Open Access Article
Open Access ArticleEvaluation of the anti-depressant potential of EGCG-loaded nanoparticles in unstressed and stressed mice
Shakti
Dahiya
 *ab,
Ruma
Rani
a,
Neeraj
Dilbaghi
a,
Dinesh
Dhingra
c,
Sant
Lal
a and
Jaya
Verma
*ab,
Ruma
Rani
a,
Neeraj
Dilbaghi
a,
Dinesh
Dhingra
c,
Sant
Lal
a and
Jaya
Verma
 *d
*d
aDepartment of Bio and Nano Technology, Guru Jambheshwar University of Science & Technology, Hisar-125001, India.
bDepartment of Surgery, Division of Pediatric General and Thoracic Surgery, Children's Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA 15244, USA. E-mail: shd156@pitt.edu
cDepartment of Pharmaceutical Sciences, Guru Jambheshwar University of Science & Technology, Hisar-125001, India
dCenter for Precision Engineering, Harbin Institute of Technology, Harbin, 150001, Heilongjiang, China. E-mail: jverma@hit.edu.cn
First published on 29th April 2024
Abstract
Epigallocatechin-3-gallate (EGCG) is a key bio-active component of green tea and has demonstrated significant antidepressant activity in laboratory animals. Nano-particulate drug delivery offers great potential to overcome drawbacks associated with EGCG i.e. its low solubility and stability by transforming it into effective deliverable drugs. In the current study, nano-formulations of EGCG alone and with piperine were synthesized using antisolvent precipitation methodology followed by evaluation of their in vivo antidepressant effect in unstressed and stressed Swiss male albino mice. The mice were exposed to distinct stressors i.e. tail pinch, induction of immobilization, etc. throughout a span of three weeks. Zein, a protein nanocarrier, was nano-encapsulated with EGCG (25 mg) and EGCG + piperine (25 mg + 5 mg). For a continuous three weeks, the mice were administered EGCG-loaded nanosuspensions (25 mg kg−1) and EGCG–piperine nanocomplexes (25 mg kg−1). To determine the impact of various medication treatments on stressed and unstressed mice, the tail suspension test (TST) was employed as a behavioural paradigm. Mice exposed to various drug treatments were also evaluated for the effect on locomotor activity. The animals were euthanized followed by further estimation of plasma corticosterone, plasma nitrite, brain malondialdehyde, brain MAO-A, brain reduced glutathione, and brain catalase levels. The EGCG–piperine nanocomplex (25 mg kg−1) and paroxetine HCl (10 mg kg−1) per se significantly reduced immobility time in unstressed and stressed mice as compared to their respective control groups treated with a vehicle. However, in the case of locomotor activity, no significant effect was observed. EGCG loaded nanosuspension, EGCG–piperine nanocomplex and paroxetine HCl significantly decreased plasma nitrite, and brain MAO-A, brain malondialdehyde and brain catalase levels. However, these drug treatments significantly increased plasma corticosterone and brain reduced glutathione levels in unstressed and stressed mice as compared to their respective control groups treated with a vehicle. So, the intraperitoneal administration of nanoformulations synthesized using EGCG alone and along with piperine significantly improves the antidepressant-like behavior in mice.
1. Introduction
Depression, a debilitating psychiatric illness, inflicts an extensive health hazard to humanity. According to WHO statistics, 3.8% of the population face depression, including 5% of the adult population (4% in males and 6% in females), and 5.7% of individuals aged 60 years and above. Approximately 280 million individuals globally experience depression.1 Although depression causes disability both in males and females, females are more prone to depression than males. It is expected to become the single leading cause of sickness burden worldwide by 2030.2 Symptomatically, it is characterized by anhedonia (loss of interest and pleasure), pervasive low mood, suicidal inclination, sleep disturbances, exhausted, feeling of worthlessness/guilt and diminished concentration.3 The brain exhibits one of the most significant rates of oxygen consumption per unit mass in the body. Even slight disruptions in the balance of antioxidant defenses and oxidative stress can have harmful effects on neurons. Molecular pathways linked to oxidative stress may play a role in the development of depressive and anxiety disorders. A disparity between the production of reactive oxygen species (ROS) from internal or external sources and the body's ability to neutralize ROS through antioxidants has been identified in these types of disorders.4 Carcinogenicity and erratic effectiveness are one of the assessed outcomes associated with antidepressant drugs as approved by the United States Food and Drug Administration (FDA).5 Consequently, to develop a nontoxic and effective antidepressant drug, traditional herbs are becoming a new area of interest for researchers.Epigallocatechin gallate (EGCG), a flavonoid compound in green tea, holds potential to protect the human body from numerous neuropsychiatric disorders such as anxiety,6 depression7 and other neurodegenerative disorders like Alzheimer's and Parkinson's disease.8 Besides neuroprotection, EGCG also offers numerous health benefits like anticancer, antioxidant, anti-atherogenic, and anti-inflammatory effects etc.9,10 Despite being efficient, features like poor bioavailability, degradation in the gastrointestinal tract, rapid metabolism, fast elimination and various bioconversions such as methylation, glucuronidation, and sulfation in the body restrict its therapeutic use.11 There are two different approaches to improve its therapeutic potential in vivo by increasing its bioavailability which includes (a) the use of combinational therapy i.e., the use of potential inhibitors along with the main drug (b) the use of nano-drug delivery systems. An increase in the bioavailability of EGCG was demonstrated by inhibiting the process of glucuronidation via co-administration of piperine with EGCG. Piperine, an alkaloid present in black