 Open Access Article
Open Access ArticleThe etiology, pathogenesis, treatment, and development of transdermal drug delivery systems for rheumatoid arthritis
Mirza Muhammad Faran Ashraf
Baig
 *ab,
Chi Hin
Kwan
b,
Hongkai
Wu
*ab,
Chi Hin
Kwan
b,
Hongkai
Wu
 *b and
Sek Ying
Chair
*a
*b and
Sek Ying
Chair
*a
aCroucher Laboratory for Human Genomics, Asia-Pacific Genomic and Genetic Nursing Center, The Nethersole School of Nursing, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China. E-mail: mirzafaran-ashraf@hotmail.com; mirzabaig@cuhk.edu.hk; sychair@cuhk.edu.hk
bDepartment of Chemistry, and the Hong Kong Branch of Chinese National Engineering Research Centre for Tissue Restoration, The Hong Kong University of Science and Technology, Hong Kong SAR 999077, China. E-mail: chhkwu@ust.hk
First published on 12th July 2024
Abstract
Rheumatoid arthritis (RA) is a long-term autoimmune disease that causes irreversible deformity of joints and disability of body parts. The symptoms include synovial tissue inflammation and cartilage and bone damage. To reduce the inflammation, therapeutic drugs are often used to target and limit the inflammation factor. Nonetheless, there are significant problems with the treatment such as a first-pass effect, gastrointestinal side effects, skin stratum corneum barrier, etc. Hence, a transdermal delivery system (TDDS) is applied for the treatment of rheumatoid arthritis as it increases the effectiveness of the drugs by overcoming the difficulties mentioned above. This paper reviews the research progress of transdermal drug delivery for the treatment of rheumatoid arthritis and explores the details of dosage forms such as gel, patch, drug microneedles, nanostructured lipid carriers and drug-loaded electrospun nanofibers, which provide numerous ideas for these dosage forms in RA treatment when using transdermal drug delivery methods.
1. Introduction
1.1. Introduction of rheumatoid arthritis (RA)
Rheumatoid arthritis (RA) is a systemic and chronic autoimmune disease with symptoms such as synovial inflammation, damage to the joints, osteopenia, etc.1 The pathophysiology of RA is accompanied by an imbalance of the immune system, which leads to the infiltration of inflammatory cells and dysregulation in the proliferation of synovial fibroblasts.2 It causes damage to synovial tissue, cartilage, and joints. The prevalence rate of RA is up to 1% of the population but still it may affect millions of patients worldwide with an economic burden on the society.3,4 RA can occur at any age and in a higher proportion of females than males.5 RA can affect people at any age but with an increased incidence rate above 40 years old and in the geriatric age group.61.2. Genetic and environmental factors involved in the pathogenesis of RA
The pathogenesis of RA is not completely understood but can be related to genetic and environmental factors, or both.7 Smoking, periodontitis, and the gut microbiome are the environmental risk factors associated with the genetic factors causing RA.8 Smoking is considered another important triggering factor for RA.9 Studies show that tobacco can stimulate antigen-presenting cells (ACPs) in the lungs and lead to the development of autoimmunity.10,11 How environmental factors exactly contribute is yet to be evaluated; however, there might be hundreds of loci related to RA that can become mutated.12,13 These loci can result in the overproduction of autoantibodies and activate B cells, T cells, and macrophages, causing the production of inflammatory factors.14,15 In between these loci, the major histocompatibility complex (MHC) of genes is tightly linked to RA as it encodes the proteins, mainly those of MHC classes I and II, that will bind to the T cell receptor and activate it.16 No wonder then that the types of loci that encode post-translational modification enzymes, co-stimulatory pathways, and intracellular regulatory pathways can cause abnormal immune activation or regulation.2,171.3. The involvement of inflammatory factors in immuno-pathogenesis of RA
These inflammatory factors lead to the damage of cartilage and bone and result in joint erosion. Synovial inflammation is very painful and directly linked to pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β).18 These immunological reactions give rise to severe pathological conditions.19 TNF-α can cause the abnormal expansion of fibroblast-like synoviocytes (FLS).20 Then, it will lead to gene overexpression of cathepsin and matrix metalloproteinases (MMPs).21 As a result, collagen and proteoglycan will start to break down and destroy the cartilage and bone.22 A high level of IL-6 can lead to unusual activation of the Janus kinase (JAK)-signal transducer and the transcription pathway.23,24 Then, it affects T cell proliferation, B cell survival, proliferation, and activation to worsen the inflammation.25 The major pathophysiological pathways, including the major hallmarks of RA, are illustrated in Fig. 1. | ||
| Fig. 1 The immuno-pathophysiological pathways of RA. The major hallmarks of RA involve genetic cues leading to the inflammatory processes. Declaration: The figure was adapted from the open-source platform of “Biorender” tools (or templates). Reuse of its tools or templates has been allowed by “Biorender” (therefore, copyright is not applicable) with relevant previous citations (if any) being incorporated.26 | ||
2. Conventional treatment of RA
2.1. Therapeutic drugs used to treat RA
In the mild and medium stages of RA, herbal drugs, biologicals, and therapeutic drugs can be used as a treatment that can relieve pain and reduce joint dysfunction.27–29 There are four main types of drugs for RA: non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs) or steroidal drugs, biologicals, and disease-modifying anti-rheumatic drugs (DMARDs).25,30–32 NSAIDs and GCs are related to inflammation symptomatic relief but are unable to provide pathophysiological relief.33 Therapeutic relief can be achieved using Janus kinase inhibitors such as baricitinib and tofacitinib.34 These drugs are very effective, especially upon combining with either of the other anti-rheumatic drugs, or more specifically with monoclonal antibody drugs such as sarilumab and golimumab.35,362.2. Mechanism of action of NSAIDs to decrease inflammation in RA
NSAIDs are commonly used in the early stages of RA for painkilling and anti-inflammation purposes,37 for example, naproxen, ibuprofen, diclofenac, even aspirin, etc. Conventional NSAIDs are non-selective cyclooxygenase (COX) inhibitors, which can inhibit both COX-1 and COX-2, whereas selective COX-2 inhibitors are celecoxib, valdecoxib, parecoxib, etc.38,39 These drugs can effectively counteract the reaction of converting arachidonic acid to inflammatory prostaglandins without affecting the levels of beneficial prostaglandins.40 However, selective COX-2 inhibitor drugs might lead to some cardiovascular complications as an adverse effect.41 Most of NSAIDs are organic acids with an aryl group and low pKa values. Due to these characteristics, NSAIDs can locate and stay in the inflammation areas with their characteristic lower pH conditions.37 As a result, the production of prostaglandins, which cause inflammation and pain, can be reduced.40,422.3. Mechanism of action of GCs to decrease inflammation in RA
GCs are drugs that contain steroidal hormones such as prednisone, dexamethasone, etc.43 Due to the lipophilic structure of GCs, they can pass through the plasma membrane of the cells and bind with the GC receptors (GRs). After the GC/GR complex forms, it is transported to the nucleus and limits conformational changes after binding with the negative GC-responsive element (GRE) in the nucleus to suppress the gene transcription of immune proteins.44 Moreover, the GC/GR complex can interact with transcription factors such as nuclear factor-κB (NF-κB). In this way, GCs can inhibit the transcription of some pro-inflammatory cytokines that are produced by the monocytes and macrophages, including IL-1β, IL-6, and TNF-α, which are related to RA.45–472.4. DMARDs for RA treatment
DMARDs are a group of first-line medications for RA treatment.48 DMARDs are further classified into a traditional class – “conventional synthetic DMARDs” (csDMARDs),49 biological DMARDs (bDMARDs),35,50 and targeted synthetic DMARDs (tsDMARDs).51,52 The csDMARDs are commonly used for treating synovial inflammation and reducing joint damage.Leflunomide has been found to target the dihydroorotate dehydrogenase (DHODH),58 which is an enzyme responsible for the biosynthesis of pyrimidine.59 The drug inhibits the DHODH enzyme and interferes with the oxidative conversion of dihydroorotate to orotate. This causes the decreased secretion of inflammatory cytokines, which is desirable for treating RA.59
