Open Access Article
Open Access ArticleOptimization of culture conditions of Scenedesmus sp. algae and catalytic performance of a NiFe2O4@SiO2/MgO magnetic nano-catalyst for biodiesel production†
Peyman Abazaria,
Saeed Masoum *a and
Seyed Ali Hosseini Tafreshib
*a and
Seyed Ali Hosseini Tafreshib
aDepartment of Analytical Chemistry, Faculty of Chemistry, University of Kashan, Kashan, Iran. E-mail: masoum@kashanu.ac.ir; Fax: +983155912397; Tel: +983155912338
bDepartment of Biotechnology, Faculty of Chemistry, University of Kashan, Kashan, Iran
First published on 19th August 2024
Abstract
This study aims to optimize the lipid content of Scenedesmus sp. for high-yield biodiesel production. Three factors affecting the culture conditions, namely salinity, nitrogen concentration, and light intensity, were selected and their effects on the maximum lipid content were investigated using the Box Behnken design. The results showed that the maximum lipid content (32.7% of algal dry weight) was obtained in the algal samples cultured under the optimized conditions. A core–shell magnetic nano-catalyst, NiFe2O4@SiO2/MgO, was synthesized and used to produce biodiesel via the transesterification reaction. The nano-catalyst was characterized by field emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDS), X-ray powder diffraction analysis (XRD), vibrating sample magnetometry (VSM), elemental mapping techniques, transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FT-IR). Using the Box Behnken design and keeping the temperature constant, the molar ratio of methanol to oil, the amount of catalyst, and the time were optimized to achieve the maximum yield of biodiesel. The maximum yield of biodiesel was 95.3% under the optimal conditions.
1. Introduction
Alternative fuels are being sought instead of fossil fuels due to increasing air pollution, acid rain, and greenhouse gases.1 It is possible to substitute fossil fuels with biofuels, such as biodiesel. Compared to gasoline, they emit fewer carbon dioxide emissions, have higher flash points, include fewer sulfur compounds, and have higher cetane numbers.2 Sources of biofuel production are divided into four generations. Biofuel production has been able to surpass edible and non-edible seeds with microalgae in the third generation. Compared to plants, this generation grows faster, doesn't require fertile soil and freshwater, and can withstand bad environments. Microalgae can grow in saline water as well as wastewater.3 One of the well-known algae with suitable lipid content for biodiesel production is Scenedesmus algae.4 This algae was first discovered by Turpin in 1828. Scenedesmus is a flat colony with 2–32 cells in one or two rows. This algae has different cell shapes but is often cylindrical and lunate.5 Biodiesel is a mono-alkyl ester produced by reacting algae oil with methanol and an acid or alkali catalyst via esterification or transesterification.6 Chemical reactions can be accelerated using substances called catalysts, which do not get depleted during the process. Catalysts come in different forms like solid, liquid, and biocatalysts. Among them, magnetic solid catalysts are preferred by scientists because they have a high recovery capacity and can be quickly separated from the reaction.7 Different methods for extracting oil from algae can be used depending on the laboratory facilities. These methods include supercritical fluid extraction, organic solvent extraction, microwave-assisted organic solvent extraction, ultrasound-assisted organic solvent extraction, and organic solvent extraction with Soxhlet apparatus.8 The amount of lipid content in algal cells is essential for biodiesel production. Algae usually do not have a significant amount of oil but at the different route, such as some abiotic stress, can increase the amount of algae oil. These abiotic stresses like salinity, metal stress, light, and nitrogen depletion can induce lipid productivity in algal cells.9 Ji et al. conducted research on Scenedesmus obliquus algae by adding varying amounts of NaCl to the culture medium at concentrations of 0, 0.01, 0.10, 0.15, and 0.20 M. After analyzing the data, it was found that the algae growth medium with 0.2 M of NaCl produced the highest oil yield, amounting to 32.26% of dry weight.10 Also, Nzayisenga et al. stated that the effect of three light intensities of 2700, 8100, and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 lux on three algae Chlorella vulgaris, Desmodesmus sp., and Scenedesmus obliquus, and different results were obtained, so that in Scenedesmus and Desmodesmus increasing the light intensity from 2700 to 16
200 lux on three algae Chlorella vulgaris, Desmodesmus sp., and Scenedesmus obliquus, and different results were obtained, so that in Scenedesmus and Desmodesmus increasing the light intensity from 2700 to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 lux increased oil production, while in Chlorella vulgaris, increasing the light intensity decreased oil production in algae cells.11
200 lux increased oil production, while in Chlorella vulgaris, increasing the light intensity decreased oil production in algae cells.11
Response surface methodology (RSM) is a statistical and mathematical method to optimize different and important factors in the response of an experiment.12 The big advantage of RSM is that it helps us get optimal solutions faster with fewer test runs.13 There are various methods for experimental designs. The most common of which are Box Behnken design, central composite design, full factorial design. Box–Behnken design (BBD) is a design that, unlike central composite design, which is done on five levels, is done only on three levels (−1, 0, +1), which increases the economic advantage of this method compared to central composite design.14
Nanocomposites have various applications, among these applications, their use as battery cathodes, nanowires, sensors, non-linear optics, and other systems can be mentioned.15 One of the latest applications of nanocomposites is the use of highly active ZIF-8@CNT composite catalysts as cathode materials for anion exchange membrane fuel cells.16
In 2019, Arul Raj et al. were able to produce biodiesel with a yield of 87.5% by using an optimized process. They achieved this by synthesizing a nano-catalyst made of polyethylene glycol (PEG) encapsulated ZnOMn2+ and extracting oil from the algae Nanochloropsis ocutala. The optimal conditions for this process were a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol to oil, 4 hours reaction time, 60 °C reaction temperature, and 3.5 wt% catalyst amount.17 Also, the optimal amount of 4% calcium oxide nano-catalyst showed the highest efficiency in producing biodiesel using Chlorella vulgaris algae oil in a transesterification reaction.18
1 molar ratio of methanol to oil, 4 hours reaction time, 60 °C reaction temperature, and 3.5 wt% catalyst amount.17 Also, the optimal amount of 4% calcium oxide nano-catalyst showed the highest efficiency in producing biodiesel using Chlorella vulgaris algae oil in a transesterification reaction.18
In 2022, Farrokheh et al. conducted a study to determine the effectiveness of two magnetic nanocatalysts, CaO/KOH–Fe3O4, and KF/KOH–Fe3O4, on the oil extracted from two types of algae, Chlorella vulgaris and Spirulina platensis. The goal of the study was to produce biofuel using the electrolysis method. Results showed that the oil extracted from Chlorella vulgaris and the KF/KOH–Fe3O4 magnetic nano-catalyst had the best performance under optimal conditions. These conditions included a molar ratio of methanol to oil of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a reaction time of 2 hours, a catalyst amount of 1.5 wt%, and a reaction temperature of 25 °C. The reaction yield was found to be 96.8%.19 Also in 2023, Mittal and Gash successfully created biodiesel with a 99% yield. They accomplished this by using a calcium methoxide nano-catalyst and oil from Spirulina algae during the trans-esterification reaction. The optimal conditions for this process were a 30
1, a reaction time of 2 hours, a catalyst amount of 1.5 wt%, and a reaction temperature of 25 °C. The reaction yield was found to be 96.8%.19 Also in 2023, Mittal and Gash successfully created biodiesel with a 99% yield. They accomplished this by using a calcium methoxide nano-catalyst and oil from Spirulina algae during the trans-esterification reaction. The optimal conditions for this process were a 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol to oil, 3 wt% of catalyst, 3 hours reaction time, and a temperature of 80 °C.20
1 molar ratio of methanol to oil, 3 wt% of catalyst, 3 hours reaction time, and a temperature of 80 °C.20
This study focused on using Scenedesmus sp. to produce high-yield biodiesel, synthesis of a nickel ferrite nano-catalyst coated with silica and functionalized with magnesium oxide, characterization of magnetic catalyst, investigation of magnetic catalyst in trans-esterification of Scenedesmus sp. to produce high-yield biodiesel and optimizing the conditions of process using BBD. The magnetic core in the synthesized catalyst increases its recycling properties and enables easy separation from the reaction environment. The core–shell structure serves to safeguard the magnetic core from physical and chemical damage. Magnesium oxide nanoparticles are used for their high stability in environmental conditions and their good track record in previous research.21,22
2. Experimental section
2.1. Materials
All chemicals were purchased from Merck (Germany) and used as received without further purification.Synthesis of NiFe2O4. To synthesize the nickel ferrite magnetic nano-catalyst, start by adding 50 ml of 0.4 M FeCl3·6H2O solution to 50 ml of 0.2 M NiCl2·6H2O solution. Pour both solutions into a round-bottom flask and heat it to 50 °C. After 15 minutes, NaOH 3 M was added drop by drop until the pH of the environment reached 13, then 3 drops of oleic acid were added and stirred for 50 minutes at a temperature of 80 °C. During this period, to prevent iron from oxidizing, nitrogen was purged inside the flask. The obtained sediments were washed three times with deionized water and ethanol and then dried in an oven at 80 °C for 12 hours. Finally, they were calcined at 600 °C for 10 hours.23
Synthesis of NiFe2O4@SiO2. One gram of synthesized nickel ferrite in the previous step was weighed. The mixture of 20 ml of ethanol and 4 ml of deionized water was added to it and was placed under nitrogen gas for 5 minutes in the mechanical recoil, then 1 ml of tetraethoxysilane (TEOS) and 2 ml of 25% ammonia was added to the reaction mixture. The reaction was carried out under room temperature for 3 hours. Finally, the resulting product was separated with an external magnet washed three times with deionized water and ethanol, and dried at a temperature of 70 °C for 8 hours.24
Synthesis of NiFe2O4@SiO2/MgO. To synthesize NiFe2O4@SiO2/MgO, Rahimi et al.'s method was used with slight modifications,25 so that a solution of 60% (w/w) of magnesium nitrate to NiFe2O4@SiO2 was prepared. After stirring 1 gr of NiFe2O4@SiO2 in the solution above at 40 °C for 1 hour sodium hydroxide (2 M) was added drop by drop until the pH reaches 11–12, and stir for an additional hour at 65 °C. Then the obtained sediment was washed three times with distilled water and dried for 10 hours at 80 °C in an oven and finally calcined at 650 °C for 2.30 hours.
