Open Access Article
Open Access ArticleEfficient phosphate removal utilizing N, Zn-doped carbon dots as an innovative nanoadsorbent†
Mina Alikhania,
Ehsan Khoshkalam *b,
Jalal Sadeghib,
Laura Bulgariuc and
Hossein Eshghi
*b,
Jalal Sadeghib,
Laura Bulgariuc and
Hossein Eshghi d
d
aDepartment of Chemistry, Payame Noor University, Tehran, Iran
bDepartment of Soil Science, Faculty of Agriculture, Ferdowsi University of Mashhad (FUM), Mashhad, Iran. E-mail: e.khoshkalam@alumni.um.ac.ir; Fax: +985138807147; Tel: +989104050217
cDepartment of Environmental Engineering and Management, Cristofor Simionescu Faculty of Chemical Engineering and Environmental Protection, Gheorghe Asachi Technical University of Iasi, 700050 Iasi, Romania
dDepartment of Chemistry, Faculty of Science, Ferdowsi University of Mashhad (FUM), Mashhad, Iran
First published on 5th August 2024
Abstract
The primary goal of this study is to examine PO43− adsorption from aqueous solutions using zinc-doped carbon dots (Zn-N-CDs) as a new adsorbent and cost-effective technique. Zn-N-CDs were produced through a hydrothermal process and subsequently identified using various techniques. The effect of reaction time, temperature, pH, ionic strength, adsorbent dosage, initial PO43− concentration, and anion competition (NO3−, Cl−, HCO3−, and SO42−) on PO43− adsorption using Zn-N-CDs were investigated. The characterization results depicted that Zn-N-CDs have a spherical structure without obvious aggregation and revealed the amorphous nature of carbon dots with many pores. Zn-N-CDs had a high affinity for adsorbing PO43−, reaching equilibrium within 5 minutes. While PO43− adsorption reduced with an increase in temperature, it increased with pH and ionic strength. The optimal conditions for PO43− adsorption were determined to be pH 8, 100 mM KCl as an ionic strength, and 5 g L−1 of Zn-N-CDs. The presence of SO42− and HCO3− as competing anions slightly decreased PO43− adsorption. Thermodynamic studies revealed that PO43− adsorption onto Zn-N-CDs was endothermic, spontaneous, and disordered, as evidenced by ΔG° < 0, ΔH° > 0, and ΔS° > 0. The experimental data fit well with a pseudo-second-order kinetic model (R2 = 0.999) and the Freundlich isotherm model (R2 = 0.9754), signifying that PO43− adsorption onto Zn-N-CDs occurs through multi-layer and chemi-sorption mechanisms. Overall, Zn-N-CDs indicated a great capability to adsorb high concentrations of PO43− across a wide range of pH values, indicating their potential for environmental remediation.
1. Introduction
Agricultural practices, mining activities, and the utilization of synthetic detergents etc. are important sources of the pollutant PO43− in the environment.1,2 Environmental PO43− abundance can be detrimental to humans, leading to kidney damage and osteoporosis.2 Increasing PO43− concentration in surface water promotes the growth of phosphate-dependent species such as algae and duckweed.3 These organisms consume a lot of oxygen and keep sunlight out of the water. This makes the water inhospitable for other species. This phenomenon is often referred to as eutrophication.2,3 Such conditions result in the demise of aquatic life, the production of odors, and the proliferation of harmful organisms.3 Total PO43− can range from 8.5 mg L−1 in landfill leachate to 740 mg L−1 in fresh urine, according to data found in the literature.1,2 In addition, the recommended levels of total PO43− in streams that reach lakes and flowing bodies of water are 0.05 and 0.1 mg L−1, respectively.1–3 Recovering PO43− from aqueous environments could reduce these issues.Over the last few decades, various techniques, including biological, chemical, and physical treatment processes have been utilized to remove PO43−. However, these methods have become expensive and ineffective due to the production of sludge, the introduction of chemical reagents to the environment, and the generation of secondary harmful materials during the treatment process.4 The biological technique, for instance, takes a long time and results in biological sludge. Additionally, some decontamination techniques, including chemical reduction, may produce hazardous byproducts.5 To address these issues, scientists have turned their attention to adsorption treatment for water and wastewater purification. Adsorption techniques have proven to be highly efficient, sensitive, and selective in removing various organic and inorganic pollutants from water and wastewater.1,2,4 Different types of adsorbents, including industrial and agricultural wastes,6 polymeric exchangers,7 EL-MNP@Zeolite,8 La-CTS-ATP,9 hydrotalcite,10 and LDH11 have been used for PO43− removal. However, the high cost of producing and consuming these compounds, the multiple steps involved in the synthesis process, introducing harmful chemicals to the environment and the low efficiency in PO43− adsorption are the disadvantages of such adsorbents.12,13 Therefore, in order to be applicable at full scale, it is desirable to have environmentally friendly adsorbents.
The use of nanotechnology with the ability to produce nanoparticles with superior properties has been considered in surface absorption studies.14,15 Among different nanoparticles, carbon based materials, including carbon dots (CDs) and graphene, have emerged as alternatives to conventional adsorbent materials. Carbon dots have been receiving increased interest due to their distinct optical properties, low toxicity, simple synthesis, extremely small size, high biocompatibility, and cost-effective precursors.13,16,17 Owing to their distinctive physicochemical characteristics and the presence of numerous hydrophilic functional groups, CDs provide a multitude of surface adsorption sites to remove pollutants from the environment.18,19
Carbon dots have been utilized in numerous studies for the removal of heavy metals.20–22 The capability of carbon dots to adsorb heavy metals primarily depends on their chemical properties, particular surface area, pore volumes, and the existence of functional groups.22 Researchers have argued that the adsorption process of metal ions can occur due to physiosorption, electrostatic attraction and chemical sorption.13 Among these reactions, electrostatic attraction is considered pivotal in the adsorption mechanism between carbon dot-based adsorbents and metal ions, primarily due to the abundance of oxygen-containing groups with negative charges on the surface of these materials.13 However, carbon dots have not been used as an adsorbent for removing PO43−, and the reaction mechanism of these compounds with PO43− ions remains unknown.
On the other hand, some research has reported that the combination of metal ions with carbon-based materials and LDH can generate additional surface functional groups and increase the efficiency of PO43− adsorption.1,10,11 For instance, Nakarmi et al.1 by synthesized a zinc oxide-biochar nanocomposite, which removed 265.5 mg g−1 of PO43− from water solutions. In another research, doping Mn2+/Zn2+/Fe3+ oxy(hydroxide) onto LDH, drastically increased the PO43− adsorption, resulting in the adsorption of 82.3 mg g−1 of PO43−. Furthermore, Koilraj and Kannan10 argued that the presence of zinc ions in the ZnAl hydrotalcite could enhance PO43− adsorption during chemi-sorption mechanisms.
Furthermore, the recent study showed that modifying carbon dots with zinc ions can alter their physical and chemical properties.23 Based on our findings, so far no studies have been conducted regarding the adsorption of PO43− by these types of nanoparticles. Therefore, to provide a precise answer to the question of whether the modification of carbon dots with zinc ions has the ability to retain PO43− or not, this study aimed to prepare zinc-doped carbon dots (Zn-N-CDs), as a novel adsorbent for PO43− adsorption from water solutions. The main objectives of this research were: (1) synthesizing and characterization of Zn-N-CDs, (2) evaluating PO43− adsorption using Zn-N-CDs at different pH, electrolyte concentrations, and temperatures to determine the optimum dosage of the adsorbent, (3) investigating the kinetics, isotherms and thermodynamics studies of PO43− adsorption, and (4) understanding the main mechanisms of PO43− adsorption using Zn-N-CDs.
2. Materials and methods
2.1 Materials
ZnCl2 (99%), citric acid (C6H8O7, 99%), urea (CH4N2O, 99%), KCl (99.5%), NaOH (98%), K2SO4 (99%), KH2PO4 (99.5%), KHCO3 (99.5%), and KNO3 (99.5%) were all purchased from Merck, Germany, and are all of analytical grade. Also, double-distilled water was used to create each solution.2.2 Preparation of Zn-N-CDs
The water-soluble Zn-N-CDs were synthesized using the previously described approach.23,24 Briefly, 0.19 g (1 mmol) of citric acid and 0.12 g (2 mmol) of urea were dissolved in 15 mL of deionized water. Subsequently, the mixture was stirred at room temperature for 15 minutes. Then, the solution was added to a 5 mL aqueous solution of ZnCl2 (1 mmol, 0.14 g) and stirred for 30 min. The aqueous solution was then placed in a Teflon autoclave, sealed, and heated for 12 h at 200 °C. The autoclave was then cooled to room temperature. The solution was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min to remove excess reactants. To separate the black, insoluble particles, the resulting brown solution was filtered through a 0.45 μm filter membrane. The solution was then freeze-dried to obtain a dark brown Zn-N-CDs powder. The schematic of Zn-N-CDs synthesis is shown in Fig. S1.†
000 rpm for 20 min to remove excess reactants. To separate the black, insoluble particles, the resulting brown solution was filtered through a 0.45 μm filter membrane. The solution was then freeze-dried to obtain a dark brown Zn-N-CDs powder. The schematic of Zn-N-CDs synthesis is shown in Fig. S1.†
2.3 Characterization of Zn-N-CDs
The infrared spectra of KBr pellets were recorded using a PerkinElmer FT-IR spectrophotometer in the 400–4000 cm−1 region at standard ambient temperature. A field emission scanning electron microscope (FESEM, TESCAN MIRA3), was utilized to examine the surface structure and perform electron dispersive X-ray (EDS) analysis on the Zn-N-CDs. The UV-vis spectrophotometer Shimadzu UV-2550 was utilized for the analysis. Dynamic light scattering (DLS) and zeta potential (Malvern Zetasizer Nano ZS-90) was used to measure the particle size and surface charge of the Zn-N-CDs. The Zn-N-CDs structure was studied using a Zeiss transmission electron microscope (TEM). Measurements of fluorescence (FL) were taken using an F-7000 Hitachi spectrophotometer. The surface area and pore size characteristics of the Zn-N-CDs were determined using a Brunauer–Emmett–Teller (BET) analysis. The measurements were conducted using an ASAP 2020 apparatus (Micromeritics Instrument Corporation, USA), which is an Accelerated Surface Area and Porosity (ASAP) system.2.4 Batch adsorption experiments
In continue, the experiment was done in different pHs (2–12). The pH of solutions was adjusted using HCl/NaOH 0.1 M. Then, the optimum pH was used for further batch experiments. For investigating the effect of ionic strength, the adsorption PO43− was tested at different KCl salt concentrations. The best ionic strength was then selected for determining of adsorbent dosage for PO43− removal. Additionally, the effect of different background solutions, including KCl, NaCl, and CaCl2, on PO43− adsorption was evaluated. Finally, the effect of initial PO43− concentration (50–500 mg L−1) was evaluated under all optimal conditions. After sorption, all samples were centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) and the equilibrium PO43− concentration was measured (using UV-spectrophotometer at a wavelength of 880 nm) at their supernatant.5 The majority of the trials were done in triplicate. eqn (1) and (2) were used to achieve PO43− adsorption in percent (R%) and qt (mg g−1), respectively.5,10
000 rpm, 5 min) and the equilibrium PO43− concentration was measured (using UV-spectrophotometer at a wavelength of 880 nm) at their supernatant.5 The majority of the trials were done in triplicate. eqn (1) and (2) were used to achieve PO43− adsorption in percent (R%) and qt (mg g−1), respectively.5,10
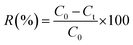 | (1) |
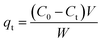 | (2) |
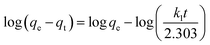 | (3) |
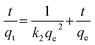 | (4) |
| qt = kpt1/2 + C | (5) |
In these equations, the terms qt, qe, k1, k2, kp, C, and t stand for the amounts of ions adsorbed at time t (mg g−1), equilibrium time (mg g−1), pseudo-first-order rate constant (min−1), pseudo-second-order rate constant (mg min−1), intra-particle diffusion rate constant (mg g−1 min−1), intercept, and time (min), respectively.
In order to determining accurately adsorption capacity of Zn-N-CDs for PO43− removal, adsorption data was fitted to the Freundlich (eqn (6)), Langmuir (eqn (7)), and Temkin (eqn (8)) isotherm models.1
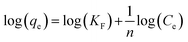 | (6) |
| Ce/qe = Ce/b + 1/KLb | (7) |
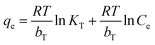 | (8) |
The equilibrium adsorption capacity and concentration of PO43− at equilibrium are denoted by qe (mg g−1) and Ce (mg L−1), respectively. The experimental constants KF and 1/n are used in the Freundlich equation. The terms b (mg g−1) and KL in the Langmuir equation refer to the highest potential for adsorption and the constant of binding energy, respectively. bT, KT are the Temkin isotherm constants. R is the gas constant (J mol−1 K−1) and T is the temperature (K). RT/BT is the heat of adsorption (J mol−1).
The adsorption process of PO43− on the Zn-N-CDs was evaluated by calculating thermodynamics parameters including ΔG° (Gibbs free energy, kJ mol−1), ΔH° (enthalpy change, kJ mol−1), and ΔS° (entropy change, J mol−1 K−1). These parameters were calculated as follow (eqn (9) and (10)).11
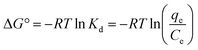 | (9) |
| ln(Kd) = −ΔH°/RT + ΔS°/R | (10) |
3. Results and discussion
3.1 Characterization
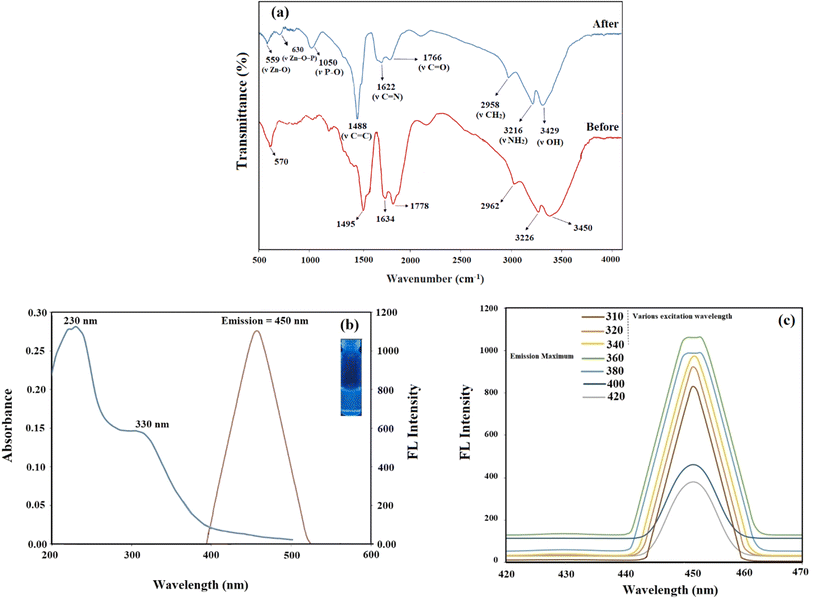
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C
N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.28 Additionally, a band at 570 cm−1 is attributed to the metal-carbon dot stretching vibration (v(Zn–O)).28 These results confirm the presence of functional groups such as –OH, –NH, C–H, C
C bonds.28 Additionally, a band at 570 cm−1 is attributed to the metal-carbon dot stretching vibration (v(Zn–O)).28 These results confirm the presence of functional groups such as –OH, –NH, C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and Zn–O in N-CDs.
C, and Zn–O in N-CDs.
After the PO43− adsorption, there was a shift towards lower wavelengths (Fig. 1a), which could have been caused by a chemical interaction resulting in the formation of complexes containing acidic oxygen and dehydration.29 Adsorption of PO43− significantly reduced the intensity on Zn–O stretching vibration, indicating the interaction between PO43− and the surface functional groups of Zn-N-CDs. Additionally, it was observed that a new band emerged at 1050 cm−1, which was attributed to the presence of new C–O–P bonds in the structure of Zn-N-CDs.11 Additionally, the peak at 630 cm−1 supported the Zn–O–P stretching vibration and demonstrated that P and the Zn-doped carbon dots can interact during of the adsorption process.30
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band and the n → π* transitions of C
C band and the n → π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C
N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in Zn-N-CDs.27,31 Furthermore, the fluorescence emission spectra showed the maximum emission intensity at 450 nm after excitation at 360 nm (Fig. 1b). Optical images of the aqueous Zn-N-CDs solution (inset of Fig. 1b) show the pale blue under 365 nm of UV-light.
O bonds in Zn-N-CDs.27,31 Furthermore, the fluorescence emission spectra showed the maximum emission intensity at 450 nm after excitation at 360 nm (Fig. 1b). Optical images of the aqueous Zn-N-CDs solution (inset of Fig. 1b) show the pale blue under 365 nm of UV-light.Fig. 1c depicts the fluorescence emission spectra of Zn-N-CDs at various excitation wavelengths (310–420 nm). The difference in the excitation wavelength only resulted in a rapid decline in the intensity of the emission wavelength, with no alteration in peak position. Hence, the findings indicate that the excitation wavelength influences the fluorescence intensity of the Zn-N-CDs. Also, this observation demonstrates the remarkably uniform surface structure and narrow size distribution of Zn-N-CDs.32 In addition, the maximum fluorescence intensity was obtained at λex = 360 nm. Furthermore, an assessment of the quantum yield (QY) of the Zn-N-CDs indicated that it exhibited a significantly high QY. The QY of Zn-N-CDs using quinine sulfate (QY = 0.54) as standard was 47.54% at λex = 360 nm. Details about this investigation are listed in Table S1 on ESI.† The quantum yield (QY) achieved for the Zn-N-CDs is deemed satisfactory for analytical applications.
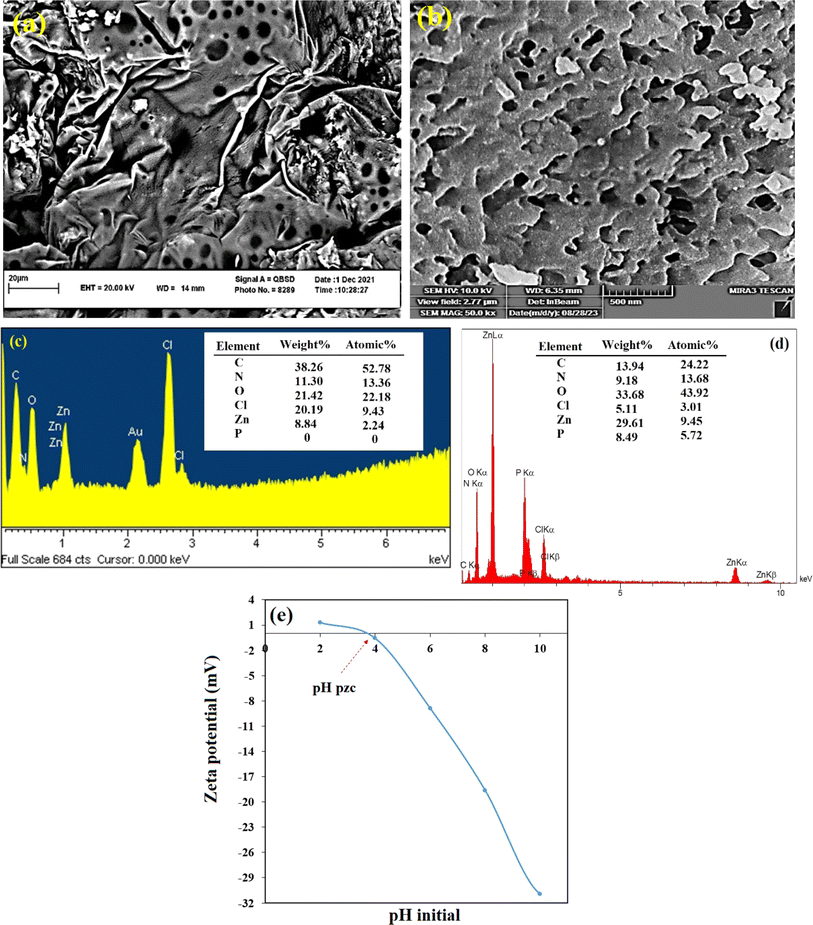
The TEM images of Zn-N-CDs showed spherical particles that were uniformly dispersed without obvious aggregation (Fig. 2S†).33 The diameter of the Zn-N-CDs varied between 4 and 5 nm, and an average diameter of 4.3 nm was determined using the particle size distribution plot and fitting the histogram to a Gaussian model (Fig. 2S†). Also, the particle size of Zn-N-CDs was determined to be about 17 nm by using DLS measurement (Fig. 3S†). The hydrodynamic diameter of particles in a solvent is typically larger than the particle size measured in a vacuum. The presence of a hydration layer on the Zn-N-CDs surface in an aqueous solution leads to an increase in particle size.34
The results of electron disperse X-ray (EDS) analysis before and after adsorption of PO43− (500 mg L−1) are shown in Fig. 2c and d. The results depict that there were decreases in carbon (38.26–13.94 wt%); chlorine (20.19–5.11 wt%) and increases in oxygen (21.42–33.68 wt%) and zinc (8.84–29.61 wt%) contents. After the PO43− adsorption, the reduction of the chloride content occurred due to the anions exchange between PO43− and chloride from the surface of Zn-N-CDs.11 The increase in the weight percentage of zinc (from 8.84 to 29.61 wt%) after PO43− adsorption suggests that some of the zinc ions in the aqueous solution may have been adsorbed onto the surface of the carbon dots along with the PO43−, which will be discuss in the following sections. Furthermore, the presence of P elements (8.49 wt%) and the increase in oxygen percentage (from 21.42 to 33.68 wt%) indicate the adsorption of PO43− ions onto the absorbent surface.
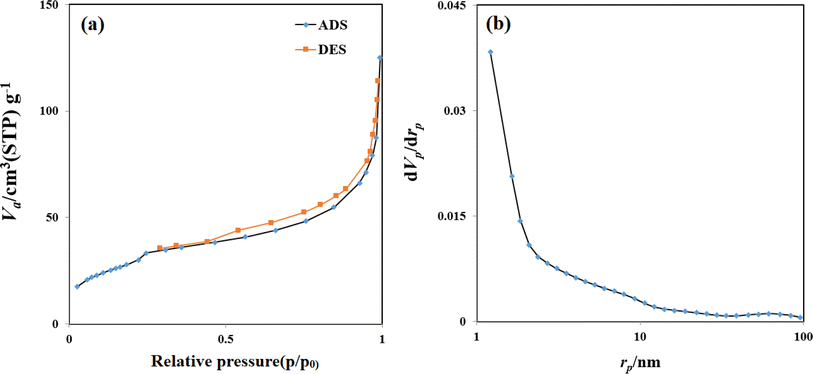
According to the BJH analysis, the carbon dots exhibited a surface area of 107.17 m2 g−1 and a total pore volume of 0.18 cm3 g−1. The average pore size was observed to be 6.76 nm. As shown in Fig. 3a, the adsorption–desorption isotherms can be classified as type IV according to the Brunauer–Deming–Deming–Teller (BDDT) classification, with a prominent hysteresis loop at medium pressure (Fig. 3a). This suggests the presence of a mesoporous structure within the carbon dots.36
The high surface area and mesopores in the Zn-N-CDs provide ample adsorption sites and efficient mass transfer, respectively, which are crucial for effectively removing PO43− from aqueous environments. The mesoporous structure facilitates the diffusion of phosphate-containing species into the porous network, while the large surface area enhances the adsorption capacity of the Zn-N-CDs.