2.5. The traditional drug delivery approaches to treat RA
The treatment of RA is aimed at preventing inflammatory processes and reducing the pain of patients.76,80,82,83 The drug delivery systems used in this treatment are in the dosage forms of tablets, capsules, and injections but with certain limitations.84–86 The major routes for administration of drugs for RA treatment are illustrated in Fig. 2. However, the oral method could, for instance, cause toxicities, adverse effects, low bioavailability, a first-pass effect, or be quickly eliminated by the liver and kidneys, while the IM/IV methods (or even intrathecal injections) may cause phlebitis, skin hypersensitivity, irritation, infections, damage to tissues, damage to vital organs, anaphylaxis, and even shock.87–89 Considering the general limitations of the traditional dosage forms, transdermal drug delivery systems (TDDSs) are becoming the latest approaches to treating RA.90–92 A desirable TDDS once developed can be used to avoid most of the side effects of traditional dosage forms, with the potential to enhance bioavailability.93,94 This report reviews the research on the TDDSs developed to treat RA and discusses the various types of TDDSs used to treat RA. | ||
| Fig. 2 Proposed drug administration routes and formulations to treat RA. Declaration: The figure was adapted from the open-source platform of “Biorender” tools (or templates). Reuse of its tools or templates has been allowed by “Biorender” (therefore, copyright is not applicable) with relevant previous citations (if any) being incorporated.95 | ||
3. Establishing the need for transdermal drug delivery systems
Drug delivery orally is the most desirable system for patients, especially those who require long-term consumption of therapeutic drugs. Therefore, it is the most usual method for the treatment of RA because of its low cost, convenience, safety, and flexibility. Moreover, injection is also one of the common ways involving intraarticular or subcutaneous methods to directly inject the drug into the joint area.96–98 Injections can reduce certain limitations and adverse effects of the conventional oral route method, where drugs undergo gastrointestinal metabolism leading to poor bioavailability at the target joints.99,100 On the other hand, the intra-articular injection can improve the drug's efficacy as it increases drug retention times in the joint and promotes more sustainable drug release.87,101Most oral NSAID formulations are formulated to have low solubility, making them less bioavailable.102–104 Nevertheless, methotrexate is the most commonly used csDMARD, and it has been shown to have a tablet dosage form with adverse effects as well as disadvantages of undergoing a first-pass effect, low bioavailability, and an effect on food consumption.105–107
bDMARDs are used through the injection method to treat RA efficiently; however, the injection method may cause injection site infection and an infusion reaction.108–110 As a result, transdermal administration (TDDS) has become a high-profile development in the treatment of RA, as TDDS can bypass the first-pass effect and the gastrointestinal side effects of oral administration, as well as adverse effects of parenteral administration to reduce the risk of the injection method.111–113
Recently, ibuprofen gel and ketoprofen gels were developed as a type of TDDS dosage form for RA treatment.94,114,115 Thus, the formulation development of an efficient TDDS for RA treatment has been attempted more frequently during the last decade.90,116,117 This research area has added advancements to the existing technology, creating and testing novel excipients, and optimizing the preparation processes.118,119
4. Transdermal administration of drugs
Most of the drugs used in TDDSs should have a molecular weight of less than 600 Da. The drugs should possess unique heterophilic properties to undergo efficient transdermal delivery. The transdermal efficacy of the drugs for RA treatment can be enhanced by developing new chemical derivatives of the drugs with the desired molecular properties. Moreover, developing new technologies such as microneedles or adhesive patches that can lead to easy bypass of the stratum corneum can increase the diffusion coefficients and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of the drugs during the transdermal delivery.120 The main barrier is the stratum corneum, since the subcutaneous layers of the skin already have very high diffusion coefficients, and log
P values of the drugs during the transdermal delivery.120 The main barrier is the stratum corneum, since the subcutaneous layers of the skin already have very high diffusion coefficients, and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (from 1 to 3).120 Considering these criteria, surges in transdermal RA treatments can be explored to develop novel TDDS approaches rather than continuing conventional therapeutic approaches for RA.
P values (from 1 to 3).120 Considering these criteria, surges in transdermal RA treatments can be explored to develop novel TDDS approaches rather than continuing conventional therapeutic approaches for RA.
4.1. The skin as a barrier for the TDDS
The skin is the largest organ of the human body, as its surface area is around 1.5–2 m2 and accounts for around 15% of a person's weight. The skin can be grouped into three areas: the epidermis, the dermis, and the subcutaneous layers, as shown in Fig. 3.121 The skin protects the underneath parts of the body from heat, germs, chemicals, etc. | ||
| Fig. 3 Schematic representation of the skin. Declaration: The figure was adapted from the open-source platform of “Biorender” tools (or templates). Reuse of its tools or templates has been allowed by “Biorender” (therefore, copyright is not applicable) with relevant previous citations (if any) being incorporated.115 | ||
4.2. The mechanism of transdermal drug absorption
The drugs can be absorbed by the skin via two pathways: the trans-epidermal route or the trans-appendage route.92 Various advanced techniques involved to facilitate the transdermal penetration of drugs are microneedles, iontophoresis, the use of skin-permeable peptides, or use of mechanical force such as with a jet injector or through irradiation such as ultrasound, as shown in Fig. 4.126 The trans-epidermal pathway can be chiefly subdivided into trans-cellular and intercellular, while the trans-appendage route can be chiefly subdivided into glandular and follicular routes.127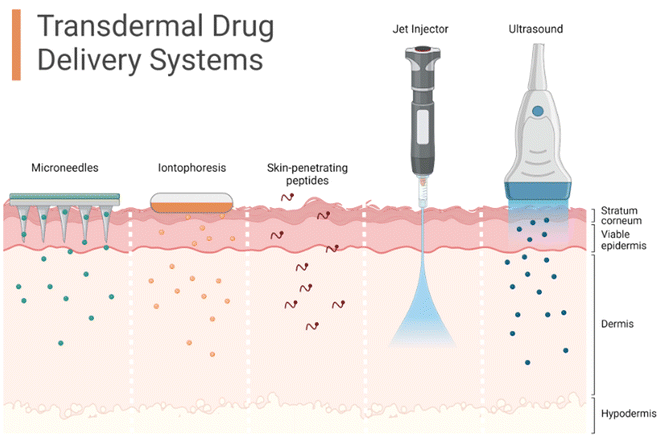 | ||
| Fig. 4 Possible drug penetration routes across the human skin. Declaration: The figure was adapted from the open-source platform of “Biorender” tools (or templates). Reuse of its tools or templates has been allowed by “Biorender” (therefore, copyright is not applicable) with relevant previous citations (if any) being incorporated.126 | ||
4.2.1.1. The trans-cellular or intracellular route. The trans-cellular route via keratinocytes and mature corneocytes can allow transport of hydrophilic, and heterophilic polar solutes.130 As hydrated keratin is present in corneocytes, an aqueous environment is provided. However, the corneocytes are bound by lipid membranes, making further drug penetration challenging. Successful drug penetration involves unique and dynamic molecular properties of the drugs or small molecules. The drugs should be capable of undergoing repetitive geometric and polarity shifts to exhibit desired heterophilic characteristics for transdermal delivery.92
4.2.1.2. The intercellular pathway. The intercellular pathway favors lipophilic substances and non-polar drugs. The drug molecules are transported and diffused through the intercellular route, which is the lipid matrix. Subsequently, hydrophobic medications can penetrate the dermis through the lipid matrix, can be absorbed by the dermis, and can reach the bloodstream. Therefore, the intercellular route can be the main pathway for the absorption of lipophilic drugs.59
5. Transdermal dosage forms for RA treatment
In this section, we have summarized various dosage forms used for the transdermal delivery of drugs for RA treatment. There are several novel transdermal delivery systems, such as patches, microneedles, nano-structured lipid carriers, and drug-loaded electrospun nanofibers, which have great potential for future application in RA treatment.5.1. Gels
Among the TDDSs used to treat RA, gels are the most common dosage forms. Gels are semisolids, comprising a three-dimensional network of structures that are widely used in food, cosmetics, biotechnology, and other industries. They are created by chemically or physically crosslinking polymers that include an additional hydrophilic or hydrophobic solvent phase.134 Gel systems with various design technologies that exhibit distinct mechanical characteristics have been designed such as hydrogels, microemulsion gels and ethosomal gels. Gels can increase the transdermal bioavailability and effectiveness of anti-rheumatic medications.5.2. Patches
The patch is a material with a multilayered three- or two dimensional design comprising four films: an impermeable film, a drug-containing matrix film, an adhesive film, and a peelable anti-adhesion protective film.146 One of the key technologies used to fabricate patches is 3D-printing technology especially employing a fluid deposition model as shown in Fig. 5.147 The patch has excellent administration compliance: nanocarriers can be mixed into the patch matrix layer to increase transdermal penetration performance.115 The transdermal patch is a very comfortable and efficient dosage form as it is non-invasive and avoids the first-pass effect of the GI tract, which is the major adverse effect of oral drug administration. It delivers drugs through the skin that directly enter the blood circulation at a slow preset rate.148 The preparation technology for patches, their structural elements, and the use of materials are improving day by day to enhance their effectiveness as TDDSs. | ||
| Fig. 5 Basic designs of a transdermal patch with 3D printing technology and using a fused deposition model. Declaration: The figure was adapted from the open-source platform of “Biorender” tools (or templates). Reuse of its tools or templates has been allowed by “Biorender” (therefore, copyright is not applicable) with relevant previous citations (if any) being incorporated.147 | ||
5.2.1.1. Celecoxib patch. The NSAID celecoxib (CXB) is a selective COX-2 inhibitor, which is a sulfonamide molecule having adverse gastrointestinal effects and poor aqueous solubility. Researchers prepared a gel-based layered patch with a transdermal microemulsion as the base that included CXB drugs.149 Use of a microemulsion can improve the skin penetration and lessen the adverse effects of the drug. In this instance, the microemulsion was composed of a co-surfactant Transcutol P, the surfactant Tween 80, and the oil phase triacetin. The pseudo-ternary phase was used to adjust the concentration of each component. Carbopol 934 was also added to the gel to increase its viscosity and adjust the skin contact and retention parameters. According to the ex vivo drug release study, the drug penetration rate of using a microemulsion gel is four times higher than that of conventional gel. Furthermore, the gel did not cause potential adverse effects to the skin, such as redness, rash, irritation, etc. Moreover, in vivo investigation showed that the test animal's inflammatory response had significantly decreased. In addition, compared to the commercial formulation, the drug concentration was enhanced with improvements in the retention period as well as overall bioavailability.149
5.2.1.2. Dexibuprofen patch. Dexibuprofen is an S-isomer of ibuprofen, which also contains the ability to be anti-inflammatory. However, dexibuprofen is forbidden for medication purposes due to its massive adverse effects, such as gastric ulceration, gastrointestinal bleeding, dyspepsia, anorexia, abdominal pain, heartburn, etc. However, researchers have created a transdermal patch based on a microemulsion that contains dexibuprofen that can lessen adverse effects and increase medication effectiveness.150 The components of the microemulsion are water, a surfactant mixture consisting of Tween 80 and propylene glycol (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and an oil phase made of ethyl oleate. The microemulsion has a pH of about 5.46, which is appropriate for skin penetration. Microemulsions are also stable chemically and physically. In addition, the in vitro release analysis shows zero-order release kinetics and up to 79.73% drug release in 24 hours. Cumulative drug permeation is observed to be up to 8174.45 μg cm−2, which is a significant increase in skin permeability. Moreover, in vivo anti-inflammatory investigations demonstrate that a hind paw rat model exhibits an enormous reduction in swelling and inflammation with use of this patch.150
1), and an oil phase made of ethyl oleate. The microemulsion has a pH of about 5.46, which is appropriate for skin penetration. Microemulsions are also stable chemically and physically. In addition, the in vitro release analysis shows zero-order release kinetics and up to 79.73% drug release in 24 hours. Cumulative drug permeation is observed to be up to 8174.45 μg cm−2, which is a significant increase in skin permeability. Moreover, in vivo anti-inflammatory investigations demonstrate that a hind paw rat model exhibits an enormous reduction in swelling and inflammation with use of this patch.150
5.2.1.3. Diclofenac sodium patch. Diclofenac sodium (DS), an NSAID, is one of the most widely used analgesic drugs via the oral route, while leflunomide (LEF) is a DMARD used to block DHODH and hence lower the production of inflammatory cytokines. Although both DS and LEF are powerful anti-inflammatory drugs, they pose various gastrointestinal tract side effects such as dyspepsia, nausea, abdominal pain, oral or gut wall ulceration, and gastric bleeding.151–154 Researchers have designed a transdermal DS/LEF patch with a microemulsion base gel matrix layer, which avoids the first-pass effect and lessens the gastrointestinal side effects of the orally administered drugs.140 The microemulsion is formulated with isopropyl myristate (oil phase), Tween 80 (surfactant), and 1-pentanol (co-surfactant). After the microemulsion is prepared, it is combined with 1% (W/V) LEF and 1% (W/V) DS with respect to the oil and aqueous phases, respectively. The microemulsion pH value is around 4.7–6.55 and it is not irritable to the skin. The cumulative drug release profiles for LEF and DS after 24 hours are 77.36% and 89.90%, respectively, according to in vitro drug release assay. Furthermore, the patch demonstrates that improved drug penetration can be achieved at high concentrations of both surfactant and co-surfactant. According to an in vivo investigation, LEF/DS therapy reduces weight loss in arthritic rats. RA normally causes the loss of lean tissues, which constitute a major portion of the body mass, so RA can be linked to weight loss. In addition, histopathology analysis demonstrates that the gel patch with the LEF/DS microemulsion when applied to arthritic rats promotes recovery from inflammatory/fibrotic symptoms.140
6. Microneedles (MNs)
MNs are drug delivery forms that are less invasive and painless, having needle lengths ranging from 25 to 100 μm and pointer sizes also in the micrometer range.118,156 MNs penetrate the SC skin-layer to create microchannels through which the drug can be directly injected into the upper dermis where the drug can diffuse further to enter the body's circulation. As the length of MNs is not sufficient to reach the skin thickness up to nerves or blood vessels in the dermis, they do not cause discomfort or pain.116,157 Nonetheless, MNs are shown to carry and deliver small particles as well as macromolecules such as proteins and peptides.158 The different types of MNs include solid MNs, hollow MNs, coated MNs, dissolving MNs, hollow MNs, degradable MNs, bio-responsive MNs, hydrogel MNs, and so on, as shown in Fig. 6.159 Moreover, the hydrogel MNs and the dissolving MNs have become the latest research topic because of their extraordinary mechanical, skin-contact, drug penetration, and sustained drug release characteristics.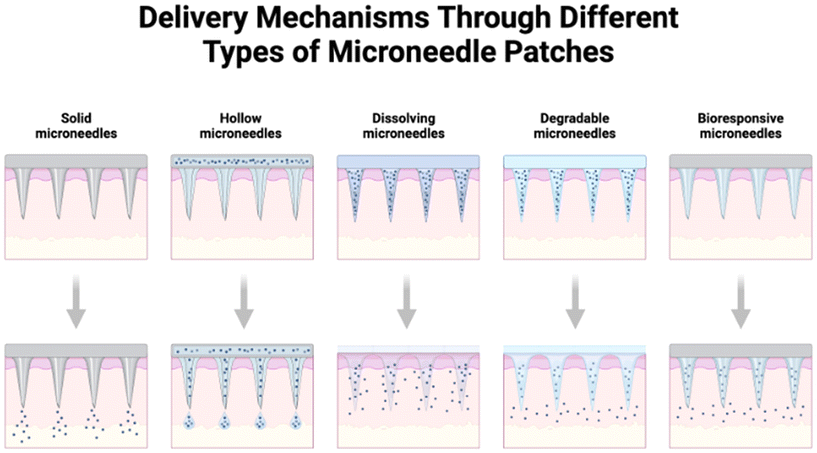 | ||
| Fig. 6 Types of MNs such as solid MNs, hollow MNs, coated MNs, dissolving MNs, degradable MNs, bioresponsive MNs, hydrogel MNs, and so on. Declaration: The figure was adapted from the open-source platform of “Biorender” tools (or templates). Reuse of its tools or templates has been allowed by “Biorender” (therefore, copyright is not applicable) with relevant previous citations (if any) being incorporated.159 | ||
6.1. Dissolving microneedles (dissolving MNs)
Dissolving MNs are made from a water-soluble substance such as maltose, chondroitin, hyaluronic acid, etc.113,160,161 The drug molecules are released into the skin by pressing the dissolving MNs against it. Since the MNs are composed of biocompatible and water-soluble substances, such as sugars and cellulose derivatives, they can be entirely dissolved within the skin over time. As a result, there will be no biohazardous remains on the skin.116,162,1636.2. Hydrogel microneedles (hydrogel MNs)
