Open Access Article
Open Access ArticleA review on vegetable oil-based non isocyanate polyurethane: towards a greener and sustainable production route
Marwah Rayung a,
Noraini Abd Ghani*bc and
Norhafizah Hasanudind
a,
Noraini Abd Ghani*bc and
Norhafizah Hasanudind
aSchool of Wood Industry, Faculty of Applied Sciences, Universiti Teknologi MARA, Cawangan Pahang Kampus Jengka, 26400 Bandar Tun Razak Pahang, Malaysia
bCentre of Research in Ionic Liquids, Universiti Teknologi PETRONAS, Seri Iskandar 32610, Perak, Malaysia. E-mail: noraini.ghani@utp.edu.my
cFundamental and Applied Science Department, Universiti Teknologi PETRONAS, Seri Iskandar 32610, Perak, Malaysia
dTerra Mineral Lab Sdn Bhd, Level 16, Perak Techno Trade Centre Bandar Meru Jaya, Off Jalan Jelapan, Ipoh 30020, Perak Darul Ridzuan, Malaysia
First published on 19th March 2024
Abstract
The transition from conventional polyurethane (PU) to non isocyanate polyurethane (NIPU) is driven mainly by safety concerns, environmental considerations, and sustainability issues associated with the current PU technology. NIPU has emerged as a promising alternative, addressing limitations related to traditional PU production. There has been increasing interest in bio-based NIPU aligning with the aspiration for green materials and processes. One important biomass resource for the development of bio-based NIPU is vegetable oil, an abundant, renewable, and relatively low cost feedstock. As such, this review aims to provide insight into the progression of NIPU derived from vegetable oils. This article highlights the synthetic and green approach to NIPU production, emphasizing the method involving the polyaddition reaction of cyclic carbonates and amines. The review includes case studies on vegetable oil-based NIPU and perspectives on their properties. Further, discussions on the potential applications and commercial importance of PU and NIPU are included. Finally, we offer perspectives on possible research directions and the future prospects of NIPU, contributing to the ongoing evolution of PU technology.
1 Introduction
Polyurethane has become an essential part of the modern lifestyle. PU was first discovered in the 1930s by Otto Bayer while looking to replace natural rubber during World War II.1 Currently, PU is an important class of polymers, along with polyethylene, polypropylene, polyvinyl chloride, and polyethylene terephthalate. The global scale production of PU in 2020 exceeded USD 70.67 billion and is expected to reach USD 94.59 billion by 2029. This remarkable scale is due to the wide range of applications of PU in diverse fields. It is mainly used as flexible and rigid foams, resins, adhesives, sealants, and coatings,2 commonly found in biomedical applications, textiles, the building, construction and automotive industries, and many others. The outstanding advantages of PU are its durability, toughness, and exceptional chemical resistance. Also, PU possesses durability comparable to metals, yet the elasticity of rubber, making it a versatile alternative to metals, plastics, and rubber in various engineering and commodity applications.3 Despite the many advantages of PU, the downside of this polymer relates to the use of toxic and moisture-sensitive isocyanate in the manufacturing process. The involvement of this hazardous component renders PU production extremely toxic and dangerous.4 In addition to the risks associated with isocyanate, the majority of PU available today was derived from non-renewable petroleum-based chemicals. These challenges motivate researchers and industries to find greener and more sustainable alternative routes to produce PU.In view of the recent trend towards green chemistry, the development of bio-based PU has been mainly directed towards the preparation of bio-based polyol, bio-based isocyanate, biomass chain extender and isocyanate-free polyurethane.5 In particular, isocyanate-free PU or commonly known as NIPU is gaining research interest due to the immense benefits this route has to offer. With the absence of isocyanate, NIPU production is deemed to be safe, have reduced environmental impact, lower volatile organic compounds emissions, and potential for cost savings. NIPU can be synthesized via a catalyst-free and solvent-free under moderate reaction conditions. This approach is considered atom-efficient and generates no by-products.6 Presently, there is a surge of interest in producing bio-based NIPU where the materials used are derived from renewable biomass sources. Different types of building blocks can be produced from renewable feedstock. In particular, vegetable oils present vast opportunities for this purpose, as they can be transformed into many novel products.7 The rationale for using vegetable oils in the production of NIPU is related to their wide availability, renewable nature, eco-friendliness, and the potential to reduce toxicity and health concerns.
Considering the significant growth of studies using vegetable oils as the starting materials to produce NIPU, this work aims to convey the current state of the art in this field. To the best of our knowledge, even though there are some reviews focusing on bio-based NIPU, there is no work specifically focusing on vegetable oil-based NIPU in detail. Therefore, this review could serve as a foundational framework and complementary to the existing literature.
2 Insight into PU and NIPU
Polyurethane is a polymer joined by the urethane linkage comprising of alternative isocyanate (mono-, di-, polyisocyanate) unit, referred to as the hard segment, and the alcohols that possess two or more hydroxyl groups (from here on refers to as polyols) as the soft segments. In the structure of PU, the hard segment contributes to the stiffness and rigidity of the polymer, whereas the soft segment is responsible for the elasticity or flexibility properties.8 It is worth to mention that the ratio of the hard segment to the soft segment affects the structure, physical, mechanical, and thermal properties of PU. An increasing amount of isocyanate leads to a more rigid and hydrophobic PU, while excess amount of polyol produces softer and more hydrophilic PU.9 Therefore, the ratio of polyol to isocyanate plays a vital role in designing PU products. In addition to polyol and isocyanate, additives are often incorporated to aid the reaction or to achieve specific requirements of end applications. Frequently used additives during PU processing include catalysts, surfactants, blowing agents, plasticizers, pigments, chain extenders, fillers, flame retardants, smoke retardants, and stabilizers. The functions of each materials and additive are presented in Table 1.10| Material/additive | Function |
|---|---|
| Isocyanate | Hard segment, responsible for rigidity in PU |
| Polyol | Soft segment, responsible for flexibility in PU |
| Catalyst | Speed up the reaction, enhance reactivity |
| Surfactant | Improve dispersion of isocyanate and polyol |
| Blowing agent | Aid the production of PU foams |
| Plasticizer | Reduce hardness |
| Pigment | Produce coloured PU |
| Chain extender | Structural modification of PU, mechanical support |
| Filler | Reduce the cost, improve mechanical properties |
| Flame retardant | Reduce flammability |
| Smoke retardant | Reduce smoke generation when material is burnt |
| Stabilizer | Protect PU from damage caused by heat, light, etc. |
PU can be produced as a thermosetting or thermoplastic, either rigid and hard or flexible and soft material, depending on the specific choice of isocyanate and polyol. In general, isocyanate is characterized by the highly reactive isocyanate (R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group and this reagent is available commercially in liquid or powder form. The R group can be aliphatic, cycloaliphatic, polycyclic, or aromatic in nature. Comparatively, aromatic isocyanate is more reactive than other types, as the negative charge gets delocalized into the R. Diisocyanate which possesses two reactive isocyanate groups in the structure, is the most widely used due to its high reactivity.11 In the case of polyols, they can be broadly divided into monomeric and polymeric polyols. Monomeric polyols are low molecular weight polyols such as glycerol, ethylene glycol, propylene glycol, and others. They can be further categorized as diols, triols, tetraols, etc. depending on the number of hydroxyl groups they possess. Meanwhile, polymeric polyols refer to high molecular weight compounds.12 Fig. 1 presents the general reaction of polyol and isocyanate to produce PU, highlighting the urethane linkage. Some common example of isocyanates and starter polyol are included.
O) group and this reagent is available commercially in liquid or powder form. The R group can be aliphatic, cycloaliphatic, polycyclic, or aromatic in nature. Comparatively, aromatic isocyanate is more reactive than other types, as the negative charge gets delocalized into the R. Diisocyanate which possesses two reactive isocyanate groups in the structure, is the most widely used due to its high reactivity.11 In the case of polyols, they can be broadly divided into monomeric and polymeric polyols. Monomeric polyols are low molecular weight polyols such as glycerol, ethylene glycol, propylene glycol, and others. They can be further categorized as diols, triols, tetraols, etc. depending on the number of hydroxyl groups they possess. Meanwhile, polymeric polyols refer to high molecular weight compounds.12 Fig. 1 presents the general reaction of polyol and isocyanate to produce PU, highlighting the urethane linkage. Some common example of isocyanates and starter polyol are included.
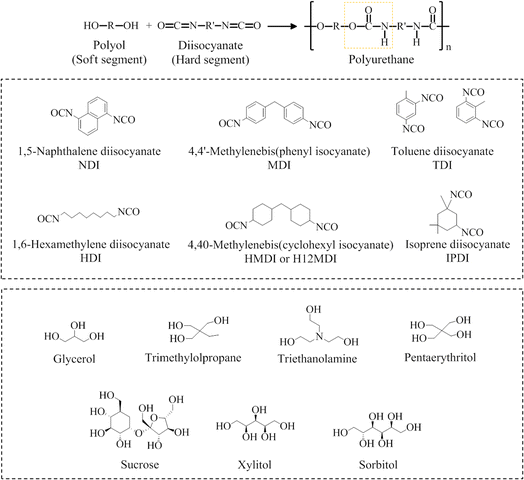 | ||
| Fig. 1 Schematic representation of polyurethane production with some common example of isocyanates and starter polyol. | ||
PU is considered to be physiologically inert and safe for many applications. However, the isocyanate monomers are not and they can pose health risks. Isocyanates, being highly reactive chemicals, may cause irritation to the skin, eyes, and respiratory tracts upon exposure. Therefore, elaborate safety procedures are necessary during handling, transport, and storage. In addition to this, the manufacturing of isocyanates involves the use of hazardous and toxic phosgene, a deadly gas used as the starting material. Moreover, isocyanate can contribute to air pollution, specifically in the form of volatile organic compounds released during the production process, impacting air quality and potentially causing environmental issues.13 Another concerns in the production of PU is their moisture sensitive nature of isocyanates. They have strong reactivity with water and can react rapidly upon contact. This reaction can caused premature curing and often results in the formation of unusable by-products.14 Furthermore, as for the vast majority of polymers, the precursors of PU, polyols and isocyanates mainly derived from chemical intermediates based on the non-renewable petroleum or natural gas resources.
To address these challenges, efforts have been directed towards the synthesis of bio-based PU. In this context, various studies have explored the production of bio-based polyol originated from renewable resources such as vegetable oil,15 fatty acid,16 fatty acid methyl esters,17 crude glycerol,18 wood,19 crop residues,20 lignin,21 and protein feedstock.22 While bio-based polyols are readily available in the market and well-documented in research, options for bio-based isocyanates are significantly limited. Despite that, commercial isocyanates with high renewable content are available including those based on fatty acids or amino acids. The main challenges in developing bio-based isocyanates are related to high production costs, low efficiency, reactivity limitations, and subpar technical performance.9 Consequently, the majority of bio-based PUs are only partially derived from renewable resources. Typically, these PUs are synthesized using bio-based polyols combined with petrochemical-based isocyanates.23 Currently, well-known companies like DuPont, BASF, BioAmber, Myriant, General Mills Co. Henkel Corporation are producing bio-based raw materials for PU synthesis.24
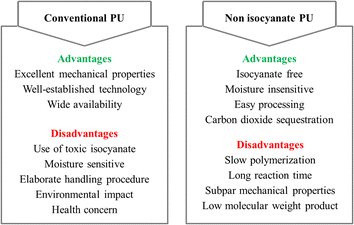
The next progressive step towards greener PU production is through the non isocyanate route. Various ways have been established to produce NIPU which will be discussed in Section 3, with the most common methods being the reaction of cyclic carbonate and amine groups. Through this method, NIPU enable to eliminate the need for isocyanates or phosgene derivatives and contribute to carbon dioxide sequestration as cyclic carbonates are commonly prepared by carbon dioxide fixation of epoxies.13 Without the use of isocyanates, NIPU synthesis is considered reliable in terms of safety and environmental impact. Other than being moisture insensitive, the production of NIPU has little-to-no use of those volatile organic compounds.25 Though promising, some technical challenges somehow hinder the commercialization of NIPU such as slow polymerization, long reaction time, subpar mechanical properties, and difficulty to synthesize high molecular weight product.26,27 Various factors affects the production of high molecular weight products, including the concentration and ratio of monomers, synthesis conditions i.e. temperature, pressure and reaction time, the present and types of catalysts, and polymerization mechanism. Nevertheless, there are trade-offs between the advantages and disadvantages of NIPU and conventional PU as depicted in Fig. 2. From this point of view, PU and NIPU differ significantly in synthesis methods, safety considerations, and environmental impact. While PU is already well-established, NIPU serves as a promising alternative with potential environmental benefits. As NIPU technology continues to evolve, it is expected to offer improvement in properties and production techniques to match or even surpass conventional PU.
3 Production routes of NIPU
The pioneer work on NIPU was reported by Dyer and Scott in 1957. In this study, they proposed the synthesis of urethanes and cyclic urea by reacting ethylene carbonate and various diamines to produce β-hydroxycarbamate. These compounds were subsequently polycondensed through a transesterification reaction.28 This early study laid the foundation for the development of NIPU and opened up new possibilities in polymer synthesis. NIPU based on cyclic carbonate-amine chemistry has since emerged as a significant area of study and is extensively explored for various applications. To date, there are four main approaches to synthesize NIPU including polycondensation, ring opening polymerization, rearrangement and polyaddition reaction29 as presented in Fig. 3. All the four methods are briefly described in this section.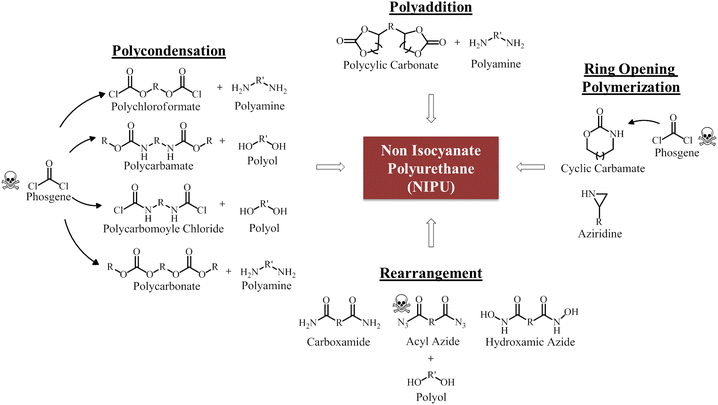 | ||
| Fig. 3 Common synthesis routes of NIPU. Adapted from ref. 2 with permission from MDPI, copyright 2023. | ||
Polycondensation reaction is one of the approaches used in NIPU synthesis, involving the condensation of suitable precursors to form the desired polymeric structure. Several routes can be used for this method such as the reaction between polychloroformate and polyamine, polycarbamate and polyol, polycarbamoyl chloride and polyol, and the interaction between polycarbonate and polyamine. However, the precursors for these routes are synthesized from toxic phosgene or its derivatives. Hence, there is not much difference in the production process of isocyanate-based polyurethane and NIPUs using polycondensation.30 Additionally, the process releases side products such as alcohol or HCl, posing significant limitation in industrial applications. Other significant drawbacks of polycondensation include the need for a catalyst, extended reaction time, the necessity for purification of end products, and the formation of low molecular weight compounds.31
Ring-opening polymerization is another method for synthesizing NIPU, where a cyclic monomer such as aziridine or cyclic carbamate, undergoes a ring-opening reaction to form the polymer chain. Although this process does not produce any undesirable side-products, it is often performed at high temperature. Further, the precursors, cyclic carbamate are commonly derived from phosgene and aziridine is toxic remains significant issues for NIPU synthesis through the ring-opening pathway.32 Rearrangement reaction can also be utilized in NIPU synthesis. The literature has reported the synthesis of NIPU through the rearrangement of acyl azides (Curtius rearrangement), carboxamides (Hoffman rearrangement), or hydroxamic azides (Lossen rearrangement).33 In these reactions, isocyanates are generated in situ, allowing the synthesis of polyurethane. However, it is worth mentioning that these routes still involve the use of isocyanates, albeit produced in situ. Furthermore, the reactants involved such as azides, carboxamides, and hydroxamic azides, are highly hazardous substances, which add to the importance of handling them with care.
Lastly, NIPU can be synthesized through the polyaddition pathway by reacting cyclic carbonates with amines. By far, this is the most general approach used due to its inherent advantages. Polyaddition reaction offer flexibility choosing reactants, allowing for a wide range of compounds to be utilized. This enables the customization of NIPU properties for specific applications. NIPUs obtained through the polyaddition of cyclic carbonates and diamines or polyamines are extensively studied in the literature, particularly concerning their mechanical properties. Furthermore, the synthesis of these NIPUs is more economically advantageous from an industrial standpoint, as it eliminates the need for solvents, catalysts, and the absence of by-products.34 Table 2 presents the comparison of synthesis methods of NIPU. It is crucial to carefully evaluate the benefits and limitations associated with each method prior to NIPU synthesis. This review focuses on the production of NIPU mostly but not limited to using cyclic carbonate with amine group through the polyaddition reaction. Further details on cyclic carbonates and types of amine will be discussed in the following section.
| Polycondensation | Ring-opening | Rearrangement | Polyaddition |
|---|---|---|---|
| - Use solvent and catalyst | - Absence of side-products | - Isocyanates are generated in situ during reaction | - Can be conducted with solvent- and catalyst-free method |
| - Longer reaction time | - Performed at high temperature | - Reactants are highly hazardous substances | - Absence of by-products |
| - Formation of low molecular weight by-products during reaction | - Toxic precursors | - Purification is not needed | |
| - Required purification of obtained polymer |
Ghasemlou et al. proposed a mechanism for the NIPU synthesis involving a tetrahedral intermediate. Fig. 4 depicts the reaction mechanism between five-membered cyclic carbonates and amines. Initially, the amine group attacks the carbonyl group in the cyclic carbonate leading to the formation of a tetrahedral intermediate. The next step involves the deprotonation of the tetrahedral intermediate, along with the elimination of hydrogen ions resulting from the attack of another amine group. Then, the presence of nitrogen atom induces the cleavage of the carbon–oxygen bond, leading to the formation of NIPU. This initiation of bond breakage is primarily attributed to the strong electron-withdrawing effect exerted by the nitrogen atom. The reaction between cyclic carbonate and amines involving a five-membered cyclic carbonate may lead to the formation of two isomers. The isomers contain a urethane moiety and a hydroxyl group in their backbone. The only difference between these isomers is the type of hydroxyl group present, whether a primary or secondary hydroxyl group. The formation of these isomers is dependent on the structure of intermediate compounds involved in the reaction.4
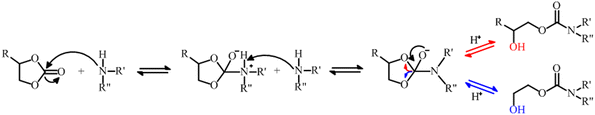 | ||
| Fig. 4 Proposed reaction mechanism between cyclic carbonates and amines to produce NIPU. Adapted from ref. 4 with permission from Elsevier, copyright 2019. | ||
4 Reactants for preparing NIPU through polyaddition reaction
4.1 Cyclic carbonates
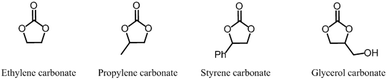
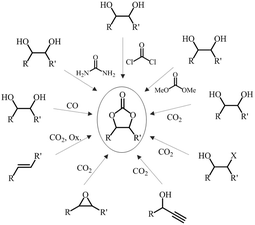
Cyclic carbonates (CC) are a class of versatile compounds that have garnered attention in both academic and industrial areas due to their attractive attributes in the context of green chemistry and sustainability, leading to the surge of interest in their synthesis and applications. In terms of properties, CC are relatively non-toxic, exhibit low volatility, and possess thermal and mechanical stability. They can be synthesized from a variety of relatively inexpensive starting materials. Owing to these advantages, cyclic carbonates have found diverse applications in various fields. Apart from their used as monomers in polymer synthesis, they can serve as reactants or green solvents in chemical reactions.35 Besides, CC are used in the formulation of electrolytes for energy storage devices like lithium ion batteries.36 Other than that, they have been used in industrial lubricants and as environmentally benign intermediates for the preparation of a wide variety of organic chemical products.37 There are several types of cyclic carbonates that can be classified based on their size and substitution patterns such as 5-, 6-, 7- and 8-membered ring cyclic carbonates (5CC, 6CC, 7CC and 8CC), with the most common being the 5CC and 6CC. It is worth mentioning that the synthesis of 5CC is much easier as the 6CC have higher reactivity and lower stability.38 Therefore, this review will be mainly focusing on the use of 5CC to synthesize NIPU. Fig. 5 depicts the structure of commonly produced 5CC.The first work described on cyclic carbonates was conducted by Carothers et al. in the early 1930s.39,40 Since then, the studies on cyclic carbonates have evolved, and they have now become commercially available with various methods, utilizing different precursors for their synthesis.41 Traditionally, the synthesis of cyclic carbonates relied on a chemical reaction between diols and phosgene or its derivatives. This method offered high yields due to the high reactivity of phosgene, albeit requiring additional purification steps. However, due to the toxicity associated with phosgene and its derivatives, alternative approaches have been developed.42 Fig. 6 displays some common routes to prepare cyclic carbonates using different starting materials. The most commonly studied and effective methods remain the cycloaddition reaction of carbon dioxide and epoxides. This reaction is favoured because it utilizes carbon dioxide, CO2 a renewable, non-toxic and abundant reactant, besides being relatively facile and straightforward. The preparation of CC can also be viewed as an efficient and practical method for CO2 fixation and consumption. Remarkably, it can be performed efficiently without the need for a solvent. The reaction is also thermodynamically favourable as the high free energy of epoxides counterbalance the high thermodynamic stability of CO2.43
 | ||
| Fig. 6 Synthesis routes of cyclic carbonates with various precursors. Adapted from ref. 38 with permission from MDPI, copyright 2020. | ||
The industrial production of cyclic carbonates primarily utilized propylene carbonate and ethylene carbonate, derived from propylene oxide and ethylene oxide, respectively. These two epoxides are obtained from non-renewable petroleum derived chemicals. Other issues that raise concerns are about the toxicity and volatility of these starting materials. To address this issue, ongoing research is exploring various approaches aimed at achieving safe and sustainable production of renewable cyclic carbonates. One focus is on establishing alternative methods for the industrial production of epoxides from bio-based materials. In view of this, various types of renewable resources can be used such as terpene, vanillin, tannin, lignin derivatives, glycerol, isosorbide, and vegetable oils.4 Among these sources, vegetable oils present as promising option as they are readily available and abundant. The presence of unsaturation sites in vegetable oils provides a wide range of possibilities for chemical modifications44 such as epoxidation, acrylation, carbonation, malenization, click chemistry and various other reactions. A detailed discussion on carbonated vegetable oils will be presented in Section 6 of this review.
4.2 Amine curing agents
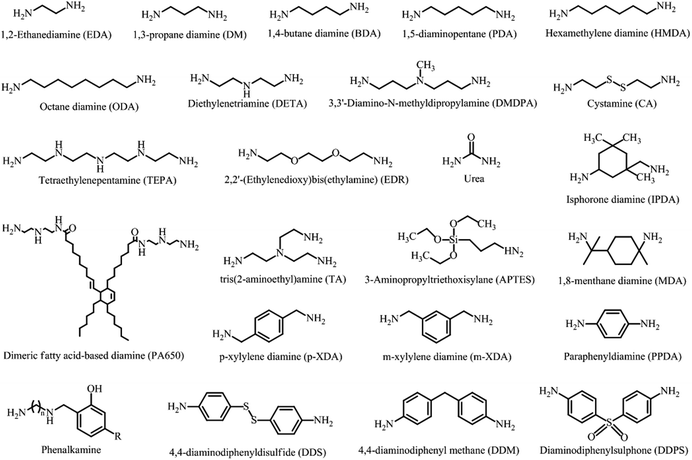
The properties of NIPU can be tailored made based on the selection of amine used during the synthesis stage. The choice of amine can affect the molecular weight, crosslink density, and degree of branching of the polymer, which in turn dictates the mechanical, thermal, and other related properties of the resulting NIPU. The properties of NIPU can also be tuned by adjusting the ratio of amine to cyclic carbonate used in the synthesis.24 A higher amine-to-cyclic carbonate ratio can lead to higher crosslinking density and improved mechanical properties, whereas a lower ratio may result to a more flexible and lower stiffness polymer. Various types of amine have been investigated such as diamine, triamine or polyamine with aliphatic, cycloaliphatic, or aromatic structure. It is important to note that, in NIPU synthesis, the polyamine precursor must possess at least two amine groups within its structure. This is crucial for increasing reactivity towards cyclic carbonates and achieving a high conversion rate.4 Primary amines are typically more reactive than secondary or tertiary amines due to their higher nucleophilicity. Diamines usually produced linear polymers, while polyamines can lead to branched or crosslinked polymers. The use of cycloaliphatic and aromatic amines can improve the chemical, mechanical, and thermal stability of the resulting polymer.45Currently, there is progressing research and development to produced bio-based amines from renewable resources such as biomass, agricultural waste, or other sustainable feedstock. Similarly to cyclic carbonates, the development of bio-based amines is driven by the desire to reduce reliance on fossil fuels and to mitigate the environmental impact associated with traditional amine production methods. The available literature suggests that amino acid, vegetable oil derivatives, sugar derivatives, lignin, and proteins can serve as feedstock for the production of amines.46 Overall, regardless of the source, the selection of amine plays a crucial role in determining the properties of NIPU, and careful consideration of the amine group and its ratio to cyclic carbonate is necessary to tailor the material properties and meet specific application requirements. Fig. 7 displays some examples of amine curing agents that has been used to prepare NIPU.
5 Properties of NIPU
The properties of NIPU can be tailor-made to meet specific requirements of the final products. The abundant options of starting materials and pathways ensure the diversity of NIPU produced.47 The properties of NIPU can be customized by selecting appropriate precursors and incorporating additives. This flexibility in properties customization allows for the optimization of NIPU for different applications. NIPU has been reported to demonstrate superior thermal stability, resistance to water and resistance to solvents compared to conventional PU.48 The presence of pendent hydroxyl groups in the NIPU structure make it capable of forming intermolecular hydrogen bonds with urethane carbonyl groups. These intramolecular hydrogen bonds lead to the decrease of the susceptibility of urethane linkages to hydrolysis. Furthermore, NIPU demonstrate enhanced thermal stability as they lack thermally labile allophanate and biuret groups that are typically present in the chemical structure of conventional PU.49 Meanwhile the mechanical properties of NIPU are significantly influenced by processing conditions, the carbonate/amine ratio, and presence of fillers. In Table 3, a comparison of isocyanate-based PU (both synthetic and bio-based) and NIPU is presented. Particularly, the mechanical properties of polyurethane vary based on the specific types of PU produced. Some interesting properties of NIPU will be briefly discussed in this section, such as self-healing, shape memory effects, and reprocessibility.| Sample | Mechanical properties | Ref. | |
|---|---|---|---|
| Tensile strength | Elongation | ||
| Isocyanate-based PU | |||
| Synthetic PU | 11.0 MPa | 440% | 50 |
| Palm oil-PU foam | 60–115 kPa | 38–80% | 51 |
| Rapeseed oil-PU foam | 82–115 kPa | 78–154% | 52 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| NIPU | |||
| Soybean oil_NIPU film | 0.35–1.35 MPa | 2.2–4.5% | 53 |
| Soybean oil_NIPU membranes | 1.7–6.2 MPa | 165–306% | 54 |
| Soybean oil_NIPU film | 0.3–11.1 MPa | 3–433% | 55 |
| Soybean oil_NIPU film | 1.8–6.2 MPa | 152–309% | 56 |
| Canola oil_NIPU film | 5–8 MPa | 103–192% | 57 |
| Jatropha oil_NIPU film | 4–17 MPa | 30–230% | 58 |
| Linseed oil_NIPU film | 2–18 MPa | 1–310% | 59 |
| Soybean oil_NIPU film | 7.2–10.1 MPa | 80–97% | 60 |
| Sunflower oil_NIPU film | 0.9–5.2 MPa | 100–240% | 61 |
| Linseed oil_NIPU film | 11.9–18.9 MPa | 26.0–57.1% | 26 |
| Linseed oil_NIPU film | 5.0–9.5 MPa | 2148–327.3% | 62 |
| Soybean oil_NIPU film | 0.45–0.97 MPa | 36.7–92.0% | 63 |
5.1 Self-healing
The concept of self-healing materials has gained attention in recent years as a way to develop repairable materials that can last longer, required less maintenance, and reduced need for replacements. Generally, self-healing polymers (SHP) can be classified based on their mechanism and working chemistry as presented in Fig. 8.64 The SHP by mechanism involves either intrinsic or extrinsic repair of the materials. Intrinsic SHP refers to polymers that can restore their own structural integrity without relying on external assistance, whereas extrinsic SHP requires external agent pre-embedded in the polymer matrix to trigger them to repair the damage. In terms of working chemistry, SHP can be categorized into autonomous and non-autonomous. Autonomous polymers can recover their shape automatically or respond to damage and recover without relying on external stimuli to initiate the healing process. Meanwhile, non-autonomous self-healing polymers require external stimuli to heal the damage surface. Without the presence of stimuli, the healing process does not occur.65–67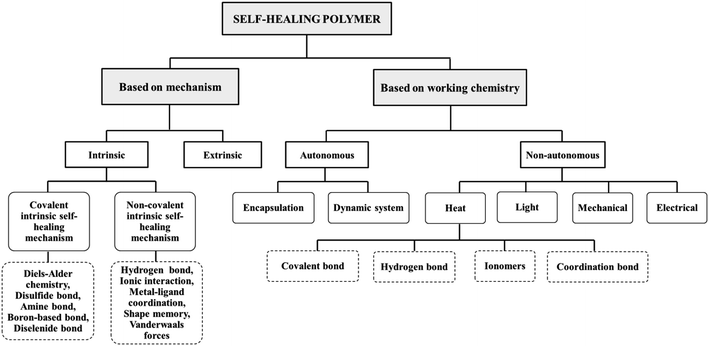 | ||
| Fig. 8 General classification of self-healing polymer. Adapted from ref. 64 with permission from Wiley, copyright 2022. | ||
Self-healing NIPU has been investigated and reported by several studies. Raut et al. described the preparation of self-healable hydrophobic NIPU based on carbonylimidazole–amine reaction and dynamic Diels–Alder click reaction employing furan-maleimide cycloaddition. The produced NIPU exhibits excellent hydrophobic and self-healing properties.68 In one study, Hu et al. produced degradable and self-healable NIPU by copolymerizing bis(6-membered cyclic carbonate) and amino-terminated liquid nitrile rubber. The report suggested that NIPU indeed possesses self-healing characteristic with a healing efficiency of more than 88%. They suggest the mechanism is due to the internal transesterification exchange between the hydroxyl and carbamate groups.69 Further, Wang et al. studied the self-healing properties of NIPU from the reaction of bio-based diglycerol bis(cyclic carbonate) with furan methylamine and trimethylolpropane tris(poly[propylene glycol], amine terminated) ether or tris(2-aminoethyl) amine under mild conditions, followed by the reaction with bismaleimide. The highest healing efficiency of NIPU was obtained at 80%.70 In other study, they prepared soft self-healing NIPU via Diels–Alder reaction between furan terminated NIPU and unsaturated polyester. The obtained NIPU achieved 100% self-healing efficiency.66
5.2 Shape memory effects
The concepts of self-healing and shape memory effects of polymers are interconnected. While self-healing polymers repair damage by restoring their molecular structure, shape memory effects (SME) refer to the ability of polymers to remember their original shape and recover it after being deformed, typically when subjected to a specific stimulus, such as heat, light, electricity, and chemical induction. SME is one approach to facilitate healing of materials. Shape memory is the consequence of molecular structure, reversible mobility changes, conformational entropy, and programming.65 To enable shape memory in a polymer, two essential structural elements are required which are; (a) permanent net-points or junctions, and (b) reversible molecular switching segments. The permanent net-points or junctions serve as stable anchor points within the polymer network that maintain the original shape of the material. They are responsible for holding the polymer chains in place and preserving the memory of the permanent shape. Meanwhile, reversible molecular switching segments allow the polymer chains to undergo reversible conformational changes when subjected to a stimulus. The reversible switching enables the transition between the temporary shape and the permanent shape. These combinations allow the polymer to exhibit shape memory behaviour.71Several studies have reported the shape memory effects of NIPU. For instance, Yin et al. synthesized semicrystalline NIPU with tunable triple shape memory properties by introducing behenic acid into the system. They were able to obtained NIPU with a shape recovery rate close to 100%.72 Another study on triple shape memory NIPU was reported by Schimpf et al. NIPU was obtained by curing pentaerythritol-based cyclic carbonates with hyperbranched, semi crystalline polyamidoamines with the subsequent addition of behenic amides. The production of nanocrystalline with programmable shapes can be attributed to the crystallization of the behenic amide side chains.73 Meanwhile, another study on lignin/NIPU hybrid polymers were synthesized by Zhang et al. using bis(6-membered cyclic carbonate) and polyetheramine. The lignin/NIPU hybrid polymers exhibited excellent shape memory effects due to the transcarbamoylation reactions in their dynamic covalent network triggered by high temperature.74 Another study based on lignin derived NIPU was presented by Sternberg and Pilla. In their study, Kraft lignin was functionalized with glycerol carbonate and subsequently reacted with Priamine 1074 curing agent. The cured NIPU samples were subjected to shape memory effect test by heating them at 105 °C for 10 minutes. A semicircular deformation was induced as the samples cooled down to room temperature. The produced lignin-based NIPU exhibits the ability to retain the deformation after cooling to room temperature and shows reversibility by returning to the pre-deformed shape upon re-heating.75 Further, Li et al. synthesized a five-membered bicyclic carbonate by reacting meso-erythritol with carbon dioxide. The as-synthesized carbonate was reacted with Jeffamine T-403, a trifunctional polyetheramine curing agent to produce thermosets NIPU. This NIPU was then incorporated with poly(2-oxo-1,3-dioxolane-4-yl)methyl methacrylate -grafted Fe3O4 nanoparticles. It was found that the nanocomposites retained excellent shape memory properties, and this shape memory behaviour could be triggered by the photothermal effect of Fe3O4 nanoparticles.76
5.3 Reprocessibility
Reprocessability is an important property of polymers that enables recycling, reshaping, and reuse of these materials. Reprocessability refers to the ability of a polymer to undergo reprocessing without significant degradation or loss of properties. Reprocessable polymers are highly sought after as they can contribute to achieving sustainability goals, waste reduction, and resource efficiency. One example of study on reprocessibility of NIPU was conducted by Chen et al. They synthesized NIPU using poly(propylene glycol) dicyclocarbonate and tris(2-aminoethyl)amine in the presence of 4-(dimethylamino)pyridine catalyst. The reprocessing ability of NIPU was performed at 140 °C for 2 hours, and the resulting NIPU elastomer showed full property recovery after multiple reprocessing steps. This study revealed that the rearrangement of NIPU networks during reprocessing involves both dissociative reversible cyclic carbonate aminolysis and associative transcarbamoylation exchange reactions. Through these reactions, NIPU can be designed to be intrinsically reprocessable with full property recovery.77 A subsequent study on NIPU reprocessibility was conducted by Hu et al. They synthesized NIPU through the reaction of carbonated soybean oil with a difunctional amine with 4-(dimethylamino)pyridine catalyst. This study achieved a complete recovery of NIPU network after multiple melt-state recycling steps under relatively mild reprocessing conditions. In addition to the reversible cyclic carbonate aminolysis and transcarbamoylation exchange reactions, the networks were found to undergo a third dynamic chemistry through a transesterification exchange reaction.69 Recently, Purwanto et al. described a rapid synthesis of reprocessable self-blowing NIPU foams through the competitive reactions of cyclic carbonate aminolysis and thiol-decarboxylation. They able to achieve a significant reduction in foaming production time, from 20 hours to 30 minutes when the gelling reaction conducted at 80 °C. The NIPU foams were capable to be melt-reprocessed into well-consolidated films for multiple cycles with a full recovery of cross-link density.78 Another recent study investigated the reprocessability of bio-based non isocyanate polythiourethane (NIPTU) conducted by Chen et al. In this study, raw materials were obtained from bio-wastes i.e. five-membered cyclic dithiocarbonates (DTC); NC-514-DTC and Cyclo-DTC were derived from cashew nutshell liquid and rice husks. They employed JEFFAMINE T-403 as the curing agent. Due to its dynamic covalent thiourethane and disulphide cross-links, this NIPTU demonstrated excellent reprocessability with full recovery after multiple reprocessing stages.79 Based on the observations from the presented studies so far, it can be deduced that NIPU has reprocessable properties, including those prepared from bio-based resources.6 Case studies on vegetable oil derived NIPU
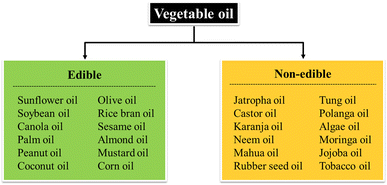
Vegetable oils are derived from plant sources such as seeds, fruits, nuts, and grains.80 They can be broadly classified as edible and non-edible oils based on their chemical content. Edible vegetable oils are derived from sources that are commonly used for direct human consumption as food intake, whereas non-edible oils are derived from sources that are not used for food purposes. Fig. 9 illustrates some examples of edible and non-edible vegetable oils available in the market and ready for modification. While it is relevant to use edible oil as precursor for polymer production, there are valid concerns and criticisms regarding its potential impact on food security. In this context, the use of non-edible oils as a feedstock for polymer production is an attractive alternative to edible oils, providing a commercially viable and sustainable solution for the polymer industry.81The next subsection presents a series of case studies on vegetable oil-based NIPU derived from both edible and non-edible oil that have been reported to this date. The case studies highlight the general characteristics of the oils, including their origin, fatty acid composition, and other related properties. They also offer insights into the preparation methods of NIPU, including the choice of curing agent, catalyst, and other additives, as well as their impact on the final properties of NIPU. Moreover, the studies probe into the properties and performance of NIPU and their suitability for various applications in diverse fields.
6.1 Soybean oil
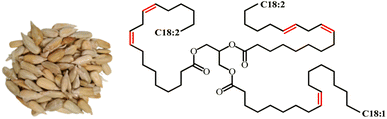
Soybean oil (SBO) is extracted from the seeds of the soybean plant (Glycine max). Soybean originated from China and now has been cultivated in other parts of the world. Currently, SBO is the second largest source of vegetable oil after palm oil. South America currently accounts for over 50% of global soybean production worldwide.82 The oil is high in unsaturated fatty acids, comprising approximately 80% of oleic, linoleic, and linolenic acids.83 In addition to its use in food applications, SBO is also gained significant interest in scientific and industrial fields to produce bioplastics, coatings, paints, and lubricants.84 Other than these applications, the use of soybean oil as a raw material in the production of polyurethane also has been extensively studied in recent years. Fig. 10 shows the image of soybean seeds and the chemical structure of SBO.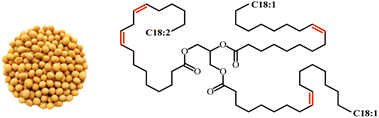 | ||
| Fig. 10 Image of soybean seeds and the chemical structure of soybean oil. The image is obtained from ref. 85 with permission from MDPI, copyright 2023. | ||
Among various types of vegetable oil reported for NIPU production, soybean oil has been the most investigated so far. The earliest study on soybean oil-based NIPU was conducted by Tamami et al. to examine the effects of different amine groups on the physical properties of the resulted NIPU. Two diamine groups, ethylenediamine (EDA) and hexamethylenediamine (HMDA), were used along with one triamine group, tris(2-aminoethyl)amine (TA). TA_NIPU display the highest Tg of 43 °C, the smallest strain at break (70%) and the lowest soluble fraction (6.8%) compared to EDA and HMDA. This can be attributed to the fact that TA has a tighter network than the diamine groups. HMDA_NIPU has the lowest Tg value of 18 °C and EDA_NIPU has 34 °C. The higher level of flexibility of HMDA moiety explains the lower Tg value of HMDA_NIPU compared to EDA_NIPU regardless being the same type of diamine, only differs in chain length.86
In another study, Javni et al. study the use of aromatic and cycloaliphatic diamines to increase the strength and rigidity of NIPU. The properties evaluated included mechanical, thermal, and swelling behaviour. The findings indicate that the mechanical properties depend on crosslinking density and the hydrogen bond between urethane, hydroxyl, and ester groups, which are controlled by the carbonate to amine structure and the amine ratio. The presence of rigid aromatic and cycloaliphatic structures increases intersegmental physical interactions, resulting in higher strength. Thermal analysis revealed that the Tg was affected by the carbonate to amine ratio. In regards to NIPU swelling in toluene, factors like the degree of crosslinking and solubility parameters of NIPU affected the swelling behaviour. Additionally, NIPU based on aromatic and cycloaliphatic diamines exhibited higher hydrophobicity and were less susceptible to water penetration compared to those based on aliphatic diamines.55
Lee and Deng described the synthesis of NIPU using soybean oil and lignin. Initially, the urethane monomer was prepared by the reaction of carbonated soybean oil (CSBO) with APTES. Following that, the monomer was reacted with various loading of lignin to produce polyurethane. In this case, lignin functions as the hard segment whereas polysiloxane linkages (–Si–O–Si–) act as the soft segments. Two different curing temperatures were used, which were drying at room temperature and curing at 60 °C. It was found that tensile strength enhanced with the increase of lignin content and curing at higher temperatures. This enhancement can be attributed to the rigidity of the lignin aromatic structure and lignin acting as the crosslinking agent. In contrast, curing at low temperature resulted in an increase in strain, as the lignin is predicted to blend with the polymer instead of acting as a crosslinking agent. This study proves that the mechanical properties of NIPU can be controlled by varying the temperature and lignin content.53
Poussard et al. prepared CSBO from epoxidized soybean oil (ESBO) under supercritical CO2 conditions at 120 °C and 100 bar. A complete conversion of ESBO to CSBO was achieved within a 9 hours reaction time. The use of supercritical CO2 conditions decreased the viscosity of soybean oil and facilitated the coupling of CO2 with epoxides leading to complete conversion in a shorter time compared to lower pressure and higher temperature conditions. In parallel, bio-based amino-telehelic oligoamides were synthesized from a dimeric fatty acid and ethylenediamine. NIPU was prepared with short 1,4-butanediamine (BDA) and 1,5-pentanediamine (PDA), and with the higher molecular weight as-prepared oligoamide by melt-copolymerizion with ESBO. Thermal stability analysis of NIPU based on CSBO and BDA or PDA showed a relatively lower thermal stability compared to NIPU containing the bio-based oligoamide. Despite this, the thermal stability of all prepared NIPUs was higher than conventional polyurethane, which recorded around 310 °C. The improvement in stability was expected due to the hydroxyl functions in the urethane generating intra- and intermolecular hydrogen bonds.87
A study on chitosan-based non isocyanate polyurethane was reported by Das et al. In their study, CSBO was mixed with chitosan solutions at different concentration (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight ratio of CSBO to chitosan) to obtain a fully bio-based NIPU. Thermal analysis based on TGA revealed NIPU_1
3 weight ratio of CSBO to chitosan) to obtain a fully bio-based NIPU. Thermal analysis based on TGA revealed NIPU_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 has the highest thermal stability due its highly crosslinked polymer network structure. This study found out that increasing chitosan content improves the thermal stability of NIPU. Meanwhile, DSC analysis shows that NIPU samples exhibit two glass transition temperatures for he hard segment, Tg(h) and soft segment, Tg(s) of the polymer, indicating phase separation phenomena. The produced NIPU also exhibit amorphous nature as there is no endothermic or exothermic behaviour observed. In addition, this study also evaluated the chemical resistance and optical properties of the NIPU_1
3 has the highest thermal stability due its highly crosslinked polymer network structure. This study found out that increasing chitosan content improves the thermal stability of NIPU. Meanwhile, DSC analysis shows that NIPU samples exhibit two glass transition temperatures for he hard segment, Tg(h) and soft segment, Tg(s) of the polymer, indicating phase separation phenomena. The produced NIPU also exhibit amorphous nature as there is no endothermic or exothermic behaviour observed. In addition, this study also evaluated the chemical resistance and optical properties of the NIPU_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in different chemical environments: acid, alkali, and neutral. The samples exhibited color changes as the pH of the environment changed, as displayed in Fig. 11. They also observed changes in the morphology of the NIPU films due to leaching of some components into the chemical media.88
3 in different chemical environments: acid, alkali, and neutral. The samples exhibited color changes as the pH of the environment changed, as displayed in Fig. 11. They also observed changes in the morphology of the NIPU films due to leaching of some components into the chemical media.88
 | ||
Fig. 11 Physical appearance of NIPU_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 films tested for 48 hours in different media; (a) HCl, (b) NaCl, (c) NaOH, and (d) no chemical environment. Adapted from ref. 88 with permission from Wiley, copyright 2020. 3 films tested for 48 hours in different media; (a) HCl, (b) NaCl, (c) NaOH, and (d) no chemical environment. Adapted from ref. 88 with permission from Wiley, copyright 2020. | ||
Further, Doley et al. prepared a hybrid NIPU from CSBO and highly branched cyclic carbonate polyester (CHPE) reacted with polyamidoamine. Thermal analysis study by TGA showed that the hybrid NIPU has good thermal stability due to the presence of rigid aromatic rings, polar–polar interactions, and hydrogen bonding interactions. The presence of hydroxyl groups in the structure also contributes to the increased thermal stability. In addition, the chemical resistance of NIPU was tested in three media; HCl (10% aq.), NaOH (2% aq.), and water for 21 days. The results show that NIPU has satisfactory resistance towards water and acidic media due to the more crosslinked polymer chain. However, NIPU's resistance to alkali media was poor due to the presence of hydrolysable ester bonds in both CSBO and CHPE.60
Gholami et al. prepared wound dressing membranes from soybean oil-based NIPU containing azetidinium groups as an antibacterial agent. The article highlights the importance of using antibacterial wound dressings to reduce the population of microbes at the wounded site and eliminate the need for oral uptake of antibiotics that can be harmful. Two types of NIPU membranes were prepared; one with an internal secondary amine group, tetraethylenepentamine (TEPA_NIPU) and one without (HMDA_NIPU). The azetidinium containing cross-linked networks were obtained by the addition of epichlorohydrin (ECH) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of ECH to TEPA_NIPU. For the antibacterial assay, HMDA_NIPU was used as a control. The antibacterial activity against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) was monitored. Overall, the dressings showed excellent antibacterial properties against both bacteria and were non-cytotoxic to fibroblast. The NIPU dressings had desirable tensile properties with ∼6 MPa tensile strength and ∼200% elongation at break that remained under a hydrated state. The dressings were capable of maintaining a moist environment over mildly to moderately exuding wounds due to their ability to absorb (reaching up to 30% equilibrium water absorption) or release them as vapour (water vapour transmission rate of 390 to 710 g per m2 per day for dry and wet dressings). Therefore, these dressings have the potential to promote the healing of injured skin tissue in a hygienic setting.54
2 molar ratio of ECH to TEPA_NIPU. For the antibacterial assay, HMDA_NIPU was used as a control. The antibacterial activity against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) was monitored. Overall, the dressings showed excellent antibacterial properties against both bacteria and were non-cytotoxic to fibroblast. The NIPU dressings had desirable tensile properties with ∼6 MPa tensile strength and ∼200% elongation at break that remained under a hydrated state. The dressings were capable of maintaining a moist environment over mildly to moderately exuding wounds due to their ability to absorb (reaching up to 30% equilibrium water absorption) or release them as vapour (water vapour transmission rate of 390 to 710 g per m2 per day for dry and wet dressings). Therefore, these dressings have the potential to promote the healing of injured skin tissue in a hygienic setting.54
Dong et al. design and prepared a self-healing CSBO derived NIPU incorporated with dynamic disulphide bonds. The NIPUs were prepared using different types of amine; bio-based dimeric fatty diamine PA650, isophorone diamine (IPDA), amino acid-derived flexible disulphide-based cystamine (CA), and the pre-mixture of these amines. The self-healing properties were adjusted by varying the amine ratio. The healing efficiency was estimated through the recovery of tensile strength, and it was observed that the self-healing capability of NIPU was improved with the addition of CA. In this case, the different diamines used have specific contribution to the properties of NIPU. The use of PA650 contributed to higher thermal stability and elasticity, while the addition of IPDA resulted in good tensile strength. The incorporation of CA significantly improved the self-healing efficiency by 45%. The efficiency was enhanced by one time when NIPU was healed at 80 °C for 4 hours. The good mobility and flexibility of NIPU networks formed by linear CA and bulky PA650 contribute to the improved healing ability. The proposed mechanism for self-healing of NIPU and the image of healed NIPU are presented in Fig. 12.56
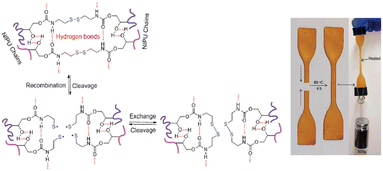 | ||
| Fig. 12 Proposed mechanisms for self-healing NIPU and image of NIPU healed at 80 °C for 4 hours. Adapted from ref. 56 with permission from RSC, copyright 2020. | ||
Another study on self-healing and potential reprocessing of soybean oil-based NIPU was reported by Yang et al. In this work, CSBO was reacted with two types of amines; 4,4′-diaminodiphenylmethane (DDM) and 4,4′-diaminodiphenyldisulfide (DDS). In essence, disulfide bonds are one kind of dynamic covalent bond that can undergo bond exchange reactions under various stimuli (light, heat, ultrasound, etc.) making them attractive for materials to reprocess and heal under relatively mild conditions. The produced NIPU have good thermal stability and the tensile strength properties were improved by the introduction of disulphide bonds in the structure. Moreover, the disulphide bond was found to be effective in promoting self-healing properties. Fig. 13 shows the image of reprocessed ESO–DDS, CSBO–DDM and CSBO–DDS samples by using hot pressing method. The study revealed that the different network structures had a significant impact on the performance of the samples, and the dual dynamic covalent bond (CSBO–DDS) was more effective in accelerating molecular chain rearrangement than either single bond type (ESO–DDS or CSBO–DDM). Overall, the results suggest that the synergistic effect of disulphide bond exchange and carbamate bond exchange provides a feasibility study for designing NIPU with milder self-healing and reprocessing behaviours.89
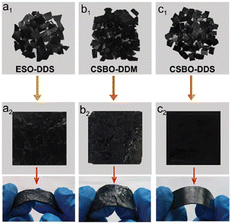 | ||
| Fig. 13 Reprocessing of (a) single-disulphide ESO-DDS, (b) single carbamate CSBO-DDM, and (c) dual dynamic CSBO-DDS by hot pressing technique. The image obtained from ref. 89 with permission from RSC, copyright 2021. | ||
Liu et al. prepared a fully bio-based NIPU from CSBO and a terpene derivative with a dynamic network structure. In their work, 1,8-menthane diamine (MDA) derived from turpentine was used as the curing agent. The ratio of CSBO to MDA was varied using the amino/cyclic carbonate molar content with ratio of 1.2, 1.0 and 0.8, respectively. Through a stress relaxation study, the produced NIPU displayed dynamic covalent bond exchange. Additionally, the NIPU possesses self-healing, recyclability, reprocessing, and shape memory effect as can be observed in Fig. 14. In fact, the recycling process can be repeated multiple times with less impact on the properties of the recovered NIPU. The mechanical properties and thermal stability of NIPU are almost fully recovered (>85%) after several reprocessing cycles, indicating that the bond exchange repairs the network. Interestingly, this study produced NIPU with triple-shape memory due to the additional reversible phase transition.90
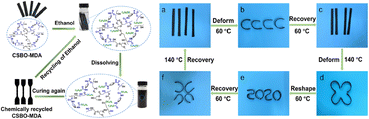 | ||
| Fig. 14 Chemical recycling process and shape memory effect of CSBO/MDA_NIPU. (a) deformed at 60 °C and then cooled at 4 °C for a new temporary (b), recovered at 60 °C to the original rectangular shape (c), heated at 140 °C and then cooled at 4 °C (d), reshaped at 60 °C and then cooled at 4 °C shape (e), recovered at 60 °C to the permanent shape (f), and reconfigured at 140 °C to the original shape I. Images obtained from ref. 90 with permission from ACS, copyright 2021. | ||
6.2 Linseed oil
Linseed oil (LSO) is extracted from the seeds of flax plant (Linum usitatissimum). The terms “linseed” and “flaxseed” are often used interchangeably, depending on the geographic location and the context in which they are being used. The chemical composition of LSO is characterized by the presence of a high concentration of polyunsaturated fatty acids (73%) made up of linolenic acid and linoleic acid; 18% monounsaturated fatty acid (oleic acid); and 9% of saturated fatty acid (stearic acid).91,92 LSO is classified as a drying oil because when coated on a non-absorbent substrate and being exposed to air, it dries promptly forming a hard, non-melting, and water insoluble film.93 The drying process occurs due to a polymerization reaction initiated by exposure to light and facilitated by the presence of atmospheric oxygen through a series of chemical reactions called autoxidation.94 These properties make LSO a popular ingredient in paints, varnishes, and wood finishes. The image of linseed and its oil chemical structure is displayed in Fig. 15. | ||
| Fig. 15 Image of linseed and the chemical structure of linseed oil. The image adapted from ref. 95 with permission from MDPI, copyright 2020. | ||
Moritz and Rolf examined the effect of homogenous and heterogeneous catalyst for CLSO synthesis. TBAB was use as the homogeneous catalyst, whereas silica-supported 4-pyrrolidinopyridinium iodide (SiO2–I) served as the heterogeneous catalyst. This study demonstrate the use of heterogeneous catalyst aids in simplifying the recovery process, however the activity is still not comparable to that of homogenous catalysts. At the same condition of 30 bar, TBAB catalyst shows complete conversion after 20 hours, whereas, SiO2–I required 45 hours to reach completion. The produced CLSO was then cured with EDA, BDA and IPDA. The high carbonate content of CLSO led to high viscosity causing processing problems. Thus, CLSO was blended with CSBO to reduce the viscosity and increase the gel time. In this case, CSBO acted as a reactive diluent. Variations in carbonate content by varying the ratio of CLSO and CSBO significantly affected the thermal and mechanical properties of NIPU. They observed a high stiffness of NIPU was produced when the pure CLSO was cured with IPDA, increasing the glass transition temperature to 60 °C. Although the stiffness of NIPU has increased, there is a significant reduction in its ability to stretch without breaking, indicating severe brittleness.59
Pouladi et al. conducted a study on the anti-corrosion performance of NIPU at various carbonate contents of CLSO. This work started with epoxidation, followed by carbonation to form cyclo-carbonate groups. During the carbonation stage, CLSO was prepared with three percentage of carbonate content which were 45% (CLSO45), 75% (CLSO75), and 100% (CLSO100) obtained at 24, 36 and 48 hours of reaction time, respectively. Each type of CLSO reacted with diethylenetriamine (DETA) to form NIPU. The results indicated an improvement in adhesion and hardness, and a reduction in flexibility with increasing carbonate content of CSLO. The increase in carbonate content contributed to a higher formation of hydroxyl groups in the NIPU structure due to the reaction of cyclo-carbonate and amine. This led to the improved adhesion strength and mechanical properties of the polymer. The corrosion study based on electrochemical impedance spectroscopy analysis revealed NIPU produced from CLSO75 exhibited superior anti-corrosion properties, comparable to conventional PU. For CLSO100_NIPU, a high content of carbonate led to more formation of hydroxyl group upon reaction with amine and thus resulted to a decrease in corrosion resistance (Fig. 16).96
 | ||
| Fig. 16 NIPU foam produced from CLSO and HMDA. Image obtained from ref. 26 with permission from ACS, copyright 2021. | ||
Zhang et al. reported a study on the preparation of waterborne non isocyanate polyurethane (NIWPU) from CLSO using different amine group 3,3′-diamino-N-methyldipropylamine (DMDPA), HMDA, m-xylylene diamine (m-XDA) and 2,2′-(ethylenedioxy)bis(ethylamine) (EDR) to produce cationic (CNIWPU), anionic (ANIWPU) and non-ionic (NNIWPU) polyurethane. The NIWPU dispersions exhibit excellent storage stability and tailorable properties. For instance, the anionic NIWPU exhibited good coating performance with the highest pencil hardness was recorded at 7H and crosshatch adhesion of 5B, whereas, the non-ionic NIWPUs showed a tailorable cloud points ranging from 60 to 80 °C. Meanwhile, the cationic NIWPU showcased good mechanical properties with tensile strength more than 9 MPa and elongation at break surpassing 200%. Notably, CNIWPU exhibited antibacterial properties against Escherichia coli and Staphylococcus aureus above 98%, as depicted in Fig. 17. Based on these findings, the authors suggested the potential use of these NIWPUs as antibacterial coatings, adhesives and soft elastomers.62
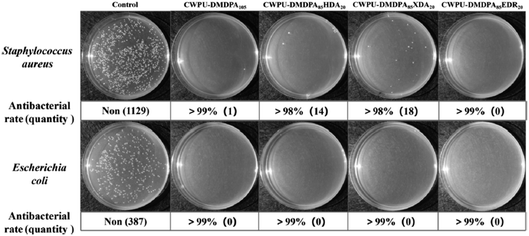 | ||
| Fig. 17 Antibacterial study of cationic NIWPU against S. aureus and E. coli. The image obtained from ref. 62 with permission from Elsevier, copyright 2023. | ||
6.3 Sunflower oil
Sunflower oil is extracted from the seeds of the sunflower plant (Helianthus annus L.). This plant is native in North America and has been cultivated around the world.97 The oil contains around 85% unsaturated and 15% saturated fatty acid, with 44–75% linoleic acids and 14–43% oleic acid content.98 In addition to its culinary uses, sunflower oil is also used in cosmetics and skincare products due to its moisturizing and nourishing properties.99 It is a common ingredient in a variety of products, including lotions, soaps, and hair care products. Sunflower oil also has been used to prepare polyurethane. A study synthesized PU films using sunflower oil-based polyol and diisocyanate with the addition of graphene oxide and reduced graphene oxide.100 Another study reported on TiO2-nanocomposites coatings from sunflower oil-based amide diol as a soft segment.101 Additionally, Asare et al. prepared a polyol from sunflower oil, reacted it with methylene diphenyl diisocyanate to produced rigid polyurethane foams.102 Fig. 18 displays the image of sunflower seeds and the chemical structure of the oil.103In the case of NIPU, there have been a few studies on sunflower oil reported so far. In one study, Boyer et al. synthesized terminal and internal epoxidized fatty acids diesters (TEFAD and IEFAD) from sunflower oil's oleic fatty acid methyl ester. TEFAD and IEFAD were then reacted with CO2 to produce terminal and internal carbonated fatty acids diesters (TCFAD and ICFAD). Subsequently, NIPU was obtained by the polyaddition reaction of TCFAD and ICFAD with EDA and IPDA. The resulting NIPU exhibits molecular weights up to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 with a glass transition temperature (Tg) of −15 °C. This study reported that NIPU produced from IPDA resulted in the absence of the side reaction that typically occurs between amine and ester functions, which usually leads to the formation of amide linkages.104
500 g mol−1 with a glass transition temperature (Tg) of −15 °C. This study reported that NIPU produced from IPDA resulted in the absence of the side reaction that typically occurs between amine and ester functions, which usually leads to the formation of amide linkages.104
A fully bio-based NIPU was prepared using carbonated sunflower oil and novel bio-based amines synthesized form castor oil and oleic acid, as conducted by Farhadian et al. Three different amine groups were synthesized; tri-ester amide/amine (TEAA), polyamine-polyol (PAPO) and di-amide amine (DAA) as shown in Fig. 19. Then, carbonated sunflower oil was prepared by the reaction of epoxidized sunflower oil with CO2 at atmospheric pressure. Subsequently, NIPU was obtained by the reaction of CSFO with the bio-based amine groups without a catalyst and solvent. Based on thermal and physical characterizations, bio-based NIPU exhibits excellent thermal stability, degradation resistance, and low water absorption. The thermal stability is enhanced is attributed to the presence of amide, ester and hydroxyl groups in their structure, as well as their comparatively higher molecular weight than short diamines.105
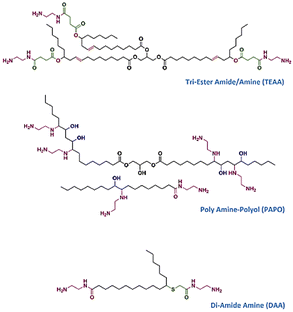 | ||
| Fig. 19 The chemical structure of synthesized bio-based amines. Reuse from ref. 105 with permission from Elsevier, copyright 2018. | ||
Farid et al. prepared NIPU a flame-retardant coating from carbonated sunflower oil and 1,4-phenyldiamine with the addition of varied concentration of zirconia/silica (ZrO2/SiO2) nanoparticles. ZrO2/SiO2 was formed by an in situ reaction which binds ZrO2 nanoparticles with SiO2 nanospheres. Through thermal stability and flammability studies, the materials showed high resistance to flammability and demonstrated the ability to withstand direct contact with fire without ignition. The use of amine with aromatic properties significantly enhanced the flame retardancy of the produced NIPU and further improved by the addition of ZrO2/SiO2 nanoparticles. The authors reported that the optimum loading of ZrO2/SiO2 was up to 3% which give the best thermal, mechanical, and chemical resistance performance. At 5% ZrO2/SiO2, agglomeration was observed, impacting the properties of NIPU.106
Doley and Dolui investigated the effects of different diamine groups on the properties of NIPU by using short chain diamine (EDA), long chain aliphatic diamine (DETA), and cyclo aliphatic diamine (EDA) for anti-corrosive coating. The findings indicate that the properties of NIPU were influenced by the diamines structure and the molar ratio of carbonate to amine used. Diamines with shorter chains resulted in a higher proportion of urethane and amide groups compared to diamines with longer aliphatic chains or cycloaliphatic diamines. In terms of coating properties, NIPU based on IPDA showed high tensile strength, hardness, thermal stability, gloss, and chemical resistance compared to NIPU based on EDA of DETA. Additionally, IPDA-based coating demonstrates the lowest corrosion rate and highest resistance due to its rigid structure.61
6.4 Castor oil
Castor oil is obtained from the seeds of castor plant (Ricinus communis) belonging to the Euphorbiaceae family. The main component of castor oil is ricinoleic (90%), an 18-carbon fatty acid with a hydroxyl group located on the 12th carbon, along with linoleic (4%), oleic (3%), palmitic (1%), stearic (1%), and linolenic (<1%) fatty acids. The oil has been used for medicinal and industrial purposes for centuries, including applications in lubricants, coatings, surfactants, printing inks, paints, etc.107 Other than being non edible, the high content of ricinoleic acid made castor oil a viable option as a monomer in the production of non isocyanate polyurethane.108 Fig. 20 displays the image of castor seeds and the chemical structure of castor oil.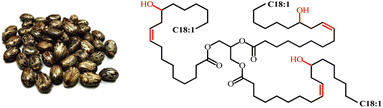 | ||
| Fig. 20 Image of castor seeds and chemical structure of castor oil. The image was obtained from ref. 109 with permission from Elsevier, copyright 2020. | ||
One of the earlier studies on NIPU from castor oil derivatives was reported by Pathak et al. The research commenced with the esterification of tris-2-hydroxy ethyl isocyanurate (THEIC) with dehydrated castor oil fatty acid (DCOFA) to produce THEIC–ester of fatty acid (TEFA). Further, TEFA was epoxidized (ETEFA) and underwent a carbonation reaction (CTEFA). The produced CTEFA was subjected to reactions with three types of amine groups; diaminodiphenylsulphone (DDPS), HMDA and IPDA to produce NIPU. The type of amine used significantly influenced the properties exhibited by the NIPU coatings. In this case, the corrosion rates for HMDA_NIPU, IPDA_NIPU and DDPS_NIPU were recorded at 0.01599, 0.006516, and 0.0001989 mmpy, respectively. This finding proved that coating cured with aromatic and cyclo-aliphatic amines resulted in a better anti-corrosion properties compared to coating cured with aliphatic amines. The aromatic hardener (DDPS) would impart rigidity and barrier protection to the coating, whereas the long aliphatic chain in the HMDA hardener produced a porous and permeable coating and thus decreasing the barrier properties.45
Following that, Rodrigues et al. prepared a simple on-pot procedure of non isocyanate poly(acyl) urethane using castor oil and urea in a presence of a catalyst.110 Since castor oil contain large amount of ricinoleic acid, it can be directly utilized to prepare NIPU without prior modification. In this study, they assess the ideal reaction parameters to synthesize NIPU by varying the oil to urea ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), synthesis temperature (50 °C to 100 °C), curing time (24, 48 and 72 hours), temperature (80 °C to 100 °C), type of catalyst (BF3· OEt2 (CH3COO)2Zn·2H2O, SnCl2·2H2O), and percentage loading of the catalyst (5% and 10% w/w). They suggested 1
4), synthesis temperature (50 °C to 100 °C), curing time (24, 48 and 72 hours), temperature (80 °C to 100 °C), type of catalyst (BF3· OEt2 (CH3COO)2Zn·2H2O, SnCl2·2H2O), and percentage loading of the catalyst (5% and 10% w/w). They suggested 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of castor oil to urea is the best ratio for synthesis with 10% w/w of BF3· OEt2 as the catalyst and the reaction proceeded at 70 °C. As for curing, the NIPU was well produced at 100 °C for 72 hours curing temperature and time. GPC analysis proved the formation of polymeric NIPU with an average molecular mass exceeding 10
2 ratio of castor oil to urea is the best ratio for synthesis with 10% w/w of BF3· OEt2 as the catalyst and the reaction proceeded at 70 °C. As for curing, the NIPU was well produced at 100 °C for 72 hours curing temperature and time. GPC analysis proved the formation of polymeric NIPU with an average molecular mass exceeding 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a polydispersity index of 1.2. Results from DSC and DMA analyses indicated a relatively uniform chemical composition, while the polymeric chains exhibited varied sizes.110 This information is important for understanding the properties and potential applications of NIPU, as the size and distribution of its polymer chains can impact its strength, flexibility, and thermal stability.
000 g mol−1 and a polydispersity index of 1.2. Results from DSC and DMA analyses indicated a relatively uniform chemical composition, while the polymeric chains exhibited varied sizes.110 This information is important for understanding the properties and potential applications of NIPU, as the size and distribution of its polymer chains can impact its strength, flexibility, and thermal stability.
6.5 Jatropha oil
Jatropha oil is derived from the seeds of Jatropha curcas plant. Jatropha curcas is belongs to the Euphorbiaceous family and is native to Central America, but has been widely cultivated in other parts of the world for its oil. Jatropha seeds typically content 35–40% oil content, whereas the kernel can contain up to 35–40%. This makes jatropha a potentially valuable crop for producing many industrial products.111 Jatropha oil contains about 79% unsaturated fatty acids and 21% saturated fatty acids, mostly oleic and linoleic acid. The presence of toxic and purgative compounds such as phorbol esters makes the oil non-edible and cannot be consumed without purification.112 Thus, jatropha oil is commonly used for biodiesel,113 lubricants,114 cosmetics, and soap production,115 and other applications. Jatropha oil has also been used for polyurethane production such as for coatings,116 waterborne-PU,117 and polymer electrolytes.118 Fig. 21 shows the image of jatropha seeds and the oil chemical structure.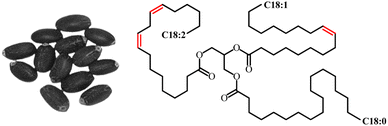 | ||
| Fig. 21 Image of jatropha seeds and the chemical structure of jatropha oil. The image is obtained from ref. 119 with permission from Elsevier, copyright 2016. | ||
Haniffa et al. conducted an optimization study on the carbonation reaction of epoxidized jatropha oil (EJO) using various parameters such as temperature and pressure. Besides, they prepared carbonated alkyd resin (CAR) from jatropha oil. The carbonated EJO (CJO) was blended with CAR at varied weight ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and reacted with 1,3-diaminopropane (DM) and IPDA to produce NIPU. Then, the samples were assessed based on their thermomechanical properties and chemical resistance. The analysis revealed optimal condition at 120 °C, 2 MPa of CO2 pressure and 30 hours reaction time, achieving 99% and 97% carbonate conversion for CJO and CAR, respectively. The blend exhibited improved thermal, mechanical, and chemical resistance compared to single CJO and CAR when cured with DM and IPDA. It was observed that NIPU based on CJO/CAR(1
4) and reacted with 1,3-diaminopropane (DM) and IPDA to produce NIPU. Then, the samples were assessed based on their thermomechanical properties and chemical resistance. The analysis revealed optimal condition at 120 °C, 2 MPa of CO2 pressure and 30 hours reaction time, achieving 99% and 97% carbonate conversion for CJO and CAR, respectively. The blend exhibited improved thermal, mechanical, and chemical resistance compared to single CJO and CAR when cured with DM and IPDA. It was observed that NIPU based on CJO/CAR(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)_IPDA showed better solvent and chemical resistance compared to CJO/CAR(1
3)_IPDA showed better solvent and chemical resistance compared to CJO/CAR(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)_DM. Other than the amine content, the blending of CJO and CAR contributed to the enhancement of the interfacial surface of NIPU.58
3)_DM. Other than the amine content, the blending of CJO and CAR contributed to the enhancement of the interfacial surface of NIPU.58
Another work on jatropha oil-based NIPU was reported by Bhakri et al. for wood composite applications. The CJO was reacted with 3-aminopropyltriethoxisylane (APTES) which known for its excellent amine activity with cyclic carbonate. The presence of silane in APTES allows for the formation of siloxane (–Si–O–Si–) bonds between molecules, producing a more hydrophobic PU. The addition of a Lewis catalyst like LiCl influenced the reaction speed by activating the cyclic carbonate while leaving the amine unaffected. They proposed mechanism of NIPU synthesis as depicted in Fig. 22.120
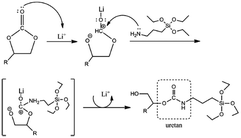 | ||
| Fig. 22 Proposed mechanisms of NIPU synthesis using CJO and APTES in the presence of LiCl catalyst. Adapted from ref. 120 with permission from Tech Science Press, copyright 2023. | ||
6.6 Rubber seed oil
Rubber seed oil (RSO) is a non-edible oil extracted from rubber seeds, a by-product of the natural rubber tree (Hevea brasiliensis). Crude rubber seed oil cannot be used as food resource without refining due to the presence of toxic cyanogenic glycoside compounds.121 The oil is rich in unsaturated fatty acids with 77–82% made up of oleic, linoleic, and linolenic acid and less content of saturated acids for around 17–20%.122 RSO is primarily utilized in non-food purposes such as biodiesels,123 coatings,124 plasticizers,125 cosmetics,126 etc. The oil also has been reported for the production of various types of polyurethane such as waterborne-PU,127 foams,128 thermosetting-PU,129 and resins.130 Fig. 23 displays the image of rubber seeds and the chemical structure for rubber seed oil.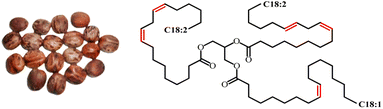 | ||
| Fig. 23 Image of rubber seeds and chemical structure of rubber seed oil. The image is obtained from ref. 131 with permission from Elsevier, copyright 2017. | ||
The current research on rubber seed oil and NIPU is limited with only two studies available to date by Raden and co-workers. In their work, a preliminary study was conducted using epoxidized linoleic acid (ELA) extracted from RSO. After converting ELA to carbonated linoleic acid (CLA), they observed increment of viscosity from 705 cP to 1471 cP. The presence of cyclic carbonate groups led to an increase in viscosity due to the enhanced intermolecular interactions involving the polar oxygen atoms in the cyclic carbonate groups. The CLA was then reacted with different percentage of EDA from 5, 10, 15, and 20 w/w% for the preparation of NIPU. An increase in EDA loading resulted in higher viscosity due to high degree of crosslinking in NIPU and a decrease in tack free time, implying a shorter curing time.132 In their recent study, cyclic carbonate from epoxidized rubber seed oil (ERSO) was synthesized using a low-pressure method. This study focused more into the synthesis part of ERSO at which they investigate the effects of different reaction temperatures and ratios of hydrogen peroxide and formic acid on the properties of ERSO. However, there was less emphasis on the carbonated RSO and NIPU in the discussion.133
6.7 Canola oil
Canola oil is an edible oil derived from a specific variety of rapeseed selectively bred to have low levels of toxic glucosinolates and erucic acid compounds that can be harmful when consumed in large quantities.134 This rapeseed variety is known as “canola” in Canada and the United States, whereas in some other parts of the world, it is known as “rapeseed”. This plant belongs to the Brassicaceae (Cruciferae) family that comprise thousands of species. Specifically, canola varieties could belong to Brassica rapa, Brassica napus or Brassica juncea. Canola oil is among important commodity oil globally and it is widely used as cooking oil. Canola oil contains high degree of monounsaturated fatty acids and very low in saturated fatty acids.135 Canola oil consists of approximately 60% oleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 20% linoleic acid (C18
1), 20% linoleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and 10% α-linolenic acid (C18
2), and 10% α-linolenic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). In comparison to other vegetable oils, canola oil has the lowest saturated fatty acid contain with 7% or less. Canola oil is considered very healthy oil owing to its fatty acid composition and the ideal 2
3). In comparison to other vegetable oils, canola oil has the lowest saturated fatty acid contain with 7% or less. Canola oil is considered very healthy oil owing to its fatty acid composition and the ideal 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 omega-6/omega-3 ratio.136 Other than being used for food purpose, canola is also being used for non-food products including biofuels, lubricants, and soaps. Fig. 24 displays the image of the seeds and the chemical structure of canola oil.
1 omega-6/omega-3 ratio.136 Other than being used for food purpose, canola is also being used for non-food products including biofuels, lubricants, and soaps. Fig. 24 displays the image of the seeds and the chemical structure of canola oil.
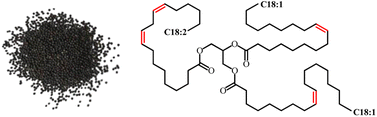 | ||
| Fig. 24 Image of Brassica napus seeds and chemical structure of the oil. The figure is adapted from ref. 137 with permission from MDPI, copyright 2021. | ||
Research has been conducted to explore the possibility of utilizing canola oil as the raw material for the preparation of polyurethane for diverse applications such as coatings,138 adhesives,139 foams,140 and elastomers.141 These studies focused on converting canola/rapeseed oil into polyols and subsequently reacting them with isocyanate groups to produce PU that meets the criteria for their proposed applications. In the case of NIPU, only one study was reported, conducted by Malik and Kaur. They used 5,10,15-tris(pentafluorophenyl)corrolato-manganese(III) complex as a novel catalyst for the preparation of carbonated canola oil (CCO) taking place under mild conditions. Fig. 25 shows the reaction scheme to synthesis the metal complex catalyst. The results indicate that the new catalyst can significantly reduce the reaction time to one-quarter of that required by conventional catalysts. The CCO was further reacted with four types of diamines; EDA, HMDA, IPDA, and paraphenyldiamine (PPDA) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, cured at 80 °C for 8 hours to produce NIPU as the end product. Based on thermal analysis, all types of NIPU exhibited a relatively similar decomposition pattern and remained thermally stable up to 200 °C. On the other hand, the structure of diamine affected the tensile strength of NIPU. This study revealed NIPU with diamine having shorter carbon chain length i.e. EDA (8 MPa) demonstrated higher strength compare to HMDA (6 MPa), whereas the swelling behaviour of EDA_NIPU was observed to be less than HMDA_NIPU.57
1 molar ratio, cured at 80 °C for 8 hours to produce NIPU as the end product. Based on thermal analysis, all types of NIPU exhibited a relatively similar decomposition pattern and remained thermally stable up to 200 °C. On the other hand, the structure of diamine affected the tensile strength of NIPU. This study revealed NIPU with diamine having shorter carbon chain length i.e. EDA (8 MPa) demonstrated higher strength compare to HMDA (6 MPa), whereas the swelling behaviour of EDA_NIPU was observed to be less than HMDA_NIPU.57
 | ||
| Fig. 25 Reaction scheme for the synthesis of 5,10,15-tris(pentafluorophenyl)corrolato-manganese(III) complex catalyst. Adapted from ref. 57 with permission from Wiley, copyright 2017. | ||
6.8 Summary of vegetable oil-based NIPU
Table 4 provides a summary of reported studies on NIPUs derived from various types of vegetable oils. The literature indicates a consistent pattern of research in the production of NIPU from both edible and non-edible oils. Soybean oil stands out as the most reported study so far. This has to do with its high unsaturation value and wide availability. All the oils used contain high unsaturated value that can be exploited for chemical modification. On the side note, palm oil has not been explored for NIPU applications despite being the largest vegetable oil produced globally. This could be related to the high saturated fatty acid content of palm oil compared to other oils used for NIPU synthesis. It is known that saturated fatty acids have stable chemical structures which make them less reactive compared to unsaturated fatty acids. While palm oil may require additional processing steps for chemical modification, it can still be subjected to chemical transformations for NIPU production. Research has investigated various types of amine groups in the form of aliphatic, cycloaliphatic and aromatic amines to produce NIPU. Additionally, apart from using bio-based cyclic carbonate, bio-based amines have also been utilized to produce fully bio-based NIPU. Based on the literature available so far, reactions were conducted with and without the presence of catalysts and solvents, depending on the nature of the experiments. Solvents typically used to reduce the viscosity of the carbonated resins, whereas catalysts are employed to accelerate the reaction rate, allowing more efficient polymerization. Catalysts are commonly used in the preparation of carbonated vegetable oils. Notably, most of studies excluded the use of catalysts in the aminolysis reactions.| No. | Vegetable oil | Amine group | Synthesis methods | Observation | Applications | References |
|---|---|---|---|---|---|---|
| 1 | Soybean oil | EDA, HMDA and TA | (1) Carbonation (CSBO) | NIPU made from triamine (TA) has the highest Tg and level of stress, smallest strain at break and lowest soluble fraction due to its tighter network structure | Not tested | 86 |
| Catalyst: TBAB | ||||||
| (2) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 2 | Soybean oil | m-XDA, p-XDA and IPDA | (1) Carbonation (CSBO) | The three NIPUs produced were elastomeric PUs and the Tg was dominantly affected by the amine-to-cyclic carbonate ratio, while the impact of the amine's structure was relatively minor | Not tested | 55 |
| Catalyst: TBAB | ||||||
| (2) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 3 | Soybean oil | APTES | (1) Carbonation (CSBO) | Tensile strength of NIPU increased with the addition of lignin by contributing to the rigidity of polymer | Not tested | 53 |
| Catalyst: TBAB | ||||||
| (2) Aminolysis (NIPU) | ||||||
| Catalyst: LiCl | ||||||
| Solvent: THF | ||||||
| (3) Addition of lignin | ||||||
| 4 | Soybean oil | BDA, PDA and oligoamide | (1) Synthesis of oligoamide | NIPU produced with short diamines exhibit low mechanical properties, whereas NIPU derived from oligoamide shows better mechanical properties | Not tested | 87 |
| (2) Carbonation (CSBO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 5 | Soybean oil | PA650, IPDA, and cystamine | (1) Carbonation (CSBO) | The self-healing properties of produced NIPU were contributed by the intrinsic hydrogen bonds existed in the matrix and enhanced by the addition of cysteine-derived disulphide-containing cystamine | Not tested | 56 |
| Catalyst: TBAB | ||||||
| (2) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 6 | Soybean oil | Chitosan | (1) Epoxidation (ESBO) | NIPU produced using chitosan as the amine source has good chemical and thermal stability | Not tested | 88 |
| (2) Carbonation (CSBO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: LiCl | ||||||
| Solvent: THF | ||||||
| 7 | Soybean oil | Polyamidoamine | (1) Epoxidation (ESBO) | NIPU exhibited good mechanical properties and thermal stability due to the increased in rigidity of the polymeric chain and dense polymer network with CSBO/CHPE blend | Not tested | 60 |
| (2) Carbonation (CSBO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Synthesis of CHPE | ||||||
| (4) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: THF | ||||||
| 8 | Soybean oil | DDS and DDM | Aminolysis (NIPU) | The self-healing properties of NIPU under UV irradiation enhanced with the presence of disulphide bonds (DDS) | Not tested | 89 |
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 9 | Soybean oil | DDM | (1) Epoxidation (ESBO) | NIPU has shape memory effect and reprocessing ability | Not tested | 142 |
| (2) Carbonation (CSBO) | ||||||
| (3) Aminolysis (NIPU) | ||||||
| 10 | Soybean oil | MDA | (1) Carbonation (CSBO) catalyst: TBAI, L-ascorbic acid | NIPU has shape memory properties, self-healing ability, chemical recyclability and physical reprocessing due to the dynamic covalent bond exchange | Not tested | 90 |
| (2) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 11 | Soybean oil | TEPA and HMDA | (1) Carbonation (CSBO) | The NIPU wound dressing membranes possess good mechanical properties, showed no toxicity against fibroblasts and high antibacterial activity against E. coli and S. aureus | Antibacterial wound dressing membranes | 54 |
| Catalyst: TBAB, CaCl2 | ||||||
| (2) Aminolysis (NIPU) | ||||||
| Catalyst: None | ||||||
| Solvent: DMF | ||||||
| (3) Addition of azetidinium | ||||||
| 12 | Soybean oil | APTES and DETA | (1) Carbonation (CSBO) | The addition of lignin causes a reduction in the adhesive properties in terms of peel force performance of NIPU | Adhesive | 143 |
| Catalyst: TBAB | ||||||
| (2) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: DMF, THF | ||||||
| (3) Addition of lignin | ||||||
| 13 | Soybean oil | Priamine 1075 | (1) Carbonation (CSBO) | The combination of reactive carbonates with epoxides reduced the occurrence of unwanted side reactions such as ester aminolysis and intramolecular cyclization | Not tested | 144 |
| (2) Aminolysis (NIPU) | ||||||
| 14 | Soybean oil | TEPA | (1) Synthesis of tetraniline (TANI) | Observation: Addition of TANI improve mechanical and anti-corrosion properties of NIPU | Anti-corrosion coating | 63 |
| (2) Carbonation (CSBO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: DMF | ||||||
| 15 | Linseed oil | Phenalkalmine | (1) Carbonation (CLSO) | Real-time FTIR spectra were utilized to measure the in situ decay of the carbonate groups and the appearance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the urethane linkages O group of the urethane linkages |
Not tested | 145 |
| Catalyst: TBAB | ||||||
| (2) Aminolysis (NIPU) | ||||||
| 16 | Linseed oil | EDA, BDA and IPDA | (1) Carbonation (CLSO) | NIPU produced has a high dimensional stability and stiffness due to high carbonate functionality of CLSO | Not tested | 59 |
| Catalyst: TBAB | ||||||
| (2) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 17 | Linseed oil | DETA | (1) Epoxidation (ELSO) | Adhesion and hardness properties of NIPU improved with the increase in carbonation number of the CLSO | Anti-corrosion coating | 96 |
| (2) Carbonation (CLSO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 18 | Linseed oil | BDA, PDA, ODA and HMDA | (1) Epoxidation (ELSO) | CLSO showed a high reactivity and this can shorten curing time for NIPU synthesis, however viscosity increases with the carbonated group content can be problematic to achieve a homogenous mixture | Foam | 26 |
| (2) Carbonation (CLSO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 19 | Linseed oil | m-XDA, HMDA | (1) Carbonation (CLSO) | Density functional theory calculation revealed the reactivity of epoxy groups at different sites of the ELSO, whereas the reaction conditions for CLSO and NIPU were optimized to achieve high atom efficiency | Not tested | 146 |
| (2) Aminolysis (NIPU) | ||||||
| (3) Computational approach | ||||||
| 20 | Linseed oil | DMDPA, m-XDA, HMDA and EDR | (1) Carbonation (CLSO) | CNIWPUs, ANIWPUs and NNIWPUs exhibited good stability and the use of aromatic, aliphatic and polyether diamines as chain extender produce NIWPUs with tailorable properties | Not tested | 62 |
| Catalyst: TBAB | ||||||
| (2) Synthesis of CNIWPUs, ANIWPUs and NNIWPUs | ||||||
| Catalyst: none | ||||||
| Solvent: MEK | ||||||
| 21 | Sunflower oil | EDA and IPDA | (1) Epoxidation (TEFAD, IEFAD) | NIPU obtained exhibit molecular weight up to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 and no side reaction occur between ester and amine for IPDA_NIPU 500 g mol−1 and no side reaction occur between ester and amine for IPDA_NIPU |
Not tested | 104 |
| (2) Carbonation (TCFAD, ICFAD) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 22 | Sunflower oil | TEAA, PAPO and DAA | (1) Synthesis of bio-based amine (TEAA, PAPO, DAA) | The used of bio-based amines produced NIPU with improved thermal stability due to the presence of amide, ester and hydroxyl groups in the amines structure, along with theirs high molecular weight | Not tested | 105 |
| (2) Epoxidation (ESFO) | ||||||
| (3) Carbonation (CSFO) | ||||||
| Catalyst: Porphyrin/TBAB | ||||||
| (4) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 23 | Sunflower oil | EDA, DETA and IPDA | (1) Epoxidation (ESFO) | IPDA_NIPU produced the best thermal stability, chemical resistance and anti-corrosive properties | Anti-corrosion coating | 61 |
| (2) Carbonation (CSFO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 24 | Sunflower oil | PPDA | (1) Epoxidation (ESFO) | Flame retardancy enhanced by the use of amine with aromatic properties and with the incorporation of ZrO2_SiO2 nanoparticles | Flame retardant composites coating | 106 |
| (2) Carbonation (CSFO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: xylene | ||||||
| (4) Addition of ZrO2_SiO2 (NIPU composites) | ||||||
| 25 | Castor oil | DDPS, HMDA and IPDA | (1) Esterification (TEFA) | Coating cured with aromatic and cyclo-aliphatic amine resulted in a better anti-corrosion properties compared to coating cured with aliphatic amine | Anti-corrosive coating | 45 |
| (2) Epoxidation (ETEFA) | ||||||
| (3) Carbonation (CTEFA) | ||||||
| Catalyst: TBAB | ||||||
| (4) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: xylene/MEK | ||||||
| 26 | Castor oil | Urea | Direct mixing of castor oil and urea | A simple route to synthesize NIPU in a solvent-free environment, one-pot under mild reaction temperature | Not tested | 110 |
| Catalyst: BF3·OEt2 | ||||||
| 27 | Jatropha oil | DM and IPDA | (1) Epoxidation (EJO, EAR) | Blend of CJO/CAR produce NIPU with excellent thermal, mechanical, and chemical resistance properties | Not tested | 58 |
| (2) Carbonation (CJO, CAR) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: MEK | ||||||
| 28 | Jatropha oil | APTES | (1) Epoxidation (EJO) | The presence of silane in APTES allows for the formation of siloxane (–Si–O–Si–) bonds between molecules, producing a more hydrophobic NIPU | Not tested | 120 |
| (2) Carbonation (CJO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: LiCl | ||||||
| Solvent: THF | ||||||
| 29 | Rubber seed oil | EDA | (1) Epoxidation (ELA) | Increase in EDA loading resulted to increase in viscosity due to high degree of crosslink in NIPU and decrease in tack free time | Not tested | 132 |
| (2) Carbonation (CLA) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 30 | Rubber seed oil | EDA | (1) Epoxidation (ERSO) | The optimum condition for epoxidation of RSO was 50 °C, and increasing molar ratio of hydrogen peroxide increased the oxirane number, whereas when molar ratio for formic acid increased, the opposite effect was observed | Not tested | 133 |
| (2) Carbonation (CRSO) | ||||||
| Catalyst: TBAB | ||||||
| (3) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none | ||||||
| 31 | Canola oil | EDA, HMDA, IPDA and PPDA | (1) Synthesis of manganese-based catalyst | The new catalyst can significantly decrease the carbonation reaction time to one-quarter of the time required by conventional catalysts | Not tested | 57 |
| (2) Epoxidation (ECO) | ||||||
| (3) Carbonation (CCO) | ||||||
| Catalyst: manganese-based | ||||||
| (4) Aminolysis (NIPU) | ||||||
| Catalyst: none | ||||||
| Solvent: none |
7 Potential applications of NIPU
NIPU shares similarities with conventional polyurethane and offers a wide range of applications owing to its versatile properties and environmental advantages. There is a surge of interest to utilize NIPU in many areas, including those currently served by traditional polyurethane as well as exploring new applications. NIPU finds application across various industries and sectors such as coatings, adhesives, packaging materials, insulation materials, biomedical applications, electronic components, and more. This section briefly reviews several applications where NIPU has been investigated, but not limited to those produced using vegetable oil-based feedstock.7.1 Coatings
NIPU has found applications as coating materials due to its excellent adhesion, durability, and chemical resistance nature. It can be used in protective coatings for various surfaces such as metals, plastics, and woods. Various studies have already reported on the use of NIPU for coating applications. In a study by Zhang et al., NIPU coating was investigated for its high hardness, transparency, strong adhesion, and superior flexibility properties. The NIPU was prepared using epoxy-oligosiloxane nanoclusters and an amine curing agent. The mixture was casted onto different substrates i.e. glass, steel, ceramic, epoxy, aluminium alloy, tinplate, titanium, and polyethylene terephthalate. This study suggested that the strong adhesion between NIPU coating and the substrate results from the high density polar groups in the NIPU. Besides, the addition of amine-terminated low-surface-tension polydimethylsiloxane endowed the coating with exceptional anti-icing, self-cleaning, and anti-smudge abilities. This work able to achieve NIPU coating that exhibits a combination of the hardness properties of ceramic and the flexibility of polymers.147Another research reports on NIPU coating using pentaerythritol glycidyl ether-based cyclic carbonate (PGC)/single-end cyclic carbonate poly(dimethylsiloxane) (PDMS-C) and ethylene imine polymer (PEI). In this case, the PDMS-C functions as a liquid-repelling agent to provide NIPU coatings with low surface energy, whereas PEI was used as a curing agent to achieve a high crosslinking density within the coating matrix. It was discovered that the produced NIPU coating displayed high repellency towards various liquids such as water, hexadecane, corn oil, pump oil, milk, fingerprint fluid, and cola. The transparent coating demonstrated excellent self-cleaning properties, allowing dust to slide off with water, leaving no residue of dust or water on the surface. The coatings also showed excellent resistance against ink deposition, maintaining anti-ink performance after 5000 abrasion cycles, making them suitable for various applications where ink smudging or deposition needs to be minimized.148
Boisaubert et al. reported a study on non-isocyanate polyurethane acrylate coatings (NIPUAs) by photocrosslinking acrylate terminated NIPU oligomers (A-Ol) with reactive diluents. The A-Ol was obtained through a transurethane polycondensation pathway, followed by an acrylation reaction of the resulting hydroxyl chain-ends. This work investigates the effect of reactive diluents content and chemical structures on the properties of the coatings. Three types of diluents were used which were hexamethylene diol diacrylate (HDDA), urethane diol dimethacrylate (UDMA), and N-hexamethylene dihydroxyethyl carbamate diacrylate (BHECA). The different types of diluent significantly impact the thermal and mechanical properties of the NIPUA coating. The properties observed align with those of flexible PU currently being develop in the market. The UV-cured coatings are deemed feasible owing to their fast curing and low-energy consumption.149
Moreover, a series of bio-based NIPU coatings were synthesized by performing ring-opening polymerization of rosin-based cyclic carbonate with amines. Rosin is natural resources derived from pine and conifer trees, available abundantly in nature. The NIPU was modified with epoxy and cyclic carbonate-functionalized polyhedral oligomeric silsesquioxanes (POSS). The results demonstrated that the addition of POSS into the NIPU networks led to significant improvements in water tolerance, pencil hardness, and thermal stability of the NIPU/POSS coatings.150 Another bio-based NIPU coating study was reported by Choong et al. with recyclable and intrinsic healing properties via three different types of healing mechanisms; thermo-healing, moisture-healing, and room temperature self-healing. These desirable properties were achieved by the addition of bismaleimide cross-linker. Interestingly, the coating has remarkable moisture-healing property attributed to the presence of hydroxyl functionalities in the NIPU structure. The unique combination of bio-based resources, recyclable properties, and the presence of triple healing sites in their structure makes these materials highly promising for coating applications.151 Nevertheless, the exploration of vegetable oil-based NIPU has also been conducted and is currently being investigated for corrosion protection coatings, flame retardant composites coating, and others, as described in Section 6.
7.2 Adhesives
PU-based adhesives are commercially available in the market and are extensively used across various sectors such as packaging, automotive, construction, woodworking, and more. The demands of these adhesives continue to grow, driven by their excellent adhesion properties, flexibility and performance at low temperatures. This serves as a motivation for the continuous NIPU-based adhesives research to seek a green alternative to the conventional PU adhesives. In the case of vegetable oil-based NIPU, only one preliminary study so far has been reported for soybean oil-based adhesive143 as mentioned in Section 6.8. Despite the lack numbers of studies using vegetable oil, many works already have been conducted on other bio-based resources. For instance, one study prepared low curing temperature tannin-based NIPU wood adhesives and evaluate its properties. In this work, commercial glycerol diglycidyl ether (GDE) was assesses as a promoter for the tannin-based NIPU adhesives. GDE facilitated the curing process at a reduced temperature and promoted the degree of crosslinking between the epoxy groups of GDE and amino groups present in the derived-products of NIPU. The inclusion of GDE in appropriate proportions was found to decrease the curing temperature, enhance bonding performance, and reduce the emission of harmful substances during the preparation of tannin-based NIPU adhesives.152Other adhesive from sucrose-based NIPU was synthesized by Xi et al. by the reaction of sucrose, dimethyl carbonate and hexamethylene diamine for bonding particleboard. A silane coupling agent was used as a crosslinking promoter to decrease the curing temperature. The results showed that the particleboards bonded with the sucrose-based NIPU adhesive at 230 °C exhibited excellent properties. However, the properties decreased as the press time was reduced. The coupling agent significantly reduced the curing temperature of the adhesive, enabling good bonding even at lower press temperatures. This study confirmed the coupling of NIPU with silane produced improved results and made a suitable adhesive for particleboard, medium-density fibreboard, and other particulate wood panels.153
A study by Gomez-Lopez et al. investigated the synergetic effect of dopamine and alkoxysilanes on the rheological and adhesive properties of NIPU. In this case, dopamine acted as an adhesive promoter, and aminopropyl trimethoxysilane functioned as a fast-curing promoter. They found out that adjusting the soft/hard ratios is crucial to achieving high lap-shear strength adhesion value. Furthermore, the addition of dopamine and silane compounds, maintained these adhesive properties even at high temperatures for optimal compositions. Adhesion tests on various substrates including polyamide, high-density polyethylene, poly(methyl methacrylate), oak wood, and aluminium showed the best performances on polar substrates, confirming the strong interactions between dopamine and hydroxyl groups of NIPU.154
Another study on wood bio-based NIPU adhesives was performed by Chen et al. The preparation involves the reaction of soy protein isolate (SPI), dimethyl carbonate (DMC), and hexamethylene diamine. The reaction resulted in the formation of both linear and branched oligomers, which could further crosslink to form a hardened network. To evaluate the adhesive's performance, plywood panels were prepared and bonded using the SPI-NIPU adhesive. The dry strength of the adhesive met the requirements of relevant standards, indicating its suitability for interior grade plywood panels.155
7.3 Foams
NIPU foams have gained interest in research and development due to their immense potential in diverse areas such as in packaging, furniture and bedding, automotive industries, etc. In the case of vegetable oil-based NIPU foams, only one study on linseed oil-based NIPU was reported.26 In contrast to the limited research on vegetable oil, other sources of bio-materials have been reported for NIPU foams production. One study constructed recyclable self-blown NIPU foams through an aminolysis approach. The method capitalizes on the divergent chemistries of amines with cyclic carbonates and thiolactone. By combining these reactants, a polymer network is created, and in situ generation of the blowing agent (CO2) occurs through the reaction of a thiol with a cyclic carbonate. This process results in the formation of multiple linkages including hydroxyurethane, thioethers, and amides within the polymer network. The foams produced exhibit open-cell morphology and can range from flexible to rigid depending on the formulation and reaction conditions. Fig. 26 shows the photographic images of produced NIPU foams at varied NAHcT content. This study reported that the foams can be easily repurposed into films or structural composites through thermal treatment, demonstrating their recyclability. Notably, both the formation and recycling of the foams can be achieved without the use of a catalyst.156 | ||
| Fig. 26 The effect of NAHcT content on NIPU foaming. Reuse from ref. 156 with permission from ACS, copyright 2022. | ||
Chen et al. described the preparation of condensed tannin-glucose-based NIPU foams with improved fire retardancy. Tannin was added as a flame-retardant agent to improve the fire resistance property of glucose-NIPU. The weight ratios of tannin (T) to glucose ratios were varied from 0 g/40 g (NIPU_Tannin0), 10 g/30 g (NIPU_Tannin10), and 20 g/20 g (NIPU_Tannin20). The mixture was reacted with dimethyl carbonate, followed by addition of hexamethylenediamine in water. The visual appearance of the foams is presented in Fig. 27. Ignition combustion experiments indicated that tannin modified NIPU foams exhibit a longer burning time and higher residual weight compared to blank NIPU foam. Additionally, the addition of condensed tannin increased the limiting oxygen index from 17.5% to 25.5%.157
 | ||
| Fig. 27 Images of tannin–glucose-based NIPU foams. Reuse from ref. 157 with permission from Elsevier, copyright 2020. | ||
Another study on flame-resistant rigid NIPU bio-foam based on chitosan and tannic acid was reported by Smith et al. The foams were prepared with the absence of solvents and catalysts. The foam's structure was achieved by incorporating glutaraldehyde and four different carboxylic acids: malic acid, maleic acid, citric acid, and aconitic acid. The resulting foam samples were compared based on their morphology, thermal degradation, and flame resistance. This study observed that the properties of the foam varied depending on the carboxylic acid used. However, in all cases, the peak thermal degradation and peak heat release for NIPU foams were delayed by more than 100 °C compared to commercial rigid PU foam. Further, when subjected to a butane torch test, the NIPU foams exhibited significant improvements as shown in Fig. 28. The foams showed an 80% higher remaining mass and a 75% reduction in after-burn time compared to conventional PU. This finding highlights the superior fire-resistant properties of the bio-based NIPU foam which eliminates the hazards associated with traditional PU.158
 | ||
| Fig. 28 Graphical images of commercial (a) PU foam and (b) NIPU foam 2 s after the removal of a butane torch flame. Reuse from ref. 158 with permission from MDPI, copyright 2022. | ||
7.4 Biomedical applications
The use of NIPU in biomedical applications has been investigated previously owing to its non-toxic and biocompatible properties. In the case of vegetable oil-based NIPU, Gholami and Yeganeh have prepared soybean-oil based NIPU containing azetidinium groups as antibacterial wound dressing membranes54 as discussed in Section 6.1. A recent study was conducted on the fabrication of photosensitive NIPU acrylate (NIPUA) resin for 3D printing of customized biocompatible orthopedic surgical guides by Wang et al. Compared to commercial photosensitive resins, NIPUA exhibited higher thermal stability, hemocompatibility, superior biocompatibility to ME3T3-E1 bone cells and C1C12 muscle cells, and a non-immunogenic effect towards macrophages. The NIPUA also did not caused inflammatory response during in vivo implantation unlike the severe response by commercial resins. Further, the transcriptome analysis revealed that NIPUA-treated MC3T3-E1 cells showed downregulation of genes associated with cell death and disruption of the cell cycle compared to those treated with commercial resin. On the other hand, there was an upregulation of genes related to autophagy and anti-tumor activity. To summarize, the NIPUA resin exhibited excellent mechanical and thermal properties as well as good biocompatibility towards bone cells, muscle cells, and macrophages suggests the suitability of this material for use in orthopedic surgical guides.159One work by Visser et al. reported on the electrospinning of NIPU by the transurethanization reaction of polycarbonate diols (PCLD) and 1,6-hexanedicarbamate. Different molecular weights of PCDL were utilized to optimize the electrospinnability of NIPU. Through scanning electron microscopy, they observed the electrospun NIPU formed fibrous scaffolds with submicron-sized fiber diameters. To assess the potential of these electrospun NIPU mats as biomimetic load-bearing pericardial substitutes in cardiac tissue engineering, their cytotoxicity was evaluated using primary human fibroblasts and a human epithelial cell line. Interestingly, the bare NIPU mats exhibited excellent cell adhesion without the need for further biofunctionalization, surpassing the performance of collagen-functionalized NIPU mats. These results indicate that the NIPU mats possess significant potential for use in biomimetic scaffolds.160 In another study, Aduba et al. prepared fibrous mats NIPU using electrospinning method. Fig. 29 depicted the photographic images of (a) NIPU fibrous mats and (b) PU control and their scanning electron microscope images (c and d). The NIPU fibrous mats were found to be non-toxic and biocompatible as demonstrated by cell viability and attachment studies with human fibroblast. These finding suggest that the mats can be safely used in various biomedical applications including tissue engineering, drug delivery, and wound dressing technology.161
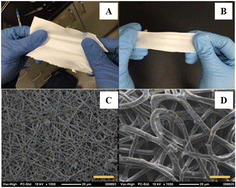 | ||
| Fig. 29 Photographic images of electrospun and their scanning electron microscopy microscale images (A and C) NIPU, (B and D) TPU control. Reproduced from ref. 161 with permission from Wiley, copyright 2018. | ||
7.5 Food preservation
One interesting study was reported by Yang et al. on the use of bio-based NIPU in food preservation application. The NIPU was prepared from sweet potato residual (SPR) from the starch industry. The crude SPR was first converted into a liquid state, forming chain-extending homogenous molecules with active hydroxyl groups. Next the liquefied SPR underwent epoxidation and carbonation reactions to produce cyclic carbonates. The cyclic carbonate precursor was then reacted with different types of amines (EDA, HMDA, IPDA, DETA and TAEA). Among the five testing groups, TAEA_NIPU film exhibited the highest tensile strength (8.5 MPa) due to the strong curing and crosslinking properties of TAEA. The authors suggested that the produced NIPU derived from SPR would be safe for use as a food cling material. To evaluate its potential, the researchers atomized the initial liquid of SPR NIPU and spray coated the film onto the surface of banana peels to preserve freshness. The viscosity of the initial NIPU liquid was reported to be 6 Pa s−1, indicating that it can be directly used for spray coating without further modifications. During the experiment, both treated and control groups were stored under similar conditions of 10–25 °C temperature, 30–65% humidity and in a ventilated dark place. After 7 days of storage, small black patches appeared on the surface of the banana peel for both treated and control groups. By the second week of storage, the control group began to spoil, whereas the banana peels in the coated with SPR-NIPU exhibited only slight darkening compared to 1 week after storage as displayed in Fig. 30. This indicates that SPR-NIPU can serve as a protective coating, effectively preserving the freshness of food.162 | ||
| Fig. 30 Experimental study on preservation of bananas using SPR-based NIPU spray coating. Reproduced from ref. 162 with permission from Elsevier, copyright 2022. | ||
8 Conclusions and future outlook
Significant progress has been made in NIPU development as outlined in this review. NIPU ability to avoid the use of isocyanates while maintaining desirable properties addresses both safety and environmental concerns associated with traditional PU production. The research and development in NIPU so far have demonstrated its potential across various applications. This is due to the wide range of properties NIPU such that it offers ample opportunities for alteration to meet diverse application requirements. Moreover, NIPU compatibility with bio-based feedstock further enhances its eco-friendliness and aligns with the growing emphasis on sustainable materials. In the case of vegetable oil-based NIPU, currently, there are still limited but continuous studies reported in this area. Both edible and non-edible vegetable oil has been utilized to prepare bio-based NIPU at which soybean oil being the most used source of oil so far. With ongoing research and development, it is likely to see an increasing number of reports on vegetable oil-based NIPU in the future. In addition, there are valid concerns related to the use of edible oil for non-food purpose applications. This has opened up the route to prepare NIPU using non-edible vegetable oil. Some studies already reported on the fully bio-based NIPU derived from bioresources. Overall, even though NIPU technology is promising, it is not yet attained the stage of industrial implementation. Presently, challenges related to synthesis issues and technical performance of NIPU hinders its commercialization. Specifically, issues like slow polymerization, extended reaction time, and technical performance pose obstacles in NIPU production. Addressing these challenges must involve a thorough consideration of various factors like the selection of monomer ratio, solvent, catalyst, and reaction parameters which collectively contribute to enhancing NIPU properties. Additionally, broader challenges like scalability, cost-effectiveness, and end-of-life considerations must be addressed for NIPU to be accepted as a suitable alternative to the traditional PU. To achieve this, the development of scalable synthesis route and the implementation of comprehensive cycle assessments are essential steps that will contribute to the competitiveness of NIPU. Furthermore, the industry is most likely to shift into NIPU only when they become cost-competitive with the current PU technology. This is perhaps the direction of future efforts to materialize NIPU as commercial products.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge Yayasan Universiti Teknologi PETRONAS (YUTP) for the financial support through grant 015LC0-142.References
- B. Bizet, B. Bizet, É. Grau, H. Cramail and J. M. Asua, Polym. Chem., 2020, 11, 3786–3799 RSC.
- J. Catalá, I. Guerra, J. M. García-Vargas, M. J. Ramos, M. T. García and J. F. Rodríguez, Polymers, 2023, 15, 1589 CrossRef PubMed.
- A. Das and P. Mahanwar, Adv. Ind. Eng. Polym. Res., 2020, 3, 93–101 Search PubMed.
- M. Ghasemlou, F. Daver, E. P. Ivanova and B. Adhikari, Eur. Polym. J., 2019, 118, 668–684 CrossRef CAS.
- X. Ma, J. Chen, J. Zhu and N. Yan, Macromol. Rapid Commun., 2021, 42, 2000492 CAS.
- M. Tryznowski, J. Izdebska-Podsiadły and Z. Żołek-Tryznowska, Prog. Org. Coat., 2017, 109, 55–60 CrossRef CAS.
- V. Ca, G. Lligadas, J. C. Ronda and M. Galia, Mater. Today, 2013, 16, 337–343 CrossRef.
- A. Patti, F. Costa, M. Perrotti, D. Barbarino and D. Acierno, Materials, 2021, 14, 1951 CrossRef CAS PubMed.
- J. Niesiobędzka and J. Datta, Green Chem., 2023, 25, 2482–2504 RSC.
- F. M. de Souza, P. K. Kahol and R. K. Gupta, in Polyurethane Chemistry: Renewable Polyols and Isocyanates, ACS Publications, 2021, pp. 1–24 Search PubMed.
- H. Choe and J. H. Kim, J. Ind. Eng. Chem., 2019, 69, 153–160 CrossRef CAS.
- Y. Li, X. Luo and S. Hu, Poyols and Polyurethanes from Vegetable Oils and Their Derivatives, in Bio-based Polyols and Polyurethane, Springer International Publishing, 2015, pp. 15–43, DOI:10.1007/978-3-319-21539-6.
- C. Pronoitis, M. Hakkarainen and K. Odelius, ACS Sustain. Chem. Eng., 2022, 10, 2522–2531 CrossRef CAS.
- M. A. Aristri, M. A. R. Lubis, S. M. Yadav, P. Antov, A. N. Papadopoulos, A. Pizzi, W. Fatriasari, M. Ismayati and A. H. Iswanto, Appl. Sci., 2021, 11, 4242 CrossRef CAS.
- G. dong Feng, Y. Ma, M. Zhang, P. you Jia, L. hong Hu, C. guo Liu and Y. hong Zhou, Prog. Org. Coat., 2019, 133, 267–275 CrossRef.
- S. Laurichesse, C. Huillet and L. Avérous, Green Chem., 2014, 16, 3958–3970 RSC.
- T. N. M. Tuan Ismail, N. A. Ibrahim, M. A. Mohd Noor, S. S. Hoong, K. D. P. Palam, S. K. Yeong, Z. Idris, C. M. Schiffman, I. Sendijarevic, E. Abd Malek, N. Zainuddin and V. Sedijarevic, J. Am. Oil Chem. Soc., 2018, 95, 509–523 CrossRef CAS.
- S. Cui, Z. Liu and Y. Li, Ind. Crops Prod., 2017, 108, 798–805 CrossRef CAS.
- R. Galhano, N. F. Acero, S. Matos, R. Carvalho, M. Vale, A. C. Marques, J. C. Bordado and M. M. Mateus, J. Polym. Environ., 2017, 1–10 Search PubMed.
- Q. Wang and N. Tuohedi, Sustainability, 2020, 12, 4214 CrossRef CAS.
- Y. Cao, Z. Liu, B. Zheng, R. Ou, Q. Fan, L. Li, C. Guo, T. Liu and Q. Wang, Composites, Part B, 2020, 200, 108295 CrossRef CAS.
- H. Tian, J. Wu and A. Xiang, J. Vinyl Addit. Technol., 2017, 24, 105–111 Search PubMed.
- R. Morales-Cerrada, R. Tavernier and S. Caillol, Polymers, 2021, 13, 1255 CrossRef CAS PubMed.
- K. Błażek and J. Datta, Crit. Rev. Environ. Sci. Technol., 2019, 49, 1–39 CrossRef.
- P. M. Paraskar, M. S. Prabhudesai, V. M. Hatkar and R. D. Kulkarni, Prog. Org. Coat., 2021, 156, 106267 CrossRef CAS.
- T. Dong, E. Dheressa, M. Wiatrowski, A. P. Pereira, A. Zeller, L. M. L. Laurens and P. T. Pienkos, ACS Sustainable Chem. Eng., 2021, 9, 12858–12869 CrossRef CAS.
- T. Liu, X. Yang, S. Zhang, Q. Wang, N. Jiang and G. Wang, RSC Adv., 2022, 30167–30173 RSC.
- E. Dyer and H. Scott, J. Am. Chem. Soc., 1957, 79, 672–675 CrossRef CAS.
- A. Z. Yu, R. A. Setien, J. M. Sahouani, J. Docken and D. C. Webster, J. Coat. Technol. Res., 2019, 16, 41–57 CrossRef CAS.
- Y. Suryawanshi, P. Sanap and V. Wani, Polym. Bull., 2019, 76, 3233–3246 CrossRef CAS.
- P. Stachak, I. Łukaszewska, E. Hebda and K. Pielichowski, Materials, 2021, 14, 3497 CrossRef CAS PubMed.
- A. Cornille, R. Auvergne, O. Figovsky, B. Boutevin and S. Caillol, Eur. Polym. J., 2017, 87, 535–552 CrossRef CAS.
- L. Filippi and M. A. R. Meier, Macromol. Rapid Commun., 2021, 42, 2000440 CrossRef CAS PubMed.
- J. Datta and M. Włoch, Polym. Bull., 2016, 73, 1459–1496 CrossRef CAS.
- N. Ismail, M. Essalhi, M. Rahmati, Z. Cui, M. Khayet and N. Tavajohi, Green Chem., 2021, 23, 2130–2147 RSC.
- C. C. Su, M. He, R. Amine, Z. Chen, R. Sahore, N. Dietz Rago and K. Amine, Energy Storage Mater., 2019, 17, 284–292 CrossRef.
- C. Claver, M. B. Yeamin, M. Reguero and A. M. Masdeu-Bultó, Green Chem., 2020, 22, 7665–7706 RSC.
- E. J. C. Lopes, A. P. C. Ribeiro and L. M. D. R. S. Martins, Catalysts, 2020, 10, 479 CrossRef CAS.
- J. W. Hill and W. H. Carothers, J. Am. Chem. Soc., 1933, 55, 5031–5039 CrossRef CAS.
- E. W. Spanagel and W. H. Carothers, J. Am. Chem. Soc., 1935, 57, 929–934 CrossRef CAS.
- F. Camara, S. Benyahya, V. Besse, G. Boutevin, R. Auvergne, B. Boutevin and S. Caillol, Eur. Polym. J., 2014, 55, 17–26 CrossRef CAS.
- C. Carré, Y. Ecochard, S. Caillol and L. Avérous, ChemSusChem, 2019, 12, 3410–3430 CrossRef PubMed.
- P. P. Pescarmona, Curr. Opin. Green Sustainable Chem., 2021, 29, 100457 CrossRef CAS.
- M. Rayung, M. M. Aung, S. C. Azhar, L. C. Abdullah, M. S. Su'ait, A. Ahmad and S. N. A. Md Jamil, Materials, 2020, 13, 838 CAS.
- R. Pathak, M. Kathalewar, K. Wazarkar and A. Sabnis, Prog. Org. Coat., 2015, 89, 160–169 CrossRef CAS.
- V. Froidevaux, C. Negrell, S. Caillol, J. P. Pascault and B. Boutevin, Chem. Rev., 2016, 116, 14181–14224 CrossRef CAS PubMed.
- C. Zhang, K.-c. Huang, H. Wang and Q. Zhou, Prog. Org. Coat., 2020, 148, 105855 CrossRef CAS.
- B. Zhang, X. Yang, X. Lin, H. Shang, Q. Liu, H. Wang, S. Liu, X. Xu and F. Dong, ACS Sustainable Chem. Eng., 2023, 11, 6100–6113 CrossRef CAS.
- G. Rokicki, P. G. Parzuchowski and M. Mazurek, Polym. Adv. Technol., 2015, 26, 707–761 CrossRef CAS.
- E. Wondu, Z. Lule and J. Kim, Polymers, 2019, 11, 1103 CrossRef PubMed.
- F. H. Yeoh, C. S. Lee, Y. B. Kang, S. F. Wong, S. F. Cheng and W. S. Ng, Polymers, 2020, 12, 1842 CrossRef CAS PubMed.
- S. Dworakowska, D. Bogdal and A. Prociak, Polymers, 2012, 4, 1462–1477 CrossRef.
- A. Lee and Y. Deng, Eur. Polym. J., 2015, 63, 67–73 CrossRef CAS.
- H. Gholami and H. Yeganeh, Eur. Polym. J., 2021, 142, 110142 CrossRef CAS.
- I. Javni, D. P. Hong and Z. S. Petrovic, J. Appl. Polym. Sci., 2013, 128, 566–571 CrossRef CAS.
- J. Dong, B. Liu, H. Ding, J. Shi, N. Liu, B. Dai and I. Kim, Polym. Chem., 2020, 11, 7524–7532 RSC.
- M. Malik and R. Kaur, Polym. Adv. Technol., 2017, 29, 1078–1085 CrossRef.
- M. A. C. M. Haniffa, Y. C. Ching, C. H. Chuah, Y. C. Kuan, D. S. Liu and N. S. Liou, Polymers, 2017, 9, 162 CrossRef PubMed.
- B. Moritz and M. Rolf, Green Chem., 2012, 14, 483–489 RSC.
- S. Doley, A. Bora, P. Saikia, S. Ahmed and S. K. Dolui, J. Polym. Res., 2021, 30–33, DOI:10.1007/s10965-021-02485-2.
- S. Doley and S. K. Dolui, Eur. Polym. J., 2018, 102, 161–168 CrossRef CAS.
- W. Zhang, T. Wang, Z. Zheng, R. L. Quirino, F. Xie, Y. Li and C. Zhang, Chem. Eng. J., 2023, 452, 138965 CrossRef CAS.
- N. Dhore, E. Prasad, R. Narayan, C. R. K. Rao and A. Palanisamy, Sustainable Chem., 2023, 4, 95–109 CrossRef CAS.
- M. Goyal, S. N. Agarwal and N. Bhatnagar, J. Appl. Polym. Sci., 2022, 139, e52816 CrossRef CAS.
- Z. Li, R. Yu and B. Guo, ACS Appl. Bio Mater., 2021, 4, 5926–5943 CrossRef CAS PubMed.
- D. Wang, S. Chen, J. Zhao and Z. Zhang, Mater. Today Commun., 2020, 23, 101138 CrossRef CAS.
- F. Zhang, P. Ju, M. Pan, D. Zhang, Y. Huang, G. Li and X. Li, Corros. Sci., 2018, 144, 74–88 CrossRef CAS.
- S. K. Raut, P. K. Behera, T. S. Pal, P. Mondal, K. Naskar and N. K. Singha, Chem. Commun., 2021, 57, 1149–1152 RSC.
- S. Hu, X. Chen and J. M. Torkelson, ACS Sustain. Chem. Eng., 2019, 7, 10025–10034 CrossRef CAS.
- D. w. Wang, S. Chen, J. b. Zhao, Z. y. Zhang and J. y. Zhang, Polym. Eng. Sci., 2021, 61, 497–505 CrossRef CAS.
- C. C. Hornat and M. W. Urban, Prog. Polym. Sci., 2020, 102, 101208 CrossRef CAS.
- X. Yin, H. Liu, R. Lin, X. Liu, Z. Huang, J. Du, Y. Gu, X. Lin, W. Lin and G. Yi, J. Appl. Polym. Sci., 2023, 140, e53705 CrossRef CAS.
- V. Schimpf, B. Heck, G. n. Reiter and R. Mülhaupt, Macromolecules, 2017, 50, 3598–3606 CrossRef CAS.
- T. Zhang, B. Xue, Q. Yan, Y. Yuan, J. Tan, Y. Guan, J. Wen, X. Li and W. Zhao, Ind. Crops Prod., 2023, 199, 116706 CrossRef CAS.
- J. Sternberg and S. Pilla, Green Chem., 2020, 22, 6922–6935 RSC.
- L. Li, B. Zhao, H. Wang, Y. Gao, J. Hu and S. Zheng, Compos. Sci. Technol., 2021, 215, 109009 CrossRef CAS.
- X. Chen, L. Li, K. Jin and J. M. Torkelson, Polym. Chem., 2017, 8, 6349–6355 RSC.
- N. S. Purwanto, Y. Chen, T. Wang and J. M. Torkelson, Polymer, 2023, 272, 125858 CrossRef CAS.
- Y. Chen, B. Chen and J. M. Torkelson, Macromolecules, 2023, 56, 3687–3702 CrossRef CAS.
- Y. Zhou, W. Zhao, Y. Lai, B. Zhang and D. Zhang, Front. Plant Sci., 2020, 11, 1315 CrossRef PubMed.
- M. Rayung, M. Min, A. Ahmad, M. Sukor, L. Chuah, S. Nurul and A. Jamil, Mater. Chem. Phys., 2019, 222, 110–117 CrossRef CAS.
- X. P. Song, M. C. Hansen, P. Potapov, B. Adusei, J. Pickering, M. Adami, A. Lima, V. Zalles, S. V. Stehman, C. M. Di Bella, M. C. Conde, E. J. Copati, L. B. Fernandes, A. Hernandez-Serna, S. M. Jantz, A. H. Pickens, S. Turubanova and A. Tyukavina, Nat Sustainability, 2021, 4, 784–792 CrossRef PubMed.
- Q. Wu, Y. Hu, J. Tang, J. Zhang, C. Wang, Q. Shang, G. Feng, C. Liu, Y. Zhou and W. Lei, ACS Sustainable Chem. Eng., 2018, 6, 8340–8349 CrossRef CAS.
- M. Qi, Y. J. Xu, W. H. Rao, X. Luo, L. Chen and Y. Z. Wang, RSC Adv., 2018, 8, 26948–26958 RSC.
- R. M. Nair, V. N. Boddepalli, M. R. Yan, V. Kumar, B. Gill, R. S. Pan, C. Wang, G. L. Hartman, R. Silva e Souza and P. Somta, Plants, 2023, 12, 609 CrossRef PubMed.
- B. Tamami, S. Sohn and G. L. Wilkes, J. Appl. Polym. Sci., 2004, 92, 883–891 CrossRef CAS.
- L. Poussard, J. Mariage, B. Grignard, C. Detrembleur, C. Je, C. Calberg, B. Heinrichs, J. D. Winter, P. Gerbaux, J. Raquez, L. Bonnaud and P. Dubois, Macromolecules, 2016, 49, 2161–2171 CrossRef.
- M. Das, B. Mandal and V. Katiyar, J. Appl. Polym. Sci., 2020, 137, 1–12 Search PubMed.
- X. Yang, S. Wang, X. Liu, Z. Huang, X. Huang, X. Xu, H. Liu, D. Wang and S. Shang, Green Chem., 2021, 23, 6349–6355 RSC.
- X. Liu, X. Yang, S. Wang, S. Wang, Z. Wang, S. Liu, X. Xu, H. Liu and Z. Song, ACS Sustainable Chem. Eng., 2021, 9, 4175–4184 CAS.
- X. Sun, Y. Wang, H. Li, J. Zhou, J. Han and C. Wei, LWT, 2021, 151, 112137 CAS.
- R. Turco, R. Tesser, V. Russo, T. Cogliano, M. Di Serio and E. Santacesaria, Ind. Eng. Chem. Res., 2021, 60, 16607–16618 CAS.
- N. Nehchiri, S. Amiri and M. Radi, Packag. Technol. Sci., 2021, 34, 283–295 CAS.
- Q. Wang and G. W. Padua, J. Agric. Food Chem., 2005, 53, 3444–3448 CrossRef CAS PubMed.
- Á. Agüero, D. Lascano, D. Garcia-Sanoguera, O. Fenollar and S. Torres-Giner, Sustainability, 2020, 12, 652 Search PubMed.
- J. Pouladi, S. M. Mirabedini, H. E. Mohammadloo and N. G. Rad, Eur. Polym. J., 2021, 153, 110502 CAS.
- B. S. Adeleke and O. O. Babalola, Food Sci. Nutr., 2020, 8, 4666–4684 CrossRef CAS PubMed.
- M. R. Akkaya, J. Food Sci. Technol., 2018, 55, 2318–2325 CrossRef CAS PubMed.
- B. Behera, V. K. Singh, S. Kulanthaivel, M. K. Bhattacharya, K. Paramanik, I. Banerjee and K. Pal, Eur. Polym. J., 2015, 64, 253–264 CrossRef CAS.
- V. Suthar, M. A. Asare, F. M. d. Souza and R. K. Gupta, Polymers, 2022, 14, 4974 CrossRef CAS PubMed.
- M. Alam, N. M. Alandis, F. Zafar, E. Sharmin and Y. M. Al-Mohammadi, J. Macromol. Sci., Part A: Pure Appl. Chem., 2018, 55, 698–708 CrossRef CAS.
- M. A. Asare, P. Kote, S. Chaudhary, F. M. d. Souza and R. K. Gupta, Poolymers, 2022, 14, 5282 CrossRef CAS PubMed.
- S. Moqadam and M. Salami-Kalajahi, e-Polym., 2016, 16, 0152 Search PubMed.
- A. Boyer, E. Cloutet, T. Tassaing, B. Gadenne, C. Alfos and H. Cramail, Green Chem., 2010, 12, 2205–2213 RSC.
- A. Farhadian, A. Ahmadi, I. Omrani and A. Babaei, Polym. Degrad. Stab., 2018, 155, 111–121 CrossRef CAS.
- M. E. Farid, M. A. El-Sockary, A. M. El-Saeed, A. I. Hashem, O. M. Abo Elenien, M. S. Selim and S. E. Park, Mater. Res. Express, 2019, 6, 065042 CrossRef CAS.
- I. Chakraborty and K. Chatterjee, Biomacromolecules, 2020, 21, 4639–4662 CrossRef CAS PubMed.
- A. F. Guzmán, D. A. Echeverri and L. A. Rios, J. Chem. Technol. Biotechnol., 2017, 92, 1104–1110 CrossRef.
- V. Subramaniyan, in Nuts and Seeds in Health and Disease Prevention: Therapeutic Importance of Castor Seed Oil, ed. V. R. Preedy and R. R. Watson, Elsevier, 2nd edn, 2020, pp. 485–495, DOI:10.1016/B978-0-12-818553-7.00034-6.
- J. D. O. Rodrigues, C. K. Z. Andrade, R. L. Quirino and M. J. A. Sales, Prog. Org. Coat., 2022, 162, 106557 CrossRef CAS.
- J. Nisar, R. Razaq, M. Farooq, M. Iqbal, R. A. Khan, M. Sayed, A. Shah and I. u. Rahman, Renewable Energy, 2017, 101, 111–119 CrossRef CAS.
- S. Saalah, L. C. Abdullah, M. M. Aung, M. Z. Salleh, D. R. A. Biak, M. Basri, E. R. Jusoh and S. Mamat, Molecules, 2017, 22, 1–17 CrossRef PubMed.
- H. Acherki, A. Bouaid and J. M. Marchetti, Biofuels, Bioprod. Biorefin., 2022, 16, 219–227 CrossRef CAS.
- L. I. Farfan-Cabrera, E. A. Gallardo-Hernández, M. Gómez-Guarneros, J. Pérez-González and J. G. Godínez-Salcedo, Renewable Energy, 2020, 149, 1197–1204 CrossRef CAS.
- M. Shahinuzzaman, Z. Yaakob and M. Moniruzzaman, J. Cosmet. Dermatol., 2016, 15, 185–193 CrossRef CAS PubMed.
- N. H. Mudri, L. C. Abdullah, M. M. Aung, M. Z. Salleh, D. R. Awang Biak and M. Rayung, Polymers, 2020, 12, 1–17 CrossRef PubMed.
- S. Saalah, L. C. Abdullah, M. M. Aung, M. Z. Salleh, D. R. A. Biak, M. Basri, E. R. Jusoh, S. Mamat and S. S. O. Al Edrus, Polymers, 2021, 13, 795 CrossRef CAS PubMed.
- M. Rayung, M. M. Aung, M. Sukor, L. C. Abdullah and A. Ahmad, ACS Omega, 2020, 5, 14267–14274 CrossRef CAS PubMed.
- S. Dharma, H. H. Masjuki, H. C. Ong, A. H. Sebayang, A. S. Silitonga, F. Kusumo and T. M. I. Mahlia, Energy Convers. Manage., 2016, 115, 178–190 CrossRef CAS.
- S. Bhakri, M. Ghozali, E. Cahyono, E. Triwulandari, R. W. Kartika, N. N. Solihat, A. H. Iswanto, P. Antov, V. Savov, L. S. Hua, E. A. Agustiany, L. Kristak and W. Fatriasari, J. Renewable Mater., 2022, 1–19 Search PubMed.
- N. T. Thuy, V. M. Duc and N. T. Liem, Vietnam J. Chem., 2018, 56, 181–186 CrossRef.
- C. F. Jisieike and E. Betiku, Biocatal. Agric. Biotechnol., 2020, 24, 101522 CrossRef.
- C. F. Uzoh, A. Nnuekwe, O. Onukwuli, S. Ofochebe and C. Ezekannagha, J. Cleaner Prod., 2021, 280, 124563 CrossRef CAS.
- J. Huang, J. Zhang, G. Zhu, X. Yu, Y. Hu, Q. Shang, J. Chen, L. Hu, Y. Zhou and C. Liu, Prog. Org. Coat., 2021, 159, 106391 CrossRef CAS.
- N. T. Thuy, B. X. Nam and V. M. Duc, Vietnam J. Chem., 2019, 57, 735–740 CrossRef.
- P. Chaikul, N. Lourith and M. Kanlayavattanakul, Ind. Crops Prod., 2017, 108, 56–62 CrossRef CAS.
- A. Saetung, A. Rungvichaniwat, P. Tsupphayakorn-ake, P. Bannob, T. Tulyapituk and N. Saetung, J. Polym. Res., 2016, 23, 64 CrossRef.
- N. Saetung, S. Somjit, P. Thongkapsri, T. Tulyapitak and A. Saetung, J. Polym. Res., 2016, 23, 58 CrossRef.
- J. Hong, D. Radojčić, X. Q. Yang, X. Wan and Z. S. Petrović, J. Appl. Polym. Sci., 2020, 173, 48509 CrossRef.
- J. Hong, X. Q. Yang, X. Wan, Z. Zheng and Z. S. Petrović, Polym. Int., 2017, 66, 126–132 CrossRef CAS.
- W. Roschat, T. Siritanon, B. Yoosuk, T. Sudyoadsuk and V. Promarak, Renewable Energy, 2017, 101, 37–944 CrossRef.
- A. H. Raden, F. M. Ahmad and S. Azemi, Adv. Mater. Res., 2013, 812, 73–79 Search PubMed.
- S. A. R. A. Raden, M. A. Faiza and A. Zuliahani, Sains Malays., 2021, 50, 2407–2417 CrossRef.
- E. Cartea, A. De Haro-Bailón, G. Padilla, S. Obregón-Cano, M. Del Rio-Celestino and A. Ordás, Foods, 2019, 8, 292 CrossRef CAS PubMed.
- S. Ghobadi, Z. Hassanzadeh-Rostami, F. Mohammadian, M. Zare and S. Faghih, J. Am. Coll. Nutr., 2019, 38, 185–196 CrossRef CAS PubMed.
- V. J. Barthet, in Reference Module in Food Science: Canola: Overview, Elsevier, 2016, pp. 1–5, DOI:10.1016/b978-0-08-100596-5.00029-9.
- N. Raboanatahiry, H. Li, L. Yu and M. Li, Agronomy, 2021, 11, 1776 CrossRef CAS.
- M. Alam, M. Altaf and N. Ahmad, Polymers, 2021, 13, 3325 CrossRef CAS PubMed.
- X. Kong, G. Liu and J. M. Curtis, Int. J. Adhes. Adhes., 2011, 31, 559–564 CrossRef CAS.
- M. Leszczyńska, E. Malewska, J. Ryszkowska, M. Kurańska, M. Gloc, M. K. Leszczyński and A. Prociak, Materials, 2021, 14, 1772 CrossRef PubMed.
- K. Mizera, J. Ryszkowska, M. Kurańska and A. Prociak, Polym. Bull., 2020, 77, 823–846 CrossRef CAS.
- X. Yang, C. Ren, X. Liu, P. Sun, X. Xu, H. Liu, M. Shen, S. Shang and Z. Song, Mater. Chem. Front., 2021, 5, 6160–6170 RSC.
- P. Naruebhorn, M. Seadan and S. Suttiruengwong, Suan Sunandha Science and Technology Journal, 2022, 9, 8–14 CrossRef.
- P. Helbling, F. Hermant, M. Pet, T. Tassain, T. Vidil and H. Cramail, Polym. Chem., 2023, 14, 500–513 RSC.
- A. R. Mahendran, N. Aust, G. Wuzella, U. Müller and A. Kandelbauer, J. Polym. Environ., 2012, 20, 926–931 CrossRef CAS.
- T. Wang, H. Deng, N. Li, F. Xie, H. Shi, M. Wu and C. Zhang, Green Chem., 2022, 24, 8355–8366 RSC.
- P. Zhang, G. Zhang, J. Pan, C. Ma and G. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 5998–6004 CrossRef CAS PubMed.
- C. Liu, J. Wu, X. Zhou, X. Zhou, Z. Wu and J. Qu, Prog. Org. Coat., 2022, 163, 106690 CAS.
- P. Boisaubert, N. Kébir, A. S. Schuller and F. Burel, Eur. Polym. J., 2020, 138, 109961 CrossRef CAS.
- G. Liu, G. Wu, J. Chen and Z. Kong, Prog. Org. Coat., 2016, 101, 461–467 CrossRef CAS.
- P. S. Choong, N. X. Chong, E. K. Wai Tam, A. M. Seayad, J. Seayad and S. Jana, ACS Macro Lett., 2021, 10, 635–641 CAS.
- X. Chen, A. Pizzi, E. Fredon, C. Gerardin, X. Zhou, B. Zhang and G. Du, Int. J. Adhes. Adhes., 2022, 112, 103001 CrossRef CAS.
- X. Xi, Z. Wu, A. Pizzi, C. Gerardin, H. Lei, B. Zhang and G. Du, Wood Sci. Technol., 2019, 53, 393–405 CrossRef CAS.
- A. Gomez-Lopez, B. Grignard, I. Calvo, C. Detrembleur and H. Sardon, Macromol. Rapid Commun., 2021, 42, 2000538 CrossRef CAS PubMed.
- X. Chen, A. Pizzi, X. Xi, X. Zhou, E. Fredon and C. Gerardin, J. Renewable Mater., 2021, 9, 1045–1057 Search PubMed.
- F. Monie, B. Grignard and C. Detrembleur, ACS Macro Lett., 2022, 11, 236–242 CrossRef CAS PubMed.
- X. Chen, J. Li, X. Xi, A. Pizzi, X. Zhou, E. Fredon, G. Du and C. Gerardin, Polym. Degrad. Stab., 2020, 175, 109121 CrossRef CAS.
- D. L. Smith, D. Rodriguez-Melendez, S. M. Cotton, Y. Quan, Q. Wang and J. C. Grunlan, Polymers, 2022, 14, 5019 CrossRef CAS PubMed.
- Y. Wang, Z. Zheng, J. L. Pathak, W. Feng, W. Wu, C. Yang, L. Wu and H. Zheng, Int. J. Bioprint., 2023, 9, 80–93 Search PubMed.
- D. Visser, H. Bakhshi, K. Rogg, E. Fuhrmann, F. Wieland, K. Schenke-Layland, W. Meyer and H. Hartmann, ACS Omega, 2022, 7, 39772–39781 CrossRef CAS PubMed.
- D. C. Aduba, K. Zhang, A. Kanitkar, J. M. Sirrine, S. S. Verbridge and T. E. Long, J. Appl. Polym. Sci., 2018, 135, 46464 CrossRef.
- Y. Yang, H. Cao, Y. Wang, J. Zhao, W. Ren, B. Wang, P. Qin, F. Chen, Y. Wang and D. Cai, Ind. Crops Prod., 2022, 186, 115224 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |