Open Access Article
Open Access ArticleAdvances in boron nitride-based nanomaterials for environmental remediation and water splitting: a review
Vishal Gadore,
Soumya Ranjan Mishra,
Ashish Kumar Singh and
Md. Ahmaruzzaman *
*
Department of Chemistry, National Institute of Technology Silchar, 788010, Assam, India. E-mail: mda2002@gmail.com
First published on 22nd January 2024
Abstract
Boron nitride has gained wide-spread attention globally owing to its outstanding characteristics, such as a large surface area, high thermal resistivity, great mechanical strength, low density, and corrosion resistance. This review compiles state-of-the-art synthesis techniques, including mechanical exfoliation, chemical exfoliation, chemical vapour deposition (CVD), and green synthesis for the fabrication of hexagonal boron nitride and its composites, their structural and chemical properties, and their applications in hydrogen production and environmental remediation. Additionally, the adsorptive and photocatalytic properties of boron nitride-based nanocomposites for the removal of heavy metals, dyes, and pharmaceuticals from contaminated waters are discussed. Lastly, the scope of future research, including the facile synthesis and large-scale applicability of boron nitride-based nanomaterials for wastewater treatment, is presented. This review is expected to deliver preliminary knowledge of the present state and properties of boron nitride-based nanomaterials, encouraging the future study and development of these materials for their applications in various fields.
1. Introduction
In today's world, boron nitride (BN) nanomaterials are the most popular, promising, and effective nanomaterials. BN is a compound with equal numbers of alternately linked boron and nitrogen atoms. Boron nitride was believed to be a type of synthetic material, but recently, it has also been found in natural minerals.1 BN is a compound that exists in several forms, including a soft hexagonal (h-BN) form having sp2 bonded layered configurations, which is similar to graphite; a hard cubic (c-BN) form, which is analogous to diamond; and an amorphous (a-BN) form with properties that are the same as those of amorphous carbon.2,3 Because of their advantageous properties, including electrical insulation, high thermal conductivity, chemical inertness, and optical transparency, hexagonal boron nitride nanoparticles have attracted special attention. These characteristics make BN appealing as a material for various uses, including optical coatings, lubricants, and protective and advanced ceramic composites.4 As shown in Fig. 1, the crystal structures of BNs are represented by the cubic boron nitride (c-BN) and hexagonal boron nitride (h-BN). The hardest form of BN is c-BN, which has high density and is similar to a diamond crystal lattice. It is the second hardest substance known to date. However, due to the lack of an easy route for the preparation of c-BN compared to h-BN, research on c-BN is limited. Similar to graphite, h-BN has a layered hexagonal structure comprising covalently bound B–N rings. It is frequently utilized as a thermally conductive substance and an electrical insulator because it is chemically and thermally stable. Several h-BN nanostructures, similar to carbon-based graphitic nanomaterials, are listed as nanowires, such as BN fullerene, BN nanotubes (BNNTs), and BN nanofibers.5–8 These h-BN nanostructures exhibit unusual band gap structures and electrical capabilities. Therefore, they are among the top research interests.9 This review concentrates on the current developments in h-BN nanostructure surface modification and applications.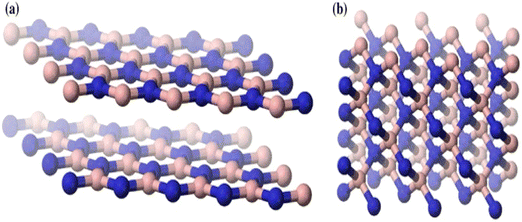 | ||
| Fig. 1 Crystal form of (a) hexagonal boron nitride and (b) cubic boron nitride.25 | ||
As a remarkable heat conductor, h-BN has 600 W m−1 K−1 of thermal conductivity and a broad band gap that varies from 5.5 to 6.4 eV depending on nanostructures. Considering this, h-BN is well known as an electrical insulator.10–14 h-BN has demonstrated a wide range of possible uses in electrocatalysts,15 thermally conductive nanocomposites,16–19 far ultraviolet light-emitting diodes,20 field emitters, nano-dimensional electronic devices,21 and polymer nanocomposites for severe environments.22 Additionally, they are utilized in biological applications23 such as orthopaedic implants,24 biosensing,25,26 medication, and gene delivery.27,28 There is a lot of interest in the use of h-BN nanoparticles as functional fillers to reinforce and modify polymer materials. For instance, BNNT-polymer composites with high BNNT fractions were created by Zhi et al.29 In this review, a variety of thermoplastics, including poly(ethylene vinyl alcohol), poly(methyl methacrylate), poly(vinyl butyral), and polystyrene, were used as a matrix. By incorporating 20 wt% to 30 wt% of BNNTs into these polymers, the resulting polymer composites achieved up to twenty times the thermal properties of polymer nanocomposites, a break of an electric field that was 20% stronger, and a temperature expansion coefficient that was 20% lower.
To create dielectric epoxy composites with excellent thermal conductivity,30 we used h-BN nanoparticles as fillers. A reduced dielectric loss factor with increased thermal conductivity of the nanocomposites demonstrated their promise for packaging and heat management in a micro-electronic gadget. Additionally, BNNF (boron nitride nanofiber)-strengthened polymer nanocomposites were created and researched. The BNNF/polymer composites, with 31.3 wt%, which show a superior in-plane heat conductivity of 60 W m−1 K−1, were demonstrated by BNNF. This makes this composite a suitable alternative for thermal interface materials (TIMs) in heat management applications. A polymer composite with boron nitride nanosheets (BNNSs) demonstrated a notable reduction in CTE and an improvement in the elastic modulus31 until the transparency of the composite films was nearly identical to that of the pure polymer films, demonstrating how they may be used in composite optical windows, optoelectronic devices, and other applications.
Even with these outstanding qualities and good uses, it is important to note that, similar to other nanomaterials, the high surface energy and significant tendency to agglomerate severely restrict the practical applications of h-BN nanoparticles. The disadvantage is problematic in composite applications due to low dispersion and surface characteristics. Most researchers concur that these two factors are crucial to the characteristics and application of polymer composites. Dissimilar graphite carbon nano-materials, whose interfacial and separation attributes may be easily tailored using a variety of covalent and non-covalent surface modification techniques, made the h-BN surface modification quite tricky. There are numerous hypotheses that explain this observation. Firstly, because of the electronegativity differences between boron and nitrogen, the covalent sp2 links between the boron and nitrogen atoms are somewhat ionic, the same as the carbon–carbon bonds in a graphitic structure, which strengthens the boron-nitrogen connections and makes them harder to separate. Secondarily, the difficulties in the surface modification of h-BNs are also due to the unique stacking order of the atomic surface, shown in Fig. 2a.31 More crystalline graphite causes a Bernal (AB) stacking sequence, at which the atomic plane is moved to half hexagon [Fig. 2a(i) and (ii)]. In h-BN, the adjoining planes, hexagons are superimposed, similar to how they are stacked in AA′; thus, the B atoms are stacked on top of and below the equivalent N atoms in h-BN [Fig. 2a(iii) and (iv)]. These characteristics may cause a “lip–lip” interaction between the nearby BN surfaces, as seen in Fig. 2b (for the sake of clarity, only the constructions made of nanotubes are displayed here; this phenomenon also occurs in other nanostructures, such as nanosheets).32 Bridges are then created by the chemical bonds that occur between the atoms of boron and nitrogen in the nearby layers. In conclusion, h-BNs are more resistant to chemical modification than graphitic materials because of their greater chemical inertness. The new material features of h-BN now make it an intriguing material in its own right, enabling a wide range of optical,20,33,34 electro-optical,35,36 and quantum optics37 functions.
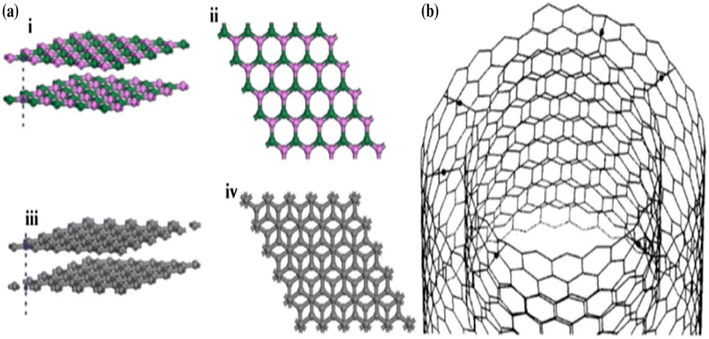 | ||
| Fig. 2 (a) Morphology of (i) and (ii) h-BN sheets and (iii) and (iv) graphite sheets showing differences in the order in which they are stacked.31 (b) Interaction between the neighbouring layers of a multi-walled nanotube.32 | ||
By creating hyperbolic phonon polaritons in a natural material, its extremely anisotropic crystal structure, characteristics, and polar chemical bonding may be used in device applications in IR nanophotonics.33,34 Even at ambient temperature, its point defects, which have single-photon emission characteristics, can produce quantum emitters in the visible to the near-IR region.37 In order to fully utilize h-BN for electronic usage, techniques for manufacturing and preparation must be advanced, encompassing thin-film and bulk crystal formation device integration, metallization, etching, and deposition. The potential for several of these emerging applications was significantly enhanced by significant advances in producing bulk, good-quality, isotopically nourishing materials.38,39 These initiatives are currently in the beginning stages. However, improvements in mass growth,40 chemical vapour deposition (CVD),41 and molecular beam epitaxy (MBE)42,43 have been observed.
Although various existing review articles discuss the applications of BN nanotubes, the present review article aims to provide comprehensive information on BN nanostructures. For the first time, the present state-of-the-art review summarizes the structural properties of BN, its synthesis, development, modifications and potential applications in the field of environmental remediation and energy production. The scope of the suture research is also discussed to promote further research on the topic.
2. Properties and structure of BN
A highly anisotropic crystal of boron and nitrogen atoms is tightly bound in the in-plane direction, known as hexagonal boron nitride.44 The B–N bond is polar covalent because the electronegativity difference between nitrogen and boron is more than 0.4 eV. Only van der Waals bonds exist between neighbouring sheets when the sheets are out of the plane, as in the case of graphite.45 Despite having an energy gap of about 6 eV, h-BN is an indirect bandgap semiconductor with a very high internal quantum efficiency for strong ultraviolet emission (up to 40%). Because of the high anisotropic crystal structure of h-BN, the frequencies of the typical lattice vibrational modes (optic phonons), which have two different branches, are likewise highly anisotropic. These are termed upper (λFS ≈ 6.2–7.3 μm) and lower (λFS ≈ 12.2–13.1 μm) bands, where λFS stands for the wavelengths in free space, and they are developed from in-plane and out-of-plane phonons respectively. Each branch's longitudinal optic and transverse optic phonons were spectrally separated because of the polar nature, creating a highly reflecting “Reststrahlen band”.46 Surface phonon polaritons are possible in this band, with the optic and acoustic phonons generating a phonon sideband in the ultraviolet luminescence spectrum.Hassel47 and Brager's48 recognized theory for BN is that it has a graphite-like arrangement with B and N atoms replacing the carbon atoms, and has been assigned structural type B.12 in the “Struckturbericht”.49 The observed and computed intensities do not correspond well with this structure, but a thorough re-evaluation has been done, as the original work is susceptible to additional criticism. Hence, the conclusion is that the Hassel structure is wrong.
Copper and manganese K-radiation were used to produce powder photographs of the crystalline structure of BN. There were lines found that corresponded to 28 diffraction planes, or these may all be classified based on a hexagonal unit cell with dimensions at 35.5 °C of a = 2.50380 ± 0.0001 A, c = 6.6600 ± 0.001 A. A calibrated microphotometer and a Geiger counter spectrometer were both used to obtain intensity readings from the photographs. It is clear from these findings and the density that the layer structure of BN has an interlayer gap of 1/2 c; every layer comprises a flat network of B3N3 hexagons. Four possible arrangements of these layers satisfy the symmetry of a unit cell. Three of these methods involve distinct arrangements of B and N atoms among the carbon atom-occupied positions for graphite. The model that Hassel and Brager chose most closely matches the measured intensities, as shown in Fig. 3 and 4, and presents the fourth way. This is a novel form of packing where the locations of the boron and nitrogen atoms are switched in the neighbouring layers, and the hexagonal rings are placed right on top of one another. A comparison of the calculated and observed intensity demonstrates that BN has a novel form of packing, and not the graphite type. A most striking result of this distinction between the packing of BN and graphite is the variation in the relative strengths of the hkl lines when h + 2k ≠ 3n. These lines become stronger when l is even and weaker when l is odd in BN. However, the opposite is true in graphite. This discrepancy is clearly evident in the powder images and cannot be explained by the carbon atoms' different atomic scattering strengths compared to B and N atoms.
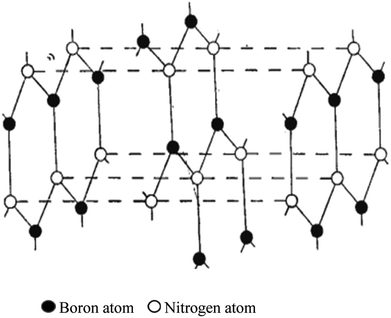 | ||
| Fig. 3 Hassel structure of BN.47 | ||
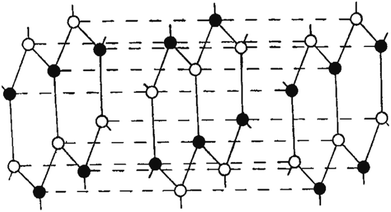 | ||
| Fig. 4 Newly suggested BN structure.47 | ||
The structural characteristics of various nanotubes are examined using theoretical and experimental means. Using molecular dynamics techniques and considering the interatomic interactions, theoretical simulations of structures were created. The Tersoff interaction potentials50 suggested for BN were used to define the structures.25 The LAMMPS software51 was used to carry out the simulation, and the Ovito52 and VMD53 programs were used to display the simulation results. These samples' (nanotubes) thicknesses ranged between 20 to 200 nm. Defocussing occurred on the range of many tens of nano-meters during electron microscope investigation (30–100 nm). These nanotube samples were generated in a gasostat using a graphite furnace for electron microscopy research.54 An h-BN crucible was used to hold a sample of Yttrium Aluminum Garnet (YAG), which was then heated at a high temperature. The mp of YAG is 1942 °C. The raw powders contained in a tantalum ampoule were sintered to create the YAG sample in a high-pressure solid container.
2.1. Singlewall nanotubes
The best carbon nanotubes are cylindrical in form; for these materials, the idea of chirality has been proposed. Chirality is defined by two integers (m, n) representing the position of the network hexagons that must line up with the hexagons at the origin of the coordinate as a consequence of the network convolution.55Under this context, tubes were divided into three categories: “zigzag” configurations (m, 0), “armchair” configurations (m, n = m), and “chiral” configurations (m, n ≠ m). The single-wall nanotube diameter may be calculated using chirality indices.
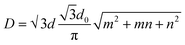 | (1) |
Fig. 5 depicts BN nanotubes with various chiralities. The ideally cylindrical nanotube having smooth walls (a feature of carbon nanotubes) was reportedly predicted. However, the minimization of energy in the BN nanotubes results in the development of distinctive “ribbed” walls (Fig. 5). Nitrogen atoms are moved outward in the tube, while boron atoms are pushed inward. The simulation shows that the B–N bond length may vary between 1.5 + 0.1 Å for tubes with various chiralities. Electron diffraction patterns were used to identify the chirality of the nanotubes.57
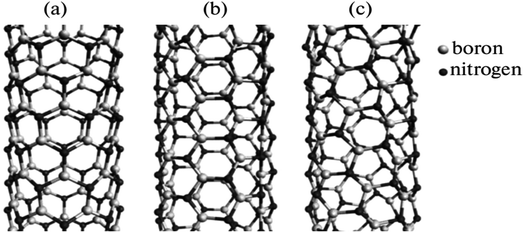 | ||
| Fig. 5 Simulation of various chiralities in BN nanotubes: (a) zigzag, (b) armchair, and (c) chiral configurations.56 | ||
2.2. Multiwall nanotubes
Regarding the structure and layout, multiwall nanotubes are more diverse than single-wall ones.56 Multiwall nanotubes of transverse structures and longitudinal structures strongly depend on their development. Different kinds of nanotubes may result from synthesis.58 Like carbon nanotubes, hexagonal BN nanotubes have an array of hexagonal networks arranged ideally below one another (as opposed to a graphite structure), with the B and N atoms alternately arranged throughout the Z axis. The two forms of nanotubes we typically saw were regular linear tubes and so-called bamboo-like tubes. The latter are frequently paired with defective ones, and their potential uses are not considered. However, it was demonstrated in ref. 59 and 60 that these nanotubes can be more advantageous than traditional, unstrained BN nanotubes in several applications. In contrast to regular tubes, bamboo-like ones are frequently twisted and interlaced. Their width range varies between 40 and 100 nm. A few tubes are filled, while others are empty (Fig. 6a). The curvaceous bands in the nanotube tips may be seen in the strong-resolution TEM images (Fig. 6b).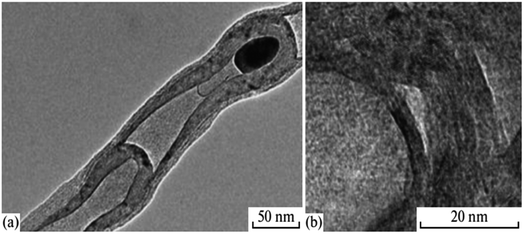 | ||
| Fig. 6 (a) TEM image and (b) HRTEM image of a bamboo-like nanotube.56 | ||
A multiwall BN nanotube is seen in Fig. 7. In experiments, nanotubes with 50–60 walls have often been seen. However, there are also specific tubes with 30 to 90 walls. As the number of walls increases, the shape deviates more from the ideal cylindrical shape. The interplanar distance may vary based on the perturbing impact on adjacent tubes. Thus, in multiwall BN nanotubes, many phenomena are present. EELS and element mapping were used to investigate the chemistry make-up of nanotubes. The nanotubes under review were demonstrated to contain a BN core covered in a carbon coating.56
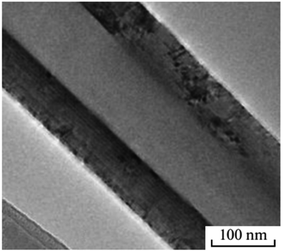 | ||
| Fig. 7 TEM image of a multiwall BN nanotube.56 | ||
Faceting causes the twisting of nanotubes. Additionally, the chance of discovering a rhombohedral BN interlayer increases with the number of walls inside a nanotube. Fig. 8 displays three nanotubes with a variety of walls: 2, 4, and 10 walls, respectively. The density of irregularities in packing various walls increases with the number of walls. For a simulated 10-wall nanotube, the beginning of faceting can be seen clearly.
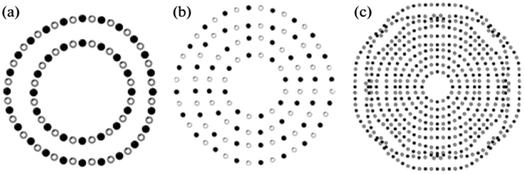 | ||
| Fig. 8 Simulation of (a) two, (b) four, and (c) ten-walled BN nanotubes.61 | ||
3. Synthesis of BN
The development of h-BNs is crucial for surface modification. This is because structural elements, including the diameter, purity level, faults, and edge structure of BN, significantly impact the surface modification of h-BNs, which have an immediate connection to the synthesis procedures. This article briefly describes a few typical synthesis techniques for various h-BN nanostructures.3.1. Mechanical exfoliation
The mechanical exfoliation process is also defined as mechanical peeling or mechanical cracking. Initially, sticky tapes were employed to separate graphene monolayers using this method.62 By using this method, layer h-BNs may also be synthesized, and very thin sheets of h-BNs were achieved.63,64 It could not maintain the van der Waals link between the adjacent h-BN layers by applying direct peeling force to the adhesive tapes throughout mechanical exfoliation. Still, the robust covalent boron–nitrogen bond structures remained intact and were detected using an optical microscope. Unfortunately, this method is not particularly effective for h-BNs due to the previously noted lip–lip interactions between the BN surfaces.32 The shear force was also used to exfoliate h-BNs mechanically instead of the direct peeling force. The production of high-quality BNNSs from ball-milling h-BN powders in an N2 environment was reported by Li et al.65 They adjusted the ball-milling parameters to produce a mild shear force. Fig. 9 shows the SEM images of peeled h-BNs, and a suggested exfoliation pathway under a shear force that occurs in response to the milling balls.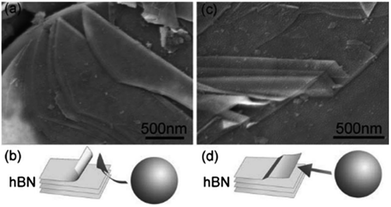 | ||
| Fig. 9 SEM images illustrating the mechanism of mechanical exfoliation; (a) and (b) cleavage at the edge of h-BN nanoparticles, and (c) and (d) peeling of h-BN thin sheets by ball milling.65 | ||
3.2. Chemical exfoliation
It has been demonstrated that chemical exfoliation, the liquid exfoliation method, makes it straightforward to produce mono- and few-layer BNNSs that are almost devoid of redundant components like catalysts and might scale up to vast numbers.66 Because of the strong interactions between polar solvent molecules and h-BNs, high polar solvents, including N,N-dimethylformamide (DMF), N-methyl-pyrrolidone (NMP), and isopropanol (IPA), were utilized to promote the synthesis of BNNSs. Large h-BNs might be divided into layered h-BNs by the cooperative action of sonication and a polar solvent. We produced pure BNNSs in thicknesses between 2 and 10 nm at the milligram scale.3.3. Chemical vapor deposition (CVD) synthesis
Chemical vapour deposition (CVD) is applied to create excellent quality and good-performance thin film nanoparticles. In a basic CVD procedure, one or many more volatile preparations are exposed to the surface, and they react with or degrade on the foundation surface to generate the desired deposit. Lourie et al.67 initially used the CVD process to generate BNNTs. They used borazine (B3H6N3) as a precursor and Ni2B molecules as a catalyst at 1110 °C in the synthesis of BNNTs. The SEM image of BNNTs is illustrated in Fig. 10. Since then, several h-BN nanostructures have also been created using CVD techniques. Significant advantages include the relatively low power input, excellent purity, and, most importantly, the extensibility of the CVD process. Currently, the CVD development of h-BN nanosheets mostly utilizes the pyrolysis of a single precursor, such as hexachloroborazine (B3N3Cl6), borazine (B3H6N3),68 or trichloroborazine (B3N3H3Cl3),69 or the combination of two compounds as the base material, like BCl3–NH3, BF3–NH3, and B2H6–NH3.70 Two typical chemical reactions involving two substrates in one case (chemical reaction (2)) and one substrate in the other (chemical reaction (3)), respectively, are given below.| BF3 + NH3 → h-BN + 3HF | (2) |
| (CIBNH)3 + NI(111) → h-BN/Ni(111) + 3(HCL) | (3) |
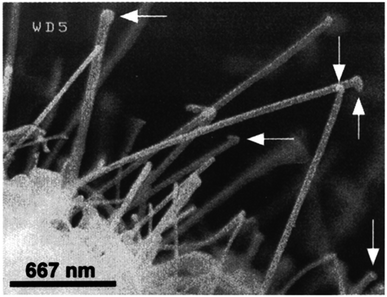 | ||
| Fig. 10 SEM images of h-BN nanotubes.67 | ||
3.4. Other synthesis techniques
In addition to the methods already described, several methods have been investigated to create h-BN nanomaterials. Among them are laser ablation,72–74 plasma-arc technique,75,76 and plasma-enhanced pulsed laser deposition.77 The investigations for all of these methods, which often demand significant input energy (>2000 °C), are not as comprehensive as those for the earlier techniques.A chemical reaction known as a substitution reaction occurs when an atom or functional group replaces one in a chemical molecule.25 Han et al. used graphene sheets as templates to create extremely crystalline h-BN utilizing a carbon substitution process.78,79
| B2O2(g) + 3C(sheets) + N(g) → 2h-BN(sheets) + 3CO(g) | (4) |
They put B2O3 powders in an open furnace, and coated them with graphene sheets and molybdenum oxide (as a booster). For 30 minutes, the furnace was kept at 1650 °C in a flowing N2 environment. The residual carbon layers were then removed by gathering the product and heating it in the air. Using electrospun polyacrylonitrile fiber as templates, the widely utilized electrospinning method was employed to create constant BN nanofibers (BNNFs). By altering the electrospinning process parameters, such as the applied electrical field and B2O3 solution concentration, the diameter of the BNNFs may be adjusted.7 The FE-SEM images of the BN nanosheets synthesized at 1200 °C are shown in Fig. 11.80
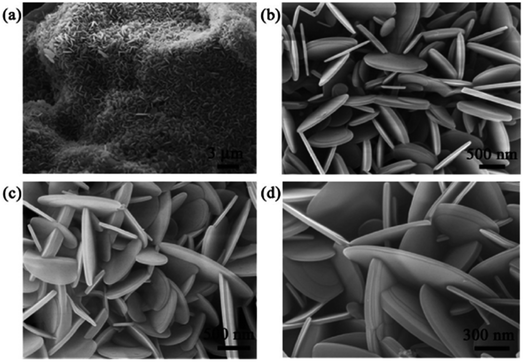 | ||
| Fig. 11 (a) FE-SEM image and high-resolution FE-SEM images at (b and c) 500 nm and (d) 300 nm of BN nanosheets synthesized at 1200 °C.80 | ||
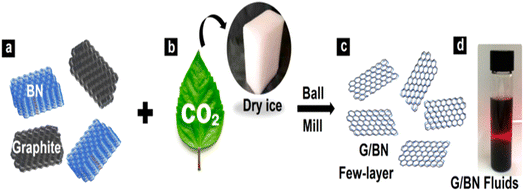 | ||
| Fig. 12 G/BN schematic diagram.83 | ||
4. Applications of BN and its nanocomposites
In various fields, including chemistry, metallurgy, high-temperature technology, electrotechnology, and electronics, h-BN has become well-established.84 Their special qualities suggest numerous prospective applications in numerous technical sectors, including high mechanical stiffness, broadband gap, superior thermal conductivity, large surface area, and thermal stability. In particular, functional fillers made from h-BN nanoparticles have been employed extensively to create high-performance polymer nanocomposites.25 The use of BNs and BN-based nanomaterials in prospective environmental applications like membrane separation, adsorption, and photocatalytic degradation to remove impurities has been thoroughly researched.85,86 BN-based nanomaterials have been used in investigations on the surface assimilation of various inorganic (bulk metals), as well as organic pollutants (mainly dyes and medicines), and the information they provide can be useful when employing these materials to treat water and wastewater.874.1. Adsorption of pollutants
| BN-based nanomaterial | Species | Initial concentration (mg L−1) | Maximum adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|---|
| BN | Cu2+, Cd2+ & Ni2+ | 10 | 18.42 ± 0.33, 12.00 ± 0.51 & 7.91 ± 0.57 | 91 |
| Activated BN | Cr(III) | 52 | 352 | 95 |
| h-BN | Cu(II) & Ni(II) | 300 to 700 | 200 & 95 | 98 |
| Activated BN nanosheet | Hg, Cu & Pb | 40 | ∼200 to 400 | 99 |
| BN-nanoribbon | Cd & Cu | 600 & 500 | 530 & 331 | 100 |
| Porous BN-4 microrod | Cu | 200 | 365 | 101 |
| Fe3O4–BN | As(III) & As(V) | 0.134 to 0.556 & 0.856 | 30.3 & 26.3 | 96 and 102 |
| Polyaniline–h-BN | PO43− & NO3− | 100 | 106 & 67.9 | 103 |
| g-C3N4/BCN | NaCl | 500 | 13.6 | 104 |
| Amine-functionalized porous BN | Cr(VI) | 20 | 120.95 | 105 |
| Diamide–pyridine-modified hierarchically porous boron nitride | U(VI) | 100 | 87.5 | 106 |
| P(AANa-co-AM)/BNNFs hydrogel | Pb2+ | 300 | 490 | 107 |
| h-BN–Fe3O4 nanocomposites | Cr(VI) | 25 | 208.6 | 108 |
| FeS@h-BN nanocomposites | U(VI) & Se(IV) | 35 & 72 | 163.11 & 196.34 | 109 |
| BCN–DAPhen | U(VI) | 100 | 2050.8 | 110 |
| Macroporous boron nitride fibers | Cd2+ & Zn2+ | 350 | 2989 & 1885 | 111 |
| Porous BN | Ni(II) | 40 | 237.6 | 112 |
Adsorption in the solo adsorption process seems to occur very quickly (2 min), and with the following removal order: Cu2+ > Cd2+ > Ni2+. However, the interaction of the metal ions in the ternary system causes variations in the adsorption performance. Cu2+ negatively impacted the elimination of Cd2+ and Ni2+, whereas Cd2+ and Ni2+ were positively impacted by Cu2+. These results point to a variety of adsorption processes, including the complex formation of metal ions with surface –NH2 functional molecules,92 ion interactions between metal ions and H of –OH,93 and electrostatic interactions related to the pairings of –O and metal ions,94 by incorporating a framework agent in the heat breakdown process of the activated BN substrate. It shows an exceptionally quick removal rate of 99.9 wt% (<6 h) and 90 wt% (<4 h) for different metal ions. It has an incredibly massive surface area (2100 m2 g−1) and a huge pore volume (1.66 cm3 g−1), effectively creating a new activated BN.95 It unquestionably demonstrates that the greatest adsorption capabilities of excited BNs are significantly higher than those of penetrable BN and adsorbents carbon reported in early works.96,97 It was found that excited BN with polar boron–nitrogen bonds exhibits considerably stronger positively attracting metal ions than excited carbon with covalent carbon–carbon bonds. This results from the rich electron density that polyelectronic nitride allots to metal ions and the “lop-sided” density feature of ionic B–N bonding. The extensive surface area and abundant interspaces in the excited boron nitride significantly contribute to the material's high adsorption capacity and efficiency.91
With additional bulk metals, Pb2+ was efficiently removed utilizing a small layer of BN nanosheets fabricated employing a low-temperature manufacturing approach.113 According to the results of interference testing, the adsorbents displayed a strong affinity for Pb2+ (845 mg g−1), while being hindered by heavy metal ions like Cd2+, Ni2+, and Cu2+ ions (their adsorption capacities were 312, 201, and 402 mg g−1, respectively). In order to explain the removal of Pb2+, both chemical and physical adsorption processes were utilized. At pH = 6, the electrostatic interaction between the negatively charged BN adsorbents and the positively charged ions may be a major factor in the Pb2+ adsorption on BN nanosheets. The “lop-sided” density features of boron-nitrogen bonds form extra –NH2 and B–OH bonds, showing that multi-electron nitrides might modify different electron densities on the metal ions. Therefore, the polarity of boron-nitrogen bonds was adequate for removing Pb2+.95 Overall, the large number of chemical linkages that form coordinate bonds with lead ions on the BN nanosheets are largely responsible for the high removal efficiency of lead ions, including the strong B–O–Pb interaction and –NH2/Pb complexation.114 Fig. 13a details the feasible mechanism of Pb2+ adsorption on BN nanosheets. Most earlier investigations on the removal of heavy metals utilizing various BNs have established that bulky metals were in opposite charge to manufactured BNs and might interact electrostatically, accelerating the adsorption response.115–117
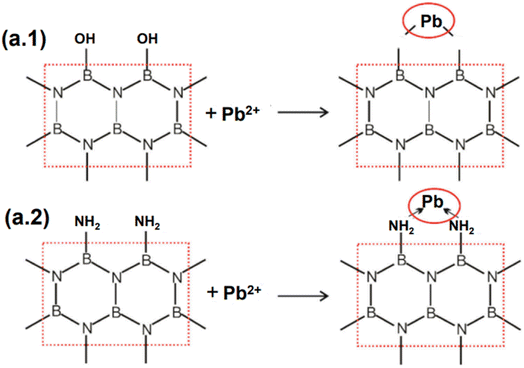 | ||
| Fig. 13 The probable mechanisms of (a.1) B–OH & (a.2) –NH2 in lead ion adsorption on the BN-550 nanostructure.113 | ||
Consequently, there is a pressing need to clean industrial effluent with dye contamination. Reverse osmosis,127 advanced oxidation processes,128 membrane filtration,129 coagulation–flocculation,130 and adsorption131 are just a few of the methods that can be used to treat industrial wastewater. Adsorption is typically favoured among them because of its benefits, including cheap operating costs, greater efficiency, and an environmentally friendly nature.132
The study of methyl orange surface assimilation onto BN nanosheets included theoretical and experimental analysis.133 The synthesized adsorbent has the greatest experimental adsorption capacity, 575 mg g−1, which is attributable to the strong Coulomb interaction between the positively charged BN nanosheet and negatively charged methyl orange. Particularly, it was found that the chemical hardness, electrophilicity, and electronegativity data of the methyl orange–BN clusters are 1.45 eV, 5.51 eV, and 4 eV, respectively. The interaction between the adsorbent surface and the dye chemical is another important aspect of adsorption. According to research based on calculations using density functional theory, the oxygen atom of a methyl orange compound is closest to the BN nanosheet with a range of 0.233 nm. The desorption and reusability of BN nanosheets are allowed by the physical feature of methyl orange adsorption on BN nanosheets. The modified structure is symmetrical, and the reactions are exothermic, according to the measured adsorption energy of methyl orange, which looked to be −296 kJ mol−1.
In a different investigation, negatively charged or activated BN fibres and the cationic dye methylene blue interacted electrostatically to reach the highest adsorption capacity of 392 mg g−1 at pH 8.0 and 30 °C.134 Since the BN filament has a “lop-sided” density feature of significant ionic boron-nitrogen bonding, and poly-electron nitrides have the potential to allocate a greater electron cloud to the positively charged solute, the polar boron–nitrogen bond is suitable for methylene blue chemical adsorption.135 At pH levels higher than 2, the surface charge of the influenced BNs shifts toward a negative charge, which enhances the adsorption of the positively charged methylene blue. The penetrable BN, which has a massive pore volume of 0.63 cm3 g−1 and a very large specific surface area of 1100 m2 g−1, enhances the adsorption performance.136 Furthermore, additional activated sites in activated BN significantly improve the adsorption of methylene blue due to the abundance of hydroxyl and amino functional groups and the large density structural defects, which offer strong binding sites and enhance the dissociation of methylene blue on the BN fibres.134
A layered BN–carbon nitride nanocomposite was successfully made by calcining a combination of BN particles and urea (NH2CONH2) at an elevated temp of 600 °C, where urea is thermally polymerized through a calcination reaction to produce CN.137 Using BN particles and urea as substrates. Fig. 14 depicts the expected creation process of the layered BN–carbon nitride nanoparticles. At starting concentrations of 220 mg L−1 and 120 mg L−1, the BN carbon nitride nanocomposites successfully removed malachite green (1040 mg g−1) and neutral red (1350 mg g−1) from H2O. Meanwhile, the anionic dyes MB (54.0 mg g−1) and MO (55.9 mg g−1) have comparatively poor adsorption capabilities. This finding suggests that neutral red withdrawal by the BN–carbon nitride nanocomposite may not be primarily caused by electrostatic attraction.
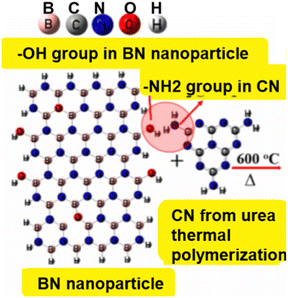 | ||
| Fig. 14 The layered BN–carbon nitride composites (BN-30-600) are thought to be generated using urea and BN nanoparticles as precursors.137 | ||
According to a prior study, the size of the pollutants significantly affects how well adsorbents remove them.138 In comparison to methylene blue (length = 1.42 nm and width = 0.54 nm) and methyl orange (length = 1.45 nm width = 0.43 nm), neutral red cationic dyes seem to have the lowest size (1.22 nm/0.49 nm). This is generally constant with their respective volumes of neutral red = 188 cm3 mol−1, methyl orange = 209 cm3 mol−1, and methylene blue = 207 cm3 mol−1. However, the size variance between these dyes is still insufficient to satisfactorily explain why neutral red's removal is around twenty times larger than that of methylene blue and methyl orange. The adsorption energy appears to have little impact on the removal of the adsorbent because the differences in adsorption energies between both the adsorbent and dye compounds (methylene blue = 35.2 kcal mol−1, neutral red = 39.1 kcal mol−1, methyl orange = 37.2 kcal mol−1) are likewise negligible.139 However, the biological neutral red molecules lack H+ ions, and are difficult to break down in H2O and precipitate in an alkaline medium. As a result, it was proven that neutral red might be eliminated by neutral red by attracting H+. By using two additional cationic dyes (crystal violet and malachite green), the high affinity of BN-30-600 for H+ has been verified.137
Using porous carbon–BN nano-scrolls resembling flower stamens, amazing dye surface assimilation capability was seen for eliminating methylene blue (250 mg g−1) and Congo red (620 mg g−1). The results were related to a variety of characteristics. Since pigments are filled in the cavities and adsorbed on the carbon–BN nano-scroll surface, their high surface area (890 m2 g−1) and porous structure are advantageous during the dye molecule's adsorption process. Following MB and Congo red adsorption, FE-SEM pictures reveal that the carbon–BN scroll feature was mostly unchanged. FTIR spectra show that the Congo red-coated carbon–BN with an aromatic benzene ring stretching band at around 1600 cm−1 is more powerful than the MB band.140 We saw strong interactions between dye molecules and C–BN nanoscrolls. Raman and XPS investigations show that the C–BN molecule has sp2 hybrid carbon and BN domains, resulting in conjugated π–π interactions with the dyes, including aromatic benzene (C6H6) rings. Furthermore, the B atom of carbon–BN interacts with a Lewis base through the nitrogen and sulfur atoms of Congo red and MB, creating Lewis-acids and Lewis-bases interactions.141 Furthermore, negatively charged ionized boron–oxygen bonding enhances the hydrophilicity between carbon–BN and pigments, facilitating enhanced adsorbing behaviour and exciting cationic dye molecules like MB due to electrostatic interactions.142 The summary of the adsorptive removal of dyes by BN-based nanomaterials is given in Table 2.
| BN-based nanomaterial | Dye compound | Initial concentration (mg L−1) | Maximum adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|---|
| BN-based nanosheet | Methyl orange | 110 | 575 | 133 |
| h-BN | Rhodamine B & sunset yellow FCF | 100 & 75 | 140 to 208 & 58 to 105 | 131 |
| Polydopamine microspheres | Methylene blue | 10 | 90.7 | 141 |
| BN carbon nitride | Neutral red & malachite green | 220 & 120 | 1350 and 1041 | 137 |
| Activated BN | Congo red & methylene orange | 50 | 300 & 400 | 95 |
| h-BN | Methylene blue | 500 to 200 | 230 | 143 |
| h-BN whiskers | Rhodamine-B & methylene blue | 3 & 60 | 210 & 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 973 973 |
144 |
| Calcium alginate immobilized graphene oxide | Methylene blue | 20 to 70 | 181.81 | 142 |
| Ag–BN | Rhodamine-B | 100 | 880 | 145 |
| Porous BN | Methylene blue | 10 to 50 | 189 | 146 |
| Hexagonal boron nitride nanosheets | Methylene blue & acridine orange | 10 to 500 | 415 & 286 | 147 |
| BNNS–PVDF | Methylene blue | 50 | 142.86 | 148 |
| BNNS | Methylene blue | 50 to 300 | 322.5 | 149 |
| Porous BN | Congo red & methyl green | 350 & 250 | 1096 & 1203 | 150 |
| Cu–BNNS | Rhodamine B | 500 | 743 | 151 |
| Boron carbon nitride | Methyl blue & basic yellow-1 | 136.4 & 101.4 | 1220 & 560 | 152 |
| Carbon-doped boron nitride | Sulfamethoxazole | 10 | 28.75 | 153 |
| BCN | Methylene blue | 10 to 50 | 212.8 | 154 |
| Oxygen-rich fish-scale-like porous boron nitride | Methylene blue | 50 | 422.6 | 155 |
| BNHSs with ultrathin shells | Basic yellow 1 | 40 | 191.7 | 156 |
Pharmaceutical compounds have been found in aquatic ecosystems since the 1990s, and Ternes,163 Heberer,160 and Kümmerer164 have identified them as new uncontrolled pollutants. According to Halling-Sørensen et al.,161 most pharmaceutical compounds are naturally biologically active and hydrophilic so that humans may easily absorb them, and persistent so that they do not degrade before having a healing impact. These substances of human origin are continuously released into aquatic habitats through various channels, treated wastewater being the main one. The outcome of these chemicals during wastewater treatment procedures is a key element in regulating their quantities in aquatic habitats. Antibiotics have been removed using a variety of methods, including ion exchange, coagulation,130,165 liquid membrane separation, photo-catalytic degradation,166 reverse osmosis, ozonation,167 and adsorption. Adsorption is favoured over these other methods because it is straightforward to design, simple to make, effective, inexpensive, and produces negligible harmful by-products. Here, we investigated the use of BN as an adsorbent in the removal of three antibiotics from water, which are tetracycline (TC), ofloxacin (OFL), and cephalexin (CFX). The summary of the adsorptive removal of pharmaceutical compounds by BN-based nanomaterials is given in Table 3.
| BN-based nanomaterial | Pharmaceutical compounds | Initial concentration (mg L−1) | Maximum adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|---|
| g-BN | Gatifloxacin | 80 | 88.5 | 168 |
| BN nanosheets | Tetracycline, ofloxacin & cephalexin | 200 | 346.66, 72.50 & 225.0 | 172 |
| BN | Tetracycline | 10 | 369.79 | 169 |
| BN nanosheets | Tetracycline | 50 to 100 | 1101 | 170 |
| BNNSs | Chlortetracycline hydrochloride & norfloxacin | 1170 & 174 | 400 & 120 | 172 |
| BN nanosheet | Estrone | 0.05 to 12 | 249 | 173 |
| BN bundles | Sulfadiazine & erythromycin | 20 to 100 | 137 and 150 | 174 |
| Ni–BN | Tetracycline | 20 to 100 | 430 | 175 |
| Graphene–BN aerogel | Ciprofloxacin | 10.5 | 185 | 176 |
| Carbon-doped BN | Tetracycline | 40 | 76.7 | 177 |
| BN with boron vacancies | Tetracycline | 200 | 370 | 178 |
| BNNS | Tetracycline | 50 to 300 | 322.5 | 149 |
| CoO/P-BNFs | Chlortetracycline | 150 | 655.474 | 179 |
| Carbon-modified HBN | Bisphenol-A & paracetamol | 35 to 100 | 49.75 & 67.56 | 180 |
| GO/BNF | Gemfibrozil | 10.5 | 55.1 | 181 |
| ZnTi LDH/h-BN | Amaranth & diazepam | 20 & 5 | 99.5% & 99.8% | 182 |
| BN nanosheet | Levofloxacin | 10 | 17.61 | 183 |
| Porous hexagonal BN | Tetracycline | 160 | 322.16 | 184 |
A possible method of removing tetracycline from sea urchin-like BN adsorbents is shown in Fig. 15, which has a maximum adsorption efficiency of 370 mg g−1. The structure that resembles a sea urchin was manufactured using fibres, and it has many mesopores that stretch by the centre toward the surroundings. BN adsorbents with mesoporous and large surface areas seemed particularly helpful for better adsorption. Additionally, the sea urchin-like structure also prevents the release of tetracycline. Contrary to scattered fibres and nanosheets, fibre clusters in BN nano-adsorbents with sea urchin-like forms allowed for the precipitation of organic pollutants in the core of the sea urchin, improving the adsorbing capacity. As the principal electromotive force on the BN adsorbent surface, π–π association plays a crucial role in the substantial adsorption of tetracycline with aromatic benzene ring geometries. Tetracycline physical formulations further showed Lewis basicity due to a lack of solid bonding with chlorine. As a result, interactions between sea urchin-like BN nanomaterials and Lewis acids might be the source of the removal driving force and lead to acid-base complexation.168 Meanwhile, the introduction of carbons in BN nano-adsorbents may successfully increase the molecular orbital energy and reduce chemical stability, increasing the adsorption capability of BN nano-adsorbents that resemble sea urchins (minor stability implies better adsorption capability).156 Moreover, the defects of the uneven BN-layered form support the improved BN adsorption performance.
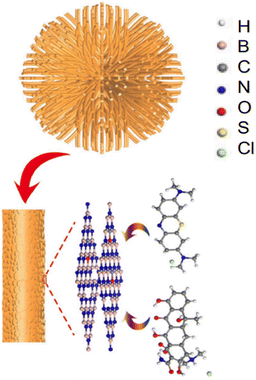 | ||
| Fig. 15 Sea urchin-like hexagonal BN's potential adsorption mechanism diagram for methylene blue and tetracycline.169 | ||
Utilizing BN nanosheets containing N defects, density functional concept calculations were performed to evaluate the adsorption process for tetracycline.170 The conventional method for chosen determining the electronic version of all linked components and interactions was the hybrid density potential with the dispersion-adjusted term, which has been successfully used in BN adsorption techniques.171 Fig. 16 depicts the adjusted forms of pure BN nanosheets, N-defective BN nanosheets containing N vacancies, and their corresponding adsorbed forms. Complexes formed by tetracycline molecule adsorption on the nano-adsorbent surfaces were also modified. After adsorption, BN nanosheets containing N-defects have a nonplanar structure, especially in the combination of N-defective BN nanosheets containing N vacancies and tetracycline, demonstrating a strong association between them. The calculated results revealed that the association energies were −37.0 and −60.2 kcal mol−1 for pure BN nanosheets and tetracycline and N-defective BN nanosheets with N vacancy and tetracycline, respectively. The construction of N-defects throughout the BN nanosheet boosted the adsorptive intensities of tetracycline, enhancing the adsorption capabilities, as shown by the significantly increased interaction energy value.170
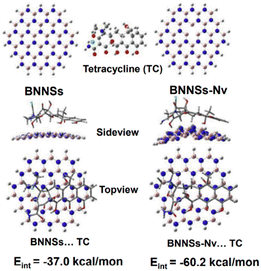 | ||
| Fig. 16 Best possible structures of BN nanosheets (BNNSs) and N-defective BNNSs with N vacancy (BNNSs–Nv) and their related adsorbed forms.170 | ||
Tetracycline, ofloxacin, and cephalexin were used to evaluate the adsorption capability of BN nanosheets with a wide surface area to other adsorbents.172 Graphene oxide (313 mg g−1), carbon nanotubes (100 mg g−1), reduced graphene oxide (35 mg g−1), and activated carbon composites (262 mg g−1) were all outperformed by BN nanosheets produced by advanced a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water/methanol mixture and a temp of 900 °C for 2 hours. The literature is quite poor in its results about ofloxacin adsorptive elimination. Activated carbon (137 mg g−1), magnetite-coated zeolite (24.9 mg g−1), bentonite (10.4 mg g−1), and natural zeolite (16.1 mg g−1) all performed less well in adsorbing cephalexin than BN nanosheets (225 mg g−1). The larger surface area (1800 m2 g−1), higher pore volume (2.75 cm3 g−1), and smaller sieve-like size (6.2 nm) of BN nanosheets may be responsible for their improved adsorption ability for the three antibiotics. The primary adsorption process is thought to include π–π association between the three target substances' aromatic benzene rings and the BN nanosheets' ring network.173 In the case of tetracycline and cephalexin, a further adsorption process is connected to the amino affinity between the substances and BN nanosheets. As a result, ofloxacin had a lower adsorption capability than tetracycline and cephalexin. Tetracycline showed the highest level of adsorption among the antibiotics because it had a greater composition of aromatic benzene rings than the other antimicrobials.
1 water/methanol mixture and a temp of 900 °C for 2 hours. The literature is quite poor in its results about ofloxacin adsorptive elimination. Activated carbon (137 mg g−1), magnetite-coated zeolite (24.9 mg g−1), bentonite (10.4 mg g−1), and natural zeolite (16.1 mg g−1) all performed less well in adsorbing cephalexin than BN nanosheets (225 mg g−1). The larger surface area (1800 m2 g−1), higher pore volume (2.75 cm3 g−1), and smaller sieve-like size (6.2 nm) of BN nanosheets may be responsible for their improved adsorption ability for the three antibiotics. The primary adsorption process is thought to include π–π association between the three target substances' aromatic benzene rings and the BN nanosheets' ring network.173 In the case of tetracycline and cephalexin, a further adsorption process is connected to the amino affinity between the substances and BN nanosheets. As a result, ofloxacin had a lower adsorption capability than tetracycline and cephalexin. Tetracycline showed the highest level of adsorption among the antibiotics because it had a greater composition of aromatic benzene rings than the other antimicrobials.
4.2. Photocatalytic applications
The elimination of organic contaminants using semiconductor-based photocatalysis, a new wastewater treatment method, may be possible by utilizing sun energy to create active species.190–192 Because of its high stability, cheap cost, and environmental friendliness, graphitic carbon nitride (g-C3N4), a metal-free semiconductor, exhibits tremendous promise for environmental remediation.193,194 Unfortunately, the poor yields and large coupling rates of photocatalytic activity result from being concealed by visible light, insufficient oxidation abilities, and small specific surface area, and these factors continue to restrict the photocatalytic efficiency of pure g-C3N4 for environmental clean-up. To address the limitations of g-C3N4, a number of techniques have been developed, including defect engineering,195 heterojunction design,196 morphological controls,197 metal/non-metal modifications,190,198 and many others.
The h-BN can work as a co-catalyst to readily create hybrid materials with nanomaterials, and increase the number of target sites as its dimensions become closer to those of quantum dots.199 Another benefit is that BNQDs have a lot of oxygen-containing functional groups that are negatively charged. These functional groups have a strong electrostatic attraction to photogenerated holes, which indicates that they have a lot of capability as hole channels to help separate photocarriers.200 The merging of flow engineering and BNQDs alteration process procedures appears to be a possible way to collaboratively improve the photooxidative capabilities and carrier generation–separation performances of g-C3N4.
Wu et al.201 developed a special BNQDs-modified reduced ultrathin g-C3N4 nanosheets (BNRU) metal-free photocatalyst for this investigation by combining self-assembly and thermal treatments as illustrated in Fig. 17. A visible light catalyst was employed to degrade typical FQs contaminants. Investigations into the photocatalysts' structural compositions, energy band structure, and optical and electrochemical characteristics were conducted in detail. The creation of numerous environmental factors (such as pollutant concentration, H2O constituents, natural water matrices, solution pH, and mixed pharmaceuticals H2O under natural solar light) was another method by which the efficiency of the BNRU technology was demonstrated. They were used to simulate actual water treatment processes.201 Finally, feasible charge transfer methods and kinetics/pathways for pollutant degradation throughout the photocatalytic process were suggested.
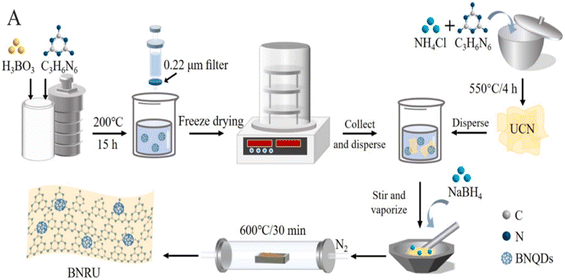 | ||
| Fig. 17 Formations of BNRU.201 | ||
NOR removal (removal of one group) studies under visible (blue-LED) light irradiation were used to assess the prepared materials' photocatalytic activity. Adsorption, direct photolysis, and photocatalysis were all shown to contribute to the elimination of NOR in Fig. 18. After 30 minutes of dark adsorption to reach adsorption–desorption equilibrium, the UCN and RUCN absorbed 20.3% and 12.2% of the NOR, respectively. With the addition of BNQDs, the NOR absorption efficiency improved, which was ascribed to chemisorption dominated by interactions between the BNQDs and NOR molecules in the form of π–π stacking interactions.172,202 After 15 minutes of exposure to visible light, 6.7% of the NOR had been degraded without using photocatalysts, proving that a direct photolysis process was not the predominant cause of the NOR removal. The UCN only degraded 59.2% of the NOR after 15 minutes. However, the removal effectiveness increased to 79.5% when the RUCN was present. Remarkably, the BNRU composites considerably improved the effectiveness of the NOR removal, allowing ten mg L−1 of the NOR to decompose under the same parameters.
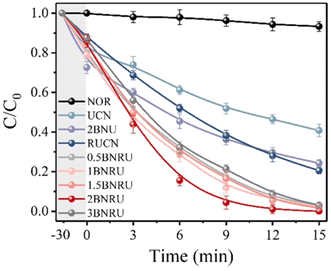 | ||
| Fig. 18 NOR degradation-based photocatalytic activity.201 | ||
A pseudo-first-order kinetic model was used to determine the kinetic rate constant (Kobs) of the well-prepared photocatalyst for NOR degradation, as shown in Fig. 19. When BNQD packing relayed 2 × 10−2 wt%, it was evident that the BNRU composite seemed to have the maximum photocatalytic activity (2BNRU), compared to the UCN (0.0514 min−1), which had a Kobs value of 0.3744 min−1, was 7.28, 4.53, and 3.83 times higher, BNU packing with 2 × 10−2 wt% BNQDs (denoted as 2BNU), and RUCN loaded with 0.0977 min−1 of RUCN, proportionately. On the other hand, too many BNQDs blocked the reaction site, decreasing the photocatalytic activity.203 As a result, 2BNRU was chosen as the best sample for future research into possible uses for the photocatalytic process.
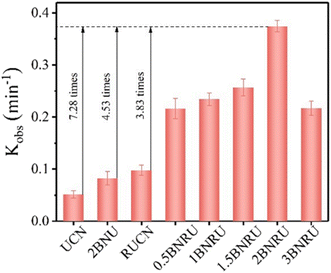 | ||
| Fig. 19 Kinetic rate constant based on the photocatalytic activity.203 | ||
The degradation performance against many common FQs, including ciprofloxacin (CIP), norfloxacin (NOR), ofloxacin (OFX), lomefloxacin (LOM), and enrofloxacin (ENX), was tested in order to assess the adaptability of the 2BNRU system. The removal efficiencies for ENX, CIP, LOM, OFX, and NOR, after 15 minutes of exposure utilizing the 2BNRU system, were determined to be 87.7%, 100%, 94.4%, 94.6%, and 100%, respectively, as shown in Fig. 20. According to calculations, the relevant kinetic rate constants are 0.1266, 0.3153, 0.1883, 0.188, and 0.3744, min−1, as shown in Fig. 21. These results revealed that the photocatalytic method was universally applicable and performed better at degrading different FQs. Additionally, due to its maximum release rate, the 2BNRU photocatalytic system's degradation kinetics and processes were examined using NOR as a sample contaminant. A summary of the photocatalytic properties of the BN-based nanomaterials is given in Table 4.
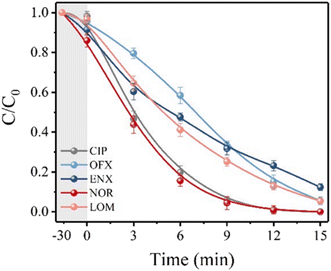 | ||
| Fig. 20 Photocatalytic degradation efficiency.201 | ||
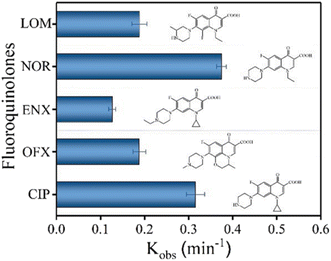 | ||
| Fig. 21 Kinetic rate constants of 2BNRU for fluoroquinolones when exposed to visible light.201 | ||
| BN-based nanomaterial | Pharmaceutical compounds | Initial concentration n (mg L−1) | Time (h) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| g-C3N4 (BNRU) | Fluoroquinolone | 10 | 0.5 | 46.9 | 201 |
| ZnTi LDH/h-BN | Diazepam | 10 | 2 | 95 | 182 |
| MnFeO3/h-BN | Ibuprofen | 15 | 2 | 66 | 204 |
| Bi2WO6/BN | Tetracycline & ofloxacin | 20 | 2 | 99.1 & 94.66 | 205 |
| Ultrathin–h-BN/Bi2MoO6 | Tetracycline, oxytetracycline & doxycycline | 20 | 2.33 | 99.19, 95.28 & 91.04 | 206 |
| AuNPs/h-BN | Levofloxacin & hydrochloride | 10 | 5 | 84.4 | 207 |
| TiO2–BN–Pd | Acetaminophen | 1 | 4 | 90 | 208 |
| BN/Fe3O4/MIL-53(Fe) ternary nanocomposite | Ibuprofen | 10 | 3 | 100 | 209 |
| BN/Bi2MoO6 | Iohexol | 20 | 2.5 | 92 | 210 |
| Carbon nitride-modified boron nitride (CNBN) | Ciprofloxacin | 10 | 4 | 98 | 211 |
| Boron nitride/3,4,9,10-perylenetetracarboxylic dianhydride | Tetracycline | 20 | 3 | 70.52 | 212 |
| SrWO4/BN/GCE | Ornidazole | 10 | 3 | 92.4 to 99 | 213 |
| Ni(OH)2–TiO2 | Tetracycline | 100 | 2 | 74 | 214 |
| MoSe2/ZnO/p-BN | Ofloxacin | 30 | 1 | 99.2 | 215 |
| BN/SnO2 | Salicylic acid | 25 | 0.66 | 82 | 216 |
| BBOB/BNQDs | Tetracycline | 10 | 1 | 76.9 | 217 |
| CuS/h-BN | Ibuprofen | 21.20 | 0.5 | 99.2 | 218 |
| Ag@CP–BNQDs | Tetracycline | 10 | 0.5 | 80 | 219 |
Various photocatalytic materials only eliminate pollutants when exposed to UV light because they have broadband gaps. It has been observed that some substances, such as dyes, have the ability to sensitize a broadband gap photocatalyst by shifting their photo-excited electron into the conduction bands of the photocatalyst when exposed to visible light. This develops a photocatalyst with a broadband gap that can break down pollutants when exposed to visible light.228,229 For instance, Gao et al. evaluated the photocatalytic sensitisation degradation of organic dyes when exposed to visible light.230 By photosensitizing TiO2 with eosin Y and rhodamine B, Diaz-Angulo et al. claim to have improved the photodegradation and absorption of acetaminophen and diclofenac.231
It has been reported that the removal of organic contaminants is significantly enhanced by the photo-deposition of platinum nanomaterials on dye-sensitized TiO2.232 Metal-based and metal-free photocatalysts are the two primary types of photocatalysts. Metal-based photocatalysts are used in environmental and energy-related applications (including S–TiO2/NH2–UiO-66,233 and Ag/AgCl@MIL-101 (ref. 234)) because of its non-toxic and high stability.235 The metal-free wide-bandgap photocatalyst (BN) is used due to its widespread application in water purification. Because of its extraordinarily high chemical stability, BN is widely used to eliminate contaminants from H2O.236 Due to its broad indirect bandgap of 5.5 eV, ultraviolet light also makes it extremely difficult to move the electron to the conduction band from the valence band in BN, so it is often not a photocatalytic material.237 However, –OH groups are available on the surfaces of BN made using the oxygen-limiting technique. The bandgap of BN has been reported to be reduced for hydroxylation from 5.5 eV to 3.94 eV.238
Under UV light irradiation, BN made by this technique can break down neutral red,239 thus implying that BN responds to UV light by acting as a photocatalytic material. We have not examined the impact of BN on how dyes break down sensitizing when subjected to visible light. Herein, the oxygen-limited technique creates BN material, and the sensitization decomposition performances of Rh-B (rhodamine B), MB (methylene blue), MO (methyl orange), Rh-B/MB and Rh-B/MO on the surface of BN under vis-light irradiation are studied.
According to Fig. 22, this removal experiment has two periods: one is adsorption, and the other is photodegradation when exposed to visible light. Using BN, Rh-B is eliminated from the water by about 87.8%, with 42.6% of that removal occurring by adsorption and the remaining 45.2% occurring via photodegradation. As a result of band structures, BN cannot absorb visible light and degrades Rh-B when exposed to it.240 Under vis-light irradiation, BN was utilized to break uncoloured AT to show if it is the photocatalytic substance that responds to vis-light. BN absorbs about 10% of AT. The amount of atrazine present at the start of the photodegradation period and the conclusion of the photodegradation period is essentially the same. Because of this, BN cannot break colourless AT when exposed to visible light. Instead, Rh-B is most likely degraded by BN through a process known as sensitization photodegradation.
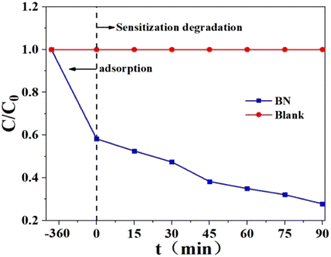 | ||
| Fig. 22 Rh-B elimination by BN under vis-light irradiation.240 | ||
When exposed to vis-light, investigations are being conducted into the sensitized decomposition of methylene blue and methyl orange by BN. In accordance with Fig. 23A, 18.3% of methylene blue has been eliminated by BN under vis-light irradiation, of which 6.1% have been eliminated by adsorption, and another 12.2% have been eliminated by photodegradation.
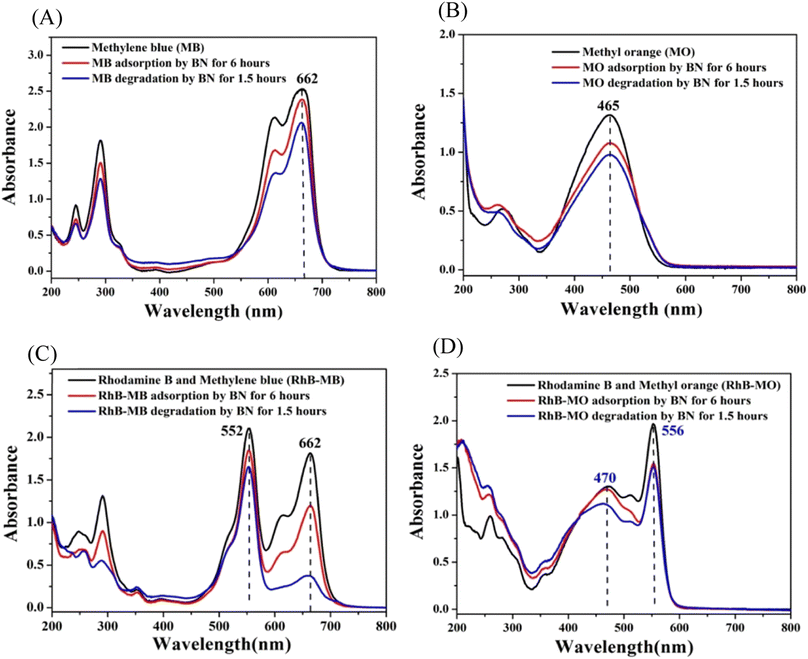 | ||
| Fig. 23 (A) The ultraviolet-visible spectra of MB elimination by BN via adsorption and photo-removal under visible light irradiation. (B) The ultraviolet-visible spectra show the elimination of MO by BN using a combination of adsorption and photo-removal when exposed to noticeable light. (C) The Rh-B/MB solution's ultraviolet-visible spectra after being subjected to BN's adsorption and photo-removal processes while exposed to visible light. (D) The Rh-B/MO solution's UV-vis spectra after being subjected to BN's adsorption and photo-removal processes while exposed to noticeable light.240 | ||
Fig. 23B shows that 25.7% of methyl orange is eliminated, with 18.3% coming through adsorption and 7.4% coming from sensitization decomposition. The findings above demonstrate that when exposed to visible light, various dyes exhibit varying sensitization effects by BN. The greatest sensitization photodegradation effects are produced by Rh-B (45.2%), MB (12.2%), and MO (7.4%). Rh-B is a cationic dye, and BN's surface is negatively charged (−31 mV). However, the interaction between Rh-B and BN mostly depends on hydrogen bonds (the hydrogen bond strength between the O atom in Rh-B and the H attached to the O in the BN model is 2.4 Å). Because MB has a planar structure and is also a positively charged dye, the interaction between methylene blue and BN is mostly caused via π–π stacking (the distance of stacking is 3.5 Å). It is vital to have a suitable e-transfer route between the BN and sensitizer so that the electron can transfer easily from the lowest unoccupied molecular orbital of a sensitizer to a conductive band of BN.241 To let the electrons pass smoothly through the sensitizer into the photocatalytic substance, they are both frequently associated in the sensitised solar cell.242–244 As a result, hydrogen bonds may be a richer electron transfer route than non-covalent π-interactions of aromatic rings, which explains why Rh-B's (45.2%) greater sensitization degradation efficiency than MB's (12.2%), as well as MO's (7.4%).
Researchers also looked at the sensitized degradation of the Rh-B/MB and Rh-B/MO mixture solution by BN when exposed to visible light. When Rh-B is combined with MB or MO, exciting outcomes are obtained. As shown in Fig. 23C, mixing MB and Rh-B did not produce a new UV absorption peak (662 nm for MB and 552 nm for Rh-B, respectively). This could be because Rh-B is a non-planar cation, and MB and Rh-B are both cationic dyes. As a result, in Rh-B/MB combinations, the connection between Rh-B and methylene blue is not much stronger to alter the UV absorption peaks of Rh-B and methylene blue. The ultraviolet absorption peaks of a combination of Rh-B and methylene blue are formed by superimposing their respective ultraviolet absorption peaks. The total removal of Rh-B from the Rh-B/MB combination is 21.4% (Fig. 23C), of which 12.2% is due to Rh-B's adsorption on BN, and another 9.2% is due to Rh-B's sensitization degradation on BN under visible light irradiation. A total of 79.2% of the MB in the Rh-B/MB combination (Fig. 23C) is eliminated, of which MB's adsorption on BN removes 33.8%, and another 45.4% is removed by MB's sensitization degradation on BN when exposed to visible light. This outcome is somewhat surprising, considering that Rh-B's sensitization photodegradation by BN is prohibited, and Rh-B's sensitization photodegradation by BN is promoted.
Similarly, studies are being conducted on the sensitization photodegradation of Rh-B/MO. According to Fig. 23D, the ultraviolet absorption peak of MO is responsible for the peak at 470 nm in the Rh-B/MO mixture, and the ultraviolet absorption peak of Rh-B is responsible for the peak at 556 nm in the Rh-B/MO mixture. As a result, the UV absorption peaks of Rh-B/MO are a superposition of those of Rh-B and MO. Rh-B is a positively charged dye, while methyl orange is an anion dye. They interact with each other with a greater energy of 21.2 kcal mol−1 than Rh-B and MB (11.5 kcal mol−1). A total of 23.1% of the Rh-B in the Rh-B/MO combination is eliminated, of which 20.5% is due to Rh-B's adsorption on BN, and the remaining 2.6% is due to Rh-B's sensitization degradation on BN when exposed to visible light. In contrast, MO is eliminated from the Rh-B/MO combination by 13.9%, of which 3.2% is due to MO's adsorption on BN, and another 10.7% is due to MO's sensitization degradation on BN when exposed to visible light. As shown, the sensitisation degradation rate of Rh-B falls from 45.2% to 2.6%, while that of MO slightly increases from 7.4% to 10.7%. The summary of the photodegradation of dyes by BN-based nanomaterials is given in Table 5.
| BN-based nanomaterial | Dyes | Initial concentration n (mg L−1) | Efficiency (%) | Ref. |
|---|---|---|---|---|
| BN | Rhodamine B | 20 | 45.2 | 240 |
| BN/SnO2 | Methyl orange | 25 | 92 | 216 |
| BN–TiO2 | Rhodamine B & methylene blue | 10 | 98 & 92 | 245 |
| ZnO/BNQD-4 | Methylene blue & methyl orange | 10 | 99 & 97.9 | 246 |
| BN, chitosan & graphene-based composite (BNCG) | Methylene blue | 10 | 87 | 247 |
| BN5–Ag3/TiO2 | Methylene blue | 20 | 98 | 248 |
| BN/C3N4 | Rhodamine B | 5 | 97.2 | 249 |
| h-BN@PbWO4 | Rhodamine B | 5 | 99.9 | 250 |
| ZnFe2O4/BN | Congo red | 10 | 92 | 251 |
| h-BN/Sb2WO6 | Rhodamine B | 25 | 80.8 | 252 |
| ZnTi LDH/h-BN | Amaranth | 20 | 100 | 182 |
| BN-modified bismuth oxyiodide | Methyl orange | 10 | 86 | 253 |
| Ni–BaTiO3/h-BN | Crystal violet | 15 | 86 | 254 |
| BN/Fe | Methyl orange | 150 | 99.1 | 255 |
| h-BN/NiS2/NiS | Rhodamine B | 10 | 98.5 | 256 |
| F–BN/TiO2 | Rhodamine B | 150 | 92 | 257 |
| BN nanotubes (BNNTs) | Methyl orange | 5 | 92 | 258 |
4.3. Hydrogen evolution reaction
Researchers are urged to identify alternative carbon-neutral energy vectors as part of efforts to mitigate the impact of global climate change.259,260 In light of this, hydrogen produced from renewable sources is a prospective negligible-emission fuel and a crucial resource for the chemical sector. Because of its beneficial characteristics, like low density combined with the maximum energy densities of all substances known to man and ease of collection and transport, this finds many uses in the current environment. The potential of newly discovered nanomaterials for photocatalytic water splitting is shown in Table 6.High manufacturing costs are one of the key obstacles to the practical use of “green” hydrogen. However, if economically reactive metal catalysts having more solar-to-hydrogen efficiency are produced, solar-driven water splitting might be a cost-effective method.261,262 Highly active photocatalytic technologies have recently been produced employing various innovative construction techniques through 2D materials as platforms.263–265 The h-BN is the most notable member of this family. It is excellent for various applications because of its extraordinary chemical stability and heat conductivity.266,267 Moreover, the unfavourable oxide–metal interactions in heterogeneous catalysis can be eliminated by replacing oxide supports with h-BN.268 This has earlier been used for things like CO2 reduction,269 H2 synthesis,270 NOx reductions,271 NH3 synthesis,272 and the oxidation of organic contaminants.273 Recently, there has been a lot of interest in using h-BN as a catalyst-assisted material combined with different nanocomposite materials for photocatalytic activities.
Charge separation and electron transport need to be made easier by a catalyst and support that are well coupled to increase the photocatalytic activities as illustrated in Fig. 24.274 The charge carrier separation is facilitated by the photoexcited holes being drawn to the negatively charged as-synthesized h-BN nanostructures. The adsorption of hydroxyl ions is predicted to cause a negative charge on the newly synthesized h-BN surface.275,276 In many photocatalytic applications, the synthetic h-BN is a good catalyst support material for composite catalysts due to its inherent behaviour.277,278 On the other hand, several methods for creating extremely effective and affordable bifunctional materials have been discovered to accelerate the hydrogen evolution process from water splitting.
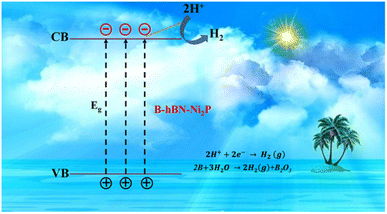 | ||
| Fig. 24 Water splitting via photocatalytic hydrogen evolution.274 | ||
Recently, NiS/Ni2P heterostructures were synthesized by Xiao et al.279 for effective electrocatalytic water splitting. Because Ni2P has the synergistic effects of the Ni vacancy trapping electrons and the P site accepting protons, the Ni–P bond does have some charge carrier separation that is advantageous for hydrogen production.280 The inclusion of h-BN assists in prolonging the charge carrier life period in the heterostructure, but Ni2P itself is hampered by the quick coupling of the photogenerated charge carriers across its small band gap. The h-BN/Ni2P catalyst's band gap is adjusted for improved hydrogen formation by solar water splitting. This properly regulated band gap increased the photocatalytic activity by regulating the recoupling process of the photogenerated carriers. Additionally, it has been observed that the activity of photocatalytic hydrogen evolution may be increased by doping specific semiconductors with boron281,282 due to B's capacity to function as a photocatalyst for the generation of hydrogen from solar water splitting, which is shown in the equation below.283
| 2B + 3H2O → 3H2 + B2O3 | (5) |
Water is photolyzed by utilizing the electrons of the electron–hole pairs produced with irradiation at low and close pH, respectively, to convert H+ or water molecules into H2. Photoexcited electrons move from the valence band to the conduction band of the narrow band-gap semiconductor Ni2P when the B/h-BN/Ni2P system is exposed to solar radiation, leaving holes in the valence band. The redox process, which produces hydrogen, is then started by the electrons moving toward the surface of Ni2P's conduction band. The ionic interaction between the anionic h-BN and the holes in the valence bands of Ni2P facilitates the further reaction. Hence, the impact of h-BN effectively boosts the activities of the photocatalyst by reducing the rate at which the charge carriers produced by photosynthesis recombine.
Similarly, in the presence of sunlight, the excess boron produced in the h-BN/Ni2P lattice further separates water into H2 through a secondary reaction, as seen in Fig. 25.283 In this case, boron is not regenerated inside the photocatalytic composite and is instead oxidized to B2O3 under illumination together with the production of hydrogen. According to the hypothesized mechanism, Ni2P, assisted by h-BN, acts as a major photocatalyst for hydrogen synthesis, whereas B can increase the total hydrogen production through a different reaction route.283 Therefore, in the current system, B may be considered a “hydrogen production booster” that is depleted as the reaction progresses under irradiation. As a result, the main photocatalyst and boron work together to increase the rate at which hydrogen is produced overall. Our investigation shows that bare Ni2P shows photocatalytic activity for producing hydrogen from splitting water.274 Moreover, the mechanism's efficiency is increased by including h-BN, perhaps by improving the charge separation caused by photogeneration.278 Therefore, the reactions would not completely stop even if boron were consumed. As a result, the overall impact of the catalysts improves the entire photocatalytic hydrogen-generating reaction through solar water splitting.
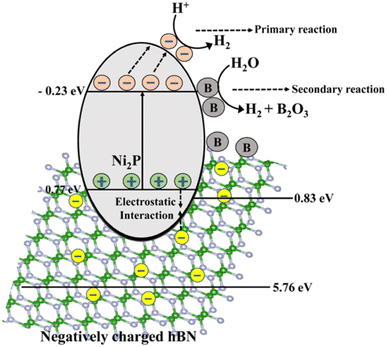 | ||
| Fig. 25 The schematic illustration of the mechanism of the photocatalytic H2 production by the B/h-BN/Ni2P nanocomposite.283 | ||
5. Future prospects
Although BN-based structures are becoming fantastic materials for various applications, including the removal of inorganic contaminants from water bodies and the removal of dyes and pharmaceuticals, several challenges remain.Due to its appealing qualities, including a wide surface area, excellent chemical and mechanical strength, a lot of structural flaws, more reactive sites, and functional groups, BN-based nanomaterials exhibit confident adsorptive capability. However, numerous other study fields may need to be examined to assess the effectiveness and applicability of BN or BN-based nanomaterials more accurately.
BN-based products have little or no toxicity, according to most research studies that are currently accessible. However, it is predicted that BN nanostructures can provide environmental risks because of the complexity of nanoparticles and the unique architectures of BN particles. Compared to carbon nanotubes, some research claims that BNNTs are more cytotoxic.290 Therefore, an in-depth investigation is required to thoroughly assess the toxicities and bio-compatibility of BN particles.
Other technological issues also present opportunities for innovation, such as creating an online tracking system for determining the purity and trace amounts of BN nanoparticles in water. Most studies on BN-based nanomaterials revealed results from laboratory tests, and the investigation is still in its early stages. Evaluating the possibilities of BN-based nanomaterials in continuous operation systems is essential. Additionally, there is enormous potential for creating brand-new, affordable, simple, profitable, and ecologically responsible processes for the high-yield preparation of BN-based products. Furthermore, it is essential to thoroughly research how BN-based products interact with contaminants. Applying analytical and characterization methods to verify the theoretically predicted adsorption mechanisms may be advantageous.
Another problem restricting the use of BN-based materials is their tendency to aggregate. The solubility of unmodified BN nanoparticles, which are often hydrophobic, in the aqueous media is another issue and a possible study topic. To develop strategies for simple dispersions, study of the surfaces of these materials must be carried out, as must research on synthetic methods. Although numerous types of research have documented the synthesis and characterization of BN materials, further research is required to broaden this research area. It is necessary to investigate suitable operationalized techniques and materials to produce BN materials with the requisite characteristics for particular applications. The solubility and the dispersibility of BN nanoparticles in water may be enhanced via chemical functionalization.291
Like most new materials, BN-based compounds have not been used in actual applications to address genuine water purification issues. Researchers need to examine whether it is possible to use these incredible materials in practical applications. This necessitates the creation of a system that can produce BN-based products in large quantities at a reasonable cost, as well as evaluating many commercial and economic factors. BN-based compounds have not been evaluated in many sections of the water purification industry. For instance, the desalination capabilities of these materials have not received much attention. Researchers might focus on using these materials in various desalination systems to assess their effectiveness.
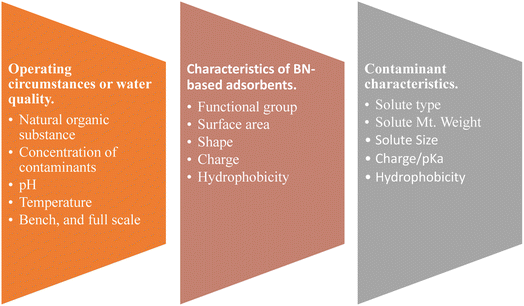
Using BN materials with antibacterial properties might create new possibilities for post- or pre-treatment water and desalination. Numerous theoretical studies have also predicted the exceptional qualities of BN materials in purifying water. Researchers must concentrate on creating novel BN structures and consider alternative environmental uses for old and new BN materials. There is a high probability that BN-based materials will soon be introduced to the market. Based on recent positive results, it may be reasonably predicted that BN-based materials may become the future water purification materials. Fig. 26 summarises the potential main areas of future research on BN-based nanomaterials from the perspective of pollutant qualities, wastewater quality conditions, and BN-based adsorbent capabilities.
6. Conclusion
Currently, BN is the most encouraging nanomaterial. This paper describes various applications of BN, which was earlier considered a synthetic material of no advantageous use. In a broad spectrum of introspection, it is widely used in heavy metals, dyes, and pharmaceutical adsorption. Basic methods to synthesise nanomaterials include mechanical exfoliation, chemical exfoliation, and chemical vapour deposition. It also avails a greener synthesis method, i.e., green dry ice-assisted ball milling. The fundamental properties that it holds enhance its beauty in the field of its application, like large surface area, mechanical stiffness, thermal stability, and superior thermal conductivity for which it is attractive for the adsorption of various inorganic (heavy metals) and organic pollutants, mainly dyes and antibiotics, and the information they give can be useful for the treatment of water and wastewater. Its uses in photodegradation are highly appreciated and engulfed to remove organic pollutants. Hydrogen evolution from water splitting is also carried out because of its low density, combined with the highest energy densities of all substances known to man and ease of storage and transportation.Conflicts of interest
There are no conflicts to declare.References
- L. F. Dobrzhinetskaya, R. Wirth, J. Yang, H. W. Green, I. D. Hutcheon, P. K. Weber and E. S. Grew, Am. Mineral., 2014, 99, 764–772 CrossRef CAS.
- Y. Shimizu, Y. Moriyoshi, H. Tanaka and S. Komatsu, Appl. Phys. Lett., 1999, 75, 929 CrossRef CAS.
- D. Golberg, Y. Bando, O. Stéphan and K. Kurashima, Appl. Phys. Lett., 1998, 73, 2441–2443 CrossRef CAS.
- C. Tang, Y. Bando and D. Golberg, Chem. Commun., 2002, 2, 2826–2827 RSC.
- Y. Saito, M. Maida and T. Matsumoto, Jpn. J. Appl. Phys., Part 1, 1999, 38, 159–163 CrossRef CAS.
- Z. G. Chen, J. Zou, G. Liu, F. Li, Y. Wang, L. Wang, X. L. Yuan, T. Sekiguchi, H. M. Cheng and G. Q. Lu, ACS Nano, 2008, 2, 2183–2191 CrossRef CAS PubMed.
- Y. Qiu, J. Yu, J. Yin, C. Tan, X. Zhou, X. Bai and E. Wang, Nanotechnology, 2009, 20, 345603 CrossRef PubMed.
- H. Zhang, J. Yu, Y. Chen and J. F. Gerald, J. Am. Ceram. Soc., 2006, 89, 675–679 CrossRef CAS.
- R. Haubner, M. Wilhelm, R. Weissenbacher and B. Lux, High Performance Non-Oxide Ceramics II, 2002, vol. 102, pp. 1–45 Search PubMed.
- M. Corso, W. Auwärter, M. Muntwiler, A. Tamai, T. Greber and J. Osterwalder, Science, 2004, 303, 217–220 CrossRef CAS PubMed.
- D. Golberg, Y. Bando, Y. Huang, T. Terao, M. Mitome, C. Tang and C. Zhi, ACS Nano, 2010, 4, 2979–2993 CrossRef CAS PubMed.
- Y. Lin, T. V. Williams and J. W. Connell, J. Phys. Chem. Lett., 2010, 1, 277–283 CrossRef CAS.
- P. Ma and J. T. Spencer, J. Mater. Sci., 2015, 50, 313–323 CrossRef CAS.
- L. Ci, L. Song, C. Jin, D. Jariwala, D. Wu, Y. Li, A. Srivastava, Z. F. Wang, K. Storr, L. Balicas, F. Liu and P. M. Ajayan, Nat. Mater., 2010, 9, 430–435 CrossRef CAS PubMed.
- G. Elumalai, H. Noguchi and K. Uosaki, Phys. Chem. Chem. Phys., 2014, 16, 13755–13761 RSC.
- H. B. Cho, Y. Tokoi, S. Tanaka, H. Suematsu, T. Suzuki, W. Jiang, K. Niihara and T. Nakayama, Compos. Sci. Technol., 2011, 71, 1046–1052 CrossRef CAS.
- X. Wang, A. Pakdel, J. Zhang, Q. Weng, T. Zhai, C. Zhi, D. Golberg and Y. Bando, Nanoscale Res. Lett., 2012, 7, 1–7 CrossRef PubMed.
- W.-L. Song, P. Wang, L. Cao, A. Anderson, M. J. Meziani, A. J. Farr and Y.-P. Sun, Angew. Chem., 2012, 124, 6604–6607 CrossRef.
- J. Yu, X. Huang, C. Wu, X. Wu, G. Wang and P. Jiang, Polymer, 2012, 53, 471–480 CrossRef CAS.
- K. Watanabe, T. Taniguchi and H. Kanda, Nat. Mater., 2004, 3, 404–409 CrossRef CAS PubMed.
- C. R. Dean, A. F. Young, I. Meric, C. Lee, L. Wang, S. Sorgenfrei, K. Watanabe, T. Taniguchi, P. Kim, K. L. Shepard and J. Hone, Nat. Nanotechnol., 2010, 5, 722–726 CrossRef CAS PubMed.
- Z. Q. Duan, Y. T. Liu, X. M. Xie and X. Y. Ye, Chin. Chem. Lett., 2013, 24, 17–19 CrossRef CAS.
- M. Emanet, Ö. Şen, Z. Çobandede and M. Çulha, Colloids Surf., B, 2015, 134, 440–446 CrossRef CAS PubMed.
- R. Agrawal, A. Nieto, H. Chen, M. Mora and A. Agarwal, ACS Appl. Mater. Interfaces, 2013, 5, 12052–12057 CrossRef CAS PubMed.
- Z. Zheng, M. C. Cox and B. Li, J. Mater. Sci., 2017, 53, 66–99 CrossRef.
- Y. L. Zhou, J. F. Zhi, P. F. Wang, Y. M. Chong, Y. S. Zou, W. J. Zhang and S. T. Lee, Appl. Phys. Lett., 2008, 92, 163105 CrossRef.
- V. Raffa, G. Ciofani and A. Cuschieri, Nanotechnology, 2009, 20, 075104 CrossRef CAS PubMed.
- Q. Weng, B. Wang, X. Wang, N. Hanagata, X. Li, D. Liu, X. Wang, X. Jiang, Y. Bando and D. Golberg, ACS Nano, 2014, 8, 6123–6130 CrossRef CAS PubMed.
- C. Zhi, Y. Bando, T. Terao, C. Tang, H. Kuwahara and D. Golberg, Adv. Funct. Mater., 2009, 19, 1857–1862 CrossRef CAS.
- X. Huang, C. Zhi, P. Jiang, D. Golberg, Y. Bando and T. Tanaka, Adv. Funct. Mater., 2013, 23, 1824–1831 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang, H. Kuwahara and D. Golberg, Adv. Mater., 2009, 21, 2889–2893 CrossRef CAS.
- T. Guo, P. Nikolaev, A. G. Rinzler, D. Tomanek, D. T. Colbert and R. E. Smalley, J. Phys. Chem., 1995, 99, 10694–10697 CrossRef CAS.
- S. Dai, Z. Fei, Q. Ma, A. S. Rodin, M. Wagner, A. S. McLeod, M. K. Liu, W. Gannett, W. Regan, K. Watanabe, T. Taniguchi, M. Thiemens, G. Dominguez, A. H. Castro Neto, A. Zettl, F. Keilmann, P. Jarillo-Herrero, M. M. Fogler and D. N. Basov, Science, 2014, 343, 1125–1129 CrossRef CAS PubMed.
- J. D. Caldwell, A. V. Kretinin, Y. Chen, V. Giannini, M. M. Fogler, Y. Francescato, C. T. Ellis, J. G. Tischler, C. R. Woods, A. J. Giles, M. Hong, K. Watanabe, T. Taniguchi, S. A. Maier and K. S. Novoselov, Nat. Commun., 2014, 5, 1–9 Search PubMed.
- A. Woessner, M. B. Lundeberg, Y. Gao, A. Principi, P. Alonso-González, M. Carrega, K. Watanabe, T. Taniguchi, G. Vignale, M. Polini, J. Hone, R. Hillenbrand and F. H. L. Koppens, Nat. Mater., 2014, 14, 421–425 CrossRef PubMed.
- S. Dai, Q. Ma, M. K. Liu, T. Andersen, Z. Fei, M. D. Goldflam, M. Wagner, K. Watanabe, T. Taniguchi, M. Thiemens, F. Keilmann, G. C. A. M. Janssen, S. E. Zhu, P. Jarillo-Herrero, M. M. Fogler and D. N. Basov, Nat. Nanotechnol., 2015, 10, 682–686 CrossRef CAS PubMed.
- T. T. Tran, K. Bray, M. J. Ford, M. Toth and I. Aharonovich, Nat. Nanotechnol., 2015, 11, 37–41 CrossRef PubMed.
- A. J. Giles, S. Dai, I. Vurgaftman, T. Hoffman, S. Liu, L. Lindsay, C. T. Ellis, N. Assefa, I. Chatzakis, T. L. Reinecke, J. G. Tischler, M. M. Fogler, J. H. Edgar, D. N. Basov and J. D. Caldwell, Nat. Mater., 2017, 17, 134–139 CrossRef PubMed.
- T. Q. P. Vuong, S. Liu, A. Van Der Lee, R. Cuscó, L. Artús, T. Michel, P. Valvin, J. H. Edgar, G. Cassabois and B. Gil, Nat. Mater., 2017, 17, 152–158 CrossRef PubMed.
- T. Taniguchi and K. Watanabe, J. Cryst. Growth, 2007, 303, 525–529 CrossRef CAS.
- X. Z. Du, J. Li, J. Y. Lin and H. X. Jiang, Appl. Phys. Lett., 2016, 108, 052106 CrossRef.
- Y. J. Cho, A. Summerfield, A. Davies, T. S. Cheng, E. F. Smith, C. J. Mellor, A. N. Khlobystov, C. T. Foxon, L. Eaves, P. H. Beton and S. V. Novikov, Sci. Rep., 2016, 6, 1–7 CrossRef PubMed.
- T. Q. P. Vuong, G. Cassabois, P. Valvin, E. Rousseau, A. Summerfield, C. J. Mellor, Y. Cho, T. S. Cheng, J. D. Albar, L. Eaves, C. T. Foxon, P. H. Beton, S. V. Novikov and B. Gil, 2D Mater., 2017, 4, 021023 CrossRef.
- J. D. Caldwell, I. Aharonovich, G. Cassabois, J. H. Edgar, B. Gil and D. N. Basov, Nat. Rev. Mater., 2019, 4, 552–567 CrossRef CAS.
- J. Koskelo, G. Fugallo, M. Hakala, M. Gatti, F. Sottile and P. Cudazzo, Phys. Rev. B, 2017, 95, 035125 CrossRef.
- E. Yoxall, M. Schnell, A. Y. Nikitin, O. Txoperena, A. Woessner, M. B. Lundeberg, F. Casanova, L. E. Hueso, F. H. L. Koppens and R. Hillenbrand, Nat. Photonics, 2015, 9, 674–678 CrossRef CAS.
- O. Hassel, BN. Norsk. Geol. Tidskr., 1926, 9, 266 Search PubMed.
- A. Brager, Acta Physicochim. URSS, 1937, 7, 699–706 CAS.
- E. A. Wood and IUCr, Acta Crystallogr., 1951, 4, 353–362 CrossRef CAS.
- J. Tersoff, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 9902 CrossRef CAS PubMed.
- S. Plimpton, J. Comput. Phys., 1995, 117, 1–19 CrossRef CAS.
- A. Stukowski, Modell. Simul. Mater. Sci. Eng., 2009, 18, 015012 CrossRef.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- V. D. Blank, D. V. Batov, B. A. Kulnitskiy, E. V. Polyakov, I. A. Perezhogin, D. A. Podgorny and Y. N. Parkhomenko, Journal of Superhard Materials, 2007, 29, 206–212 CrossRef.
- P. M. Ajayan and T. W. Ebbesen, Rep. Prog. Phys., 1997, 60, 1025 CrossRef CAS.
- Y. S. Buranova, B. A. Kulnitskiy, I. A. Perezhogin and V. D. Blank, Crystallogr. Rep., 2015, 60, 90–94 CrossRef CAS.
- D. Golberg, Y. Bando, K. Kurashima and T. Sato, Solid State Commun., 2000, 116, 1–6 CrossRef CAS.
- R. Ma and Y. Bando, Sci. Technol. Adv. Mater., 2003, 4, 403–407 CrossRef CAS.
- L. Y. Heng, A. Chou, J. Yu, Y. Chen and J. J. Gooding, Electrochem. Commun., 2005, 7, 1457–1462 CrossRef CAS.
- R. Ma, Y. Bando, T. Sato, D. Golberg, H. Zhu, C. Xu and D. Wu, Appl. Phys. Lett., 2002, 81, 5225 CrossRef CAS.
- T. Oku, K. Hiraga, T. Matsuda, T. Hirai and M. Hirabayashi, Diamond Relat. Mater., 2003, 12, 1138–1145 CrossRef CAS.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- J. C. Meyer, A. Chuvilin, G. Algara-Siller, J. Biskupek and U. Kaiser, Nano Lett., 2009, 9, 2683–2689 CrossRef CAS PubMed.
- R. V. Gorbachev, I. Riaz, R. R. Nair, R. Jalil, L. Britnell, B. D. Belle, E. W. Hill, K. S. Novoselov, K. Watanabe, T. Taniguchi, A. K. Geim and P. Blake, Small, 2011, 7, 465–468 CrossRef CAS PubMed.
- L. H. Li, Y. Chen, G. Behan, H. Zhang, M. Petravic and A. M. Glushenkov, J. Mater. Chem., 2011, 21, 11862–11866 RSC.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H. Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- O. R. Lourie, C. R. Jones, B. M. Bartlett, P. C. Gibbons, R. S. Ruoff and W. E. Buhro, Chem. Mater., 2000, 12, 1808–1810 CrossRef CAS.
- W. Auwärter, H. U. Suter, H. Sachdev and T. Greber, Chem. Mater., 2004, 16, 343–345 CrossRef.
- F. Müller, K. Stöwe and H. Sachdev, Chem. Mater., 2005, 17, 3464–3467 CrossRef.
- X. Song, J. Gao, Y. Nie, T. Gao, J. Sun, D. Ma, Q. Li, Y. Chen, C. Jin, A. Bachmatiuk, M. H. Rümmeli, F. Ding, Y. Zhang and Z. Liu, Nano Res., 2015, 8, 3164–3176 CrossRef CAS.
- P. Ahmad, M. U. Khandaker, Y. M. Amin and Z. R. Khan, AIP Conf. Proc., 2015, 1657, 100010 CrossRef.
- R. Arenal, O. Stephan, J. Lou Cochon and A. Loiseau, J. Am. Chem. Soc., 2007, 129, 16183–16189 CrossRef CAS PubMed.
- D. Golberg, Y. Bando, M. Eremets, K. Takemura, K. Kurashima and H. Yusa, Appl. Phys. Lett., 1998, 69, 2045 CrossRef.
- D. P. Yu, X. S. Sun, C. S. Lee, I. Bello, S. T. Lee, H. D. Gu, K. M. Leung, G. W. Zhou, Z. F. Dong and Z. Zhang, Appl. Phys. Lett., 1998, 72, 1966 CrossRef CAS.
- O. Stephan, P. M. Ajayan, C. Colliex, P. Redlich, J. M. Lambert, P. Bernier and P. Lefin, Science, 1994, 266, 1683–1685 CrossRef CAS PubMed.
- J. Cumings and A. Zettl, Chem. Phys. Lett., 2000, 316, 211–216 CrossRef CAS.
- C. Y. Zhang, X. L. Zhong, J. B. Wang and G. W. Yang, Chem. Phys. Lett., 2003, 370, 522–527 CrossRef CAS.
- W. Q. Han, H. G. Yu and Z. Liu, Appl. Phys. Lett., 2011, 98, 203112 CrossRef.
- W. Han, Y. Bando, K. Kurashima and T. Sato, Appl. Phys. Lett., 1998, 73, 3085 CrossRef CAS.
- R. Gao, L. Yin, C. Wang, Y. Qi, N. Lun, L. Zhang, Y. X. Liu, L. Kang and X. Wang, J. Phys. Chem. C, 2009, 113, 15160–15165 CrossRef CAS.
- B. T. Branson, P. S. Beauchamp, J. C. Beam, C. M. Lukehart and J. L. Davidson, ACS Nano, 2013, 7, 3183–3189 CrossRef CAS PubMed.
- M. Hemmat Esfe, A. Naderi, M. Akbari, M. Afrand and A. Karimipour, J. Therm. Anal. Calorim., 2015, 121, 1273–1278 CrossRef CAS.
- H. Zhao, J. Ding, B. Xu, X. Zhao, Y. Zheng, Z. Shao and H. Yu, ACS Sustain. Chem. Eng., 2019, 7, 14266–14272 CrossRef CAS.
- A. Lipp, K. A. Schwetz and K. Hunold, J. Eur. Ceram. Soc., 1989, 5, 3–9 CrossRef CAS.
- V. Vatanpour, S. A. Naziri Mehrabani, B. Keskin, N. Arabi, B. Zeytuncu and I. Koyuncu, Ind. Eng. Chem. Res., 2021, 60, 13391–13424 CrossRef CAS.
- C. Zhou, C. Lai, C. Zhang, G. Zeng, D. Huang, M. Cheng, L. Hu, W. Xiong, M. Chen, J. Wang, Y. Yang and L. Jiang, Appl. Catal., B, 2018, 238, 6–18 CrossRef CAS.
- Y. G. Park, S. N. Nam, M. Jang, C. Min Park, N. Her, J. Sohn, J. Cho and Y. Yoon, Sep. Purif. Technol., 2022, 288, 120637 CrossRef CAS.
- R. Arora, Mater. Today: Proc., 2019, 18, 4745–4750 CAS.
- T. Meng, C. Wang, W. J. Florkowski and Z. Yang, Aquac. Econ. Manag., 2023, 27, 1–24, DOI:10.1080/13657305.2021.1996480.
- M. K. Sharma, C. K. Jain, V. K. Choubey and D. C. Singhal, Environ. Monit. Assess., 2008, 157, 11–21 CrossRef PubMed.
- F. Han, Y. Zong, D. Jassby, J. Wang and J. Tian, Environ. Res., 2020, 183, 109240 CrossRef CAS PubMed.
- Q. Zhu and Z. Li, Chem. Eng. J., 2015, 281, 69–80 CrossRef CAS.
- Y. Xie, X. Yuan, Z. Wu, G. Zeng, L. Jiang, X. Peng and H. Li, J. Colloid Interface Sci., 2019, 536, 440–455 CrossRef CAS PubMed.
- X. Zhao, Y. Wang, H. Wu, L. Fang, J. Liang, Q. Fan and P. Li, J. Mol. Liq., 2017, 248, 1030–1038 CrossRef CAS.
- J. Li, X. Xiao, X. Xu, J. Lin, Y. Huang, Y. Xue, P. Jin, J. Zou and C. Tang, Sci. Rep., 2013, 3, 1–7 Search PubMed.
- R. S. Bangari, V. K. Yadav, J. K. Singh and N. Sinha, ACS Omega, 2020, 5, 10301–10314 CrossRef CAS PubMed.
- W. Shen, Q. Guo, Y. Zhang, Y. Liu, J. Zheng, J. Cheng and J. Fan, Colloids Surf., A, 2006, 273, 147–153 CrossRef CAS.
- W. Da Oh, M. G. H. Lee, W. D. Chanaka Udayanga, A. Veksha, Y. Bao, A. Giannis, J. W. Lim and G. Lisak, J. Environ. Chem. Eng., 2019, 7, 102872 CrossRef.
- J. Li, P. Jin, W. Dai, C. Wang, R. Li, T. Wu and C. Tang, Mater. Chem. Phys., 2017, 196, 186–193 CrossRef CAS.
- T. Liu, Y. L. Li, J. Y. He, Y. Hu, C. M. Wang, K. S. Zhang, X. J. Huang, L. T. Kong and J. H. Liu, New J. Chem., 2019, 43, 3280–3290 RSC.
- M. Wang, Y. Bai, B. Zhang, B. Zhong, Y. Yu, J. Zhang, X. Huang and G. Wen, Ceram. Int., 2019, 45, 6684–6692 CrossRef CAS.
- R. S. Bangari, A. K. Singh, S. Namsani, J. K. Singh and N. Sinha, ACS Appl. Mater. Interfaces, 2019, 11, 19017–19028 CrossRef CAS PubMed.
- P. Karthikeyan, S. S. D. Elanchezhiyan, J. Preethi, S. Meenakshi and C. M. Park, Appl. Surf. Sci., 2020, 511, 145543 CrossRef CAS.
- S. Wang, G. Wang, T. Wu, Y. Zhang, F. Zhan, Y. Wang, J. Wang, Y. Fu and J. Qiu, J. Mater. Chem. A, 2018, 6, 14644–14650 RSC.
- Z. Song, K. Guo, W. Bai and C. Tang, J. Solid State Chem., 2023, 317, 123720 CrossRef CAS.
- P. Zhang, Y. Chen, Q. Guo, Y. Liu, H. Chong, H. Weng, X. Zhao, Y. Yang and M. Lin, Sep. Purif. Technol., 2023, 305, 122538 CrossRef CAS.
- X. Gao, X. Huang, J. Lin, C. Yu, C. Tang and Y. Huang, Soft Matter, 2023, 19, 973–982 RSC.
- U. Lawal Usman, B. Kumar Allam, N. Bahadur Singh and S. Banerjee, J. Mol. Liq., 2022, 354, 118833 CrossRef CAS.
- J. Kou, X. Wei, H. Wu, W. Linghu, G. Sheng and Y. Pan, J. Mol. Liq., 2022, 359, 119355 CrossRef CAS.
- Y. Chen, P. Zhang, L. Jiao, G. Chen, Y. Yang, H. Chong and M. Lin, Chem. Eng. J., 2022, 446, 137337 CrossRef CAS.
- S. Li, F. Liu, Y. Su, N. Shao, D. Yu, Y. Liu, W. Liu and Z. Zhang, J. Hazard. Mater., 2019, 378, 120669 CrossRef CAS PubMed.
- L. Li, K. Chang, P. Fang, K. Du, C. Chen, S. Zhou, C. Shen, W. Linghu, G. Sheng, T. Hayat and X. Guo, Appl. Surf. Sci., 2020, 521, 146373 CrossRef CAS.
- T. Liu, Y. Li, J. He, K. Zhang, Y. Hu, X. Chen, C. Wang, X. Huang, L. Kong and J. Liu, J. Mater. Sci., 2019, 54, 5366–5380 CrossRef CAS.
- L. L. Ling, W. J. Liu, S. Zhang and H. Jiang, Environ. Sci. Technol., 2017, 51, 10081–10089 CrossRef CAS PubMed.
- F. khan, N. Abdul, I. U. Din, T. Saeed, M. A. Alotaibi, A. I. Alharthi, A. Habib and T. Malik, Ceram. Int., 2021, 47, 4749–4757 CrossRef CAS.
- D. Peng, W. Jiang, F. F. Li, L. Zhang, R. P. Liang and J. D. Qiu, ACS Sustain. Chem. Eng., 2018, 6, 11685–11694 CrossRef CAS.
- Y. Zhang, G. Wang, S. Wang, J. Wang and J. Qiu, J. Colloid Interface Sci., 2019, 552, 604–612 CrossRef CAS PubMed.
- G. Yadav, S. R. Mishra, V. Gadore, N. Yadav and M. Ahmaruzzaman, Sci. Rep., 2023, 13, 1–21 CrossRef PubMed.
- A. K. Dey, S. R. Mishra and M. Ahmaruzzaman, Environ. Sci. Pollut. Res., 2023, 30, 53887–53903 CrossRef CAS PubMed.
- Y. Wang, Y. Mu, Q. B. Zhao and H. Q. Yu, Sep. Purif. Technol., 2006, 50, 1–7 CrossRef CAS.
- H. Liu, P. Li, T. Zhang, Y. Zhu and F. Qiu, Food Bioprod. Process., 2020, 119, 257–267 CrossRef CAS.
- V. Gadore, S. R. Mishra and M. Ahmaruzzaman, Sci. Rep., 2023, 13, 7708 CrossRef CAS PubMed.
- S. Nethaji and A. Sivasamy, Chemosphere, 2011, 82, 1367–1372 CrossRef CAS PubMed.
- J. Wang, T. Tsuzuki, B. Tang, X. Hou, L. Sun and X. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 3084–3090 CrossRef CAS PubMed.
- M. Ghaedi, M. Ghayedi, S. N. Kokhdan, R. Sahraei and A. Daneshfar, J. Ind. Eng. Chem., 2013, 19, 1209–1217 CrossRef CAS.
- A. Mirzaei, A. Ebadi and P. Khajavi, Chem. Eng. J., 2013, 231, 550–560 CrossRef CAS.
- L. Malaeb and G. M. Ayoub, Desalination, 2011, 267, 1–8 CrossRef CAS.
- F. Han, V. S. R. Kambala, M. Srinivasan, D. Rajarathnam and R. Naidu, Appl. Catal., A, 2009, 359, 25–40 CrossRef CAS.
- A. Lee, J. W. Elam and S. B. Darling, Environ. Sci.: Water Res. Technol., 2016, 2, 17–42 RSC.
- C. Sheng, A. G. A. Nnanna, Y. Liu and J. D. Vargo, Sci. Total Environ., 2016, 550, 1075–1083 CrossRef CAS PubMed.
- M. Ptaszkowska-Koniarz, J. Goscianska and R. Pietrzak, J. Colloid Interface Sci., 2017, 504, 549–560 CrossRef CAS PubMed.
- Y. D. Liang, Y. J. He, T. T. Wang and L. H. Lei, Journal of Water Process Engineering, 2019, 27, 77–88 CrossRef.
- R. S. Bangari, A. Yadav and N. Sinha, Soft Matter, 2021, 17, 2640–2651 RSC.
- J. Li, Y. Huang, Z. Liu, J. Zhang, X. Liu, H. Luo, Y. Ma, X. Xu, Y. Lu, J. Lin, J. Zou and C. Tang, J. Mater. Chem. A, 2015, 3, 8185–8193 RSC.
- Z. Zhou, J. Zhao, Z. Chen, X. Gao, T. Yan, B. Wen and P. Von Ragué Schleyer, J. Phys. Chem. B, 2006, 110, 13363–13369 CrossRef CAS PubMed.
- M. X. Zhu, L. Lee, H. H. Wang and Z. Wang, J. Hazard. Mater., 2007, 149, 735–741 CrossRef CAS PubMed.
- Y. Guo, R. Wang, P. Wang, L. Rao and C. Wang, ACS Sustain. Chem. Eng., 2019, 7, 5727–5741 CrossRef CAS.
- P. Wang, C. Wu, Y. Guo and C. Wang, Phys. Chem. Chem. Phys., 2016, 18, 30196–30203 RSC.
- P. Wang, Y. Yin, Y. Guo and C. Wang, RSC Adv., 2016, 6, 10615–10624 RSC.
- Y. Yan, M. Zhang, K. Gong, L. Su, Z. Guo and L. Mao, Chem. Mater., 2005, 17, 3457–3463 CrossRef CAS.
- J. Fu, Z. Chen, M. Wang, S. Liu, J. Zhang, J. Zhang, R. Han and Q. Xu, Chem. Eng. J., 2015, 259, 53–61 CrossRef CAS.
- Y. Li, Q. Du, T. Liu, J. Sun, Y. Wang, S. Wu, Z. Wang, Y. Xia and L. Xia, Carbohydr. Polym., 2013, 95, 501–507 CrossRef CAS PubMed.
- T. Shen, S. Liu, W. Yan and J. Wang, J. Mater. Sci., 2019, 54, 8852–8859 CrossRef CAS.
- Q. Li, T. Yang, Q. Yang, F. Wang, K. C. Chou and X. Hou, Ceram. Int., 2016, 42, 8754–8762 CrossRef CAS.
- J. Pang, Y. Chao, H. Chang, H. Li, J. Xiong, Q. Zhang, G. Chen, J. Qian, W. Zhu and H. Li, ACS Sustain. Chem. Eng., 2018, 6, 4948–4957 CrossRef CAS.
- V. Ugraskan, B. Isik and O. Yazici, Chem. Eng. Commun., 2022, 209, 1111–1129 CrossRef CAS.
- A. S. Krishna Kumar, J. Warchol, J. Matusik, W. L. Tseng, N. Rajesh and T. Bajda, npj Clean Water, 2022, 5, 1–12 CrossRef.
- R. S. Bangari, A. Yadav, J. Bharadwaj and N. Sinha, J. Environ. Chem. Eng., 2022, 10, 107052 CrossRef CAS.
- R. S. Bangari, A. Yadav, P. Awasthi and N. Sinha, Colloids Surf., A, 2022, 634, 127943 CrossRef CAS.
- C. Xu, J. Zeng, X. Gu, Y. Wang, E. Li, C. Zhang, C. Ge, C. Jin, M. Miao, Z. Jin, T. Gao, X. Jiang, P. Dai, Y. Bando, R. Li, J. Rong and X. Bin Wang, J. Mater. Chem. A, 2022, 10, 846–854 RSC.
- F. Liu, Q. Zhou, Y. Li and J. Pang, Nanomaterials, 2022, 12, 318 CrossRef CAS PubMed.
- C. Yang, D. Bu and S. Huang, Ceram. Int., 2022, 48, 27658–27663 CrossRef CAS.
- Y. Sun, J. Bian and Q. Zhu, J. Mol. Liq., 2022, 349, 118216 CrossRef CAS.
- B. Isik, V. Ugraskan, F. Cakar and O. Yazici, Res. Chem. Intermed., 2022, 48, 4249–4268 CrossRef CAS.
- J. Li, M. Xia, L. Zhang, J. Tao, C. Wang and T. Wu, Funct. Mater. Lett., 2021, 15, 2251009 CrossRef.
- G. Lian, X. Zhang, S. Zhang, D. Liu, D. Cui and Q. Wang, Energy Environ. Sci., 2012, 5, 7072–7080 RSC.
- S. A. Sassman and L. S. Lee, Environ. Sci. Technol., 2005, 39, 7452–7459 CrossRef CAS PubMed.
- A. Sapkota, A. R. Sapkota, M. Kucharski, J. Burke, S. McKenzie, P. Walker and R. Lawrence, Environ. Int., 2008, 34, 1215–1226 CrossRef PubMed.
- V. Gadore, S. Ranjan Mishra and M. Ahmaruzzaman, Chem. Eng. J., 2023, 475, 146157 CrossRef CAS.
- T. Heberer, Toxicol. Lett., 2002, 131, 5–17 CrossRef CAS PubMed.
- B. Halling-Sørensen, S. Nors Nielsen, P. F. Lanzky, F. Ingerslev, H. C. Holten Lützhøft and S. E. Jørgensen, Chemosphere, 1998, 36, 357–393 CrossRef PubMed.
- P. Grenni, V. Ancona and A. Barra Caracciolo, Microchem. J., 2018, 136, 25–39 CrossRef CAS.
- T. A. Ternes, Water Res., 1998, 32, 3245–3260 CrossRef CAS.
- K. Kümmerer, Chemosphere, 2001, 45, 957–969 CrossRef PubMed.
- W. Yang, Y. Wu, L. Zhang, J. Jiang and L. Feng, Desalin. Water Treat., 2015, 54, 1134–1140 CrossRef CAS.
- S. R. Mishra, V. Gadore, R. Verma, K. R. B. Singh, J. Singh and M. Ahmaruzzaman, Chem. Eng. J., 2023, 146207 CrossRef CAS.
- J. Gomes, R. Costa, R. M. Quinta-Ferreira and R. C. Martins, Sci. Total Environ., 2017, 586, 265–283 CrossRef CAS PubMed.
- Y. Chao, W. Zhu, J. Chen, P. Wu, X. Wu, H. Li, C. Han and S. Yan, Green Chem. Lett. Rev., 2014, 7, 330–336 CrossRef.
- Z. Liu, K. Zhao, D. Li and Y. Tang, J. Am. Ceram. Soc., 2021, 104, 1601–1610 CrossRef CAS.
- Y. Chao, J. Zhang, H. Li, P. Wu, X. Li, H. Chang, J. He, H. Wu, H. Li and W. Zhu, Chem. Eng. J., 2020, 387, 124138 CrossRef CAS.
- N. Lv, L. Sun, L. Chen, Y. Li, J. Zhang, P. Wu, H. Li, W. Zhu and H. Li, Phys. Chem. Chem. Phys., 2019, 21, 21867–21874 RSC.
- D. Liu, W. Lei, S. Qin, K. D. Klika and Y. Chen, Phys. Chem. Chem. Phys., 2015, 18, 84–88 RSC.
- G. Liu, Z. Zhang, C. Yan, Y. Wang, X. Ma, P. Gao and Y. Feng, Chemosphere, 2018, 207, 534–542 CrossRef CAS PubMed.
- Q. Song, J. Liang, Y. Fang, C. Cao, Z. Liu, L. Li, Y. Huang, J. Lin and C. Tang, J. Hazard. Mater., 2019, 364, 654–662 CrossRef CAS PubMed.
- Q. Song, J. Liang, Y. Fang, Z. Guo, Z. Du, L. Zhang, Z. Liu, Y. Huang, J. Lin and C. Tang, Chem. Eng. J., 2020, 394, 124985 CrossRef CAS.
- L. Han, A. M. E. Khalil, J. Wang, Y. Chen, F. Li, H. Chang, H. Zhang, X. Liu, G. Li, Q. Jia and S. Zhang, Sep. Purif. Technol., 2021, 278, 119605 CrossRef.
- Y. Guo, C. Yan, P. Wang, L. Rao and C. Wang, Chem. Eng. J., 2020, 387, 124136 CrossRef CAS.
- Y. Chao, B. Tang, J. Luo, P. Wu, D. Tao, H. Chang, X. Chu, Y. Huang, H. Li and W. Zhu, J. Colloid Interface Sci., 2021, 584, 154–163 CrossRef CAS PubMed.
- B. Wang, W. Bai, G. Wang, K. Guo, H. Duan, Y. Xue and C. Tang, Colloids Surf., A, 2022, 632, 127749 CrossRef CAS.
- N. S. Mishra, S. Chandra and P. Saravanan, J. Mol. Liq., 2023, 369, 120969 CrossRef CAS.
- A. M. E. Khalil, L. Han, I. Maamoun, T. A. Tabish, Y. Chen, O. Eljamal, S. Zhang, D. Butler and F. A. Memon, Adv. Sustainable Syst., 2022, 6, 2200016 CrossRef CAS.
- E. Omrani, A. Ahmadpour, M. Heravi and T. R. Bastami, Journal of Water Process Engineering, 2022, 47, 102581 CrossRef.
- A. Goyal, D. Aggarwal, S. Kapoor, N. Goel, S. Singhal and J. Shukla, New J. Chem., 2020, 44, 3985–3997 RSC.
- Q. Song, Y. Fang, Z. Liu, L. Li, Y. Wang, J. Liang, Y. Huang, J. Lin, L. Hu, J. Zhang and C. Tang, Chem. Eng. J., 2017, 325, 71–79 CrossRef CAS.
- L. J. Zhou, G. G. Ying, J. L. Zhao, J. F. Yang, L. Wang, B. Yang and S. Liu, Environ. Pollut., 2011, 159, 1877–1885 CrossRef CAS PubMed.
- J. Lu, Y. Ji, J. M. Chovelon and J. Lu, Water Res., 2021, 198, 117136 CrossRef CAS PubMed.
- J. Gibs, H. A. Heckathorn, M. T. Meyer, F. R. Klapinski, M. Alebus and R. L. Lippincott, Sci. Total Environ., 2013, 458–460, 107–116 CrossRef CAS PubMed.
- S. Raha, D. Mohanta and M. Ahmaruzzaman, Sci. Rep., 2021, 11, 1–19 CrossRef PubMed.
- A. Pruden, R. Pei, H. Storteboom and K. H. Carlson, Environ. Sci. Technol., 2006, 40, 7445–7450 CrossRef CAS PubMed.
- J. Cai, J. Huang, S. Wang, J. Iocozzia, Z. Sun, J. Sun, Y. Yang, Y. Lai and Z. Lin, Adv. Mater., 2019, 31, 1806314 CrossRef PubMed.
- Y. Wang, B. Jing, F. Wang, S. Wang, X. Liu, Z. Ao and C. Li, Water Res., 2020, 180, 115925 CrossRef CAS PubMed.
- M. Ahmaruzzaman and V. Gadore, J. Environ. Chem. Eng., 2021, 9, 105836 CrossRef CAS.
- S. R. Mishra and M. Ahmaruzzaman, Mater. Today Commun., 2021, 28, 102562 CrossRef CAS.
- L. Lin, Z. Yu and X. Wang, Angew. Chem., 2019, 131, 6225–6236 CrossRef.
- C. Feng, L. Tang, Y. Deng, J. Wang, J. Luo, Y. Liu, X. Ouyang, H. Yang, J. Yu, J. Wang, C. Feng, L. Tang, J. Wang, J. Luo, Y. Liu, X. Ouyang, H. Yang, J. Yu and Y. Deng, Green Chem. Lett. Rev., 2020, 30, 2001922 CAS.
- M. Ahmaruzzaman and S. R. Mishra, Mater. Res. Bull., 2021, 143, 111417 CrossRef CAS.
- F. Wang, Y. Feng, P. Chen, Y. Wang, Y. Su, Q. Zhang, Y. Zeng, Z. Xie, H. Liu, Y. Liu, W. Lv and G. Liu, Appl. Catal., B, 2018, 227, 114–122 CrossRef CAS.
- Q. Xu, B. Zhu, B. Cheng, J. Yu, M. Zhou and W. Ho, Appl. Catal., B, 2019, 255, 117770 CrossRef CAS.
- S. Angizi, S. A. A. Alem, M. Hasanzadeh Azar, F. Shayeganfar, M. I. Manning, A. Hatamie, A. Pakdel and A. Simchi, Prog. Mater. Sci., 2022, 124, 100884 CrossRef CAS.
- Y. Yang, C. Zhang, D. Huang, G. Zeng, J. Huang, C. Lai, C. Zhou, W. Wang, H. Guo, W. Xue, R. Deng, M. Cheng and W. Xiong, Appl. Catal., B, 2019, 245, 87–99 CrossRef CAS.
- Y. Wu, X. Jin, H. Liu, W. Lv and G. Liu, Sep. Purif. Technol., 2022, 303, 122185 CrossRef CAS.
- S. Yu, X. Wang, H. Pang, R. Zhang, W. Song, D. Fu, T. Hayat and X. Wang, Chem. Eng. J., 2018, 333, 343–360 CrossRef CAS.
- L. Liang, S. Gao, J. Zhu, L. Wang, Y. Xiong, X. Xia and L. Yang, Chem. Eng. J., 2020, 391, 123599 CrossRef CAS.
- Z. Balta and E. B. Simsek, J. Alloys Compd., 2022, 898, 162897 CrossRef CAS.
- T. Yan, Z. Du, J. Wang, H. Cai, D. Bi, Z. Guo, Z. Liu, C. Tang and Y. Fang, J. Alloys Compd., 2022, 894, 162487 CrossRef CAS.
- Z. Du, L. Feng, Z. Guo, T. Yan, Q. Hu, J. Lin, Y. Huang, C. Tang and Y. Fang, J. Colloid Interface Sci., 2021, 589, 545–555 CrossRef CAS PubMed.
- Y. He, N. Xu, L. B. Junior, X. Hao, B. Yao, Q. Yang, D. Liu and Z. Ma, Appl. Surf. Sci., 2020, 520, 146336 CrossRef CAS.
- S. Sayegh, F. Tanos, A. Nada, G. Lesage, F. Zaviska, E. Petit, V. Rouessac, I. Iatsunskyi, E. Coy, R. Viter, D. Damberga, M. Weber, A. Razzouk, J. Stephan and M. Bechelany, Dalton Trans., 2022, 51, 2674–2695 RSC.
- N. Liu, Y. Dang, B. Hu, M. Tian, H. Jiang, G. Quan, R. Qiao, J. Lei and X. Zhang, Surf. Interfaces, 2022, 35, 102472 CrossRef CAS.
- H. He, W. Wang, C. Xu, S. Yang, C. Sun, X. Wang, Y. Yao, N. Mi, W. Xiang, S. Li and G. Liu, Sci. Total Environ., 2020, 730, 139100 CrossRef CAS PubMed.
- Z. Xiao, Y. Zheng, P. Chen, H. Liu, Z. Fang, J. Zhang, Z. Lin, Y. Zhang, J. Luo, W. Zhang, W. Lv and G. Liu, Environ. Sci.: Nano, 2022, 9, 3110–3125 RSC.
- X. Ji, X. Liu, Y. Guo and J. Zhang, Chem. Eng. J., 2021, 425, 131260 CrossRef CAS.
- B. Muthukutty, A. Krishnapandi and S. M. Chen, New J. Chem., 2020, 44, 2489–2499 RSC.
- L. Lin, W. Jiang, M. Bechelany, M. Nasr, J. Jarvis, T. Schaub, R. R. Sapkota, P. Miele, H. Wang and P. Xu, Chemosphere, 2019, 220, 921–929 CrossRef CAS PubMed.
- H. Cai, J. Wang, Z. Du, Z. Zhao, Y. Gu, Z. Guo, Y. Huang, C. Tang, G. Chen and Y. Fang, Colloids Surf., A, 2023, 663, 131050 CrossRef CAS.
- B. Singh, K. Singh, M. Kumar, S. Thakur and A. Kumar, Chem. Phys., 2020, 531, 110659 CrossRef CAS.
- T. Han, Y. Chen and H. Shi, Surf. Interfaces, 2022, 35, 102402 CrossRef CAS.
- P. Borthakur, P. K. Boruah and M. R. Das, J. Environ. Chem. Eng., 2021, 9, 104635 CrossRef CAS.
- S. A. Idrees, L. A. Jamil and K. M. Omer, ACS Omega, 2022, 7, 37620–37628 CrossRef CAS PubMed.
- S. Mosleh, K. Dashtian, M. Ghaedi and M. Amiri, RSC Adv., 2019, 9, 30100–30111 RSC.
- T. Sinha, M. Ahmaruzzaman and A. Bhattacharjee, J. Environ. Chem. Eng., 2014, 2, 2269–2279 CrossRef CAS.
- D. Wang, H. Shen, L. Guo, C. Wang and F. Fu, ACS Omega, 2016, 1, 566–577 CrossRef CAS PubMed.
- Q. Liu, Y. Li, H. Chen, J. Lu, G. Yu, M. Möslang and Y. Zhou, J. Hazard. Mater., 2020, 382, 121040 CrossRef CAS PubMed.
- N. J. Ismail, M. H. D. Othman, R. Kamaludin, M. I. M. Esham, N. A. Ali, M. A. Rahman, J. Jaafar and S. A. Bakar, Arabian J. Sci. Eng., 2019, 44, 10031–10040 CrossRef CAS.
- H. Ouyang, H. Huang, H. Wang and X. Zheng, J. Mater. Sci., 2020, 55, 976–989 CrossRef CAS.
- V. Gadore, S. R. Mishra and M. Ahmaruzzaman, J. Hazard. Mater., 2024, 461, 132458 CrossRef CAS PubMed.
- P. Garcia-Muñoz, F. Fresno, V. A. de la Peña O'Shea and N. Keller, Heterog. Photocatal., 2020, 378, 107–162 Search PubMed.
- V. Gadore, S. R. Mishra and M. Ahmaruzzaman, Environ. Sci. Pollut. Res., 2023, 30, 90410–90457 CrossRef CAS PubMed.
- D. Mohanta, S. V. Gupta, V. Gadore, S. Paul and M. Ahmaruzzaman, ACS Omega, 2022, 7, 20357–20368 CrossRef CAS PubMed.
- D. Gao, S. Zheng, L. Wang, C. Wang, H. Zhang and Q. Wang, Sep. Purif. Technol., 2019, 224, 308–314 CrossRef CAS.
- J. Diaz-Angulo, I. Gomez-Bonilla, C. Jimenez-Tohapanta, M. Mueses, M. Pinzon and F. Machuca-Martinez, Photochem. Photobiol. Sci., 2019, 18, 897 CrossRef CAS PubMed.
- E. Bae and W. Choi, Environ. Sci. Technol., 2003, 37, 147–152 CrossRef CAS PubMed.
- Y.-X. Li, X. Wang, C.-C. Wang, H. Fu, Y. Liu, P. Wang and C. Zhao, J. Hazard. Mater., 2020, 399, 123085 CrossRef CAS PubMed.
- S. Gao, T. Feng, C. Feng, N. Shang and C. Wang, J. Colloid Interface Sci., 2016, 466, 284–290 CrossRef CAS PubMed.
- C. Li, Y. Xu, W. Tu, G. Chen and R. Xu, Green Chem., 2017, 19, 882–899 RSC.
- J. Qu, Q. Li, C. Luo, J. Cheng and X. Hou, Coatings, 2018, 8, 214 CrossRef.
- A. Pakdel, Y. Bando and D. Golberg, Chem. Soc. Rev., 2014, 43, 934–959 RSC.
- Y. Guo, R. Wang, P. Wang, L. Rao and C. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 4640–4651 CrossRef CAS PubMed.
- Y. Guo, R. Wang, C. Yan, P. Wang, L. Rao and C. Wang, Chemosphere, 2019, 229, 112–124 CrossRef CAS PubMed.
- X. Ji, Y. Guo, S. Hua, H. Li and S. Zhang, New J. Chem., 2020, 44, 9238–9247 RSC.
- C. Cheng, W.-H. Fang, R. Long and O. V. Prezhdo, JACS Au, 2021, 1, 550–559 CrossRef CAS PubMed.
- J. J. Guerard and Y. P. Chin, J. Agric. Food Chem., 2012, 60, 9801–9806 CrossRef CAS PubMed.
- H. Lee, J. Kim, D. Y. Kim and Y. Seo, Org. Electron., 2018, 52, 103–109 CrossRef CAS.
- Y. J. Jang, S. Thogiti, K. Y. Lee and J. H. Kim, Crystals, 2019, 9, 452 CrossRef CAS.
- Y. Sheng, J. Yang, F. Wang, L. Liu, H. Liu, C. Yan and Z. Guo, Appl. Surf. Sci., 2019, 465, 154–163 CrossRef CAS.
- D. Liu, J. Song, J. S. Chung, S. H. Hur and W. M. Choi, Molecules, 2022, 27, 6833 CrossRef CAS PubMed.
- R. V. Khose, K. D. Lokhande, M. A. Bhakare, P. S. Dhumal, P. H. Wadekar and S. Some, ChemistrySelect, 2021, 6, 7956–7963 CrossRef CAS.
- M. Nasr, L. Soussan, R. Viter, C. Eid, R. Habchi, P. Miele and M. Bechelany, New J. Chem., 2018, 42, 1250–1259 RSC.
- W. Liu and W. Hu, Mater. Charact., 2022, 191, 112165 CrossRef CAS.
- Q. P. Zhang, D. M. Liang, W. F. Zhu, J. H. Liu, Y. Wu, D. G. Xu, X. Y. Bai, M. Wei and Y. L. Zhou, J. Solid State Chem., 2019, 269, 594–599 CrossRef CAS.
- E. Erusappan, S. Thiripuranthagan, M. Durai, S. Kumaravel and T. Vembuli, New J. Chem., 2020, 44, 7758–7770 RSC.
- U. Rafiq, M. Wahid and K. Majid, ChemistrySelect, 2020, 5, 11637–11647 CrossRef CAS.
- W. Li, Y. Liu, J. Di, M. Ji, J. Xia and H. Li, Phys. Status Solidi, 2018, 215, 1800146 CrossRef.
- S. Sankeetha, R. Muralidharan, N. Abirami, H. Leelavathi, S. Tamizharasan, A. Kumarasamy and R. Arulmozhi, Ceram. Int., 2023, 49, 6125–6138 CrossRef CAS.
- Q. Zhang, W. Zhang, B. Liang, J. Liu, D. Lan and M. Jiao, J. Inorg. Organomet. Polym. Mater., 2022, 32, 4434–4440 CrossRef CAS.
- W. Wang, L. Song, H. Zhang, G. Zhang and J. Cao, Front. Chem. Sci. Eng., 2021, 15, 1537–1549 CrossRef CAS.
- A. ul Ahmad, A. Abbas, S. Ali, M. F. e-alam, Z. Farooq, Q. Abbas, M. Ahmad, A. Farid, A. Muhammad afzal, H. M. Umair Arshad, M. Javid and M. Iqbal, Ceram. Int., 2021, 47, 10089–10095 CrossRef.
- A. Khalid, P. Ahmad, A. Khan, M. U. Khandaker, I. Kebaili, M. M. Alam, I. U. Din, S. Muhammad, Z. Razzaq, I. U. Rehman, H. A. Abbasi and D. Hayat, RSC Adv., 2022, 12, 6592–6600 RSC.
- C. Jiang, S. J. A. Moniz, A. Wang, T. Zhang and J. Tang, Chem. Soc. Rev., 2017, 46, 4645–4660 RSC.
- T. Yao, X. An, H. Han, J. Q. Chen and C. Li, Adv. Energy Mater., 2018, 8, 1800210 CrossRef.
- J. Jia, L. C. Seitz, J. D. Benck, Y. Huo, Y. Chen, J. W. D. Ng, T. Bilir, J. S. Harris and T. F. Jaramillo, Nat. Commun., 2016, 7, 1–6 Search PubMed.
- A. Hossain, K. Sakthipandi, A. K. M. Atique Ullah and S. Roy, Nano-Micro Lett., 2019, 11, 1–26 CrossRef PubMed.
- Z. He, J. Zhang, X. Li, S. Guan, M. Dai and S. Wang, Small, 2020, 16, 2005051 CrossRef CAS PubMed.
- G. Zuo, Y. Wang, W. L. Teo, A. Xie, Y. Guo, Y. Dai, W. Zhou, D. Jana, Q. Xian, W. Dong and Y. Zhao, Angew. Chem., 2020, 132, 11383–11388 CrossRef.
- Y. Li, B. Sun, H. Lin, Q. Ruan, Y. Geng, J. Liu, H. Wang, Y. Yang, L. Wang and K. Chiu Tam, Appl. Catal., B, 2020, 267, 118702 CrossRef CAS.
- H. Si, G. Lian, J. Wang, L. Li, Q. Wang, D. Cui and C. P. Wong, ACS Appl. Mater. Interfaces, 2016, 8, 1578–1582 CrossRef CAS PubMed.
- C. Gautam, C. S. Tiwary, S. Jose, G. Brunetto, S. Ozden, S. Vinod, P. Raghavan, S. Biradar, D. S. Galvao and P. M. Ajayan, ACS Nano, 2015, 9, 12088–12095 CrossRef CAS PubMed.
- A. P. Farkas, Á. Szitás, D. Jurdi, K. Palotás, J. Kiss and Z. Kónya, Appl. Catal., A, 2020, 592, 117440 CrossRef CAS.
- Y. Cao, R. Zhang, T. Zhou, S. Jin, J. Huang, L. Ye, Z. Huang, F. Wang and Y. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 9935–9943 CrossRef CAS PubMed.
- X. Xing, M. Zhang, L. Hou, L. Xiao, Q. Li and J. Yang, Int. J. Hydrogen Energy, 2017, 42, 28434–28444 CrossRef CAS.
- J. T. Grant, C. A. Carrero, F. Goeltl, J. Venegas, P. Mueller, S. P. Burt, S. E. Specht, W. P. McDermott, A. Chieregato and I. Hermans, Science, 2016, 354, 1570–1573 CrossRef CAS PubMed.
- P. F. Sun, W. L. Wang, X. Zhao and J. S. Dang, Phys. Chem. Chem. Phys., 2020, 22, 22627–22634 RSC.
- M. N. Ivanova, Y. A. Vorotnikov, E. E. Plotnikova, M. V. Marchuk, A. A. Ivanov, I. P. Asanov, A. R. Tsygankova, E. D. Grayfer, V. E. Fedorov and M. A. Shestopalov, Inorg. Chem., 2020, 59, 6439–6448 CrossRef CAS PubMed.
- M. S. Meera, S. K. Sasidharan, A. Hossain, J. Kiss, Z. Konya, L. Elias and S. M. A. Shibli, ACS Appl. Energy Mater., 2022, 5, 3578–3586 CrossRef CAS.
- A. Pendse, S. Cetindag, M. H. Lin, A. Rackovic, R. Debbarma, S. Almassi, B. P. Chaplin, V. Berry, J. W. Shan and S. Kim, Small, 2019, 15, 1904590 CrossRef CAS PubMed.
- X. Wen, Y. Wang and J. Zhao, New J. Chem., 2018, 42, 12838–12844 RSC.
- J. Peng, S. Wang, P. H. Zhang, L. P. Jiang, J. J. Shi and J. J. Zhu, J. Biomed. Nanotechnol., 2013, 9, 1679–1685 CrossRef CAS PubMed.
- S. Meng, X. Ye, X. Ning, M. Xie, X. Fu and S. Chen, Appl. Catal., B, 2016, 182, 356–368 CrossRef CAS.
- X. Xiao, D. Huang, Y. Fu, M. Wen, X. Jiang, X. Lv, M. Li, L. Gao, S. Liu, M. Wang, C. Zhao and Y. Shen, ACS Appl. Mater. Interfaces, 2018, 10, 4689–4696 CrossRef CAS PubMed.
- M. Tahir, Energy Fuels, 2021, 35, 14197–14211 CrossRef CAS.
- J. C. Wang, Y. Hou, F. D. Feng, W. X. Wang, W. Shi, W. Zhang, Y. Li, H. Lou and C. X. Cui, Appl. Surf. Sci., 2021, 537, 148014 CrossRef CAS.
- S. Thaweesak, S. Wang, M. Lyu, M. Xiao, P. Peerakiatkhajohn and L. Wang, Dalton Trans., 2017, 46, 10714–10720 RSC.
- B. Wahbeh, T. A. Hamed and R. Kasher, Renewable Energy, 2012, 48, 10–15 CrossRef CAS.
- Q. Liu, C. Chen, M. Du, Y. Wu, C. Ren, K. Ding, M. Song and C. Huang, ACS Appl. Nano Mater., 2018, 1, 4566–4575 CrossRef CAS.
- D. P. Kumar, J. Choi, S. Hong, D. A. Reddy, S. Lee and T. K. Kim, ACS Sustain. Chem. Eng., 2016, 4, 7158–7166 CrossRef CAS.
- T. C. Bhagya, A. Krishnan, S. Arunima Rajan, M. Ameen Sha, B. R. Sreelekshmy, P. Jineesh and S. M. A. Shibli, Photochem. Photobiol. Sci., 2019, 18, 1716–1726 CrossRef CAS PubMed.
- G. J. Lee, Y. H. Hou, C. Y. Chen, C. Y. Tsay, Y. C. Chang, J. H. Chen, T. L. Horng, S. Anandan and J. J. Wu, Int. J. Hydrogen Energy, 2021, 46, 5938–5948 CrossRef CAS.
- S. Mao, J. W. Shi, G. Sun, Y. Zhang, X. Ji, Y. Lv, B. Wang, Y. Xu and Y. Cheng, Chem. Eng. J., 2021, 404, 126533 CrossRef CAS.
- A. Sarilmaz, G. Yanalak, E. Aslan, F. Ozel, I. H. Patir and M. Ersoz, Renewable Energy, 2021, 164, 254–259 CrossRef CAS.
- A. Merlo, V. R. S. S. Mokkapati, S. Pandit and I. Mijakovic, Biomater. Sci., 2018, 6, 2298–2311 RSC.
- S. M. Sharker, Int. J. Nanomed., 2019, 14, 9983 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |