Open Access Article
Open Access ArticleDBU-catalyzed diastereoselective 1,3-dipolar [3+2] cycloaddition of trifluoroethyl amine-derived isatin ketimines with chalcones: synthesis of 5′-CF3-substituted 3,2′-pyrrolidinyl spirooxindoles†
Feng-Ji Zhou‡
,
Bao-Lei Zhu‡,
Zhen-Hui Huang,
Ning Lin* and
Zhen-Wei Zhang *
*
College of Pharmacy, Guangxi University of Chinese Medicine, Guangxi Zhuang Yao Medicine Center of Engineering and Technology, Nanning 530200, China. E-mail: zhenweizhang@gxtcmu.edu.cn; linning@gxtcmu.edu.cn
First published on 2nd January 2024
Abstract
A diastereoselective 1,3-dipolar cycloaddition reaction between trifluoroethyl amine-derived isatin ketimines and chalcones was successfully achieved in the presence of DBU. A series of 5′-CF3-substituted 3,2′-pyrrolidinyl spirooxindoles were efficiently synthesized with high yields and excellent diastereoselectivities (up to 89% yield, and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). The in vitro anticancer activities of these highly functionalized spiro[pyrrolidin-3,2′-oxindole] derivatives were evaluated.
1 dr). The in vitro anticancer activities of these highly functionalized spiro[pyrrolidin-3,2′-oxindole] derivatives were evaluated.
Introduction
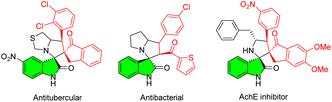
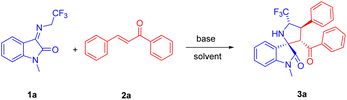
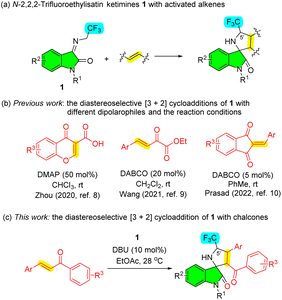
Pyrrolidinyl spirooxindoles have emerged as one class of privileged scaffolds commonly encountered in diverse natural products and biologically active compounds, thus establishing their medicinal significance in drug discovery and development. As a notable subtype, 3,2′-pyrrolidinyl spirooxindoles have demonstrated significant pharmacological relevance in the field of pharmaceutical research (Fig. 1).1On the other side, the presence of fluorine atoms has been found in up to 20% of commercially available medications.2 Fluorine substitutions are often applied for the strategic modifications of lead compounds. In particular, it is generally acknowledged that the introduction of a trifluoromethyl group into the ɑ-position of the pyrrolidine can effectively enhance the binding affinity of drug receptors.3 Consequently, synthetic protocols of 5′-CF3-containing 3,2′-pyrrolidinyl spirooxindoles have garnered much more attention. Among them, 1,3-dipolar [3 + 2] cycloadditions of N-2,2,2-trifluoroethylisatin ketimines 1 with various activated alkenes have been extensively reported in the past few years (Scheme 1a).4,5 As we know, chalcones are a well-studied group of naturally occurring aromatic ketones with biological properties including anti-inflammatory and anti-cancer.6 Additionally, owing to their facile synthetic accessibility and ɑ,β-unsaturated carbonyl conjugated system containing two electrophilic centers, they are frequently used as valuable intermediates for the formation of heterocycles, particularly five- and six-membered heterocyclic molecules.7 Considering the importance of spirooxindoles, fluorine compounds, and chalcones, we hypothesized that their integration could augment the bioactivities. However, to date, there have been few successful examples employing the chalcone-type compounds as the dipolarophiles for the diastereoselective 1,3-dipolar [3 + 2] cycloaddition involving the ketimines 1 (Scheme 1b).8–10 Despite the aborted cycloaddition of chalcone or its analogue with the ketimines 1 in chloroform catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU),9 we proposed that the alternative reaction condition would enable the utilization of chalcones for this cycloaddition (Scheme 1c).
 | ||
| Scheme 1 1,3-Dipolar [3 + 2] cycloaddition reactions of N-2,2,2-trifluoroethylisatin ketimines with various activated alkenes. | ||
Herein, we disclosed the DBU-catalyzed diastereoselective 1,3-dipolar [3 + 2] cycloaddition of N-2,2,2-trifluoroethylisatin ketimines with chalcones in ethyl acetate to construct diverse functionalized 5′-CF3-containing 3,2′-pyrrolidine spirooxindole derivatives exhibiting promising anticancer activities.
Results and discussion
N-2,2,2-Trifluoroethylisatin ketimine 1a and chalcone 2a were chosen as the model substrates for the envisaged [3 + 2] cycloaddition reaction (Table 1). Initially, this cyclization sequence proceeded smoothly in dichloromethane at 28 °C in the presence of K2CO3, affording the spirocyclic product 3a in 68% yield with high dr (Table 1, entry 1). Encouraged by the outcome, a range of common inorganic and organic bases such as Cs2CO3, Et3N, DABCO, DBU, t-BuOK and DMAP were tested (entries 2–7). It was found that DBU was the best choice (entry 5).| Entry | Base | Solvent | Time/h | Yieldb/% | drc |
|---|---|---|---|---|---|
| a Unless otherwise noted, all the reactions were performed on a 0.10 mmol scale of 1a and 2a (1.2 equiv.), using 20 mol% catalyst in solvent (2 mL) at 28 °C.b Isolated yields.c Determined by 1H NMR spectroscopy of the crude mixture.d 10 mol% catalyst.e 5 mol% catalyst.f 2 mol% catalyst.g Reaction was carried out at 35 °C. | |||||
| 1 | K2CO3 | DCM | 28 | 68 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 2 | Cs2CO3 | DCM | 22 | 72 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Et3N | DCM | 72 | 30 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | DABCO | DCM | 72 | 49 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | DBU | DCM | 24 | 83 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 6 | t-BuOK | DCM | 38 | 41 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 7 | DMAP | DCM | 16 | trace | — |
| 8 | DBU | EtOAc | 40 min | 89 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 9 | DBU | MeCN | 2 | 82 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 10 | DBU | MeOH | 72 | — | — |
| 11 | DBU | PhMe | 18 | 67 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | DBU | DCE | 26 | 62 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 13d | DBU | EtOAc | 50 min | 87 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 14e | DBU | EtOAc | 2.5 | 80 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 15f | DBU | EtOAc | 48 | 51 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 16d,g | DBU | EtOAc | 45 min | 86 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
The subsequent screening of different solvents, including DCM, EtOAc, MeCN, MeOH, PhMe and DCE (Table 1, entries 5 and 8–12) indicated that EtOAc was the optimal solvent, resulting in 89% yield and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr within 40 minutes (entry 8). To our delight, the reaction in MeCN could sustain high levels of yield and diastereoselectivity for a short time of 2 hours (entry 9). However, no reaction was observed in a protic solvent such as MeOH even after 72 hours (entry 10). The utilization of PhMe and DCE in the screening process resulted in diminished product yields over an extended reaction time, albeit with excellent diastereoselectivities (entries 11 and 12). With EtOAc as the solvent, upon reducing the catalyst loading to 10 mol%, both the yield and diastereoselectivity were maintained (Table 1, entry 13). Nevertheless, a significant reduction in yield and an increase in reaction time were observed when using much lower catalyst loading (entries 14 and 15). The elevation of the reaction temperature to 35 °C led to a slight decrease in yield and diastereoselectivity (entry 16). Therefore, the employment of DBU (10 mol%) as the catalyst and EtOAc as the solvent at 28 °C was identified as the optimal reaction conditions for this cycloaddition reaction.
2 dr within 40 minutes (entry 8). To our delight, the reaction in MeCN could sustain high levels of yield and diastereoselectivity for a short time of 2 hours (entry 9). However, no reaction was observed in a protic solvent such as MeOH even after 72 hours (entry 10). The utilization of PhMe and DCE in the screening process resulted in diminished product yields over an extended reaction time, albeit with excellent diastereoselectivities (entries 11 and 12). With EtOAc as the solvent, upon reducing the catalyst loading to 10 mol%, both the yield and diastereoselectivity were maintained (Table 1, entry 13). Nevertheless, a significant reduction in yield and an increase in reaction time were observed when using much lower catalyst loading (entries 14 and 15). The elevation of the reaction temperature to 35 °C led to a slight decrease in yield and diastereoselectivity (entry 16). Therefore, the employment of DBU (10 mol%) as the catalyst and EtOAc as the solvent at 28 °C was identified as the optimal reaction conditions for this cycloaddition reaction.
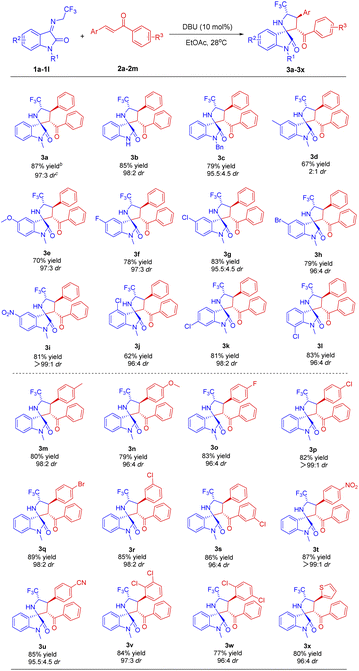
Under the established optimum conditions, the reaction generality was next explored by conducting the [3 + 2] cycloaddition of various N-2,2,2-trifluoroethylisatin ketimines and chalcones (Table 2). As can be seen from Table 2, all reactions were remarkably completed within 50 minutes and demonstrated good tolerance towards a diverse array of functional groups, affording the desired products in moderate to good yields and with up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivities.
1 diastereoselectivities.
At first, the cycloaddition of different ketimines 1a–1l with chalcone 2a was examined. As shown in Table 2, the reactions proceeded smoothly under the optimal reaction conditions to obtain the corresponding spirocyclic products 3a–3l with high yields and excellent diastereoselectivities (up to 87% yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr), except for the product 3d with relatively lower diastereoselectivity while employing the 5-methyl substituted ketimine 1d. Particularly, N-unsubstituted ketimine 1b also exhibited notable reactivity, affording the product 3b in high yield and exceptional diastereoselectivity (85% yield and 98
1 dr), except for the product 3d with relatively lower diastereoselectivity while employing the 5-methyl substituted ketimine 1d. Particularly, N-unsubstituted ketimine 1b also exhibited notable reactivity, affording the product 3b in high yield and exceptional diastereoselectivity (85% yield and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr). Gratifyingly, both electron-donating group (–OMe) and electron-withdrawing groups (–F, –Cl, –Br and –NO2) at the 5-, 6-, or 7-position of the ketimines displayed favourable compatibility (3e–3l). Furthermore, the cycloaddition reactions between the ketimine 1a and a variety of chalcones 2b–2m could furnish the cycloadducts 3m–3w with high yields and excellent diastereoselectivities, indicating that both electron-donating substituents (–Me and –OMe) and electron-withdrawing groups (–F, –Cl, –Br, –NO2, and –CN) at para- or meta-position on the two phenyl rings of chalcones have only a negligible impact on yield and diastereoselectivity. Notably, chalcones containing the dichloro-substituted phenyl were also tolerant in the catalytic system to afford the cycloadducts 3v and 3w, respectively, with good yields and excellent diastereoselectivities. In addition, the substitution of phenyl in chalcone with thienyl (3x) also gave satisfactory results.
2 dr). Gratifyingly, both electron-donating group (–OMe) and electron-withdrawing groups (–F, –Cl, –Br and –NO2) at the 5-, 6-, or 7-position of the ketimines displayed favourable compatibility (3e–3l). Furthermore, the cycloaddition reactions between the ketimine 1a and a variety of chalcones 2b–2m could furnish the cycloadducts 3m–3w with high yields and excellent diastereoselectivities, indicating that both electron-donating substituents (–Me and –OMe) and electron-withdrawing groups (–F, –Cl, –Br, –NO2, and –CN) at para- or meta-position on the two phenyl rings of chalcones have only a negligible impact on yield and diastereoselectivity. Notably, chalcones containing the dichloro-substituted phenyl were also tolerant in the catalytic system to afford the cycloadducts 3v and 3w, respectively, with good yields and excellent diastereoselectivities. In addition, the substitution of phenyl in chalcone with thienyl (3x) also gave satisfactory results.
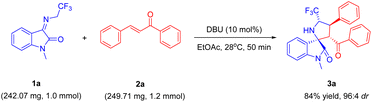
A scale-up experiment was executed under the standard reaction conditions, thereby demonstrating the feasibility of performing this reaction on a 10-fold amplification (Scheme 2). The yield and diastereoselectivity of compound 3a remained consistently at a high level (84% yield and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dr), despite a slight decrease.
4 dr), despite a slight decrease.
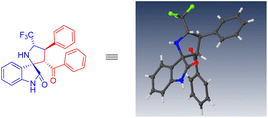
To establish the absolute configuration of the products, a single crystal of compound 3b was obtained for X-ray crystallographic analysis (Fig. 2). The absolute configurations of other products were determined by analogy to 3b.
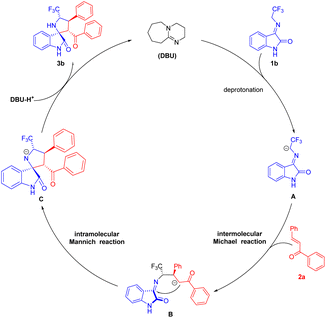
Based on the experimental results and related literatures, we proposed a Michael/Mannich cascade reaction mechanism. As illustrated in Scheme 3, DBU initially promoted the formation of azomethine ylide A through deprotonation of the precursor 1b. Then activated 1b attacked the β-position of chalcone 2a to form B. The α-position of 2a was negatively charged due to the breakage of the double bond, and then attacked the carbocation of 1b to undergo a cyclization reaction to form a negatively charged transition state C of the nitrogen. Subsequently, 3,2′-pyrrolidinyl spirooxindole product 3b was obtained after hydrogenation.
With a diverse range of functionalized 5′-CF3-3,2′-pyrrolidine spirooxindoles successfully derived from chalcones in hand, we finally endeavored to evaluate their anticancer activities. All 24 targeted products were subjected to in vitro cytotoxicity tests against human gastric cancer cell line (SCG7901), followed by assessment of cell viability using MTT-based assays (for details, see ESI†). As listed in Table 3, the preliminary experimental data revealed that the selected 6 spirocyclic compounds exhibited certain cytotoxicity to SCG7901 cells with acceptable IC50 values (all <35.00 μM). Based on these results, it is anticipated that the availability of chalcone-derived CF3-containing spirooxindoles might provide promising lead compounds for further structural modification and bioactive assays.
Conclusions
In conclusion, we have developed an efficient DBU-catalyzed diastereoselective 1,3-dipolar [3 + 2] cycloaddition reaction of N-2,2,2-trifluoroethylisatin ketimines with chalcones in ethyl acetate. This methodology enables the construction of chalcone-derived functionalized 5′-CF3-containing 3,2′-pyrrolidine spirooxindoles in good yields and excellent diastereoselectivities (up to 89% yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
Author contributions
Conceptualization, Z.-W. Zhang and N. Lin; funding acquisition, Z.-W. Zhang and N. Lin; writing—original draft preparation, F.-J. Zhou, B.-L. Zhu, and Z.-H. Huang; writing—review and editing, Z.-W. Zhang and N. Lin.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We sincerely thank Guangxi Natural Science Foundation (2021GXNSFDA075016 and 2020GXNSFAA297215); Gui Style Xinglin Top Talent Funding Project of Guangxi University of Chinese Medicine (2022C005); Thousands of Young and Middle-aged Backbone Teachers Training Project of Guangxi Colleges and Universities (Gui-Jiao 2019-81); Discipline and Platform Construction Funds (Guangxi University of Chinese Medicine Science of Chinese Materia Medica Platform Construction); and Innovation Project of Guangxi Graduate Education (YCSW2021231).Notes and references
- For selected reviews, see: (a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 (Angew. Chem., 2007, 119, 8902–8912) CrossRef CAS PubMed; (b) J. J. Badillo, N. V. Hanhan and A. K. Franz, Curr. Opin. Drug Discov. Dev., 2010, 13, 758–776 CAS; (c) B. Yu, D.-Q. Yu and H.-M. Liu, Eur. J. Med. Chem., 2015, 97, 673–698 CrossRef CAS PubMed; (d) N. Ye, H. Chen, E. A. Wold, P.-Y. Shi and J. Zhou, ACS Infect. Dis., 2016, 2, 382–392 CrossRef CAS PubMed; (e) T. L. Pavlovska, R. G. Redkin, V. V. Lipson and D. V. Atamanuk, Mol. Div., 2016, 20, 299–344 CrossRef CAS PubMed; (f) A. J. Boddy and J. A. Bull, Org. Chem. Front., 2021, 8, 1026–1084 RSC.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- For recent reviews on [3+2] cycloaddition of N-2,2,2-trifluoroethylisatin ketimines, see: (a) H.-Z. Gui, Y. Wei and M. Shi, Chem.–Asian J., 2020, 15, 1225–1233 CrossRef CAS PubMed; (b) Z. Sun, C. Zhang, L. Chen, H. Xie, B. Liu and D. Liu, Chin. J. Org. Chem., 2021, 41, 1789–1803 CrossRef CAS; (c) Y. Liu, L. Wang, D. Ma and Y. Song, Molecules, 2023, 28, 2990 CrossRef CAS PubMed; (d) B. Borah, N. S. Veeranagaiah, S. Sharma, M. Patat, M. S. Prasad, R. Pallepogub and L. R. Chowhan, RSC Adv., 2023, 13, 7063–7075 RSC.
- For recent papers on [3+2] cycloaddition of N-2,2,2-trifluoroethylisatin ketimines, see: (a) W.-C. Yuan, L. Yang, J.-Q. Zhao, H.-Y. Du, Z.-H. Wang, Y. You, Y.-P. Zhang, J. Liu, W. Zhang and M.-Q. Zhou, Org. Lett., 2022, 24, 4603–4608 CrossRef CAS PubMed; (b) R. Ma, J.-L. Zhang, X.-Q. Hu and P.-F. Xu, Synthesis, 2023, 55, 1929–1939 CrossRef CAS; (c) L.-Q. Li, J.-Q. Zhao, Y.-P. Zhang, Y. You, Z.-H. Wang, Z.-Z. Ge, M.-Q. Zhou and W.-C. Yuan, Molecules, 2023, 28, 5372 CrossRef CAS PubMed.
- (a) G. Rajendran, D. Bhanu, B. Aruchamy, P. Ramani, N. Pandurangan, K. N. Bobba, E. J. Oh, H. Y. Chung, P. Gangadaran and B. C. Ahn, Pharmaceuticals, 2022, 15, 1250 CrossRef CAS PubMed; (b) M. A. Shalaby, S. A. Rizk and A. M. Fahim, Org. Biomol. Chem., 2023, 21, 5317–5346 RSC.
- (a) S. Mastachi-Loza, T. I. Ramírez-Candelero, L. J. Benítez-Puebla, A. Fuentes-Benítes, C. González-Romero and M. A. Vázquez, Chem.–Asian J., 2022, 17, e202200706 CrossRef CAS PubMed; (b) Y. N. Nayak, S. L. Gaonkar and M. Sabu, J. Heterocycl. Chem., 2023, 60, 1301–1325 CrossRef CAS; (c) L. Maram and F. Tanaka, Org. Lett., 2020, 22, 2751–2755 CrossRef CAS PubMed.
- X. W. Liu, J. Yue, Z. Li, D. Wu, M. Y. Tian, Q. L. Wang and Y. Zhou, Tetrahedron, 2020, 76, 131678 CrossRef CAS.
- Y. Xiong, X.-X. Han, Y. Lu, H.-J. Wang, M. Zhang and X.-W. Liu, Tetrahedron, 2021, 87, 132112 CrossRef CAS.
- M. S. Prasad, S. Bharani, S. M. Sharief, M. Ravi, M. Sivaprakash, B. Borah and L. R. Chowhan, RSC Adv., 2022, 12, 34941–34945 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2310278. For ESI see DOI: https://doi.org/10.1039/d3ra08127c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |