Open Access Article
Open Access ArticleElectrochemically triggered rational design of bismuth copper sulfide for wearable all-sulfide semi-solid-state supercapacitor with a wide operational potential window (1.8 V) and ultra-long life†
Edugulla
Girija Shankar
,
Bhimanaboina
Ramulu
 ,
Manchi
Nagaraju
and
Jae Su
Yu
,
Manchi
Nagaraju
and
Jae Su
Yu
 *
*
Department of Electronics and Information Convergence Engineering, Institute for Wearable Convergence Electronics, Kyung Hee University, 1732 Deogyeong-daero, Giheung-gu, Yongin-si, Gyeonggi-do 17104, Republic of Korea. E-mail: jsyu@khu.ac.kr
First published on 30th April 2024
Abstract
There has been a growing need for supercapacitors (SCs) as a promising energy storage device with high energy and power densities. For this reason, electrodes with a wide operational potential window (OPW) and high capacity are desirable for energy storage. Previously, carbon-based electrodes were mostly used as a negative electrode in SCs, but their low capacity and narrow OPW hindered the performance of the SCs. Hence, a novel binder-free faradaic type bismuth copper sulfide (BCS) electrode has been synthesized in 600 s at room temperature via a simple and swift electrodeposition (ED) method. The effect of the Bi and Cu molar concentrations in the growth solution (BixCu3−xS3) on its crystal structure and surface morphology is investigated during the ED process to further understand the synergistic benefits of Bi and Cu ions for enhanced electrochemical performance. The optimized BCS electrode exhibits excellent electrochemical properties with a wide OPW of 1.2 V and a specific capacity of 290 mA h g−1. Additionally, nickel sulfide (NS) as a faradaic type positive electrode is synthesized via the ED. The BCS and NS electrodes are employed as negative and positive electrodes, respectively, to fabricate a novel gel electrolyte-based wearable all-sulfide semi-solid-state SC (ASSSC). The ASSSC device maintains ∼100% capacitance retention across the 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles. Furthermore, two serially connected ASSSC devices are attached to the human fingers to power the electronic gadgets using various finger movements.
000 charge–discharge cycles. Furthermore, two serially connected ASSSC devices are attached to the human fingers to power the electronic gadgets using various finger movements.
1. Introduction
Demand for economical, highly efficient, flexible, lightweight, and wearable energy storage devices is increasing in day-to-day life. Supercapacitors (SCs) have attracted much attention due to their advantages such as rapid charge–discharge rates, low cost, eco-friendliness feature, safe operation, and long life.1 For modern wearable electronics, carbon cloth (CC) is being extensively used as a current collector for flexible SCs because of its high electrical conductivity, flexibility, and mechanical toughness.2 However, despite their merits, the SCs suffer from incompetent energy density when compared to batteries, limiting their real-life application as an efficient energy storage device.3 The energy density (E) can be calculated using E = 0.5 × CV2, where C and V represent the capacitance and operational potential window (OPW), respectively. Considering the following, the SCs comprised of electrodes with a wide OPW and high capacitance can enhance the energy density. This evolved into the concept of asymmetric SCs (ASCs) consisting of different electrodes with high electroactive sites, wide OPW, and fast ion-electron transfer kinetics. So far, various metal oxide and chalcogen-based positive electrode materials for SCs have been reported with satisfactory results.4 Graphene-based materials as well as typical carbonaceous materials such as activated carbon and biomass-derived carbon have been reported.5 However, a limited specific capacitance (Cs) and OPW fails to enhance the energy density of the ASC, impeding its commercialization. Therefore, a negative electrode with a wide OPW and high Cs is extremely desirable to assemble an ASC with enhanced energy density.In this respect, electrode materials with a multielectron transfer can offer a promising way to enhance the capacitance in an aqueous alkaline medium.6,7 Bismuth (Bi)-based electrode materials show multiple reversible redox reactions in an aqueous alkaline medium, unveiling a distinct “quasi-conversion reaction” mechanism (Bi2O3 ⇔ Bi0).8 According to previous studies, Bi2O3 exhibits a very high specific capacity (Cts) of 345.11 mA h g−1. Recently, copper-based materials have been employed as a negative electrode due to their unique property and high theoretical capacity in an alkaline medium with a wide OPW below 0 V (vs. H/H+).9,10 Furthermore, the addition of sulfur (S) to the crystal structure of a bimetallic structure is anticipated to boost the electrochemical properties due to the lower electronegativity and higher electrical conductivity (S = 5 × 10−28 S m−1) of S when compared to O.11,12 Inspired by the following, in this work, novel bismuth copper sulfide (BCS) was synthesized and employed as a negative electrode in the SC device.
Recently, nickel sulfide (NS) electrode materials have been intensively studied for use as a positive electrode in an SC device. The Heazlewoodite structure of NS consists of four Ni–Ni bonds, and the following bonds are shorter compared to the Ni–Ni bond in the Ni metal, further leading to the demonstration of enhanced electrochemical properties and electrical conductivity when compared to the other Ni-based compounds.13 The NS has been used as a positive electrode in the SC device. Generally, metal chalcogenide (Bi2S3, Ni2S3, CoS2, and so on) electrode materials have been synthesized using complicated hydrothermal and solvothermal methods.14 However, the following synthesis method involves long synthesis time, high temperature, low mass loading, and no growth control. In this respect, the electrodeposition (ED) method has emerged as a promising and simple binder-free synthesis technique to synthesize metal chalcogenides under room temperature conditions. The advantages of the ED method, such as single-step synthesis, growth control, short duration, and room temperature synthesis, make it a potential technique for bulk synthesis.15 In addition, binder-free methodology further enhances the electron transport and electroactive sites.
Encouraged by the following, the present work demonstrated the synthesis and fabrication of the novel BCS/CC and NS/CC as negative and positive electrodes, respectively via a simple ED method to produce wearable all-sulfide semi-solid-state SC (ASSSC). The effect of the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar concentration ratio in the BixCu3−xS3 growth solution on the crystal structure, the surface morphology, and electrochemical properties was investigated. An interconnected three-dimensional (3D) network of nanosheet arrays was obtained for Bi1Cu2S3 (x = 1), further benefiting the electrolyte interaction with the electroactive site. Moreover, uniformly distributed arrays of nanoparticle morphology were obtained for NS, and their electrochemical properties were studied. Furthermore, a novel ASSSC device was fabricated by employing optimized BCS and NS electrodes as negative and positive electrodes, respectively, using a poly(vinyl alcohol) (PVA)–KOH gel-based semi-solid-state electrolyte. The structural and mechanical well-integrated free-standing electrode materials on CC (the ASSSC device) demonstrated an ultra-long life with an excellent cycling stability performance. Also, a wearable ASSSC device attached to human fingers was employed as a power source to power a digital display and an electric motor under various finger movements, demonstrating a real-life practical application. The following results suggest the potential application of the ASSSC device for next-generation advanced energy storage applications.
Cu molar concentration ratio in the BixCu3−xS3 growth solution on the crystal structure, the surface morphology, and electrochemical properties was investigated. An interconnected three-dimensional (3D) network of nanosheet arrays was obtained for Bi1Cu2S3 (x = 1), further benefiting the electrolyte interaction with the electroactive site. Moreover, uniformly distributed arrays of nanoparticle morphology were obtained for NS, and their electrochemical properties were studied. Furthermore, a novel ASSSC device was fabricated by employing optimized BCS and NS electrodes as negative and positive electrodes, respectively, using a poly(vinyl alcohol) (PVA)–KOH gel-based semi-solid-state electrolyte. The structural and mechanical well-integrated free-standing electrode materials on CC (the ASSSC device) demonstrated an ultra-long life with an excellent cycling stability performance. Also, a wearable ASSSC device attached to human fingers was employed as a power source to power a digital display and an electric motor under various finger movements, demonstrating a real-life practical application. The following results suggest the potential application of the ASSSC device for next-generation advanced energy storage applications.
2. Experimental section
2.1. Preparation of a BixCu3−xS3/CC negative electrode and a NS positive electrode
Initially, an efficient amount of the CC substrate (2 × 1 pieces) was functionalized with oxygen by treating it with concentrated HNO3 at 70 °C for 6 h and it was further cleaned with deionized water (DIW) and ethanol to remove impurities, followed by drying at 70 °C for 6 h. The following enhances the hydrophilicity of the CC substrate, which anchors the uniform growth of the electrode material by enhancing nucleation sites and the surface activity of the CC substrate, which results in an improved electrode–electrolyte interaction by facilitating rapid absorption and desorption, helping to boost the electrochemical properties. The CC exposing the 1 × 1 cm2 area was attached to the poly(ethylene terephthalate) (PET) sheet to prevent the backside coating. To synthesize the BiCu2S3 on the CC, bismuth(III) nitrate pentahydrate (1 mmol), copper nitrate trihydrate (2 mmol), sodium sulfate anhydrous (3 mmol), and lithium chloride (8 mmol) were dissolved in 40 ml of DIW and the pH of the growth solution was adjusted to ∼3 using concentrated hydrochloric acid. In addition, the growth solution was bubbled with nitrogen gas to remove the dissolved oxygen. A conventional three-electrode setup was used for the ED, where CC, Ag/AgCl, and platinum (Pt) wire served as the working, reference, and counter electrodes, respectively. The BiCu2S3 nanosheet arrays on the CC were grown at a constant voltage of −1 V for 600 s using a chronoamperometry method. The fabricated electrode was flushed with DIW and ethanol to remove the unreacted elements and dried at 70 °C for 6 h. The following electrode was denoted as BCS-x (x = 1, where ‘x’ denotes the mmol concentration of Bi in the growth solution). To further analyze the effect of the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar concentration ratio in the growth solution on the crystal structure and electrochemical properties, additional two electrodes were synthesized by varying the ‘x’ value to 0.5 and 2 under the same conditions. The electrodes obtained were denoted as BCS-0.5 and BCS-2. An almost similar electrode material mass was acquired by all three electrodes (∼3 mg cm−2). Furthermore, a similar ED technique was employed to prepare the NS electrode as a positive electrode by changing the growth solution. For the growth solution, 2 mmol nickel sulfate hexahydrate, 3 mmol sodium sulfate anhydrous, and 8 mmol lithium chloride were dissolved in 40 ml of DIW and the ED parameters and conditions were kept similar to those of the negative electrode. The obtained electrode was denoted as NS.