2.2. Instrumentation
The IR spectra were recorded on an ATR Bruker Tensor II (Germany) in the range of 400–4000 cm−1. A Philips instrument with Cu anode (λ = 0.154056 nm) was used to record XRD patterns of the synthesized catalyst from 10° to 80° (2θ). Field emission scanning electron microscopy (FE-SEM) of the nano-catalyst was conducted using a TESCAN MIRA 3 (Czech Republic) at a 15 kV accelerating voltage. The magnetic properties of the produced nano-catalyst were evaluated using the MDKB instrument from Iran. The morphological features of the sample were investigated with a Zeiss (Germany) Transmission Electron Microscope (TEM) operating at 100 kV. The optical density of the algae sample was measured using OPTIZEN 3220UV (South Korea), and the identification of FAMEs was carried out using GC-MS equipment (GC 6890, MS 5973 from Agilent (USA)).2.3. Microalgal culture conditions
Scenedesmus sp. was obtained from algal culture collection of University of Kashan. The alga was cultured and maintained in Johnson medium (0.5 mM NaCl) in sterile conditions at 25 °C and under 1500 lux light intensity with a photoperiod of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 hours of dark
12 hours of dark![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) light cycles.26
light cycles.26
2.4. Experimental design of lipid content and trans-esterification reaction
To optimize the amount of lipid in Scenedesmus, the response surface methodology (RSM) based on Box–Behnken design (BBD) was used. Design-Expert software (Ver. 13.0.5.0) designed 15 experiments with 3 central points according to the following equation.27| N = 2k(k − 1) + C0 | (1) |
After investigating the factors affecting lipid production, three factors were selected. Light intensity (A), nitrate concentration (B), and salinity (C), were arranged in three levels (−1, 0, +1) (see Table 1). These factors were applied to the algal growth media on the 14th day of culture when the algae located at logarithmic phase of growth. 5 days after treating, the amount of lipid was measured.
| Variable | Factor | Range and level | ||
|---|---|---|---|---|
| Low level (−1) | Central point (0) | High level (+1) | ||
| Light intensity (lux) | A | 800 | 5000 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Nitrate concentration (mM) | B | 0.5 | 2.5 | 5 |
| Salinity concentration (mM) | C | 0.5 | 100 | 500 |
Box–Behnken design can be fitted to the quadratic model to show the interactive effects between the experimental variables to optimize the factors that affect the yield of lipid production for achieving a high yield of lipid production. The following quadratic equation model can be expressed.28
| Y = α0 + α1A + α2B + α3C + α4AB + α5AC + α6BC + α7A2 + α8B2 + α9C2 + ε | (2) |
For the trans-esterification reaction three factors such as: methanol to oil molar ratio (A), amount of catalyst (B), time (C), were chosen to optimize the yield of biodiesel production at constant temperature (60 °C). The range and level of these factors are displayed in Table 2. According to Design-Expert software, 15 experiments with 3 central points were run. During the designed experiment, each test was conducted inside a round-bottom balloon equipped with a mechanical stirrer, a condenser, and a heater. After the reaction, an external magnet removed the catalyst and the mixture was separated in a funnel into the upper phase (biodiesel) and bottom phase (glycerol) as a byproduct of the trans-esterification reaction. Biodiesel yield was calculated according to eqn (3). Also, in this case, eqn (2) as a quadratic equation was used to show the interactive effects between independent variables and optimization of biodiesel production yield.18
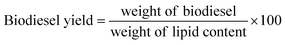 | (3) |
| Variable | Factor | Range and level | ||
|---|---|---|---|---|
| Low level (−1) | Central point (0) | High level (+1) | ||
| Methanol to oil molar ratio | A | 6 | 12 | 18 |
| Amount of catalyst (% wt) | B | 0.5 | 2 | 4 |
| Time (min) | C | 60 | 120 | 180 |
2.5. Algal growth
Optical densities of the control and 15 different samples at 750 nm were read by UV/Vis spectrophotometer at various time points. The specific growth rate (μ) was also calculated based on the following equation:29
 | (4) |
2.6. Lipid content
To measure the lipid content of Scenedesmus algae, the modified method of Bligh and Dyer was used.30 Chloroform and methanol were added to 1 g of dry algae powder in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, so that the first 5 ml of chloroform and 5 ml of methanol were added to the dry algae powder, and vortexed for 5 minutes, then another 5 ml of chloroform was added to the algae sample and vortexed again for five minutes. After that, for better separation of chloroform and methanol, 10 ml of sodium chloride 1% (w/w) was added to the above sample, and the sample was centrifuged for 15 minutes at a speed of 6000 rpm. After centrifuge, chloroform and methanol are completely separated, the upper phase is the methanol and the lower one is the chloroform, which contains the lipid content. The chloroform content was poured into a pre-weighed test tube and placed in the oven for 24 hours at a temperature of 55 °C. The percentage of lipid content was calculated according to the following equation:
1, so that the first 5 ml of chloroform and 5 ml of methanol were added to the dry algae powder, and vortexed for 5 minutes, then another 5 ml of chloroform was added to the algae sample and vortexed again for five minutes. After that, for better separation of chloroform and methanol, 10 ml of sodium chloride 1% (w/w) was added to the above sample, and the sample was centrifuged for 15 minutes at a speed of 6000 rpm. After centrifuge, chloroform and methanol are completely separated, the upper phase is the methanol and the lower one is the chloroform, which contains the lipid content. The chloroform content was poured into a pre-weighed test tube and placed in the oven for 24 hours at a temperature of 55 °C. The percentage of lipid content was calculated according to the following equation:
 | (5) |
2.7. GC-MS analysis of produced biodiesel by NiFe2O4@SiO2/MgO
The total extracted lipids from Scenedesmus sp. were converted to fatty acid methyl esters (FAMEs) by treating methanol and synthesized catalyst in the optimized condition in transesterification reaction at 60 °C. For analyzing FAMEs, gas chromatography-mass spectroscopy with HP-5ms capillary column (60 m × 250 μm × 0.25 μm) was used. 1 μl of FAMEs sample was injected at 250 °C in splitless mode. The temperature of the oven was initially maintained at 50 °C without holding time and increased to 250 °C at a ramping of 3 °C min−1, and hold for 5 min at this temperature. By analyzing retention times and library search reports (NISTIL.S database), identification of FAMEs was performed.3. Result and discussion
3.1. Characterization of NiFe2O4@SiO2/MgO core–shell magnetic nano-catalyst
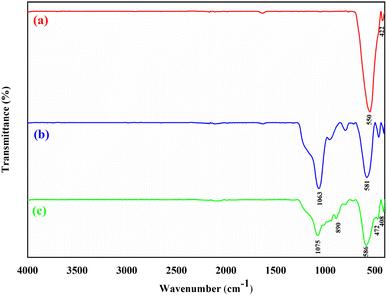
NiFe2O4@SiO2/MgO core–shell magnetic nano-catalyst was synthesized in three main steps as shown in Fig. 1. In the first step NiFe2O4 was synthesized by the co-precipitation method. In the second step, a layer of silica was coated on the surface of the nickel ferrite synthesized in the previous step. The synthesized nano-catalyst was finally modified with MgO on its surface for functionalization. The core–shell magnetic nano-catalyst was characterized by FT-IR, XRD, VSM, FE-SEM and TEM.The FT-IR spectrum of NiFe2O4 (a), NiFe2O4@SiO2 (b), and NiFe2O4@SiO2/MgO (c) in the range 400–4000 cm−1 is shown in Fig. 2. The bands at 550 and 422 cm−1 are related to the stretching vibration of Fe–O and Ni–O, respectively (Fig. 2a).31 For all three steps, the related peaks of NiFe2O4 were observed at 408–586 cm−1. The peaks at 1075 cm−1 and 1063 cm−1 in Fig. 2b and c are attributed to the Si–O–Si stretching vibration that shows silica coated on the surface of nickel ferrite.32 The peak at 472 cm−1 is assigned to Mg–O–Si vibration.33 The MgO cubic face peak is exhibited at 890 cm−1.34
The XRD patterns of NiFe2O4 (a), MgO (b), NiFe2O4@SiO2 (c), and NiFe2O4@SiO2/MgO (d) are presented in Fig. 3. NiFe2O4, NiFe2O4@SiO2 XRD patterns are matched with standard characteristic peaks reported in JCPDS card number 01-086-2267. Also NiFe2O4@SiO2/MgO XRD pattern is matched with card number 01-074-1225. The characteristic peak at 2θ = 25° shows that amorphous SiO2 coated on the surface of nickel ferrite successfully.
The synthesized NiFe2O4, NiFe2O4@SiO2, and NiFe2O4@SiO2/MgO nanoparticles had a crystallite size of 18.96, 26.73, and 27.43 nm, respectively, according to the Debye Scherrer formula and information related to Debye Scherrer calculation for NiFe2O4, NiFe2O4@SiO2, and NiFe2O4@SiO2/MgO are shown in Tables S1, S2† and 3, respectively.35
 | (6) |
| Peak position 2θ (°) | 30.46 | 35.86 | 43.54 | 57.60 | 63.19 |
| FWHM β (°) | 0.29 | 0.41 | 0.35 | 0.35 | 0.29 |
| Size (nm) | 29.67 | 21.28 | 25.54 | 27.07 | 33.61 |
| λ = 0.154 nm, K = 0.94 | |||||
The magnetic properties of NiFe2O4 (a) and NiFe2O4@SiO2/MgO (b) are shown in Fig. 4. The magnetic properties of NiFe2O4 were detected at 38.94 emu g−1 while after coating with SiO2 and functionalizing with MgO magnetic properties of the synthesized nano-catalyst reached 30.76 emu g−1 with a decrease of about 8.18 emu g−1. The findings suggest that the magnetic nano-catalyst that was synthesized has robust magnetic properties, which enable effortless separation from the reaction mixture by utilizing an external magnet. This feature enhances the economic viability of the reaction by allowing the catalyst to be reused multiple times.
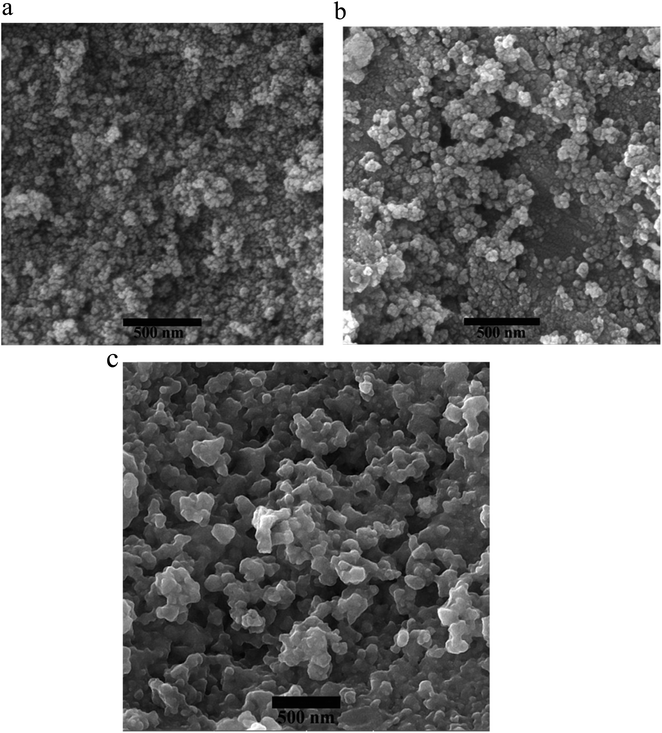
FE-SEM analyses were employed to examine the synthesized nanoparticles' size and morphology, as shown in Fig. 5. The average nanoparticle size for NiFe2O4 (Fig. 5a), NiFe2O4@SiO2 (Fig. 5b), and NiFe2O4@SiO2/MgO (Fig. 5c) was determined to be 16, 25, and 40 nm. As depicted in the SEM image of the final catalyst, the three-dimensional structure and the voids between the manufactured nano-catalyst have increased the surface area of contact with the environment, leading to a significant increase in the efficiency and effectiveness of the catalyst.
The TEM image of NiFe2O4 (a), NiFe2O4@SiO2 (b), and NiFe2O4@SiO2/MgO (c) was demonstrated in Fig. 6. As shown in Fig. 6b the structure of core and shell is well represented. Fig. 6c was related to the final catalyst, showing a weaker core and shell structure due to the deposition of magnesium oxide nanoparticles on the silica surface.
The elemental energy-dispersive X-ray (EDS) spectrum of the NiFe2O4@SiO2/MgO nano-catalyst is displayed in Fig. 7. This nano-catalyst contains Si, Fe, Ni, Mg, and O with the following weight percentages according to the spectrum; Si: 8.81 (% w/w), Fe: 34.86 (% w/w), Ni: 23.31 (% w/w), Mg: 3.91 (% w/w), and O: 29.12 (% w/w). The small amount of Mg in the catalytic will increase the trans-esterification reaction efficiency.36
The elemental mapping of the NiFe2O4@SiO2/MgO is shown in Fig. 8. These images demonstrate uniform dispersion of constituent elements throughout the synthesized nano-catalyst, creating active sites across the entire catalyst surface. As a result, the efficiency of this catalyst increases in the trans-esterification reaction.
3.2. Investigating the growth of algae in the control medium and stressed medium
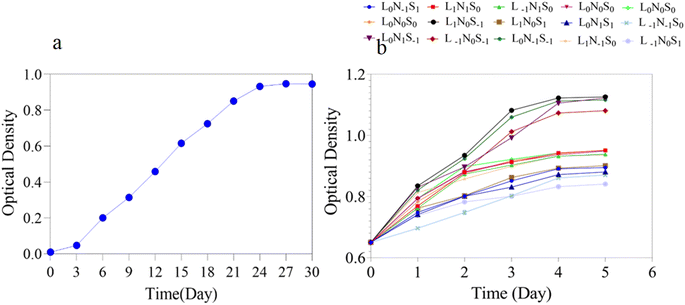
The growth curves of Scenedesmus sp. algae under the control medium (a) and 15 treatments (b) related to the optimization of the lipid content are shown in Fig. 9, and the specific growth rate (μ) is calculated and shown in Table 4. The results of biomass measurement only showed a negligible decrease in biomass. The final biomass of the control sample was equal to 0.877 g l−1 and in the optimized condition with the highest amount of lipid content was equal to 0.730 g l−1 after five days of culture.| Sample | μ (d−1) | Sample | μ (d−1) | Sample | μ (d−1) | Sample | μ (d−1) |
|---|---|---|---|---|---|---|---|
| Control | 0.090 | L0N0S0 | 0.082 | L0N1S1 | 0.077 | L1N−1S0 | 0.062 |
| L0N−1S1 | 0.060 | L0N0S0 | 0.079 | L0N1S−1 | 0.100 | L0N0S0 | 0.081 |
| L1N1S0 | 0.070 | L1N0S−1 | 0.111 | L−1N0S−1 | 0.106 | L−1N−1S0 | 0.076 |
| L−1N1S0 | 0.077 | L1N0S1 | 0.052 | L0N−1S−1 | 0.119 | L−1N0S1 | 0.059 |
3.3. Result of the Box–Behnken design for optimization of the lipid content
To determine the optimum conditions for producing high lipid content, the response surface methodology and Box–Behnken design method were applied. In the first section of the design experiment, three factors (A: light intensity, B: nitrate concentration, and, C: salinity concentration) were chosen as the independent variables that affected lipid production, and the percentage of lipid was selected as the response. Table 5 presents ANOVA results for the influencing factors on lipid production, which shows that the factors with p-value < 0.05 have a significant impact on lipid production. Consequently, the A (light intensity), B (KNO3 concentration), C (salinity concentration), B2, C2, and BC (NO3 concentration × salinity concentration) have p-values smaller than 0.05 and significantly increased lipid productivity.| Source | Sum of squares | df | Mean square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 516.35 | 7 | 73.76 | 72.28 | <0.0001 | Significant |
| A-Light intensity | 38.06 | 1 | 38.06 | 37.30 | 0.0005 | |
| B-Nitrate concentration | 51.16 | 1 | 51.16 | 50.13 | 0.0002 | |
| C-Salinity | 304.80 | 1 | 304.80 | 298.67 | <0.0001 | |
| AB | 5.55 | 1 | 5.55 | 5.43 | 0.0525 | |
| BC | 10.18 | 1 | 10.18 | 9.97 | 0.0160 | |
| B2 | 83.22 | 1 | 83.22 | 81.55 | <0.0001 | |
| C2 | 29.98 | 1 | 29.98 | 29.38 | 0.0010 | |
| Residual | 7.14 | 7 | 1.02 | |||
| Lack of fit | 6.85 | 5 | 1.37 | 9.44 | 0.0986 | Not significant |
| Pure error | 0.2905 | 2 | 0.1452 | |||
| Cor total | 523.49 | 14 | ||||
| R-Squared | 0.9864 | |||||
| Adj R-squared | 0.9727 | |||||
| Pred R-squared | 0.8884 |
Scenedesmus sp. algae lipid content can be predicted using a reduced quadratic model using the following equation:
| Y = 15.81 − 2.18A − 2.53B + 6.17C + 1.18AB + 1.60BC + 4.73B2 + 2.84C2 | (7) |
In Table 6, calculated results from the reduced quadratic model are summarized based on experimental ranges and levels of independent variables.
| Run | Factor A | Factor B | Factor C | Percentage of lipid content | |
|---|---|---|---|---|---|
| Light intensity (lux) | NO3 (mM) | Salinity (mM) | Experimental | Predicted | |
| 1 | 800 | 0.5 | 100 | 25.8 | 26.4 |
| 2 | 5000 | 5 | 500 | 28.0 | 28.6 |
| 3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.5 | 0.5 | 10.4 | 10.3 |
| 4 | 5000 | 5 | 0.5 | 12.9 | 13.0 |
| 5 | 5000 | 2.5 | 100 | 15.8 | 15.8 |
| 6 | 5000 | 0.5 | 500 | 31.2 | 30.5 |
| 7 | 800 | 5 | 100 | 19.8 | 19.0 |
| 8 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.5 | 500 | 22.6 | 22.6 |
| 9 | 5000 | 2.5 | 100 | 16.6 | 15.8 |
| 10 | 5000 | 2.5 | 100 | 16.2 | 15.8 |
| 11 | 5000 | 0.5 | 0.5 | 22.5 | 21.3 |
| 12 | 800 | 2.5 | 0.5 | 13.5 | 14.7 |
| 13 | 800 | 2.5 | 500 | 26.9 | 27.0 |
| 14 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5 | 100 | 17.0 | 17.0 |
| 15 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.5 | 100 | 18.4 | 19.7 |
Fig. 10 illustrates response surface plots between pairs of two experimental variables. As a result of all 15 experimental runs using response surface methodology, the following conditions produce maximum lipid content (32.7% of algal dry weight): A = 800 lux (light intensity), B = 0.5 mM (KNO3 concentration), C = 500 mM (salinity concentration).
3.4. Result of the Box–Behnken design for optimization of the biodiesel yield
During the second part of this design experiment, at a constant temperature (60 °C), three independent variables that affect the reaction conditions of biodiesel production were considered, including methanol to oil molar ratio (A), amount of catalyst (B), and reaction time (C). This was accomplished by designing 15 experiments and selecting biodiesel yield as the response variable. Table 7 provides the ANOVA table for variables affecting biodiesel yield. As a result, A (molar ratio methanol to oil), B (amount of catalyst), C (reaction time), AB (molar ratio methanol to oil × amount of catalyst), A2 and C2 factors significantly improved biodiesel yields.| Source | Sum of squares | df | Mean square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 7865.62 | 6 | 1310.94 | 210.23 | <0.0001 | Significant |
| A-Methanol to oil | 190.03 | 1 | 190.03 | 30.47 | 0.0006 | |
| B-Amount of catalyst | 896.55 | 1 | 896.55 | 143.77 | <0.0001 | |
| C-Reaction time | 312.50 | 1 | 312.50 | 50.11 | 0.0001 | |
| AB | 59.91 | 1 | 59.91 | 9.61 | 0.0147 | |
| A2 | 5497.80 | 1 | 5497.80 | 881.64 | <0.0001 | |
| C2 | 1250.76 | 1 | 1250.76 | 200.58 | <0.0001 | |
| Residual | 49.89 | 8 | 6.24 | |||
| Lack of fit | 47.84 | 6 | 7.97 | 7.80 | 0.1180 | Not significant |
| Pure error | 2.05 | 2 | 1.02 | |||
| Cor total | 7915.51 | 14 | ||||
| R-Squared | 0.9937 | |||||
| Adj R-squared | 0.9890 | |||||
| Pred R-squared | 0.9794 |
According to the Box–Behnken design and response surface methodology, eqn (8) represents the correlation between experiment variables and response (biodiesel yield, Y).
| Y = 87.39 − 4.87A + 10.59B + 6.25C − 3.87AB − 38.47A2 − 18.35C2 | (8) |
In Table 8, the experimental conditions and the obtained results are presented. Response surface plots between pairs of two experimental variables are shown in Fig. 11. Using response surface methodology, the following conditions have been found to produce high biodiesel yields (95.34%): A = 11.3 (methanol to oil molar ratio), B = 4 (amount of catalyst), C = 130 (time).
| Run | Factor A | Factor B | Factor C | Biodiesel yield | |
|---|---|---|---|---|---|
| Methanol to oil molar ratio | Amount of catalyst (% wt) | Reaction time (min) | Experimental | Predicted | |
| 1 | 6 | 2 | 180 | 44.7 | 41.7 |
| 2 | 6 | 2 | 60 | 27.5 | 29.2 |
| 3 | 12 | 4 | 180 | 84.3 | 85.9 |
| 4 | 18 | 2 | 60 | 17.1 | 19.4 |
| 5 | 12 | 4 | 60 | 74.3 | 73.4 |
| 6 | 12 | 0.5 | 60 | 55.3 | 52.2 |
| 7 | 6 | 0.5 | 120 | 38.7 | 39.3 |
| 8 | 12 | 0.5 | 180 | 61.2 | 64.7 |
| 9 | 12 | 2 | 120 | 87.0 | 87.4 |
| 10 | 6 | 4 | 120 | 67.6 | 68.2 |
| 11 | 12 | 2 | 120 | 87.3 | 87.7 |
| 12 | 18 | 4 | 120 | 50.9 | 50.8 |
| 13 | 18 | 0.5 | 120 | 37.4 | 37.3 |
| 14 | 18 | 2 | 180 | 34.0 | 31.9 |
| 15 | 12 | 2 | 120 | 88.9 | 87.4 |
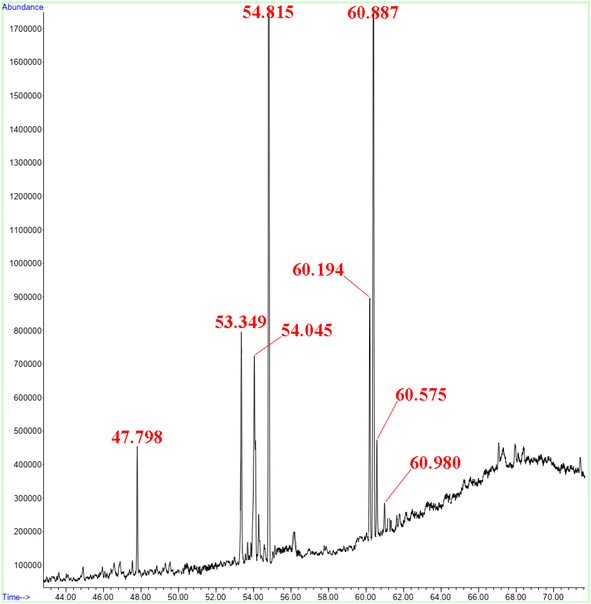
As shown in Table 9 and Fig. 12, the main FAME composition and GC-MS spectrum of Scenedesmus sp. biodiesel synthesized under ideal conditions were reported. The results of GC-MS analysis show that the high presence of methyl esters with carbon numbers of 16 and 18 can reduce oxidation of biodiesel and increase the storage capacity of biodiesel.37
| Number | Retention time | Compound name | Percent |
|---|---|---|---|
| 1 | 47.8 | Myristic acid, methyl ester | 4.1 |
| 2 | 53.3 | Methyl 4,7,10,13-hexadecatetranoate | 7.0 |
| 3 | 54.0 | Palmitoleic acid, methyl ester | 14.0 |
| 4 | 54.8 | Palmitic acid, methyl ester | 32.7 |
| 5 | 60.2 | Linoleic acid, methyl ester | 9.0 |
| 6 | 60.4 | Linolenic acid, methyl ester | 28.6 |
| 7 | 60.6 | Elaidic acid, methyl ester | 3.5 |
| 8 | 60.9 | Stearic acid, methyl ester | 1.1 |
As shown in Table 10 NiFe2O4@SiO2/MgO nano-catalyst were compared with other reported catalysts.
3.5. Proposed reaction mechanism of transesterification reaction
The proposed mechanism of transesterification reaction using NiFe2O4@SiO2/MgO nano-catalyst is shown in Fig. S1.† In step 1, methanol is activated on the surface of the catalyst, and intermediate (I) is formed. Then, the negative charge of oxygen attacks the carbonyl group of triglyceride, and the carbonyl group is removed from the compound, therefore, methyl ester is produced (II). In the following, the above reaction is repeated in two consecutive steps and finally, 3 moles of methyl ester and glycerol molecule are formed (III).3.6. Reusability of the NiFe2O4@SiO2/MgO nano-catalyst
Considering the importance of environmental and economic considerations, reusable catalysts have attracted considerable attention. An attempt was made in this work to study the reusability of a NiFe2O4@SiO2/MgO nano-catalyst in the transesterification reaction. For this purpose, after separating the nano-catalyst by an external magnet, the catalyst was washed with acetone and deionized water and dried at 60 °C for 3 hours. This method makes the synthesized catalyst show the highest efficiency in optimized conditions with the least reduction in reaction efficiency after reuse. As shown in Fig. 13, the catalyst reusability tests were conducted after six runs.FE-SEM image (Fig. 14a) and an XRD pattern (Fig. 14b) were obtained after several runs of NiFe2O4@SiO2/MgO recycling and no noticeable changes in morphology and XRD patterns were observed. As evidenced by morphological and XRD patterns, the catalyst produced excellent stability and retained catalytic activity after several recovery steps.
4. Conclusion
This research aimed to optimize Scenedesmus sp. lipid content to produce high lipid content and high-yield biodiesel using the response surface methodology and Box–Behnken design. The catalyst used in the transesterification reaction was NiFe2O4@SiO2/MgO. The reason for using this catalyst is the availability of cheap nanocatalyst ingredients and the high magnetic properties of this nanocatalyst, which adds to the economic features of this nanocatalyst. Also, the use of MgO due to its insensitivity to the water content of the reactants and SiO2 due to the increase in the reaction surface, increase the efficiency of the transesterification reaction. Box–Behnken design calculations show that the results obtained from the laboratory data were accurate enough, also the efficiency of 95.3 for the production of biodiesel shows that the used catalyst has a good effect on the production of biodiesel and the transesterification reaction.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the University of Kashan for supporting this work by Grant No. 890412/3.References
- B. Changmai, C. Vanlalveni, A. P. Ingle, R. Bhagat and S. L. Rokhum, RSC Adv., 2020, 10, 41625–41679 RSC.
- F. C. De Oliveira and S. T. Coelho, Renewable Sustainable Energy Rev., 2017, 75, 168–179 CrossRef CAS.
- B. Sajjadi, W.-Y. Chen, A. A. A. Raman and S. Ibrahim, Renewable Sustainable Energy Rev., 2018, 97, 200–232 CrossRef CAS.
- A. G. Ishaq, H. M. Matias-Peralta and H. Basri, Pertanika J. Trop. Agric. Sci., 2016, 39, 1–16 Search PubMed.
- E. H. Hegewald, Algae, 1997, 12, 235–246 Search PubMed.
- D. Y. C. Leung, X. Wu and M. K. H. Leung, Appl. Energy, 2010, 87, 1083–1095 CrossRef CAS.
- S. G. Krishnan, F. Pua and F. Zhang, Biomass Bioenergy, 2021, 149, 106099 CrossRef CAS.
- R. Halim, M. K. Danquah and P. A. Webley, Biotechnol. Adv., 2012, 30, 709–732 CrossRef CAS.
- D. G. Giarikos, J. Brown, R. Razeghifard, D. Vo, A. Castillo, N. Nagabandi, J. Gaffney, M. Zelden, A. Antakshinova, S. Rodriguez and S. Muhammad, Appl. Water Sci., 2021, 11, 39 CrossRef CAS.
- X. Ji, J. Cheng, D. Gong, X. Zhao, Y. Qi, Y. Su and W. Ma, Sci. Total Environ., 2018, 633, 593–599 CrossRef CAS.
- J. C. Nzayisenga, X. Farge, S. L. Groll and A. Sellstedt, Biotechnol. Biofuels, 2020, 13, 4 CrossRef CAS PubMed.
- M. A. Bezerra, R. E. Santelli, E. P. Oliveira, L. S. Villar and L. A. Escaleira, Talanta, 2008, 76, 965–977 CrossRef CAS.
- K. Sundareswaran, S. Peddapati and S. Palani, IET Renewable Power Generation, 2014, 8, 670–678 CrossRef.
- S. Beg and S. Akhter, in Design of Experiments for Pharmaceutical Product Development, Springer Singapore, Singapore, 2021, pp. 77–85 Search PubMed.
- C. C. Okpala, International Journal of Advanced Engineering and Technology, 2014, 12, 18 Search PubMed.
- R. Kumar, M. Mooste, Z. Ahmed, S. Akula, I. Zekker, M. Marandi, M. Käärik, J. Leis, A. Kikas, A. Treshchalov, M. Otsus, J. Aruväli, V. Kisand, A. Tamm and K. Tammeveski, Ind. Chem. Mater., 2023, 1, 526–541 RSC.
- J. Vinoth Arul Raj, B. Bharathiraja, B. Vijayakumar, S. Arokiyaraj, J. Iyyappan and R. Praveen Kumar, Bioresour. Technol., 2019, 282, 348–352 CrossRef CAS.
- M. Davoodbasha, A. Pugazhendhi, J.-W. Kim, S.-Y. Lee and T. Nooruddin, Fuel, 2021, 300, 121018 CrossRef CAS.
- A. Farrokheh, K. Tahvildari and M. Nozari, Biomass Convers. Biorefin., 2022, 12, 403–417 CrossRef CAS.
- V. Mittal and U. K. Ghosh, Bioresour. Technol. Rep., 2023, 23, 101504 CrossRef CAS.
- S. Alaei, M. Haghighi, B. Rahmanivahid, R. Shokrani and H. Naghavi, Renewable Energy, 2020, 154, 1188–1203 CrossRef CAS.
- T. Amani, M. Haghighi and B. Rahmanivahid, J. Ind. Eng. Chem., 2019, 80, 43–52 CrossRef CAS.
- H. Naeimi and F. Kiani, Appl. Organomet. Chem., 2019, 33, 1–12 CrossRef.
- A. Limouzadeh and H. Naeimi, J. Coord. Chem., 2020, 73, 1907–1924 CrossRef CAS.
- T. Rahimi, D. Kahrizi, M. Feyzi, H. R. Ahmadvandi and M. Mostafaei, Ind. Crops Prod., 2021, 159, 113065 CrossRef CAS.
- M. K. Johnson, E. J. Johnson, R. D. MacElroy, H. L. Speer and B. S. Bruff, J. Bacteriol., 1968, 95, 1461–1468 CrossRef CAS PubMed.
- G. Zhang, H. Zhang, D. Yang, C. Li, Z. Peng and S. Zhang, Catal. Sci. Technol., 2016, 6, 6417–6430 RSC.
- A. Marwaha, P. Rosha, S. K. Mohapatra, S. K. Mahla and A. Dhir, Energy Rep., 2019, 5, 1580–1588 CrossRef.
- G. E. Fogg and B. Thake, Algal cultures and phytoplankton ecology, University of Wisconsin Press, 1987 Search PubMed.
- E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS.
- N. Hosseini Nasab and J. Safari, J. Mol. Struct., 2019, 1193, 118–124 CrossRef CAS.
- E. Al-Bermany, A. T. Mekhalif, H. A. Banimuslem, K. Abdali and M. M. Sabri, Silicon, 2023, 15, 4095–4107 CAS.
- J. T. Kloprogge and R. L. Frost, Vib. Spectrosc., 2000, 23, 119–127 CrossRef CAS.
- T. Somanathan, V. M. Krishna, V. Saravanan, R. Kumar and R. Kumar, J. Nanosci. Nanotechnol., 2016, 16, 9421–9431 CrossRef CAS.
- M. Qiu, Y. Zhang and B. Wen, J. Mater. Sci.: Mater. Electron., 2018, 29, 10437–10444 CrossRef CAS.
- B. Rahmani Vahid, M. Haghighi, S. Alaei and J. Toghiani, Energy Convers. Manage., 2017, 143, 23–32 CrossRef CAS.
- Y. Chisti, Biotechnol. Adv., 2007, 25, 294–306 CrossRef CAS PubMed.
- S. Ahmad, S. Chaudhary, V. V. Pathak, R. Kothari and V. V. Tyagi, Renewable Energy, 2020, 160, 86–97 CrossRef CAS.
- E. Safakish, H. Nayebzadeh, N. Saghatoleslami and S. Kazemifard, Algal Res., 2020, 49, 101949 CrossRef.
- T. T. Mamo and Y. S. Mekonnen, Appl. Biochem. Biotechnol., 2020, 190, 1147–1162 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02904f |
| This journal is © The Royal Society of Chemistry 2024 |