Open Access Article
Open Access ArticleCombustion properties and pollutant analysis of coal-blended bio-heavy oil fuel
Yuan Baiac,
Yuqing Sun *b,
Haojun Panb,
Sheng Wanga,
Yuehong Donga,
Bin Chenb,
Jian Qiub and
Wenheng Jing
*b,
Haojun Panb,
Sheng Wanga,
Yuehong Donga,
Bin Chenb,
Jian Qiub and
Wenheng Jing *b
*b
aState Key Laboratory of Low-Carbon Smart Coal-Fired Power Generation and Ultra-Clean Emission, China Energy Science and Technology Research Institute Co., Ltd, Nanjing, China
bState Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing, China. E-mail: sunyq@njtech.edu.cn; jingwh@njtech.edu.cn
cGuodian Environmental Protection Research Institute Co., Ltd, Nanjing, China
First published on 31st January 2024
Abstract
Excessive carbon-dioxide emissions drive global climate change and environmental challenges. Integrating renewable biomass fuels with coal in power units is crucial for achieving low-carbon emission reductions. Coal blending with bio-heavy oil enhances the combustion calorific value of the fuel, improves combustion characteristics, and decreases pollutant emissions. This study found that bio-heavy oil with low sulfur (0.073%), low nitrogen (0.18%), low ash, and high oxygen (11.005%) content exhibits excellent fuel performance, which can be attributed to the abundant oxygen-containing functional groups (such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the alcohols, aldehydes, and ketones present in bio-heavy oil. Additionally, the residual moisture in coal-blended bio-heavy oil reduces the fuel's calorific value. The calorific value increases with a higher proportion of blended bio-heavy oil (28.1, 28.9, 32.1, 34.7, 40.6 MJ kg−1). Experiments on combustion flame shooting reveal that the combustion time of bio-heavy oils is significantly shorter than that of coal. As the proportion of blended bio-heavy oil increases, the flame height increases. Coal blending with bio-heavy oil involves three stages: water evaporation, volatile-matter decomposition, fixed-carbon combustion and mineral decomposition. This advances the combustion process and improves coal's ignition performance. Furthermore, the amount of gaseous pollutants (sulfur dioxide and nitrogen dioxide) in coal mixed with bio-heavy oil is relatively low, which is in alignment with the green environmental protection guidelines. The blending of coal with biomass fuel holds significant practical and strategic importance for developing high-efficiency, low-carbon, coal power units.
O) in the alcohols, aldehydes, and ketones present in bio-heavy oil. Additionally, the residual moisture in coal-blended bio-heavy oil reduces the fuel's calorific value. The calorific value increases with a higher proportion of blended bio-heavy oil (28.1, 28.9, 32.1, 34.7, 40.6 MJ kg−1). Experiments on combustion flame shooting reveal that the combustion time of bio-heavy oils is significantly shorter than that of coal. As the proportion of blended bio-heavy oil increases, the flame height increases. Coal blending with bio-heavy oil involves three stages: water evaporation, volatile-matter decomposition, fixed-carbon combustion and mineral decomposition. This advances the combustion process and improves coal's ignition performance. Furthermore, the amount of gaseous pollutants (sulfur dioxide and nitrogen dioxide) in coal mixed with bio-heavy oil is relatively low, which is in alignment with the green environmental protection guidelines. The blending of coal with biomass fuel holds significant practical and strategic importance for developing high-efficiency, low-carbon, coal power units.
1. Introduction
The 75th session of the United Nations General Assembly proposed the two-step goal of “carbon peaking and carbon neutrality” in China. Peak CO2 emissions are intended to occur by 2030, and carbon neutrality is to be achieved by 2060.1–3 The energy-intensive power industry is a major CO2 emitter, and the current energy consumption structure of coal power units is dominated by coal.4–6 Coal emissions from China's primary energy consumption account for approximately 80% of the country's total emissions, with coal power units accounting for 50% or more of the coal consumption.7,8 Coal power units serve as China's electric power safety measure, ensuring stable production of “ballast”, and are its basic secure power supply. The depth of the peak power status is difficult to shake.9,10 Therefore, development of a clean and low-carbon power industry is an important part of realizing China's “carbon peak, carbon neutral” goal.11,12Biomass, which is an abundant renewable resource with low sulfur and ash content, is the fourth largest energy source after coal, oil, and natural gas, and occupies an important position in the energy system.13–15 The biomass resources available for development and utilization in China are estimated to be equivalent to approximately 750 million tons of standard coal.16,17 Biomass has a lower ash content (<10%) than coal (generally 10–15%) and lower nitrogen content. Particularly, its sulfur content (less than 0.4%) is much less than that of coal (0.5–1.5%).18,19 The chemical energy released during biomass combustion can be utilized to heat water and other media, producing low-carbon and environmentally friendly steam.20,21 Coal-biomass co-combustion is regarded as a low-risk, low-cost, sustainable, and renewable energy option that is promising for reducing CO2, SOx, and NOx emissions.22,23 Moreover, coal-biomass co-combustion allows for truly low NO emissions and higher heat of combustion compared to conventional coal combustion.24,25
Large amounts of bio-heavy oil are constantly and simultaneously produced during biodiesel production, constituting an inexpensive and readily available source.26,27 Currently, the annual production of bio-heavy oil in China is one million tons, however, it is typically treated as industrial waste during production. Bio-heavy oil, is an environmentally friendly and carbon-neutral alternative fuel.28 It mainly comprises high-boiling alcohols, ketones, esters, and ethers of O1–O12 with a sulfur content less than 0.05%.29,30 This accounts for the favorable fuel properties of bio-heavy oil, which has been reported to possess a high calorific value for combustion, even exceeding that of coal.31 Therefore, the use of bio-heavy oil as fuel blended with coal not only helps reduce the CO2, SOx, and NOx emissions generated during the combustion process but also increases the combustion calorific value of the fuel, reduces the ash content, and improves the combustion characteristics of the fuel.32,33
This study employs a technology involving the blending of coal with bio-heavy oil to increase the combustion calorific value of the fuel, improve the fuel miscibility characteristics, and reduce the emission of gaseous pollutants. The composition and content of coal and bio-heavy oil are analyzed, and the effects of the blending ratio on the combustion calorific value and miscibility characteristics (such as flame morphology, ignition characteristics, combustion rate, and combustion process) of coal and bio-heavy oil fuels are investigated. Furthermore, the composition of gaseous pollutants in coal blended with bio-heavy oil is analyzed using mass spectrometry and a flue gas detector. Coal-blending biomass technology will facilitate the transformation and upgrading of the energy structure of coal power units, providing an effective route for carbon reduction at the source in coal power units.
2. Materials and characterization
Coal powder was purchased from Hebei Shijiazhuang Mineral Products Factory. And bio-heavy oil was provided by Sinopec Qilu Petrochemical Company. The coal and bio-heavy oil were thoroughly mixed in a mortar using the grinding method.The organic element contents of coal and bio-heavy oil were analyzed using an organic element analyzer (Elementar Vario EL, Elementar, Germany). The organic matter from coal and bio-heavy oils was determined using an industrial analyzer (SX2-10-12N). The viscosities of bio-heavy oil and coal-blended bio-heavy oil were measured using a high-temperature Brookfield viscometer (NDJ-1F, Shanghai Qigao Instrument Co., Ltd, China). The functional groups in the fuels were analyzed by Fourier transform infrared spectroscopy (FTIR, Nicolet8700, Thermo Fisher Scientific). The calorific values were determined using a microcomputerized automatic touch calorimeter (ZDHW-8E, Henan Hebixintianke Coal Quality Instrument Co., Ltd, China). The combustion flames of the fuels were captured using a high-speed camera (AcutEye V4.0, Hunan Ketianjian Photoelectric Techco. Co., Ltd, China). The combustion process of the fuel was analyzed by thermogravimetry-derivative thermogravimetry (TG-DTG, NETZSCH STA 449 F5, NETZSCH, Germany). The composition and amount of gaseous products released during the combustion of the fuels were detected using thermogravimetry-mass spectrometry (TG-MS, TG (Hitachi 7300, Hitachi, Japan), MS (LC-D200M PRO)). The on-line flue gas detection during fuel combustion was performed using a flue gas analyzer (HP-GAS, Nanjing Hope Techco. Co., Ltd, China) and a tube furnace (GSL-1500X-OTF, Hefei Kejing Materials Techco. Co., Ltd, China).
3. Results and discussion
3.1. Characterization of coal, bio-heavy oil and coal-bio-heavy oil blend
The morphologies of coal, bio-heavy oil, and coal-bio-heavy oil blend are presented in Fig. 1. Coal, with its small particle size of 1–2 μm (Fig. 1a–c), blends seamlessly with bio-heavy oil with good fluidity, ensuring optimal combustion of the fuel mixture. In addition, there is a big difference between the chemical compositions of bio-heavy oil and coal, which affects the fuel combustion process.29,34,35 As shown in Table 1, bio-heavy oil has lower sulfur (0.073%) and nitrogen (0.18%) contents and higher oxygen (11.005%) content than coal. This contributes to cleaner exhaust emissions and enhanced fuel performance. Specifically, blending coal with bio-heavy oil helps reduce the emissions of pollutants such as CO2, SOx, and NOx. As shown in Table 2, the contents of moisture, ash, and fixed carbon in coal are significantly higher than those of the bio-heavy oil, and the volatile matter content in coal is significantly lower than that of bio-heavy oil. Coal blending with bio-heavy oil diminishes the ash content and reduces the waste residue, thereby elevating the overall fuel utilization rate. The exceptionally low fixed-carbon content of bio-heavy oil, recorded at a mere 0.97%, stands in stark contrast to the value of 52.34% observed in coal. Thus, bio-heavy oil ignites and burns more rapidly during combustion, and the coal blending of bio-heavy oil is conducive to improving fuel combustion efficiency. Additionally, bio-heavy oil boasts a volatile matter content exceeding 90%, rendering it highly combustible. It is worth noting that during the initial stages of combustion, the volatile analysis of bio-heavy oil tends to be larger. Under conditions of insufficient air and low temperatures, this can lead to the generation of a flame with black smoke, a crucial factor when conducting combustion experiments.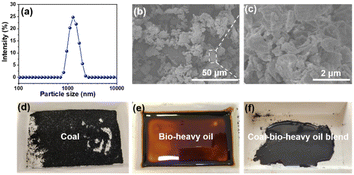 | ||
| Fig. 1 (a) Size distribution and (b and c) scanning electron microscopic images of coal. The morphologies of (d) coal, (e) bio-heavy oil, and (f) coal-bio-heavy oil blend are also presented. | ||
| Samples | N (%) | C (%) | H (%) | S (%) | O (%) |
|---|---|---|---|---|---|
| Coal | 1.16 | 65.11 | 3.853 | 0.625 | 9.676 |
| Bio-heavy oil | 0.18 | 77.06 | 9.824 | 0.073 | 11.005 |
| Samples | Moisture Mad (%) | Ash A (%) | Volatile matter V (%) | Fixed carbon FCad (%) | |||
|---|---|---|---|---|---|---|---|
| Aad | Ad | Vad | Vd | Vdaf | |||
| Coal | 3.39 | 14.04 | 14.53 | 30.23 | 34.80 | 36.61 | 52.34 |
| Bio-heavy oil | 0.49 | 1.61 | 1.62 | 96.93 | 97.89 | 99.01 | 0.97 |
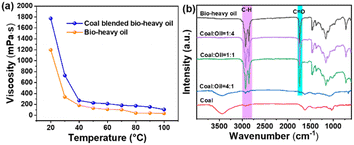
The contrast in fluidity between coal and bio-heavy oil is a pivotal aspect, with bio-heavy oil's blending significantly improving its fluidity. In Fig. 2a, the viscosity of pure bio-heavy oil is 1200 mPa s at room temperature. When coal is blended with bio-heavy oil in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the fuel exhibited fluidity, and its viscosity becomes as high as 1800 mPa s. Notably, temperature variations during combustion play a crucial role in altering the molecular energy and, consequently the viscosity of the fuel. The impact of temperature is illustrated as the blended fuel viscosity decreases significantly from 1800 to 300 mPa s from 20 to 40 °C. This phenomenon is attributed to the increased energy of bio-heavy oil molecules, which intensifies their movement. This reduces the intermolecular friction and viscosity of the blended fuel. As the temperature further increases to 100 °C, the viscosity of the blended fuel remains essentially unchanged and is comparable to that of bio-heavy oil.
1, the fuel exhibited fluidity, and its viscosity becomes as high as 1800 mPa s. Notably, temperature variations during combustion play a crucial role in altering the molecular energy and, consequently the viscosity of the fuel. The impact of temperature is illustrated as the blended fuel viscosity decreases significantly from 1800 to 300 mPa s from 20 to 40 °C. This phenomenon is attributed to the increased energy of bio-heavy oil molecules, which intensifies their movement. This reduces the intermolecular friction and viscosity of the blended fuel. As the temperature further increases to 100 °C, the viscosity of the blended fuel remains essentially unchanged and is comparable to that of bio-heavy oil.
Analyzing functional groups through FTIR, as depicted in Fig. 2b, provides valuable insights into coal, bio-heavy oil, and blends of coal and bio-heavy oil at different mixing ratios. Absorption peaks at 2919 and 2852 cm−1 for coal, bio-heavy oil, and coal-bio-heavy oil blends correspond to alkane stretching vibrations. Compared to coal, bio-heavy oil and coal-bio-heavy oil blend exhibit a more pronounced stretching vibration peak at 1739 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds present in the aldehydes or ketones within bio-heavy oil. Additionally, the higher the proportion of bio-heavy oil doping, the more significant the peak is. This is mainly because coal is a fossil raw materials, which is a product obtained during the processing of crude oil, and thus, has no oxygen-containing functional group.34,36 While bio-heavy oil is a raw material obtained from biomass processing and is enriched with oxygen-containing functional groups, enhancing its combustion potential.29
O bonds present in the aldehydes or ketones within bio-heavy oil. Additionally, the higher the proportion of bio-heavy oil doping, the more significant the peak is. This is mainly because coal is a fossil raw materials, which is a product obtained during the processing of crude oil, and thus, has no oxygen-containing functional group.34,36 While bio-heavy oil is a raw material obtained from biomass processing and is enriched with oxygen-containing functional groups, enhancing its combustion potential.29
Utilizing a micro-computerized automatic touch calorimeter, we probed the impact of both moisture content and various bio-heavy oil blending ratios on the combustion calorific value of the fuels. As can be observed in Fig. 3a, the calorific values of the combustion of coal, bio-heavy oil, and coal-bio-heavy oil blended fuels after drying at 120 °C for 24 h are higher than those of the fuel without drying. The calorific values of coal, dry coal, coal-bio-heavy oil, dry coal-bio-heavy oil, bio-heavy oil, and dry bio-heavy oil fuel are 26.1, 28.1, 27.3, 28.9, 38.3, 40.6 MJ kg−1, respectively. This indicates that the moisture residue reduces the calorific value of fuels, and fuels treated by drying exhibit higher combustion calorific values.37 As shown in Fig. 3b, the calorific value of coal blended with bio-heavy oil is higher than that of coal. Moreover, the higher the ratio of coal blended with bio-heavy oil, the higher the calorific value. Specifically, the calorific values of the fuels are 28.1, 28.9, 32.1, 34.7, 40.6 MJ kg−1, when the mixing ratios of coal and bio-heavy oil were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 4
0, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 0
4, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. As seen in Table 3, the calorific value of coal blended with bio-heavy oil is higher than that of ordinary biomass such as wood chips. Moreover, the higher the proportion of bio-heavy oil blending, the closer the calorific value of the fuel is to that of petroleum.19,31
1, respectively. As seen in Table 3, the calorific value of coal blended with bio-heavy oil is higher than that of ordinary biomass such as wood chips. Moreover, the higher the proportion of bio-heavy oil blending, the closer the calorific value of the fuel is to that of petroleum.19,31
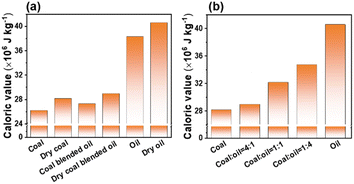 | ||
| Fig. 3 Effect of (a) moisture and (b) bio-heavy oil blending ratio on the calorific value of the fuels. | ||
| Samples | Wood chips | Coal | Petroleum | Coal blended bio-heavy oil (this work) |
|---|---|---|---|---|
| Calorific value (MJ kg−1) | 19.2 | 29 | 42 | 28.1–40.6 |
3.2. Combustion characteristics of coal blended with bio-heavy oil
To further analyze the effect of bio-heavy oil blending on the coal combustion intensity, a high-speed camera was used to capture the height and brightness of the combustion flame of the fuel at a shooting speed of 500 fps and resolution of 1280 × 1024 (Fig. 4a). Fig. 4b–f show that fuel combustion can be divided into three stages: initiation, steady combustion, and decay. In the combustion process, the electric-resistance wire becomes a high-temperature heat source. Upon heating, part of the fuel on the silicon wafer heats rapidly and gradually, generating a flame accompanied by black smoke. During the combustion experiment, we found that the amount of black smoke produced when coal was burned greater than that produced when bio-heavy oil was burned. Clearly, coal remained almost unburnt (Fig. 4b), whereas the bio-heavy oil burned vigorously (Fig. 4f), which is mainly because the bio-heavy oil has abundant oxygen-containing functional groups. The higher the blending ratio of bio-heavy oil, the higher the combustion flame of the fuel would be (Fig. 4c–e). In addition, the combustion time required for coal blended with bio-heavy oil was lesser than that required for the combustion of coal alone, which indicates that blending bio-heavy oil with coal helps in the complete combustion of coal, thereby improving its utilization.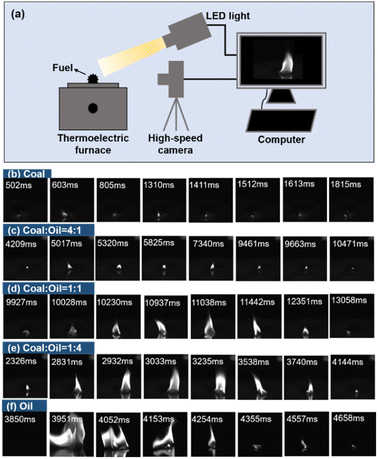 | ||
| Fig. 4 (a) Schematic diagram of the flame shooting device for fuels, and (b–f) photographs of the combustion flame of coal-bio-heavy oil at different blending ratios. | ||
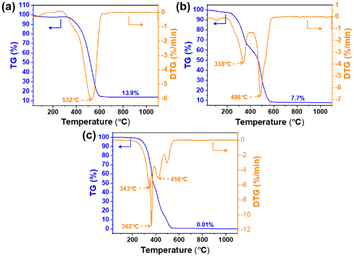
TG-DTG characterization was used to analyze the combustion process of the fuel.38 As shown in Fig. 5, the combustion of bio-heavy oil and coal is divided into three stages: water evaporation, volatile matter decomposition, fixed-carbon combustion, and mineral decomposition. As the fuel is dried in an oven at 120 °C for 24 h prior to TG experiments, most of the moisture is removed, therefore, the fuel weight loss in the first stage is minimal (<5%). The weight loss of coal at this stage is higher than that of the bio-heavy oils because of the higher moisture content in coal (Table 2, Fig. 5a and c).
In the second stage, the volatile components of the bio-heavy oil continuously decompose with a gradual increase in temperature, and the chemical reactions between the volatile components and oxygen accelerate with an increase in temperature. The decomposition of volatile matter and combustion of fixed carbon in bio-heavy oil specifically occur within the range of 250–520 °C, whereas in coal, these processes are concentrated in the range of 300–600 °C. Three peaks occur at 343, 362, and 416 °C during the combustion of bio-heavy oil, which are lower than the combustion temperature of coal (532 °C). Obviously, the ignition temperature of bio heavy oil is lower than that of coal, mainly due to the stronger reactivity of oxygen-containing functional groups in bio-heavy oil compared to alkanes in coal, making it easier to decompose, oxidize, and burn. In addition, coal-blended bio-heavy oil catches fire earlier compared to coal. This may be because the thermal decomposition of substances such as aldehydes or ketones in bio-heavy oil generates a large number of reactive free radicals which also promotes the decomposition of alkanes in coal.39 The DTG curve of bio-heavy oil is narrow and the peak is high, indicating that the volatile release is intense and concentrated, making it the most combustible. In comparison, no obvious peak is observed in the volatile release during the combustion of coal alone. The DTG peaks formed when a mixture of bio-heavy oil and coal undergoes combustion similar to those obtained in the case of bio-heavy oil alone, except that the peaks are lower (Fig. 5b).
In the last stage, a portion of the fixed carbon in coal remains unburnt, resulting in some residue, which is consistent with the ash results in Table 2. In contrast, the bio-heavy oil leaves almost no residue. The residual content of coal combustion (13.9%) is much higher than that of bio-heavy oil combustion (0.01%), and the residual content of coal mixed with bio-heavy mass is 7.7%. These results show that after blending bio-heavy oil with coal, the combustion characteristics of the coal and bio-heavy oil blend gradually resemble those of bio-heavy oil. As the volatile components of bio-heavy oil can be resealed at lower temperatures, the volatile components and water contained in bio-heavy oil are released to form differently sized gaps inside the fuel, which is conducive to achieving full contact with oxygen.40 Therefore, the blending of bio-heavy oil can advance the coal combustion process, thereby improving the ignition performance of coal.
3.3. Pollutant release characteristics of coal blended with bio-heavy oil
Mass spectrometry was used to detect the composition and content of the gaseous products released during the combustion of bio-heavy oil and coal blended with bio-heavy oil (coal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bio-heavy oil = 1
bio-heavy oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), including the mass spectral curves of CO (m/z = 28), NO (m/z = 30), O2 (m/z = 32), CO2 (m/z = 44), NO2 (m/z = 46), and SO2 (m/z = 64).41 As shown in Fig. 6, the amount of gaseous pollutants in bio-heavy oil after combustion is in the order of CO > CO2 > NO > SO2 ≈ NO2. The peak of CO2 is significantly higher than that of the other gases, mainly because of the precipitation of organic matter and coke combustion in the air. Similar to the mass spectral curve of CO2, the curve of NO shows obvious peaks, whereas that of SO2 and NO2 do not show obvious peaks, which is related to the low content of nitrogen and sulfur in bio-heavy oil. This indicates that the SO2 and NO2 emissions during the combustion of bio-heavy oil are relatively low. Therefore, the blending of coal and bio-heavy oil is expected to reduce the emissions of gaseous pollutants during the combustion process.22 The content of gaseous pollutants in coal blended with bio-heavy oil after combustion, presented in Fig. 7, are also in the order of CO > CO2 > NO > SO2 ≈ NO2. Obviously, the emissions of NO, NO2 and SO2 during the combustion of coal blended with bio-heavy oil are relatively low, which is in line with the guidelines of green chemical industries.
1), including the mass spectral curves of CO (m/z = 28), NO (m/z = 30), O2 (m/z = 32), CO2 (m/z = 44), NO2 (m/z = 46), and SO2 (m/z = 64).41 As shown in Fig. 6, the amount of gaseous pollutants in bio-heavy oil after combustion is in the order of CO > CO2 > NO > SO2 ≈ NO2. The peak of CO2 is significantly higher than that of the other gases, mainly because of the precipitation of organic matter and coke combustion in the air. Similar to the mass spectral curve of CO2, the curve of NO shows obvious peaks, whereas that of SO2 and NO2 do not show obvious peaks, which is related to the low content of nitrogen and sulfur in bio-heavy oil. This indicates that the SO2 and NO2 emissions during the combustion of bio-heavy oil are relatively low. Therefore, the blending of coal and bio-heavy oil is expected to reduce the emissions of gaseous pollutants during the combustion process.22 The content of gaseous pollutants in coal blended with bio-heavy oil after combustion, presented in Fig. 7, are also in the order of CO > CO2 > NO > SO2 ≈ NO2. Obviously, the emissions of NO, NO2 and SO2 during the combustion of coal blended with bio-heavy oil are relatively low, which is in line with the guidelines of green chemical industries.
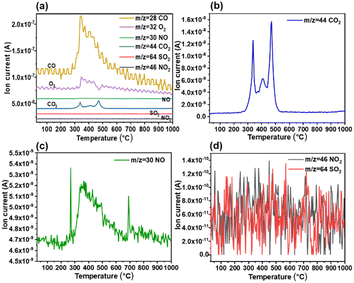 | ||
| Fig. 6 (a) Mass spectrometry results of gaseous pollutant emissions from bio-heavy oil combustion. Mass spectral curves of (b) CO2, (c) NO, and (d) NO2 and SO2. | ||
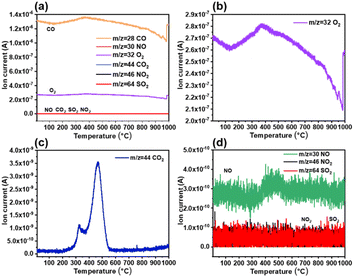 | ||
| Fig. 7 (a) Mass spectrometry results of gaseous pollutant emissions from the combustion of coal blended with bio-heavy oil. Mass spectral curves of (b) O2, (c) CO2, and (d) NO, NO2, and SO2. | ||
Online flue gas detection during fuel combustion was performed using a flue gas analyzer and tube furnace (Fig. 8a). The fuel combustion (coal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bio-heavy oil = 1
bio-heavy oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was performed in an air atmosphere, and the combustion temperature was increased from room temperature to 1100 °C at a rate of 10 °C min−1. The gaseous pollutants' composition and concentration were monitored in real-time using a flue gas analyzer. For safety considerations, the test was initiated by opening the outlet valve of the tubular furnace when the outlet pressure of the tubular furnace reached 0.05 MPa. During combustion (Fig. 8b), the cumulative concentrations of SO2 were 127 (480 °C), 12 (810 °C), and 12 ppm (1100 °C), respectively. The cumulative concentrations of NO2 were 18 (480 °C), 0.1 (810 °C), and 0 ppm (1100 °C), respectively. The results showed that the SO2 and NO2 gaseous pollutant contents of coal blended with bio-heavy oil were relatively low, making it green and environmentally friendly.
1) was performed in an air atmosphere, and the combustion temperature was increased from room temperature to 1100 °C at a rate of 10 °C min−1. The gaseous pollutants' composition and concentration were monitored in real-time using a flue gas analyzer. For safety considerations, the test was initiated by opening the outlet valve of the tubular furnace when the outlet pressure of the tubular furnace reached 0.05 MPa. During combustion (Fig. 8b), the cumulative concentrations of SO2 were 127 (480 °C), 12 (810 °C), and 12 ppm (1100 °C), respectively. The cumulative concentrations of NO2 were 18 (480 °C), 0.1 (810 °C), and 0 ppm (1100 °C), respectively. The results showed that the SO2 and NO2 gaseous pollutant contents of coal blended with bio-heavy oil were relatively low, making it green and environmentally friendly.
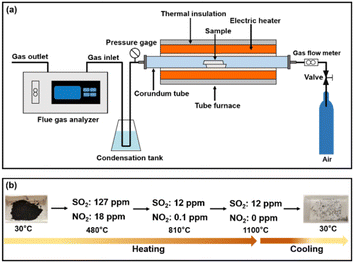 | ||
Fig. 8 (a) Devices for combustion and flue-gas analysis. (b) Combustion process of fuel (coal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bio-heavy oil = 1 bio-heavy oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and analysis of gas concentration. 1) and analysis of gas concentration. | ||
4. Conclusions
In this study, blending coal with bio-heavy oil was found to increase the combustion calorific value of the fuel, improve the fuel combustion characteristics, and reduce pollutant emissions. As alcohols, aldehydes, and ketones in bio-heavy oils were rich in oxygenated functional groups (e.g., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), the oxygen content of bio-heavy oils was relatively high (11.005%), indicating good fuel properties. In addition, moisture residues reduce the calorific value of the fuel. The higher the percentage of blended bio-heavy oil, the higher the calorific value of the fuel. Specifically, the calorific values of the fuels were 28.1 (coal
O), the oxygen content of bio-heavy oils was relatively high (11.005%), indicating good fuel properties. In addition, moisture residues reduce the calorific value of the fuel. The higher the percentage of blended bio-heavy oil, the higher the calorific value of the fuel. Specifically, the calorific values of the fuels were 28.1 (coal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bio-heavy oil = 1
bio-heavy oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), 28.9 (4
0), 28.9 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 32.1 (1
1), 32.1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 34.7 (1
1), 34.7 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), and 40.6 (0
4), and 40.6 (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) MJ kg−1. In addition, blending bio-heavy oil resulted in an increased the flame height and enhanced the ignition performance of the coal. The amounts of sulfur dioxide and nitrogen dioxide gaseous pollutants when burning the blend of coal and bio-heavy oil were relatively low, which was in line with the green environmental protection requirements. Biomass fuel-coal blending technology is of considerable significance for the development of high-efficiency, low-carbon coal-power units.
1) MJ kg−1. In addition, blending bio-heavy oil resulted in an increased the flame height and enhanced the ignition performance of the coal. The amounts of sulfur dioxide and nitrogen dioxide gaseous pollutants when burning the blend of coal and bio-heavy oil were relatively low, which was in line with the green environmental protection requirements. Biomass fuel-coal blending technology is of considerable significance for the development of high-efficiency, low-carbon coal-power units.
Author contributions
Yuan Bai: methodology, investigation, validation, formal analysis. Yuqing Sun: formal analysis, investigation, data curation, writing-review & editing, funding acquisition. Haojun Pan: data curation, formal analysis. Sheng Wang: methodology, investigation. Yuehong Dong: validation, formal analysis. Bin Chen: formal analysis, investigation. Jian Qiu: methodology, formal analysis. Wenheng Jing: conceptualization, resources, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the State Key Laboratory of Low-Carbon Smart Coal-Fired Power Generation and Ultra-Clean Emission/China Energy R&D Center of Energy Saving, Emission Reduction and Pollution Control Technology in Coal-fired Power Generation (202340835), the State Key Laboratory of Materials-Oriented Chemical Engineering (SKL-MCE-22A03), the National Key Research and Development Program (2021YFB3801303), Jiangsu Provincial Key Research and Development Program (BE2022033-2), and Jiangsu Funding Program for Excellent Postdoctoral Talent (2023ZB241).Notes and references
- L. Sun, H. Cui and Q. Ge, Adv. Clim. Change Res., 2022, 13, 169–178, DOI:10.1016/j.accre.2022.02.002.
- Y. Wei, K. Chen, J. Kang, W. Chen, X. Wang and X. Zhang, Engineering, 2022, 14, 52–63, DOI:10.1016/j.eng.2021.12.018.
- K. Jeong, T. Hong and J. Kim, Energy Build., 2018, 158, 86–94, DOI:10.1016/j.enbuild.2017.10.015.
- D. Wang, X. Xie, Y. Zhou, X. Han and L. Si, Appl. Therm. Eng., 2023, 230, 120850, DOI:10.1016/j.applthermaleng.2023.120850.
- P. Zhang, K. Feng, L. Yan, Y. Guo, B. Gao and J. Li, Environ. Sci. Ecotechnology., 2024, 17, 100295, DOI:10.1016/j.ese.2023.100295.
- Y. Dong, X. Jiang, Z. Liang and J. Yuan, Resour., Conserv. Recycl., 2018, 134, 184–195, DOI:10.1016/j.resconrec.2018.03.012.
- X. Teng, F. P. Liu and Y. H. Chiu, Nat. Resour. Forum, 2020, 44, 334–352, DOI:10.1111/1477-8947.12207.
- X. Tang, Y. Jin, B. C. McLellan, J. Wang and S. Li, Resour., Conserv. Recycl., 2018, 129, 307–313, DOI:10.1016/j.resconrec.2016.07.018.
- Y. Ding, M. Li, A. Abdulla, R. Shan, S. Gao and G. Jia, Environ. Res. Lett., 2021, 16, 64043, DOI:10.1088/1748-9326/abfd5a.
- J. Zhou and W. Zhang, Energy, 2023, 273, 126996, DOI:10.1016/j.energy.2023.126996.
- H. Liang, Y. Zeng, X. Jiang and Y. Li, Heliyon, 2023, 9, e13467, DOI:10.1016/j.heliyon.2023.e13467.
- B. Tang, R. Li, B. Yu and Y. Wei, J. Cleaner Prod., 2019, 230, 720–733, DOI:10.1016/j.jclepro.2019.05.151.
- Y. Zhang, Y. Liang, S. Li, Y. Yuan, D. Zhang, Y. Wu, H. Xie, K. Brindhadevi, A. Pugazhendhi and C. Xia, Fuel, 2023, 347, 128461, DOI:10.1016/j.fuel.2023.128461.
- Z. Li, Y. Liang, W. Ni, Q. Liao, N. Xu, L. Li, J. Zheng and H. Zhang, Appl. Energy, 2022, 311, 118684, DOI:10.1016/j.apenergy.2022.118684.
- J. A. Mathews, Energy Policy, 2008, 36, 940–945, DOI:10.1016/j.enpol.2007.11.029.
- B. A. Farhan, L. Zhihe, S. Ali, T. A. Shah, L. Zhiyu, A. Zhang, S. Javed and M. Asad, Environ. Sci. Pollut. Res. Int., 2023, 30, 64904–64931, DOI:10.1007/s11356-023-27098-8.
- M. Yaseen, F. Abbas, M. B. Shakoor, A. A. Farooque and M. Rizwan, Arabian J. Geosci., 2020, 13, 77, DOI:10.1007/s12517-019-5049-x.
- G. Song, L. Shen, J. Xiao and L. Chen, Energy Sources, Part A, 2013, 35, 809–816, DOI:10.1080/15567036.2011.586983.
- Z. Lu, X. Chen, S. Yao, H. Qin, L. Zhang, X. Yao, Z. Yu and J. Lu, Fuel, 2019, 258, 116150, DOI:10.1016/j.fuel.2019.116150.
- B. Zhao, Y. Su, D. Liu, H. Zhang, W. Liu and G. Cui, Energy, 2016, 113, 821–830, DOI:10.1016/j.energy.2016.07.107.
- J. Moon, J. Lee and U. Lee, Bioresour. Technol., 2011, 102, 9550–9557, DOI:10.1016/j.biortech.2011.07.041.
- L. Baxter, Fuel, 2005, 84, 1295–1302, DOI:10.1016/j.fuel.2004.09.023.
- J. Dai, S. Sokhansanj, J. R. Grace, X. Bi, C. J. Lim and S. Melin, Can. J. Chem. Eng., 2008, 86, 367–386, DOI:10.1002/cjce.20052.
- L. Dong, S. Gao, W. Song, J. Li and G. Xu, Energy Fuels, 2009, 23, 224–228, DOI:10.1021/ef800589c.
- Y. Xu, K. Yang, J. Zhou and G. Zhao, Sustain, 2020, 12, 3692, DOI:10.3390/su12093692.
- J. Kim, J. Jung, S. Cho, Y. F. Tsang, C. Wang, J. Lee and E. E. Kwon, J. Cleaner Prod., 2019, 217, 633–638, DOI:10.1016/j.jclepro.2019.01.289.
- G. Knothe and L. F. Razon, Prog. Energy Combust. Sci., 2017, 58, 36–59, DOI:10.1016/j.pecs.2016.08.001.
- J. Kim and J. Choi, Renewable Energy, 2023, 219, 119543, DOI:10.1016/j.renene.2023.119543.
- S. Jung, M. Kim, K. A. Lin, Y. Park and E. E. Kwon, J. Cleaner Prod., 2021, 294, 126347, DOI:10.1016/j.jclepro.2021.126347.
- H. Seo, H. Kim and E. Jeon, Energy Environ., 2020, 31, 424–439, DOI:10.1177/0958305X19874317.
- R. Gao, J. Li, S. Wang, Y. Zhang, L. Zhang, Z. Ye, Z. Zhu, W. Yin and S. Jia, Anal. Methods, 2023, 15, 1168–1674, 10.1039/d2ay02086f.
- P. Ndayishimiye and M. Tazerout, Energy, 2011, 36, 1790–1796, DOI:10.1016/j.energy.2010.12.046.
- J. Dharmaraja, D. D. Nguyen, S. Shobana, G. D. Saratale, S. Arvindnarayan, A. E. Atabani, S. W. Chang and G. Kumar, Fuel, 2019, 239, 153–161, DOI:10.1016/j.fuel.2018.10.123.
- F. Gao, Z. Jia, Y. Shan, Y. Teng, Y. Li and X. Pu, ACS Omega, 2022, 7, 39830–39839, DOI:10.1021/acsomega.2c03824.
- W. Wilczyńska-Michalik, R. Gasek, M. Michalik, J. Dańko and T. Plaskota, Mineralogia, 2018, 49, 67–97, DOI:10.2478/mipo-2018-0008.
- T. Q. Donaghy, N. Healy, C. Y. Jiang and C. P. Battle, Energy Res. Social Sci., 2023, 100, 103104, DOI:10.1016/j.erss.2023.103104.
- L. Ma, J. L. Goldfarb, J. Song, C. Chang and Q. Ma, J. Cleaner Prod., 2022, 366, 132991, DOI:10.1016/j.jclepro.2022.132991.
- R. Lin, R. Yuan, Z. Yang, C. Zhai and X. Wang, Energy Sources, Part A, 2021, 1–12, DOI:10.1080/15567036.2021.1956018.
- Y. Wang, L. Zou, H. Shao, Y. Bai, Y. Liu, Q. Zhao and F. Li, Sci. Total Environ., 2022, 838, 156489, DOI:10.1016/j.scitotenv.2022.156489.
- A. E. Farrell and A. R. Gopal, MRS Bull., 2008, 33, 373–380, DOI:10.1557/mrs2008.76.
- N. E. Magida, L. L. Bolo, S. P. Hlangothi, G. Dugmore and A. S. Ogunlaja, Combust. Sci. Technol., 2021, 193, 419–436, DOI:10.1080/00102202.2019.1658577.
| This journal is © The Royal Society of Chemistry 2024 |