Hydrogel MNs comprise a cross-linked network of hydrophilic polymers. Hydrogel MNs patch, upon expanding, slightly penetrates on the skin surface, while in contact with the epithelium, can further penetrate to the stratum corneum. Then, they absorb tissue fluid and transport the drug through diffusion. Biomedical applications are increasingly being focused on hydrogel MNs due to their great biocompatibility, degradability, and non-toxicity.93,1667. Flexible liposomes (FLs)
Liposomes are lipid-vesicular, bilayer-shaped nanocarriers. As they contain both a hydrophilic head and a hydrophobic tail, they are used to deliver both hydrophilic and hydrophobic drugs.167 Liposomes are mostly formed from phospholipids and cholesterol. They have high adaptability, as the formulation, surface charge, and properties, as well as the vesicle size, can be modified. FLs are a novel type of liposome that have edge activators that can improve the flexibility of the lipid layer. As a result, compared to rigid liposomes, they can disrupt the lipid barrier and penetrate deeper into the epidermis layers.168 Also, they do not fuse with skin lipids and dehydrate on the skin's surface due to their osmotic concentration gradient.1697.1. FLs for steroidal drug delivery
Researchers have designed dexamethasone (DEX)- and dextran sulfate (DS)-loaded flexible liposomes (DS-FLs/DEX) to treat RA via TDDSs.170 DEX and DS are both anti-inflammatory and immunosuppressive GCs. In this design, DS-FLs/DEX are combined with hydrogels to provide a transdermal administration route. In the characterization study of DS-FLs/DEX, the penetration rate of DS-FLs/DEX was 5.4 times higher than that when using the regular liposome (DS-RLs/DEX) under the same pressure of 0.5 MPa. According to in vitro studies, the DS-FLs/DEX can achieve an initial burst release of 33% within the first 5 hours and maintain a steady release of 90% of DEX at 48 hours in a slightly acidic environment (pH = 6.5, inflammatory pH). In pharmacokinetics studies, the plasma half-life of the DS-FLs/DEX hydrogel is the longest among all the groups, which are DEX (oral), DEX hydrogel, DS-RLs/DEX, and DS-FLs/DEX. As the plasma half-life is prolonged, the bioavailability of DEX is improved. Nevertheless, in vivo studies show that the level of pro-inflammatory cytokines is lower with the application of DS-FLs/DEX compared to DS-RLs/DEX. As a result, DS-RLs/DEX and DS-FLs/DEX both have great potential for RA treatment.1708. Electrospun and electrosprayed nanofibers (NFs)
Electrospun and electrosprayed nanofibers (NFs) are produced by electrospinning or electrospraying techniques where a polymer solution layer is coated over a backing layer, as shown in Fig. 7.171 Due to their huge volume and surface area, the inter-woven fibers can more effectively deliver both hydrophilic and hydrophobic medicines. The drug release characteristics can be modified by altering the drug-to-polymer ratio, fiber diameter, and structure.172,173 | ||
| Fig. 7 A method of electrospinning and electrospraying by which DMAP, AAP, and HPβCD can be deposited to formulate patches with wound healing properties.174 Declaration: The figure was adapted from the open-source platform of “Biorender” tools (or templates). Reuse of its tools or templates has been allowed by “Biorender” (therefore, copyright is not applicable) with relevant previous citations (if any) being incorporated.171 | ||
Researchers developed a novel drug delivery structure by using electrospun cyclodextrin NFs.174 The inclusion complexes (IC) between NSAID and 2-hydroxypropyl-β-cyclodextrin (HPβCD) are used to create the NFs. The NSAIDs utilized in this situation are 4-aminoantipyride (AAP) and 4-dimethylaminoantipyrine (DMAP). Since HPβCD is non-toxic and biodegradable, it is an ideal material to create a biopolymer. After the fabrication is done, the DMAP/HPβCD-IC-NF and AAP/HPβCD-IC-NF forms a meshwork or a web-like layer of exceptional flexibility for biomedical applicability.174
The in vitro analysis indicates that there is excellent biocompatibility between DMAP/HPβCD-IC-NF and AAP/HPβCD-IC-NF. Cell viability was up to 100% 24 and 48 hours after the application of both DMAP/HPβCD-IC-NF (5 μg mL−1) and AAP/HPβCD-IC-NF (5 μg mL−1). Hence, sealing NSAIDs with electrospun nanofibers can improve their stability, and this technique also improves the cell survival, which promotes the growth of skin fibroblast cells. Also, both types of NF show excellent effects on the skin fibroblast group, with remarkable migration of the fibroblasts compared to fibroblasts without treatment. Although there are very few in vivo studies, addressing biocompatibility issues, but NSAIDs-HPβCD-IC-NFs can still be considered as potential candidates for RA treatment for further exploration.174
9. Conclusion
Rheumatoid arthritis (RA) is a chronic disease due to the dysfunction of the autoimmune system. The dysfunction causes secretion of inflammatory factors, which lead to cartilage damage and bone erosion. The conventional drug delivery systems that have been previously reported, such as oral and injection administration, have had their drawbacks and limitations shown in treating RA. In comparison, transdermal drug delivery systems (TDDSs) have certain advantages and efficacies, and have been proved safe in treating RA. This paper reviewed the ability of the TDDS and the drugs that are used in TDDSs for RA treatment. TDDSs can be used to improve the percutaneous penetration and solubility of drugs to attain sustainable drug release and reduce the systemic adverse reactions of currently available dosage forms. Thus, the skin-route bioavailability of the drug is enhanced and localized inhibition for the expression of IL-1β, IL-6, and TNF-α inflammatory factors is efficient. The use of synthetic materials to develop TDDSs in the context of treating RA will appeal to readers; nevertheless, a few studies have already mentioned TDDSs in detail or discussed the biological safety and feasibility of using TDDSs for RA treatment. The challenges of TDDSs are short-term and long-term skin damage due to toxic reactions, metabolic pathways, and the cytotoxicity of the active group when the drug enters the skin. Although the TDDSs used resulted in great performances in the RA animal models, more clinical data need to be tested in the future as the human pathological characteristics of RA are more complicated. There are numerous chemical substances and cells involved in the process of inflammation. Only a few currently targeted groups of chemicals have been used in TDDS technology. Histamine, bradykinin, E-selectin, and platelet β3 integrin-affecting drugs are some other examples of the potential target group of chemicals to be used in developing different types of TDDSs. This article will appeal to acupuncture specialists, traditional Chinese medical practitioners, biologists, clinicians, chemists, and drug delivery specialists to develop novel skin-penetration and drug-bioavailability improvement technologies, such as microneedles and patch techniques, by designing further possible transdermal drug administration technologies. There are very few reviews specifically focusing on transdermal drug delivery systems to treat RA. This one will reveal broader opportunities for readers to design and develop sustainable and efficient TDDSs for RA treatment.Author contributions
Mirza Muhammad Faran Ashraf Baig: initial draft writing, major re-writing, conceptualization, editing, review, and grammar revision. Chi Hin Kwan: initial draft writing, investigation and validation. Hongkai Wu and Sek Ying Chair: revision.Data availability
The data have been appropriately cited. The data will be made available on request.Conflicts of interest
The authors declare no competing interests including any of a financial or personal nature.Acknowledgements
This work was financially supported by the Hong Kong Research Grant Council (#16309920, #16300622, #16309421 and #16300323), Hong Kong ITC (Grant ITC-CNERC14SC01), the Guangdong Basic and Applied Basic Research Foundation (2022B1515130010 and 2024A1515011810), the Guangdong Natural Science Foundation (GDST23SC01) and the National Natural Science Foundation of China (82372345 and 82202582).References
- M. I. Edilova, A. Akram and A. A. Abdul-Sater, Biomed. J., 2021, 44, 172–182 CrossRef CAS PubMed.
- M. V. Sokolova, G. Schett and U. Steffen, Clin. Rev. Allergy Immunol., 2022, 63, 138–151 CrossRef CAS PubMed.
- D. Rosselli, J. D. Rueda, N. Tarazona and C. E. Díaz, Value Health, 2014, 17, A48 CrossRef.
- A. Al Jedai, H. Al-Mudaiheem, P. Pathak, N. Awad, O. Mohamed, N. Alghanim, W. Hussain, A. Qamar and T. Alama, Value Health, 2019, 22, S47 CrossRef.
- J. Tesser, I. Lin, N. J. Shiff, S. D. Chakravarty, G. Schmajuk, N. Hammam and S. Desai, Clin. Rheumatol., 2022, 41, 2319–2327 CrossRef PubMed.
- C. Bes, Ther. Adv. Musculoskeletal Dis., 2018, 10, 3–11 CrossRef PubMed.
- A. M. Wheeler, J. F. Baker, J. A. Poole, D. P. Ascherman, Y. Yang, G. S. Kerr, A. Reimold, G. Kunkel, G. W. Cannon, K. D. Wysham, N. Singh, D. Lazaro, P. Monach, S. L. Bridges, T. R. Mikuls and B. R. England, Semin. Arthritis Rheum., 2022, 57, 152098 CrossRef PubMed.
- E. W. Karlson, B. Ding, B. T. Keenan, K. Liao, K. H. Costenbader, L. Klareskog, L. Alfredsson and L. B. Chibnik, Arthritis Care Res., 2013, 65, 1147–1156 CrossRef PubMed.
- C. I. Amos, W. V. Chen, E. Remmers, K. A. Siminovitch, M. F. Seldin, L. A. Criswell, A. T. Lee, S. John, N. D. Shephard, J. Worthington, F. Cornelis, R. M. Plenge, A. B. Begovich, T. D. Dyer, D. L. Kastner and P. K. Gregersen, BMC Proc., 2007, 1, S3 CrossRef PubMed.
- N. Petrovská, K. Prajzlerová, J. Vencovský, L. Šenolt and M. Filková, Autoimmun. Rev., 2021, 20(5), 102797 CrossRef PubMed.
- F. Wouters, M. P. Maurits, L. Van Boheemen, M. Verstappen, K. Mankia, X. M. E. Matthijssen, A. L. Dorjée, P. Emery, R. Knevel, D. Van Schaardenburg, R. E. M. Toes and A. H. M. Van Der Helm-Van Mil, Ann. Rheum. Dis., 2022, 81, 48–55 CrossRef CAS PubMed.
- A. C. Y. Yau and R. Holmdahl, Dis. Models Mech., 2016, 9, 1111–1123 CrossRef CAS PubMed.
- S. Rantapää Dahlqvist and F. Andrade, J. Intern. Med., 2019, 286, 627–643 CrossRef PubMed.
- H. Yamada, Immunol. Med., 2022, 45, 1–11 CrossRef PubMed.
- L. A. Ridgley, A. E. Anderson and A. G. Pratt, Curr. Opin. Rheumatol., 2018, 30, 207–214 CrossRef CAS PubMed.
- H. U. Scherer, T. Häupl and G. R. Burmester, J. Autoimmun., 2020, 110, 102400 CrossRef CAS PubMed.
- M. A. M. van Delft and T. W. J. Huizinga, J. Autoimmun., 2020, 110, 102392 CrossRef CAS PubMed.
- P. D. Kiely and E. Nikiphorou, Medicine, 2018, 46, 216–221 CrossRef.
- D. Gao, X. Gao, F. Yang and Q. Wang, Int. J. Mol. Sci., 2022, 23(15), 8158 CrossRef CAS PubMed.
- L. Yi, J. Ke, J. Liu, H. Lai, Y. Lv, C. Peng, Y. Zhi, Q. Du, L. Liu, P. Wang, H. Zhou and Y. Dong, J. Leukocyte Biol., 2021, 110, 1113–1120 CrossRef CAS PubMed.
- G. Deyab, T. M. Reine, T. T. Vuong, T. Jenssen, G. Hjeltnes, S. Agewall, K. Mikkelsen, Ø. Førre, M. W. Fagerland, S. O. Kolset and I. Hollan, PLoS One, 2021, 16, e0253247 CrossRef CAS PubMed.
- J. R. Martinez, J. Cresta, G. DeSantis, M. Thoonkuzhy, S. A. Jundi, F. Yousefi, R. L. Mauck and G. R. Dodge, Osteoarthrotic Cartilage, 2020, 28, S487 CrossRef.
- Y. Tanaka, Rheumatology, 2021, 60, VI12–VI20 CrossRef PubMed.
- D. M. Schwartz, M. Bonelli, M. Gadina and J. J. O'Shea, Nat. Rev. Rheumatol., 2016, 12, 25–36 CrossRef CAS PubMed.
- P. Rein and R. B. Mueller, Rheumatol. Ther., 2017, 4, 247–261 CrossRef PubMed.
- D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646–674 CrossRef CAS PubMed.
- P. D. W. Kiely and E. Nikiphorou, Medicine, 2022, 50, 143–148 CrossRef.
- S. Y. Ranade and R. S. Gaud, Int. J. Pharma Sci. Res., 2013, 4, 3782–3794 Search PubMed.
- E. Zampeli and K. Gerasimidou, in Comprehensive Pharmacology, 2022, vol. 5, pp. 427–446 Search PubMed.
- M. Okazaki, H. Kobayashi, Y. Ishii, M. Kanbori and T. Yajima, Rheumatol. Ther., 2018, 5, 185–201 CrossRef PubMed.
- A. Kerschbaumer, A. Sepriano, J. S. Smolen, D. Van Der Heijde, M. Dougados, R. Van Vollenhoven, I. B. McInnes, J. W. J. Bijlsma, G. R. Burmester, M. De Wit, L. Falzon and R. Landewé, Ann. Rheum. Dis., 2020, 79, S744–S759 CrossRef PubMed.
- G. Zhao, R. Ren, X. Wei, Z. Jia, N. Chen, Y. Sun, Z. Zhao, S. M. Lele, H. A. Zhong, M. B. Goldring, S. R. Goldring and D. Wang, J. Controlled Release, 2021, 339, 484–497 CrossRef CAS PubMed.
- R. Deshmukh, Mater. Today Commun., 2023, 35, 106351 CrossRef.
- A. Mogul, K. Corsi and L. McAuliffe, Ann. Pharmacother., 2019, 53, 947–953 CrossRef CAS PubMed.
- H. P. Tony, E. Feist, P. M. Aries, S. Zinke, K. Krüger, J. Ahlers, I. Albrecht, C. Barrionuevo, S. Kalus and H. Burkhardt, Rheumatol. Adv. Pract., 2022, 6(1), rkac002 CrossRef PubMed.
- J. S. Smolen, J. Kay, E. L. Matteson, R. Landewé, E. C. Hsia, S. Xu, Y. Zhou and M. K. Doyle, Ann. Rheum. Dis., 2014, 73, 1811–1818 CrossRef CAS PubMed.
- L. J. Crofford, Arthritis Res. Ther., 2013, 15, S2 CrossRef PubMed.
- S. Bhattacharya and B. G. Prajapati, Asian J. Pharm. Clin. Res., 2017, 10, 353–365 CrossRef CAS.
- R. Huelin, T. Pokora, T. S. Foster and J. F. Mould, Expert Rev. Pharmacoeconomics Outcomes Res., 2012, 12, 505–523 CrossRef PubMed.
- D. Clemett and K. L. Goa, Drugs, 2000, 59, 957–980 CrossRef CAS PubMed.
- I. E. Van Der Horst Bruinsma and M. T. Nurmohamed, Ther. Adv. Musculoskeletal Dis., 2012, 4, 413–422 CrossRef CAS PubMed.
- O. G. Quiñones and M. B. R. Pierre, Curr. Cancer Drug Targets, 2018, 19, 5–16 CrossRef PubMed.
- U. Baschant, L. Frappart, U. Rauchhaus, L. Bruns, H. M. Reichardt, T. Kamradt, R. Braüer and J. P. Tuckermann, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 19317–19322 CrossRef CAS PubMed.
- J. M. Ehrchen, J. Roth and K. Barczyk-Kahlert, Front. Immunol., 2019, 10, 2028 CrossRef CAS PubMed.
- A. Achuthan, FASEB J., 2022, 36(S1), r4392 Search PubMed.
- M. Zewail, N. Nafee and N. Boraie, J. Pharm. Sci., 2021, 110, 2808–2822 CrossRef CAS PubMed.
- S. Timmermans, J. Souffriau and C. Libert, Front. Immunol., 2019, 10, 1545 CrossRef CAS PubMed.
- A. F. Radu and S. G. Bungau, Cells, 2021, 10(11), 2857 CrossRef CAS PubMed.
- C. T. de Castro, M. J. de Queiroz, F. C. Albuquerque, C. C. Brandão, L. F. Gerlack, D. C. R. Pereira, S. C. Barros, W. W. Andrade, E. de A. Bastos, J. de N. B. Azevedo, R. Carreiro, M. L. Barreto and D. B. dos Santos, Front. Pharmacol., 2022, 13, 1–16 Search PubMed.
- L. M. Verhoef, L. Tweehuysen, M. E. Hulscher, B. Fautrel and A. A. den Broeder, Rheumatol. Ther., 2017, 4, 1–24 CrossRef PubMed.
- P. Shi, L. Wang, J. He and Y. Lu, Int. J. Biol. Life Sci., 2023, 3, 35–42 CrossRef.
- M. Massalska, W. Maslinski and M. Ciechomska, Cells, 2020, 9(8), 1876 CrossRef CAS PubMed.
- B. Brynedal, N. Yoosuf, T. B. Ulfarsdottir, D. Ziemek, M. Maciejewski, L. Folkersen, H. Westerlind, M. Müller, P. Sahlström, S. A. Jelinsky, A. Hensvold, L. Padyukov, N. V. Pomiano, A. Catrina, L. Klareskog and L. Berg, Front. Med., 2023, 10, 1–12 Search PubMed.
- F. Taktak and A. P. T. Yiğen, J. Mol. Struct., 2002, 1252, 132133 CrossRef.
- J. M. Kremer, Semin. Arthritis Rheum., 1999, 29, 14–26 CrossRef CAS PubMed.
- I. Padjen, M. R. Crnogaj and B. Anić, Reumatologia, 2021, 58, 390–400 CrossRef PubMed.
- M. Cutolo, A. Sulli, C. Pizzorni, B. Seriolo and R. H. Straub, Ann. Rheum. Dis., 2001, 60, 729–735 CrossRef CAS PubMed.
- F. C. Breedveld and J. M. Dayer, Ann. Rheum. Dis., 2000, 59, 841–849 CrossRef CAS PubMed.
- J. Bae and J. W. Park, Drug Dev. Ind. Pharm., 2016, 42, 254–262 CrossRef CAS PubMed.
- M. F. Tsoi and K. L. Hyrich, Medicine, 2022, 50, 173–177 CrossRef.
- E. Pelechas, P. V. Voulgari and A. A. Drosos, J. Clin. Med., 2019, 8(3), 387 CrossRef CAS PubMed.
- B. Serrano-Benavente, L. Valor, T. del Río Blasco, I. Janta, R. González Benítez, J. C. Nieto-González, J. Martínez-Barrio, J. G. O. Bonilla, A. Ariza, F. J. López-Longo, J. M. Álvaro-Gracia, I. Monteagudo and C. M. González-Fernández, J. Clin. Rheumatol., 2022, 28, E150–E155 CrossRef PubMed.
- J. R. Curtis, B. Stolshek, P. Emery, B. Haraoui, E. Karis, G. Kricorian, D. H. Collier, P. K. Yen and V. P. Bykerk, J. Clin. Rheumatol., 2023, 29, 16–22 CrossRef PubMed.
- A. Khan and D. Scott, Open Access Rheumatol.: Res. Rev., 2011, 3, 63–71 CAS.
- R. N. Maini and M. Feldmann, Arthritis Res., 2002, 4(Suppl. 2), S22–S28 CrossRef PubMed.
- K. Lauper, M. Iudici, D. Mongin, S. A. Bergstra, D. Choquette, C. Codreanu, R. Cordtz, D. De Cock, L. Dreyer, O. Elkayam, E. M. Hauge, D. Huschek, K. L. Hyrich, F. Iannone, N. Inanc, L. Kearsley-Fleet, E. K. Kristianslund, T. K. Kvien, B. F. Leeb, G. Lukina, D. C. Nordström, K. Pavelka, M. Pombo-Suarez, Z. Rotar, M. J. Santos, A. Strangfeld, P. Verschueren, D. S. Courvoisier and A. Finckh, Ann. Rheum. Dis., 2022, 81, 1358–1366 CrossRef CAS PubMed.
- C. Derambure, G. Dzangue-Tchoupou, M. A. D'Agostino, T. Lequerre and O. Vittecoq, PLoS One, 2020, 15(8), e0237143 CrossRef CAS PubMed.
- R. Sanmartí and H. Corominas, J. Clin. Med., 2023, 12(5), 1734 CrossRef PubMed.
- S. Lee, H. Lee and E. Y. Kim, BioDrugs, 2019, 33, 469–483 CrossRef PubMed.
- C. Salliot, M. Dougados and L. Gossec, Ann. Rheum. Dis., 2009, 68, 25–32 CrossRef CAS PubMed.
- M. J. H. Boumans and P. P. Tak, Arthritis Res. Ther., 2009, 11(6), 134 CrossRef PubMed.
- P. Youssef, B. Marcal, P. Button, M. Truman, P. Bird, H. Griffiths, L. Roberts, K. Tymms and G. Littlejohn, J. Rheumatol., 2020, 47, 1174–1181 CrossRef CAS PubMed.
- K. L. Winthrop, M. Harigai, M. C. Genovese, S. Lindsey, T. Takeuchi, R. Fleischmann, J. D. Bradley, N. L. Byers, D. L. Hyslop, M. Issa, A. Nishikawa, T. P. Rooney, S. Witt, C. L. Dickson, J. S. Smolen and M. Dougados, Ann. Rheum. Dis., 2020, 79, 1290–1297 CrossRef CAS PubMed.
- S. Ren, I. Bermejo, E. Simpson, R. Wong, D. L. Scott, A. Young and M. Stevenson, Pharmacoeconomics, 2018, 36, 769–778 CrossRef PubMed.
- K. Sonomoto and Y. Tanaka, Jpn. J. Clin. Immunol., 2015, 38, 443–447 CrossRef CAS PubMed.
- C. S. Thudium, A. C. Bay-Jensen, S. Cahya, E. R. Dow, M. A. Karsdal, A. E. Koch, W. Zhang and R. J. Benschop, Arthritis Res. Ther., 2020, 22, 235 CrossRef CAS PubMed.
- V. Huss, H. Bower, H. Wadström, T. Frisell and J. Askling, Rheumatology, 2022, 61, 1810–1818 CrossRef CAS PubMed.
- K. D. Deane, D. Aletaha, J. M. Bathon, P. Emery, G. E. Fragoulis, V. M. Holers, T. W. J. Huizinga, J. R. Kolfenbach, J. R. O'Dell, D. W. Pearson, E. Park, J. Smolen, Y. Tanaka, P. C. Taylor, A. van der Helm-van Mil, R. F. van Vollenhoven and E. W. St. Clair, in A Clinician's Pearls and Myths in Rheumatology, 2nd edn, 2023, pp. 1–23 Search PubMed.
- Y. Tanaka, Mod. Rheumatol., 2020, 779–787 CrossRef CAS PubMed.
- S. Kubo, S. Nakayamada and Y. Tanaka, Expert Opin. Invest. Drugs, 2023, 32, 333–344 CrossRef CAS PubMed.
- S. Chokesuwattanaskul, M. Fresneda Alarcon, S. Mangalakumaran, R. Grosman, A. L. Cross, E. A. Chapman, D. Mason, R. J. Moots, M. M. Phelan and H. L. Wright, Metabolites, 2022, 12(7), 650 CrossRef CAS PubMed.
- P. Kawalec, K. Śladowska, I. Malinowska-Lipień, T. Brzostek and M. Kózka, Ther. Clin. Risk Manage., 2018, 14, 15–29 CrossRef CAS PubMed.
- I. B. McInnes and G. Schett, Lancet, 2017, 389, 2328–2337 CrossRef CAS PubMed.
- B. Liu, H. Yuan, H. Wang, J. Sun, Q. Zhao and B. Han, Drug Eval. Res., 2021, 44, 561–565 Search PubMed.
- J. S. Smolen, J. Kay, M. K. Doyle, R. Landewé, E. L. Matteson, J. Wollenhaupt, N. Gaylis, F. T. Murphy, J. S. Neal, Y. Zhou, S. Visvanathan, E. C. Hsia and M. U. Rahman, Lancet, 2009, 374, 210–221 CrossRef CAS PubMed.
- K. Pathak, P. Shahi and N. Kumari, Int. J. Pharm. Invest., 2015, 5, 161 CrossRef PubMed.
- M. C. Bruno, M. C. Cristiano, C. Celia, N. D'Avanzo, A. Mancuso, D. Paolino, J. Wolfram and M. Fresta, ACS Nano, 2022, 16, 19665–19690 CrossRef CAS PubMed.
- Y. Tanaka, Mod. Rheumatol., 2023, 33, 633–639 CrossRef PubMed.
- N. Tank, B. Karelia and B. Vegada, J. Pharmacol. Pharmacother., 2017, 8, 92–105 Search PubMed.
- S. Chakraborty, N. V. Gupta, K. T. Sastri, S. M, P. Chand, H. Kumar, R. A. M. Osmani, D. V. Gowda and V. Jain, J. Drug Delivery Sci. Technol., 2022, 73, 103476 CrossRef CAS.
- P. Quan, B. Jiao, R. Shang, C. Liu and L. Fang, J. Ethnopharmacol., 2021, 265, 113294 CrossRef CAS PubMed.
- Y. Xu, M. Zhao, J. Cao, T. Fang, J. Zhang, Y. Zhen, F. Wu, X. Yu, Y. Liu, J. Li and D. Wang, Acta Pharm. Sin. B, 2023, 13, 4417–4441 CrossRef CAS PubMed.
- M. Li, H. Cui, Y. Cao, Y. Lin, Y. Yang, M. Gao, W. Zhang and C. Wang, J. Controlled Release, 2023, 354, 664–679 CrossRef CAS PubMed.
- J. Yi, Y. Liu, H. Xie, H. An, C. Li, X. Wang and W. Chai, Front. Bioeng. Biotechnol., 2022, 10, 1014543 CrossRef PubMed.
- S. Adepu and S. Ramakrishna, Molecules, 2021, 26(19), 5905 CrossRef CAS PubMed.
- R. M. Salem, A. El-deeb, M. Elsergany, H. Elsaadany and R. El-khouly, Clin. Rheumatol., 2021, 40, 557–564 CrossRef PubMed.
- S. K. Agarwal, K. Farheen, G. M. Oderda, C. E. Marion and L. M. Balfe, J. Manage. Care Pharm., 2011, 17, 9-b Search PubMed.
- R. J. Dehoratius, L. H. Brent, J. R. Curtis, L. A. Ellis and K. L. Tang, Patient, 2018, 11, 361–369 CrossRef PubMed.
- M. J. Ho, S. R. Kim, Y. W. Choi and M. J. Kang, J. Pharm. Invest., 2019, 49, 9–15 CrossRef CAS.
- W. Chen, Z. Li, Z. Wang, H. Gao, J. Ding and Z. He, J. Pain Res., 2020, 13, 3315–3329 CrossRef CAS PubMed.
- J. Pradal, O. Jordan and E. Allémann, J. Drug Delivery Sci. Technol., 2012, 22, 409–419 CrossRef CAS.
- A. Arslan, B. Yet, E. Nemutlu, Y. Akdağ Çaylı, H. Eroğlu and L. Öner, Pharmaceutics, 2023, 15(2), 363 CrossRef CAS PubMed.
- V. Promelle, V. Goeb and J. Gueudry, J. Clin. Med., 2021, 10(10), 2118 CrossRef CAS PubMed.
- F. Atzeni, I. F. Masala, M. Bagnasco, L. Lanata, F. Mantelli and P. Sarzi-Puttini, Pain Ther., 2021, 10, 577–588 CrossRef PubMed.
- J. Wohlrab, R. H. H. Neubert, J. Michael and S. Naumann, J. Ger. Soc. Dermatol., 2015, 13, 891–902 Search PubMed.
- B. Amarji, N. K. Garg, B. Singh and O. P. Katare, J. Drug Targeting, 2016, 24, 147–160 CrossRef CAS PubMed.
- A. Dehshahri, A. Kumar, V. S. Madamsetty, I. Uzieliene, S. Tavakol, F. Azedi, H. S. Fekri, A. Zarrabi, R. Mohammadinejad and V. K. Thakur, Gels, 2021, 7, 1–20 Search PubMed.
- E. J. Park, H. Kim, S. M. Jung, Y. K. Sung, H. J. Baek and J. Lee, J. Rheum. Dis., 2020, 27, 4–21 CrossRef.
- J. L. Nam, K. Takase-Minegishi, S. Ramiro, K. Chatzidionysiou, J. S. Smolen, D. Van Der Heijde, J. W. Bijlsma, G. R. Burmester, M. Dougados, M. Scholte-Voshaar, R. Van Vollenhoven and R. Landewé, Ann. Rheum. Dis., 2017, 76, 1108–1113 CrossRef CAS PubMed.
- Y. Kadota, K. Nishida, K. Hashizume, Y. Nasu, R. Nakahara, T. Kanazawa, M. Ozawa, R. Harada, T. Machida and T. Ozaki, Mod. Rheumatol., 2016, 26, 68–74 CrossRef PubMed.
- S. Jagtap, P. Badhe, N. Gujarathi, A. Jadhav, S. Daware and D. Shewale, Int. J. Pharm. Sci. Rev. Res., 2018, 49, 65–70 CAS.
- G. Chen, K. Xu, J. J. Dou, J. H. Yan, D. H. Ju, H. Y. Zhao, M. J. Liu and B. H. Hao, Chin. Pharm. J., 2012, 47, 435–438 CAS.
- X. Song, Y. Wang, H. Chen, Y. Jin, Z. Wang, Y. Lu and Y. Wang, J. Drug Deliv. Sci. Technol., 2021, 63, 102537 CrossRef CAS.
- I. M. Oliveira, D. C. Fernandes, I. F. Cengiz, R. L. Reis and J. M. Oliveira, J. Mater. Sci. Mater. Med., 2021, 32, 1–13 CrossRef PubMed.
- Y. Zhang, Z. Gao, S. Chao, W. Lu and P. Zhang, Drug Delivery, 2022, 29, 1934–1950 CrossRef CAS PubMed.
- W. Yao, C. Tao, J. Zou, H. Zheng, J. Zhu, Z. Zhu, J. Zhu, L. Liu, F. Li and X. Song, Int. J. Pharm., 2019, 563, 91–100 CrossRef CAS PubMed.
- J. Da Ma, J. Jing, J. W. Wang, T. Yan, Q. H. Li, Y. Q. Mo, D. H. Zheng, J. L. Gao, K. A. Nguyen and L. Dai, Arthritis Res. Ther., 2019, 21, 153 CrossRef PubMed.
- Y. Li, Y. Sun, S. Wei, L. Zhang and S. Zong, Drug Dev. Ind. Pharm., 2021, 47, 878–886 CrossRef CAS PubMed.
- L. A. Alwan and E. J. Al-Akkam, J. Drug Delivery Sci. Technol., 2021, 11, 656–662 Search PubMed.
- E. Larrañeta, R. E. M. Lutton, A. D. Woolfson and R. F. Donnelly, Mater. Sci. Eng., R, 2016, 104, 1–32 CrossRef.
- S. Narasimha Murthy and H. N. Shivakumar, in Handbook of Non-Invasive Drug Delivery Systems, 2010, pp. 1–36 Search PubMed.
- M. Kitaoka and M. Goto, in Methods in Pharmacology and Toxicology, 2016, vol. 39, pp. 349–367 Search PubMed.
- D. K. Mishra, V. Pandey, R. Maheshwari, P. Ghode and R. K. Tekade, in Basic Fundamentals of Drug Delivery, 2018, pp. 595–650 Search PubMed.
- J. E. Lai-Cheong and J. A. McGrath, Medicine, 2017, 45, 347–351 CrossRef.
- A. M. Römgens, D. L. Bader, J. A. Bouwstra, F. P. T. Baaijens and C. W. J. Oomens, J. Mech. Behav. Biomed. Mater., 2015, 50, 215–222 CrossRef PubMed.
- R. Wang, Q. Bian, Y. Xu, D. Xu and J. Gao, Int. J. Pharm., 2021, 602, 120598 CrossRef CAS PubMed.
- F. Hueber, J. Wepierre and H. Schaefer, Skin Pharmacol., 1992, 5, 99–107 CrossRef CAS PubMed.
- G. Damiani, A. Pacifico, D. M. Linder, P. D. M. Pigatto, R. Conic, A. Grada and N. L. Bragazzi, Dermatol. Ther., 2019, 32(6), e13113 Search PubMed.
- Y. Javadzadeh and L. Azharshekoufeh Bahari, in Nano- and Microscale Drug Delivery Systems: Design and Fabrication, 2017, pp. 131–146 Search PubMed.
- N. K. Garg, B. Singh, R. K. Tyagi, G. Sharma and O. P. Katare, Colloids Surf., B, 2016, 147, 17–24 CrossRef CAS PubMed.
- A. Z. Alkilani, M. T. C. McCrudden and R. F. Donnelly, Pharmaceutics, 2015, 7, 438–470 CrossRef CAS PubMed.
- J. Juan, I. Marlen, C. Luisa, R. Diaz-, A. Luisa and N. Casas, in Recent Advances in Novel Drug Carrier Systems, 2012 Search PubMed.
- Y. Shahzad, R. Louw, M. Gerber and J. Du Plessis, J. Controlled Release, 2015, 202, 1–13 CrossRef CAS PubMed.
- B. Demir, L. Rosselle, A. Voronova, Q. Pagneux, A. Quenon, V. Gmyr, D. Jary, N. Hennuyer, B. Staels, T. Hubert, A. Abderrahmani, V. Plaisance, V. Pawlowski, R. Boukherroub, S. Vignoud and S. Szunerits, Nanoscale Horiz., 2022, 7, 174–184 RSC.
- I. A. Tekko, G. Chen, J. Domínguez-Robles, R. R. S. Thakur, I. M. N. Hamdan, L. Vora, E. Larrañeta, J. C. McElnay, H. O. McCarthy, M. Rooney and R. F. Donnelly, Int. J. Pharm., 2020, 586, 119580 CrossRef CAS PubMed.
- W. Preedalikit, P. Leelapornpisid and U. Vinijketkhumnua, Acta Hortic., 2014, 1023, 179–184 CrossRef.
- M. A. Sallam, A. M. Motawaa and S. M. Mortada, Drug Dev. Ind. Pharm., 2015, 41, 141–147 CrossRef CAS PubMed.
- N. K. Garg, N. Tandel, S. K. Bhadada and R. K. Tyagi, Front. Pharmacol., 2021, 12, 1–10 Search PubMed.
- S. Heuschkel, A. Goebel and R. H. H. Neubert, J. Pharm. Sci., 2008, 97, 603–631 CrossRef CAS PubMed.
- M. A. Shewaiter, T. M. Hammady, A. El-Gindy, S. H. Hammadi and S. Gad, J. Drug Deliv. Sci. Technol., 2021, 61, 102110 CrossRef CAS.
- M. D. Chatzidaki, S. Demisli, E. Zingkou, P. G. V. Liggri, D. P. Papachristos, G. Balatsos, V. Karras, F. Nallet, A. Michaelakis, G. Sotiropoulou, S. E. Zographos and V. Papadimitrioum, Colloids Surfaces A Physicochem. Eng. Asp., 2022, 654, 130159 CrossRef CAS.
- Y. S. R. Elnaggar, W. M. El-Refaie, M. A. El-Massik and O. Y. Abdallah, J. Controlled Release, 2014, 180, 10–24 CrossRef CAS PubMed.
- S. K. Babasahib, R. W. Born and N. M. Raghavendra, Artif. Cells, Nanomed., Biotechnol., 2022, 50, 59–70 CrossRef CAS PubMed.
- E. Touitou, N. Dayan, L. Bergelson, B. Godin and M. Eliaz, J. Controlled Release, 2000, 65, 403–418 CrossRef CAS PubMed.
- M. M. A. Elsayed, O. Y. Abdallah, V. F. Naggar and N. M. Khalafallah, Int. J. Pharm., 2007, 332, 1–16 CrossRef CAS PubMed.
- M. N. Pastore, Y. N. Kalia, M. Horstmann and M. S. Roberts, Br. J. Pharmacol., 2015, 172, 2179–2209 CrossRef CAS PubMed.
- M. Sirbubalo, A. Tucak, K. Muhamedagic, L. Hindija, O. Rahić, J. Hadžiabdić, A. Cekic, D. Begic-Hajdarevic, M. C. Husic, A. Dervišević and E. Vranić, Pharmaceutics, 2021, 13, 924 CrossRef CAS PubMed.
- Y. Lin, Y. Chen, R. Deng, H. Qin, N. Li, Y. Qin, H. Chen, Y. Wei, Z. Wang, Q. Sun, W. Qiu, J. Shi, L. Chen, Y. Wang, G. Nie and R. Zhao, Nano Today, 2023, 49, 101791 CrossRef CAS.
- M. Cao, L. Ren and G. Chen, AAPS PharmSciTech, 2017, 18, 1960–1971 CrossRef CAS PubMed.
- F. R. Ali, M. H. Shoaib, R. I. Yousuf, S. A. Ali, M. S. Imtiaz, L. Bashir and S. Naz, Biomed Res. Int., 2017, 2017, 4654958 Search PubMed.
- P. G. Xu, X. F. Lei, B. Di Ren, S. Y. Lv and J. L. Zhang, Trop. J. Pharm. Res., 2017, 16, 477–482 CrossRef CAS.
- N. R. de Barros, P. A. M. Chagas, F. A. Borges, J. L. P. Gemeinder, M. C. R. Miranda, B. C. Garms and R. D. Herculano, J. Mater., 2015, 2015, 1–7 Search PubMed.
- Q. Dai, L. Xu and X. Yu, Int. J. Rheum. Dis., 2019, 22, 1498–1505 CrossRef CAS PubMed.
- W. Xi, J. H. Hu, Q. G. Zhu and J. Y. Liu, Pharm. Care Res., 2004, 4, 240–242 CAS.
- L. Djekic, M. Martinovic, R. Stepanović-Petrović, A. Micov, M. Tomić and M. Primorac, Eur. J. Pharm. Sci., 2016, 92, 255–265 CrossRef CAS PubMed.
- J. Cao, J. Su, M. An, Y. Yang, Y. Zhang, J. Zuo, N. Zhang and Y. Zhao, Mol. Pharm., 2021, 18, 305–316 CrossRef CAS PubMed.
- P. R. Yadav, M. N. Munni, L. Campbell, G. Mostofa, L. Dobson, M. Shittu, S. K. Pattanayek, M. J. Uddin and D. Bhusan Das, Pharmaceutics, 2021, 13(8), 1132 CrossRef CAS PubMed.
- M. Kirkby, A. R. J. Hutton and R. F. Donnelly, Pharm. Res., 2020, 37 CAS.
- Y. Zhang, J. Yu, A. R. Kahkoska, J. Wang, J. B. Buse and Z. Gu, Adv. Drug Delivery Rev., 2019, 139, 51–70 CrossRef CAS PubMed.
- W. Zhao, L. Zheng, J. Yang, Y. Li, Y. Zhang, T. Ma and Q. Wang, Eur. J. Pharm. Sci., 2023, 184, 106409 CrossRef CAS PubMed.
- W. Zhao, L. Zheng, J. Yang, Z. Ma, X. Tao and Q. Wang, Drug Delivery, 2023, 30, 121–132 CrossRef CAS PubMed.
- W. J. Lee, M. R. Han, J. S. Kim and J. H. Park, Expert Opin. Drug Delivery, 2019, 16, 199–206 CrossRef CAS PubMed.
- K. Ita, Biomed. Pharmacother., 2017, 93, 1116–1127 CrossRef CAS PubMed.
- E. Korkmaz, E. E. Friedrich, M. H. Ramadan, G. Erdos, A. R. Mathers, O. Burak Ozdoganlar, N. R. Washburn and L. D. Falo, Acta Biomater., 2015, 24, 96–105 CrossRef CAS PubMed.
- E. Korkmaz, E. E. Friedrich, M. H. Ramadan, G. Erdos, A. R. Mathers, O. B. Ozdoganlar, N. R. Washburn and L. D. Falo, J. Pharm. Sci., 2016, 105, 3453–3457 CrossRef CAS PubMed.
- Y. Li, D. Bi, Z. Hu, Y. Yang, Y. Liu and W. K. Leung, Materials, 2023, 16(13), 4805 CrossRef CAS PubMed.
- R. Tenchov, R. Bird, A. E. Curtze and Q. Zhou, ACS Nano, 2021, 15, 16982–17015 CrossRef CAS PubMed.
- M. Badran, K. Shalaby and A. Al-Omrani, Sci. World J., 2012, 2012, 134876 Search PubMed.
- T. Amnuaikit, T. Limsuwan, P. Khongkow and P. Boonme, Asian J. Pharm. Sci., 2018, 13, 472–484 CrossRef PubMed.
- Y. P. Zhao, J. F. Han, F. Y. Zhang, T. T. Liao, R. Na, X. F. Yuan, G. Bin He and W. Ye, Drug Delivery, 2022, 29, 2269–2282 CrossRef CAS PubMed.
- Z. Liu, S. Ramakrishna and X. Liu, APL Bioeng., 2020, 4, 1–8 CAS.
- R. Goyal, L. K. Macri, H. M. Kaplan and J. Kohn, J. Controlled Release, 2016, 240, 77–92 CrossRef CAS PubMed.
- J. Xue, J. Xie, W. Liu and Y. Xia, Acc. Chem. Res., 2017, 50, 1976–1987 CrossRef CAS PubMed.
- V. Meenatchi, A. Sood, R. Bhaskar and S. S. Han, J. Mol. Liq., 2024, 393, 123558 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |
