Open Access Article
Open Access ArticleStudy on anionic–nonionic mixed surfactant for enhanced oil recovery in a hypersaline reservoir
Luxuan Maa,
Ping Xub,
Lei Wanga,
Kai Xiaa,
Hui Du a,
Ruitong Gaoa and
Zhaojun Chen
a,
Ruitong Gaoa and
Zhaojun Chen *a
*a
aCollege of Chemistry and Chemical Engineering, Institute for Sustainable Energy and Resources, Qingdao University, Qingdao 266071, China. E-mail: tingyvxuan@126.com
bDepartment of Economic Management, Yantai Engineering & Technology College, Yantai 264006, China
First published on 5th January 2024
Abstract
Hypersaline reservoirs are characterized by high salinity and high calcium and magnesium concentration. In order to enhance oil recovery of the hypersaline reservoirs, a specialized ternary mixed surfactant system composed of nonionic alkanolamide surfactants and anionic surfactant was developed in this study. Through careful analysis and optimization, lauric acid diethanolamide (LDEA), octanoic acid diethanolamide (ODEA), and sodium dodecyl sulfonate (SDS) were identified as promising candidates for the surfactant compounding system, and formed a ternary surfactant system composed of LDEA, ODEA, and SDS with the mass ratio of 4.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66
0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00. Experimental results revealed that the interfacial tension of the system was consistently below 10−2 mN m−1 and could even reach ultra-low levels (10−3 mN m−1) under conditions of calcium and magnesium ion content of 2000 mg L−1, surfactant concentrations ranging from 0.05 to 0.3 wt%, temperature ranging from 50 to 80 °C, and salinity ranging from 20
1.00. Experimental results revealed that the interfacial tension of the system was consistently below 10−2 mN m−1 and could even reach ultra-low levels (10−3 mN m−1) under conditions of calcium and magnesium ion content of 2000 mg L−1, surfactant concentrations ranging from 0.05 to 0.3 wt%, temperature ranging from 50 to 80 °C, and salinity ranging from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 50
000 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1. Furthermore, the mixed surfactant system exhibited favorable wetting capacity and emulsifying power. The static adsorption capacities of the mixed surfactant on oil sands were less than 2 mg g−1. This study offered a novel strategy for the actual exploitation of reservoirs with high calcium-magnesium and high salinity.
000 mg L−1. Furthermore, the mixed surfactant system exhibited favorable wetting capacity and emulsifying power. The static adsorption capacities of the mixed surfactant on oil sands were less than 2 mg g−1. This study offered a novel strategy for the actual exploitation of reservoirs with high calcium-magnesium and high salinity.
1. Introduction
Crude oil remains the primary strategic energy source, despite extensive research and attention given to new energy alternatives in recent years.1 After primary and secondary oil recovery, there are still large amounts of unexploited crude oil in the ground, especially for heavy oil reservoirs. Enhanced oil recovery (EOR) technologies have proven to be the highly effective approach for those reservoirs.2,3 Surfactant flooding, as a fundamental EOR method, has garnered significant interest as a promising approach to improve oil recovery.4,5 However, the existing surfactant flooding technologies are mainly suitable for reservoirs with low oil viscosity, and there are scarcely any suitable surfactant displacement agents for hypersaline heavy oil reservoirs.6,7Anionic surfactants have been most widely used in tertiary oil recovery due to their favorable characteristics, such as high temperature resistance, less adsorption on sandstone surface, lower cost, and high interfacial activity.8–12 Up to now, many researchers have reported the application of anionic surfactants in EOR.13–15 However, due to the presence of anions in structure, the salt resistance of anionic surfactants is usually relatively poor, and it is easy to react with multivalent metal ions such as calcium and magnesium to produce precipitation, making them unsuitable for use in high salinity, especially high calcium and magnesium oil reservoirs.16,17 Nonionic alkanolamide surfactants also have gained widespread use in EOR due to their unique properties, including high surface activity, strong salt resistance, good resistance to multivalent cations, good compatibility with other types of surfactants, and good solubility.18,19 But nonionic surfactants also have the disadvantages of less resistance to high temperature due to their low cloud point, and relatively poor stability in reservoirs. Previous studies have verified that the combination of anionic and nonionic surfactants can both increase the salt tolerance of the system and enhance the temperature resistance, which will be very beneficial for improving the recovery efficiency of hypersaline heavy oil reservoirs.20,21
The Bamianhe reservoir, located in Shouguang, China, is characterized by high viscosity (up to 154 mPa s−1), high salinity in formation water (reaching 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 712 mg L−1), and high calcium and magnesium ions (up to 1787 mg L−1). In this study, a novel ternary mixed surfactant system composed of nonionic alkanolamide surfactants and anionic surfactant was developed for the enhanced oil recovery of Bamianhe reservoir, and the physical and chemical properties of the new surfactant system were investigated in detail. The results will undoubtedly have positive significance for improving oil recovery in old oil fields with high calcium and magnesium.
712 mg L−1), and high calcium and magnesium ions (up to 1787 mg L−1). In this study, a novel ternary mixed surfactant system composed of nonionic alkanolamide surfactants and anionic surfactant was developed for the enhanced oil recovery of Bamianhe reservoir, and the physical and chemical properties of the new surfactant system were investigated in detail. The results will undoubtedly have positive significance for improving oil recovery in old oil fields with high calcium and magnesium.
2. Experimental section
2.1. Materials
All the reagents, including octanoic acid diethanolamide (ODEA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, HLB: 14.0), decylic acid diethanolamide (DDEA, 1
1.5, HLB: 14.0), decylic acid diethanolamide (DDEA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 HLB: 13.1), lauric acid diethanolamide (LDEA, 1
1.5 HLB: 13.1), lauric acid diethanolamide (LDEA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, HLB: 12.1), myristic acid diethanolamide (MDEA, 1
1.5, HLB: 12.1), myristic acid diethanolamide (MDEA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 HLB: 11.2), palmitic acid diethanolamide (PDEA, 1
1.5 HLB: 11.2), palmitic acid diethanolamide (PDEA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 HLB: 10.2), stearic acid diethanolamide (SDEA, 1
1.5 HLB: 10.2), stearic acid diethanolamide (SDEA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, HLB: 9.3), and sodium dodecyl sulfonate (SDS), were procured from Tianjin Fuyu Fine Chemical Co. Ltd, Tianjin, China. The samples of crude oil, oil sands and oil rock slices used in the experiments were all obtained from the Bamianhe Oil Field, Shouguang, China. The composition and characteristics of the crude oil utilized for the experimental analysis are detailed in Table 1.
1.5, HLB: 9.3), and sodium dodecyl sulfonate (SDS), were procured from Tianjin Fuyu Fine Chemical Co. Ltd, Tianjin, China. The samples of crude oil, oil sands and oil rock slices used in the experiments were all obtained from the Bamianhe Oil Field, Shouguang, China. The composition and characteristics of the crude oil utilized for the experimental analysis are detailed in Table 1.
| Properties | Test | |
|---|---|---|
| Density (g cm−1) | 0.9537 | |
| Viscosity (50 °C) (mm2 s−1) | 315.7 | |
| Carbon residue (wt%) | 10.2 | |
| HLB | About 8.5 | |
| Elemental composition (wt%) | C | 84.07 |
| H | 12.05 | |
| S | 1.88 | |
| N | 0.57 | |
| O | 1.43 | |
| SARA (wt%) | Saturates | 40.80 |
| Aromatics | 30.12 | |
| Resin | 27.21 | |
| Asphaltene | 1.87 | |
2.2. Preparation of anionic–nonionic mixed surfactant solutions
Firstly, the simulated formation water with different salinities was prepared based on Table 2. Then, a certain amount of anionic surfactant and nonionic surfactant with different mass radio was added into the above simulated formation water. After complete dissolution, the anionic-nonionic mixed surfactant solutions with different concentrations and different salinities could be obtained.| Salinity (mg L−1) | Ca2+ (mg L−1) | Mg2+ (mg L−1) | Na+ (mg L−1) | Cl− (mg L−1) |
|---|---|---|---|---|
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1000 | 1000 | 5238 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762 762 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1000 | 1000 | 9172 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828 828 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1000 | 1000 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 106 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894 894 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1000 | 1000 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1000 | 1000 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 709 709 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291 291 |
2.3. Characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 and the Ca2+ and Mg2+ contents of 2000 mg L−1) within conical flasks with cover. The mixed solution was heated up to 50 °C (or 80 °C) with stirring for 20 minutes, subsequently, maintained the temperature and stayed for 2 hours. So the test solution samples with a certain concentration would be obtained. Then the solution samples were measured by a turbidimeter (HACH, 1720E).
000 mg L−1 and the Ca2+ and Mg2+ contents of 2000 mg L−1) within conical flasks with cover. The mixed solution was heated up to 50 °C (or 80 °C) with stirring for 20 minutes, subsequently, maintained the temperature and stayed for 2 hours. So the test solution samples with a certain concentration would be obtained. Then the solution samples were measured by a turbidimeter (HACH, 1720E).The oil film peeling time, which assessed the time of oil film detached from the oiled quartz slide, was determined based on the method described in our previous literature.23
3. Results and discussion
3.1. Water solubility test
Table 3 showed the solubility characteristics of each surfactant. It was observed that when the surfactant concentrations were ranging 0.05 wt% to 0.3 wt%, only ODEA, DDEA and LDEA had good solubility in simulated formation water at the temperature ranging of 50 to 80 °C. While the solutions of MDEA, PDEA and SDEA appeared turbid when the concentration increased to 0.3 wt%. As for anionic surfactant SDS, there was no doubt that it showed good water solubility throughout the experiment conditions. Therefore, ODEA, DDEA, LDEA and SDS were used for further analysis in this work.| Surfactants | 0.05 wt% | 0.3 wt% | ||
|---|---|---|---|---|
| 50 °C | 80 °C | 50 °C | 80 °C | |
| a Note: when the turbidity of surfactant solutions was lower than 1 NTU, the solubility of surfactant was defined as g (good), otherwise was defined as w (worse). | ||||
| ODEA | g | g | g | g |
| DDEA | g | g | g | g |
| LDEA | g | g | g | g |
| MDEA | g | g | w | w |
| PDEA | g | g | w | w |
| SDEA | w | w | w | w |
| SDS | g | g | g | g |
3.2. Oil/aqueous interfacial tension analyses
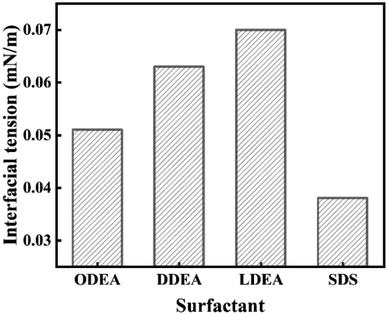
Fig. 1 showed the Oil/Aqueous interfacial tension values for ODEA, DDEA, LDEA and SDS. It could be seen that the four samples all had low oil/aqueous interfacial tension values (<0.1 mN m−1). However, in practical applications, it is generally required surfactant to have ultra-low interfacial tension (<10−2 mN m−1). Therefore, it is imperative to decrease the interfacial tension through mixed surfactant system. This corroborated previous conclusions that independent surfactants do not yield satisfactory EOR effects.24 What's more, the HLB value of Bamianhe crude oil was about 8.5, so among the six nonionic surfactants, LDEA was chosen to mix with SDS for preparing mixed surfactant system after comprehensive considerations of water solubility, interfacial tension and HLB values.3.3. Analyses of mixed surfactant system
Fig. 2a illustrated the impact of varying the mass ratio of LDEA and SDS on the oil–aqueous interfacial tension values. It could be seen that with the mass ratio of LDEA and SDS increased, the oil–aqueous interfacial tension of system decreased gradually. When the LDEA-SDS combination ratio was 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
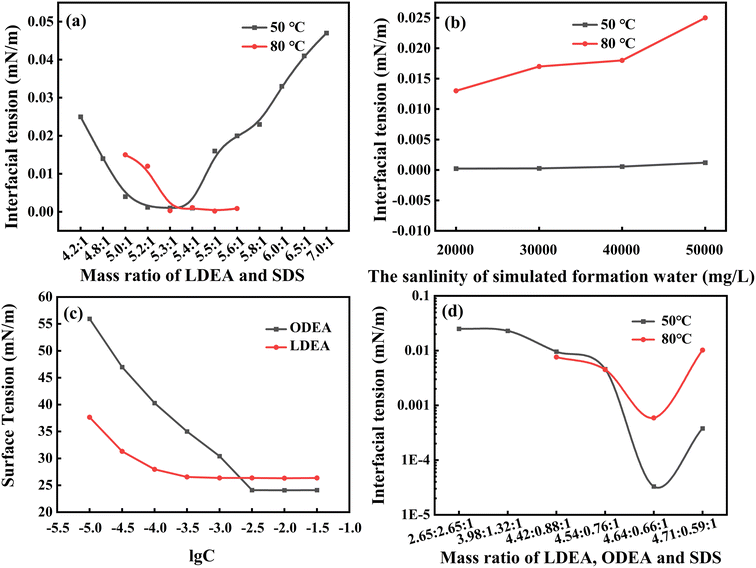
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the system had the lowest oil–aqueous interfacial tension values as 10−3 (50 °C) and 3 × 10−4 mN m−1 (80 °C). Subsequently, as the mass ratio further increased, the interfacial tension of the system gradually increased. However, when the surfactant concentration decreased from 0.2 wt% to 0.05 wt%, the oil–aqueous interfacial tension values of the system were slightly higher than 10−2 mN m−1 at 80 °C (see Fig. 2b). The reason might be owing to that the adsorption capacity of LDEA on the surface of oil droplets was relatively large under high temperature conditions. This could be mitigated through mixing different alkanolamide surfactants to adjust polarity.
1, the system had the lowest oil–aqueous interfacial tension values as 10−3 (50 °C) and 3 × 10−4 mN m−1 (80 °C). Subsequently, as the mass ratio further increased, the interfacial tension of the system gradually increased. However, when the surfactant concentration decreased from 0.2 wt% to 0.05 wt%, the oil–aqueous interfacial tension values of the system were slightly higher than 10−2 mN m−1 at 80 °C (see Fig. 2b). The reason might be owing to that the adsorption capacity of LDEA on the surface of oil droplets was relatively large under high temperature conditions. This could be mitigated through mixing different alkanolamide surfactants to adjust polarity.
From Fig. 2c, the surface tension of LDEA and ODEA solutions basically no longer decreased when the solution concentrations reached 1 mmol L−1 and 3.16 mmol L−1, respectively, which corresponded to their CMC values. Notably, the attained CMC values of LDEA and ODEA aligned with the literature for SDS (with a CMC value of 1.78 mmol L−1).25 The consistency of the composite system were thus ensured.
Based on the mass ratio of LDEA and SDS as 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a certain amount ODEA was used to instead LDEA for preparing the new mixed surfactant system. From Fig. 2d, when the ternary system with a ratio of LDEA
1, a certain amount ODEA was used to instead LDEA for preparing the new mixed surfactant system. From Fig. 2d, when the ternary system with a ratio of LDEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ODEA
ODEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS = 4.64
SDS = 4.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66
0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the oil/aqueous interfacial tension values were both below 10−3 mN m−1 at 50 °C and 80 °C. Therefore, the ternary mixed surfactant system was finally set as LDEA: ODEA: SDS = 4.64
1, the oil/aqueous interfacial tension values were both below 10−3 mN m−1 at 50 °C and 80 °C. Therefore, the ternary mixed surfactant system was finally set as LDEA: ODEA: SDS = 4.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66
0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
3.4. Interfacial tension of ternary mixed surfactant system
Fig. 3 showed the oil–aqueous interfacial tension of ternary mixed surfactant system (LDEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ODEA
ODEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS = 4.64
SDS = 4.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66
0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under different conditions. The results indicated that the interfacial tension of mixed surfactant system were all below 10−2 mN m−1 in the concentration range of 0.05–0.3 wt% and the solution salinity range of 20
1) under different conditions. The results indicated that the interfacial tension of mixed surfactant system were all below 10−2 mN m−1 in the concentration range of 0.05–0.3 wt% and the solution salinity range of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
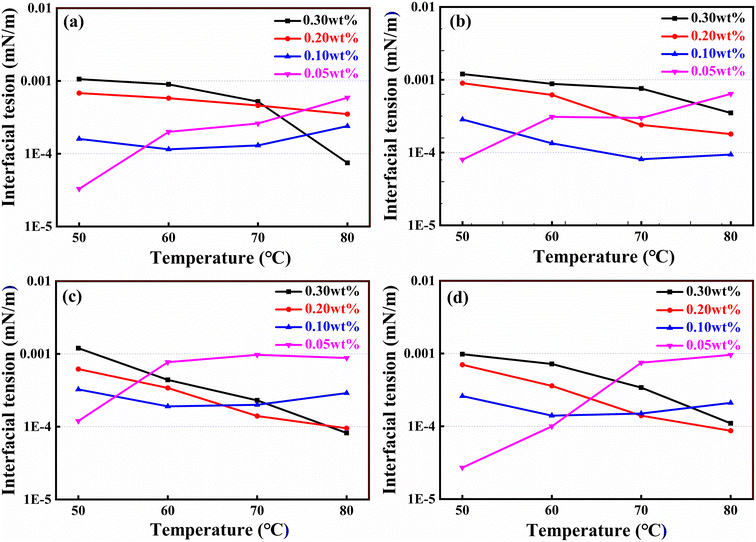
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 at the temperature range of 50–80 °C. It was worth mentioning that the interfacial tension of mixed surfactant system in present work was much smaller than that of the surfactants reported in literature under similar experimental conditions.26,27 The reason might be that when the anionic surfactants and nonionic surfactants mixed, the system could form mixed micelles and mixed adsorption layers, thereby weakening the repulsive effect between ions of anionic surfactants and letting the micelles easier to be formed. As for the system with concentration of 0.05 wt%, the interfacial tension increased with increasing temperature, mainly because high temperature would lead to the acceleration of thermal movement of the surfactant in the two phases, and the mass transfer between oil and water accelerated. The ternary mixed surfactant system could significantly reduce the oil–aqueous interfacial tension and had good salt and temperature resistance, which would greatly facilitate oil displacement in the Bamianhe oil field.
000 mg L−1 at the temperature range of 50–80 °C. It was worth mentioning that the interfacial tension of mixed surfactant system in present work was much smaller than that of the surfactants reported in literature under similar experimental conditions.26,27 The reason might be that when the anionic surfactants and nonionic surfactants mixed, the system could form mixed micelles and mixed adsorption layers, thereby weakening the repulsive effect between ions of anionic surfactants and letting the micelles easier to be formed. As for the system with concentration of 0.05 wt%, the interfacial tension increased with increasing temperature, mainly because high temperature would lead to the acceleration of thermal movement of the surfactant in the two phases, and the mass transfer between oil and water accelerated. The ternary mixed surfactant system could significantly reduce the oil–aqueous interfacial tension and had good salt and temperature resistance, which would greatly facilitate oil displacement in the Bamianhe oil field.
3.5. Wetting capacity of ternary mixed surfactant system
The contact angle of ternary mixed surfactant system (LDEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ODEA
ODEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS = 4.64
SDS = 4.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66
0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on both quartz and oil rock surfaces under the calcium and magnesium ion contents of 2000 mg L−1 and solution salinity of 20
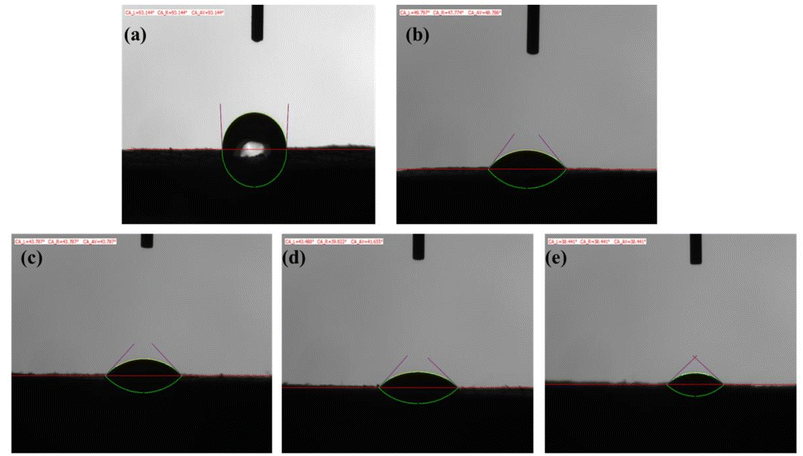
1) on both quartz and oil rock surfaces under the calcium and magnesium ion contents of 2000 mg L−1 and solution salinity of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 were measured and the results are shown in Table 4 and Fig. 4. The data revealed that the contact angles of the salt solution on quartz and oil rock surfaces, in the absence of surfactant, were 44.4° and 93.1°, respectively. As the mixed surfactant concentration increased from 0 to 0.3 wt%, the contact angle on oil rock surface decreased from 93.1° to 38.4°. This indicated that the ternary mixed surfactant system could lead to a greater reduction of contact angle on oil rock surface, and the ternary mixed surfactant had a very good wettability to oil rock surface.
000 mg L−1 were measured and the results are shown in Table 4 and Fig. 4. The data revealed that the contact angles of the salt solution on quartz and oil rock surfaces, in the absence of surfactant, were 44.4° and 93.1°, respectively. As the mixed surfactant concentration increased from 0 to 0.3 wt%, the contact angle on oil rock surface decreased from 93.1° to 38.4°. This indicated that the ternary mixed surfactant system could lead to a greater reduction of contact angle on oil rock surface, and the ternary mixed surfactant had a very good wettability to oil rock surface.
| Surfactant concentration (wt%) | Contact angle (degree) | |
|---|---|---|
| Quartz surface | Oil rock surface | |
| 0 | 44.4 | 93.1 |
| 0.05 | 27.8 | 48.8 |
| 0.10 | 27.7 | 43.8 |
| 0.20 | 0 | 41.7 |
| 0.30 | 0 | 38.4 |
Fig. 5 demonstrated that the oil film peeling time decreased with the mixed surfactant concentration increase. When the surfactant concentration was 0.3 wt%, the oil film peeling time was just for 3 minutes. The result indicated that the ternary mixed surfactant system had a good wetting reversal ability for Bamianhe crude oil.
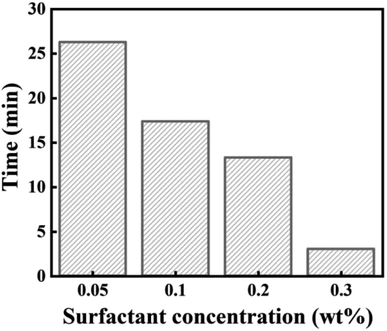 | ||
Fig. 5 The influence surfactant concentration on oil film peeling time. Experimental conditions: calcium and magnesium ion contents = 2000 mg L−1, total salinity = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1. 000 mg L−1. | ||
3.6. Emulsifying power
Fig. 6 presented the emulsifying power (EP) of the mixed surfactant system (LDEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ODEA
ODEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS = 4.64
SDS = 4.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66
0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
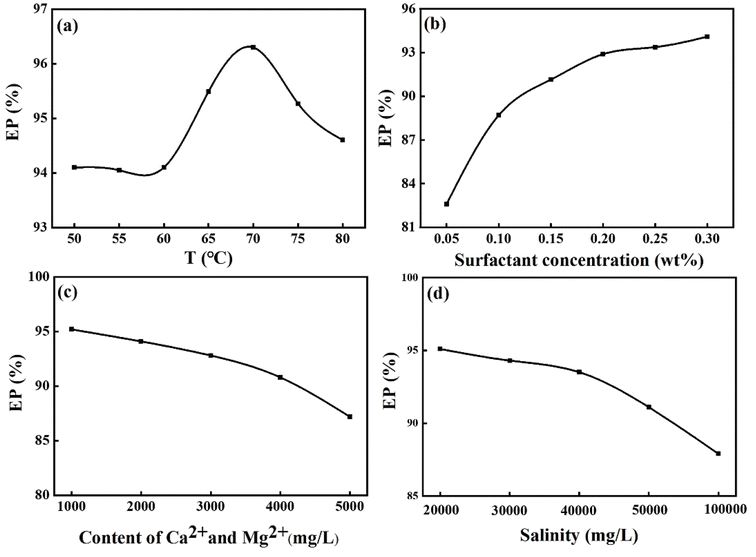
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a function of temperature, surfactant concentration, content of calcium and magnesium ions, and total salinity. It could be observed from Fig. 6a that the mixed surfactant system exhibited the highest EP values (96.4%) at 70 °C under same experimental conditions. As shown in Fig. 6b–d, the EP of the system gradually increased with the increase of surfactant concentration, but decreased with increasing solution salinity and the content of calcium and magnesium ions under same experimental conditions. Under the whole experimental conditions, the EP values was all more than 80%, demonstrating that the ternary mixed surfactant system had a strong emulsification ability for Bameanhe crude oil.
1) as a function of temperature, surfactant concentration, content of calcium and magnesium ions, and total salinity. It could be observed from Fig. 6a that the mixed surfactant system exhibited the highest EP values (96.4%) at 70 °C under same experimental conditions. As shown in Fig. 6b–d, the EP of the system gradually increased with the increase of surfactant concentration, but decreased with increasing solution salinity and the content of calcium and magnesium ions under same experimental conditions. Under the whole experimental conditions, the EP values was all more than 80%, demonstrating that the ternary mixed surfactant system had a strong emulsification ability for Bameanhe crude oil.
3.7. Static adsorption
Fig. 7 illustrated the static adsorption capacity of the mixed surfactant system (LDEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ODEA
ODEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS = 4.64
SDS = 4.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66
0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on oil sands with different concentration at different total salinity (20
1) on oil sands with different concentration at different total salinity (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
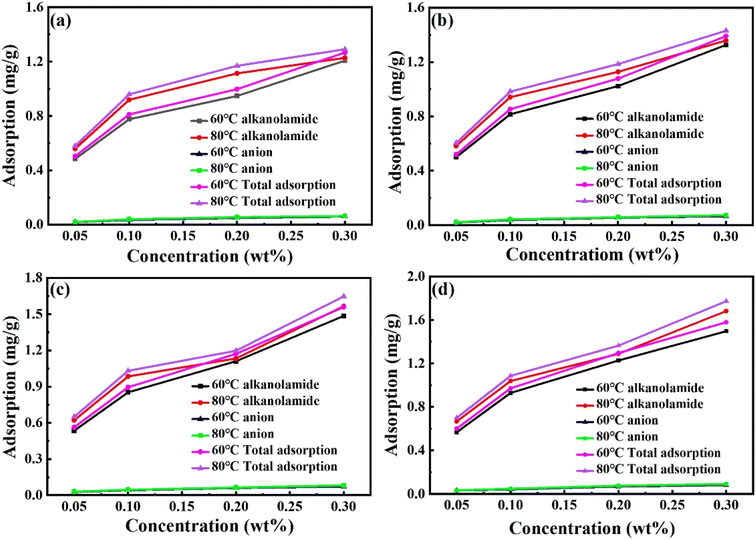
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1) and temperature (60 and 80 °C). It could be observed that the adsorption amounts of LDEA and ODEA, and SDS both increased with the increase of the surfactant concentration and salinity. However, the adsorption capacity of LDEA and ODEA on oil sands was much higher than that of SDS under the same experimental conditions. This should be owing to that oil sands had negative charge in mixed surfactant solutions, and anionic surfactant SDS also has a negative charge, so they would attempt to repel each other, in contrast, the nonionic surfactants LDEA and ODEA could not be ionized in the solution, resulting in a relatively higher adsorption capacity. Within the experimental range, the adsorption capacities of samples were all kept below 2 mg g−1, which were smaller than other reported surfactants.28,29 This indicated that the mixed surfactant system had less adsorption loss in high salinity oil reservoirs.
000 mg L−1) and temperature (60 and 80 °C). It could be observed that the adsorption amounts of LDEA and ODEA, and SDS both increased with the increase of the surfactant concentration and salinity. However, the adsorption capacity of LDEA and ODEA on oil sands was much higher than that of SDS under the same experimental conditions. This should be owing to that oil sands had negative charge in mixed surfactant solutions, and anionic surfactant SDS also has a negative charge, so they would attempt to repel each other, in contrast, the nonionic surfactants LDEA and ODEA could not be ionized in the solution, resulting in a relatively higher adsorption capacity. Within the experimental range, the adsorption capacities of samples were all kept below 2 mg g−1, which were smaller than other reported surfactants.28,29 This indicated that the mixed surfactant system had less adsorption loss in high salinity oil reservoirs.
4. Conclusions
In present work, a specialized ternary mixed surfactant system composed of nonionic alkanolamide surfactants and anionic surfactant was successfully developed with the optimal LDEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ODEA
ODEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS ratio of 4.64
SDS ratio of 4.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66
0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Within the experimental conditions that temperature ranging from 50 to 80 °C, the total salinity range of 20
1. Within the experimental conditions that temperature ranging from 50 to 80 °C, the total salinity range of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 50
000 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1, and calcium and magnesium ion content of 2000 mg L−1, the interfacial tension values between mixed surfactant solution and crude oil were all found to be below 10−2 mN m−1 and even lower than 10−3 mN m−1. The static adsorption amounts of mixed surfactant onto oil sands remained below 2 mg g−1 when the surfactant concentration ranged from 0.05 to 0.3 wt%. The mixed surfactant system also exhibited favorable wetting capacity and emulsifying power. In summary, the investigated ternary mixed surfactant system exhibited significant potential for EOR in Bamianhe Oil Field.
000 mg L−1, and calcium and magnesium ion content of 2000 mg L−1, the interfacial tension values between mixed surfactant solution and crude oil were all found to be below 10−2 mN m−1 and even lower than 10−3 mN m−1. The static adsorption amounts of mixed surfactant onto oil sands remained below 2 mg g−1 when the surfactant concentration ranged from 0.05 to 0.3 wt%. The mixed surfactant system also exhibited favorable wetting capacity and emulsifying power. In summary, the investigated ternary mixed surfactant system exhibited significant potential for EOR in Bamianhe Oil Field.
Author contributions
Conceptualization, Luxuan Ma, Ping Xu, Lei Wang, Kai Xia, Ruitong Gao, Hui Du and Zhaojun Chen; methodology, Luxuan Ma, Ping Xu, Lei Wang, Kai Xia, Ruitong Gao, Hui Du and Zhaojun Chen; software, Luxuan Ma and Zhaojun Chen; validation, Ruitong Gao and Hui Du; writing – original draft, Luxuan Ma and Zhaojun Chen; writing – review & editing, Luxuan Ma, Ping Xu, Lei Wang, Kai Xia, Ruitong Gao, Hui Du and Zhaojun Chen. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by the Research and Development Center for the Sustainable Development of Continental Sandstone Mature Oilfield by National Energy Administration (RH220000290235).References
- D. Abraham, O. Orodu, V. Efeovbokhan, E. Okoro, T. Ojo and L. Keshinro, in SPE Nigeria Annual International Conference and Exhibition, 2020 Search PubMed.
- V. Alvarado and E. Manrique, Energies, 2010, 3(9), 1529–1575 CrossRef.
- N. Kumar, T. Gaur and A. Mandal, J. Ind. Eng. Chem., 2017, 54, 304–315 CrossRef CAS.
- N. Pal, N. Saxena, K. V. D. Laxmi and A. Mandal, Chem. Eng. Sci., 2018, 187, 200–212 CrossRef CAS.
- H. Gong, H. Zhang, L. Xu, K. Li, L. Yu, Y. Li and M. Dong, RSC Adv., 2017, 7(63), 39564–39575 RSC.
- U. K. Bhui, S. Sanyal, R. Saha, S. Rakshit and S. K. Pal, Fuel, 2018, 234, 1081–1088 CrossRef CAS.
- S. Kumar and A. Mandal, Appl. Surf. Sci., 2016, 372, 42–51 CrossRef CAS.
- S. Jain, H. Pachisia, A. Sharma, S. Patel, S. Patel and B. Ragunathan, International Conference on Additive Manufacturing and Advanced Materials (AM2), 2022, pp. 7220–7223 Search PubMed.
- X.-M. Jiang, L. Zhang, W.-Q. Zhang and S. Zhao, J. Surfactants Deterg., 2015, 18(1), 41–45 CrossRef CAS.
- P. Koreh, M. Lashkarbolooki, M. Peyravi and M. Jahanshahi, J. Pet. Sci. Eng., 2022, 218, 110960 CrossRef CAS.
- J. Lv, W. Qiao and C. Xiong, Langmuir, 2014, 30(28), 8258–8267 CrossRef CAS.
- M. Salehi, S. J. Johnson and J.-T. Liang, Surfactants Deterg., 2010, 13(3), 243–246 CrossRef CAS.
- J. Barman, S. B. Gogoi, J. Viswanathan and D. Konwar, Formulation of Chemical Slug for EOR Application of Moran Oil Field of Upper Assam Basin, India, Sustainability Issues in Environmental Geotechnics, Springer International Publishing, Cham, 2018, vol. 289, pp. 137–150 Search PubMed.
- S.-S. Sheng, X.-L. Cao, Y.-W. Zhu, Z.-Q. Jin, L. Zhang, Y. Zhu and L. Zhang, J. Mol. Liq., 2020, 313, 112772 CrossRef CAS.
- K. Hazarika, R. Yadav, S. B. Gogoi and U. K. Bhui, Int. J. Ambient Energy, 2019, 40(6), 645–656 CrossRef CAS.
- H. ShamsiJazeyi, R. Verduzco and G. J. Hirasaki, Colloids Surf., A, 2014, 453, 168–175 CrossRef CAS.
- S. Mohammadi, S. Kord and J. Moghadasi, J. Mol. Liq., 2019, 281, 352–364 CrossRef CAS.
- W.-F. Pu, D.-J. Du, Y.-L. Tang and S. Wang, J. Surfactants Deterg., 2018, 21(5), 687–697 CrossRef CAS.
- Y. He, K. Liao, J. Bai, L. Fu, Q. Ma, X. Z. Ren and W. Wang, ACS Omega, 2021, 6(16), 11068–11076 CrossRef CAS PubMed.
- N. A. M. Saad, D. F. Mohshim and A. Abdul, 3rd Symposium on Industrial Science and Technology (SISTEC), 2022, Vol. 51, pp. 1282–1287 Search PubMed.
- C. Xue, H. Zhao, Q. Wang, K. Zhang and Y. Li, J. Dispersion Sci. Technol., 2018, 39(10), 1427–1434 CrossRef CAS.
- M. J. Qazi, S. J. Schlegel, E. H. G. Backus, M. Bonn, D. Bonn and N. Shahidzadeh, Langmuir, 2020, 36(27), 7956–7964 CrossRef CAS PubMed.
- Z. Chen, H. Mi, X. Liu, K. Xia, F. Ge, X. Li and X. Zhang, J. Surfactants Deterg., 2019, 22(6), 1309–1317 CrossRef CAS.
- J. Vilasau, C. Solans, M. J. Gomez, J. Dabrio, R. Mujika-Garai and J. Esquena, Colloids Surf., A, 2011, 389(1–3), 222–229 CrossRef CAS.
- D.-Y. Zhu, F. Cheng, Y. Chen and S.-C. Jiang, Colloids Surf., A, 2012, 397, 1–7 CrossRef CAS.
- J. Liu, L. Zhong, T. Hao, Y. Liu and S. Zhang, J. Mol. Liq., 2022, 349, 118207 CrossRef CAS.
- A. A. Dehghan, M. Masihi and S. Ayatollahi, Energy Fuels, 2013, 27(10), 5852–5860 CrossRef CAS.
- T. Yuan, Z. Liu, R. Gao, G. Hu, G. Zhang and J. Zhao, J. Appl. Polym. Sci., 2018, 135(14), 46086 CrossRef.
- S. E. I. Lebouachera, R. Chemini, M. Khodja, B. Grassl, M. A. Ghriga, D. Tassalit and N. Drouiche, Eur. Phys. J. Plus, 2019, 134(9), 436 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |