, A. Siva Sesha Reddy, J. Ashok, N. Veeraiah and B. Appa Rao, Appl. Phys. A, 2018, 124, 755 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03515a |
| This journal is © The Royal Society of Chemistry 2024 |






