Open Access Article
Open Access ArticleGraphitic carbon nitride supported silver nanoparticles (AgNPs/g-C3N4): synthesis and photocatalytic behavior in the degradation of 2,4-dichlorophenoxyacetic acid†
Phung Thi Lan *a,
Nguyen Hoang Haob,
Nguyen Trung Hieua,
Nguyen Thi Thu Ha
*a,
Nguyen Hoang Haob,
Nguyen Trung Hieua,
Nguyen Thi Thu Ha a,
C. Trevor Brownc and
Le Minh Camad
a,
C. Trevor Brownc and
Le Minh Camad
aFaculty of Chemistry, Hanoi National University of Education, 136 Xuan Thuy, Cau Giay, Hanoi, Vietnam. E-mail: lanpt@hnue.edu.vn
bCollege of Education, Vinh University, 182 Le Duan, Vinh, Nghe An, Vietnam
cThe UNE, Armidale, Australia
dThanh Do University, QL 32, Kim Chung, Hoai Duc, Ha Noi, Vietnam
First published on 13th June 2024
Abstract
Graphitic carbon nitride supported silver nanoparticles (AgNPs/g-C3N4) with 1%, 3%, and 5% AgNPs were successfully synthesized by an “ex situ” method with ultrasound of a mixture of AgNP solution and g-C3N4. The AgNP solution was prepared by chemical reduction with trisodium citrate, and g-C3N4 was synthesized from the urea precursor. The supported nanoparticles were characterized by X-ray diffraction spectroscopy (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen adsorption–desorption (BET), Fourier transformation infrared (FTIR) and Raman spectroscopy, ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS), photoluminescence spectroscopy (PL), electron paramagnetic resonance (EPR) and electrochemical impedance spectroscopy (EIS) Nyquist plots. The visible light-driven photocurrent measurement was performed by three on–off cycles of intermittent irradiation. The analyses show that AgNPs were evenly dispersed on g-C3N4, and have sizes ranging from 40 to 50 nm. The optical properties of the AgNPs/g-C3N4 material were significantly enhanced due to the plasmonic effect of AgNPs. The photocatalytic activity of catalysts was evaluated by 2,4-D degradation under visible light irradiation (λ > 420 nm). In the reaction conditions: pH 2.2; Co (2,4-D) 40 ppm; a m/v ratio of 0.5 g L−1, AgNPs/g-C3N4 materials exhibit superior photocatalytic activity compared to the pristine g-C3N4. The studies on the influence of free radicals and photogenerated holes, h+, show that ˙OH, O2˙−, and h+ play decisive roles in the photocatalytic activity of AgNPs/g-C3N4. The TOC result indicates the minimal toxicity of the by-products formed during the 2,4-D degradation. In addition, the AgNPs/g-C3N4 catalytic activity under direct sunlight irradiation was similar to that under artificial UV irradiation. Based on these results, a possible mechanism is proposed to explain the enhanced photocatalytic activity and stability of AgNPs/g-C3N4. Theoretical calculations on the interaction between 2,4-D and g-C3N4, Ag/g-C3N4 was also performed. The calculated results show that the adsorption of 2,4-D on Ag-modified g-C3N4 is significantly more effective compared to pristine g-C3N4.
Introduction
Herbicides, especially chemicals that control undesired plants and protect crops, have played a crucial role in agriculture. However, excessive use and misuse of herbicides not only affects the quality of agricultural products, due to the proliferation of herbicide residues, but also directly contributes to environmental pollution in soil, water, and the atmosphere. 2,4-Dichlorophenoxyacetic acid (2,4-D) is a commonly used herbicide, and, if used excessively, can moderately persist, particularly in anaerobic environments, such as soil and water.1 Bioaccumulation of 2,4-D can be highly toxic to aquatic life and has been associated with Parkinson's disease and autism in humans.2 New methods to completely treat persistent organic chlorine compounds like 2,4-D are of great interest to the agriculture industry.Currently, two main methods are used for 2,4-D treatment: adsorption3–6 and advanced oxidation processes (AOPs) such as ozonation, Fenton oxidation, and photocatalysis.7–10 Adsorption methods remove 2,4-D primarily from water. The 2,4-D is retained on the surface of adsorbent materials without degradation into environmentally friendly products. In contrast, AOPs have the potential to completely remove toxic chemicals from the environment. Photocatalysis is particularly attractive because it harnesses natural sunlight as an energy source.7,11,12
Graphite carbon nitride (g-C3N4), the metal-free semiconductor has been widely used in many applications such as chemical sensors, water splitting, and pollutant degradation13–17 Graphitic carbon nitride (g-C3N4) is a promising material for the photocatalysis of 2,4-D.12 It is synthesized from readily available, low-cost precursors (urea, melamine, thiourea, etc.), has high chemical and thermal stability, is not toxic, and has a moderate band gap energy of approximately 2.7 eV.11 However, g-C3N4 has limitations, such as rapid electron–hole recombination rates and a small specific surface area. To enhance the catalytic activity of g-C3N4, it is combined with nano-sized metal semiconductor oxides like TiO2,18,19 WO3,20 or d-band metal nanoparticles like Ag, Au (AgNPs, AuNPs). Such additives cause plasmon effects, which enhance light absorption and thus photocatalytic activity.21,22
Nanoparticles (NPs) of noble metals (i.e., Ag, Au, Pt) can strongly absorb visible light due to their surface plasmon resonance (SPR), which can be tuned by varying their size, shape and surrounding. Furthermore, noble metal NPs can also work as both electron traps and active reaction sites. These factors gave rise to a new approach to efficient visible-light photocatalysts. Recently, a variety of Ag-based semiconductor photocatalysts have been capturing considerable attention because of the surface plasmonic resonance (SPR) effect of noble metal nanoparticles under light irradiation. Han et al.23 synthesized Ag@AgCl nanoframe photocatalyst. The as-synthesized samples showed superior photocatalytic activity and photo-generated charge carrier separation efficiency under visible light irradiation. Dong et al.24 prepared uniform cubic Ag@AgCl photocatalysts and indicated that O2˙− and Cl0 are likely to be reactive species leading to the degradation of pollutants.
There have been few methods of Ag nano synthesis applied by research groups, for example Yao and co-authors25 synthesized AgCl and then converted to Ag0 by irradiation method (photolysis). The source of Cl− ions to create AgCl is geothermal water. In the work of Zhou et al.26 g-C3N4 was synthesized from calcining dicyandiamide in the N2 stream. Ag/AgCl/gC3N4 was synthesized by a two-stage oxidation method. Zhang and co-authors27 synthesized g-C3N4 from calcined melamine combined with hydrothermal then Ag@AgCl/g-C3N4 was synthesized by ion exchange method. In these synthesis methods the Ag contents were often not controlled resulting a quite high Ag content (10–40%) and therefore it could be the reason for low plasmon effect.
In this work, we aimed to enhance the photocatalytic performance of g-C3N4 for the photodegradation of 2,4-D by incorporating noble metal silver. The addition of Ag was intended to reduce electron–hole pair recombination and induce surface plasmon resonance, thereby boosting charge carrier generation. g-C3N4 was synthesized with varying amounts of Ag nanoparticles (3 wt%, and 5 wt% AgNPs/g-C3N4) using an “ex situ” ultrasound-assisted method. The structure, morphology, and optical properties of the synthesized samples were thoroughly investigated. The findings demonstrated that Ag nanoparticles were uniformly dispersed on the g-C3N4 surface, resulting in Ag/g-C3N4 photocatalysts with high photocatalytic activity and photochemical stability for 2,4-D degradation under visible light irradiation. The potential photocatalytic mechanism of charge transfer in Ag/g-C3N4 composites under visible light irradiation was explored in detail, considering energy levels and trapping experiments. This work offers a straightforward yet highly effective synthesis method for AgNPs/g-C3N4 and contributes to the development of novel visible light-driven photocatalysts.
Experimental
The main chemicals used in this research are from Sigma Aldrich with purity levels greater than 99.9%: urea, (NH2)2CO; trisodium citrate dihydrate, Na3C6H5O7·2H2O; silver nitrate, AgNO3; citric acid hydrate, C6H8O7·H2O; sodium borohydride, NaBH4; hydrochloric acid, HCl 37%; sodium hydroxide, NaOH. For 2,4-Dichlorophenoxyacetic acid C8H6Cl2O3 (2,4-D), its purity level is greater than 98%.Synthesis of g-C3N4
Urea is finely ground and enclosed in aluminium foil, and then heated to 550 °C in a nitrogen atmosphere for 4 hours, with a heating rate of 5 °C min−1. After heating, it is cooled to room temperature, washed three times with a 0.1 M nitric acid (HNO3) solution, and then washed with distilled water until pH 7. Finally, the sample is dried at 80 °C for 12 hours, resulting in a pale-yellow powder.Synthesis of AgNPs solution
AgNPs solutions are synthesized through reaction between silver nitrate and trisodium citrate at 60 °C, 70 °C, and 80 °C, and with reaction times of 45 minutes and 60 minutes. The synthesis process for the formation of stable AgNPs is optimized by varying AgNO3 concentrations (0.1 M, 0.01 M, and 0.001 M) and trisodium citrate dihydrate (Na3C6H5O7·2H2O) concentrations (0.3 M and 0.03 M).Synthesis of AgNPs/g-C3N4
The AgNPs/g-C3N4 catalyst is prepared by accurately weighing g-C3N4, and then sonicating with 100 mL of AgNPs solution for 1 hour at 20 °C. After sonication, the solution is allowed to settle, and the upper liquid portion is removed. Subsequently, the sample is dried at 40 °C for 12 hours, resulting in AgNPs/g-C3N4 material.Three AgNPs/g-C3N4 catalysts with different weight percentages of AgNPs were synthesized. The mass of g-C3N4 was varied to produce 3 wt%, and 5 wt% AgNPs samples (1 wt% AgNPs was also prepared for comparison).
The crystalline phases of the synthesized catalyst samples were determined using X-ray diffraction (XRD) on a Bruker D8 Advance instrument (Germany) with a wavelength (λ) of 1.5406 Å. Light absorption capability and bandgap energies (Eg) of the samples were assessed using UV-visible diffuse reflectance spectroscopy (UV-Vis DRS) performed on a Shimadzu UV-2600 device. Surface morphology and the dispersion of AgNPs over g-C3N4 were identified using scanning electron microscopy (SEM) performed on FESEM S-4800 equipment system and transmission electron microscopy (TEM) using JEM1010 device. Elemental compositions were determined through X-ray energy dispersive spectroscopy (EDX) using FESEM S-4800 equipment system. The textile properties of the samples were estimated from N2 adsorption and desorption isotherms at 77 K using TriStar 3000 V6 07A equipment. Raman spectra were obtained using a Renishaw Raman system model 2000 spectrometer. Electron paramagnetic resonance (EPR) spectroscopy with center field of 3320 G, microwave frequency of 9.399 GHz and power of 0.05 mW was employed on Bruker EMX-Micro device to evaluate the presence of unpaired electrons.
The photoluminescence (PL) spectra of the photocatalysts were measured using FLS1000 Photoluminescence Spectrometer. The Fourier transform infrared (FTIR) spectra were recorded using FTIR spectrometer (model: Nicolet iS10, Thermo Scientific, USA).
The photocatalytic degradation reactions of 2,4-D over the synthesized catalyst samples were carried out in a photoreaction system. A quartz glass beaker is placed in a temperature-controlled bath at 25 °C, and a 250 W Xenon lamp is located 20 cm above the solution surface.
The experimental procedure was as follows: a specific volume (V mL) of the 2,4-D solution with an initial concentration of Co (ppm), was mixed with a predetermined amount (m, mg) of AgNPs/g-C3N4. This mixture was stirred in the dark for 30 minutes, and then subjected to continuous illumination for a total of 210 minutes. At 30 minute intervals during irradiation, the 2,4-D concentration was monitored, using UV-visible absorption spectroscopy at a wavelength of 283 nm, to assess the catalytic activity of the synthesized samples. Additionally, the samples were analyzed using a TOC-VCSN analyzer (multi NC 2100S device) to quantify the residual total organic carbon content. To investigate the active species generated during the photocatalytic degradation process, capture experiments for free radicals (hydroxyl radicals (˙OH), superoxide radicals (O2˙−), and holes (h+)) were conducted using tert-butyl alcohol (t-BuOH), ascorbic acid (AA), and ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), respectively.
Photoelectrochemical measurements were carried out using a precision potentiostat/galvanostat (model: AUTOLAB 302 N) with a standard three-electrode system, employing a Pt plate as the counter electrode and a saturated Ag/AgCl electrode as the reference electrode. A phosphate buffer solution (pH = 7) was used as the electrolyte. The working electrode, with an area of 1 cm2, was prepared by coating FTO conductor glass with viscous pastes of pristine or Ag-doped graphitic carbon nitride using the doctor blade method and Scotch tape as a spacer. The pastes were prepared by mixing 30 mg of carbon nitride, 10 mg of ethyl cellulose, 10 mg of lauric acid, and 150 mg of terpineol. The coated films were subsequently dried overnight at 70 °C to improve their adhesion.
Electrochemical impedance spectra (EIS) were recorded over a frequency range of 0.1 Hz to 105 Hz. Photocurrent density measurements were performed within a potential range from −0.4 to 1.2 V vs. Ag/AgCl, with a potential scanning speed of 0.01 V s−1.
Results and discussion
Optimization of the AgNPs synthesis process
For CAgNO3 = 0.01 M mixed with 0.03 M and 0.3 M trisodium citrate and then heated at 80 °C for 1 hour resulted in the precipitation of large free silver particles.
For CAgNO3 = 0.001 M mixed with 0.3 M trisodium citrate and then heated at 80 °C for 1 hour resulted in free silver particles. Mixing with a 0.03 M trisodium citrate solution, however, resulted in a colloidal silver solution with an orange-silver hue, indicating the formation of AgNPs.
Reaction temperature. Three temperatures 60 °C, 70 °C, and 80 °C, were investigated. At 60 °C and 70 °C, the reaction rate is slow when 0.001 M AgNO3 and 0.03 M trisodium citrate are mixed, and no AgNPs appeared after more than 1 hour. When the temperature is increased to 80 °C, a noticeable yellow colour change in the solution is observed within 20 minutes.
Reaction time. The extent of AgNPs formation when 0.001 M AgNO3 was mixed with 0.03 M trisodium citrate, and heated at 80 °C was measured after 45 minutes and after 1 hour. After 45 minutes, 6.0 M HCl was added to the solution, and a white precipitate of AgCl was observed. This indicated that unreacted Ag+ ions remain in the solution, and therefore incomplete reduction. After 1 hour, 6.0 M HCl was added, and there was no apparent white AgCl precipitate. The solubility product of AgCl ≈10−10, and so the Ag+ concentration in the solution is very low, approximately 10−11 M. By 1 hour, Ag+ ions had been completely reduced to Ag0 nanoparticles.
From these findings, the process to synthesize AgNPs solutions is as follows: A mixture of 250 mL of 0.001 M AgNO3 and 0.03 M trisodium citrate is heated at 80 °C and maintained for 1 hour in the absence of light. AgNPs form in accordance with the overall reaction:29–31
| 4Ag+ + Na3C6H5O7 + 2H2O → 4Ag0 + H3C6H5O7 + 3Na+ + H+ + O2 |
The ratio of Ag+ to Na3C6H5O7 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and so the amount of citrate used is approximately 2.38 times the amount needed to completely reduce Ag+. This excess citrate serves two purposes. The first is to ensure that the reaction proceeds to completion, with all Ag+ ions reduced to free AgNPs, and the second is that citrate acts as a stabilizing agent for the AgNPs colloid system.30
4, and so the amount of citrate used is approximately 2.38 times the amount needed to completely reduce Ag+. This excess citrate serves two purposes. The first is to ensure that the reaction proceeds to completion, with all Ag+ ions reduced to free AgNPs, and the second is that citrate acts as a stabilizing agent for the AgNPs colloid system.30
UV-Vis absorption spectrum of AgNPs in solution. The characteristic UV-Vis absorption spectrum of AgNPs in solution is presented in Fig. S1,† covering the wavelength range from 300 to 700 nm.
The spectrum of the AgNPs solution reveals a maximum absorption peak at a wavelength (λmax) of 441.7 nm, which falls within the visible light region. This result is entirely consistent with previous studies,32–34 where nano Ag was successfully synthesized using a reduction method with trisodium citrate and β-D-glucose. Kamyar et al.34 synthesized nano Ag using a reduction method with an extract from the turmeric plant. All studies32–34 showed a UV-Vis maximum absorption peak of AgNPs in the wavelength range of 400–450 nm. The λmax value shifts slightly within this wavelength range depending on reaction conditions such as the reducing agent used, the stabilizing agent for the AgNPs colloid system, reaction time, temperature, and Ag+ concentration. Thus, in this work, by employing trisodium citrate as the reducing agent with a silver nitrate solution under the specified reaction conditions, AgNPs solutions were successfully synthesized.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the 2,4-D molecule. During photodegradation of 2,4-D, intermediate products such as six-membered ring-containing compounds may appear and may absorb light at wavelengths smaller than 283 nm. Therefore, to ensure that the analysis of 2,4-D concentrations during photocatalysis is not affected by these intermediate products, a calibration curve at 283 nm is used for the quantification. The resultant 2,4-D calibration curve is shown in (Fig. S2b†). Linear regression of these data gives the following equation for the concentration of 2,4-D, C2,4-D:
O group in the 2,4-D molecule. During photodegradation of 2,4-D, intermediate products such as six-membered ring-containing compounds may appear and may absorb light at wavelengths smaller than 283 nm. Therefore, to ensure that the analysis of 2,4-D concentrations during photocatalysis is not affected by these intermediate products, a calibration curve at 283 nm is used for the quantification. The resultant 2,4-D calibration curve is shown in (Fig. S2b†). Linear regression of these data gives the following equation for the concentration of 2,4-D, C2,4-D:| C2,4-D = mAbs − bC2,4-D |
Characteristics of AgNPs/g-C3N4
| Samples | C | N | Ag | Total |
|---|---|---|---|---|
| 3% AgNPs/g-C3N4 | 31.50 | 65.90 | 2.60 | 100.00 |
| 5% AgNPs/g-C3N4 | 29.81 | 66.20 | 4.00 | 100.00 |
Table 1 shows that there is a good agreement between the AgNPs content, measured from the EDX analyses (2.60 wt% and 4.00 wt%) and the stoichiometry of the synthesis mixtures. In addition, only three elements (C, N, and Ag) are clearly apparent in the spectra, and so the purity of each sample is high. The EDX result confirms that AgNPs successfully adsorbed the g-C3N4 surface. For convenience, the labels 3 wt% and 5 wt% AgNPs are used throughout this paper.
Pure g-C3N4 and two AgNPs/g-C3N4 samples have a dominant peak at 2θ = 27.4° indexed to g-C3N4 (JCPDS 87-1526).35 This peak is ascribed to the (002) plane of reflection in the g-C3N4 structure. In the XRD patterns of both 3 wt% AgNPs/g-C3N4 and 5 wt% AgNPs/g-C3N4 samples, four additional peaks appear at 2θ angles of 38.18°, 44.25°, 64.38°, and 77.27°, corresponding to the (111), (200), (220), and (311) planes, respectively, which are characteristic of the AgNPs crystal structure (JCPDS 87-717). These peaks have been previously reported.33,34,36 These reflected planes indicate that AgNPs crystals have a face-centered cubic structure. Only Ag crystals exhibit plasmonic effects. No other crystalline impurities can be observed.
| Samples | SBET (m2 g−1) | Smi (m2 g−1) | Sex (m2 g−1) | Vmi (cm3 g−1) | Vex (cm3 g−1) | Vtot (cm3 g−1) | D (nm) |
|---|---|---|---|---|---|---|---|
| a SBET (m2 g−1): BET surface area; Smi (m2 g−1): micropore surface area; Sex (m2 g−1): external surface area; Vmi (cm3 g−1): micropore volume; Vex (cm3 g−1): external pore volume; Vtot (cm3 g−1): total pore volume; D (nm): average pore dimension. | |||||||
| g-C3N4 | 58 ± 2 | 2 ± 0.5 | 56 ± 2 | 0.0012 | 0.4699 | 0.4711 | 33.675 |
| 3% AgNPs/g-C3N4 | 69 ± 2 | 9 ± 0.5 | 59 ± 2 | 0.0036 | 0.4049 | 0.4085 | 27.4619 |
| 5% AgNPs/g-C3N4 | 78 ± 5 | 10 ± 0.5 | 68 ± 3 | 0.0042 | 0.4683 | 0.4725 | 27.6820 |
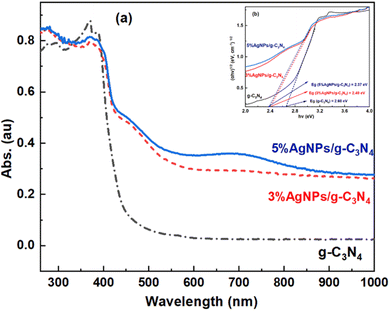 | ||
| Fig. 5 UV-Vis DRS spectra of g-C3N4, 3 wt% AgNPs/g-C3N4 and 5 wt% AgNPs/g-C3N4 (a); plots of (αhv)1/2 against hv for g-C3N4, 3 wt% AgNPs/g-C3N4 and 5 wt% AgNPs/g-C3N4 (b). | ||
It is observed that pristine g-C3N4 can absorb visible light with an absorption edge at around 460 nm. The composites show a redshift in the absorption edge towards longer wavelengths. Compared to pristine g-C3N4, AgNPs/g-C3N4 composites exhibit an additional weak and broad peak around 450–750 nm, characteristic of the silver surface plasmon resonance band. Additionally, a much higher absorbance in the range of 500–800 nm is observed. These results indicate the positive influence of the plasmon effect generated by Ag NPs on the visible light absorption capability of AgNPs/g-C3N4 compared to pristine g-C3N4.
Energy bandgaps (Eg) are determined using the Kubelka–Munk equation:
| (αhv)n = A(hv − Eg) |
Fig. 5b shows plots of (αhν)1/2 against hν, and from this, the band gap energy is the intersection of the tangent, dashed line with the hν axis. The resultant Eg values of g-C3N4, 3% AgNPs/g-C3N4 and 5% AgNPs/g-C3N4 are 2.60 eV, 2.40 eV, and 2.37 eV, respectively. The Eg values of the AgNPs/g-C3N4 composites are significantly lower than those of pristine g-C3N4 which extends the absorption band into the visible light range. This phenomenon is likely to be a consequence of the plasmon effect on AgNPs. Remarkable absorption enhancement in visible light region is beneficial for improving photocatalytic behavior in this irradiation region.
Fig. 6 presents the PL spectra of pristine g-C3N4 and AgNPs/g-C3N4. Pristine g-C3N4 exhibits a strong emission peak at approximately 460 nm. Compared to pristine g-C3N4, the PL intensity of AgNPs/g-C3N4 composites decreases significantly. The weaker PL peak intensity indicates a slower recombination rate of photogenerated carriers, resulting in an increased availability of electrons and holes for the photocatalytic process of Ag/g-C3N4. However, excessive loading of Ag on g-C3N4, such as in the case of 5%-Ag/g-C3N4, causes the PL intensity to increase again, indicating a higher recombination rate of photogenerated carriers. It is evident that Ag/g-C3N4 composites with optimal amounts of Ag loading have the potential to serve as highly active photocatalysts. These findings strongly align with the results of the transient photocurrent density and EIS experiments.
 | ||
| Fig. 8 SEM images and Energy dispersive X-ray (EDX) mapping analysis of carbon, nitrogen, silver and mixed for (A) g-C3N4, (B) 3% AgNPs/g-C3N4 and (b) 5% AgNPs/g-C3N4. | ||
TEM images of 3 wt% and 5 wt% AgNPs/g-C3N4 show that AgNPs are observed as black dots with an average size of 40–50 nm and are uniformly dispersed on the surface of g-C3N4. These results are consistent with previous studies32,34 that demonstrated the successful synthesis of AgNPs composites on g-C3N4.
Photocatalytic degradation of 2,4-D by AgNPs/g-C3N4
The result shows that after 180 minutes of illumination, the characteristic absorption peak at 283 nm in the 2,4-D solution has completely disappeared, indicating that 2,4-D has decomposed into simpler compounds, including oxidation into CO2 and H2O. At 283 nm, the optical absorption values (A) for the mixtures with AgNPs/g-C3N4 are lower than the mixture with pure g-C3N4, and the lowest absorption is seen over the 3% AgNPs/g-C3N4.
The UV-Vis absorption spectra of the 2,4-D solution with 3% AgNPs/g-C3N4 at different illumination times were recorded (Fig. S5†).
After 60 minutes of illumination, optical absorption at 283 nm had decreased significantly and continued to decrease after 120, 150 and 180 minutes. After 180 minutes of illumination, the degradation of 2,4-D remained approximately constant, fluctuating around 94%. From the UV-Vis spectra, it is also apparent that as 2,4-D decomposes, the A increases over the wavelength range of 240–260 nm. For the initial 2,4-D solution, the A values over this range are relatively low, but after 60 minutes of illumination, they increase. This indicates that 2,4-D has degraded into simpler organic structures that more strongly absorb radiation in the 240–260 nm range. As illumination time increases, the A values in this range gradually decrease and approach zero, suggesting that these intermediate products further decompose over time. To optimize the adsorption, and photocatalytic decomposition of 2,4-D, an illumination time of 210 minutes is chosen for the next analysis of the conditions.
The variation of the 2,4-D concentration relative to the initial concentration C/Co over illumination time t (min) is presented in Fig. 10.
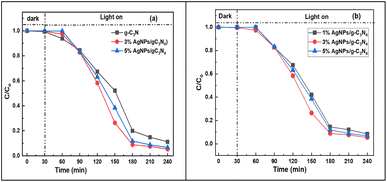 | ||
| Fig. 10 Relative concentrations of 2,4-D as a function of illumination time in g-C3N4 and in 3% and 5% AgNPs/g-C3N4. Illumination commenced at 30 minutes. | ||
The percentage efficiency, H% of treating 2,4-D is calculated from:
 | (1) |
The experimental results show that the materials weakly adsorb 2,4-D. In the first 50 minutes of illumination, the decrease in 2,4-D concentration is not significant, and H% increases at most by 10–15% for all samples. This indicates that 2,4-D only weakly adsorbs to the samples and is consistent with an induction period for the photocatalytic reaction to commence. Time is initially required to form free radicals, O2˙−, ˙OH, and photo-induced electrons and holes. After 90 minutes of illumination (at the 120 minute mark in Fig. 10), the H% of 2,4-D over the samples starts to become significant. The 2,4-D degradation efficiency over g-C3N4 and 1% AgNPs/g-C3N4 is equivalent, at 32.6%. The 3% and 5% AgNPs/g-C3N4 samples exhibit better performance, reaching 41.7% and 36.8% efficiencies, respectively. After 120 minutes of illumination (at the 150 minutes mark in Fig. 10), the efficiency differences become more pronounced, with H% values following the sequence: g-C3N4 < 1% AgNPs/g-C3N4 < 5% AgNPs/g-C3N4 < 3% AgNPs/g-C3N4 (at 47.9%, 57.9%, 61.6%, and 73.6%, respectively). After 150 minutes of illumination, the 2,4-D degradation efficiency over g-C3N4 reaches 79.97%, while over % AgNPs/g-C3N4 an efficiency of 91.0% is achieved. After 210 minutes of illumination, when the concentrations of 2,4-D in the solutions are very low, and the differences in degradation efficiencies between the samples are less significant. However, the 3% AgNPs/g-C3N4 sample still exhibits the largest H% value at 92.4%. Therefore, it can be concluded that the photocatalytic activity of the samples increases in the order of g-C3N4 < 1% AgNPs/g-C3N4 < 5% AgNPs/g-C3N4 < 3% AgNPs/g-C3N4. The superior 2,4-D degradation efficiencies of AgNPs/g-C3N4 when compared to pure g-C3N4, reflect the role of AgNPs, and are consistent with published findings. Kashyap et al.36 proposed a photocatalytic mechanism for 2,4-D decomposition over g-C3N4 supported by Ag NPs and Au NPs and explained that Ag NPs facilitate electron transfer between the conduction band of g-C3N4 and the reaction agents through surface plasmon resonance (SPR). This causes a reduction in the rate of recombination of photogenerated electrons and holes. Also, Ag NPs exhibit strong absorption in the visible light range, narrowing the bandgap of the g-C3N4 material, allowing it to be more active in the visible light spectrum.
Fig. 11 showed the dependence of ln(Co/C) vs. time (t) over synthesized materials.
 | ||
| Fig. 11 The dependence of ln(Co/C) vs. time (t) for photocatalytic degradation of 2,4-D over synthesized materials. | ||
As shown in Fig. 11, the photodegradation of 2,4-D followed a first-order kinetic model over the 3% AgNPs/g-C3N4 composites under the experimental conditions established. From the linear form of ln(Co/C) vs. time (t), the rate constants were calculated. The kinetic parameters of the photocatalytic degradation of 2,4-D are presented in Table 3.
| Materials | g-C3N4 | 1%AgNPs/g-C3N4 | 3%AgNPs/g-C3N4 | 5%AgNPs/g-C3N4 |
|---|---|---|---|---|
| a The findings showed that the photocatalytic rate constant changes slightly when g-C3N4 is coupled with AgNPs, increasing in the following order: g-C3N4 < 1%AgNPs/g-C3N4 < 5% AgNPs/g-C3N4 < 3% AgPNs/g-C3N4. | ||||
| kap (min−1) | 0.0131 | 0.0152 | 0.0182 | 0.0169 |
| R2 | 0.9380 | 0.9463 | 0.9522 | 0.9459 |
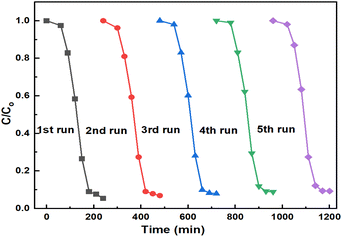 | ||
| Fig. 12 Stability study for the photocatalytic 2,4-D degradation by 3%AgNP/g-C3N4 under visible light illumination. | ||
On the other hand, after the reuse cycles, an XRD study was conducted (Fig. 13) to determine if there were any changes in the material. However, no significant changes were found in the structure of the catalyst.
As 3% AgNPs/g-C3N4 is the most efficient material for 2,4-D degradation, it was used to investigate other factors in the degradation process.
Influence of 2,4-D initial concentration. Three experiments were conducted concurrently using 20 mg of 3% AgNPs/g-C3N4, 40 mL of 2,4-D solution with concentrations of 20, 40, and 60 ppm, and a pH of 2.2. The mixtures were stirred in the dark for 30 minutes, followed by 210 minutes of illumination. The concentrations of 2,4-D are measured after 30 minutes in the dark and after 210 minutes of illumination. The results are shown in Fig. 14A.
At the lower initial concentrations of 2,4-D (20 ppm and 40 ppm), the treatment efficiency is high, reaching 93.5%, and 92.4%, respectively. However, at the highest initial concentration (60 ppm), the degradation efficiency dropped significantly to 70.50%. This decrease in treatment efficiency as the pollutant concentration increases is consistent with previous studies.40,41 In the subsequent experiments, an initial 2,4-D concentration of 40 ppm is used.
Influence of pH. Three parallel experiments were conducted with three 2,4-D solutions with a concentration of 40 ppm, and a pH: 2.2, 5.5, or 8.2. In each solution, 20 mg of 3% AgNPs/g-C3N4 is added. The solutions were stirred in the dark for 30 minutes, followed by 210 minutes of illumination. The degradation efficiency results are presented in Fig. 14B.
The degradation efficiency of 2,4-D with 3% AgNPs/g-C3N4 decreases significantly as the pH of the solution increases. The higher the pH value, the lower the 2,4-D treatment efficiency. Efficiency decreases from 93.5% to 78.2% to 58.2% when the pH increases from 2.2 to 5.5 to 8.2. This result is consistent with previous publications18 and can be explained by:
(1) At pH below 4.88, the following chain reactions are initiated that produce more O2˙− and ˙OH free radicals:18
| O2˙− + H+ ↔ HO2˙ pKa = 4.88 |
| HO2− + H+ → H2O2 |
| H2O2 + e− → ˙H2O2 |
| ˙H2O2 + O2˙− → ˙OH + OH− + O2 |
| H2O2 → 2˙OH |
(2) As pH increases, a portion of 2,4-D exist in the form of salt anions, which may affect its ability to participate in the photocatalytic reaction:
| C8H6Cl2O3 + OH− → C8H5Cl2O3− + H2O |
Therefore, a pH of 2.2 is chosen to maximize the photocatalytic activity of 2,4-D.
From Fig. 14C, at mass-to-volume ratios of 0.75 g L−1, 0.5 g L−1, and 0.25 g L−1, the degradation efficiency of 2,4-D is 94.5%, 92.4%, and 87.3%, respectively. Larger catalyst masses provide more surface area for the absorption of radiation, resulting in more photogenerated electron–hole pairs and free radicals. As a consequence, 2,4-D degradation improves. For this study, a ratio of 0.50 g L−1 is chosen, because, at higher ratios, the degradation efficiency did not differ significantly, and so conserved catalyst material and energy.
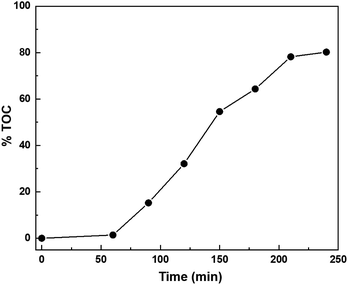
Mineralization of 2,4-D. The potential viability of the AgNPs/g-C3N4 photocatalyst is assessed through the mineralization efficiency of the 2,4-D. TOC during the photocatalytic reactions was recorded to investigate the mineralization of 2,4-D. The result is shown in Fig. 15. After 240 min irradiation about 80.2% TOC are removed, indicating that 2,4-D has not only been degradation but also efficiently mineralized over AgNPs/g-C3N4 under visible light irradiation.
The transient photocurrent responses via three on–off cycles of g-C3N4 and AgNPs/g-C3N4 under visible light illumination are shown in Fig. 16. The photocurrent intensity of g-C3N4 increases after introducing Ag, indicating Ag plays an important role in electronic transmission. As revealed by the PL analysis, the result confirms that there is a lower rate of recombination of photogenerated electron–hole pairs on AgNPs/g-C3N4 compared to that on pristine g-C3N4. Under light irradiation, the generated photocurrent of 3%AgNPs/g-C3N4 electrode is highest, thus 3%AgNPs/g-C3N4 holds strongest visible light irradiation, which is well in agreement with photocatalytic activities.
The Nyquist plots with pristine g-C3N4, and 3% (5%) AgNPs/g-C3N4 as photoanodes under visible light illumination are shown in Fig. 16. In general, a smaller arc radius in an EIS Nyquist plot corresponds to an effective separation and faster transfer of charges at the semiconductor/electrolyte interface.42 The arc radii of AgNPs/g-C3N4 composites were smaller than that of pristine g-C3N4, signifying AgNPs/g-C3N4 composites have the higher efficiency in charge separation and electron transfer. The photoelectrochemical results confirm that the addition of Ag considerably improves the electro–hole pairs transfer and enhances the photocatalytic activity.
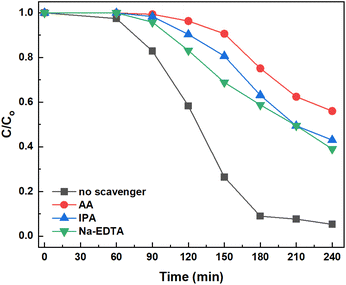
Radical trapping experiments. Three free radicals O2˙−, ˙OH, and photogenerated holes were investigated to reveal the contribution of these radicals to the 2,4-D degradation in the reaction. The radical scavengers used are ascorbic acid-AA (for O2˙−), isopropyl alcohol (IPA) (for ˙OH), and Na-EDTA (for photogenerated h+ holes). Each experiment used 80 mg of 3%AgNPs/g-C3N4 in 160 mL of a 40 ppm 2,4-D solution at pH 2.2. The mixtures are stirred in the dark for 30 minutes. Then, 16 mL of pure isopropyl alcohol radical scavenger is added to one of the solutions, and the mixture is exposed to light. After illumination times of 60, 90, 120, 150, and 180 minutes, the remaining 2,4-D concentration in the solution is determined. The same procedure is repeated with 16 mL of 0.1 M Na-EDTA, and 16 mL of 0.01 M ascorbic acid as radical scavengers. The results are presented in Fig. 17.
From Fig. 17, the degradation efficiency of 2,4-D with 3% AgNPs/g-C3N4 significantly decreased when introducing the radical scavengers AA, IPA and Na-EDTA into the reaction system. When no radical scavengers are added, the 2,4-D removal efficiency reached 92.4%, which is 1.83 times higher compared to when radical scavengers are present, indicating that O2˙−, ˙OH and photogenerated h+ holes are integral to the decomposition of 2,4-D. During the irradiation period from 60 to 180 minutes, AA display an inhibiting effect much stronger than IPA and Na-EDTA, suggesting that the O2˙− has a more pronounced effect than ˙OH radical and h+ holes in the degradation of 2,4-D. Our result is in agreement with Kashyap et al.,36 who have proposed a mechanism for the photocatalytic oxidation and decomposition of 2,4-D over Ag/g-C3N4. This mechanism includes the direct participation of O2˙−, ˙OH radicals, and photogenerated h+ holes.
A possible mechanism discussion. A mechanism for the charge separation and transport of the electron–hole pairs over AgNPs/g-C3N4 under visible light illumination was proposed, based on the results of this study. The transfer of photoexcited e− and h+ depends on the relative band edge positions of the valence band (VB) and conduction band (CB) potentials, and Eg. In our work, the CB and VB potentials of the samples are estimated using eqn (2) and (3) (ref. 43–45)
| EVB = χ − Ee + 0.5Eg | (2) |
| ECB = EVB − Eg | (3) |
When stimulated with a suitable light source the electrons are excited from VB to CB on g-C3N4, resulting in a CB rich in electrons (e−) and leaving behind in the VB holes (h+). The accumulated electrons on g-C3N4 migrate to the surface of Ag because the Fermi level of g-C3N4 is lower than that of Ag.46 These photoelectrons react with water-dissolved oxygen (the reduction potential of oxygen −0.046 eV vs. NHE) to generate superoxide radicals (O2˙−). On the other hand, the potential for the ˙OH/H2O (2.68 eV vs. NHE) is too positive for the holes (h+) in the VB of g-C3N4 to react with water to produce ˙OH radicals. However, the scavenger experiments confirmed the existence of ˙OH implying that ˙OH should be produced through the multiple-electron reduction reaction of ˙O2− conversion.47 Finally, based on the result of trap experiments the VB h+ of g-C3N4 would directly react the 2,4-D.
In this work, all the experiments were conducted at pH value of 2.1 then the effect of pH value could be described as eqn (4)–(11):45,48,49
| g-C3N4. + hν → g-C3N4 (e− + h+) | (4) |
| g-C3N4 (e−) → Ag (e−) | (5) |
| Ag (e−) + O2 → ˙O2− | (6) |
 | (7) |
 | (8) |
| H2O2 + e− → ˙OH + HO− | (9) |
| H2O2 + hv → 2˙OH | (10) |
| 2,4-D + ˙O2−/˙OH/h+ → products | (11) |
 | ||
| Fig. 18 2,4-D photodegradation over 3% AgNPs/g-C3N4 and g-C3N4 under UV irradiation and direct sunlight irradiation. | ||
Theoretical calculations on the interaction between 2,4-D and g-C3N4, Ag/g-C3N4. The theoretical simulation is also conducted to confirm a good interaction between 2,4-D and studied photocatalysts. The g-C3N4 model employed in theoretical calculations is the corrugated g-C3N4 model as described in ref. 50 and 51. All optimization and energy calculations were performed using the GFN2-xTB method, an accurate and broadly parameterized self-consistent tight-binding quantum chemical method incorporating multipole electrostatics and density-dependent dispersion contributions52 This method has demonstrated high efficiency and effectiveness for calculations involving large systems.
The optimized adsorption configurations of 2,4-D on g-C3N4 and Ag/g-C3N4 are presented in Fig. S6 and S7,† respectively. Table S1† summarizes the calculated results for the adsorption processes. The adsorption of 2,4-D on Ag-modified g-C3N4 is significantly more effective compared to pristine g-C3N4. This is evident from various adsorption parameters such as adsorption energy, the shortest distance (dmin) from 2,4-D to g-C3N4, the charge transfer, and the bond order (BO) formed between 2,4-D and g-C3N4, as presented in Table S1.† The adsorption energy of 2,4-D on Ag/g-C3N4 is −271.3 kJ mol−1, which is substantially more negative than the adsorption energy of 2,4-D on pristine g-C3N4, which is −95.9 kJ mol−1. The dmin distance from 2,4-D to pristine g-C3N4 is 3.098 Å, significantly larger than the sum of the covalent radii of C and Cl (0.730 + 1.020 = 1.750 Å) or N and Cl (0.710 + 1.020 = 1.730 Å). In contrast, the dmin distance from 2,4-D to Ag-modified g-C3N4 is 2.254 Å, approximately equal to the sum of the covalent radii of Ag and O (1.450 + 0.660 = 2.110 Å).53 There is a charge transfer (q) from Ag/g-C3N4 to 2,4-D (0.243 e), and the bond order (BO) formed between 2,4-D and Ag atoms is 0.780. This indicates that the adsorption of 2,4-D on Ag-modified g-C3N4 is a chemisorption process, involving the formation of covalent bonds between atoms in 2,4-D and the Ag atoms. In contrast, the adsorption process of 2,4-D on pristine g-C3N4 is physical adsorption, dominated by van der Waals interactions.
Conclusions
Three samples of AgNPs/g-C3N4 were successfully synthesized with AgNPs content of 3%, and 5% using an “ex situ” method with the assistance of ultrasound from AgNPs solutions and g-C3N4. The AgNPs solutions were synthesized using a citrate-assisted reduction method, and g-C3N4 was synthesized from a urea precursor. The characterization of the samples was carried using X-ray diffraction spectroscopy (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM), Nitrogen adsorption–desorption (BET), Fourier transformation infrared spectroscopy (FTIR), ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS) and photoluminescence spectroscopy, electron paramagnetic resonance (EPR). The visible-light-driven photocurrent measurement was performed by three on–off cycles of intermittent irradiation. The AgNPs were evenly distributed on g-C3N4 and have relatively small nanoscale sizes ranging from 40 to 50 nm. It was revealed that the plasmonic effect of AgNPs played an important role in broadened visible-light absorption and efficient electron–hole separation, mainly accounting for the improved photocatalytic activity. The investigation on the influence of ˙OH, O2˙− radicals and photogenerated holes (h+) demonstrating that h+, ˙OH, and O2˙− play crucial roles in the photocatalytic activity of the AgNPs/g-C3N4 catalysts.Under the optimized conditions of pH = 2.2, Co (2,4-D) = 40 ppm, and a catalyst-to-volume ratio of 0.5 g L−1, the 3% AgNPs/g-C3N4 showed a weak propensity for 2,4-D adsorption, while almost complete oxidation and degradation of 2,4-D were observed after 210 minutes of illumination. The AgNPs/g-C3N4 composites display a good stability and maintain a high photocatalytic performance during five reaction cycles. That indicate AgNPs/g-C3N4 catalyst is stable for the photocatalytic 2,4-D degradation, which is significant for its practical applications.
Theoretical calculations on the interaction between 2,4-D and g-C3N4, Ag/g-C3N4 was also performed. The calculated results show that the adsorption of 2,4-D on Ag-modified g-C3N4 is significantly more effective compared to pristine g-C3N4.
Data availability
Data for this article are available upon request from the corresponding author.Author contributions
Phung Thi Lan, Nguyen Hoang Hao and Le Minh Cam conceived of the study and designed the experiments. Nguyen Trung Hieu, Nguyen Thi Thu Ha, synthesized the materials. Nguyen Hoang Hao and C. Trevor Brown performed the material characterization and data analysis. Phung Thi Lan, C. Trevor Brown and Le Minh Cam wrote the manuscript. Nguyen Hoang Hao and Le Minh Cam supervised the project. All the authors contributed to the final version of the manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
This work is funded by the Ministry of Science and Technology in Vietnam under grant no. ĐTĐL.CN-66/19 and the Ministry of Education and Training under grant no. B2022-TDV-06.References
- J. Walters, Environmental fate of 2,3-dichlorophenoxyacetic acid, Environ. Monit. Pest. Manage., 2021, 3510, 1–18 Search PubMed.
- N. R. Zuanazzi, N. d C. Ghisi and E. C. Oliveira, Analysis of global trends and gaps for studies about 2,4-D herbicide toxicity: A scientometric review, Chemosphere, 2020, 241, 125016 CrossRef CAS.
- H. K. Hue, L. V. Anh and D. B. Trong, Study of the adsorption of 2,4-dichlorophenoxyacetic acid from the aqueous solution onto activated carbon, Vietnam J. Chem., 2018, 56(2), 208–213 CrossRef CAS.
- J. Goscianska and A. Olejnik, Removal of 2,4-D herbicide from aqueous solution by amino silane grafted mesoporous carbons, Adsorption, 2019, 25, 345–355 CrossRef CAS.
- S. F. A. Shattar, N. A. Zakaria and K. Y. Foo, Preparation of a montmorillonite-derived adsorbent for the practical treatment of ionic and nonionic pesticides, J. Mater. Res. Technol., 2019, 8(5), 4713–4724 CrossRef CAS.
- M. S. Fernando, A. A. D. S. Onélia and G. A. V. Melissa, Adsorption of herbicide 2,4-D from aqueous solution using organo-modified bentonite clay, Environ. Sci. Pollut. Res., 2019, 26(18), 18329–18342 CrossRef PubMed.
- N. S. Trivedi and S. A. Mandavgane, Fundamentals of 2, 4 Dichlorophenoxyacetic Acid Removal from Aqueous Solutions, Sep. Purif. Rev., 2018, 47(4), 337–354 CrossRef CAS.
- X. Lü, Q. Zhang, W. Yang, X. Li, L. Zeng and L. Li, Catalytic ozonation of 2,4-dichlorophenoxyacetic acid over novel Fe–Ni/AC, RSC Adv., 2015, 5, 10537–10545 RSC.
- J. L. Rodríguez, T. Poznyak, M. A. Valenzuela, H. Tiznado and I. Chairez, Surface interactions and mechanistic studies of 2,4-dichlorophenoxyacetic acid degradation by catalytic ozonation in presence of Ni/TiO2, Chem. Eng. J., 2013, 222, 426–434 CrossRef.
- G. C. González, C. Julcour, H. Chaumat, U. Jáuregui-Haza, H. Delmas and Henri, Degradation of 2,4-dichlorophenoxyacetic acid by photolysis and photo-Fenton oxidation, J. Environ. Chem. Eng., 2018, 6(1), 874–882 CrossRef.
- W. Jiuqing, X. Jun, C. Xiaobo and X. Li, A review on gC3N4-based photocatalysts, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef.
- G. Dong, Y. Zhang, Q. Pan and J. Qiu, A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties, J. Photochem. Photobiol., C, 2014, 20, 33–50 CrossRef CAS.
- L. Xibao, K. Bangbang, D. Fan, Z. Zhiqiang, L. Xudong, H. Lu, H. Juntong, F. Zhijun, C. Zhi, X. Jilin, P. Biaolin and L. W. Zhong, Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies, Nano Energy, 2021, 81, 105671 CrossRef.
- X. Li, T. Han, Y. Zhou, Y. Xie, Y. Luo, J. Huang, Z. Chen and F. Deng, Photoelectrocatalytic hydrogen evolution and synchronous degradation of organic pollutants by pg-C3N4/β-FeOOH S-scheme heterojunction, Sci. China: Technol. Sci., 2024, 67(4), 1238–1252, DOI:10.1007/s11431-023-2604-x.
- W. Yong, L. Qiang, W. NgieHing, S. Jaka, H. Juntong, D. Guoliang, H. Xifeng and L. Xibao, Near-infrared (NIR) light responsiveness of CuS/S–C3N4 heterojunction photocatalyst with enhanced tetracycline degradation activity, Ceram. Int., 2022, 48(1S), 2454–2469 Search PubMed.
- L. Runlu, C. Zhixin, Y. Yao, L. Yao, A. C. Waqas, W. Dawei and Z. Shenmin, Recent advancements in g-C3N4-based photocatalysts for photocatalytic CO2 reduction: a mini review, RSC Adv., 2020, 10, 29408 RSC.
- Y. Hu, X. Li, W. Wang, F. Deng, L. Han, X. Gao, Z. Feng, Z. Chen, J. Huang, F. Zeng and F. Dong, Bi and S Co-doping g-C3N4 to Enhance Internal Electric Field for Robust Photocatalytic Degradation and H2 Production, Chin. J. Struct. Chem., 2022, 41(6), 2206069–2206078 CAS.
- R. Liu, Y. Liu, C. Liu, S. Luo, Y. Teng, L. Yang, R. Yang and Q. Cai, Enhanced photoelectrocatalytic degradation of 2,4-dichlorophenoxyacetic acid by CuInS2 nanoparticles deposition onto TiO2 nanotube arrays, J. Alloys Compd., 2011, 509(5), 2434–2440 CrossRef CAS.
- Y. P. Zang, L. P. Li, X. G. Li, R. Lin and G. S. Li, Hybridization of brookite TiO2 with g-C3N4: a visible-light-driven photocatalyst for As3+ oxidation, MO degradation and water splitting for hydrogen evolution, J. Mater. Chem. A, 2014, 2, 15774–15780 RSC.
- M. Hepel and J. Luo, Photoelectrochemical mineralization of textile diazo dye pollutants using nanocrystalline WO3 electrodes, Electrochim. Acta, 2001, 47(5), 729–740 CrossRef CAS.
- S. Patnaik, D. P. Sahoo and K. Parida, An overview on Ag modified g-C3N4 based nanostructured materials for energy and environmental applications, Renewable Sustainable Energy Rev., 2018, 82(1), 1297–1312 CrossRef CAS.
- S. Tonda, S. Kumar and V. Shanker, Surface plasmon resonance-induced photocatalysis by Au nanoparticles decorated mesoporous g-C3N4 nanosheets under direct sunlight irradiation, Mater. Res. Bull., 2016, 75, 51–58 CrossRef CAS.
- C. Han, L. Ge, C. Chen, Y. Li, Z. Zhao, X. Xiao and J. Zhang, Site-selected synthesis of novel Ag@AgCl nanoframes with efficient visible light induced photocatalytic activity, J. Mater. Chem. A, 2014, 2(31), 12594–12600 RSC.
- R. F. Dong, B. Z. Tian, C. Y. Zeng, T. Y. Li, T. T. Wang and J. L. Zhong, Ecofriendly Synthesis and Photocatalytic Activity of Uniform Cubic Ag@AgCl Plasmonic Photocatalyst, J. Phys. Chem. C, 2013, 117, 213–220 CrossRef CAS.
- X. Yao, X. Liu and X. Hu, Synthesis of the Ag/AgCl/g-C3N4 Composite with High Photocatalytic Activity under Visible Light Irradiation, ChemCatChem, 2014, 6(12), 1–11, DOI:10.1002/cctc.201402487.
- T. Zhou, Y. Xu, H. Xu, H. Wang, Z. Da, S. Huang, H. Ji and H. Li, In situ oxidation synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl/g-C3N4 and its activity, Ceram. Int., 2014, 40, 9293–9301 CrossRef CAS.
- S. Zhang, J. Li, X. Wang, Y. Huang, M. Zeng and J. Xu, In Situ Ion Exchange Synthesis of Strongly Coupled Ag @AgCl/g C3N4 Porous Nanosheets as Plasmonic Photocatalyst for Highly Efficient Visible-Light Photocatalysis, ACS Appl. Mater. Interfaces, 2014, 6(24), 22116–22125 CrossRef CAS PubMed.
- S. Djokic, Synthesis and Antimicrobial Activity of Silver Citrate Complexes, Bioinorg. Chem. Appl., 2018, 7, DOI:10.1155/2008/436458.
- L. Oprica, M. Andries, L. Zaharescu, L. Popescu, D. Pricop, D. Creanga and M. Balasoiu, Citrate-Silver nanoparticles and their impact on some environmentally beneficial fungi, Saudi J. Biol. Sci., 2020, 27(12), 3365–3375 CrossRef CAS.
- R. L. Spina, D. Mehn, F. Fumagalli, M. Holland, F. Reniero, F. Rossi and D. Gilliland, Synthesis of Citrate-Stabilized Silver Nanoparticles Modified by Thermal and pH Preconditioned Tannic Acid, Nanomaterials, 2020, 10(10), 2031 CrossRef.
- M. U. Rashid, M. Khairul and M. E. Quayum, Synthesis of Silver Nanoparticles (Ag-NPs) and their uses for Quantitative Analysis of Vitamin C Tablets, J. Appl. Polym. Sci., 2013, 118, 312–319 Search PubMed.
- P. S. Yerragopu, S. Hiregoudar, U. Nidoni and K. T. Ramappa, Chemical Synthesis of Silver Nanoparticles Using Trisodium Citrate, Stability Study and Their Characterization, Int. Res. J. Pure Appl. Chem., 2020, 21(3), 37–50 CrossRef.
- K. Shameli, Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method, Int. J. Mol. Sci., 2012, 13(6), 6639–6650 CrossRef CAS PubMed.
- K. Shameli, M. B. Ahmad, A. Zamanian, P. Sangpour, P. Shabanzadeh, Y. Abdollahi and M. Zargar, Mohsen, Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder, Int. J. Nanomed., 2012, 7, 5603–5610 CrossRef CAS PubMed.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Polymeric Photocatalysts Based on Graphitic Carbon Nitride, J. Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS.
- T. Kashyap, S. Biswasi, A. R. Pal and C. Biswajit, Unraveling the Catalytic and Plasmonic Roles of g-C3N4 Supported Ag and Au Nanoparticles Under Selective Photoexcitation, ACS Sustain. Chem. Eng., 2019, 7(23), 19295–19302 CrossRef CAS.
- X. Bai, L. Wang, R. Zong and Y. Zhu, Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods, J. Phys. Chem. C, 2013, 117, 9952 CrossRef CAS.
- L. Hao, J. Yue, M. Xinlong, L. Tongyao, Y. Linfeng, L. Bin, Y. Shu, W. Yongzhi and W. Yuhua, Construction of a well-dispersed Ag/graphene-like g-C3N4 photocatalyst and enhanced visible light photocatalytic activity, RSC Adv., 2017, 7, 8688 RSC.
- G. Devipriya, K. S. Adit, Q. Mohammad, K. G. Animes and R. P. Nageswara, Silver grafted graphitic-carbon nitride ternary hetero-junction Ag/gC3N4(Urea)-gC3N4(Thiourea) with efficient charge transfer for enhanced visible-light photocatalytic green H2 production, Appl. Surf. Sci., 2021, 558, 149900 CrossRef.
- H. Ji, F. Chang, X. F. Hu, W. Qin and J. W. Shen, Photocatalytic degradation of 2,4,6-trichlorophenol over g-C3N4 under visible light irradiation, Chem. Eng. J., 2013, 218, 183–190 CrossRef CAS.
- A. Adak, I. Das, B. Mondal, S. Koner, P. Datta and L. Blaney, Degradation of 2,4-dichlorophenoxyacetic acid by UV 253.7 and UV-H2O2: Reaction kinetics and effects of interfering substances, Emerging Contam., 2019, 5, 53–60 CrossRef.
- M. Aleksandrzak, K. Sielicki and E. Mijowska, Enhancement of photocatalytic hydrogen evolution with catalysts based on carbonized MOF-5 and g-C3N4, RSC Adv., 2020, 10(7), 4032–4039 RSC.
- M. Roškarič, G. Žerjav, M. Finšgar, J. Zavašnik and A. Pintar, Influence of the calcination duration of g-C3N4/TiO2 “veggie-toast-like” photocatalyst on the visible-light triggered photocatalytic oxidation of bisphenol A, J. Alloys Compd., 2023, 947, 169585 CrossRef.
- R. Hao, G. Wang, H. Tang, L. Sun, C. Xu and D. Han, Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity, Appl. Catal., B, 2016, 187, 47–58 CrossRef CAS.
- P. T. Lan, N. H. Hao and L. M. Cam, Study on the synthesis and photocatalytic performance of modified TiO2 supported by g-C3N4 in the degradation of 2,4-dichlorophenoxyacetic acid, ChemistrySelect, 2024, 9(1 of 13), e202305026, DOI:10.1002/slct.202305026.
- R. Liu, W. Yang, G. He, W. Zheng, M. Li, W. Tao and M. Tian, Ag-Modified g-C3N4 Prepared by a One-Step Calcination Method for Enhanced Catalytic Efficiency and Stability, ACS Omega, 2020, 5, 19615–19624 CrossRef CAS PubMed.
- C. Lin, Y. Sudong, Q. Wensheng, Z. Qian, Z. Jie and Z. Peng, Supramolecular Self-Assembly of Nitrogen-Deficient Ag/g-C3N4 Nanofiber Films with Enhanced Charge Transfer Dynamics for Efficient Visible-Light Photocatalytic Activity, ACS Appl. Mater. Interfaces, 2021, 13, 49993–50004 CrossRef PubMed.
- H. Ji, F. Chang, X. Hu, W. Qin and J. Shen, Photocatalytic degradation of 2,4,6-trichlorophenol over g-C3N4 under visible light irradiation, Chem. Eng. J., 2013, 218, 183–190 CrossRef CAS.
- R. Liu, Y. Liu, C. Liu, S. Luo, Y. Teng, L. Yang, R. Yang and Q. Cai, Enhanced photoelectrocatalytic degradation of 2,4-dichlorophenoxyacetic acid by CuInS2 nanoparticles deposition onto TiO2 nanotube arrays, J. Alloys Compd., 2011, 509, 2434–2440 CrossRef CAS.
- N. T. T. Ha, P. T. Be, P. T. Lan, N. T. Mo, L. M. Cam and N. N. Ha, Whether planar or corrugated graphitic carbon nitride combined with titanium dioxide exhibits better photocatalytic performance?, RSC Adv., 2021, 11, 16351–16358 RSC.
- T. T. H. Nguyen, M. C. Le and N. N. Ha, Understanding the influence of single metal (Li, Mg, Al, Fe, Ag) doping on the electronic and optical properties of g-C3N4: a theoretical study, Mol. Simul., 2021, 47(1), 10–17 CrossRef CAS.
- B. Christoph, E. Sebastian and G. Stefan, GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions, J. Chem. Theory Comput., 2019, 15(3), 1652–1671 CrossRef.
- B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revé, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Covalent radii revisited, Dalton Trans., 2008, 2832–2838 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02658f |
| This journal is © The Royal Society of Chemistry 2024 |