3.2 Kinetic and thermodynamic studies of PO43− adsorption
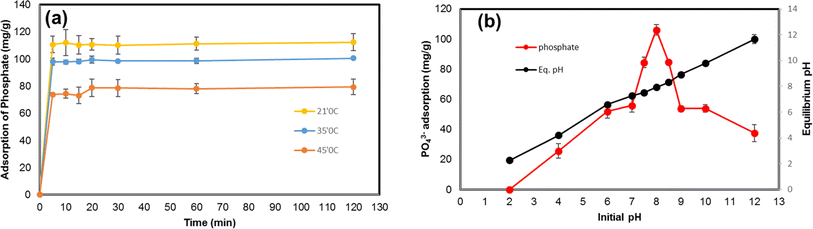
The effect of contact time and temperature on PO43− adsorption using Zn-N-CDs was evaluated (Fig. 4a). PO43− underwent a fast reaction by Zn-N-CDs and the amount of PO43− adsorption at temperatures 21, 35, and 45 °C were 110.62, 98.02, and 73.2 mg g−1, respectively. In fact, the process achieved equilibrium in within 5 minutes, and no additional substantial adsorption was found. This fast reaction could be attributed to high surface area and the structure of Zn-N-CDs. Carbon dots contain numerous active adsorption sites, as indicated by the FT-IR spectrum (Fig. 1a). Furthermore, by doping Zn2+ onto the carbon dots particles and creating a new composite, the number of adsorption sites capable of binding with PO43 increased.11 Hence, PO43− adsorption on Zn-N-CDs exhibits a high reaction rates. Various interactions such as physical adsorption, anion exchange, precipitation, and complexation may account for the rapid adsorption of PO43−,10,11,37 as will be explained later.The experimental results were described by three noticeable kinetic models (Table 1). A key element in establishing which kinetic model is suitable for explaining experimental results is the regression coefficient (R2).5 As a result, the high regression coefficient (0.999) pseudo-second-order model was better suited to describe data. At 21, 35, and 45 °C, the regression coefficients of the pseudo-second-order model were significantly higher than those of the pseudo-first-order and intra-particle diffusion kinetic models. This strong match indicates that chemisorption interactions which entail the creation of chemical bonds between the adsorbate and the adsorbent are primarily responsible for the PO43− adsorption onto Zn-N-CDs.8,9 Furthermore, comparing the adsorption capacity predicted by the kinetic models (qe.cal) with the adsorption capacity determined from experimental data (qe.exp) is also important.5 Table 1 shows that the qe.cal values for the pseudo-second-order at temperatures 21, 35, and 45 °C are 112.36, 100, and 79.37 mg g−1, respectively, which are much closer to qe.exp values obtained from experimental data. In addition, at 21 °C, 35 °C, and 45 °C, the pseudo-second-order kinetic model's rate constants (K values) were found to be 0.038, 0.033, and 0.023, respectively. The pseudo-second-order model's lower K values emphasize the relative speed of the adsorption process in addition to demonstrating the model's adequacy in explaining the experimental results.8 Therefore, the high regression coefficient, adsorption capacity (qe.cal), and lower K values of the pseudo-second-order indicate that the PO43− adsorption by Zn-N-CDs follows the pseudo-second-order kinetic equation. Comparable results for green-synthesized iron nanoparticles,8 Zinc Oxide Betaine-Modified Biochar Nanocomposites (ZnOBBNC),1 halloysite nanotubes (HNTs),3 and Mg-Al-LDH11 have been documented in the literature.
| Pseudo-first-order | Pseudo-second-order | Intraparticle diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | qe.exp | qe.cal (mg g−1) | K1 (min−1) | R2 | qe.cal (mg g−1) | K2 (g min−1 min−1) | R2 | C | Kp (mg g−1 min−1/2) | R2 |
| 21 | 110.62 | 5.37 | −0.0003 | 0.1615 | 112.36 | 0.038 | 0.999 | 65.553 | 6.6668 | 0.3305 |
| 35 | 98.02 | 7.09 | −0.0003 | 0.2271 | 100 | 0.033 | 0.999 | 57.902 | 6.0064 | 0.3393 |
| 45 | 79.46 | 10.84 | −0.0004 | 0.4255 | 79.37 | 0.023 | 0.999 | 43.45 | 4.9799 | 0.3841 |
| T (K) | ΔG° (KJ mol−1) | ΔH° (KJ mol−1) | ΔS° (J mol−1 K−1) | |||||||
| 294 | −16.16 | 0.163 | 54.14 | |||||||
| 308 | −15.87 | |||||||||
| 318 | −14.8 | |||||||||
Table 1 shows the thermodynamic parameters of PO43− adsorption onto Zn-N-CDs. The negative values of ΔG°, at 21 °C, 35 °C, and 45 °C specify that the PO43− adsorption onto Zn-N-CDs is a spontaneous process. This means that the process may occur without the need for external energy input, emphasizing an essential aspect of efficient adsorption processes. As the temperature increased, the ΔG° values decreased, indicating that higher temperatures were less favorable for adsorption. The endothermic feature of adsorption was confirmed by the positive result of ΔH° (0.163). The increase in randomness at the solid/solution interface during the adsorption of PO43− onto Zn-N-CDs is indicated by the positive value of ΔS° (54.14).8,11,38,39 As is common for adsorption events where new configurations between adsorbate and adsorbent are produced, these results suggest that the adsorption process is not only energy-dependent (endothermic) but also leads to a more disordered state at the interface.1,8,11 These finding also align with the adsorption of PO43− onto other adsorbents, such as Mg-Al-LDH,11 IONP,38 FeMg2Mn-LDH,39 and green-synthesized iron nanoparticles.8
3.3 Effect of pH on PO43− adsorption and possible mechanisms
A variation of pH not only could change the speciation of ions in the solution but also can affect the surface chemistry of the adsorbent. The evaluation of this phenomenon can illustrate the absorbent behavior in the natural environment. For this reason, the effect of pH on PO43− adsorption onto Zn-N-CDs is investigated and depicted in Fig. 4b. At acidic pH (≈2) no significant adsorption of PO43− was detected. Moreover, by increasing pH, the PO43− adsorption dramatically increased and the maximum adsorption was reached 105.96 mg g−1 at pH 8. After pH 8, the process of PO43− adsorption decreased drastically. For explanation of reactions between adsorbent and adsorbate, some factors should be noted, including: (1) physicochemical structure of Zn-N-CDs, (2) speciation of PO43− ions in solution, and (3) the role of zinc ions in the reaction.As FT-IR spectrum (see Fig. 1a), Zn-N-CDs contain numerous active adsorption sites such as –OH, –NH2, and zinc hydroxide. These functional groups have a decisive role in PO43− adsorption.1,4,10,11 In acidic pH, the functional groups can be protonated, which helps to retain PO43− electrostatically.1 However, the surface zeta potential of the adsorbent at different pH (Fig. 2e) depicts that the Zn-N-CDs contain negligible surface positive charge at pH 2 and at pH 4 (also known as pHpzc), and the net surface charge is almost neutral. Therefore, the capability of the adsorbent to adsorb PO43− electrostatically, at highly acidic pH, is relatively low and the Zn-N-CDs, in a wide variety of pH, including negative surface charge. It means that increasing pH accelerates the deprotonation of the functional groups of the adsorbent, and the net surface charge is negative. For this reason, electrostatic attraction might not be the main mechanism to remove PO43− from the solution. On the other hand, by increasing pH and deprotonation of the surface functional groups, it is expected that the electrostatic repulsion force between PO43− and Zn-N-CDs increases, which can reduce the adsorption of PO43− on the negative surface charge of particles. However, by increasing pH, the amount of adsorption dramatically increased. Therefore, to explain this occurrence, other possible interactions between adsorbent and adsorbate should be mentioned.
Accordingly, the distribution of PO43− species in a solution as a function of pH is crucial (eqn (11)–(13)).3,7 At pH = 2, the dominant species is H3PO4. Meanwhile, the H3PO4 is uncharged, the low adsorption capacity at highly acidic pH could attributed to this phenomenon.1,9 By increasing pH, the concentration of H2PO4− and HPO42− gradually increase. Since, the net surface charge of Zn-N-CDs is negative, PO43− adsorption mainly occurs through chemisorption interactions,1,10,40 which will be discussed in the following lines.
| H3PO4 ↔ H2PO4− + H+pKa1 = 2.15 | (11) |
| H2PO4− ↔ HPO42− + H+pKa2 = 7.2 | (12) |
| HPO42− ↔ PO43− + H+pKa3 = 12.35 | (13) |
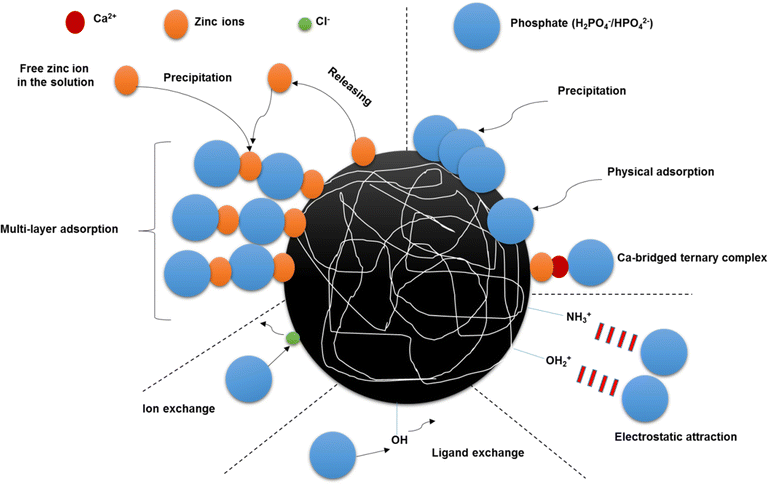
Generally, metal oxide (hydroxides) have high affinity to adsorb PO43− ions.10 PO43− could react with zinc hydroxides through ligand exchange. In addition, active sites of zinc hydroxides and PO43− ions act as Lewis base and Lewis acid, respectively. As a result of these reactions, Zn–O form bonds with oxygen ions of PO43−. Consequently, monodentate-mononuclear (MM), bidentate-mononuclear (BM), and bidentate-binuclear (BB) inner-sphere complexes could be formed (eqn (14)–(16)).10,11 The fact that PO43− adsorption increases with increasing pH might therefore be due in part to these processes. Additionally, inner–sphere complex interactions could raise the equilibrium pH by releasing OH ions into the solution.2,11 However, based on Fig. 4b, the equilibrium pH remained steady during the adsorption process. This discrepancy might be explained through the role of zinc and PO43− ions in the solution. Exploring these roles could unravel the main mechanisms of PO43− adsorption.
| ≡Zn–OH + H2PO4− ↔ ≡Zn–H2PO4− + OH− (MM) | (14) |
| ≡Zn–(OH)2 + HPO42− ↔ ≡Zn–HPO4 + 2OH− (BM) | (15) |
| (≡Zn)2–OH + HPO42− → (≡Zn)2–HPO4 + OH− (BB) | (16) |
Most likely, a portion of the zinc cations might release into the solution from the carbon dots structure. The EDS spectrum (see Fig. 2c and d) also confirmed that the percentage of zinc ions in the solution, during the adsorption of PO43−, was increased. Free zinc cations are unstable in the solution, which are quickly surrounded by water molecules, resulting in the formation of water–zinc complexes. Proton ions generated by these processes could buffer the solution (eqn (17) and (18)).41 On the other hand, dissociation of PO43− ions, due to increasing pH, might help to buffer the system.7
| [Zn(H2O)6]2+ + H2O ↔ Zn(OH)aq+ + H+ | (17) |
| Zn(OH)aq+ + H2O ↔ Zn(OH)2(aq) + H+ | (18) |
By improving pH from 2 to 8, the concentration of zinc species in the solution such as Zn(OH)+ and Zn(OH)2 gradually increase.41 The adsorbed PO43− on the Zn-N-CDs surface, might act as a new adsorption site for zinc hydroxides in the solution forming a surface precipitate. Meanwhile, the zinc ion that has been adsorbed could play as a sorption site for the PO43− which is still in the solution.10,11 As a result of these reactions (anion exchange, complexation, and precipitation), hopeite (Zn3(PO4)2·4H2O) can develop on the surface of Zn-N-CDs structure.10 In addition, there is the possibility of ion exchange between PO43− in the solution and chloride ions that are adsorbed onto the Zn-N-CDs (eqn (19)) [5]. These interactions and possibilities indicate that the adsorption of PO43− using the synthesized particles might occurred through multi-layer adsorption, which will be discussed in the Sections 3–6. Furthermore, in another study, when Zn-Mg-Al-LDH was utilized to remove PO43, zinc ions played a crucial role in the buffering of a system and multi-layer adsorption of PO43−.
| ≡2CD–Zn–OH + ZnCl2 + 3H2PO4− → ≡3CD–Zn–H2PO4 + 2Cl− + 2OH− | (19) |
The results of FT-IR spectrum, FESEM, and EDS spectrum confirmed these potential mechanisms. Moreover, PO43− can react with monovalent and divalent cations in the solution and form precipitation on the surface of Zn-N-CDs, which will be discussed in the following section. After reaching pH 8, the amount of PO43− adsorption drastically decreased. Indeed, in basic pH, the functional groups on the surface of Zn-N-CDs are dissociated, leading to an increase in the negative charge on the surface. As a result, the adsorption of PO43− is reduced due to the enhanced repulsion between the negatively charged surface of Zn-N-CDs and the phosphate ions.1,9 Furthermore, OH− ions in the basic pH, compete with PO43− ions on active adsorption sites.9 Other research also confirmed this possibility. When HA520E-Fe (hybrid polymeric anion exchanger impregnated with hydrated Fe(III) oxide) was used to remove PO43−, by increasing pH, the adsorption process also increased; however, after pH 7 the efficiency of PO43− adsorption drastically decreased.7 By explaining the above phenomena and clarifying the interactions, the probable mechanisms of PO43− adsorption on Zn-N-CDs are illustrated in Fig. 5.
3.4 Effect of monovalent and divalent cations on PO43− adsorption
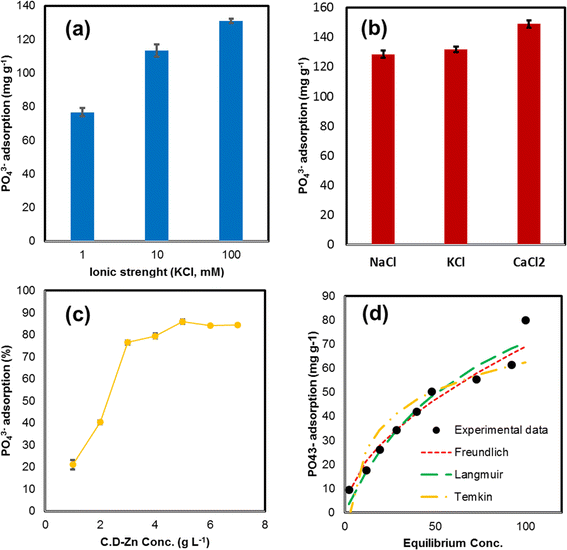
The background solution, by affecting the thickness of the electrical double layer of an adsorbent and increase/decrease of surface charge, has a decisive role in interactions between adsorbent and adsorbate. The evaluation of these situations could unravel the behavior of the adsorbent in the natural environment. For this reason, PO43− adsorption was investigated in a background solution of 10–100 mM KCl (Fig. 6a). The results from the experiments indicate that the adsorption increases by increasing electrolyte concentration and high PO43− adsorption capacity (130.93 mg g−1) occurred at 100 mM KCl concentration. The presence of K+ ions in the reaction could change the electric potential at the interface of Zn-N-CDs. Indeed, through increasing electrolyte concentration and adsorption of K+ ions on the surface of Zn-N-CDs, the thickness of electrical double layer (EDL) decreases.40,42,43 Therefore, the electrostatic repulsion between PO43− ions and the charged surface decline, which favors adsorption and formation of inner-sphere complexations.40,42,43 Furthermore, K+ ions that adsorbed on the surface of Zn-N-CDs, can act as a bridge (between PO43− and the charged surface) to enhance the adsorption process through outer-sphere complexation.40,43 Also, in previous research, by increasing the concentration of KNO3, as a background solution, PO43− adsorption increased.40In addition, the PO43− adsorption capacity on Zn-N-CDs was evaluated in different background solution (concentration = 100 mM) including NaCl, KCl and CaCl2 salts (Fig. 6b). The results show that the PO43− adsorption in the presence of CaCl2 > KCl > NaCl. The adsorption capacity of PO43− in the CaCl2, KCl, and NaCl were acquired 148.89, 131.71, and 128.46 mg g−1, respectively. The surface charge density of the cations could explain this occurrence, which Na+ > K+ > Ca2+ has a surface charge density, respectively. Therefore, when these cations expose into the solution, they are surrounding with water molecules and from H2O-cations complexes. Any cation that has a lower surface charge density has a relatively smaller hydration radius. This means that the cation with a smaller hydration radius can better approach the surface of the adsorbent and neutralize the negative surface charge of the particles. The neutralization of the negative charge on the surface means the decline of the thickness of the electric double layer (EDL) of the absorbent, and also the reduction of the electrostatic repulsion force between the absorbent and the adsorbate.42,44 Ca2+ compared to K+ and Na+ has lower hydration radius, which at the solid–solution interface could decrease surface negative charge and electrostatic repulsion forces. Therefore, PO43− ions can easily approach the surface of the adsorbent to form chemical adsorption.44 This reaction was confirmed in a previous study by Zong et al.2 who investigated PO43− adsorption onto SCBC-La. They reported that increasing NaCl concentration as a background solution did not increase the adsorption capacity of PO43−. However, in another study, when Ca2+ ions were chosen as a background solution, the PO43− adsorption improved.42
As mentioned before, by decreasing the electrical double layer and approaching PO43− to the charged surface, the adsorption capacity increased. This issue leads to cascades of reactions including electrostatic adsorption, precipitation, and complexation.44 Based on literature, metal oxyhydroxides have high affinity for adsorbing Ca2+.42 The adsorption of Ca2+ on charged surface could stimulate the adsorption of PO43− and vice versa.42 The adsorbed Ca2+ by producing surface positive charge increase the electrostatic adsorption of PO43−. Furthermore, the Mendez & Hiemstra44 argued that ternary complex formation could occur on the surface of metal oxyhydroxides. Indeed, Ca2+ act as a bridge and cation-bridged ternary complex (M–Ca–PO4) could develop on the surface. On the other hand, by increasing pH, the PO43− surface speciation can change from monodentate-protonated to bidentate-deprotonated leading to increase the surface negative charge.42,44 Consequently, PO43− act as a bridge and PO4-bridged ternary complex could form (M-PO4-Ca).44 Furthermore, interaction of Ca-PO4 increase with rising pH and solid phase can precipitate as octa-Ca-PO4 (Ca4H(PO4)3·2.5H2O), β-tri-Ca-PO4 (Ca3(PO4)2), and hydroxyapatite (HPA) on the surface of Zn-N-CDs.44 Additionally, some researches claimed that both ions, Ca2+ and PO43−, are bonded monodentately to the charged surface, but there is also an additional chemical reaction (lateral interaction) between the adsorbed cations and anions.44,45
3.5 Effect of adsorbent dosage
The optimal level of Zn-N-CDs dosage for PO43− adsorption is illustrated in Fig. 6c. The experimental results indicate that by increasing the adsorbent dosage, due to enhancement specific surface area and active adsorption sites, the PO43− adsorption increased, and by using 5 g L−1 of adsorbent, 85.81% of initial PO43− concentration (500 mg L−1) was adsorbed (maximum adsorption). However, the adsorption capacity at higher concentrations of Zn-N-CDs dosage remains steady. This behavior is due to the large number of Zn-N-CDs active sites compared to the PO43− concentration. In addition, this may be due to the PO43− mass transfer resistance at high adsorbent dosages from the bulk liquid to solid surface sites.1,43 Acquiring the optimal dosage of adsorbent could help avoid over-consumption of Zn-N-CDs and increase the adsorbent efficiency in aquatic and terrestrial environments.46 Therefore, 5 g L−1 of Zn-N-CDs was chosen as the optimal dosage for further experiments.3.6 Adsorption isotherms
Adsorption isotherm models can explain the interactions between adsorbent and adsorbate. Therefore, in order to describe and clarify the adsorption mechanism, the isotherm models such as Freundlich, Langmuir, and Temkin were applied in different initial PO43− concentrations (50–500 mg L−1). The Freundlich model indicates that the adsorption process is multi-layer, which happens on heterogeneous sites. This model deduces that there are non-homogeneous affinities and heat distribution of adsorption toward the heterogeneous surface.9,43,47 Unlike the Freundlich model, the Langmuir isotherm model has some assumptions, monolayer adsorption occurs on homogenous sites, and adsorption energy is constant. In addition, in this model, no lateral interaction happens between adsorbed molecules.9,43,47 The Temkin isotherm model also presumes multi-layer adsorption; however, it cannot be utilized for very low and high concentration values. Furthermore, the Temkin model proposes that all of the molecules in the layer experience a linear decrease in heat of adsorption.47 As shown in the experimental data (Fig. 6d), PO43− adsorption capacity using Zn-N-CDs increased with increasing initial PO43− concentration. The adsorbent indicated the high adsorption capacity under optimal condition. These data depict that the Zn-N-CDs can be used to remove PO43− from aquatic solutions, both at low and high concentrations. Based on Langmuir model, the maximum adsorption capacity is 122.417 mg g−1. This value is higher than that of some other adsorbents discussed in Section 3.8.Based on the data obtained from the isotherm models (Table 2), the experimental data well fitted to the Freundlich model when R2 is 0.9754, and this value is higher than the R2 values of the Langmuir (R2 = 0.906) and the Temkin model (R2 = 0.8368). As mentioned above, the Freundlich model suggests that the surface of Zn-N-CDs is heterogeneous and that the adsorption of PO43− ions occurs in multiple layers.43 Additionally, the high value of KF (5.477) in the Ferundlich model indicates that PO43− could easily adsorb onto Zn-N-CDs. Furthermore, the 1/n value in the Ferundlich model indicates the adsorption intensity or surface heterogeneity. When 1/n > 1 and 1/n = 1, it presumes that the adsorption process is unfavorable and irreversible, respectively.43,47 However, when 0 < 1/n < 1 demonstrates that the adsorption process is favorable even at high concentrations of PO43−. Constantly, these obtained data specify that the Ferundlich model might clarify the adsorption mechanism. Since this model assumes multilayer adsorption, this assumption can confirm the previously discussed adsorption mechanisms (Section 3.3). As we mentioned before, PO43− ions adsorb onto the surface of Zn-N-CDs via electrostatic and/or ligand exchange. Furthermore, the adsorbed PO43− ions could serve as new adsorption sites for zinc ions in the solution. These processes can continue with PO43− adsorption by adsorbed zinc ions. These reactions involve multi-layer adsorption procedures, which are consistent with the information provided by the Freundlich model.
| Isotherm models | ||||||||
|---|---|---|---|---|---|---|---|---|
| Freundlich | Langmuir | Temkin | ||||||
| KF (mg g−1) | 1/n | R2 | Qmax (mg g−1) | KL (L mg−1) | R2 | BT (KJ mol−1) | KT | R2 |
| 5.477 | 0.550 | 0.9754 | 122.417 | 0.014 | 0.906 | 16.999 | 0.395 | 0.8368 |
3.7 The effect of competing anions
Evaluating the adsorption selectivity of Zn-N-CDs for practical applications is important. Therefore, the effect of coexisting anions including NO3−, SO42−, HCO3−, and Cl− on PO43− adsorption using 5 g L−1 of Zn-N-CDs was investigated (Fig. 7). The results showed that 85.22% of initial PO43− concentration (5.2 mM) was adsorbed in the blank situation (without competing anions), and in the presence of competing anions (NO3−, SO42−, HCO3−, and Cl−), this amount reached around 74.58%. Additionally, SO42− and HCO3− competed with PO43− ions on active adsorption sites, resulting in the removal of approximately 76.36% and 93.67% of these anions from the solution, respectively. Furthermore, no significant adsorption of NO3− and Cl− was detected. The data indicates that Zn-N-CDs have a high PO43− adsorption capacity in the presence of other anions and the slight decrease in PO43− adsorption could be attributed to the competition between PO43− and coexisting anions (SO42− and HCO3−) on the charged surface. Additionally, a recent study evaluated PO43− adsorption using LA-CTS-ATP and the researchers reported that SO42− had no significant change on PO43− adsorption. Furthermore, HCO3− drastically decreased the amount of adsorption of PO43−.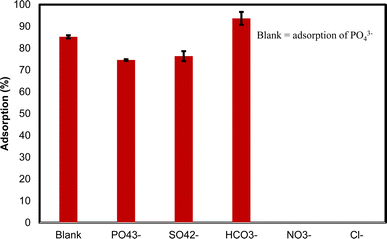 | ||
| Fig. 7 The effect of competing anions on PO43− adsorption ([Zn-N-CDs] = 5 g L−1, [PO43−] = [HCO3−] = [SO42−] = [NO3−] = [Cl−] = 5.2 mM, I = 100 mM KCl, shaking time = 5 min, 21 °C). | ||
Moreover, the occupying of active adsorption sites by PO43−, SO42−, and HCO3− through chemisorption, especially PO43− ions, could increase the net negative surface charge of the nanoparticles, which leads to the increasing electrostatic force repulsion between adsorbent and adsorbate. While ions such as NO3− and Cl− have electrostatic adsorption, and this kind of adsorption is highly sensitive to the presence of cations, anions, and concentration of media solution, the increase of negative surface charge prevents the adsorption of NO3− and Cl− ions.5 In addition, in other studies, NO3− and Cl− ions have been reported not to be a good competitor for PO43− adsorption due to outer-sphere complexes.5,11 On the other hand, some literature has reported the presence of coexisting anions, resulting from increasing ionic strength and declining the thickness of electrical double layer, could increase PO43− adsorption.2,5 As mentioned before, PO43− can adsorb through monodentate-mononuclear (MM), bidentate-mononuclear (BM), and bidentate-binuclear (BB) inner-sphere complexes. These interactions not only occupy the active adsorption sites but also increase net negative surface charge, which improves the colloidal stability of nanoparticles in an environment. Since the Zn-N-CDs have a high capability to adsorb PO43−, even in the presence of competing anions, this phenomenon shows that the Zn-N-CDs are promising nano-adsorbents for application in water and wastewater treatment.
3.8 Comparative studies
Table 3 compared the maximum PO43− adsorption capacity using Zn-N-CDs with the other PO43−adsorbents previously described in the literature. The adsorption capacity is greater than other sorbents such as Feryhidrate,43 La-CTS-ATP,9 EL-MNP@Zeolite,8 SCBC-La,2 Ca-BC,48 CTAB-modified IONP,38 Mn2+/Zn2+/Fe3+/Mg-Al-LDH composite,11 and ZnAlZr4-HT.10 On the other hand, Zn-N-CDs adsorption capacity is lower than ZnOBBNC1 and silica and ligand embedded composite.4 However, it appears that the synthesis of Zn-N-CDs is significantly simpler, more affordable, and more environmentally friendly. The maximum PO43− adsorption capacity by Zn-N-CDs was obtained in alkaline pH. This indicates the capability of Zn-N-CDs to remove PO43− in wide ranges of pH. In addition, the removing of PO43− in basic pH reveals that Zn-N-CDs can be used in aquatic and terrestrial environments. Zn-N-CDs also have a more rapid equilibrium time when compared to other adsorbents, which demonstrates the potential of this sorbent for the purification process.| Materials | Maximum adsorption capacity (mg g−1) | pH | Equilibrium time (min) | References |
|---|---|---|---|---|
| ZnOBBNC | 265.50 | 7 | 15 | 1 |
| Ferrihydrite | 50 | 7 | 120 | 43 |
| La-CTS-ATP | 102.9 | 5 | 50 | 9 |
| EL-MNP@Zeolite | 59.88 | 2–8 | 15 | 8 |
| Silica and ligand embedded composite | 159.12 | 4 | 60 | 4 |
| SCBC-La | 58.8 | 3 | 1080 | 2 |
| Ca-BC | 13.61 | 8.5 | 4320 | 48 |
| CTAB-modified IONP | 18.69 | 2 | 90 | 38 |
| Mn2+/Zn2+/Fe3+/Mg-Al-LDH composite | 82.3 | 7.5 | 60 | 11 |
| ZnAlZr4-HT | 91 | 2.3 | 120 | 10 |
| Zn-N-CDs | 122.417 | 8 | 5 | Current study |
4. Conclusion
The results of this research demonstrate that the modified carbon dots, Zn-N-CDs, have a high capability to remove PO43− from aqueous solutions. The key findings are summarized as follows: (i) The adsorption of PO43− was strongly pH-dependent, with the highest adsorption capacity observed at pH 8. Increasing the ionic strength, up to around 100 mM KCl, significantly enhanced the adsorption capacity, with up to 78.82% of PO43− removed. (ii) The adsorption process was found to be endothermic and spontaneous in nature. (iii) Zinc ions played a crucial role in buffering the solution and increasing PO43− removal. The PO43− was adsorbed in multi-layers on the surface of Zn-N-CDs through ligand exchange and precipitation reactions.Overall, the data presented in this study indicate that Zn-N-CDs could be a promising material for the removal of PO43− from aquatic and terrestrial environments. However, the low yield and challenges in obtaining carbon dots as a solid powder due to their hydrophilic surface groups pose limitations on their large-scale use. Further research is needed to fully explore the potential of carbon dots in this field. Future studies should focus on assessing the impact of carbon dots on various biological species, such as microbes and aquatic organisms, in field-scale trials. Additionally, the effectiveness of phosphate removal by carbon dots in the presence of other impurities and potential compounds that may be present in real wastewater, such as organic matter and heavy metals, should be investigated.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Mina Alikhani and Ehsan Khoshkalam designed and performed experiments and did all the data analysis and wrote this manuscript. Jalal Sadeghi did some laboratory experiments. Laura Bulgariu and Hossein Eshghi verified the analytical methods. All authors contributed to revising the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. We are grateful to all of those with whom we have had the pleasure to work during this research. Also, we thank the chemistry department of Payame Noor University of Mashhad for providing laboratory space.References
- A. Nakarmi, S. E. Bourdo, L. Ruhl, S. Kanel, M. Nadagouda, P. Kumar, I. Pavel and T. Viswanathan, J. Environ. Manage., 2020, 272, 111048 CrossRef CAS PubMed.
- E. Zong, Y. Shen, J. Yang, X. Liu and P. Song, ACS Omega, 2023, 8, 14177–14189 CrossRef CAS PubMed.
- D. A. Almasri, N. B. Saleh, M. A. Atieh, G. McKay and S. Ahzi, Sci. Rep., 2019, 9, 1–13 CrossRef CAS PubMed.
- M. R. Awual, J. Clean. Prod., 2019, 228, 1311–1319 CrossRef CAS.
- E. Khoshkalam, A. Fotovat, A. Halajnia, H. Kazemian and H. Eshghi, J. Mol. Liq., 2023, 375, 121366 CrossRef CAS.
- Y. Dai, Q. Sun, W. Wang, L. Lu, M. Liu, J. Li, S. Yang, Y. Sun, K. Zhang, J. Xu, W. Zheng, Z. Hu, Y. Yang, Y. Gao, Y. Chen, X. Zhang, F. Gao and Y. Zhang, Chemosphere, 2018, 211, 235–253 CrossRef CAS PubMed.
- S. Wiriyathamcharoen, S. Sarkar, P. Jiemvarangkul, T. T. Nguyen, W. Klysuban and S. Padungthon, Chem. Eng. J., 2020, 381, 122671 CrossRef CAS.
- Q. Xu, W. Li, L. Ma, D. Cao, G. Owens and Z. Chen, Sci. Total Environ., 2019, 703, 135003 Search PubMed.
- H. Kong, Q. Li, X. Zheng, P. Chen, G. Zhang and Z. Huang, Int. J. Biol. Macromol., 2023, 224, 984–997 CrossRef CAS PubMed.
- P. Koilraj and S. Kannan, J. Colloid Interface Sci., 2010, 341, 289–297 CrossRef CAS PubMed.
- D. Guaya, H. Cobos, C. Valderrama and J. L. Cortina, Nanomaterials, 2022, 12(20), 12203680 CrossRef PubMed.
- G. Gallareta-Olivares, A. Rivas-Sanchez, A. Cruz-Cruz, S. M. Hussain, R. B. González-González, M. F. Cárdenas-Alcaide, H. M. N. Iqbal and R. Parra-Saldívar, Chemosphere, 2023, 312(2022), 137190 CrossRef CAS PubMed.
- C. Long, Z. Jiang, J. Shangguan, T. Qing, P. Zhang and B. Feng, Chem. Eng. J., 2021, 406, 126848 CrossRef CAS.
- S. Sikiru, O. J. A. Abiodun, Y. K. Sanusi, Y. A. Sikiru, H. Soleimani, N. Yekeen and A. B. A. Haslija, J. Environ. Chem. Eng., 2022, 10, 108065 CrossRef CAS.
- R. Bhateria and R. Singh, J. Water Process Eng., 2019, 31, 100845 CrossRef.
- S. J. Park, J. Y. Park, J. W. Chung, H. K. Yang, B. K. Moon and S. S. Yi, Chem. Eng. J., 2020, 383, 123200 CrossRef CAS.
- P. Devi, P. Rajput, A. Thakur, K. H. Kim and P. Kumar, TrAC – Trends in Anal. Chem., 2019, 114, 171–195 CrossRef CAS.
- A. Mehta, A. Mishra, S. Basu, N. P. Shetti, K. R. Reddy, T. A. Saleh and T. M. Aminabhavi, J. Environ. Manage., 2019, 250, 109486 CrossRef CAS.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- M. Sabet and K. Mahdavi, Appl. Surf. Sci., 2019, 463, 283–291 CrossRef CAS.
- L. Wang, C. Cheng, S. Tapas, J. Lei, M. Matsuoka, J. Zhang and F. Zhang, J. Mater. Chem. A, 2015, 3, 13357–13364 RSC.
- M. Y. Pudza, Z. Z. Abidin, S. A. Rashid, F. M. Yasin, A. S. M. Noor and M. A. Issa, Nanomaterials, 2020, 10(2), 10020315 Search PubMed.
- M. Alikhani, A. Mirbolook, J. Sadeghi and A. Lakzian, Plant Physiol. Biochem., 2023, 200, 107783 CrossRef CAS.
- J. Cheng, C. F. Wang, Y. Zhang, S. Yang and S. Chen, RSC Adv., 2016, 6, 37189–37194 RSC.
- Y. Chen, H. Lian, Y. Wei, X. He, Y. Chen, B. Wang, Q. Zeng and J. Lin, Nanoscale, 2018, 10, 6734–6743 RSC.
- D. W. Li, X. F. Zhang, X. T. Zhang, X. Sen lv and N. You, Opt Mater., 2023, 137, 1–7 Search PubMed.
- R. Atchudan, T. N. J. I. Edison, S. Mani, S. Perumal, R. Vinodh, S. Thirunavukkarasu and Y. R. Lee, Dalton Trans., 2020, 49, 17725–17736 RSC.
- S. K. Tammina, Y. Wan, Y. Li and Y. Yang, J. Photochem. Photobiol., B, 2020, 202, 111734 CrossRef CAS PubMed.
- B. O. Isiuku, C. E. Enyoh, C. E. Duru and F. C. Ibe, Curr. Res. Green Sustainable Chem., 2021, 4, 100136 CrossRef CAS.
- B. Biswas, T. Rahman, M. Sakhakarmy, H. Jahromi, M. Eisa, J. Baltrusaitis, J. Lamba, A. Torbert and S. Adhikari, Heliyon, 2023, 9, e19830 CrossRef CAS PubMed.
- Z. E. T. Teymoorian, N. Hashemi and M. H. Mousazadeh, SN Appl. Sci., 2021, 305(3) DOI:10.1007/s42452-021-04287-z.
- Q. Xu, Y. Liu, R. Su, L. Cai, B. Li, Y. Zhang, L. Zhang, Y. Wang, Y. Wang, N. Li, X. Gong, Z. Gu, Y. Chen, Y. Tan, C. Dong and T. S. Sreeprasad, Nanoscale, 2016, 8, 17919–17927 RSC.
- Q. He, J. Ren and Y. Liu, Nanotechnology, 2022, 33(17) DOI:10.1088/1361-6528/ac4b7a.
- K. Yao, X. Lv, G. Zheng, Z. Chen, Y. Jiang, X. Zhu, Z. Wang and Z. Cai, Environ. Sci. Technol., 2018, 52, 14445–14451 CrossRef CAS.
- J. D. Clogston and A. K. Patri, Methods Mol. Biol., 2011, 697, 63–70 CrossRef CAS PubMed.
- X. Chen, H. Mi, C. Ji, C. Lei, Z. Fan, C. Yu and L. Sun, J. Mater. Sci., 2020, 55, 5510–5521 CrossRef CAS.
- C. Duan, T. Ma, J. Wang and Y. Zhou, J. Water Process Eng., 2020, 37, 101339 CrossRef.
- D. Cao, X. Jin, L. Gan, T. Wang and Z. Chen, Chemosphere, 2016, 159, 23–31 CrossRef CAS PubMed.
- H. Zhou, Y. Tan, Y. Yang, Y. Zhang, X. Lei and D. Yuan, Appl. Clay Sci., 2021, 200, 105903 CrossRef CAS.
- J. Antelo, M. Avena, S. Fiol, R. López and F. Arce, J. Colloid Interface Sci., 2005, 285, 476–486 CrossRef CAS PubMed.
- H. Kaya, R. B. Karabacak, Y. Çelik, J. Peake, S. Watkins, R. Sayer and E. Suvacı, Microchem. J., 2023, 191, 108772 CrossRef CAS.
- M. Talebi, R. Rahnemaie, E. Goli and M. Hossein, Chem. Geol., 2016, 437, 19–29 CrossRef.
- Z. Ajmal, A. Muhmood, M. Usman, S. Kizito, J. Lu, R. Dong and S. Wu, J. Colloid Interface Sci., 2018, 528, 145–155 CrossRef CAS.
- J. C. Mendez and T. Hiemstra, ACS Earth Space Chem., 2020, 4, 545–557 CrossRef CAS.
- C. Tiberg and J. P. Gustafsson, J. Colloid Interface Sci., 2016, 471, 103–111 CrossRef CAS PubMed.
- S. M. Alardhi, A. H. Abdalsalam, A. A. Ati, M. H. Abdulkareem, A. A. Ramadhan, M. M. Taki and Z. Y. Abbas, Polym. Bull., 2023, 81, 1131–1157 CrossRef.
- S. Kalam, S. A. Abu-Khamsin, M. S. Kamal and S. Patil, ACS Omega, 2021, 6, 32342–32348 CrossRef CAS PubMed.
- Y. K. Choi, H. M. Jang, E. Kan, A. R. Wallace and W. Sun, Environ. Eng. Res., 2019, 24, 434–442 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02428a |
| This journal is © The Royal Society of Chemistry 2024 |