Open Access Article
Open Access ArticleNanofiber applications in microbial fuel cells for enhanced energy generation: a mini review
Fatma Yalcinkaya *a,
Rafael Torres-Mendieta
*a,
Rafael Torres-Mendieta b,
Jakub Hruzaa,
Andrea Vávrová
b,
Jakub Hruzaa,
Andrea Vávrová c,
Lucie Svobodová
c,
Lucie Svobodová d,
Andrea Pietrelli
d,
Andrea Pietrelli e and
Ioannis Ieropoulos
e and
Ioannis Ieropoulos *f
*f
aDepartment of Environmental Technology, Institute for Nanomaterials, Advanced Technologies and Innovation, Technical University of Liberec, Studentská 1402/2, 461 17 Liberec, Czech Republic. E-mail: fatma.yalcinkaya@tul.cz
bDepartment of Chemistry, Faculty of Science, Humanities and Education, Technical University of Liberec, Studentská 1402/2, 46117 Liberec, Czech Republic
cDepartment of Nursing and Emergency Care, Faculty of Health Studies, Technical University of Liberec, Studentská 1402/2, 46117 Liberec, Czech Republic
dDepartment of Material Science, Faculty of Mechanical Engineering, Technical University of Liberec, Studentská 1402/2, 46117 Liberec, Czech Republic
eUniversité de Lyon, INSA Lyon, Université Lyon 1, Ecole Centrale de Lyon, CNRS, Ampère, UMR5005, F-69621 Villeurbanne, France
fCivil, Maritime and Environmental Engineering Department, University of Southampton, Southampton, SO16 7QF, UK
First published on 18th March 2024
Abstract
Microbial fuel cells (MFCs) represent simple devices that harness the metabolic activities of microorganisms to produce electrical energy from diverse sources such as organic waste and sustainable biomass. Because of their unique advantage to generate sustainable energy, through the employment of biodegradable and repurposed waste materials, the development of MFCs has garnered considerable interest. Critical elements are typically the electrodes and separator. This mini-review article presents a critical assessment of nanofiber technology used as electrodes and separators in MFCs to enhance energy generation. In particular, the review highlights the application of nanofiber webs in each part of MFCs including anodes, cathodes, and membranes and their influence on energy generation. The role of nanofiber technology in this regard is then analysed in detail, focusing on improved electron transfer rate, enhanced biofilm formation, and enhanced durability and stability. In addition, the challenges and opportunities associated with integrating nanofibers into MFCs are discussed, along with suggestions for future research in this field. Significant developments in MFCs over the past decade have led to a several-fold increase in achievable power density, yet further improvements in performance and the exploration of cost-effective materials remain promising areas for further advancement. This review demonstrates the great promise of nanofiber-based electrodes and separators in future applications of MFCs.
1. Introduction
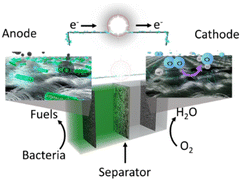
The utilization of biomass, particularly organic waste, is considered an environmentally friendly and sustainable approach to energy production, making it a valuable alternative source of renewable energy.1 Microbial fuel cells (MFCs) have gained increasing attention as promising bio-electrochemical systems that can convert chemical energy stored in organic compounds, such as acetate, sugars, nitrate, and ethanol,2,3 into electricity through the metabolic activity of microorganisms.4,5 MFCs offer numerous advantages over conventional fuel cells, including negating the need for expensive or exotic catalysts, such as platinum, and generating electricity from renewable sources of organic matter, including waste streams.6 Furthermore, MFCs have the potential to remove pollutants via the microbial metabolism thereby finding application in wastewater treatment, bioenergy generation, and biosensors.5,7,8By definition, the MFC is a device that converts the energy from organic compounds into electrical energy through the metabolic processes of microorganisms.9–12 The operation of MFCs is based on the transfer of electrons from the anode electrode to the cathode. This is achieved by electrochemically active bacteria, which oxidize organic matter in the anode compartment, releasing electrons and cations, eqn (1). The electrons flow through the external circuit to the cathode, where they combine with an oxidant to produce water (eqn (2)).7,13,14 Meanwhile, the protons migrate through the membrane to the cathode compartment, where they combine with the electrons and an oxidant (e.g., O2) to complete the reaction, as per the given chemical eqn (2).13
| C2H4O2 + 2H2O → 2CO2 + 8e− + 8H+ | (1) |
| 2O2 + 8H+ + 8e− → 4H2O (E0 = 1.23 V) | (2) |
In a conventional MFC, two half-cells – an anode and a cathode, are separated by an ion exchange membrane, as depicted in Fig. 1. The process of electricity generation in the MFC is sustained through a continuous consumption of an oxidising agent, e.g., oxygen, as indicated by eqn (1) and (2). The cathode compartment can work with either aqueous or atmospheric oxygen.15 Due to its high redox potential, oxygen is considered to be a suitable electron acceptor for the cathode in MFCs. The interest in microbial fuel cells has been consistently increasing over the last two decades.16
Despite these promising features and significant interest, the performance of MFCs is currently characterized by lower power density, when compared with chemical fuel cells, whose rates of reaction are naturally higher than biological processes; this has however driven the need for innovation to enhance performance. As with every real system, MFCs produce energy output that is lower than their theoretical maximum due to different electrochemical losses. The losses are due to resistance in materials, separator material, and electrolytes, leading to the lower power production of MFCs compared to their potential. According to Torres et al.,17 the primary obstacle for maximizing power output in microbial fuel cells (MFCs) is the reactor design, which must integrate anodes with a high surface area, low ohmic resistances, and minimal cathode potential losses. Developments in electrode and membrane materials are focused on enhancing MFC performance by seeking novel materials with improved capabilities.18–21 In recent years, nanofibers in MFCs have emerged as a possible pathway to enhancing performance. Carbon nanofibers (CNFs) are widely utilised as MFC electrodes due to their unique network structure and exceptional structural stability. The main challenges for MFC systems are cost reduction and productivity enhancement. Using nanofibers offers a viable option to tackle the main issues of reducing costs and increasing productivity in microbial fuel cell (MFC) systems.22,23 Nanofibers can be produced economically employing efficient methods and resources, leading to decreased production expenses. The customisable features enhance the optimisation of electrode and membrane materials, hence enhancing the performance and lifespan of MFC systems. Due to their small size, highly porous structure, tight pore size, and high specific surface area, nanofiber webs are ideal for integration into MFCs.24,25 Such properties offer several advantages in MFCs, including enhanced bacterial adhesion, mass transfer, and electron transfer efficiency.26,27 Fig. 2 illustrates the superior power generation advantages of CNFs anode compared to commercial carbon felt. For instance, incorporating nanofibers into the anode can promote microbial adhesion and increase surface area, resulting in faster electron transfer rates and higher power production.28,29 Tao et al.30 used a hierarchically structured textile polypyrrole/poly(vinyl alcohol-co-polyethylene) nanofibers/poly(ethylene terephthalate) (referred to PPy/NFs/PET) as an anode of MFC. The results showed the high surface roughness, porous and three-dimensional interconnecting conductive scaffold improved the colonization of Escherichia coli and electron transfer to the anode. The maximum power and current densities were 2420 mW m−2 and 5500 mA m−2, which is approximately 17 times higher compared to anode prepared without a nanofiber layer (144 mW m−2). It is clearly shown that the nanofiber effect on the colonization of bacteria is non-negligible.
 | ||
| Fig. 2 Composite anode of electrospun carbon nanofibers and hybrid carbon nanotubes facilitates microbial attachment, electron transfer, and exhibits superior conductivity and biocompatibility compared to commercial carbon felt (this figure has been reproduced from ref. 26 with permission from Elsevier publisher, copyright 2024). | ||
Integrating nanofibers in MFCs has demonstrated potential benefits for improving power density, current output, and durability of these cells. Nanofibers can be used as an anode material to facilitate electron transfer from bacteria to the electrode surface, a cathode material to enhance oxygen reduction, or a membrane material to separate the anode and cathode compartments. However, more research is needed to optimize the fabrication and integration of nanofibers in MFCs and to understand their long-term stability and performance under different operating conditions.
In the literature, CNFs have been widely studied in MFCs due to their excellent electrical conductivity and biocompatibility. One such study,31 employed activated electrospun carbon nanofibers (ACNFs) in an MFC as an alternative cathode catalyst to platinum (Pt) and conducted a performance comparison with plain carbon paper. It was found that chemically ACNFs showed better catalytic activity than that of the physically activated one with 78% more power generation. Chemically ACNFs with 8 M KOH generated oxygen reduction reaction (ORR) performance levels that contributed to 3.17 times more power than that of the carbon paper, 1.78 and 1.16 times more power generation than that of the physically activated ACNFs and the chemically activated ACNFs with 4 M KOH, respectively. Karra et al.32 utilized ACNFs as the anode material to stimulate bacterial biofilm growth, and improve MFC performance. The analysis of biofilm adhesion, both qualitatively and quantitatively, indicated that ACNFs outperformed other commonly used carbon anodes. The power density of the ACNFs was 1.13 and 3.18 times higher than that from granular activated carbon and carbon cloth anodes, respectively. Metal doped carbon nanofibers (MDCNFs) have also been explored for their use in MFCs due to their high electrical conductivity and catalytic activity. Bosch-Jimenez et al.33 have successfully prepared CNFs doped with metals such as Co, Ni or Fe which increased surface areas up to 573 m2 g−1. Adding metals increased mesoporosity and catalytic activity of cathode material. Manickam et al.29 used activated carbon nanofiber anodes in MFC. The preliminary tests in a single chamber MFC demonstrated a 10% increase in current densities to ∼2715 A m−3 compared to the highest maximum obtained so far. The bio-electrochemical performance of activated carbon nanofiber anodes was compared to commonly-used anodes like carbon cloth and granular activated carbon, and this anode architecture is expected to help overcome low power density issues that have limited the widespread adoption of MFCs.
Polymer nanofibers have been investigated for their use in MFCs due to their high surface area and flexibility. Polymeric polyvinylidene fluoride (PVDF)/Nafion composite membranes are good candidates as proton exchange membranes in MFCs due to their porosity, high specific surface area, tight pore size, chemical resistance, good electrical insulation, good thermal properties and its biocompatibility34,35 as shown in Fig. 2. When combining carbon nanofibers (CNFs) with Nafion 117, a commonly utilized membrane in Microbial Fuel Cells (MFCs), can alter membrane roughness, pore size, and porosity, consequently enhancing the power generated by the MFCs.36 The reduction in pore size and roughness of these nanocomposite membranes leads to the blockage of oxygen transfer from the cathode to the anode and impedes the migration of bacteria and other components from the anode to the cathode. Consequently, higher power production can be achieved. Chae et al.37 developed a sulfonated polyether ether ketone (SPEEK)-based composite proton exchange membrane reinforced with polyimide nanofibers for use in microbial electrolysis cells. The addition of the nanofiber layer not only enhances the dimensional stability of the SPEEK membrane but also improves its affinity for protons, all while reducing costs. Additionally, the composite membrane demonstrated superior hydrogen efficiency (electron to hydrogen) of 86.4 ± 14.7%, compared to 77.2 ± 10.3% observed with Nafion membranes.
In addition to the potential improvement of MFC performance, nanofiber technology can also contribute to the sustainability of MFCs by utilizing renewable feedstocks in nanofiber production. Nanofibers can be fabricated from various materials, such as carbon, metal, polymer, and ceramic, using different fabrication techniques, including electrospinning,38,39 melt spinning,40 force spinning,41 chemical vapour deposition,42 and template synthesis.43 The most common nanofiber production process is electrospinning due to several benefits compared to conventional techniques for producing nanofibers, including flexibility in choosing materials, ability in controlling fiber size and structure, and capacity for large-scale production. Electrospinning is a method that can produce continuous and uniform nanofibers from various polymers and composite materials, making it a popular choice for applications in various applications, including fuel cells.
2. Nanofiber technology for microbial fuel cells
Nanofiber technology has shown promise in the development of microbial fuel cells (MFCs) due to several advantages over traditional electrode materials. Nanofibers are ultra-thin fibers with diameters ranging from 1 to 100 nanometers that can be made from a variety of materials, including composites, polymers, metals, and ceramics.Fig. 3 shows the polymeric nanofibers which have fiber diameters between 67–185 nm (diameter measured using ImageJ program). Their high surface area-to-volume ratio, small diameter, and porosity render these suitable as electrode materials in MFCs. The high surface area of nanofibers allows for a larger number of microorganisms to attach to the electrode, resulting in improved performance.44 The small fiber diameter of nanofibers enhances the accessibility of microorganisms to the electrode surface, improving their metabolic activity and resulting in increased energy generation. The high porosity of nanofibers facilitates the diffusion of nutrients and oxygen to the microorganisms, which is essential for their growth and metabolism.45 Additionally, certain types of nanofiber materials, such as carbon nanotubes and graphene, have high electrical conductivity, facilitating the transfer of electrons between the microorganisms and the electrode and resulting in improved energy generation.46 Ho-Young et al.47 included conductive nanocomposite into anode of MFCs. Compared to commercial graphite felt, the nanocomposite anode showed a 1.8-fold increase in power density. The role of carbon nanofibers on MFCs is not only because of their conductivity, but also their adoption properties. The conductive carbon nanofibers can improve bacterial attachment and extracellular electron transfer simultaneously. For instance, Zhang et al.48 fabricated a bacteria/Multi-Walled Carbon Nanotube (MWCNT) hybrid biofilm by inserting the MWCNTs into the anode biofilm. The bacteria/MWCNTs biofilm was formed via an adsorption–filtration method. The start-up time was shortened by 53.8% while the current density, power density, and coulombic efficiency increased by 46.2%, 58.8% and 84.6%, respectively compared to naturally grown biofilm. Apparently, carbon-based nanofibers, including carbon nanotubes and graphene, are attractive due to their high electrical conductivity and adsorption properties. Other commonly used nanofiber materials in MFCs include metal oxides, such as titanium dioxide, and conductive polymers, such as polyaniline.
3. Applications of nanofiber technology in microbial fuel cells
Nanofiber technology can have different applications in MFCs, including the development of nanofiber-based anodes, cathodes, and separator membranes. Low charge transfer efficiency of electrodes and costly catalysts are limiting the development of MFC technology. Due to the unique properties of nanofibers explained in the previous section, these materials are promising alternative to conventional materials, which significantly impact the efficiency and performance of MFCs. In the following section, the application of nanofiber webs in various components of MFC is summarised.3.1. Nanofiber-based anodes
The anode part of MFCs mostly influences the microorganism attachment, biofilm formation, substrate oxidation, and electron transfer rate. For this reason, the anode half-cell has been the focus of research. A desirable anode should exhibit; high surface area and porosity to enhance bacterial attachment, capacity to enhance biofilm formation via a strong interconnection between microorganisms and the material, and good electrical conductivity.29,44,49 The ideal anode selection is summarized in Table 1. The material should have a networked structure to ensure stable attachment of the biofilm. Qualitative and quantitative biofilm adhesion analysis exhibited that activated carbon nanofibers showed better performance compared to granular activated carbon and carbon cloth anode.32 The combination of high porosity and short distances between the free surface and the bulk allows for improved nutrient access to the deep inner layers, optimizing the utilization of the available surface area. However, to enhance the properties of nanofibers in MFCs, the addition of various materials such as reduced graphene oxide (rGO), carbon nanotubes (CNTs), metals, and metal–organic frameworks is necessary. This addition provides improved conductivity, thermal stability, mechanical strength, and corrosion resistance to the nanofibers. For instance, using electrospinning and calcination techniques, nitrogen-doped carbon nanofibers anchored with iron nanoparticles (Fe/N-x@CNFs) have been developed as anode electrocatalysts with good electrocatalytic activity and biocompatibility.50 In another study,51 Mo-doped carbon nanofibers were prepared by using electrostatic spinning, followed by stabilization and carbonization. The Mo-doped carbon nanofibers anode delivered a maximum power density of 1287.38 mW m−2 while pristine carbon nanofiber delivered 649.69 mW m−2. Conductive nanofiber with a higher surface area could improve the conductivity of the anode for microorganism adherence on anodic electrodes.52 By using activated carbon nanofiber with carbon nanotubes in anode, the power density of the could increase by 180% compared to that of the commercial graphite felt,47 and 40% higher maximum power density compared to unmodified carbon cloth.53 The aligned carbon nanofiber-bacteria (ACNF-bacteria) hybrid exhibits a rich porous structure and a large specific surface area, resulting in significantly enhanced electrocatalytic performance compared to pristine carbon cloth (CC) anodes.22 The ACNF-bacteria hybrid has a maximum power density of 704 mW m−2, surpassing the performance of ACNF, CNF, and CC anodes by 1.7, 2.1, and 2 times, respectively. Jiang et al.54 employed a manganese cobalt metal–organic framework (MOFs) derived carbon nanofiber (CNF) anode electrode to improve electricity generation as well as pollutant removal. The modified CNF provides the anode's conductivity and increases the surface area and porous structure, allowing for more attachment sites for electroactive bacteria. This enhancement improved the electrocatalytic activity of bioanode and catalytic reduction capability of anaerobic microorganisms for Sb(V), leading to increased performance in microbial fuel cells. Wu et al.51 employed molybdenum (Mo) metal-doped carbon nanofiber (CNF) anode electrodes. The Mo–CNF anodes were produced through electrospinning and high-temperature carbonization processes. These Mo–CNF II anodes demonstrated an accelerated electron transfer rate and achieved a maximum power density of 1287.38 mW m−2, which was twice that of the pristine CNF anode. The enhancement is due to the improved microbial colonisation, electrocatalytic activity, and larger reaction surface areas enabled by the Mo–CNF structure. These characteristics not only enable direct electron transfer but also promote flavin-like mediated indirect electron transfer mechanisms.| Ideal properties | Effect on MFC performance | References |
|---|---|---|
| Anode electrode | ||
| Conductivity | Reduce resistance, improve electron transfer, lower losses | 66 and 67 |
| Improve electrochemical performance over plain carbon paper | ||
| Surface area | Enhance bacterial attachment | 68 and 69 |
| More biocatalysts from organic compounds oxidation (e.g., graphite felt yields higher output power than a graphite rod because of its increased surface area) | ||
| Porosity and pore structure | Maintain anoxic conditions for electricity generation in the anode | 68 and 70 |
| Large bio-accessible surface area | ||
| Thickness | Minimise resistance to electron transport from the biofilm to the anode | 71 |
| Stability and durability | pH shift tolerant conditions | 72 |
| Biocompatibility | Facilitate bacteria–electrode interaction and higher biomass | 73–75 |
| Electro catalytic activity | Enhancement on in situ oxidation of the microbial metabolites | 76 |
| Low cost | Feasibility of scale-up and commercial application | 77 |
| Mechanical strength | Better mechanical strength under a range of conditions by using carbonaceous and metallic materials (e.g., carbon paper, carbon rods, graphite felt, reticulated vitreous carbon, nickel sheets, stainless steel mesh, and copper sheets) | 68 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Cathode electrode | ||
| Conductivity | Polarization loss reduction | 78 |
| Oxygen reduction reaction enhancement | ||
| Stability and durability | pH shift tolerant | 72 |
| Low cost | Feasibility of scale-up and commercial application | 77 |
| Catalytic activity | Oxygen reduction reaction enhancement | 74 and 79 |
| Lower cathodic activation energy and increase the rate of reaction | ||
| Biocompatibility | Improve the biocathode biocompatibility | 78 |
| Active sites | Enhancement in number of active sites (e.g. pyridinic and pyrrolic nitrogen) to facilitate a more efficient transfer of electrons during the oxygen reduction reaction | 74 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Separator membrane | ||
| Stability | To be resilient and stable in acidic and alkaline conditions | 80 |
| Conduction | To conduct the protons to cathode, not electrons to fulfil the eqn (1) and (2) for energy generation | 81 |
| Impermeability to gases | To allow H+ to pass from the anode to the cathode side and be impermeable to gases like H2, O2, and N2 | 82 |
| Low cost | Feasibility of scale-up and commercial application | 80 and 83 |
| Hydrophilicity | To facilitate cationic transport and should also inhibit oxygen diffusion | 84 |
In another work,55 electrospun metal-doped CNF were employed as anode electrodes. The CNF served as carriers for metals, enhancing the surface area and creating a highly porous structure. Among the three different metals (Fe, Ni, Cu), the iron-doped CNF showed the maximum output power (641.96 mW m−2), providing a 7.62-fold increase compared to pristine CNF (Fig. 4). Differences in power output across various metals are due to variances in active sites on the carbon nanofiber surface, as well as differences in surface morphology, structure, and electronegativity. These differences influence the direct contact between the anode interface and extracellular proteins of electricity-producing microorganisms, affecting the degree to which the diffusion limit is surpassed. Therefore, significant differences have been observed in the improvement of bioelectrocatalytic performance with different metal anode materials. Furthermore, combining iron cobalt bimetallic metal–organic frameworks (FeCo-MOFs) with CNFs can enhance the power density up to 5300 mW m−2 since the synergistic effect between different metals in bimetallic MOFs improves the catalytic performance of MFC.56,57 The incorporation of bimetallic MOFs enhances both the strength and flexibility of CNFs. This combination enhances the electrocatalytic efficiency of bimetallic MOFs and avoids the agglomeration of nanoparticles. Barakat et al.58 conducted a comparative analysis of various anode materials, including cobalt (Co)-doped carbon nanofibers (CNFs), single and double layer active Co-free CNF mats, carbon cloth, and carbon paper. The findings indicated that the inclusion of additional Co significantly increased the power density, reaching a maximum of 21 mW m−2, as illustrated in Fig. 5. Adding cobalt (Co) to carbon nanofibers (CNFs) helps reduce the negative impact of the metal on microorganisms and lowers the chance of metal dissolution, while simultaneously utilising the advantageous features of cobalt.
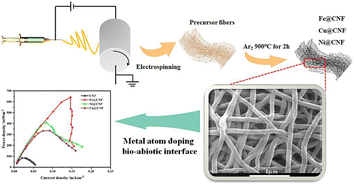 | ||
| Fig. 4 Electrospun metal-doped carbon nanofibers at the bio–abiotic interface enable rapid bioelectrocatalysis (this figure has been reproduced from ref. 55 with permission from Elsevier publisher, copyright 2024). | ||
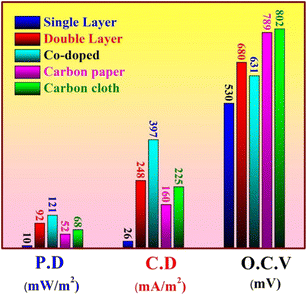 | ||
| Fig. 5 Power density, open circuit potential, and current density after 24 h batching of MFCs using different anodes (this figure has been reproduced from ref. 58 with permission from MDPI publisher, copyright 2024). | ||
It can be concluded that employing conductive nanofibers as anodes can alter the surface morphology and porosity of the anode material, thereby impacting the performance of MFC units. The incorporation of metals onto the nanofiber layer has shown significant potential to enhance power generation performance several-fold. This improvement is attributed to better attachment of electroactive bacteria, enhanced electrocatalytic activity, and catalytic reduction processes facilitated by the modified nanofiber structure.
3.2. Nanofiber-based cathodes
Currently, carbon cloth, carbon paper, and graphite are the most commonly used cathode materials. It is expected that a good cathode material should capture protons easily and have a high redox potential. The power density and the electrical performance of the cathode can be improved by modifying it with a highly active catalyst.59 It is possible to use carbon60 or metal-based catalysts such as platinum (Pt)61 or biocatalysts by attachment of microorganisms on the cathode.62,63 The main limitation of noble metal–electrode cathodes for scaled-up applications is their high cost.64 The drawback of the biocatalyst is accumulation of metabolites which limits ions transferred through cell membranes.65 An ideal oxygen reduction reaction catalyst should be extremely active, durable, long-lasting, scalable, and, most significantly, inexpensive. To maximize the technoeconomic potential of microbial fuel cells, it is critical to choose a cost-effective cathode material.Nanofiber-based cathodes have been developed as a viable option to improve MFCs' performance. Nanofibers' high surface area, porosity, and electrical conductivity make them suitable for replacing traditional electrode materials such as graphite and platinum. Carbon-based nanofibers, such as graphene and carbon nanotubes, are particularly attractive due to their high electrical conductivity and potential for enhanced electron transfer.
Xu et al.100 used nitrogen-doped reduced graphene oxide–carbon nanofiber hybrid membranes as cathode material which showed superior MFC performance with maximum power density reached 826 mW m−2 and oxygen reduction reaction activity compared to the pristine nitrogen-doped carbon nanofibers and commercial activated carbon (Fig. 6). Ghasemi et al.31 studied activated electrospun carbon nanofibers as a cathode electrode and compared performance to plain carbon paper. To increase surface area and catalytic activity of the cathode, chemical and physical activation was done by KOH reagents and CO2 gas, respectively. Chemically activated carbon electrodes have a better catalytic activity than physically activated ones with 78% more power generation. On the other hand, the cost of chemically activated carbon nanofibers was 2.65 times greater than that of the traditionally used platinum cathode. Eom et al.101 used polyacrylonitrile (PAN)-based carbon nanofibers in both anode and cathode electrode. Palladium (Pd) was used at various ratios to enhance the catalytic activity of MFC together with carbon nanofibers in the cathode electrodes. Results indicated that the performance of MFC increased as the content of Pd increased. Pd incorporated nanofiber showed current density and power density 17.2 times and 283 times higher than pristine carbon nanofiber. A novel cathode, electrospun zeolitic imidazolate framework-67/polyacrylonitrile carbon nanofiber (ZIF-67/CNFs), has been developed to improve the oxygen reduction reaction (ORR) performance, pollutant removal, and bioelectricity output of microbial fuel cells (MFCs).102 This innovative cathode achieved the highest output voltage (607 ± 9 mV) and maximum power density (1191 mW m−2). The porous structure of the nanofiber composite electrode effectively decreased the internal resistance of the MFC cathode. Nandy et al.23 conducted a comparative study on the performance of soil microbial fuel cell (SMFC) technology utilizing different cathode materials: Fe-doped carbon nanofiber (CNF), Pt-doped carbon cloth (PtC), carbon cloth, and graphite felt (GF). The study showed that Fe-doped CNF and PtC had steady performance, reaching peak power densities of 25.5 mW m−2 and 30.4 mW m−2, respectively, in relation to the cathode's geometric area. The graphite felt (GF) showed superior electrochemical performance, with a peak power density of 87.3 mW m−2. The increased performance of GF was due to its larger surface area, which improved biofilm adhesion and resulted in higher oxygen reduction reaction (ORR) activity. Gong et al.103 synthesized carbon nanofiber membranes (CNMs) incorporated with palladium nanoparticles (Pd-CNMs) using polyimide as the primary material. Palladium nanoparticles are evenly spread and highly active on the surface of carbon nanofibers in the Pd-CNMs structure. The Pd-CNMs exhibit outstanding electrocatalytic performance for the oxygen reduction process (ORR) in alkaline electrolytes due to their large specific surface area. Comparing the electrocatalytic power of Pd-CNMs to commercial Pd/C, the half-wave potential fell by approximately 0.03 V and 0.042 V, respectively after 4000 CV. Commercial Pd/C showed greater electrocatalytic activity but lower ORR performance than Pd-CNMs.
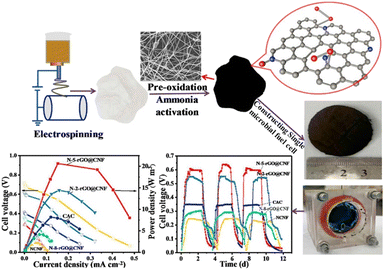 | ||
| Fig. 6 Nitrogen-doped reduced graphene oxide@carbon nanofiber (N-rGO@CNF) hybrid membranes, with varying amounts of rGO embedded into CNF, serving as high-performance integrated air cathodes in microbial fuel cells (this figure has been reproduced from ref. 100 with permission from Elsevier publisher, copyright 2024). | ||
On the other hand, Santoro et al.104 proposed using nitric acid (HNO3) activated CNFs cathodes (ACNF) as a substitute for platinum (Pt)-based cathodes in single chamber microbial fuel cells. The nitrogen functional groups attached to the nanofiber surfaces likely enhanced the properties of the ACNF. The findings demonstrated that CNFs activated by HNO3 exhibited greater stability in voltage output and power production over extended periods compared to a Pt-based cathode, which experienced deterioration and detachment of the catalyst with time. While Cong et al.105 found that nitrogen-doped carbon is not electrochemically stable under prolonged potential scanning, employing hot-pressing of ACNF led to gradual degradation over time. Furthermore, it was suggested that the ACNF cathode serves as a dependable and cost-effective alternative to Pt-based cathodes. Similarly, Yang et al.106 synthesized nitrogen-doped porous carbon nanofibers (CNFs) as a substitute for platinum-based catalysts in fuel cells. Depending on the carbonization temperature, variations in nitrogen content and the degree of graphitic phase were observed. The optimal carbonization temperature for achieving a desirable graphitic phase and a nitrogen content of 3.5% (atomic percentage) in the carbon fibers was determined to be 1000 °C. Among the samples with varying pore volumes (0.09, 0.52, and 0.94 cm3 g−1), the medium porous sample (C-PEOPAN-11-1000) exhibited the highest performance in the oxygen reduction reaction (ORR), with a total pore volume of 0.79 cm3 g−1. The measured H2O2 yields for C-PEOPAN-11-1000 and commercial Pt/C catalyst were approximately 8% and 2%, respectively, at a potential of 0.5 V. Furthermore, C-PEOPAN-11-1000 demonstrated superior tolerance to methanol crossover and excellent stability in KOH solution compared to Pt/C catalyst. The remarkable electrocatalytic activity, with an onset potential of approximately 0.09 V for the C-PEOPAN-11-1000 sample (compared to 0.07 V for commercial Pt/C catalyst), was attributed to the balance between nitrogen content, electrical conductivity, and active site density on the surface. Their analysis showed that all nitrogen atoms primarily existed in active pyridinic and quaternary-N bonding configurations across all carbon fibers. Similarly, nitrogen-doped carbon-based nanofibers (N-CNFs) were synthesized as a catalyst layer at the cathode in Single Chamber Microbial Fuel Cells (SCMFCs) with an air-cathode, using a carbonization temperature of 900 °C.107 The presence of nitrogen defects, combined with their high surface area, makes them a promising catalyst layer for the oxygen reduction reaction (ORR). The results obtained confirmed that SCMFCs equipped with N-CNFs achieved a higher maximum power density (1.15 W g−1) compared to those with a reference Pt/C layer (0.571 W g−1). This highlights the significant potential of N-CNFs to replace noble metal-based catalysts. The Pt metal group-free oxygen reduction reaction (ORR) catalysts based on Fe, Co, Ni, and Mn demonstrated superior electrochemical performance and power generation compared to activated carbon, with the following order of effectiveness: Fe > Co > Ni > Mn as shown in Fig. 7.108 This suggests a promising avenue for synthesizing Pt metal group-free catalysts for microbial fuel cell (MFC) applications.
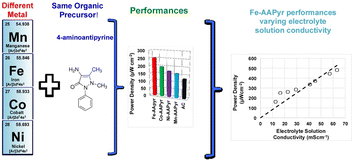 | ||
| Fig. 7 Air breathing cathodes for MFCs using Mn-, Fe-, Co- and Ni-containing platinum group metal-free catalysts (this figure has been reproduced from ref. 108 with permission from Elsevier publisher, copyright 2024). | ||
Besides carbon, polymeric nanofibers started to be used in MFCs. Silver (Ag) anchored PVDF nanofiber membrane was prepared for efficient oxygen reduction reaction in MFC's cathode.109 Neither supporting carbon cloth, nor coating catalyst material has been used to fabricate MFC cathode. The Ag showed catalyst role. Compared to commercial Pt/carbon (C) (20% Pt) cathode, the PVDF@Ag nanofiber cathode showed higher power density, chemical oxygen demand (COD) removal rate, and coulombic efficiency as 72%, 57.44%, and 25.7%, respectively. This work showed, without carbon cloth or paper and catalyst coating, it is possible to get high MFC performance by using modified nanofibers.
It is very common to use nanofibers together with a catalyst. Graphene/nickel (Ni) nanofiber hybrid was prepared for cathode catalyst by the decoration of Ni nanofibers on the graphene flakes.110 Compared to pure Ni or graphene catalysts, graphene/Ni nanofiber showed almost 2-fold higher power density. Ahmed et al.111 prepared polyaniline (PANI) nanofiber/carbon black composite as an oxygen reduction catalyst in MFC. The power density of pristine PANI nanofiber increased 2.6-fold by adding carbon black and 1.2-fold lower than commercial Pt catalyst. However, the lower cost of PANI/C would suggest an alternative to Pt catalyst for the bulk application. Other researchers used non-precious metal (Co, Ni or Fe) doped carbon nanofiber air-cathode for MFC.33 Using metals not only improved the catalytic activity but also increased the surface area of the electrode up to 573 m2 g−1. In terms of the catalytic potential of electrodes, the ascending sort was Fe1 > Co > Ni > pristine carbon nanofibers. On the other hand, for the long-term stability (6000 h) and high performance of the low-cost, Co-dopped carbon nanofibers showed the best performance with a current density of 27.4 A m−3, power output up to 14.4 W m−3 with COD removal of 70–85%.
3.3. Nanofiber-based membranes
MFCs equipped with high-resistance membranes tend to have poor performance due to limited proton diffusion between the anode and cathode, resulting in low current and power densities.112 Besides the influence on performance, the membrane type is a significant factor in the development of MFCs, accounting for approximately 60% of the total cost. Currently, Nafion membranes possess the ideal qualities needed for MFCs.80,113 Nevertheless, despite being the top choice for MFC membranes, their high cost hinders their widespread adoption as MFCs are scaled-up. Therefore, novel membrane designs focus on low cost and maintaining low internal resistance to facilitate efficient proton transfer with enhanced characteristics. When selecting a membrane for MFC applications, several criteria must be fulfilled. These include exceptional mechanical and chemical stability, absence of electronic conduction, low permeability to gases like H2, O2, and N2, high ionic conductivity, ease of acquisition, superior species selectivity, minimal oxygen and fuel crossover, and affordability with low electrical resistance.80 Besides providing low internal resistance, the separator used on the anolyte side of the cathode should have a high hydrophilicity to facilitate cationic transport and should also inhibit oxygen diffusion.84Functionalized nanofibers can be used to selectively transport specific ions or molecules, such as proton-selective membranes that enhance proton transfer in MFCs. Either including metals/inorganics or proton-conducting groups (e.g., –COOH, –SO3H, and –PO3H2), the nanofibers can be suitable to use as proton exchange membranes.
Limited research has been conducted regarding the utilization of nanofiber membranes in the separator component of MFCs. More research is being conducted on the application of nanofiber membranes in lithium cells, direct methanol fuel cells (DMFCs), biomedical materials, sensors, and electronic devices.31,114 Due to pore size, nanofibers act as microfiltration membranes. It was found that using a microfiltration membrane reduces the internal resistance of MCFs compared to Nafion proton exchange membranes (PEM).115 Dong et al.116 compared the proton conductivity of pristine Nafion film membrane and high-purity Nafion nanofiber (99.9 wt%) via electrospinning with the use of 0.1 wt% poly(ethylene oxide). It was found that proton conductivity increased with decreasing fiber diameter. Compared to pristine Nafion film, Nafion nanofibers showed 15-fold higher proton conductivity. Based on the previous finding it can be expected that nanofiber membranes can help reduce internal resistance with a lower cost. Shahgaldi et al.34 prepared PVDF/Nafion composite membranes as proton exchange membranes. The results indicate that maximum power density was attained with 0.4 g concentration of Nafion in composite, which was even higher than that of pristine Nafion. By this method, it is possible to reduce the price of separator membrane cost. Similarly, electrospun activated carbon nanofiber/Nafion membranes were fabricated and their power production was compared to Nafion 112 and Nafion 117 membranes.36 Results indicated that, the nanofiber membrane produced the highest power density of 57.64 mW m−2 while it was 13.99 mW m−2 and 38.30 mW m−2 for Nafion 112 and Nafion 117, respectively. The carbon electrospun structure changed the roughness, lowered pore size and increased porosity of membranes which resulted in higher generation of power in MFC. Li et al.35 functionalized the PVDF nanofiber with Nafion, as shown in Fig. 8. The addition of Nafion chains to PVDF-based nanofibers improved their compatibility with the Nafion matrix and created a PVDFNF-Nafion reinforced Nafion composite membrane (Nafion-CM1). Functionalizing the nanofiber surfaces created proton-conducting channels and enhanced the proton conductivity of Nafion-CM1. Nafion-CM1 demonstrated a maximum power density of 700 mW cm2 in single cell testing with H2/O2, exceeding the 500 mW cm2 of the commercial Nafion 212 membrane. In another work, PVDF nanofiber was coated with perfluorinated sulfuric acid ionomer (PVDF-PFSA) to be used in MFCs as a replacement for the Nafion 117 membrane and their power densities were compared.117 The nanofiber-based proton exchange membrane showed superior properties compared to Nafion 117, such as; lower dimensional change, lower water uptake, with a max power density of 548 mW m−2. Liu et al.118 engineered proton-conductive membranes by reinforcing PVDF nanofibers with aromatic ionomers. The resulting composite nanofiber membrane (SPP-TFP-4.0-PVDF) exhibited a highly porous and isotropic structure enhanced by partially fluorinated aromatic ionomers. With excellent chemical stability and consistent rupture energy levels at both high and low relative humidity (RH) levels and higher proton conductivity compared to commercial Nafion membrane, the SPP-TFP-4.0-PVDF membrane presents itself as a promising alternative proton-conductive membrane. Similarly, PVDF nanofibers were used as a proton exchange membrane after being functionalized with polydopamine/polyethyleneimine (PDA/PEI) and filled with sulfonated poly (ether ether ketone) (SPEEK) (SPEEK-PDA/PEI@PVDF).119 Undoubtedly, the membrane exhibited exceptional mechanical properties, particularly in wet conditions. Additionally, the SPEEK-PDA/PEI@PVDF composite membrane demonstrated excellent long-term stability and durability. The proton conductivity was measured at 48 mS cm−1 at 80 °C, and the highest power density was recorded at 58.9 mW cm−2. The greatest power density attained was 58.9 mW cm−2 using 2 M methanol as fuel, in comparison to the pristine SPEEK membrane (47 mW cm−2) and Nafion115 (48.4 mW cm−2). The durability test showed the excellent stability of the composite membrane, showing just a 2.6% loss in open circuit voltage after working at 80 °C for 100 hours.
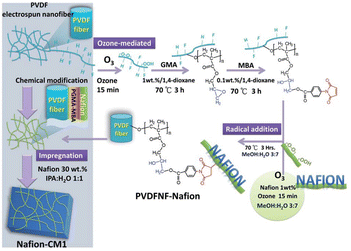 | ||
Fig. 8 Synthetic route of Nafion-functionalized PVDF nanofibers (PVDFNF-Nafion) and preparation of Nafion composite membrane (Nafion-CM1): ozone treatment introduces peroxide groups in Nafion chains, facilitating the formation of highly reactive radicals. These radicals react with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C unsaturated groups. Surface functionalization of PVDF nanofibers involves a two-step process: radical polymerization of GMA followed by addition reaction with MBA. Maleimide groups anchored on the PVDF nanofibers serve as active sites for reaction with radical-containing Nafion chains, resulting in Nafion-functionalized PVDF nanofibers (PVDFNF-Nafion). GMA: glycidylmethacrylate, MBA: maleimidobenzoic acid (this figure has been reproduced from ref. 35 with permission from Royal Society of Chemistry publisher, copyright 2024). C unsaturated groups. Surface functionalization of PVDF nanofibers involves a two-step process: radical polymerization of GMA followed by addition reaction with MBA. Maleimide groups anchored on the PVDF nanofibers serve as active sites for reaction with radical-containing Nafion chains, resulting in Nafion-functionalized PVDF nanofibers (PVDFNF-Nafion). GMA: glycidylmethacrylate, MBA: maleimidobenzoic acid (this figure has been reproduced from ref. 35 with permission from Royal Society of Chemistry publisher, copyright 2024). | ||
Based on the previous studies, it can be concluded that, the proton exchange membranes based on nanofibers demonstrate exceptional MFC performance attributes surpassing those of the Nafion membrane, suggesting their potential as a viable substitute for Nafion membranes in MFC reactors. Future studies are required to optimize the properties of nanofibers and their potential applications in MFCs and other energy-related fields.
4. Challenges and future directions
The implementation of nanofibers into MFC components offers promise for the enhancement of performance. However, there are several limitations that must be addressed for extensive application at larger scale. Table 2 shows the main challenges and possible solutions to overcome the limitations of nanofiber technology to use in energy applications.| Limitations | Solution | Reference |
|---|---|---|
| Cost | The increase of industrial scale production devices reduces the price of nanofiber webs | 85–87 |
| Un-spinnability | Using polymer mixture or using additives can help to fabricate nanofiber web | 88 and 89 |
| Defect-free surface | Optimizing both system and process parameters is essential to prevent uneven web surface, which can lead to energy generation failure | 90–92 |
| Low conductivity | The conductivity can be improved by using conductive polymers, carbon nanofibers, or additives | 32 and 93–95 |
| Biocompatibility | Focusing on biocompatible polymers and using non-toxic chemicals can be the solution | 96–98 |
| Stability | Using chemical resistance nanofiber webs such as PVDF or PSU are beneficial to enhance stability and durability under acidic and alkaline conditions | 39 and 99 |
The improvement in bulk production of nanofibers accelerate their application area in the market. Especially after COVID19, nanofibers have been widely used in face masks.120,121 Future research should focus on optimizing the properties of nanofibers and exploring their potential applications in MFCs and other energy-related fields. The challenges mentioned in Table 2 are not insurmountable but require great research and effort. For instance, for cost-effectiveness production, the equipment, polymers, solvents, additives need to be selected carefully. Otherwise, the fabrication of nanofibers with high-quality raw materials can be costly. The scalability of nanofiber production for large-scale MFC applications needs to be addressed to make this technology economically viable.
The other limitations, such as un-spinnability, defect-free surface or low conductivity can be solved by using polymer mixture, solvent mixture, polymer type, additives, changing process and system parameters. The defect-free surface is important in MFC. For instance, large defects or low conductivity can cause electron loss, high internal-resistance, low proton conductivity or low impermeability to gases.
The biocompatibility of nanofiber-based materials is another challenge that needs to be addressed. Some nanofibers might negatively affect microbial development and activity, which would prevent their usage in MFCs. To ensure that nanofibers can be used safely in MFCs, a detailed investigation into their toxicity is required.
Regarding their long-term efficacy, the stability and durability of nanofiber-based electrodes and membranes in MFCs also pose challenges. The polymer selection must be done carefully. For instance, polyacrylonitrile (PAN) is one of the most commonly used polymers for the production of nanofibers. However, the sensitivity of this polymer to alkaline restrict its application. It is found that the nitrile groups of PAN hydrolysis and swells under alkaline condition and pores getting smaller.122 It is necessary to evaluate the mechanical stability and long-term durability of these components to ensure their efficacy over extended periods.
Recent research has demonstrated that the incorporation of nanofibers into MFCs holds great potential for enhancing the performance of MFCs in the future years.28,29,32,123 These studies have highlighted the remarkable capability of nanofibers to significantly enhance power generation, potentially by several orders of magnitude. In this respect, future research should focus on optimizing the properties of nanofibers according to MFCs application. The advancement of nanofiber materials and their composites through the exploration of novel synthesis methods holds the potential to significantly enhance their performance characteristics and expand the scope of their applications. In order to improve the overall power generation of MFC systems, it is also critical to give priority to the exploration and development of MFC stacks. This factor becomes essential in order to meet the power requirements required for the future implementation of large-scale MFC operations.
In summary, the incorporation of nanofiber technology into MFCs exhibits considerable potential for improving their performance and extending their applicability.28,29,102 Nevertheless, certain obstacles relating to cost-effectiveness, biocompatibility, and long-term stability must be addressed. The focus of future research should be on improving nanofiber characteristics and investigating potential uses for them in MFCs and other areas related to energy production.
5. Conclusions
This article described the significance of nanofiber technology in revolutionizing MFCs as a sustainable energy source. The proper and efficient selection of the material from which MFCs are constructed is a crucial element in the effort to produce high-performance MFCs. Nanofiber technology has been shown to enhance the performance of MFCs through improved electron transfer rate, enhanced biofilm formation and microbial activity, and increased durability and stability. Nanofiber-based anodes, cathodes, and membranes have been investigated in MFCs, with promising results. However, the incorporation of nanofiber technology into MFCs poses various challenges encompassing cost-effectiveness, biocompatibility, and long-term stability. Future research should focus on the optimizing the properties of nanofiber and the exploration of their potential applications not only in MFCs but also in other energy-related domains. Utilizing MFCs as a viable source of sustainable energy presents an opportunity to reduce reliance on non-renewable resources and address the impacts of climate change. Additionally, the potential of nanofiber technology to improve MFC performance and expand their applications holds great promise in advancing a future driven by sustainable energy solutions.Author contributions
Fatma Yalcinkaya, Ioannis Ieropoulos: conceptualization, methodology, supervision, data curation, writing – original draft, preparation, writing – review & editing, visualization. Rafael Torres-Mendieta: conceptualization, methodology, data curation, writing – original draft, preparation, writing – review & editing, visualization. Andrea Pietrelli, Jakub Hruza, Andrea Vávrová, Lucie Svobodová: conceptualization, writing – review & editing, visualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors acknowledge the COST Action [Protection, Resilience, Rehabilitation of damaged environment (PHOENIX), CA19123], supported by COST (European Cooperation in Science and Technology; www.cost.eu). Ioannis Ieropoulos is a Bill & Melinda Gates Foundation grantee (grant #: INV-042655).Notes and references
- T. Pham, K. Rabaey, P. Aelterman, P. Clauwaert, L. De Schamphelaire, N. Boon and W. Verstraete, Microbial fuel cells in relation to conventional anaerobic digestion technology, Eng. Life Sci., 2006, 6, 285–292 CrossRef CAS.
- D. Pant, G. Van Bogaert, L. Diels and K. Vanbroekhoven, A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production, Bioresour. Technol., 2010, 101, 1533–1543, DOI:10.1016/j.biortech.2009.10.017.
- A. Tsipa, C. K. Varnava, P. Grenni, V. Ferrara and A. Pietrelli, Bio-electrochemical system depollution capabilities and monitoring applications: Models, applicability, advanced bio-based concept for predicting pollutant degradation and microbial growth kinetics via gene regulation modelling, Processes, 2021, 9, 1038 CrossRef CAS.
- C. Santoro, C. Arbizzani, B. Erable and I. Ieropoulos, Microbial fuel cells: From fundamentals to applications. A review, J. Power Sources, 2017, 356, 225–244, DOI:10.1016/j.jpowsour.2017.03.109.
- V. Ancona, A. B. Caracciolo, D. Borello, V. Ferrara, P. Grenni and A. Pietrelli, Microbial fuel cell: an energy harvesting technique for environmental remediation, Int. J. Environ. Impacts, 2020, 3, 168–179 CrossRef.
- K. Obileke, H. Onyeaka, E. L. Meyer and N. Nwokolo, Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review, Electrochem. Commun., 2021, 125, 107003, DOI:10.1016/j.elecom.2021.107003.
- M. Rahimnejad, A. Adhami, S. Darvari, A. Zirepour and S.-E. Oh, Microbial fuel cell as new technology for bioelectricity generation: A review, Alexandria Eng. J., 2015, 54, 745–756, DOI:10.1016/j.aej.2015.03.031.
- A. Z. Imoro, N. A. Acheampong, S. Oware, H. Okrah, V. T. Coulibaly, A. G. Ali, F. Asare-Amegavi, D. Krah and F. Offei, The Potential Benefits of Microbial Fuel Cells in the Context of the Sustainable Development Goals, in, Microbial Fuel Cells for Environmental Remediation, ed. A. Ahmad, M. N. Mohamad Ibrahim, A. A. Yaqoob and S. H. Mohd Setapar, Springer Nature, Singapore, 2022, pp. 167–182, DOI:10.1007/978-981-19-2681-5_9.
- M. C. Potter, Electrical effects accompanying the decomposition of organic compounds, Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character, 1911, 84, 260–276 Search PubMed.
- N. Lovecchio, V. Di Meo and A. Pietrelli, Customized Multichannel Measurement System for Microbial Fuel Cell Characterization, Bioengineering, 2023, 10(5), 624, DOI:10.3390/bioengineering10050624.
- A. Pietrelli, I. Bavasso, N. Lovecchio, V. Ferrara and B. Allard, MFCs as biosensor, bioreactor and bioremediator, in, 2019 IEEE 8th International Workshop on Advances in Sensors and Interfaces (IWASI), 2019, pp. 302–306, DOI:10.1109/IWASI.2019.8791412.
- H. P. Bennetto, D. K. Ewart, A. M. Nobar and I. Sanderson, Microbial Fuel Cell Studies of Iron-Oxidising Bacteria, in, Charge and Field Effects in Biosystems—2, ed. M. J. Allen, S. F. Cleary and F. M. Hawkridge, Springer US, Boston, MA, 1989, pp. 339–349, DOI:10.1007/978-1-4613-0557-6_29.
- D. Ucar, Y. Zhang and I. Angelidaki, An Overview of Electron Acceptors in Microbial Fuel Cells, Front. Microbiol., 2017, 8, 643 Search PubMed , https://www.frontiersin.org/articles/10.3389/fmicb.2017.00643.
- A. Pietrelli, V. Ferrara, F. Khaled, B. Allard, F. Buret and F. Costantini, Electrical characterization of MFC for low power applications, in, 2016 IEEE 16th International Conference on Environment and Electrical Engineering (EEEIC), 2016, pp. 1–5, DOI:10.1109/EEEIC.2016.7555624.
- I. Ieropoulos, C. Melhuish and J. Greenman, Artificial gills for robots: MFC behaviour in water, Bioinspiration Biomimetics, 2007, 2, S83, DOI:10.1088/1748-3182/2/3/S02.
- Analyze Results, https://www.webofscience.com/wos/woscc/analyze-results/35133ee5-ba02-4ca8-b500-901ac57150bf-7fabea52, accessed April 6, 2023.
- C. I. Torres, A. Kato Marcus and B. E. Rittmann, Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria, Biotechnol. Bioeng., 2008, 100, 872–881 CrossRef CAS PubMed.
- G. Palanisamy, H.-Y. Jung, T. Sadhasivam, M. D. Kurkuri, S. C. Kim and S.-H. Roh, A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes, J. Cleaner Prod., 2019, 221, 598–621, DOI:10.1016/j.jclepro.2019.02.172.
- H. M. A. Sharif, M. Farooq, I. Hussain, M. Ali, M. A. Mujtaba, M. Sultan and B. Yang, Recent innovations for scaling up microbial fuel cell systems: Significance of physicochemical factors for electrodes and membranes materials, J. Taiwan Inst. Chem. Eng., 2021, 129, 207–226, DOI:10.1016/j.jtice.2021.09.001.
- M. Mashkour, M. Rahimnejad, M. Mashkour and F. Soavi, Increasing bioelectricity generation in microbial fuel cells by a high-performance cellulose-based membrane electrode assembly, Appl. Energy, 2021, 282, 116150, DOI:10.1016/j.apenergy.2020.116150.
- P. Surti, S. K. Kailasa, A. Mungray, T. J. Park and A. K. Mungray, Vermiculite nanosheet augmented novel proton exchange membrane for microbial fuel cell, Fuel, 2024, 357, 130046, DOI:10.1016/j.fuel.2023.130046.
- M. Zhang, Y. Liu and C. Li, Enhanced performance of microbial fuel cells with a bacteria/shape-controllable aligned carbon nanofibers hybrid biofilm, Int. J. Hydrogen Energy, 2023, 48, 1107–1119, DOI:10.1016/j.ijhydene.2022.09.300.
- A. Nandy, D. Farkas, B. Pepió-Tárrega, S. Martinez-Crespiera, E. Borràs, C. Avignone-Rossa and M. Di Lorenzo, Influence of carbon-based cathodes on biofilm composition and electrochemical performance in soil microbial fuel cells, Environ. Sci. Ecotechnology, 2023, 16, 100276, DOI:10.1016/j.ese.2023.100276.
- B. Yalcinkaya, F. C. Callioglu and F. Yener, Measurement and analysis of jet current and jet life in roller electrospinning of polyurethane, Text. Res. J., 2014, 84, 1720–1728 CrossRef CAS.
- B. Yalcinkaya, F. Yener, O. Jirsak and F. Cengiz-Callioglu, On the nature of electric current in the electrospinning process, J. Nanomater., 2013, 2013, 6 Search PubMed.
- T. Cai, M. Huang, Y. Huang and W. Zheng, Enhanced performance of microbial fuel cells by electrospinning carbon nanofibers hybrid carbon nanotubes composite anode, Int. J. Hydrogen Energy, 2019, 44, 3088–3098, DOI:10.1016/j.ijhydene.2018.11.205.
- F. D. Cesare, E. D. Mattia, E. Zussman and A. Macagnano, A study on the dependence of bacteria adhesion on the polymer nanofibre diameter, Environ. Sci.: Nano, 2019, 6, 778–797, 10.1039/C8EN01237G.
- Y. Liu, X. Zhang, H. Li, L. Peng, Y. Qin, X. Lin, L. Zheng and C. Li, Porous α-Fe2O3 nanofiber combined with carbon nanotube as anode to enhance the bioelectricity generation for microbial fuel cell, Electrochim. Acta, 2021, 391, 138984, DOI:10.1016/j.electacta.2021.138984.
- S. S. Manickam, U. Karra, L. Huang, N.-N. Bui, B. Li and J. R. McCutcheon, Activated carbon nanofiber anodes for microbial fuel cells, Carbon, 2013, 53, 19–28, DOI:10.1016/j.carbon.2012.10.009.
- Y. Tao, Q. Liu, J. Chen, B. Wang, Y. Wang, K. Liu, M. Li, H. Jiang, Z. Lu and D. Wang, Hierarchically Three-Dimensional Nanofiber Based Textile with High Conductivity and Biocompatibility As a Microbial Fuel Cell Anode, Environ. Sci. Technol., 2016, 50, 7889–7895, DOI:10.1021/acs.est.6b00648.
- M. Ghasemi, S. Shahgaldi, M. Ismail, B. H. Kim, Z. Yaakob and W. R. Wan Daud, Activated carbon nanofibers as an alternative cathode catalyst to platinum in a two-chamber microbial fuel cell, Int. J. Hydrogen Energy, 2011, 36, 13746–13752, DOI:10.1016/j.ijhydene.2011.07.118.
- U. Karra, S. S. Manickam, J. R. McCutcheon, N. Patel and B. Li, Power generation and organics removal from wastewater using activated carbon nanofiber (ACNF) microbial fuel cells (MFCs), Int. J. Hydrogen Energy, 2013, 38, 1588–1597, DOI:10.1016/j.ijhydene.2012.11.005.
- P. Bosch-Jimenez, S. Martinez-Crespiera, D. Amantia, M. Della Pirriera, I. Forns, R. Shechter and E. Borràs, Non-precious metal doped carbon nanofiber air-cathode for Microbial Fuel Cells application: Oxygen reduction reaction characterization and long-term validation, Electrochim. Acta, 2017, 228, 380–388, DOI:10.1016/j.electacta.2016.12.175.
- S. Shahgaldi, M. Ghasemi, W. R. Wan Daud, Z. Yaakob, M. Sedighi, J. Alam and A. F. Ismail, Performance enhancement of microbial fuel cell by PVDF/Nafion nanofibre composite proton exchange membrane, Fuel Process. Technol., 2014, 124, 290–295, DOI:10.1016/j.fuproc.2014.03.015.
- H.-Y. Li and Y.-L. Liu, Nafion-functionalized electrospun poly(vinylidene fluoride) (PVDF) nanofibers for high performance proton exchange membranes in fuel cells, J. Mater. Chem. A, 2014, 2, 3783–3793, 10.1039/C3TA14264G.
- M. Ghasemi, S. Shahgaldi, M. Ismail, Z. Yaakob and W. R. W. Daud, New generation of carbon nanocomposite proton exchange membranes in microbial fuel cell systems, Chem. Eng. J., 2012, 184, 82–89, DOI:10.1016/j.cej.2012.01.001.
- K.-J. Chae, K.-Y. Kim, M.-J. Choi, E. Yang, I. S. Kim, X. Ren and M. Lee, Sulfonated polyether ether ketone (SPEEK)-based composite proton exchange membrane reinforced with nanofibers for microbial electrolysis cells, Chem. Eng. J., 2014, 254, 393–398, DOI:10.1016/j.cej.2014.05.145.
- F. Yalcinkaya, B. Yalcinkaya and O. Jirsak, Analysis of the effects of rotating roller speed on a roller electrospinning system, Text. Res. J., 2017, 87, 913–928 CrossRef CAS.
- F. Yalcinkaya, Preparation of various nanofiber layers using wire electrospinning system, Arabian J. Chem., 2019, 12, 5162–5172, DOI:10.1016/j.arabjc.2016.12.012.
- A. Patnaik and R. D. Anandjiwala, An optimized melt spinning process to increase the productivity of nanofiber materials, J. Ind. Text., 2016, 45, 1026–1037 CrossRef CAS.
- S. Padron, A. Fuentes, D. Caruntu and K. Lozano, Experimental study of nanofiber production through forcespinning, J. Appl. Phys., 2013, 113, 024318 CrossRef.
- Z. He, L. Liu, C. Gao, Z. Zhou, X. Liang, Y. Lei, Z. He and S. Liu, Carbon nanofibers grown on the surface of graphite felt by chemical vapour deposition for vanadium redox flow batteries, RSC Adv., 2013, 3, 19774–19777, 10.1039/C3RA22631J.
- J. Hulteen, H. Chen, C. Chambliss and C. Martin, Template synthesis of carbon nanotubule and nanofiber arrays, Nanostruct. Mater., 1997, 9, 133–136 CrossRef CAS.
- G. Massaglia, V. Margaria, M. R. Fiorentin, K. Pasha, A. Sacco, M. Castellino, A. Chiodoni, S. Bianco, F. C. Pirri and M. Quaglio, Nonwoven mats of N-doped carbon nanofibers as high-performing anodes in microbial fuel cells, Mater. Today Energy, 2020, 16, 100385, DOI:10.1016/j.mtener.2020.100385.
- Y. Qin, H. Li, Y. Sun, S. Guo, Y. Liu, Z. Zhai and C. Li, Directional assembly of multi-catalytic sites CoCu-MOFs with porous carbon nanofiber templates as efficient catalyst for microbial fuel cells, J. Environ. Chem. Eng., 2023, 11, 109662, DOI:10.1016/j.jece.2023.109662.
- H. Jiang, L. J. Halverson and L. Dong, A miniature microbial fuel cell with conducting nanofibers-based 3D porous biofilm, J. Manuf. Syst., 2015, 25, 125017, DOI:10.1088/0960-1317/25/12/125017.
- H.-Y. Jung and S.-H. Roh, Carbon Nanofiber/Polypyrrole Nanocomposite as Anode Material in Microbial Fuel Cells, J. Nanosci. Nanotechnol., 2017, 17, 5830–5833, DOI:10.1166/jnn.2017.14149.
- P. Zhang, J. Liu, Y. Qu, J. Zhang, Y. Zhong and Y. Feng, Enhanced performance of microbial fuel cell with a bacteria/multi-walled carbon nanotube hybrid biofilm, J. Power Sources, 2017, 361, 318–325, DOI:10.1016/j.jpowsour.2017.06.069.
- K. Guo, A. Prévoteau, S. A. Patil and K. Rabaey, Engineering electrodes for microbial electrocatalysis, Curr. Opin. Biotechnol., 2015, 33, 149–156 CrossRef CAS PubMed.
- Y. Liu, J. Wang, Y. Sun, H. Li, Z. Zhai, S. Guo, T. Ren and C. Li, Nitrogen-doped carbon nanofibers anchoring Fe nanoparticles as biocompatible anode for boosting extracellular electron transfer in microbial fuel cells, J. Power Sources, 2022, 544, 231890 CrossRef CAS.
- X. Wu, X. Li, Z. Shi, X. Wang, Z. Wang and C. M. Li, Electrospinning Mo-Doped Carbon Nanofibers as an Anode to Simultaneously Boost Bioelectrocatalysis and Extracellular Electron Transfer in Microbial Fuel Cells, Materials, 2023, 16 DOI:10.3390/ma16062479.
- J. M. Moradian, F.-Q. Yang, N. Xu, J.-Y. Wang, J.-X. Wang, C. Sha, A. Ali and Y.-C. Yong, Enhancement of bioelectricity and hydrogen production from xylose by a nanofiber polyaniline modified anode with yeast microbial fuel cell, Fuel, 2022, 326, 125056, DOI:10.1016/j.fuel.2022.125056.
- S.-H. Roh, Electricity Generation from Microbial Fuel Cell with Polypyrrole-Coated Carbon Nanofiber Composite, J. Nanosci. Nanotechnol., 2015, 15, 1700–1703, DOI:10.1166/jnn.2015.9317.
- N. Jiang, M. Yan, Q. Li, S. Zheng, Y. Hu, X. Xu, L. Wang, Y. Liu and M. Huang, Bioelectrocatalytic reduction by integrating pyrite assisted manganese cobalt-doped carbon nanofiber anode and bacteria for sustainable antimony catalytic removal, Bioresour. Technol., 2024, 395, 130378, DOI:10.1016/j.biortech.2024.130378.
- W. Lin, S. Wu, T. Tang, Y. Liao, W. Miao, Z. Shi and X. Wu, Tuning metal atom doped interface of electrospinning nanowires to toward fast bioelectrocatalysis, Bioelectrochemistry, 2024, 157, 108664, DOI:10.1016/j.bioelechem.2024.108664.
- C. Hou, W. Chen, L. Fu, S. Zhang, C. Liang and Y. Wang, Facile synthesis of a Co/Fe bi-MOFs/CNF membrane nanocomposite and its application in the degradation of tetrabromobisphenol A, Carbohydr. Polym., 2020, 247, 116731, DOI:10.1016/j.carbpol.2020.116731.
- N. Jiang, J. Song, M. Yan, Y. Hu, M. Wang, Y. Liu and M. Huang, Iron cobalt-doped carbon nanofibers anode to simultaneously boost bioelectrocatalysis and direct electron transfer in microbial fuel cells: Characterization, performance, and mechanism, Bioresour. Technol., 2023, 367, 128230, DOI:10.1016/j.biortech.2022.128230.
- N. A. M. Barakat, M. T. Amen, R. H. Ali, M. M. Nassar, O. A. Fadali, M. A. Ali and H. Y. Kim, Carbon Nanofiber Double Active Layer and Co-Incorporation as New Anode Modification Strategies for Power-Enhanced Microbial Fuel Cells, Polymers, 2022, 14, 1542, DOI:10.3390/polym14081542.
- K. Watanabe, Recent Developments in Microbial Fuel Cell Technologies for Sustainable Bioenergy, J. Biosci. Bioeng., 2008, 106, 528–536, DOI:10.1263/jbb.106.528.
- K. Scott, I. Cotlarciuc, I. Head, K. P. Katuri, D. Hall, J. B. Lakeman and D. Browning, Fuel cell power generation from marine sediments: Investigation of cathode materials, J. Chem. Technol. Biotechnol., 2008, 83, 1244–1254, DOI:10.1002/jctb.1937.
- S. Cheng, H. Liu and B. E. Logan, Power Densities Using Different Cathode Catalysts (Pt and CoTMPP) and Polymer Binders (Nafion and PTFE) in Single Chamber Microbial Fuel Cells, Environ. Sci. Technol., 2006, 40, 364–369, DOI:10.1021/es0512071.
- A. Bergel, D. Féron and A. Mollica, Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm, Electrochem. Commun., 2005, 7, 900–904, DOI:10.1016/j.elecom.2005.06.006.
- Y. Zhang, J. Sun, Y. Hu, S. Li and Q. Xu, Bio-cathode materials evaluation in microbial fuel cells: A comparison of graphite felt, carbon paper and stainless steel mesh materials, Int. J. Hydrogen Energy, 2012, 37, 16935–16942, DOI:10.1016/j.ijhydene.2012.08.064.
- B. Logan and J. Regan, Microbial fuel cell: challenges and technology, Environ. Sci. Technol., 2006, 40, 5172–5180 CrossRef CAS.
- H. Luo, S. Jin, P. H. Fallgren, H. J. Park and P. A. Johnson, A novel laccase-catalyzed cathode for microbial fuel cells, Chem. Eng. J., 2010, 165, 524–528, DOI:10.1016/j.cej.2010.09.061.
- M. Ghasemi, W. R. W. Daud, N. Mokhtarian, A. Mayahi, M. Ismail, F. Anisi, M. Sedighi and J. Alam, The effect of nitric acid, ethylenediamine, and diethanolamine modified polyaniline nanoparticles anode electrode in a microbial fuel cell, Int. J. Hydrogen Energy, 2013, 38, 9525–9532, DOI:10.1016/j.ijhydene.2012.12.016.
- U. Schröder, J. Nießen and F. Scholz, A generation of microbial fuel cells with current outputs boosted by more than one order of magnitude, Angew. Chem., Int. Ed., 2003, 42, 2880–2883 CrossRef PubMed.
- R. Kaur, A. Marwaha, V. A. Chhabra, K.-H. Kim and S. K. Tripathi, Recent developments on functional nanomaterial-based electrodes for microbial fuel cells, Renewable Sustainable Energy Rev., 2020, 119, 109551, DOI:10.1016/j.rser.2019.109551.
- A. Mehdinia, E. Ziaei and A. Jabbari, Facile microwave-assisted synthesized reduced graphene oxide/tin oxide nanocomposite and using as anode material of microbial fuel cell to improve power generation, Int. J. Hydrogen Energy, 2014, 39, 10724–10730, DOI:10.1016/j.ijhydene.2014.05.008.
- S. Wu, W. He, W. Yang, Y. Ye, X. Huang and B. E. Logan, Combined carbon mesh and small graphite fiber brush anodes to enhance and stabilize power generation in microbial fuel cells treating domestic wastewater, J. Power Sources, 2017, 356, 348–355, DOI:10.1016/j.jpowsour.2017.01.041.
- Z. He, S. D. Minteer and L. T. Angenent, Electricity Generation from Artificial Wastewater Using an Upflow Microbial Fuel Cell, Environ. Sci. Technol., 2005, 39, 5262–5267, DOI:10.1021/es0502876.
- S. M. Haile, Fuel cell materials and components☆☆☆The Golden Jubilee Issue—Selected topics in Materials Science and Engineering: Past, Present and Future, edited by S. Suresh, Acta Mater., 2003, 51, 5981–6000, DOI:10.1016/j.actamat.2003.08.004.
- C. Zhao, Y. Wang, F. Shi, J. Zhang and J.-J. Zhu, High biocurrent generation in Shewanella-inoculated microbial fuel cells using ionic liquid functionalized graphene nanosheets as an anode, Chem. Commun., 2013, 49, 6668–6670, 10.1039/C3CC42068J.
- H. Yuan and Z. He, Graphene-modified electrodes for enhancing the performance of microbial fuel cells, Nanoscale, 2015, 7, 7022–7029, 10.1039/C4NR05637J.
- D.-D. Zhai, Z. Fang, H. Jin, M. Hui, C. J. Kirubaharan, Y.-Y. Yu and Y.-C. Yong, Vertical alignment of polyaniline nanofibers on electrode surface for high-performance microbial fuel cells, Bioresour. Technol., 2019, 288, 121499, DOI:10.1016/j.biortech.2019.121499.
- M. Rosenbaum, F. Zhao, U. Schröder and F. Scholz, Interfacing Electrocatalysis and Biocatalysis with Tungsten Carbide: A High-Performance, Noble-Metal-Free Microbial Fuel Cell, Angew. Chem., Int. Ed., 2006, 45, 6658–6661, DOI:10.1002/anie.200602021.
- S. Tao and J. T. Irvine, A redox-stable efficient anode for solid-oxide fuel cells, Nat. Mater., 2003, 2, 320–323 CrossRef CAS.
- C. Li, L. Ding, H. Cui, L. Zhang, K. Xu and H. Ren, Application of conductive polymers in biocathode of microbial fuel cells and microbial community, Bioresour. Technol., 2012, 116, 459–465, DOI:10.1016/j.biortech.2012.03.115.
- M. Zhou, M. Chi, J. Luo, H. He and T. Jin, An overview of electrode materials in microbial fuel cells, J. Power Sources, 2011, 196, 4427–4435, DOI:10.1016/j.jpowsour.2011.01.012.
- J. Ramirez-Nava, M. Martínez-Castrejón, R. L. García-Mesino, J. A. López-Díaz, O. Talavera-Mendoza, A. Sarmiento-Villagrana, F. Rojano and G. Hernández-Flores, The Implications of Membranes Used as Separators in Microbial Fuel Cells, Membranes, 2021, 11(10), 738, DOI:10.3390/membranes11100738.
- G. Hernández-Flores, A. Andrio, V. Compañ, O. Solorza-Feria and H. M. Poggi-Varaldo, Synthesis and characterization of organic agar-based membranes for microbial fuel cells, J. Power Sources, 2019, 435, 226772, DOI:10.1016/j.jpowsour.2019.226772.
- A. Brunetti, E. Fontananova, A. Donnadio, M. Casciola, M. L. Di Vona, E. Sgreccia, E. Drioli and G. Barbieri, New approach for the evaluation of membranes transport properties for polymer electrolyte membrane fuel cells, J. Power Sources, 2012, 205, 222–230, DOI:10.1016/j.jpowsour.2012.01.108.
- M. Shabani, H. Younesi, M. Pontié, A. Rahimpour, M. Rahimnejad and A. A. Zinatizadeh, A critical review on recent proton exchange membranes applied in microbial fuel cells for renewable energy recovery, J. Cleaner Prod., 2020, 264, 121446 CrossRef CAS.
- S. Singh, A. Modi and N. Verma, Enhanced power generation using a novel polymer-coated nanoparticles dispersed-carbon micro-nanofibers-based air-cathode in a membrane-less single chamber microbial fuel cell, Int. J. Hydrogen Energy, 2016, 41, 1237–1247, DOI:10.1016/j.ijhydene.2015.10.099.
- A. Nadaf, A. Gupta, N. Hasan, S. Ahmad, P. Kesharwani and F. J. Ahmad, Recent update on electrospinning and electrospun nanofibers: current trends and their applications, RSC Adv., 2022, 12, 23808–23828 RSC.
- Z. Li, Z. Cui, L. Zhao, N. Hussain, Y. Zhao, C. Yang, X. Jiang, L. Li, J. Song and B. Zhang, High-throughput production of kilogram-scale nanofibers by Kármán vortex solution blow spinning, Sci. Adv., 2022, 8, eabn3690 CrossRef CAS.
- R. HMTShirazi, T. Mohammadi, A. A. Asadi and M. A. Tofighy, Electrospun nanofiber affinity membranes for water treatment applications: A review, J. Water Proc.engineering, 2022, 47, 102795, DOI:10.1016/j.jwpe.2022.102795.
- F. Yener, O. Jirsak and R. Gemci, Using a Range of PVB Spinning Solution to Acquire Diverse Morphology for Electrospun Nanofibres, 2012 Search PubMed.
- F. Yener and O. Jirsak, in, Improving Performance of Polyvinyl Butyral Electrospinning, 2011, pp. 356–361 Search PubMed.
- F. Yalcinkaya, Experimental study on electrospun polyvinyl butyral nanofibers using a non-solvent system, Fibers Polym., 2015, 16, 2544–2551, DOI:10.1007/s12221-015-5525-1.
- B. Yalcinkaya, F. Yener, F. Cengiz-Çallıoğlu and O. Jirsak, in, Effect of Concentration and Salt Additive on Taylor Cone Structure, 2012, pp. 200–203 Search PubMed.
- F. Yener and O. Jirsak, in, Effect of Nonsolvent on Electrospinning Performance and Nanofiber Properties, 2012, pp. 471–475 Search PubMed.
- J. Choi, R. Wycisk, W. Zhang, P. N. Pintauro, K. M. Lee and P. T. Mather, High Conductivity Perfluorosulfonic Acid Nanofiber Composite Fuel-Cell Membranes, ChemSusChem, 2010, 3, 1245–1248, DOI:10.1002/cssc.201000220.
- T. Tamura and H. Kawakami, Aligned Electrospun Nanofiber Composite Membranes for Fuel Cell Electrolytes, Nano Lett., 2010, 10, 1324–1328, DOI:10.1021/nl1007079.
- H. Wang, X. Li, X. Zhuang, B. Cheng, W. Wang, W. Kang, L. Shi and H. Li, Modification of Nafion membrane with biofunctional SiO2 nanofiber for proton exchange membrane fuel cells, J. Power Sources, 2017, 340, 201–209, DOI:10.1016/j.jpowsour.2016.11.072.
- M. R. Yusof, R. Shamsudin, S. Zakaria, M. Azmi Abdul Hamid, F. Yalcinkaya, Y. Abdullah and N. Yacob, Electron-Beam Irradiation of the PLLA/CMS/β-TCP Composite Nanofibers Obtained by Electrospinning, Polymers, 2020, 12(7), 1593, DOI:10.3390/polym12071593.
- M. R. Yusof, R. Shamsudin, Y. Abdullah, F. Yalcinkaya and N. Yaacob, Electrospinning of carboxymethyl starch/poly(L-lactide acid) composite nanofiber, Polym. Adv. Technol., 2018, 29, 1843–1851, DOI:10.1002/pat.4292.
- N. Khan, A. H. Anwer, S. Sultana, A. Ibhadon and M. Z. Khan, Effective toxicity assessment of synthetic dye in microbial fuel cell biosensor with spinel nanofiber anode, J. Environ. Chem. Eng., 2022, 10, 107313, DOI:10.1016/j.jece.2022.107313.
- L. Huang, N.-N. Bui, S. S. Manickam and J. R. McCutcheon, Controlling electrospun nanofiber morphology and mechanical properties using humidity, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1734–1744, DOI:10.1002/polb.22371.
- M. Xu, L. Wu, M. Zhu, Z. Wang, Z.-H. Huang and M.-X. Wang, Self-supporting nitrogen-doped reduced graphene oxide@carbon nanofiber hybrid membranes as high-performance integrated air cathodes in microbial fuel cells, Carbon, 2022, 193, 242–257, DOI:10.1016/j.carbon.2022.03.024.
- H. Eom, H. J. Joo, S. C. Kim and S. S. Kim, Properties of carbon-based nanofiber with Pd and its application to microbial fuel cells electrode, Environ. Technol. Innovation, 2020, 19, 100800, DOI:10.1016/j.eti.2020.100800.
- N. Jiang, M. Huang, J. Li, J. Song, S. Zheng, Y. Gao, M. Shao and Y. Li, Enhanced bioelectricity output of microbial fuel cells via electrospinning zeolitic imidazolate framework-67/polyacrylonitrile carbon nanofiber cathode, Bioresour. Technol., 2021, 337, 125358, DOI:10.1016/j.biortech.2021.125358.
- M. Gong, X. Li, L. Hu, H. Xu, C. Yang, Y. Luo, S. Li, C. Yin, M. Gan and L. Zhou, Preparation and Characterization of Palladium Nanoparticle-Embedded Carbon Nanofiber Membranes via Electrospinning and Carbonization Strategy, ( 2024), DOI:10.2139/ssrn.4720490.
- C. Santoro, A. Stadlhofer, V. Hacker, G. Squadrito, U. Schröder and B. Li, Activated carbon nanofibers (ACNF) as cathode for single chamber microbial fuel cells (SCMFCs), J. Power Sources, 2013, 243, 499–507, DOI:10.1016/j.jpowsour.2013.06.061.
- K. Cong, M. Radtke, S. Stumpf, B. Schröter, D. G. G. McMillan, M. Rettenmayr and A. Ignaszak, Electrochemical stability of the polymer-derived nitrogen-doped carbon: an elusive goal?, Mater. Renew. Sustain. Energy, 2015, 4, 5, DOI:10.1007/s40243-015-0046-9.
- D.-S. Yang, S. Chaudhari, K. P. Rajesh and J.-S. Yu, Preparation of Nitrogen-Doped Porous Carbon Nanofibers and the Effect of Porosity, Electrical Conductivity, and Nitrogen Content on Their Oxygen Reduction Performance, ChemCatChem, 2014, 6, 1236–1244, DOI:10.1002/cctc.201400035.
- G. Massaglia, V. Margaria, A. Sacco, M. Castellino, A. Chiodoni, F. C. Pirri and M. Quaglio, N-doped carbon nanofibers as catalyst layer at cathode in single chamber Microbial Fuel Cells, Int. J. Hydrogen Energy, 2019, 44, 4442–4449, DOI:10.1016/j.ijhydene.2018.10.008.
- M. Kodali, C. Santoro, A. Serov, S. Kabir, K. Artyushkova, I. Matanovic and P. Atanassov, Air Breathing Cathodes for Microbial Fuel Cell using Mn-, Fe-, Co- and Ni-containing Platinum Group Metal-free Catalysts, Electrochim. Acta, 2017, 231, 115–124, DOI:10.1016/j.electacta.2017.02.033.
- Y. Sun, H. Li, J. Wang, Y. Liu, S. Guo and C. Li, One-piece adhesive-free molding polyvinylidene fluoride @Ag nanofiber membrane for efficient oxygen reduction reaction in microbial fuel cells, J. Environ. Chem. Eng., 2022, 10, 108898, DOI:10.1016/j.jece.2022.108898.
- B. Kartick, S. K. Srivastava and A. Chandra, Graphene/Nickel Nanofiber Hybrids for Catalytic and Microbial Fuel Cell Applications, J. Nanosci. Nanotechnol., 2016, 16, 303–311, DOI:10.1166/jnn.2016.10667.
- J. Ahmed, H. J. Kim and S. Kim, Polyaniline Nanofiber/Carbon Black Composite as Oxygen Reduction Catalyst for Air Cathode Microbial Fuel Cells, J. Electrochem. Soc., 2012, 159, B497, DOI:10.1149/2.049205jes.
- J. X. Leong, W. R. W. Daud, M. Ghasemi, K. B. Liew and M. Ismail, Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: a comprehensive review, Renewable Sustainable Energy Rev., 2013, 28, 575–587, DOI:10.1016/j.rser.2013.08.052.
- L. Koók, G. Dörgő, P. Bakonyi, T. Rózsenberszki, N. Nemestóthy, K. Bélafi-Bakó and J. Abonyi, Directions of membrane separator development for microbial fuel cells: A retrospective analysis using frequent itemset mining and descriptive statistical approach, J. Power Sources, 2020, 478, 229014, DOI:10.1016/j.jpowsour.2020.229014.
- D. J. Khadem, Z. Yaakob, S. Shahgaldi, M. Ghasemi and W. R. Wan Daud, in, Synthesis and Characterization of PES/TiO2 Nanofibers Membrane, Trans Tech Publ, 2011, pp. 613–619 Search PubMed.
- X. Tang, K. Guo, H. Li, Z. Du and J. Tian, Microfiltration membrane performance in two-chamber microbial fuel cells, Biochem. Eng. J., 2010, 52, 194–198, DOI:10.1016/j.bej.2010.08.007.
- B. Dong, L. Gwee, D. Salas-de la Cruz, K. I. Winey and Y. A. Elabd, Super Proton Conductive High-Purity Nafion Nanofibers, Nano Lett., 2010, 10, 3785–3790, DOI:10.1021/nl102581w.
- H.-Y. Jung and S.-H. Roh, Polyvinylidene fluoride nanofiber composite membrane coated with perfluorinated sulfuric acid for microbial fuel cell application, J. Nanosci. Nanotechnol., 2020, 20, 5711–5715, DOI:10.1166/jnn.2020.17622.
- F. Liu, I. S. Kim and K. Miyatake, Proton-conductive aromatic membranes reinforced with poly(vinylidene fluoride) nanofibers for high-performance durable fuel cells, Sci. Adv., 2023, 9, eadg9057, DOI:10.1126/sciadv.adg9057.
- J. Chu, Y. Ou, F. Cheng, H. Liu, N. Luo, F. Hu, S. Wen and C. Gong, Achieving better balance on the mechanical stability and conduction performance of sulfonated poly(ether ether ketone) proton exchange membranes through polydopamine/polyethyleneimine co-modified poly(vinylidene fluoride) nanofiber as support, Int. J. Hydrogen Energy, 2024, 50, 1381–1390, DOI:10.1016/j.ijhydene.2023.10.298.
- W. K. Essa, S. A. Yasin, I. A. Saeed and G. A. M. Ali, Nanofiber-Based Face Masks and Respirators as COVID-19 Protection: A Review, Membranes, 2021, 11(4), 250, DOI:10.3390/membranes11040250.
- R. and M. ltd, Nanofiber Market – Growth, Trends, COVID-19 Impact, and Forecasts, 2023–2028, https://www.researchandmarkets.com/reports/4514872/nanofiber-market-growth-trends-covid-19, accessed May 25, 2023.
- M. Bryjak, H. Hodge and B. Dach, Modification of porous polyacrylonitrile membrane, Angew. Makromol. Chem. Chemie, 1998, 260, 25–29 CrossRef CAS.
- H. Li, Y. Sun, J. Wang, Y. Liu and C. Li, Nanoflower-branch LDHs and CoNi alloy derived from electrospun carbon nanofibers for efficient oxygen electrocatalysis in microbial fuel cells, Appl. Catal., B, 2022, 307, 121136, DOI:10.1016/j.apcatb.2022.121136.
| This journal is © The Royal Society of Chemistry 2024 |