Open Access Article
Open Access ArticleAcetohydroxamic acid salts: mild, simple and effective degradation reagents to counter Novichok nerve agents†
Boris Smolkin *a,
Victoria Nahum
*a,
Victoria Nahum *a,
Eugenia Bloch-Shilderman
*a,
Eugenia Bloch-Shilderman b,
Uri Nilib,
Gil Fridkin
b,
Uri Nilib,
Gil Fridkin a and
Nissan Ashkenazi
a and
Nissan Ashkenazi *ac
*ac
aDepartment of Organic Chemistry, IIBR-Israel Institute for Biological Research, PO Box 19, 7410001, Ness Ziona, Israel. E-mail: boriss@iibr.gov.il; victorian@iibr.gov.il; nissan.ashkenazi@iibr.gov.il
bDepartment of Pharmacology, IIBR-Israel Institute for Biological Research, PO Box 19, 7410001, Ness Ziona, Israel
cDepartment of Chemical Sciences, Ariel University, 4070000 Ariel, Israel
First published on 8th May 2024
Abstract
Novichoks is the latest known class of organophosphorus nerve agents to be developed. These highly lethal persistent agents, which exert their toxicity mainly through dermal exposure, pose new major challenges in mitigating their effect, mainly in respect to decontamination and medical countermeasures. Herein we report on the effective degradation of Novichok agents (A-230, A-232 and A-234) by hydroxamic acid salts. This class of α-nucleophiles, with emphasis on the FDA approved drug acetohydroxamic acid, were found to promote rapid hydrolysis of these extremely toxic agents. Using 31P NMR the Novichoks degradation rates were determined to be in time scale of minutes with the following order of reactivity A-230>A-232>A-234. The degradation efficiency was found to be dependent on the nucleophiles, their counter-cations and the specific solvent mixture used. Hence, these scavengers can serve as efficient and mild decontaminants in various scenarios including surfaces, dermal decontamination (as an alternative to active lotions such as the RSDL® kit) and also as a medical countermeasure in the form of “catch-up therapy”.
1 Introduction
Novichok chemical warfare agents (CWAs), also named the A-series, were developed by the Soviet Union during the cold war (1970s–1990s) and are considered as the 4th generation of highly toxic organophosphorus compounds (OPs).1–3 General public attention towards these agents was obtained, however, only during early 2018, after a Novichok agent was used in a poisoning incident in England.4 This event, prompted a revision of the OPCWs' convention on the prohibition of the development, production, stockpiling, use and destruction of chemical weapons. Specifically, by 2020 these agents were explicitly added to schedule 1 of the annex of chemicals.5 This fact, combined with the knowledge gained from the recent poisoning events regarding the extreme toxicity and stability of these agents, led to the realization that new countermeasures must be developed to effectively tackle Novichoks.6,7 These include analytical detection,8–16 decontamination (vide infra) methodologies and medical treatments. Structurally, Novichoks resemble the G series more than the V-series of nerve agents, since both Novichoks and G agents possess a fluoride leaving group (Fig. 1), which is replaced by the hydroxyl of serine at the acetyl choline esterase (AChE) active site during the initial step of intoxication.17 However, although they share a similar biochemical mechanism, as was published also in a recent case report,18 G-agents’ and Novichoks' hydrolytic properties were found to markedly differ, the latter being more similar to V-agents, in this respect.19,20 Specifically, while G-agents rapidly hydrolyse in aqueous media even at a mildly basic pH (Sarin t1/2 = 5.5 min at pH 10,21 Soman t1/2 = 12 min at pH 10),22,23 Novichok agents are extremely stable at this pH (we measured the t1/2 to be ≫110 h at pH 11). In fact, their stability at neutral pH is even greater than that of VX and VR.19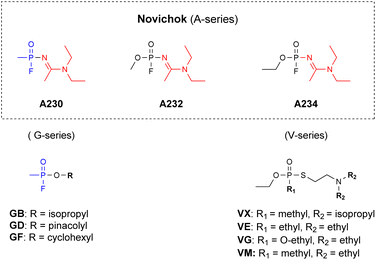 | ||
| Fig. 1 The structure of the Novichok agents as compared to known CWAs (the unique amidine moiety is marked in red). | ||
As part of our ongoing research to develop CWAs decontamination methodologies, we were interested in developing effective detoxification agents that would be universal. Namely, effective towards all types of OP nerve agents including Novichoks. Specifically, we were interested in the decontamination of delicate surfaces such as electronic devices and most importantly human skin for which highly corrosive solutions, such as bleach or the organic decontamination solution DS2 cannot be implied.23–28
To the best of our knowledge, to date, only three reports studied Novichoks' stability towards hydrolysis and possible methods for their degradation (enzymatic, using MOFs or standard decontaminants) using the actual agents.19,20,29 All reports were published after the Salisbury incident. Thus, there is still much to be done to thoroughly examine their effective decontamination using existing protocols, as well as to develop effective medical countermeasures against them.
