Open Access Article
Open Access ArticleA green bio-organic catalyst (taurine) promoted one-pot synthesis of (R/S)-2-thioxo-3,4-dihydropyrimidine(TDHPM)-5-carboxanilides: chiral investigations using circular dichroism and validation by computational approaches†
Mehul P. Parmar a,
Disha P. Vala
a,
Disha P. Vala a,
Savan S. Bhalodiya
a,
Savan S. Bhalodiya a,
Dipti B. Upadhyay
a,
Dipti B. Upadhyay a,
Chirag D. Patel
a,
Chirag D. Patel a,
Subham G. Patel
a,
Subham G. Patel a,
Srinivasa R. Gandholib,
Althaf H. Shaik
a,
Srinivasa R. Gandholib,
Althaf H. Shaik b,
Amy Dunne Miller
b,
Amy Dunne Miller c,
Joaquina Nogales
c,
Joaquina Nogales c,
Sourav Banerjee
c,
Sourav Banerjee c,
José M. Padrón
c,
José M. Padrón d,
Nasser Amri
d,
Nasser Amri e,
Nagesh Kumar Kandukuri
e,
Nagesh Kumar Kandukuri b and
Hitendra M. Patel
b and
Hitendra M. Patel *a
*a
aP. G. Department of Chemistry, Sardar Patel University, Near University Circle, Vallabh Vidyanagar, 388120, Gujarat, India. E-mail: hm_patel@spuvvn.edu; mpparmar1997@gmail.com; dishuahir1999@gmail.com; sa1bhalodiya@gmail.com; diptik705@gmail.com; chiragkumarp23@gmail.com; subhamp666@gmail.com
bYMC Application Lab, Plot No. 78/A/6, Phase VI, Industrial Park Jeedimetla, Gajularamaram Village, Quthbullapur, Medchal, Hyderabad-500055, Telangana, India. E-mail: vasuraj3@yahoo.com; althaf.2000@gmail.com; kknkumar@gmail.com
cDepartment of Cellular and Systems Medicine, School of Medicine, University of Dundee, Dundee, UK. E-mail: 140001249@dundee.ac.uk; jnogalesdiaz002@dundee.ac.uk; s.y.banerjee@dundee.ac.uk
dBioLab, Instituto Universitario de Bio-Orgánica Antonio González, Universidad de La Laguna, Avda. Astrofísico Francisco Sánchez 2, 38206 La Laguna, Spain. E-mail: jmpadron@ull.es
eDepartment of Chemistry, College of Science, Jazan University, P.O. Box 2097, Jazan 45142, Saudi Arabia. E-mail: namri@jazanu.edu.sa
First published on 19th March 2024
Abstract
Owing to the massive importance of dihydropyrimidine (DHPMs) scaffolds in the pharmaceutical industry and other areas, we developed an effective and sustainable one-pot reaction protocol for the synthesis of (R/S)-2-thioxo-DHPM-5-carboxanilides via the Biginelli-type cyclo-condensation reaction of aryl aldehydes, thiourea and various acetoacetanilide derivatives in ethanol at 100 °C. In this protocol, taurine was used as a green and reusable bio-organic catalyst. Twenty-three novel derivatives of (R/S)-TDHPM-5-carboxanilides and their structures were confirmed by various spectroscopy techniques. The aforementioned compounds were synthesized via the formation of one asymmetric centre, one new C–C bond, and two new C–N bonds in the final product. All the newly synthesized compounds were obtained in their racemic form with up to 99% yield. In addition, the separation of the racemic mixture of all the newly synthesized compounds was carried out by chiral HPLC (Prep LC), which provided up to 99.99% purity. The absolute configuration of all the enantiomerically pure isomers was determined using a circular dichroism study and validated by a computational approach. With up to 99% yield of 4d, this one-pot synthetic approach can also be useful for large-scale industrial production. One of the separated isomers (4R)-(+)-4S developed as a single crystal, and it was found that this crystal structure was orthorhombic.
1 Introduction
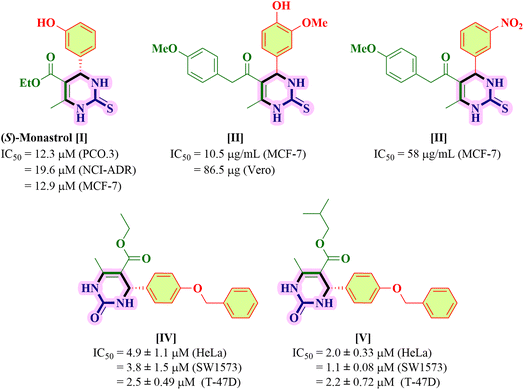
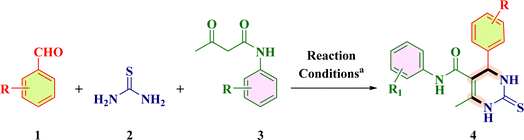
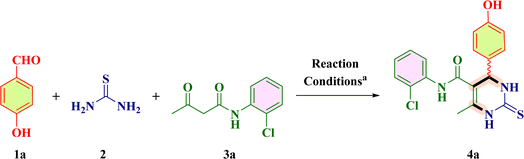
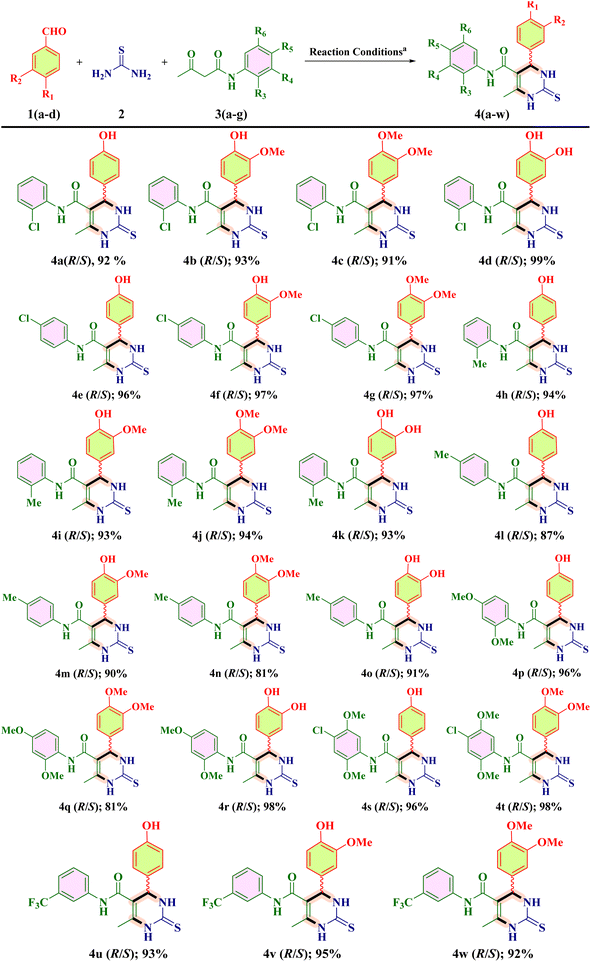
The current organic synthesis research is mainly focused on competency, creating synthetic libraries, and constitutional structural diversity.1,2 In this regard, one-pot multicomponent reactions (MCRs) constitute an incredible method for producing bioactive heterocycles because of their versatility, cost-effectiveness, sustainability, ability to minimize waste, as well as potential to simplify a variety of chemical processes.3–7 Heterocyclic compounds play a pivotal role in medicinal chemistry as well as in drug discovery and in the regulation of biological activities. Among these heterocycles, pyrimidine is the most studied ring system as it is present in vitamins, nucleic acids, drugs and chemotherapeutic agents.8 Comparing the two main structural isomers of pyrimidine (i.e., 3,4-dihydropyrimidine-2(1H)-one and 5,6-dihydropyrimidine-2(1H)-one), 3,4-DHPM has received tremendous attention. 3,4-DHPMs were first synthesized by Pietro Biginelli through a single-pot cyclo-condensation reaction of an aryl aldehyde, urea, and β-ketoester using an acid catalyst in ethanol at reflux temperature.9–11 The diversely substituted products obtained were correctly identified by Biginelli as 3,4-dihydro-2(1H)-pyrimidinones (DHPMs). Because of their wide range of pharmacological characteristics, 3,4-DHPM-5-carboxanilide scaffolds are effective chemical moieties that fascinate chemical innovations in both synthetic and medicinal context. The synthetic and medicinal uses of 3,4-DHPM-5-carboxanilide scaffolds are fascinating due to their wide range of pharmacological properties, such as anticancer,2 anti-inflammatory,12 antimicrobial and antimycobacterial,13 antibacterial,13 antiretroviral,14 anti-HIV-1,15 and anti-HSV16 effects. Some of the biologically active 2-thioxo/2-oxo-3,4-DHPM nucleus-bearing scaffolds such as monastrol17 (antitumoral), [II],1 [III],2 [IV] and [V]5 are shown in Fig. 1.Later on, the scope of this reaction was expanded by the variation in reagents and reaction conditions with the change in catalyst, reaction temperature, and reaction time that generated different multifunctionalized 2-thioxo-3,4-DHPM-5-carboxanilides (TDHPM-5-carboxanilides). In the past few decades, enormous attention has been paid to the synthesis of TDHPM-5-carboxanilides using a variety of catalysts such as zirconium(IV) tetrachloride,18 concentrated HCl,19–21 uranyl nitrate hexahydrate,22 and p-TSA.1 Table 1 describes the comparative study of the previously reported work towards the construction of (R/S)-TDHPM-5-carboxanilide derivatives 4 using the one-pot reaction of aryl aldehyde 1, thiourea 2, and acetoacetanilides 3. These previously reported reaction protocols have suffered from several shortcomings such as the use of harsh reaction conditions, potentially hazardous and corrosive catalysts, and a limited substrate scope with a lower yield as well as a longer reaction time. Some of these methods required the use of classical purification techniques such as column chromatography to obtain pure products. There are very few research studies which utilized taurine as a catalyst with a limited number of substrates. Moreover, the racemic mixture of all developed compounds has not been properly separated and characterized as its AC.23,24 According to the reported method,24 the synthesis of (R/S)-TDHPM-5-carboxanilides 4(a–w) has been carried out and all the parameters have been checked, which clearly indicate that it does not produce the claimed percentage yield within the given time periods, while the present protocol offers the corresponding (R/S)-TDHPM-5-carboxanilides in good to excellent yields in the shortest reaction time compared to the previously reported works. This reaction protocol provides the titled (R/S)-TDHPM-5-carboxanilides in pure form without any need for a classical purification technique. Furthermore, green metric calculations were also included for the present protocol. The reaction occurred in the presence of taurine as a green bioorganic catalyst in ethanol. Taurine (2-aminomethanesulphonic acid) is a key β-amino acid, which is found in large amounts in the living organisms, particularly in animals, and it also exists in a zwitterionic form, which enhances the reaction rate as well as leads to essential biological properties.25 It is an important part of the bile and it forms one-tenth per cent of the total human weight as well as also used in energy drinks and diet supplements, and possesses several biological properties in the human body. The easy availability in nature, good to excellent yields, eco-friendliness, commercial availability, cost-effectiveness as well as easy recyclability of taurine inspire us to use it for the studied transformation.
| Sr. no. | Reaction conditionsa | Yieldb (%) | Time (h) | References |
|---|---|---|---|---|
| a Reaction conditions: aryl aldehyde (1 mmol) 1, thiourea (1.5 mmol) 2, acetoacetanilides (1 mmol).b Isolated yield. | ||||
| 1 | Zirconium(IV)tetrachloride, ACN, reflux | 35–41 | 6–8 | 18 |
| 2 | Concentrated HCl, EtOH, reflux | 58–80 | 20–24 | 19–21 |
| 3 | UO2NO3·6H2O, ACN, reflux | 77–87 | 7–8 | 22 |
| 4 | p-TSA, EtOH, RT | 67–97 | 24–30 | 1 |
| 5 | Taurine, EtOH, 100 °C | 92 | 3 | Our work |
The construction and isolation of optically active/chiral molecules have received significant attention in various fields such as food products, functional and fragrant materials (ferroelectric liquid crystals and optically active non-linear molecules), agrochemicals and pharmaceutical fields. There are two different ways to obtain optically active compounds: the first called the asymmetric synthesis and the second the resolution of racemates into distinct enantiomers.26 It might occasionally be a challenge to acquire both the isomers by employing the first method. However, by implementing the second method, both isomers are obtained. There are different methods to resolve the racemic mixture, but the direct enantioseparation by utilizing chiral stationary phases (CSPs) in HPLC is a simple, practical, and more significantly used method from both analytical and preparative perspectives.27,28 In the past, to determine the absolute configuration (AC), generally single-crystal XRD, stereoselective synthesis, and NMR have been mostly used.29 These conventional approaches have several drawbacks including the need for high-quality crystals, chiral synthesis processes, and diastereomeric substances, respectively. However, the chiroptic spectroscopic techniques do away with the aforementioned requirements, and the analyses can be performed in either a native solution phase or a vapour phase. By relating their chirotopic properties, these empirical methods for finding the AC have attracted remarkable attention that involve the findings of the Cotton effect through the utilization of the octant rule based on ORD (optical rotary dispersion) via circular dichroism.30 Furthermore, by comparing the theoretical and calculated results of one of the enantiomerically pure isomer with the experimental results, the AC can be found through the use of chiroptical properties such as optical rotation/specific optical rotation (OR/SOR), electronic circular dichroism (ECD), and vibrational circular dichroism (VCD), as determined by DFT (density functional theory) calculations.31,32 Since ECD has been proved to be a valuable tool for stereochemical assignment, time-dependent DFT (TDDFT) is most frequently used to generate UV and ECD spectra. TDDFT has shown to be a highly effective technique for chirality assignment over a wide variety of organic scaffolds.33–36
As per the green chemistry perspectives, it is inevitable to alter the hazardous and volatile solvents for any organic transformations. Therefore, in the present work, we avoided the use of dichloromethane, 1,4-dioxane, N,N-dimethyl formamide, chloroform, carbon tetrachloride and diethyl ether, as they are potentially harmful in nature. Due to the magnificent versatility, here in the present article, we delineate a water-tolerant taurine as a green-bioorganic catalyst for the environmentally safe and cost-efficacious one-pot synthetic procedure of TDHPM-5-carboxanilides 4(a–w) in ethanol. In order to synthesize 4(a–w), we employed aryl aldehydes 1(a–d), thiourea 2 and diverse acetoacetanilides 3(a–g) as key precursors. We chose N-(2-chlorophenyl)-4(R/S)-(4-hydroxyphenyl)-6-methyl-2-thioxo-3,4-DHPM-5-carboxamide 4a as a representative and in ethanol, the implementation of taurine as a promoter enhanced the productivity of (R/S)-4a (92%). Furthermore, we successfully isolated all of the racemic mixtures 4(a–w) for the first time using Prep-LC and achieved up to 99.99% purity for pure (S)- and (R)-enantiomers, respectively. Thereafter, their absolute configuration was correctly identified using an experimental electronic circular dichroism (ECD) study and validated through utilization of a computational approach, i.e., TD-DFT calculation utilizing the B3LYP method with the 6-311G++(d,p) basis set. In addition, we developed a single crystal of one of the separated isomer, 4s, which has (R)-configuration. The main attraction of this protocol includes an economic and reusable bio-organic catalyst, low environmental factor values, a shorter reaction time, exquisite atom economy, a large substrate scope with good to excellent yields up to 99%, industrial scale application, and chiral resolution of all the synthesized 4(a–w), which gave up to 99.99% of purity.
2 Result and discussion
2.1 Chemistry
In order to build the sustainable approach for the titled derivatives, we first chose the one-pot three-component treatment of 4-hydroxybenzaldehyde 1a (1 mmol), thiourea 2 (1.5 mmol), and acetoacetanilide derivative 3a as a determinant reaction. In the beginning, we carried out the reaction using 15 mol% catalyst under solvent-free conditions. The results from TLC observation showed that the reaction did not initiate with taurine under solvent-free conditions (Table 2, Entry 1). Hence, we synthesized 4(a–w) using various preferred and useable solvents such as t-butyl alcohol, methanol, isopropyl alcohol, water, water–ethanol, acetone, ethanol, and acetonitrile and acetic acid respectively. Recommended solvents mean “solvents to be tested first in a screening exercise if, of course, there is no chemical incompatibility in the process condition”.37 At the beginning, the model reaction was performed using polar aprotic solvent acetonitrile and acetone (Table 4, Process D and H). The reaction was also carried out in polar protic solvents such as t-butyl alcohol, methanol, IPA, water, water–ethanol, and ethanol (Table 4, Process A–C, F, G–I). The greenness and comparability of these recommended solvents were expressed utilizing green metrics to yield 4(a–w).| Entry | Catalyst | Solvent | Temperature | Yieldb (%) | Time (h) | Conversation concerning aldehydec |
|---|---|---|---|---|---|---|
| a Reaction condition: 4-hydroxybenzaldehyde (1 mmol) 1a, Thiourea (1.5 mmol) 2, 2-chloro-acetoacetanilide (1 mmol) 3a, 15 mol% taurine, 4 mL EtOH.b Isolated yield.c Observed from TLC analysis. | ||||||
| 1 | 15 mol% taurine | — | 100 °C | 74 | 24 | Completed |
| 2 | 15 mol% p-TSA | EtOH | 100 °C | 52 | 8 | Mixture of products |
| 3 | 15 mol% AcOH | EtOH | 100 °C | 74 | 4 | Mixture of product |
| 4 | 15 mol% D-10 camphor sulphonic acid | EtOH | 100 °C | 90 | 4.5 | Complete |
| 5 | 10 mol% taurine | EtOH | 100 °C | 77 | 5 | Complete |
| 6 | 12 mol% taurine | EtOH | 100 °C | 81 | 4 | Complete |
| 7 | 14 mol% taurine | EtOH | 100 °C | 86 | 3.5 | Complete |
| 8 | 15 mol% taurine | EtOH | 100 °C | 92 | 3 | Complete |
| 9 | 15 mol% taurine | EtOH | 80 °C | 83 | 4 | Complete |
| 10 | 15 mol% taurine | EtOH | 90 °C | 87 | 3.5 | Complete |
| 11 | 18 mol% taurine | EtOH | 120 °C | 92 | 3 | Complete |
Thereafter, we further performed the model reaction by utilizing different organo-acid catalysts such as p-TSA, AcOH, D-10 camphor sulphonic acid, and taurine (Table 2, Entries 1–12), in which we found that the catalyst plays a dominant role in the accomplishment of the reaction. Among them, p-TSA- and acetic acid-catalyzed reactions yielded a mixture of products (Table 2, Entries 2 and 3), while the D-camphor sulphonic acid-catalyzed reaction gave complete conversion of aldehydes, but it took more time (Table 2, Entry 4). For the successful conversion of the reaction utilizing ethanol as the green reaction media, taurine was found to be an efficacious catalyst among all of the screening catalysts. We observed that during the reaction, a yellowish solid mass started to fall out within half an hour and then ethanol was added and the reaction was continued. After 3 h, product 4a fell out inside the reaction pot which made the workup procedure easy (Table 2, Entry 9). Thereafter, we often investigated the effect of the amount of catalysts on the isolated yield, which showed that the decrease in the amount of catalyst caused a decrease in the isolated yield of 4a, while compared to this, an increase in catalyst amount did not significantly affect the isolated yield of 4a (Table 2, Entries 5–9). After that, to reach the ideal temperature, we then conducted several different temperature tests (Table 2, Entries 9–12). The results from the temperature examination showed that by decreasing the temperature, the isolated yield of 4a decreased. In contrast, an increment in the temperature from 100 °C to 120 °C did not show any sensible change in the isolated yield of 4a. The TLC investigation indicated distinct spot formation. Therefore, in order to provide the most optimal conditions for the model reaction, we selected ethanol as the solvent system at 100 °C and 15 mol% taurine as the catalyst.
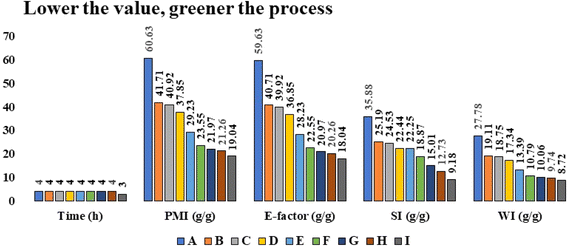
2.2 Evaluation of green chemistry metrics
The green metrics enable us to compare the environmental impact of each simulated approach. Thus, from the greenness and sustainability point of view, we assess the green metrics38–40 calculation by utilizing well-known parameters, namely, atom economy (AE), atom efficiency (AE), carbon efficiency (CE), reaction mass efficiency (RME), optimum efficiency (OE), mass productivity (MP), and mass intensity (MI) as described in Table 4 (Process A–H) and process mass intensity (PMI), E-factor, solvent and water intensity (SI & WI) as described in Table 3 (Process A–H). The lower values of the parameters included in Table 3 indicate the greenness of the process. It could be observed that the PMI, E-factor, SI, and WI of processes E–I are higher among the processes E–I. Among the two polar aprotic solvents (Table 3, Processes D & H), acetone was found to have a greener parameter as compared to acetonitrile. In the processes E & G involving polar protic solvents water and aqueous alcohol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the aqueous alcohol-mediated process appeared greener than the water-mediated process (Table 3, Processes E & G). Then we carried out the process with various polar protic alcohols and found that the long-chain alcohols produced 4a less efficiently than small and sterically less hindered alcohols (Table 3, Processes A–C & I). We observed that Process I (ethanol) showed the lowest values of PMI, E-factor, SI, and WI and was completed in a shorter reaction time than the remaining processes (Table 3). Thus, process I which proceeded in ethanol is found to be greener as than others.
1), the aqueous alcohol-mediated process appeared greener than the water-mediated process (Table 3, Processes E & G). Then we carried out the process with various polar protic alcohols and found that the long-chain alcohols produced 4a less efficiently than small and sterically less hindered alcohols (Table 3, Processes A–C & I). We observed that Process I (ethanol) showed the lowest values of PMI, E-factor, SI, and WI and was completed in a shorter reaction time than the remaining processes (Table 3). Thus, process I which proceeded in ethanol is found to be greener as than others.
| Process | Solvent | Time (h) | PMI (g g−1) | E-factor (g g−1) | SI (g g−1) | WI (g g−1) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 4-hydroxybenzaldehyde (1 mmol) 1a, thiourea (1.5 mmol) 2, and 2-chloro-acetoacetanilide (1 mmol) 3a, 15 mol% taurine, 100 °C. | ||||||
| A | t-Butyl alcohol | 4 | 60.63 | 59.63 | 35.88 | 27.78 |
| B | Methanol | 4 | 41.71 | 40.71 | 25.19 | 19.11 |
| C | Isopropyl alcohol | 4 | 40.92 | 39.92 | 24.53 | 18.75 |
| D | Acetonitrile | 4 | 37.85 | 36.85 | 22.44 | 17.34 |
| E | Water | 4 | 29.23 | 28.23 | 22.25 | 13.39 |
| F | Acetic acid | 4 | 23.55 | 22.55 | 18.87 | 10.79 |
| G | Water–ethanol | 4 | 21.97 | 20.97 | 15.01 | 10.06 |
| H | Acetone | 4 | 21.26 | 20.26 | 12.73 | 9.74 |
| I | Ethanol | 3 | 19.04 | 18.04 | 9.18 | 8.72 |
 |
||||||
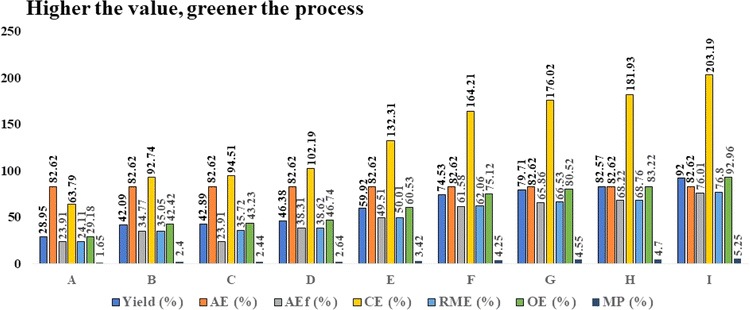
Followed by this, the green perspective of the ongoing protocol was explored in terms of AE, RME, OE, and CE.41 The higher value of these parameters as in Table 4 indicates the sustainability of the process. The calculated result from Table 4 (Processes A–I) indicates that in comparison to processes A–H, the ethanol-mediated process I exhibited the greatest values in percentage for the parameters, yield, AE, AEf, CE, RME, OE, and MP.
| Process | Solvent | Yieldb (%) | AE (%) | AEf (%) | CE (%) | RME (%) | OE (%) | MP (%) |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 4-hydroxybenzaldehyde (1 mmol) 1a, thiourea (1.5 mmol) 2, and 2-chloro-acetoacetanilide (1 mmol) 3a, 15 mol% taurine, 100 °C.b Isolated yield. | ||||||||
| A | t-Butyl alcohol | 28.95 | 82.62 | 23.91 | 63.79 | 24.11 | 29.18 | 1.65 |
| B | Methanol | 42.09 | 82.62 | 34.77 | 92.74 | 35.05 | 42.42 | 2.40 |
| C | Isopropyl alcohol | 42.89 | 82.62 | 23.91 | 94.51 | 35.72 | 43.23 | 2.44 |
| D | Acetonitrile | 46.38 | 82.62 | 38.31 | 102.19 | 38.62 | 46.74 | 2.64 |
| E | Water | 59.92 | 82.62 | 49.51 | 132.31 | 50.01 | 60.53 | 3.42 |
| F | Acetic acid | 74.53 | 82.62 | 61.58 | 164.21 | 62.06 | 75.12 | 4.25 |
| G | Water–ethanol | 79.71 | 82.62 | 65.86 | 176.02 | 66.53 | 80.52 | 4.55 |
| H | Acetone | 82.57 | 82.62 | 68.22 | 181.93 | 68.76 | 83.22 | 4.70 |
| I | Ethanol | 92 | 82.62 | 76.01 | 203.19 | 76.80 | 92.96 | 5.25 |
 |
||||||||
Following the computation of green chemistry metrics and the identification of optimal reaction conditions, we explored the substrate scope for the synthesis of TDHPM-5-carboxanilides 4(a–w), which involves one-pot three-component treatment of discrete aryl aldehydes 1(a–d), thiourea 2 and acetoacetanilides 3(a–g) (Table 5). Then we investigated the effect of various electron-donating and electron-withdrawing groups on the aryl ring of acetoacetanilides on the yield of 4(a–w). To our delight, the reaction goes fluently with different aryl aldehydes 1(a–d) bearing different electron-donating substituents only and acetoacetanilides 3(a–g) bearing various electron-donating and electron-withdrawing groups, furnishing good to excellent yields of 4(a–w) (Table 5). We also found that the presence of different ERGs on an aryl ring of aldehyde did show considerable effects on an isolated yield of 4(a–w). EWGs containing acetoacetanilides gave higher yields than that of ERGs. In addition, the position of either EWGs or ERGs on acetoacetanilides affects the product yield significantly. All the synthesized compounds were isolated from the reaction mixture by simple filtration in the pure form without requiring any classical purification technique. The highest yield of 99% and 98% were obtained for products 4d, 4r and 4t. All the newly synthesized (R/S)-TDHPM-5-carboxanilides 4(a–w) were characterized by various spectroscopy techniques (ESI file†).
2.3 Isolation of the racemic mixture
The chiral resolution of racemates of 4(a–w) was successfully carried out with polysaccharide-based CSPs under normal phase conditions. For sample preparation, a racemic mixture of the sample accurately weighing 0.5 mg mL−1 was dissolved in n-hexane/ethanol, and we observed that the product was not very soluble in it and precipitated in HPLC sample vials. Making a homogeneous solution is important for the accurate determination of enantiomeric ratios. To a vial containing sample, a minimal amount of DCM (30%) was added until the solution became homogeneous. Then ethanol (70%) was added. Thereafter, enantiomers of 4a were separated on YMC Chiral ART Cellulose-SZ (4.6 × 250 mm) column of 5 μm size, [cellulose-tris-(3-chloro-4-methylphenylcarbamate)] under normal phase conditions. Separations were conducted by isocratic elution with a solvent mixture of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 n-hexane
0.1 n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol
ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diethylamine under normal phase conditions. In run, 10 μL of the sample was injected and the flow rate was set at 1.0 mL min−1 with a detection wavelength of 215 nm. The column temperature was ambient. All the synthesized compounds are racemic mixtures having a percent enantiomeric ratio of 50/50 (R/S). In viewing the CD spectra, it is evident that for all compounds designated 4(a–w), the S-isomer always emerges as the first peak to elute, followed by the R-isomer. Fig. 2(a) shows the separation of the racemates of 4a(R/S) into two individual isomers R and S with a retention time of 7.37 min and 11.18 minutes, respectively. Moreover, Fig. 2(b) and (c) show the separated (S) and (R) isomers of 4a with a purity of 99.78% and 99.58%, respectively.
diethylamine under normal phase conditions. In run, 10 μL of the sample was injected and the flow rate was set at 1.0 mL min−1 with a detection wavelength of 215 nm. The column temperature was ambient. All the synthesized compounds are racemic mixtures having a percent enantiomeric ratio of 50/50 (R/S). In viewing the CD spectra, it is evident that for all compounds designated 4(a–w), the S-isomer always emerges as the first peak to elute, followed by the R-isomer. Fig. 2(a) shows the separation of the racemates of 4a(R/S) into two individual isomers R and S with a retention time of 7.37 min and 11.18 minutes, respectively. Moreover, Fig. 2(b) and (c) show the separated (S) and (R) isomers of 4a with a purity of 99.78% and 99.58%, respectively.
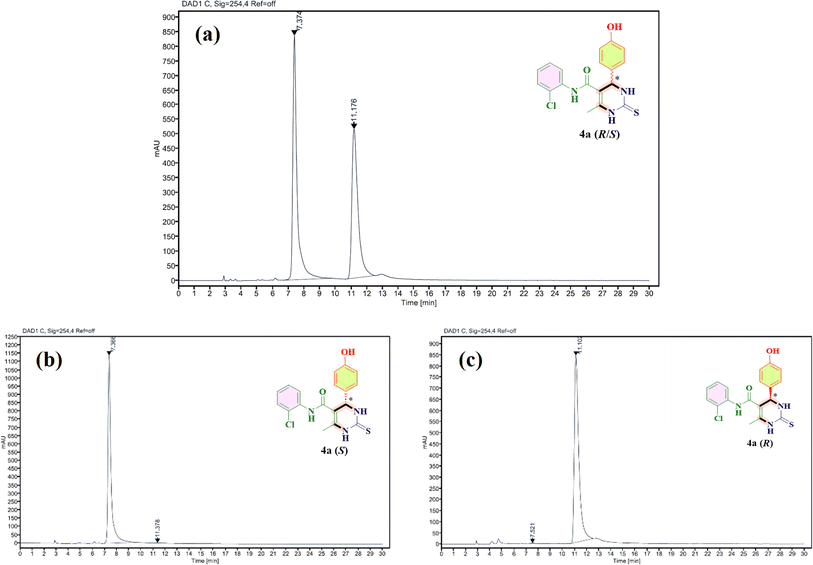 | ||
| Fig. 2 Chromatogram shows the separation of racemate of 4a (a) in its two enantiomers (b) and (c) using the YMC Chiral ART Cellulose-SZ column. | ||
Similarly, we separated the remaining 22 racemates, 4(b–w) into enantiomerically pure (S)- and (R)-isomers. The chromatograms of all the remaining racemates 4(b–w) are shown in Fig. S46 to S67 (ESI file).† Table 6 displays the percentage purity of each identified enantiomer.
| Entry | % purity | Entry | % purity | ||
|---|---|---|---|---|---|
| R | S | R | S | ||
| 4a | 97.98 | 99.63 | 4m | 98.30 | 99.90 |
| 4b | 98.04 | 99.52 | 4n | 98.18 | 99.83 |
| 4c | 99.81 | 99.96 | 4o | 99.69 | 99.99 |
| 4d | 99.58 | 99.78 | 4p | 99.10 | 99.99 |
| 4e | 95.20 | 99.72 | 4q | 99.80 | 99.95 |
| 4f | 91.92 | 99.17 | 4r | 99.47 | 99.90 |
| 4g | 99.67 | 99.94 | 4s | 99.71 | 99.82 |
| 4h | 96.59 | 99.70 | 4t | 99.30 | 99.57 |
| 4i | 96.83 | 98.62 | 4u | 95.51 | 93.72 |
| 4j | 98.76 | 98.85 | 4v | 80.33 | 94.17 |
| 4k | 98.44 | 93.85 | 4w | 93.49 | 93.37 |
| 4l | 99.17 | 99.92 | |||
2.4 Identification of the absolute configuration
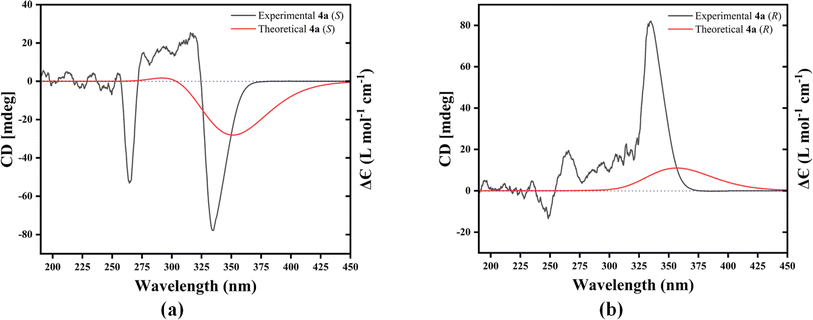
The most crucial task after resolving the racemic mixture is to find out the absolute configuration (AC) of the enantiomerically pure isomer through their chirotopic properties. To assign the AC of the enantiomerically pure isomers of 4a, we compared the theoretical/calculated and experimental/observed ECD spectra (Fig. 3(a) and (b)). The experimental ECD spectra of enantiopure isomers of 4a were obtained according to the procedure mentioned in Section 2.3 (ESI file†). The TDDFT calculations were performed with the B3LYP/6-311++G(d,p) level of computations in order to generate the theoretical/calculated ECD spectra of each of the resolved isomers. Fig. 3(a) and (b) demonstrate that the experimental (Black) and theoretical (Red) ECD spectra of the enantiomerically pure isomers recorded in DMSO closely match one another. These results from both the experimental and theoretical ECD spectra validate and confirm that the (−)- and (+)- enantiomers of 4a have 4S and 4R absolute configurations, respectively. In a similar manner, we discovered the ACs of every resolved enantiomerically pure molecule 4(b–w), as illustrated in Fig. S68 to S89 (ESI file).†2.5 Crystal data and structure refinement for 4s
To develop a single-crystal compound, (4R)-(+)-4s, 50 mg was dissolved in 60 mL of ethyl acetate: methanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solution and heated until (4R)-(+)-4s dissolved completely and the solution was reduced to half (30 mL), followed by the addition of 50 mg of activated charcoal to remove coloured impurities from the compound. After filtering out the content, the charcoal treatment solution was kept in a clean beaker covered with aluminium foil for a few days. When ethyl acetate and methanol were evaporated, a single crystal of compound (4R)-(+)-4s formed over the course of 10–15 days. It evolved into a yellow rectangular shape with approximate dimensions of 0.330 mm × 0.250 mm × 0.130 mm.
3) solution and heated until (4R)-(+)-4s dissolved completely and the solution was reduced to half (30 mL), followed by the addition of 50 mg of activated charcoal to remove coloured impurities from the compound. After filtering out the content, the charcoal treatment solution was kept in a clean beaker covered with aluminium foil for a few days. When ethyl acetate and methanol were evaporated, a single crystal of compound (4R)-(+)-4s formed over the course of 10–15 days. It evolved into a yellow rectangular shape with approximate dimensions of 0.330 mm × 0.250 mm × 0.130 mm.
Compound (4R)-(+)-4s crystallises (Fig. 4) with the orthorhombic crystal system, space group P21212 (http://it.iucr.org/cgi-bin/gotosgtable.pl?number=18%26tabletype=S), and unit cell parameter: a = 14.6984(8) Å, b = 17.4270(8) Å, c = 19.6342(10) Å, α = 90°, β = 90°, γ = 90° and Z = 8. The density calculated is 1.379 g cm−3. The single crystal image, ORTEP diagram, unit cell, and packing configurations are shown in Fig. 4, and other observed data for the single crystal investigation including crystal data and structure refinement for compound (4R)-(+)-4s (CCDC 2324474) are provided in Table 7.
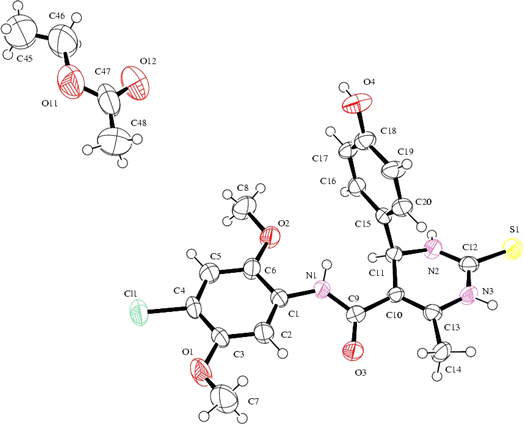 | ||
| Fig. 4 Molecular structure of compound (4R)-(+)-4s, showing the atom-labelling scheme and displacement ellipsoids at a 40% probability level. | ||
| Crystal description | yellow |
| Crystal size | 0.330 × 0.250 × 0.130 mm |
| Empirical formula | C24H28ClN3O6S |
| Formula weight | 522.00 |
| Radiation, wavelength | Mo Kα, 0.71073 Å |
| Unit cell dimension | a = 14.6984(8) Å α = 90° |
| b = 17.4270(8) Å β = 90° | |
| c = 19.6342(10) Å γ = 90° | |
| Crystal system | Orthorhombic |
| Space group | P21212 (http://it.iucr.org/cgi-bin/gotosgtable.pl?number=18%26tabletype=S) |
| Unit cell volume | 5029.28 Å3 |
| No. of molecule per unit cell, Z | 8 |
| Temperature | 296(2) K |
| Absorption coefficient | 0.280 mm−1 |
| F(000) | 2192 |
| Scan mode | Multi-scan |
| θ range for the entire data collection | 2.38 to 21.36° |
| Density (calculated) | 1.379 |
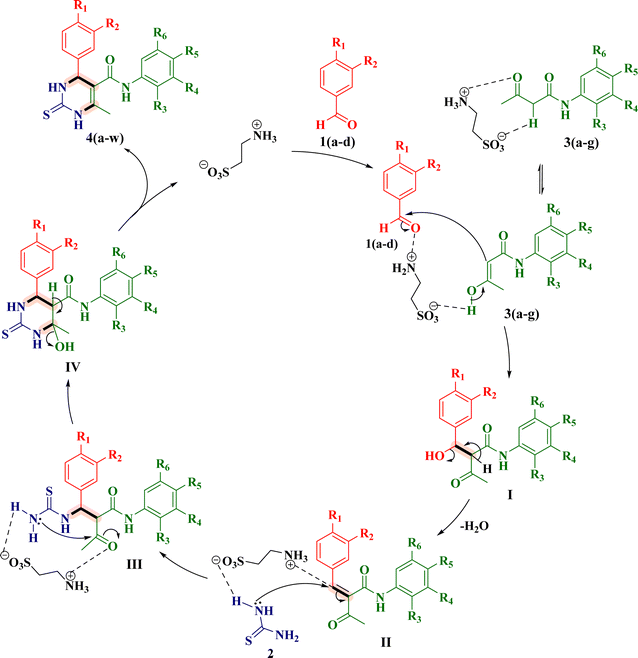
O. Kappe42 provided mechanistic investigations of the Biginelli reaction. On the basis of that, a plausible reaction mechanism for the taurine-promoted synthesis of (R/S)-TDHPM-5-carboxanilides 4(a–w) is depicted in Fig. 5.23 As taurine exists in its more stable zwitterionic form initially, it can simultaneously activate the carbonyl group of an aryl aldehyde 1(a–d) and the active methylene (–CH2–) group of acetoacetanilides 3(a–g). This makes the Knoevenagel condensation reaction easier, where activated 3(a–g) attacks the activated carbonyl group of 1(a–d) to generate I. Now, the removal of water molecules from I forms the Knoevenagel adduct II. Here, taurine can further activate thiourea 2 which attacks on benzylidene carbon of adduct II, which forms intermediate III. Here, taurine can further activate the N-atom of thiourea and the ketocarbonyl carbon in intermediate III followed by the Michael addition to obtain the intermediate IV. Afterwards, the removal of water molecules from the intermediate IV gives the final product 4(a–w) and the catalyst was recovered. As taurine is a metal-free bio-organic catalyst, its separation procedure is quite simple and environmentally friendly. It should be noted that taurine has a number of notable benefits over other homogeneous Lewis acid and organo-acid catalysts that have been identified. According to the literature, when O. Kappe had treated aldehyde, urea and ethyl acetoacetate, he obtained the desired dihydropyrimidine-2-one. Moreover, when he treated aldehyde, thiourea and ethyl acetoacetate, he obtained 2-amino-1,3-thiazine and not the desired dihydropyrimidine-2-thione. However, in our case, when we treated aldehyde, acetoacetanilide and thiourea, we obtained the desired dihydropyrimidine-2-thione instead of 2-amino-1,3-thiazine. We confirmed the formation of the desired DHPM-2-thione by utilizing the NMR spectra of each of the synthesized compounds 4(a–w) [see ESI†]. For Kappe's product, 1H NMR = 7.13 ppm (NH2), 13.38 ppm (NH–Me) and 2.81 (CH3–N); 13C NMR = 159.3 ppm [N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S in ring (compound 10a, R
C–S in ring (compound 10a, R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H)] and 166.5 ppm [N
H)] and 166.5 ppm [N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S in ring (compound 10b, R
C–S in ring (compound 10b, R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3)]. However, in our case, 1H NMR peaks appeared at 9.38 (=C–NH–C
CH3)]. However, in our case, 1H NMR peaks appeared at 9.38 (=C–NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) and 9.31 (chiral C–NH–C
S) and 9.31 (chiral C–NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), in which both N-atoms were present in the ring, and 13C NMR peaks appeared at 174.03 ppm (NH–C(=S)–NH) in which S is not a part of the ring instead of Kappe's compounds 10a and 10b. Furthermore, one of the separated isomer (4R)-(+)-4S developed as a single crystal, and it was found that this crystal structure was orthorhombic. This confirms that, our reaction proceeded through the plausible mechanism, as illustrated in Fig. 5.
S), in which both N-atoms were present in the ring, and 13C NMR peaks appeared at 174.03 ppm (NH–C(=S)–NH) in which S is not a part of the ring instead of Kappe's compounds 10a and 10b. Furthermore, one of the separated isomer (4R)-(+)-4S developed as a single crystal, and it was found that this crystal structure was orthorhombic. This confirms that, our reaction proceeded through the plausible mechanism, as illustrated in Fig. 5.
2.6 Recycling procedure for taurine
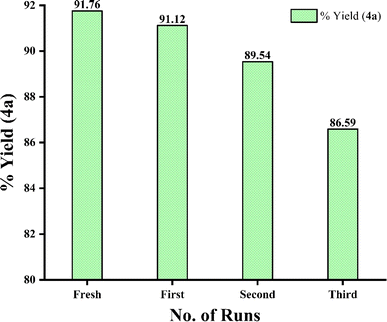
Once the reaction was completed (as indicated by TLC), water was added to the crude reaction mixture for the purpose of full solidification of the product 4a and the mixture was stirred and then filtered. Taurine was then recycled from the filtrate by loading the filtrate in a rotary evaporator under vacuum at 65 °C. As the solvent was evaporated from the filtrate, absolute alcohol was added to it in which taurine remains insoluble and the remaining impurities also get solubilised in it. Thereafter, it was again filtered to attain pure taurine. Considering the fresh run, the recovered catalyst was reused for up to four consecutive runs without measurable change in the isolated yield of 4a (Fig. 6).3 Conclusion
In summary, we effectively outlined a simple, eco-competent and environmentally friendly one-pot procedure for the synthesis of novel derivatives of (R/S)-TDHPM-5-carboxanilide scaffolds. This is achieved by treating aryl aldehydes with thiourea and acetoacetanilides using taurine as a green bioorganic catalyst in ethanol as a reaction medium. All the synthesized compounds were obtained with up to 99% yield. From the industrial application point of view, we have also performed a gram-scale procedure, which gives a yield of 99% (4d). The shorter reaction time, low catalyst loading, mild reaction conditions, higher atom economy, use of reusable bio-organic catalysts, good to excellent yields, and a large substrate scope are some of the vital benefits of the protocol presented here. All the newly synthesized (R/S)-TDHPM-5-carboxanilides were well characterized by various spectroscopy techniques. After synthesizing the aforementioned scaffolds, all the racemates 4(a–w) were isolated into their individual isomers using a Prep-LC column at room temperature on CSPs. Cellulose-tris-(3-chloro-4-methylphenylcarbamate), cellulose-tris-(3,5-dichlorophenylcarbamate), and cellulose-tris(3,5-dimethyl-phenylcarbamate) are the various CSPs employed under normal phase conditions. The purity of the resolved isomers is up to 99.99%. Thereafter, the ACs of all the isolated enantiopure compounds were assigned by analyzing and comparing the theoretical and experimental ECD spectra. Practical ECD spectra were recorded using a circular dichroism spectrometer, while theoretical ECD spectra were recorded with TDDFT calculations using the B3LYP method with the 6-311++G(d,p) basis set. It is anticipated that, with the improvement in computational technologies, ECD calculations will evolve into a routine tool for the assignment of AC. Furthermore, we have also developed a single crystal of one of the separated isomer 4s, which has an orthorhombic crystal structure and a (R)-configuration. An anti-proliferative study of all the enantiopure isomers of the title scaffold on six solid tumor cell lines is under investigation.Author contributions
Mehul P. Parmar: method development, investigation, validation, spectral analysis, writing original draft, writing – review and editing, data analysis, software visualization; Disha P. Vala: writing – review and editing, formal analysis, software visualization; Savan S. Bhalodiya: writing – review and editing, spectral analysis; Dipti B. Upadhyay: writing – review and editing, spectral analysis, software visualization; Chirag D. Patel: review and editing, spectral analysis; Subham G. Patel: software visualization; Srinivasa R. Gandholi: chiral separation; Althaf H. Shaik: chiral separation; Amy Dunne Miller: biological activity; Joaquina Nogales: biological activity; Sourav Banerjee: biological activity; José M. Padrón: biological activity; Nasser Amri: review and editing; Nagesh Kumar Kandukuri: writing – review and editing, chiral separation; Hitendra M. Patel: conceptualization, data analysis, writing – review and editing, supervision.Conflicts of interest
The authors declare no conflict of interest.Abbreviations
| DHPM | Dihydropyrimidines |
| TDHPM | 2-Thioxo-3,4-Dihydropyrimidine |
| THPM | Tetrahydropyrimidine |
| HPLC | High-Performance Liquid Chromatography |
| LC | Liquid Chromatography |
| AC | Absolute Configuration |
| CSPs | Chiral Stationary Phases |
| NMR | Nuclear Magnetic Resonance |
| HRMS | High Resolution Mass Spectroscopy |
| EWG | Electron Withdrawing Group |
| ERG | Electron Releasing Group |
| TLC | Thin Layer Chromatography |
| CD | Circular Dichroism |
| ECD | Electronic Circular Dichroism |
| VCD | Vibrational Circular Dichroism |
| ORD | Optical Rotary Dispersion |
| OR | Optical Rotation |
| SOR | Specific Optical Rotation |
| DFT | Density Functional Theory |
| TDDFT | Time-Dependent Density Functional Theory |
| AE | Atom Economy |
| AEf | Atom Efficiency |
| CE | Carbon Efficiency |
| RME | Reaction Mass Efficiency |
| OE | Optimum Efficiency |
| MP | Mass Productivity |
| MI | Mass Intensity |
| SI | Solvent Intensity |
| WI | Water Intensity |
| DMSO | Dimethyl Sulphoxide |
Acknowledgements
All the authors are thankful to the Department of Chemistry, Sardar Patel University, Vallabh Vidyanagar for providing us necessary research facilities and computational studies. MPP, DPV, DBU are thankful to the Knowledge Consortium of Gujarat for SHODH fellowship (Student Reference No. 2021016434, 2021016429, 2021016413, respectively). SSB is thankful to the CSIR-NET fellowship (Reference No. Nov/06/2020(i)EU-V). CDP is thankful to National fellowship for scheduled Tribe (Award No. 202223-NFST-GUJ-00003). SGP is grateful to the UGC, New Delhi for a UGC-JRF (NTA Ref. No. 201610157514; dated: 01.04.2021). SB is funded by a United Kingdom Research and Innovation Future Leaders Fellowship (MR/W008114/1). We would like to extend our heartfelt gratitude to the YMC India Lab for their invaluable support in the development of chiral methods for our samples and the isolation of isomers through prep-LC. Their expertise and assistance were instrumental in the successful completion of this research.References
- N. L. Naishima, S. Faizan, R. M. Raju, A. S. V. L. Sruthi, V. Ng, G. K. Sharma, K. S. Vasanth, V. K. Shivaraju, R. Ramu and B. R. P. Kumar, Design, Synthesis, Analysis, Evaluation of Cytotoxicity Against MCF-7 Breast Cancer Cells, 3D QSAR Studies and EGFR, HER2 Inhibition Studies on Novel Biginelli 1,4-Dihydropyrimidines, J. Mol. Struct., 2023, 1277, 134848 CrossRef CAS.
- B. R. Prashantha Kumar, G. Sankar, R. B. Nasir Baig and S. Chandrashekaran, Novel Biginelli dihydropyrimidines with potential anticancer activity: A parallel synthesis and CoMSIA study, Eur. J. Med. Chem., 2009, 44(10), 4192–4198 CrossRef CAS PubMed.
- D. M. Patel, H. J. Patel, J. M. Padrón and H. M. Patel, A novel substrate directed multicomponent reaction for the syntheses of tetrahydro-spiro [pyrazolo [4, 3-f] quinoline]-8, 5′-pyrimidines and tetrahydro-pyrazolo [4, 3-f] pyrimido [4, 5-b] quinolines via selective multiple C–C bond formation under metal-free conditions, RSC Adv., 2020, 10(33), 19600–19609 RSC.
- M. G. Sharma, R. M. Vala and H. M. Patel, Pyridine-2-carboxylic acid as an effectual catalyst for rapid multi-component synthesis of pyrazolo [3, 4-b] quinolinones, RSC Adv., 2020, 10(58), 35499–35504 RSC.
- R. M. Vala, M. G. Sharma, D. M. Patel, A. Puerta, J. M. Padrón, V. Ramkumar, R. L. Gardas and H. M. Patel, Synthesis and in vitro study of antiproliferative benzyloxy dihydropyrimidinones, Arch. Pharm., 2021, 354(6), 2000466 CrossRef CAS PubMed.
- D. P. Vala, R. M. Vala and H. M. Patel, Versatile Synthetic Platform for 1,2,3-Triazole Chemistry, ACS Omega, 2022, 7(42), 36945–36987 CrossRef CAS PubMed.
- S. S. Bhalodiya, M. P. Parmar, D. B. Upadhyay, C. D. Patel, D. P. Vala, D. Rajani and H. M. Patel, Cs2CO3-promoted one-pot synthesis of novel tetrahydrobenzofuran-4(2H)-ones: In vitro antimicrobial, antimalarial activity and in silico docking study, Results Chem., 2024, 7, 101304 CrossRef CAS.
- S. Manimekalai, K. Radhakrishnan, P. Mohandass and S. C. Mohan, Antimicrobial Activity of 6-Aryl-4-methyl-2-oxo-1, 2, 3, 6-tetrahydropyrimidine-5-(N-aryl) carboxamides: A Structure-Reactivity Study, Int. J. Pharm., Chem. Biol. Sci., 2017, 7(4), 357–363 CAS.
- O. Muñoz-Muñiz and E. Juaristi, An enantioselective approach to the Biginelli dihydropyrimidinone condensation reaction using CeCl3 and InCl3 in the presence of chiral ligands, Arkivoc, 2003, 11, 16–26 Search PubMed.
- M. G. Sharma, J. Pandya, D. M. Patel, R. M. Vala, V. Ramkumar, R. Subramanian, V. K. Gupta, R. L. Gardas, A. Dhanasekaran and H. M. Patel, One-Pot Assembly for Synthesis of 1,4-Dihydropyridine Scaffold and Their Biological Applications, Polycyclic Aromat. Compd., 2021, 41(7), 1495–1505 Search PubMed.
- M. G. Sharma, R. M. Vala, D. P. Rajani, V. Ramkumar, R. L. Gardas, S. Banerjee and H. M. Patel, Crystal structure, antibacterial and antifungal evaluation of 5-bromothiophene based 3,4-dihydropyrimidin-2-(1H)-(thi)ones, Phosphorus, Sulfur, Silicon Relat. Elem., 2023, 198(2), 145–153 CrossRef CAS.
- R. L. Sawant, C. A. Bansode and J. B. Wadekar, In vitro anti-inflammatory potential and QSAR analysis of oxazolo/thiazolo pyrimidine derivatives, Med. Chem. Res., 2013, 22(4), 1884–1892 CrossRef CAS.
- P. K. Chaudhari, Synthesis and Biological Studies of 1, 2, 3, 4-Tetrahydro-6-Methyl-2-Oxopyrimidine-5-Carboxamide Derivative, Int. J. Pharma Bio Sci., 2012, 3(2), 333–341 CAS.
- R. Zabihollahi, R. Vahabpour, C. Hartoonian, B. Sedaghati, S. Sadat, M. Soleymani, M. Ranjbar, A. Fassihi, M. Aghasadeghi and H. Memarian, Evaluation of the in vitro antiretroviral potential of some Biginelli-type pyrimidines, Acta Virol., 2012, 56(1), 11 CrossRef CAS PubMed.
- S. Sepehri, S. Gharagani, L. Saghaie, M. R. Aghasadeghi and A. Fassihi, QSAR and docking studies of some 1,2,3,4-tetrahydropyrimidines: evaluation of gp41 as possible target for anti-HIV-1 activity, Med. Chem. Res., 2015, 24(4), 1707–1724 CrossRef CAS.
- R. Zabihollahi, A. Fassihi, M. R. Aghasadeghi, H. R. Memarian, M. Soleimani and K. Majidzadeh-A, Inhibitory effect and structure–activity relationship of some Biginelli-type pyrimidines against HSV-1, Med. Chem. Res., 2013, 22(3), 1270–1276 CrossRef CAS.
- T. U. Mayer, T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber and T. J. Mitchison, Small Molecule Inhibitor of Mitotic Spindle Bipolarity Identified in a Phenotype-Based Screen, Science, 1999, 286(5441), 971–974 CrossRef CAS PubMed.
- M. Soleymani and H. R. Memarian, Synthesis of 3,4-Dihydropyrimidin-2(1H)-one-5-carboxamides, Z. Naturforsch. B, 2010, 65(4), 485–492 CrossRef CAS.
- P. K. Chaudhari, Synthesis and Biological Studies of 1, 2, 3, 4-Tetrahydro-6-Methyl-2-Oxopyrimidine-5-Carboxamide Derivative, Int. J. Pharma Bio Sci., 2012, 3(2), 333–341 CAS.
- C. C. Rathod and M. J. Solanki, Studies, Synthesis and Antimicrobial Activity of 6-Methyl-4-(3-Phenyl-Pyridine-4-Yl-Methanone-1-H-Pyrazoyl)-2-Thio-N-Substituted Phenyl Pyrimide-5-Carboxamide, Int. j. sci. res. chem., 2018, 3(2), 28–36 Search PubMed.
- J. N. Lalpara, S. D. Hadiyal, A. J. Radia, J. M. Dhalani and G. G. Dubal, Design and Rapid Microwave Irradiated One-Pot Synthesis of Tetrahydropyrimidine Derivatives and Their Screening In Vitro Antidiabetic Activity, Polycyclic Aromat. Compd., 2022, 42(6), 3063–3078 CrossRef CAS.
- K. Venkatesan, V. Satyanarayana and A. Sivakumar, Synthesis of pyrimidine carboxamide derivatives catalyzed by uranyl nitrate hexa Hydrate with their antibacterial and antioxidant studies, Bull. Chem. Soc. Ethiop., 2016, 30(1), 119–128 CrossRef CAS.
- N. Daneshvar, F. Shirini, M. S. N. Langarudi and R. Karimi-Chayjani, Taurine as a green bio-organic catalyst for the preparation of bio-active barbituric and thiobarbituric acid derivatives in water media, Bioorg. Chem., 2018, 77, 68–73 CrossRef CAS.
- M. Abd El Aleem Ali Ali El-Remaily and O. M. Elhady, Green Bio-organic and Recoverable Catalyst Taurine (2-aminoethanesulfonic acid) for Synthesis of Bio-active Compounds 3,4-Dihydropyrimidin Derivatives in Aqueous Medium, ChemistrySelect, 2020, 5(39), 12098–12102 CrossRef CAS.
- M. P. Parmar, R. M. Vala and H. M. Patel, Importance of Hybrid Catalysts toward the Synthesis of 5H-Pyrano[2,3-d]pyrimidine-2-ones/2,4-diones (Thiones), ACS Omega, 2023, 8(2), 1759–1816 CrossRef CAS PubMed.
- Y. Okamoto and T. Ikai, Chiral HPLC for efficient resolution of enantiomers, Chem. Soc. Rev., 2008, 37(12), 2593–2608 RSC.
- G. Subramanian, Chiral Separation Techniques: A Practical Approach, John Wiley & Sons, 2008 Search PubMed.
- A. Dondoni, A. Massi and S. Sabbatini, Improved synthesis and preparative scale resolution of racemic monastrol, Tetrahedron Lett., 2002, 43(34), 5913–5916 CrossRef CAS.
- P. L. Polavarapu, Determination of the absolute configurations of chiral drugs using chiroptical spectroscopy, Molecules, 2016, 21(8), 1056 CrossRef PubMed.
- Ö. V. Rúnarsson, C. Benkhäuser, N. J. Christensen, J. A. Ruiz, E. Ascic, M. Harmata, V. Snieckus, K. Rissanen, P. Fristrup, A. Lützen and K. Wärnmark, Resolution and Determination of the Absolute Configuration of a Twisted Bis-Lactam Analogue of Tröger’s Base: A Comparative Spectroscopic and Computational Study, J. Org. Chem., 2015, 80(16), 8142–8149 CrossRef PubMed.
- P. L. Polavarapu, Optical rotation: Recent advances in determining the absolute configuration, Chirality, 2002, 14(10), 768–781 CrossRef CAS PubMed.
- C. Diedrich and S. Grimme, Systematic Investigation of Modern Quantum Chemical Methods to Predict Electronic Circular Dichroism Spectra, J. Phys. Chem. A, 2003, 107(14), 2524–2539 CrossRef CAS.
- X.-C. Li, D. Ferreira and Y. Ding, Determination of absolute configuration of natural products: theoretical calculation of electronic circular dichroism as a tool, Curr. Org. Chem., 2010, 14(16), 1678–1697 CrossRef CAS PubMed.
- T. D. Crawford, M. C. Tam and M. L. Abrams, The Current State of Ab Initio Calculations of Optical Rotation and Electronic Circular Dichroism Spectra, J. Phys. Chem. A, 2007, 111(48), 12057–12068 CrossRef CAS PubMed.
- S.-D. Zhao, L. Shen, D.-Q. Luo and H.-J. Zhu, Progression of absolute configuration determination in natural product chemistry using optical rotation (dispersion), matrix determinant and electronic circular dichroism methods, Curr. Org. Chem., 2011, 15(11), 1843–1862 CrossRef CAS.
- A. Wakai, H. Fukasawa, C. Yang, T. Mori and Y. Inoue, Theoretical and Experimental Investigations of Circular Dichroism and Absolute Configuration Determination of Chiral Anthracene Photodimers, J. Am. Chem. Soc., 2012, 134(10), 4990–4997 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 selection guide of classical-and less classical-solvents, Green Chem., 2016, 18(1), 288–296 RSC.
- D. B. Upadhyay, R. M. Vala, S. G. Patel, P. J. Patel, C. Chi and H. M. Patel, Water mediated TBAB catalyzed synthesis of spiro-indoline-pyrano[3,2-c]quinolines as α-amylase inhibitor and in silico studies, J. Mol. Struct., 2023, 1273, 134305 CrossRef CAS.
- P. J. Patel, D. M. Patel, R. M. Vala, S. G. Patel, D. B. Upadhyay, Y. Pannerselvam and H. M. Patel, Catalyst-Free, Room-Temperature Accessible Regioselective Synthesis of Spiroquinolines and Their Antioxidant Study, ACS Omega, 2023, 8(1), 444–456 CrossRef CAS PubMed.
- S. G. Patel, A. González-Bakker, R. M. Vala, P. J. Patel, A. Puerta, A. Malik, R. K. Sharma, J. M. Padrón and H. M. Patel, Microwave-assisted multicomponent synthesis of antiproliferative 2, 4-dimethoxy-tetrahydropyrimido [4, 5-b] quinolin-6 (7 H)-ones, RSC Adv., 2022, 12(47), 30404–30415 RSC.
- S. Abou-Shehada, P. Mampuys, B. Maes, J. Clark and L. Summerton, An evaluation of credentials of a multicomponent reaction for the synthesis of isothioureas through the use of a holistic CHEM21 green metrics toolkit, Green Chem., 2017, 19(1), 249–258 RSC.
- C. O. Kappe, A Reexamination of the Mechanism of the Biginelli Dihydropyrimidine Synthesis. Support for an N-Acyliminium Ion Intermediate1, J. Org. Chem., 1997, 62(21), 7201–7204 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2324474. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra01391c |
| This journal is © The Royal Society of Chemistry 2024 |