pepper, acts by inhibiting the effect of modulations resulting in an increase in its bioavailability.12 Piperine has also been used to enhance the bioavailability of other polyphenols (curcumin and resveratrol) by increasing their absorption.13,14 Thus, a combination strategy is a very reliable approach that reduces the adverse side effects and increases the therapeutic potential of polyphenols. Another way to improve the bioavailability of polyphenols is the development of nano-drug delivery systems wherein herbal bioactive compounds are encapsulated into nanoparticles. This new approach offers benefits like target specific delivery, enhanced solubility, improved bioavailability, low dose, sustained release, reduced elimination, and metabolism of phytochemicals. The nano-drug delivery system not only increases the selectivity and efficacy of the phytonutrients but also protects them from thermal and photo-degradation by minimizing their side effects too.15
Keeping in view the above findings, the current study was intended to prepare nanoformulations of EGCG alone (EGCG loaded nanosuspension) and with piperine (EGCG–piperine nanocomplex) loaded into protein nanoparticles and evaluate their potential as an antidepressant agent on Swiss male albino mice. The effect of the synthesized nanoformulation(s) on depression was investigated by evaluating their effect on catecholaminergic and serotonergic/5-hydroxytryptamine (5-HT) systems, monoamine oxidase (MAO) system(s) enzymes such as glutathione oxidase (GSH) and catalase and lipid peroxidation by employing behavioral model (tail suspension test, TST), biochemical, and neurochemical approaches. This is the first report of the evaluation of the anti-depressant potential of a nanoformulation prepared using EGCG in unstressed and stressed mice.
2. Materials and methods
2.1. Drugs and chemicals
Paroxetine HCl hemihydrate was received as a gift sample from Jubilant Generics Limited, Noida (U.P.). Epigallocatechin gallate was procured from MP Biomedicals, India. Zein and tween-80 were procured from Sigma Aldrich, St Louis, USA and S D-fine-chem, Mumbai, respectively. Lecithin, piperine, disodium hydrogen phosphate, sodium dihydrogen phosphate, Tris, EDTA disodium (AR), sucrose, sulfanilamide, 5-hydroxytryptamine creatinine sulphate, N-(1-naphthyl) ethylenediamine dihydrochloride, and meta-phosphoric acid were obtained from HiMedia Lab. Pvt. Ltd, Mumbai, India. Total protein was estimated using a kit from Erba Chem, Mumbai, India.2.2. Synthesis of the EGCG loaded nanosuspension and EGCG–piperine nanocomplex
Zein was used as a nano carrier in the anti-solvent precipitation process to prepare EGCG-loaded nanoparticles.16,17 Zein-polymer and lecithin, which serve as stabilizers, were included in the organic phase and dispersed in 70% (v/v) ethanol before being filtered to remove contaminants. The antisolvent phase is made up of Tween-80 dissolved in ultrapure water. Blank nanoparticles are produced when the organic phase is added to the anti-solvent phase while being vigorously stirred at 1200 rpm (Fig. 1). | ||
| Fig. 1 Synthesis of zein encapsulated nanoparticles using the anti-solvent precipitation methodology.17 | ||
The same procedure utilized to prepare blank nanoparticles was also used to prepare drug-loaded nanoparticles. When EGCG (25 mg), EGCG + piperine (in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) were combined in the organic phase, the formation of the EGCG-loaded nanosuspension and EGCG–piperine nanocomplex occurred, respectively. In order to increase the stability of the synthesized nanoformulations, the lyophilized nanosuspensions were cryoprotected with 1% (w/v) mannitol in a lab model freeze drier (Freezone 6-Plus Labconco, USA). Software with full-factorial (three levels three-factor 33) design expertise was used to optimise the nano-formulations. Particle size (PS), polydispersity index (PDI), and zeta potential (ZP) were among the physiochemical characterization methods used to evaluate the optimized nano-formulations.
1 ratio) were combined in the organic phase, the formation of the EGCG-loaded nanosuspension and EGCG–piperine nanocomplex occurred, respectively. In order to increase the stability of the synthesized nanoformulations, the lyophilized nanosuspensions were cryoprotected with 1% (w/v) mannitol in a lab model freeze drier (Freezone 6-Plus Labconco, USA). Software with full-factorial (three levels three-factor 33) design expertise was used to optimise the nano-formulations. Particle size (PS), polydispersity index (PDI), and zeta potential (ZP) were among the physiochemical characterization methods used to evaluate the optimized nano-formulations.
2.3. In vivo study of the anti-depressant activity of the synthesised nanosuspensions
| Weeks | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|---|---|---|---|---|---|---|
| I—induction of immobilization for a duration of 2 hours; E—exposure to empty water bottles for a period of 1 hour; F—subjected to foreign object exposure for 24 hours (such as a piece of plastic); O—continual illumination throughout the night; T2—application of tail pinch stimulus for 60 seconds; X—placement of cage at a 45-degree tilt for 7 hours; T1—application of tail pinch stimulus for 30 seconds. | |||||||
| 1st week | I | E | F | O | T2 | X | T1 |
| 2nd week | I | O | X | T2 | E | T1 | F |
| 3rd week | O | F | T1 | X | T2 | I | E |
Groups 1–7: Three weeks of intraperitoneal administration of the following substances: vehicle, paroxetine HCl (10 mg kg−1), EGCG (25 mg kg−1), EGCG loaded nanosuspension (containing 25 mg kg−1 EGCG), EGCG–piperine nanocomplex (containing 25 mg kg−1 EGCG), blank nanosuspension (containing 25 mg kg−1 void drug particles) and piperine (5 mg kg−1), respectively. The locomotor activity scores of mice were documented on the 21st day, 60 minutes after the vehicle or medication was administered. The immobility period in mice was then evaluated using the TST on the 22nd day after that.
Groups 8–14: For three weeks in sequence, the following medications were intraperitoneally administered: vehicle, paroxetine HCl (10 mg kg−1), EGCG (25 mg kg−1), EGCG loaded nanosuspension (containing 25 mg kg−1 EGCG), EGCG–piperine nanocomplex (containing 25 mg kg−1 EGCG), blank nanosuspension (containing 25 mg kg−1) and piperine (5 mg kg−1). On the 21st day, scores of the locomotor activity of mice were taken post 60 minutes of inducing stress. Mice were used to perform a TST on day 22.
2.4. Models employed for behavioral study
2.5. Biochemical estimation
On days 22 and 23, drug administration was also resumed. Animals were tested on the 22nd day. Mice were used in biochemical tests on the 23rd day, an hour after the medication was given. Blood samples (0.5–0.8 ml) from the retro-orbital plexus of mice were obtained, and then they were centrifuged for 10 minutes at 4 °C and 2500 rpm using a chilled centrifuge (C-30 Plus model, REMI, Mumbai, India) to separate the plasma. The corticosterone and nitrite levels were measured using separate plasma samples.After the collection of blood samples, the animals were euthanized using cervical dislocation and their brains were isolated. Cold buffer containing 0.25 M sucrose, 0.1 M Tris, and 0.02 M EDTA (pH 7.4) was used to wash the isolated brain samples before weighing them. The brain sample was homogenised in cold buffer containing 0.25 M sucrose, 0.1 M Tris, and 0.02 M EDTA (pH 7.4), and then centrifuged twice using a refrigerator centrifuge at 2500 rpm for 10 min at 4°. The resulting pellet was removed, and the supernatant was centrifuged once more for 20 minutes at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C. The resulting supernatant was split into two portions:
000 rpm and 4 °C. The resulting supernatant was split into two portions:
Part I: The precipitates i.e., the mitochondrial fraction was utilized to estimate the level of MAO-A.
Part II: The residual supernatant was used for evaluating the reduced glutathione, catalase, and lipid peroxidase activity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and mixed well for 20 min at 4 °C after being rinsed two times with sucrose-Tris-EDTA buffer (100 ml). After centrifugation, (15 000 rpm, 30 minutes, 0 °C), the obtained pellets were re-dissolved in ice-cold sodium phosphate buffer. A solution of 5-hydroxytryptamine (100 ml, 4 mM) and sodium phosphate buffer (2.75 ml, 100 mM, pH 7.4) was taken in a quartz cuvette and stored in a UV-visible spectrophotometer (SPECTROstar Nano, BMG LABTECH, Germany). 150 cc of mitochondrial fraction were added to begin the enzymatic process, and for 5 minutes, the alteration in absorbance at 280 nm wavelength was recorded. The 5-HT solution and sodium phosphate buffer were used as references.
1) and mixed well for 20 min at 4 °C after being rinsed two times with sucrose-Tris-EDTA buffer (100 ml). After centrifugation, (15 000 rpm, 30 minutes, 0 °C), the obtained pellets were re-dissolved in ice-cold sodium phosphate buffer. A solution of 5-hydroxytryptamine (100 ml, 4 mM) and sodium phosphate buffer (2.75 ml, 100 mM, pH 7.4) was taken in a quartz cuvette and stored in a UV-visible spectrophotometer (SPECTROstar Nano, BMG LABTECH, Germany). 150 cc of mitochondrial fraction were added to begin the enzymatic process, and for 5 minutes, the alteration in absorbance at 280 nm wavelength was recorded. The 5-HT solution and sodium phosphate buffer were used as references.
3. Results
3.1. Synthesis and optimization of the EGCG loaded nanosuspension and EGCG–piperine nanocomplex
The anti-solvent precipitation method was employed to prepare EGCG-loaded nanosuspensions and EGCG–piperine nanocomplexes utilising protein nanocarriers. Full factorial design expert software (Version 8.0.7.1, Stat-Ease Inc., Minneapolis, MN) was used for optimization.31–33 The EGCG-loaded nanosuspension and EGCG–piperine nanocomplex were discovered to have particle sizes of 118.3 nm, 0.125, and −36.4 mV and 184.2 nm, 0.231, and −38.3 mV, respectively. In the EGCG–piperine nanocomplex, the increase in particle size was brought about by the encapsulation of two drug moieties into the polymeric matrix.3.2. Behavioral models utilized for the assessment of the antidepressant effects of various drug treatments
4. Discussion
Epigallocatechin gallate, a polyphenol present in green tea, possesses numerous therapeutic benefits that provide protection against life-threatening conditions such as cancer and cardiovascular diseases and neurodegenerative disorders30,34 and also in the prevention of psychiatric ailments by attenuating oxidative stress.35 Besides all these benefits, a number of drawbacks like short shelf life, gastro-intestinal degradation, and inefficient permeability limit its therapeutic efficacy.30 In addition, a significant variation in the bioavailability of catechins (EC, ECG, EGC, and EGCG) has been reported, with EGCG being the least bioavailable.36 One of the reasons behind the low bioavailability of catechins is their large size.37 The other reason for its low bioavailability is severe bioconversions during absorption, comprising methylation, sulfonation, and glucuronidation.11There are two alternative approaches to combat the poor bioavailability of phytochemicals. One is the use of potential inhibitors that hinders the process of bioconversions and the other is the encapsulation of herbal bioactive into nanoparticles. According to a study, co-administration of EGCG with an inhibitor (piperine) slows down the process of elimination and prevents glucuronidation.12 However, research has shown that encapsulating EGCG in chitosan nanoparticles greatly increased its bioavailability.38,39 Additionally, mice who received an oral EGCG-loaded nanosuspension had plasma EGCG concentrations that were 1.5 times higher than those of free EGCG.40 In rats, EGCG's oral bioavailability was more than doubled when it was enclosed in nanolipidic particles41 compared to free EGCG.
Depression is becoming a new threat to the modern society. In order to decrease its prevalence and to antagonize the effects linked to detrimental psychiatric conditions, there is a need to explore new agents that also overcome the side effects associated with antidepressant agents. The combination strategy for treating depression has gained popularity due to a range of potential benefits, including reduced demoralization from psychiatric effects in depressed patients experiencing therapeutic failure, diminished withdrawal syndrome, and the potential for faster and more effective clinical responses.42,43 To estimate the stress pathology in animals at the pre-clinical level, chronic unpredictable mild stress (CUMS) is generally considered as the most reliable and valuable experimental model.44 After CUMS simulation, the behavioral and biochemical changes were observed in mice such as an increase in immobility time, a decline in activity of antioxidant enzymes (GSH, SOD and CAT) and variations in the activity of monoaminergic responses confirming their depressive state of mind. Similar depressive symptoms were noticed when animals were exposed to multiple stressors for a very long time.45,46 The present study suggests that intraperitoneal administration of nanoformulations synthesized using EGCG significantly improves the antidepressant-like behavior in mice subjected to chronic unpredictable mild stress (CUMS) for 3 successive weeks and the results far exceeded when a combination of EGCG and piperine were administered to mice than by either compound when used alone.47–49
One of the most popular behavioural models used to assess the effectiveness of antidepressants is the tail suspension test (TST).50 The current study revealed that when the CUMS technique was used on the mice, their immobility duration increased considerably compared to controls, indicating their depressive-like behaviour. In comparison to vehicle-treated controls, the intraperitoneal administration of paroxetine HCl (10 mg kg−1), EGCG-loaded nanosuspension (comprising 25 mg kg−1 EGCG), and EGCG–piperine nanocomplex (comprising 25 mg kg−1 EGCG) for three consecutive weeks significantly reduced the immobility time in both unstressed and stressed mice. Additionally, the administration of paroxetine HCl (10 mg kg−1), EGCG (25 mg kg−1), EGCG loaded nanosuspension (comprising 25 mg kg−1 EGCG) and EGCG–piperine nanocomplex (comprising 25 mg kg−1 EGCG) did not exhibit any effect on the locomotor activity of unstressed and stressed mice; hence this confirmed their CNS stimulant activity and indicated that the anti-depressant effect of EGCG in unstressed and stressed mice is specific. These results are in line with earlier studies.51
Besides behavioral abnormalities, chronic unpredictable mild stress induction also activates the hypothalamic–pituitary–adrenal (HPA) axis and is responsible for the synthesis and secretion of glucocorticoids from the adrenal cortex. The hyper-secretion of glucocorticoids in blood such as corticosterone in rodents or cortisol in primates disturbs the hormonal balance and ultimately leads to depression.52 The studies denoted that antidepressant drugs produce therapeutic effect in depression patients by suppressing the activity of the HPA axis53 and thus by restoring the functioning of the HPA axis to normal can be used as a treatment for depression.52 In the present study, induction of the CUMS procedure resulted in an increase in the corticosterone level compared to the naïve animals and other observations also support this finding.54 The administration of EGCG loaded nanosuspension significantly reduced the plasma corticosterone level in stressed mice. No significant effect was observed in the case of unstressed mice signifying that overactivity of HPA axis only occurred in stressed conditions.
These findings align with previous studies that the administration of EGCG prevents the increase in the corticosterone level occurring due to the induction of CUMS.51,55
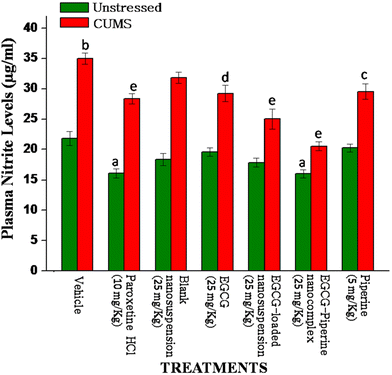
Additionally, persistent unpredictable mild stress is thought to encourage oxidative stress and undermine the brain's antioxidant defense system.56 Reactive oxygen species are produced as a result of oxidative stress, which causes oxidative damage to macromolecules like lipids, proteins, and DNA. This damage eventually results in neural malfunction and depression.57 Since their levels reverted to normal post antidepressant treatment, antioxidant enzymes and lipid peroxidation can be viewed as important depression markers.58 Chronic unpredictable mild stress that was induced caused significant oxidative damage, such as a rise in plasma nitrite levels and lipid peroxidation, as well as a decrease in the functioning of the antioxidant enzymes GSH, SOD, and CAT that are present in the brain tissue.59 The current findings suggest that mice exposed to various stressors for three weeks in a row increased the levels of lipid peroxidation and nitrite while decreasing the activity of an endogenous antioxidant enzyme. Chronic administration of EGCG (25 mg kg−1), EGCG–piperine nanocomplex (comprising 25 mg kg−1 EGCG), and EGCG-loaded nanosuspension reversed these parameters, resulting in a significant reduction in lipid peroxidation levels in stressed mice, an increase in GSH in unstressed mice, and a decrease in catalase levels in the brains of both unstressed and stressed mice. As a result, EGCG demonstrated considerable antioxidant activity in mice, and the findings support earlier research.60,61 When compared to their naive animals, plasma nitrite levels of stressed and unstressed mice showed a significant decline after treatment with EGCG loaded nanosuspension (25 mg kg−1) and EGCG–piperine nanocomplex. Therefore, EGCG demonstrated a strong protective effect on mice against oxidative stress brought on by CUMS. It has also been noted that mice exposed to various stressful conditions have higher plasma nitrite levels.20
Monoamine oxidase (MAO) is a flavin enzyme situated within the outer mitochondrial membrane of cells throughout the body. MAO plays a crucial role in the metabolic breakdown of biogenic and xenobiotic amines within both the central nervous system and peripheral tissues. It is further characterized into two different forms – MAO-A and MAO-B. MAO-A exhibits a substrate preference for serotonin, epinephrine, and norepinephrine, while MAO-B shows a substrate preference for dopamine. During major depression, activity of MAO-A in the brain was elevated in the brain resulting in a decrease in the monoamine level.62 Therefore, in the present study only the MAO-A level in the brain was measured. An increase in the activity of MAO-A was observed due to the exposure to different stressors. A significant inhibition in the brain MAO-A activity was observed in both unstressed and stressed mice upon administration of the EGCG loaded nanosuspension (25 mg kg−1) and EGCG–piperine nanocomplex (25 mg kg−1) for 3 successive weeks. The findings are in concurrence with the previous research demonstrating that EGCG exhibits monoamine oxidase inhibiting activity.63 The present findings indicate that more inhibition of MAO-A activity was observed when the combination of EGCG and piperine was administered and this is also in alignment with a previous study where a combination of ferulic acid and piperine displayed a better inhibitory effect on MAO-A than the treatment with single drug.64 The relative order of effects of various drug treatments (paroxetine HCl, blank nanosuspension, EGCG, EGCG-loaded nanosuspension, EGCG–piperine nanocomplex and piperine on behavioral model (TST) and biochemical parameters (plasma nitrite, plasma corticosterone, brain MAO-A, brain lipid peroxidation, brain GSH and brain catalase) is shown in Table 2.
| S. no. | Parameters | Order of significance in unstressed mice based on the ‘q’ value | Order of significance in stressed mice based on the ‘q’ value |
|---|---|---|---|
| 1 | Tail suspension test | Paroxetine HCl (19.343) > EGCG–piperine nanocomplex (11.384) > EGCG loaded nanosuspension (5.584) | Paroxetine HCl (18.533) > EGCG–piperine nanocomplex (9.064) > EGCG loaded nanosuspension (7.338) |
| 2 | Plasma nitrite | EGCG–piperine nanocomplex (5.810) > paroxetine HCl (5.757) | EGCG–piperine nanocomplex (14.452) > EGCG loaded nanosuspension (9.950) > paroxetine HCl (6.619) > EGCG (5.739) > piperine (5.428) |
| 3 | Plasma corticosterone | No effect was observed | EGCG–piperine nanocomplex (6.726) > EGCG loaded nanosuspension (6.058) > paroxetine HCl (5.674) > EGCG (4.973) |
| 4 | Brain MAO-A activity | EGCG–piperine nanocomplex (6.752) > EGCG loaded nanosuspension (5.539) > paroxetine HCl (5.531) > EGCG (5.189) > piperine (4.886) | EGCG–piperine nanocomplex (15.226) > EGCG loaded nanosuspension (12.551) > paroxetine HCl (7.752) > EGCG (5.935) |
| 5 | Brain lipid peroxidation | EGCG–piperine nanocomplex (6.275) > paroxetine HCl (5.945) > EGCG loaded nanosuspension (4.905) | EGCG–piperine nanocomplex (9.363) > EGCG loaded nanosuspension (8.191) > paroxetine HCl (8.158) > EGCG (6.275) > piperine (5.747) |
| 6 | Brain reduced glutathione | EGCG–piperine nanocomplex (28.457) > EGCG loaded nanosuspension (10.301) > paroxetine HCl (6.366) > EGCG (5.874) | EGCG–piperine nanocomplex (7.644) > EGCG loaded nanosuspension (6.127) > paroxetine HCl (6.045) > EGCG (5.533) |
| 7 | Brain catalase | EGCG–piperine nanocomplex (6.608) > paroxetine HCl (6.177) > EGCG loaded nanosuspension (5.459) | EGCG–piperine nanocomplex (13.719) > EGCG loaded nanosuspension (11.995) > paroxetine HCl (8.619) > EGCG (9.337) > piperine (6.823) |
In conclusion, the administration of synthesized nanoformulations shows a significant anti-depressant effect in unstressed and stressed mice possibly by decreasing the plasma nitrite level; inhibiting the activity of the MAO-A enzyme; and increasing the activity of the antioxidant enzyme in the brain. Antidepressant-like activity of the synthesized nanoformulation using EGCG was found comparable to paroxetine HCl, a standard antidepressant drug used in the current study. Indeed, the encapsulation of drug moieties into nanoparticles increases the bioavailability by improving the solubility and stability of phytochemicals65,66 and it was also concluded from present findings that nano-encapsulation enhances the efficacy of a herbal drug (EGCG) and these effects were more pronounced when the combinational strategy (EGCG along with piperine) was used. Piperine (5 mg kg−1) also displayed an anti-depressant effect, but it was less significant due to its low concentration. However, the anti-depressant effect was increased when it was combined with EGCG. It is concluded that the simultaneous administration of EGCG and piperine has the potential to produce a synergistic effect on depression-like behavior in mice. This combination could serve as a natural alternative for mitigating psychiatric disorders, offering improved efficacy and minimal side effects.
5. Conclusion
The bioavailability of polyphenols is very poor which restricts their therapeutic potential, and improving their systemic absorption is the real challenge. The use of bioavailability enhancers and their encapsulation into nanoparticles are the two approaches that can overcome the bioavailability issue. In this current investigation, EGCG was loaded into nanoparticles alone and with piperine and their anti-depressant potential in mice was evaluated. The antisolvent precipitation methodology was employed for the synthesis of nanoparticles loaded with EGCG and EGCG–piperine nanocomplex using zein as a nanocarrier. These findings show that the encapsulation of EGCG into nanoparticles produces a significant antidepressant effect on Swiss male albino mice and the results obtained are comparable to those with the standard anti-depressant drug, paroxetine HCl. The antidepressant effect of EGCG is due to its antioxidant nature and these results are further amplified when it is combined with piperine. Moreover, the carrier i.e. zein used for the encapsulation of EGCG is biocompatible, biodegradable, non-toxic, FDA approved and safe for human use.Conflicts of interest
The authors report no conflicts of interest in this work.Acknowledgements
The first author, Shakti Dahiya (IF 130021), expresses gratitude to the Department of Science & Technology for granting the “INSPIRE” Fellowship. Additionally, the authors acknowledge DST-Nanomission for furnishing the necessary infrastructure to carry out this study.References
- Institute of Health Metrics and Evaluation, Global Health Data Exchange (GHDx).
- A. Hughes-Morley and B. Young, et al., Factors affecting recruitment into depression trials: systematic review, meta-synthesis and conceptual framework, J. Affect. Disord., 2015, 172, 274–290 CrossRef PubMed.
- R. Bianchi and I. S. Schonfeld, et al., Burnout–depression overlap: A review, Clin. Psychol. Rev., 2015, 36, 28–41 CrossRef PubMed.
- Y. Xu and C. Wang, et al., Novel therapeutic targets in depression and anxiety: antioxidants as a candidate treatment, Curr. Neuropharmacol., 2014, 12(2), 108–119 CrossRef CAS PubMed.
- A. Amerio and J. F. Gálvez, et al., Carcinogenicity of psychotropic drugs: A systematic review of US Food and Drug Administration–required preclinical in vivo studies, Aust. N. Z. J. Psychiatry, 2015, 49(8), 686–696 CrossRef PubMed.
- M. Vignes and T. Maurice, et al., Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG), Brain Res., 2006, 1110(1), 102–115 CrossRef CAS PubMed.
- A. Di Lorenzo and S. F. Nabavi, et al., Antidepressive-like effects and antioxidant activity of green tea and GABA green tea in a mouse model of post-stroke depression, Mol. Nutr. Food Res., 2016, 60(3), 566–579 CrossRef CAS PubMed.
- O. Weinreb and S. Mandel, et al., Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases, J. Nutr. Biochem., 2004, 15(9), 506–516 CrossRef CAS PubMed.
- D. Mereles, et al., Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises?, Int. J. Mol. Sci., 2011, 12(9), 5592–5603 CrossRef CAS PubMed.
- D. G. Nagle and D. Ferreira, et al., Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives, Phytochemistry, 2006, 67(17), 1849–1855 CrossRef CAS PubMed.
- X. Meng and S. Sang, et al., Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats, Chem. Res. Toxicol., 2002, 15(8), 1042–1050 Search PubMed.
- J. D. Lambert and J. Hong, et al., Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice, J. Nutr., 2004, 134(8), 1948–1952 CrossRef CAS PubMed.
- P. Rinwa and A. Kumar, et al., Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression, PLoS One, 2013, 8(4), e61052 CrossRef CAS PubMed.
- Y. Xu and L. Zhang, et al., Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: behavioral and neurobiological analyses, Metab. Brain Dis., 2013, 28, 571–583 CrossRef CAS PubMed.
- B. Hu and X. Liu, et al., Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols, J. Food Drug Anal., 2017, 25(1), 3–15 CrossRef CAS PubMed.
- D. Yadav, et al., Nanonization of curcumin by antisolvent precipitation: process development, characterization, freeze drying and stability performance, Int. J. Pharm., 2014, 477(1–2), 564–577 CrossRef CAS PubMed.
- S. Dahiya and R. Rani, et al., Potentiation of nootropic activity of EGCG loaded nanosuspension by piperine in swiss male albino mice, Future J. Pharm. Sci., 2018, 4(2), 296–302 CrossRef.
- T. J. Shors, et al., Estrogen-mediated effects on depression and memory formation in females, J. Affect. Disord., 2003, 74(1), 85–96 CrossRef CAS PubMed.
- S. Li and C. Wang, et al., Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms, Life Sci., 2007, 80(15), 1373–1381 CrossRef CAS PubMed.
- M. Kolasa and J. Solich, et al., Paroxetine and low-dose risperidone induce serotonin 5-HT1A and Dopamine D2 receptor heteromerization in the mouse prefrontal cortex, Neuroscience, 2018, 377, 184–196 CrossRef CAS PubMed.
- H. Ueno and Y. Takahashi, et al., Effect of simultaneous testing of two mice in the tail suspension test and forced swim test, Sci. Rep., 2022, 12(1), 9224 CrossRef CAS PubMed.
- L. Steru and R. Chermat, et al., The tail suspension test: a new method for screening antidepressants in mice, Psychopharmacology, 1985, 85, 367–370 CrossRef CAS PubMed.
- D. Dhingra, et al., Behavioral and biochemical evidences for antidepressant-like activity of palmatine in mice subjected to chronic unpredictable mild stress, Pharmacol. Rep., 2014, 66, 1–9 CrossRef CAS PubMed.
- L. C. Green and D. A. Wagner, et al., Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids, Anal. Biochem., 1982, 126(1), 131–138 CrossRef CAS PubMed.
- J. Bartos, et al., Colorimetric and fluorimetric determination of steroids, Pure Appl. Chem., 1979, 51(10), 2157–2159 Search PubMed.
- A. Schurr, et al., Differential inhibition of mitochondrial monoamine oxidase from brain by hashish components, Biochem. Pharmacol., 1976, 25(10), 1201–1203 CrossRef CAS PubMed.
- Y. Liang and X. Yang, et al., Antidepressant-like effect of the saponins part of ethanol extract from SHF, J. Ethnopharmacol., 2016, 191, 307–314 CrossRef CAS PubMed.
- E. J. Wills, Mechanisms of lipid peroxide formation in tissues role of metals and haematin proteins in the catalysis of the oxidation of unsaturated fatty acids, Biochim. Biophys. Acta, 1965, 98(2), 238–251 CrossRef CAS PubMed.
- D. Jollow and J. Mitchell, et al., Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite, Pharmacology, 1974, 11(3), 151–169 CrossRef CAS PubMed.
- S. Dahiya and R. Rani, et al., Chitosan-gellan gum bipolymeric nanohydrogels—a potential nanocarrier for the delivery of epigallocatechin gallate, BioNanoScience, 2017, 7, 508–520 CrossRef.
- H. Abbas and Y. A. El-Feky, et al., Development and optimization of curcumin analog nano-bilosomes using 21.31 full factorial design for anti-tumor profiles improvement in human hepatocellular carcinoma: In vitro evaluation, in vivo safety assay, Drug Delivery, 2022, 29(1), 714–727 CrossRef CAS PubMed.
- N. Rigopoulos and C. M. Gkaliouri, et al., Full Factorial Design Synthesis of Silver Nanoparticles Using Origanum vulgare, Reactions, 2023, 4(3), 505–517 CrossRef.
- M. Liu and M. Sharma, et al., Full factorial design, physicochemical characterization, ex vivo investigation, and biological assessment of glutathione-loaded solid lipid nanoparticles for topical application, Int. J. Pharm., 2023, 630, 122381 CrossRef CAS PubMed.
- J. Xie and L. Jiang, et al., Neuroprotective effects of Epigallocatechin-3-gallate (EGCG) in optic nerve crush model in rats, Neurosci. Lett., 2010, 479(1), 26–30 CrossRef CAS PubMed.
- A. K. Sachdeva and A. Kuhad, et al., Epigallocatechin gallate ameliorates behavioral and biochemical deficits in rat model of load-induced chronic fatigue syndrome, Brain Res. Bull., 2011, 86(3–4), 165–172 CrossRef CAS PubMed.
- B. A. Warden and L. S. Smith, et al., Catechins are bioavailable in men and women drinking black tea throughout the day, J. Nutr., 2001, 131(6), 1731–1737 CrossRef CAS PubMed.
- C. A. Lipinski and F. Lombardo, et al., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Delivery Rev., 2012, 64, 4–17 CrossRef.
- B. Hu and Y. Ting, et al., Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability, Chem. Commun., 2012, 48(18), 2421–2423 RSC.
- A. Dube and J. A. Nicolazzo, et al., Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate, Eur. J. Pharm. Sci., 2010, 41(2), 219–225 CrossRef CAS PubMed.
- A. Dube and J. A. Nicolazzo, et al., Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability, Eur. J. Pharm. Sci., 2011, 44(3), 422–426 CrossRef CAS PubMed.
- A. Smith and B. Giunta, et al., Nanolipidic particles improve the bioavailability and α-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer's disease, Int. J. Pharm., 2010, 389(1–2), 207–212 CrossRef CAS PubMed.
- R. W. Lam and D. D. Wan, et al., Combining antidepressants for treatment-resistant depression: a review, J. Clin. Psychiatry, 2002, 63(8), 685–693 CrossRef CAS PubMed.
- G. Rubio and L. San, et al., Reboxetine adjunct for partial or nonresponders to antidepressant treatment, J. Affect. Disord., 2004, 81(1), 67–72 CrossRef CAS PubMed.
- M. K. Bhutani and M. Bishnoi, et al., Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes, Pharmacol., Biochem. Behav., 2009, 92(1), 39–43 CrossRef CAS PubMed.
- P. J. Willner, Animal models as simulations of depression, Trends Pharmacol. Sci., 1991, 12, 131–136 CrossRef CAS PubMed.
- P. J. N. Willner, Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS, Neuropsychobiology, 2005, 52(2), 90–110 CrossRef CAS PubMed.
- A. Mannan and T. G. Singh, et al., Insights into the mechanism of the therapeutic potential of herbal monoamine oxidase inhibitors in neurological diseases, Curr. Drug Targets, 2022, 23(3), 286–310 CrossRef CAS PubMed.
- V. Singh and G. Chauhan, et al., Anti-depressant like effects of quercetin 4′-O-glucoside from Allium cepa via regulation of brain oxidative stress and monoamine levels in mice subjected to unpredictable chronic mild stress, Nutr. Neurosci., 2021, 24(1), 35–44 CrossRef CAS PubMed.
- K. Randhawa and V. Singh, et al., Isolation of Pleurotus florida derived acetylcholinesterase inhibitor for the treatment of cognitive dysfunction in mice, Food Sci. Hum. Wellness., 2021, 10(4), 490–496 CrossRef CAS.
- J. F. Cryan and C. Mombereau, et al., The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice, Neurosci. Biobehav. Rev., 2005, 29(4–5), 571–625 CrossRef CAS PubMed.
- W. L. Zhu and H. S. Shi, et al., Green tea polyphenols produce antidepressant-like effects in adult mice, Pharmacol. Res., 2012, 65(1), 74–80 CrossRef CAS PubMed.
- Y. Pan and W. Y. Zhang, et al., Effects of icariin on hypothalamic-pituitary-adrenal axis action and cytokine levels in stressed Sprague-Dawley rats, Biol. Pharm. Bull., 2006, 29(12), 2399–2403 CrossRef CAS PubMed.
- H. Himmerich and P. Zimmermann, et al., Changes in the hypothalamic-pituitary-adrenal axis and leptin levels during antidepressant treatment, Neuropsychobiology, 2007, 55(1), 28–35 CrossRef CAS PubMed.
- Q. Q. Mao and S. P. Ip, et al., Peony glycosides produce antidepressant-like action in mice exposed to chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2009, 33(7), 1211–1216 CrossRef CAS PubMed.
- N. Adachi and S. Tomonaga, et al., Epigallocatechin gallate attenuates acute stress responses through GABAergic system in the brain, Eur. J. Pharmacol., 2006, 531(1–3), 171–175 CrossRef CAS PubMed.
- G. Lucca and C. M. Comim, et al., Effects of chronic mild stress on the oxidative parameters in the rat brain, Neurochem. Int., 2009, 54(5–6), 358–362 CrossRef CAS PubMed.
- H. K. Manji, et al., Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics, Psychopharmacol. Bull., 2001, 35(2), 5–49 CAS.
- M. Bilici and H. Efe, et al., Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments, J. Affect. Disord., 2001, 64(1), 43–51 CrossRef CAS PubMed.
- B. Kumar and A. Kuhad, et al., Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences, Psychopharmacology, 2011, 214, 819–828 CrossRef CAS PubMed.
- B. Frei, et al., Antioxidant activity of tea polyphenols in vivo: evidence from animal studies, J. Nutr., 2003, 133(10), 3275S–3284S CrossRef CAS PubMed.
- Y. Levites and O. Weinreb, et al., Green tea polyphenol (–)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration, J. Neurochem., 2001, 78(5), 1073–1082 CrossRef CAS PubMed.
- J. H. Meyer and N. Ginovart, et al., Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression, Arch. Gen. Psychiatry, 2006, 63(11), 1209–1216 CrossRef CAS PubMed.
- Y. Bandaruk and R. Mukai, et al., Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria, J. Agric. Food Chem., 2012, 60(41), 10270–10277 CrossRef CAS PubMed.
- G. Li, L. Ruan and R. Chen, et al., Synergistic antidepressant-like effect of ferulic acid in combination with piperine: involvement of monoaminergic system, Metab. Brain Dis., 2015, 30, 1505–1514 CrossRef CAS PubMed.
- R. Rani and S. Dahiya, et al., Evaluation of anti-diabetic activity of glycyrrhizin-loaded nanoparticles in nicotinamide-streptozotocin-induced diabetic rats, Eur. J. Pharm. Sci., 2017, 106, 220–230 CrossRef CAS PubMed.
- V. Kakkar, et al., Antidepressant activity of curcumin loaded solid lipid nanoparticles (C-SLNs) in mice, Am. J. PharmTech Res., 2012, 2(3), 729–736 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |