Open Access Article
Open Access ArticleElectron transfer at the heterojunction interface of CoP/MoS2 for efficient electrocatalytic hydrogen evolution reaction†
Lili Zhanga,
Aijiao Xua,
Xinxing Shia,
Huanhuan Zhanga,
Zongpeng Wanga,
Shijie Shen a,
Jitang Zhang
a,
Jitang Zhang *abc and
Wenwu Zhong
*abc and
Wenwu Zhong *a
*a
aZhejiang Key Laboratory for Island Green Energy and New Materials, Taizhou University, Taizhou 318000, China. E-mail: zhangjt@tzc.edu.cn; zhongww@tzc.edu.cn
bERA Co, Ltd., Taizhou 318020, China
cZhejiang University, College of Chemical and Biological Engineering, Hangzhou 310027, China
First published on 17th June 2024
Abstract
Modulating the electronic states of electrocatalysts is critical for achieving efficient hydrogen evolution reaction (HER). However, how to develop electrocatalysts with superior electronic states is an urgent challenge that must be addressed. Herein, we prepared the CoP/MoS2 heterojunction with a microsphere morphology consisting of thin nanosheets using a facile two-step method. The catalyst's ultrathin nanosheet structure not only provides an extensive surface area for exposing active sites, but it also enables ion transport and bubble release. Electron transfer occurs between CoP and MoS2, optimizing the heterojunction's charge distribution and enhancing the intermediates' adsorption capabilities. As a result, the CoP/MoS2 heterojunction exhibits outstanding electrocatalytic hydrogen evolution activity with an overpotential of only 88 mV at a current density of 10 mA cm−2, which exceeds both the sulfide heterojunction Co9S8/MoS2 and the phosphide heterojunction CoP/CoMoP2. The experimental results and DFT calculation results show that the former has stronger synergistic effects and higher HER activity. This work sheds light on the exploration of efficient heterojunction electrocatalysts with excellent electronic structures.
Introduction
Zero carbon emission is a crucial goal in the context of addressing environment pollution and climate warming.1–3 Hydrogen energy produced by the electrocatalytic hydrogen evolution reaction (HER) is regarded as an excellent alternative fuel to traditional fossil fuel sources.4–10 At present, Pt based materials are the most efficient catalysts for the HER. Nevertheless, the low quantity and high price of noble metals hinders their widespread use in HER. Developing highly-efficient and cost-effective catalysts is of great importance in shifting from fossil fuel-based energy to renewable hydrogen.11–17 Electrocatalysts' electronic states can influence their ability to adsorb and activate water molecules, as well as the subsequent electron transfer process. As a result, studying and understanding the electronic states of electrocatalysts is critical for designing effective electrocatalysts for efficient hydrogen evolution reaction. The presence of a heterointerface between two materials with different electronic properties can create a gradient in the electron density. This gradient promotes charge transfer across the interface, influencing the redox behavior of the active sites and altering their valence states during HER.Transition metal sulfides,18–21 selenides,22–24 phosphates,25–27 carbides28–30 and other compounds with high activity and conductivity have drawn a lot of attention in the field of hydrogen evolution catalysts. CoP is a promising catalyst for hydrogen evolution among transition metal compounds due to its high HER activity, excellent stability and excellent conductivity.31,32 Nonetheless, some research indicates that slow reaction kinetics in HER process are caused by the significant binding energy of CoP and reaction intermediates, which may hinder the further improvement of its hydrogen evolution absorption capacity.33–35 MoS2 has been demonstrated to be a potential HER electrocatalyst among transition metal sulfides.36–41 2D MoS2 exhibits unique electrical properties due to significant interaction between atoms, which allows the nanosheet to expose a large number of active sites. Unfortunately, MoS2 is a semiconductor with the limited catalytic active site and low electrical conductivity prevent its widespread use in the electrolytic water industry. Heterostructures have unique catalytic properties of HER due to the advantages of synergistic effect and electronic structural control.42,43 Therefore, the combination of CoP with MoS2 to further boost catalytic performance by utilizing the synergistic effect of the two has been widely used. Guo et al.44 developed a hollow CoP@MoS2 heterogeneous nanoframework by combining MoS2 on a CoP nanoframework obtained from a metal–organic framework nanostructure (ZIF-67). The overpotential of CoP@MoS2 is 119 mV and the corresponding Tafel slope is only 49 mV dec−1 at 10 mA cm−2. The remarkable catalytic activity is due to its unique structure, moderate composition ratio and strong contact between CoP and MoS2, and also the large number of defects and disordered configurations. Although some reports on CoP/MoS2 heterojunction hydrogen evolution catalysts have been published, the synthesis process is more complex.45,46 Therefore, to preparation of simple and excellent CoP/MoS2 catalyst for HER hydrogen evolution and the exploration of its related mechanism still need to be further carried out.
Herein, we fabricated phosphide and sulfide heterojunction CoP/MoS2 with microsphere morphology. Meanwhile, the catalytic performance and mechanism of CoP/MoS2 were described by experimentation and DFT calculation. The hydrogen evolution features of hybrid heterojunctions of phosphide CoP/CoMoP2 and sulfide Co9S8/MoS2 fabricated by the same precursor (CoMo-LDH) were compared with CoP/MoS2. The results demonstrate that CoP/MoS2 has better hydrogen evolution performance than CoP/CoMoP2 and Co9S8/MoS2.47,48 At the current density of 10 mA cm−2, the required overpotential of CoP/MoS2 is only 88 mV. Various characterizations and DFT simulations reveal that heterojunctions consisting of phosphide and sulfide have stronger synergistic effects and better HER activities than phosphide heterojunctions and sulfide heterojunctions.
Results and discussions
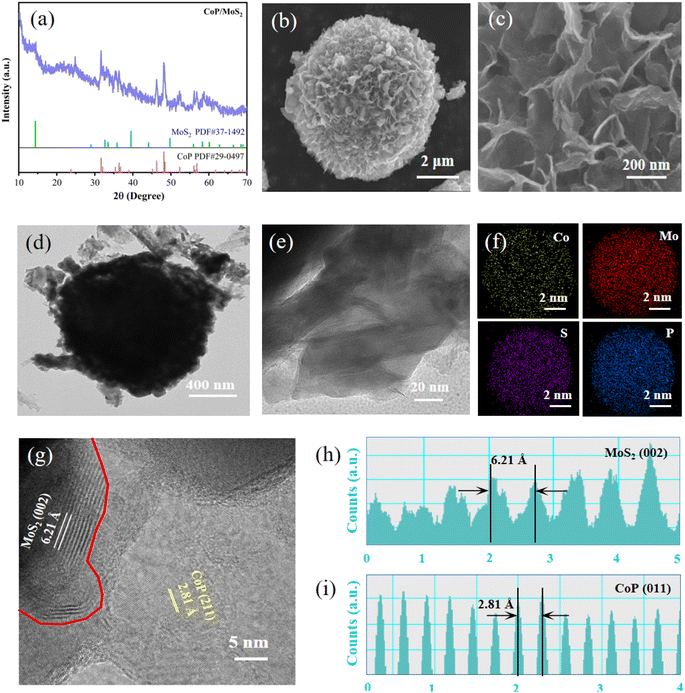
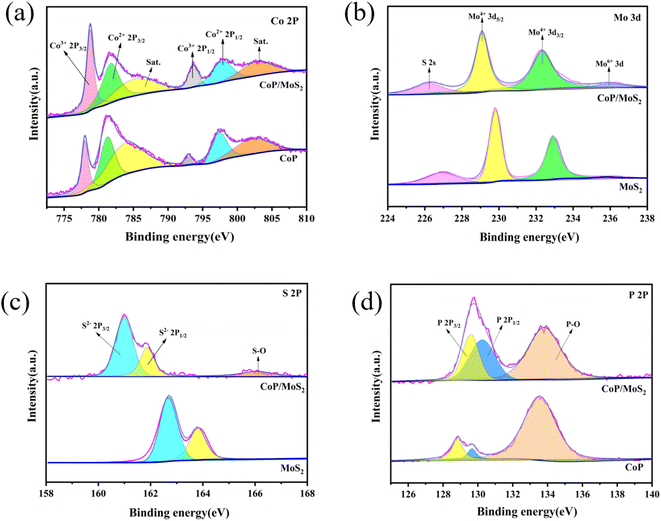
The XRD pattern of CoP/MoS2 hydrogen evolution catalyst prepared at 550 °C is shown in Fig. 1(a). By contrast to CoP standard data(PDF #29-0947), different samples exhibit discernible diffraction peaks at 2θ of 48.1° and 46.2°, matching the (211) and (112) crystal planes of CoP. Furthermore, all three samples exhibit comparable intensity diffraction peaks at the 2θ of 14.4°, which match to the (002) crystal face of MoS2 (PDF #37-1492). Additionally, samples at 450 °C and 650 °C were prepared to investigate the effect of sulfuration and phosphorization temperatures on the products. As shown in Fig. S1(a),† samples prepared at 450 °C and 650 °C have no significant diffraction peaks, whereas the sample prepared at 550 °C have significant diffraction peaks and the best crystallinity, these results show that 550 °C is a suitable temperature to obtain the product. The EDS spectrum (Fig. S1(b)†) provides additional proof that the constituent components of the sample are Co, Mo, P and S. The presence of a small amount of O may be due to the insufficient vacuum degree in the quartz tube of the tube furnace, resulting in slight oxidation of the sample. SEM images of CoP/MoS2 at different scales are displayed in Fig. 1(b and c). The sample has a microsphere-like shape and is covered in nanosheet structures. The cross-linked nanosheet structure can offer a better heterogeneous interface as well as the improved electronic conductivity. Fig. S2† displays SEM images of samples prepared at various temperatures and magnifications. It is clear that samples at 450, 550 and 650 °C retain the flower ball shape of the precursor. At 450 °C, the ultra-thin nanosheets on the surface of the flower ball are connected to each other and the sample nanosheets are more densely arranged. When the temperature reached 650 °C, the thickness of the nanosheets on the microspherical surface increased. Contraction and aggregation also took place at this temperature, which led to a reduction in the quantity of active sites and a decline in catalytic efficiency. The CoP/MoS2 heterostructures prepared at 550 °C can be observed in TEM images with microspherical structures (Fig. 1(d)), and ultrathin nanosheet structures can be observed in enlarged images (Fig. 1(e)). The distribution of the Co, Mo, S and P elements in the sample is uniform, as seen in Fig. 1(f). High-resolution TEM images (Fig. 1(g)) reveal distinct fringes with the 6.21 Å fringe matching to the (002) crystal face of MoS2 (Fig. 1(h)) and the 2.81 Å fringe corresponding to the (011) crystal face of CoP (Fig. 1(i)). There is a distinct interface between them, indicating the successful construction of a heterojunction.Chemical compositions and elemental states of the samples were profiled using X-ray photoelectron spectroscopy (XPS). Fig. S3† displays the observed spectra of CoP, MoS2 and CoP/MoS2. Elemental components including Co, Mo, S, P and O are present in CoP/MoS2 sample. The O element is formed by the oxidation of the sample surface, and the C element is formed by the toner added with the correction data. The Co2+ electrons are represented by the peaks near 781.9 eV and 798.1 eV in the high-resolution XPS spectrum of Co 2p (Fig. 2(a)), while the electrons of Co3+ are represented by the peaks at 777.8 eV and 793.8 eV. Furthermore, the peaks from Co at 786.1 eV and 803.5 eV are satellite peaks. There is evidence that some electrons in CoP/MoS2 are transported from Co to Mo, as the Co3+ peak area of CoP/MoS2 is substantially greater than the Co3+ peak area of CoP. Four peaks are visible at 226.3 eV, 229.1 eV, 232.3 eV and 231.1 eV in the high-resolution XPS spectra of Mo (Fig. 2(b)). These peaks are assigned to S 2s, Mo4+ 3d5/2, Mo4+ 3d3/2 and Mo6+ 3d, respectively. In contrast to MoS2, the Mo4+ 3d peak of CoP/MoS2 moves in the direction of a lower binding energy, suggesting that Mo in CoP/MoS2 receives electrons. The emergence of Mo6+ is caused by the oxidation of some Mo elements on the surface of the sample. P 2p3/2, P 2p1/2 and P–O are represented by the three peaks in the XPS spectra of high-resolution P 2p electrons (Fig. 2(c)), which are situated at 129.6 eV, 130.2 eV and 133.8 eV, respectively. CoP/MoS2 exhibits an increase in P 2p peak area and a decrease in P–O peak area, a shift in peak location towards the direction of higher binding energy. The peaks between 160 and 165 eV in the XPS spectra of S 2p electrons (Fig. 2(d)) can be fitted with two peaks, which are S2− 2p3/2 and S2− 2p1/2 electrons, respectively. The S 2s peaks of CoP/MoS2 migrate substantially in the direction of low binding energy as compared to MoS2. The aforementioned findings demonstrate that the electrical structure of CoP/MoS2 is optimized and that charge transfer occurs between CoP and MoS2.
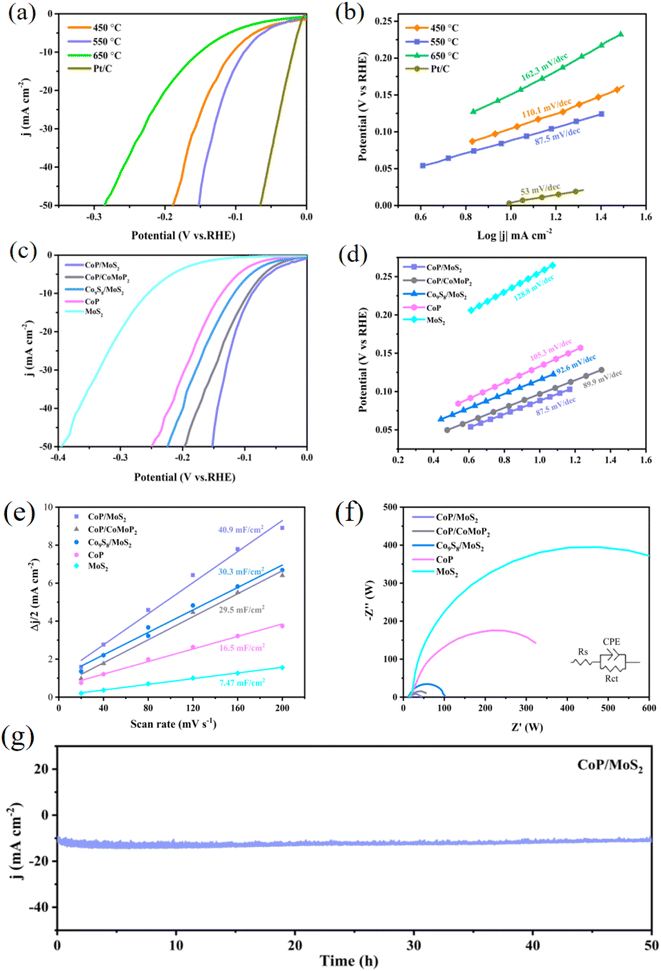
In general, the catalytic activity of HER of various materials is reflected by the corresponding overpotential at a current density of 10 mA cm−2. As demonstrated by Fig. 3(a and b), samples at 450 °C, 550 °C and 650 °C have overpotentials of 104, 88 and 151 mV at a current density of 10 mA cm−2, with corresponding Tafel slopes of 110.1, 87.5 and 162.3 mV dec−1, respectively. According to the results of the LSV polarization curve and the Tafel slope results, the CoP/MoS2 sample synthesized at 550 °C has the best catalytic performance. Meantime, the catalytic characteristics of phosphide/sulfide heterojunction CoP/MoS2, phosphide heterojunction CoP/CoMoP2, sulfide heterojunction Co9S8/MoS2, as well as CoP and MoS2 samples were compared. At 10 mA cm−2 current density, the LSV of each sample was 88, 94, 117, 137 and 254 mV, with the corresponding Tafel slopes of 85.7, 89.9, 92.6, 105.3 and 128.8 mV dec−1. The results show that the catalytic performance of the CoP/MoS2 hydrogen evolution catalyst is superior to the CoP/CoMoP2 and Co9S8/MoS2 hydrogen evolution catalysts, indicating that the CoP/MoS2 sample has excellent HER kinetics. In order to determine the relatively effective electrochemically active surface area, the double-layer capacitance (Cdl) which is linearly proportional to the electrochemically active specific surface area (ECSA) is further measured. In previous research, Cdl of CoP/CoMoP2, Co9S8/MoS2, CoP and MoS2 were evaluated and ECSA fitted at scanning rates of 0.02, 0.04, 0.08, 0.12, 0.16 and 0.20 V s−1. Fig. 3(e) shows that their Cdl values are 30.3, 29.5, 16.5 and 7.47 mF cm−2, respectively. CoP/MoS2 samples was 40.9 mF cm−2, showing that the CoP/MoS2 heterojunction hydrogen evolution catalyst had a greater specific surface area of electrochemical activity, which considerably improved its hydrogen evolution performance. The electrochemical impedance of the prepared catalyst was measured in the range of 0.01 to 105 Hz. As shown in Fig. 3(f), the Rct values of each sample are in the order from small to large: CoP/MoS2(14.4 Ω), CoP/CoMoP2(24.2 Ω), Co9S8/MoS2(50.6 Ω), CoP(63.6 Ω) and MoS2(189.8 Ω) indicate that the phosphide/sulfide heterojunction CoP/MoS2 hydrogen evolution catalyst has lower charge transfer resistance than the sulfide and phosphide hydrogen evolution catalyst, which contributes to the rapid electron transfer in HER process. The stability of CoP/MoS2 samples prepared at 550 °C in 1 M KOH solution was tested by constant point electrolysis method. As shown in Fig. 3(g), at the overpotential of 88 mV, the current density did not change significantly for more than 50 consecutive hours, indicating that the prepared CoP/MoS2 heterojunction hydrogen evolution catalyst had excellent electrocatalytic hydrogen evolution stability, which was mainly due to the good binding force between CoP and MoS2 substrate.
To further understand the mechanism of the enhancement of HER activity after CoP/MoS2 forms heterogeneous structures, we use the Vienna Ab initio Simulation Package (VASP) for DFT calculation, Fig. S5† shows the optimized structure model of CoP, MoS2 and CoP/MoS2. Fig. S6(a)† shows the integral value of the density difference in XY cross sections of CoP/MoS2 at different Z positions, which facilitates the quantification of the density difference distribution. It can be seen that the isosurface value of charge transfer at a fixed position is isosurface value = 0.005 e A−3. Fig. S6(b)† depicts the CoP/MoS2 differential charge density, where yellow and blue represent positive and negative charges, respectively. The yellow representing charge accumulation and blue representing charge consumption. It can be seen that electrons flow from CoP to MoS2 and the charge distribution is optimized at the CoP/MoS2 interface after forming a heterojunction. The improvement in heterojunction performance can be attributed to electronic structural optimization, which is consistent with the results of XPS results. Gibbs free energy (ΔGH*) is a appropriate parameter to evaluate HER activity of catalyst. To further investigate the HER activity of CoP/MoS2 heterostructure hydrogen evolution catalysts, ΔGH* of CoP/MoS2, CoP/CoMoP2, Co9S8/MoS2, CoP and MoS2 were calculated (Fig. 4(a)). It can be seen that the ΔGH* of CoP/CoMoP2, Co9S8/MoS2, CoP and MoS2 are 0.39 eV, −0.24 eV, 0.61 eV and 2.5 eV respectively, whereas CoP/MoS2 is only 0.16 eV, which is very close to the ideal 0 eV, indicating that CoP/MoS2 is the most favorable for HER reaction. The adsorption energy of the catalyst on water molecules is another parameter that reflects HER activity. As shown in Fig. 4(b), the adsorption energies of CoP/MoS2, CoP/CoMoP2, Co9S8/MoS2, CoP and MoS2 samples are −1.03 eV, −0.72 eV, −0.3 eV, −0.16 eV and 0.05 eV, respectively. The results indicate that Co9S8/MoS2 exhibits the best adsorption of water molecules out of the three. This suggests that Co9S8/MoS2 performs the best among these three in adsorbing water molecules. Both of hydrogen evolution free energy and water absorption energy of phosphating and sulfide heterojunction CoP/MoS2 are superior to phosphating heterojunction CoP/CoMoP2 and sulfide heterojunction Co9S8/MoS2, indicating that phosphating/sulfide heterojunction CoP/MoS2 have better synergistic effect and higher HER activity. Furthermore, we calculated Co, Mo, P and S atoms' partial density of states (PDOS) of CoP, MoS2 and CoP/MoS2. The systems all exhibit metal-like electronic characteristics (Fig. S7†). The density of states near the Fermi level of CoP/MoS2 is lower than that of CoP and MoS2. This suggests that it is not the conductivity of the heterojunction but the adsorption properties of its active sites that contribute to the superior performance.
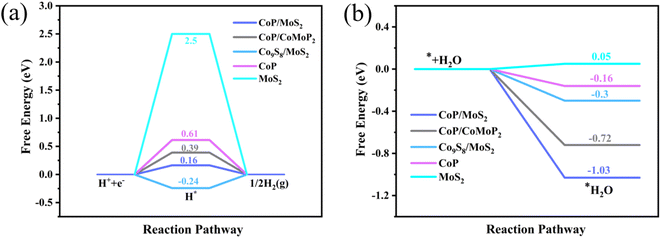 | ||
| Fig. 4 (a) The ΔGH* and (b) for water adsorption energy for CoP/MoS2, CoP/CoMoP2, Co9S8/MoS2, CoP and MoS2. | ||
Conclusion
In summary, we successfully synthesized CoP/MoS2 microsphere heterojunction HER catalysts with a large number of nanosheets by phosphating and vulcanizing the CoMo-LDH precursor at different temperatures. The sample prepared at 550 °C shows the best crystallinity and surface morphology. The nanosheet structure on the microsphere's surface has an extensive surface area and fully exposed active sites. In addition, the charge transfer between CoP and MoS2 optimizes the electronic state of the catalyst. As a result, the overpotential of CoP/MoS2 is only 88 mV at a current density of 10 mA cm−2, which is superior to that of the phosphides heterojunction CoP/CoMoP2 and the sulfides heterojunction Co9S8/MoS2. The findings suggest that CoP/MoS2 has a stronger synergistic effect than the latter two. This work proposes a novel and simple way to fabricating high-performance transition metal-based compound heterojunctions.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52072255, 22379104), Natural Science Foundation of Zhejiang Province, China (LY24E020004), China Postdoctoral Science Foundation (2022M723546), Taizhou University Excellent Youth Project (Z2020073).References
- O. Geden, Nat. Geosci., 2016, 9, 340–342 CrossRef CAS.
- J. Rogelj, M. Schaeffer, M. Meinshausen, R. Knutti, J. Alcamo, K. Riahi and W. Hare, Environ. Res. Lett., 2015, 10, 105007 CrossRef.
- P. A. Koudakan, C. Wei, A. Mosallanezhad, B. Liu, Y. Y. Fang, X. B. Hao, Y. T. Qian and G. M. Wang, Small, 2022, 18, 2107974 CrossRef CAS PubMed.
- F. Qureshi, M. Yusuf, A. A. Pasha, H. W. Khan, B. Imteyaz and K. Irshad, Int. J. Hydrogen Energy, 2022, 47, 41397–41420 CrossRef CAS.
- R. K. Mishra, V. Kumar, L. G. Trung, G. J. Choi, J. W Ryu, P. Kumar, R. Bhardwaj, S. H. Lee and J. S. Gwag, Mater. Lett., 2023, 338, 134026 CrossRef CAS.
- F. Almomani, A. Al-Rababah, M. Tawalbeh and A. Al-Othman, Fuel, 2023, 332, 125905 CrossRef CAS.
- P. K. Pathak, A. K. Yadav and S. Padmanaban, Int. J. Hydrogen Energy, 2023, 48, 9921–9927 CrossRef CAS.
- M. Kim, Y. Kim, M. Y. Ha, E. Shin, S. J. Kwak, M. Park, I. D. Kim, W. B. Jun, W. B. Lee, Y. J. Kim and H. T. Jun, Adv. Mater., 2023, 2211497 CrossRef CAS PubMed.
- J. M. Xiao, S. S. Zhang, Y. Y. Sun, X. T. Liu, G. L. He, H. Liu, J. Khan, Y. L. Zhu, Y. Q. Su, S. Y. Wang and L. Han, Small, 2023, 19, 2206472 CrossRef CAS PubMed.
- H. B. He, L. B. Zeng, X. Y. Peng, Z. B. Liu, D. S. Wang, B. Yang, Z. J. Li, L. C. Lei, S. B. Wang and Y. Hou, Chem. Eng. J., 2023, 451, 138628 CrossRef CAS.
- L. L. Zhang, S. J. Shen, J. T. Zhang, Z. P. Lin, Z. P. Wang, Q. H. Zhang, W. W. Zhong, L. Zhu and G. F. Wu, Small Methods, 2022, 2200900 CrossRef CAS PubMed.
- L. L. Zhang, Z. P. Wang, J. T. Zhang, Z. P. Lin, Q. H. Zhang, W. W. Zhong and G. F. Wu, Nano Res., 2023, 16(5), 6552–6559 CrossRef CAS.
- Z. P. Lin, Z. P. Wang, J. J. Gong, T. C. Jin, S. J. Shen, Q. H. Zhang, J. C. Wang and W. W. Zhong, Adv. Funct. Mater., 2023, 33, 2307510 CrossRef CAS.
- S. J. Shen, Z. Y. Hu, H. H. Zhang, K. Song, Z. P. Wang, Z. P. Lin, Q. H. Zhang, L. Gu and W. W. Zhong, Angew. Chem., Int. Ed., 2022, 61, e202206460 CrossRef CAS PubMed.
- Z. X. Wu, P. F. Yang, Q. C. Li, W. P. Xiao, Z. J. Li, G. R. Xu, F. Liu, B. H. Jia, T. Y. Ma, S. H. Feng and L. Wang, Angew. Chem., Int. Ed., 2023, 62, e2023004 Search PubMed.
- K. Song, H. H. Zhang, Z. P. Lin, Z. P. Wang, L. L. Zhang, X. X. Shi, S. J. Shen, S. C. Chen and W. W. Zhong, Adv. Funct. Mater., 2023, 2312672 Search PubMed.
- S. J. Shen, H. H. Zhang, K. Song, Z. P. Wang, T. T. Shang, A. Gao, Q. H. Zhang, L. Gu and W. W. Zhong, Angew. Chem., Int. Ed., 2024, 63, e20231534 Search PubMed.
- Y. M. Ding, N. W. Li, S. Yuan and L. Yu, Chem.–Asian J., 2022, 17, e202200178 CrossRef PubMed.
- J. Xu, G. L. Shao, X. Tang, F. Lv, H. Y. Xiang, C. F. jing, S. Liu, S. Dai, Y. G. Li, J. Luo and Z. Zhou, Nat. Commun., 2022, 13, 2193 CrossRef CAS PubMed.
- M. Dar, K. Majid and M. Wahid, New J. Chem., 2022, 46, 22427–22440 RSC.
- B. F. Chen, Y. C. Wang, S. J. Shen, W. W. Zhong, H. S. Lu and Y. Pan, Small Methods, 2024, 2301598 CrossRef PubMed.
- X. Xiao, S. J. Shen, L. L. Zhang, Z. P. Lin, Z. P. Wang, Q. H. Zhang, W. W. Zhong and B. S. Zhan, Chem.–Asian J., 2023, 18, e202201182 CrossRef CAS PubMed.
- X. W. Pan, W. J. He, D. Cao, Y. Li, C. C. Liu, L. M. Liang, Q. Y. Hao and H. Liu, ACS Appl. Nano Mater., 2023, 6, 1724–1731 CrossRef CAS.
- A. Majumdar, P. Dutta, A. Sikdar, H. Lee, D. Ghosh, S. N. Jha, S. Tripathi, Y. Oh and U. N. Maiti, Small, 2022, 18, 2200622 CrossRef CAS PubMed.
- T. W. Zhao, S. H. Wang, Y. B. Li, C. Jia, Z. Su, D. Hao, B. J. Ni, Q. Zhang and C. Zhao, Small, 2022, 18, 2204758 CrossRef CAS PubMed.
- D. I. Jeong, H. W. Choi, J. Kim, U. Y. Lee, B. K. Koo, B. K. Kang and D. H. Yoon, Appl. Surf. Sci., 2023, 614, 156189 CrossRef CAS.
- Z. D. Li, C. Y. Zhang, Y. Yang, S. S. Pi, Y. J. Yu, C. F. Wan, B. Q. Zhou, W. X. Chao and L. Lu, Inorg. Chem. Front., 2023, 10, 325 RSC.
- Y. F. Ma, M. Chen, H. B. Geng, H. F. Dong, P. Wu, X. M. Li, G. Q. Guan and T. J. Wang, Adv. Funct. Mater., 2020, 30, 2000561 CrossRef CAS.
- D. S. Baek, J. Y. Lee, J. J. Kim and S. H. Joo, ACS Catal., 2022, 12, 7415–7426 CrossRef CAS.
- S. Upadhya and O. P. Pandey, J. Electrochem. Soc., 2022, 169, 016511 CrossRef.
- S. J. Shen, Z. P. Wang, Z. P. Lin, K. Song, Q. H. Zhang, F. Q. Meng, L. Gu and W. W. Zhong, Adv. Mater., 2022, 34, 2110631 CrossRef CAS PubMed.
- J. Q. Guo, C. Ouyang, Z. X. Zhan, T. Lei and P. Yin, Int. J. Hydrogen Energy, 2022, 47, 181–196 CrossRef CAS.
- X. Z. Song, W. Y. Zhu, J. C. Ni, Y. H. Zhao, T. Zhang, Z. Q. Tan, L. Z. Liu and X. F. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 33151–33160 CrossRef CAS PubMed.
- L. H. Zhi, J. B. Tu, J. X. Li, M. Li and J. C. Liu, J. Colloid Interface Sci., 2022, 616, 379–388 CrossRef CAS PubMed.
- M. D. Wang, X. Y. Liu and X. Wu, Nano Energy, 2023, 114, 108681 CrossRef CAS.
- X. Liu, Z. Q. Li, H. L. Jiang, X. Wang, P. F. Xia, Z. J. Duan, Y. Z. Ren, H. Y. Xiang, H. M. Li, J. Zeng, Y. G. Zhou and S. Liu, Small, 2023, 2307293 Search PubMed.
- H. J. Liu, S. Zhang, Y. M. Chai and B. Dong, Angew. Chem., Int. Ed., 2023, 62, e202313845 CrossRef CAS PubMed.
- K. J. Wang, Y. Jing, S. Gao, X. R. Liu, B. X. Liu, Y. C. Li, P. Zhang and B. H. Xu, J. Colloid Interface Sci., 2023, 648, 709–718 CrossRef CAS PubMed.
- J. Y. Shen, J. L. Zhang, G. N. Zhang, W. H. Li, M. Zheng, F. Y. Guo and Q. Q. Chen, New J. Chem., 2022, 46, 16419–16425 RSC.
- K. Cao, S. W. Sun, A. Y. Song, J. X. Ba, H. W. Lin, X. H. Yu, C. Q. Xu, B. J. Jin, J. Huang and D. H. Fan, J. Alloys Compd., 2022, 907, 164539 CrossRef CAS.
- Y. Xu, J. Cheng, H. K. Lv, L. W. Ding, K. Zhang, A. N. Hu and X. Yang, Chem. Eng. J., 2023, 470, 144344 CrossRef CAS.
- G. Q. Zhao, K. Rui, S. X. Dou and W. P. Sun, Adv. Funct. Mater., 2018, 28, 1803291 CrossRef.
- H. X. Wang, W. W. Fu, X. H. Yang, Z. Y. Huang, J. Li, H. J Zhang and Y. Wang, J. Mater. Chem. A, 2020, 8, 6926 RSC.
- T. Y. Xia, L. Zhou, S. Q. Gu, H. Gao, X. Y. Ren, S. F. Li, R. M. Wang and H. Z. Guo, Mater. Des., 2021, 211, 110165 CrossRef CAS.
- C. L. Zhang, Y. Xie, J. T. Liu, F. H. Cao, H. P. Cong and H. Li, Chem. Eng. J., 2021, 419, 129977 CrossRef CAS.
- J. G. Li, K. F. Xie, H. C. Sun, Z. S. Li, X. Ao, Z. H. Chen, K. K. Ostrikov, C. D. Wang and W. J. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 36649–36657 CrossRef CAS PubMed.
- L. L. Zhang, X. X. Shi, A. J. Xu, W. W. Zhong, J. T. Zhang and S. J. Shen, Nano Res., 2024, 17(3), 2061–2069 CrossRef.
- L. L. Zhang, J. T. Zhang, A. J. Xu, Z. P. Lin, Z. P. Wang, W. W. Zhong, S. J. Shen and G. F. Wu, Biomimetics, 2023, 8, 104 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02712d |
| This journal is © The Royal Society of Chemistry 2024 |