Cu molar concentration ratio in the growth solution on the crystal structure and electrochemical properties, additional two electrodes were synthesized by varying the ‘x’ value to 0.5 and 2 under the same conditions. The electrodes obtained were denoted as BCS-0.5 and BCS-2. An almost similar electrode material mass was acquired by all three electrodes (∼3 mg cm−2). Furthermore, a similar ED technique was employed to prepare the NS electrode as a positive electrode by changing the growth solution. For the growth solution, 2 mmol nickel sulfate hexahydrate, 3 mmol sodium sulfate anhydrous, and 8 mmol lithium chloride were dissolved in 40 ml of DIW and the ED parameters and conditions were kept similar to those of the negative electrode. The obtained electrode was denoted as NS.
2.2. Electrochemical properties
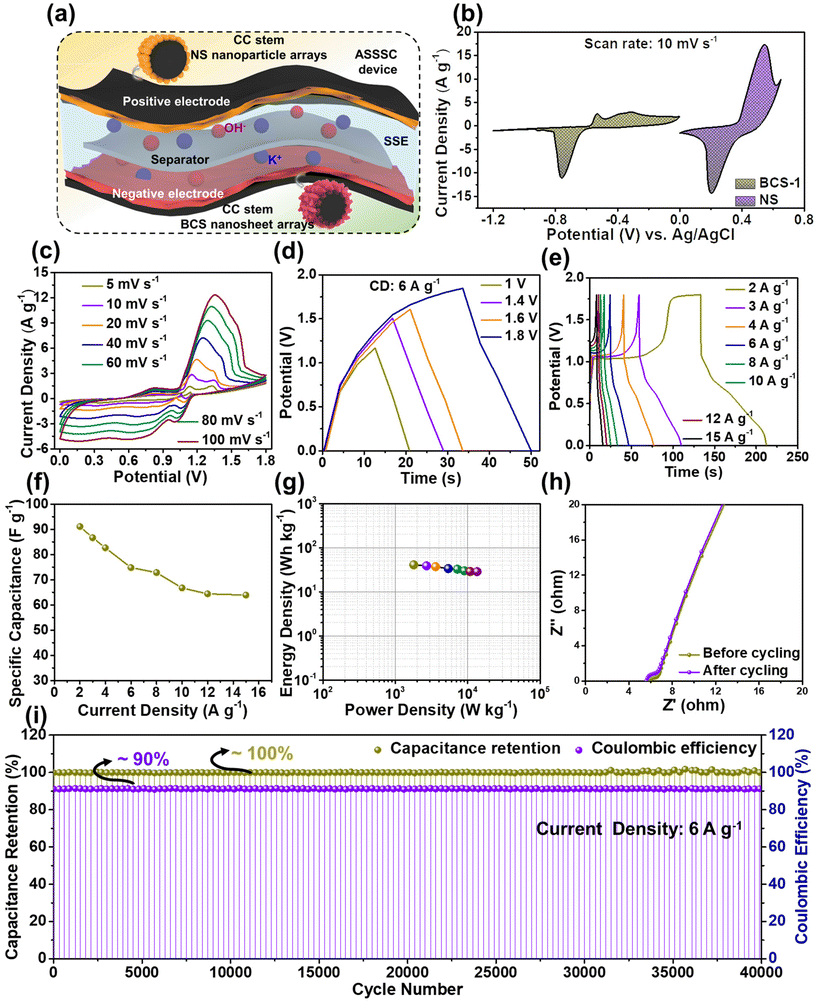
Cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) (frequency range from 100 kHz to 0.01 Hz) measurements were performed individually for all the as-prepared electrodes to study the electrochemical properties in a three-electrode system. Here, Pt wire, Ag/AgCl, and the as-prepared electrodes served as counter, reference, and working electrodes, respectively. Fig. 1 shows a schematic illustration for the synthesis of the BCS-1 and NS electrodes.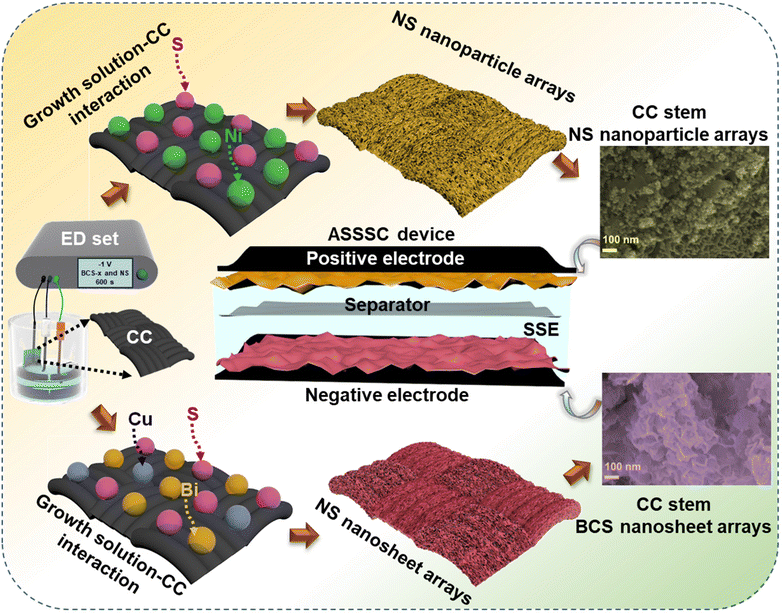 | ||
| Fig. 1 A schematic representation displaying the simple synthesis of BCS-x and NS on a flexible CC current collector and assembly of the ASSSC device. | ||
2.3. Assembly of wearable ASSSC device
Prior to assembly, the PVA-KOH gel-based semi-solid-state electrolyte was prepared as reported in a previous report.16 A wearable ASSSC device based on two electrodes was assembled as shown in Fig. 1, with the BCS-1 and NS as the negative and positive electrodes, respectively. Whatman 42 filter paper (used as the separator) was soaked in a semi-solid-state electrolyte and then sandwiched between the negative and positive electrodes. After the solid-state electrolyte was semi-solidified, parafilm tape was employed to seal the assembly. The shape adaptability of the semi-solid-state electrolyte fits well with the wearable ASSSC device, thus avoiding the leakage problem of using liquid electrolytes and dissolution/pealing out during electrochemical analysis. To maximize the electrochemical performance of the ASSSC device, a well-known charge balance equation was employed to obtain the optimized mass ratios of the negative and positive electrodes:17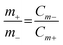 | (1) |
3. Results and discussion
The present work was designed to fabricate an entirely electrochemically triggered all-sulfide SC as a facile and low-cost advanced wearable energy storage device. The overall Cs of the ASSSC device is wholly dependent on the individual Cs of the negative and positive electrodes, according to the following relationship:18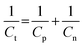 | (2) |
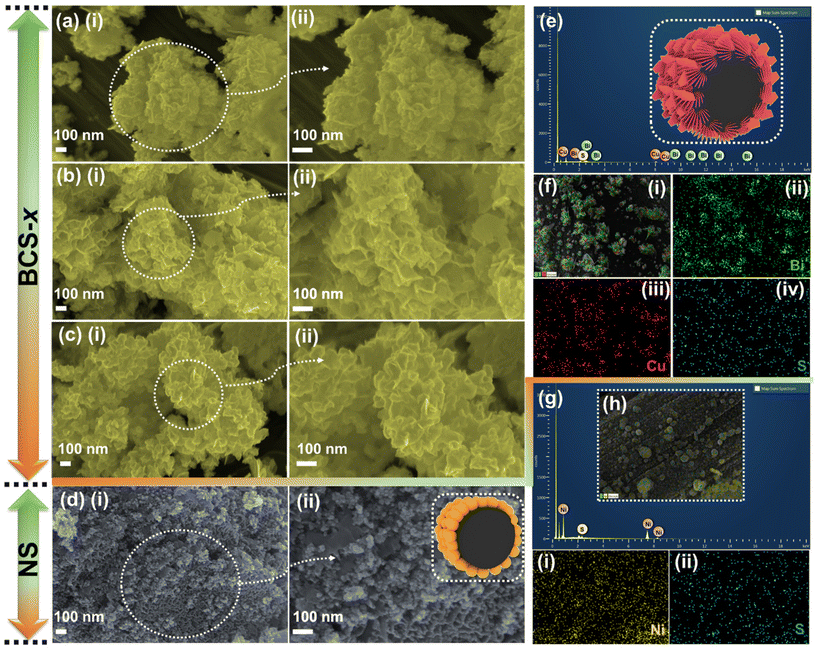
To study the surface morphology of the as-prepared BCS-x and NS electrodes, field-emission scanning electron microscope (FE-SEM) analysis was employed as shown in Fig. 2. The effect on the surface morphology with different molar concentration ratios of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu in growth solution was studied using the FE-SEM images. The surface morphology of BCS-0.5 is shown in Fig. 2(a) (i and ii). The FE-SEM images revealed the mixed morphology of nanosheets infused with coagulated nanoparticle arrays. The FE-SEM images of the BCS-1 showed the uniform growth of the densely packed vertically aligned nanosheet arrays on CC, as shown in Fig. 2(b) (i and ii). This result suggests that the woven CC fiber acted as an excellent scaffold for uniform growth of the nanosheet arrays with strong adhesion of electrode material and CC, making it a robust electrode, which may further support the long stability of the electrochemical cycles. The high magnification image showed that the average thickness of the nanosheets is ∼35 nm and they are interconnected with each other, creating a 3D porous structure. This may benefit the accessibility of the electroactive sites for the electrolyte during the electrochemical process. The FE-SEM images (Fig. 2(c) (i and ii)) of BCS-2 revealed an increase in the thickness and non-uniformity of the sheets upon increasing the Bi molar concentration in the growth solution. The FE-SEM images verified that the BiCu2S3 was the optimized growth solution to obtain the uniform and strongly adhered nanosheet arrays with CC fiber. Next, the uniformly distributed nanoparticles with an uneven size are adhesively supported on CC fiber as shown by the FE-SEM images of NS in Fig. 2(d) (i and ii). The interconnected nanoparticles further act as a bridge for electronic transmission, which significantly benefits the charge transfer process. Furthermore, the strongly adhered integrated EM-CC fiber can provide enhanced conductivity and short reaction path during the redox reaction of the EC process. Additionally, energy-dispersive X-ray spectroscopy (EDS) was performed to determine the elemental presence and distribution in BCS-1 and NS. Fig. 2(e and g) show the presence of Bi, Cu and S in the BCS-1 electrode and Ni and S in the NS electrode. Furthermore, elemental analysis suggests the uniform distribution of the detected elements in both BCS-1 and NS electrodes, as shown in Fig. 2(f) (i–iv) and (h) (i, ii). In addition, transmission electron microscope (TEM) and high-resolution (HR)-TEM were employed to study the internal structure of the BCS-x samples, as shown in Fig. S1 of the ESI.† The obtained results were in good agreement with those of the FE-SEM results. From Fig. S1(a and b) of the ESI,† the BCS-0.5 sample confirmed the presence of both clusters of nanoparticles and nanosheets in an infused form. For the BCS-1 sample, the presence of interconnected nanosheets with decent transparency suggests a thin 2D morphology, as shown in Fig. S1(d and e) of the ESI.† From Fig. S1(g and h) of the ESI,† a decrease in the sheet length with a low transparency was observed in the BCS-2 sample, suggesting an increase in the sheet thickness. Moreover, the HR-TEM analysis was performed for all the BCS-x samples together with the selected area electron diffraction (SAED) pattern. From the HR-TEM results (Fig. S1(c, f and i) of the ESI†), all the BCS-x samples demonstrated that the lattice fringes were aligned in various orientations with the SAED pattern containing the bright rings, which indicated the polycrystalline nature. The insets of the HR-TEM images showed the corresponding SAED pattern of each BCS-x sample.
Cu in growth solution was studied using the FE-SEM images. The surface morphology of BCS-0.5 is shown in Fig. 2(a) (i and ii). The FE-SEM images revealed the mixed morphology of nanosheets infused with coagulated nanoparticle arrays. The FE-SEM images of the BCS-1 showed the uniform growth of the densely packed vertically aligned nanosheet arrays on CC, as shown in Fig. 2(b) (i and ii). This result suggests that the woven CC fiber acted as an excellent scaffold for uniform growth of the nanosheet arrays with strong adhesion of electrode material and CC, making it a robust electrode, which may further support the long stability of the electrochemical cycles. The high magnification image showed that the average thickness of the nanosheets is ∼35 nm and they are interconnected with each other, creating a 3D porous structure. This may benefit the accessibility of the electroactive sites for the electrolyte during the electrochemical process. The FE-SEM images (Fig. 2(c) (i and ii)) of BCS-2 revealed an increase in the thickness and non-uniformity of the sheets upon increasing the Bi molar concentration in the growth solution. The FE-SEM images verified that the BiCu2S3 was the optimized growth solution to obtain the uniform and strongly adhered nanosheet arrays with CC fiber. Next, the uniformly distributed nanoparticles with an uneven size are adhesively supported on CC fiber as shown by the FE-SEM images of NS in Fig. 2(d) (i and ii). The interconnected nanoparticles further act as a bridge for electronic transmission, which significantly benefits the charge transfer process. Furthermore, the strongly adhered integrated EM-CC fiber can provide enhanced conductivity and short reaction path during the redox reaction of the EC process. Additionally, energy-dispersive X-ray spectroscopy (EDS) was performed to determine the elemental presence and distribution in BCS-1 and NS. Fig. 2(e and g) show the presence of Bi, Cu and S in the BCS-1 electrode and Ni and S in the NS electrode. Furthermore, elemental analysis suggests the uniform distribution of the detected elements in both BCS-1 and NS electrodes, as shown in Fig. 2(f) (i–iv) and (h) (i, ii). In addition, transmission electron microscope (TEM) and high-resolution (HR)-TEM were employed to study the internal structure of the BCS-x samples, as shown in Fig. S1 of the ESI.† The obtained results were in good agreement with those of the FE-SEM results. From Fig. S1(a and b) of the ESI,† the BCS-0.5 sample confirmed the presence of both clusters of nanoparticles and nanosheets in an infused form. For the BCS-1 sample, the presence of interconnected nanosheets with decent transparency suggests a thin 2D morphology, as shown in Fig. S1(d and e) of the ESI.† From Fig. S1(g and h) of the ESI,† a decrease in the sheet length with a low transparency was observed in the BCS-2 sample, suggesting an increase in the sheet thickness. Moreover, the HR-TEM analysis was performed for all the BCS-x samples together with the selected area electron diffraction (SAED) pattern. From the HR-TEM results (Fig. S1(c, f and i) of the ESI†), all the BCS-x samples demonstrated that the lattice fringes were aligned in various orientations with the SAED pattern containing the bright rings, which indicated the polycrystalline nature. The insets of the HR-TEM images showed the corresponding SAED pattern of each BCS-x sample.
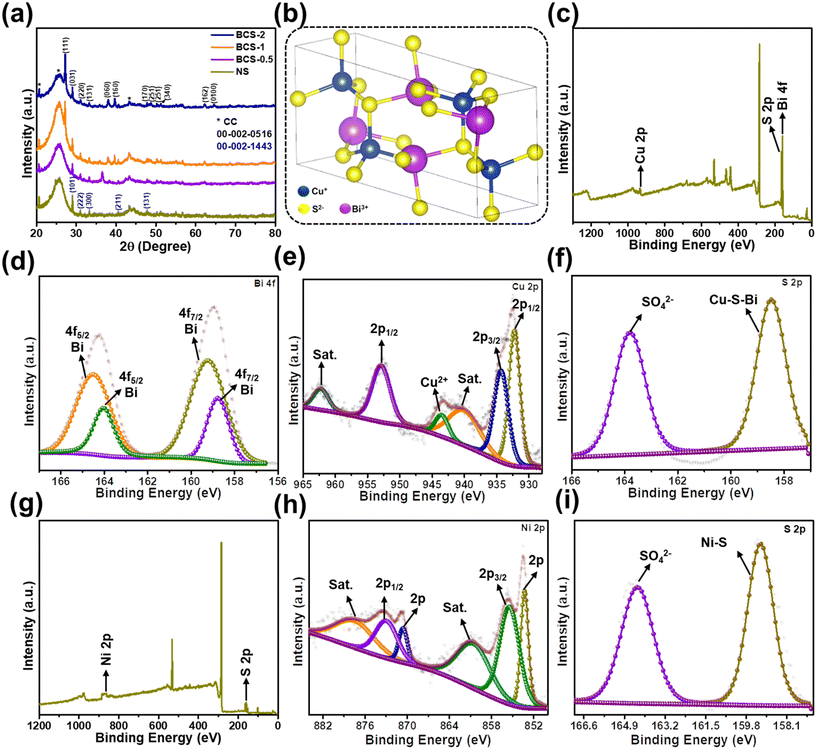
The X-ray diffraction (XRD) technique was employed to investigate the crystal structure and phase of all the negative electrodes with different molar concentration ratios of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu in growth solution (BCS-x), together with the positive (NS) electrode, as shown in Fig. 3(a and b). From the results, the BCS-1 and BCS-2 materials exhibited similar diffraction patterns with the characteristic diffraction peaks of orthorhombic BiCuS2. The diffraction peaks at 27.19°, 29.02°, 30.98°, 33.26°, 37.98°, 39.72°, 47.70°, 48.83°, 50.17°, 51.19°, 62.19°, and 64.56° are indexed as (111), (031), (220), (131), (060), (160), (170), (251), (251), (340), (162), and (100), respectively according to the JCPDS card no. 00-002-0516 (Fig. 3(a)). Furthermore, lattice parameters (a, c), full width at half maximum (FWHM), volume (V), and crystallite size (D) of the BCS-1 and BCS-2 were calculated using the diffraction peak at 37.98° to investigate the effect of the molar concentration ratio of Bi
Cu in growth solution (BCS-x), together with the positive (NS) electrode, as shown in Fig. 3(a and b). From the results, the BCS-1 and BCS-2 materials exhibited similar diffraction patterns with the characteristic diffraction peaks of orthorhombic BiCuS2. The diffraction peaks at 27.19°, 29.02°, 30.98°, 33.26°, 37.98°, 39.72°, 47.70°, 48.83°, 50.17°, 51.19°, 62.19°, and 64.56° are indexed as (111), (031), (220), (131), (060), (160), (170), (251), (251), (340), (162), and (100), respectively according to the JCPDS card no. 00-002-0516 (Fig. 3(a)). Furthermore, lattice parameters (a, c), full width at half maximum (FWHM), volume (V), and crystallite size (D) of the BCS-1 and BCS-2 were calculated using the diffraction peak at 37.98° to investigate the effect of the molar concentration ratio of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu in growth solution on the crystal structure using the well-known relationship:19
Cu in growth solution on the crystal structure using the well-known relationship:19
a = λ/√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ; c = λ/sin θ; c = λ/sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ; D = kλ/βhkl θ; D = kλ/βhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ; V = 0.85(a2c) θ; V = 0.85(a2c) | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu in the growth solution affected the crystal structure. However, for BCS-0.5, the characteristic diffraction peaks of BiCuS2 were not matched. It is evident that a minimum of 1 mmol and 2 mmol of Bi and Cu, respectively, should be present in the growth solution to form the BiCuS2 phase. Additionally, the NS exhibited the peaks at 29.10°, 31.06°, 33.37°, 40.50°, and 47.83°, which is matched with the rhombohedral NS and indexed as (101), (222), (300), (211), and (131), respectively, according to the JCPDS card no. 00-002-1443. Furthermore, the lattice parameters (a, c), FWHM, V, and D were calculated using a peak at 31.06° for NS and this is shown in Table 1. Fig. 3(b) shows the crystal structure retrieved from the vista software, where each Cu1+ atom is connected to four S2− atoms, forming CuS4 tetrahedra corners connected with eight equivalent BiS5 square pyramids and corners connected with four equivalent CuS4 tetrahedra.
Cu in the growth solution affected the crystal structure. However, for BCS-0.5, the characteristic diffraction peaks of BiCuS2 were not matched. It is evident that a minimum of 1 mmol and 2 mmol of Bi and Cu, respectively, should be present in the growth solution to form the BiCuS2 phase. Additionally, the NS exhibited the peaks at 29.10°, 31.06°, 33.37°, 40.50°, and 47.83°, which is matched with the rhombohedral NS and indexed as (101), (222), (300), (211), and (131), respectively, according to the JCPDS card no. 00-002-1443. Furthermore, the lattice parameters (a, c), FWHM, V, and D were calculated using a peak at 31.06° for NS and this is shown in Table 1. Fig. 3(b) shows the crystal structure retrieved from the vista software, where each Cu1+ atom is connected to four S2− atoms, forming CuS4 tetrahedra corners connected with eight equivalent BiS5 square pyramids and corners connected with four equivalent CuS4 tetrahedra.
| Sample name | a (Å) | c (Å) | FWHM (degree) | V (Å3) | D (nm) |
|---|---|---|---|---|---|
| BCS-1 | 2.73 | 4.73 | 0.41 | 0.272 | 21.27 |
| BCS-2 | 2.72 | 4.72 | 0.45 | 0.271 | 19.41 |
| NS | 3.32 | 5.75 | 0.73 | 0.267 | 11.76 |
The X-ray photoelectron spectroscopy (XPS) analysis was performed to investigate the surface elemental composition of the BCS-1 and NS electrodes. The full survey scan spectrum of the BCS-1 is shown in Fig. 3(c), confirming the presence of Bi, Cu, and S elements. The high-resolution (HR)-XPS spectrum of the Bi 4f is shown in Fig. 3(d). The spectrum consists of dual contributions due to the presence of various valences. The results suggest the presence of the trivalent and pentavalent Bi in BCS-1. The peaks at 158.75 and 164.52 eV correspond to Bi3+ and the peaks at 159.25 and 164.04 eV are related to Bi5+.20 The HR-XPS spectrum of Cu 2p is illustrated in Fig. 3(e). The strong peak at 934.01 eV corresponds to Cu 2p3/2, whereas the peaks at 953.99 and 932.33 eV correspond to the Cu 2p1/2 which confirms the presence of metallic copper in two different states (+1, +2) and successively verifies the successful reduction of copper nitrate trihydrate by the applied voltage during the ED process.21 Furthermore, ∼20 eV difference was observed between the peaks of Cu 2p1/2 and Cu 2p3/2, which shows the presence of Cu2+ in the BCS-1 electrode.22 Two strong satellite peaks (denoted as sat.) were observed at 962.34 and 943.57 eV, which is attributed to the paramagnetic chemical state of Cu2+.22 In addition, the typical characteristic peak of copper oxide was observed at 940.4 eV, indicating the uniform oxidation of the copper crystals upon exposure to the atmosphere.23 The HR-XPS spectrum of S 2p (Fig. 3(f)) shows two strong peaks at 158.46 and 163.8 eV which correspond to the SO42− ions and the Bi–S–Cu, respectively. The presence of the Bi–S–Cu peak confirms the bonding of the S with both the Cu+ and Bi+ sites. Moreover, the FWHM value of the Bi–S–Cu peak was observed to be greater than that of the SO42− ions, which may benefit the electrochemical properties due to the synergistic effect.21 Next, the XPS was also performed for the NS electrode to study the elemental composition and oxidation. The survey scan spectrum confirmed the presence of the Ni and S elements as shown in Fig. 3(g). The HR-XPS spectrum of Ni 2p is shown in Fig. 3(h), and the peaks at 855.62 and 873.12 eV correspond to Ni 2p3/2 and 2p1/2, respectively, together with the satellite peaks at 877.61 and 860.65 eV, which suggested the presence of the Ni species as Ni2+ and Ni3+. In addition, two peaks at 870.71 and 853.35 eV were observed, corresponding to zero valent metallic Ni.24,25 The HR-XPS spectrum of the S 2p of the NS electrode was similar to that of the BCS-1 electrode, as shown in Fig. 3(i). The two strong peaks at 159.20 and 164.3 eV correspond to the SO42− ions and Ni–S, respectively. The Ni–S peak confirmed the bonding of the S with the Ni+ sites. Furthermore, the greater FWHM value of the Ni–S peak compared to the SO42− ion peak may further benefit the electrochemical properties due to the synergistic effect.21
To further determine the elemental ratio of the as-prepared BCS-1 and NS samples, inductively coupled plasma (ICP) spectroscopy was performed. It is worth noting that the detection of the sulfur content in the sample was not possible using the ICP technique because sulfur has a high ionization potential, reducing the sensitivity and further intense polyatomic interferences affect all the isotopes of sulfur. In this respect, the atomic ratio percentages of Bi and Cu were found to be 19.7% and 80.2%, respectively, for the BCS-1 sample, and the Ni content in the NS sample was also determined, as shown in Fig. S2(a and b) in the ESI.† The ICP spectroscopy confirmed the presence of the Bi and Cu contents in the BCS-1 sample, whereas the Ni content in the NS sample also agreed well with the EDS and XPS results.
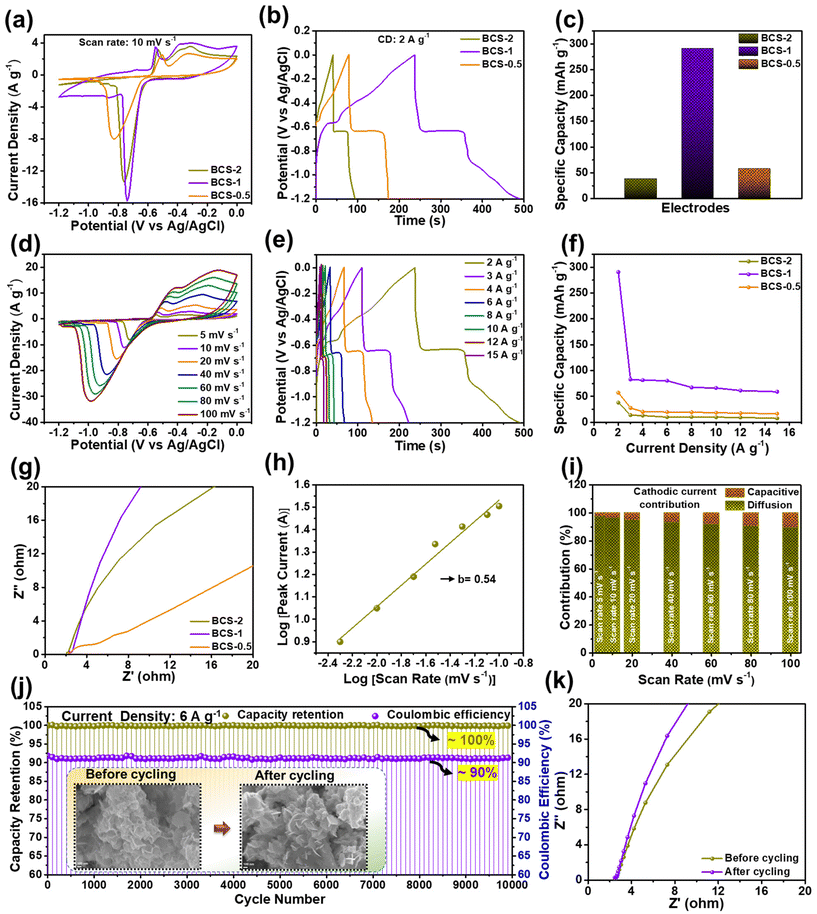
The electrochemical properties of all the as-prepared BCS-x were evaluated as discussed in the Experimental section. Initially, comparative CV analysis was performed with a fixed scan rate of 10 mV s−1 and the OPW of 0 to −1.2 V in the three-electrode cell is illustrated in Fig. 4(a). From these results, it was seen that the BCS-x electrodes demonstrated a similar redox CV nature. It was evident that the redox peaks were predominantly generated by the reaction of BiCuS2 (BiCuS2 + OH− ⇌ BiCuS2OH + H2O + e−), indicating the dominant faradaic type charge storage behavior.26 However, a change in the integral area of the CV curves was observed in the BCS-x samples, verifying the effect of the molar concentration ratio of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu in the growth solution. Among the BCS-x electrodes, the BCS-1 electrode demonstrated the superior area under the CV curve, which implied that there was a better capacity comparatively. Furthermore, a comparative GCD test was performed at a constant current density of 2 A g−1 to study the charge–discharge behavior, as shown in Fig. 4(b). The asymmetric and non-linear GCD curves were demonstrated by all the BCS-x electrodes, which indicated the faradaic type of energy storage behavior occurring from the redox reactions at the electrode–electrolyte interface. The GCD curves demonstrated two distinguishable plateaus showing the two redox transitions of the electrons. These results exactly coincided with the obtained CV results. The BCS-1 electrode exhibited the superior discharge time among the BCS-x electrodes. Considering the integral area under discharge time, the Cts values were calculated as 38, 290, and 57 mA h g−1 for the BCS-0.5, BCS-1, and BCS-2 electrodes, respectively, and these are shown as a bar graph in Fig. 4(c). Next, the CV test was performed for all the prepared BCS-x electrodes with a constant OPW (−1.2 to 0 V) at various scan rates ranging from 5 to 100 mV s−1, as shown in Fig. 4(d), and Fig. S2(c and d) in the ESI.† A gradual increase in the current peak, together with no considerable variation in the curves of respective voltammograms, was observed for all the prepared electrodes. This means that there is low equivalent series resistance and outstanding rate performance for all of the BCS-x electrodes.27,28 A considerable shift in the redox peaks was observed towards the higher sites of the anodic and cathodic sides with an increase in the scan rate, probably due to the concentration polarization during the electrochemical activity.29 The GCD assessment was performed at various current densities ranging from 2 to 15 A g−1 for all the BCS-x electrodes as shown in Fig. 4(e), and Fig. S2(e and f) in the ESI.† All the electrodes retained the GCD curves shape at high current densities, indicating excellent rate capability. Furthermore, the Cts values for individual current densities were calculated from the discharge times of the GCD curves for all the BCS-x electrodes, and the comparative graph was plotted as shown in Fig. 4(f). Because of the integrity of the suitable nanosheet arrays, the BCS-1 electrode offered the highest Cts at all the individual current densities among the BCS-x electrodes. The impressive Cts of the BCS-1 is probably due to the synergistic effects of strong adhesion, interconnected nanosheet arrays, and optimal Bi/Cu molar concentration. Additionally, the TEM results demonstrated that the nanosheets with satisfactory transparency suggest that there is a thin 2D morphology. Low-dimensional nanostructures are an important candidate for surface/interface related applications such as SCs due to their interesting properties such as a high surface-to-volume ratio, low radius of curvature, high electron kinetics, and better thermal and chemical stabilities. According to a previous report, nanosheet-like surface morphology had a larger specific surface area (SSA) when compared to the nanorods- and particle-like structures.15 In this respect, the thin nanosheet arrays of the BCS-1 provided enhanced accessible reaction sites and electrolyte diffusion, favoring the surface reactions, which lead to rapid ion/electron kinetics. The relatively low Cts of the BCS-0.5 and BCS-2 can be explained by the respective FE-SEM and TEM images. The nanosheets infused with coagulated nanoparticle arrays of BCS-0.5 and increased thickness with shortening of length and non-uniformity of sheets of the BCS-2 hinder the electron transport, leading to the limitation of electron transfer.
Cu in the growth solution. Among the BCS-x electrodes, the BCS-1 electrode demonstrated the superior area under the CV curve, which implied that there was a better capacity comparatively. Furthermore, a comparative GCD test was performed at a constant current density of 2 A g−1 to study the charge–discharge behavior, as shown in Fig. 4(b). The asymmetric and non-linear GCD curves were demonstrated by all the BCS-x electrodes, which indicated the faradaic type of energy storage behavior occurring from the redox reactions at the electrode–electrolyte interface. The GCD curves demonstrated two distinguishable plateaus showing the two redox transitions of the electrons. These results exactly coincided with the obtained CV results. The BCS-1 electrode exhibited the superior discharge time among the BCS-x electrodes. Considering the integral area under discharge time, the Cts values were calculated as 38, 290, and 57 mA h g−1 for the BCS-0.5, BCS-1, and BCS-2 electrodes, respectively, and these are shown as a bar graph in Fig. 4(c). Next, the CV test was performed for all the prepared BCS-x electrodes with a constant OPW (−1.2 to 0 V) at various scan rates ranging from 5 to 100 mV s−1, as shown in Fig. 4(d), and Fig. S2(c and d) in the ESI.† A gradual increase in the current peak, together with no considerable variation in the curves of respective voltammograms, was observed for all the prepared electrodes. This means that there is low equivalent series resistance and outstanding rate performance for all of the BCS-x electrodes.27,28 A considerable shift in the redox peaks was observed towards the higher sites of the anodic and cathodic sides with an increase in the scan rate, probably due to the concentration polarization during the electrochemical activity.29 The GCD assessment was performed at various current densities ranging from 2 to 15 A g−1 for all the BCS-x electrodes as shown in Fig. 4(e), and Fig. S2(e and f) in the ESI.† All the electrodes retained the GCD curves shape at high current densities, indicating excellent rate capability. Furthermore, the Cts values for individual current densities were calculated from the discharge times of the GCD curves for all the BCS-x electrodes, and the comparative graph was plotted as shown in Fig. 4(f). Because of the integrity of the suitable nanosheet arrays, the BCS-1 electrode offered the highest Cts at all the individual current densities among the BCS-x electrodes. The impressive Cts of the BCS-1 is probably due to the synergistic effects of strong adhesion, interconnected nanosheet arrays, and optimal Bi/Cu molar concentration. Additionally, the TEM results demonstrated that the nanosheets with satisfactory transparency suggest that there is a thin 2D morphology. Low-dimensional nanostructures are an important candidate for surface/interface related applications such as SCs due to their interesting properties such as a high surface-to-volume ratio, low radius of curvature, high electron kinetics, and better thermal and chemical stabilities. According to a previous report, nanosheet-like surface morphology had a larger specific surface area (SSA) when compared to the nanorods- and particle-like structures.15 In this respect, the thin nanosheet arrays of the BCS-1 provided enhanced accessible reaction sites and electrolyte diffusion, favoring the surface reactions, which lead to rapid ion/electron kinetics. The relatively low Cts of the BCS-0.5 and BCS-2 can be explained by the respective FE-SEM and TEM images. The nanosheets infused with coagulated nanoparticle arrays of BCS-0.5 and increased thickness with shortening of length and non-uniformity of sheets of the BCS-2 hinder the electron transport, leading to the limitation of electron transfer.
By virtue of the electrochemical analysis, an increase in the current density leads to insufficient time for full utilization of the electroactive site by the electrolyte, and a gradual decrease in the Cts was observed for all the electrodes at high current densities. From the electrochemical results, an excellent Cts with a wide OPW (0 to −1.2 V) enabled the potential application of the BCS-1 electrode as a negative electrode for high-performance SC. To further investigate the total resistance offered by the electrochemical system for individual electrodes, EIS analysis was performed on individual electrodes. Comparative Nyquist plots of the BCS-x electrodes are shown in Fig. 4(g). All the electrodes demonstrated a straight line in the lower frequency region, which is related to the resistance offered by the diffusion limiting process (Warburg impedance). The EIS results were consistent with the obtained CV and GCD results. Among the BCS-x electrodes, the BCS-1 electrode demonstrated a straight line inclined towards the y-axis. This implied that there was high electron transfer, ion diffusion, and electrical conductivity at the interface of the electrode and electrolyte, which is beneficial for enhancing the electrochemical properties. From the CV results, the BCS-1 electrode demonstrated redox peaks at all the scan rates, which shows the excellent electrochemical reversibility and rapid charge transfer of the electrode. However, all the nanostructure-based electrodes store charge by both surface-controlled capacitive and diffusion-controlled processes. To further investigate the dominant energy storage by the BCS-1 electrode, a modified power law equation was employed:29
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = log i = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a + blog a + blog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v v | (4) |
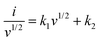 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles were performed at a current density of 6 A g−1 for the BCS-1 electrode, as shown in Fig. 4(j). From the results, the BCS-1 electrode demonstrated excellent cycling stability with ∼100% capacity retention and ∼90% coulombic efficiency over 10
000 GCD cycles were performed at a current density of 6 A g−1 for the BCS-1 electrode, as shown in Fig. 4(j). From the results, the BCS-1 electrode demonstrated excellent cycling stability with ∼100% capacity retention and ∼90% coulombic efficiency over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles. In order to study the morphological stability of the electrode, the FE-SEM images were observed for the BCS-1 electrode before and after the cycling test, as shown in the inset of Fig. 4(j). After the cycling, the electrode retained almost the original morphology of the nanosheet arrays, suggesting that there was good morphological stability and strong adhesion of the electrode material with the CC, which exactly coincides with the cycling results. Furthermore, the EIS analysis of the electrode was performed before and after the cycling test, which demonstrated that the Nyquist plots were almost identical with a marginal shift of the straight line towards the y-axis in the lower frequency region after the cycling as shown in Fig. 4(k). This suggests that there was improved conductivity over the cycling period. The excellent cycling stability can be attributed to the interconnected vertically aligned nanosheet arrays and the strong material adhesion to the current collector, which benefits the fast electrolyte ion/electron transportation. Furthermore, the electrochemical performance of the BCS-1 electrode was compared with previous literature as summarized in Table 2.
000 GCD cycles. In order to study the morphological stability of the electrode, the FE-SEM images were observed for the BCS-1 electrode before and after the cycling test, as shown in the inset of Fig. 4(j). After the cycling, the electrode retained almost the original morphology of the nanosheet arrays, suggesting that there was good morphological stability and strong adhesion of the electrode material with the CC, which exactly coincides with the cycling results. Furthermore, the EIS analysis of the electrode was performed before and after the cycling test, which demonstrated that the Nyquist plots were almost identical with a marginal shift of the straight line towards the y-axis in the lower frequency region after the cycling as shown in Fig. 4(k). This suggests that there was improved conductivity over the cycling period. The excellent cycling stability can be attributed to the interconnected vertically aligned nanosheet arrays and the strong material adhesion to the current collector, which benefits the fast electrolyte ion/electron transportation. Furthermore, the electrochemical performance of the BCS-1 electrode was compared with previous literature as summarized in Table 2.
| Sample name | Potential window (V) | Specific capacity (mA h g−1) | No. of cycles | Cycling stability (%) | Ref. |
|---|---|---|---|---|---|
| CuS@MnS | 1.1 | 89.77 | 3000 | 95.9 | 34 |
| CuS | 1.1 | 45.73 | 3000 | 80 | 34 |
| BGNS-1 | 0.65 | 237 | 100 | 75 | 26 |
| Bi2S3/FGS | 0.9 | 262 | 5000 | 75 | 35 |
| Co-S-80 | 0.6 | 147 | 1000 | 86.5 | 36 |
| MoS2/Mn3O4 | 0.5 | 50 | 2000 | 76 | 37 |
| CoNi2S4 | 0.55 | 247 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
82 | 38 |
| BCS-1 | 1.2 | 290 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
100 | Present work |
Furthermore, a comparison of the XRD and Raman spectroscopy analyses for the BCS-1 electrode was performed before and after cycling. From Fig. S2(g) in the ESI,† the Raman spectrum of the BCS-1 electrode before cycling demonstrated prominent peaks at 1364 and 1584 cm−1 which originated from the substrate (CC), and in addition, two peaks were observed at 610 and 306 cm−1 corresponding to the metal–sulfur bond stretching.31 Similarly, the Raman spectrum of the BCS-1 electrode after cycling exhibited peaks at the same positions as those before cycling without considerable peak shift, but with a prominent decrease in the peak intensity which may be due to the continuous charge–discharge cycles. Additionally, the a comparative XRD analysis was performed for the BCS-1 electrode sample before and after cycling, as shown in Fig. S2(h) in the ESI.† Both samples demonstrated a similar XRD pattern without any significant peak shift, but there was a slight decrease in the FWHM value for the sample after cycling, which may be due to the continuous 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles. The results obtained suggest the excellent cycling stability of the prepared BCS-1 electrode in the long-term test.
000 GCD cycles. The results obtained suggest the excellent cycling stability of the prepared BCS-1 electrode in the long-term test.
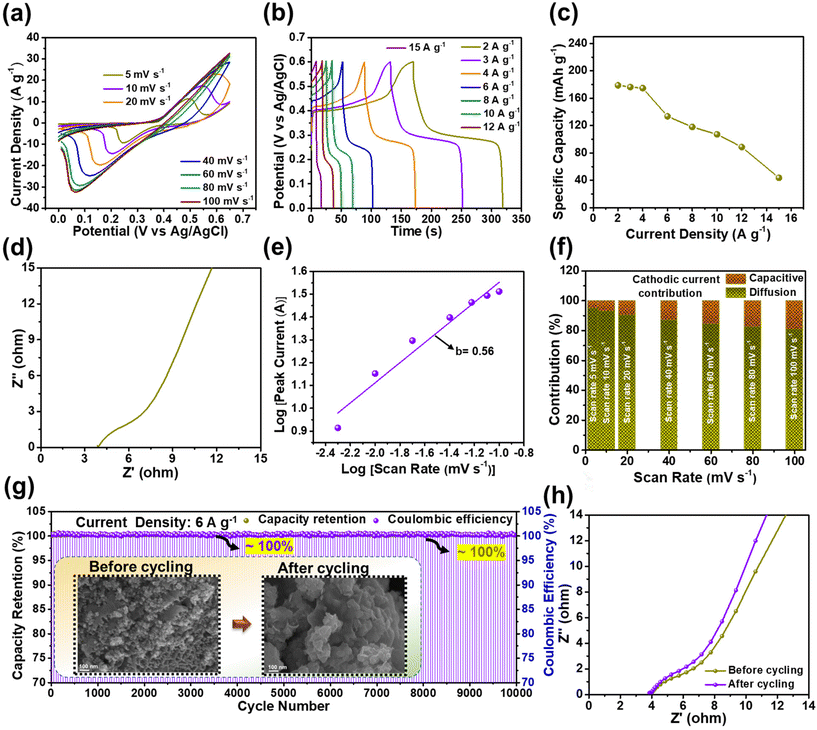
Motivated by the excellent electrochemical performance of the BCS-1 electrode, the ASSSC device was fabricated using the electrode as a negative electrode, and a Ni element-based NS electrode was employed as a positive electrode. Prior to the device fabrication, the electrochemical properties of the NS electrode were determined. The CV curves were measured at various scan rates ranging from 5 to 10 mV s−1 with an OPW of 0 to 0.65 V as shown in Fig. 5(a). A significant increase in the current peak was observed with the increasing the scan rate, implying that there was an excellent rate capability. However, the shift of both anodic and cathodic peaks was observed upon the increase in scan rate, which typically corresponds to the concentration polarization at the interface of the electrode and electrolyte.32 The GCD analysis was performed with a sequence of OPWs between 0 to 0.6 V at various current densities (2–15 A g−1), and non-linear triangular symmetric GCD curves were observed at all the current densities, as shown in Fig. 5(b). This implies that the NS electrode has faradaic type of capacitive behavior. In Fig. 5(c), the Cts value with respect to current density was calculated from the GCD analysis, demonstrating the maximum Cts value of 178 mA h g−1 at 2 A g−1. Fig. 5(d) shows the EIS plot of the NS electrode. A linear straight line at a lower frequency was observed, indicating high ion diffusion, electron transfer, and electrical conductivity of the electrode, which improved the electrochemical properties. Fig. 5(e) shows the relationship between the peak current density and scan rate to determine the b value. To evidence the faradaic type of the charge storage mechanism of the NS electrode, the b value was calculated using eqn (4) from the cathodic peaks, and the value obtained was 0.56. Furthermore, the ratio of diffusion-controlled and surface-controlled charge storages was calculated using eqn (5) and these are shown in the bar graph of individual scan rates (Fig. 5(f)). From the results, a superior diffusion-controlled charge storage mechanism was observed at all the scan rates, suggesting that the dominant faradaic type charge storage was by the NS electrode. Furthermore, the long-term cycling stability of the NS electrode was also determined, as shown in Fig. 5(g). As expected from the beneficial nanoparticle array morphology, the NS electrode demonstrated outstanding cycling stability with ∼100% capacity retention and ∼100% coulombic efficiency over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles. Consecutively, the morphological stability of the electrode was determined using the FE-SEM images of the sample before and after cycling, as shown in the inset of Fig. 5(g). After the cycling, the NS electrode retained almost the initial morphology of the nanoparticle arrays, which indicates excellent morphological stability and strong adhesion of the electrode material, which exactly overlapped with the cycling results. In addition, the EIS analysis of the NS electrode was performed before and after the cycling test, and identical Nyquist plots with a minimal shift of the straight line towards the y-axis in the lower frequency were observed after the cycling, as shown in Fig. 5(h), suggesting that there was enhanced conductivity over the cycling and supporting the cycling stability.
000 GCD cycles. Consecutively, the morphological stability of the electrode was determined using the FE-SEM images of the sample before and after cycling, as shown in the inset of Fig. 5(g). After the cycling, the NS electrode retained almost the initial morphology of the nanoparticle arrays, which indicates excellent morphological stability and strong adhesion of the electrode material, which exactly overlapped with the cycling results. In addition, the EIS analysis of the NS electrode was performed before and after the cycling test, and identical Nyquist plots with a minimal shift of the straight line towards the y-axis in the lower frequency were observed after the cycling, as shown in Fig. 5(h), suggesting that there was enhanced conductivity over the cycling and supporting the cycling stability.
Fig. 6(a) shows the schematic representation of the electrolyte ion diffusion over the electrodes making up the ASSSC device during electrochemical analysis. The electrochemical properties of the ASSSC device were evaluated. To check the OPW of the fabricated ASSSC device, the CV curves of BCS-1 (−1.2 to 0 V) and NS (0 to 0.65 V) electrodes in a three-electrode system at a scan rate of 10 mV s−1 were plotted together, as shown in Fig. 6(b). This result suggests 0 to 1.8 V as an optimum OPW for the ASSSC device. Furthermore, the charge storage performance of the ASSSC device was initially investigated using CV analysis performed at various scan rates ranging from 5 to 100 mV s−1 with a fixed OPW (0 to 1.8 V) as shown in Fig. 6(c). The CV curves obtained at all the scan rates were composed of redox current peaks, indicating the faradaic type charge storage behavior of the ASSSC device, and a gradual increase in current response and no change in the shape of CV curve was observed upon increasing the scan rate, showing that the good rate performance resulted from beneficial ion/electron transport kinetics in the constituents of the electrodes of the ASSSC device. In addition, the GCD analysis was employed to investigate the charge–discharge performance of the ASSSC device. Previously, an optimum OPW was determined by performing the GCD analysis at various OPWs (1 to 1.8 V) with a fixed current density of 6 A g−1 (Fig. 6(d)). This result indicated that the optimum OPW of 0 to 1.8 V exactly coincided with the CV result. Furthermore, the GCD analysis was also recorded for the ASSSC device at various current densities (2 to 15 A g−1) at the optimized OPW as shown in Fig. 6(e). The non-linear GCD curves indicate the faradaic type charge storage behavior by the ASSSC device, which is coincident with the CV result. Considering the discharge time, the Cs value was calculated at an individual current density as shown in Fig. 6(f). The ASSSC device delivered the maximum Cs value of 91 F g−1 at 2 A g−1 and retained 69% of the initial Cs value even at a high current density of 15 A g−1, which indicates the good rate performance of the ASSSC device. From the electrochemical results, the energy and power density values were calculated for the ASSSC device, as illustrated in the Ragone plot (Fig. 6(g)). The ASSSC device successfully demonstrated the maximum power and energy density values of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 W kg−1 and 41 W h kg−1, respectively, which results from the synergetic effect of the constituents of the electrode of the ASSSC device. For practical applications, long-term cycling stability plays a crucial role. To verify this, a continuous 40
500 W kg−1 and 41 W h kg−1, respectively, which results from the synergetic effect of the constituents of the electrode of the ASSSC device. For practical applications, long-term cycling stability plays a crucial role. To verify this, a continuous 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycling test was performed for the ASSSC device at a current density of 6 A g−1. Typically, the SCs exhibit a dip in the capacity value during the initial GCD cycles due to the formation of substances containing OH− causing changes in the morphological structures.33 However, the ASSSC device demonstrated a negligible dip during the initial GCD cycles, which shows the excellent morphological stability of the constituent electrodes and the overall maintenance of the ∼100% capacitance retention and ∼90% coulombic efficiency across the 40
000 GCD cycling test was performed for the ASSSC device at a current density of 6 A g−1. Typically, the SCs exhibit a dip in the capacity value during the initial GCD cycles due to the formation of substances containing OH− causing changes in the morphological structures.33 However, the ASSSC device demonstrated a negligible dip during the initial GCD cycles, which shows the excellent morphological stability of the constituent electrodes and the overall maintenance of the ∼100% capacitance retention and ∼90% coulombic efficiency across the 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles, as shown in Fig. 6(i). Moreover, the EIS analysis was performed using the ASSSC device before and after the cycling test, which implied there was no distinct degradation (Fig. 6(h)). However, the straight line in the lower frequency shifted slightly towards the y-axis, indicating the excellent conductivity over the cycling. In addition, a comparative cycling stability test was performed at a low current density (2 A g−1) and a high current density (6 A g−1) for the ASSSC device by performing 20
000 GCD cycles, as shown in Fig. 6(i). Moreover, the EIS analysis was performed using the ASSSC device before and after the cycling test, which implied there was no distinct degradation (Fig. 6(h)). However, the straight line in the lower frequency shifted slightly towards the y-axis, indicating the excellent conductivity over the cycling. In addition, a comparative cycling stability test was performed at a low current density (2 A g−1) and a high current density (6 A g−1) for the ASSSC device by performing 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous GCD cycles. The ASSSC device demonstrated almost overlapping cycling stability performance with a capacitance retention of ∼100% at both low and high current densities over 20
000 continuous GCD cycles. The ASSSC device demonstrated almost overlapping cycling stability performance with a capacitance retention of ∼100% at both low and high current densities over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles, showing the good long-term cycling stability of the ASSSC device at both low and high current densities, as shown in Fig. S3 of the ESI.† Finally, the electrochemical performance of the ASSSC device was compared with previously published reports, as summarized in Table 3.
000 GCD cycles, showing the good long-term cycling stability of the ASSSC device at both low and high current densities, as shown in Fig. S3 of the ESI.† Finally, the electrochemical performance of the ASSSC device was compared with previously published reports, as summarized in Table 3.
| Device name | Potential window (V) | ED (W h kg−1) | No. of cycles | Cycling stability (%) | Ref. |
|---|---|---|---|---|---|
| BMP-2//AC | 1.8 | 31.9 | 6000 | 86.5 | 39 |
| CuS@CD-GOH | 1.4 | 28 | 5000 | 90 | 40 |
| Bi2O3@MnO2//Bi2O3@MnO2 | 1.2 | 28 | 2000 | 72 | 41 |
| TCBOC//TCBOC | 1.2 | 15.2 | 5000 | 85 | 42 |
| (Fe(III)/BiOCl)//(Fe(III)/BiOCl) | 1 | 17 | 5000 | 72 | 43 |
| BM//AC | 1.2 | 14.4 | 5000 | 90 | 44 |
| H-CuS//AC | 1.6 | 15.97 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
89 | 9 |
| ASSSC | 1.8 | 41 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
100 | Present work |
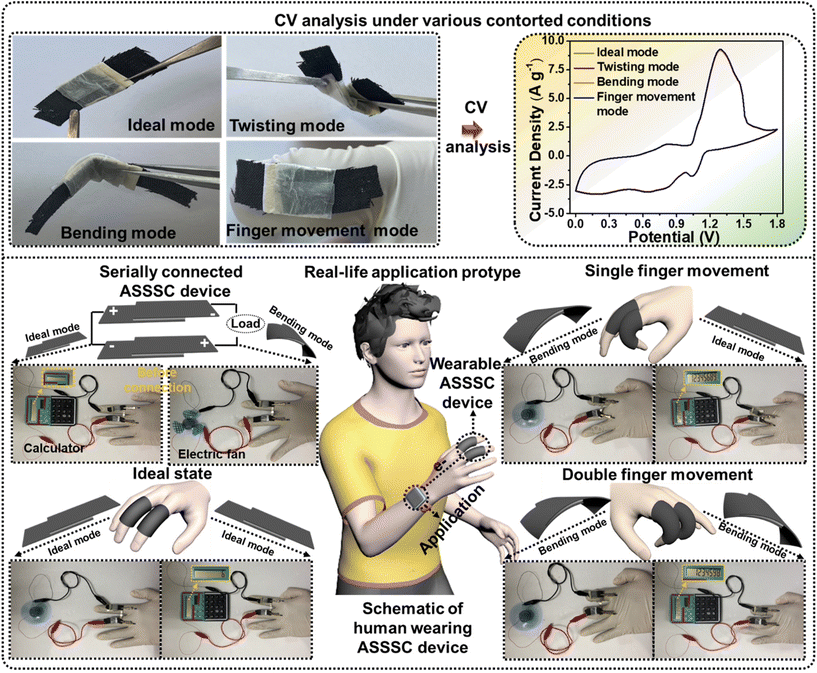
With the developments in advanced portable electronics, flexible SCs are becoming more desirable in energy storage systems, which are anticipated to operate under a mechanically contorted state. Next, to determine the flexibility effect on the electrochemical properties, the CV measurements were performed for the ASSSC device under various mechanically contorted states (ideal, twisting, bending, and finger movement modes). The obtained CV curves were almost identical to each other, validating the mechanical stability of the ASSSC device without sacrificing any capacitive performance. To further verify the performance and practical application under a contorted state, serially connected two ASSSC devices were attached to human fingers and further employed to power the liquid crystal display (LCD), and the electrical fan with various finger movements (single finger movement and double finger movement) and these were compared with the ideal state. As expected from an excellent electrochemical performance by the ASSSC device, serially connected devices successfully powered the LCD and electrical fan under all the finger movements without any power loss, as shown in Fig. 7. The results indicate the excellent flexibility and mechanical stability of the ASSSC device, suggesting the potential application of a wide OPW ASSSC device with high power and energy densities for flexible energy storage systems.
4. Conclusions
The present study demonstrated the synthesis of flexible BCS via the ED method for use as a negative electrode. The effect of the molar concentration ratio of Bi and Cu in the growth solution on the crystal structure and surface morphology was also investigated as BixCu3−xS3. The optimum surface morphology of the nanosheet arrays was obtained with “x = 1” in the growth solution of BixCu3−xS3. The BCS-1 electrode revealed excellent electrochemical properties with a Cts value of 290 mA h g−1. The BCS-1 electrode material with a vertically aligned interconnected 3D network of nanosheet arrays provided continuous channels for rapid electron/ion transfer and firm adhesion with CC, eventually exhibiting an excellent cycling stability of ∼100% capacity retention across 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles to the initial cycle. Furthermore, a novel all-sulfide gel electrolyte-based ASSSC device was fabricated using the BCS-1 and NS as the negative and positive electrodes, respectively. The ASSSC device delivered the maximum power and energy density values of 13
000 GCD cycles to the initial cycle. Furthermore, a novel all-sulfide gel electrolyte-based ASSSC device was fabricated using the BCS-1 and NS as the negative and positive electrodes, respectively. The ASSSC device delivered the maximum power and energy density values of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 W kg−1 and 41 W h kg−1, respectively and overall maintained ∼100% capacity retention across the 40
500 W kg−1 and 41 W h kg−1, respectively and overall maintained ∼100% capacity retention across the 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles. To validate the application of the ASSSC device under contorted conditions, two serially connected ASSSC devices were attached to human fingers and further employed to power the LCD and electrical fan with various finger movements. As a result, the ASSSC device successfully powered them without any power loss, verifying the application of the ASSSC device as a wearable energy storage device. The prototype of the ASSSC device is cost-effective, scalable, and simple, showing the synthesis of novel all-sulfide flexible electrodes for advanced flexible, portable, and wearable electronic applications.
000 GCD cycles. To validate the application of the ASSSC device under contorted conditions, two serially connected ASSSC devices were attached to human fingers and further employed to power the LCD and electrical fan with various finger movements. As a result, the ASSSC device successfully powered them without any power loss, verifying the application of the ASSSC device as a wearable energy storage device. The prototype of the ASSSC device is cost-effective, scalable, and simple, showing the synthesis of novel all-sulfide flexible electrodes for advanced flexible, portable, and wearable electronic applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) (2018R1A6A1A03025708).References
- A. Vlad, N. Singh, J. Rolland, S. Melinte, P. M. Ajayan and J. F. Gohy, Hybrid supercapacitor-battery materials for fast electrochemical charge storage, Sci. Rep., 2014, 4, 1–7 CrossRef PubMed.
- X. Liu, W. Xu, D. Zheng, Z. Li, Y. Zeng and X. Lu, Carbon cloth as an advanced electrode material for supercapacitors: progress and challenges, J. Mater. Chem. A, 2020, 8, 17938–17950 RSC.
- G. Xiong, P. He, B. Huang, T. Chen, Z. Bo and T. S. Fisher, Graphene nanopetal wire supercapacitors with high energy density and thermal durability, Nano Energy, 2017, 38, 127–136 CrossRef CAS.
- N. S. Shaikh, S. B. Ubale, V. J. Mane, J. S. Shaikh, V. C. Lokhande, S. Praserthdam, C. D. Lokhande and P. Kanjanaboos, Novel electrodes for supercapacitor: Conducting polymers, metal oxides, chalcogenides, carbides, nitrides, MXenes, and their composites with graphene, J. Alloys Compd., 2022, 893, 161998 CrossRef CAS.
- N. Hillier, S. Yong and S. Beeby, The good, the bad and the porous: A review of carbonaceous materials for flexible supercapacitor applications, Energy Rep., 2020, 6, 148–156 CrossRef.
- K. K. Upadhyay, T. Nguyen, T. M. Silva, M. J. Carmezim and M. F. Montemor, Electrodeposited MoOx films as negative electrode materials for redox supercapacitors, Electrochim. Acta, 2017, 225, 19–28 CrossRef CAS.
- R. Sahoo, D. T. Pham, T. H. Lee, T. H. T. Luu, J. Seok and Y. H. Lee, Redox-Driven Route for Widening Voltage Window in Asymmetric Supercapacitor, ACS Nano, 2018, 12, 8494–8505 CrossRef CAS PubMed.
- M. Xu, Y. Niu, X. Teng, S. Gong, L. Ji and Z. Chen, High-capacity Bi2O3 anode for 2.4 V neutral aqueous sodium-ion battery-supercapacitor hybrid device through phase conversion mechanism, J. Energy Chem., 2022, 65, 605–615 CrossRef CAS.
- Y. Liu, Z. Zhou, S. Zhang, W. Luo and G. Zhang, Controllable synthesis of CuS hollow microflowers hierarchical structures for asymmetric supercapacitors, Appl. Surf. Sci., 2018, 442, 711–719 CrossRef CAS.
- Z. Pan, F. Cao, X. Hu and X. Ji, A facile method for synthesizing CuS decorated Ti3C2 MXene with enhanced performance for asymmetric supercapacitors, J. Mater. Chem. A, 2019, 7, 8984–8992 RSC.
- S. Ghosh, P. Samanta, N. C. Murmu and T. Kuila, Investigation of electrochemical charge storage in nickel-cobalt-selenide/reduced graphene oxide composite electrode and its hybrid supercapacitor device, J. Alloys Compd., 2020, 835, 155432 CrossRef CAS.
- B. Qin, X. Zhao, Q. Wang, W. Yao, Y. Cai, Y. Chen, P. Wang, Y. C. Zou, J. Cao, X. Zheng, J. Qi and W. Cai, A tandem electrocatalyst with dense heterointerfaces enabling the stepwise conversion of polysulfide in lithium-sulfur batteries, Energy Storage Mater., 2023, 55, 445–454 CrossRef.
- F. Qi, L. Shao, X. Shi, F. Wu, H. Huang, Z. Sun and A. Trukhanov, “Carbon quantum dots-glue” enabled high-capacitance and highly stable nickel sulphide nanosheet electrode for supercapacitors, J. Colloid Interface Sci., 2021, 601, 669–677 CrossRef CAS PubMed.
- Y. Yan, J. Lin, K. Huang, X. Zheng, L. Qiao, S. Liu, J. Cao, S. C. Jun, Y. Yamauchi and J. Qi, Tensile Strain-Mediated Spinel Ferrites Enable Superior Oxygen Evolution Activity, J. Am. Chem. Soc., 2023, 145, 24218–24229 CrossRef CAS PubMed.
- E. G. Shankar, A. K. Das and J. S. Yu, Facile one-step electrodeposition synthesis of binder-free CoxFe3(-xSe4 ultrathin nanosheet arrays towards high-performance quasi-solid-state supercapacitors, Appl. Surf. Sci., 2022, 596, 153613 CrossRef CAS.
- E. G. Shankar, A. K. Das and J. S. Yu, Biomass-derived ant colony-like ion diffused redox porous carbon toward economical and high-performance quasi-solid-state supercapacitor, Int. J. Energy Res., 2022, 46, 1593–1608 CrossRef CAS.
- A. K. Das, E. G. Shankar, B. Ramulu and J. S. Yu, Electrochemical performance of asymmetric supercapacitor with binder-free CoxMn3(-xSe4 and radish-derived carbon electrodes using K3[Fe(CN)6] additive in electrolyte, Chem. Eng. J., 2022, 448, 137725 CrossRef.
- E. G. Shankar, A. K. Das and J. S. Yu, Entire onion source-derived redox porous carbon electrodes towards efficient quasi-solid-state solar charged hybrid supercapacitor, J. Mater. Sci. Technol., 2022, 125, 118–127 CrossRef CAS.
- E. G. Shankar, M. Aishwarya, A. Khan, A. B. V. K. Kumar and J. S. Yu, Efficient solar light photocatalytic degradation of commercial pharmaceutical drug and dye using rGO-PANI assisted c-ZnO heterojunction nanocomposites, Ceram. Int., 2021, 47, 23770–23780 CrossRef.
- D. A. Zatsepin, A. F. Zatsepin, D. W. Boukhvalov and N. V. Gavrilov, Bi-doped silica glass: A combined XPS – DFT study of electronic structure and pleomorphic imperfections, J. Alloys Compd., 2020, 829, 154459 CrossRef CAS.
- N. K. Gupta, S. Kim, J. Bae and K. S. Kim, Chemisorption of hydrogen sulfide over copper-based metal-organic frameworks: Methanol and UV-assisted regeneration, RSC Adv., 2021, 11, 4890–4900 RSC.
- S. Adhikari, D. Sarkar and G. Madras, Hierarchical Design of CuS Architectures for Visible Light Photocatalysis of 4-Chlorophenol, ACS Omega, 2017, 2, 4009–4021 CrossRef CAS PubMed.
- Z. Jin, C. Liu, K. Qi and X. Cui, Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst, Sci. Rep., 2017, 7, 1–9 CrossRef PubMed.
- B. Ye, X. Cao, Q. Zhao, A. Zhou and J. Wang, Free-standing NiCoSe2 nanostructure on Ni foam via electrodeposition as high-performance asymmetric supercapacitor electrode, Nanotechnology, 2020, 31, 335706 CrossRef CAS PubMed.
- G. Zhang, H. Xuan, J. Yang, R. Wang, Z. Xie, X. Liang, P. Han and Y. Wu, Preparation and characterization of novel 2D/3D NiSe2/MnSe grown on rGO/Ni foam for high-performance battery-supercapacitor hybrid devices, J. Power Sources, 2021, 506, 230255 CrossRef CAS.
- G. Nie, X. Lu, J. Lei, L. Yang and C. Wang, Facile and controlled synthesis of bismuth sulfide nanorods-reduced graphene oxide composites with enhanced supercapacitor performance, Electrochim. Acta, 2015, 154, 24–30 CrossRef CAS.
- S. Zheng, Y. Fu, L. Zheng, Z. Zhu, J. Chen, Z. Niu and D. Yang, PEDOT-engineered Bi2O3 nanosheet arrays for flexible asymmetric supercapacitors with boosted energy density, J. Mater. Chem. A, 2019, 7, 5530–5538 RSC.
- A. K. Das, B. Ramulu, E. G. Shankar and J. S. Yu, Binder-free CuS@PEDOT and Co–V–Se electrodes for flexible quasi-solid-state asymmetric supercapacitor, Chem. Eng. J., 2022, 429, 132486 CrossRef.
- A. K. Das, U. N. Pan, V. Sharma, N. H. Kim and J. H. Lee, Nanostructured CeO2/NiV–LDH composite for energy storage in asymmetric supercapacitor and as methanol oxidation electrocatalyst, Chem. Eng. J., 2021, 417, 128019 CrossRef CAS.
- A. Ajay, A. Paravannoor, J. Joseph, V. Amruthalakshmi, S. S. Anoop, S. V. Nair and A. Balakrishnan, 2 D amorphous frameworks of NiMoO4 for supercapacitors: Defining the role of surface and bulk controlled diffusion processes, Appl. Surf. Sci., 2015, 326, 39–47 CrossRef.
- O. Rabin, J. M. Perez, J. Grimm, G. Wojtkiewicz and R. Weissleder, An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles, Nat. Mater., 2006, 5, 118–122 CrossRef CAS PubMed.
- V. Tezyk, C. Rossignol, N. Sergent, E. Djurado, J. Laurencin and E. Siebert, Cyclic voltammetry and high-frequency series resistance of La0.6Sr0.4Co0.2Fe0.8O3-δ electrode deposited on GDC: Effect of the electrode microstructure and the oxygen partial pressure, Electrochim. Acta, 2019, 304, 312–322 CrossRef CAS.
- Q. Fang, M. Sun, X. Ren, Y. Sun, Y. Yan, Z. Gan, J. Huang, B. Cao, W. Shen, Z. Li and Y. Q. Fu, Hydrothermal synthesis of cobalt sulfide nanotubes: The size control and its application in supercapacitors, J. Colloid Interface Sci., 2022, 611, 503–512 CrossRef CAS PubMed.
- P. Himasree, I. K. Durga, T. N. V. Krishna, S. S. Rao, C. V. V. Muralee Gopi, S. Revathi, K. Prabakar and H. J. Kim, One-step hydrothermal synthesis of CuS@MnS on Ni foam for high performance supercapacitor electrode material, Electrochim. Acta, 2019, 305, 467–473 CrossRef CAS.
- H. Lu, Q. Guo, F. Zan and H. Xia, Bi2S3 nanoparticles anchored on graphene nanosheets with superior electrochemical performance for supercapacitors, Mater. Res. Bull., 2017, 96, 471–477 CrossRef CAS.
- H. Wan, X. Ji, J. Jiang, J. Yu, L. Miao, L. Zhang, S. Bie, H. Chen and Y. Ruan, Hydrothermal synthesis of cobalt sulfide nanotubes: The size control and its application in supercapacitors, J. Power Sources, 2013, 243, 396–402 CrossRef CAS.
- M. Wang, H. Fei, P. Zhang and L. Yin, Hierarchically Layered MoS2/Mn3O4 Hybrid Architectures for Electrochemical Supercapacitors with Enhanced Performance, Electrochim. Acta, 2016, 209, 389–398 CrossRef CAS.
- S. Rafai, C. Qiao, M. Naveed, Z. Wang, W. Younas, S. Khalid and C. Cao, Microwave-anion-exchange route to ultrathin cobalt-nickel-sulfide nanosheets for hybrid supercapacitors, Chem. Eng. J., 2019, 362, 576–587 CrossRef CAS.
- F. Wu, X. Wang, W. Zheng, H. Gao, C. Hao and C. Ge, Synthesis and characterization of hierarchical Bi2MoO6/Polyaniline nanocomposite for all-solid-state asymmetric supercapacitor, Electrochim. Acta, 2017, 245, 685–695 CrossRef CAS.
- B. De, T. Kuila, N. H. Kim and J. H. Lee, Carbon dot stabilized copper sulphide nanoparticles decorated graphene oxide hydrogel for high performance asymmetric supercapacitor, Carbon, 2017, 122, 247–257 CrossRef CAS.
- Z. A. Shaikh, P. V. Shinde, S. F. Shaikh, A. M. Al-Enizi and R. S. Mane, Facile synthesis of Bi2O3@MnO2 nanocomposite material: A promising electrode for high performance supercapacitors, Solid State Sci., 2020, 102, 106158 CrossRef CAS.
- Q. X. Xia, N. M. Shinde, J. M. Yun, T. Zhang, R. S. Mane, S. Mathur and K. H. Kim, Bismuth Oxychloride/MXene symmetric supercapacitor with high volumetric energy density, Electrochim. Acta, 2018, 271, 351–360 CrossRef CAS.
- R. Rameshbabu, M. Sandhiya and M. Sathish, Fe(III) ions grafted bismuth oxychloride nanosheets for enhanced electrochemical supercapacitor application, J. Electroanal. Chem., 2020, 862, 113958 CrossRef CAS.
- A. M. Teli, T. S. Bhat, S. A. Beknalkar, S. M. Mane, L. S. Chaudhary, D. S. Patil, S. A. Pawar, H. Efstathiadis and J. Cheol Shin, Bismuth manganese oxide based electrodes for asymmetric coin cell supercapacitor, Chem. Eng. J., 2022, 430, 133138 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi00675e |
| This journal is © the Partner Organisations 2024 |