In light of the above-mentioned stability of Novichoks, we envisioned that only strong nucleophiles in an appropriate solvent mixture would effectively react with the Novichok agents to provide efficient degradation. Recently, following a thorough investigation, we found that α-nucleophiles like hydroxamic acid salts degrade VX effectively on time scale of a few minutes.30 Moreover, we also demonstrated the therapeutic value and dermal and systemic safety of one of our lead lotions as a “catch-up therapy” against dermal intoxication by VX in live pigs.31 Decades ago, these types of nucleophiles were already shown to effectively hydrolyze G-agents like sarin.32 Herein we report our findings regarding the ability of hydroxamate salts to degrade Novichoks in a relevant time frame, which is applicable for both surface, dermal and transdermal detoxification. The study was conducted using 31P NMR spectroscopy, which allowed an accurate time-dependent monitoring of the reaction progress in respect to the agents and their degradation products, thus enabling the extraction of the reactions' kinetic parameters.
2 Experimental section
Caution! OP's are extremely toxic compounds, with special emphasis on Novichok agents. Experiments with these compounds should only be performed by trained personal using applicable safety procedures.2.1 NMR spectroscopy
31P{1H} spectra were obtained at 202 MHz at room temperature on a 11.7T (500 MHz) Bruker spectrometer (Avance III HD). Chemical shifts were calibrated to trimethyl phosphate as 0 ppm. The spectra were recorded using standard parameters of the TopSpin software (version 3.5). Each data point was obtained from 32 scans with a spectral width of 200 ppm and 4 s recycle delay. All spectra were taken under identical conditions and integration was done automatically using TopSpin 3.5.2 software and the built in AU multi_integ3.2.2 Degradation kinetics
Hydroxamic acids (26 equiv.) and the appropriate base (KOH, NaOH, amine) were added to water, mPEG, DMSO or PG solution (1 mL) and stirred for 1 min until completely dissolved. Novichok agents (A230, A232 or A234) (2 μL, 1 equiv.) were carefully added, and the solution was transferred to an NMR tube for kinetic analysis. Kinetic NMR acquisition started as soon as possible (without lock and shimming) until full degradation or no further change in reaction conversion were observed. Analysis of the results was performed by the GraphPad Prism 5 software to determine half-life times.3 Results and discussion
This current study commenced by examining the Novichok decontamination activity of four selected hydroxamic acid derivatives, which were previously shown to effectively degrade VX and its simulant.30 A-232, was chosen as the first agent to be examined. The hydroxamic acids, i.e., acetohydroxamic, salicylhydroxamic, caprylhydroxamic and bufexamac (Table 1) were converted to the various salts using different inorganic and organic bases. Notably, all these compounds are FDA-approved for topical skin applications. In addition, the solvent mixtures tested were chosen based on common solvents used in pharmaceutical products. Specifically, the degradation reactions in this study were performed by dissolving 1 eq. of the Novichok agent in the selected solvent mixtures and adding it to a solution containing 26 eq. of the hydroxamate salt, in order to obtain pseudo-first order conditions. It should be noted that a much higher ratio is expected to be used in real-life scenarios. For example in our “catch-up therapy” protocol an excess of ∼103 of the AHA salt is used,31 similar to the skin decontamination protocol using RSDL® (Reactive Skin Decontamination Lotion) where a ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 ratio is also desirable.33 Such a high ratio should obviously further accelerate the reactions (see the kinetic equations below). Directly after mixing, the samples were consecutively examined using 31P NMR spectroscopy to study the reaction's progress and the resulting kinetic data was analysed via integration using the TOPSPIN software and GraphPad Prism 5 (Fig. 2 and S1–S48†), to provide half-life times (t1/2) of these reactions, which are summarized in Table 1. In previous work we showed that adding up to 20% DMSO to the lotion dramatically enhances the skin penetration capability of the hydroxamic acid salts and also increases the solubility of the more lipophilic hydroxamate derivatives in the aqueous media, while still maintaining reasonable degradation rates of the persistent nerve agent VX.30 Thus, lotions holding H2O/DMSO mixtures should be preferred for skin (including in-skin) decontamination.
103 ratio is also desirable.33 Such a high ratio should obviously further accelerate the reactions (see the kinetic equations below). Directly after mixing, the samples were consecutively examined using 31P NMR spectroscopy to study the reaction's progress and the resulting kinetic data was analysed via integration using the TOPSPIN software and GraphPad Prism 5 (Fig. 2 and S1–S48†), to provide half-life times (t1/2) of these reactions, which are summarized in Table 1. In previous work we showed that adding up to 20% DMSO to the lotion dramatically enhances the skin penetration capability of the hydroxamic acid salts and also increases the solubility of the more lipophilic hydroxamate derivatives in the aqueous media, while still maintaining reasonable degradation rates of the persistent nerve agent VX.30 Thus, lotions holding H2O/DMSO mixtures should be preferred for skin (including in-skin) decontamination.
| # | Compound name | Counter cation | Solvent | t1/2 (min) |
|---|---|---|---|---|
| a CHA and BUF salts have a very poor solubility in DMSO and its mixtures with water, therefore the reaction with A232 couldn't be performed in these solvents.b Due to low solubility of the degradation products in the reaction mixture the reaction did not proceed to completion.c N. R. – no reaction. | ||||
| 1 | Acetohydroxamate (AHA) | Na+ (1a) | DMSO | 30.4 |
| Na+ (1a) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.9 | ||
| Na+ (1a) | PG/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
28.1 | ||
| Na+ (1a) | mPEG/H2O 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
≫300 | ||
| K+ (1b) | H2O | <2 | ||
| K+ (1b) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.4 | ||
| Diethylamine (1c) | PG/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
36.4 | ||
| Diethylamine (1c) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
41.7 | ||
| Olamine (1d) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
128.0 | ||
| Epolamine (1e) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
161.9 | ||
| Piperazine (1f) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
124.3 | ||
| 2 | Salicylhydroxamate (SHA) | 2Na+ (2a) | DMSO | <3 |
| 2Na+ (2a) | PG/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.4 | ||
| 2Diethylamine (2c) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
111.2 | ||
| 2Olamine (2d) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N.Rc | ||
| 2Piperazine (2f) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N.R | ||
| 3 | Caprylhydroxamate (CHA)a | Na+ (3a) | PG/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
∼20b |
| Diethylamine (3c) | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
69.1 | ||
| 4 | Bufexamac (BUF)a | Na+ (4a) | PG/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
∼20b |
As may be seen in Table 1, among the tested hydroxamic acid derivatives, salts of acetohydroxamic acid 1 and salicylhydroxamic acid 2 in DMSO, H2O and their mixtures were found to be the most effective, rapidly degrading A-232 with a t1/2 of a few minutes (1a-b and 2a). The degradation of A-232 by organic salts of 1, namely, diethylamine, olamine, epolamine and piperazine (1c–f) respectively, was however much slower, with half-life values ranging between 36 and 162 min, while organic salts of 2, olamine (2d) and piperazine (2f) didn't affect A-232 degradation at all. Among the organic salts examined in a DMSO/H2O mixture, diethylammonium salt of AHA (1c) showed the best activity. Surprisingly, the degradation of A-232 using the AHA Na salt (1a) in a mPEG/H2O 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, a solution used in the RSDL lotion, proceeded extremely slowly with t1/2 of more than 300 min, while under the same conditions VX was degraded much faster, with a t1/2 value of 7.45 min.30
1 mixture, a solution used in the RSDL lotion, proceeded extremely slowly with t1/2 of more than 300 min, while under the same conditions VX was degraded much faster, with a t1/2 value of 7.45 min.30
The more hydrophobic hydroxamic acid salts, Caprylhydroxamate 3a and Bufexamac 4a, were found to have poor solubility in DMSO and its mixtures with water, therefore the reaction of these derivatives with A-232 was performed in a PG/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture to show that they are less active than inorganic salts of 1 and 2, degrading A-232 with a t1/2 of ∼20 min.
1 mixture to show that they are less active than inorganic salts of 1 and 2, degrading A-232 with a t1/2 of ∼20 min.
Next, we proceeded to determine the effectiveness of the best hydroxamic acid derivative, identified, to degrade other members of the Novichok family i.e., A-230 and A-234. In this respect, the AHA salts (Na+, K+ and DEA+) were found to be more suitable than the SHA (2Na+ salt), although showing similar activity. This is because the latter acid derivative holds two labile protons, which need to be deprotonated in order to react efficiently with A-232, as ascertained from the salt partial stability in aqueous mixtures. Water or DMSO/H2O mixtures were selected as the solvents of choice as they provided the best kinetic results for A-232 degradation among the solvents and solvent mixtures evaluated.
A summary of the Novichok agents' degradation kinetics using AHA salts is presented in Table 2. As can be seen, in addition to three different AHA counter ions, namely, sodium, potassium and diethylamine, each experiment was also performed in three solvent conditions, i.e., water and two DMSO/H2O mixtures at room temperature.
| Agent | AHA salt | % DMSOa | kobs (min−1) | t1/2 (min) |
|---|---|---|---|---|
| a Percent of DMSO in a solution of DMSO/H2O (v/v).b Due to NMR time scale limitations, more accurate values of t1/2 and kobs couldn't be determined. | ||||
| A-230 | AHA Na | 0 | —b | <2b |
| AHA Na | 20 | — | <2 | |
| AHA Na | 50 | — | <2 | |
| AHA K | 0 | — | <2 | |
| AHA K | 20 | — | <2 | |
| AHA K | 50 | — | <2 | |
| AHA DEA | 0 | — | <2 | |
| AHA DEA | 20 | — | <2 | |
| AHA DEA | 50 | — | <2 | |
| A-232 | AHA Na | 0 | 0.1924 | 3.6 |
| AHA Na | 20 | — | <3.5 | |
| AHA Na | 50 | 0.0872 | 7.9 | |
| AHA K | 0 | — | <2 | |
| AHA K | 20 | — | <3 | |
| AHA K | 50 | 0.0938 | 7.4 | |
| AHA DEA | 0 | 0.1474 | 4.7 | |
| AHA DEA | 20 | 0.1440 | 4.8 | |
| AHA DEA | 50 | 0.0166 | 41.7 | |
| A-234 | AHA Na | 0 | 0.07825 | 8.86 |
| AHA Na | 20 | 0.03905 | 17.75 | |
| AHA Na | 50 | 0.02068 | 33.51 | |
| AHA K | 0 | 0.08161 | 8.49 | |
| AHA K | 20 | 0.04566 | 15.18 | |
| AHA K | 50 | 0.02007 | 34.53 | |
| AHA DEA | 0 | 0.06982 | 9.93 | |
| AHA DEA | 20 | 0.03003 | 23.08 | |
| AHA DEA | 50 | 0.00489 | 141.88 | |
The experiments described reflect a significant difference in the stability of the three Novichok agents tested, under the conditions examined. The phosphonate derivative A-230 is degraded in less than a few minutes (t1/2 < 2 min) regardless of the solvent or counter-cation used. The degradation of A-232 was also rather quick, albeit slower than that of A-230, specifically in the case of the AHA DEA salt and a mixture of DMSO/water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A-234 was found be the most stable, with half-life values ranging between 8 and 140 min, depending on the counter-cation and the percent of DMSO in the solution. Interestingly, the same tendency of Novichok agents' susceptibility to hydrolysis was previously observed also at pH 7.2, where the hydrolysis rate of A-230 agent was the fastest between the Novichok agents and the hydrolysis rate of A-234 was 50 times slower.19 Importantly, the degradation rates of all three agents, using a small and simple scavenger, such as the AHA salt, do not fall short of the rates obtained with complicated structures as Zr based MOFs (reported by de Koning et al.).20 The nucleophile ionization state was found to have a significant impact on the effectiveness of the degradation reactions, as was nicely demonstrated in the A-234 experiments, and to a lesser extent in the A-232 experiments, Table 2. Specifically, as the percentage of DMSO in the solution mixture was increased the concentration of the ionized nucleophile decreased, leading to slower reaction rates. Importantly, this trend was observed only when degradation in water alone was not immediate, as inferred from the experiments with A-230.
1. A-234 was found be the most stable, with half-life values ranging between 8 and 140 min, depending on the counter-cation and the percent of DMSO in the solution. Interestingly, the same tendency of Novichok agents' susceptibility to hydrolysis was previously observed also at pH 7.2, where the hydrolysis rate of A-230 agent was the fastest between the Novichok agents and the hydrolysis rate of A-234 was 50 times slower.19 Importantly, the degradation rates of all three agents, using a small and simple scavenger, such as the AHA salt, do not fall short of the rates obtained with complicated structures as Zr based MOFs (reported by de Koning et al.).20 The nucleophile ionization state was found to have a significant impact on the effectiveness of the degradation reactions, as was nicely demonstrated in the A-234 experiments, and to a lesser extent in the A-232 experiments, Table 2. Specifically, as the percentage of DMSO in the solution mixture was increased the concentration of the ionized nucleophile decreased, leading to slower reaction rates. Importantly, this trend was observed only when degradation in water alone was not immediate, as inferred from the experiments with A-230.
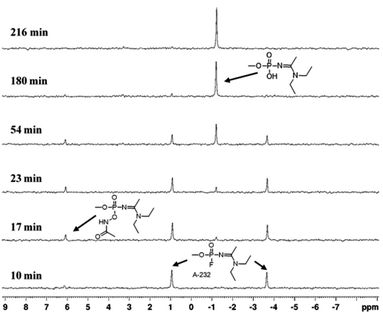
A plausible degradation mechanism of Novichok agents by AHA salts is implied from the 31P NMR spectra of the reaction progress. Specifically, in all experiments, the Novichok agent first appears as a distinct doublet stemming from the phosphorus–fluoride (P–F) coupling. As the reaction with the AHA nucleophile progresses, the doublet intensity decreases while two new signals arising from the degradation products appear. This pattern is nicely seen in Fig. 2, which presents 31P NMR spectra of the reaction of A-232 with AHA DEA in DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
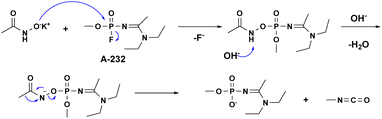
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where A-232 signals appear at 0.97, −3.75 ppm, and the products signals at 6.2 and −1 ppm, while at the end of the reaction only the signal, at −1 ppm, remains. Initially, the AHA nucleophile attacks the phosphorous center to obtain an acetamidophosphoate intermediate, which is observed as an emerging signal at 6.2 ppm. Then, at a second step, this intermediate converts via Lossen rearrangement to the final stable product, phosphate acid, observed at −1 ppm, Scheme 1. The fact that the acetamidophosphoate intermediate is observed indicates that the Lossen rearrangement step is slower than the initial step of the nucleophilic attack. Although it is not clear which specific step of the Lossen reaction is decelerated, the deprotonation or the concerted deposphorylation-alkyl migration, one may speculate on the former because the solution is only mildly basic (pH = 11). Importantly, this suggested degradation pathway of Novichoks, promoted by hydroxamic acid salts was previously reported for other organophosphorus nerve agents such as the persistent VX, Tabun and Sarin.30,34 Notably, although toxicological data regarding Novichok degradation products has not been published, it is safe to assume that their toxicity would be drastically reduced compared to the parent agent, exactly as was found for other nerve agents (i.e., ∼103 decrease in lethality).35
1, where A-232 signals appear at 0.97, −3.75 ppm, and the products signals at 6.2 and −1 ppm, while at the end of the reaction only the signal, at −1 ppm, remains. Initially, the AHA nucleophile attacks the phosphorous center to obtain an acetamidophosphoate intermediate, which is observed as an emerging signal at 6.2 ppm. Then, at a second step, this intermediate converts via Lossen rearrangement to the final stable product, phosphate acid, observed at −1 ppm, Scheme 1. The fact that the acetamidophosphoate intermediate is observed indicates that the Lossen rearrangement step is slower than the initial step of the nucleophilic attack. Although it is not clear which specific step of the Lossen reaction is decelerated, the deprotonation or the concerted deposphorylation-alkyl migration, one may speculate on the former because the solution is only mildly basic (pH = 11). Importantly, this suggested degradation pathway of Novichoks, promoted by hydroxamic acid salts was previously reported for other organophosphorus nerve agents such as the persistent VX, Tabun and Sarin.30,34 Notably, although toxicological data regarding Novichok degradation products has not been published, it is safe to assume that their toxicity would be drastically reduced compared to the parent agent, exactly as was found for other nerve agents (i.e., ∼103 decrease in lethality).35
According to the suggested mechanism the reaction kinetics may be described by the following equation:
| Rate = kobs [A-agent] | (1) |
| kobs = k0 + kOH [OH−] + k2 [AHA−] | (2) |
As the hydroxamate ion is a very strong nucleophile the agent's hydrolysis by the hydroxide ions present in the water basic solutions may be essentially neglected. Indeed, in a buffer of pH 11, i.e. a similar value to that of the AHA solutions, A-232 and A-234 were found highly stable with half-life exceeding 4 days (data not shown). Therefore, the rate constant, kobs, can be expressed as:
| kobs = k2 [AHA−] | (3) |
Moreover, it is assumed that the reaction kinetics is highly dependent on the ionization state of the active nucleophile in the solution, which is present in large excess and kept at a constant pH (∼11). The addition of DMSO, an organic solvent, to the aqueous reaction mixture leads to an increase in the pKa value of the acetohydroxamic acid (from 9.55 in case of 0% DMSO to 13.3 in case of 50% DMSO, as was previously shown).30 This leads to a decrease in the concentration of the ionized nucleophile, and accordingly to a decrease in the reaction rate. Therefore, eqn (3) can actually be expressed by:
| kobs = k2 [AHA]t × αAHA− | (4) |
4 Conclusions
In conclusion, the experiments performed in this work demonstrate that AHA salts are effective nucleophilic scavengers that lead to fast and full Novichok agents' degradation. Our previous results demonstrated the therapeutic value and dermal and systemic safety of one of our AHA salts as a potential “catch-up therapy” against dermal intoxication by VX in live pigs.31 Combined, the above suggest that DMSO/H2O solutions of AHA salts have a high potential to serve as a generic tool for the decontamination of low-volatility CWAs. Specifically, they may be useful for both skin surface decontamination and within-skin “catch-up therapy” (manuscript in preparation).Conflicts of interest
There are no conflicts to declare.Notes and references
- V. S. Mirzayanov, STATE SECRETS: An Insider's Chronicale of the Russian Chemical Weapons Program, Outskirts Press, 2008 Search PubMed.
- M. Kloske and Z. Witkiewicz, Chemosphere, 2019, 221, 672–682 CrossRef CAS PubMed.
- G. Bauer, A. Wildauer, G. Provoden, B. Menzi and C. Curty, Forensic Sci., 2023, 3, 231–244 CrossRef.
- M. Peplow, Chem. Eng. News, 2018, 96, 3–4 Search PubMed.
- Schedule 1, https://www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/schedule-1 Search PubMed.
- T. L. Blom and T. T. Wingelaar, Mil. Med., 2023, usad464 CrossRef PubMed.
- M. Hrabinova, J. Pejchal, V. Hepnarova, L. Muckova, L. Junova, J. Opravil, J. Zdarova Karasova, T. Rozsypal, A. Dlabkova, H. Rehulkova, T. Kucera, Z. Vecera, F. Caisberger, M. Schmidt, O. Soukup and D. Jun, Arch. Toxicol., 2024, 98, 1135–1149 CrossRef CAS PubMed.
- M. Otsuka, A. Yamaguchi and H. Miyaguchi, Forensic Toxicol., 2023, 41, 221–229 CrossRef CAS PubMed.
- J. Y. Lee, J. Y. Shin and H. S. Kim, J. Anal. Toxicol., 2023, 47, 81–88 CrossRef CAS PubMed.
- A. Yamaguchi, H. Miyaguchi and M. Tokeshi, Anal. Chem., 2022, 94, 4658–4665 CrossRef CAS PubMed.
- F. Mirbabaei, A. Mohammad-Khah, M. T. Naseri, M. Babri, S. M. Faraz, S. E. Hosseini and D. Ashrafi, Anal. Bioanal. Chem., 2022, 414, 3429–3442 CrossRef CAS PubMed.
- J. H. Lee, W. E. Jang, J. H. Park, H. B. Mohammad, J.-Y. Lee, W.-H. Jeong and M.-S. Kim, Bull. Korean Chem. Soc., 2022, 43, 444–449 CrossRef CAS.
- H. John, M. Dentzel, M. Siegert and H. Thiermann, Anal. Chem., 2022, 94, 2048–2055 CrossRef CAS PubMed.
- M. Eskandari, S. M. Faraz, S. E. Hosseini, S. Moradi and H. Saeidian, Int. J. Mass Spectrom., 2022, 473, 116794 CrossRef CAS.
- D. Noort, A. Fidder, D. van der Riet-van Oeveren, R. Busker and M. J. van der Schans, Chem. Res. Toxicol., 2021, 34, 1926–1932 Search PubMed.
- M. de Bruin-Hoegée, L. Lamriti, J. P. Langenberg, R. C. M. Olivier, L. F. Chau, M. J. van der Schans, D. Noort and A. C. van Asten, Anal. Methods, 2023, 15, 142–153 RSC.
- S. Costanzi, J.-H. Machado and M. Mitchell, ACS Chem. Neurosci., 2018, 9, 873–885 CrossRef CAS PubMed.
- D. Steindl, W. Boehmerle, R. Koerner, D. Praeger, M. Haug, J. Nee, A. Schreiber, F. Scheibe, K. Demin, P. Jacoby, R. Tauber, S. Hartwig, M. Endres and K.-U. Eckardt, Lancet, 2021, 397, 249–252 CrossRef CAS PubMed.
- S. P. Harvey, L. R. McMahon and F. J. Berg, Heliyon, 2020, 6, e03153 CrossRef PubMed.
- M. C. de Koning, C. Vieira Soares, M. van Grol, R. P. T. Bross and G. Maurin, ACS Appl. Mater. Interfaces, 2022, 14, 9222–9230 CrossRef CAS PubMed.
- R. L. Gustafson and A. E. Martell, J. Am. Chem. Soc., 1962, 84, 2309–2312 CrossRef CAS.
- J. R. Ward, Y. C. Yang, R. B. Wilson Jr. and W. D. Burrows, Bioorg. Chem., 1988, 16, 12–16 CrossRef CAS.
- Y.-C. Yang, J. A. Baker and J. R. Ward, Chem. Rev., 1992, 92, 1729–1743 CrossRef CAS.
- H.-J. Altman and A. Richardt, Decontamination of chemical warfare agents, in Decontamination of Warfare Agents: Enzymatic Methods for the Removal of B/C Weapons, ed. A. Richardt and M.-M. Blum, Wiley-VCH Verlag GmbH & Co. KGaA, 2008, ch. 7, pp. 83–115 Search PubMed.
- K. Kim, O. G. Tsay, D. A. Atwood and D. G. Churchill, Chem. Rev., 2011, 111, 5345–5403 CrossRef CAS PubMed.
- S. S. Talmage, A. P. Watson, V. Hauschild, N. B. Munro and J. King, Curr. Org. Chem., 2007, 11, 285–298 CrossRef CAS.
- A. W. Khan, S. Kotta, S. H. Ansari, J. Ali and R. K. Sharma, Def. Sci. J., 2013, 63, 487–496 CrossRef CAS.
- B. Picard, I. Chataigner, J. Maddaluno and J. Legros, Org. Biomol. Chem., 2019, 17, 6528–6537 RSC.
- H. Jung, J. Heo, N. Park, K. C. Lim and H. Jung, J. Hazard. Mater., 2023, 451, 131150 CrossRef CAS PubMed.
- V. Nahum, U. Nili, E. Bloch-Shilderman, B. Smolkin and N. Ashkenazi, Int. J. Pharm., 2021, 603, 120689 CrossRef CAS PubMed.
- E. Bloch-Shilderman, U. Nili, V. Nahum, B. Smolkin and N. Ashkenazi, Arch. Toxicol., 2023, 97, 2771–2783 CrossRef CAS PubMed.
- M. A. Stolberg and W. A. Mosher, J. Am. Chem. Soc., 1956, 79, 2618–2620 CrossRef.
- Reactive Skin Decontamination Lotion (RSDL) – Medical Countermeasures Database – CHEMM (and references therein), https://chemm.hhs.gov/countermeasure_RSDL.htm, (accessed 30 April 2024) Search PubMed.
- A. Bierwisch, M. Koller, F. Worek and S. Kubik, Eur. J. Org Chem., 2016, 5831–5838 CrossRef CAS.
- N. B. Munro, S. S. Talmage, G. D. Griffin, L. C. Waters, A. P. Watson, J. F. King and V. Hauschild, Environ. Health Perspect., 1999, 107, 933–974 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02038c |
| This journal is © The Royal Society of Chemistry 2024 |