 Open Access Article
Open Access ArticleCancer immunotherapy boosted by layered double hydroxide nanoparticles
Xiaochun
Deng†
a,
Gaoming
Li†
a,
Mingwu
Shen
 *a and
Xiangyang
Shi
*a and
Xiangyang
Shi
 *ab
*ab
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Shanghai Engineering Research Center of Nano-Biomaterials and Regenerative Medicine, College of Biological Science and Medical Engineering, Donghua University, Shanghai 201620, China. E-mail: xshi@dhu.edu.cn; mwshen@dhu.edu.cn
bCQM – Centro de Química da Madeira, Universidade da Madeira, Campus Universitário da Penteada, 9020-105 Funchal, Portugal
First published on 14th August 2024
Abstract
The development of innovative nanoplatforms for cancer immunotherapy has garnered considerable attention in biomedical research. Layered double hydroxide (LDH) is a two-dimensional inorganic nanomaterial consisting of positively charged brucite-like cationic layers and negatively charged anions intercalated in the interlayer space. LDH-based nanoplatforms have been emerging as promising candidates for enhancing the efficacy of cancer immunotherapy. This review highlights the latest advancements in the application of LDH in cancer immunotherapy. The unique physicochemical properties of LDH, such as a high surface area, tunable porosity, and facile surface modification, entail it to be a versatile platform to deliver antigens, drugs, and other therapeutic agents. In addition, LDH's inherent biocompatibility and biodegradability contribute to its suitability for in vivo applications. Moreover, the nanoplatform formed by the integration of self-adjuvant LDH with tumor antigen and immunomodulatory components has shown promising results in enhancing antigen presentation, promoting immune cell activation and regulating the immune suppressive tumor microenvironment. In this review, we discuss the application of LDH as a carrier-supported immune modulator in immunotherapy and the application of LDH as an adjuvant to construct tumor vaccines. Finally, future research challenges of LDH in immunotherapy are briefly discussed. Conclusively, the versatility and adaptability of LDH-based nanoplatforms make them promising candidates for the next generation of cancer immunotherapeutics.
1. Introduction
Immunotherapy is a powerful treatment modality to tackle a variety of advanced cancer types, aiming to regulate the patient's immune system to create tumor-specific immune cells capable of seeking out and destroying malignant cells and preventing tumor progression and recurrence.1,2 In addition, immunotherapy improves the antitumor immune response to achieve the desired treatment efficacy with fewer side effects and off-target processes than other conventional therapeutic modes.3,4 Immune cells, including lymphocytes, mononuclear macrophages, dendritic cells, granulocytes, hematopoietic stem cells, etc., play important roles in cancer immunotherapy and can be divided into antigen-specific lymphocytes, antigen-presenting cells (APCs) and other cells involved in immune response according to the different action mechanisms.5,6 Antigens released from necrotic or apoptotic cancer cells can be taken up and processed into shorter peptides by APCs and presented in the major histocompatibility complex class (MHC) as an MHC–peptide complex. Then, the MHC–peptide complex binds to the T cell receptor on the surface of naive T cells, thus promoting tumor-specific cytotoxic T lymphocytes (CTLs) to infiltrate the local tumor sites, kill tumor cells, and release additional tumor antigens.7,8 Unfortunately, antitumor immune cells always face recruitment barriers, depletion, and off-target immune attack due to the presence of immunosuppressive tumor microenvironments (TMEs),9 and hence, the therapeutic effect of immunotherapy for solid tumors is not ideal in clinical treatment, and only a small number of patients can be treated to have immune responses.8,10 Therefore, it is necessary to develop more efficient immunotherapy approaches to fight cancer.With the booming development of nanotechnology, nanomaterials have been often applied to construct versatile platforms with effective cancer immunotherapeutic efficacy.3 As a clay material, layered double hydroxide (LDH) has a high specific surface area and a two-dimensional inorganic layered structure11 and has been widely used as a drug loading carrier.12 LDH is composed of a cationic layer of positively charged octahedral metal hydroxide M(OH)6 and an interlayer channel containing anions and water molecules, whose general formula is defined as [M1−x2+Mx3+(OH)2]x+(An−x/n)·mH2O, where M2+ and M3+ are the divalent and trivalent metal ions, respectively. Some of the M2+ ions can be replaced by M3+, while the water molecules and exchangeable guest anions (An−) are located in the sandwich channel to maintain the charge balance.13,14 The surface of LDH is positively charged, allowing for the adsorption of negatively charged proteins, nucleic acids and cell membranes through electrostatic interactions, and the formed nanohybrids can be effectively taken up by cells through clathrin-mediated or non-receptor-mediated pathways.15,16 Anion exchange is another method that can be used to functionalize LDH with different substances in the interlayer space of LDH to improve immunotherapy effects, and even adjust the performance of LDH by changing the layer spacing.17,18
In addition, as a weakly alkaline nanomaterial, LDH can be easily degraded in acidic environments with low toxicity, which empowers it to regulate the pH of the TME to facilitate the transformation of tumor-associated macrophages (TAMs) from the M2 type to M1 and specifically block the autophagy pathway of tumor cells by disrupting the lysosomal functions.19–21 Moreover, LDH with a composition of Al similar to commercial aluminum adjuvants (Alum) has been shown to have potential adjuvant activity in vivo,22 can stimulate stronger humoral immunity and can cause less local inflammation than Alum, thus inducing robust cellular immunity.12,23,24 Because of these advantages, LDH has been used to combine with other species, such as proteins,23 nucleic acids,25 bacteria,26 and other nanomaterials27 to build up drug delivery systems or tumor vaccines for improved cancer immunotherapy by promoting antigen presentation, stimulating APC cell activation and other effective pathways.
LDH has great potential for the construction of tumor immunotherapy platforms. Here, we summarize the research progress of LDH as a carrier and as an adjuvant in the construction of cancer immunotherapy drug delivery systems (Fig. 1). It should be noted that this is not a comprehensive review covering all aspects of LDH-based nanoplatforms for cancer immunotherapy, but rather a discussion of important developments in the structural design of tumor nanovaccine and immunotherapy applications. Finally, the challenges and outlooks of LDH-based nanoplatforms for cancer immunotherapy are also briefly discussed.
2. LDH as a carrier for cancer immunotherapy applications
LDH with a large specific surface area and positively charged surface can load negatively charged substances, including proteins, nucleic acids, and inorganic nanomaterials. In addition, LDH possesses the characteristic of high biocompatibility and can be simply mass prepared at low cost. This endows it with great potential for the construction of cancer immunotherapy drug delivery systems for clinical translation.2.1. LDH loaded with nanomaterials
Nanomaterials having different fascinating characteristics, such as optical properties (e.g., graphene quantum dots),27 magnetic properties (e.g., iron oxide),28 and enzymatic catalysis properties29 can be designed for photothermal/photodynamic therapy, hypothermia, or catalysis of hydrogen peroxide in the TME to generate reactive oxygen species (ROS) to tackle cancer.In a recent work, copper (Cu)-containing LDH was developed as a carrier to load ultrasmall FeOOH nanodots within the LDH interlayer space inserted with a heat-shock protein (Hsp90) inhibitor (Fig. 2A). In such a design, FeOOH produces highly cytotoxic hydroxyl radicals through a Fenton reaction, and the Cu-LDH nanocarrier acts as a highly efficient near-infrared (NIR) photothermal conversion agent that can enhance the ability of FeOOH nanodots to catalyze ROS generation, while the addition of ganetespib (STA, an Hsp90 inhibitor) prompts the decreased threshold temperature for photothermal therapy (PTT)-mediated cancer cell apoptosis from above 45 °C to 38–42 °C and enhances the bioactivity of antigens released from dying cells. The triple functions of the nanocomposite platform led to the immunogenic cell death (ICD) of tumor cells, characterized by the exposure of calreticulin (CRT) on the tumor cell surface, an “Eat Me” signal and one of the main signals of ICD (Fig. 2B) for dendritic cell (DC) maturation and CTL infiltration in tumors and spleens. Then, the 4T1 (murine breast carcinoma cell line) tumor-bearing mouse model was applied to establish primary and abscopal tumors to prove the immunotherapeutic efficacy of the designed nanosystem. The results showed that the primary tumor was eradicated, the abscopal tumor growth upon fever-type heating was restrained, and more cytotoxic T lymphocytes were induced in the abscopal tumors and spleens after just one treatment for one week with the designed LDH therapeutics.30
 | ||
| Fig. 2 (A) Schematic illustration of therapeutic effects induced by FeOOH@STA/Cu-LDH nanohybrid on both primary and abscopal 4T1 tumors. (B) In vitro biological characterization using cultured 4T1 cancer cells through confocal laser scanning microscopic imaging (scale bar represents 20 μm).30 Copyright 2020, Wiley-VCH. | ||
In another study, a pH-responsive, self-destructive intelligent nanoplatform was constructed based on LDH and applied for breast cancer treatment through magnetic resonance/fluorescence dual-mode imaging-guided mitochondrial membrane potential damage (MMPD), photodynamic therapy (PDT), PTT, and immunotherapy under external NIR light irradiation (Fig. 3A). LDH was effectively loaded with indocyanine green (ICG) through electrostatic interactions and coated with calcium phosphate (Ca3(PO4)2) through biomineralization (M-LDH/ICG@Ca3(PO4)2, MICaP). The pH-sensitivity of Ca3(PO4)2 rendered the dissociation of the platform in the TME to release calcium ions, thus leading to the reduction of mitochondrial membrane potential and the elevated permeability of the mitochondrial membrane and consequently reducing oxygen consumption to solve the TME hypoxia situation and destroying mitochondrial functions to activate the intrinsic apoptosis pathway.31 In the presence of oxygen and laser irradiation, the incorporated ICG as a photosensitizer was able to generate cytotoxic ROS, which directly disrupted cellular components and tumor blood vessels to initiate tumor cell necrosis for subsequent triggering of ICD.32 As shown in Fig. 3B, the level of CD8+ T cells in the MICaP + L (L represents laser) group was the highest and 1.03 times and 1.72 times higher than those in the M-LDH@ICG (MI) + L group and the MICaP group, respectively. Eventually, the immune response generated by the LDH-based multifunctional therapeutic platform greatly boosts the CTL response, thereby limiting tumor growth and metastasis.33
 | ||
| Fig. 3 (A) Preparation of MICaP and the use of MICaP for Ca2+-induced MMPD and phototherapy (PDT/PTT) to realize the cancer immune response. (B) Flow cytometry analyses of the percentages (%) of CD8+ T cells in the spleens of mice after various treatments.33 Copyright 2023, American Chemical Society. | ||
2.2. LDH loaded with RNA
RNA, such as messenger RNA (mRNA), microRNA, and small interfering RNA (siRNA), has been regarded as a class of highly attractive drugs for the treatment and prevention of numerous diseases. Besides, some RNAs play an important role in regulating the polarization of macrophages. However, RNAs are easily degraded by exonuclease and RNA enzymes in the human body.34–36 LDH, with many excellent properties, can be applied to deliver RNA as a vector to protect RNA from degradation and increase the expression of a given protein or knock out the target gene or promote the polarization of TAMs to enhance systemic antitumor immunity.34Recent studies have reported that LDH loaded with siRNA could block the intracellular immune checkpoint NR2F6 to synergistically induce robust immunotherapy effects when accompanied by the anti-PD-L1 antibody. Furthermore, it was found that PTT produced by LDH after laser irradiation could alter the “cold” tumor to a “hot” one for enhanced immunotherapy of hepatocellular carcinoma.37 Besides, LDH was also applied to deliver microRNA-155 (miR155) for development and function in both innate and adaptive immune cells, specifically to modulate the immunosuppressive TME by repolarizing TAMs to the M1 phenotype via STAT3, ERK1/2, and NF-κB pathways, induce the infiltration of activated CTLs, and inhibit the myeloid-derived suppressor cell infiltration in the TME (Fig. 4).25
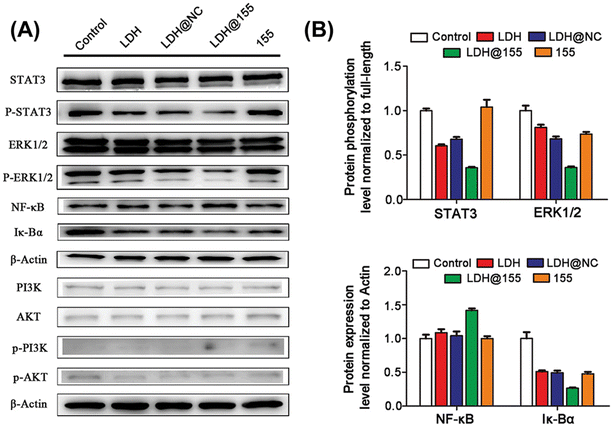 | ||
| Fig. 4 Signalling pathways and mechanism of LDH@155 on M2 macrophage repolarization to the M1 type. (A) Western blot assay of the selected proteins for the determination of phosphorylation and full-length antibody array. (B) Quantitative analysis of the relative protein expression levels after different treatments.25 Copyright 2019, Wiley-VCH. | ||
2.3. LDH/bacteria nanohybrids
It is well known that some anaerobic bacteria such as Propionibacterium acnes (P. acnes) can target and colonize tumors on account of the hypoxic characteristics of the TME.38 In addition, the immunogenicity and metabolites of bacteria can strongly activate the immune response and induce the production of cytotoxic substances, respectively.39 However, naked bacteria in the blood are easily captured and killed by erythrocytes, and exposure to antigens can lead to systemic inflammation.40 Studies have shown that using LDH as a carrier to form LDH/bacteria nanohybrids can avoid the above problems.26 In a proof of concept study,26 negatively charged bacteria were interacted with positively charged LDH to form nanohybrids, thus avoiding clearance of bacteria during the blood circulation for tumor targeting and colonization with a good safety profile. Degradation of LDH occurred when the LDH/bacterial nanohybrids reached the acidic TME, exposing P. acnes to trigger immune responses. At the same time, the metal ions released from LDH under the acidic TME participated in the process of tumor inhibition. Specifically, P. acnes activated and induced macrophages to express nitric oxide synthase and release nitric oxide (NO), which accessed tumor cells and was oxidized to form cytotoxic reactive nitrogen species (ONOO−). Zn2+ released from LDH played a key role in inhibiting the rapid proliferation of tumor cells and was tightly regulated by the intracellular metallothionein (MT) with abundant sulfhydryl groups and free radical scavenging ability. In addition, NO3− released from the LDH provided the substrate for the denitrification of P. acnes. Hence, the concentrations of Zn2+ and NO3− gradually increased with the degradation of LDH in the acidic TME. The former broke the homeostasis of intercellular Zn, while the latter was conducive to bacterial denitrification, which prompted the greater production of cytotoxic ONOO−, compromised the free radical scavenging ability of MT, and ultimately caused ROS accumulation and DNA damage, leading to apoptosis of cancer cells (Fig. 5A). In addition, flow cytometry showed that the LDH–bacterial nanohybrid could induce the transformation of M2-type TAMs to tumor-inhibiting M1-one (Fig. 5B), which further promoted the immune response and realized effective antitumor activity.26 | ||
| Fig. 5 (A) Schematic illustration of P. acnes@LDHs for inhibition of tumor growth and metastasis. (B) Flow cytometry analysis of F4/80+ CD80+ M1 macrophages and F4/80+ CD206+ M2 macrophages of tumor tissues after treatment for 14 days.26 Copyright 2023, Wiley-VCH. | ||
In another study, P. acnes@LDH nanohybrids were similarly prepared except for that the cations of LDH were changed from Zn2+ to cobalt ions (Co2+). The release of Co2+ under acidic conditions inhibited the activity of superoxide dismutase (SOD), thereby increasing the level of intracellular superoxide anion free radicals (˙O2−). The reaction of ˙O2− with NO and the production of bacterial denitrification promoted the production of high and stable active nitrogen ONOO−, leading to mitochondrial damage and DNA breakage, thereby inducing tumor cell apoptosis.39 However, the reported LDH/bacteria nanohybrids have not been used for antitumor immunotherapy to generate systemic antitumor immunity.
