In situ built nanoconfined Nb2O5 particles in a 3D interconnected Nb2C MXene@rGO conductive framework for high-performance potassium-ion batteries†
Cong
Liu
ab,
Zhitang
Fang
a,
Weizhi
Kou
a,
Xiaoge
Li
c,
Jinhua
Zhou
d,
Gang
Yang
 d,
Luming
Peng
d,
Luming
Peng
 a,
Xuefeng
Guo
a,
Xuefeng
Guo
 a,
Weiping
Ding
a,
Weiping
Ding
 a and
Wenhua
Hou
a and
Wenhua
Hou
 *a
*a
aKey Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023, P. R. China. E-mail: whou@nju.edu.cn
bSchool of Materials Engineering, Jiangsu University of Technology, Changzhou 213001, P.R. China
cSchool of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225009, P. R. China
dSuzhou Key Laboratory of Functional Ceramic Materials, Changshu Institute of Technology, Changshu, 215500, P. R. China
First published on 10th November 2023
Abstract
Exploring novel anode materials with excellent electrochemical performance is of great significance for the development of potassium-ion batteries (KIBs). Here, a 3D interconnected Nb2C/rGO conductive framework with in situ generated Nb2O5 nanoparticles (Nb2O5/Nb2C/rGO) is successfully constructed by a simple one-step hydrothermal method and subsequent freeze-drying and annealing treatments. The unique structure formed by the intimate contact of the three components has a 3D conductive network, abundant pores and a large specific surface area, which can not only inhibit the self-restacking of Nb2C nanosheets and the agglomeration of Nb2O5 nanoparticles and alleviate the volume change during the charge–discharge process, but also expose more active sites and provide unimpeded channels for the diffusion of K+ and infiltration of the electrolyte. Meanwhile, Nb2O5 nanoparticles produced by in situ oxidation of surface Nb2C and the residual subsurface Nb2C with a low potassium ion diffusion barrier and a high conductivity can shorten the diffusion distance and promote the diffusion kinetics of electrons/ions. Benefiting from the elaborately designed structure and synergistic effects of three different components, as an anode for KIBs, the resulting Nb2O5/Nb2C/rGO exhibits a superior specific capacity of 410.6 mA h g−1 after 100 cycles at 0.1 A g−1, an exceptional rate performance of 159.0 mA h g−1 at 5 A g−1, a capacity retention of 88.8% and a coulombic efficiency over 99.8% after 1000 cycles at 2.0 A g−1. Moreover, Nb2O5/Nb2C/rGO also shows a good potassium storage performance in a KIB full-cell. Furthermore, the combined potassium storage mechanism of K+ intercalation/deintercalation is revealed by CV and in/ex situ analyses. This work can provide more meaningful guidance for the rational design and construction of anode materials for high-performance KIBs.
1. Introduction
Potassium-ion batteries (KIBs) are promising candidates for replacing lithium-ion batteries (LIBs) as large-scale energy storage devices due to their low cost and K/K+ potential (−2.93 V), abundant potassium resources, fast ionic conductivity in the electrolyte and similar “rocking chair” mechanism.1,2 Unfortunately, the widespread application of KIBs is still hindered owing to the large radius of K+ (1.38 Å), which will result in sluggish kinetics, low discharge capacity and large volume changes of the electrode material during repeated charge–discharge processes.3 Therefore, it remains a great challenge to develop suitable electrode materials with improved diffusion kinetics, a high specific discharge capacity and the capability of accommodating large volume changes.Undoubtedly, the intrinsic phase structure of electrode materials has an important influence on the electrochemical performance.3–6 Transition metal oxides (TMOs) are the research focus because of their advantages such as low cost, abundant resources, high theoretical specific capacity, safety and adjustable interlayer spacing.7,8 In particular, Nb2O5 is an ideal electrode material with merits of a relatively small volume change, good electrochemical stability, nontoxicity and environmental friendliness.9,10 In fact, Nb2O5 has been extensively studied in the field of ion batteries, showing considerable electrochemical performance.11 Nevertheless, the intrinsic low electrical conductivity (∼3 × 10−6 S cm−1) of Nb2O5 seriously limits its specific capacity and high rate capability.12
To address this problem, various strategies have been devised, such as controlling the morphology and structure, hybridization with highly conductive materials, heteroatom doping, etc.13–15 For instance, Zhang et al. synthesized a Nb2O5/C@N-coated carbon nanocomposite through a combination of hydrothermal and multi-step annealing treatments.16 As an anode for sodium ion batteries (SIBs) and KIBs, the obtained material showed relatively high specific capacities of 201 and 175 mA h g−1 after 100 cycles at a current density of 0.1 A g−1, respectively. Li et al. designed an urchin-like Nb2O5 nanocomposite encapsulated in nitrogen-doped carbon sheaths (Nb2O5/C), showing an enhanced rate capability and cycling stability as an anode of KIBs.17 Therefore, nanostructured Nb2O5-based composites with a short transport distance of electrons/ions, high electronic conductivity and excellent electrolyte accessibility are powerful guarantees for obtaining superior electrochemical performance.
Transition metal carbides (MXenes), another kind of typical layered material, have shown great competitiveness in efficient energy storage due to their unique 2D structure, high conductivity, large interlayer spacing, low diffusion barrier of ions (Li+, Na+, K+ and Mg2+) and so on.18 However, the large number of surface functional groups and self-restacking properties of MXenes significantly affect their electrochemical performance, leading to a limited potassium storage capacity (50–150 mA h g−1).19
In general, the electrochemical performance of MXenes can be effectively improved by changing the surface state of MXenes or constructing MXene-based nanocomposites.20,21 For instance, Gogotsi and co-workers used TiO2 and Ti3C2 as raw materials to synthesize TiO2/Ti3C2 nanocomposites through van der Waals interactions, showing improved lithium storage performance.22 The tightly coupled Ti3C2/NiCoP nanocomposite reported by Yin et al. has a unique structure with high conductivity and a large specific surface area, thus exhibiting increased sodium storage performance.23 Zhang et al. fabricated Ti3C2/MoS2 with a low concentration of surface functional groups by hydrothermal and annealing methods, possessing a relatively high specific capacity as an anode of KIBs.24 Besides, other nanomaterials, such as MnO2, Sn, Bi, Si, SnO2, etc., have also been introduced into MXenes to form the corresponding nanocomposites to improve the electrochemical performance.18,25 Nevertheless, the practical application of the above-mentioned methods is limited by the complex and cumbersome steps. On the other hand, the specific capacity and cycling stability of the obtained MXene-based nanocomposites still need to be further improved to meet the practical requirements. Therefore, developing simple and effective methods for the preparation of well-structured MXene-based nanocomposites with good electrochemical properties is of great significance.
Recent studies have shown that MXenes can be oxidized in situ to form composites of TMO nanoparticles and MXenes nanosheets due to the presence of thermodynamically metastable transition metal atoms on the surface.26,27 For example, Gogotsi et al. once prepared Nb2O5@Nb4C3Tx and TiO2@Ti3C2Tx nanocomposites by simple one-step oxidation of the corresponding MXenes in a CO2 atmosphere, exhibiting a rather good cycle performance in Li-ion capacitors.26 Yuan's group constructed an accordion-like TiO2/Ti3C2Tx composite by the hydrothermal treatment of Ti3C2Tx MXenes, exhibiting high specific capacities of 267 and 101 mA h g−1 at a current density of 0.2 A g−1 as anodes for LIBs and SIBs, respectively.27 Therefore, the in situ oxidation of MXenes is an effective strategy to obtain TMO/MXene nanocomposites, which not only greatly suppresses the self-restacking of MXenes, but also fully utilizes the properties of TMO nanoparticles.
On the other hand, the introduction of 3D structures with abundant pores and a large specific surface area into MXenes, TMOs and their composites can further improve their specific capacity and diffusion kinetics.28,29 For example, Tong et al. once designed 3D porous Nb2O5/rGO composites, showing reversible capacities of 225 and 160 mA h g−1 as anodes of SIBs and KIBs at a current density of 0.2 A g−1.29 The sandwich-structured TiO2/Ti3C2/rGO prepared by Zhang et al. shows high energy and power densities as an anode for Li-ion capacitors.28 In addition, other 3D porous structures, such as Ti3C2 hollow spheres and Ti3C2/rGO aerogels, have also been reported.30 These results indicate that well-designed 3D TMO/MXene/rGO nanocomposites with high conductivity, a small crystal size and an abundant porous structure can overcome the limitations of TMOs and MXenes to obtain high specific capacity and excellent cycling stability.
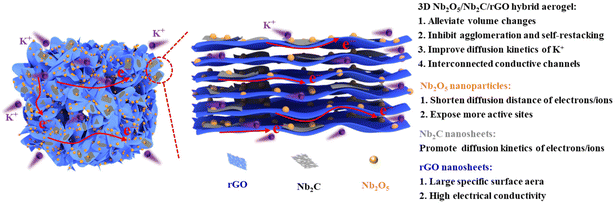
To date, Ti3C2-derived TiO2/Ti3C2 nanocomposites have been intensively studied in different fields.31–34 Compared with Ti3C2, Nb2C has a relatively higher practical capacity (170 mA h g−1vs. 100.0 mA h g−1 for Ti3C2) and a much lower diffusion barrier of K+ (0.004 eV vs. 0.103 eV for Ti3C2).35 Therefore, it is reasonable to expect that a better electrochemical performance can be obtained by using Nb2C as the matrix material. For example, Nb2C/rGO hybrid aerogels were synthesized by a low-temperature hydrothermal self-assembly method in our previous work, achieving an excellent potassium storage performance.36 However, the presence of thermodynamically metastable transition metal atoms on the surface of Nb2C imposes extremely strict requirements on the practical use and storage conditions of Nb2C/rGO. This problem could be effectively solved by converting Nb2C/rGO into Nb2O5/Nb2C/rGO with good environmental stability through in situ oxidation. Meanwhile, the in situ generated Nb2O5 nanoparticles with a small volume effect can also expose more active sites and shorten the diffusion distance of ions/electrons. Undoubtedly, it can be expected that 3D Nb2O5/Nb2C/rGO hybrid aerogels with the above advantages will have a potential application in KIBs. Nevertheless, until now there is no report on Nb2O5/Nb2C/rGO as an anode for KIBs and the related energy storage mechanisms.
Based on the above considerations, in this work, the 3D interconnected Nb2O5/Nb2C/rGO conductive framework is successfully synthesized by a simple one-step hydrothermal method and subsequent freeze-drying and annealing treatments. The morphology, microstructure and chemical composition of the 3D porous aerogel, Nb2O5 nanoparticles formed by in situ oxidation of Nb2C and residual Nb2C nanosheets are characterized in detail. The electrochemical properties and potassium ion diffusion behaviors of the prepared samples are investigated thoroughly, and the structure–property relationship is disclosed. Moreover, the synergistic potassium storage mechanism of K+ intercalation/deintercalation is also revealed explicitly through CV and in/ex situ analyses.
2. Experimental section
2.1 Materials
Tetramethylammonium hydroxide ((CH3)4NOH, TMAOH, AR), ethanol (C2H5OH, AR) and hydrofluoric acid (HF, 49 wt%) were obtained from Sinopharm Chemical Reagent Co., Ltd. Nb2AlC was purchased from Nanjing Mission New Materials Co., Ltd.2.2 Synthesis of the Nb2O5/Nb2C/rGO hybrid aerogel
Firstly, Nb2CTx and rGO suspensions were obtained according to our previous report.36 Subsequently, the mixed suspensions of Nb2CTx (50.0 mL, 5.6 mg mL−1), rGO (9.5 mL, 15.2 mg mL−1) and deionized water (10.0 mL) were stirred for 1 h, sonicated for 1 h, and then transferred to a 100 mL reactor and hydrothermally treated at 180 °C for 12 h. After cooling to room temperature, the obtained Nb2O5/Nb2CTx/rGO hybrid hydrogel was washed three times with deionized water and then freeze-dried to obtain the Nb2O5/Nb2CTx/rGO hybrid aerogel. Finally, the Nb2O5/Nb2CTx/rGO aerogel was annealed at 600 °C for 3 h to obtain the Nb2O5/Nb2C/rGO hybrid aerogel with high crystallinity and a low concentration of surface functional groups.For comparison, the Nb2O5/Nb2CTx/rGO aerogel and Nb2CTx nanosheets were respectively soaked in deionized water containing H2O2 (10 mL) for 24 h, resulting in a fully oxidized Nb2O5/rGO hybrid aerogel and Nb2O5 nanoparticles. The other steps were consistent with those for the preparation of the Nb2O5/Nb2C/rGO hybrid aerogel.
2.2 Characterization
The structure, surface morphology and composition of the as-prepared samples were identified using an X-ray diffractometer (XRD, Cu Kα, λ = 1.5418 Å, Philips X′Pert), a scanning electron microscope (SEM, JEOL JEM-6300F), a transmission electron microscope (TEM, JEOL JEM-2100F) and an X-ray photoelectron spectrometer (XPS, ESCALAB250Xi). The specific surface area and pore-size distribution were measured using a Micromeritics ASAP 2020 surface area analyzer. TG analyses (Netzsch STA449C) were performed to determine the content of rGO in the hybrid aerogel. Raman spectra were collected on an automatic laser confocal Raman microspectrometer (LabRAM Aramis).2.3 Electrochemical measurements
The working electrode was prepared by mixing the active material (70 wt%), Super P (15 wt%) and polyvinylidene difluoride (PVDF, 15 wt%) in N-methyl-2-pyrrolidone (NMP) and stirring for about 24 h to obtain a uniform dispersion slurry. The mixed slurry was coated on copper foil and dried at 100 °C for 10 h. Subsequently, the obtained working electrode (0.7–1.0 mg cm−2) was assembled into a 2032 type coin cell, in which potassium foil was used as the counter electrode, a glass microfiber filter (Whatman, GF/D) was utilized as the separator and 0.8 M KPF6 EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) was employed as the electrolyte (0.02 mL). The assembly of the KIB full cell is consistent with the above-mentioned half cells except that Prussian blue (PB, KxFe[Fe(CN)6]) was used as the cathode material. The cathode was prepared by coating a slurry mixture of Prussian blue (70 wt%), carbon black (15 wt%) and PVDF (15 wt%) in NMP onto aluminium foil. The designed amount of the cathode material was 1.1 times that of the anode material. Moreover, in order to better reveal the characteristics of the full cell, the anode and cathode electrodes were first discharged and charged for 3 cycles to form a stable SEI.
1 by volume) was employed as the electrolyte (0.02 mL). The assembly of the KIB full cell is consistent with the above-mentioned half cells except that Prussian blue (PB, KxFe[Fe(CN)6]) was used as the cathode material. The cathode was prepared by coating a slurry mixture of Prussian blue (70 wt%), carbon black (15 wt%) and PVDF (15 wt%) in NMP onto aluminium foil. The designed amount of the cathode material was 1.1 times that of the anode material. Moreover, in order to better reveal the characteristics of the full cell, the anode and cathode electrodes were first discharged and charged for 3 cycles to form a stable SEI.
Galvanostatic charge–discharge (GCD) and galvanostatic intermittent titration technique (GITT) tests were performed on the LAND test instrument (CT2001A, Wuhan) within the voltage range of 0.01–3.0 V. Electrochemical impedance spectra (EIS, 0.01–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz, 5 mV) and cyclic voltammetry (CV) curves were collected at the electrochemical workstation (CHI 660E, Chenhua).
000 Hz, 5 mV) and cyclic voltammetry (CV) curves were collected at the electrochemical workstation (CHI 660E, Chenhua).
3. Experimental section
3.1 Synthesis and characterization
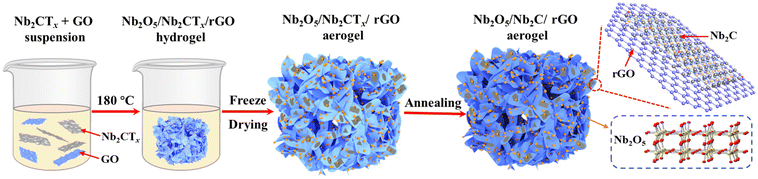
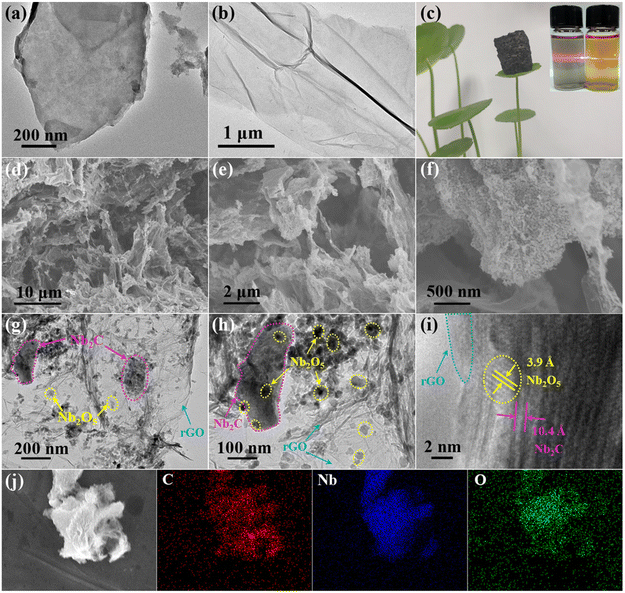
Fig. 1 shows a schematic illustration for the preparation of the Nb2O5/Nb2C/rGO aerogel. The suspension of Nb2CTx nanosheets was obtained by etching Nb2AlC powder in HF, followed by exfoliation through intercalation and sonication.36 To assemble Nb2CTx nanosheets into a 3D porous structure, GO nanosheets with a strong gelling ability were selected as the gelling agent due to the weak self-gelling ability of Nb2CTx. Benefiting from the similar hydrophilicity and negative charge of Nb2CTx and GO nanosheets, a stable homogeneously mixed suspension of Nb2CTx and GO nanosheets can be easily formed in water. During the subsequent hydrothermal reaction, the hydrophilic GO was reduced to rGO with a more hydrophobic surface, and the resulting rGO nanosheets formed a 3D interconnected framework structure. Meanwhile, Nb2CTx nanosheets were self-assembled onto the surface of rGO nanosheets in a face-to-face form via interfacial interaction, thus generating the Nb2O5/Nb2CTx/rGO hybrid hydrogel, in which Nb2O5 nanoparticles came from the in situ surface oxidation of Nb2CTx nanosheets at high temperature. Finally, the Nb2O5/Nb2C/rGO hybrid aerogel was obtained through freeze-drying and annealing the Nb2O5/Nb2CTx/rGO hybrid hydrogel.SEM and (HR)TEM were applied to observe the morphology of the prepared samples. As shown in Fig. S1,† the raw material Nb2AlC has a densely-packed bulk structure, which can be transformed into multilayered Nb2CTx (m-Nb2CTx) with a typical accordion-like structure after HF etching. Fig. 2a depicts the TEM images of single- and few-layered Nb2CTx nanosheets obtained by exfoliating m-Nb2CTx with TMAOH intercalation and sonication. It can be observed that they have a smooth surface. Besides, the translucent state of Nb2CTx nanosheets indicates that they have an ultrathin layer thickness. By contrast, GO nanosheets not only have an ultrathin thickness, but also a wrinkled surface with large lateral dimensions (Fig. 2b). The large GO nanosheets can easily wrap small Nb2CTx nanosheets under the interfacial interaction.37 It is verified by the Tyndall effect that Nb2CTx and GO nanosheets with abundant surface functional groups can be stably dispersed in water (the inset of Fig. 2c), which is the prerequisite for the preparation of electrode materials.38Fig. 2c demonstrates the ultra-light property of the obtained Nb2O5/Nb2C/rGO aerogel.
As detailed in Fig. 2d, Nb2O5/Nb2C/rGO has a randomly distributed porous structure. The pores are interconnected, with a size of 10 to 20 μm, which can not only promote the diffusion of ions/electrons, but also accommodate volume changes. From the magnified SEM image of the Nb2O5/Nb2C/rGO aerogel (Fig. 2e and f), it can be seen that the aerogel is composed of wrinkled nanosheets and the nanosheets are interconnected, being beneficial for the transport of electrons.
As shown in Fig. S2 (ESI†), the fully oxidized Nb2O5/rGO sample exhibits a similar aerogel structure to Nb2O5/Nb2C/rGO. However, a large amount of Nb2O5 nanoparticles almost completely cover rGO, and even lead to the blockage of the porous structure, being not conducive to the transport of electrolyte ions. On the other hand, in the Nb2O5 sample without rGO (Fig. S3, ESI†), severe agglomeration of Nb2O5 nanoparticles is observed. This further indicates that the addition of graphene effectively prevents the self-restacking of Nb2C nanosheets and the agglomeration of Nb2O5 nanoparticles.
From Fig. 2g it is once again proved that Nb2O5/Nb2C/rGO has a rich porous structure. It is also observed that Nb2C nanosheets adhered tightly to rGO nanosheets in a face-to-face form (Fig. 2h). Being relative to the initial Nb2C nanosheets (Fig. 2a), the smaller Nb2C nanosheets in Nb2O5/Nb2C/rGO indicate that Nb2C nanosheets undergo structural degradation and partial oxidation under high temperature conditions. As a result, it can be observed that Nb2O5 nanoparticles with an average diameter of 20–40 nm are tightly and homogeneously anchored on the surface of Nb2C and/or rGO nanosheets (Fig. 2h), thus facilitating the electron transport of Nb2O5 nanoparticles through the interconnected highly conductive aerogel structure.
In the HRTEM image (Fig. 2i), the clear lattice fringes indicate the high crystallinity of Nb2O5/Nb2C/rGO. The interlayer spacings of 10.4 and 3.9 Å match well with the (002) plane of Nb2C and the (001) plane of orthorhombic Nb2O5, respectively.29,35,39 Besides, the HRTEM image also shows good integration of Nb2C nanosheets and Nb2O5 nanoparticles within the amorphous rGO network. Generally speaking, the diffusion barrier of the electrolyte ions in the (001) plane of Nb2O5 is much lower than that of other directions.39 Therefore, the combination of nano-sized Nb2O5 with a preferentially exposed (001) plane is expected to provide a high energy storage performance. In addition, the elemental mapping images in Fig. 2j show the uniform distribution of C, Nb and O in the Nb2O5/Nb2C/rGO aerogel, proving that Nb2C and Nb2O5 are evenly adhered to the rGO nanosheets.
To investigate the crystalline structure of the as-prepared samples, XRD was employed. As displayed in Fig. S4,† Nb2AlC has typical characteristic diffraction peaks (JCPDS No. 30-0033).35 As expected, the diffraction peaks of Nb2AlC undergo obvious changes after HF treatment. For example, the (002) diffraction peak of the resulting m-Nb2CTx shifts to a low 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ angle, which indicates the successful removal of Al layers from Nb2AlC.35
θ angle, which indicates the successful removal of Al layers from Nb2AlC.35
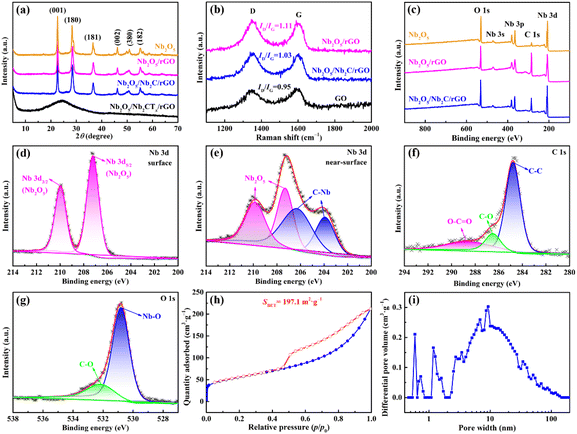
Fig. 3a presents XRD patterns of the as-prepared samples. The obtained Nb2O5/Nb2CTx/rGO aerogel has a distinct diffraction peak of rGO at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 26.0°, indicating that GO is successfully reduced to rGO.40,41 Nevertheless, no diffraction peaks of Nb2O5 and Nb2CTx are detected in the Nb2O5/Nb2CTx/rGO aerogel without annealing. It could be related to the amorphous nature of Nb2O5 and highly dispersed ultrathin Nb2CTx nanosheets.42–44 After annealing at 600 °C, new peaks centered at 22.63°, 28.35°, 36.59°, 46.14°, 50.89° and 55.15° appear in the obtained Nb2O5/Nb2C/rGO aerogel. These peaks correspond to the (001), (180), (181), (002), (380) and (182) crystal planes of Nb2O5, respectively, proving the formation of well-crystalline orthorhombic Nb2O5 (T-Nb2O5, JCPDS No. 30-0873).15,29,42 In addition, Nb2O5 and Nb2O5/rGO also exhibit typical diffraction peaks of T-Nb2O5, suggesting that Nb2CTx in the raw materials of these two samples is successfully oxidized to Nb2O5.
θ = 26.0°, indicating that GO is successfully reduced to rGO.40,41 Nevertheless, no diffraction peaks of Nb2O5 and Nb2CTx are detected in the Nb2O5/Nb2CTx/rGO aerogel without annealing. It could be related to the amorphous nature of Nb2O5 and highly dispersed ultrathin Nb2CTx nanosheets.42–44 After annealing at 600 °C, new peaks centered at 22.63°, 28.35°, 36.59°, 46.14°, 50.89° and 55.15° appear in the obtained Nb2O5/Nb2C/rGO aerogel. These peaks correspond to the (001), (180), (181), (002), (380) and (182) crystal planes of Nb2O5, respectively, proving the formation of well-crystalline orthorhombic Nb2O5 (T-Nb2O5, JCPDS No. 30-0873).15,29,42 In addition, Nb2O5 and Nb2O5/rGO also exhibit typical diffraction peaks of T-Nb2O5, suggesting that Nb2CTx in the raw materials of these two samples is successfully oxidized to Nb2O5.
To determine the structural characteristics of the prepared samples, Raman spectra were recorded. As shown in Fig. 3b, two broad peaks are observed at 1350 and 1590 cm−1, which can be indexed to the D-band (disordered carbon) and the G-band (ordered crystalline graphite), respectively.29,45 In general, the intensity ratio of the D- to G-band (ID/IG) can be used to measure the degree of disorder and defects in the prepared materials.35 The increased ID/IG value from 0.95 for GO to 1.03 for Nb2O5/Nb2C/rGO and 1.11 for Nb2O5/rGO indicates that GO has been successfully reduced in the hydrothermal process.46 Compared with Nb2O5/rGO, the higher graphitization degree of Nb2O5/Nb2C/rGO is beneficial for improving the electronic conductivity.35 Furthermore, according to TGA (Fig. S5, ESI†), the content of rGO in the Nb2O5/Nb2C/rGO sample is calculated to be 27.0 wt%.
To reveal the surface and near-surface elemental compositions of Nb2O5, Nb2O5/rGO and Nb2O5/Nb2C/rGO samples, XPS tests were carried out. As shown in Fig. 3c, the survey spectra prove the existence of C, O and Nb elements in all prepared samples. Compared to Nb2O5, the significant increase of the C element in Nb2O5/rGO and Nb2O5/Nb2C/rGO indicates the successful introduction of rGO. The absence of the F element indicates that it is successfully removed during the synthetic process, which can greatly reduce side reactions and facilitate the transport of electrolyte ions.47
As illustrated in Fig. 3d, the high-resolution Nb 3d XPS spectrum for the surface of the Nb2O5/Nb2C/rGO sample shows two peaks at 210.0 eV (Nb 3d3/2) and 207.2 eV (Nb 3d5/2), consistent with the binding energies of Nb2O5.29 This suggests that a substantial amount of Nb2C on the surface of Nb2C/rGO samples is converted into Nb2O5, being in agreement with the above XRD results. The surface layer of the Nb2O5/Nb2C/rGO sample is removed by Ar+ sputtering for 300 s, and it is obvious that the high-resolution Nb 3d XPS spectrum for the near-surface of Nb2O5/Nb2C/rGO shows a great difference. Specifically, the near-surface of Nb2O5/Nb2C/rGO exhibits four peaks at 210.0, 207.2, 206.3 and 204.0 eV, respectively (Fig. 3e). The first two peaks are assigned to Nb2O5, while the last two peaks can be indexed to C–Nb bonds.29,35 This indicates that the interior of the Nb2O5/Nb2C/rGO sample is closely related to Nb2C. That is, after hydrothermal and annealing treatments, the surface Nb2C is oxidized to Nb2O5, while the pristine Nb2C is still present in the interior bulk of the material. In contrast, Nb 3d spectra of Nb2O5/rGO and Nb2O5 (Fig. S6, ESI†) show that only Nb2O5 exists on the surface and near-surface, which further proves that Nb2C is completely oxidized with the assistance of H2O2.
As shown in Fig. 3f, the C 1s spectrum of Nb2O5/Nb2C/rGO can be deconvoluted into three peaks centered at 288.6 eV, 286.5 eV and 284.8 eV, which are assigned to O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O and C–C, respectively.35 As displayed in Fig. 3g, the O 1s spectrum can only be divided into two peaks, namely the C–O peak (532.2 eV) and the Nb–O peak (530.8 eV).35 These results suggest that –OH is successfully removed after the annealing process, which is in favor of the transport of electrolyte ions.47
O, C–O and C–C, respectively.35 As displayed in Fig. 3g, the O 1s spectrum can only be divided into two peaks, namely the C–O peak (532.2 eV) and the Nb–O peak (530.8 eV).35 These results suggest that –OH is successfully removed after the annealing process, which is in favor of the transport of electrolyte ions.47
The specific surface area and porous nature of Nb2O5/Nb2C/rGO, Nb2O5/rGO and Nb2O5 samples were examined using N2 adsorption–desorption isotherms. As displayed in Fig. 3h, typical type I and IV isotherms indicate that there are abundant micropores and mesopores in Nb2O5/Nb2C/rGO.16,47 Obviously, Nb2O5/Nb2C/rGO exhibits a larger specific surface area of 197.1 m2 g−1 compared with that of Nb2O5/rGO (127.6 m2 g−1) and Nb2O5 (70.8 m2 g−1) (Fig. S7a, ESI†). The largest specific surface area of Nb2O5/Nb2C/rGO may be attributed to the 3D interconnected porous structure of the aerogel and the well-dispersed Nb2O5 nanoparticles on the surface of Nb2C and/or rGO. The significant decrease in the specific surface area of the Nb2O5/rGO sample is related to the large number of aggregated Nb2O5 nanoparticles generated by the complete oxidation of Nb2C, which severely blocked the porous structure of the resulting aerogel. The smallest specific surface area of Nb2O5 is caused by the serious agglomeration of Nb2O5 nanoparticles and the self-restacking of Nb2C. As shown in Fig. 3i and Fig. S7b (ESI†), the Nb2O5/Nb2C/rGO sample contains abundant mesopores that may originate from the 3D porous structure of the aerogel, while both Nb2O5/rGO and Nb2O5 lack this important feature. In addition, Nb2O5/Nb2C/rGO also has the largest total pore-volume (0.33 cm3 g−1vs. 0.17 cm3 g−1 for Nb2O5/rGO and 0.16 cm3 g−1 for Nb2O5). Benefiting from the abundant mesopores, large pore-volume and high specific surface area of Nb2O5/Nb2C/rGO, it can ensure sufficient contact area between the active material and electrolyte and promote the migration of electrolyte ions, being conducive to the improvement of specific capacity and rate capability.
3.2 Potassium storage performance
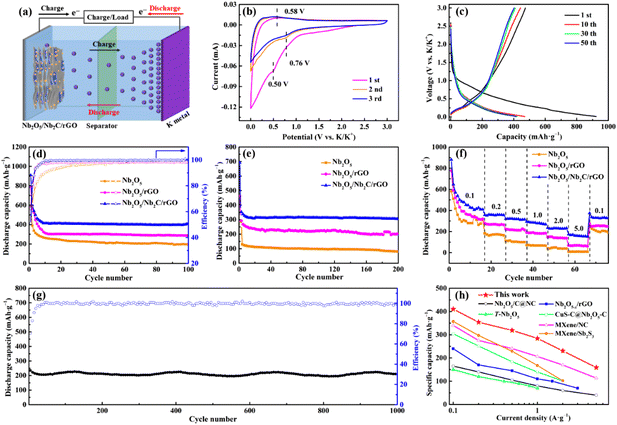
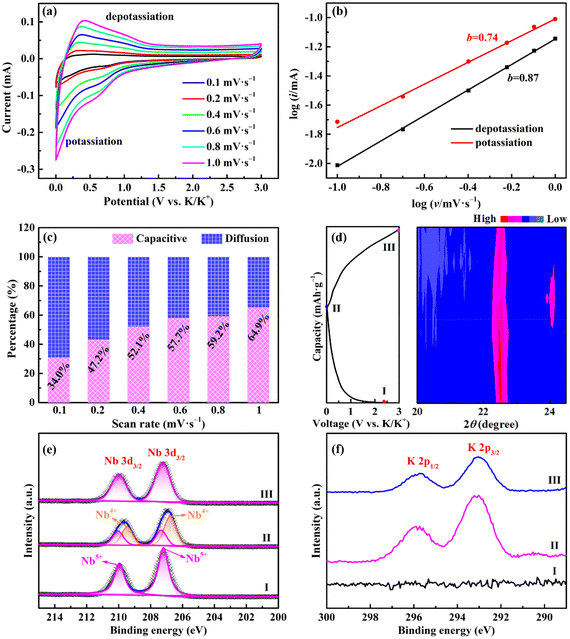
To assess the advantages of the as-prepared samples, KIBs were assembled and tested under different conditions. Fig. 4a is a schematic illustration of the working principle of KIBs. K+ ions will be intercalated/adsorbed to the host material in the initial discharging process along with the gradual decrease of the voltage of KIBs. During the subsequent charging process, the intercalated/adsorbed K+ ions will be deintercalated/desorbed from the host material and move to the counter electrode simultaneously.1Fig. 4b presents typical CV curves of Nb2O5/Nb2C/rGO at a scan rate of 0.1 mV s−1. A distinct cathodic peak appears at 0.50 V in the first cycle and disappears during the subsequent cycles, which can be attributed to the formation of a SEI film.23,35 Another cathodic peak at 0.76 V is derived from the intercalation of K+ into Nb2C (Nb2C + xK+ + xe− → KxNb2C). Moreover, the anodic peak at 0.58 V corresponds to the deintercalation of K+ from Nb2O5/Nb2C/rGO during the charging process.23 Encouragingly, after the first cycle, the almost completely overlapped CV curves suggest that the electrochemical reaction of Nb2O5/Nb2C/rGO is highly reversible during potassiation/depotassiation processes.
Fig. 4c shows GCD profiles of Nb2O5/Nb2C/rGO at a current density of 0.05 A g−1. There is no obvious charge–discharge plateau throughout the potassiation/depotassiation process, which is consistent with previous reports.15,16,35 A high specific discharge capacity of 473.0 mA h g−1 is achieved in the first cycle with a corresponding coulombic efficiency (CE) of 51.3%. The large capacity loss in the first cycle is quite normal.48 On the one hand, it is caused by the formation of a SEI, and on the other hand, it may be ascribed to the side reactions of K+ with the –O group and carbon defects on rGO nanosheets and the irreversible insertion/adsorption of K+ in the aerogel with a porous structure.49 However, the CE of Nb2O5/Nb2C/rGO rapidly reaches above 98.0% in the 10th cycle and remains stable during the subsequent cycles, implying the good reversibility of Nb2O5/Nb2C/rGO.50,51
Fig. 4d displays the specific discharge capacities and CEs of Nb2O5/Nb2C/rGO, Nb2O5/rGO and Nb2O5 at 0.1 A g−1. Notably, the initial CE of Nb2O5/Nb2C/rGO (50.9%) is lower than those of Nb2O5 (55.2%) and Nb2O5/rGO (62.5%), which may be caused by the larger specific surface area of Nb2O5/Nb2C/rGO. However, Nb2O5/Nb2C/rGO renders a high reversible capacity of 410.6 mA h g−1 after 100 cycles, far exceeding 287.1 mA h g−1 of Nb2O5/rGO and 195.4 mA h g−1 of Nb2O5 in this paper and 352.3 mA h·g−1 of Nb2C/rGO-2 in our previous work.36 In addition, Nb2O5/Nb2C/rGO also exhibits good cycling stability. As shown in Fig. 4e, the specific capacity of Nb2O5/Nb2C/rGO remains at 304.8 mA h g−1 after 200 cycles at a current density of 0.5 A g−1, the corresponding capacity retention is 90.3% relative to the 5th cycle. By comparison, the capacities of Nb2O5/rGO and Nb2O5 decrease significantly to 200.3 and 80.4 mA h g−1, and the corresponding capacity retentions are 74.7 and 62.7% relative to the 5th cycle, respectively. Overall, Nb2O5/Nb2C/rGO has the best specific discharge capacity and cycling stability.
The rate capabilities of Nb2O5/Nb2C/rGO, Nb2O5/rGO and Nb2O5 were also tested to evaluate the practical application potential. Fig. S8 (ESI†) reveals the GCD curves of Nb2O5/Nb2C/rGO at different current densities. It can be seen that the shape of GCD curves does not notably change with the increase of current density, except for the gradual decrease of specific capacity, which further proves the reversibility of the electrochemical reaction. As shown in Fig. 4f, Nb2O5/Nb2C/rGO presents a high reversible capacity of 410.2 mA h g−1 at 0.1 A g−1. As the current density is gradually increased to 0.2, 0.5, 1.0 and 2.0 A g−1, Nb2O5/Nb2C/rGO delivers good capacity retention with specific discharge capacities of 354.0, 318.7, 284.4 and 230.5 mA h g−1, respectively. Even at a high current density of 5.0 A g−1, a discharge capacity of 159.0 mA h g−1 can still be obtained. More strikingly, Nb2O5/Nb2C/rGO still provides a high specific capacity of 329.5 mA h g−1 when the current density is restored to 0.1 A g−1. However, for Nb2O5/rGO, it just displays the discharge capacities of 320.1, 265.0, 213.1, 184.0, 139.6 and 69.4 mA h g−1 at 0.1, 0.2, 0.5, 1.0, 2.0 and 5.0 A g−1. Even worse, Nb2O5 has almost lost its capacity when the current density is increased to 5 A g−1. The discharge capacity of Nb2O5/rGO and Nb2O5 degrades sharply with the current density, which is mainly caused by the limited transfer ability of ions and/or electrons at a high current density.
Furthermore, Nb2O5/Nb2C/rGO also exhibits promising long-term cycling stability, as illustrated in Fig. 4g. Distinctly, Nb2O5/Nb2C/rGO realizes an ultra-stable capacity of 208.9 mA h g−1 over 1000 cycles at 2 A g−1. Not only that, it also has a high capacity retention of 88.8% and CE of over 99.5%. It is worth mentioning that the preeminent electrochemical performance of Nb2O5/Nb2C/rGO as an anode of KIBs in this work exceeds those of all Nb2O5-based materials, as well as most anode materials in the literature (Fig. 4h and Table S1†).15,16,29,52–54
3.3 Potassium storage mechanism
To gain a deep insight into the excellent K+ storage performance of Nb2O5/Nb2C/rGO, a series of CV tests were carried out. Fig. 5a depicts CV curves at different scan rates (0.1–1.0 mV s−1). According to the previous literature, the overall specific capacity usually comes from the contribution of capacitive- and diffusion-controlled behaviors. The capacitive-controlled behavior can be qualitatively analyzed by the value of b in the following equation:15,16Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (i) = blog (i) = blog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (v) + log (v) + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (a) (0.5 ≤ b ≤ 1) (a) (0.5 ≤ b ≤ 1) | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (i) versus log
(i) versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (v). Typically, the value of b varies between 0.5 for diffusion-controlled behavior and 1.0 for capacitive-controlled behavior. As shown in Fig. 5b, the b-values of potassiation and depotassiation peaks of Nb2O5/Nb2C/rGO are 0.74 and 0.87, respectively, indicating that the electrochemical reaction combines capacitive and diffusion behaviors.
(v). Typically, the value of b varies between 0.5 for diffusion-controlled behavior and 1.0 for capacitive-controlled behavior. As shown in Fig. 5b, the b-values of potassiation and depotassiation peaks of Nb2O5/Nb2C/rGO are 0.74 and 0.87, respectively, indicating that the electrochemical reaction combines capacitive and diffusion behaviors.
The contribution of diffusion and capacitive behaviors in the total specific capacity can also be quantified using the following equation:15,16
| i(v) = k1v + k2v1/2 | (2) |
To further illustrate the structural evolution of Nb2O5/Nb2C/rGO during potassiation/depotassiation, in situ XRD and ex situ XPS were employed at selected potentials. As shown in Fig. 5d, along with the proceeding of the potassiation process, the typical (001) diffraction peak of Nb2O5 gradually shifts to a low angle. Meanwhile, the intensity reduces greatly. This indicates that K+ is successfully intercalated into the Nb2O5 lattice, and the intercalation of K+ may lead to a decrease in crystallinity.16,17 In contrast, the (001) diffraction peak returns to the original position during the subsequent charging process, corresponding to the reversible deintercalation of K+ from the Nb2O5 lattice.17 In addition, a new peak appears at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 24.0° (KC8) upon discharging to 0.01 V and disappears during the subsequent charging process, indicating that K+ can also be reversibly intercalated/deintercalated in rGO.49
θ = 24.0° (KC8) upon discharging to 0.01 V and disappears during the subsequent charging process, indicating that K+ can also be reversibly intercalated/deintercalated in rGO.49
Combined CV (Fig. 4b), HRTEM (Fig. S10, ESI†) and in situ XRD results, it can be suggested that the following reactions occur in the potassiation/depotassiation process:
| Nb2C + xK+ + xe− ↔ KxNb2C | (3) |
| Nb2O5 + yK+ + ye− ↔ KyNb2O5 | (4) |
| 8C + zK+ + ze− ↔ KzC8 | (5) |
Fig. 5e and Fig. S11 (ESI†) show the Nb 3d high-resolution XPS spectra of Nb2O5/Nb2C/rGO at different states. The binding energies of Nb 3d3/2 and Nb 3d5/2 are shifted from 210.0 and 207.2 eV (fresh electrode, spectrum I in Fig. 5e) to lower binding energies of 209.7 and 206.9 eV (fully potassiated electrode, spectrum II), indicating the formation of Nb4+.29 After charging to 3.0 V (spectrum III), the two peaks almost return to their original positions, revealing a reversible electrochemical redox reaction of Nb.15,55
The K 2p high-resolution XPS spectra were also recorded at different states. As shown in Fig. 5f, compared with the fresh electrode (spectrum I), two peaks can be observed at 295.9 eV (K 2p1/2) and 293.0 eV (K 2p3/2) for the fully potassiated electrode (spectrum II), proving the intercalation of K+.49,56 On the other hand, the intensity of K 2p peak is significantly weakened in the fully depotassiated electrode (spectrum III), once again demonstrating the reversible deintercalation of K+ during the cycling.57 The remaining K 2p peaks may be attributed to different potassium salts in the SEI film.58
3.4 Diffusion kinetics
To investigate the diffusion capability of K+ inside the electrode material, the K+ diffusion coefficients (DK+s) of Nb2O5/Nb2C/rGO, Nb2O5/rGO and Nb2O5 were obtained through GITT. As shown in Fig. 6a, the GITT curves were recorded at a current density of 0.1 A g−1 with a galvanostatic pulse of 10 min and a relaxation of 30 min. DK+ values of the prepared samples were calculated based on the GITT curves using the following equation:59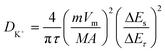 | (6) |
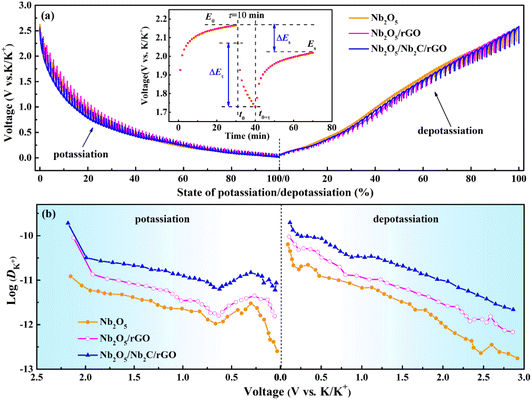 | ||
| Fig. 6 (a) GITT curves (inset shows the single-step GITT titration of Nb2O5/Nb2C/rGO) and (b) diffusion coefficients of K+ for Nb2O5/Nb2C/rGO, Nb2O5/rGO and Nb2O5. | ||
The improved diffusion kinetics of ions/electrons was further verified by EIS. As shown in Fig. S12,† the EIS spectra of Nb2O5/Nb2C/rGO, Nb2O5/rGO and Nb2O5 have similar compositions. It can be fitted using an equivalent circuit including solution resistance (Rs), charge transfer resistance (Rct) and Warburg impedance (Ws).40 All samples exhibit similar Rs, but their Rct values have a great difference. Compared with 875 Ω of Nb2O5/rGO and 1160 Ω of Nb2O5, Nb2O5/Nb2C/rGO displays the smallest Rct value of 720 Ω, suggesting the fastest charge transfer in Nb2O5/Nb2C/rGO. This may be ascribed to the tight coupling of Nb2C, Nb2O5 and rGO, and the high conductivity of the residual Nb2C and rGO. In addition, the largest linear slope of Nb2O5/Nb2C/rGO in the low frequency region implies the smallest Ws, i.e., the smallest diffusion resistance of K+ inside the Nb2O5/Nb2C/rGO electrode, being in good agreement with the above GITT results.35 Hence, the optimized electron/ion diffusion capability helps Nb2O5/Nb2C/rGO achieve preeminent rate performance.
3.5 Structural stability
To intuitively verify the structural stability of Nb2O5/Nb2C/rGO, Nb2O5/rGO and Nb2O5, the SEM images after 100 cycles at a current density of 0.1 A g−1 were recorded. As illustrated in Fig. S13 (ESI†), the structural integrities of Nb2O5/rGO and Nb2O5 electrodes are severely damaged. For example, the porous structure of the Nb2O5/rGO electrode undergoes partial collapse and the agglomeration of Nb2O5 nanoparticles further blocks the pores; and the Nb2O5 electrode shows obvious cracks. However, compared with the initial morphology (Fig. 2d–f), the 3D porous structure of the Nb2O5/Nb2C/rGO electrode as well as the Nb2O5 nanoparticles are basically preserved, highlighting the structural stability of Nb2O5/Nb2C/rGO.3.6 Electrochemical performance of the KIB full cell
Nb2O5/Nb2C/rGO shows excellent electrochemical performance in the above-mentioned half-cells. To demonstrate the feasibility of Nb2O5/Nb2C/rGO for practical applications, a full-cell was assembled by using Nb2O5/Nb2C/rGO as the anode and Prussian blue (PB, KxFe[Fe(CN)6]) as the cathode. The GCD curves of the full cell at a current density of 0.1 A g−1 are presented in Fig. S14b (ESI†), the charge and discharge capacities are 160.0 and 86.8 mA h g−1 (based on the weight of the cathode), respectively. Moreover, the assembled full-cell also exhibits good cycling stability. As shown in Fig. S14c (ESI†), the specific capacity of Nb2O5/Nb2C/rGO//PB remains at 75.1 mA h g−1 after 100 cycles at a current density of 0.1 A g−1, and the corresponding capacity retention is 86.5%. Interestingly, the fully charged full-cell can successfully light up 34 light-emitting diodes (LEDs), proving that the Nb2O5/Nb2C/rGO anode has a good prospect for practical applications in energy storage devices.The excellent electrochemical performance of the Nb2O5/Nb2C/rGO aerogel can be attributed to the rationally designed unique aerogel structure and the synergistic effects of three different components (Fig. 7): (1) the 3D porous structure with a large specific surface area can ensure the full contact between the electrolyte and the active material, exposing more active sites. In addition, the Nb2O5 nanoparticles in the composite can further expose more active sites. These are beneficial for the improvement of specific capacity. (2) The 3D interconnected conductive network formed by the intimate contact of three components, the Nb2O5 nanoparticles generated by in situ oxidation, and the residual Nb2C with a low potassium diffusion barrier and a high conductivity can shorten the diffusion distance and promote the diffusion kinetics of ions/electrons, which is conducive to obtaining outstanding rate performance. (3) The porous aerogel structure can accommodate the volume change during the charge–discharge process, and suppress the self-restacking of Nb2C and agglomeration of Nb2O5 nanoparticles, thereby retaining the integrity and stability of the Nb2O5/Nb2C/rGO structure.
4. Conclusions
In summary, the Nb2C/rGO conductive framework with in situ generated Nb2O5 nanoparticles (Nb2O5/Nb2C/rGO) was successfully synthesized by a simple one-step high-temperature hydrothermal method and subsequent freeze-drying and annealing treatments, in which small-sized Nb2C nanosheets are encapsulated by large-sized rGO nanosheets, and the Nb2O5 nanoparticles generated by in situ oxidation of Nb2C are uniformly dispersed on the surface of Nb2C and/or rGO, and the intimate contact of three components makes full use of the advantages of the 3D conductive network. The resulting 3D porous hybrid aerogel with abundant pores and a large specific surface area can not only inhibit the self-restacking of Nb2C nanosheets and the agglomeration of Nb2O5 nanoparticles and alleviate the volume change during the charge–discharge process to improve the cycling stability, but also provide unimpeded channels for the diffusion of K+ and the infiltration of the electrolyte and expose more active sites to improve the specific capacity and rate performance. Meanwhile, Nb2O5 nanoparticles and residual Nb2C nanosheets with a low potassium diffusion barrier and a high conductivity can shorten the diffusion distance and facilitate diffusion kinetics of electrons/ions. As a result, compared with Nb2O5/rGO, Nb2O5 and previous reports, the obtained Nb2O5/Nb2C/rGO exhibits a much better potassium storage behavior as an anode of KIBs. Specifically, Nb2O5/Nb2C/rGO presents a large specific capacity of 410.6 mA h g−1 after 100 cycles at 0.1 A g−1, and an attractive rate performance of 159.0 mA h g−1 at 5 A g−1. In addition, after 1000 consecutive high-rate cycles at 2.0 A g−1, it still has a specific capacity of 208.9 mA h g−1, with an ultralow capacity decay of 0.011% per cycle. More strikingly, Nb2O5/Nb2C/rGO also shows a good potassium storage performance in a KIB full-cell. We believe that this facile synthesis method and the unique electrode structure can pave a new way to improve the performance of MXenes and accelerate the application of MXenes.Author contributions
Cong Liu: conceptualization, methodology, software, investigation, data curation, visualization, and writing – original draft. Zhitang Fang: methodology, software, validation, and data curation. Weizhi Kou: methodology, validation, and data curation. Xiaoge Li: methodology, validation, and data curation. Jinhua Zhou: validation and investigation. Gang Yang: conceptualization, formal analysis, resources, and supervision. Luming Peng: conceptualization, formal analysis, resources, and supervision. Xuefeng Guo: conceptualization, formal analysis, resources, and supervision. Weiping Ding: conceptualization, formal analysis, resources, and supervision. Wenhua Hou: conceptualization, formal analysis, resources, writing – review & editing, supervision, project administration, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support of the National Natural Science Foundation of China (22379063) and the Modern Analysis Center of Nanjing University.References
- T. Hosaka, K. Kubota, A. S. Hameed and S. Komaba, Research development on K-Ion batteries, Chem. Rev., 2020, 120, 6358 CrossRef CAS PubMed.
- L. Fan, H. Xie, Y. Hu, Z. Caixiang, A. M. M. Rao, J. Zhou and B. Lu, A tailored electrolyte for safe and durable potassium ion batteries, Energy Environ. Sci., 2023, 16, 305 RSC.
- W. Zhang, Y. Liu and Z. Guo, Approaching high-performance potassium-ion batteries via advanced design strategies and engineering, Sci. Adv., 2019, 5, eaav7412 CrossRef CAS PubMed.
- P. Du, L. Cao, B. Zhang, C. Wang, Z. Xiao, J. Zhang, D. Wang and X. Ou, Recent progress on heterostructure materials for next-generation sodium/potassium ion batteries, Renewable Sustainable Energy Rev., 2021, 151, 111640 CrossRef CAS.
- P. Li, H. Kim, K. H. Kim, J. Kim, H. G. Jung and Y. K. Sun, State-of-the-art anodes of potassium-ion batteries: synthesis, chemistry, and applications, Chem. Sci., 2021, 12, 7623 RSC.
- M. Shen, H. Ding, L. Fan, A. M. Rao, J. Zhou and B. Lu, Neuromorphic carbon for fast and durable potassium storage, Adv. Funct. Mater., 2023, 33, 2213362 CrossRef CAS.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Mixed transition-metal oxides: design, synthesis, and energy-related applications, Angew. Chem., Int. Ed., 2014, 53, 1488 CrossRef CAS PubMed.
- G. Maduraiveeran, M. Sasidharan and W. Jin, Earth-abundant transition metal and metal oxide nanomaterials: Synthesis and electrochemical applications, Prog. Mater. Sci., 2019, 106, 100574 CrossRef CAS.
- J. Ma, X. Guo, H. Xue, K. Pan, C. Liu and H. Pang, Niobium/tantalum-based materials: Synthesis and applications in electrochemical energy storage, Chem. Eng. J., 2020, 380, 122428 CrossRef CAS.
- M. Zhang, D. A. Kitchaev, Z. Lebens-Higgins, J. Vinckeviciute, M. Zuba, P. J. Reeves, C. P. Grey, M. S. Whittingham, L. F. J. Piper, A. van der Ven and Y. S. Meng, Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage, Nat. Rev. Mater., 2022, 522 CrossRef.
- F. Shen, Z. Sun, Q. He, J. Sun, R. B. Kaner and Y. Shao, Niobium pentoxide based materials for high rate rechargeable electrochemical energy storage, Mater. Horiz., 2021, 8, 1130 RSC.
- L. Yan, X. Rui, G. Chen, W. Xu, G. Zou and H. Luo, Recent advances in nanostructured Nb-based oxides for electrochemical energy storage, Nanoscale, 2016, 8, 8443 RSC.
- J. Mei, Y. Zhang, T. Liao, Z. Sun and S. X. Dou, Strategies for improving the lithium-storage performance of 2D nanomaterials, Natl. Sci. Rev., 2018, 5, 389 CrossRef CAS.
- K. T. Kang, J. Park, D. Suh and W. S. Choi, Synergetic behavior in 2D layered material/complex oxide heterostructures, Adv. Mater., 2019, 31, e1803732 CrossRef PubMed.
- N. Li, F. Zhang and Y. Tang, Hierarchical T-Nb2O5 nanostructure with hybrid mechanisms of intercalation and pseudocapacitance for potassium storage and high-performance potassium dual-ion batteries, J. Mater. Chem. A, 2018, 6, 17889 RSC.
- J. Yuan, X. Li, J. Liu, S. Zuo, X. Li, F. Li, Y. Gan, H. He, X. Xu, X. Zhang and J. Meng, Pomegranate-like structured Nb2O5/carbon@N-doped carbon composites as ultrastable anode for advanced sodium/potassium-ion batteries, J. Colloid Interface Sci., 2022, 613, 84 CrossRef CAS PubMed.
- Z. Zhao, J. Cheng, K. Li, C. Li, S. Zhang, X. Pei, Z. Niu, Z. Liu, Y. Fu and D. Li, Heterojunction interfacial promotion of fast and prolonged alkali-ion storage of urchin-like Nb2O5@C nanospheres, J. Mater. Chem. A, 2021, 9, 23467 RSC.
- Y. Wu, Y. Sun, J. Zheng, J. Rong, H. Li and L. Niu, MXenes: Advanced materials in potassium ion batteries, Chem. Eng. J., 2021, 404, 126565 CrossRef CAS.
- M. K. Aslam and M. Xu, A mini-review: MXene composites for sodium/potassium-ion batteries., Nanoscale, 2020, 12, 15993 RSC.
- Z. Hong, H. Maleki, T. Ludwig, Y. Zhen, M. Wilhelm, D. Lee, K.-H. Kim and S. Mathur, New insights into carbon-based and MXene anodes for Na and K-ion storage: A review, J. Energy Chem., 2021, 62, 660 CrossRef CAS.
- S. Sun, C. Liao, A. M. Hafez, H. Zhu and S. Wu, Two-dimensional MXenes for energy storage, Chem. Eng. J., 2018, 338, 27 CrossRef CAS.
- Y. T. Liu, P. Zhang, N. Sun, B. Anasori, Q. Z. Zhu, H. Liu, Y. Gogotsi and B. Xu, Self-assembly of transition metal oxide nanostructures on MXene nanosheets for fast and stable lithium storage, Adv. Mater., 2018, 30, e1707334 CrossRef PubMed.
- D. Zhao, R. Zhao, S. Dong, X. Miao, Z. Zhang, C. Wang and L. Yin, Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries, Energy Environ. Sci., 2019, 12, 2422 RSC.
- J. Li, B. Rui, W. Wei, P. Nie, L. Chang, Z. Le, M. Liu, H. Wang, L. Wang and X. Zhang, Nanosheets assembled layered MoS2/MXene as high performance anode materials for potassium ion batteries, J. Power Sources, 2020, 449, 227481 CrossRef CAS.
- J. Pang, R. G. Mendes, A. Bachmatiuk, L. Zhao, H. Q. Ta, T. Gemming, H. Liu, Z. Liu and M. H. Rummeli, Applications of 2D MXenes in energy conversion and storage systems, Chem. Soc. Rev., 2019, 48, 72 RSC.
- C. J. Zhang, S. J. Kim, M. Ghidiu, M.-Q. Zhao, M. W. Barsoum, V. Nicolosi and Y. Gogotsi, Layered orthorhombic Nb2O5@Nb4C3Tx and TiO2@Ti3C2Tx hierarchical composites for high performance Li-ion batteries, Adv. Funct. Mater., 2016, 26, 4143 CrossRef CAS.
- C. Yang, Y. Liu, X. Sun, Y. Zhang, L. Hou, Q. Zhang and C. Yuan, In situ construction of hierarchical accordion-like TiO2/Ti3C2 nanohybrid as anode material for lithium and sodium ion batteries, Electrochim. Acta, 2018, 271, 165 CrossRef CAS.
- Z. Li, G. Chen, J. Deng, D. Li, T. Yan, Z. An, L. Shi and D. Zhang, Creating sandwich-like Ti3C2/TiO2/rGO as anode materials with high energy and power density for Li-ion hybrid capacitors, ACS Sustainable Chem. Eng., 2019, 7, 15394 CrossRef CAS.
- Z. Tong, R. Yang, S. Wu, D. Shen, T. Jiao, K. Zhang, W. Zhang and C. S. Lee, Surface-engineered black niobium oxide@graphene nanosheets for high-performance sodium-/potassium-ion full batteries, Small, 2019, 15, e1901272 CrossRef PubMed.
- Z. Wu, T. Shang, Y. Deng, Y. Tao and Q. H. Yang, The assembly of MXenes from 2D to 3D, Adv. Sci., 2020, 7, 1903077 CrossRef CAS PubMed.
- C. Song, W. Zhang, Q. Jin, Y. Zhang, X. Wang and Z. Bakenov, In situ constructed accordion-like Nb2C/Nb2O5 heterostructure as efficient catalyzer towards high-performance lithium-sulfur batteries, J. Power Sources, 2022, 520, 230902 CrossRef CAS.
- K. Rajavel, Y. Hu, P. Zhu, R. Sun and C. Wong, MXene/metal oxides-Ag ternary nanostructures for electromagnetic interference shielding, Chem. Eng. J., 2020, 399, 125791 CrossRef CAS.
- Y. Wang, X. Hu, H. Song, Y. Cai, Z. Li, D. Zu, P. Zhang, D. Chong, N. Gao, Y. Shen and C. Li, Oxygen vacancies in actiniae-like Nb2O5/Nb2C MXene heterojunction boosting visible light photocatalytic NO removal, Appl. Catal., B, 2021, 299, 120677 CrossRef CAS.
- H. Cheng and W. Zhao, Regulating the Nb2C nanosheets with different degrees of oxidation in water lubricated sliding toward an excellent tribological performance, Friction, 2022, 10, 398 CrossRef CAS.
- C. Liu, J. Zhou, X. Li, Z. Fang, R. Sun, G. Yang and W. Hou, Surface modification and in situ carbon intercalation of two-dimensional niobium carbide as promising electrode materials for potassium-ion batteries, Chem. Eng. J., 2022, 431, 133838 CrossRef CAS.
- C. Liu, Z. Fang, X. Li, J. Zhou, G. Yang, L. Peng, X. Guo, W. Ding and W. Hou, Rational design of 3D porous niobium carbide MXene/rGO hybrid aerogels as promising anode for potassium-ion batteries with ultrahigh rate capability, Nano Res., 2023, 16, 2463 CrossRef CAS.
- X. Zhang, R. Lv, A. Wang, W. Guo, X. Liu and J. Luo, MXene aerogel scaffolds for high-rate lithium metal anodes, Angew. Chem., Int. Ed., 2018, 57, 15028 CrossRef CAS PubMed.
- S. Zhao, H. B. Zhang, J. Q. Luo, Q. W. Wang, B. Xu, S. Hong and Z. Z. Yu, Highly electrically conductive three-dimensional Ti3C2Tx MXene/reduced graphene oxide hybrid aerogels with excellent electromagnetic interference shielding performances, ACS Nano, 2018, 12, 11193 CrossRef CAS PubMed.
- Y. Li, R. Wang, W. Zheng, Q. Zhao, S. Sun, G. Ji, S. Li, X. Fan and C. Xu, Design of Nb2O5/graphene hybrid aerogel as polymer binder-free electrodes for lithium-ion capacitors, Mater. Technol., 2020, 35, 625 CrossRef CAS.
- Q. Wang, S. Wang, X. Guo, L. Ruan, N. Wei, Y. Ma, J. Li, M. Wang, W. Li and W. Zeng, MXene–reduced graphene oxide aerogel for aqueous Zinc–ion hybrid supercapacitor with ultralong cycle life, Adv. Electron. Mater., 2019, 5, 1900537 CrossRef CAS.
- H. An, L. Jiang, F. Li, P. Wu, X. Zhu, S. Wei and Y. Zhou, Hydrogel-derived three-dimensional porous Si-CNT@G nanocomposite with high-performance lithium storage, Acta Phys.-Chim. Sin., 2020, 36, 1905034 Search PubMed.
- H. Sun, L. Mei, J. Liang, Z. Zhao, C. Lee, H. Fei, M. Ding, J. Lau, M. Li, C. Wang, X. Xu, G. Hao, B. Papandrea, I. Shakir, B. Dunn, Y. Huang and X. Duan, Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage, Science, 2017, 356, 599 CrossRef CAS PubMed.
- Y. Li, S. Yang, Z. Liang, Y. Xue, H. Cui and J. Tian, 1T-MoS2 nanopatch/Ti3C2 MXene/TiO2 nanosheet hybrids for efficient photocatalytic hydrogen evolution, Mater. Chem. Front., 2019, 3, 2673 RSC.
- S. Zong, J. Liu, Z. Huang, L. Liu, J. Liu, J. Zheng and Y. Fang, Mxene-TiO2 composite with exposed {101} facets for the improved photocatalytic hydrogen evolution activity, J. Alloys Compd., 2022, 896, 163039 CrossRef CAS.
- J. Wang, B. Yin, T. Gao, X. Wang, W. Li, X. Hong, Z. Wang and H. He, Reduced graphene oxide modified few-layer exfoliated graphite to enhance the stability of the negative electrode of a graphite-based potassium ion battery, Acta Phys.-Chim. Sin., 2022, 38, 2012088 Search PubMed.
- Z. Sui, Q. Meng, X. Zhang, R. Ma and B. Cao, Green synthesis of carbon nanotube-graphene hybrid aerogels and their use as versatile agents for water purification, J. Mater. Chem., 2012, 22, 8767 RSC.
- J. Li, X. Yuan, C. Lin, Y. Yang, L. Xu, X. Du, J. Xie, J. Lin and J. Sun, Achieving high pseudocapacitance of 2D titanium carbide (MXene) by cation intercalation and surface modification, Adv. Energy Mater., 2017, 7, 1602725 CrossRef.
- B. Wang, Z. Zhang, F. Yuan, D. Zhang, Q. Wang, W. Li, Z. Li, Y. A. Wu and W. Wang, An insight into the initial Coulombic efficiency of carbon-based anode materials for potassium-ion batteries, Chem. Eng. J., 2022, 428, 131093 CrossRef CAS.
- R. Zhao, H. Di, X. Hui, D. Zhao, R. Wang, C. Wang and L. Yin, Self-assembled Ti3C2 MXene and N-rich porous carbon hybrids as superior anodes for high-performance potassium-ion batteries, Energy Environ. Sci., 2020, 13, 246 RSC.
- X. Yi, A. M. M. Rao, J. Zhou and B. Lu, Trimming the degrees of freedom via a K+ flux rectifier for safe and long-Life potassium-ion batteries, Nano-Micro Lett., 2023, 15, 200 CrossRef CAS PubMed.
- X. Yi, Y. Feng, A. M. M. Rao, J. Zhou, C. Wang and B. Lu, Quasi-solid aqueous electrolytes for low-cost sustainable alkali-metal batteries, Adv. Mater., 2023, 35, 2302280 CrossRef CAS PubMed.
- K. Cao, R. Zheng, S. Wang, J. Shu, X. Liu, H. Liu, K. J. Huang, Q. S. Jing and L. Jiao, Boosting coulombic efficiency of conversion–reaction anodes for potassium–ion batteries via confinement effect, Adv. Funct. Mater., 2020, 30, 2007712 CrossRef CAS.
- J. Cao, Z. Sun, J. Li, Y. Zhu, Z. Yuan, Y. Zhang, D. Li, L. Wang and W. Han, Microbe-assisted assembly of Ti3C2Tx MXene on fungi-derived nanoribbon heterostructures for ultrastable sodium and potassium ion storage., ACS Nano, 2021, 15, 3423 CrossRef CAS PubMed.
- T. Wang, D. Shen, H. Liu, H. Chen, Q. Liu and B. Lu, A Sb2S3 nanoflower/MXene composite as an anode for potassium-ion batteries, ACS Appl. Mater. Interfaces, 2020, 12, 57907 CrossRef CAS PubMed.
- Y. Zhang, L. Fang, W. Sun, B. Shi, X. Chen, Y. Gu, K. Ding, Z. Wang and K. Sun, A novel synthesis of Nb2O5@rGO nanocomposite as anode material for superior sodium storage, Chin. Chem. Lett., 2021, 32, 1144 CrossRef CAS.
- Z. Li, X. Wu, W. Luo, C. Wang, W. Feng, X. Hong and L. Mai, Dual sulfur-doped sites boost potassium storage in carbon nanosheets derived from low-cost sulfonate, Chem. Eng. J., 2022, 431, 134207 CrossRef CAS.
- H. Bi, X. He, L. Yang, H. Li, B. Jin and J. Qiu, Interconnected carbon nanocapsules with high N/S co-doping as stable and high-capacity potassium-ion battery anode, J. Energy Chem., 2022, 66, 195 CrossRef CAS.
- J. Li, W. Qin, J. Xie, H. Lei, Y. Zhu, W. Huang, X. Xu, Z. Zhao and W. Mai, Sulphur-doped reduced graphene oxide sponges as high-performance free-standing anodes for K-ion storage, Nano Energy, 2018, 53, 415 CrossRef CAS.
- L. Qin, Y. Liu, S. Xu, S. Wang, X. Sun, S. Zhu, L. Hou and C. Yuan, In–plane assembled single–crystalline T–Nb2O5 nanorods derived from few–layered Nb2CTx MXene nanosheets for advanced Li–ion capacitors, Small Methods, 2020, 4, 2000630 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi01775c |
| This journal is © the Partner Organisations 2024 |