Open Access Article
Open Access ArticleHaloarchaeal poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] composite films reinforced with graphene nanoplatelets as a biomaterial for skin tissue engineering†
Prajakta Praveen Bhendea,
Joephil D. Dias a,
Honey Srivastavab,
Rashmi Chauhanb,
Sachin Waigaonkarc,
Anasuya Gangulya and
Judith M. Braganςa
a,
Honey Srivastavab,
Rashmi Chauhanb,
Sachin Waigaonkarc,
Anasuya Gangulya and
Judith M. Braganςa *a
*a
aDepartment of Biological Sciences, Birla Institute of Technology and Science, Pilani KK Birla Goa Campus, Goa, India. E-mail: judith@goa.bits-pilani.ac.in
bDepartment of Chemistry, Birla Institute of Technology and Science, Pilani KK Birla Goa Campus, Goa, India
cDepartment of Mechanical Engineering, Birla Institute of Technology and Science, Pilani KK Birla Goa Campus, Goa, India
First published on 5th August 2024
Abstract
Halophilic archaea are an untapped source for a wide range of applications. This study explores the potential of a copolymer poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate) (PHBV), naturally synthesized by the halophilic archaeon, Halogeometricum borinquense E3, as a potential candidate for a tissue engineering biomaterial. Composites and blends from natural PHBV were fabricated with poly(L-lactic acid) (PLLA), poly(ε-caprolactone) (PCL) and graphene nanoplatelets (GNP) to enhance the properties of the material. This significantly improved the tensile strength of the blend to 4.729 MPa (359%). The reinforcement with 0.3% w/v of GNP further increased the tensile strength to 13.268 MPa (981%). Characterization of the films was done using ATR-FTIR, XRD, TGA, and SEM. The haloarchaeal PHBV exhibited the highest porosity ergo the highest swelling percentage while the PHBV/GNP showed the least. All the films showed good biocompatibility compared to tissue culture plastic (TCP). The viability of HaCaT cells and L929 fibroblast cells was maximum on the PHBV/PLLA/PCL blend albeit no significant change in the cell viability was observed in the graphene-reinforced nanocomposite. The films were also highly hemocompatible (<5% hemolysis).
1 Introduction
The field of regenerative medicine and tissue engineering depends on the selection of an appropriate biomaterial to be used as a scaffold, cells of the tissue to be engineered and the factors required for growth and differentiation of the cells. Natural-origin polymers are often preferred to be used as a biomaterial.1,2 Any polymer to be used as a biomaterial should be non-toxic, biocompatible, and biodegradable. It should be porous and have a wide surface area to promote cell–cell interaction and facilitate the transport of nutrients and waste products. It should possess suitable mechanical strength to withhold the developing tissue and thus promote cell adhesion, growth, and differentiation.3,4Polyhydroxyalkanoates (PHAs) are a group of naturally occurring polyesters synthesized by microorganisms due to an imbalance in essential nutrients with excessive carbon and stored as insoluble cytoplasmic inclusions or “carbonosomes”. These granules can be enzymatically depolymerized and used as a source of carbon.5,6 PHAs are versatile and their properties change according to the number of carbon atoms present in the polymer chain. PHAs with 3–5 carbon atoms are classified as short chain length polymers (scl); 6–14 carbon atoms are classified under medium chain length (mcl) and more than 14 carbon atoms are grouped as long chain length (lcl). Based on the available carbon source, the microorganisms can synthesize either a homopolymer containing the same repeating unit e.g., poly(3-hydroxybutyrate) or copolymers e.g., poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)].7,8
In this study, we explored the application of intrinsically produced copolymer, poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] by haloarchaeon, Halogeometricum borinquense E3, as a potential biomaterial for skin tissue engineering. Haloarchaea belong to the family Halobacteriaceae, within the third Domain of Life, Archaea. These are extremely halophilic organisms requiring about 100–300 g L−1 of NaCl for their growth.9,10 Haloarchaea are advantageous to be used for PHA production as they are non-pathogenic and do not contain lipopolysaccharide (LPS) as in Gram negative bacteria. LPS endotoxin gets co-extracted with the polymer thereby causing immunogenic and pyrogenic reactions.11
PHAs are an intriguing option for biomaterial owing to their lack of toxicity towards cells and blood, capacity for biodegradation, and non-carcinogenicity. A lot of research is being done investigating the applications of PHA in hard tissue engineering (bone,12 cartilage,13 and tendon), wound healing,14 nerve tissue,15 vascular tissue repair16,17 and as bio-absorbable sutures.18 Due to its lower degree of crystallinity, PHBV is preferred over PHB for medical applications. The degradation of heteropolymers is also higher than homopolymers in the human body.19 PHBV as calcium silicate composites have been studied for cartilage tissue engineering20 while blends of PHBV with PCL, pullulan, chitosan or hydroxyapatite based scaffolds have been explored for bone tissue engineering applications.21–23 Besides some studies have explored the use of PHBV composites with fibronectin or collagen in wound healing and vascular tissue engineering.24,25
Despite its remarkable properties, PHAs (especially scl's) have drawbacks like high fragility, low impact resistance, reduced elongation at break, and poor mechanical strength. In this study, we have blended PHBV with 1 wt% poly(L-lactic acid) (PLLA) and 1 wt% poly(ε-caprolactone) (PCL). PLLA is a homopolymer obtained from natural resources like sugarcane and corn starch using sustainable catalysts like sorbitol. It provides improved mechanical properties to the blend for effective support in prolonged regenerative processes. PLLA is also non-toxic, it degrades to form lactic acid which is a natural metabolite and is excreted as carbon dioxide and water.22,23 Jamal et al. 2024 (ref. 24) used PHBV and PLLA to develop a bilayer composite membrane for growth and proliferation of MC3T3 pre-osteoblastic cells. PCL is an aliphatic polyester synthesized by the ring-opening polymerization of ε-caprolactone. Its porous nature provides an increased surface area for cell–cell interaction. It is also known to enhance the growth and differentiation of fibroblasts.25
Graphene has a sp2 hybridized carbon in a 2D honeycomb lattice structure. This along with electronic distribution gives rise to a large surface area, unique electrical conductivity, and excellent mechanical properties. The availability of a large surface area facilitates the adsorption of proteins and growth factors thereby promoting cell adhesion and growth.26,27 It is critical to assess the cytotoxicity and biocompatibility of any material before its use in biomedical applications. The cytotoxicity of graphene is directly related to its concentration used, the length of incubation and the structure and type of graphene used.28 Salesa et al. 2022 (ref. 29) reported that graphene nanoplatelets (GNPs) at non-cytotoxic levels showed significant proliferative activity of HaCaT cells. Moreover GNPs were capable of upregulating almost half of the thirteen genes which were required for wound healing and skin tissue engineering.
The use of graphene for various biomedical applications is gaining momentum. Suvarnaphaet et al. 2019 (ref. 30) developed a biodegradable electrode patch made of graphene and PHA which could detect an electrocardiogram signal triggered by electrical activity through the heart. Moschetta et al. 2021 (ref. 31) made a biocompatible neuronal interface using P(3HB) and GNP which showed potential for interfacing with primary neurons in order to target disorders of the central nervous system.
This study is the first report to analyse archaeal derived PHBV/PLLA/PCL-graphene nanoplatelets composite for the proliferation of keratinocytes and fibroblasts with potential for skin tissue engineering. Haloarcheal PHBV (1% w/v), PLLA (1% w/v), PCL (1% w/v), and GNP (0.3% w/v) were used to prepare a blend/nanocomposite using the solvent casting method. The synthesised blends and graphene nanocomposites were further characterised to assess their ultrastructure, chemical composition, functional group analysis, thermal and mechanical properties. Concomitantly, their biological characterisation was aimed to analyse the hemocompatibility, biocompatibility and proliferative capacity of human skin keratinocytes HaCaT cells and mouse fibroblast L929 cells for potential applications in skin tissue engineering.
2 Materials
The polymer, PCL of practical grade having molecular weight 9000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 with melt flow index of 22–24 g/10 min was purchased from Otto Chemie Pvt. Ltd. Mumbai, India, and medical grade PLLA of molecular weight 100–140 kDa was purchased from Nomisma Healthcare Pvt. Ltd. Gujarat, India. The graphene nanoplatelets of particle size 5 μm were purchased from Sigma-Aldrich. Stains used for confocal microscopy were Fluoroshield with DAPI from Sigma-Aldrich; and Acti-stain™ 555 Fluorescent Phalloidin from Cytoskeleton, Inc. Analytical grades of chloroform and diethyl ether from Thomas Baker were used. Sodium cacodylate and osmium tetroxide were purchased from Sigma-Aldrich. The haloarchaeal strain Halogeometricum borinquense E3 (AB904833) was isolated previously in our laboratory.32 All cell culture materials, media components and sodium hypochlorite were purchased from Himedia, India unless mentioned otherwise. The HaCaT cell line was a kind gift from NBIL, Bengaluru, India while the L929 fibroblasts were purchased from NCCS Pune, India.
000 g mol−1 with melt flow index of 22–24 g/10 min was purchased from Otto Chemie Pvt. Ltd. Mumbai, India, and medical grade PLLA of molecular weight 100–140 kDa was purchased from Nomisma Healthcare Pvt. Ltd. Gujarat, India. The graphene nanoplatelets of particle size 5 μm were purchased from Sigma-Aldrich. Stains used for confocal microscopy were Fluoroshield with DAPI from Sigma-Aldrich; and Acti-stain™ 555 Fluorescent Phalloidin from Cytoskeleton, Inc. Analytical grades of chloroform and diethyl ether from Thomas Baker were used. Sodium cacodylate and osmium tetroxide were purchased from Sigma-Aldrich. The haloarchaeal strain Halogeometricum borinquense E3 (AB904833) was isolated previously in our laboratory.32 All cell culture materials, media components and sodium hypochlorite were purchased from Himedia, India unless mentioned otherwise. The HaCaT cell line was a kind gift from NBIL, Bengaluru, India while the L929 fibroblasts were purchased from NCCS Pune, India.
3 Methods
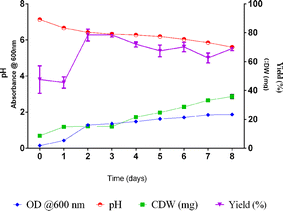
3.1 Production of PHBV polymer and preparation of blends
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C (Thermo Scientific Heraeus Multifuge X3 FR Centrifuge) to obtain the biomass. 4% NaOCl was added to the biomass and incubated on a rocker for 2–3 h. The mixture was centrifuged again using the same conditions. The supernatant was discarded, and the pellet was washed with distilled water and diethyl ether followed by reflux with hot chloroform. The chloroform-polymer mixture was poured into a clean glass Petri dish and kept in the cold room for the evaporation of the solvent to obtain the polymer film.
000 rpm for 15 min at 4 °C (Thermo Scientific Heraeus Multifuge X3 FR Centrifuge) to obtain the biomass. 4% NaOCl was added to the biomass and incubated on a rocker for 2–3 h. The mixture was centrifuged again using the same conditions. The supernatant was discarded, and the pellet was washed with distilled water and diethyl ether followed by reflux with hot chloroform. The chloroform-polymer mixture was poured into a clean glass Petri dish and kept in the cold room for the evaporation of the solvent to obtain the polymer film.3.2 Preparation of PHBV derived blend and composite
Halogeometricum borinquense E3 derived PHBV, was blended by using 1% (w/v) of the polymer with 1% (w/v) PLLA, 1% (w/v) PCL, and 0.3% (w/v) GNP. The polymers were dissolved in chloroform at 60 °C till homogenous using a vortex mixer. The mixture was poured into a clean dry glass Petri dish. The composite containing the GNPs was sonicated for 5 minutes before pouring to obtain a uniform dispersion of the GNPs in the solution. The dispersions were left undisturbed for 24 hours in the cold room for the solvent to evaporate and form a film. The average thickness of each film was measured using a micrometer screw gauge.3.3 Physicochemical characterization
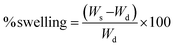 | (A1) |
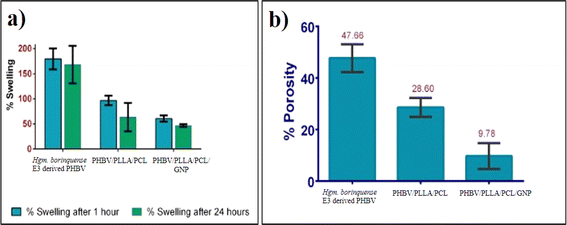
To calculate the porosity of the films, the films of known weight were immersed in absolute ethanol for 1 h and incubated at 30 °C. After incubation, the films were taken out and the excess ethanol was removed using a blotting paper. The films were weighed again. Porosity was calculated in terms of percentage using the formula below:33
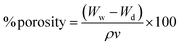 | (A2) |
3.4 Biological characterisation
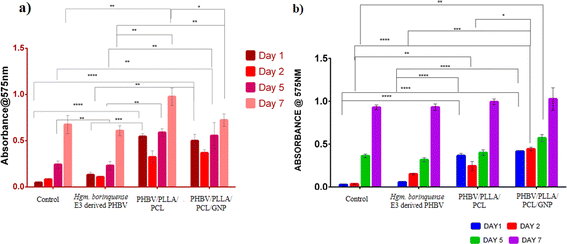
The cell lines were maintained in Dulbecco's Minimal Essential Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 0.1% antibiotic–antimycotic solution. The polymer films were sterilized by immersing in 70% ethanol and UV irradiated for 1 h on each side. Two washings with sterile PBS were given and the films were placed inside a tissue culture grade 48-well plate. A 70% confluent flask of each cell line was trypsinized. The cells were counted using a cell counter (Bio-Rad TC-20). 1 × 104 cells were seeded on the sterile films. The plate was incubated in a humidified environment of 5% CO2 at 37 °C. Media change was given every alternate day. The cell viability was evaluated spectrophotometrically by (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) (MTT) assay after day 1, day 2, day 5, and day 7. A concentration of 5 mg per ml MTT was added to the wells and incubated for 4 h. The purple formazan crystals were solubilized using dimethyl sulfoxide (DMSO). The plate was shaken for 5 min and the contents were transferred to a 96-well microtiter dish. The absorbance was read at 575 nm. Cells grown in a tissue culture grade 48 well were considered as control.34,35
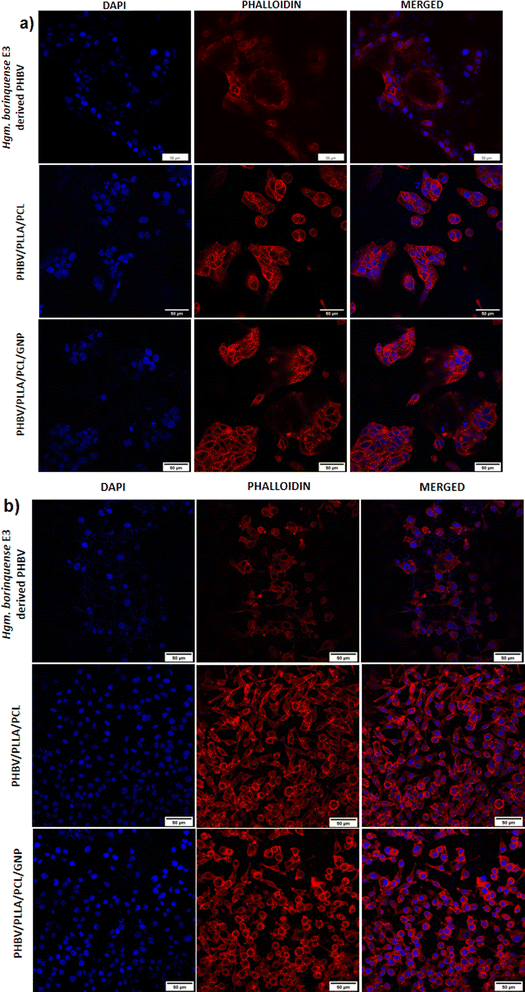
For confocal microscopy, the films containing the cells were fixed in 4% paraformaldehyde (pH 7.0) in PBS. The samples were washed 2–3 times with PBS. The cells were permeabilized with 0.1% Triton-X-100 followed by another round of washing. The samples were stained with Acti-stain 555 Fluorescent Phalloidin in the dark for 30 min at room temperature. 2–3 washes of PBS were given, and the samples were counterstained with DAPI and observed under a confocal microscope (Olympus Corporation FV3000).37,38
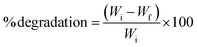 | (A3) |
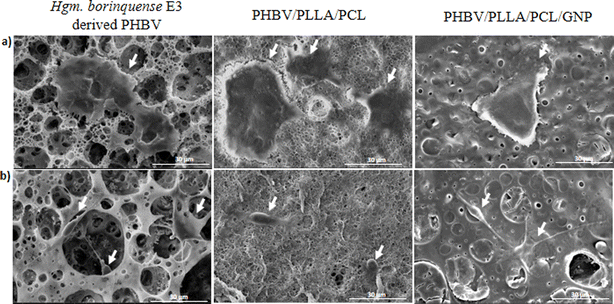
The ultrastructure of the degraded films was observed by SEM (ESI data S3†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution of the blood was made with sterile normal saline (0.85% NaCl). The films were incubated in normal saline for 30 min at 37 °C. The saline was carefully decanted out and diluted blood was added to the films. The films were incubated at 37 °C for 60 min. After the incubation, the mixture was centrifuged at 2000 rpm for 10 min. The absorbance of the supernatant was read at 545 nm. Blood incubated with sodium carbonate and normal saline was considered a positive and negative control respectively. The degree of hemolysis was evaluated as follows:
10 dilution of the blood was made with sterile normal saline (0.85% NaCl). The films were incubated in normal saline for 30 min at 37 °C. The saline was carefully decanted out and diluted blood was added to the films. The films were incubated at 37 °C for 60 min. After the incubation, the mixture was centrifuged at 2000 rpm for 10 min. The absorbance of the supernatant was read at 545 nm. Blood incubated with sodium carbonate and normal saline was considered a positive and negative control respectively. The degree of hemolysis was evaluated as follows:
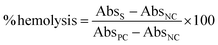 | (A4) |
4 Statistics
All experiments were conducted in triplicates. The quantitative data is expressed as mean ± standard error of mean. Statistical analysis was done using Two-way ANOVA with Tukey's multiple comparisons test in GraphPad Prism 6 (GraphPad Software Inc) and Origin Pro 2023b (Origin Lab Corporation). Values of p < 0.05 were considered significant.5 Results and discussions
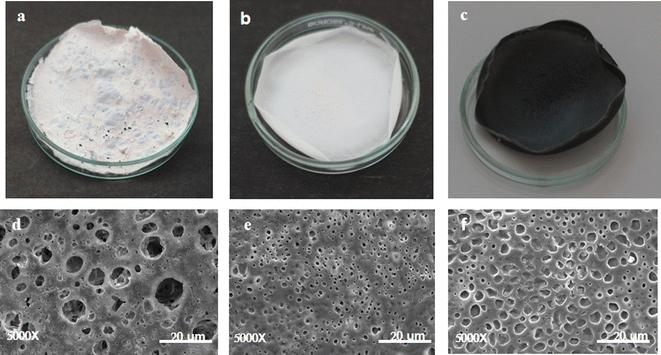
5.1 Production of PHBV polymer and preparation of blends and composites
| Sr. no | Blend | Composition | Average thickness (μm) |
|---|---|---|---|
| 1 | Native polymer | Halogeometricum borinquense E3 derived polymer | 77 |
| 2 | PHBV/PLLA/PCL | 1% w/v Halogeometricum borinquense E3 derived polymer + 1% w/v poly-L-Lactic acid + 1% w/v poly-ε-caprolactone (PCL) | 110 |
| 3 | PHBV/PLLA/PCL/GNP | 1% w/v Halogeometricum borinquense E3 derived polymer + 1% w/v poly-L-Lactic acid + 1% w/v poly-ε-caprolactone (PCL) + 0.3% w/v graphene nanoplatelets (GNP) | 120 |
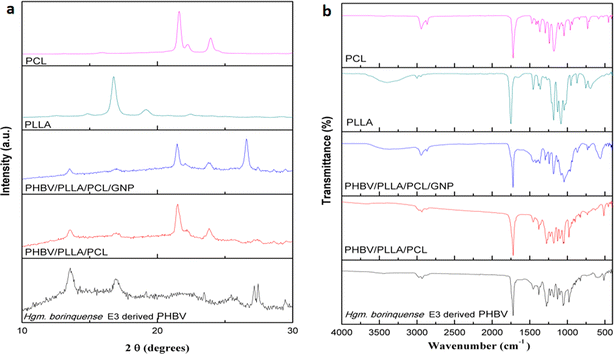
5.2 Physicochemical characterisation
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of ester bonds. The characteristic peak for symmetrical wagging of CH3 group and C–O stretch were observed at 1386 cm−1 and in the region 1283 to 980 cm−1, respectively.51
O of ester bonds. The characteristic peak for symmetrical wagging of CH3 group and C–O stretch were observed at 1386 cm−1 and in the region 1283 to 980 cm−1, respectively.51In the FT-IR spectra of PHBV/PLLA/PCL/GNP nanocomposite the intense peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of ester group at 1726 cm−1 shows the presence of PHBV. The medium intensity C–O stretching peaks are broadened due to interaction with GNP and are observed in the region 980–1350 cm−1. Multiple small peaks were obtained in the region of 1500 cm−1 contributed by GNP. This is in agreement with the findings of Ahmed J. et al. (2021).52 The changes in the peak intensity of the PHBV/PLLA/PCL/GNP could be related to the action of weak van der Waals forces.53
O of ester group at 1726 cm−1 shows the presence of PHBV. The medium intensity C–O stretching peaks are broadened due to interaction with GNP and are observed in the region 980–1350 cm−1. Multiple small peaks were obtained in the region of 1500 cm−1 contributed by GNP. This is in agreement with the findings of Ahmed J. et al. (2021).52 The changes in the peak intensity of the PHBV/PLLA/PCL/GNP could be related to the action of weak van der Waals forces.53
The FT-IR spectra of PLLA shows the characteristic broad peak corresponding to O–H stretch at 3400 cm−1. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of ester and C–O stretching peaks were observed in the region 1757 cm−1 and 1200–1069 cm−1, respectively and the characteristic peak for symmetrical wagging of CH3 group with medium intensity was observed at 1374 cm−1. Similarly for PCL the C
O of ester and C–O stretching peaks were observed in the region 1757 cm−1 and 1200–1069 cm−1, respectively and the characteristic peak for symmetrical wagging of CH3 group with medium intensity was observed at 1374 cm−1. Similarly for PCL the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of ester and C–O stretching peaks were observed in the region 1725 cm−1 and 1246–1050 cm−1 respectively. In FT-IR spectra, the C–H stretching peaks were observed in the region 2975–2865 cm−1 with the characteristic asymmetric and symmetric stretch of C–H corresponding to CH3 at 2931 cm−1 and 2865 cm−1 respectively. The C
O of ester and C–O stretching peaks were observed in the region 1725 cm−1 and 1246–1050 cm−1 respectively. In FT-IR spectra, the C–H stretching peaks were observed in the region 2975–2865 cm−1 with the characteristic asymmetric and symmetric stretch of C–H corresponding to CH3 at 2931 cm−1 and 2865 cm−1 respectively. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of PHBV, PLLA and PCL merged together and gave an intense peak at 1723 cm−1 showing the highly ordered crystalline structure. The peak for symmetrical wagging of CH3 group with medium intensity was observed at 1374 cm−1 and the medium intensity C–O stretch were observed in the region 1282–982 cm−1.
O of PHBV, PLLA and PCL merged together and gave an intense peak at 1723 cm−1 showing the highly ordered crystalline structure. The peak for symmetrical wagging of CH3 group with medium intensity was observed at 1374 cm−1 and the medium intensity C–O stretch were observed in the region 1282–982 cm−1.
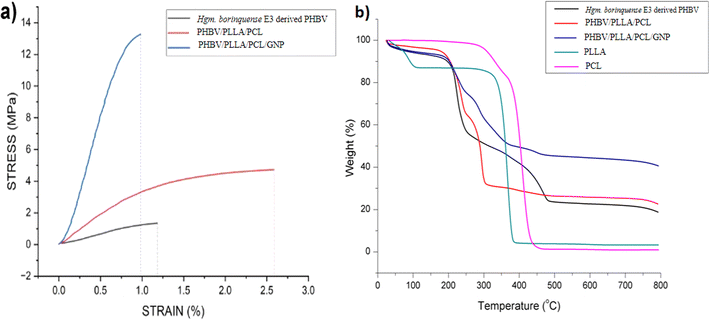 | ||
| Fig. 4 (a) Stress vs. strain curve of PHBV derived from Halogeometricum borinquense E3 and its blend/composite. (b) Thermogravimetric curve of the haloarchaeal PHBV and its blend/composite45 from 25 °C to 800 °C. | ||
| Sample ID | Young's modulus (MPa) | Ultimate tensile strength (UTS) (MPa) | Elongation at break (%) |
|---|---|---|---|
| Halogeometricum borinquense E3 derived PHBV | 148.25 ± 2.1 | 1.42 ± 0.3 | 1.03 ± 0.2 |
| PHBV/PLLA/PCL | 352.75 ± 30 | 5.1 ± 0.5 | 4.11 ± 2.2 |
| PHBV/PLLA/PCL/GNP | 396.25 ± 42 | 13.94 ± 1.2 | 0.88 ± 0.1 |
The DSC curves for PHBV and its blends are shown in ESI data S2.† PCL shows an endothermic peak corresponding to its melting point (Tm) at 68 °C. For PLLA glass transition temperature (Tg) is observed at 49 °C and Tm at 156 °C. The absence of cold crystallization peak shows the absence of less stable α′ state in PLLA. The presence of an endothermic peak at 73 °C may be due to vaporization of solvent molecule which is supported by ∼13% wt loss in TGA curve below 100 °C. The DSC scan of PHBV shows Tc at 64 °C and a premelting peak near 133 °C which is just before the Tm at 144 °C. For PHBV/PLLA/PCL blend a shift in baseline corresponding to its Tg is observed at 57 °C and a flow temperature at 157 °C. The absence of a sharp peak corresponding to the melting shows an increase in the amorphous nature of the blend. Similarly for PHBV/PLLA/PCL/GNP composites Tg is observed at a higher temperature i.e. at 59 °C as compared to PHBV which is usually between −5 to 5 °C. This shows the more ordered state of polymeric chains. The flow temperature is observed at 157 °C.
5.3 Biological characterisation
| Sample | Absorbance | % hemolysis | Remarks |
|---|---|---|---|
| Hgm. borinquense E3 derived PHBV | 0.01 | 0.0391 | Highly hemocompatible |
| PHBV/PLLA/PCL | 0.018 | 0.3522 | Highly hemocompatible |
| PHBV/PLLA/PCL/GNP | 0.037 | 1.0958 | Highly hemocompatible |
| Positive control | 2.564 | NA | |
| Negative control | 0.009 | NA |
6 Conclusion
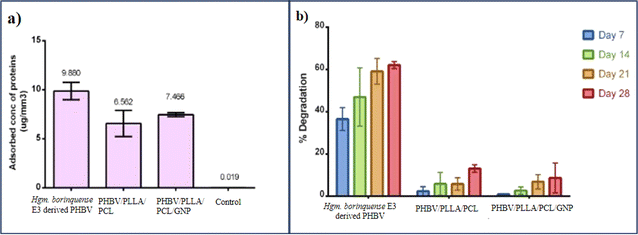
The current study focussed on the development of a blend/nanocomposite using an innately synthesized copolymer PHBV by Halogeometricum borinquense E3. The mechanical strength of the haloarchaeal PHBV was successfully improved by blending with PLLA, PCL and/or reinforcing it with 0.3% w/v of GNP. A comparative study of its physicochemical and biological properties was carried out. The haloarchaeal PHBV had a higher porosity (47.67%) and demonstrated greater swelling than the PHBV/PLLA/PCL blend, followed by the PHBV/GNP composite. Halogeometricum borinquense E3 derived PHBV also adsorbed the maximum proteins per mm3 of its surface area. The trend that was seen in the case of protein adsorption is native PHBV > PHBV/PLLA/PCL/GNP composite > PHBV/PLLA/PCL. The cell viability of the PHBV/PLLA/PCL was significantly higher than the tissue culture grade control and even native PHBV. The incorporation of GNP into the composite did not cause any significant change in the viability of the cells or hemocompatibility. Therefore, this nanocomposite can be used as potential novel biomaterial in skin tissue regeneration.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Anasuya Ganguly and Judith M. Braganςa are grateful to Department of Biotechnology, Ministry of Science and Technology, Government of India for funding under DBT Builder – BITS Pilani KK Birla Goa Campus Interdisciplinary Life Science Programme for Advance Research and Education (Level III) BT/INF/22/SP2543/2021. Judith M. Braganca also thanks DST&WM, Government of Goa for the Research Grant. The authors are grateful to the Central Sophisticated Instrumentation Facility (CSIF), BITS Pilani Goa Campus. The authors would like to acknowledge Ms Mamta Mestry, Technical Assistant, Department of Chemical Engineering BITS Pilani KK Birla Goa Campus, for her help in DSC-TGA and FTIR analysis. The authors are grateful to Ms Navodipa Bhattacharya and Prof. Dibakar Chakrabarty for their help in bicinchoninic acid (BCA) assay. Prajakta Praveen Bhende thanks Birla Institute of Technology and Sciences, Pilani, KK Birla Goa Campus for the Institute Fellowship.References
- D. Kucera, I. Pernicová and A. Kovalcik, et al., Characterization of the promising poly(3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila, Bioresour. Technol., 2018, 256, 552–556, DOI:10.1016/j.biortech.2018.02.062.
- M. R. Singh, S. Patel and D. Singh, Natural Polymer-based Hydrogels as Scaffolds for Tissue Engineering, Elsevier Inc., 2016, DOI:10.1016/B978-0-323-42865-1.00009-X.
- H. M. Chang, Z. H. Wang and H. N. Luo, et al., Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)- based scaffolds for tissue engineering, Braz. J. Med. Biol. Res., 2014, 47(7), 533–539, DOI:10.1590/1414-431X20143930.
- P. X. Ma, Scaffolds for tissue fabrication, Mater. Today, 2004, 7(5), 30–40, DOI:10.1016/S1369-7021(04)00233-0.
- J. Możejko-Ciesielska and R. Kiewisz, Bacterial polyhydroxyalkanoates: Still fabulous?, Microbiol. Res., 2016, 192(2016), 271–282, DOI:10.1016/j.micres.2016.07.010.
- S. Mohapatra, S. Maity and H. R. Dash, et al., Bacillus and biopolymer: Prospects and challenges, Biochem. Biophys. Rep., 2017, 12, 206–213, DOI:10.1016/j.bbrep.2017.10.001.
- M. E. Grigore, R. M. Grigorescu, L. Iancu, R. M. Ion, C. Zaharia and E. R. Andrei, Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: a review, J. Biomater. Sci., Polym. Ed., 2019, 30(9), 695–712, DOI:10.1080/09205063.2019.1605866.
- A. Anjum, M. Zuber, K. M. Zia, A. Noreen, M. N. Anjum and S. Tabasum, Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements, Int. J. Biol. Macromol., 2016, 89, 161–174, DOI:10.1016/J.IJBIOMAC.2016.04.069.
- S. DasSarma, J. A. Coker and P. DasSarma, Archaea (overview), Encyclopedia of Microbiology, 3rd edn, 2009, pp. 1–23, DOI:10.1016/B978-012373944-5.00108-5.
- B. Stres, M. J. Bonete, R. M. Martínez-Espinosa, I. Mahne and H. Bothe, Organisms of the Nitrogen Cycle Under Extreme Conditions: Low Temperature, Salinity, pH Value and Water Stress, Biology of the Nitrogen Cycle, 2007, 369–379, DOI:10.1016/B978-044452857-5.50025-4.
- G. Y. A. Tan, C. L. Chen and L. Li, et al., Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review, Polymers, 2014, 6(3), 706–754, DOI:10.3390/POLYM6030706.
- J. Ramier, T. Bouderlique and O. Stoilova, et al., Biocomposite scaffolds based on electrospun poly(3-hydroxybutyrate) nanofibers and electrosprayed hydroxyapatite nanoparticles for bone tissue engineering applications, Mater. Sci. Eng., C, 2014, 38(1), 161–169, DOI:10.1016/j.msec.2014.01.046.
- J. Sun, J. Wu, H. Li and J. Chang, Macroporous poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrices for cartilage tissue engineering, Eur. Polym. J., 2005, 41(10), 2443–2449, DOI:10.1016/J.EURPOLYMJ.2005.04.039.
- N. Pramanik, T. Mitra and M. Khamrai, et al., Characterization and evaluation of curcumin loaded guar gum/polyhydroxyalkanoates blend films for wound healing applications, RSC Adv., 2015, 5(78), 63489–63501, 10.1039/C5RA10114J.
- S. J. Armstrong, M. Wiberg, G. Terenghi and P. J. Kingham, ECM Molecules Mediate Both Schwann Cell Proliferation and Activation to Enhance Neurite Outgrowth, Tissue Eng., Part A, 2007, 13(12), 2863–2870, DOI:10.1089/TEN.2007.0055.
- F. Opitz, K. Schenke-Layland and W. Richter, et al., Tissue engineering of ovine aortic blood vessel substitutes using applied shear stress and enzymatically derived vascular smooth muscle cells, Ann. Biomed. Eng., 2004, 32(2), 212–222, DOI:10.1023/B:ABME.0000012741.85600.F1/METRICS.
- G. Q. Chen and Q. Wu, The application of polyhydroxyalkanoates as tissue engineering materials, Biomaterials, 2005, 26(33), 6565–6578, DOI:10.1016/J.BIOMATERIALS.2005.04.036.
- Y. He, Z. Hu and M. Ren, et al., Evaluation of PHBHHx and PHBV/PLA fibers used as medical sutures, J. Mater. Sci.: Mater. Med., 2014, 25(2), 561–571, DOI:10.1007/S10856-013-5073-4/METRICS.
- Ł. Kaniuk and U. Stachewicz, Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications, ACS Biomater. Sci. Eng., 2021, 7(12), 5339–5362, DOI:10.1021/ACSBIOMATERIALS.1C00757.
- J. Wu, J. Sun and J. Liu, Evaluation of PHBV/calcium silicate composite scaffolds for cartilage tissue engineering, Appl. Surf. Sci., 2014, 317, 278–283, DOI:10.1016/j.apsusc.2014.08.101.
- A. D. Dalgic, D. Atila, A. Karatas, A. Tezcaner and D. Keskin, Diatom shell incorporated PHBV/PCL-pullulan co-electrospun scaffold for bone tissue engineering, Mater. Sci. Eng., C, 2019, 100, 735–746, DOI:10.1016/j.msec.2019.03.046.
- R. Naseem, L. Zhao, Y. Liu and V. V. Silberschmidt, Experimental and computational studies of poly-L-lactic acid for cardiovascular applications: recent progress, Mech. Adv. Mater. Mod. Process., 2017, 3(1), 1–18, DOI:10.1186/S40759-017-0028-Y.
- E. Capuana, F. Lopresti, M. Ceraulo and V. La Carrubba, Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications, Polym, 2022, 14, 1153, DOI:10.3390/POLYM14061153.
- M. Jamal, F. Sharif and M. M. Shozab, et al., Development of Biocompatible Electrospun PHBV-PLLA Polymeric Bilayer Composite Membranes for Skin Tissue Engineering Applications, Molecules, 2024, 29(9), 2049, DOI:10.3390/molecules29092049.
- P. Goodarzi, K. Falahzadeh and M. Nematizadeh, et al., Tissue engineered skin substitutes, Adv. Exp. Med. Biol., 2018, 1107, 143–188, DOI:10.1007/5584_2018_226/COVER.
- S. R. Shin, Y. C. Li and H. L. Jang, et al., Graphene-based materials for tissue engineering, Adv. Drug Delivery Rev., 2016, 105, 255–274, DOI:10.1016/J.ADDR.2016.03.007.
- S. Darvishi, S. Ahadian, H. Savoji and H. Savoji, Graphene-Based Nanomaterials in Tissue Engineering and Regenerative Medicine, Handb Graphene, 2019, vol. 2, pp. 637–658, DOI:10.1002/9781119468455.ch38.
- Y. Chang, S. T. Yang and J. H. Liu, et al., In vitro toxicity evaluation of graphene oxide on A549 cells, Toxicol. Lett., 2011, 200(3), 201–210, DOI:10.1016/j.toxlet.2010.11.016.
- B. Salesa, A. Tuñón-Molina, A. Cano-Vicent, M. Assis, J. Andrés and Á. Serrano-Aroca, Graphene Nanoplatelets: In Vivo and In Vitro Toxicity, Cell Proliferative Activity, and Cell Gene Expression, Appl. Sci., 2022, 12(2), 720, DOI:10.3390/app12020720.
- P. Suvarnaphaet, S. Sasivimolkul, C. Sukkasem, et al., Biodegradable Electrode patch made of Graphene/PHA for ECG detecting Applications, in 2019 12th Biomedical Engineering International Conference (BMEiCON), IEEE, 2019, pp. 1–5, DOI:10.1109/BMEiCON47515.2019.8990243.
- M. Moschetta, M. Chiacchiaretta and F. Cesca, et al., Graphene Nanoplatelets Render Poly(3-Hydroxybutyrate) a Suitable Scaffold to Promote Neuronal Network Development, Front. Neurosci., 2021, 15, 1–12, DOI:10.3389/fnins.2021.731198.
- B. B. Salgaonkar and J. M. Bragança, Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricum borinquense strain E3, Int. J. Biol. Macromol., 2015, 78, 339–346, DOI:10.1016/j.ijbiomac.2015.04.016.
- A. I. Aghmiuni, S. Heidari Keshel, F. Sefat and A. Akbarzadeh Khiyavi, Quince seed mucilage-based scaffold as a smart biological substrate to mimic mechanobiological behavior of skin and promote fibroblasts proliferation and h-ASCs differentiation into keratinocytes, Int. J. Biol. Macromol., 2020, 142, 668–679, DOI:10.1016/J.IJBIOMAC.2019.10.008.
- E. I. Shishatskaya, E. D. Nikolaeva, O. N. Vinogradova and T. G. Volova, Experimental wound dressings of degradable PHA for skin defect repair, J. Mater. Sci.: Mater. Med., 2016, 27(11), 1, DOI:10.1007/s10856-016-5776-4.
- P. Sangsanoh, N. Israsena, O. Suwantong and P. Supaphol, Effect of the surface topography and chemistry of poly(3-hydroxybutyrate) substrates on cellular behavior of the murine neuroblastoma Neuro2a cell line, Polym. Bull., 2017, 74(10), 4101–4118, DOI:10.1007/s00289-017-1947-9.
- A. Suslu, A. Z. Albayrak, E. Bayir, A. Sendemir Urkmez and U. Cocen, In Vitro Biocompatibility and Antibacterial Activity of Electrospun Ag Doped HAp/PHBV Composite Nanofibers, Int. J. Polym. Mater. Polym. Biomater., 2015, 64(9), 465–473, DOI:10.1080/00914037.2014.977892.
- A. L. Rivera-Briso, F. L. Aachmann, V. Moreno-Manzano and Á. Serrano-Aroca, Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications, Int. J. Biol. Macromol., 2020, 143, 1000–1008, DOI:10.1016/J.IJBIOMAC.2019.10.034.
- K. T. Shalumon, K. H. Anulekha, K. P. Chennazhi, H. Tamura, S. V. Nair and R. Jayakumar, Fabrication of chitosan/poly(caprolactone) nanofibrous scaffold for bone and skin tissue engineering, Int. J. Biol. Macromol., 2011, 48(4), 571–576, DOI:10.1016/j.ijbiomac.2011.01.020.
- M. F. Abazari, Z. S. Karizi, N. Hajati-Birgani, M. Kohandani, S. Torabinejad, F. Nejati, N. Nasiri, M. H. Maleki, H. Mohajerani and V. Mansouri, Curcumin-loaded PHB/PLLA nanofibrous scaffold supports osteogenesis in adipose-derived stem cells in vitro, Polym. Adv. Technol., 2021, 32(9), 3563–3571, DOI:10.1002/pat.5366.
- F. Schlottmann, V. Bucan, P. M. Vogt and N. Krezdorn, A Short History of Skin Grafting in Burns: From the Gold Standard of Autologous Skin Grafting to the Possibilities of Allogeneic Skin Grafting with Immunomodulatory Approaches, Medicina, 2021, 57(3), 225, DOI:10.3390/medicina57030225.
- S. Malfatti, B. J. Tindall and S. Schneider, et al., Complete genome sequence of Halogeometricum borinquense type strain (PR3T), Stand. Genomic Sci., 2009, 1(2), 150, DOI:10.4056/SIGS.23264.
- R. Mahansaria, S. Bhowmik and A. Dhara, et al., Production enhancement of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Halogeometricum borinquense, characterization of the bioplastic and desalination of the bioreactor effluent, Process Biochem., 2020, 94, 243–257, DOI:10.1016/J.PROCBIO.2020.04.004.
- B. Salgaonkar and J. Bragança, Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), Bioengineering, 2017, 4(2), 50, DOI:10.3390/bioengineering4020050.
- L. Suamte, A. Tirkey, J. Barman and P. Jayasekhar Babu, Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications, Smart Mater. Manuf., 2023, 1, 100011, DOI:10.1016/j.smmf.2022.100011.
- R. Naseem, G. Montalbano, M. J. German, A. M. Ferreira, P. Gentile and K. Dalgarno, Influence of PCL and PHBV on PLLA Thermal and Mechanical Properties in Binary and Ternary Polymer Blends, Molecules, 2022, 27, 7633, DOI:10.3390/MOLECULES27217633.
- G. Lutzweiler, A. Ndreu Halili and N. Engin Vrana, The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation, Pharmaceutics, 2020, 12(7), 602, DOI:10.3390/pharmaceutics12070602.
- Z. Zhang, Y. Feng, L. Wang, D. Liu, C. Qin and Y. Shi, A review of preparation methods of porous skin tissue engineering scaffolds, Mater. Today Commun., 2022, 32, 104109, DOI:10.1016/j.mtcomm.2022.104109.
- Y. Zhou, M. Zhao, H. Guo, Y. Li, Q. Liu and B. Deng, Morphology and crystallization behavior of poly(3-hydroxybutyrate- co -3-hydroxyvalerate)/polyhedral oligomeric silsesquioxane hybrids, RSC Adv., 2019, 9(15), 8146–8158, 10.1039/C8RA09281H.
- R. Balu, T. S. S. Kumar, M. Ramalingam and S. Ramakrishna, Electrospun Polycaprolactone/Poly(1,4-butylene adipate-co-polycaprolactam) Blends: Potential Biodegradable Scaffold for Bone Tissue Regeneration, J. Biomater. Tissue Eng., 2011, 1(1), 30–39, DOI:10.1166/jbt.2011.1004.
- J. Wang, L. Zhan, X. Zhang, R. Wu, L. Liao and J. Wei, Silver Nanoparticles Coated Poly(L-Lactide) Electrospun Membrane for Implant Associated Infections Prevention, Front. Pharmacol, 2020, 11, 431, DOI:10.3389/fphar.2020.00431.
- R. Naseem, G. Montalbano, M. J. German, A. M. Ferreira, P. Gentile and K. Dalgarno, Influence of PCL and PHBV on PLLA Thermal and Mechanical Properties in Binary and Ternary Polymer Blends, Molecules, 2022, 27(21), 7633, DOI:10.3390/molecules27217633.
- J. Ahmed, M. Z. Mulla, A. Vahora, A. Bher and R. Auras, Polylactide/graphene nanoplatelets composite films: Impact of high-pressure on topography, barrier, thermal, and mechanical properties, Polym. Compos., 2021, 42(6), 2898–2909, DOI:10.1002/PC.26023.
- S. Khasim, Polyaniline-Graphene nanoplatelet composite films with improved conductivity for high performance X-band microwave shielding applications, Results Phys., 2019, 12, 1073–1081, DOI:10.1016/J.RINP.2018.12.087.
- P. Kuppan, K. S. Vasanthan, D. Sundaramurthi, U. M. Krishnan and S. Sethuraman, Development of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibers for skin tissue engineering: Effects of topography, mechanical, and chemical stimuli, Biomacromolecules, 2011, 12(9), 3156–3165, DOI:10.1021/bm200618w.
- D. Alsafadi, O. Al-Mashaqbeh, A. Mansour and M. Alsaad, Optimization of nitrogen source supply for enhanced biosynthesis and quality of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by extremely halophilic archaeon Haloferax mediterranei, Microbiologyopen, 2020, 9(8), e1055, DOI:10.1002/MBO3.1055.
- J. J. Elsner and M. Zilberman, Novel antibiotic-eluting wound dressings: An in vitro study and engineering aspects in the dressing's design, J. Tissue Viability, 2010, 19(2), 54–66, DOI:10.1016/j.jtv.2009.11.001.
- W. J. Chong, S. Shen and Y. Li, et al., Biodegradable PLA-ZnO nanocomposite biomaterials with antibacterial properties, tissue engineering viability, and enhanced biocompatibility, Smart Mater. Manuf., 2023, 1, 100004, DOI:10.1016/J.SMMF.2022.100004.
- B. Mensah, D. S. Konadu, F. Nsaful, P. N. Angnunavuri and S. Kwofie, A systematic study of the effect of graphene oxide and reduced graphene oxide on the thermal degradation behavior of acrylonitrile-butadiene rubber in air and nitrogen media, Sci. Afr., 2023, 19, e01501, DOI:10.1016/j.sciaf.2022.e01501.
- B. Sitharaman, S. Kanakia and J. Toussaint, et al., Physicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agent, Int. J. Nanomed., 2013, 2821, DOI:10.2147/IJN.S47062.
- J. Li and D. J. Mooney, Designing hydrogels for controlled drug delivery, Nat. Rev. Mater., 2016, 1(12), 1–17, DOI:10.1038/NATREVMATS.2016.71.
- B. Yang, B. Nagarajan and P. Mertiny, Characterization of swelling behavior of carbon nano-filler modified polydimethylsiloxane composites, J. Elastomers Plast., 2021, 53(8), 955, DOI:10.1177/00952443211006156.
- A. O. Basar, S. Castro, S. Torres-Giner, J. M. Lagaron and H. Turkoglu Sasmazel, Novel poly($ε$-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug, Mater. Sci. Eng., C, 2017, 81, 459–468, DOI:10.1016/J.MSEC.2017.08.025.
- P. P. Bhende, R. Chauhan, S. Waigaonkar, J. M. Bragança and A. Ganguly, Composites of Bacillus megaterium H16 derived poly-3-hydroxybutyrate as a biomaterial for skin tissue engineering, Int. J. Biol. Macromol., 2023, 244, 125355, DOI:10.1016/j.ijbiomac.2023.125355.
- K. B. Narayanan, G. T. Park and S. S. Han, Electrospun poly(vinyl alcohol)/reduced graphene oxide nanofibrous scaffolds for skin tissue engineering, Colloids Surf., B, 2020, 191, 110994, DOI:10.1016/j.colsurfb.2020.110994.
- N. Mahmoudi, N. Eslahi and A. Mehdipour, et al., Temporary skin grafts based on hybrid graphene oxide-natural biopolymer nanofibers as effective wound healing substitutes: pre-clinical and pathological studies in animal models, J. Mater. Sci.: Mater. Med., 2017, 28(5), 73, DOI:10.1007/s10856-017-5874-y.
- S. N. Kalva, Y. B. Dalvi and N. K. P, et al., Air-jet spun PHBV/PCL blend tissue engineering scaffolds exhibit improved mechanical properties and cell proliferation, Results Mater., 2023, 19, 100415, DOI:10.1016/j.rinma.2023.100415.
- S. G. Hu, C. H. Jou and M. C. Yang, Protein adsorption, fibroblast activity and antibacterial properties of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) grafted with chitosan and chitooligosaccharide after immobilized with hyaluronic acid, Biomaterials, 2003, 24(16), 2685–2693, DOI:10.1016/S0142-9612(03)00079-6.
- A. Zonari, M. T. Cerqueira and S. Novikoff, et al., Poly(hydroxybutyrate-co-hydroxyvalerate) Bilayer Skin Tissue Engineering Constructs with Improved Epidermal Rearrangement, Macromol. Biosci., 2014, 14(7), 977–990, DOI:10.1002/MABI.201400005.
- N. Kumar, D. Desagani and G. Chandran, et al., Biocompatible agarose-chitosan coated silver nanoparticle composite for soft tissue engineering applications, Artif. Cells, Nanomed., Biotechnol., 2018, 46(3), 637–649, DOI:10.1080/21691401.2017.1337021.
- A. M. Pinto, J. A. Moreira, F. D. Magalhães and I. C. Gonçalves, Polymer surface adsorption as a strategy to improve the biocompatibility of graphene nanoplatelets, Colloids Surf., B, 2016, 146, 818–824, DOI:10.1016/J.COLSURFB.2016.07.031.
- P. C. Caracciolo, M. I. Rial-Hermida, F. Montini-Ballarin, G. A. Abraham, A. Concheiro and C. Alvarez-Lorenzo, Surface-modified bioresorbable electrospun scaffolds for improving hemocompatibility of vascular grafts, Mater. Sci. Eng., C, 2017, 75, 1115–1127, DOI:10.1016/J.MSEC.2017.02.151.
- A. A. Shitole, P. S. Giram, P. W. Raut, P. P. Rade, A. P. Khandwekar, N. Sharma and B. Garnaik, Clopidogrel eluting electrospun polyurethane/polyethylene glycol thromboresistant, hemocompatible nanofibrous scaffolds, J. Biomater. Appl., 2019, 33(10), 1327–1347, DOI:10.1177/0885328219832984.
- J. Horakova, P. Mikes and A. Saman, et al., Comprehensive assessment of electrospun scaffolds hemocompatibility, Mater. Sci. Eng., C, 2018, 82, 330–335, DOI:10.1016/J.MSEC.2017.05.011.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00713a |
| This journal is © The Royal Society of Chemistry 2024 |