2.4. LDH as a carrier of metal ions
It is well reported that bioactive metal ions are involved in the regulation of tumors and inflammation through inducing ferroptosis41 or activating the CTLs.42 The cationic laminate of LDH consists of divalent and trivalent metal ions, and the type of metal ion can be changed by replacing the cation precursor, which empowers LDH with the peculiarity to load a variety of metal ions and an adjustable band gap to achieve highly efficient tumor therapy (Table 1). For example, MgCaFe-LDH (MCF) was prepared by reacting three cationic precursors with a base solution (Fig. 6A). The introduced Ca2+ modulated the energy band of LDH and effectively promoted the separation of electron–hole pairs, thus equipping LDH with outstanding sonodynamic therapy (SDT) performance. The release of Fe3+ from LDH in the TME exhausted GSH to generate Fe2+ that could induce a Fenton reaction and activate the ferroptosis pathway (Fig. 6B), prompting LDH to overcome the immune suppressive TME with increased oxidative stress. | ||
| Fig. 6 (A) The construction of Ca2+-introduced MCF to fulfil high-performance oxidation stress and outstanding antitumor immunotherapy by activating the T-cell mediated immunity. (B) Schematic illustration of the MCF-induced ferroptosis pathway and the sonodynamic mechanism.44 Copyright 2023, Elsevier. (C) Schematic illustration of the local element rearrangement strategy to synthesize MgMnFe-LDH for efficient SDT in combination with immunotherapy.46 Copyright 2023, Elsevier. | ||
| Samples | Ions | Functionalities | Ref. |
|---|---|---|---|
| Co-LDH | Co2+ | Inhibiting the activity of SOD, thus increasing the level of intracellular superoxide anions | 39 |
| Cu-LDH | Cu2+ | As an efficient NIR photothermal conversion agent | 30 |
| Fe–Ni-LDH | Fe3+; Ni2+ | Fe3+ can be reduced to Fe2+ by intracellular GSH to achieve a Fenton-like reaction, and Ni2+ can mediate the Fenton-like reaction. Both Fe2+ and Ni2+ enable CDT through ROS production | 43 |
| MgCaFe-LDH | Mg2+; Ca2+; Fe3+ | Ca2+ renders LDH with SDT performance and Fe3+ depletes GSH to generate Fe2+ for Fenton reaction mediation. The combined pro-inflammatory effects of Ca2+ and Fe2+ promote a higher ratio of M1 polarization of TAMs | 44 |
| Zn-LDH | Zn2+ | Zn2+ activates the cGAS-STING signaling pathway | 45 |
| MgMnFe-LDH | Mg2+; Mn2+; Fe3+ | Mn2+ promotes the generation of ROS and oxygen and maturates DCs; Fe3+ is used to react with GSH to form Fe2+ for Fenton reaction mediation and ferroptosis of cancer cells | 46 |
| MgAl-LDH | Mg2+; Al3+ | Al3+ is used to activate immunity, similar to traditional Alum adjuvants | 23 |
In addition, the expression of the natural killer activating receptor NKG2D in CD8+ T cells was downregulated in response to intracellular Mg2+ lessening, which hindered the antitumor immune response.47 Thus, the presence of Mg2+ conferred the ability of LDH to activate T cells. Moreover, LDH exhibited the ability to polarize TAMs toward the M1 phenotype and excellent immunotherapy function on account of the combined proinflammatory effects of the incorporated Ca2+ and Fe2+/Fe3+. Furthermore, the integrated Mg2+ and Ca2+ in LDH synergistically activated CD8+ and CD4+ T cells and enhanced T cell infiltration into tumor tissues. Overall, this work addressed the use of LDH as a carrier of multiple bioactive metal ions for integrated TME regulation, SDT of tumor cells, and immune activation to effectively treat cancer.44
In another study,46 a local element rearrangement strategy was employed to reconstruct iron-based LDH by partially replacing Mg2+ with Mn2+ to form MgMnFe-LDH as a novel ultrasound-triggered catalyst for efficient SDT of tumors (Fig. 6C). In the authors’ design, Mn2+ changed the length of the Fe–O bond and generated active metal sites with a higher electron density, which improved the catalytic performance of LDH to realize the self-supply of oxygen and strong ROS generation abilities and reduced the band gap of LDH to promote the formation of e–h+ pairs to form ˙O2− and 1O2 for improved SDT performance. In addition, the doped Mn2+ as an active agent can effectively promote DC maturation and regulate immunosuppression. Overall, the developed MgMnFe-LDH enabled efficient treatment of a murine breast cancer model through the synergistic strategy of ferroptosis brought by Fe2+, SDT and immunotherapy.46
2.5. Endothelial barrier-crossing of LDH-based nanoplatforms
Tumor tissue targeting mainly utilizes tumor vascular leakage and tumor lymphatic system defects to passively accumulate nano-sized particles in solid tumors through enhanced permeability and retention (EPR) effects.48 LDH nanomaterials can cross the endothelial barrier through the EPR effect. For instance, Lu et al.49 demonstrated that LDH-based composite nanoplatforms can effectively accumulate in the tumor area, and the fluorescence intensity can reach a peak at 24 h post-injection due to the long blood circulation time of nanoparticles (NPs) and the EPR effect. In another study, Zhu et al.33 proved that MICaP NPs could reach the tumor site through the EPR effect, leading to the decomposition of Ca3(PO4)2 and the release of ICG.However, the EPR effect, as one of the main mechanisms by which solid tumor accumulates nanomaterials after systemic administration, has become controversial due to its diversity across tumors and species (insufficient vascular endothelial space in human tumors compared to mouse models).50 In a recent study, Warren Chan et al.51 found that endothelial cell gaps in tumor blood vessels were 60 times smaller than the injected NPs and that NPs can be accumulated in tumors through a trans-endothelial pathway (transcytosis). These findings challenge the early understanding of EPR effects and could impact the rationale for developing cancer nanomedicines. However, the transcytosis pathway as well as the so-called nanomaterial-induced endothelial leakiness (NanoEL)52 have not been found or explored for LDH-based nanoplatforms. Alternatively, LDH is often functionalized with different targeting ligands (e.g., folate,53 hyaluronic acid,54 or peptides55) or camouflaged by cancer cell membranes56,57 to achieve active targeting of tumors along with the passive EPR effect.
3. LDH-based nanovaccines for cancer immunotherapy
Vaccines have been widely adopted for cancer immunotherapy because of their ability to induce tumor-specific immune response.58 Vaccines involve in the cancer immune cycle, which begins with the release of tumor antigens, the uptake of antigens and then the stimulation of cross-presentation of tumor antigens by APCs, the initiation and activation of T cells, the transport and infiltration of tumor antigen-specific T cells, the recognition of tumor cells by T cells, and finally the destruction of tumor cells by CTLs.59 Compared to traditional vaccines with the characteristics of low efficiency of APC antigen presentation and less accumulation in lymph nodes caused by direct administration of naked antigens in vivo, nanovaccines show many potential advantages, such as enhanced stability of antigens through the packaging and shielding effects of the carrier, improved uptake of antigens by APCs, accumulation and retention of antigens/adjuvants in lymph nodes through size regulation or targeting ligand modification of nanomaterials, enhanced immunogenicity by the addition of an immune adjuvant,60 as well as improved tumor antigen-specific CTL response through antigen cross-presentation.59,61 Thus, nanovaccines have a very broad prospect in immunotherapy.Adjuvants, as one of the important components of vaccines, can improve the strength, breadth, and persistence of the immune response (Fig. 7).62 Adjuvants, a kind of immune stimulants that are dangerous signaling molecules with similar functions as pathogen-associated molecular patterns or damage-associated molecular patterns, can trigger the innate immune responses to induce activation and maturation of APCs by targeting pattern recognition receptors (PRRs) on APCs. After maturation, APCs terminate antigen phagocytosis to enhance their ability to present and express high levels of co-stimulatory signals and cytokines, initiating and enhancing adaptive immune responses.63,64 Different types of immunostimulants generated signals by targeting different PRRs, leading to secretion of different cytokines. Currently, the immunostimulants are mainly divided into four categories according to the different APC receptors: toll-like receptors,65 cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING),66 C-type lectins67 and others.64,68 Common adjuvants included aluminum, oil-in-water emulsions, and liposomes, which have been approved for use in human vaccines but still face many obstacles, including the highly immunosuppressive TME, the anisotropic structure and poor dispersion of adjuvants, downregulation of MHC, failed activation of APCs, and non-biodegradability, which limit their therapeutic effects.12,23,69 The process of activating APCs is often accompanied by the transformation of TAMs from the M2 type with the pro-tumor immunosuppressive state to the M1 type with the antitumor immunoactivation state, thus building up a multi-directional pro-inflammatory network to trigger powerful antitumor immunity.70
 | ||
| Fig. 7 (A) Vaccines without adjuvants induce modest production of T helper-polarizing cytokines, antibodies, and activated T cells. (B) In contrast, vaccines with adjuvants promote the maturation of APCs, increase the interaction between APCs and T cells, and promote the production of greater numbers and more types of T helper-polarizing cytokines, multifunctional T cells, and antibodies, leading to broad and durable immunity.64 Copyright 2023, Springer Nature. | ||
LDH with an aluminum component has been proven to be a better alternative to the traditional Alum adjuvant.71 It could induce effective immune responses for more than four months with a more active immunostimulatory effect, attributed to its abilities of effective drug loading, sustained drug release, and constant immune stimulation,23,72 promotion of TAMs repolarization from M2 to M1 by blocking the autophagy of TAMs via efficiently disrupting endosomes and lysosomes, and inhibition of the formation of myeloid-derived suppressor cells.73
3.1. LDH–antigen vaccines
BSA-Cy7, a model antigen, was devoted to investigating the properties of different adjuvants. The LDH–antigen vaccine was shown to form loose nodules as biodegradable depots in situ after intravenous administration, kept releasing antigens and recruited more leukocytes and macrophages than the aluminum adjuvant with tight agglomerates (Fig. 8A).23 Moreover, the application of LDH was proven to induce more efficient cellular responses, with spleen cells in the LDH group secreting approximately 1.6 times more interferon-γ (IFN-γ) than the aluminum adjuvant group and durable immune responses on account of continuous stimulation and increased memory T-cell responses. These results demonstrated the great potential of LDH as a new type of adjuvant in immunotherapy.23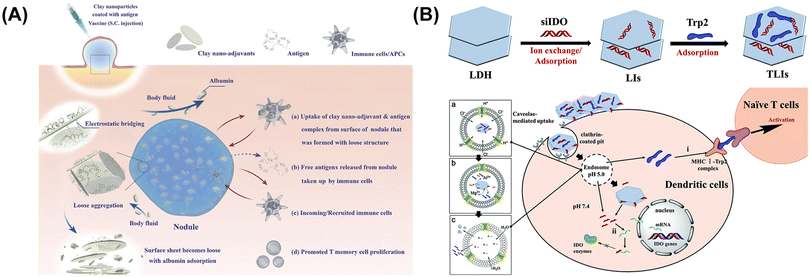 | ||
| Fig. 8 (A) Clay nanoadjuvant complexed with antigens was able to form nodules with a loose structure at the site of injection. The low aggregations were connected with electrostatic bridging that became destabilized and detached from the surface of nodules when it was exposed to a body fluid (containing albumin).23 Copyright 2018, Wiley-VCH. (B) Schematic representation showing the construction of Trp2-LDH-siIDO NPs and their endocytosis and cellular tracking pathways.15 Copyright 2017, The Royal Society of Chemistry. | ||
In another research,74 ovalbumin (OVA) was used as a model antigen and complexed with LDH to form an LDH–antigen vaccine. The designed vaccine stimulated DC maturation by greatly increasing the MHC II complex on DCs. At the same time, the OVA antigen was released from the LDH–OVA complex into the cytoplasm of tumor cells and then enzymatically degraded into functional epitopes, which interacted with MHC class I complexes, leading to the presentation of the MHC class I-functional epitope complexes on the surface of DCs and eventually activating T cells to achieve the desired immunotherapy effect in vitro.
3.2. LDH–nucleic acid vaccines
DNA (encoding a specific protein antigen) vaccines can induce humoral and cellular immunity.75 However, the effect of DNA vaccines is limited due to the limited bioavailability of DNA.76 In addition, only a tiny fraction of the DNA injected enters the nucleus of DCs.77 Therefore, it is necessary to develop effective vectors to improve the efficiency of DNA delivery, induce effective immunity, and increase the intensity of immune responses.LDH, as both a carrier and an adjuvant, has been used to enhance the antitumor ability of DNA vaccines. In 2011,75 Li et al. reported an LDH–DNA vaccine loaded with DNA (stably transfected with cDNA of OVA) through electrostatic interactions and showed that LDH can protect DNA from degradation in the presence of DNase I enzyme, deliver DNA to draining lymphoid tissue and DCs for endocytosis through intradermal administration, improve the effectiveness of the vaccine in producing specific antibodies, and overcome the physiological barrier of lymph node tunica and lymph-vessel gap via increasing the overall momentum of the LDH–DNA vaccine. Moreover, the LDH–DNA vaccine enabled DC maturation to trigger an antigen-specific Th1 immune response and TAMs to be repolarized into an M1-like phenotype, ultimately achieving efficient antitumor immunity.75 In addition, LDH as an adjuvant has also been used to construct an LDH–RNA vaccine for immunotherapy of melanoma.15 Specifically, the Trp2/LDH/siIDO vaccine was prepared by inserting indoleamine 2,3-dioxygenase siRNA (siIDO) into the interlayer of LDH to avoid RNase A degradation and loading tyrosinase-related protein 2 (Trp2) on the surface of LDH (Fig. 8B). The Trp2/LDH/siIDO complexes entered the cells through the endosome pathway and then escaped from the endosome by destroying the endosome membrane through proton buffering in an acidic environment to release Trp2 and siIDO. The former combined with MHC class I molecules presented on the DC surface and stimulated the activation of T cells, while the latter combined with the mRNA transcribed by IDO genes to inhibit the translation of IDO enzymes to overcome the immunosuppressive TME.15 LDH not only acts as an adjuvant to promote cellular immunity but also acts as a positive charge carrier to load protein and nucleic acids, which has a great prospect in constructing highly efficient nucleic acid vaccines.
3.3. LDH–CpG dual-adjuvant vaccines
Compared with a single LDH adjuvant, LDH combined with other adjuvants can synergistically improve the antitumor immune response of the vaccine.78 CpG, as a bioadjuvant, is reported to maturate DCs by interacting with toll-like receptor-9 and enhance cell-mediated immune responses.79 By integrating CpG with LDH, a dual-adjuvant vaccine has been constructed.78–80 For example, Xu et al. proposed a new “Trojan horse” scheme to construct an LDH–CpG dual-adjuvant vaccine, in which cancer cell membranes (CCMs) were assembled on the surface of the LDH–CpG dual-adjuvant vaccine and then coated with mannose-modified BSA. Among them, LDH combined with CpG acted as dual-immunostimulant adjuvants to trigger DC maturation, CCMs were considered as an antigen due to their expression of abundant tumor-associated antigens (TAAs) and tumor-specific antigens to also stimulate DC maturation, mannose was applied for targeting APCs to induce strong antitumor immune responses, and BSA prevented the immune-escape of the CCMs in APCs by masking CCM antigens. In addition, the LDH–CpG dual-adjuvant vaccine could migrate to the draining lymph nodes (dLNs) to trigger tumor-specific CD8+ T cell responses in vivo and eventually significantly inhibit tumor growth.81Many other functional therapeutic platforms have been developed based on LDH–CpG dual-vaccines. For example, Gu et al. reported the use of Cu2+-doped LDH to load CpG. The addition of Cu2+ imparted the LDH–CpG vaccine with the chemodynamic therapy (CDT) ability through the Fenton-like reaction with the high-level H2O2 in the TME, thus enabling synergistic CDT-immunotherapy to ultimately inhibit the growth of both primary and distant tumors through the created immune responses.82 In another study,83 Liu et al. designed a multifunctional LDH–CpG dual-adjuvant vaccine for the synergistic PTT/chemotherapy/immunotherapy of a murine breast cancer model (Fig. 9A). In brief, ICG was intercalated into LDH interlayers for PTT, while CpG and doxorubicin (Dox)/DNA prodrug with the characteristics of stable and low side effects were loaded onto the LDH particle surface for immune activation and chemotherapy, respectively (Fig. 9B), thus effectively preventing the recurrence and metastasis of invasive breast cancer (Fig. 9C).
 | ||
| Fig. 9 (A) Hybrid nanovaccine constructed by first coating with BSA and then orderly loading ICG, Dox/DNA prodrug, and CpG ODN 1826. (B) Nanovaccine with 808 nm NIR irradiation heats the tumor tissues and releases Dox at a temperature above 41 °C to kill tumor cells through efficient PTT and subsequent chemotherapy (for short, CTX). (C) Matured DC activate naive T cells in the dLNs and induce potent CTLs (CD8+ T Cells).83 Copyright 2019, American Chemical Society. | ||
3.4. In situ vaccines using LDH as an adjuvant
The vaccines described above were all pre-prepared ones in vitro, which required the identification of tumor-specific antigens. Antigens were generally derived from killed tumor cells, tumor cell lysates, tumor-derived exosomes, and a few previously identified peptide and protein antigens for certain types of cancer. Besides, neoantigens from the genetic mutation of tumor cells have also been used to construct cancer vaccines to trigger tumor-specific CTL responses. However, the low frequency of neoantigens expressed by heterogeneous tumor cells reduced the effectiveness of cancer vaccines. In situ vaccines can be formed directly using endogenous antigens produced by tumors, which is promising for personalized medicine development.In a recent work,45 Ling et al. applied Zn2+-doped LDH (Zn-LDH) to design an in situ vaccine (Fig. 10A). In such a design, Zn2+ released from Zn-LDH in TME produced reactive oxygen species (ROS) to accelerate mitochondrial damage and combined with DNA released from damaged mitochondria to activate the cGAS-STING signaling pathway in tumor cells and induce the ICD. Subsequently, TAAs released by ICD induced through Zn2+-mediated CDT were captured by Zn-LDH to form an in situ vaccine that was delivered to tumor-associated dLNs to induce the CTLs (Fig. 10B). In another study,21 LDH was shown to promote immune response by neutralizing the acidic TME and destroying lysosomes of tumor cells, thus leading to autophagy destruction and tumor cell death. Subsequently, LDH captures tumor antigens released from dying tumor cells to form an in situ vaccine to effectively inhibit the growth of both melanoma and colon tumors. Lastly, TAAs resulting from radiofrequency ablation could be captured by cGAMP-loaded LDH to form an advanced in situ vaccine. The created LDH–cGAMP can be effectively ingested by cancer cells or immune cells, inducing a strong type I interferon response, and the formed in situ vaccine can produce sustained immune stimulation and effectively promote the activation of DCs. Moreover, this therapeutic nanoplatform significantly altered the TME, improved the response efficiency of the anti-programmed death ligand 1-based immunotherapy effect, and made it possible to treat liver cancer with poor immunogenicity.84
 | ||
| Fig. 10 (A) Synthesis of Zn-LDH by substituting Mg2+ with Zn2+ in the brucite layers of LDH through a metal ion exchange method. (B) TME modulation and tumor-specific immunity induction.45 Copyright 2022, Wiley-VCH. | ||
3.5. The mechanism of LDH-enhanced antigen presentation and immune cell activation
In general, the mechanism of LDH to induce a strong immune response can be summarized as follows: LDH serves as a carrier to load antigens, forming a delivery system with loose structure clumps with antigens at the injection site (the depot effect).23 Due to the slow acid-triggered degradation process of LDH, the antigen can be continuously released for immune cell recruiting, thus effectively promoting the proliferation of memory T cells and then promoting the internalization of antigen to generate intracellular danger signals for APC maturation and immune response stimulation. A recent study of the LDH–OVA nanovaccine74 showed that LDH coordinated with activation signals of cytokines and chemokines, co-stimulators, and proteases to help OVA maturate DCs. Due to the rapid endosomal escape, the LDH–antigen complex enables antigen processing into epitopes complexed with MHC class II/I complexes for subsequent (cross)-presentation.LDH also carries metal ions that activate the immune response. For example, Zn2+ in Zn-LDH activates the cGAS-STING signaling pathway by binding to DNA released by damaged mitochondria, thereby stimulating powerful antitumor immunity.45 Reduction of intracellular Mg2+ leads to downregulation of the natural killer activating receptor NKG2D in CD8+ T cells, hindering their antitumor response.44
3.6. Comparison with other immunotherapy nanoplatforms
At present, the widely used delivery platforms are lipid NPs (LNPs), mesoporous silica NPs, gold NPs and so on. LNPs are a potent RNA carrier,85 which can envelope RNA and promote RNA transport to the cytoplasm. LNPs are also a potent multifunctional adjuvant to combine with multiple antigens and induce a robust T follicular helper cell response and a durable protective antibody titer.86 However, the synthesis of LNPs is quite complicated, while the preparation of LDH is simple and convenient. In addition, LDH is stable and has a longer storage time period than LNPs. Gold NPs can be synthesized to have controlled size, optical properties and surface functionality. They have a high surface area to volume ratio and a tunable loading of biomolecules, drugs and imaging contrast agents.87 However, gold NPs can be easily bound with various proteins in the human body to form agglomerates that are difficult to be cleared out of the body, while LDH is a biodegradable material and there is no issue regarding its body clearance. Mesoporous silica NPs,88 as another inorganic material, are rich in functionalizable silanol groups, have an adjustable pore structure (pore size > 0.6 cm3 g−1) and a large surface area (>600 m2 g−1), and are easy to be synthesized on a large scale. Although mesoporous silica NPs can resist a wide range of stresses, including pH, mechanical and thermal stresses for promising therapeutic and diagnostic applications, they do not have intrinsic immune adjuvant properties, which are instead possessed by LDH for convenient nanovaccine construction.4. Conclusion and prospects
The immunotherapy platform based on LDH has made remarkable progress. On the one hand, the inherent advantages of a large plane structure, weak base composition, rich positive charge, and special interlayer structure make it an excellent carrier for constructing a range of immunotherapy platforms. LDH can be integrated with nanomaterials, RNA, or bacteria through electrostatic interactions, or be doped with metal ions with different functions by changing the type of salt. On the other hand, as a substitute for traditional adjuvants, LDH can directly boost cancer immunotherapy and has a great prospect in constructing LDH-based nanovaccines through integration with antigens, nucleic acids, CpG adjuvants/antigens (as a dual-adjuvant vaccine), or newly formed TAAs during the tumor treatment process (as an in situ vaccine).Despite these promising advances in building LDH-based immunotherapy platforms/vaccines to inhibit tumor growth, there are still some challenges and spaces to be explored. For instance, it is necessary to precisely control the size, charge, and internal space of LDH to ensure a consistent treatment effect. In this case, in order to better control the quality of the material, the microfluidic method may be used to synthesize specific LDH by optimizing the ratio of metal salt to alkali solution and the channel size and the flow rate of the microfluidic chip. In addition, there are few studies on targeted delivery of LDH-based immunotherapy platforms/vaccines, and it is necessary to develop LDH-based platforms or vaccines that are modified with targeting ligands or cell membranes to achieve the desired targeting specificity of cancer cells or immune cells or to realize the targeted delivery to typical tumor types (e.g., brain glioma) after blood–brain barrier crossing. In addition, the complex diversity of the TME often affects the effectiveness of immunotherapy; so it is critical to develop multi-approach combination therapy platforms, such as the development of PTT–immunotherapy–CDT combination therapy platforms, to achieve multi-directional stimulation of immune responses. Lastly, personalized therapeutic nanovaccines should also be developed through the formation of in situ vaccines or use of all-cell-component TAAs to tackle different tumor types.
Abbreviations
| Alum | Aluminum adjuvants |
| APCs | Antigen-presenting cells |
| CCM | Cancer cell membranes |
| CTLs | Cytotoxic T lymphocytes |
| CRT | Calreticulin |
| CTX | Chemotherapy |
| cGAS-STING | Cyclicguanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes |
| DCs | Dendritic cells |
| dLNs | Draining lymph nodes |
| Dox | Doxorubicin |
| Hsp90 | Heat-shock protein 90 |
| ICG | Indocyanine green |
| ICD | Immunogenic cell death |
| IFN-γ | Interferon-γ |
| LDH | Layered double hydroxide |
| MCF | MgCaFe-LDH |
| MMPD | Mitochondrial membrane potential damage |
| MHC | Major histocompatibility complex |
| MICaP | M-LDH/ICG@Ca3(PO4)2 |
| miR155 | MicroRNA-155 |
| mRNA | Messenger RNA |
| MT | Metallothionein |
| NIR | Near-infrared |
| ONOO− | Cytotoxic reactive nitrogen species |
| ˙O2− | Superoxide anion free radical |
| OVA | Ovalbumin |
| P. acnes | Propionibacterium acnes |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| PRRs | Pattern recognition receptors |
| ROS | Reactive oxygen species |
| siRNA | Small interfering RNA |
| SDT | Sonodynamic therapy |
| silDO | Indoleamine 2,3-dioxygenase siRNA |
| TAA | Tumor-associated antigen |
| TAMs | Tumor-associated macrophages |
| TME | Tumor microenvironments |
| Trp2 | Tyrosinase-related protein 2 |
| Zn-LDH | Zn2+-doped LDH |
Author contributions
Xiaochun Deng: software and writing – original draft. Gaoming Li: software and writing – original draft. Mingwu Shen: supervision, resources, funding acquisition, and project administration. Xiangyang Shi: supervision, resources, funding acquisition, project administration, and writing – review and editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (U23A2096 and 52350710203), the Science and Technology Commission of Shanghai Municipality (23520712500, and 20DZ2254900), and the National Key R&D Program (2022YFE0196900).References
- D. M. Francis and S. N. Thomas, Adv. Drug Delivery Rev., 2017, 114, 33–42 CrossRef CAS PubMed.
- M. P. Manspeaker and S. N. Thomas, Adv. Drug Delivery Rev., 2020, 160, 19–35 CrossRef CAS PubMed.
- J. Li, W. Lu, Y. Yang, R. Xiang, Y. Ling, C. Yu and Y. Zhou, Adv. Sci., 2022, 10, 2204932 CrossRef PubMed.
- Z. Duan and Y. Luo, Signal Transduction Targeted Ther., 2021, 6, 127 CrossRef CAS PubMed.
- F. Conceição-Silva, C. S. M. Reis, P. M. De Luca, J. Leite-Silva, M. A. Santiago, A. Morrot and F. N. Morgado, Cells, 2021, 10, 1891 CrossRef PubMed.
- J. Shao, Q. Xu, S. Su, J. Wei, F. Meng, F. Chen, Y. Zhao, J. Du, Z. Zou, X. Qian and B. Liu, Cell. Immunol., 2018, 334, 78–86 CrossRef CAS PubMed.
- J. Li, W. Lu, Y. Yang, R. Xiang, Y. Ling, C. Yu and Y. Zhou, Adv. Sci., 2023, 10, 2204932 CrossRef CAS PubMed.
- H. Yu, M. Wu, S. Chen, M. Song and Y. Yue, Front. Bioeng. Biotechnol., 2022, 10, 989881 CrossRef PubMed.
- T. K. Kim, E. N. Vandsemb, R. S. Herbst and L. Chen, Nat. Rev. Drug Discovery, 2022, 21, 529–540 CrossRef CAS PubMed.
- H. Phuengkham, L. Ren, I. W. Shin and Y. T. Lim, Adv. Mater., 2019, 31, 1803322 CrossRef PubMed.
- Y. Cao, D. Zheng, F. Zhang, J. Pan and C. Lin, J. Mater. Sci. Technol., 2022, 102, 232–263 CrossRef CAS.
- L. Zhang, J. Hu, Y. Jia, R. Liu, T. Cai and Z. P. Xu, Nanoscale, 2021, 13, 7533–7549 RSC.
- V. K. Ameena Shirin, R. Sankar, A. P. Johnson, H. V. Gangadharappa and K. Pramod, J. Controlled Release, 2021, 330, 398–426 CrossRef CAS PubMed.
- G. Liu, X. Zhang, H. Liu, Z. He, P. L. Show, Y. Vasseghian and C. Wang, Environ. Res., 2023, 234, 116534 CrossRef CAS PubMed.
- L. Zhang, D. Liu, S. Wang, X. Yu, M. Ji, X. Xie, S. Liu and R. Liu, J. Mater. Chem. B, 2017, 5, 6266–6276 RSC.
- J. Oh, S. Choi, S. Kim and J. Choy, Bioconjugate Chem., 2006, 17, 1411–1417 CrossRef CAS PubMed.
- R. Djaballah, A. Bentouami, A. Benhamou, B. Boury and E. H. Elandaloussi, J. Alloys Compd., 2018, 739, 559–567 CrossRef CAS.
- M. Duan, S. Liu, Q. Jiang, X. Guo, J. Zhang and S. Xiong, Chin. Chem. Lett., 2022, 33, 4428–4436 CrossRef CAS.
- C. Chen, W. Zhang, S. Lu, J. Wang, Y. Tan, S. Zhao, Y. Ouyang, L. Xu, B. Zhou, X. Yin, H. Ran and H. Liu, Colloids Surf., B, 2023, 223, 113157 CrossRef CAS PubMed.
- C. Zhu, J. Jiang, Y. Jia, Z. P. Xu and L. Zhang, Acc. Mater. Res., 2023, 4, 758–771 CrossRef CAS.
- L. Zhang, Y. Jia, J. Yang, L. Zhang, S. Hou, X. Niu, J. Zhu, Y. Huang, X. Sun, Z. P. Xu and R. Liu, ACS Nano, 2022, 16, 12036–12048 CrossRef CAS PubMed.
- G. R. Williams, K. Fierens, S. G. Preston, D. Lunn, O. Rysnik, S. De Prijck, M. Kool, H. C. Buckley, B. N. Lambrecht, D. O'Hare and J. M. Austyn, J. Exp. Med., 2014, 211, 1019–1025 CrossRef CAS PubMed.
- W. Chen, H. Zuo, B. Li, C. Duan, B. Rolfe, B. Zhang, T. J. Mahony and Z. P. Xu, Small, 2018, 14, 1704465 CrossRef PubMed.
- A. Li, L. Qin, D. Zhu, R. Zhu, J. Sun and S. Wang, Biomaterials, 2010, 31, 748–756 CrossRef CAS PubMed.
- L. Yang, J. Sun, Q. Liu, R. Zhu, Q. Yang, J. Hua, L. Zheng, K. Li, S. Wang and A. Li, Adv. Sci., 2019, 6, 1802012 CrossRef PubMed.
- P. Zhu, C. Zhou, J. Chen, Q. Chu, F. Li, Y. Fu and X. Li, Adv. Funct. Mater., 2023, 33, 2214105 CrossRef CAS.
- Z. Wang, H. Yang, Y. Bai, L. Cheng and R. Zhu, Biomed. Mater., 2022, 17, 024101 CrossRef PubMed.
- N. Guo, F. Cang, Z. Wang, T. Zhao, X. Song, S. Farris, Y. Li and Y. Fu, Mater. Sci. Eng., C, 2021, 126, 112143 CrossRef CAS PubMed.
- B. Nasseri, E. Alizadeh, F. Bani, S. Davaran, A. Akbarzadeh, N. Rabiee, A. Bahadori, M. Ziaei, M. Bagherzadeh, M. R. Saeb, M. Mozafari and M. R. Hamblin, Appl. Phys. Rev., 2022, 9, 011317 CAS.
- B. Li, G. Hao, B. Sun, Z. Gu and Z. P. Xu, Adv. Funct. Mater., 2020, 30, 1909745 CrossRef CAS.
- Z. Yang, J. Wang, S. Liu, X. Li, L. Miao, B. Yang, C. Zhang, J. He, S. Ai and W. Guan, Biomaterials, 2020, 229, 119580 CrossRef CAS PubMed.
- X. Duan, C. Chan and W. Lin, Angew. Chem., Int. Ed., 2019, 58, 670–680 CrossRef CAS PubMed.
- B. Zhu, F. Qu, D. Bi, R. Geng, S. Chen and J. Zhu, ACS Appl. Mater. Interfaces, 2023, 15, 9135–9149 CrossRef CAS PubMed.
- Y. Lin, Y. Wang, S. Blake, M. Yu, L. Mei, H. Wang and J. Shi, Theranostics, 2020, 10, 281–299 CrossRef CAS PubMed.
- C. Chakraborty, A. R. Sharma, G. Sharma, C. G. P. Doss and S.-S. Lee, Mol. Ther.–Nucleic Acids, 2017, 8, 132–143 CrossRef CAS PubMed.
- R. Rupaimoole and F. J. Slack, Nat. Rev. Drug Discovery, 2017, 16, 203–222 CrossRef CAS PubMed.
- Y. Lu, J. Zhou, Q. Zhou, X. Yang, X. Wang, J. Yu, J. Zhang, Y. Du and R. Yu, J. Nanobiotechnol., 2022, 20, 351 CrossRef CAS PubMed.
- C. Clairmont, K. C. Lee, J. Pike, M. Ittensohn, K. B. Low, J. Pawelek, D. Bermudes, S. M. Brecher, D. Margitich, J. Turnier, Z. Li, X. Luo, I. King and L. M. Zheng, J. Infect. Dis., 2000, 181, 1996–2002 CrossRef CAS PubMed.
- C. Zhou, J. Chen, B. Zheng, P. Zhu, Q. Chu, F. Li, Y. Fu, X. Li and J. Luo, ACS Appl. Mater. Interfaces, 2023, 15, 44731–44741 CrossRef CAS PubMed.
- H. Minasyan and F. Flachsbart, Int. Rev. Immunol., 2019, 38, 3–17 CrossRef CAS PubMed.
- S. He, Y. Jiang, J. Li and K. Pu, Angew. Chem., Int. Ed., 2020, 59, 10633–10638 CrossRef CAS PubMed.
- F. Li, B. Chaigne Delalande, C. Kanellopoulou, J. C. Davis, H. F. Matthews, D. C. Douek, J. I. Cohen, G. Uzel, H. C. Su and M. J. Lenardo, Nature, 2011, 475, 471–476 CrossRef CAS PubMed.
- C. Chen, W. Zhang, S.-Y. Lu, J. Wang, Y. Tan, S. Zhao, Y. Ouyang, L. Xu, B. Zhou, X. Yin, H. Ran and H. Liu, Colloids Surf., B, 2023, 223, 113157 CrossRef CAS PubMed.
- J. Wu, L. Wang, W. Tang, X. Cui, K. Wei, S. Cheng, Z. Pei, H. Lei, Z. Liu and L. Cheng, Mater. Today, 2023, 68, 164–176 CrossRef CAS.
- L. Zhang, J. Zhao, X. Hu, C. Wang, Y. Jia, C. Zhu, S. Xie, J. Lee, F. Li and D. Ling, Adv. Mater., 2022, 34, 2206915 CrossRef CAS PubMed.
- L. Wang, Z. Mao, J. Wu, X. Cui, Y. Wang, N. Yang, J. Ge, H. Lei, Z. Han, W. Tang, S. Guan and L. Cheng, Nano Today, 2023, 49, 101782 CrossRef CAS.
- B. Chaigne Delalande, F. Li, G. M. O'Connor, M. J. Lukacs, P. Jiang, L. Zheng, A. Shatzer, M. Biancalana, S. Pittaluga, H. F. Matthews, T. J. Jancel, J. J. Bleesing, R. A. Marsh, T. W. Kuijpers, K. E. Nichols, C. L. Lucas, S. Nagpal, H. Mehmet, H. C. Su, J. I. Cohen, G. Uzel and M. J. Lenardo, Science, 2013, 341, 186–191 CrossRef CAS PubMed.
- D. Fan, Y. Cao, M. Cao, Y. Wang, Y. Cao and T. Gong, Signal Transduction Targeted Ther., 2023, 8, 293 CrossRef PubMed.
- Y. Lu, J. Zhou, Q. Zhou, X. Yang, X. Wang, J. Yu, J. Zhang, Y. Du and R. Yu, J. Nanobiotechnol., 2022, 20, 351 CrossRef CAS PubMed.
- Z. Jing, Q. Du, X. Zhang and Y. Zhang, Chem. Eng. J., 2022, 446, 137147 CrossRef CAS.
- S. Sindhwani, A. M. Syed, J. Ngai, B. R. Kingston, L. Maiorino, J. Rothschild, P. MacMillan, Y. Zhang, N. U. Rajesh, T. Hoang, J. L. Y. Wu, S. Wilhelm, A. Zilman, S. Gadde, A. Sulaiman, B. Ouyang, Z. Lin, L. Wang, M. Egeblad and W. C. W. Chan, Nat. Mater., 2020, 19, 566–575 CrossRef CAS PubMed.
- M. I. Setyawati, C. Y. Tay, B. H. Bay and D. T. Leong, ACS Nano, 2017, 11, 5020–5030 CrossRef CAS PubMed.
- D. Park, J. Cho, O. Kwon, C. Yun and J. Choy, Angew. Chem., Int. Ed., 2016, 55, 4582–4586 CrossRef CAS PubMed.
- A. Dong, X. Li, W. Wang, S. Han, J. Liu, J. Liu, J. Zhao, S. Xu and L. Deng, Trans. Tianjin Univ., 2016, 22, 237–246 CrossRef CAS.
- Y. Ye, D. H. Bremner, H. Zhang, X. Chen, J. Lou and L.-M. Zhu, Colloids Surf., B, 2022, 210, 112261 CrossRef CAS PubMed.
- J. Wang, L. Sun, J. Liu, B. Sun, L. Li and Z. P. Xu, J. Nanobiotechnol., 2021, 19, 351 CrossRef CAS PubMed.
- G. Li, Y. Guo, C. Ni, Z. Wang, M. Zhan, H. Sun, G. Choi, J.-H. Choy, X. Shi and M. Shen, J. Mater. Chem. B, 2024, 12, 7429–7439 RSC.
- M. Yarchoan, B. A. Johnson, E. R. Lutz, D. A. Laheru and E. M. Jaffee, Nat. Rev. Cancer, 2017, 17, 569–569 CrossRef CAS PubMed.
- X. Xie, T. Song, Y. Feng, H. Zhang, G. Yang, C. Wu, F. You, Y. Liu and H. Yang, Chem. Eng. J., 2022, 437, 135505 CrossRef CAS.
- Q. Wang, Z. Wang, X. Sun, Q. Jiang, B. Sun, Z. He, S. Zhang, C. Luo and J. Sun, J. Controlled Release, 2022, 351, 102–122 CrossRef CAS PubMed.
- J. S. Weber and J. J. Mulé, Nat. Biotechnol., 2015, 33, 44–45 CrossRef CAS PubMed.
- J. C. Cox and A. R. Coulter, Vaccine, 1997, 15, 248–256 CrossRef CAS PubMed.
- A. M. Hafner, B. Corthésy and H. P. Merkle, Adv. Drug Delivery Rev., 2013, 65, 1386–1399 CrossRef CAS PubMed.
- T. Zhao, Y. Cai, Y. Jiang, X. He, Y. Wei, Y. Yu and X. Tian, Signal Transduction Targeted Ther., 2023, 8, 283 CrossRef CAS PubMed.
- M. S. Duthie, H. P. Windish, C. B. Fox and S. G. Reed, Immunol. Rev., 2011, 239, 178–196 CrossRef CAS PubMed.
- S. Van Herck, B. Feng and L. Tang, Adv. Drug Delivery Rev., 2021, 179, 114020 CrossRef CAS PubMed.
- T. B. H. Geijtenbeek and S. I. Gringhuis, Nat. Rev. Immunol., 2009, 9, 465–479 CrossRef CAS PubMed.
- A. M. Kvarnhammar, T. Petterson and L. Cardell, Immunology, 2011, 134, 314–325 CrossRef CAS PubMed.
- Z. Liao, J. Huang, P. Lo, J. F. Lovell, H. Jin and K. Yang, J. Nanobiotechnol., 2022, 20, 345 CrossRef PubMed.
- Y. Guo, Q. Bao, P. Hu and J. Shi, Nat. Commun., 2023, 14, 7306 CrossRef CAS PubMed.
- D. Shi, B. Fan, B. Sun, J. Zhou, Y. Zhao, R. Guo, Z. Ma, T. Song, H. Fan, J. Li, L. Li and B. Li, Virology, 2022, 565, 58–64 CrossRef CAS PubMed.
- W. Chen, B. Zhang, T. Mahony, W. Gu, B. Rolfe and Z. P. Xu, Small, 2016, 12, 1627–1639 CrossRef CAS PubMed.
- G. Jing, L. Yang, H. Wang, J. Niu, H. Wang, Y. Gao, Y. Li, B. Wei, Y. Qian and S. Wang, Adv. Healthcare Mater., 2023, 12, 2301471 CrossRef CAS PubMed.
- S. Yan, K. Xu, L. Li, W. Gu, B. E. Rolfe and Z. P. Xu, Front. Pharmacol., 2018, 9, 1060 CrossRef PubMed.
- A. Li, L. Qin, W. Wang, R. Zhu, Y. Yu, H. Liu and S. Wang, Biomaterials, 2011, 32, 469–477 CrossRef CAS PubMed.
- R. Wang, D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman and S. L. Hoffman, Science, 1998, 282, 476–480 CrossRef CAS PubMed.
- M. E. Barry, D. Pinto Gonzalez, F. M. Orson, G. J. McKenzie, G. R. Petry and M. A. Barry, Hum. Gene Ther., 1999, 10, 2461–2480 CrossRef CAS PubMed.
- L. Zhang, Y. Jia, Y. Huang, H. Liu, X. Sun, T. Cai, R. Liu and Z. Xu, Nano Res., 2021, 14, 1326–1334 CrossRef CAS.
- S. Yan, W. Gu, B. Zhang, B. E. Rolfe and Z. P. Xu, Dalton Trans., 2018, 47, 2956–2964 RSC.
- L. Zhang, X. Xie, D. Liu, Z. Xu and R. Liu, Biomaterials, 2018, 174, 54–66 CrossRef CAS PubMed.
- J. Wang, B. Sun, L. Sun, X. Niu, L. Li and Z. P. Xu, Biomater. Sci., 2023, 11, 2020–2032 RSC.
- Y. Yin, H. Wang, J. Xue, C. Yin, Y. Xing and W. Gu, Adv. Healthcare Mater., 2023, 12, 2301269 CrossRef CAS PubMed.
- L. Zhang, X. Sun, Z. Xu and R. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 35566–35576 CrossRef CAS PubMed.
- Z. Tian, Q. Hu, Z. Sun, N. Wang, H. He, Z. Tang and W. Chen, ACS Nano, 2023, 17, 19441–19458 CrossRef CAS PubMed.
- Y. Lee, M. Jeong, J. Park, H. Jung and H. Lee, Exp. Mol. Med., 2023, 55, 2085–2096 CrossRef CAS PubMed.
- M.-G. Alameh, I. Tombácz, E. Bettini, K. Lederer, S. Ndeupen, C. Sittplangkoon, J. R. Wilmore, B. T. Gaudette, O. Y. Soliman, M. Pine, P. Hicks, T. B. Manzoni, J. J. Knox, J. L. Johnson, D. Laczkó, H. Muramatsu, B. Davis, W. Meng, A. M. Rosenfeld, S. Strohmeier, P. J. C. Lin, B. L. Mui, Y. K. Tam, K. Karikó, A. Jacquet, F. Krammer, P. Bates, M. P. Cancro, D. Weissman, E. T. Luning Prak, D. Allman, B. Z. Igyártó, M. Locci and N. Pardi, Immunity, 2021, 54, 2877–2892 CrossRef CAS PubMed.
- M. Norouzi, Pharmacol. Res., 2020, 156, 104753 CrossRef CAS PubMed.
- E. Rastegari, Y. Hsiao, W. Lai, Y. Lai, T. Yang, S. Chen, P. I. Huang, S. Chiou, C. Mou and Y. Chien, Pharmaceutics, 2021, 13, 1067 CrossRef CAS PubMed.
Footnote |
| † Authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |





