Open Access Article
Open Access ArticleFrom waste to wealth: iron oxide doped hydroxyapatite-based biosensor for the colorimetric detection of ascorbic acid
Amir Badshaha,
Sadaf Noreena,
Mohibullah Shahb,
Muhammad Asada,
Riaz Ullahc,
Essam A. Alid,
Jibran Iqbale,
Wei Sun*f and
Umar Nishan *a
*a
aDepartment of Chemistry, Kohat University of Science and Technology, Kohat 26000, KP, Pakistan. E-mail: umarnishan85@gmail.com
bDepartment of Biochemistry, Bahauddin Zakariya University, Multan 66000, Pakistan
cDepartment of Pharmacognosy, College of Pharmacy, King Saud University Riyadh, Saudi Arabia
dDepartment of Pharmaceutical Chemistry, College of Pharmacy, King Saud University Riyadh, Saudi Arabia
eCollege of Interdisciplinary Studies, Zayed University, Abu Dhabi, 144534, United Arab Emirates
fHainan International Joint Research Center of Marine Advanced Photoelectric Functional Materials, College of Chemistry and Chemical Engineering, Hainan Normal University, Haikou 571158, P. R. China. E-mail: sunwei@hainnu.edu.cn
First published on 18th June 2024
Abstract
Ascorbic acid plays a pivotal role in the human body. It maintains the robustness, enlargement, and elasticity of the collagen triple helix. However, the abnormal concentration of ascorbic acid causes various diseases, such as scurvy, cardiovascular diseases, gingival bleeding, urinary stones, diarrhea, stomach convulsions, etc. In the present work, an iron-doped hydroxyapatite (HAp@Fe2O3)-based biosensor was developed for the colorimetric detection of ascorbic acid based on a low-cost, biocompatible, and ubiquitous material. Due to the catalytic nature of HAp owing to the acidic and basic moieties within the structure, it was used as a template for HAp@Fe2O3 synthesis. This approach provides an active as well as large surface area for the sensing of ascorbic acid. The synthesized platform was characterized by various techniques, such as UV-Vis, FTIR, SEM, XRD, TGA, EDX, etc. The HAp@Fe2O3 demonstrated inherent peroxidase-like activity in the presence of 3,3′,5,5′-tetramethylbenzidine (TMB) oxidized with the assistance of H2O2. It resulted in the color changing to blue-green, and after the addition of ascorbic acid, the color changed to colorless, resulting in the reduction of TMB. To achieve optimal sensing parameters, experimental conditions were optimized. The quantity of HAp@Fe2O3, H2O2, pH, TMB, time, and the concentration of ascorbic acid were fine-tuned. The linear range for the proposed sensor was 0.6–56 μM, along with a limit of detection of 0.16 μM and a limit of quantification of 0.53 μM. The proposed sensor detects ascorbic acid within 75 seconds at room temperature. The proposed platform was also applied to quantitatively check the concentration of ascorbic acid in a physiological solution.
1 Introduction
Ascorbic acid (AA), also known as vitamin C, plays an important role in various redox metabolic reactions in the human body. As an efficient antioxidant, it helps in scavenging free radicals in the body to protect the cells from oxidative damage, enhance immunity, prevent scurvy, the common cold, cancer, etc. Its main source is fruits and vegetables, and it cannot be synthesized in the human body.1 It is highly desirable to develop a robust, low-cost, and reliable platform for the sensing and monitoring of AA.Various techniques have been used for the detection of ascorbic acid, such as electrochemical techniques,2 fluorometric methods,3 high-performance liquid chromatography, etc.4 These techniques, despite their merits, have some drawbacks, such as complicated sample preparation steps that are time-consuming, costly, and require highly sophisticated instrumentation. These techniques require highly skilled operators, and acquiring and sustaining them in laboratories with limited resources is a big challenge. On the other hand, the colorimetric method has great potential for ascorbic acid detection owing to its simplicity, rapid operation, and cost-effectiveness.5 The added advantage associated with this approach is that its results can be visualized by the unaided eye.6
Hydroxyapatite (HAp) holds a prime position among biomaterials and has been utilized for bone implants. HAp comprises the major component of bone mineral and is expressed through the chemical formula Ca10(PO4)6(OH).7 HAp has been produced utilizing a variety of precursors as well as extracted from natural sources. The most common natural sources of HAp extraction are marine organisms, animals, algae, and minerals.8 For the extraction of HAp, both marine and terrestrial animal bones, scales, and shells have been employed. Similarly, fruit peels, wood, flowers, leaves, and stalks, are considered other suitable sources of HAp.9 In the present work, we have used waste chicken bones as a precursor for HAp extraction. Chicken is a highly consumed product worldwide, and it is estimated that its consumption generates around 68 billion tons of waste annually. This enormous amount of waste needs to be used in a beneficial manner.10 The extraction of HAp and fabrication of the colorimetric sensor reported in this work are an attempt to sustainably and beneficially use chicken waste. Various techniques have been reported to isolate HAp from waste materials, such as mechanochemical,11 wet chemical methods, etc.12 However, the above-mentioned methods are laborious and require sophisticated equipment and apparatus. Therefore, in the present work, the calcination method has been used for the preparation of HAp because of its easy handling and the use of common instruments for the isolation of HAp.13
HAp and its nanocomposites have been used in various fields, such as diagnosis and overcoming environmental hazards.14 These composites are efficient in the removal of dyes like Congo red, methylene blue, etc.15 According to reports, HAp/chitosan nanocomposite is a promising option for tissue engineering, especially for scaffolds made of bone and cartilage.16 Novel immunosensors have been reported using HAp nanocrystals, gold nanoparticles, and chitosan mixed thoroughly with anti-PSA antibodies for the diagnosis of prostate cancer.17
Various methods, such as ion-beam sputter coating18 and electrophoretic deposition19 are used for adding metal ions to HAp. In comparison to these methods, doping is more efficient because ionic doping can result in crystallinity of HAp, changes in thermal stability, grain size, lattice parameters, crystal structure phase decomposition, microstructure, and solubility depending on the amount and size of the dopant.20
In the current work, we synthesized HAp@Fe2O3 using the calcination method. The novelty of this work lies in the use of HAp@Fe2O3 for the sensing of ascorbic acid using chromogenic substrate (TMB) with the assistance of H2O2. In this process, the mimic enzyme (HAp@Fe2O3) provides a platform for the conversion of TMB into an oxidized form with the help of the oxidizing agent H2O2. Upon addition of AA, the oxidized form of TMB reversed back to its reduced (colorless) form, as observed with the naked eye and confirmed through a UV-Vis spectrophotometer. A new, fast, extremely sensitive, and selective AA detection method was developed. Different reaction conditions, such as HAp@Fe2O3 quantity, pH, TMB concentration, and incubation time, were optimized to get the best performance of the proposed platform. The proposed sensor was also applied to quantitatively check the concentration of ascorbic acid in physiological samples.
2 Experimental
2.1. Reagents-and-materials
All of the studies employed analytical-grade compounds; no additional purification was carried out. Double-distilled water was utilized to prepare the solutions. Ascorbic acid (≥97.0%), iron nitrate nonahydrate (Fe(NO3)3·9H2O), NaOH (≥97.0%), hydrogen chloride (98%), H2O2 (35%), and TMB were utilized. We acquired all of the chemicals from Sigma-Aldrich.2.2. Instrumentation
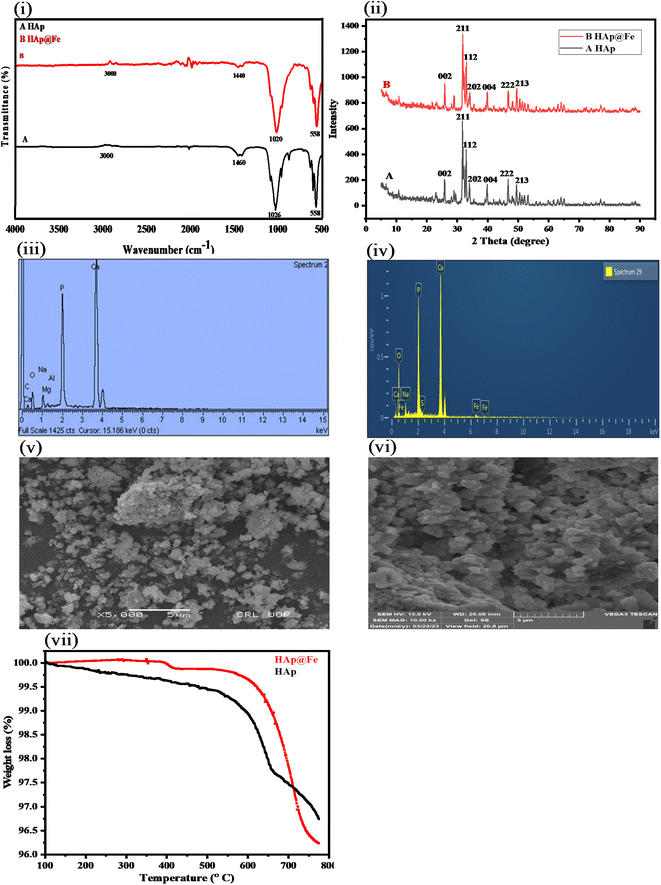
Fourier transform infrared spectroscopy (FTIR) was used for the identification of the characteristic peaks of HAp@Fe2O3 in the range 4000–500 cm−1 (Agilent Technologies, Danbury, Conn, USA). The resolution of 4 cm−1 and 256 scans per sample were selected. A scanning electron microscope coupled to an energy dispersive X-ray spectrophotometer (SEM-EDS) with a TESCAN VEGA (LMU) SEM with INCAx-act (Oxford Instruments) was used to study the morphology and elemental composition of the synthesized platform. Phase identification of the prepared HAp@Fe2O3 was confirmed by X-ray diffraction (Shimadzu, LabX XRD-6100). The thermal stability was observed by thermogravimetric analysis of TGA (Pyris-1, V-3.81 PerkinElmer). A UV-Vis spectrophotometer was used for taking the absorption spectra (Shimadzu, UV, 1,800, Japan).2.3. Synthesis of hydroxyapatite
Chicken waste bones were collected from a local restaurant. To remove any visible fleshy tissues adhering to bones, a thorough cleansing procedure was carried out. First of all, the bones were washed thoroughly with distilled water. Subsequently, the bones were crushed into small pieces and subjected to distilled water and acetone treatment. The meticulously cleaned waste bones were immersed in a 1 M NaOH solution. This was followed by proper washing and drying for 24 hours at 70 °C.21 The extraction of inorganic HAp from the processed chicken bones was achieved through calcination at 800 °C. Pre-treated and dried bone pieces (10 g) were placed in alumina crucibles and subjected to calcinations in order to remove all the organic components. The temperature was gradually increased at a heating rate of 5 °C per minute and heated to 800 °C for a period of 8 hours.2.4. Synthesis of iron doped HAp
The process involves treating iron nitrate nonahydrate (Fe(NO3)3·9H2O) with HA powder derived from broiler chicken bone in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 by weight. The resulting mixture of powdered substances was gradually heated and fused within high-alumina crucibles while being covered. This fusion was performed at a rate of 5 °C per minute and maintained for three hours at a temperature of 800 °C. Subsequently, the crucibles were allowed to cool down to room temperature.22
9 by weight. The resulting mixture of powdered substances was gradually heated and fused within high-alumina crucibles while being covered. This fusion was performed at a rate of 5 °C per minute and maintained for three hours at a temperature of 800 °C. Subsequently, the crucibles were allowed to cool down to room temperature.22
2.5. Detection of ascorbic acid
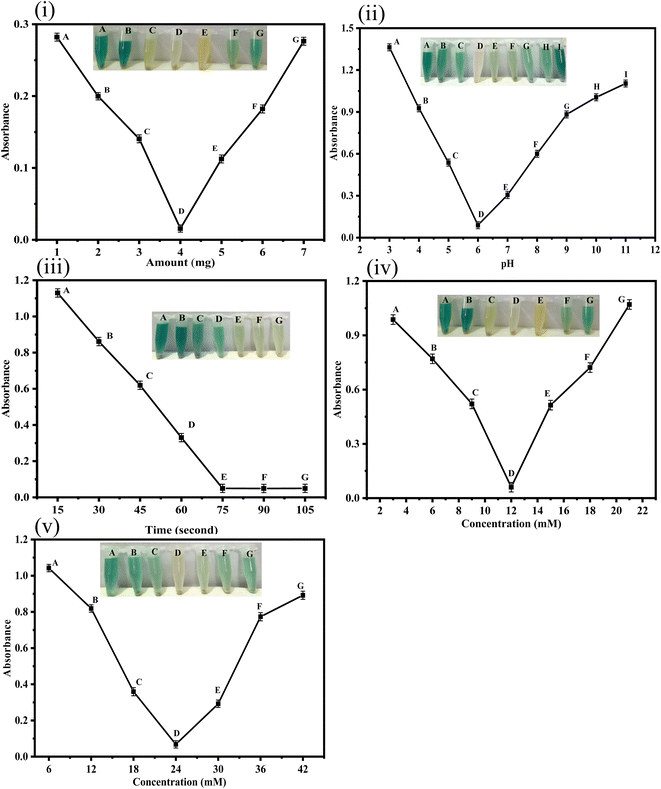
For the colorimetric detection of ascorbic acid, TMB was used as a chromogenic substrate in the presence of HAp@Fe2O3. The experiment was performed as follows: TMB underwent oxidation assisted by H2O2 in the presence of the synthesized platform. This resulted in a color change from colorless to blue-green. The protocol involves the mixing of 4 mg of HAp@Fe2O3, 500 μL of PBS buffer solution (6 mM), 200 μL of TMB (12 mM), and 100 μL of hydrogen peroxide (24 mM). Subsequently, 100 μL of ascorbic acid (0.6–56 μM) were added to the reaction mixture. This was followed by the incubation of the reaction mixture for 75 seconds. After the addition of ascorbic acid, the color changes from blue-green to colorless owing to the reduction of TMB. The anticipated change in visible range was also authenticated through a UV-Vis spectrophotometer.233 Results and discussions
3.1. Characterization result
| Element | HAp | HAp@Fe2O3 | ||
|---|---|---|---|---|
| Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | |
| C | 6.87 | 13.84 | — | — |
| O | 26.72 | 40.41 | 47.68 | 67.13 |
| Na | 3.70 | 3.89 | 2.59 | 2.54 |
| Mg | 0.57 | 0.57 | — | — |
| Al | 0.27 | 0.24 | — | — |
| P | 20.76 | 16.22 | 15.54 | 11.30 |
| Ca | 41.12 | 24.83 | 32.08 | 18.03 |
| S | — | — | 0.50 | 0.35 |
| Fe | — | — | 1.61 | 0.65 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
3.2. Colorimetric determination of ascorbic acid
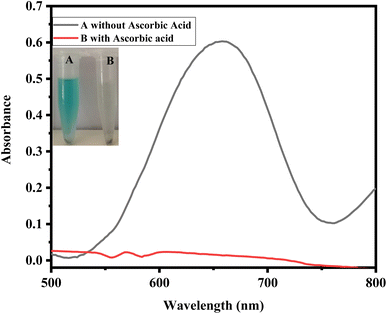
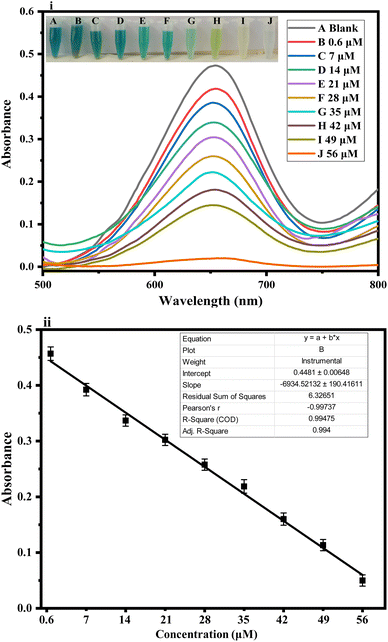
Fig. 2 shows the colorimetric detection of ascorbic acid. The following optimum experimental conditions were followed: HAp@Fe2O3 (4 mg) was mixed with 500 μL of PBS solution (6 mM) and 200 μL of TMB solution (12 mM), followed by the addition of 100 μL of hydrogen peroxide solution (24 mM) in an Eppendorf tube. This combination led to a color change from colorless to blue-green, which was observed with the naked eye. After the addition of ascorbic acid to 120 μL (56 μM), the blue-green color changed to a colorless form. Fig. 6 presents both the UV-Vis spectra and a visual colorimetric change.293.3. Proposed mechanism of the reaction
In the current work, the proposed sensor, HAp@Fe2O3, functions as a mimic enzyme. With the assistance of H2O2, the mimic enzyme converts the TMB into oxidized TMB, resulting in the formation of a blue-green color. Here, H2O2 helps in the generation of hydroxyl free radicals, which attack TMB and generate a colored complex. The generation of hydroxyl free radicals from H2O2 is catalyzed by the mimic enzyme. The addition of ascorbic acid to this complex results in the reduction of TMB, which becomes colorless in its reduced form. The oxidation of ascorbic acid results in the formation of dehydroascorbic acid. With the addition of ascorbic acid, the free radical generated from hydrogen peroxide by the catalytic action of HAp@Fe2O3 was scavenged. This scavenging of free radicals by ascorbic acid results in no oxidation of TMB. This colorimetric change was also confirmed with a UV-Vis spectrophotometer. The oxidized TMB showed maximum absorption at 652 nm (Scheme 1).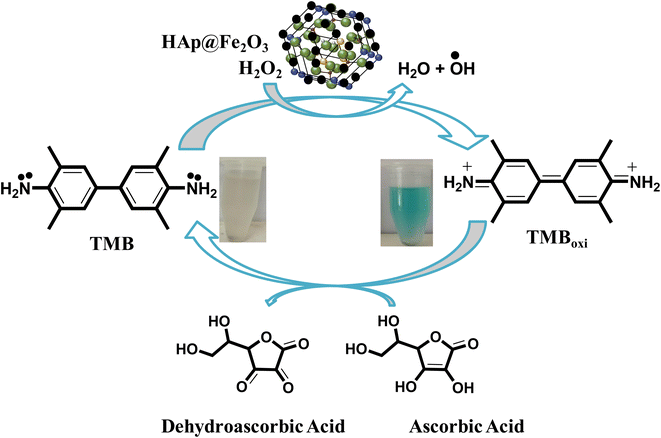 | ||
| Scheme 1 Proposed mechanism for the sensing of ascorbic acid based on the HAp@Fe2O3 mimic enzyme assisted by H2O2. | ||
3.4. Optimization of various parameters
| Catalysts | Substrate | Vmax (10−8 M s−1) | Km (mM) | Reference |
|---|---|---|---|---|
| HRP | TMB | 17.19 | 0.424 | 35 |
| HRP | H2O2 | 10.55 | 3.240 | |
| HAp@Fe2O3 | TMB | 15.4 | 3.11 | This work |
| HAp@Fe2O3 | H2O2 | 23.5 | 6.90 |
| S. no. | Materials used | Method applied | Linear range (μM) | LOD (μM) | References |
|---|---|---|---|---|---|
| 1 | CQDs | Colorimetric | 1.0–105 | 0.14 | 36 |
| 2 | Cu–Ag/rGO | Colorimetric | 5–30 | 3.8 | 37 |
| 3 | Papain-Ag NPs | Colorimetric | 0.25–50 | 0.079 | 38 |
| 4 | Pt/CeO2 nanocomposites | Colorimetric | 0.5–30 | 0.08 | 39 |
| 5 | Smartphone based CD spectrometer | Colorimetric | 0.6250–40 | 0.4946 | 40 |
| 6 | Silica coated Au nanorods | Colorimetric | 0.1–2.5 | 0.049 | 41 |
| 7 | Graphene quantum dots | Colorimetric | 0.3–10 | 0.094 | 42 |
| 8 | M-CQDs | Colorimetric | 10–70 | 3.26 | 43 |
| 9 | HAp@Fe2O3 | Colorimetric | 0.6–56 | 0.16 | Present work |
3.5. Selectivity study of the proposed method
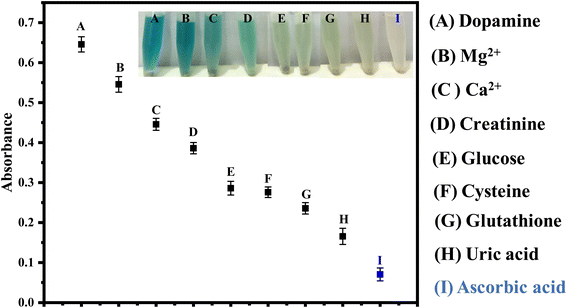
The selectivity of the proposed sensor, HAp@Fe2O3, is shown in Fig. 5. In the presence of commonly co-existing substances, including dopamine, uric acid, Mg2+, glucose, creatinine, and Ca2+, the selectivity of the proposed sensor was found to be excellent. The results illustrate that with the addition of ascorbic acid, the color completely changed from blue-green to colorless, while with the addition of dopamine, uric acid, Mg2+, glucose, creatinine, and Ca2+, no color change was observed. The proposed biosensor showed significant selectivity for the detection of ascorbic acid, as indicated in the results.Under the aforementioned experimental conditions, the reproducibility of the proposed sensors was tested after each preparation. We observed a high level of reproducibility in the results. The response of the fabricated sensor was tested over a period of 6 months. For this purpose, periodically each month, the sensor response was evaluated. There was no considerable difference in the response of the sensor over the mentioned period of time. This highlighted the stability of the proposed sensing platform.
3.6. Application of the fabricated sensor
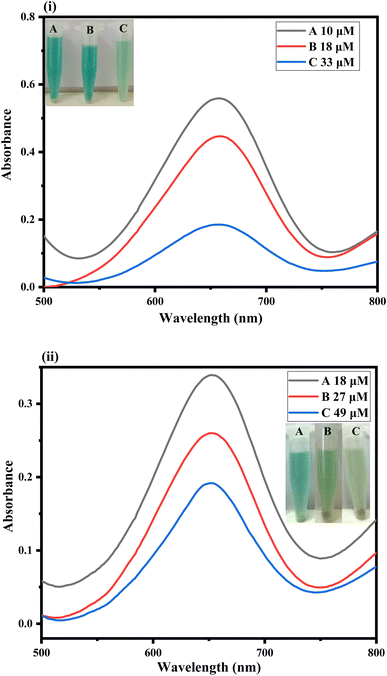
To examine the practical use of the fabricated platform, we employed the proposed sensor for detecting ascorbic acid in physiological solutions, as shown in Fig. 6(i). We applied the proposed sensor for the detection of ascorbic acid concentrations within the range of 0.6–56 μM in physiological solutions. Different concentrations of ascorbic acid in physiological solutions, such as 10, 18, and 33 μM, were prepared and introduced to the fabricated sensor. Using a UV-Vis spectrophotometer, we investigated the alteration in colorimetric detection of ascorbic acid within physiological solutions. We observed an absorption peak at 652 nm that exhibited a linear decrease in absorbance with the rise in ascorbic acid concentration within the optimized time.34 It proves that our suggested sensor is highly effective for detecting ascorbic acid in physiological solutions. Subsequently, blood plasma samples were collected from the Al-Habib clinical laboratory near the DHQ hospital in Kohat, KP, Pakistan. The samples were screened for the detection of ascorbic acid in the linear range of the proposed sensor. Strategic dilution of the real samples was performed to bring the analyte concentration into the linear range of our proposed sensor as and when needed. The response was observed with the naked eye and confirmed through a UV-Vis spectrophotometer, as shown in Fig. 6(ii).4 Conclusion
In conclusion, a new, straight-forward, highly thermally stable, low-cost, biocompatible, highly sensitive, and selective sensing platform for ascorbic acid was fabricated. All the characterizations confirmed the successful synthesis of the mimic enzyme (HAp@Fe2O3). The fabricated sensor detected ascorbic acid with high sensitivity and selectivity in the presence of a bunch of potential interfering species. Comprehensive optimization studies were performed to fine-tune the sensing parameters, such as the amount of mimic enzyme (4 mg), pH (6), time (75 seconds), TMB concentration (12 mM), and H2O2 concentration (24 mM). The proposed sensor exhibited an extensive linear range of 0.6–56 μM, with a very low limit of detection (0.16 μM) and limit of quantification (0.53 μM) and an R2 value of 0.998. These results indicate the fabricated platform is highly sensitive, and there is a strong correlation between absorption and concentration. The fabricated sensor was successfully used to detect ascorbic acid in physiological solutions. The sensing platform has the potential to be used for the diagnosis of diseases caused by the change in ascorbic acid. It can also be used in the food industry for the monitoring of different food samples.Conflicts of interest
The authors declare that there is no conflict of interests.Acknowledgements
Authors wish to thanks Researchers Supporting Project Number (RSP2024R45) at King Saud University Riyadh Saudi Arabia for financial support.References
- H. Guan, B. Han, D. Gong, Y. Song, B. Liu and N. Zhang, Colorimetric sensing for ascorbic acid based on peroxidase-like of GoldMag nanocomposites, Spectrochim. Acta, Part A, 2019, 222, 117277 CrossRef CAS PubMed.
- C.-L. Sun, H.-H. Lee, J.-M. Yang and C.-C. Wu, The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites, Biosens. Bioelectron., 2011, 26(8), 3450–3455 CrossRef CAS PubMed.
- Y. Matsuoka, M. Yamato and K.-i. Yamada, Fluorescence probe for the convenient and sensitive detection of ascorbic acid, J. Clin. Biochem. Nutr., 2016, 58(1), 16–22 CrossRef CAS PubMed.
- B. Chandrashekar and B. K. Swamy, Simultaneous cyclic voltammetric determination of norepinephrine, ascorbic acid and uric acid using TX-100 modified carbon paste electrode, Anal. Methods, 2012, 4(3), 849–854 RSC.
- U. Nishan, S. Rehman, R. Ullah, A. Bari, S. Afridi, M. Shah, J. Iqbal, M. Asad, A. Badshah and N. Khan, Fabrication of a colorimetric sensor using acetic acid-capped drug-mediated copper oxide nanoparticles for nitrite biosensing in processed food, Front. Mater., 2023, 10, 1169945 CrossRef.
- M. Asad, N. Muhammad, N. Khan, M. Shah, M. Khan, M. Khan, A. Badshah, Z. Latif and U. Nishan, Colorimetric acetone sensor based on ionic liquid functionalized drug-mediated silver nanostructures, J. Pharm. Biomed. Anal., 2022, 221, 115043 CrossRef CAS PubMed.
- B. X. V??ng and T. H. J. M. Linh, The Extraction of pure hydroxyapatite from porcine bone by thermal process, Metall. Mater. Eng., 2019, 25(1), 47–58 CrossRef PubMed.
- N. Muhammad, Y. Gao, F. Iqbal, P. Ahmad, R. Ge, U. Nishan, A. Rahim, G. Gonfa and Z. Ullah, Extraction of biocompatible hydroxyapatite from fish scales using novel approach of ionic liquid pretreatment, Sep. Purif. Technol., 2016, 161, 129–135 CrossRef CAS.
- N. M. Pu’ad, P. Koshy, H. Abdullah, M. Idris and T. Lee, Syntheses of hydroxyapatite from natural sources, Heliyon, 2019, 5(5), e01588 CrossRef PubMed.
- T. McGauran, N. Dunne, B. M. Smyth and E. Cunningham, Feasibility of the use of poultry waste as polymer additives and implications for energy, cost and carbon, J. Cleaner Prod., 2021, 291, 125948 CrossRef CAS.
- M. V. Khvostov, M. S. Borisova, N. V. Bulina, S. V. Makarova, N. B. Dumchenko, T. G. Tolstikova and N. Z. Lyakhov, The influence of zinc and silicate ions on biological properties of hydroxyapatite synthesized by a mechanochemical method, Ceram. Int., 2021, 47(7), 9495–9503 CrossRef CAS.
- K. W. Goh, Y. H. Wong, S. Ramesh, H. Chandran, S. Krishnasamy, A. Sidhu and W. Teng, Effect of pH on the properties of eggshell-derived hydroxyapatite bioceramic synthesized by wet chemical method assisted by microwave irradiation, Ceram. Int., 2021, 47(7), 8879–8887 CrossRef CAS.
- R. A. Ramli, R. Adnan, M. A. Bakar and S. a. M. Masudi, Synthesis and characterisation of pure nanoporous hydroxyapatite, J. Phys. Sci., 2011, 22(1), 20–37 Search PubMed.
- S. Pai, M. S. Kini and R. Selvaraj, A review on adsorptive removal of dyes from wastewater by hydroxyapatite nanocomposites, Environ. Sci. Pollut. Res., 2021, 28, 11835–11849 CrossRef CAS PubMed.
- D. C. Manatunga, R. M. de Silva, K. Nalin de Silva, N. de Silva and E. Premalal, Metal and polymer-mediated synthesis of porous crystalline hydroxyapatite nanocomposites for environmental remediation, R. Soc. Open Sci., 2018, 5(1), 171557 CrossRef PubMed.
- M. Nikpour, S. Rabiee and M. Jahanshahi, Synthesis and characterization of hydroxyapatite/chitosan nanocomposite materials for medical engineering applications, Composites, Part B, 2012, 43(4), 1881–1886 CrossRef CAS.
- G. Shen, C. Cai and J. Yang, Fabrication of an electrochemical immunosensor based on a gold–hydroxyapatite nanocomposite–chitosan film, Electrochim. Acta, 2011, 56(24), 8272–8277 CrossRef CAS.
- I. Pana, A. C. Parau, C. M. Cotrut, M. Dinu, D. M. Vranceanu, A. E. Kiss, G. Serratore, D. A. Böhner, C. Vitelaru and G. Ambrogio, Influence of deposition temperature on the structure and functional properties of Mg doped hydroxyapatite coatings deposited on manufactured AZ31B alloy substrates by RF magnetron sputtering, Ceram. Int., 2023, 49(13), 22340–22354 CrossRef CAS.
- L.-Y. Li, L.-Y. Cui, B. Liu, R.-C. Zeng, X.-B. Chen, S.-Q. Li, Z.-L. Wang and E.-H. Han, Corrosion resistance of glucose-induced hydrothermal calcium phosphate coating on pure magnesium, Appl. Surf. Sci., 2019, 465, 1066–1077 CrossRef CAS.
- I. Ratha, P. Datta, V. K. Balla, S. K. Nandi and B. Kundu, Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review, Ceram. Int., 2021, 47(4), 4426–4445 CrossRef CAS.
- E. Barua, A. B. Deoghare, P. Deb, S. D. Lala and S. J. M. T. P. Chatterjee, Effect of pre-treatment and calcination process on micro-structural and physico-chemical properties of hydroxyapatite derived from chicken bone bio-waste, Mater. Today: Proc., 2019, 15, 188–198 CAS.
- N. T. Weissmueller, H. A. Schiffter, A. J. Pollard and A. C. J. M. L. Tas, Molten salt synthesis of potassium-containing hydroxyapatite microparticles used as protein substrate, Mater. Lett., 2014, 128, 421–424 CrossRef CAS.
- J. Peng, J. Ling, X.-Q. Zhang, L.-Y. Zhang, Q.-E. Cao, Z.-T. J. S. Ding and A. B. Chemical, A rapid, sensitive and selective colorimetric method for detection of ascorbic acid, Sens. Actuators, B, 2015, 221, 708–716 CrossRef CAS.
- H. Sahana, D. K. Khajuria, R. Razdan, D. R. Mahapatra, M. Bhat, S. Suresh, R. R. Rao and L. Mariappan, Improvement in bone properties by using risedronate adsorbed hydroxyapatite novel nanoparticle based formulation in a rat model of osteoporosis, J. Biomed. Nanotechnol., 2013, 9(2), 193–201 CrossRef CAS PubMed.
- E. R. Kramer, A. M. Morey, M. Staruch, S. L. Suib, M. Jain, J. I. Budnick and M. J. J. O. M. S. Wei, Synthesis and characterization of iron-substituted hydroxyapatite via a simple ion-exchange procedure, J. Mater. Sci., 2013, 48, 665–673 CrossRef CAS.
- A. Khaliq, R. Nazir, M. Khan, A. Rahim, M. Asad, M. Shah, M. Khan, R. Ullah, E. A. Ali and A. Khan, Co-doped CeO2/activated C nanocomposite functionalized with ionic liquid for colorimetric biosensing of H2O2 via peroxidase mimicking, Molecules, 2023, 28(8), 3325 CrossRef CAS PubMed.
- U. Nishan, I. Ullah, N. Muhammad, S. Afridi, M. Asad, S. U. Haq, M. Khan, M. Soylak and A. J. A. J. f. S. Rahim, Engineering, Investigation of Silver-Doped Iron Oxide Nanostructures Functionalized with Ionic Liquid for Colorimetric Sensing of Hydrogen Peroxide, Arabian J. Sci. Eng., 2023, 48(6), 7703–7712 CrossRef CAS.
- M. Khalid, S. S. B. Jikan, S. Adzila, Z. Murni, N. Badarulzaman, R. Rosley and M. U. J. B. R. A. C. Hameed, Synthesis and characterizations of hydroxyapatite using precursor extracted from chicken egg shell waste, Biointerface Res. Appl. Chem., 2022, 12(4), 5663–5671 CAS.
- U. Nishan, W. Ullah, N. Muhammad, M. Asad, S. Afridi, M. Khan, M. Shah, N. Khan and A. J. A. o. Rahim, Development of a nonenzymatic colorimetric sensor for the detection of uric acid based on ionic liquid-mediated nickel nanostructures, ACS Omega, 2022, 7(30), 26983–26991 CrossRef CAS PubMed.
- D. Uzunoğlu and A. Özer, Facile Synthesis of Magnetic Iron-Based Nanoparticles from the Leach Solution of Hyperaccumulator Plant Pinus brutia for the Antibacterial Activity and Colorimetric Detection of Ascorbic Acid, ACS Appl. Bio Mater., 2022, 5(11), 5465–5476 CrossRef PubMed.
- M. Drozd, M. Pietrzak, P. G. Parzuchowski, E. J. A. Malinowska and B. Chemistry, Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles, Anal. Bioanal. Chem., 2016, 408, 8505–8513 CrossRef CAS PubMed.
- M. Rong, L. Lin, X. Song, Y. Wang, Y. Zhong, J. Yan, Y. Feng, X. Zeng and X. J. B. Chen, Bioelectronics, Fluorescence sensing of chromium (VI) and ascorbic acid using graphitic carbon nitride nanosheets as a fluorescent “switch”, Biosens. Bioelectron., 2015, 68, 210–217 CrossRef CAS PubMed.
- E. Davidson, Z. Xi, Z. Gao and X. Xia, Ultrafast and sensitive colorimetric detection of ascorbic acid with Pd-Pt core-shell nanostructure as peroxidase mimic, Sens. Int., 2020, 1, 100031 CrossRef.
- U. Nishan, U. Sabba, A. Rahim, M. Asad, M. Shah, A. Iqbal, J. Iqbal and N. J. M. C. Muhammad, Physics, Ionic liquid tuned titanium dioxide nanostructures as an efficient colorimetric sensing platform for dopamine detection, Mater. Chem. Phys., 2021, 262, 124289 CrossRef CAS.
- W. Xue, T. Cheng-Ling, L. Jia-Jun, H.-Z. ZHANG and W. Jian, Ultra-small CuS nanoparticles as peroxidase mimetics for sensitive and colorimetric detection of uric acid in human serum, Chin. J. Anal. Chem., 2018, 46(5), e1825–e1831 Search PubMed.
- X. Shu, Y. Chang, H. Wen, X. Yao and Y. Wang, Colorimetric determination of ascorbic acid based on carbon quantum dots as peroxidase mimetic enzyme, RSC Adv., 2020, 10(25), 14953–14957 RSC.
- G. Darabdhara, B. Sharma, M. R. Das, R. Boukherroub and S. Szunerits, Cu-Ag bimetallic nanoparticles on reduced graphene oxide nanosheets as peroxidase mimic for glucose and ascorbic acid detection, Sens. Actuators, B, 2017, 238, 842–851 CrossRef CAS.
- J. Peng, J. Ling, X.-Q. Zhang, L.-Y. Zhang, Q.-E. Cao and Z.-T. Ding, A rapid, sensitive and selective colorimetric method for detection of ascorbic acid, Sens. Actuators, B, 2015, 221, 708–716 CrossRef CAS.
- X. Liu, X. Wang, C. Qi, Q. Han, W. Xiao, S. Cai, C. Wang and R. Yang, Sensitive colorimetric detection of ascorbic acid using Pt/CeO2 nanocomposites as peroxidase mimics, Appl. Surf. Sci., 2019, 479, 532–539 CrossRef CAS.
- L. Kong, Y. Gan, T. Liang, L. Zhong, Y. Pan, D. Kirsanov, A. Legin, H. Wan and P. Wang, A novel smartphone-based CD-spectrometer for high sensitive and cost-effective colorimetric detection of ascorbic acid, Anal. Chim. Acta, 2020, 1093, 150–159 CrossRef CAS PubMed.
- G. Wang, Z. Chen and L. Chen, Mesoporous silica-coated gold nanorods: towards sensitive colorimetric sensing of ascorbic acid via target-induced silver overcoating, Nanoscale, 2011, 3(4), 1756–1759 RSC.
- J.-J. Liu, Z.-T. Chen, D.-S. Tang, Y.-B. Wang, L.-T. Kang and J.-N. Yao, Graphene quantum dots-based fluorescent probe for turn-on sensing of ascorbic acid, Sens. Actuators, B, 2015, 212, 214–219 CrossRef CAS.
- S. Chandra, V. K. Singh, P. K. Yadav, D. Bano, V. Kumar, V. K. Pandey, M. Talat and S. H. Hasan, Mustard seeds derived fluorescent carbon quantum dots and their peroxidase-like activity for colorimetric detection of H2O2 and ascorbic acid in a real sample, Anal. Chim. Acta, 2019, 1054, 145–156 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |