Transition-metal catalyzed reactions of diazo compounds and N,N-dialkylnitrosoamines†
Yuan
Chen
,
Rui-jun
Peng
,
Xue-jing
Zhang
 and
Ming
Yan
and
Ming
Yan
 *
*
Guangdong Provincial Key Laboratory of Chiral Molecules and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, China. E-mail: yanming@mail.sysu.edu.cn
First published on 21st November 2023
Abstract
In this study, we present a Ru(II)-catalyzed reaction involving diazoindandiones and N,N-dialkylnitrosoamines, leading to the efficient preparation of a diverse array of isoquinoline-1,3,4-trione derivatives in moderate to excellent yields. The reaction was proposed to occur via the rearrangement of the nitroso ylide intermediates. Additionally, we report a Rh(III)-catalyzed reaction employing dimethyl diazomalonate and N,N-dialkylnitrosoamines, yielding stable nitroso ylide products. These findings offer valuable insights into the synthetic potential of diazo compounds and N,N-dialkylnitrosoamines in transition-metal-catalyzed transformations.
Introduction
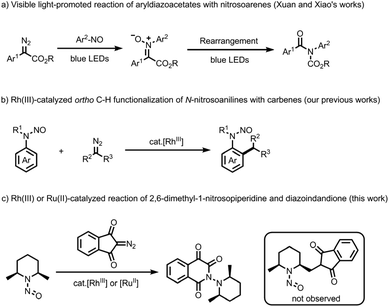
Transition-metal catalyzed reactions of carbenes with tertiary amines and nitrogen-heterocycles have undergone significant development for the synthesis of drugs, fine chemicals and natural products.1 These transformations prominently entail the formation of ammonium ylides and the subsequent rearrangements. The typical transformations involve 1,2-Steves rearrangement and 2,3-sigmatropic rearrangements. While aziridines have been employed, the cheletropic extrusion of olefins, as well as ring expansion, may ensue from the resulting aziridinium ylides, yielding imines or other complex amines.2 The reactions of carbenes with pyridines yield the corresponding pyridinum ylides, serving as active intermediates in 1,3-dipolar cycloadditions with olefins and alkynes.3 Furthermore, Xuan, Xiao and co-workers reported a visible light-promoted reaction of aryldiazoacetates with nitrosoarenes. The generation of nitrones and subsequent rearrangement provided amides efficiently (Scheme 1a).4 Despite notable strides in this area, the rearrangement reactions of carbenes with electron-deficient nitrogen-containing compounds remain less explored. Such transformations also hold considerable promise for applications in organic synthesis.Over the past decades, transition-metal-catalyzed C–H activation, aided by directing groups and subsequent carbene transfer reactions, has emerged as a robust method for C–C bond construction.5 Among these directing groups, N-nitrosoamino has proven to be particularly adept, known for its facile removal and versatility.6 Highly regioselective insertions into ortho aromatic C(sp2)–H bonds of N-nitrosoanlines were achieved with a range of carbenes.7 Recently, our group successfully developed a Rh(III)-catalyzed ortho C–H functionalization of N-nitrosoanilines employing α-sulfonylcarbenes and naphthoquinone carbenes (Scheme 1b).8 Despite this success in aromatic C(sp2)–H bond functionalization, migratory insertions of carbenes into aliphatic C(sp3)–H bonds remain a formidable challenge, with only limited progress achieved thus far.9 Given the efficacy of the nitrosoamino group as a directing moiety, we sought to extend this strategy to the migratory insertion of carbenes into aliphatic C(sp3)–H bonds. As a preliminary investigation, we conducted a test using diazoindandione and cis-2,6-dimethyl-1-nitrosopiperidine (Scheme 1c). Unexpectedly, we did not observe the anticipated C(sp3)–H insertion product; instead, a significant yield of isoquinoline-1,3,4-trione product was isolated.
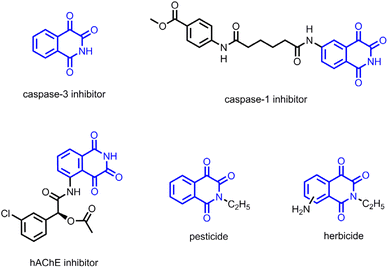
Isoquinoline-1,3,4-triones represent crucial structural motifs present in numerous bioactive compounds and pharmaceuticals associated with neuronal apoptosis and neurodegenerative diseases (Scheme 2).10 However, the existing synthetic methodologies for these compounds are constrained,11 typically necessitating multi-step sequences involving harsh conditions, hazardous reagents, or exhibiting a restricted substrate scope. In this study, we present a Ru(II)-catalyzed rearrangement reaction of diazoindandiones and N,N-dialkylnitrosoamines. A series of isoquinoline-1,3,4-triones were efficiently prepared in good yields. Additionally, we explore transition-metal-catalyzed reactions of other diazo compounds with N,N-dialkylnitrosoamines.
Results and discussion
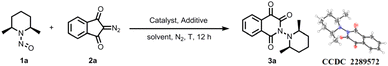
In the initial investigation, we examined the reaction between cis-2,6-dimethyl-1-nitrosopiperidine 1a and diazoindandione 2a, and the results are summarized in Table 1. Referring to our previous study,8a [Cp*RhCl2]2/AgSbF6/AgOAc was used as the catalyst. Notably, no product 3a was observed at room temperature (Table 1, entry 1). However, upon raising the reaction temperature to 60 °C, we obtained product 3a in a yield of 60% (Table 1, entry 2). The structural identity of 3a was verified through comprehensive analysis including NMR, HRMS, and X-ray diffraction. Notably, two stereoisomers were identified in the product (ratio = 1/1, as determined by 13C NMR analysis). We postulated that the steric bulk imposed by the 2,6-dimethyl substituents on the piperidine ring hinders rotation around the N–N bond. Further, based on X-ray diffraction analysis of one of the isomers, the plane of the isoquinoline-1,3,4-trione scaffold and piperidine ring exhibited nearly orthogonal orientations. In this isomer, two methyl groups of the piperidine ring projected forward towards the plane of the isoquinoline-1,3,4-trione. Conversely, in the alternate stereoisomer, these methyl groups were inferred to be directed backward relative to the plane of the isoquinoline-1,3,4-trione.| Entry | Catalyst | Additive | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), catalyst (2.5 mol%), additive (15 mol%) in solvent (2.0 mL) for 12 h under a N2 atmosphere. b Yield determined by 1H NMR using dimethyl terephthalate as the internal standard. c See the ESI† for more details. d 1a (0.4 mmol), 2a (0.2 mmol). e 1a (0.3 mmol), 2a (0.2 mmol). f Isolated yield after column chromatography. g 1a (0.2 mmol), 2a (0.2 mmol). h 1a (0.2 mmol), 2a (0.4 mmol). | |||||
| 1 | [Cp*RhCl2]2 | AgSbF6 | DCE | r.t. | 0 |
| AgOAc | |||||
| 2 | [Cp*RhCl2]2 | AgSbF6 | DCE | 60 | 60 |
| AgOAc | |||||
| 3 | [Cp*RhCl2]2 | AgSbF6 | DCE | 60 | 64 |
| 4 | Other catalystsc | AgSbF6 | DCE | 60 | 0–20 |
| 5 | [RuCl2(p-cymene)]2 | AgSbF6 | DCE | 60 | 81 |
| 6 | None | AgSbF6 | DCE | 60 | 0 |
| 7 | [RuCl2(p-cymene)]2 | None | DCE | 60 | 0 |
| 8d | [RuCl2(p-cymene)]2 | AgSbF6 | DCE | 60 | 82 |
| 9e | [RuCl2(p-cymene)]2 | AgSbF6 | DCE | 60 | 96 (96f) |
| 10g | [RuCl2(p-cymene)]2 | AgSbF6 | DCE | 60 | 68 |
| 11h | [RuCl2(p-cymene)]2 | AgSbF6 | DCE | 60 | 72 |
| 12e | [RuCl2(p-cymene)]2 | Other Ag saltsc | DCE | 60 | 35–87 |
| 13e | [RuCl2(p-cymene)]2 | AgSbF6 | Other solventsc | 60 | 0–60 |
A slight improvement in yield was observed in the absence of AgOAc (Table 1, entry 3). Various other transition metal catalysts, including Cu(OTf)2, Rh2(OAc)4, Pd(OAc)2, IPrAuCl, [Cp*IrCl2]2 and Cp*Co(CO)I2 were evaluated, but were found to be ineffective for this reaction (Table 1, entry 4).12 Encouragingly, when [Cp*RhCl2]2 was replaced with [RuCl2(p-cymene)]2, the yield increased to 81% (Table 1, entry 5). The reaction did not proceed in the absence of either [RuCl2(p-cymene)]2 or AgSbF6 (Table 1, entries 6 and 7), underscoring their essential roles in the transformation. We then investigated the influence of different molar ratios of the two substrates on the reaction (Table 1, entries 8–11). Notably, when the molar ratio of substrate 1a to 2a was adjusted to 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield significantly improved to 96% (Table 1, entry 9). Despite examining various silver salts and solvents (Table 1, entries 12 and 13), inferior yields were consistently obtained.12 Additionally, reducing the loading of [RuCl2(p-cymene)]2 or adjusting the reaction temperature resulted in diminished yields.12
1, the yield significantly improved to 96% (Table 1, entry 9). Despite examining various silver salts and solvents (Table 1, entries 12 and 13), inferior yields were consistently obtained.12 Additionally, reducing the loading of [RuCl2(p-cymene)]2 or adjusting the reaction temperature resulted in diminished yields.12
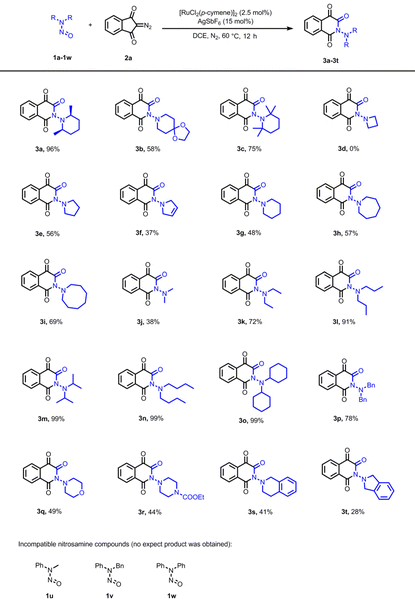
Having established the optimized reaction conditions, a comprehensive examination of various nitrosoamines was conducted, with summarized outcomes provided in Table 2. The reaction involving 8-nitroso-1,4-dioxa-8-azaspiro[4.5] decane (1b) gave product 3b in a 58% yield. Comparatively, 2,2,6,6-tetramethylpiperidine-derived substrate 1c exhibited a lower yield than 1a, suggesting that steric hindrance exerted an adverse influence on the reaction. Subsequent investigation into the impact of ring size revealed that the reaction of N-nitroso-azetidine 1d did not yield the anticipated product 3d; instead, a complex mixture was observed, likely due to the strain associated with the highly-tense four-membered ring. The reaction of N-nitrosopyrrolidine (1e) proceeded smoothly, affording product 3e in moderate yield. Conversely, a lower yield was obtained for the reaction of N-nitroso-2,5-dihydro-1H-pyrrole (1f), potentially attributed to side reactions stemming from the carbon–carbon double bond. Unexpectedly, unsubstituted N-nitroso-piperidine (1g) yielded product 3g in a considerably lower yield. However, an increase in ring size (1h–1i) appeared to have a favorable effect on the yield. A range of acyclic N,N-dialkylnitrosoamines were also explored. While the reaction of N-nitroso-dimethylamine 1j afforded the product 3j in a lower yield (38%) due to its inherent instability, a notably higher yield of 72% was achieved for N-nitroso-diethylamine 1k. Substitution with higher alkyl groups substantially enhanced the yields, with nearly quantitative yields achieved for N,N-diisopropyl, N,N-dibutyl, and N,N-dicyclohexyl nitrosoamines (1m–1o). N,N-Dibenzyl nitrosoamine 1p proved to be a suitable substrate, yielding product 3p in good yield. However, reactions involving morpholine, piperazine, and tetrahydroisoquinoline-derived nitrosoamines (1q–1s) yielded products in lower yields. Additionally, poor yield was observed for the isoindoline-derived nitrosoamine (1t). These results suggest that electron-rich nitrosoamines are more amenable to the reaction, with substrate 1c constituting an exception due to pronounced steric hindrance. Notably, no stereoisomerism was observed for products 3b–3s. It is noteworthy that N-aryl nitrosamines (1u–1w) did not exhibit reactivity with diazoindandione under the catalysis of [RuCl2(p-cymene)]2. No nitroso ylide was isolated and most of the substrates (N-aryl nitrosamines and diazoindandione) were recovered after the reaction. In contrast, Zhu's group reported a Cp*Rh(III)-catalyzed migratory insertion of carbenes into ortho aromatic C(sp2)–H bonds of N-aryl nitrosamines.7a
| a Reaction conditions: 1a–1w (0.3 mmol), 2a (0.2 mmol), [RuCl2(p-cymene)]2 (2.5 mol%) and AgSbF6 (15 mol%) in DCE (2 mL) at 60 °C under N2 for 12 h. Isolated yields. |
|---|
|
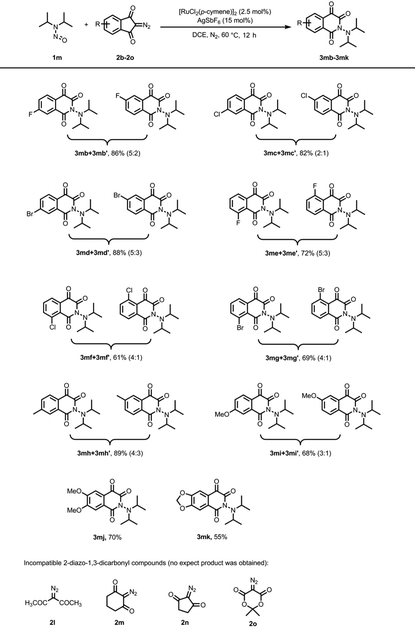
Due to the generation of atropisomeric products in the reaction of cis-2,6-dimethyl-1-nitrosopiperidine 1a, we subsequently explored the scope of diazoindandiones utilizing N,N-diisopropylnitrosoamine 1m as the model substrate. The outcomes are summarized in Table 3. Substitution on the phenyl ring with either electron-withdrawing or electron-donating groups was well-tolerated, affording the expected products in moderate to good yields. Notably, when meta-F, Cl, or Br-substituted diazoindandiones were employed, the reaction yielded two regioisomeric products (3mb/3mb′, 3mc/3mc′, 3md/3md′) in yields of 86%, 82%, and 88%, respectively. Similarly, the reaction of ortho-halogen-substituted diazoindandiones provided two regioisomeric products (3me/3me′, 3mf/3mf′, 3mg/3mg′) in good yields. Diazoindandiones bearing meta-CH3 and OCH3 groups underwent the transformation smoothly, yielding the regioisomeric products (3mh/3mh′, 3mi/3mi′) in 89% and 68% yield, respectively. Additionally, the reaction of 3,4-dimethoxy and methylenedioxy diazoindandiones afforded the target products 3mj and 3mk in yields of 70% and 55%. In parallel, we explored other 2-diazo-1,3-dicarbonyl compounds such as 3-diazopentane-2,4-dione (2l), 2-diazocyclohexane-1,3-dione (2m), 2-diazocyclopentane-1,3-dione (2n), and 5-diazo-2,2-dimethyl-1,3-dioxane-4,6-dione (2o), but these substrates displayed no reactivity under the current conditions. Most of diazo compounds (2l–2o) and N,N-diisopropylnitrosoamine 1m could be recovered after the reaction. This observation suggests that this rearrangement reaction is exclusive to diazoindandiones.
| a Reaction conditions: 1m (0.3 mmol), 2b–2o (0.2 mmol), [RuCl2(p-cymene)]2 (2.5 mol%) and AgSbF6 (15 mol%) in DCE (2 mL) at 60 °C under N2 for 12 h. Isolated yields. |
|---|
|
Furthermore, we studied the reaction of cis-2,6-dimethyl-1-nitrosopiperidine 1a and dimethyl diazomalonate 2p. [Cp*RhCl2]2/AgSbF6 and [RuCl2(p-cymene)]2/AgSbF6 were used as the catalyst respectively. Again, the migratory insertion of the carbene into aliphatic C(sp3)–H bond was not observed. Instead, a nitroso ylide 4a was obtained in a moderate yield. The structure was confirmed by NMR, HRMS, and X-ray diffraction analyses. While the generation of nitrogen ylides from the reaction of carbenes and amines is well-documented,13 only a light-promoted reaction of aryldiazoacetates with nitrosoarenes was developed.4 The electron-deficient nature of the nitroso group was expected to hinder its reactivity with electrophilic carbenes. We conducted an optimization of reaction conditions,14 and identified [CpRhCl2]2/AgSbF6 as the most effective catalyst, while [RuCl2(p-cymene)]2/AgSbF6 yielded lower yields in this transformation. Notably, Cu(OTf)2 and Rh2(OAc)4, commonly employed catalysts for generating nitrogen ylides from diazo compounds and amines, proved to be inefficient for this particular transformation. We also evaluated several N,N-dialkylnitrosoamines, with summarized results in Table 4. A moderate yield was obtained in the reaction involving 2-methyl-1-nitrosopiperidine. However, complex mixtures were observed in the reactions of N,N-diethylnitrosoamine and N,N-dipropylnitrosoamine, resulting in only low yields of ylide products. Additionally, we explored the reaction of 1a with ethyl diazoacetate (EDA). No expected ylide product was observed, instead, a nitrone product was obtained in a low yield.15 The absence of one ester group might shift the product towards a more stable nitrone. Lastly, the reaction with methyl phenyldiazoacetate yielded no ylide or nitrone products, only dimerized products from methyl phenyldiazoacetate were observed.15
| a Reaction conditions: 1 (0.2 mmol), 2p (0.4 mmol), [Cp*RhCl2]2 (2.5 mol%) and AgSbF6 (15 mol%) in DCE (2 mL) at 60 °C under N2 for 12 h. Isolated yields. |
|---|
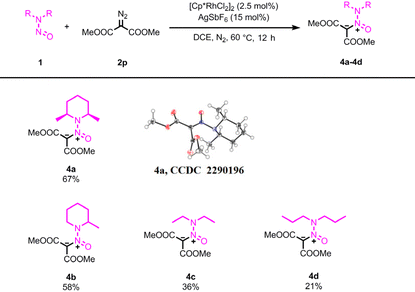
|
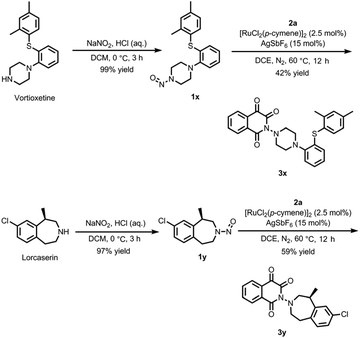
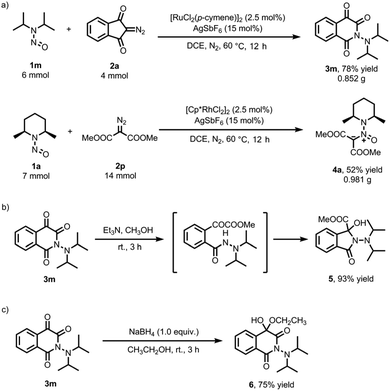
To explore the potentials of the reaction in the structural modifications of drugs, antidepressant Vortioxetine and weight loss drug Lorcaserin were selected as the examples (Scheme 3). The nitrosation and subsequent reaction with diazoindandione 2a provided products 3x and 3y, respectively. The results confirmed the availability of the method in the modification of drugs containing secondary amino groups.
To showcase the practicality of this approach, we conducted two gram-scale reactions (Scheme 4a). This resulted in a 78% yield for product 3m and a 52% yield for product 4a. Additionally, we delved into various derivatizations of product 3m. Treating it with methanol and triethylamine afforded product 5 in an excellent yield, likely through a keto-ester intermediate (Scheme 4b).16 Reduction of 3m with NaBH4 in ethanol provided hemiacetal 6 in a good yield (Scheme 4c).17
To investigate the impact of both electronic and steric properties of the substrate on the rearrangement reaction, two competitive experiments were conducted as outlined in Scheme 5. It was observed that almost equivalent of 3g and 3q was generated according to 1H NMR analysis (Scheme 5a). Furthermore, the reaction rate of substrate 1k (N,N-diethyl-substituted) is nearly same with that of substrate 1m (N,N-diisopropyl-substituted) (Scheme 5b). This outcome indicates that electronic and steric effect have a slight influence on the reaction rate.
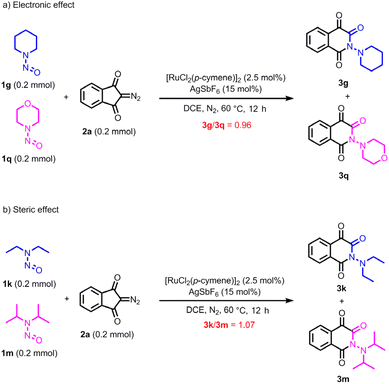 | ||
| Scheme 5 Influence of electronic and steric properties of the substrate on the rearrangement reaction. | ||
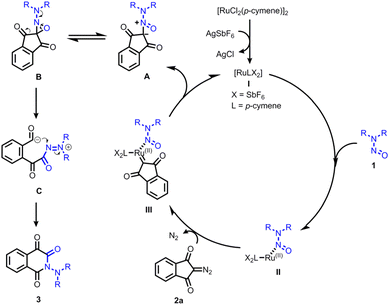
Based on the current findings and prior research,18 we propose a plausible reaction mechanism outlined in Scheme 6. Initially, the formation of the active catalyst I occurs through the reaction of [RuCl2(p-cymene)]2 with AgSbF6. Coordination of I with N,N-dialkylnitrosoamines leads to the generation of intermediate II. Subsequently, the decomposition of diazoindandione results in the formation of the ruthenium carbene species III. The subsequent attack from the nitrosoamine to give the nitroso ylide A and to regenerate the active Ru(II) catalyst I. Nitroso ylide A undergoes tautomeric conversion to oxaziridine B. The rearrangement, involving the cleavage of N–O and C–C bonds, yields the acylazo cationic intermediate C. The electron-donating capability of N,N-dialkyl moieties plays a pivotal role in stabilizing C. An intramolecular addition event then furnishes the final product 3. Notably, in the case of dimethyl diazomalonate, the nitroso ylide remains stable and can be isolated.
Conclusions
In summary, we have discovered an unexpected rearrangement reaction involving diazoindandiones and N,N-dialkylnitrosoamines, employing [RuCl2(p-cymene)]2/AgSbF6 as an efficient catalyst. This methodology exhibits notable tolerance towards both cyclic and acyclic nitrosoamines. The synthesis of isoquinoline-1,3,4-triones was achieved in good to excellent yields under mild reaction conditions. Additionally, the reaction of N,N-dialkylnitrosoamines with diazomalonates led to the formation of nitroso ylides. We have proposed a tentative reaction mechanism. These findings hold significant promise for further investigations into novel reactions involving nitrosoamines and carbenes.Author contributions
Yuan Chen performed the experiments, interpreted the data, and prepared the manuscript. Rui-jun Peng gave useful helps in the experiments. Xue-jing Zhang contributed to the confirmation of data and the ESI. Ming Yan came up with the idea for this research, created the experiments, and reviewed the manuscript and ESI thoroughly.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (82061128001), the Natural Science Foundation of Guangdong (2021A1515010503, 2021A1515010186, 2022A1515010744) for the financial support.References
- (a) C. Y. Shi, B. Zhou, M. Y. Teng and L. W. Ye, Recent Advances in Asymmetric [1,2]-Stevens-Type Rearrangement via Metal Carbenes, Synthesis, 2023, 55, 2118–2127 CrossRef CAS; (b) Z. Schwartz, C. Valiton, M. Lovasz and A. G. Roberts, Recent Applications of Ammonium Ylide Based [2,3]-Sigmatropic and [1,2]-Stevens Rearrangements To Transform Amines into Natural Products, Synthesis, 2023 DOI:10.1055/s-0042-1751446; (c) S. Jana, Y. J. Guo and R. M. Koenigs, Recent Perspectives on Rearrangement Reactions of Ylides via Carbene Transfer Reactions, Chem. – Eur. J., 2021, 27, 1270–1281 CrossRef CAS; (d) Z. Sheng, Z. K. Zhang, C. H. Chu, Y. Zhang and J. B. Wang, Transition metal-catalyzed [2,3]-sigmatropic rearrangements of ylides: An update of the most recent advances, Tetrahedron, 2017, 73, 4011–4022 CrossRef CAS.
- H. J. Dequina and J. M. Schomaker, Aziridinium Ylides: Underused Intermediates for Complex Amine Synthesis, Trends Chem., 2020, 2, 874–887 CrossRef CAS PubMed.
- (a) S. L. Dong, X. Fu and X. F. Xu, [3 + 2]-Cycloaddition of Catalytically Generated Pyridinium Ylide: A General Access to Indolizine Derivatives, Asian J. Org. Chem., 2020, 9, 1133–1143 CrossRef CAS; (b) L. D. Funt, M. S. Novikov and A. F. Khlebnikov, New applications of pyridinium ylides toward heterocyclic synthesis, Tetrahedron, 2020, 76, 131415 CrossRef CAS.
- (a) B. G. Cai, S. S. Luo, L. Li, L. Li, J. Xuan and W. J. Xiao, Visible Light-Promoted Amide Bond Formation via One-Pot Nitrone in Situ Formation/Rearrangement Cascade, CCS Chem., 2020, 2, 2764–2771 Search PubMed; (b) B. G. Cai, L. Li, G. Y. Xu, W. J. Xiao and J. Xuan, Visible-light-promoted nitrone synthesis from nitrosoarenes under catalyst- and additive-free conditions, Photochem. Photobiol. Sci., 2021, 20, 823–829 CrossRef CAS.
- (a) Y. Xia, D. Qiu and J. B. Wang, Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion, Chem. Rev., 2017, 117, 13810–13889 CrossRef CAS; (b) Y. Y. Xiang, C. Wang, Q. P. Ding and Y. Y. Peng, Diazo Compounds: Versatile Synthons for the Synthesis of Nitrogen Heterocycles via Transition Metal-Catalyzed Cascade C-H Activation/CarbeneInsertion/Annulation Reactions, Adv. Synth. Catal., 2019, 361, 919–944 CrossRef CAS; (c) S. Kumar, S. Nunewar, S. Oluguttula, S. Nandur and V. Kanchupalli, Recent advances in Rh(III)/Ir(III)-catalyzed C-H functionalization/annulation via carbene migratory insertion, Org. Biomol. Chem., 2021, 19, 1438–1458 RSC; (d) S. Nunewar, S. Kumar, S. Talakola, S. Nanduri and V. Kanchupalli, Co(III), Rh(III) & Ir(III)-Catalyzed Direct C-H Alkylation/Alkenylation/Arylation with Carbene Precursors, Chem. – Asian J., 2021, 16, 443–459 CrossRef CAS PubMed; (e) N. Jha, N. P. Khot and M. Kapur, Transition-Metal-Catalyzed C-H Bond Functionalization of Arenes/Heteroarenes via Tandem C-H Activation and Subsequent Carbene Migratory Insertion Strategy, Chem. Rec., 2021, 21, 4088–4122 CrossRef CAS PubMed.
- (a) J. P. Huang, Z. Y. Chen, J. J. Yuan and Y. Y. Peng, Recent Advances in Highly Selective Applications of Nitroso Compounds, Asian J. Org. Chem., 2016, 5, 951–960 CrossRef CAS; (b) G. Rani, V. Luxami and K. Paul, Traceless directing groups: a novel strategy in regiodivergent C-H functionalization, Chem. Commun., 2020, 56, 12479–12521 RSC; (c) S. R. De, N-Nitroso, As A Novel Directing Group in Transition-Metal-Catalyzed C(sp2)-H Bond Functionalizations of N-Nitrosoanilines, Asian J. Org. Chem., 2021, 10, 980–1011 CrossRef CAS; (d) P. Chaudhary, J. Kandasamy, A. P. G. Macabeo, R. J. I. Tamargo and Y. R. Lee, Recent Advances and Strategies for the Transition-Metal-Catalyzed C-H Functionalization of N-Nitrosoanilines, Adv. Synth. Catal., 2021, 363, 2037–2060 CrossRef CAS.
- (a) J. Wang, M. Y. Wang, K. H. Chen, S. K. Zha, C. Song and J. Zhu, C-H Activation-Based Traceless Synthesis via Electrophilic Removal of a Directing Group. Rhodium(III)-Catalyzed Entry into Indoles from N-Nitroso and α-Diazo-β-keto Compounds, Org. Lett., 2016, 18, 1178–1181 CrossRef CAS PubMed; (b) X. W. Hu, X. Chen, Y. X. Shao, H. S. Xie, Y. F. Deng, Z. F. Ke, H. F. Jiang and W. Zeng, Co(III)-Catalyzed Coupling-Cyclization of Aryl C-H Bonds with α-Diazoketones Involving Wolff Rearrangement, ACS Catal., 2018, 8, 1308–1312 CrossRef CAS; (c) K. L. Wang, X. Song, Y. D. Xin, X. Y. Zhang and X. S. Fan, Condition-Controlled Selective Synthesis of Pyranone-Tethered Indazoles or Carbazoles through the Cascade Reactions of N-Nitrosoanilines with Iodonium Ylides, Org. Lett., 2023, 25, 4422–4428 CrossRef CAS PubMed.
- (a) R. J. Peng, L. Chen, X. J. Zhang and M. Yan, Rh(III)-Catalyzed C-H Functionalization of N-Nitrosoanilines with α-Sulfonylcarbenes, Adv. Synth. Catal., 2022, 364, 1–7 CrossRef; (b) R. J. Peng, Y. Chen, X. J. Zhang and M. Yan, Regioselective ortho C-H Insertion of N-Nitrosoanilines with Naphthoquinone Carbenes, Org. Biomol. Chem., 2023, 21, 7525–7529 RSC.
- (a) A. G. Bonet, F. J. Hernández, B. de Luis and R. Martin, Pd-Catalyzed C(sp3)-H Functionalization/Carbenoid Insertion: All-Carbon Quaternary Centers via Multiple C-C Bond Formation, J. Am. Chem. Soc., 2016, 138, 6384–6387 CrossRef; (b) H. S. Xie, Z. R. Ye, Z. F. Ke, J. Y. Lan, H. F. Jiang and W. Zeng, Rh(III)-catalyzed regioselective intermolecular N-methylene Csp3-H bond carbenoid insertion, Chem. Sci., 2018, 9, 985–989 RSC; (c) B. Ghosh and R. Samanta, Rh(III)-Catalyzed straightforward arylation of 8-methyl/formylquinolines using diazo compounds, Chem. Commun., 2019, 55, 6886–6889 RSC; (d) B. Audic and N. Cramer, Rhodium(III)-Catalyzed Cyclopropane C-H/C-C Activation Sequence Provides Diastereoselective Access to α-Alkoxylated γ-Lactams, Org. Lett., 2020, 22, 5030–5034 CrossRef CAS; (e) P. Zhang, J. Zeng, P. Pan, X. J. Zhang and M. Yan, Palladium-Catalyzed Migratory Insertion of Carbenes and C-C Cleavage of Cycloalkanecarboxamides, Org. Lett., 2022, 24, 536–541 CrossRef CAS.
- (a) Y. H. Zhang, H. J. Zhang, F. Wu, Y. H. Chen, X. Q. Ma, J. Q. Du, Z. L. Zhou, J. Y. Li, F. J. Nan and J. Li, Isoquinoline-1,3,4-trione and its derivatives attenuate β-amyloid-induced apoptosis of neuronal cells, FEBS J., 2006, 273, 4842–4852 CrossRef CAS; (b) Y. H. Chen, Y. H. Zhang, H. J. Zhang, D. Z. Liu, M. Gu, J. Y. Li, F. Wu, X. Z. Zhu, J. Li and F. J. Nan, Design, Synthesis, and Biological Evaluation of Isoquinoline-1,3,4-trione Derivatives as Potent Caspase-3 Inhibitors, J. Med. Chem., 2006, 49, 1613–1623 CrossRef CAS PubMed; (c) X. Q. Ma, H. J. Zhang, Y. H. Zhang, Y. H. Chen, F. Wu, J. Q. Du, H. P. Yu, Z. L. Zhou, J. Y. Li, F. J. Nan and J. Li, Novel irreversible caspase-1 inhibitor attenuates the maturation of intracellular interleukin-1β, Biochem. Cell Biol., 2007, 85, 56–65 CrossRef CAS; (d) S. P. Singh and D. Gupta, Discovery of potential inhibitor against human acetylcholinesterase: a molecular docking and molecular dynamics investigation, Comput. Biol. Chem., 2017, 68, 224–230 CrossRef CAS PubMed.
- (a) J. S. Yadav, B. V. S. Reddy, U. V. S. Reddy and K. Praneeth, Azido-Schmidt reaction for the formation of amides, imides and lactams from ketones in the presence of FeCl3, Tetrahedron Lett., 2008, 49, 4742–4745 CrossRef CAS; (b) A. D. Mola, C. Tedesco and A. Massa, Metal-Free Air Oxidation in a Convenient Cascade Approach for the Access to Isoquinoline-1,3,4(2H)-triones, Molecules, 2019, 24, 2177 CrossRef PubMed; (c) M. B. Reddy, K. Prasanth and R. Anandhan, Controlled Photochemical Synthesis of Substituted Isoquinoline-1,3,4(2H)-triones, 3-Hydroxyisoindolin-1-ones, and Phthalimides via Amidyl Radical Cyclization Cascade, Org. Lett., 2022, 24, 3674–3679 CrossRef CAS PubMed; (d) X. L. Lin, Y. Yu, L. Zhang, L. J. Leng, D. R. Xiao, T. Cai and Q. L. Luo, Switchable synthesis of 1,4-bridged dihydro isoquinoline-3-ones and isoquinoline-1,3,4-triones through radical oxidation of isoquinolinium salts with phenyliodine(III) diacetate, Org. Chem. Front., 2022, 9, 4676–4684 RSC.
- See Table S1 in ESI† for the details.
- (a) D. Zhang and W. H. Hu, Asymmetric Multicomponent Reactions Based on Trapping of Active Intermediates, Chem. Rec., 2017, 17, 739–753 CrossRef CAS PubMed; (b) Y. Y. Ren, S. F. Zhu and Q. L. Zhou, Chiral proton-transfer shuttle catalysts for carbene insertion reactions, Org. Biomol. Chem., 2018, 16, 3087–3094 RSC.
- See Table S2 in ESI† for the details.
- See Scheme S16 in ESI† for the details.
- P. B. Wakchaure, S. Easwar, V. G. Puranik and N. P. Argade, Facile air-oxidation of N-homopiperonyl-5,6-dimethoxy homophthalimide: simple and effificient access to nuevamine, Tetrahedron, 2008, 64, 1786–1791 CrossRef CAS.
- S. Maniam, S. Sandanayake, E. I. Izgorodina and S. J. Langford, Unusual Products from Oxidation of Naphthalene Diimides, Asian J. Org. Chem., 2016, 5, 490–493 CrossRef CAS.
- (a) J. Li, H. Li, D. Q. Fang, L. J. Liu, X. Han, J. N. Sun, C. P. Li, Y. Zhou, D. J. Ye and H. Liu, Sulfoximines Assisted Rh(III)-Catalyzed C-H Activation/Annulation Cascade to Synthesize Highly Fused Indeno-1,2-benzothiazines, J. Org. Chem., 2021, 86, 15217–15227 CrossRef CAS PubMed; (b) S. Y. Chen, Y. C. Zheng, X. G. Liu, J. L. Song, L. Xiao and S. S. Zhang, Synthesis of Indole-Substituted Trifluoromethyl Sulfonium Ylides by Cp*Rh(III)-Catalyzed Diazo-carbenoid Addition to Trifluoromethylthioether, J. Org. Chem., 2023, 88, 5512–5519 CrossRef CAS; (c) K. L. Wang, X. Song, Y. D. Xin, X. Y. Zhang and X. S. Fan, Condition-Controlled Selective Synthesis of Pyranone-Tethered Indazoles or Carbazoles through the Cascade Reactions of N-Nitrosoanilines with Iodonium Ylides, Org. Lett., 2023, 25, 4422–4428 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2289572 and 2290196. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01607b |
| This journal is © the Partner Organisations 2024 |