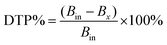Open Access Article
Open Access ArticleInsight into central nervous system targeted nanostructured lipid carriers via the nose to brain pathway
Mridusmita
Das
a,
Anupam
Sarma
 *a,
Himakshi
Baruah
ab and
Debojeet
Basak
a
*a,
Himakshi
Baruah
ab and
Debojeet
Basak
a
aAdvanced Drug Delivery Laboratory, School of Pharmaceutical Sciences, Girijananda Chowdhury University, Guwahati-781017, Assam, India. E-mail: anupampharmacy@gmail.com; Tel: +9101386317
bDepartment of Pharmaceutics, School of Pharmacy, The Assam Kaziranga University, Jorhat, Assam
First published on 13th September 2024
Abstract
In nanomedicine, targeting the central nervous system (CNS) is one of the biggest challenges. The presence of the blood–brain barrier (BBB) leads to the failure of drugs to reach the brain; hence, CNS-related diseases are challenging to treat. Various invasive and noninvasive methods have been established to overcome the difficulty of passing through the BBB. Delivery of drugs by using nanoparticles through the nasal route is one of the noninvasive methods developed to treat CNS disorders. The nose to brain pathway allows direct transport to the brain without crossing the BBB. Among the nanocarriers designed to target the CNS, nanostructured lipid carriers (NLC) are the focus of this review. NLCs appeared as a newer generation of solid lipid nanoparticles (SLN) developed to get over SLN's limitations. They are novel pharmaceutical preparations made of lipids, surfactants and co-surfactants that are physiologic and biocompatible. Liquid lipids (oil) are added to the solid lipid to create a matrix which results in structural flaws in the solid lipids and creates a less ordered crystalline framework that prevents leakage of the drug and provides high drug loading. The imperfection in the internal arrangement of NLCs aids more drug accommodation. A systematic search was performed across the main databases like PubMed, Springer, Scopus, Taylor and Francis, Google Scholar and Wiley. The search applied terms and keywords related to nose to brain delivery, nanostructured lipid carriers and neurodegenerative diseases. This review discusses the anatomy of the nose, associated pathways, advantages and limitations of NLCs, and preparation techniques and recent developments of NLCs delivered via the nose to brain route. The reported records demonstrated the feasibility and potential of NLCs for innovative uses for treatment in the future via the nose to brain route.
1. Introduction
Drug buildout for central nervous system (CNS) diseases and psychiatric conditions is arduous due to the possible side effects caused by the drug, the complexity of the brain and especially the lack of systematic strategies for delivering drugs across the blood–brain barrier (BBB).1 A relevant obstacle in the entry of active molecules into the CNS is the BBB. More than 98% of CNS-active medications are unable to penetrate the barrier because their physiochemical characteristics do not fit the entrance requirements for molecules entering the CNS.2 The CNS is made up of the brain and spinal cord, which are the body's processing center. Any abnormality of these neurons causes various neurological dysfunctions. The term neurodegenerative disease refers to a group of deteriorating, incurable conditions that affect neurons and are characterized by a progressive, slow decline in the structure and performance of the central or peripheral nervous system. These neurons are nonreproducible and nonrenewable, so they cannot be replaced if the body loses them. These disorders are one of the most important medical and socioeconomic issues of our time, impacting a wide variety of elderly people and having a substantial impact on patients’ professional, social and family lives, ultimately rendering them completely incapable of performing any daily activity. These are a few examples: Alzheimer's disease (AD), Huntington's disease (HD), Parkinson's disease (PD), prion disease and amyotrophic lateral sclerosis (ALS).3 The BBB provides a great obstacle to deliver drugs into the CNS. Routes such as parenteral and transdermal have been known to provide insufficient results that favor the nasal route as being superior when confirmed by a comparative study.4 Thus, recent research focuses on searching for alternative routes for improving drugs that treat CNS conditions.5 Comparing intranasal delivery, a noninvasive drug delivery approach to traditional drug delivery methods which are unable to cross the BBB provides a remarkable possibility to deliver drugs to the CNS in a focused and noninvasive way to provide direct nose-to-brain transport.6 Intranasal administration can give direct access to drugs in the CNS through the different pathways present in the nose to brain route.7 This route offers various advantages over other routes of administration: the convenience of administration, patient acceptance, non-invasiveness, a quick beginning of action, decreased enzymatic activity, a comparatively broad and permeable absorption surface and minimization of hepatic first-pass metabolism. As a result, there are more products on the market that use the nose to deliver small and large molecules (peptides, proteins and vaccinations) systemically. Therefore, nasal administration has been suggested as a treatment for several CNS illnesses including migraine, sleep difficulties, viral infections, brain tumors and conditions like multiple sclerosis (MS), schizophrenia, Parkinson's disease (PD), Alzheimer's disease (AD), and even obesity. Although the nasal route provides a large number of advantages, the lack of established translational animal models, inadequate olfactory area deposition from typical nasal devices, limited residence time, insufficient bioavailability of hydrophilic or big compounds, mucosal irritation and tiny nasal cavity spaces are some examples of potential constraints.8Nanotechnology-based approaches to conquer the limitations of nasal drug delivery have attained the attention of researchers. Due to the unique properties of nanoparticles, they can circumvent the BBB, but the adverse effects of nanoparticles in the CNS is unknown. The nasal route is one way for nanoparticles to enter the brain. However, once inside the brain, they may concentrate and not be removed by the systemic circulation, leading to neurotoxicity.9 To overcome these restrictions, researchers focus on creating advanced drug delivery systems. Nanostructured lipid carriers (NLCs) are advanced lipid-based nanoparticle carriers in drug delivery systems. They are composed of a binary mixture of solid and liquid lipids, strengthened with surfactants. The ratio of solid to liquid lipids ranges from 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to 99.9
30 to 99.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
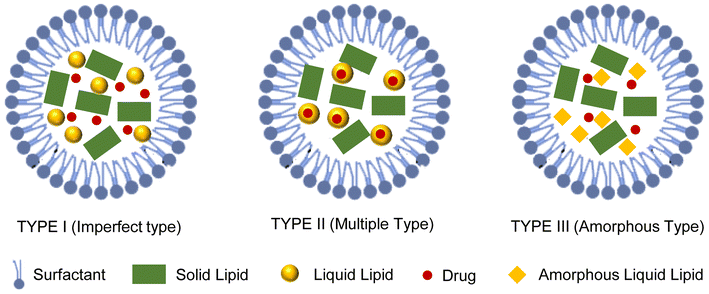
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, while surfactant concentrations vary from 1.5% to 5% (w/v). NLCs are spherical, with oil droplets embedded in the solid lipid matrix. Deficiencies in the lipid matrix create amorphous regions that enhance drug encapsulation and payload. The average size of NLCs is 10–500 nm. NLCs offer significant advantages over other lipid nanocarriers. They exhibit excellent biocompatibility, avoid organic solvents, and are easier to sterilize and scale up. As the second generation of solid lipid nanoparticles (SLNs), NLCs improve drug loading and storage time, addressing issues like drug expulsion. They are more stable, have a lower water content, and are cost-effective, providing better pharmaceutical stability and higher drug content. NLCs can encapsulate both lipophilic and hydrophilic drugs, are simple to manufacture and have improved aqueous dispersibility and excellent drug entrapment. They offer extended drug release, and their solid lipid matrices are widely acknowledged as safe.10 In recent years, NLCs have gained more interest as potential drug carrier systems as they have a vast capacity to deliver therapeutic agents such as those that act specially on treating and managing neurological disorders.11
0.1, while surfactant concentrations vary from 1.5% to 5% (w/v). NLCs are spherical, with oil droplets embedded in the solid lipid matrix. Deficiencies in the lipid matrix create amorphous regions that enhance drug encapsulation and payload. The average size of NLCs is 10–500 nm. NLCs offer significant advantages over other lipid nanocarriers. They exhibit excellent biocompatibility, avoid organic solvents, and are easier to sterilize and scale up. As the second generation of solid lipid nanoparticles (SLNs), NLCs improve drug loading and storage time, addressing issues like drug expulsion. They are more stable, have a lower water content, and are cost-effective, providing better pharmaceutical stability and higher drug content. NLCs can encapsulate both lipophilic and hydrophilic drugs, are simple to manufacture and have improved aqueous dispersibility and excellent drug entrapment. They offer extended drug release, and their solid lipid matrices are widely acknowledged as safe.10 In recent years, NLCs have gained more interest as potential drug carrier systems as they have a vast capacity to deliver therapeutic agents such as those that act specially on treating and managing neurological disorders.11
The main focus of this systematic review is the exploration of aspects of CNS-targeted nanostructured lipid carriers for nose-to-brain delivery. The literature search was conducted through various databases such as PubMed, Scopus, Science Direct, Taylor and Francis, Google Scholar, Web of Science, RSC, Springer and Wiley, using the keywords nose to brain delivery, nanostructured lipid carriers and neurodegenerative diseases. In this article different obstacles in CNS drug delivery and the potential of nose to brain drug delivery were discussed, including the anatomy of the nose, the pathways available for drug delivery through the intranasal route and the difficulties faced in delivering drugs intranasally and how to overcome them. The various advantages and disadvantages of NLCs, the different types of excipient used, NLC types, manufacturing and evaluation methods, as well as recent developments of NLCs for effective nose-to-brain delivery, pharmacokinetic considerations, stability, patents, and regulatory aspects of NLCs were summarized. Additionally, future prospects in the field of nose-to-brain delivery of NLCs were discussed.
2. Obstacles in drug delivery to the CNS
The brain and CNS are incredibly intricate systems that naturally protect the body against potentially damaging or contagious bloodborne pathogens.12 The human brain's molecular and cellular biochemical structure is continually changing due to developmental processes and life experiences, which affect how information is processed and flows through the brain.13 Its regular operation depends on the CNS's ability to maintain homeostasis.9 The drug delivery system towards the brain is challenging since the blood capillary endothelial cells of the brain have a strong defense mechanism against xenobiotics. The same defense systems that keep the brain safe from outside influences also prevent the admission of potentially useful drug molecules.14 Drug movement to the brain parenchyma is impeded by three causes. The blood–brain barrier (BBB) is found in vessels in the brain, the blood–cerebrospinal fluid barrier (BCSFB) is displayed by ventricles having a choroid plexus epithelium, and the cerebrospinal fluid–brain barrier is where ependymal cells cover the brain tissue inside the ventricles by an epithelial layer of cells and restrict the passage of substances to the brain tissue via the CSF (Fig. 1).15,16 | ||
| Fig. 1 Obstacles in drug delivery to the central nervous system (CNS). The CNS barrier system includes the blood–brain barrier (BBB), the blood–cerebrospinal fluid barrier (BCSFB), and the cerebrospinal fluid–brain barrier (CBB). The BBB is composed of pericytes, a basement membrane (BM), and astrocyte endfeet encircling tightly connected capillary endothelial cells, all surrounded by neurons, microglia, and other cells. The BCSFB exists in arachnoid granulations protruding into veins and the choroid plexus (CP), and is made up of a BM and tightly connected epithelial cells. The CBB consists of the pia mater and a glial boundary membrane composed of astrocytes. Additionally, a perivascular (PV) space is formed between the blood vessels extending into the brain parenchyma and the pia mater. As arterioles penetrate deep into the brain parenchyma and become capillaries, the surrounding pia mater gradually transforms into a BM. This figure has been adapted from the open access article from ref. 29. The figure is licensed under https://creativecommons.org/licenses/by-nc-nd/4.0/. | ||
2.1. Blood–brain barrier
Drug development for brain diseases is difficult and more complex than for other therapeutic areas. Many drugs cannot enter the brain, complicating brain targeting.17 The blood–brain barrier is a natural semipermeable edge between the brain and the rest of the body. This barrier is shaped by astrocytes, tight-jointed endothelial cells, pericytes, neurons and basal membrane.18 Endothelial cells linked by tight junctions separate the brain parenchyma and blood. The tight junctions are the reason for the strict permeability, as they enclose the intercellular spaces between endothelial cells. Pericytes cover the endothelial cells by supplying a type of additional layer or second layer to the BBB.19 Astrocytes contribute by secreting chemical reagents modifying the permeability through the BBB in response to neuronal signals, providing a cellular linkage between the neuronal circuitry and blood vessels, and stabilizing the BBB.20 The existence of efflux transporters in the BBB is the cause of the poor penetrating ability of drugs.26 The primary function of the BBB is to protect the brain tissue and maintain the exchanges within the brain and blood circulation.21 The BBB confines the passageway of large hydrophilic molecules but then consents to the passage of small lipophilic molecules of molecular weight <400 Da to the brain22,23 To prevent the influx of random complexes into the brain, the BBB acts as a barrier and allows selective passage to the brain. Several transport processes ensure the particular entry of molecules, such as diffusion, carrier-mediated transport, paracellular transport, adsorptive transcytosis, receptor-mediated transport and proton pump efflux transporters.232.2. Blood–cerebrospinal fluid barrier
When delivering a drug to CNS, the blood–cerebrospinal fluid barrier (BCSFB) prevents drugs from being administered. This barrier is formed by the discordance between the CSF and interstitial fluid.24 It is located in the choroid plexus epithelium, the modified ependymal lining of the ventricles in the brain secreting cerebrospinal fluid.25 The transporters in the BCSFB are recognized for maintaining the permeation of various molecules between the systemic circulation, CSF and brain interstitial space. Like the BBB, this barrier also comprises tight junctions restraining molecules’ paracellular diffusion. Even though, when compared with the BBB, the BCSFB is much leakier, it is exceedingly capable of monitoring the CSF's molecular composition by selective transport mechanisms.22 CSF serves as the foundation of the extracellular fluid surrounding neurons, playing a crucial role in supporting and sustaining their synapses. The BCSFB also significantly provides ions, micronutrients, and growth factors to the CNS. It also serves as a potential entry point for pathogens because of the secretion of CSF, causing the BCSFB to be more permeable, resulting in a lower electric resistance between the epithelial cells making it more vulnerable to diffusion from the blood.272.3. Cerebrospinal fluid–brain barrier
The cerebrospinal fluid–brain barrier (CBB) outlines the spinal canal and cerebral ventricles by an ependymal cell monolayer. The CBB is formed by these ependymal cells linked together with cell junctional proteins adjacent to the apical surface. They enable debris movement and CSF bulk flow by acting as a barrier that is protective by defending the periventricular white matter tissues and juxtaposed basal subependymal gray from CSF-laden toxins.28 This barrier promotes water, exogenous tracer and CSF protein entry from the CSF by selective permeation due to the presence of gap junctions. Drugs can enter brain parenchyma through the CSF by two routes, diffusion and convection. Diffusion takes place by the drug diffusing from the CSF to the brain by the ependymal cells, and convection involves penetration of the drug in the CSF to the brain by the perivascular surfaces originating at the CSF exterior. Drugs injected into the CSF distribute to the blood and brain surface but do not diffuse into the brain parenchyma beyond a distance of 1–2 mm from the CSF compartment, according to studies, and the distribution of drugs in the CSF does not reflect the number of drugs permeating the brain parenchyma.293. Potential of nose to brain delivery
The BBB prevents chemicals from entering the brain or CNS, making direct administration of therapeutics to the brain extremely difficult. Many researchers developed substitute routes and methodologies to subdue this hurdle, but nose to brain routes emerged as an excellent help for CNS targeting.30 Comparing the nose to brain route with the conventional route, it is a noninvasive method that provides access to the CNS with the olfactory pathway or trigeminal pathway. Another potential of nose to brain delivery is through the systemic pathway which is undesired while delivering the drug through the nose to the brain. The systemic pathway is an indirect pathway that reaches the systemic circulation by nasal blood vessels to arteries and from arteries to the brain and spinal cord but the chances of reaching the brain by crossing the BBB is very low. Even though small-sized molecules can eventually enter, the dose reaching the brain will be too low for any therapeutic efficacy.31 The nasal route prevents encounters with the BBB, which eventually does not lead to any obstacle related to crossing the BBB.32 Due to its proximity to the CSF, the olfactory area has a direct connection to the CNS.33 The nasal cavity has benefits that offer effective absorption and better permeability of small molecules and biopharmaceuticals. In order to safeguard the nerves that extend to the brain, the nasal cavity's roof has been built nearby. Nasal delivery would be able to supply a direct and less invasive strategy for treating CNS diseases including neurological disorders like Alzheimer's disease or Parkinson's disease and others by targeting the site of action.344. Anatomy of nose
The nose is considered to be a complicated creation and before studying drug absorption and penetration of the nose, it is necessary to understand the structure of the nasal cavity and its functions. The cavity is divided to three parts: the first is the vestibule region (with a surface area of ∼0.6 cm2), the second being olfactory region (with a reported surface area of 2–12.5 cm2) and lastly the respiratory region (Fig. 2).5 It has various functions, which include respiration and olfaction.35 It also involves functions such as maintenance of the humidity and temperature of the inhaled air in addition to exclusion of external pathogens.6,36The vestibule region of the nasal cavity prevents the entry of pathogens and has no absorption activity in its anterior part. It includes mucus and nasal hair that filter air particles entering the nose. Also, it is less porous owing to minimal vasculature and a reduced surface area.36
The olfactory region comprises basal cells, olfactory receptor cells and sustentacular cells.9 The olfactory receptor neurons that are bipolar neurons transmit data through the epithelium to the olfactory bulb.37
The third part of the nasal cavity, i.e., the respiratory region, surrounds the lateral walls of the nasal cavity and is considered to be the most vascular region. It has an area of ∼130 cm2.35 The respiratory epithelium of the respiratory region is comprised of ciliated columnar cells and non-ciliated columnar cells, goblet cells that secrete mucus and basal cells. This area plays a major role in the absorption of drugs.9
5. Pathways of nose to brain delivery
The features of the nasal cavity, including its highly vascularized and permeable mucosal lining, facilitate the rapid and efficient absorption of drugs. Additionally, the availability of the olfactory and trigeminal nerve pathways is crucial in this context. The olfactory region is directly connected to the brain, particularly the olfactory bulb, via the olfactory nerves. The respiratory region, on the other hand, is supplied with trigeminal sensory neurons and blood vessels. Drugs can potentially reach different brain regions through a direct neuronal pathway, offering a strategy to bypass the blood–brain barrier (BBB) (Fig. 2).38 Although the exact mechanism of drug transport from the nasal cavity to the brain remains a topic of ongoing research, some authors suggest that transporters present in both the olfactory bulb and the respiratory mucosa of the nasal cavity may play a significant role.39,40 The pharmacokinetics of nose to brain targeted drugs through various pathways has been illustrated in Fig. 3. | ||
| Fig. 3 Pathways of nose to brain drug delivery and pharmacokinetics. This figure has been adapted from the open access article from ref. 64. The figure is licensed under https://creativecommons.org/licenses/by/4.0/. | ||
5.1. Olfactory pathway
For a drug to be delivered to the brain from the nasal cavity it must pass through the olfactory epithelium through the olfactory nerve pathway.41 This pathway is separated into various parts: olfactory tract, olfactory epithelium, piriform cortex, anterior olfactory nucleus, amygdala and hypothalamus. It is considered a significant route for brain delivery via intranasal administration.42 This pathway is further subdivided into two categories. They are the intracellular or intraneuronal and extracellular or extraneuronal pathways.43 In the intraneuronal pathway, through mechanisms like endocytosis, olfactory neurons ingest the molecules or drugs in the olfactory epithelium, after which they enter the olfactory bulb.44 In the extraneuronal pathway, the drugs delivered intranasally cross the olfactory nerves and reach the olfactory bulb. For targeting various CNS disorders, the olfactory area allows a transparent connection between the nose and the brain.455.2. Trigeminal pathway
The second pathway of the nasal route is the trigeminal pathway, the most significant nerve pathway joining the CNS by innervating the respiratory epithelium and olfactory epithelium of the nasal passage.46 The dorsum of the head, a portion of the eye, a large portion of the nasal mucosa, cerebral blood vessels, and the meninges are all innervated by means of the ophthalmic division of the trigeminal nerve.47 The trigeminal nerve transfers sensory information through the nasal cavity, oral cavity, eyelids and cornea to the CNS.48 The trigeminal nerve pathway comprises three branches: the ophthalmic nerve, the maxillary nerve and the mandibular nerve.49 This pathway is important for the nose to brain delivery of drugs because the ophthalmic branches and maxillary branches of the trigeminal nerve pass directly through the nasal mucosa.50,51 The drug targeted to the brain administered from the nose uses this pathway to channel drugs from the pons of the brainstem and to the hindbrain.46 The transfer of many agents has been the subject of numerous research studies, for instance, insulin-like growth factor 1 (IGF-1) and interferon-β-1b (IFNβ-1b) through the trigeminal route.52,535.3. Systemic pathway
The systemic pathway is an indirect route for drug delivery to the brain that reaches the brain from the lungs and blood circulation. Being an indirect pathway it requires the drug to transport from the BBB to reach the brain.31 Crossing the BBB is a rate-limiting stage because it requires more time to acquire the desired beneficial outcome and limits the quantity of drug entering the brain.54 Hence, this pathway is suitable for small and lipophilic molecules as they can cross the BBB transcellularly.55 Through the nasal blood vessels, the drug enters and reaches the carotid artery, after which the drug reaches the brain and, finally, the spinal cord. This pathway is least ideal because of various peripheral effects that can be caused.56 A few observations on animals have claimed that medications can penetrate the brain via a local balance pathway from the venous to the carotid artery.5.4. Lymphatic pathway
For drug transport at the submucosal level, several extracellular pathways exist in the olfactory nerve, spreading to the bulb of the olfactory region to the brain, consisting of perivascular, perineural or lymphatic channels.57 The lymphatic pathway connects the subarachnoid space to the nasal lymphatic network.58 Some drugs administered through the nasal route permeate the nasal mucosa and can get circulated through lymph nodes of the well-developed lymphatic network situated beneath the nasal mucosa.59 This route finally ends in the neck's cervical lymph nodes, maintains water balance in the brain, and exports antigens to the cervical lymph nodes via the cerebrospinal fluid (CSF). Through this lymphatic pathway, drugs can be transported to the CSF which will successfully reach the brain tissue. It can also be said that this pathway may potentially overcome BBB limitations by maintaining the concentration in the brain and reducing possible peripheral side effects.60 Although these connections among the subarachnoid space, nasal mucosa, and deep cervical lymph nodes are not entirely understood, a study describing them has been published that reports CSF clearance occurring through the lymphatic system, and evidence showing a relationship to the lymphatic vessels after intranasal administration for transporting substances to the CNS is deficient.61–636. Challenges in formulating nose to brain delivery
Although the nose to brain route offers many rewards, some limitations limit the delivery of drugs to the brain.6.1. Mucociliary clearance
An essential mechanism of cleaning the nasal cavity during inhalation to eliminate foreign particles, likely dust, allergens and bacteria trapped in the nasal cavity's mucosa, is the activity of nasal mucociliary clearance.65 When the nasal preparation does not match the physiological conditions of the nasal cavity, the residence time in the cavity shortens, leading to decreased drug bioavailability. This limitation can be overcome by reducing mucociliary clearance (Cecilia de Barros et al., 2022).666.2. Permeation
The nasal route befits the passage of small molecules; hence, comparatively larger molecules, like proteins and peptides, need the advantages of absorption enhancers.67 These absorption enhancers upgrade the permeability leading to a rise in the bioavailability.68 Excipients used as mucoadhesives greatly increase the time of interaction through the nasal mucosa for better permeation of the formulation.696.3. Enzymatic degradation
The presence of certain enzymes such as exopeptidases (monoaminopeptidase and diaminopeptidase) and endopeptidases (cysteine, serine) in the lumen and epithelial barrier of the nasal cavity debases various proteins and peptides.70 Also, the presence of the peptidase enzyme restricts the bioavailability of the proteins and peptides as the enzyme cleaves these large molecules.71 To protect the bioactive substance from enzymatic degradation, enzyme inhibitors or novel drug carriers are used to improve bioavailability in the nasal cavity.726.4. pH and nasomucosal toxicity
A significant concern for CNS disorders is toxicity. A certain bacterium available in the nasal secretions is known to be killed in acidic pH by lysozyme, which in alkaline pH seems to be inactive and the tissue of the nasal mucosa is liable to microbial infection.73 For successful drug delivery, the pH of the formulations should be around 5.5–6.5 to avoid nasal irritation and tissue damage that renders the formulation toxic.74 The application of mucoadhesive agents that increase the interaction period with the nasal epithelium produces toxicity. Modifying the absorption enhancers used in the formulation can help diminish toxicity.757. Nanostructured lipid carriers
Nanostructured lipid carriers (NLCs) fall under the lipidic drug delivery system category and have gained awareness in the area of nanoparticulate delivery of drugs (Fig. 4). The primary factor, as compared with alternative nanoparticulate delivery techniques, is the recognized biocompatibility.76 NLCs are a new delivery system that proved to be more stable and capable of forming concentrated dispersions.77 Among the point mentioned above, NLCs exhibit several other advantages over other drug delivery systems.7.1. Advantages and disadvantages of NLCs over other nanoparticles
NLCs are known to have excellent advantages over other lipid nanocarriers. Some of them show excellent biocompatibility. Organic solvents can be avoided, and they are easier to sterilize and scale up. NLCs are the second generation of SLNs, which were discovered as an alternative for liposomes and other nanoparticles. Due to the distinct structure of NLCs, they cover up the disadvantages of other nanoparticles available like drug expulsion and are seen to improve the drug loading along with storage time.78 Also, NLCs can be more stable and have a lower water content as compared with others.79 When compared with polymeric or surfactant-based carriers, NLCs are inexpensive, provide better pharmaceutical stability, and deliver a higher content of drug as related to different carriers available in the market. NLCs have the opportunity to transport both lipophilic drugs and hydrophilic drugs at the same time.77 Additionally, NLCs are simple to make, and have improved aqueous dispersibility, excellent drug entrapment for hydrophilic and lipophilic substances and a controllable particle size. They are a cutting-edge and effective drug delivery method, particularly for lipophilic drugs, and show extended drug release. Due to their solid lipid matrices, which are also widely acknowledged as safe or have regulatory acceptance, these carriers are extremely effective systems (Jaiswal et al., 2016).80 Also, surface modifications are possible to accomplish easily.81 Looking over the advantages of NLCs over other nanoparticles, it can be justified that they are a suitable nanocarrier for the nose to brain delivery of therapeutics. Apart from the several advantages of NLCs, there are also certain limitations. Some limitations or demerits embrace a lower loading capacity for hydrophilic drugs and the combination of two or more therapeutic agents can cause a decrease in the encapsulation efficiency.82 Also, the type and number of surfactants used can sometimes lead to irritation, sensitivity or cytotoxicity.837.2. Composition of NLCs
Lipids, surfactants, organic solvents and other agents are the main components in formulating NLCs (Table 1).84| Components | Examples | Ref. |
|---|---|---|
| Solid lipid | Stearic acid, Dynasan 116, Dynasan 118, lauric acid, glyceryl monostearate, cetyl palmitate, Compritol 888 ATO, tristearin, Tefose 63 | 101–104 |
| Natural liquid lipid | Sunflower oil, castor oil, lavender oil, cinnamon oil, clove oil, jojoba oil, soyabean oil, sesame oil, olive oil, garlic oil, almond oil, shark liver oil (squalene) | 105–108 |
| Synthetic liquid lipid | Vitamin E, isopropyl myristate, Capmul MCM, liquid paraffin, Lauroglycol 90, isopropyl palmitate, Miglyol 829, Miglyol 840, Captex 300, Miglyol 812 | 109–112 |
| Hydrophilic surfactants | Tween 60, Tween 80, sodium oleate, polyvinyl alcohol, Pluronic F-68, Poloxamer 407, Poloxamine 908, sodium cholate, tyloxapol | 113–115 |
| Lipophilic surfactants | Span 20, Span 40, Span 60, Span 80, Myverol 18-04K | 116 and 117 |
| Amphiphilic surfactants | Egg lecithin, soy lecithin, phosphatidylcholine, Gelucire 50/13, phosphatidylethanolamines | 118–120 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 up to 99.9
30 up to 99.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1.86 The kind and structure of lipids influence the different properties of the nanocarriers. It has been claimed that the most appropriate parameters for selecting a suitable lipid are the solubility or the lipid's apparent partition coefficient of bioactives in it. The solubility of drug molecule in lipids determines the drug loading and encapsulation efficiency. The evaluation parameters of NLC formulations, such as drug entrapment, drug loading, size of NLC and charge of NLC, are affected by the degree of crystallization of the different lipids used. The particle size of nanodispersion increases due to the increased viscosity in the dispersed phase and high melting point lipids. Other lipid-related characteristics that could affect the standard of the NLC include lipid hydrophilicity, lipid crystal shape, and composition fluctuation.87
0.1.86 The kind and structure of lipids influence the different properties of the nanocarriers. It has been claimed that the most appropriate parameters for selecting a suitable lipid are the solubility or the lipid's apparent partition coefficient of bioactives in it. The solubility of drug molecule in lipids determines the drug loading and encapsulation efficiency. The evaluation parameters of NLC formulations, such as drug entrapment, drug loading, size of NLC and charge of NLC, are affected by the degree of crystallization of the different lipids used. The particle size of nanodispersion increases due to the increased viscosity in the dispersed phase and high melting point lipids. Other lipid-related characteristics that could affect the standard of the NLC include lipid hydrophilicity, lipid crystal shape, and composition fluctuation.87
The lipid at room temperature is solid but it melts at temperatures above 80 °C. It should be biodegradable, stable chemically without any toxic or hazardous effects, physiologically acceptable and a generally-recognized-as-safe (GRAS) status lipid. The lipids used include triglycerides, fatty acids, waxes and partial glycerides. Some commonly used solid lipids are Compritol 888 ATO, Precirol ATO5, etc.88
The solubility of drugs in liquid lipids is typically greater than in solid lipids.89 Liquid lipids are selected or obtained from natural sources, which should be cost effective and non-irritating. Some liquid lipids used are sunflower oil, oleic acid etc. Oil-based liquid lipid assimilation leads to flaws in a solid lipid's structural integrity. These structural imperfections help in more drug loading as they cause the crystalline arrangement of solid lipid to be less ordered, leading to minimized drug leakage.90
7.3. Types of NLC
From studies on suppositories, it is well recognized that well-ordered crystalline lipid matrices will cause pharmacological ejection. This can occur with solid lipid nano- and microparticle mixes particularly when nanoparticles are ordered from very pure lipids such as tristearin.121 The NLC matrix is a disorganized structure where the various lipid molecules are combined to produce a matrix with as many flaws as possible. The combination of both the lipids results in a core that is solid at body and room temperature. Different forms of NLC are produced, each with a unique morphology depending on the varied production methods and the makeup of the lipid mixes (Fig. 4). The primary idea is to give the lipid matrix a specific nanostructure to maximize the payload for active chemicals and decrease compound ejection during storage.1227.4. Surface modification
Surface modification is coating the NLCs with hydrophilic polymers, ensuring the ease of transport across the epithelium, and its stability and targeting ability.134 During the transport of drug-loaded NLC via the nose to brain route, the surface of the NLC is modified to improve the limitations of the nasal cavity, mainly mucociliary clearance and penetration. To increase the adhesion property of the NLC to the nasal mucosa, the NLCs are coated with chitosan,135Delonix regia gum136 and gelatin.137 Cell-penetrating peptides (CPP), for example, can be added to the surfaces of NPs to make them more capable of travelling from the nose to the brain.138 The penetration of chitosan-coated NLCs, PLGA NPs and NLCs in a study utilizing an in vitro model of olfactory cell monolayers is 0.7%, 8% and 22%, respectively. After surface modification with CPPs, Tat or penetratin, Tat-chitosan-NLCs and penetratin-PLGA-NPs displayed 46% and 7% penetration, respectively.139 As a result, ligand conjugation affects the rate at which molecules pass over the epithelium of the nose. Lectins, including wheat germ agglutinin, are a relevant targeting ligand for NP delivery from the nose to the brain.140 For nasal administration applications, lactoferrin is potentially a target ligand.1417.5. Fabrication techniques
There are numerous protocols available for formulating NLCs. The methods for creating SLNs can also be used to create NLCs. The process that makes use of both high pressure and high temperature is known as the high-pressure homogenization method. It is further subclassified as hot homogenization and cold homogenization.142 Hot homogenization involves drug and lipid melting which then at the same temperature is joined with an aqueous surfactant.143 The cold homogenization technique is applied to or developed to solve the limitations observed by the hot homogenization technique (Jaiswal et al., 2016).80,144 Other techniques include the microemulsion technique which involves keeping the temperature equivalent while melting and heating the lipid in an aqueous phase.145 The double emulsion method helps prepare NLCs that will be loaded with hydrophilic drugs. The process involves dissolving the drug in the aqueous solvent, further dispersion in the lipid phase forming a primary emulsion where both phases are maintained under the same temperature,146 and emulsification by the solvent evaporation method, where an aqueous phase dissolves and emulsifies the lipid in a water-insoluble organic solvent.34 Low-pressure evaporation of the solvent allows for lipid precipitation and dispersion in the aqueous solution.147 The membrane contactor method includes the usage of a specific membrane contactor to produce NLC. The process involves pressing the lipids above their melting point temperature, permitting the development of tiny droplets over the membrane.148 The aqueous phase flows inside the membrane and eradicates droplets that are formed in the outlets of the pores. After this step, by cooling the preparation at room temperature NLCs are formed.149 The melt extrusion method involves using an extruder self-possessed of three parts: the feeding zone, barrel and screw assembly.150 The feeding zone is the entry point of materials into the extruder and the barrel is the main body of the extruder, passing material from the feeder to the discharge unit.151 The screw assembly is comprised of three different screws, which are conveying screws that help in pushing the material in the forward direction, mixing screws for mixing the materials to form a homogeneous mixture that is arranged at different angles (0 and 90°) and a discharge screw that extrudes out the final product which is the NLC. This technique comprises two stages.152 In the first step, the amalgamation of both solid and liquid lipids, an aqueous phase and the drug causes the development of a pre-emulsion by extrusion. The second step follows the reduction in the size of the formed pre-emulsion with the help of a probe sonicator.153 The microfluidization method involved in formulating NLCs requires a microfluidizer device and is a mixing technique.154 The process involves applying a very high pressure in the range of 500 up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi to force out the liquid over the interaction chamber, and the interaction chamber contains microchannels that are small channels of a special configuration.155
000 psi to force out the liquid over the interaction chamber, and the interaction chamber contains microchannels that are small channels of a special configuration.155
7.6. Evaluation of nose to brain targeted NLCs
The prepared NLC formulations can be evaluated by a variety of characterization techniques like particle size, for which two methods can be used, photon correlation spectroscopy (PCS) or laser diffractometry (LD).156 A typical NLC particle diameter lies between 10 and 1000 nm. The particle size of NLCs must be lower than 200 nm for easy permeation through the nasal mucosa.157 Photon correlation spectroscopy uses the dynamic light scattering (DLS) technique.158 The polydispersity index determines whether the particles are monodispersed or polydispersed, and is a measurement of the dispersion quality of the particles.159 The size distribution measurement of NLCs ranges from 0.0000 to 1.0000, with the recommended PDI value below 0.5 for the uniform drug distribution through the nasal mucosa.157,160 A greater value could result in pharmacokinetic irregularity and unpredictability in the therapeutic outcome. In contrast, a lower value suggests an increased likelihood of an additional undeviating absorption via the nasal mucosa.161 The zeta potential relates to the evaluation of non-aggregation and physical stability and is crucial for determining how NLC particles repel one another. The zeta potential is estimated using Zetasizer and is based on electrophoretic mobility under an electric field.162 A higher zeta potential represents values greater than +30 mV and less than −30 mV. These values represent increased stability upon storage.162,163 The zeta potential is preferred to be positively charged as the particles can form electrostatic bonds along with the negatively charged nasal mucosa membrane leading to increased accumulation of drug in the brain.157 Morphology is a characterization method to determine the form of the particles and to see the potential aggregation.164 It can be determined using two approaches, scanning electron microscopy (SEM) and transmission electron microscopy (TEM), providing precise details on particle shape.165 SEM makes use of electron transmission from the sample surface while TEM makes use of electron transmission through the sample. Another approach for determining the morphology of the NLC is atomic force microscopy (AFM).166 AFM gives a three-dimensional surface profile of a sample, in contrast to electron microscopy, which only generates a two-dimensional image of a material.167 It can resolve surface features as small as 0.01 nm.164 The molecules’ ability to exist in different crystalline forms due to diverse lattice arrangements is known as polymorphism. Some lipids with low melting points used in NLC preparation might exist in their different polymorphic forms.168 For evaluating the crystallinity of NLCs, two useful tools applied are differential scanning calorimetry (DSC) and X-ray diffraction (XRD).169 The DSC method can assess physical and chemical changes, such as the degree of crystallinity and melting behavior. It is the quantity of thermally-induced physical and chemical alterations to solid nanoparticles within a sample. The ratio of the enthalpy of the NLCs to the bulk enthalpy is used to determine the NLCs’ degree of crystallinity calculated based on the total weight of the preparation.170 XRD uses the spacings between various polymorphic forms to identify them. The length of the lipid lattice's long and short spacings can be measured. DSC may be used to distinguish between amorphous solids and liquids. Still, X-ray diffraction distinguishes between crystalline and amorphous materials and evaluates the oily ingredient's impact on the sub-cell parameters and long spacings of the solid lipid nanocrystals,171 drug loading efficiency is defined as the ratio between drugs and lipids in NLCs. It depends on the drug's ability to dissolve in melted lipid, the lipid's and the drug's miscibility, the physical and chemical makeup of the matrix of solid lipid (partition coefficient, water solubility), the polymorphic state of the lipid material,172 and the entrapment efficiency (EE) which is defined as the number of active components integrated into the particles over the overall amount present in the dispersion, also called the encapsulation efficiency.173 The process of centrifugation determines the drug entrapment efficiency. The entrapment efficiency should be more than 80% for the intranasal delivery of lipid-based nanocarriers.157 The supernatant obtained by centrifugation of the formulation is evaluated for drug concentration. During centrifugation, the free drug will stay in the supernatant after the centrifugation forms an NLC sediment.145 The entrapment efficiency can be measured by the equation given below:1748. Recent developments in nose to brain targeted NLCs
Alzheimer's disease is a progressive neurological condition that affects older people resulting in a person losing their memory and thinking capability. It makes it difficult for the person to fulfil their daily tasks. Dementia is caused by AD, and is the cause of the deprivation of thoughts, identification, and cognitive developments and the behavioral changes that hamper a person's everyday life. A carbamate anticholinesterase inhibitor active only in the brain is rivastigmine hydrogen tartrate, which effectively manages AD. Anand et al. designed NLCs loaded with rivastigmine hydrogen tartrate by employing Compritol 888 ATO, Ryoto Sugar Ester and triacetin as its components for treating dementia caused by Alzheimer's disease. By modifying the preparation technique of the solvent emulsification–diffusion method, RHT-NLCs were formulated. For optimization a Box–Behnken design was applied. The values obtained for particle size, polydispersity index, zeta potential and percent entrapment efficiency were observed to be 266 ± 0.94 nm, 0.233, −16.58 mV and 61.82 ± 2.52%, respectively. For drug diffusion through goat nasal mucosa, RHT-NLC showed 81.23 ± 2.14% in 12 h. Noteworthy development was observed afterwards in the treatment with the formulated NLC in escape and transfer latency in a rat model.175Similarly, Cunha et al. optimized two NLC formulations targeting rivastigmine from nose to brain. For optimizing the formulations in two steps, the QbD approach was used because of QTPP and CQAs for intranasal delivery. Optimization designs used were the Box–Behnken and central composite designs. The parameters permitting the most significant values of CQAs were designated. Rivastigmine-loaded NLCs were formulated by the method of ultrasound and high-pressure homogenization and showed particle size values of 114.0 ± 1.9 nm and 109.0 ± 0.9 nm; the polydispersity index showed values of 0.221 ± 0.003 and 0.196 ± 0.007, the zeta potential −30.6 ± 0.3 mV and −30.5 ± 0.3 mV and the entrapment efficiency 97.0 ± 0.5% and 97.2 ± 0.3%. In vitro drug release specified sustained release, and stability studies directed rivastigmine-loaded NLCs to be stable after storage of 90 days.176
Again, for treating AD, Wavikar et al. developed in situ gelling rivastigmine-loaded NLC for nose to brain delivery prepared by an ethanol injection method with the components glyceryl monostearate, Capmul MCM C8, lecithin and Tween 80. They displayed a particle size and entrapment efficiency of 123.2 ± 2.3 nm and 68.34 ± 3.4%, respectively.177
Another research study on Alzheimer's was performed by Cunha et al., who developed in situ thermosensitive gels with NLC and nanoemulsion loaded with an acetylcholine inhibitor (rivastigmine-RVG). The evaluated values for rivastigmine-loaded NLC for particle size, PDI, zeta potential and EE were seen to be 114.00 ± 1.91 nm, 0.45 ± 0.00, −3.58 ± 1.62 mV and 95.13 ± 0.34% respectively. The stability study showed long-term stability, good nasal mucoadhesion, and prolonged drug release.178
Pioglitazone is a diabetic drug found to improve brain insulin signaling and can directly control multiple targets that are included in AD. It falls under the PPARγ agonist insulin sensitizer class. Jojo et al. prepared NLCs loaded with pioglitazone intranasally for targeting the brain and optimized via a Box–Behnken design. The formulation showed values of particle size, zeta potential, polydispersity index and entrapment efficiency of 211.4 ± 3.54 nm, 14.9 ± 1.09 mV, 0.257 ± 0.108 and 70.18 ± 4.5%, respectively. Stability of the formulation upon storage was seen to be at 4 °C and 25 °C. It showed sustained release in an in vitro drug release study. An ex vivo permeability study showed an improvement in nasal permeability. Toxicity and in vivo biodistribution studies ensured the formulation's safety and direct transport from nose to brain in rats.179
Resveratrol is a compound found naturally that shows many anti-inflammatory, antioxidant, and neuroprotective effects. It is found that resveratrol can be used in preventing and treating AD because a neuroprotective effect can be seen due to the ability of the drug to remove Aβ peptide and the breaking down of APP. Rajput et al. prepared an in situ hydrogel of NLCs loaded with resveratrol for its administration intranasally by the melt emulsification–probe sonication method optimized by the Plackett–Burman design. They characterized the hydrogel after loading the NLC into the in situ gel. Evaluating the formulation showed a particle size of 132 ± 12 30 nm, a polydispersity index of 0.165 ± 0.002, a zeta potential of −23 ± 4 mV, drug loading of 10 ± 3% and an entrapment efficiency of 74 ± 6%. A high amount of drug distribution in the brain was confirmed by a pharmacokinetic study showing the safety along with the efficacy of the formulation over the nose to brain route, indicating a hopeful method to treat Alzheimer's disease (AD).180
Lamotrigine shows efficacy and tolerability in patients experiencing epilepsy. It offers fewer complications like dizziness, asthenia and somnolence than phenytoin and carbamazepine. Alam et al. prepared and investigated lamotrigine-loaded NLC for brain targeting following intranasal administration. A Box–Behnken design optimization method was used to optimize the formulation and estimates made for particle size, PDI, zeta potential and EE; the observed values were 151.6 ± 7.6 nm, 0.249 ± 0.035, 11.75 ± 2.96 mV, 96.64 ± 4.27% respectively. After 24 h of treatment, a study of drug release in vitro revealed sustained release.181
Similarly, Nair et al. prepared phenytoin-loaded NLCs by the nose-to-brain route to experiment with the release of phenytoin sodium in the brain for treating acute epileptic seizures. Three phenytoin sodium-loaded NLCs were prepared of size of <50, size ranging in between 50–100 nm and size of >100 nm by the method of melt emulsification. In vitro drug release, in vivo pharmacokinetic studies and ex vivo permeation studies of three NLCs showed that NLC of particle size <50 nm offered immediate drug release, a higher concentration of drug in the brain and CSF, with greater permeation within 5 min of administration intranasally. The results ensured that phenytoin sodium NLCs could treat acute epileptic seizures.182
Also, Khan et al. fabricated carbamazepine-loaded NLCs for better brain delivery to treat epilepsy. CBZ-NLC were prepared using trilaurin and oleic acid stabilized with usage of Poloxamer 188, Tween 80 and Span 80 and optimized. CBZ-NLCs showed a particle size, zeta potential and % drug incorporation of 97.7 nm, −22 mV and 85%, respectively. A biphasic release pattern with an initial fast release followed by a prolonged release was demonstrated by an in vitro release study.183
Depression is a health issue affecting individuals of all age groups worldwide and is caused by the disproportion in neurotransmitter levels that transfer information from the presynaptic to the postsynaptic neuron. Thymoquinone, obtained from Nigella sativa seeds, shows various pharmacological activities. It provides peroxidation to lipid membranes, delaying neuroinflammation by obstructing the inflammatory mediators’ development known to be causing depression. Qizilbash et al. prepared NLCs loaded with thymoquinone-enriched naringenin for the treatment of depression by the nasal pathway. For preparing naringenin-loaded NLCs, thymoquinone was used as the liquid lipid established by the method of ultrasonication and optimized by a central composite rotatable design. The formulation exhibited particle size: 84.17 to 86.71 nm, PDI: 0.258 to 0.271, zeta potential: −8.15 to −8.21 mV and %EE: 87.58 to 88.21%. Comparing the in vitro drug release profile of TQ-NGN-NLC with NGN suspension, it was found that the NLC had a cumulative drug release of 82.42 ± 1.88% while the drug suspension had a release of 38.20 ± 0.82%.184
Flibanserin was primarily established as an antidepressant, but it was found to show increased libido in females and was adapted for treating female sexual interest/arousal disorder. Ahmed et al. fabricated flibanserin-loaded NLC in situ gel to enhance the delivery of flibanserin in the brain by a hot emulsification method, and optimization was done by the Box–Behnken method. After being prepared, FLB-NLC was fused into gellan gum in situ gel and subjected to rat histological evaluation, in vitro drug release and in vivo pharmacokinetic performance. The vesicular size was determined to be 114.63 nm, with a spherical shape. When compared with raw FLB control gel, in vitro release demonstrated improved release. In vivo studies improved the drug concentration in the plasma and brain compared with control gel. Lastly, the histological analysis confirmed that there were no abnormal indications. These outcomes confirmed that the FLB-NLC in situ gel could be administered nasally.185 Besides these, several other nose-to-brain targeted NLCs have been summarized in Table 2.
| Formulation | Solid lipid | Liquid lipid | Disease | Characteristics | Ref. |
|---|---|---|---|---|---|
| Zopiclone-loaded NLC | Palmitic acid | Cod liver oil | Insomnia | Particle size was found to be 71.27 ± 13.57 nm with PDI of 0.097 ± 0.15, ZP ranging from −24.75 ± 3.04 to −34.1 ± 4.86 mV along with %EE showing 94.31 ± 2.44%. | 186 |
| Results showed superior delivery of zopiclone intranasally with faster and higher brain uptake of 6.9 ± 1.02% ID g−1 at 5 min post-administration. | |||||
| Chitosan-coated buspirone-loaded NLC | Glyceryl monostearate | Oleic acid | — | Particle size was found to be 190.98 ± 4.72 nm along with ZP of 17.47 mV and %EE of 80.53 ± 1.26%. TEM showed incorporation of drug into the NLC adequately and DSC studies proved the presence of drug in an amorphous state. Results showed shelf life of 27.61 months with high DTP value of 1462.49%. | 187 |
| Agomelatine-loaded NLC | Stearyl alcohol | Oleic acid | Depression | Particle size was shown to be 99.8 ± 2.6 nm with PDI of 0.142 ± 0.017, ZP of −23.2 ± 1.2 mV and %EE of 97.1 ± 2.1%. TEM showed a spherical shape of the particle and DSC showed an amorphous state of the drug within the NLC matrix. DTE value was reported to be 321.21% and DTP value to be 74.55% showing an effective strategy for brain targeting. | 188 |
| Chitosan-coated donepezil-loaded NLC | Compritol | Capryol 90 | Alzheimer's disease | Particle size was found to be 192.5 ± 7.3 nm along with PDI of 0.298 ± 0.021, ZP of 38.9 mV and %EE of 89.85 ± 2.17%. Spherical shape was observed by TEM and DSC showed an amorphous state of the drug in the NLC matrix. DTE value of 321.21% and DTP value of 74.55% showed efficient brain targeting by intranasal administration. | 189 |
| Chitosan-coated rotigotine-loaded NLC | Compritol 888 ATO | Caproyl 90 | Parkinson disease | Particle size was found to be 170.48 ± 8.37 nm, PDI of 0.19 ± 0.03, ZP of 26.73 mV and %EE of 82.37 ± 2.48%. In vitro studies showed a sustained release pattern with DTE value of 422.03% and DTP value of 76.03% after intranasal administration. Results of confocal laser scanning microscopy proved better targeting of brain by the prepared formulation. | 190 |
9. Pharmacokinetic considerations of NLCs
Pharmacokinetics plays a significant role in the delivery of NLC inside the body. It tackles the delivery of drugs inside the body and is subject to the drug's physicochemical properties, like molecular weight, size, shape, charge and aqueous solubility. The development of the carrier system, which in this case is NLC, also plays an important part in pharmacokinetic considerations (release and absorption of drug) that determine the drug's bioavailability. The therapeutic effect is mainly expressed by the pharmacokinetics and tissue distribution of NLC.191 Different in vivo models are used to determine the PK profile. Choosing an appropriate in vivo model is crucial for studying the anatomy of the nasal cavity. Directing the intranasal study, the first model used was the rat, and later on other animal models of sheep, monkeys, mice and dogs were used for advancement of the absorption data. For the initial absorption studies, mouse and rat models are used.192 Different nose to brain delivery strategies’ efficiency is expressed using specific metrics. Drug targeting efficiency % (DTE%) and drug transport percentage (DTP%) are the parameters used. DTE% signifies the competence of drug to reach the brain when compared with blood, following intranasal administration vs. parenteral administration. DTP% denotes the drug percentage entering the brain by the nasal route directly in relation to the complete quantity of the drug found in the brain.19310. Stability of NLCs
NLCs are the second generation of solid lipid nanoparticles (SLNs) developed to solve problems related to the delivery of therapeutics with the help of SLNs. Though NLCs provide many advantages, they are somewhat limited by the stability problem. The issue of stability arises because of less water.194 Other reasons for the instability of NLCs include increased particle size, the expulsion of drugs from the matrix, and aggregation alongside long-term storage. Upon storage, highly concentrated NLCs link with each other, forming a pearl-like structure, causing movement restriction within the dispersion. Low-concentration NLCs, when stored, show the collision of particles with each other, resulting in aggregation.195 To overcome the instability of NLC, three methods are usually employed. One approach is the application of the freeze drying method. The others are the addition of preservatives or stabilizers in the formulation and spray drying.196 Freeze drying or lyophilization is the water removal process to improve stability. It involves the sublimation of ice followed by desorption in a vacuum. This process ensures storage for an extended period without the risk of damage to the original properties of the formulation.197 The second approach of stabilizers/preservatives involves the addition of primary surfactants into the formulation. Surfactants can lower the interfacial tension between two phases of NLC by gathering in the interface of the binding surface, producing a layer around the particles, thus improving the physical stability of the prepared formulation upon storage.198 Preservatives are categorized into four kinds based on their capacity of maintaining NLC stability: (a) propylene glycol is a preservative that has no stabilizing effects, (b) caprylyl glycol is a preservative that has minor stabilizing effects, (c) Euxyl K700 is a preservative having major stabilizing effects and (d) pentylene glycol is a preservative showing a stability effect.133 For spray drying the formulated NLC, it is required that the lipid matrix should have a melting point above 70 °C.14411. Regulatory and toxicity aspects of nose to brain targeted NLCs
For the widespread acceptability of NLCs, one of the accepted modest regulatory hurdles by the regulatory authorities is using GRAS (generally regarded as safe) excipients.199 These systems enlist excipients with physiological, non-toxic, biodegradable and compatible profiles.200 In addition to having the GRAS designation, excipients must be used following the regulatory authorities’ frequently acceptable concentrations. A toxicity study must prove an excipient's safety at a given concentration when used in more significant quantities.201 It has been found that the minimization of toxicity and possible side effects is effective because of nanocarriers achieved by reducing the dose required. When the molecules are transformed from natural to nanoscale, they may change completely or notably in both physicochemical and biological properties and may show toxicity.202 It is essential to thoroughly investigate the toxicity of the bioactive molecules and excipients used in the formulation before targeting it to the desired site of action. NLCs are a blend of hydrophilic and lipophilic bioactive compounds stabilized by surfactants. These excipients may have some responsibility in causing toxicity to the brain. Surfactants can trigger the immune system; thus, in vitro toxicity studies may be performed to deliver favorable safety data.203 Testing the toxicity of NLC administered through the nose to brain route can be done by conducting a nasal tissue toxicity test. A nasal tissue toxicity study inspects the probable toxic properties to be shown on the nasal mucosa by the formulated preparation. It can be performed on the nasal mucosa of sheep, pigs, or other suitable animals obtained freshly. This study showed the pathological changes in the nasal mucosa after administering the formulation intranasally and is compared with untreated nasal mucosa. The treated mucosa (mucosa with the formulation) is examined under a microscope to determine whether the formulation is showing safe or toxic effects.20412. Patents on NLCs
So far numerous studies have been conducted on NLCs for drug delivery applications. Many of them are patented. This section summarized a few important patents for various drug delivery approaches (Table 3).| Patent number | Title of patent | Inventors/assignee | Claims | Ref. |
|---|---|---|---|---|
| CN101632638B | Silybin nanostructured lipid carrier and preparation method thereof | Shandong University | Silybin NLC comprises silibinin 0.05–0.5 wt%, solid lipid material 0.1–5 wt%, liquid lipid 0.02–2 wt%, fat-soluble emulsifier 0.2–5 wt%, water-soluble emulsifier 0.2–5 wt%, surplus is water. | 205 |
| The described organic solvent is selected from one of the following: chloroform, acetone, ethanol, ethyl acetate. | ||||
| WO2016065444A1 | Method for producing nanostructured lipid carriers on triblock copolymers, nanostructured lipid carriers thereby produced and uses thereof. | Nelson Eduardo Duran Caballero, Alzira Xavier Pinto DINI | NLC in a block copolymer comprises two solid fused lipids selected from cupuassu butter and lanolin, a photoprotective property liquid lipid and a triblock copolymer. | 206 |
| The photoprotective liquid lipid is selected from the group of buriti oil, coconut oil, passion fruit oil, chamomile oil, sunflower oil, avocado oil or any photoprotective liquid lipid | ||||
| CN101658493B | Azithromycin nanostructured lipid carrier and preparation method thereof | Suzhou Nanohealth Biotech Co Ltd. | Azithromycin NLC has the composition of: azithromycin 0.1–1%, emulsifying agent 20–40%, complex lipid material 3–8%, all the remainder is water. | 207 |
| The particle size range is found 40–100 nm. | ||||
| CN101658468B | Coenzyme Q nanostructured lipid carrier and preparation method thereof | Suzhou Nanohealth Biotech Co Ltd. | Coenzyme Q nanostructured lipid carrier has the composition of: ubiquinone 1–5%, emulsifying agent 0.5–25%, tristerin and suffering/caprin 0.5–25%. | 208 |
| Particle size range of described compositions is at 40–120 nm. | ||||
| BR102019013856A2 | Solid lipid composition for production of nanostructured lipid carrier | Nágila Maria Pontes Silva Ricardo, Tamara Gonçalves De Araújo, Bianca Oliveira Louchard | Production of NLC contained at least one oil, a wax, a butter and a surfactant that are fused and recrystallized. | 210 |
| NLC characterized obtained from 2 to 20% of solid lipid composition through an emulsification method followed by sonication. | ||||
| CN115054578A | Tumor-targeting norcantharidin nanostructured lipid carrier and preparation method thereof | Panzhihua central hospital | The tumor-targeting norcantharidin nanostructured lipid carrier is characterized by comprising the following raw materials in parts by weight: 0.1–6 parts of norcantharidin, 5–15 parts of polyethylene glycol-400, 8–16 parts of polyoxyethylene castor oil, 20–50 parts of ethyl oleate, 0.4–2 parts of tripalmitin, 1–5 parts of phospholipid, 2–5 parts of glyceryl monostearate, 801-5 parts of Tween-801 and 72–308 parts of a water phase. | 209 |
| The tumor-targeted norcantharidin NLC is round and oval, and has an average particle diameter less than 100 nm and encapsulation efficiency of 87.41 ± 0.16%. |
Shandong University developed a silybin nanostructured lipid carrier using improved microemulsion technology-emulsification and evaporation-low-temperature curing methods. This invention establishes and relates to the silybin NLC preparation method. The invention claims to provide good compatibility and stability and release controllable biological silybin NLC.205
Nelson Eduardo Duran Caballero et al. developed NLC on triblock copolymers by the preparation technique of hot homogenization under high pressure. The invention includes cosmetic usage precisely for hair that contributes moisture retention on the hair fibres for a prolonged time, reducing the frizz in the hair strands and boosting the shine.206
A simple and controllable preparation method and good repeatability can be applied to preparing azithromycin antibacterials for eye doses. Suzhou Nanohealth Biotech Co. Ltd developed azithromycin NLC providing good stability and higher bioavailability. The components involve 0.1–1% of azithromycin, 20–40% of emulsifier, 3–8% of lipid materials and the balance as water.
Suzhou Nanohealth Biotech Co. Ltd developed coenzyme Q NLC to provide high stability and good compatibility and has been able to solve problems like the stability of ubiquinone medicine, whose bioavailability is low.207
Nágila Maria Pontes Silva Ricardo developed NLC. The invention aimed to acquire a mixture of lipid components comprising wax, butter and oil that is melted and recrystallized to form a single lipid element entitled solid lipid. This invention ideally has the lipid balance required to produce NLC effectively in one step.208
Panzhihua Central Hospital invented a tumor-targeting norcantharidin NLC to overcome the problems associated with the low bioavailability and poor tumour targeting of the current delivery carrier of norcantharidin.209
13. Conclusion and future prospects
In the field of research, nanotechnology has emerged as a promising area for scientists to target and treat specific areas and diseases. The presence of the BBB limits brain targeting, for which the nose to brain pathway has arisen as the substitute route to target the brain. Advancement in medicine and nanotechnology has led to the development of many nanoplatforms, NLC being one of them. This review delivers information about nose to brain targeted NLCs, including different formulations of NLC prepared over a period administered via the nose to brain route. NLCs are becoming of interest because of their superiority above other lipid nanoparticles, such as their extended release of drug, enhanced bioavailability in the CNS and increased therapeutic efficacy. Also, the involvement of both solid and liquid lipids provides an advantage in high drug loading capacity, inhibiting drug expulsion. Despite the availability of several benefits, there is still a requirement to achieve appropriate, affordable, cost-effective dosage forms. All the latest works have been done in recent years; it can be resolved that nanotechnology will be achieving intense attention in the future. Because of the several advantages of NLC, it will have the upper hand compared with SLN, liposomes and other lipid nanoparticles.Further research is needed since it is unclear exactly how nanosystems go from the nasal mucosa to the olfactory and trigeminal nerves, and then to the central nervous system (CNS), as well as how they are distributed and interact with receptors in various sections of the brain. Before moving to clinical trials, it will also be crucial to conduct additional research on the toxicological effects of nanosystems administered by IN, including the brain as well as neural pathways in addition to the nasal mucosa. In conclusion, nanosystems administered intranasally have the potential to enhance patient quality of life and advance our understanding of the pathophysiology behind neurological diseases; however, additional research must be done before these technologies may be commercialized as drugs. We can also say that nasal formulations may be available to treat CNS disorders.
Author contributions
Mridusmita Das: writing – original draft, writing – review & editing. Anupam Sarma: conceptualization, writing – original draft, writing – review & editing, supervision. Debojeet Basak: writing – original draft. Himakshi Baruah: writing – original draft.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
Authors are thankful to DST-FIST [Department of Science and Technology-Fund for Improvement of S&T Infrastructure (DST-FIST), Government of India] for infrastructural facility and Girijananda Chowdhury University, Guwahati for support in writing this article.References
- T. T. L. Nguyen and H. J. Maeng, Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery, Pharmaceutics, 2022, 14(3), 572, DOI:10.3390/pharmaceutics14030572.
- K. Selvaraj, K. Gowthamarajan and V. V. S. Reddy Karri, Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting, Artif. Cells, Nanomed., Biotechnol., 2018, 46(8), 2088–2095, DOI:10.1080/21691401.2017.1420073.
- H. Akel, R. Ismail and I. Csóka, Progress and perspectives of brain-targeting lipid-based nanosystems, Eur. J. Pharm. Biopharm., 2020, 148, 38–53, DOI:10.1016/j.ejpb.2019.12.014.
- M. M. Farag, et al., Comparative pharmacodynamic study delineating the efficacy of amantadine loaded nano-emulsified organogel via intranasal versus transdermal route in rotenone-induced Parkinson's disease rat model, J. Drug Delivery Sci. Technol., 2023, 86, 104765, DOI:10.1016/j.jddst.2023.104765.
- C. P. Costa, J. N. Moreira, J. M. S. Lobo and A. C. Silva, Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies, Acta Pharm. Sin. B, 2021, 11(4), 925–940, DOI:10.1016/j.apsb.2021.02.012.
- V. Bourganis, O. Kammona, A. Alexopoulos and C. Kiparissides, Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics, Eur. J. Pharm. Biopharm., 2018,(128), 337–362, DOI:10.1016/j.ejpb.2018.05.009.
- T. T. L. Nguyen and H. J. Maeng, Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery, Pharmaceutics, 2022, 14(3), 572, DOI:10.3390/pharmaceutics14030572.
- F. Sonvico, et al., Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting, Pharmaceutics, 2018, 10(1), 34, DOI:10.3390/pharmaceutics10010034.
- K. Selvaraj, K. Gowthamarajan and V. V. S. Reddy Karri, Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting, Artif. Cells, Nanomed., Biotechnol., 2018, 46(8), 2088–2095, DOI:10.1080/21691401.2017.1420073.
- A. Sharma and A. Baldi, Nanostructured Lipid Carriers: A Review, J. Dev. Drugs, 2018, 7(1), 1–12 Search PubMed.
- J. Ahmad, M. Md Rizwanullah, S. Amin, M. H. Warsi, M. Z. Ahmad and Md. A. Barkat, Nanostructured Lipid Carriers (NLCs): Nose-to-Brain Delivery and Theranostic Application, Curr. Drug Metab., 2020, 21(14), 1136–1143, DOI:10.2174/1389200221666200719003304.
- H. Peluffo, et al., BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the CNS, Biotechnol. Adv., 2015, 33(2), 277–287, DOI:10.1016/j.biotechadv.2015.02.004.
- A. M. Grabrucker, et al., Nanoparticles as Blood–Brain Barrier Permeable CNS Targeted Drug Delivery Systems, In: The Blood Brain Barrier (BBB), ed. G. Fricker, M. Ott and A. Mahringer, 20141071–89, DOI:10.1007/7355_2013_22.
- H. Karanth and M. Rayasa, Nanotechnology in Brain Targeting, Int. J. Pharm. Sci. Nanotechnol., 2008, 1(1), 9–24 CAS.
- A. G. De Boer and P. J. Gaillard, Drug Targeting to the Brain, Annu. Rev. Pharmacol. Toxicol., 2007, 47, 323–355, DOI:10.1146/ANNUREV.PHARMTOX.47.120505.105237.
- A. Jamal, et al., Insights into Infusion-Based Targeted Drug Delivery in the Brain: Perspectives, Challenges and Opportunities, Int. J. Mol. Sci., 2022, 23(6), 3139, DOI:10.3390/ijms23063139.
- K. Zhi, et al., PLGA Nanoparticle-Based Formulations to Cross the Blood – Brain Barrier for Drug Delivery: From R & D to cGMP, Pharmaceutics, 2021, 13(4), 500, DOI:10.3390/pharmaceutics13040500.
- N. Montegiove, E. Calzoni, C. Emiliani and A. Cesaretti, Biopolymer Nanoparticles for Nose-to-Brain Drug Delivery: A New Promising Approach for the Treatment of Neurological Diseases, J. Funct. Biomater., 2022, 13(3), 125, DOI:10.3390/jfb13030125.
- C. Copeland and S. E. Stabenfeldt, Leveraging the Dynamic Blood-Brain Barrier for Central Nervous System Nanoparticle-based Drug Delivery Applications, Curr. Opin. Biomed. Eng., 2020, 14, 1–8, DOI:10.1016/j.cobme.2020.04.001.
- R. A. Razzak and G. J. Florence, Approaches to CNS Drug Delivery with a Focus on Transporter-Mediated Transcytosis, Int. J. Mol. Sci., 2019, 20(12), 3108, DOI:10.3390/ijms20123108.
- R. Awad, A. Avital and A. Sosnik, Polymeric nanocarriers for nose-to-brain drug delivery in neurodegenerative diseases and neurodevelopmental disorders, Acta Pharm. Sin. B, 2022, 13(5), 1866–1886, DOI:10.1016/j.apsb.2022.07.003.
- L. A. Khawli and S. Prabhu, Drug delivery across the blood-brain barrier, Mol. Pharmaceutics, 2013, 10(5), 1471–1472, DOI:10.1021/mp400170b.
- D. Sohel, T. Sultana and H. Kawsar, Largest Obstacle of Drug Delivery to the Blood Brain Barrier and Current Largest Obstacle of Drug Delivery to the Blood Brain Barrier and Current Approach to Solve this Problem: Recent Comprehensive Review, Glob. J. Nanomed., 2018, 4(2), 555–633, DOI:10.19080/GJN.2018.04.555633.
- K. P. Kumar, et al., Role of Lipid Based Nanoparticles in Brain Targeted Drug Delivery System, Int. J. Health Sci., 2022, 6, 2924–2940, DOI:10.53730/ijhs.v6ns4.8628.
- N. J. Abbott, Blood-brain barrier structure and function and the challenges for CNS drug delivery, J. Inherited Metab. Dis., 2013, 36(3), 437–449, DOI:10.1007/s10545-013-9608-0.
- R. D. Betterton, T. P. Davis and P. T. Ronaldson, Organic Cation Transporter (OCT/OCTN) Expression at Brain Barrier Sites: Focus on CNS Drug Delivery, Handb. Exp. Pharmacol., 2021, 266, 301–328, DOI:10.1007/164_2021_448.
- M. N. Lima, R. J. R. X. Freitas, B. A. B. R. Passos, A. M. G. Darze, H. C. Castro-Faria-Neto and T. Maron-Gutierrez, Neurovascular Interactions in Malaria, NeuroImmunoModulation, 2021, 28(3), 108–117, DOI:10.1159/000515557.
- J. F. Dunn and A. M. Isaacs, The impact of hypoxia on blood-brain, blood-CSF, and CSF-brain barriers, J. Appl. Physiol., 2021, 131(3), 977–985, DOI:10.1152/japplphysiol.00108.2020.
- S. Zhang, et al., The barrier and interface mechanisms of the brain barrier, and brain drug delivery, Brain Res. Bull., 2022, 190, 69–83, DOI:10.1016/j.brainresbull.2022.09.017.
- M. Handa, A. Singh, D. Bisht, P. Kesharwani and R. Shukla, Potential of particle size less than 15 nm via olfactory region for direct brain delivery via intranasal route, Health Sci. Rev., 2022, 4, 100038, DOI:10.1016/j.hsr.2022.100038.
- A. Sarma and M. K. Das, Nose to brain delivery of antiretroviral drugs in the treatment of neuroAIDS, Mol. Biomed., 2020, 1(1), 1–23, DOI:10.1186/s43556-020-00019-8.
- L. A. Keller, O. Merkel and A. Popp, Intranasal drug delivery: opportunities and toxicologic challenges during drug development, Drug Delivery Transl. Res., 2022, 12, 735–757, DOI:10.1007/s13346-020-00891-5.
- R. Boyuklieva and B. Pilicheva, Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders, Biomedicines, 2022, 10(7), 1706, DOI:10.3390/biomedicines10071706.
- S. Gänger and K. Schindowski, Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa, Pharmaceutics, 2018, 10(3), 116, DOI:10.3390/PHARMACEUTICS10030116.
- F. Erdő, L. A. Bors, D. Farkas, Á. Bajza and S. Gizurarson, Evaluation of intranasal delivery route of drug administration for brain targeting, Brain Res. Bull., 2018, 143, 155–170, DOI:10.1016/J.BRAINRESBULL.2018.10.009.
- C. P. Costa, J. N. Moreira, J. M. S. Lobo and A. C. Silva, Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies, Acta Pharm. Sin. B, 2021, 11(4), 925–940, DOI:10.1016/j.apsb.2021.02.012.
- J. L. Sobiesk and S. Munakomi, Anatomy, Head and Neck, Nasal Cavity, StatPearls, 2021 Search PubMed.
- R. Cassano, C. Servidio and S. Trombino, Biomaterials for drugs nose–brain transport: A new therapeutic approach for neurological diseases, Materials, 2021, 14(7), 1802, DOI:10.3390/ma14071802.
- E. J. Patharapankal, A. L. Ajiboye, C. Mattern and V. Trivedi, Nose-to-Brain (N2B) Delivery: An Alternative Route for the Delivery of Biologics in the Management and Treatment of Central Nervous System Disorders, Pharmaceutics, 2024, 16(1), 66, DOI:10.3390/pharmaceutics16010066.
- M. Elmowafy and M. M. Al-Sanea, Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies, Saudi Pharm. J., 2021, 29(9), 999–1012, DOI:10.1016/j.jsps.2021.07.015.
- S. S. Hong, K. T. Oh, H. G. Choi and S. J. Lim, Liposomal formulations for nose-to-brain delivery: Recent advances and future perspectives, Pharmaceutics, 2019, 11(10), 1–18, DOI:10.3390/pharmaceutics11100540.
- L. Kozlovskaya, M. Abou-Kaoud and D. Stepensky, Quantitative analysis of drug delivery to the brain via nasal route, J. Controlled Release, 2014, 189, 133–140, DOI:10.1016/j.jconrel.2014.06.053.
- R. S. Hirlekar and A. M. Momin, Advances in Drug Delivery from Nose to Brain: An Overview, Curr. Drug Ther., 2018, 13(1), 4–24, DOI:10.2174/1574885512666170921145204.
- B. A. Witika, et al., Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date, Pharmaceutics, 2022, 14(4), 836, DOI:10.3390/pharmaceutics14040836.
- S. Chattopadhyay, S. Das and K. N. Sarma, Nose-to-brain drug delivery: An update to the alternative path to successful targeted anti-migraine drugs, Int. J. Appl. Pharm., 2021, 13(2), 67–75, DOI:10.22159/ijap.2021v13i2.40404.
- N. J. Johnson, L. R. Hanson and W. H. Frey, Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures, Mol. Pharm., 2010, 7(3), 884–893, DOI:10.1021/mp100029t.
- M. L. Schaefer, B. Böttger, W. L Silver and T. E. Finger, Trigeminal Collaterals in the Nasal Epithelium and Olfactory Bulb: A Potential Route for Direct Modulation of Olfactory Information by Trigeminal Stimuli, J. Comp. Neurol., 2002, 444(3), 221–226, DOI:10.1002/cne.10143.
- Q. Liu and Q. Zhang, Nanoparticle systems for nose-to-brain delivery. In: Brain Targeted Drug Delivery SystemA Focus on Nanotechnology and Nanoparticulates, ed. H. Gao and X. Gao, Elsevier Ltd. 2019, pp. 219-239. DOI:10.1016/B978-0-12-814001-7.00010-X.
- A. Mistry, S. Stolnik and L. Illum, Nanoparticles for direct nose-to-brain delivery of drugs, Int. J. Pharm., 2009, 379(1), 146–157, DOI:10.1016/j.ijpharm.2009.06.019.
- S. Savale and H. Mahajan, Nose to brain a versatile mode of drug delivery system, Asian J. Biomater. Res., 2017, 3(1), 16–38 Search PubMed.
- M. Vohra, M. Amir, A. Sharma and S. Wadhwa, Formulation Strategies for Nose-to-Brain Drug Delivery in Alzheimer’s Disease, Health Sci. Rev., 2023, 6, 100075, DOI:10.1016/j.hsr.2023.100075.
- R. G. Thorne, G. J. Pronk, V. Padmanabhan and W. H. Frey, Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration, Neuroscience, 2004, 127(2), 481–496, DOI:10.1016/j.neuroscience.2004.05.029.
- T. M. Ross, P. M. Martinez, J. C. Renner, R. G. Thorne, L. R. Hanson and W. H. Frey, Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: A non-invasive treatment strategy for multiple sclerosis, J. Neuroimmunol., 2004, 151(1–2), 66–77, DOI:10.1016/j.jneuroim.2004.02.011.
- S. H. Jeong, J. H. Jang and Y. B. Lee, Drug delivery to the brain via the nasal route of administration: exploration of key targets and major consideration factors, J. Pharm. Investig., 2023, 53, 119–152, DOI:10.1007/s40005-022-00589-5.
- D. Lee and T. Minko, Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier, Pharmaceutics, 2021, 13(12), 2049, DOI:10.3390/pharmaceutics13122049.
- M. L. Formica, D. A. Real, M. L. Picchio, E. Catlin, R. F. Donnelly and A. J. Paredes, On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles, Appl. Mater. Today, 2022, 29, 101631, DOI:10.1016/j.apmt.2022.101631.
- S. Bahadur, D. M. Pardhi, J. Rautio, J. M. Rosenholm and K. Pathak, Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders, Pharmaceutics, 2020, 12(12), 1230, DOI:10.3390/pharmaceutics12121230.
- R. Hondadakatti, D. Shinde, H. R. Khandelwal, A. K. Pawar and G. S. Sanap, Intranasal Drug Delivery: Novel Delivery Route for Management of Parkinson’s and Depression Neurological Disorders, Letters in Applied NanoBioScience, 2022, 11(3), 3640–3651, DOI:10.33263/LIANBS113.36403651.
- T. Furubayashi, D. Inoue, S. Kimura, A. Tanaka and T. Sakane, Evaluation of the Pharmacokinetics of Intranasal Drug Delivery for Targeting Cervical Lymph Nodes in Rats, Pharmaceutics, 2021, 13(9), 1363, DOI:10.3390/pharmaceutics13091363.
- S. H. Jeong, J. H. Jang and Y. B. Lee, Drug delivery to the brain via the nasal route of administration: exploration of key targets and major consideration factors, J. Pharm. Investig., 2023, 53, 119–152, DOI:10.1007/s40005-022-00589-5.
- J. N. Norwood, Q. Zhang, D. Card, A. Craine, T. M. Ryan and P. J. Drew, Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate, eLife, 2019, 8, 1–32, DOI:10.7554/eLife.44278.
- Q. Ma, et al., Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain, Acta Neuropathol., 2019, 137(1), 151–165, DOI:10.1007/s00401-018-1916-x.
- J. H. Ahn, et al., Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid, Nature, 2019, 572(7767), 62–66, DOI:10.1038/s41586-019-1419-5.
- A. Sarma and M. K. Das, Nose to brain delivery of antiretroviral drugs in the treatment of neuroAIDS, Mol. Biomed., 2020, 1(1), 1–23, DOI:10.1186/s43556-020-00019-8.
- S. Gizurarson, The Effect of Cilia and the Mucociliary Clearance on Successful Drug Delivery, Biol. Pharm. Bull., 2015, 38(4), 497–506, DOI:10.1248/bpb.b14-00398.
- C. de Barros, I. Portugal, F. Batain, D. Portella, P. Severino, J. Cardoso, P. Arcuri, M. Chaud and T. Alves, Formulation, design and strategies for efficient nanotechnology-based nasal delivery systems, RPS Pharmacy and Pharmacology Reports, 2022, 1(1), 1–23, DOI:10.1093/rpsppr/rqac003.
- M. Pandey, N. Jain, J. Kanoujia, Z. Hussain and B. Gorain, Advances and Challenges in Intranasal Delivery of Antipsychotic Agents Targeting the Central Nervous System, Front. Pharmacol., 2022, 13, 1–14, DOI:10.3389/fphar.2022.865590.
- L. Illum, Nasal drug delivery: new developments and strategies, Drug Discovery Today, 2002, 7(23), 1184–1189, DOI:10.1016/s1359-6446(02)02529-1.
- Y. Feng, H. He, F. Li, Y. Lu, J. Qi and W. Wu, An update on the role of nanovehicles in nose-to-brain drug delivery, Drug Discovery Today, 2018, 23(5), 1079–1088, DOI:10.1016/j.drudis.2018.01.005.
- B. Hemalatha, M. Kalpana, B. S. Rekha, A. Varalakshmi and K. Padmalatha, An Overview on Nasal Drug Delivery System, Asian J. Pharm. Res., 2022, 249–258, DOI:10.52711/2231-5691.2022.00041.
- M. Agrawal, S. Saraf, S. Saraf, S. K. Dubey, A. Puri and U. Gupta, et al., Stimuli-responsive In situ gelling system for nose-to-brain drug delivery, J. Controlled Release, 2020, 327, 235–265, DOI:10.1016/j.jconrel.2020.07.044.
- A. K. Singh, A. Singh and N. V. S. Madhv, Nasal Cavity, a Promising Transmucosal Platform for Drug Delivery and Research Approaches From Nasal To Brain Targetting, J. Drug Delivery Ther., 2012, 2(3), 22–33, DOI:10.22270/jddt.v2i3.163.
- R. C. Dhakar, S. D. Maurya, V. K. Tilak and A. K. Gupta, A review on factors affecting the design of nasal drug delivery system, Int. J. Drug Delivery, 2011, 3, 194–208 CAS.
- A. Misra and G. Kher, Drug delivery systems from nose to brain, Curr. Pharm. Biotechnol., 2012, 13(12), 2355–2379, DOI:10.2174/138920112803341752.
- A. Lofts, F. Abu-Hijleh, N. Rigg, R. K. Mishra and T. Hoare, Using the Intranasal Route to Administer Drugs to Treat Neurological and Psychiatric Illnesses: Rationale, Successes, and Future Needs, CNS Drugs, 2022, 36, 739–770, DOI:10.1007/s40263-022-00930-4.
- A. Khosa, S. Reddi and R. N. Saha, Nanostructured lipid carriers for site-specific drug delivery, Biomed. Pharmacother., 2018, 103, 598–613, DOI:10.1016/j.biopha.2018.04.055.
- A. Sharma and A. Baldi, Nanostructured Lipid Carriers: A Review, J. Dev. Drugs, 2018, 7(1), 1–12 Search PubMed.
- S. Javed, B. Mangla, Y. Almoshari, M. H. Sultan and W. Ahsan, Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery, Nanotechnol. Rev., 2022, 11(1), 1744–1777, DOI:10.1515/ntrev-2022-0109.
- M. Elmowafy and M. M. Al-Sanea, Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies, Saudi Pharm. J., 2021, 29(9), 999–1012, DOI:10.1016/j.jsps.2021.07.015.
- P. Jaiswal, B. Gidwani and A. Vyas, Nanostructured lipid carriers and their current application in targeted drug delivery, Artif. Cells, Nanomed., Biotechnol., 2016, 44(1), 27–40, DOI:10.3109/21691401.2014.909822.
- A. S. Bornare and R. B. Saudagar, Nanostructured lipid carrier (NLC): A modern approach for transdermal drug delivery, Res. J. Pharm. Technol., 2017, 10(8), 2784–2792, DOI:10.5958/0974-360X.2017.00493.0.
- D. Lee and T. Minko, Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier, Pharmaceutics, 2021, 13(12), 2049, DOI:10.3390/pharmaceutics13122049.
- M. Agrawal, et al., Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting, J. Controlled Release, 2020, 321, 372–415, DOI:10.1016/j.jconrel.2020.02.020.
- E. B. Souto, et al., Physicochemical and biopharmaceutical aspects in fl uencing skin permeation and role of SLN and NLC for skin drug delivery, Heliyon, 2022, 8(2), e08938, DOI:10.1016/j.heliyon.2022.e08938.
- I. Chauhan, M. Yasir, M. Verma and A. P. Singh, Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery, Adv. Pharm. Bull., 2020, 10(2), 150–165, DOI:10.34172/apb.2020.021.
- C. Puglia and F. Bonina, Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals, Expert Opin. Drug Delivery, 2012, 9(4), 429–441, DOI:10.1517/17425247.2012.666967.
- D. Eldridge, Production Techniques, In ASTM Manual on Zirconium and Hafnium, ed. J. H. Schemel, 2009, DOI:10.1520/stp28002s.
- M. Elmowafy and M. M. Al-Sanea, Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies, Saudi Pharm. J., 2021, 29(9), 999–1012, DOI:10.1016/j.jsps.2021.07.015.
- S. Selvamuthukumar and R. Velmurugan, Nanostructured Lipid Carriers: A potential drug carrier for cancer chemotherapy, Lipids Health Dis., 2012, 11(1), 159, DOI:10.1186/1476-511X-11-159.
- N. B. Ndemazie, et al., Multi-disciplinary Approach for Drug and Gene Delivery Systems to the Brain, AAPS PharmSciTech, 2022, 23(1), 1–21, DOI:10.1208/s12249-021-02144-1.
- F. Han, S. Li, R. Yin, H. Liu and L. Xu, Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: Nanostructured lipid carriers, Colloids Surf., A, 2008, 315(1–3), 210–216, DOI:10.1016/j.colsurfa.2007.08.005.
- V. A. Duong, T. T. L. Nguyen and H. J. Maeng, Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method, Molecules, 2020, 25(20), 1–36, DOI:10.3390/molecules25204781.
- I. Chauhan, M. Yasir, M. Verma and A. P. Singh, Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery, Adv. Pharm. Bull., 2020, 10(2), 150–165, DOI:10.34172/apb.2020.021.
- K. Karn-Orachai, et al., The effect of surfactant composition on the chemical and structural properties of nanostructured lipid carriers, J. Microencapsulation, 2014, 31(6), 609–618, DOI:10.3109/02652048.2014.911374.
- F. Han, S. Li, R. Yin, H. Liu and L. Xu, Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: Nanostructured lipid carriers, Colloids Surf., A, 2008, 315(1–3), 210–216, DOI:10.1016/j.colsurfa.2007.08.005.
- K. V. Chandana, N. Vishal Gupta and S. Kanna, Nanostructured Lipid Carriers: the Frontiers in Drug Delivery, Asian J. Pharm. Clin. Res., 2019, 12, 8–12, DOI:10.22159/ajpcr.2019.v12i7.33595.
- G. Rajalakshmi, C. K. Dhanapal and R. Sundhararajan, An Insight to Nanostructured Lipid Carrier System, J. Drug Delivery Ther., 2020, 10(6 s), 173–182, DOI:10.22270/jddt.v10i6-s.4589.
- K. V. M. Surya Tej, et al., Nano structured lipid carrier based drug delivery system, J. Chem. Pharm. Res., 2016, 8(2), 627–643 Search PubMed.
- D. Eldridge, Production Techniques, In ASTM Manual on Zirconium and Hafnium, ed. J. H. Schemel, 2009, DOI:10.1520/stp28002s.
- M. Üner and G. Yener, Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspective, Int. J. Nanomed., 2007, 2(3), 289–300 Search PubMed.
- R. K. Shirodkar, L. Kumar, S. Mutalik and S. Lewis, Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Emerging Lipid Based Drug Delivery Systems, Pharm. Chem. J., 2019, 53(5), 440–453, DOI:10.1007/s11094-019-02017-9.
- J. S. Negi, P. Chattopadhyay, A. K. Sharma and V. Ram, Development of solid lipid nanoparticles (SLNs) of lopinavir using hot self nano-emulsification (SNE) technique, Eur. J. Pharm. Sci., 2013, 48(1–2), 231–239, DOI:10.1016/j.ejps.2012.10.022.
- V. Saez, I. D. L. Souza and C. R. E. Mansur, Lipid nanoparticles (SLN & NLC) for delivery of vitamin E: a comprehensive review, Int. J. Cosmet. Sci., 2018, 40(2), 103–116, DOI:10.1111/ics.12452.
- K. Manjunath and V. Venkateswarlu, Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration, J. Controlled Release, 2005, 107(2), 215–228, DOI:10.1016/j.jconrel.2005.06.006.
- D. Psimadas, P. Georgoulias, V. Valotassiou and G. Loudos, Molecular Nanomedicine Towards Cancer, J. Pharm. Sci., 2012, 101(7), 2271–2280, DOI:10.1002/jps.
- M. Muchow, P. Maincent, R. H. Mller and C. M. Keck, Production and characterization of testosterone undecanoate-loaded NLC for oral bioavailability enhancement, Drug Dev. Ind. Pharm., 2011, 37(1), 8–14, DOI:10.3109/03639045.2010.489559.
- J. Pardeike, et al., Development of an Itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application, Int. J. Pharm., 2011, 419(1–2), 329–338, DOI:10.1016/j.ijpharm.2011.07.040.
- J. Y. Fang, C. L. Fang, C. H. Liu and Y. H. Su, Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC), Eur. J. Pharm. Biopharm., 2008, 70(2), 633–640, DOI:10.1016/j.ejpb.2008.05.008.
- M. Sun, S. Nie, X. Pan, R. Zhang, Z. Fan and S. Wang, Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro, Colloids Surf., B, 2014, 113, 15–24, DOI:10.1016/j.colsurfb.2013.08.032.
- M. Elmowafy, et al., Enhancement of Bioavailability and Pharmacodynamic Effects of Thymoquinone Via Nanostructured Lipid Carrier (NLC) Formulation, AAPS PharmSciTech, 2016, 17(3), 663–672, DOI:10.1208/s12249-015-0391-0.
- S. Khan, M. Shaharyar, M. Fazil, M. Q. Hassan, S. Baboota and J. Ali, Tacrolimus-loaded nanostructured lipid carriers for oral delivery-in vivo bioavailability enhancement, Eur. J. Pharm. Biopharm., 2016, 109, 149–157, DOI:10.1016/j.ejpb.2016.10.011.
- N. V. Shah, A. K. Seth, R. Balaraman, C. J. Aundhia, R. A. Maheshwari and G. R. Parmar, Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: Design and in vivo study, J. Adv. Res., 2016, 7(3), 423–434, DOI:10.1016/j.jare.2016.03.002.
- I. Lacatusu, N. Badea, A. Murariu, O. Oprea, D. Bojin and A. Meghea, Antioxidant activity of solid lipid nanoparticles loaded with umbelliferone, Soft Mater., 2013, 11(1), 75–84, DOI:10.1080/1539445X.2011.582914.
- S. M. Martins, B. Sarmento, C. Nunes, M. Lúcio, S. Reis and D. C. Ferreira, Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration, Eur. J. Pharm. Biopharm., 2013, 85(3 PART A), 488–502, DOI:10.1016/j.ejpb.2013.08.011.
- J. Akbari, et al., The design of naproxen solid lipid nanoparticles to target skin layers, Colloids Surf., B, 2016, 145, 626–633, DOI:10.1016/j.colsurfb.2016.05.064.
- J. Akbari, et al., The design of naproxen solid lipid nanoparticles to target skin layers, Colloids Surf., B, 2016, 145, 626–633, DOI:10.1016/j.colsurfb.2016.05.064.
- J. Y. Fang, C. L. Fang, C. H. Liu and Y. H. Su, Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC), Eur. J. Pharm. Biopharm., 2008, 70(2), 633–640, DOI:10.1016/j.ejpb.2008.05.008.
- A. Narala and K. Veerabrahma, Preparation, Characterization and Evaluation of Quetiapine Fumarate Solid Lipid Nanoparticles to Improve the Oral Bioavailability, J. Pharm., 2013, 1–7, DOI:10.1155/2013/265741.
- T. H. Tran, et al., Hyaluronic acid-coated solid lipid nanoparticles for targeted delivery of vorinostat to CD44 overexpressing cancer cells, Carbohydr. Polym., 2014, 114, 407–415, DOI:10.1016/j.carbpol.2014.08.026.
- T. H. Tran, et al., Formulation and optimization of raloxifene-loaded solid lipid nanoparticles to enhance oral bioavailability, J. Nanosci. Nanotechnol., 2014, 14(7), 4820–4831, DOI:10.1166/jnn.2014.8722.
- H. Bunjes, K. Westesen and M. H. J. Koch, Crystallization tendency and polymorphic transitions in triglyceride nanoparticles, Int. J. Pharm., 1996, 129(1–2), 159–173, DOI:10.1016/0378-5173(95)04286-5.
- A. Sable, T. Shangrapawar and A. Bhosale, Nanostructured lipid carrier: an advancement in topical drug delivery system, World J. Pharm. Res., 2020, 9(7), 1221–1239 CAS.
- K. Bhise, S. K. Kashaw, S. Sau and A. K. Iyer, Nanostructured Lipid Carriers Employing Polyphenols as Promising Anticancer Agents: Quality by Design (QbD) approach, Int. J. Pharm., 2017, 526(1–2), 506–515, DOI:10.1016/j.ijpharm.2017.04.078.
- P. K. Reddy A, A. Kumar and B. B. Rao P, Carrier Systems to Pass Through Bbb-A Boon to Treat Multiple Sclerosis, J. Drug Alcohol Res., 2021, 9(1), 236154 Search PubMed.
- A. R. Tekade, P. S. Mittha and C. S. Pisal, Nanostructured Lipid Carriers for Nose to Brain Delivery Targeting CNS: Diversified Role of Liquid Lipids for Synergistic Action, Adv. Pharm. Bull., 2022, 12(4), 763–771, DOI:10.34172/apb.2022.078.
- B. Gaba, M. Fazil, A. Ali, S. Baboota, J. K. Sahni and J. Ali, Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration, Drug Delivery, 2015, 22(6), 691–700, DOI:10.3109/10717544.2014.898110.
- G. Sharma, K. Thakur, K. Raza, B. Singh and O. P. Katare, Nanostructured lipid carriers: A new paradigm in topical delivery for dermal and transdermal applications, Crit. Rev. Ther. Drug Carrier Syst., 2017, 34(4), 355–386, DOI:10.1615/CritRevTherDrugCarrierSyst.2017019047.
- B. Gaba, M. Fazil, A. Ali, S. Baboota, J. K. Sahni and J. Ali, Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration, Drug Delivery, 2015, 22(6), 691–700, DOI:10.3109/10717544.2014.898110.
- L. Battaglia and E. Ugazio, Lipid nano- and microparticles: An overview of patent-related research, J. Nanomater., 2019, 834941, DOI:10.1155/2019/2834941.
- G. Sharma, K. Thakur, K. Raza, B. Singh and O. P. Katare, Nanostructured lipid carriers: A new paradigm in topical delivery for dermal and transdermal applications, Crit. Rev. Ther. Drug Carrier Syst., 2017, 34(4), 355–386, DOI:10.1615/CritRevTherDrugCarrierSyst.2017019047.
- S. Khan, S. Sharma and V. Jain, An overview of Nanostructured Lipid Carriers and its application in drug delivery through different routes, Adv. Pharm. Bull., 2023, 13(3), 446–460, DOI:10.34172/apb.2023.056.
- S. Lade, N. Shah and S. Burle, Nanostructured Lipid Carriers: A Vital Drug Carrier for Migraine Treatment, Res. J. Pharm. Technol., 2022, 15(7), 3309–3316, DOI:10.52711/0974-360X.2022.00554.
- A. Khosa, S. Reddi and R. N. Saha, Nanostructured lipid carriers for site-specific drug delivery, Biomed. Pharm., 2018, 103, 598–613, DOI:10.1016/j.biopha.2018.04.055.
- D. H. Hassan, J. N. Shohdy, M. A. El-Nabarawi, D. A. El-Setouhy and M. M. Abdellatif, Nanostructured Lipid Carriers for Transdermal Drug Delivery, Int. J. Appl. Pharm., 2022, 14(4), 88–93, DOI:10.22159/ijap.2022v14i4.44564.
- O. Gartziandia, E. Herran, J. L. Pedraz, E. Carro, M. Igartua and R. M. Hernandez, Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration, Colloids Surf., B, 2015, 134, 304–313, DOI:10.1016/j.colsurfb.2015.06.054.
- T. B. Devkar, A. R. Tekade and K. R. Khandelwal, Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using Delonix regia gum as a natural mucoadhesive polymer, Colloids Surf., B, 2014, 122, 143–150, DOI:10.1016/j.colsurfb.2014.06.037.
- Y. Z. Zhao, et al., Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats, Nanomedicine, 2014, 10(4), 755–764, DOI:10.1016/j.nano.2013.10.009.
- T. Kanazawa, H. Taki, K. Tanaka, Y. Takashima and H. Okada, Cell-penetrating peptide-modified block copolymer micelles promote direct brain delivery via intranasal administration, Pharm. Res., 2011, 28(9), 2130–2139, DOI:10.1007/s11095-011-0440-7.
- O. Gartziandia, et al., Nanoparticle transport across in vitro olfactory cell monolayers, Int. J. Pharm., 2016, 499(1–2), 81–89, DOI:10.1016/j.ijpharm.2015.12.046.
- E. Ahmad, et al., Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles, Nanoscale, 2017, 9(3), 1174–1183, 10.1039/c6nr07581a.
- C. C. Bi, et al., Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson's disease treatment, Int. J. Nanomed., 2016, 11, 6547–6559, DOI:10.2147/IJN.S120939.
- E. B. Souto, et al., Nanopharmaceutics: Part II—Production Scales and Clinically Compliant Production Methods, Nanomaterials, 2020, 10(3), 455, DOI:10.3390/nano10030455.
- M. K. Katual, G. Singh and S. L. Harikumar, Novel frontiers in intranasal drug delivery of nanocarriers for Parkinson’s treatment: A recent update, Pharmaspire, 2019, 11(1), 1–9 RSC.
- P. Jaiswal, B. Gidwani and A. Vyas, Nanostructured lipid carriers and their current application in targeted drug delivery, Artif. Cells, Nanomed., Biotechnol., 2016, 44(1), 27–40, DOI:10.3109/21691401.2014.909822.
- V. R. Salvi and P. Pawar, Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier, J. Drug Delivery Sci. Technol., 2019, 51, 255–267, DOI:10.1016/J.JDDST.2019.02.017.
- C. H. S. Kumari, et al., Formulation techniques of lipid based nanoparticles: SLN/NLCS, its evaluation and applications, Int. J. Res. Pharm. Chem., 2020, 10(1), 111–124, DOI:10.33289/IJRPC.10.1.2020.10(26).
- M. Üner, Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems, Pharmazie, 2006, 61(5), 375–386 Search PubMed.
- V. A. Duong, T. T. L. Nguyen and H. J. Maeng, Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method, Molecules, 2020, 25(20), 1–36, DOI:10.3390/molecules25204781.
- J. Kaur, G. Singh, S. Saini and A. C. Rana, Innovative Growth in Developing New Methods for Formulating Solid Lipid Nanoparticles and Microparticles, J. Drug Delivery Ther., 2012, 2(5), 146–150, DOI:10.22270/jddt.v2i5.295.
- A. M. Bhagurkar, M. A. Repka and S. N. Murthy, A Novel Approach for the Development of a Nanostructured Lipid Carrier Formulation by Hot-Melt Extrusion Technology, J. Pharm. Sci., 2017, 106(4), 1085–1091, DOI:10.1016/j.xphs.2016.12.015.
- S. Khan, S. Sharma and V. Jain, An overview of Nanostructured Lipid Carriers and its application in drug delivery through different routes, Adv. Pharm. Bull., 2023, 13(3), 446–460, DOI:10.34172/apb.2023.056.
- G. Shadambikar, et al., Formulation development of itraconazole PEGylated nano-lipid carriers for pulmonary aspergillosis using hot-melt extrusion technology, Int. J. Pharm.: X, 2021, 3, 100074, DOI:10.1016/j.ijpx.2021.100074.
- D. Muhindo, E. A. Ashour, M. Almutairi, P. H. Joshi and M. A. Repka, Continuous production of raloxifene hydrochloride loaded nanostructured lipid carriers using hot-melt extrusion technology, J. Drug Delivery Sci. Technol., 2021, 65, 102673, DOI:10.1016/j.jddst.2021.102673.
- G. V. Gajanan, G. M. Milind and Y. Adhikrao, Different techniques for preparation of nanoemulsion with characterisation and various application of IT - a review, World J. Pharm. Res., 2017, 6(15), 112–128 CAS.
- Ş. Dima, C. Dima and G. Iordăchescu, Encapsulation of Functional Lipophilic Food and Drug Biocomponents, Food Eng. Rev., 2015, 7(4), 417–438, DOI:10.1007/s12393-015-9115-1.
- J. Pardeike, A. Hommoss and R. H, Müller, Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products, Int. J. Pharm., 2009, 366(1–2), 170–184, DOI:10.1016/j.ijpharm.2008.10.003.
- C. P. Costa, J. N. Moreira, J. M. S. Lobo and A. C. Silva, Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies, Acta Pharm. Sin. B, 2021, 11(4), 925–940, DOI:10.1016/j.apsb.2021.02.012.
- F. Tamjidi, M. Shahedi, J. Varshosaz and A. Nasirpour, Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules, Innovative Food Sci. Emerging Technol., 2013, 19, 29–43, DOI:10.1016/J.IFSET.2013.03.002.
- B. Subramaniam, Z. H. Siddik and N. H. Nagoor, Optimization of nanostructured lipid carriers: understanding the types, designs, and parameters in the process of formulations, J. Nanopart. Res., 2020, 22(6), 141, DOI:10.1007/s11051-020-04848-0.
- J. Natarajan, V. V. S. R. Karri and D. Anindita, Nanostructured Lipid Carrier (NLC): A Promising Drug Delivery System, Global J. Nanomed., 2017, 1(5), 555575, DOI:10.19080/gjn.2017.01.555575.
- P. C. Pires and A. O. Santos, Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies, J. Controlled Release, 2018, 270, 89–100, DOI:10.1016/J.JCONREL.2017.11.047.
- A. Czajkowska-Kośnik, M. Szekalska and K. Winnicka, Nanostructured lipid carriers: A potential use for skin drug delivery systems, Pharmacol. Rep., 2019, 71(1), 156–166, DOI:10.1016/j.pharep.2018.10.008.
- R. Arora, S. S. Katiyar, V. Kushwah and S. Jain, Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: a comparative study, Expert Opin. Drug Delivery, 2017, 14(2), 165–177, DOI:10.1080/17425247.2017.1264386.
- V. Saez, I. D. L. Souza and C. R. E. Mansur, Lipid nanoparticles (SLN & NLC) for delivery of vitamin E: a comprehensive review, Int. J. Cosmet. Sci., 2018, 40(2), 103–116, DOI:10.1111/ics.12452.
- S. Das and A. Chaudhury, Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery, AAPS PharmSciTech, 2011, 12(1), 62–76, DOI:10.1208/s12249-010-9563-0.
- A. A. Attama, SLN, NLC, LDC: State of the Art in Drug and Active Delivery, Recent Pat. Drug Delivery Formulation, 2011, 5(3), 178–187, DOI:10.2174/187221111797200524.
- M. A. Iqbal, S. Md, J. K. Sahni, S. Baboota, S. Dang and J. Ali, Nanostructured lipid carriers system: Recent advances in drug delivery, J. Drug Targeting, 2012, 20(10), 813–830, DOI:10.3109/1061186X.2012.716845.
- N. Kathe, B. Henriksen and H. Chauhan, Physicochemical characterization techniques for solid lipid nanoparticles: Principles and limitations, Drug Dev. Ind. Pharm., 2014, 40(12), 1565–1575, DOI:10.3109/03639045.2014.909840.
- H. Mu and R. Holm, Solid lipid nanocarriers in drug delivery: characterization and design, Expert Opin. Drug Delivery, 2018, 15(8), 771–785, DOI:10.1080/17425247.2018.1504018.
- S. C. Arora, Chahat and A. Kush, Nanostructured Lipid Carriers: An Excellent Tool for Drug Delivery, IOSR J. Pharm. Biol. Sci., 2020, 15(5), 45–52 Search PubMed.
- M. Üner, Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems, Pharmazie, 2006, 61(5), 375–386 Search PubMed.
- L. Battaglia and M. Gallarate, Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery, Expert Opin. Drug Delivery, 2012, 9(5), 497–508, DOI:10.1517/17425247.2012.673278.
- A. Gordillo-Galeano and C. E. Mora-Huertas, Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release, Eur. J. Pharm. Biopharm., 2018, 133, 285–308, DOI:10.1016/j.ejpb.2018.10.017.
- M. Elmowafy, K. Shalaby, M. M. Badran, H. M. Ali, M. S. Abdel-Bakky and H. M. Ibrahim, Multifunctional carbamazepine loaded nanostructured lipid carrier (NLC) formulation, Int. J. Pharm., 2018, 550(1–2), 359–371, DOI:10.1016/j.ijpharm.2018.08.062.
- A. Anand, M. Arya, G. Kaithwas, G. Singh and S. A. Saraf, Sucrose stearate as a biosurfactant for development of rivastigmine containing nanostructured lipid carriers and assessment of its activity against dementia in C. elegans model, J. Drug Delivery Sci. Technol., 2019, 49, 219–226, DOI:10.1016/j.jddst.2018.11.021.
- S. Cunha, et al., Double optimization of rivastigmine-loaded nanostructured lipid carriers (NLC) for nose-to-brain delivery using the quality by design (QbD) approach: Formulation variables and instrumental parameters, Pharmaceutics, 2020, 12(7), 1–27, DOI:10.3390/pharmaceutics12070599.
- P. R. Wavikar and P. R. Vavia, Rivastigmine-loaded in situ gelling nanostructured lipid carriers for nose to brain delivery, J. Liposome Res., 2015, 25(2), 141–149, DOI:10.3109/08982104.2014.954129.
- S. Cunha, et al., Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: characterisation, biocompatibility, and drug deposition studies, Int. J. Pharm., 2022, 620, 121720, DOI:10.1016/J.IJPHARM.2022.121720.
- G. M. Jojo, G. Kuppusamy, A. De and V. V. S. Narayan Reddy Karri, Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design, Drug Dev. Ind. Pharm., 2019, 45(7), 1061–1072, DOI:10.1080/03639045.2019.1593439.
- A. P. Rajput and S. B. Butani, Resveratrol anchored nanostructured lipid carrier loaded in situ gel via nasal route: Formulation, optimization and in vivo characterization, J. Drug Delivery Sci. Technol., 2019, 51, 214–223, DOI:10.1016/j.jddst.2019.01.040.
- T. Alam, J. Pandit, D. Vohora, M. Aqil and A. Ali, Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: in vitro characterization and in vivo efficacy in epilepsy, Expert Opin. Drug Delivery, 2014, 12(2), 181–194, DOI:10.1517/17425247.2014.945416.
- S. C. Nair, K. P. Vinayan and S. Mangalathillam, Nose to Brain Delivery of Phenytoin Sodium Loaded Nano Lipid Carriers: Formulation, Drug Release, Permeation and In Vivo Pharmacokinetic Studies, Pharmaceutics, 2021, 13(10), 1640, DOI:10.3390/pharmaceutics13101640.
- N. Khan, et al., Nanostructured lipid carriers-mediated brain delivery of carbamazepine for improved in vivo anticonvulsant and anxiolytic activity, Int. J. Pharm., 2020, 577, 119033, DOI:10.1016/j.ijpharm.2020.119033.
- F. F. Qizilbash, et al., Thymoquinone-Enriched Naringenin-Loaded Nanostructured Lipid Carrier for Brain Delivery via Nasal Route: In Vitro Prospect and In Vivo Therapeutic Efficacy for the Treatment of Depression, Pharmaceutics, 2022, 14(3), 656, DOI:10.3390/pharmaceutics14030656.
- U. A. Fahmy, et al., Optimized Nanostructured Lipid Carriers Integrated into In Situ Nasal Gel for Enhancing Brain Delivery of Flibanserin, Int. J. Nanomedicine, 2020, 5253–5264, DOI:10.2147/IJN.S258791.
- E. Taha, et al., Cod liver oil nano-structured lipid carriers (Cod-NLCs) as a promising platform for nose to brain delivery: Preparation, in vitro optimization, ex vivo cytotoxicity & in vivo biodistribution utilizing radioiodinated zopiclone, Int. J. Pharm.: X, 2023, 5, 100160, DOI:10.1016/J.IJPX.2023.100160.
- K. M. Noorulla, et al., Intranasal delivery of chitosan decorated nanostructured lipid carriers of Buspirone for brain targeting: Formulation development, optimization and In vivo preclinical evaluation, J. Drug Delivery Sci. Technol., 2022, 67, 102939, DOI:10.1016/J.JDDST.2021.102939.
- M. Gul, et al., Formulation optimization, in vitro and in vivo evaluation of agomelatine-loaded nanostructured lipid carriers for augmented antidepressant effects, Colloids Surf., B, 2022, 216, 112537, DOI:10.1016/J.COLSURFB.2022.112537.
- M. Yasir, et al., Nose to brain delivery of donepezil through surface modified NLCs: Formulation development, optimization, and brain targeting study, J. Drug Delivery Sci. Technol., 2022, 75, 103631, DOI:10.1016/J.JDDST.2022.103631.
- A. Zafar, et al., Formulation of intranasal surface engineered nanostructured lipid carriers of rotigotine: Full factorial design optimization, in vitro characterization, and pharmacokinetic evaluation, Int. J. Pharm., 2022, 627, 122232, DOI:10.1016/J.IJPHARM.2022.122232.
- S. E. Leucuta, Nanotechnology for Delivery of Drugs and Biomedical Applications, Curr. Clin. Pharmacol., 2010, 5(4), 257–280, DOI:10.2174/157488410793352003.
- F. Sabir, R. Ismail and I. Csoka, Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: status quo and outlook, Drug Discovery Today, 2020, 25(1), 185–194, DOI:10.1016/j.drudis.2019.10.005.
- E. Samaridou and M. J. Alonso, Nose-to-brain peptide delivery – the potential of nanotechnology, Bioorg. Med. Chem., 2018, 26(10), 2888–2905, DOI:10.1016/j.bmc.2017.11.001.
- S. Javed, B. Mangla, Y. Almoshari, M. H. Sultan and W. Ahsan, Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery, Nanotechnol. Rev., 2022, 11(1), 1744–1777, DOI:10.1515/ntrev-2022-0109.
- J. Garg, K. Pathania, S. P. Sah and S. V. Pawar, Nanostructured lipid carriers: a promising drug carrier for targeting brain tumours, Future J. Pharm. Sci., 2022, 8, 1–31, DOI:10.1186/s43094-022-00414-8.
- L. Sahoo, C. S. Patro, G. K. Jena, Ch. N. Patro and S. Satapathy, A Focus on Fabrication, Characterization, Stability, Skin Targeting, Patent, Safety and Toxicity of Nanostructured Lipid Carrier, J. Pharm. Res. Int., 2022, 34, 49–76, DOI:10.9734/jpri/2022/v34i17b35771.
- E. Trenkenschuh and W. Friess, Freeze-drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization, Eur. J. Pharm. Biopharm., 2021, 165, 345–360, DOI:10.1016/j.ejpb.2021.05.024.
- A. Gordillo-Galeano and C. E. Mora-Huertas, Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release, Eur. J. Pharm. Biopharm., 2018, 133, 285–308, DOI:10.1016/j.ejpb.2018.10.017.
- R. H. Müller, M. Radtke and S. A. Wissing, Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations, Adv. Drug Delivery Rev., 2002, 54(Suppl.), S131–S155, DOI:10.1016/S0169-409X(02)00118-7.
- R. H. Muller, R. Shegokar and C. M. Keck, 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications, Curr. Drug Discovery Technol., 2011, 8(3), 207–227, DOI:10.2174/157016311796799062.
- M. Muchow, P. Maincent and R. H. Müller, Lipid nanoparticles with a solid matrix (SLN®, NLC®, LDC®) for oral drug delivery, Drug Dev. Ind. Pharm., 2008, 34(12), 1394–1405, DOI:10.1080/03639040802130061.
- N. A. Emad, B. Ahmed, A. Alhalmi, N. Alzobaidi and S. S. Al-Kubati, Recent progress in nanocarriers for direct nose to brain drug delivery, J. Drug Delivery Sci. Technol., 2021, 64, 102642, DOI:10.1016/j.jddst.2021.102642.
- N. Dhiman, R. Awasthi, B. Sharma, H. Kharkwal and G. T. Kulkarni, Lipid Nanoparticles as Carriers for Bioactive Delivery, Front. Chem., 2021, 9, 580118, DOI:10.3389/fchem.2021.580118.
- D. G. Gadhave, A. A. Tagalpallewar and C. R. Kokare, Agranulocytosis-Protective Olanzapine-Loaded Nanostructured Lipid Carriers Engineered for CNS Delivery: Optimization and Hematological Toxicity Studies, AAPS PharmSciTech, 2019, 20, 22, DOI:10.1208/s12249-018-1213-y.
- D. Zhang, L. Jia, H. Cheng, C. Duan, Y. Wang and F. Feng, Silybin nanostructured lipid carrier and preparation method thereof, CN101632638B, 2009.
- N. Araci Bou Chacra, P. Cesar Cotrim, L. Marie Monteiro, N. Fotaki and R. Löbenberg, Method for producing nanostructured lipid carriers on triblock copolymers, nanostructured lipid carriers thereby produced, and uses thereof, WO2019068161A1, 2017.
- Q. Xia and J. Wu, Azithromycin nanostructured lipid carrier and preparation method thereof, CN101658493B, 2009.
- A-Q. Xia and H. Wang, Coenzyme Q nanostructured lipid carrier and preparation method thereof – google patents, CN101658468B, 2009.
- Y. Zijun, Z. Liangming, C. Tong, W. Xiaoping and Y. Kun, Tumor-targeting norcantharidin nanostructured lipid carrier and preparation method thereof, CN115054578A, 2022.
- N. M. P. S. Ricardo, T. G. De Araujo and B. O. Louchard, Solid lipid composition for production of nanostructured lipid carrier, BR102019013856A2, 2019.
| This journal is © The Royal Society of Chemistry 2024 |