Open Access Article
Open Access ArticleAll-inorganic lead halide perovskites for photocatalysis: a review
Yajie Huang,
Jiaxing Yu,
Zhiyuan Wu,
Borui Li and
Ming Li *
*
College of Forestry, Northeast Forestry University, Harbin 150040, China. E-mail: liming1986@nefu.edu.cn; Tel: +86-451-82192120
First published on 7th February 2024
Abstract
Nowadays, environmental pollution and the energy crisis are two significant concerns in the world, and photocatalysis is seen as a key solution to these issues. All-inorganic lead halide perovskites have been extensively utilized in photocatalysis and have become one of the most promising materials in recent years. The superior performance of all-inorganic lead halide perovskites distinguish them from other photocatalysts. Since pure lead halide perovskites typically have shortcomings, such as low stability, poor active sites, and ineffective carrier extraction, that restrict their use in photocatalytic reactions, it is crucial to enhance their photocatalytic activity and stability. Huge progress has been made to deal with these critical issues to enhance the effects of all-inorganic lead halide perovskites as efficient photocatalysts in a wide range of applications. In this manuscript, the synthesis methods of all-inorganic lead halide perovskites are discussed, and promising strategies are proposed for superior photocatalytic performance. Moreover, the research progress of photocatalysis applications are summarized; finally, the issues of all-inorganic lead halide perovskite photocatalytic materials at the current state and future research directions are also analyzed and discussed. We hope that this manuscript will provide novel insights to researchers to further promote the research on photocatalysis based on all-inorganic lead halide perovskites.
1 Background and development of perovskite materials
1.1 Non-perovskite photocatalysts and oxide perovskite photocatalysts
Globally, the challenges of the energy crisis and environmental pollution are becoming more severe.1 An increasing number of studies have investigated the efficient use of solar energy to overcome these problems.2–4 Among these methods, visible light catalysis technique has demonstrated considerable promise. A wide range of photocatalysts has been extensively studied in photocatalytic processes.TiO2 was originally used as a photocatalyst to break down water into H2 and O2.5 Different catalysts have been employed, including metal oxides (ZnO, WO3, Co2O, CeO2),6–8 metal sulfides (MoS2, WS2, Co3S4),9–14 nitrides (C3N4, GaN),15,16 phosphides (InP, Ni2P, CoP, FeP),17–26 and carbides (C3N4, rGO, MoC).27–31 Photocatalysts with band gap (Eg) larger than 3 eV, such as TiO2, ZnO, and MnS, have a fatal flaw: they can only be activated by UV light and cannot respond to a wide range of visible light regions. However, independent photocatalysts with Eg less than 3 eV, such MoS2, WO3, and C3N4, are impacted by the quick recombination of photogenerated carriers even though they exhibit photocatalytic activity under visible light irradiation. In short, these conventional photocatalysts still have several issues such as poor light absorption,32 low quantum efficiency,33–36 high interfacial charge transfer resistance,37 and rapid photogenerated carrier complexation,38–41 thus significantly restricting the development and practical applications of photocatalysis. A range of efficient techniques, such as doping, implementing cocatalysts, building heterostructures, as well as controlling the morphology and crystallography, have been employed to conquer the aforementioned drawbacks and enhance the photocatalytic activity (Table 1).
| No. | Sample | Application | Synthesis method | Photocatalytic performance | Ref. |
|---|---|---|---|---|---|
| a MB: methylene blue, PMMA: polymethyl methacrylate, TC: tetracycline hydrochloride, RhB: rhodamine B. | |||||
| 1 | Ag/TiO2 | Degradation of MB wastewater | Sol–gel method | Degradation efficiency of MB is 98.86% (5 mg per L MB, 250 min) | 49 |
| CsPbBr3/PMMA | Degradation of MB wastewater | Electrospinning technology | Degradation efficiency of MB is 99.18% (5 mg per L MB, 60 min) | 50 | |
| 2 | TiO2/PCN-224 | H2 production | Vacuum filtration | H2 production rate (1.88 mmol g−1 h−1) | 51 |
| CsPbBr3 | H2 production | Hot injection | H2 evolution rate is 133.3 μmol g−1 h−1 (saturated HBr aqueous solution) | 52 | |
| 3 | ZnS/ZnIn2S4 | CO2 reduction | Hydrothermal | Acetaldehyde yield 367.63 μmol g−1 (selectivity 99.6%) | 53 |
| Co/CsPbBr3/TiOx | CO2 reduction | Electrostatic self-assembling | The rate of CO2 reduction reaches 405.2 μmol g−1 h−1 | 54 | |
| 4 | TiO2/rGO/Cu2O | Degradation of antibiotic wastewater | Hummers method | Removal rate of TC is 99.38% (100 mg L−1, 40 min) | 55 |
| CsPbBr3–TiO2 | Degradation of antibiotic wastewater | Solvothermal method | Removal rate of TC is 94% (20 mg L−1, 60 min) | 56 | |
| 5 | InP/InPS/ZnS | H2 evolution | Hot injection | The rate of H2 production reached 102 μmol mg−1 h−1 | 57 |
| CsPbX3@ZIF-8 | H2 evolution | Mechanical milling | The rate of H2 production reached 7.852 μmol g−1 h−1 | 58 | |
| 6 | MoS2 | Heavy metal degradation | Ball milling | Cr(VI) removal rate can reach 98.9% (180 min) | 59 |
| CsPbI3/ZnO | Heavy metal degradation | Hot injection | Degradation efficiency of heavy metal is 52% | 60 | |
| 7 | g-C3N4/CeO2 | Degradation of RhB wastewater | Calcination and hydrothermal | The RhB degradation rate reached 99.07% (7 mg L−1 60 min) | 61 |
| CsPbBr3/PMMA | Degradation of RhB wastewater | Ball milling | The RhB degradation rate reached 92.2% (10 mg L−1 60 min) | 62 | |
Perovskite materials have drawn considerable attention as suitable photocatalysts. The general chemical formula of perovskites is ABX3, where A, B, and X represent monovalent cations (La+, Bi+, CH(NH2)2+, CH3NH3+, Cs+, etc.), divalent cations (Pb2+, Mn2+, Co2+, Ti2+, etc.), and oxygen, halogen, etc. (O, Cl−, I−, Br−, etc.), respectively.42 When X is O, ABO3 is called an oxide perovskite; organic–inorganic hybrid perovskites generally use CH3NH3+ (MA) or CH(NH2)2+ (FA) as the A-site cation. When Pb2+ is the B-site cation and Cl−, I−, Br− or their mixtures are the halide ions (X), CsPbX3 is called an all-inorganic lead halide perovskite.43 The three-dimensional structure of a perovskite is formed by connecting the vertex corners of the (BX6)n− octahedron, which is made up of X− and B2+, with B2+ occupying the middle position within the octahedron. The photocatalytic performance of perovskites will be influenced by its crystal structure. The hybridization of the O 2p orbital of the cation in the crystal structure and the 3d orbital of the transition metal in the (BO6) octahedron will not only induce lattice distortion but also induce changes in the dipole and electronic structure, thereby affecting the separation of photogenerated carriers, finally leading to perovskites that have unique and variable photocatalytic properties. Furthermore, researchers can specifically build the electronic structure to enhance the stability, charge migration, and light absorption because of a variety of A and B site ions.44,45 Perovskite materials can also enhance the photocatalytic activity by combining ferroelectric and piezoelectric phenomena.46,47
Due to differences in the photoelectric characteristics, different perovskites exhibit varying photocatalytic efficiency. The following characteristics are mostly indicative of the attributes that influence photocatalytic efficiency: (i) light absorption coefficient. The light absorption ability of catalysts affects their photocatalytic performance. The intensity and range of light absorption are crucial markers. (ii) The length of carrier diffusion. Long carrier diffusion length improves photocatalytic efficacy, decreases charge recombination, and helps separate electrons and holes. (iii) Bandgap width. The narrower the band gap of the perovskite, the worse its stability. With an appropriate Eg (2.4–2.7 eV), the all-inorganic perovskite CsPbX3 exhibits excellent stability.
ABO3 perovskite has been the target of most studies, with impressive findings being obtained in the field of photocatalysis. Mulani et al. prepared Ni-doped LaFe0.6Ni0.4O3 through a hydrothermal method to degrade crystal violet (CV), Congo red (CR), and their mixtures under visible light. The degradation efficiencies were 99.2% (210 min), 99.1% (100 min), and 98.4% (70 min), respectively, which were far higher than that of LaFeO3. LaFe0.6Ni0.4O3 degraded CV dye through dealkylation and benzene ring cleavage, while azo bond cleavage and oxidation lead to the production of low molecular weight intermediate products of CR dye.48 In recent years, oxide perovskites have been rapidly developed in the field of photocatalysis (Fig. 1). Table 2 summarizes the synthesis methods, reaction conditions, and photocatalytic performances of oxide perovskites compared with traditional photocatalysts.
| Catalysts | Synthesis method | Eg (eV) | Application | Photocatalytic performance | Ref. |
|---|---|---|---|---|---|
| a ARS: alizarin red S, BP: phenol, BPA: bisphenol A, PNP: p-nitrophenol, DCP: dichlorophenol, MO: methyl orange. | |||||
| LaFeO3/g-C3N4/ZnO | Hydrothermal | 2.43 | Degradation of BP wastewater | Degraded 97.43% of BP (120 min, 20 mg L−1), 90.14% (BPA), 93.68% (PNP), and 92.37% (DCP) (1 h, 20 mg L−1) | 63 |
| BaTiO3@SrTiO3 | Ultrasonic and hydrothermal method | 3.23 | Water spitting | H2 evolution rate is 18 μmol g−1 min−1 | 64 |
| MgFe2O4/Bi2WO6 | Wet ball milling | 2.5 | Degradation of TC wastewater | Degradation rate of TC is 95.82% (90 min, 20 mg L−1) | 65 |
| Ag/CaZrO3 | Solid-phase method | 5.8 | CO2 reduction | CO production rate (286 μmol g−1 h−1) | 66 |
| Fe–SrWO4 | Hydrothermal | 3.39 | Nitrogen fixation | NH3 production rate is 385 μmol g−1 h−1 | 67 |
| LaCoO3/MoS2 | Sol–gel, hydrothermal, and ultrasonication method | 2.10 | Degradation of dye wastewater | Degraded 96% ARS and 90% RhB in 40 min and 80 min, respectively | 68 |
| BiFeO3/Bi2O3 | Auto-combustion method | 2.27 | Degradation of nitrobenzene | Degraded 100% nitrobenzene | 69 |
| La0.9Sr0.1Fe0.8Co0.2O3 | Pechini method | 2.17 | Degradation of dye wastewater | Degraded 98% reactive black 5 (20 ppm, 160 min) | 70 |
| NaTiO3/g-C3N4 | Hydrothermal method | 2.3 | Degradation of mixed dye wastewater | Degraded 100% mixture of MB and CV in 25 min | 71 |
| Pt–LaMnO3 | Green solid-state combustion method | 2.82 | H2 production | H2 production rate (40 μmol g−1 h−1) (20 vol% methanolic aqueous) solution | 72 |
| BaTiO3 | Ceramic and complex polymerization methods | 3.24 | Diclofenac degradation | Presented the DIC removal (61%) (25 mg L−1, 180 min) | 73 |
| Bi2O4/NaBiO3 | Hydrothermal method | 1.77 | Degradation of MO and BP | Degraded 96.3% MO/BP (20 mg L−1, 60 min) | 74 |
| BiVO4/BaSnO3 | Facile precipitation method | 2.38 | Degradation of MB wastewater | With decolorization rate of 94.1% (20 mg L−1, 2 h) | 75 |
| TiO2/CaTi4O9/CaTiO3 | Solvothermal combination with calcination | 3.21 | H2 production and Cr(VI) reduction | Produced H2 at a rate of 25.28 mmol h−1 g−1 (methanol solution) Cr(VI) reduction rates reached 30.1% (10 mg L−1, 150 min) | 76 |
1.2 Oxide perovskite to all-inorganic lead halide perovskites
ABO3 perovskite exhibited structural flexibility and stability compared with metal oxides; however, it also has shortcomings including large band gap, rapid carrier recombination, small specific surface area, and poor selectivity. Due to the high tunability of band gap, excellent charge mobility, and suitable redox capabilities, organic–inorganic hybrid lead halide perovskites stand out from various materials. Studies have demonstrated that MAPbI3 is consistently and effectively capable of producing H2 from HI under visible light irradiation, and the solar HI separation efficiency by MAPbI3 is 0.81% when Pt is utilized as a noble catalyst.77Due to their poor stability, organic–inorganic hybrid lead halide perovskites have limited application in photocatalysis. In contrast, all-inorganic lead halide perovskites exhibit highly efficient semiconductor properties. They are employed in various applications, including X-ray fluorescence imaging,78–82 visible light communication,83–85 solar cells,86–89 light-emitting diodes (LEDs),90–92 lasers,93,94 photodetectors,95–97 and photocatalysis. However, most of the previous studies have focused on the optoelectronic properties of all-inorganic lead halide perovskites; a thorough overview of the all-inorganic lead halide perovskites in photocatalysis is urgently required to provide a guide for future research. Herein, all-inorganic lead halide perovskites are summarized in detail with an emphasis on the approaches of synthesis, modification, and applications in photocatalysis. Moreover, the problems and the future development in the field of photocatalysis are summarized and prospected.
2 Synthesis methods of all-inorganic lead halide perovskites
The properties of all-inorganic lead halide perovskites largely depend on their structures, which are directly related to the synthesis methods. Numerous techniques have been investigated to synthesize all-inorganic lead halide perovskites, such as hot injection,98–100 room-temperature ligand-assisted deposition,101–103 microwave synthesis,104–106 ultrasound synthesis,107 template method,108–110 laser irradiation-assisted method,111 ball milling method,112 and solvothermal method.113 These methods can be broadly classified as “bottom-up” or “top-down” depending on the growth process.114 The two techniques covered in this paper are the hot-injection and ligand-assisted deposition in room-temperature method (LARP), which are widely used for creating all-inorganic lead halide perovskites. The synthesis methods, advantages, and disadvantages of all-inorganic lead halide perovskites are summarized in Tables 3 and 4.| No. | All-inorganic lead halide perovskite | Method | Reaction conditions | Ref. |
|---|---|---|---|---|
| 1 | CsPbX3 nanocubes (410–530 nm) (X = Cl, Br, I, and mixed systems) | Hot injection method | 140–200 °C inert gas protection | 115 |
| 2 | CsPbBr3 nanocrystals (6.8–13.6 nm) | LARP | Room temperature (25 °C) octylphosphonic acid | 116 |
| 3 | CsPbBr3 (film) | Anti-solvent method | Room temperature (varies with season) toluene as an antisolvent | 117 |
| 4 | CsPbBr3 (bulk) | Ball milling method | Ball mill (500 rpm) | 112 |
| 5 | CsPbI3 (8–12 nm) | Ultrasonic method | Room temperature tip ultrasound (power 30 W) | 107 |
| 6 | CsPbX3 (X = Cl/Br, Br and Br/I) nanowires (diameters as small as 2.6 nm) | Solvothermal method | 160 °C (high temperature reactor) | 113 |
| 7 | CsPbBr3 quantum dots | Template method | Kaolin as template | 118 |
| 8 | CsPbBr3 nanocrystals (nanoplates, nanocubes) | Microwave synthesis | Microwave reactor 80 °C (nano-plate) 160 °C (nano-cube) | 119 |
| Synthesis method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Hot injection method | The reaction process is simple and less time-consuming, and the nanocrystals are relatively uniform in morphology and size | Inert gas protection is required during high temperature processes, which will increase the costs in practical applications | 134 |
| LARP method | Low cost and simple operation | Nanocrystals have different sizes, poor crystallinity, and low experimental repeatability, making them unsuitable for mass production | 135 |
| Anti-solvent method | Small particle size, uniform particle distribution, high dispersion, and controllable morphology | There are many factors that affect the preparation effect of this method (such as the selection and dosage of antisolvent) | 136 |
| Ultrasonic method | Low reaction temperature, high purity, small particle size | The purity of synthetic materials differs | 129 |
| Solvothemal method | The synthesis conditions are mild, the product purity is high, the crystal grains are fully developed, the particle size is small and evenly distributed, and the morphology is highly controllable | Factors affecting the nucleation process and crystal growth process are difficult to be controlled; highly dependent on equipment | 137 |
| Template method | Get good nanoarrays | Templates have the problem of low yield and difficulty in separation. The templates need to be removed, which will destroy the structure of the nanomaterials to a certain extent | 138 |
2.1 Hot injection method
The hot-injection method is a typical complex decomposition procedure that involves developing CsPbX3 nanocrystals by completely mixing the PbX2 precursor material with oleic acid, oleylamine, and octadecene and then rapidly cooling them at high temperatures (140–200 °C). In general, perovskite precursors crystallize into nanoplate-like morphologies at lower reaction temperatures, while they frequently generate cubic nanocrystals at higher reaction temperatures. When the temperature cools down, the size of the perovskite nanocubes also minimizes. The hot injection method can usually obtain dispersed CsPbX3 nanocubes, and the halide composition of the nanocubes can be easily adjusted by changing the proportion of the PbX2 precursor.Nedelcu et al. firstly achieved the successful hot-injection synthesis of the all-inorganic lead halide perovskite CsPbX3, which also indicated that CsPbBr3 and CsPbI3 had higher photoluminescence quantum yield (PLQY) compared to CsPbCl3.120 For instance, the PLQY of CsPbBr3 nanocrystals reached 90%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 (Fig. 2). The hot injection method not only synthesizes pure CsPbX3 but also yields CsPbX3-based composites. Wang et al. designed CsPbBr3–CdZnS heterojunctions to photocatalytically reduce CO2 by the hot-injection method; it was found that the CsPbBr3–CdZnS heterojunction achieved a higher CO yield (55.8 μmol g−1 h−1) than that of the pristine CsPbBr3 (13.9 μmol g−1 h−1).121
115 (Fig. 2). The hot injection method not only synthesizes pure CsPbX3 but also yields CsPbX3-based composites. Wang et al. designed CsPbBr3–CdZnS heterojunctions to photocatalytically reduce CO2 by the hot-injection method; it was found that the CsPbBr3–CdZnS heterojunction achieved a higher CO yield (55.8 μmol g−1 h−1) than that of the pristine CsPbBr3 (13.9 μmol g−1 h−1).121
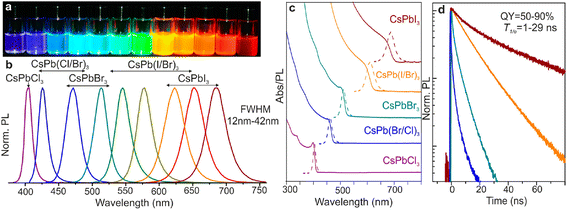 | ||
| Fig. 2 Colloidal perovskite CsPbX3 (X = Cl, Br, I) exhibit size- and composition-tunable bandgap energies covering the entire visible spectral region with narrow and bright emission: (a) colloidal solutions in toluene under UV lamp (λ = 365 nm); (b) representative PL spectra (λexc = 400 nm for all but 350 nm for CsPbCl3 samples); (c) typical optical absorption and PL spectra; (d) time-resolved PL decays for all samples shown in (c) except CsPbCl3.115 | ||
The hot-injection method for synthesizing all-inorganic lead halide perovskite nanocrystals has been continuously optimized using different precursors and ligands to achieve the controlment of shape and size as well as better stability over the years.122 Leng et al. successfully synthesized highly stable nanocrystals by designing a “modified hot-injection method” to maximize the number of ligand molecules and lowering the temperature (<40 °C) during precursor mixing to separate the nucleation and growth processes.123 However, in large-scale production, the inability to adjust the high temperature when combining precursor solutions leads to poor material reproducibility.
2.2 Room-temperature ligand-assisted deposition
Even though the hot-injection method has been widely used to produce all-inorganic perovskite nanocrystals, it is a laborious process that needs extra steps in the synthesis of the Cs-oleate precursor and is typically carried out in an inert environment. LARP is an appropriate remedy for these limitations. Organic ligands and perovskite precursors are dissolved in solvent, and then spontaneous crystallization in a supersaturated state of the crystal can be achieved by lowering the temperature, evaporating the solvent, or adding a lean solvent with a lower solubility of the substance.124 If crystallization is carried out in the presence of ligands, crystal nucleation and growth can be controlled, which is LARP.Guvenc et al. produced CsPbX3 (X = Br, Cl, I) by LARP; the PLQY of synthesized CsPbBr3 was 85%.125 By altering the experimental conditions, the LARP method can be used to manufacture all-inorganic lead halide perovskite with various morphologies. Deng et al. formed all-inorganic CsPbX3 perovskite in a variety of shapes, including 0D spherical quantum dots, 1D nanorods, and 2D nanoplates126 (Fig. 3). Zhang et al. discovered that stable CsPbBr3 nanocrystals could be manufactured by adding small amounts of water to the reaction mixture, and the PLQY of the CsPbBr3 nanocrystals was 90%.127
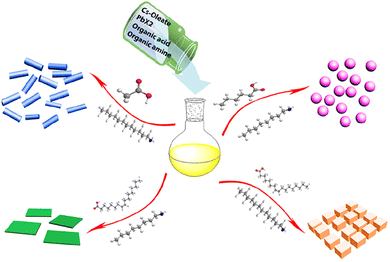 | ||
| Fig. 3 Schematic illustrating the formation process for different CsPbX3 (X = Cl, Br, I) nanocrystals mediated by organic acid and amine ligands at room temperature. Spherical quantum dots represent hexanoic acid and octylamine; nanocubes represent oleic acid and dodecylamine; nanorods represent acetate acid and dodecylamine; few unit cell-thick nanoplatelets represent oleic acid and octylamine.126 | ||
It is worth noting that perovskite nanocrystals are sensitive to polar solvents; therefore, the synthesized nanocrystals have different sizes, poor crystallinity, and low experimental repeatability, which are not suitable for large-scale production. To create high-performance all-inorganic lead halide perovskites, the researchers modified the original room-temperature ligand-assisted deposition method. Zeng et al. developed a comparable room-temperature supersaturation recrystallization technique to produce CsPbX3 quantum dots.118 A highly supersaturated state is produced as soon as the halide salt precursor is transferred from a polar (N,N-dimethylformamide) to a nonpolar solvent (toluene), and the process can be finished without using a protective environment, heating, or injection in only a few seconds. Pan et al. investigated the ligand-mediated synthesis of all-inorganic CsPbBr3 perovskite nanocrystals, and the results revealed that CsOAc was a more versatile precursor substance than the commonly used Cs2CO3, displaying a greater solubility and a wider temperature.122
2.3 Other synthesis methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132 and discovered that these materials were more stable after 30 days than those that were thermally synthesized under the same circumstances.
132 and discovered that these materials were more stable after 30 days than those that were thermally synthesized under the same circumstances.3 Modification of all-inorganic lead halide perovskite
Although all-inorganic lead halide perovskites have a great deal of potential for photocatalysis, however, pure halide perovskites typically have some issues, such as low stability, lack of active sites, and ineffective carrier extraction, which restrict the utilization of photocatalytic materials.139–142 To improve the photocatalytic activity, several techniques have been developed, including ion doping,143,144 metal loading,145,146 creation of heterojunctions,147,148 and anion exchange.149–1523.1 Ion-doping
Doping is a valuable modification strategy that adjusts the chemical structure of the lattice by incorporating some “impurities” to broaden the photoresponse range or enhance the separation of photogenerated carriers.153–156 To this end, researchers have explored methods of incorporating metal ions at the A-site and B-site. Generally speaking, A-site and B-site doping can be achieved through cation exchange or in situ synthesis. Mn2+ is the most common dopant ion in the B-site of all-inorganic lead halide perovskites.157–162 Liu et al. prepared Mn2+-doped CsPbBr3 to improve the efficiency of CO2 reduction163 (Fig. 4). The results showed that the yields of CO and CH4 reached 1917 μmol g−1 and 82 μmol g−1, which were 14.2 and 1.4 times that of CsPbBr3. In addition, other metal atoms have also been successfully doped into all-inorganic lead-halide perovskites. Patil et al. doped CsPbI2Br nanocrystals with Sr2+ to obtain more stable solar cells,164 and the CsPb0.98Sr0.02I2Br device maintained more than 85% of its initial efficiency for 100 hours under ambient conditions. Shyamal et al. partially substituted Pb2+ with Fe2+ to create Fe2+-doped CsPbBr3,165 which was utilized in ethyl acetate/aqueous solution for the photocatalytic reduction of CO2. Dong et al. produced Co2+-doped CsPbBr3/Cs4PbBr6 for CO2 reduction with 1835 mol per g CO2 yield.166 All-inorganic lead-halide perovskites are also doped with new ions. In addition to equivalent doping, heterovalent ions can also be introduced. Zhou et al. stabilized CsPbBr3 by co-doping Na+, and the results showed that co-doping metal ions significantly improved the thermal stability and optical properties of quantum dots.167 Using calculations based on density functional theory, Wang et al. investigated how Bi3+ doping affected the structural,168 electrical, and optical characteristics of CsPbBr3, and the results revealed that Bi3+ doping can improve the stability.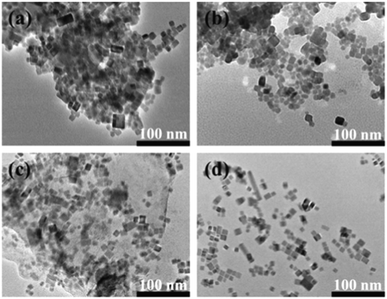 | ||
Fig. 4 TEM images of Mn-doped CsPb(Br/Cl)3 mixed-halide perovskites (a) sample 1: the ratio of PbBr2/MnCl2 is 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) sample 2: the ratio of PbBr2/MnCl2 is 2 1, (b) sample 2: the ratio of PbBr2/MnCl2 is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) sample 3: the ratio of PbBr2/MnCl2 is 2 1, (c) sample 3: the ratio of PbBr2/MnCl2 is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (d) sample 4: the ratio of PbBr2/MnCl2 is 3 3, (d) sample 4: the ratio of PbBr2/MnCl2 is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.163 7.163 | ||
Studies on A-site doping are becoming increasingly prevalent. Ru+ and K+ are a couple of alkali metal ions that have drawn increased interest as possible dopants. These dopants can be applied to boost the photoluminescence efficiency and stability of perovskite nanocrystals. For example, Huang et al. created K–CsPbBr3 nanocrystals that maintained 97.9% of their initial intensity after 30 hours.169 Ouaaka et al. assessed the electrical, optical, thermoelectric, and elastic characteristics of Rb-doped CsPbBr3 by density functional theory. The findings demonstrated that RbxCs1−xPbBr3 had excellent ultraviolet absorption capabilities, a low range of Eg, and enhanced thermal–electrical conductivity.170
In brief, ion doping has been thoroughly investigated to modify the electronic structure or create new energy and charge transfer routes to enhance the optical, electrical, and structural stability of all-inorganic lead halide perovskites. By the interaction of excitons and dopants, doping can bring additional optical, electronic, and magnetic features in addition to increasing the photocatalytic efficiency.171,172
3.2 Heterojunction construction
The formation of a heterojunction at the interface between two distinct materials can efficiently improve the separation efficiency of photogenerated carriers, thus enhancing the photocatalytic performance. There are three types of heterojunctions suitable for photocatalysis: type II heterojunction, direct Z-type heterojunction, and S-type heterojunction. Different types of heterojunctions and their applications are summarized in Table 5.| No. | Photocatalyst | Type | Application | Reference |
|---|---|---|---|---|
| 1 | WO3/CsPbBr3 | S | CO2 reduction | 173 |
| 2 | C3N4@CsPbBr3 | Z | CO2 reduction | 174 |
| 3 | CsPbBr3–rGO/Bi2WO6 | S | Norfloxacin degradation | 175 |
| 4 | α-Fe2O3/rGO/CsPbBr3 | Z | CO2 reduction | 176 |
| 5 | CsxWO3/CsPbBr3 | Z | Hydrogen generation | 177 |
| 6 | CsPbBr3–CdS | II | Contaminant degradation | 178 |
| 7 | CsPbBr3/GO | II | Photoconductivity | 179 |
| 8 | CsPbBr3/Bi2WO6 | Z | Pyrotechnic tar reduction | 180 |
| 9 | Bi2O2Se/CsPbBr3 | S | Light detection | 181 |
| 10 | CsPb(Br/I)3/SiO2 | II | LED | 90 |
| 11 | CsPbBr3/GeSn | II | Photodetector | 182 |
| 12 | CsPbBr3/TiO2 | S | CO2 reduction | 183–186 |
| 13 | CsPbBr3/PbS | Z | Light detection | 187 |
| 14 | CsPbBr3/CsPb2Br5 | Z | LED | 188 |
| 15 | CsPbBrCl2/g-C3N4 | II | Photodegradable dyes | 189 and 190 |
| 16 | CsPbBr3/GO–Pt | Z | Hydrogen generation | 191 |
| 17 | CsPbBr3/Cs4PbBr6@COF | Z | Water spilt | 192 |
| 18 | CsPbBr3–CdZnS | S | CO2 reduction | 121 |
| 19 | CsPbBr3/UiO-66 | Z | Methyl orange degradation | 193 |
| 20 | CsPbBr3@SnO2 | S | CO2 reduction | 194 |
| 21 | Ag/CsPbBr3/Bi2WO6 | Z | Rhodamine B degradation | 195 |
| 22 | PCN-222/CsPbBr3 | Z | CO2 reduction | 196 |
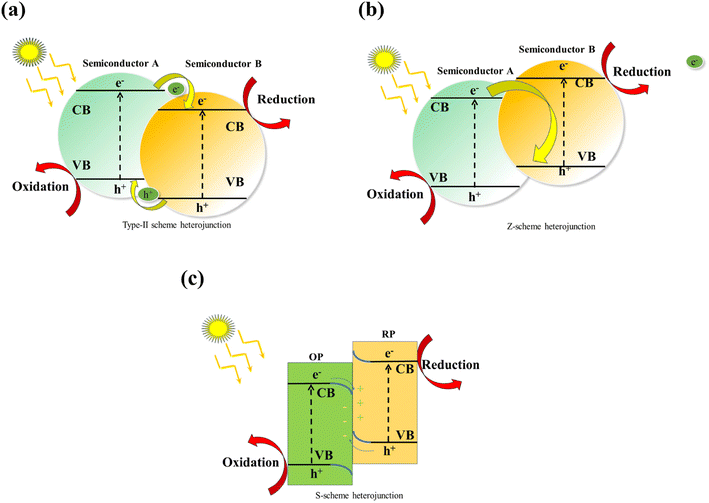 | ||
| Fig. 5 Mechanism diagram of type-II heterojunction (a); mechanism diagram of Z-scheme heterojunction (b); mechanism diagram of S-scheme heterojunction (c). | ||
However, the type II heterojunction still has some issues: on the one hand, the oxidizing capacity of two semiconductor photocatalysts decreased by the separation of photogenerated electron–holes; on the other hand, the presence of holes prevents the transfer of electron–holes between surfaces in other catalysts.
It should be noted that unanticipated side effects will result from Z-type heterojunction. The Z-type heterojunction increases the electron and hole-driving force of photogenerated chemical reactions; however, half of the electrons and holes produced during the photosynthesis process are lost. Therefore, when designing and building all-inorganic lead-halide perovskite-based heterojunction photocatalysts, the reaction process and the necessary redox potential should be taken into account.
In conclusion, all-inorganic halide perovskites are highly advantageous for photocatalysis due to their high absorption coefficient, defect tolerance, and variable band position. An extensive density of active sites, strong stability, and recyclability are also necessary for photocatalysts in addition to effective charge separation and transfer. In general, it is difficult for single-component halide perovskite photocatalysts to meet all these requirements. Integrating heterostructures of different functional materials into a single system through precise design is a common strategy to improve semiconductor performance because interactions between different components create synergistic effects. Consequently, halide-based perovskite heterostructures exhibit higher photocatalytic performance.
3.3 Other modification methods
In addition to ion doping and heterostructure, other modification methods such as anion exchange, surface ligand modification, and encapsulation can improve the stability of all-inorganic lead halide perovskites while maintaining high photocatalytic activity. All-inorganic lead halide perovskites typically consist of an all-inorganic core capped with organic ligands. We hope to find the most suitable ligands for surface passivation that will bring out the best and unique properties of the perovskite, thus broadening its applicability. Furthermore, encapsulation with materials has been proven to be a feasible and effective way to prevent decomposition and improve stability to survive water, light, and heat treatment conditions.
The two problems of low photocatalytic efficiency and poor stability of all-inorganic lead halide perovskites and the proposed solutions were already mainly discussed. As mentioned above, (1) adding dopants to the photocatalytic system can increase the active sites of the photocatalyst to promote adsorption capacity and redox reactions. By introducing appropriate doping metal ions, especially transition metal ions, the Eg of the catalyst can be adjusted, and the recombination of electrons and holes can be suppressed; (2) the construction of heterojunction nanocomposites is considered as an alternative strategy to promote all-inorganic lead halide perovskite photocatalysts. Coupling different types of cocatalysts with all-inorganic lead halide perovskites can induce the separation of photogenerated carriers and maximize the photocatalytic efficiency; (3) the instability problem of all-inorganic lead halide perovskites could be solved. The top priority is to build a protective layer or encapsulate ligands. The existence of a protective layer can isolate the perovskite from the external environment and prevent its interaction.
4 Applications of all-inorganic halide perovskites
Environmental protection and sustainable energy development are two major problems. Green, efficient storage, and renewable solar energy can be achieved using solar radiation to form chemical fuels, which are hydrogen and oxygen (from water splitting) or methane (from CO2 reduction). The attractive optical properties of all-inorganic halide perovskites (e.g., high absorption coefficient in the UV-visible region, tunable band gap, and high PLQY) make them suitable candidates for solar-driven photocatalytic applications. This section will provide an overview of the current state of development for all-inorganic lead halide perovskites in photocatalytic pollutant degradation, CO2 reduction, and H2 evolution.4.1 Photocatalytic hydrogen production
H2, as an ideal clean energy source in the 21st century, is considered as an alternative to fossil fuels. Photocatalytic hydrolysis for hydrogen production has potential applications, in which the photocatalyst CsPbX3 has been widely utilized.223–225 The balance between photocatalytic activity and stability of CsPbBr3 is achieved through the rational control of surface ligands. Wang et al. successfully achieved photocatalytic hydrogen evolution by synthesizing CsPbBr3/Pt–TiO2 composites.226 Song et al. prepared the CsPbBr3@polyaniline, which was stable even in an aqueous solution and had a photocatalytic hydrogen generation efficiency of 4.81 mmol (h−1 g−1).227 Xiang et al. utilized Zn2+-doped CsPbBr3 nanocrystals to directly reduce hydrogen atoms in water, which exhibited excellent photocatalytic activity and enhanced hydrogen production efficiency.228 Sun et al. developed a new CsPbBr3 nanocrystalline coupled NiFe-LDH photocatalyst for high-efficiency photocatalytic CO2 reduction,229 and the photocatalyst's optimum electron consumption rate was 39.58 mol g−1 h−1, which was higher than that of pristine NiFe-LDH.Low-polarity solvents can be utilized to produce stable reaction conditions for photocatalytic hydrogen production. Sun et al. produced 0.8 mol per g hydrogen during 8 hours of simulated solar irradiation using ethyl acetate.230 In hydrogen halide (HX, X = Br, I) solutions, all-inorganic lead halide perovskites also show high photocatalytic hydrogen generation efficiency, for example, CsPbBr3−xIx/Pt exhibited excellent hydrogen production performance with 224 mol h−1 in saturated aqueous HBr solution.231
The attractive optical properties of all-inorganic halide perovskites (e.g., high absorption coefficient in the UV-visible region, tunable Eg, and high PLQY) make them suitable candidates to produce hydrogen. Despite the exciting progress, the field is still in its infancy and there is still much room for development in designing targeted reaction systems, improving the stability and efficiency, and eliminating the toxicity of halide systems in converting solar into chemical energy.
4.2 Photocatalytic reduction of CO2
CO2 and other greenhouse gases have been rapidly accumulating in the atmosphere232 due to mankind's overreliance on fossil fuels, leading to severe and permanent effects on the ecosystem.233,234 China is supposed to reach a “carbon peak” by 2030,235 indicating that greenhouse gas emissions play an important role in environmental issues,; thus, photocatalytic reduction of CO2 is considered the most promising method.236–239 Due to their outstanding qualities, such as affordability, simplicity of the synthesis method, visible light absorption, high CO2 adsorption surface area, and adjustable structure, all-inorganic lead halide perovskites have been proved as effective catalysts.240–246 The all-inorganic lead-halide perovskite materials generate electron–hole pairs by absorbing solar energy, and the generated electrons can reduce CO2 to high value-added chemicals such as CO,247 CH4,248 CH3OH,249 HCHO,250 HCOOH,196 and C2H4.251Hou et al. accomplished CO2 reduction with CsPbBr3 quantum dots in ethyl acetate/H2O solution with reduction product yields of 4.3, 1.5, and 0.1 mol g−1 h−1 for CO, CH4, and H2, respectively, where the CO2 reduction selectivity was approximately 100%230 (Fig. 6). Currently, the surface modification of CsPbX3 or the creation of multi-component composites is the most cost-effective and promising solution to several issues with pure CsPbX3 in the photocatalytic reduction of CO2. Xu et al. fabricated CsPbBr3/GO nanocomposites, which showed a CO2 reduction rate of 23.7 mol h−1 g−1.176 Additionally, to increase the effectiveness of CO2 reduction, all-inorganic lead halide perovskites combined with other substances could generate heterojunctions. Wang et al. created a 0D/2D heterojunction photocatalyst with CsPbBr3/Bi2WO6 nanosheets for the photocatalytic reduction of CO2; the yield was 503 mol g−1, which was 9.5 times greater than that of a single CsPbBr3.252
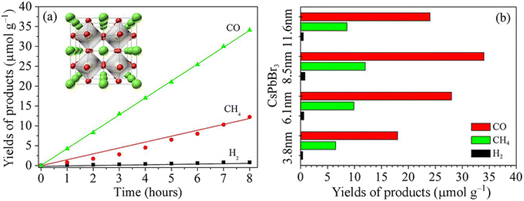 | ||
| Fig. 6 Solar reduction CO2 into fuels under 300 W Xe lamp irradiation by CsPbBr3 quantum dots: 8.5 nm CsPbBr3 quantum dots (a) and tunable CsPbBr3 quantum dots with different particle sizes (b).230 | ||
The humidity stability, CO2 capture capacity, and charge separation efficiency were significantly increased when CsPbBr3 nanocrystals combined with metal–organic frameworks (MOFs) for the photocatalytic reduction of CO2.253 The performance of perovskite nanocrystals can be significantly influenced by the special structure of MOFs. Wan et al. combined CsPbBr3 and UiO-66(NH2)254 to create a composite photocatalyst with CsPbBr3 QDs/UiO-66(NH2) MOF structure, which was able to convert CO2 to chemical fuel in a non-aqueous system. The reported photocatalytic reduction of CO2 by all-inorganic lead halide perovskites is summarized in Table 6.
| No. | Photocatalyst | Solution | Light source | Products and yield (μmol g−1 h−1) | Ref. |
|---|---|---|---|---|---|
| 1 | CsPbBr3 | Ethyl acetate/water | 300 W xenon lamp | CO (4.25) | 230 |
| CH4 (1.5) | |||||
| H2 (0.1) | |||||
| 2 | CsPbBr3–GO | Ethyl acetate | 500 W xenon lamp | CH4 (6.9) | 255 |
| 3 | CsPbBr3/g-C3N4 | Acetonitrile/water | 300 W xenon lamp | CO (149) | 256 |
| 4 | CsPbBr3/monolayered Ti3C2Tx | Ethyl acetate | 300 W xenon lamp | CO (26.32) | 257 |
| CH4 (7.25) | |||||
| 5 | CsPbBr3/TiO2 | Ethyl acetate | 300 W xenon lamp | H2 (5.08) | 258 |
| CO (7.73) | |||||
| CH4 (10.12) | |||||
| 6 | CsPbBr3@zeolitic imidazolate | CO2 and H2O vapor | 100 W xenon lamp | CO (0.67) | 253 |
| CH4 (3.33) | |||||
| 7 | Fe–CsPbBr3 | Ethyl acetate/water | 450 W xenon lamp | CO (3.2) | 165 |
| CH4 (6.1) |
All-inorganic lead halide perovskites are sensitive to water or other polar solvents, resulting in insufficient activity and low selectivity of all-inorganic lead halide perovskites. Therefore, it is considered a feasible method to improve the activity and stability of photocatalytic CO2 reduction by means of element doping, morphology control, and construction of heterojunctions.
4.3 Contaminant degradation
Many harmful pollutants are emitted into the air, water, and soil as a result of the growth of the industry.259–261 Photocatalysis is an effective method to degrade pollutants.262–264 However, the efficiency of photocatalytic degradation of pollutants in water is still limited due to the low efficiency of solar energy utilization and the rapid recombination of photogenerated holes and electrons.265–269 Among these materials for the photocatalytic degradation of pollutants, all-inorganic lead halide perovskite photocatalysts can efficiently utilize solar energy and have many advantages in terms of efficiency, greenness, and cost.60 In particular, the photocatalytic degradation of organic dyes,270–272 phenols,273–275 and antibiotics276–278 in water has generated a lot of interest in all-inorganic lead halide perovskites.Among the various pollutants, synthetic dyes such as rhodamine B,279–281 Sudan red III,282–285 methyl red,286–288 methyl violet,289–293 malachite green,294,295 acid black dye,296–299 Congo red,300 and methyl orange301–303 are common. Feng et al. created CsPb(Br1−xClx)3-Au nanoheterojunction materials,271 which were used to decompose Sudan red III pollutants, and the results showed that after 6 hours of visible light exposure, the heterostructures had degraded 71% of Sudan dye. Liu et al. prepared CsPbBr3 perovskite quantum dots/polymethyl methacrylate composites by ball milling; the degradation rate of rhodamine B by the composites under visible light reached 92.2% within 60 min.304
Besides dyes, the abuse of broad-spectrum antibiotics (ciprofloxacin,305–307 norfloxacin,308–311 2-mercaptobenzothiazole,312 and tetracycline hydrochloride313–315) causes harm to ecosystems and poses a threat to human health. Using all-inorganic lead halide perovskites, antibiotics can be successfully removed from water sources. Chen et al. produced all-inorganic CsPbBr3 calcium titanite quantum dot materials by anti-solvent precipitation,316 which was successfully used to remove antibiotic residues from ethanol, showing excellent photocatalytic activity. Zhao et al. synthesized Ag–CsPbBr3/g-C3N4 ternary composites with excellent photocatalytic activity by loading nano-Ag on the surface of CsPbBr3/bulky g-C3N4 composites317 (Fig. 7), which were applied to the visible light catalytic degradation of 7-aminocephalosporanic acid; 7%-Ag–CsPbBr3/g-C3N4 composites showed excellent photocatalytic activity, and 92.79% of 7-aminocephalosporanic acid was degraded. The stability and electron–hole separation rate of photocatalytic reactions can generally be improved by the heterostructures. Zhao et al. created type II CsPbCl3 heterostructures,276 and the catalysts showed excellent photocatalytic performance for the degradation of penicillin–6-aminopenicillins acid. Liu et al. synthesized stable CsPbBr3–TiO2 heterojunction, which was applied in the visible light-driven photodegradation of tetracycline hydrochloride;56 80% of tetracycline hydrochloride was degraded in this experiment.
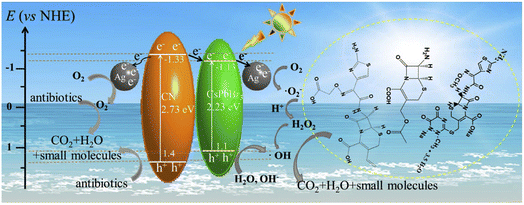 | ||
| Fig. 7 Possible mechanism diagram for cephalosporin antibiotics degradation with Ag–CsPbBr3/CN composite under visible light irradiation.317 | ||
Oxygen activation typically initiates photodegradation reactions by generating reactive oxygen species (such as ·OH and ·O2−), which react with the target pollutants. When all-inorganic lead halide perovskites are used as photocatalysts to breakdown organic dyes, some encouraging results have been obtained. All-inorganic lead halide perovskites have attracted great interest due to their excellent optoelectronic properties. Table 7 summarizes the application of all-inorganic lead halide perovskites in the field of photocatalysis, including CO2 reduction, H2 evolution, and degradation of pollutants in water. Inspired by natural photosynthesis, artificial photocatalysts that can convert solar energy into useable fuels and chemicals have been extensively studied. The structure of photocatalytic systems has been extensively studied to improve the photocatalytic activity; however, the problems of poor light absorption and conversion efficiency still need to be improved. Researchers are investigating possible strategies to improve the light harvesting and conversion efficiency of efficient photocatalysts. In addition to the effectiveness of the photocatalyst, the environmental friendliness, durability, and recyclability of the materials have also been focused on.
| Application | Sample | Synthesis method | Photocatalytic performance | Stability | Ref. |
|---|---|---|---|---|---|
| CO2 reduction | CsPb(Br0.5/Cl0.5)3 | Hot injection | Total yield of CO and CH4 up to 875 μmol g−1 (99% selectivity) (ethyl acetate solution) | The PXRD patterns of CsPb(Br0.5/Cl0.5)3 did not change significantly after photocatalysis | 207 |
| CsPbBr3 | Hot injection | CO evolution rate was 204.4 μmol g−1 h−1 and (100% selectivity) | Under 35 hours of irradiation, the production of CO increased with the extension of irradiation time | 318 | |
| CsPbBr3@SnO2 | Solvothermal method and hot-injection | It showed a total production yield of 208.3 μmol g−1 (192.3 μmol g−1 for CO, and 16.0 μmol g−1 for CH4) after 4 h | After four catalytic runs (4 h each). The result showed that no obvious decrease in activity was perceived for CsPbBr3@SnO2 | 194 | |
| CsPbBr3/MoS2 | Ultrasonic method | CO yield of 74.9 μmol g−1 g−1 (ethyl acetate solution) | The conversion of CO2 to CO and CH4 decreased slightly by about 3.4% and 9.1%, respectively, after three cycles | 319 | |
| CsPbI3/rGO | Hot injection | Formate generation with high selectivity (>90% FEformate) (KHCO3 solution) | Remaining stable in aqueous environments, rGO films provide significant protection for CsPbI3 from the aqueous environment | 320 | |
| CsPbBr3/alpha-Fe2O3 | LARP | Showed electron consumption rate of 1.42 mmol g−1 h−1 | The activity can be maintained at 90% after three cycles | 206 | |
| Wastewater degradation | CsPbBr3 | Emulsion and demulsion method | 1 mg of catalyst can decompose an MO solution into a colorless solution in 100 min | The photocatalytic activity of CsPbBr3 did not change significantly after two cycles | 272 |
| Ti3C2-MXene/NiO/CsPbI3 | Hydrothermal | 92.8% degradation of CV dye (15 mg L−1) achieved in 90 min | After eight consecutive cycles of degradation, the photocatalytic degradation activity of CV dye slightly decreased by 2.9% | 321 | |
| CsPbCl3 | Facile solution-based technique | Degraded 90% of eosin-B dye in 180 min | Photocatalytic activity did not change after 3 cycles | 322 | |
| Co/CsPbCl3 | Solvothermal | Degraded 90% of MB dye in 180 min under visible light conditions | After three cycles of experiment, the degradation rate dropped by only 6% | 323 | |
| CsPbCl3/CN | Hot injection | The degradation rate of 6-APA was 83.31% in 120 min | The photocatalytic activity did not change significantly after three cycles | 276 | |
| CsPbBr3/MOF-808 | Mechanochemical method | Cr(VI) reduction rate reached 92%; antibiotic degradation rate reached 97% (120 min) | After three runs, the PXRD patterns, FTIR spectra, and SEM image confirmed the stability of CsPbBr3/MOF-808 | 324 | |
| CsPbI3/g-C3N4 | Ultrasonic method | The degradation rate of RhB was 100% in 80 min (5 mg L−1) | After four cycles, the degradation efficiency was not reduced | 325 | |
| CsPbI3 | Hydrothermal method | The degradation rate of methyl violet was 81.7% in 120 min (5 mg L−1) | After three cycles, the degradation efficiency was slightly reduced | 326 | |
| H2 production | CsPbBr3@ZIF-8 | Ball milling | Exhibited an H2 yield of 19.63 μmol g−1 after 2.5 h | Photoluminescence intensity remained around 86.7% after 8 weeks | 58 |
| CsPbBr3 | Hot injection | Exhibited an H2 evolution rate of 13.3 μmol g−1 h−1 | Photocatalytic activity did not change significantly after 20 h | 57 | |
| CsPbBr3/GO–Pt | Anti-solvent precipitation method | Exhibited an H2 evolution rate of 1060 μmol g−1 h−1 with high selectivity (>99%) | The photocatalyst showed no obvious deactivation with consistent H2 after 20 h | 191 | |
| CsPbBr3 | Hot-injection | The amount of H2 was 132 μmol g−1 within 4 h | H2 generation with outstanding stability (≥160 h) | 223 | |
| Pt@δ-CsPbI3 | Solvothermal method | H2 production reached 12.6 mmol g−1 h−1 | The activity of the material was constant within 24 h | 327 |
5 Challenges and prospects
All-inorganic lead halide perovskites have made significant progress in the field of photocatalysis. However, the strong ionic character of this substance makes its interior structure less stable. In addition, the existing reports have insufficient research on the growth kinetics of all-inorganic lead halide perovskite in the actual manufacturing process, which makes it difficult to control the formation and leads to low practical application efficiency, mainly due to the following aspects.(1) The toxicity of lead is the main reason that restricts the actual production and large-scale commercial application of all-inorganic lead halide perovskites. Lead poisoning in humans can result in major health issues such as renal failure, nerve damage, and other disorders even at very low exposure levels.
(2) Due to the inherent ionic nature of all-inorganic lead halide perovskites, the resistance to moisture, oxygen, light, and high temperatures remains a significant challenge, and the perovskite structure is destroyed when exposed to polar solvents or water, which reduces its photocatalytic activity and limits practical application.
(3) The corresponding mechanism of photocatalysis has been extensively studied, but the precise redox pathway is still unknown.
(4) Inorganic lead halide perovskites have weak thermal and light stability and are easily harmed by external stimuli like heat, light, and oxygen.
Future enhancements will mostly focus on the following areas in response to the aforementioned issues.
(1) To reduce the toxicity of inorganic lead halide perovskites, it is necessary to develop efficient and environment-friendly lead-free metal halide perovskites by replacing Pb with other transition metals (such as Sn, Ge, Bi, and Ag). However, the activity of lead-free perovskites will be reduced. Therefore, the development of such lead-free photocatalysts should be combined with several improvement strategies.
(2) To simultaneously maintain high stability and catalytic activity, the A-site is modified with mixed ions. Mixing organic cations and inorganic cations to prepare organic–inorganic hybrid halide perovskites is a promising method. By this way, organic components can be added to improve the photocatalytic activity while retaining the stability of inorganic components. Common hybrid organic cations include FA+ and MA+; however, the ratio between organic and inorganic ions needs to be further refined.
(3) The development of novel protective layers or ligands to form a core–shell structure should be taken into consideration to address the deficiencies of inorganic perovskite's poor stability. Stable co-catalysts can be developed to form the same structure as various heterojunctions that match the energy bands of inorganic lead halide perovskites.
(4) Photocatalysis can be widely used in various fields. Exploring novel photocatalytic applications, such as (i) removal of organic matter and heavy metals in soil. Inorganic lead halide perovskite catalysts have strong redox capabilities and have broad application prospects in remediating soil organic matter and heavy metals. (ii) Organic synthesis: photocatalytic organic synthesis is an economical and green synthesis method that uses sunlight to drive chemical reactions under mild conditions. Many photoreduction reactions proceed via free radicals or radical ion intermediates. This reactive intermediate can be easily and efficiently generated by visible light. (iii) Environmental monitoring: with the continuous development of the chemical industry, the discharge of various pollutants has led to an increasing demand for the detection of various heavy metals and other pollutants present in waters. Utilizing the luminescent properties of all-inorganic lead halide perovskites is considered a highly sensitive detection method.
In summary, the development of catalysts with excellent catalytic properties is crucial for practical applications. However, how to improve the activity of the catalyst while maintaining the stability of the catalyst requires more research. The problems and challenges encountered so far will motivate researchers to develop more effective perovskite catalysts in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Fundamental Research Funds for the Central Universities (2572022BA09), Natural Science Foundation of Heilongjiang Province of China (LH2023D003).References
- L. Wang, S. Li, I. M. Ahmad, G. Zhang, Y. Sun, Y. Wang, C. Sun, C. Jiang, P. Cui and D. Li, Sci. Total Environ., 2023, 887, 164055 CrossRef CAS PubMed.
- S. Kuskaya, F. Bilgili, E. Mugaloglu, K. Khan, M. E. Hoque and N. Toguc, Renewable Energy, 2023, 206, 858–871 CrossRef CAS.
- A. Wazeer, A. Das and S. Vidya, Trans. Indian Inst. Met., 2023, 76, 1155–1163 CrossRef CAS.
- J. Zheng and B. Zeng, Sustain. Energy Technol. Assess., 2023, 57, 103303 Search PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- J. Luo, Z. Wang, H. Jiang, S. Liu, F.-Q. Xiong and J. Ma, Langmuir, 2020, 36, 4637–4644 CrossRef CAS PubMed.
- C. Xie, W. Chen, S. Du, D. Yan, Y. Zhang, J. Chen, B. Liu and S. Wang, Nano Energy, 2020, 71, 104653 CrossRef CAS.
- T. Paik, M. Cargnello, T. R. Gordon, S. Zhang, H. Yun, J. D. Lee, H. Y. Woo, S. J. Oh, C. R. Kagan, P. Fornasiero and C. B. Murray, ACS Energy Lett., 2018, 3, 1904–1910 CrossRef CAS.
- L. Yin, X. Hai, K. Chang, F. Ichihara and J. Ye, Small, 2018, 14, 1704153 CrossRef PubMed.
- M. Shen, Z. Yan, L. Yang, P. Du, J. Zhang and B. Xiang, Chem. Commun., 2014, 50, 15447–15449 RSC.
- L. Guo, C. Zhong, L. Shi, L. Ju, X. Wang, D. Yang, K. Bi, Y. Hao and Y. Yang, Adv. Opt. Mater., 2019, 7, 1801403 CrossRef.
- Y. Zou, J. W. Shi, D. Ma, Z. Fan, L. Cheng, D. Sun, Z. Wang and C. Niu, ChemSusChem, 2018, 11, 1187–1197 CrossRef CAS PubMed.
- P. Kumar, S. Kataria, S. Roy, A. Jaiswal and V. Balakrishnan, ChemistrySelect, 2018, 3, 7648–7655 CrossRef CAS.
- D. Zeng, L. Xiao, W. J. Ong, P. Wu, H. Zheng, Y. Chen and D. L. Peng, ChemSusChem, 2017, 10, 4624–4631 CrossRef CAS PubMed.
- F. He, Z. Wang, Y. Li, S. Peng and B. Liu, Appl. Catal., B, 2020, 269, 118828 CrossRef CAS.
- C. Xu, P. Ravi Anusuyadevi, C. Aymonier, R. Luque and S. Marre, Chem. Soc. Rev., 2019, 48, 3868–3902 RSC.
- E. E. Barton, D. M. Rampulla and A. B. Bocarsly, J. Am. Chem. Soc., 2008, 130, 6342–6344 CrossRef CAS PubMed.
- T. Arai, S. Sato, T. Kajino and T. Morikawa, Energy Environ. Sci., 2013, 6, 1274–1282 RSC.
- Y. Chen, B. Jia, G. Qin, H. Zhao, L. Han and P. Lu, RSC Adv., 2023, 13, 15055–15062 RSC.
- A. Dabbous, E. Colson, D. Chakravorty, J.-M. Mouesca, C. Lombard, S. Caillat, J.-L. Ravanat, F. Dubois, F. Denes, P. Renaud and V. Maurel, Chem.–Eur. J., 2023, 29, e202300303 CrossRef CAS.
- M. Asim, S. Zhang, B. Maryam, J. Xiao, C. Shi, L. Pan and J.-J. Zou, Appl. Surf. Sci., 2023, 620, 156787 CrossRef CAS.
- X. Li, J. Hu, Y. Deng, T. Li, Z.-Q. Liu and Z. Wang, Appl. Catal., B, 2023, 324, 122243 CrossRef CAS.
- F. Wang, J. Li, X. Yu, H. Tang, J. Xu, L. Sun and Q. Liu, J. Mater. Sci. Technol., 2023, 146, 49–60 CrossRef CAS.
- X. Feng and L. Zhang, J. Mater. Sci. Technol., 2023, 156, 54–63 CrossRef CAS.
- A. U. Hasanah, P. L. Gareso, N. Rauf and D. Tahir, ChemBioEng Rev., 2023, 10, 698–710 CrossRef CAS.
- A. Sinha, S. K. Sahu, S. Biswas and T. K. Ghorai, RSC Adv., 2023, 13, 22029–22042 RSC.
- Y. Xue, Y. Guo, Z. Liang, H. Cui and J. Tian, J. Colloid Interface Sci., 2019, 556, 206–213 CrossRef CAS PubMed.
- C. Lu, E. Wu, C. Li, W. Dou, Y. Lian, Y. Liang, X. Xiang and H. Wang, J. Phys. Chem. Solids, 2021, 158, 110228 CrossRef CAS.
- R. Ranjan, M. Kumar and A. S. K. Sinha, Int. J. Hydrogen Energy, 2019, 44, 16176–16189 CrossRef CAS.
- N. Sharma, S. Swami, V. Shrivastava, R. Nair and R. Shrivastava, Mater. Today: Proc., 2021, 43, 3309–3317 CAS.
- F. Cai, J. J. Ibrahim, Y. Fu, W. Kong, J. Zhang and Y. Sun, Appl. Catal., B, 2020, 264, 118500 CrossRef.
- L. Gui, Y. Xu, Q. Tang, X. Shi, J. Zhang, B. He and L. Zhao, J. Colloid Interface Sci., 2021, 603, 252–258 CrossRef CAS PubMed.
- X. Wang, Z. Zhu, J. Jiang, R. Li and J. Xiong, Chemosphere, 2023, 337, 139206 CrossRef CAS PubMed.
- T. Xu, R. Wang, C. Gu and T. Jiang, Spectrochim. Acta, Part A, 2023, 299, 122801 CrossRef CAS PubMed.
- W. Ashraf, S. H. Parvez and M. Khanuja, Environ. Res., 2023, 236, 116715 CrossRef CAS PubMed.
- L. Li, H. Yang and P. Yang, J. Colloid Interface Sci., 2023, 650, 1312–1318 CrossRef CAS PubMed.
- S. Liu, X. Zhou, J. Qin, C. Wei and Y. Hu, J. Colloid Interface Sci., 2023, 635, 59–71 CrossRef CAS PubMed.
- Q. Ding, X. Zou, J. Ke, Y. Dong, Y. Cui and H. Ma, J. Colloid Interface Sci., 2023, 649, 148–158 CrossRef CAS PubMed.
- Z. Jiang, Q. Long, B. Cheng, R. He and L. Wang, J. Mater. Sci. Technol., 2023, 162, 1–10 CrossRef.
- R. Del Sole, C. Lo Porto, S. Lotito, C. Ingrosso, R. Comparelli, M. L. Curri, G. Barucca, F. Fracassi, F. Palumbo and A. Milella, Molecules, 2023, 28, 5131 CrossRef CAS PubMed.
- A. Saleem, F. F. Alharbi, M. N. Ashiq, S. Manzoor, S. I. Abbas Shah, K. Z. Khan, S. Aman, N. Ahmad, H. A. Alzahrani and M. Messali, Phys. Status Solidi A, 2023, 220, 2200734 CrossRef CAS.
- Y. Yang, Y. Sun and Y. Jiang, Mater. Chem. Phys., 2006, 96, 234–239 CrossRef CAS.
- P. Priyadarshini, S. Das and R. Naik, RSC Adv., 2022, 12, 9599–9620 RSC.
- X. Zhu, Y. Lin, J. San Martin, Y. Sun, D. Zhu and Y. Yan, Nat. Commun., 2019, 10, 2843 CrossRef PubMed.
- P. Priyadarshini, S. Senapati and R. Naik, Renewable Sustainable Energy Rev., 2023, 186, 113649 CrossRef CAS.
- J. M. Frost, K. T. Butler, F. Brivio, C. H. Hendon, M. van Schilfgaarde and A. Walsh, Nano Lett., 2014, 14, 2584–2590 CrossRef CAS PubMed.
- A. Walsh, J. Phys. Chem. C, 2015, 119, 5755–5760 CrossRef CAS PubMed.
- S. R. Mulani, S. Bimli, E. Choudhary, R. Bunkar, U. A. Kshirsagar and R. S. Devan, Chemosphere, 2023, 340, 139890 CrossRef CAS PubMed.
- D. Komaraiah, E. Radha, J. Sivakumar, M. V. Ramana Reddy and R. Sayanna, Opt. Mater., 2020, 108, 110401 CrossRef CAS.
- Q. Zhang, X. Deng, C. Tan, Y. Zhou, X. Chen, X. Bai, J. Li, B. Tang, S. Li and H. Lin, J. Chem. Phys., 2020, 153, 024703 CrossRef CAS PubMed.
- Z. Lu, J. Gao, S. Rao, C. Jin, H. Jiang, J. Shen, X. Yu, W. Wang, L. Wang, J. Yang and Q. Liu, Appl. Catal., B, 2024, 342, 123374 CrossRef CAS.
- Q. Guo, J.-D. Zhang, Y.-J. Chen, K.-Y. Zhang, L.-N. Guo, Q.-C. Shan, J.-L. Lu, X.-H. Duan and L.-Z. Wu, Chem. Commun., 2023, 59, 4189–4192 RSC.
- A. Sabbah, I. Shown, M. Qorbani, F.-Y. Fu, T.-Y. Lin, H.-L. Wu, P.-W. Chung, C.-I. Wu, S. R. M. Santiago, J.-L. Shen, K.-H. Chen and L.-C. Chen, Nano Energy, 2022, 93, 106809 CrossRef CAS.
- K. Su, S.-X. Yuan, L.-Y. Wu, Z.-L. Liu, M. Zhang and T.-B. Lu, Small, 2023, 19, 2301192 CrossRef CAS PubMed.
- H. Peng, L. Duan, W. Xie, C. Shao, H. Cao, D. Wang, S. Rao and H. Guo, Chemosphere, 2024, 346, 140614 CrossRef CAS PubMed.
- C. Liu, X. Qian, Q. Wei, Z. Chen, J. Chen, W. Wang, X. Chen, J. Gao, Y. Liu and L. Xie, J. Cleaner Prod., 2022, 365, 132830 CrossRef CAS.
- R.-J. Huang, Z.-K. Qin, L.-L. Shen, G. Lv, F. Tao, J. Wang and Y.-J. Gao, J. Mater. Chem. A, 2023, 11, 6217–6225 RSC.
- S. Feng, S. Ning, L. Wang, J. Zhao, J. Ou, Z. Wu, S. Luo, Z. Lin, K. Yan, C. Wu and Y. Xu, ACS Appl. Energy Mater., 2022, 5, 6248–6255 CrossRef CAS.
- N. Luo, C. Chen, D. Yang, W. Hu and F. Dong, Appl. Catal., B, 2021, 299, 120664 CrossRef CAS.
- S. Gull, S. Batool, G. Li and M. Idrees, Front. Chem., 2022, 10, 1020484 CrossRef CAS PubMed.
- D. Wang, C. Miao, H. Li, B. Yu, W. Wang, Y. Wang, G. Che, C. Liu and B. Hu, Mater. Res. Bull., 2024, 170, 112552 CrossRef CAS.
- W. Liu, H. Xie, X. Guo, K. Wang, C. Yang, N. Wang and C. Ge, Opt. Mater., 2023, 136, 113398 CrossRef CAS.
- J. Cui, C. Xu, Z. Jin, H. Liu, R. Hu and F. Liu, Environ. Sci. Pollut. Res., 2023, 30, 96875–96890 CrossRef CAS PubMed.
- H. Mohan, S. Vadivel and T. Shin, Ultrason. Sonochem., 2023, 101, 106650 CrossRef CAS PubMed.
- H. Zhang, F. Meng, H. Wei, W. Yu and S. Yao, J. Colloid Interface Sci., 2023, 652, 1282–1296 CrossRef CAS PubMed.
- T. Ishii, A. Anzai, A. Yamamoto and H. Yoshida, Appl. Catal., B, 2020, 277, 119192 CrossRef CAS.
- Q. Li, X. Bai, J. Luo, C. Li, Z. Wang, W. Wu, Y. Liang and Z. Zhao, Nanotechnology, 2020, 31, 375402 CrossRef CAS PubMed.
- K. Sathiyamoorthy, A. Silambarasan, M. Navaneethan and S. Harish, Chemosphere, 2024, 348, 140575 CrossRef CAS PubMed.
- D. E. Mazouzi, F. Djani, A. Soukeur, W. Bouchal, A. Manseri, K. Derkaoui, A. Martínez-Arias, A. Ksouri, F. Şen and M. M. Kaci, Surf. Interfaces, 2024, 44, 103581 CrossRef CAS.
- G. E. de la Huerta-Hernández, J. Chávez-Carvayar, T. Rodríguez-Flores, I. Castro-Cisneros, A. Reyes-Montero and I. Hernández-Pérez, Environ. Sci. Pollut. Res., 2023, 30, 102986–103000 CrossRef PubMed.
- V. K. Amritha and S. Badhulika, New J. Chem., 2023, 47, 17897–17907 RSC.
- A. H. Jawhari, N. Hasan, I. A. Radini, K. Narasimharao and M. A. Malik, Nanomaterials, 2022, 12, 2985 CrossRef CAS PubMed.
- A. S. Rodrigues, J. E. Silveira, J. Carbajo, J. A. Zazo, J. A. Casas, A. Fernandes, M. J. Pacheco, L. Ciriaco and A. Lopes, Environ. Sci. Pollut. Res., 2021, 28, 23822–23832 CrossRef CAS PubMed.
- Y. Zhang, W. Wang, Y. Guo, Q. Shen and Z. Liu, J. Phys. Chem. Solids, 2021, 149, 109766 CrossRef CAS.
- X. Chen, Q. Dong, S. Chen, Z. Zhang, X. Zhang, Y. Di, A. Jiang, D. Zhang and T. Li, Colloids Surf., A, 2023, 664, 131143 CrossRef CAS.
- F. Li, Z. Wu, X. Tang, X. Li, X. Liu, S. Yu, L. Wei and C. Yu, J. Water Process. Eng., 2023, 56, 104470 CrossRef.
- S. Park, W. J. Chang, C. W. Lee, S. Park, H.-Y. Ahn and K. T. Nam, Nat. Energy, 2016, 2, 16185 CrossRef.
- W. Ma, T. Jiang, Z. Yang, H. Zhang, Y. Su, Z. Chen, X. Chen, Y. Ma, W. Zhu, X. Yu, H. Zhu, J. Qiu, X. Liu, X. Xu and Y. Yang, Advanced Science, 2021, 8, 2003728 CrossRef CAS PubMed.
- F. Cao, D. Yu, W. Ma, X. Xu, B. Cai, Y. M. Yang, S. Liu, L. He, Y. Ke, S. Lan, K.-L. Choy and H. Zeng, ACS Nano, 2020, 14, 5183–5193 CrossRef CAS PubMed.
- J. H. Heo, D. H. Shin, J. K. Park, D. H. Kim, S. J. Lee and S. H. Im, Adv. Mater., 2018, 30, 1801743 CrossRef PubMed.
- L. Wang, K. Fu, R. Sun, H. Lian, X. Hu and Y. Zhang, Nano-Micro Lett., 2019, 11, 52 CrossRef PubMed.
- Y. Zhou, J. Chen, O. Bakr and O. Mohammed, ACS Energy Lett., 2021, 6, 739–768 CrossRef CAS.
- X. Li, W. Cai, H. Guan, S. Zhao, S. Cao, C. Chen, M. Liu and Z. Zang, Chem. Eng. J., 2021, 419, 129551 CrossRef CAS.
- Y. Huang, L. Zhang, J. Wang, B. Zhang, L. Xin, S. Niu, Y. Zhao, M. Xu, X. Chu, D. Zhang, C. Qu and F. Zhao, Opt. Lett., 2019, 44, 1908–1911 CrossRef CAS PubMed.
- Q. Mo, C. Chen, W. Cai, S. Zhao, D. Yan and Z. Zang, Laser Photonics Rev., 2021, 15, 2100278 CrossRef CAS.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- Y. Zhou, Q. Gu, Y. Li, L. Tao, H. Tan, K. Yin, J. Zhou and S. Guo, Nano Lett., 2021, 21, 4861–4867 CrossRef CAS PubMed.
- Y. Zhao, J. Zhu, B. He and Q. Tang, Chem. Commun., 2021, 57, 7577–7580 RSC.
- C. Ponti, G. Nasti, D. Di Girolamo, I. Cantone, F. A. Alharthi and A. Abate, Trends Ecol. Evol., 2022, 37, 281–283 CrossRef CAS PubMed.
- C. Sun, Y. Zhang, C. Ruan, C. Yin, X. Wang, Y. Wang and W. W. Yu, Adv. Mater., 2016, 28, 10088–10094 CrossRef CAS PubMed.
- H. C. Yoon, H. Kang, S. Lee, J. H. Oh, H. Yang and Y. R. Do, ACS Appl. Mater. Interfaces, 2016, 8, 18189–18200 CrossRef CAS PubMed.
- G. Jin, T. Liu, Y. Li, J. Zhou, D. Zhang, P. Pang, Z. Ye, Z. Xing, G. Xing, J. Chen and D. Ma, Nanoscale, 2022, 14, 919–929 RSC.
- G. Xing, N. Mathews, S. S. Lim, N. Yantara, X. Liu, D. Sabba, M. Grätzel, S. Mhaisalkar and T. C. Sum, Nat. Mater., 2014, 13, 476–480 CrossRef CAS PubMed.
- S. Chen, K. Roh, J. Lee, W. K. Chong, Y. Lu, N. Mathews, T. C. Sum and A. Nurmikko, ACS Nano, 2016, 10, 3959–3967 CrossRef CAS PubMed.
- L. Dou, Y. M. Yang, J. You, Z. Hong, W. H. Chang, G. Li and Y. Yang, Nat. Commun., 2014, 5, 5404 CrossRef CAS PubMed.
- J. Ding, S. Du, Z. Zuo, Y. Zhao, H. Cui and X. Zhan, J. Phys. Chem. C, 2017, 121, 4917–4923 CrossRef CAS.
- W. Zhai, J. Lin, C. Li, S. Hu, Y. Huang, C. Yu, Z. Wen, Z. Liu, Y. Fang and C. Tang, Nanoscale, 2018, 10, 21451–21458 RSC.
- A. Bhardwaj, K. Kundu, R. Sasmal, P. Acharyya, J. Pradhan, S. Kalita, S. S. Agasti and K. Biswas, Chem. Sci., 2023, 14, 7161–7169 RSC.
- M. Wang, S. Wang, R. Chen, M. Zhu, Y. Liu, H. Ding, J. Ren, T. Xuan and H. Li, Coatings, 2023, 13, 1062 CrossRef CAS.
- H. Wei, T. Si, F. Xu, W. Fan, T. Yang, B. Cao, F. Juan, J. Xu and Y. Wu, Opt. Express, 2023, 31, 25298–25306 CrossRef CAS PubMed.
- Y. Chen, S. Lu, M. Nan, J. Xie, W. Shen, A. N. Aleshin, G. Cheng, S. Chen and W. Huang, Adv. Opt. Mater., 2023, 11, 2300473 CrossRef CAS.
- Y. Ma, F. Zheng, S. Li, Y. Liu, J. Ren, Y. Wu, Q. Sun and Y. Hao, ACS Appl. Mater. Interfaces, 2023, 15, 34862–34873 CrossRef CAS PubMed.
- A. Pieniazek, F. Dybala, M. P. Polak, L. Przypis, A. P. Herman, J. Kopaczek and R. Kudrawiec, J. Phys. Chem. Lett., 2023, 14, 6470–6476 CrossRef CAS PubMed.
- J. Hou, Z. Wang, P. Chen, V. Chen, A. K. Cheetham and L. Wang, Angew. Chem., Int. Ed. Engl., 2020, 59, 19434–19449 CrossRef CAS PubMed.
- C. Zhang, W. Li and L. Li, Angew. Chem., Int. Ed. Engl., 2021, 60, 7488–7501 CrossRef CAS PubMed.
- J. Cai, K. Gu, Y. Zhu, J. Zhu, Y. Wang, J. Shen, A. Trinchi, C. Li and G. Wei, Chem. Commun., 2018, 54, 8064–8067 RSC.
- Y. Tong, E. Bladt, M. F. Aygüler, A. Manzi, K. Z. Milowska, V. A. Hintermayr, P. Docampo, S. Bals, A. S. Urban, L. Polavarapu and J. Feldmann, Angew. Chem., Int. Ed. Engl., 2016, 55, 13887–13892 CrossRef CAS PubMed.
- J. Yan, Q. Wang, T. Wei, L. Jiang, M. Zhang, X. Jing and Z. Fan, ACS Nano, 2014, 8, 4720–4729 CrossRef CAS PubMed.
- M. S. Sander, M. J. Côté, W. Gu, B. M. Kile and C. P. Tripp, Adv. Mater., 2004, 16, 2052–2057 CrossRef CAS.
- M. Fu, J. Zhou, Q. Xiao, B. Li, R. Zong, W. Chen and J. Zhang, Adv. Mater., 2006, 18, 1001–1004 CrossRef CAS.
- R. Wu, S. Gong, L. Wu, H. Yu, Q. Han and W. Wu, Phys. Chem. Chem. Phys., 2022, 24, 8303–8310 RSC.
- L. Protesescu, S. Yakunin, O. Nazarenko, D. N. Dirin and M. V. Kovalenko, ACS Appl. Nano Mater., 2018, 1, 1300–1308 CrossRef CAS PubMed.
- M. Chen, Y. Zou, L. Wu, Q. Pan, D. Yang, H. Hu, Y. Tan, Q. Zhong, Y. Xu, H. Liu, B. Sun and Q. Zhang, Adv. Funct. Mater., 2017, 27, 1701121 CrossRef.
- V. A. Hintermayr, A. F. Richter, F. Ehrat, M. Döblinger, W. Vanderlinden, J. A. Sichert, Y. Tong, L. Polavarapu, J. Feldmann and A. S. Urban, Adv. Mater., 2016, 28, 9478–9485 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- A. A. M. Brown, P. Vashishtha, T. J. N. Hooper, Y. F. Ng, G. V. Nutan, Y. Fang, D. Giovanni, J. N. Tey, L. Jiang, B. Damodaran, T. C. Sum, S. H. Pu, S. G. Mhaisalkar and N. Mathews, Chem. Mater., 2021, 33, 2387–2397 CrossRef CAS.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, Adv. Funct. Mater., 2016, 26, 2435–2445 CrossRef CAS.
- X. l. Zeng, L. x. Yu, K. l. Peng, Y. Yu, Y. k. Deng, Y. j. Zhao and Y. n. Xu, J. Alloys Compd., 2023, 945, 169213 CrossRef CAS.
- Q. Pan, H. Hu, Y. Zou, M. Chen, L. Wu, D. Yang, X. Yuan, J. Fan, B. Sun and Q. Zhang, J. Mater. Chem. C, 2017, 5, 10947–10954 RSC.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635–5640 CrossRef CAS PubMed.
- Y. Wang, J. Wang, M. Zhang, S. Zheng, J. Wu, T. Zheng, G. Jiang and Z. Li, Small, 2023, 19, e2300841 CrossRef PubMed.
- A. Pan, B. He, X. Fan, Z. Liu, J. J. Urban, A. P. Alivisatos, L. He and Y. Liu, ACS Nano, 2016, 10, 7943–7954 CrossRef CAS PubMed.
- J. Leng, T. Wang, Z. K. Tan, Y. J. Lee, C. C. Chang and K. Tamada, ACS Omega, 2022, 7, 565–577 CrossRef CAS PubMed.
- Y. Yang, S. Wei, X. Kang, O. Tegus and D. Pan, J. Lumin., 2019, 211, 26–31 CrossRef CAS.
- C. M. Guvenc, A. Kocabas and S. Balci, J. Mater. Chem. C, 2023, 11, 3039–3049 RSC.
- S. Sun, D. Yuan, Y. Xu, A. Wang and Z. Deng, ACS Nano, 2016, 10, 3648–3657 CrossRef CAS PubMed.
- X. Zhang, X. Bai, H. Wu, X. Zhang, C. Sun, Y. Zhang, W. Zhang, W. Zheng, W. W. Yu and A. L. Rogach, Angew. Chem., Int. Ed. Engl., 2018, 57, 3337–3342 CrossRef CAS PubMed.
- L. Rao, Y. Tang, C. Song, K. Xu, E. T. Vickers, S. Bonabi Naghadeh, X. Ding, Z. Li and J. Z. Zhang, Chem. Mater., 2018, 31, 365–375 CrossRef.
- L. Rao, X. Du, G. Liang, Y. Tang, K. Tang and Z. Jin, Beilstein J. Nanotechnol., 2019, 10, 666–676 CrossRef CAS PubMed.
- Y. El Ajjouri, F. Palazon, M. Sessolo and H. Bolink, Chem. Mater., 2018, 30, 7423–7427 CrossRef CAS.
- G. Jiang, C. Guhrenz, A. Kirch, L. Sonntag, C. Bauer, X. Fan, J. Wang, S. Reineke, N. Gaponik and A. Eychmüller, ACS Nano, 2019, 13, 10386–10396 CrossRef CAS PubMed.
- J. Kim, N. T. Manh, H. T. Thai, S. K. Jeong, Y. W. Lee, Y. Cho, W. Ahn, Y. Choi and N. Cho, Nanomaterials, 2022, 12, 920 CrossRef CAS PubMed.
- Q. Zhang, W. Zheng, Q. Wan, M. Liu, X. Feng, L. Kong and L. Li, Adv. Opt. Mater., 2021, 9, 2002130 CrossRef CAS.
- Z. Long, S. Yang, J. Pi, D. Zhou, Q. Wang, Y. Yang, H. Wu and J. Qiu, Ceram. Int., 2022, 48, 35474–35479 CrossRef CAS.
- H. T. Ramolahloane, G. B. Nair and H. C. Swart, Mater. Res. Bull., 2023, 165, 112285 CrossRef CAS.
- X. Wu, K. Yin, M. Yang, Y. Hu and H. Peng, Flexible Printed Electron., 2023, 8, 035008 CrossRef.
- S. Ye, M. Huang, Q. Han, J. Song and J. Qu, J. Alloys Compd., 2023, 965, 171442 CrossRef CAS.
- Y. Ji, L. Liang, T. Ma, J. Hu, Z. Chen and J. Chen, Opt. Mater., 2023, 143, 114254 CrossRef CAS.
- P. V. Kamat, J. Phys. Chem. C, 2007, 111, 2834–2860 CrossRef CAS.
- J. Yang, D. Wang, H. Han and C. Li, Acc. Chem. Res., 2013, 46, 1900–1909 CrossRef CAS PubMed.
- J. Low, S. Cao, J. Yu and S. Wageh, Chem. Commun., 2014, 50, 10768–10777 RSC.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603–2636 RSC.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- U. Diebold, Surf. Sci. Rep., 2003, 48, 53–229 CrossRef CAS.
- V. Subramanian, E. E. Wolf and P. V. Kamat, J. Am. Chem. Soc., 2004, 126, 4943–4950 CrossRef CAS PubMed.
- J. Low, J. Yu, Q. Li and B. Cheng, Phys. Chem. Chem. Phys., 2014, 16, 1111–1120 RSC.
- M. S. Akple, J. Low, S. Wageh, A. A. Al-Ghamdi, J. Yu and J. Zhang, Appl. Surf. Sci., 2015, 358, 196–203 CrossRef CAS.
- Y. Bessekhouad, D. Robert and J. V. Weber, J. Photochem. Photobiol., A, 2004, 163, 569–580 CrossRef CAS.
- N. Lamers, Z. Zhang, I. G. Scheblykin and J. Wallentin, Adv. Opt. Mater., 2023, 2300435 CrossRef.
- S. Wang, Z. Wei, L. Yu, J. Hou, G. Wu and Y. Xiao, Chem. Eng. J., 2023, 468, 143503 CrossRef CAS.
- L. Yu, Y. Wei, Y. Lei, C. Liu, Y. Liu and M. Hong, CCS Chem., 2023 DOI:10.31635/ccschem.023.202302845.
- C. Zhang, M. Wang, J. Shi, J. Wang, Z. Da, Y. Zhou, Y. Xu, N. V. Gaponenko and A. S. Bhatti, Front. Chem., 2023, 11, 1199863 CrossRef CAS PubMed.
- D. J. Norris, N. Yao, F. T. Charnock and T. A. Kennedy, Nano Lett., 2001, 1, 3–7 CrossRef CAS.
- N. Pradhan and X. Peng, J. Am. Chem. Soc., 2007, 129, 3339–3347 CrossRef CAS PubMed.
- A. Malavika, S. Suresh, M. R. Subramaniam and S. K. Batabyal, New J. Chem., 2023, 47, 13783–13788 RSC.
- Z. Wei, Y. Lv and X. Su, Microchem. J., 2023, 192, 108940 CrossRef CAS.
- A. Du, W. Zhao, Y. Peng, X. Qin, Z. Lin, Y. Ye, E. Chen, S. Xu and T. Guo, Nanomaterials, 2023, 13, 17 CrossRef CAS PubMed.
- L. Jing, Q. Cen, Q. Pang and J. Z. Zhang, J. Phys. Chem. C, 2023, 127, 2448–2455 CrossRef CAS.
- Y. Li, W. Li, Y. Yu and C. Zheng, Opt. Mater. Express, 2023, 13, 1488–1496 CrossRef CAS.
- D. Patra and S. P. Singh, J. Phys. Chem. C, 2023, 127, 9397–9406 CrossRef CAS.
- R. Yun, H. Yang, W. Sun, L. Zhang, X. Liu, X. Zhang and X. Li, Laser Photonics Rev., 2023, 17, 2200524 CrossRef CAS.
- R. Zhang, G. Chen, H. Liu, J. Zhang, Y. Yuan, Z. Lv, L. Zhang, C. Wang, Z. Qin, W. Yang and J. Wang, Opt. Mater., 2023, 135, 113308 CrossRef CAS.
- Y.-W. Liu, S.-H. Guo, S.-Q. You, C.-Y. Sun, X.-L. Wang, L. Zhao and Z.-M. Su, Nanotechnology, 2020, 31, 215605 CrossRef CAS PubMed.
- J. V. Patil, S. S. Mali and C. K. Hong, J. Energy Chem., 2021, 62, 451–458 CrossRef CAS.
- S. Shyamal, S. K. Dutta and N. Pradhan, J. Phys. Chem. Lett., 2019, 10, 7965–7969 CrossRef CAS PubMed.
- G. X. Dong, W. Zhang, Y. F. Mu, K. Su, M. Zhang and T. B. Lu, Chem. Commun., 2020, 56, 4664–4667 RSC.
- X. Zhou, Q. Chang, G. Xiang, S. Jiang, L. Li, X. Tang, F. Ling, Y. Wang, J. Li, Z. Wang and X. Zhang, Spectrochim. Acta, Part A, 2023, 300, 122773 CrossRef CAS PubMed.
- W. Wang, S. Song, B. Cao and J. Li, J. Lumin., 2022, 247, 118901 CrossRef CAS.
- S. Huang, B. Wang, Q. Zhang, Z. Li, A.-d. Shan and L. Li, Adv. Opt. Mater., 2018, 6, 1701106 CrossRef.
- E. Ouaaka, M. Aazza, A. Bouymajane and F. Cacciola, Molecules, 2023, 28, 2880 CrossRef CAS PubMed.
- S. Paul, E. Bladt, A. F. Richter, M. Doeblinger, Y. Tong, H. Huang, A. Dey, S. Bals, T. Debnath, L. Polavarapu and J. Feldmann, Angew. Chem., Int. Ed., 2020, 59, 6794–6799 CrossRef CAS PubMed.
- N. Pradhan, J. Phys. Chem. Lett., 2019, 10, 2574–2577 CrossRef CAS PubMed.
- S. Jiang, Y. Song, H. Kang, B. Li, K. Yang, G. Xing, Y. Yu, S. Li, P. Zhao and T. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 3385–3394 CrossRef CAS PubMed.
- H. Bian, D. Li, S. Wang, J. Yan and S. F. Liu, Chem. Sci., 2022, 13, 1335–1341 RSC.
- Y. Zhao, X. Liang, X. Hu and J. Fan, Colloids Surf., A, 2021, 626, 127098 CrossRef CAS.
- Y. F. Xu, M. Z. Yang, B. X. Chen, X. D. Wang, H. Y. Chen, D. B. Kuang and C. Y. Su, J. Am. Chem. Soc., 2017, 139, 5660–5663 CrossRef CAS PubMed.
- J.-Y. Li, H.-Y. Chen, Y. Jiang and D.-B. Kuang, Sol. RRL, 2021, 5, 2100036 CrossRef CAS.
- A. Kipkorir, J. DuBose, J. Cho and P. Kamat, Chem. Sci., 2021, 12, 14815–14825 RSC.
- H. Jin, Y. Chen, L. Zhang, R. Wan, Z. Zou, H. Li and Y. Gao, Nanotechnology, 2021, 32, 085202 CrossRef CAS PubMed.
- R. Huang, M. Zhang, Z. Zheng, K. Wang, X. Liu, Q. Chen and D. Luo, Nanomaterials, 2021, 11, 2422 CrossRef CAS PubMed.
- M. T. Hossain, M. Das, J. Ghosh, S. Ghosh and P. K. Giri, Nanoscale, 2021, 13, 14945–14959 RSC.
- H. Cong, X. Chu, F. Wan, Z. Chu, X. Wang, Y. Ma, J. Jiang, L. Shen, J. You and C. Xue, Small Methods, 2021, 5, e2100517 CrossRef PubMed.
- E. Zhu, Y. Zhao, Y. Dai, Q. Wang, Y. Dong, Q. Chen and Y. Li, Chin. J. Chem., 2020, 38, 1718–1722 CrossRef CAS.
- Y. Liu, Y. Yang, P. Chen, Y. Shan, Y. Li, J. Shi, J. Hou, N. Zhang, G. Zhao, J. Xu, Y. Fang and N. Dai, Small, 2020, 16, e2004126 CrossRef PubMed.
- Q. Huang, Z. Liang, F. Qi, N. Zhang, J. Yang, J. Liu, C. Tian, C. Fu, X. Tang, D. Wu, J. Wang, X. Wang and W. Chen, J. Phys. Chem. Lett., 2022, 13, 2418–2427 CrossRef CAS PubMed.
- X. Xiao, S. Guo, C. Ding, Z. Zhang, H. Huang and J. Xu, J. Inorg. Mater., 2021, 36, 507–512 CrossRef.
- H. Zhao, Y. Zhang, T. Li, Q. Li, Y. Yu, Z. Chen, Y. Li and J. Yao, Nanotechnology, 2020, 31, 035202 CrossRef CAS PubMed.
- Z. P. Huang, B. Ma, H. Wang, N. Li, R. T. Liu, Z. Q. Zhang, X. D. Zhang, J. H. Zhao, P. Z. Zheng, Q. Wang and H. L. Zhang, J. Phys. Chem. Lett., 2020, 11, 6007–6015 CrossRef CAS PubMed.
- T. Paul, D. Das, B. K. Das, S. Sarkar, S. Maiti and K. K. Chattopadhyay, J. Hazard. Mater., 2019, 380, 120855 CrossRef CAS PubMed.
- M. Shu, J. Lu, Z. Zhang, T. Shen and J. Xu, J. Inorg. Mater., 2021, 36, 1217 CrossRef.
- T. Chen, M. Li, L. Shen, M. B. J. Roeffaers, B. Weng, H. Zhu, Z. Chen, D. Yu, X. Pan, M.-Q. Yang and Q. Qian, Front. Chem., 2022, 10, 833784 CrossRef CAS PubMed.
- P. Kour and S. P. Mukherjee, J. Mater. Chem. A, 2021, 9, 6819–6826 RSC.
- C. Zhang, J. Miao, L. Guo, T. Tan, W. Cai, X. Lei, Y. Li and Z. Wang, Appl. Organomet. Chem., 2023, 37, e7122 CrossRef CAS.
- Z. Dong, Y. Shi, Y. Jiang, C. Yao and Z. Zhang, J. CO2 Util., 2023, 72, 102480 CrossRef CAS.
- J.-R. Xue, R.-C. Xue, P.-Y. Chen, Y. Wang and L.-P. Yu, J. Chin. Chem. Soc., 2023, 70, 1481–1492 CrossRef CAS.
- P. Wang, X. Ba, X. Zhang, H. Gao, M. Han, Z. Zhao, X. Chen, L. Wang, X. Diao and G. Wang, Chem. Eng. J., 2023, 457, 141248 CrossRef CAS.
- H. McDaniel, P. E. Heil, C.-L. Tsai, K. Kim and M. Shim, ACS Nano, 2011, 5, 7677–7683 CrossRef CAS PubMed.
- C. H. Wang, H. W. Huang, B. Weng, D. Verhaeghe, M. Keshavarz, H. D. Jin, B. Liu, H. P. Xie, Y. Ding, Y. J. Gao, H. F. Yuan, J. A. Steele, J. Hofkens and M. B. J. Roeffaers, Appl. Catal., B, 2022, 301, 120760 CrossRef CAS.
- G. Yin, X. Qi, Y. Chen, Q. Peng, X. Jiang, Q. Wang, W. Zhang and X. Gong, J. Mater. Chem. A, 2022, 10, 22468–22476 RSC.
- Y. Jiang, J. F. Liao, H. Y. Chen, H. H. Zhang, J. Y. Li, X. D. Wang and D. B. Kuang, Chem, 2020, 6, 766–780 CAS.
- D.-E. Lee, N. Mameda, K. P. Reddy, B. M. Abraham, W.-K. Jo and S. Tonda, J. Mater. Sci. Technol., 2023, 161, 74–87 CrossRef.
- J. Lu, S. Gu, H. Li, Y. Wang, M. Guo and G. Zhou, J. Mater. Sci. Technol., 2023, 160, 214–239 CrossRef.
- M. Luo, G. Jiang, M. Yu, Y. Yan, Z. Qin, Y. Li and Q. Zhang, J. Mater. Sci. Technol., 2023, 161, 220–232 CrossRef.
- Z. Zhang, L. Li, Y. Jiang and J. Xu, Inorg. Chem., 2022, 61, 3351–3360 CrossRef CAS PubMed.
- F. Xu, K. Meng, B. Cheng, S. Wang, J. Xu and J. Yu, Nat. Commun., 2020, 11, 4613 CrossRef CAS PubMed.
- Y.-C. Pu, Y.-H. Chuang, M.-W. Zheng, Y.-J. Chang and S.-H. Liu, J. Environ. Chem. Eng., 2023, 11, 109103 CrossRef CAS.
- S.-H. Guo, J. Zhou, X. Zhao, C.-Y. Sun, S.-Q. You, X.-L. Wang and Z.-M. Su, J. Catal., 2019, 369, 201–208 CrossRef CAS.
- Y. Zhao, Y. Dai, Q. Wang, Y. Dong, T. Song, A. Mudryi, Q. Chen and Y. Li, ChemCatChem, 2021, 13, 2592–2598 CrossRef CAS.
- R. Das, A. Patra, S. K. Dutta, S. Shyamal and N. Pradhan, J. Am. Chem. Soc., 2022, 144, 18629–18641 CrossRef CAS PubMed.
- F.-B. Chiu, Y.-W. Wu and S.-H. Yang, ACS Omega, 2023, 8, 19109–19118 CrossRef CAS PubMed.
- M. Giancaspro, R. Grisorio, G. Alò, N. Margiotta, A. Panniello, G. P. Suranna, N. Depalo, M. Striccoli, M. L. Curri and E. Fanizza, Mater. Chem. Front., 2023, 7, 2637–2650 RSC.
- Y. Duan, K. Chordiya, M. U. Kahaly, F. E. Oropeza, V. A. de la Pena O’shea, D.-Y. Wang and R. D. Costa, Adv. Opt. Mater., 2022, 10, 2201176 CrossRef CAS.
- C. H. Tien, H.-H. Tsai and L. C. Chen, J. Mater. Res. Technol., 2022, 19, 3842–3851 CrossRef CAS.
- M. Wang, D. Liang, W. Ma, Q. Mo, Z. Zang, Q. Qian and W. Cai, Opt. Lett., 2022, 47, 4512–4515 CrossRef CAS PubMed.
- S. Bera, R. K. Behera, S. K. Dutta and N. Pradhan, ACS Mater. Lett., 2023, 5, 1332–1339 CrossRef CAS.
- M.-G. Jeon, A. Kirakosyan, C. Shin, S. Yun, J. Kim, L. Li and J. Choi, Chem. Eng. J., 2023, 462, 142120 CrossRef CAS.
- H. Wang, W. Lu, P. Xu, J. Luo, K. Yao, J. Zhang, X. Wei, S. Peng, H. Cheng, H. Hu and K. Sun, ACS Sustainable Chem. Eng., 2023, 11, 5963–5972 CrossRef CAS.
- Y. He, L. Zhang, G. Chen, Y. Liu, S. Shi, P. Jiang, J. Ding, S. Xu and C. Geng, Appl. Surf. Sci., 2023, 611, 155724 CrossRef CAS.
- C. Wang, M. Zhang, H. Yu, W. Zhang, G. Shi, X. Zhang, Z. Cui, P. Fu, M. Liu, X. Qiao, Y. He and X. Pang, ACS Appl. Nano Mater., 2023, 6, 3416–3424 CrossRef CAS.
- L. Wu, H. Hu, Y. Xu, S. Jiang, M. Chen, Q. Zhong, D. Yang, Q. Liu, Y. Zhao, B. Sun, Q. Zhang and Y. Yin, Nano Lett., 2017, 17, 5799–5804 CrossRef CAS PubMed.
- A. Pan, M. J. Jurow, F. Qiu, J. Yang, B. Ren, J. J. Urban, L. He and Y. Liu, Nano Lett., 2017, 17, 6759–6765 CrossRef CAS PubMed.
- X. Liang, W. Chen, J. Yang, X. Liu, X. Fang and W. Xiang, ACS Appl. Electron. Mater., 2023, 5, 2309–2317 CrossRef.
- M. Xiao, M. Hao, M. Lyu, E. G. Moore, C. Zhang, B. Luo, J. Hou, J. Lipton-Duffin and L. Wang, Adv. Funct. Mater., 2019, 29, 19056831–19056838 Search PubMed.
- X. Zhang, M. He, H. Fang, J. Bao, X. Bu, C. Yang, X. Sheng, B. Wu, Z. Zhang and Y. Zhou, Int. J. Hydrogen Energy, 2022, 47, 40860–40871 CrossRef CAS.
- M. Knezevic, V.-D. Quach, I. Lampre, M. Erard, P. Pernot, D. Berardan, C. Colbeau-Justin and M. N. Ghazzal, J. Mater. Chem. A, 2023, 11, 6226–6236 RSC.
- H. Wang, X. M. Wang, R. T. Chen, H. F. Zhang, X. L. Wang, J. H. Wang, J. Zhang, L. C. Mu, K. F. Wu, F. T. Fan, X. Zong and C. Li, Int. J. Hydrogen Energy, 2019, 4, 40–47 CAS.
- W. Song, Y. Wang, C. Wang, B. Wang, J. Feng, W. Luo, C. Wu, Y. Yao and Z. Zou, ChemCatChem, 2021, 13, 1711–1716 CrossRef CAS.
- L. Ding, C. Shen, Y. Zhao, Y. Chen, L. Yuan, H. Yang, X. Liang, W. Xiang and L. Li, Mol. Catal., 2020, 483, 110764 CrossRef CAS.
- H. Sun, R. Tang, X. Zhang, S. Zhang, W. Yang, L. Wang, W. Liang, F. Li, R. Zheng and J. Huang, Catal. Sci. Technol., 2023, 13, 1154–1163 RSC.
- J. Hou, S. Cao, Y. Wu, Z. Gao, F. Liang, Y. Sun, Z. Lin and L. Sun, Chem.–Eur. J., 2017, 23, 9481–9485 CrossRef CAS PubMed.
- Z. Guan, Y. Wu, P. Wang, Q. Zhang, Z. Wang, Z. Zheng, Y. Liu, Y. Dai, M.-H. Whangbo and B. Huang, Appl. Catal., B, 2019, 245, 522–527 CrossRef CAS.
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- M. E. El-Khouly, E. El-Mohsnawy and S. Fukuzumi, J. Photochem. Photobiol., C, 2017, 31, 36–83 CrossRef CAS.
- A. Crake, Mater. Sci. Technol., 2017, 33, 1737–1749 CrossRef CAS.
- W. Dong, Y. Ji, J. Wang, B. Li, F. Yang and J. Yang, ACS Omega, 2021, 6, 34675–34686 CrossRef CAS PubMed.
- Y.-H. Chen, K.-A. Tsai, T.-W. Liu, Y.-J. Chang, Y.-C. Wei, M.-W. Zheng, S.-H. Liu, M.-Y. Liao, P.-Y. Sie, J.-H. Lin, S.-W. Tseng and Y.-C. Pu, J. Phys. Chem. Lett., 2023, 14, 122–131 CrossRef CAS PubMed.
- H. Xiao, Q. Qian and Z. Zang, Sci. China Mater., 2023, 66, 1810–1819 CrossRef CAS.
- Y.-J. Dong, Y. Jiang, J.-F. Liao, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, Sci. China Mater., 2022, 65, 1550–1559 CrossRef CAS.
- D. Laishram, S. Zeng, K. M. Alam, A. P. Kalra, K. Cui, P. Kumar, R. K. Sharma and K. Shankar, Appl. Surf. Sci., 2022, 592, 153276 CrossRef CAS.
- C. Han, X. Zhu, J. S. Martin, Y. Lin, S. Spears and Y. Yan, ChemSusChem, 2020, 13, 4005–4025 CrossRef CAS PubMed.
- J. Fan, B. Jia and M. Gu, Photonics Res., 2014, 2, 111–120 CrossRef.
- S. A. Kulkarni, S. G. Mhaisalkar, N. Mathews and P. P. Boix, Small Methods, 2019, 3, 1800231 CrossRef.
- A. Tabish, A. M. Varghese, M. A. Wahab and G. N. Karanikolos, Catalysts, 2020, 10, 95 CrossRef CAS.
- M. Singh and I. Sinha, Sol. Energy, 2020, 208, 296–311 CrossRef CAS.
- S. Shyamal and N. Pradhan, J. Phys. Chem. Lett., 2020, 6921–6934 CrossRef CAS PubMed.
- H. Huang, B. Pradhan, J. Hofkens, M. B. J. Roeffaers and J. A. Steele, ACS Energy Lett., 2020, 5, 1107–1123 CrossRef CAS.
- X. X. Chang, T. Wang and J. L. Gong, Energy Environ. Sci., 2016, 9, 2177–2196 RSC.
- H.-L. Wu, X.-B. Li, C.-H. Tung and L.-Z. Wu, Adv. Mater., 2019, 31, 1900709 CrossRef PubMed.
- W. Zhang, Z. Wang, P. Lin, D. Wu, Z. Shi, X. Chen, T. Xu, Y. Tian and X. Li, J. Mater. Sci., 2020, 55, 6826–6833 CrossRef CAS.
- G.-X. Dong, M.-R. Zhang, K. Su, Z.-L. Liu, M. Zhang and T.-B. Lu, J. Mater. Chem. A, 2023, 11, 9989–9999 RSC.
- X. Liu, J. Qiu, Q. Huang, X. Chen, J. Yu and J. Bao, Phys. Chem. Chem. Phys., 2023, 25, 11620–11629 RSC.
- J. Wang, J. Wang, N. Li, X. Du, J. Ma, C. He and Z. Li, ACS Appl. Mater. Interfaces, 2020, 12, 31477–31485 CrossRef CAS PubMed.
- Z.-C. Kong, J.-F. Liao, Y.-J. Dong, Y.-F. Xu, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, ACS Energy Lett., 2018, 3, 2656–2662 CrossRef CAS.
- S. Wan, M. Ou, Q. Zhong and X. Wang, Chem. Eng. J., 2019, 358, 1287–1295 CrossRef CAS.
- Y.-H. Chen, J.-K. Ye, Y.-J. Chang, T.-W. Liu, Y.-H. Chuang, W.-R. Liu, S.-H. Liu and Y.-C. Pu, Appl. Catal., B, 2021, 284, 119751 CrossRef CAS.
- M. Ou, W. Tu, S. Yin, W. Xing, S. Wu, H. Wang, S. Wan, Q. Zhong and R. Xu, Angew. Chem., Int. Ed. Engl., 2018, 57, 13570–13574 CrossRef CAS PubMed.
- A. Pan, X. Ma, S. Huang, Y. Wu, M. Jia, Y. Shi, Y. Liu, P. Wangyang, L. He and Y. Liu, J. Phys. Chem. Lett., 2019, 10, 6590–6597 CrossRef CAS PubMed.
- Y.-F. Xu, X.-D. Wang, J.-F. Liao, B.-X. Chen, H.-Y. Chen and D.-B. Kuang, Adv. Mater. Interfaces, 2018, 5, 1801015 CrossRef.
- O. Lefebvre and R. Moletta, Water Res., 2006, 40, 3671–3682 CrossRef CAS PubMed.
- J. Wang, J. Liu, Z. Du and Z. Li, J. Energy Chem., 2021, 54, 770–785 CrossRef CAS.
- D. Wang, S. C. Pillai, S.-H. Ho, J. Zeng, Y. Li and D. D. Dionysiou, Appl. Catal., B, 2018, 237, 721–741 CrossRef CAS.
- V. N. Salomone, J. M. Meichtry, G. Zampieri and M. I. Litter, Chem. Eng. J., 2015, 261, 27–35 CrossRef CAS.
- P. A. K. Reddy, P. V. L. Reddy, E. Kwon, K.-H. Kim, T. Akter and S. Kalagara, Environ. Int., 2016, 91, 94–103 CrossRef CAS PubMed.
- X. Yang and D. Wang, ACS Appl. Energy Mater., 2018, 1, 6657–6693 CrossRef CAS.
- B. Ai, M. Luo and I. Khan, Processes, 2023, 11, 1398 CrossRef CAS.
- I. Bolognino, R. Pelosato, G. Marci and I. Natali Sora, Molecules, 2023, 28, 3807 CrossRef CAS PubMed.
- M. Humayun, A. Bahadur, A. Khan and M. Bououdina, Catalysts, 2023, 13, 907 CrossRef CAS.
- Y.-Y. Lin, P.-H. Lu, F.-Y. Liu, C.-S. Lu and C.-C. Chen, Catalysts, 2023, 13, 812 CrossRef CAS.
- J. Wang, N. Li, Y. Tang, Y. He and K. Shan, Int. J. Electrochem. Sci., 2022, 17, 22081 CrossRef CAS.
- X. Hu, Y. Xu, J. Wang, J. Ma, L. Wang and W. Jiang, Chem. Eng. J., 2023, 451, 139031 CrossRef CAS.
- X. Feng, H. Ju, T. Song, T. Fang, W. Liu and W. Huang, ACS Sustainable Chem. Eng., 2019, 7, 5152–5156 CrossRef CAS.
- G. Gao, Q. Xi, H. Zhou, Y. Zhao, C. Wu, L. Wang, P. Guo and J. Xu, Nanoscale, 2017, 9, 12032–12038 RSC.
- E. Fan, H. Xu, S. Sun, C. Huang, M. Li, B. Fan, G. Shao, W. Liu, H. Wang, H. Lu and R. Zhang, Colloids Surf., A, 2023, 669, 131510 CrossRef CAS.
- X. Jia, C. Liu, X. Xu, F. Wang, W. Li, L. Zhang, S. Jiao, G. Zhu and X. Wang, RSC Adv., 2023, 13, 19140–19148 RSC.
- R. Sun, X. Yu, J. Chen, W. Zhang, Y. Huang, J. Zheng and Y. Chi, Anal. Chem., 2022, 94, 17142–17150 CrossRef CAS PubMed.
- Y. Zhao, H. Shi, X. Hu, E. Liu and J. Fan, Chem. Eng. J., 2020, 381, 122692 CrossRef CAS.
- Y. Zhao, Y. Xu, X. Jing and W. Ma, Food Chem., 2023, 412, 135420 CrossRef CAS PubMed.
- S. Sharma, S. K. Mehta, A. O. Ibhadon and S. K. Kansal, J. Colloid Interface Sci., 2019, 533, 227–237 CrossRef CAS PubMed.
- G. V. S. R. P. Kumar, Y. B. Pydiraju, G. V. Lokesh and U. V. S. Likith, Water Conserv. Sci. Eng., 2023, 8, 17 CrossRef.
- E. A. Namikuchi, R. D. L. Gaspar, I. M. Raimundo Jr and I. O. Mazali, Spectrochim. Acta, Part A, 2023, 300, 122915 CrossRef CAS PubMed.
- A. Solinska, J. Marchewka, M. Sitarz and T. Bajda, Spectrochim. Acta, Part A, 2023, 299, 122758 CrossRef CAS PubMed.
- K. Guo, P. Lin, D. Wu, Z. Shi, X. Chen, Y. Han, Y. Tian and X. Li, Chem.–Eur. J., 2023, 29, e202300400 CrossRef CAS PubMed.
- A. Jimenez-Almarza, A. Lopez-Magano, R. Cano, B. Ortin-Rubio, D. Diaz-Garcia, S. Gomez-Ruiz, I. Imaz, D. Maspoch, R. Mas-Balleste and J. Aleman, Mater. Today Chem., 2021, 22, 100548 CrossRef CAS.
- H. Ju, T. Fang, Y. Zhou, X. Feng, T. Song, F. Lu and W. Liu, Appl. Surf. Sci., 2021, 551, 149452 CrossRef CAS.
- X.-F. Qi, F. Zhang, Z.-P. Chen, X. Chen, M.-C. Jia, H.-F. Ji and Z.-F. Shi, J. Mater. Chem. C, 2023, 11, 3715–3725 RSC.
- E. D. M. Isa, N. W. C. Jusoh and A. A. M. Rodzi, Environ. Sci. Pollut. Res., 2023, 30, 116921–116933 CrossRef CAS PubMed.
- Y. Khan, U. Sharafat, S. Gul, M. I. Khan, M. Ismail, M. A. Khan, R. Younus and S. B. Khan, Green Process. Synth., 2023, 12, 20228128 CrossRef CAS.
- F. Yang, X. He, T. Xin, H. Yang, L. Bai, L. Gao and Y. Wang, Molecules, 2023, 28, 3968 CrossRef CAS PubMed.
- P. Nehra, P. S. Rana and S. Singh, Environ. Sci. Pollut. Res. Int., 2023, 30, 70094–70108 CrossRef CAS PubMed.
- A. Panahi, R. Monsef, E. A. Dawi, A. S. Hussein and M. Salavati-Niasari, Sol. Energy, 2023, 258, 372–382 CrossRef CAS.
- S. Sharma, P. Jakhar and H. Sharma, J. Appl. Polym. Sci., 2023, 140, e54038 CrossRef CAS.
- U. A. A. Yasin, M. M. Ahmed, J. Zhang, Z. Jia, T. Guo, R. Zhao, J. Shi and J. Du, J. Mater. Sci., 2023, 58, 7333–7346 CrossRef CAS.
- F. Yousefzadeh, M. Ghanbari, E. A. Dawi and M. Salavati-Niasari, Arabian J. Chem., 2023, 16, 104904 CrossRef CAS.
- S. Liu, M. Li, Y. Tang and X. Wen, J. Alloys Compd., 2023, 959, 170528 CrossRef CAS.
- S. M. M. Yakout and M. E. E. El-Zaidy, J. Sol-Gel Sci. Technol., 2023, 107, 417–429 CrossRef CAS.
- S. S. Ahmed, J. Nanostruct., 2023, 13, 48–58 CAS.
- K. Balu, E. Chicardi, R. Sepulveda, M. Durai, F. Ishaque, D. Chauhan and Y.-H. Ahn, Sep. Purif. Technol., 2023, 309, 122998 CrossRef CAS.
- S. Moradi, M. Farhadian, A. R. S. Nazar and M. Moghadam, J. Mol. Liq., 2023, 377, 121520 CrossRef CAS.
- S. Ravikumar, D. Mani, E. Chicardi, R. Sepulveda, K. Balu, V. Pandiyan and Y. H. Ahn, Ceram. Int., 2023, 49, 9551–9559 CrossRef CAS.
- A. Moulahi, J. Inorg. Organomet. Polym. Mater., 2023, 33, 3948–3960 CrossRef CAS.
- I. Ahmad, M. Muneer, A. S. Khder and S. A. Ahmed, ACS Omega, 2023, 25, 22708–22720 CrossRef PubMed.
- S. Asgari, G. M. Ziarani, A. Badiei, R. S. Varma, S. Iravani and F. Mohajer, RSC Adv., 2023, 13, 17324–17339 RSC.
- K. Layek, Catal. Surv. Asia, 2023, 27, 349–362 CrossRef CAS.
- W. Liu, H. Xie, X. Guo, K. Wang, C. Yang, N. Wang and C. Ge, Opt. Mater., 2023, 136, 113398 CrossRef CAS.
- P. Li, Z. Wang, S. Yang, G. Lyu, Y. Gu, J. Chen and G. Yang, Int. J. Biol. Macromol., 2023, 242, 125137 CrossRef CAS PubMed.
- M. Lv, H. Liu, L. He, B. Zheng, Q. Tan, M. Hassan, F. Chen and Z. Gong, Environ. Res., 2023, 231, 116218 CrossRef CAS PubMed.
- M. Sharma, D. Rajput, V. Kumar, I. Jatain, T. M. Aminabhavi, G. Mohanakrishna, R. Kumar and K. K. Dubey, Environ. Res., 2023, 231, 116132 CrossRef CAS PubMed.
- D.-E. Lee, S. Moru, K. P. Reddy, W.-K. Jo and S. Tonda, J. Mater. Sci. Technol., 2023, 148, 19–30 CrossRef CAS.
- J. Lv, X. Lu, C. Zhou, X. Zeng and Y.-y. Li, J. Alloys Compd., 2023, 960, 170514 CrossRef CAS.
- D. Song, M. Li, L. Liao, L. Guo, H. Liu, B. Wang and Z. Li, Nanomaterials, 2023, 13, 1841 CrossRef CAS PubMed.
- Q. Sun, X. Hu, Y. Zhao, J. Zhang and J. Sheng, Environ. Sci. Pollut. Res., 2023, 30, 75247–75261 CrossRef CAS PubMed.
- D. Cardenas-Morcoso, A. F. Gualdrón-Reyes, A. B. Ferreira Vitoreti, M. García-Tecedor, S. J. Yoon, M. Solis de la Fuente, I. Mora-Seró and S. Gimenez, J. Phys. Chem. Lett., 2019, 10, 630–636 CrossRef CAS PubMed.
- L. Wang, M. Niu, Y. Liu, Y. Xie, Z. Ma, M. Zhang and C. Hou, J. Colloid Interface Sci., 2023, 645, 639–653 CrossRef CAS PubMed.
- Y. Xiao, Y. Jiang, E. Zhou, W. Zhang, Y. Liu, J. Zhang, X. Wu, Q. Qi and Z. Liu, J. Mater. Sci. Technol., 2023, 153, 205–218 CrossRef CAS.
- J. Xing, J. Huang, X. Wang, F. Yang, Y. Bai, S. Li and X. Zhang, J. Environ. Manage., 2023, 343, 118210 CrossRef CAS PubMed.
- X. Qian, Z. Chen, X. Yang, W. Zhao, C. Liu, T. Sun, D. Zhou, Q. Yang, G. Wei and M. Fan, J. Cleaner Prod., 2020, 249, 119335 CrossRef CAS.
- Y. Zhao, Y. Wang, X. Liang, H. Shi, C. Wang, J. Fan, X. Hu and E. Liu, Appl. Catal., B, 2019, 247, 57–69 CrossRef CAS.
- P. Wang, X. Diao, X. Zhang, Z. Zhao, H. Gao, J. T. S. Irvine and G. Wang, Mater. Today Phys., 2023, 35, 101043 CrossRef CAS.
- X. Wang, J. He, L. Mao, X. Cai, C. Sun and M. Zhu, Chem. Eng. J., 2021, 416, 128077 CrossRef CAS.
- M. T. Hoang, C. Han, Z. Ma, X. Mao, Y. Yang, S. S. Madani, P. Shaw, Y. Yang, L. Peng, C. Y. Toe, J. Pan, R. Amal, A. Du, T. Tesfamichael, Z. Han and H. Wang, Nano-Micro Lett., 2023, 15, 161 CrossRef CAS PubMed.
- A. A. Alothman, M. R. Khan, M. D. Albaqami, S. Mohandoss, Z. A. Alothman, N. Ahmad and K. N. Alqahtani, Nanomaterials, 2023, 13, 3026 CrossRef CAS PubMed.
- S. Das, T. Paul, S. Maiti and K. K. Chattopadhyay, Mater. Lett., 2020, 267, 127501 CrossRef CAS.
- A. A. Bhat, S. A. Khandy, I. Islam and R. Tomar, Sci. Rep., 2021, 11, 16473 CrossRef CAS PubMed.
- A. Asadi, N. Khosroshahi, M. Hosseinpour and V. Safarifard, Mater. Sci. Semicond. Process., 2023, 165, 107707 CrossRef CAS.
- Y. Liu and Z. Ma, Colloids Surf., A, 2021, 628, 127310 CrossRef CAS.
- M. Karami, M. Ghanbari, O. Amiri and M. Salavati-Niasari, Sep. Purif. Technol., 2020, 253, 117526 CrossRef CAS.
- G. Yuan, S. Feng, Q. Yang, F. Yi, X. Li, Y. Yuan, C. Wang and H. Yan, J. Mater. Chem. C, 2023, 11, 7570–7574 RSC.
| This journal is © The Royal Society of Chemistry 2024 |