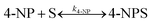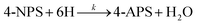Open Access Article
Open Access ArticleReview on polyaniline-based nanocomposite heterogeneous catalysts for catalytic reduction of hazardous water pollutants
Parmeshwar Lal Meena * and
Ajay Kumar Surela
* and
Ajay Kumar Surela
Department of Chemistry, University of Rajasthan, Jaipur 302004, India. E-mail: parmeshwar1978@gmail.com
First published on 23rd August 2024
Abstract
Water contamination by highly toxic substances has generated serious ecological disturbances and health problems for humans. Hence, decontamination of toxic pollutants using advanced, inexpensive, and eco-friendly approaches is the current demand. Heterogeneous catalyst-based catalytic reduction processes have offered the opportunity to transform hazardous water pollutants into non-hazardous products via sustainable, eco-friendly, and efficient routes and might be a competitive substitute for existing traditional water purification techniques. However, the key challenges linked with pure heterogeneous catalysts include agglomeration and poor dispersion, stability, recovery, and reusability, which result in poor activity and efficiency. Thus, it is essential to produce multipurpose polymer-based composite catalysts using conducting polymers, which are exceptionally good supportive and matrix materials. The blending of metal-based nanomaterials with polyaniline conducting polymers produces highly stable and efficient heterogeneous nanocomposite catalysts with amazing catalytic activity against a wide range of water pollutants. The heterogeneous catalytic reductive degradation of immensely toxic pollutant water has gained substantial curiosity because of its excellent physicochemical and surface characteristics, porous structure, recoverability, and recyclability. Therefore, this review presents the latest efforts to generate various polyaniline-based nanocomposite catalysts using a polyaniline matrix and various nanofiller materials and their potential applications in heterogeneous catalytic reduction degradation of water pollutants.
1. Introduction
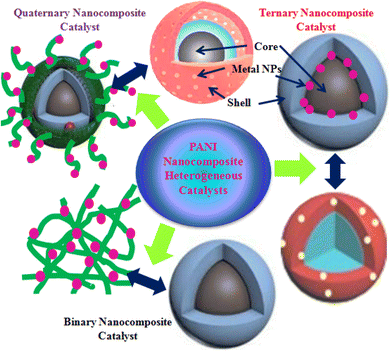
Water does not have any nutritional value but is essential for the survival of all living creatures. Currently, water shortage and purity have become critical and challenging tasks worldwide. However, 70% of Earth is covered with water, but approximately 3% of this water is fresh and adequate for drinking.1–3 The fast-growing population across the globe and intensified level of industrial and urban activities have introduced a new stage of stress to the supply of fresh water.4,5 It is estimated that by 2025, approximately 50% of the total global population will struggle for drinking water.2 Moreover, the continuous decline of water quantity and quality has significantly influenced the advantageous applications of water. This has demanded highly efficient, inexpensive, eco-friendly, and effective water purification processes to discard toxic water pollutants and improve water quality to the recommended levels of regulatory bodies. It is observed that traditional water treatment processes are mainly designed to remove bio-organisms such as bacteria, viruses, and protozoans or inactivate them using chemicals to trim down the threats of water-borne diseases.2 However, various challenges are still associated with the above-mentioned techniques. For instance, physical treatment methods, such as adsorption and air stripping processes generate waste or sludge, which needs to be further treated, and in chemical methods, the formation of toxic byproducts is a significant issue due to poor selectivity for targeted pollutants.6,7 Biological methods consume a longer time; however, they are widely employed and maintain a long-term activity.8 Additionally, the toxicity of discharged pollutants inhibits the activity of biological processes in some cases.9 The limitations associated with traditional methods and the progressive contraction of water standards and guidelines10 have inspired research communities to discover new, green, and efficient water treatment methods.Compared to the traditional approaches for water treatment, the use of advanced reduction techniques (ARTs), which include the catalytic reduction of pollutants, is favored to convert highly toxic pollutants into less hazardous or nonhazardous products.2 The reductive degradation is one the most efficient and significant traditional processes to eliminate the noxious and carcinogenic pollutants from polluted water.11,12 However, catalysis is largely concerned with the chemical industry but not as a water treatment technique.2 Currently, the catalytic reduction method of water purification is widely investigated at the laboratory and field scale and displayed thrilling results in both groundwater and industrial wastewater treatments.13 The chemical reduction process shows better activity and greater selectivity, generates less or non-toxic products and more eagerly eco-friendly products,14 and consumes fewer reagents.2 Based on the physical state of the utilized catalyst, the catalytic reduction process can be categorized as a homogeneously and heterogeneously catalyzed reduction process; when the solid catalyst is utilized, it is called heterogeneous catalytic reduction,15 and in homogeneous catalysis, a liquid phase catalyst is utilized. Usually, heterogeneous catalysis in water treatment is preferred because the isolation of the utilized catalyst in homogenous catalysis of water pollutants is technically and/or economically unfeasible and the soluble catalyst can generate serious ecological issues. In heterogeneous catalysis, the catalyst can be recovered via filtration, centrifugation, or other approaches easily, and a small amount of catalyst can effectively speed up the water purification process.16 The commonly explored heterogeneous nano-catalysts in catalytic reduction processes are metal-based nanomaterials that exhibit extraordinary reduction potential against various water pollutants under normal reaction conditions. However, pure metal-based catalysts (metal and metal oxide NPs) suffer from serious drawbacks, such as poor stability, aggregation, dispersion, recovery, and reusability. Hence, to overcome these limitations and advance the characteristics of pure catalysts, they are immobilized and blended with effective supporting materials, such as polymers, carbon-based materials, and silica. Among polymers, conducting polymers (CPs) have been extensively explored as supporting materials for numerous nanomaterials. Polyaniline (PANI) is a well-known CP with distinctive combinational features because of its effective electronic characteristics and good chemical and photostability, making it a suitable candidate for various uses in multi-disciplinary fields.17,18 Using PANI as a supporting matrix or shelter to various nanomaterials, different types of binary, ternary, and quaternary heterogeneous catalysts have been produced with significantly improved features and effectively utilized for the catalytic degradation of various water pollutants in the presence of a reducing agent. The current review presents the categorization of PANI nanocomposites as binary, ternary, and quaternary composites (Fig. 1), their synthesis utilizing different synthesis techniques, and their application as heterogeneous catalysts for the catalytic reduction degradation of various toxic water pollutants. Moreover, this review study will help in understudying new findings of the heterogeneous catalysis process, adding new aspects to the literature, and presenting information to the public to thrill them for the potential practical application of this technique for water treatment.
2. Characteristics of polyaniline and its synthesis
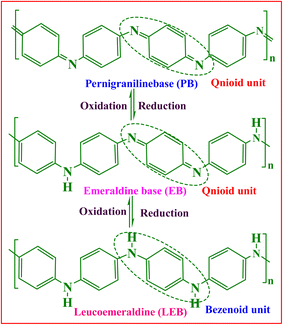
Historically, PANI was first prepared 190 years ago in 1834 by heating aniline nitrate with CuCl2 at 100 °C on a porcelain plate.19 Since then, it has become the most widely studied conducting polymer due to its facile and simple preparation process,20 and extensive acid–base doping and dedoping ability.21 PANI is a heterogeneous and semi-crystalline polymer in which an ordered section (crystalline) is dispersed in a disordered section (amorphous). The crystalline part is conducting and metallic, whereas the amorphous part is non-conducting and nonmetallic, and the crystalline region is accountable for the conducting nature of PANI.22 PANI is a homopolymer of aniline monomer that exists in three basic forms, which are structurally differing and show different oxidation states. These states are leucoemeraldine base (LEB), emeraldine base (EB), and pernigraniline (PNB) (Scheme 1). The PANI backbone structurally consists of aromatic diamine functionalities quinoid and benzenoid in a well-ordered manner. Among the PANI forms, the emeraldine form consists of both benzoid and quinoid units, half oxidized, neutral in undoped form while cationic in doped form, and has polaron and bipolaron structures.23 It is regarded as most suitable for doping due to its higher stability.24 The leucoemeraldine form is completely reduced and structurally consists of benzoid units, and pernigraniline is fully oxidized and consists of benzoid and quinoid units.23 The acid doping or protonation of EB produces polaron or bipolaron structures.25PANI is prepared via polymerization of an aniline monomer using various methods. Frequently, the method used for PANI synthesis is chemical oxidative polymerization (COP) of aniline in an acidic medium, using various oxidizing agents, and acids (organic or inorganic acids) as dopants. The most frequently employed oxidizing agents are ammonium persulphate (APS: (NH4)2S2O8) and Fe(III). However, other oxidizing agents such as transition metal ions, such as Mn3+, Mn6+, Mn7+, Cr6+, Ce4+, V5+, and Cu2+; noble metal compounds, such as Au3+, Pt6+, Pd2+, Ag+, KIO3, H2O2, and benzoyl peroxide; a mixture of oxidants FeCl3/H2O2 and KIO3/NaClO; in situ produced oxidants (Mo5+ compounds); enzymes such as horseradish peroxidase and oxidoreductase with H2O2; laccase with K3[Mo(CN)8]; and Fe(II) salts are also utilized.21 Through this procedure, PANI is obtained in the precipitate (solid powdered) form in morphologically different nanostructures, such as fiber, particle, sphere, rod, sheet, thread, tube, and micelle flower. The oxidation of aniline is influenced by several factors, such as concentration of oxidant, concentration of monomer, reaction medium, duration of the reaction, and temperature.26
Besides this procedure, other methods are also employed for the synthesis of PANI, including solid-state polymerization,27,28 electroless polymerization,29 electrochemical oxidative polymerization,30,31 plasma polymerization,32 sonochemical,33 photoinduced polymerization,34 solution polymerization,35 colloidal dispersion polymerization,22 seed polymerization,36 self-assembling polymerization,37 metathesis polymerization,38 interfacial polymerization,39,40 template synthesis,41,42 enzymatic synthesis,43 and emulsion polymerization,44 as shown in Table 1. The electrochemical oxidative polymerization (EOP) procedure is carried out by passing an electric current through an inert electrode immersed in an acidic aniline solution in which polymerization of monomers occurs on the surface of the cationic electrode as a thin film. The EOP process of aniline is usually performed under constant conditions of potential (potentiostatic), current (galvanostatic), and potential sweeping/scanning. The electrode (anode) material utilized is Pt or glass,22 and many other materials, such as Fe,47 Cu,48 Au,49 graphite,25 vitreous carbon,50 and stainless steel22,51 are also explored as electrode materials in the EOP process of PANI synthesis.
| Synthesis technique | Description | References |
|---|---|---|
| Colloidal dispersion polymerization | Aniline polymerization produces a water-soluble polymer, which produces colloidal PANI particles instead of precipitation. Macroscopic precipitation is prevented by using a steric stabilizer | 22 |
| Solid state polymerization | Polymerization of aniline at the solid surface occurs on the surface of reactant molecules | 27 and 28 |
| Electroless polymerization | Polymerization of aniline without applying external potential in the acid medium in the metal surface (Pt, Pd) via an electrochemical pathway in which reduction of dissolved O2 and oxidation of aniline take place via cathodic and anodic half-reactions, respectively, at the interface of metal/solution | 29 |
| Electrochemical oxidative polymerization | Aniline polymerization in an acidic solution by passing of current without using an oxidant produces homogeneous and pure products as film | 30 and 31 |
| Plasma polymerization | Injection of aniline in plasma produced by a DC glow discharge in which electrons or ions present in plasma induce polymerization, and polymerization takes place in solvent and chemical oxidant-free conditions | 32 |
| Sonochemical | Polymerization of aniline with drop-wise addition of oxidant under ultrasonic irradiation conditions produces PANI with higher solubility and scalability | 33 |
| Photoinduced polymerization | Polymerization of aniline in the presence of transition metal salts on illumination with light radiation produces PANI in composite form | 34 |
| Solution polymerization | Electo- or chemical polymerization of aniline in the solvent in which the produced PANI is obtained in solution form | 35 |
| Seed polymerization | Polymerization of aniline is carried out in the presence of foreign material (seed) | 36 |
| Self-assembling polymerization | Polymerization of aniline vapor on oxidant-coated substrate surface as PANI film | 37 |
| Metathesis polymerization | Obtaining PANI without using aniline by heating 1,4-dichlorobenzene in organic solvent (benzene) for 12 h at 220 °C with sodium amide | 38 |
| Interfacial polymerization | Aniline polymerization is carried out at the interface of two immiscible solvents using acid dopant and oxidant with a construable and slow polymerization rate | 39 and 40 |
| Template synthesis | Polymerization of monomer into the polymer in compliance with information received from the template present in the reaction mixture | 41 and 42 |
| Enzymatic synthesis | Polymerization of aniline is carried out by enzyme at low temperature and neutral pH producing small chain length PANI with a lower degree of doping | 43 |
| Emulsion polymerization | Polymerization of monomer in a water-dispersed phase which is dispersed in an aqueous phase that contains a surfactant | 44 |
| Chemical oxidative polymerization | Polymerization of aniline monomer is initiated by a chemical oxidant in an acidic solution that produces a bulk product in a short time | 45 and 46 |
3. Synthesis of polyaniline nanocomposites
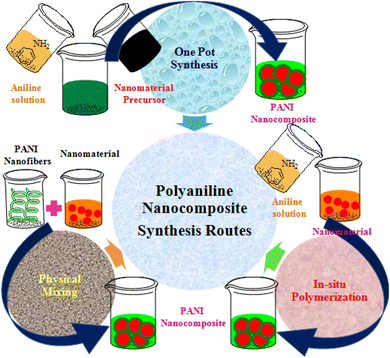
Usually, polymer-based nanocomposite formation is carried out using in situ or ex situ polymerization procedures,52 as shown in Fig. 2. The in situ process includes the synthesis of a composite simultaneously combining the filler nanomaterial and polymerization of the monomer because the nanomaterials are excellently dispersed in the matrix phase, while in ex situ polymerization already prepared PANI is mixed with nanomaterials.53 Thus, in the ex situ process, nanocomposites are prepared with poor dispersion, lower density, and non-uniform distribution of particles in the matrix,52 while in the in situ process, homogeneously dispersed composites with a higher density are prepared. During the synthesis process, the nanocomposite particles can be adorned at the surface of the polymer or immobilized in the polymer matrix.54 The deposition of nanomaterials on the surface of the polymer is cost-effective, creates higher active sites, improves surface properties, and reduces particle agglomeration, and it can be achieved via plasma polymerization, chemical vapor deposition, grafting, and dip-coating processes; however, there is a greater possibility of leaching of nanomaterial particles.55–58Although the distribution of nanoparticles inside the polymer matrix reduces the leaching of particles from the matrix and improves the recoverability of particles, agglomeration may be higher,54,58 and it can be achieved via in situ techniques that may be in situ polymerization or sol–gel process.59 The in situ polymerization process entails a controlled immobilization of elected nanomaterial with the pure monomer solution before succeeding polymerization.54 Except in situ and ex situ routes, physical mixing of polymeric structure (such as PANI) and nanomaterials already prepared are mixed by milling, solution, or melt mixing procedures. However, the components in the nanocomposite materials derived through this method are associated with weak physical interactions and thus possess poor attractions. All the processes adopted for the synthesis of PANI-nanocomposites are based on these three synthesis routes. The commonly employed synthesis methods of PANI-nanocomposites are summarized in Table 2.
| Preparation method | Description | Reference |
|---|---|---|
| In situ polymerization | Already fabricated nanomaterials are mixed in an acidic solution of aniline monomer. Next, polymerization is initiated by the drop-wise addition of an oxidant solution under constant stirring conditions, generating a nanocomposite with better dispersal of nanomaterials in the PANI matrix | 60 |
| Electrochemical polymerization | Pre-synthesized nanomaterial is dispersed in an aqueous/solvent solution of aniline that possesses a dopant, and the polymerization reaction of aniline is commenced by passing the current | 61 |
| Thermal reflux | Refluxing nanomaterial precursor and aniline in a suitable solvent at the apposite temperature generates PANI nanocomposite | 62 |
| Mixing | Physical mixing of pre-synthesized nanomaterial and PANI via milling, solution (in the same solvent or different solvents), or melt mixing | 63 |
| Sonochemical polymerization | Pre-synthesized nanomaterial is mixed in an aniline solution through ultrasonication and the polymerization reaction is initiated by adding of oxidizing agent | 64 |
| Chemical vapor deposition | Vapor phase polymerization of aniline monomers on the surface of a substrate that is coated with oxidant and nanocomposite obtained in the film form | 65 |
| Electrospinning | Formation of fibrous composite using a micro syringe pump by applying a voltage | 66 |
| Hydrothermal method | Formation of composite via hydrolysis at high temperature | 67 |
| Interfacial polymerization | Polymerization in a biphasic system, i.e. an aqueous/organic that splits the byproducts upon the basis of their solubility | 68 and 69 |
4. PANI nanocomposite heterogeneous catalysts for degradation of water pollutants
Several contemporary studies have discovered that homogenous and heterogeneous catalysis processes are incredibly competent in eradicating pollutants.70–72 In the homogenous or heterogeneous catalytic reduction process of various environmental pollutants, metal complexes or metal nanoparticles, particularly noble metal NPs, are usually employed.73,74 Although in comparison to homogenous catalysts, the heterogeneous catalysts are more significant due to their non-solubility in solvents, providing wide surface support to reactants, greater stability, and good reusability.75Due to a higher surface-to-volume quotient, the metal NPs show the unique ability of heterogeneous and homogenous catalysis potential during catalysis reactions. Heterogeneous catalysts display good revitalization and reusability features, while homogeneous catalysis shows excellent selectivity and comparatively small catalyst loadings.76 Therefore, in many catalysis reactions, the applicability of metal NPs significantly increased in current times,77 and in catalysis processes, the use of metal nanoparticles stems as a potential contender in various catalytic reactions due to their remarkable catalytic potential owing to specific crystalline features and higher specific surface area.78 However, the high surface area and surface energy due to their small size enable them to form aggregates and generate large-sized particles so that they can minimize surface energy, which accordingly reduces their catalytic ability.79 Noble metal NPs are frequently used NPs in catalytic reduction processes because of their exceptional physicochemical traits and the presence of a wide number of binding sites due to the presence of bare atoms,80,81 large surface area, and lower size.82 However, noble metal catalysts exhibit low mass transport rate, unstable deactivation, and deficient catalyst use coefficient are other challenges that limit their application.82 Thus, under numerous conditions, the use of metallic nanoparticles would stumble upon the aggregation of NPs owing to the higher surface energy in a smaller size,83,84 poor separation and recovery from the reaction medium,78 and lower stability and reusability, resulting in poor activity and complications in sustainable use. Numerous methods have been adopted and executed to overcome these limitations of pure metal-based nanoparticle catalysts, among which the immobilization of metal nanoparticles on the solid supporting surface has been considered an effective mechanism,85–87 helping in the reduction of agglomeration of nanoparticles and isolation from the reaction medium. Embedding or immobilizing pure metal NPs into the polymer matrix may improve the reaction rate.88 Various conducting polymers have been explored as supporting surfaces for immobilizing pure metal-based NPs, such as PPy and PANI. Among them, PANI is a widely studied and utilized conducting polymer as a supporting surface for immobilizing metal-based nanoparticles owing to its strong interaction with metal-based nanomaterials, and it has drawn vital interest in research due to the various effective immobilization of the metal NPs.89 Moreover, as a shell material, PANI has largely studied conducting polymer due to its distinctive electrical features, and the presence of effective anchoring sites for metal ion binding, which helps in the prevention of aggregation, easy synthesis, ecological stability, and biocompatibility, is another charismatic feature of PANI.79,90,91 Since 1989, when the first report on the catalytic reduction of water pollutants was published,92 various research groups reported numerous studies for the reduction of pollutants.2,93 The key aimed water contaminants in the catalytic reduction studies are organic dyes (RhB, MO, CR, MG, EY, and MB), nitro- and nitroso-compounds (nitrophenols, trinitrotoluene, RDX, and NDMA), organic halides (bromi/chlori/fluorinated hydrocarbons), and hazardous oxyanions.94 In the catalysis process, metal NPs lower the kinetic barrier for redox reactions and the decomposition of water pollutants.95,96
PANI in pure form with dopants can act as a catalyst due to its ease of conversion of benzenoid and quinoid rings in the PANI chain; however, its activity is comparatively low. PANI nanofibers are prepared using granite waste as an oxidant for the catalytic degradation of water pollutants, particularly 4-nitrophenol (4-NP) and Rhodamine B (RhB) dye, in the presence of NaBH4. The reduction processes of 4-NP and RhB dye were completed in 40 and 5 min, with reaction rate constants of 0.0488 and 0.958 min−1, respectively.97 This reflects that pure PANI can act as a catalyst for the reduction of pollutants but suffers from poor activity.
PANI in comparison to conventional supporting materials for metal-based NPs is beneficial due to facile and inexpensive large-scale synthesis using oxidants, and the synthesis process can be made efficient using clean oxidizing agents, such as H2O2 and molecular O2. Moreover, PANI properties can be modified by introducing various functionalities, making it a versatile support compared to inorganic materials. Using PANI as a supporting material for metal NPs, mainly noble metals (Au, Ag, Pd, and Pt), numerous composite catalysts have been produced and successfully explored for the degradation of various organic and inorganic chemical pollutants as well as biological water pollutants. Usually, the preparation of hybrids of noble metal NPs and conducting polymer can be achieved through in situ polymerization of monomer in the presence of metal NPs,98–100 simultaneous reduction of metal ions, polymerization of aniline monomer with and/or without using oxidant,101,102 direct blending of CP and metal NPs,78,103,104 in situ reduction of noble metal ions by CP.,97,105,106 one-pot synthesis of CP and noble metal NPs,107 γ-radiolysis process in which the reaction mixture is exposed to γ-rays.,108,109 interfacial polymerization in which a biphasic system i.e. an aqueous/organic phase splits the byproducts upon the basis of their solubility is utilized,110 electropolymerization that proceeds by passing electric current in the reaction mixture of aniline and metal ion or NPs using solvent,111,112 template-based synthesis process utilizing structure-guiding models to state the physical features of the resulting nanocomposite,113 and self-assembly including physically mixing and/or grinding of PANI and metal NPs.114
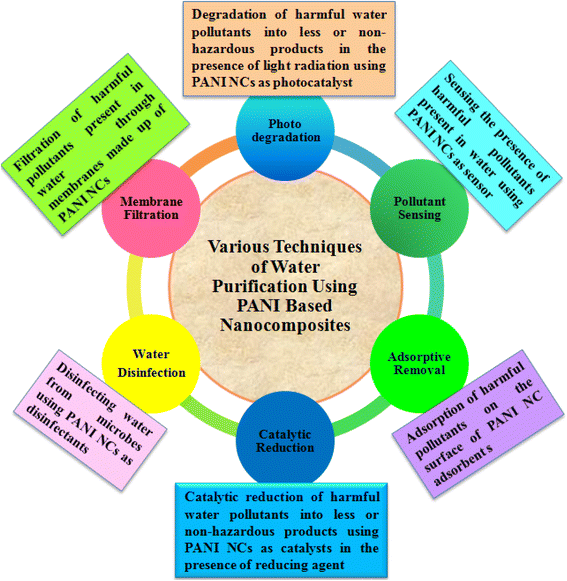
PANI nanocomposites prepared via different methods using various nanomaterials, such as metal NPs, carbon materials, organic materials, and polymeric substances, are frequently utilized for the treatment of water in various ways, including heterogeneous catalytic degradation,123 and other processes which include the photocatalytic degradation of pollutants using photocatalysts under light exposure,124,125 adsorptive removal of pollutants using composites as adsorbent materials,126–128 nano-filtration of pollutants using PANI-based materials as membranes,129–131 sensing of pollutants using as sensors,132–134 and disinfecting water from bio-organisms employing PANI composites as antimicrobial agents135,136 (Fig. 3). Among them, the application of PANI nanocomposites as heterogeneous catalysts for the catalytic reduction of toxic water pollutants into less and/or non-toxic products has drawn immense attention. Using different types of metal-based nanomaterials, various PANI binary nanocomposites have been developed, as summarized in Table 3 and efficiently utilized as heterogeneous catalysts for the catalytic reduction of various water pollutants, such as nitroaromatics, organic dyes, pesticides, metal ions, and phenolic compounds.
| PANI nanocomposite | Pollutant type | Catalyst dose | Rate constant | Time | References |
|---|---|---|---|---|---|
| a Catalyst dose amount (g = gram, μL = microlitre, mg = milligram, and mol% = mole percentage), reaction time (min = minute and s = second). | |||||
| PANI/Ni(0) | BG | 0.025 g | 0.113 min−1 | 120 min | 82 |
| Au-PANI | MB | 1 mg | 33 × 10−2 s−1 | 10 min | 83 |
| CR | 1 mg | 30 × 10−2 s−1 | 10 min | ||
| PANI/Au | 4-NP | 0.0315 g | 11.8 × 10−3 s−1 | 270 s | 84 |
| 3-NP | 0.0315 g | 28.9 × 10−3 s−1 | 90 s | ||
| 2-NP | 0.0315 g | 22.3 × 10−3 s−1 | 100 s | ||
| PANI/Ag | 4-NP | 2.7 mg | 21 × 10−3 s−1 | 3 min | 115 |
| PANIsphere-Ag | 4-NP | 15.27 | 16 × 10−2 min−1 | 23 min | 116 |
| PANI/Bi2O3 | 4-NP | 0.1 g | — | 15 min | 117 |
| Pd-PANI | 4-NP | 30 μL | 20 × 10−3 s−1 | 4 min | 118 |
| MB | 29 × 10−3 s−1 | 2.5 min | |||
| PANI/Ag | 4-NP | — | 9.5 × 10−3 s−1 | — | 119 |
| Pd/PANI | 4-NP | 30 mg | 6.4 × 10−4 s−1 | 40 min | 120 |
| PANI/Pt | 4-NP | 0.4 mol% | — | 120 min | 121 |
| Ag-PANI | 4-NP | 1 mg | 3.9 × 10−3 s−1 | 300 s | 122 |
The in situ polymerization process produces amorphous nanocomposite in which nanoparticles are homogeneously dispersed in a polymer matrix, but the unreacted reactant present with nanocomposite may affect its characteristics.137 Through the in situ reduction process, the metal NPs are present at the surface of CP instead of embedded in the matrix; hence, they are available for direct interaction with the substrate, thus demonstrating good heterogeneous catalysis,99,138 and the catalytic activity of the nanocomposite catalyst depends on the size of metal NPs.106 Chen et al.139 synthesized PANI/Pt hybrid nanocomposite catalyst via an in situ reduction process in which Pt6+ ions were reduced by citric acid doped PANI into small-size Pt NPs at the surface of PANI and dispersed uniformly, which exhibited excellent heterogeneous catalytic activity for the reduction of nitroaromatics, high reusability, and durability (Fig. 4). Through the direct blending process of CP and metal NPs, noble metal NPs can be deposited on the PANI surface of composites.140
 | ||
| Fig. 4 Reduction of Pt6+ ions into Pt NPs.139 | ||
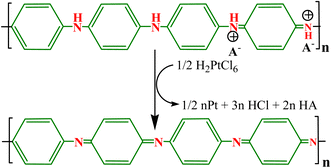
To provide support to Au NPs, Sun et al.84 reported Au NPs immobilized in PANI microtube (PANI/Au) composite via a template-free process that demonstrated effective catalysis of nitrophenols in the aqueous medium due to the higher surface area of microtubes and better dispersion, and the effective prevention of agglomeration of Au particles enhanced the stability and recovery potential of Au NPs (Fig. 5(a)). The nitrophenol reduction activity was observed in the order of 3-NP > 2-NP > 4-NP. A binary Au encapsulated (AuNP-PANI) water-soluble nanocatalyst was synthesized in a two-step process through the formation of micelle for stabilization of PANI NPs using surfactant CTAB, and the produced AuNP-PANI catalyst showed effective reduction for CR and MB dyes.83 Similarly, a binary PANI/Au heterogeneous catalyst was prepared via interfacial polymerization using AuCl4− as a source of Au NPs for the catalytic reduction of RhB dye. The AuCl4− initiated the polymerization of aniline, and itself was reduced into Au NPs deposited on the surface of PANI.142 Pd NP-loaded binary Pd@PANI catalyst with a very low amount of Pd NPs was produced via joint method interfacial polymerization and in situ process using camphor sulfonic acid ((+)-CSA) as a dopant, and the obtained catalyst displayed effective reduction potential for nitroarenes in a water solution with a small quantity of reducing agent NaBH4. The Pd@PANI catalyst exhibited good reusability and stability for numerous cyclic reuses, and the reused catalyst demonstrated an improved catalytic rate (11.2 × 10−4 s−1) compared to the fresh catalyst (6.4 × 10−4 s−1).120
 | ||
| Fig. 5 (a) Synthesis scheme for PANI microtubes and the PANI/Au catalyst,84 (b) SEM and (c) TEM image of PANI/Ni0 NCs,82 and (d–g) TEM images of the Fe3O4@Au-PANI ternary nanocomposite.141 | ||
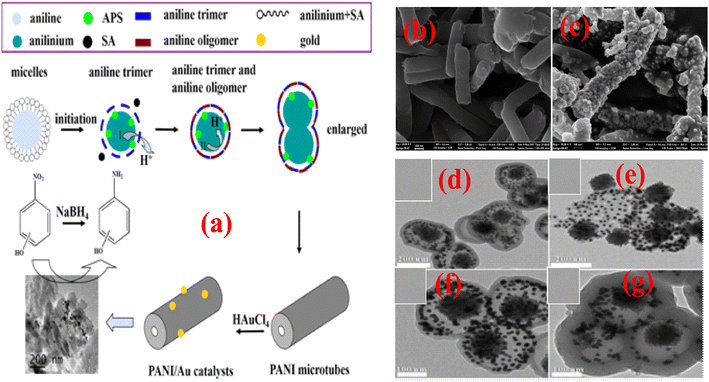
A phytic acid (PA) used as a dopant and crosslinker Ag NP-immobilized PANI nanocomposite recyclable (PANI/Ag) catalyst with a 3D structure was fabricated via an in situ redox reaction between silver nitrate and PANI. The characteristic 3D porous structure of the prepared catalyst is formed by good dispersion of Ag NPs in the coral-shaped PANI, demonstrating remarkable catalytic performance toward 4-NP with a fast rate of reduction reaction (9.5 × 10−3 s−1) due to constructive mass/electron transfer of the 3D porous structure and good attraction of Ag with PA; additionally, the magnetic features improve catalyst recyclability using an external magnetic field and retained high-level activity of up to six cyclic runs.119 In another study, Chang et al.115 prepared an Ag/PANI composite by depositing Ag NPs on the supporting surface of PANI achieved by in situ chemical reduction of AgNO3 and further utilized for the catalytic reduction of 4-NP. The reduction of 4-NP occurred on the surface of Ag/PANI due to the stacking of 4-NP via π–π interactions with PANI, which is not possible with pure Ag NPs. Ni NPs are utilized as catalysts for the degradation of various pollutants owing to their eco-friendly nature, better electron-donating potential, excellent magnetic characteristics, and inexpensiveness. However, as a Fenton-type heterogeneous catalyst, Ni NP application is limited due to the lower stability and formation of aggregates. Various nanomaterials have been successfully used as supporting materials for Ni(0) NPs, resulting in catalytic applications in numerous reactions. Bhaumik et al.82 developed Ni(0) metal NPs supported on a nanotubular porous PANI matrix (PANI/Ni0 NCs) nanocomposite catalyst (illustrated in Fig. 5(b) (SEM image) and Fig. 5(c) (TEM image)) via a reductive formation process that exhibited Fenton-like catalytic activity for the BG dye in water samples in the presence of H2O2. The PANI/Ni0 NC catalyst exhibited supremacy compared to pristine Ni0 NPs for BG degradation and degraded 100% of the BG dye within 120 min. Moreover, it retained its activity (100%) in five cyclic experiments.
Ultrafine Pt NPs immobilized on surfaces of PANI nanofibers catalyst (PANI/Pt) were fabricated via a facile solution procedure. The Pt NPs were deposited on PANI fibers via an in situ reduction process, and the catalyst illustrated greater activity against nitrobenzene and showed excellent stability, reusability, and durability.139 Ag NPs loaded on citric-acid (CA)-modified PANI catalyst (Ag@PCA) were produced through an in situ reduction process and utilized for the reduction of nitrophenols. The obtained electrocatalyst showed effective activity for the oxidization of 2-NP and 4-NP.143 Pd NPs immobilized PANI (Pd-PANI) catalyst prepared using surfactant-based liquid crystalline mesophase by employing chemical oxidation, followed by in situ radiolytic reduction of Pd2+ ions on the PANI nanowire surfaces. The developed catalyst demonstrated good reduction potential against 4-NP and MB dye due to the effective immobilization of Pd NPs and homogenous dispersion and showed better stability.118 Metal oxides, such as CuO144 and Bi2O3,145,146 alone have been explored for the catalytic degradation of numerous water pollutants. Recently, green synthesized CuO NPs utilized for the reduction of 4-NP144 and biogenic pure Bi2O3 (ref. 145 and 146) demonstrated effective catalytic ability for nitroaromatics and organic dyes, similar to Ag2O in pristine form or as a composite utilized for catalytic reduction of nitroaromatics and dye pollutants.147 However, in pure form, metal oxide NPs demonstrate relatively poor activity, recovery, reusability, and stability. By providing support or immobilizing with polymeric materials, especially PANI polymers, the activity of metal oxide NPs can be enhanced. For instance, immobilization of Bi2O3 NPs in PANI (PANI/Bi2O3) significantly empowered the reduction ability of Bi2O3 for 4-NP in an aqueous medium and reduced about 95.4% of 4-NP within a short time (10 min) by a small amount of catalyst (0.1 g) and also enhanced the stability of Bi2O3 particles.117
However, the application of PANI as a support material suffers from recovery and reuse issues.93 This limitation can be overcome by introducing magnetic characteristics to the PANI-supported metal-based NPs, which can be easily and effectively isolated and recovered by applying an external magnet. Producing magnetic characteristics in the nanoparticles by supporting magnetic materials prevents particle aggregation and causes ease of isolation and revival from the reaction solution by applying an external magnetic field.78 However, in an acid solution, the magnetic material particles exhibit poor stability and may react with catalyst(s) to be immobilized or even with the substrate; thus, their surface should be covered by forming a core–shell composite structure in which the shell can effectively protect the magnetic core particles. For the shielding of the magnetic core, numerous materials, such as carbon, silica, TiO2, other inorganic materials, and polymers, are widely utilized and have successfully helped in the prevention of corrosion, enhanced interaction, and improved inertness and stability. However, the use of polymeric shells enhances stability and magnifies the biocompatibility and solubility of magnetic material particles in water.78,148 PANI is one of the most widely studied and employed polymer materials as a shell for magnetic and other cores.78
In recent years, extensive research has been carried out on the development of magnetic and nonmagnetic PANI-supported metal-based ternary and quaternary nanocomposite catalysts for water treatment. A few of them that have been successfully employed for the catalytic degradation of various pollutants are listed in Table 4. For example, bifunctional Au immobilized Fe3O4@C (Fe3O4@C@Au) composite microspheres developed by coating Au NPs on the surface of functionalized Fe3O4@C microspheres showed excellent degradation ability for MB dye due to synergistic effects of small particles of Au NPs, excellent adsorption tendency of C-layers and better dispersion of the microspheres. The Fe3O4@C@Au catalyst degraded MB dye with a reaction rate of 0.331 min−1.160 Chen et al.78 protected the magnetic core using a PANI polymer shell and prepared Fe3O4@PANI core–shell nanostructure via an in situ polymerization process, which was utilized as solid support for Au nanoparticles to produce Fe3O4@PANI@Au composite catalyst. The Au NPs were adsorbed on the PANI surface via electrostatic interactions. The magnetic core shell-supported Au-based composite catalyst prepared in this way was utilized for the reduction of CR dye using NaBH4 as a reducing agent. After completion of the reaction, the utilized catalyst was separated and recovered effectively via an external magnetic field. Within 6 min of reaction time, the CR dye was reduced by 10 mg of catalyst, and it demonstrated good activity for up to five cyclic uses without losing significant activity. Ternary magnetic PANI-supported Ag (MnFe2O4@PANI@Ag) nanocatalyst synthesized via a simple reflux process demonstrated good activity for the degradation of organic dyes MO, EY, MB, and RhB in the presence of NaBH4; converted into colorless non-toxic forms quickly within 9, 16, 7, and 23 min, respectively; easily recovered via magnetic separation and exhibited good activity up to four cycles of reuses. The magnetic core and PANI fibrous linker shell effectively stabilized Ag particles with good dispersion and minimum aggregation.149 Zhang et al.103 used cobalt spinel (CoFe2O4) as a magnetic core material to produce ternary core shell CoFe2O4/PANI/Au nanocomposite catalyst for the catalytic reduction of 4-NP, which demonstrated excellent catalytic reactivity (100% reduction after 8 min) in a short time, and good recovery and stability.103
| PANI nanocomposite | Pollutant type | Catalyst dose | Rate constant | Time | References |
|---|---|---|---|---|---|
| a Catalyst dose amount (mg = milligram; ug = microgram), reaction time (min = minute; s = second). | |||||
| AgCl/PANI/D-Dex | 4-NP | 10 mg | 0.365 min−1 | 6 min | 69 |
| Fe3O4@PANI@Au | CR | 10 mg | 0.80 min−1 | 6 min | 78 |
| Au-PANI@mGO | 4-NP | 10 mg | 86.3 × 10−2 min−1 | 5 min | 93 |
| CR | 10 mg | 28.64 × 10−2 min−1 | 20 min | ||
| MB | 10 mg | 68.19 × 10−2 min−1 | 5 min | ||
| RhB | 10 mg | 94.02 × 10−2 min−1 | 4 min | ||
| Fe3O4@PANI/Ni@Pd | O-NA | 1 mg mL−1 | — | 3 min | 97 |
| Fe3O4@PANI/Au/m-SiO2 | 4-NP | 0.9 mL | 25.49 × 10−3 min−1 | 40 min | 99 |
| PS/PANI@Au | 4-NP | 0.375 mL | 36 × 10−3 s−1 | — | 104 |
| Ag-PANI-MWCNT | 4-NP | 1 mg | 5.4 × 10−3 s−1 | 240 s | 122 |
| PANI/ZnO/MnO2 | 4-NP | 10 mg | 21.9 × 10−2 min−1 | 10 min | 123 |
| rGO-PANI/Pd:Au | 4-NP | 2 mg | 5.8 × 10−3 s−1 | 8 min | 136 |
| O-NA | 2 mg | 5.4 × 10−3 s−1 | 8 min | ||
| RhB | 2 mg | 7.3 × 10−3 s−1 | 30 min | ||
| MG | 2 mg | 9.5 × 10−3 s−1 | 30 min | ||
| Cr(VI) | 2 mg | — | 30 min | ||
| PS/PANI-Au | 4-NP | 0.12 mL | 9.5 × 10−3 s−1 | 13 min | 140 |
| MnFe2O4@PANI@Ag | MB | 1 mg | 61 × 10−2 min−1 | 7 min | 149 |
| MO | 1 mg | 22 × 10−2 min−1 | 9 min | ||
| EY | 1 mg | 14 × 10−2 min−1 | 16 min | ||
| RhB | 1 mg | 11 × 10−2 min−1 | 11 min | ||
| Fe3O4@PANI@Au | 4-NP | 20 ug | 8.63 × 10−3 s−1 | 210 s | 150 |
| CR | 20 ug | 11.91 × 10−3 s−1 | 90 s | ||
| MO | 20 ug | 16.11 × 10−3 s−1 | 90 s | ||
| MB | 20 ug | 10.59 × 10−3 s−1 | 150 s | ||
| RhB | 20 ug | 6.41 × 10−3 s−1 | 240 s | ||
| Au@Fe3O4@PANI | 4-NP | 25 uL | 43.3 × 10−2 min−1 | 5 min | 151 |
| Fe3O4@PANI-Au | MO | 1 mg | 2.21 × 10−2 s−1 | 60 s | 152 |
| MB | 1 mg | 3.17 × 10−2 s−1 | 80 s | ||
| Fe3O4@Au-PANI | 4-NP | 3 mg mL−1 | 12.4 × 10−2 min−1 | 28 min | 141 |
| Cu(0)-PANI-ZrSiO4 | 4-NP | 1 mg | 81.1 × 10−2 min−1 | 10 min | 153 |
| 3-NP | 1 mg | 24.9 × 10−2 min−1 | 7 min | ||
| 2-NP | 1 mg | 58.4 × 10−2 min−1 | 5 min | ||
| PANI/MnO2/TiO2 | Cr(VI) | 1 mg mL−1 | 15.97 × 10−2 min−1 | 5 min | 154 |
| PS/PANI/Au | 4-NP | — | 58.66 × 10−3 s−1 | 60 s | 155 |
| Ag3PO4/Ppy/PANI | 4-NP | — | 5.3 × 10−3 min−1 | — | 156 |
| Ni(OH)2@NSA-PANI | 4-NP | 5 mg | 82.98 × 10−3 min−1 | 24 min | 157 |
| Ag@PANI–CS–Fe3O4 | 4-NP | 1 mg | 2 × 10−3 s−1 | 22 min | 158 |
| Pd@PANI–CS–Fe3O4 | 4-NP | 1 mg | 5.0 × 10−3 s−1 | 22 min | 159 |
Zhu et al.150 synthesized core–shell magnetic Fe3O4@PANI@Au nanocomposite catalyst via electrostatic self-assembly and seed growth processes that exhibited good reduction potential for organic pollutants (4-NP, MO, MB, and RhB) and inorganic pollutant (Cr(VI)) in an aqueous medium and demonstrated good stability, recovery, and reusability. Via controllable coating of PANI, Jin et al.151 prepared a magnetic ternary Au@Fe3O4@PANI nanocomposite catalyst for the catalytic reduction of 4-NP using NaBH4 as the reducing agent. The presence of PANI and iron oxide showed good stability and recyclability and improved the catalytic performance of Au NPs owing to their synergetic effects. The catalyst reduced 4-NP with a high rate of reaction and demonstrated excellent reusability of up to 10 cycles. Moreover, Fe3O4@PANI@Au exhibited good selectivity toward the degradation of cationic dyes and rapidly degraded CR dye. Bioinspired reduction of Au3+ ions using plant Allium sp. extract at the surface of magnetic core–shell Fe3O4@PANI nanocomposite generated Fe3O4@PANI-Au catalyst for reduction of hazardous dyes MB and MO using reducing agent. With a small amount of biosynthesized catalyst (1 mg and 2 mg), the dyes were reduced in a very short time. In the presence of 1 mg of catalyst dose, the MB and MO dyes took 50 and 60 s, respectively, while with 2 mg of catalyst dose, they decolorized in 60 and 80 s, respectively. The catalyst retained its activity for a long time (up to 8 cyclic reuses) and displayed good stability.152
Core–shell composite structures that possess a movable core inside a hollow capsule represent yolk–shell structures; they are extensions of conventional core shell structures.142,161 In comparison to traditional core shell structures, empty parts of yolk–shell structures possess a higher unoccupied space, low density, and a greater surface area. Various types of yolk–shell composites composed of a magnetic core and a functional shell have drawn wide attention as catalyst-supporting structures. The outer shell of yolk–shell type composite structures demonstrating greater surface area and effective dispersion features enables them to provide better support to the catalyst and maximize their catalytic potential to metal NPs.
Qiao et al.141 reported a yolk–shell type ternary composite Fe3O4@Au-PANI catalyst (TEM images shown in Fig. 5(d–g)) in which Au NPs were immobilized in a PANI shell that has a magnetic core via a SiO2 sacrificial template procedure. The Au NPs were anchored onto the inner layer of the PANI shell to avoid direct interaction with the substrate molecules. Thus, the presence of a magnetic core allowed facile recovery of the catalyst, and the PANI supported and protected the catalyst from an outer atmosphere. The catalyst with a PANI protection shell (Fe3O4@Au-PANI) demonstrated higher reduction potential against 4-NP than the catalyst without a PANI shell. The multistep synthesis procedure used to prepare Ni@Pd core–shell NPs was immobilized on magnetic Fe3O4@PANI yolk–shell nanocomposite. The yolk–shell was prepared via ultrasound-mediated in situ polymerization of aniline on Fe3O4@SiO2. Then, the silica layer was etched selectively to obtain a yolk–shell structure. Finally, the Ni@Pd core was inserted by dispersion in an aqueous medium with a reducing agent and metal precursors. The catalytic performance of the prepared yolk–shell quaternary Fe3O4@PANI/Ni@Pd catalyst was investigated against o-nitroaniline, which reduced by 99% in 3 min.79
Magnetic materials suffer from aggregation issues, which can be prevented by carbon-based materials. Recently, the use of carbon materials (GO, rGO) as support for metal NPs to produce heterogeneous catalysts can effectively prevent the aggregation of particles, facilitate dispersion in an aqueous medium, enhance the accessibility of the catalyst to pollutants, and improve the surface area and electron transfer process efficiently. By following multiphase, Pourjavadi et al.93 produced Au NPs supported on magnetic PANI@mGO composite catalyst that showed remarkable reduction ability for CR, RhB, MB, and 4-NP in aqueous phase due to good dispersion ability; higher surface area due to the presence of GO; and facile electron transfer during catalysis process due to better attraction with PANI, and also displayed good recoverability on applying an external magnetic field.79 In nonmagnetic PANI-based ternary nanocomposite, the PANI provided better support surface to metal-based NPs and was exploited for catalytic degradation of water pollutants. Ternary composite containing polystyrene core and PANI shell, such as PS/PANI/Au catalyst, developed by Sun et al.155 using sulfonated polystyrene, Au(en)2Cl3, and aniline for the degradation of 4-NP that completely reduced 4-NP into 4-AP within a very short time (60 s). Similarly, a heterogeneous catalyst PS/PANI@Au developed via the seed swelling polymerization process and in situ reduction method was employed for the effective reduction of toxic pollutant 4-NP. In the prepared catalyst, PS survived as the core and template, and the shell was formed by PANI and Au NPs. Moreover, the dedoping of the PS/PANI@Au catalyst was carried out using acid and showed a higher reduction performance for 4-NP than that of the doped catalyst.104
Through the direct blending of core–shell PS/PANI binary composites and metal NPs produced from noble metals NPs (Au, Ag, Pd, Pt) deposited on PANI shell surface due to attraction between positive surface of PANI and negative charge on metal NPs under weak acidic medium through simple electrostatic self-assembly process, the produced PS/PANi-MNPs composite represented raspberry-like morphology (Fig. 6 SEM images). The PS/PANi-Au nanocomposite catalysts were prepared in three types using three sizes (32.4, 17.5, and 3.1 nm) of Au NPs and applied for the reduction of 4-NP, which reduced it with reduction rates of 3.8 × 10−3, 7.0 × 10−3, and 1.3 × 10−2 s−1, respectively. The results of the reaction indicated that the reaction rate decreases as the particle size of Au NPs increases in the PS/PANi-Au nanocomposite catalyst. This is because the reduction reaction of 4-NP chiefly progresses on the surface of Au NPs, and smaller-size NPs (Au) possess greater reduction ability owing to the enhanced density of active edges and corner sites at the surface of Au NPs.140 Sun et al.162 utilized a copolymerization process for the synthesis of a (P(ANI-co-Py)) copolymer and produced Au NP-immobilized (P(ANI-co-Py)) composite catalyst by deposition of Au NPs using a sol–gel process. Similarly, binary Au/PANI and Au/PPy were also synthesized. The copolymer effectively stabilized small Au NPs and enhanced the catalytic activity of 4-NP. Due to the good dispersion of Au NPs in the support material, the strong interaction of metal-support and the effective adsorption of 4-NP on the catalyst surface owing to the negative surface of Au NPs increased the catalytic ability of the prepared catalyst.
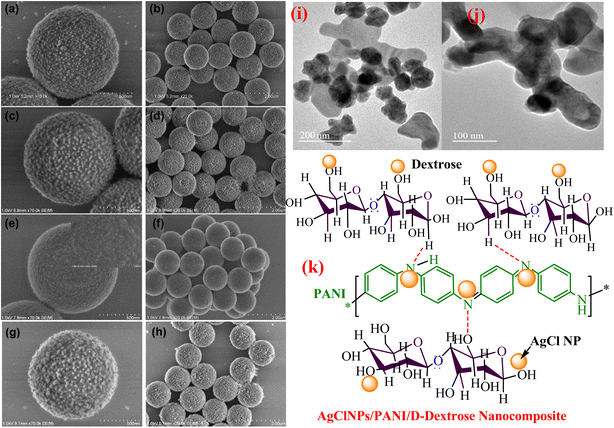 | ||
| Fig. 6 (a and b) PS/PANi-Ag, (c and d) PS/PANi-Pt, (e and f) PS/PANi-Pd, (g and h) PS/PANi-Au composite SEM images,140 (i and j) HR-TEM images of the PANI/MnO2/TiO2 nanocomposite,154 and (k) structure of AgClNPs/PANI/D-Dex.69 | ||
Recently, a core–shell type ternary catalyst in which a CNT-supported Pt (Pt/CNT) core was covered with a PANI shell to obtain Pt/CNT@PANI catalyst for the catalytic hydrogenation of Cr(VI). The Pt NPs completely wrapped with PANI exhibited excellent reduction performance against Cr(VI) in a liquid medium because of the redox characteristics of PANI, in which initially the Cr(VI) oxidized PANI and itself turned into Cr(III), followed by the catalytic hydrogenation in which PANI (oxidized) again transformed into PANI (reduced). A reusability of 92.4 was reported after the fourth catalytic recycle, which indicated good stability of the Pt/CNT@PANI.163 PANI-supported ternary metal oxide in which one of the components is MnO2 demonstrated good catalytic reduction ability against organic and inorganic water pollutants because Mn4+ can oxidize and reduce into higher and lower oxidation states. PANI-supported metal oxide ternary nanocomposites containing MnO2 as one component were synthesized and employed for the reductive degradation of pollutants.123,154 PANI/ZnO/MnO2 catalyst prepared by Meena and Saini123 via a three-step process and utilized for the catalytic degradation of 4-NP demonstrated a good catalytic reduction in an aqueous medium in a short time (10 min) due to a synergistic relationship and better stability. In another study, MnO2 containing PANI-supported ternary nanocomposite PANI/MnO2/TiO2 catalyst (Fig. 6(i and j) HRTEM images) was utilized for the catalytic reduction of toxic Cr(VI) ions.154 The composite was produced by employing a one-pot redox polymerization process at ambient temperature. In comparison to binary (PANI/MnO2, MnO2/TiO2, PANI/TiO2) and bare (MnO2, TiO2, PANI), the ternary composite effectively (99.9%) reduced Cr(VI) to Cr(III) in the presence of HCOOH.
Ma and co-workers156 tested a PANI-based ternary nanocomposite Ag3PO4/PPy/PANI as a heterogeneous catalyst for the catalytic reduction of 4-NP and 2-NA using reducing agent NaBH4 and reported that the as-prepared Ag3PO4/PPy/PANI can be used as a potential catalyst for the reductive degradation of water pollutants. Ag NP-supported PANI/MWCNT ternary composite catalyst obtained by the immobilization of Ag NPs on the PANI surface and then added with the MWCNTs showed excellent reduction ability against 4-NP with 0.054 s−1 of rate constant and good antibacterial activity towards pathogenic bacteria, which revealed many uses of Ag NPs-PANI/MWCNTs and showed a wide scope of applications in the healthcare and environmental segments.122 Copper metal NP-based ternary composite catalyst Cu0-PANI-ZrSiO4 synthesized via surface immobilization of Cu(0) NPs on the nanozirconium silicate modified-PANI surface in a solvent-less microwave preparation process and applied for the reduction of various nitrophenols using NaBH4 as a reducing agent that effectively reduced 4-NP, 3-NP, and 2-NP nitrophenols with the reduction rate of 0.811, 0.249, and 0.584 min−1, respectively at pH = 2.153
Prabakaran and Pilla69 integrated AgCl NPs into a PANI-grafted D-dextrose composite via an interfacial polymerization process, and synthesized AgClNPs/PANI/D-Dex (Fig. 6(k)) was applied for the degradation of 4-NP using NaBH4 a reducing agent that displayed remarkable activity and decolorized yellow color of 4-NP in a short time due to reduction into 4-AP. During the degradation process of 4-NP by AgClNP/PANI/D-Dex nanocomposite catalyst, the 4-NP molecules and reducing agent ions were adsorbed on the active center of the catalyst AgCl NPs, which acted as an electron transferring surface. Pd NPs anchored on PANI were supported on mesoporous SBA-15 catalyst (Pd-NPs/PANI/SBA-15) fabricated via an in situ chemical process for catalytic reduction of BrO3− ions. In the catalyst, the Pd NPs were efficiently immobilized on the amino functional groups of the PANI skeleton. The reduction of BrO3− ion was analyzed via an electrochemical process, and during the reduction process, BrO3− ions were diffused from the solution to active sites of Pd NPs, where they were catalytically reduced.164 Ni(OH)2 NPs decorated 2-NSA-doped PANI nanotube (Ni(OH)2@NSA-PANI) nanocomposites (Fig. 7) used for the catalytic reduction of nitroaromatics. The catalyst was prepared by immobilized Ni(OH)2 NPs on the surface of 2-NSA-doped PANI nanotubes. The catalyst effectively degraded (94%) 4-NP with a small amount of catalyst and showed recyclability without declining ability over ten repeating cycles. Ni(OH)2 NPs present in the (Ni(OH)2@NSA-PANI) nanocomposites acted as a reducing surface for the pollutant, and the pollutant ions along with BH4− were adsorbed on Ni(OH)2 NPs, which was the electron relay center for the reduction process.157
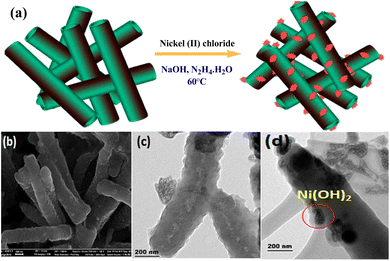 | ||
| Fig. 7 (a) Synthesis process, (b) SEM image, (c) HR-TEM image and (d) STEM image in the dark field of Ni(OH)2@NSA-PANI NCs.157 | ||
Al-saida and coauthors165 reported the synthesis of four component quaternary magnetic core–shell type Fe3O4@TiO2-PANI-Ag nanocomposite catalyst via the polymerization reaction of aniline onto magnetic core–shell structure Fe3O4@TiO2 by APS as an oxidant and then attaching Ag NPs to it. The as-prepared Fe3O4@TiO2-PANI-Ag catalyst was used in the reduction process of 4-NP and MB dye water pollutants. Moreover, >98% catalytic reduction of 4-NP was completed within 20 min when 1 mg of Fe3O4@TiO2-PANI-Ag catalyst was utilized. Besides, it is reported that the Fe3O4@TiO2-PANI-Ag catalyst showed higher competence than that of the binary PANI-Ag, TiO2–Ag, and TiO2/PANI composites due to the synergistic effect of PANI and Ag with TiO2 shell, providing effective support to Ag NPs by PANI and effectively stabilizing them. Additionally, due to magnetic characteristics, the catalyst showed good recovery and reusability. With 2 and 3 mg of composite catalysts within 12 and 6 min of reaction time, the reduction of 4-NP was achieved due to the increase in the density of binding sites of the catalyst. The reduction time was reduced with an increase in the catalyst dose amount.165
Ayad et al.158,159 in their studies reported the synthesis of quaternary composite catalysts of noble metal Pd and Ag NPs by encasing properties of conducting polymer PANI, biopolymer CS, and magnetic material Fe3O4. PANI and CS polymeric materials were utilized as supporting surfaces for noble metal (Pd and Ag) NPs, while magnetic Fe3O4 enhanced the isolation and recovery of the catalysts. The catalyst obtained in a multistep procedure with magnetic features and effective functionalities (hydroxyl and amino) present with CS and PANI effectively coordinated with Pd and Ag ions and enabled their reduction and deposition. Thus, the quaternary Pd@PANI–CS–Fe3O4 (ref. 158) and Ag@PANI–CS–Fe3O4 (ref. 159) nanocomposite catalysts were fabricated via reductive deposition of Pd and Ag NPs, respectively, on the surface of a ternary (PANI–CS–Fe3O4) composite utilized for the catalytic degradation of 4-NP, which showed good reduction potential. Pd@PANI–CS–Fe3O4 catalyst reduced 4-NP within 14 and 8 min of reaction times using 2 mg and 3 mg of catalysts, respectively, while Ag@PANI–CS–Fe3O4 catalyst took 22, 8, and 6 min with 1, 2, and 3 mg of catalysts, respectively.159
In comparison to mono metals, the bimetallic NPs exhibit higher catalytic potential and better permanence.79,136 In this regard, rGO-PANI supported bimetallic (palladium (Pd): gold (Au)) NP composite catalysts developed for the catalytic degradation of numerous organic (RhB, MG, 4-NP, 4-NA) and inorganic (Cr(VI)) water pollutants, which showed improved catalytic ability compared to mono metallic NPs due to better electrostatic attractions between PANI, rGO and (Pd: Au) NPs via a synergistic effect.136 In another study, a bimetallic quaternary yolk shell type Fe3O4@PANI/Ni@Pd composite synthesis was reported for the reduction of o-nitroaniline (O-NA) using NaBH4 as a reducing agent and converted 99% of O-NA within 3 min. Initially, using a solvothermal process, Fe3O4 NPs were prepared, followed by coating the SiO2 shell on the surface of Fe3O4 through the sol–gel process. Then, the PANI layer was introduced by ultrasound-mediated in situ polymerization of aniline and by selective etching of SiO2 layer generated yolk–shell Fe3O4@PANI composite. The Ni@Pd NPs were immobilized on the yolk–shell Fe3O4@PANI composite by reduction of Pd2+ and Ni2+ salts using NaBH4. The prepared Fe3O4@PANI/Ni@Pd exhibited a good conversion efficiency for O-NA for up to eight cycles, demonstrating good stability and reusability.79
Magnetic Fe3O4/PANI/m-SiO2 core/shell spheres were used for the support of Au NPs to prepare highly stable, reusable, robust, and reactive quaternary composite catalyst Fe3O4/PANI/Au/m-SiO2. The Au3+ ions were anchored on ternary Fe3O4/PANI/m-SiO2 core/shell spheres via a reduction reaction that occurred between the metal ions and PANI. The magnetic core and mesoporous m-SiO2 shell appreciably advanced the recycling competence and deeply strengthened the stability of Au metal NPs against aggregation, and PANI provided better attraction and reduced Au3+ ions. The presence of an m-SiO2 shell reduces agglomeration and modifies the stability remarkably because it acted as a barrier against the nucleation of NPs and also worked as a conduit in the mass transfer process of reagents. The Fe3O4/PANI/Au/m-SiO2 showed better activity and stability in the liquid phase, which designated its potential uses as competent heterogeneous catalysts in liquid-phase catalytic reactions. The reduction ability was tested with nitrophenols (4-NP, 3-NP, and 2-NP) and nitroanilines (4-NA, 3-NA, and 2-NA), which were degraded by the order of 4-NP > 2-NP > 3-NP and 4-NA > 2-NA > 3-NA, respectively.99
5. Mechanisms of catalytic reduction of water pollutants in the presence of PANI-based catalysts
PANI nanocomposites have been successfully explored as heterogeneous catalysts for the reductive elimination of numerous water pollutants, such as nitroaromatics (nitrophenols and nitroaniline), organic dyes, and oxyanions. Usually, the reduction of nitroaromatics into the corresponding amino products is kinetically restricted under normal conditions using a reducing agent such as NaBH4 (ref. 102); this barrier cannot be crushed alone by a reducing agent only. Moreover, catalysts alone cannot change nitro compounds into aminophenol products. Thus, it is essential to break down this kinetic barrier that provides a surface for electron reliance, which is possible by adding a catalyst. Thus, the reduction of nitroaromatics and other pollutants occurs on the surface of the utilized catalyst, and the resulting product is desorbed from the surface and discharged in solution. Hence, for the effective catalytic reduction of pollutants, the presence of a suitable catalyst and reducing agent is needed, making the reduction process thermodynamically and kinetically favorable. During reduction reactions, the reducing agent (BH4−) works as an electron donor, while pollutant molecules work as an electron-acceptor centre.1664-NP is a highly toxic nitrophenol that has severe health problems and environmental issues because of its lethal and mutagenic ability in living creatures, including humans.167 The major health impacts of 4-NP are on the central nervous system (CNS), liver, and blood.168 The catalytic reduction of the nitroaromatics into amino products is a significant industrial reaction; amines are the starting materials for numerous synthetic procedures to generate various dyes, pharmaceuticals, agrochemicals, and polymers.169 Moreover, amino-products are moderately less hazardous, can be eliminated more easily, and are more mineralized than those of the nitroaromatics.170 Nevertheless, the catalytic reductions of nitroaromatics, including 4-NP, occur at high-temperature conditions, take longer times and prominently use expensive catalysts. Recently, the use of polyaniline-based catalysts in the catalytic reduction process of 4-NP has gained wide interest.
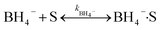
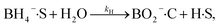
The heterogeneous catalytic reduction process can precede either the L–H (Langmuir–Hinshelwood) or the E–R (Eley–Rideal mechanism). The L–H mechanism proceeds first to adsorb both the reducing agent (BH4−) and reactant (nitroaromatics, dyes) on the catalyst surface; then, the reaction is performed. According to the E–R mechanism, adsorption of one reactant molecule occurs, which reacts with the molecules of another reactant. Additionally, in the L–H pathway, with an increase in pollutant (nitroaromatics, dyes) value, the value of the apparent rate constant (kapp) decreases as the NaBH4 concentration increases. However, in the E–R mechanism, the value of kapp enhances as the 4-NP amount enhances.171 In the literature, it has been reported that the Langmuir–Hinshelwood (L–H) reaction mechanism model is widely followed for the catalytic reduction of nitrophenols and other dye pollutants. In addition to reducing agent NaBH4 and catalyst to the reaction medium, the NaBH4 produced Na+ and BH4− ions, and the produced BH4− ions were adsorbed on the catalyst surface along with pollutant molecules and bound with the active part of catalysts, such as noble metal NPs, metal oxides or hydroxide or chloride NPs, and donated electrons to the active centre. After that, H2 is produced by the reduction of water; then, active hydrogen is generated at the surface of the catalyst.69,172 The generated H2 gas helps in stirring the reaction mixture, enhances the reaction rate, reduces reaction time, and refreshes the catalyst by removing any layer formed by oxide or other impurities on the NP surface by hydrogen flux.166 In this way, due to the transfer of electrons from a donor (reducing agent) to an acceptor (reactant) via the catalyst surface, which acts as a relay centre, and the transfer of the required hydrogen atoms from the catalyst surface to the reactant, the reactant transforms it into its reduced state at the NP catalyst surface. The reduction of 4-NP into 4-AP occurs via the formation of a stable intermediate 4-hydroxyaminophenol, and the produced 4-AP molecules discharged from the catalyst surface provide a free catalyst surface and enter into the next catalytic cycle (Fig. 8). Hydrogen generation by the reduction of water molecules occurs as follows:
| NaBH4 + 2H2O → NaBO2 + 4H2 |
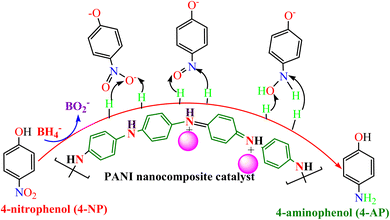 | ||
| Fig. 8 4-NP reduction mechanism in the presence of polyaniline-based nanocomposite catalysts and reducing agent (BH4−). | ||
It is assumed that the adsorption of pollutants and borohydride on the NP surface is reversible and rapid. Additionally, the transformation reaction at the NP surface is slow and a rate-determining stage, and the desorption of the product from the catalyst surface is tacit to be speedy and irreversible; thus, it does not influence the kinetics of the reduction process.173 It is well established from studies that the reduction reactions of nitroaromatics and dyes follow first-order kinetics concerning 4-NP/dye. Hence, the values of kapp for the catalytic reduction reaction are proportional to the catalyst surface area. Thus, it can be determined.167
where kBH4− is the adsorption equilibrium constant for BH4− and kH is the hydrolysis rate constant for BH4−, and S is the catalyst surface.
The reduction of 4-NP into 4-AP via an intermediate 4-hydroxyaminophenol can be represented as follows:
| 4-APS → 4-AP + S |
The adsorption and desorption processes of BH4− and 4-NP at the surface of the catalyst are fast and thus do not influence the kinetics of the reduction process. The slowest step, which is the rate-determining step, is the conversion of 4-NP into 4-AP by active hydrogen species.
The catalytic reduction of 4-NP by Ni(OH)2@NSA-PANI nanocomposite catalyst followed the Langmuir–Hinshelwood mechanism, and the Ni(OH)2 NPs on the NSA-PANI surface acted as electron-acceptors, which reversibly adsorbed hydride onto the NPs from NaBH4, followed by adsorption of the substrate with the expulsion of hydrogen, thereby transferring surface-bound hydride to the –NO2 group of nitro compounds in the rate-determining step of the reduction reaction. The hydrogen atoms on the surface of the nickel hydroxide in the Ni(OH)2@NSA-PANI nanocomposite coordinate with d orbitals of Ni via 1s electron and form metal hydride bonds via chemisorption.157 The catalytic reduction of the mechanism of 4-NP by PANI/Bi2O3 nanocomposite used Bi2O3 as the active centre, which transformed 4-NP into 4-AP via the formation of an intermediate 4-hydroxyaminophenol. The PANI acts as a stabilizer and capping agent preventing the agglomeration of Bi2O3 particles and acts as better hosts that suitably hold the Bi2O3 NPs.117 The reduction of 4-NP by AgClNPs/PANI/D-Dex nanocomposite catalyst occurs on the active centre AgCl NPs, which provides an electron transferring surface from an electron donor to the acceptor and requires hydrogen atoms for reduction of 4-NP to 4-AP via an intermediate 4-hydroxyaminophenol.69
The catalytic reduction mechanism of organic dyes and other pollutants in the presence of PANI-based nanocomposite catalysts and reducing agents is shown in Fig. 9. Dye molecules (MB, RhB, and MG dye) were adsorbed on the catalyst surface and received electrons and hydrogen ions from the reducing agent via the catalyst surface because they transform into nontoxic and colorless leuco forms, which desorb and regenerate catalysts that enter a fresh cycle of catalysis. The catalytic reduction mechanism of toxic Cr(VI) metal ions uses PANI-based nanocomposites in the presence of formic acid.136,154 In the presence of a catalyst, formic acid produces hydrogen that interacts with Cr(VI) ions and transforms into Cr(III) ions.136
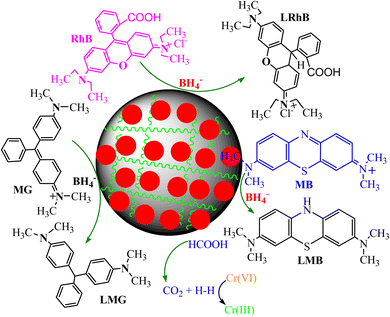 | ||
| Fig. 9 Catalytic reduction process of various water pollutant polyaniline-based nanocomposite catalysts. | ||
6. Conclusion
The catalytic degradation of hazardous water pollutants, such as organic dyes, nitroaromatics, and metal ions, using heterogeneous catalysts is a facile, efficient, inexpensive, rapid, and greener method by which toxic materials can be transformed into less toxic or nontoxic under normal atmospheric conditions. Common heterogeneous nanocatalysts used for the decontamination of water pollutants or other catalysis reactions are metal-based nanoparticles. However, in the pure state, metal-based NPs demonstrate poor activity and stability due to aggregation. To reduce agglomeration and improve the surface area, catalytic activity, and stability, the catalysts can be immobilized with polymeric materials, especially conducting polymers, which provide support and cover shells to NPs. The application of PANI conducting polymers as supporting materials to metal-based NPs has gained wide attention. The prepared metal containing PANI-based nanocomposites via numerous techniques was effectively explored for the catalytic degradation of various water pollutants under ambient conditions. Using PANI as a matrix material, the heterogeneous catalyst suffers from recovery and reusability issues, which can be improved by introducing magnetic characteristics to PANI-based nanocomposites. Magnetic PANI-based nanocomposite catalysts with core–shell structures displayed good stability, recoverability, and activity in the catalysis reactions. In the magnetic core–shell, PANI-based composites with yolk–shell structures were used more efficiently in catalytic reactions due to their high surface characteristics, vacant area, and low density, and yolk–shell nanocomposites made of bimetallic nanoparticles exhibited higher reduction potential ability towards water pollutants. Among the various environmental pollutants, such as organic dyes, nitrophenols, and metal ions, nitrophenols, such as 4-NP, have wide industrial importance, but they exhibit serious mutagenic and hazardous impacts. Thus, the reduction of 4-NP into a less hazardous amino-product has numerous industrial applications. The catalytic degradation process of water pollutants, including nitroaromatics, in the presence of heterogeneous catalysts follows pseudo-first-order kinetics in which the reaction rate depends on the surface area of the catalyst covered by the pollutant and reducing agent. In the reduction reaction, the catalyst acts as an electron relay surface through which electrons are transferred from the reducing agent (electron donor) to the pollutant (electron acceptor). Consequently, the pollutant molecules adsorbed on the catalyst surface are reduced to a nontoxic form. Hence, the synthesis of PANI-based nanocomposite heterogeneous catalysts with improved life span, stability, reusability, and recovery that can display effective reduction ability against a wide range of water pollutants is indispensable for sustainable and eco-friendly water purification techniques.Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Conflicts of interest
The author declares that he has no known competing financial interests.Acknowledgements
Authors dedicate their sincere thanks to Department of Chemistry of Rajasthan, Jaipur (India).References
- S. L. Postel, G. C. Daily and P. R. Ehrlich, Science, 1996, 271, 785–788 CrossRef CAS.
- K. N. Heck, S. Garcia-Segura, P. Westerhoff and M. S. Wong, Chem. Res., 2019, 52, 906–915, DOI:10.1021/acs.accounts.8b00642.
- P. L. Meena, J. K. Saini, A. K. Surela, K. Poswal and L. K. Chhachhia, Biomass Convers. Biorefin., 2024, 14, 1711–1730, DOI:10.1007/s13399-021-02267-2.
- P. L. Meena, J. Geol. Soc. India, 2022, 98, 1455–1465, DOI:10.1007/s12594-022-2193-9.
- S. Yadav, A. Yadav, N. Bagotia, A. K. Sharma and S. Kumar, J. Water Process Eng., 2021, 42, 102148 CrossRef.
- Y. Wang, K. Wang and X. Wang, J. Colloid Interface Sci., 2016, 466, 178–185 CrossRef CAS PubMed.
- X. Li, H. Shi, K. Li and L. Zhang, Front. Environ. Sci. Eng., 2015, 9(6), 1076–1083 CrossRef CAS.
- Y. Zhang, Z. He, H. Wang, L. Qi, G. Liu and X. Zhang, Front. Environ. Sci. Eng., 2015, 9(5), 770–783 CrossRef.
- J. Wang and Z. Bai, Chem. Eng. J., 2017, 312, 79–98 CrossRef CAS.
- S. Khamparia and D. K. Jaspal, Front. Environ. Sci. Eng., 2017, 11(1), 8 CrossRef.
- P. Wang and M. C. Lo Irene, Water Res., 2009, 43, 3727–3734 CrossRef CAS PubMed.
- Z. Xiong, J. J. Ma, W. Ng, T. D. Waite and X. S. Zhao, Water Res., 2011, 45, 2095–2103 CrossRef CAS PubMed.
- H. Zhu, F. Xu, J. Zhao, L. Jia and K. Wu, Environ. Sci. Pollut. Res. Int., 2015, 22(18), 14299–14306 CrossRef CAS PubMed.
- J. Li, H. He, C. Hu and J. Zhao, Front. Environ. Sci. Eng., 2013, 7(3), 302–325 CrossRef CAS PubMed.
- Z. He, M. Hu and X. Wang, Catal. Today, 2018, 302, 136–145 CrossRef CAS.
- X. Chu, G. Shan, C. Chang, Y. Fu, L. Yue and L. Zhu, Front. Environ. Sci. Eng., 2016, 10(2), 211–218 CrossRef CAS.
- S. Komathi, A. I. Gopalan, N. Muthuchamy and K. P. Lee, RSC Adv., 2017, 7(25), 15342–15351 RSC.
- G. Saianand, A.-I. Gopalan, L. Wang, K. Venkatramanan, V. A. L. Roy, P. Sonar, D.-E. Lee and R. Naidu, Environ. Technol. Innovat., 2022, 28, 102698, DOI:10.1016/j.eti.2022.102698.
- F. F. Runge, Ann. Phys. Chem., 1834, 107, 65 CrossRef.
- J. Stejskal and R. G. Gilbert, Pure Appl. Chem., 2002, 74, 857–867 CrossRef CAS.
- G. Ciric-Marjanovic, Synth. Met., 2013, 177, 1–47 CrossRef CAS.
- S. Bhadra, D. Khastgir, N. K. Singha and J. H. Lee, Prog. Polym. Sci., 2009, 34, 783–810 CrossRef CAS.
- D. D. Zhou, X. T. Cui, A. Hines and R. J. Greenberg, In Implantable Neural Prostheses 2: Techniques and Engineering Approaches, Springer, New York, NY, USA, 2009, pp. 217–252 Search PubMed.
- H. T. Das, S. Dutta, R. Beura and N. Das, Environ. Sci. Pollut. Res., 2022, 29, 49598–49631 CrossRef CAS PubMed.
- S. Bhadra, N. K. Singha and D. Khastgir, Polym. Int., 2007, 56, 919–927 CrossRef CAS.
- A. A. Syed and M. K. Dinesan, Talanta, 1991, 38, 815–837 CrossRef CAS PubMed.
- J. Gong, X.-J. Cui, Z.-W. Xie, S.-G. Wang and L.-Y. Qu, Synth. Met., 2002, 129, 187–192, DOI:10.1016/S0379-6779(02)00052-8.
- T. Abdiryim, Z. Xiao-Gang and R. Jamal, Mater. Chem. Phys., 2005, 90, 367–372, DOI:10.1016/j.matchemphys.2004.10.036.
- Y. Chen, E. T. Kang and K. G. Neoh, Appl. Surf. Sci., 2002, 185, 267–276, DOI:10.1016/S0169-4332(01)00817-0.
- A. F. Diaz and J. A. Logan, J. Electroanal. Chem., 1980, 111, 111–114 CrossRef CAS.
- S. K. Mondal, K. R. Prasad and N. Munichandraiah, Synth. Met., 2005, 148, 275 CrossRef CAS.
- C. Nastase, F. Nastase, A. Dumitru, M. Ionescu and I. Stamatin, Compos. Appl. Sci. Manuf., 2005, 36, 481–485 CrossRef.
- X. Jing, Y. Y. Wang, D. Wu and J. P. Qiang, Ultrason. Sonochem., 2007, 14, 75–80 CrossRef CAS PubMed.
- N. Kobayashi, K. Teshima and R. Hirohashi, J. Mater. Chem., 1998, 8, 497–506 RSC.
- N. Kuramoto and A. Tomita, Synth. Met., 1997, 88, 147–151 CrossRef CAS.
- S. Xing, C. Zhao, S. Jing and Z. Wang, Polymer, 2006, 47, 2305–2313 CrossRef CAS.
- J. Y. Kim, J. H. Lee and S. J. Kwon, Synth. Met., 2007, 157, 336–342 CrossRef CAS.
- Q. Guo, C. Yi, L. Zhu, Q. Yang and Y. Xie, Polymer, 2005, 46, 3185–3189 CrossRef CAS.
- P. Dallas, D. Stamopoulos, N. Boukos, V. Tzitzios, D. Niarchos and D. Petridis, Polymer, 2007, 48, 3162–3169 CrossRef CAS.
- J. Chen, D. Chao, X. Lu and W. Zhang, Mater. Lett., 2007, 61, 1419–1423 CrossRef CAS.
- R. V. Parthasarathy and C. R. Martin, Chem. Mater., 1994, 6, 1627–1632 CrossRef CAS.
- J. Choi, S. J. Kim, J. Lee, J. H. Lim, S. C. Lee and K. J. Kim, Electrochem. Commun., 2007, 9, 971–975 CrossRef CAS.
- S. Kobayashi and A. Makino, Chem. Rev., 2009, 109, 5288–5353 CrossRef CAS PubMed.
- V. R. Gowariker, N. V. Viswanathan and J. Sreedhar, Polymer Science, New Delhi, India, New Age International (P) Limited, 1996, 73 Search PubMed.
- J. Stejskal, I. Sapurina, J. Prokeš and J. Zemek, Synth. Met., 1999, 105, 195–202, DOI:10.1016/S0379-6779(99)00105-8.
- N. Kumari Jangid, S. Jadoun and N. Kaur, Eur. Polym. J., 2020, 125, 109485, DOI:10.1016/j.eurpolymj.2020.109485.
- J. L. Camalet, J. C. Lacroix, S. Aeiyach, K. Chane-Ching and P. C. Lacaze, Synth. Met., 1998, 93, 133–142 CrossRef CAS.
- G. Mengoli, M. T. Munari and C. Folonari, J. Electroanal. Chem., 1981, 124, 237–246 CrossRef CAS.
- E. W. Paul, A. J. Ricco and M. S. Wrighton, J. Phys. Chem., 1985, 89, 1441–1447 CrossRef CAS.
- R. L. Hand and R. F. Nelson, J. Electrochem. Soc., 1978, 125, 1059–1069 CrossRef CAS.
- A. M. P. Hussain and A. Kumar, Bull. Mater. Sci., 2003, 26, 329–334 CrossRef CAS.
- L. R. Vargas, A. K. Poli, R. D. C. L. Dutra, C. B. D. Souza, M. R. Baldan and E. S. Gonçalves, J. Aero. Technol. Manag., 2017, 9, 29–38 CrossRef CAS.
- S. Goswami, S. Nandy, E. Fortunato and R. Martins, J. Solid State Chem., 2023, 317, 123679, DOI:10.1016/j.jssc.2022.123679.
- M. J. Silva, J. Gomes, P. Ferreira and R. C. Martins, Water, 2022, 14(5), 1–34, DOI:10.3390/w14050825.
- M. H. Alhaji, K. Sanaullah, A. Khan, A. Hamza, A. Muhammad, M. S. Ishola, A. R. H. Rigit and S. A. Bhawani, Int. J. Environ. Sci. Technol., 2017, 14(9), 2039–2052 CrossRef CAS.
- A. Bouarioua and M. Zerdaoui, J. Environ. Chem. Eng., 2017, 5(2), 1565–1574 CrossRef CAS.
- C. W. Lin, S. Xue, C. Ji, S. C. Huang, V. Tung and R. B. Kaner, Nano Lett., 2021, 21(9), 3699–3707, DOI:10.1021/acs.nanolett.1c00968.
- H. S. Zakria, M. H. D. Othman, R. Kamaludin, S. H. S. A. Kadir, T. A. Kurniawan and A. Jilani, RSC Adv., 2021, 11(12), 6985–7014 RSC.
- N. K. Jangid, S. Jadoun, A. Yadav, M. Srivastava and N. Kaur, Polym. Bull., 2021, 78, 4743–4777, DOI:10.1007/s00289-020-03318-w.
- X. Liu and L. Cai, Appl. Surf. Sci., 2019, 483, 875–887 CrossRef CAS.
- K. Luo, X. Guo, N. Shi and C. Sun, Synth. Met., 2005, 151, 293–296 CrossRef CAS.
- A. A. Athawale, S. V. Bhagwat and P. P. Katre, Sens. Actuators, B, 2006, 114, 263–267 CrossRef CAS.
- A. Arzac, G. P. Leal, R. Fajgar and R. Tomovska, Part. Part. Syst. Char., 2014, 31, 143–151 CrossRef CAS.
- S. Mandal, S. K. Saha and P. Chowdhury, Int. J. Curr. Microbiol. Appl. Sci., 2017, 6, 2309–2321 CAS.
- A. Asatekin, M. C. Barr, S. H. Baxamusa, K. K. Lau, W. Tenhaeff, J. Xu and K. K. Gleason, Mater. Today, 2010, 13, 26–33 CrossRef CAS.
- D. Bharti and S. P. Tiwari, Synth. Met., 2016, 221, 186–191 CrossRef CAS.
- M. R. U. D. Biswas, K. Y. Cho, C.-H. Jung and W.-C. Oh, Process Saf. Environ. Prot., 2015, 126, 348–355 CrossRef.
- X. Zhu, Z. Song, Z. Wang, W. Liu, B. Hong, J. Bao, C. Gao and S. Sun, Appl. Catal., B, 2020, 274, 119010 CrossRef CAS.
- E. Prabakaran and K. Pilla, J. Mol. Liq., 2019, 283, 6–29, DOI:10.1016/j.molliq.2019.03.014.
- S. Liu, B. Yu, S. Wang, Y. Shen and H. Cong, Adv. Colloid Interface Sci., 2020, 281, 102165 CrossRef CAS PubMed.
- K. Dayanidhi, P. Vadivel, S. Jothi and N. S. Eusuff, J. Environ. Manage., 2020, 271, 110962 CrossRef CAS PubMed.
- M. E. Kalkan, Colloid Interface Sci. Commun., 2020, 34(20), 100222, DOI:10.1016/j.colcom.2019.100222.
- R. Das, V. S. Sypu, H. K. Paumo, M. Bhaumik, V. Maharaj and A. Maity, Appl. Catal., B, 2019, 244, 546–558 CrossRef CAS.
- M. Nasrollahzadeh, M. Sajjadi, J. Dadashi and H. Ghafuri, Adv. Colloid Interface Sci., 2020, 276, 102103 CrossRef CAS PubMed.
- S. A. Akintelu, A. S. Folorunso, F. A. Folorunso and A. K. Oyebamiji, Heliyon, 2020, 6, e04508 CrossRef PubMed.
- N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732735 CrossRef PubMed.
- D. Astruc, Nanoparticles and Catalysis, Transition-Metal Nanoparticles in Catalysis: from Historical Background to the State-Of-The Art, Wiley-VCH, Weinheim, 2008 Search PubMed.
- M. Chen, P. Liu, C. Wang, W. Ren and G. Diao, New J. Chem., 2014, 38, 4566–4573, 10.1039/C4NJ00806E.
- M. R. Nabid, Y. Bide, N. Ghalavand and M. Niknezhad, Appl. Organomet. Chem., 2014, 28(6), 389–395, DOI:10.1002/aoc.3133.
- H. Mou, C. Song, Y. Zhou, B. Zhang and D. Wang, Appl. Catal., B, 2018, 221, 565–573 CrossRef CAS.
- O. Ramirez, S. Bonardd, C. Saldias, D. Radic and A. Leiva, ACS Appl. Mater. Interfaces, 2017, 9, 16561–16570 CrossRef CAS PubMed.
- M. Bhaumik, A. Maity and H. G. Brink, J. Colloid Interface Sci., 2022, 611, 408–420 CrossRef CAS PubMed.
- P. Mondal, C. Guo and J. L. Yarger, Arab. J. Chem., 2020, 13, 4009–4018 CrossRef CAS.
- L. Sun, L. Jiang, J. Zhang, T. Murayama, M. Zhang, Y. Zheng, H. Su and C. Qi, Top. Catal., 2021, 64, 215–223, DOI:10.1007/s11244-020-01385-x.
- Y. Wang, Y. Shen, A. Xie, S. Li, X. Wang and Y. Cai, J. Phys. Chem. C, 2010, 114, 4297–4301 CrossRef CAS.
- M. Ma, Q. Zhang, D. Yin, J. Dou, H. Zhang and H. Xu, Catal. Commun., 2012, 17, 168–172 CrossRef CAS.
- K. D. Vorlop and T. Tacke, Chemieingenieurtechnik, 1989, 61(10), 836–837 CrossRef CAS.
- Y. B. Yin, S. Guo, K. N. Heck, C. A. Clark, C. L. Conrad and M. S. Wong, ACS Sustain. Chem. Eng., 2018, 6(9), 11160–11175 CrossRef CAS.
- J. Liu and J. Gao, Environ. Sci. Eng., 2023, 17(2), 26, DOI:10.1007/s11783-023-1626-z.
- Y. Zhang, P. Zhu, G. Li, W. Wang, L. Chen, D. D. Lu, R. Sun, F. Zhou and C. Wong, Nanoscale, 2015, 7, 13775–13783 RSC.
- P. Boomi, H. G. Prabu and J. Mathiyarasu, Eur. J. Med. Chem., 2014, 72, 18–25 CrossRef CAS PubMed.
- X. Sun, H. Su, Q. Lin, C. Han, Y. Zheng, L. Sun and C. Qi, Appl. Catal., A, 2016, 527, 19–29 CrossRef CAS.
- A. Pourjavadi, M. Doroudian, A. Abedin-Moghanaki and C. Bennett, Appl. Organomet. Chem., 2017, 31, e3881 CrossRef.
- J. Lee, J. C. Park and H. Song, Adv. Mater., 2008, 20(8), 1523–1528 CrossRef CAS.
- V. Georgakilas, D. Gournis, V. Tzitzios, L. Pasquato, D. M. Guldi and M. Prato, J. Mater. Chem., 2007, 17, 2679–2694 RSC.
- C. Xu, X. Wang and J. Zhu, J. Phys. Chem. C, 2008, 112, 19841–19845 CrossRef CAS.
- P. L. Meena, J. K. Saini and A. K. Surela, Inorg. Chem. Commun., 2023, 152, 110688, DOI:10.1016/j.inoche.2023.110688.
- B. Zhang, T. Cai, S. Li, X. Zhang, Y. Chen, K. G. Neoh, E. T. Kang and C. Wang, J. Mater. Chem. C, 2014, 2, 5189–5197 RSC.
- J. Han, S. Lu, C. Jin, M. Wang and R. Guo, J. Mater. Chem. A, 2014, 2, 13016 RSC.
- J. Han, R. Chen, M. Wang, S. Lu and R. Guo, Chem. Commun., 2013, 49, 11566–11568 RSC.
- V. Divya and M. V. Sangaranarayanan, Eur. Polym. J., 2012, 48, 560 CrossRef CAS.
- Z. Lu, W. Dai, B. Liu, G. Mo, J. Zhang, J. Ye and J. Ye, J. Colloid Interface Sci., 2018, 525, 86 CrossRef CAS PubMed.
- Z. Zhang, Y. Jiang, M. Chi, Z. Yang, G. Nie, X. Lu and C. Wang, Appl. Surf. Sci., 2016, 363, 578–585 CrossRef CAS.
- J. Yu, W. Guo, M. Yang, Y. Luan, J. Tao and X. Zhang, Sci. China Chem., 2014, 57, 1211–1217 CrossRef CAS.
- N. H. Mack, J. A. Bailey, S. K. Doorn, C. A. Chen, H. M. Gau, P. Xu, D. J. Williams, E. A. Akhadov and H. L. Wang, Langmuir, 2011, 27, 4979–4985 CrossRef CAS PubMed.
- J. Han, L. Li and R. Guo, Macromolecules, 2010, 43, 10636–10644 CrossRef CAS.
- S. K. Arya, A. Dey and S. Bhansali, Biosens. Bioelectron., 2011, 28, 166–173 CrossRef CAS PubMed.
- H. H. Saleh, Z. I. Ali and T. A. Afify, Adv. Polym. Technol., 2016, 35, 335 CrossRef CAS.
- S. K. Pillalamarri, F. D. Blum, A. T. Tokuhiro and M. F. Bertino, Chem. Mater., 2005, 17, 5941 CrossRef CAS.
- K. Takemura, J. Satoh, J. Boonyakida, S. Park, A. D. Chowdhury and E. Y. Park, J. Nanobiotechnol., 2020, 18, 152 CrossRef CAS PubMed.
- N. Shoaie, M. Forouzandeh and K. Omidfar, Microchim. Acta, 2018, 185, 217 CrossRef PubMed.
- P. Chakraborty, Y.-A. Chien, T.-F. M. Chang, M. Sone and T. Nakamoto, Sensors, 2020, 20, 13 CrossRef PubMed.
- W. Jin, X. Huang, H. Cheng, T. Xu, F. Wang, X. Guo, Y. Wu, Y. Ying, Y. Wen and H. Yang, Appl. Surf. Sci., 2019, 483, 489 CrossRef CAS.
- G. N. Abdelrasoul, F. Pignatelli, I. Liakos, R. Cingolani and A. Athanassiou, Composites, Part B, 2018, 149, 178 CrossRef CAS.
- G. Chang, Y. Luo, W. Lu, X. Qin, A. M. Asiri, A. O. Al-Youbib and X. Sun, Catal. Sci. Technol., 2012, 2, 800–806 RSC.
- K. M. Manesh, A. I. Gopalan, K. P. Lee and S. Komathi, Catal. Commun., 2010, 11, 913–918 CrossRef CAS.
- K. J. Kshirasagar, U. S. Markad, A. Saha, K. K. K. Sharma and G. K. Sharma, Mater. Res. Express, 2017, 4, 025015, DOI:10.1088/2053-1591/aa5947.
- G. Joseph, D. Pinheiro, M. K. Mohan and S. D. K. R. Pai, Polym. Bull., 2023, 80, 8467–8481, DOI:10.1007/s00289-022-04457-y.
- H. Huang, C. Li, Q. Zhang, J. Huang, J. Ji, Y. Liu and L. Li, Polym. Compos., 2023, 44(11), 7674–7686, DOI:10.1002/pc.27655.
- G. Wang, S. Yuan, Z. Wu, W. Liu, H. Zhan, Y. Liang, X. Chen, B. Ma and S. Bi, Appl. Organomet. Chem., 2019, e5159, DOI:10.1002/aoc.5159.
- Y. Chen, S. Lu, W. Liu and J. Han, Colloid Polym. Sci., 2015, 293, 2301–2309 CrossRef CAS.
- S. P. Deshmukh, A. G. Dhodamani, S. M. Patil, S. B. Mullani, K. V. More and S. D. Delekar, ACS Omega, 2020, 5, 219–227 CrossRef CAS PubMed.
- P. L. Meena and J. K. Saini, Results Chem., 2023, 5, 100764, DOI:10.1016/j.rechem.2023.100764.
- T. Fatima, S. Husain and M. Khanuja, Chem. Eng. J. Adv., 2022, 12, 100373 CrossRef CAS.
- J. Ma, J. Dai, Y. Duan, J. Zhang, L. Qiang and J. Xue, Renewable Energy, 2020, 56, 1008–1018 CrossRef.
- P. L. Meena and A. K. Surela, Reference Module in Materials Science and Materials Engineering, 2024, DOI:10.1016/B978-0-323-95486-0.00003-X.
- P. L. Meena, J. K. Saini, A. K. Surela, B. Mordhiya, L. Kumari Chhachia and K. S. Meena, ChemistrySelect, 2023, 8, e202300724, DOI:10.1002/slct.202300724.
- P. L. Meena, J. K. Saini, A. K. Surela and K. Poswal, Holist. Approach Environ., 2022, 12(4), 131–143, DOI:10.33765/thate.12.4.1.
- M. Alhoshan, J. Alam, A. K. Shukla and A. A. Hamid, J. Mater. Res. Technol., 2023, 4, 6034–6047, DOI:10.1016/j.jmrt.2023.04.200.
- J. Alam, A. K. Shukla, M. A. Ansari, F. A. A. Ali and M. Alhoshan, Membranes, 2021, 11(1), 25, DOI:10.3390/membranes11010025.
- I. Chajanovsky and R. Y. Suckeveriene, Processes, 2020, 8(11), 1503, DOI:10.3390/pr8111503.
- C. Zhang, S. Govindaraju, K. Giribabu, Y. S. Huh and K. Yun, Sens. Actuators, B, 2017, 252, 616–623 CrossRef CAS.
- Y. Kong, T. Wu, D. Wu, Y. Zhang, Y. Wang, B. Du and Q. Wei, Anal. Methods, 2018, 10, 4784–4792, 10.1039/C8AY01245H.
- A. Khan, A. A. P. Khan, M. M. Rahman, A. M. Asiri, Inamuddin, K. A. Alamry and S. A. Hameed, Appl. Surf. Sci., 2018, 433, 696–704 CrossRef CAS.
- S. Zhao, L. Huang, T. Tong, W. Zhang, Z. Wang, J. Wang and S. Wang, Environ. Sci.: Water Res. Technol., 2017, 3, 710–719, 10.1039/C6EW00332J.
- K. Sivaranjan, O. Padmaraj, J. Santhanalakshmi, M. Sathuvan, A. Sathiyaseelan and S. Sagadevan, Sci. Rep., 2020, 10, 2586, DOI:10.1038/s41598-020-59491-5.
- H. Gul, A.-H. A. Shah, U. Krewer and S. Bilal, Nanomaterials, 2020, 10, 1 CrossRef PubMed.
- Y. Chen, S. Lu, W. Liu and J. Han, Colloid Polym. Sci., 2015, 293, 2301–2309 CrossRef CAS.
- Y. Chen, L. Li, L. Zhang and J. Han, Colloid Polym. Sci., 2018, 296, 567–574, DOI:10.1007/s00396-018-4276-0.
- Y. Li, Y. Hu, S. Ye, Y. Wu, C. Yang and L. Wang, New J. Chem., 2016, 40, 10398 RSC.
- X. Qiao, X. Liu, X. Li and S. Xing, New J. Chem., 2015, 39, 8588–8593 RSC.
- B. Zhang, B. Zhao, S. Huang, R. Zhang, P. Xu and H. L. Wang, CrystEngComm, 2012, 14, 1542–1544 RSC.
- M. Khani, R. Sammynaiken and L. D. Wilson, Catalysts, 2023, 13, 465, DOI:10.3390/catal13030465.
- P. L. Meena, L. K. Chhachhia and A. K. Surela, J. Mol. Struct., 2024, 1303, 137575, DOI:10.1016/j.molstruc.2024.137575.
- P. L. Meena, A. K. Surela, K. Poswal, J. K. Saini and L. K. Chhachhia, Environ. Sci. Pollut. Res., 2022, 29, 79253–79271, DOI:10.1007/s11356-022-21435-z.
- P. L. Meena, A. K. Surela, K. Poswal, J. K. Saini and L. K. Chhachhia, Biomass Convers. Biorefin., 2024, 14, 3793–3809, DOI:10.1007/s13399-022-02605-y.
- P. L. Meena, K. Poswal, A. K. Surela, B. Mordhiya and K. S. Meena, Environ. Sci. Pollut. Res., 2023, 30, 68770–68791, DOI:10.1007/s11356-023-27215-7.
- K. Shang, B. Sun, J. Sun, J. Li and S. Ai, New J. Chem., 2013, 37, 2509–2514 RSC.
- Md. Amir, U. Kurtan, A. Baykal and H. Sözeri, J. Mater. Sci. Technol., 2016, 32(2), 134–141 CrossRef CAS.
- Y. Zhu, X. Zhou, D. Chen, F. Li, T. Xue and A. S. Farag, Sci. China Technol. Sci., 2017, 60, 749–757, DOI:10.1007/s11431-016-0663-0.
- C. Jin, J. Han, F. Chu, X. Wang and R. Guo, Langmuir, 2017, 33(18), 4520–4527 CrossRef CAS PubMed.
- M. S. Najafinejad, P. Mohammadi, M. M. Afsahi and H. Sheibani, Mater. Sci. Eng. C, 2019, 98, 19–29 CrossRef CAS PubMed.
- M. E. Mahmoud, M. F. Amira, M. E. Abouelanwar and S. M. Seleim, J. Mol. Liq., 2020, 299, 112192, DOI:10.1016/j.molliq.2019.112192.
- B. Vellaichamy, P. Periakaruppan and B. Nagulan, ACS Sustainable Chem. Eng., 2017, 5, 9313–9324, DOI:10.1021/acssuschemeng.7b02324.
- L. Sun, X. Sun, Y. Zheng, Q. Lin, H. Su and C. Qi, Synth. Met., 2017, 224, 1–6 CrossRef CAS.
- J. Ma, H. Deng, Z. Zhang, L. Zhang, Z. Qin, Y. Zhang, L. Gao and T. Jiao, Colloids Surf., A, 2022, 632(2), 127774, DOI:10.1016/j.colsurfa.2021.127774.
- V. S. Sypu, M. Bhaumik, K. Raju and A. Maity, J. Colloid Interface Sci., 2021, 581, 979–989 CrossRef CAS PubMed.
- M. M. Ayad, W. A. Amer, M. G. Kotp, I. M. Minisy, A. F. Rehab, D. Kopecky and P. Fitl, RSC Adv., 2017, 7, 18553 RSC.
- M. M. Ayad, W. A. Amer and M. G. Kotp, Mol. Catal., 2017, 439, 72–80 CrossRef CAS.
- Z. Gan, A. Zhao, M. Zhang, W. Tao, H. Guo, Q. Gao, R. Mao and E. Liu, Dalton Trans., 2013, 42, 8597–8605, 10.1039/C3DT50341K.
- J. Liu, S. Z. Qiao, S. Budi Hartono and G. Lu, Angew. Chem., Int. Ed., 2010, 49, 4981–4985 CrossRef CAS PubMed.
- L. Sun, Z. Yin, J. Zhang, X. Ren, M. Zhang, W. Song, Z. Xu and C. Qi, Mol. Catal., 2022, 525, 112362 CrossRef CAS.
- L. Yu, D. Li, Z. Xu and S. Zheng, Chemosphere, 2023, 310(10), 136685, DOI:10.1016/j.chemosphere.2022.136685.
- C. Sun, N. Deng, H. An, H. Cui and J. Zhai, Chemosphere, 2015, 141, 243–249 CrossRef CAS PubMed.
- B. Al-saida, W. A. Amer, E. E. Kandyel and M. M. Ayad, J. Photochem. Photobiol., A, 2020, 392, 112423, DOI:10.1016/j.jphotochem.2020.112423.
- M. I. Din, R. Khalid, Z. Hussain, T. Hussain, A. Mujahid, J. Najeeb and F. Izhar, Crit. Rev. Anal. Chem., 2020, 50(4), 322–338, DOI:10.1080/10408347.2019.1637241.
- A. Serrà, R. Artal, M. Pozo, J. Garcia-Amoros and E. Gom, Catalysts, 2020, 10, 458, DOI:10.3390/catal10040458.
- Z. M. El-Bahy, Appl. Catal., A, 2013, 468, 175–183 CrossRef CAS.
- S. R. Thawarkar, B. Thombare, B. S. Mundec and N. D. Khupse, RSC Adv., 2018, 8, 38384–38390 RSC.
- J. Y. Shen, X. P. Xu, X. B. Jiang, C. X. Hua, L. B. Zhang, X. Y. Sun, J. S. Li, Y. Mu and L. J. Wang, Water Res., 2014, 67, 11–18 CrossRef CAS PubMed.
- S. R. Thawarkar, B. Thombare, B. S. Munde and N. D. Khupse, RSC Adv., 2018, 8, 38384 RSC.
- M. Kohantorabi and M. R. Gholami, Ind. Eng. Chem. Res., 2017, 56, 1159–1167, DOI:10.1021/acs.iecr.6b04208.
- N. Bingwa and R. Meijboom, J. Phys. Chem. C, 2014, 118, 19849–19858, DOI:10.1021/jp505571p.
| This journal is © The Royal Society of Chemistry 2024 |