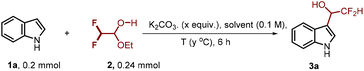Divergent synthesis of difluoromethylated indole-3-carbinols, bisindolylmethanes and indole-3-methanamines†
Yifei
Qu‡
,
Xiaojia
Cai‡
,
Yuzhuang
Guan‡
,
Jiamin
Tan
,
Zhangping
Cai
,
Minyun
Liu
,
Yasi
Huang
,
Jinhui
Hu
 *,
Wen-Hua
Chen
*,
Wen-Hua
Chen
 * and
Jia-Qiang
Wu
* and
Jia-Qiang
Wu
 *
*
School of Biotechnology and Health Sciences, Wuyi University, No. 99 Yingbin Road, Jiangmen 529020, China. E-mail: wyuchemwjq@wyu.edu.cn; wyuchemhjh@wyu.edu.cn; whchen@wyu.edu.cn
First published on 24th November 2023
Abstract
Indole-3-carbinol, bisindolylmethanes (BIMs) and indole-3-methanamines exhibit diverse therapeutic activities. Fluorinated molecules are widely used in pharmaceuticals. Herein we report a facile and straightforward method for the successful synthesis of difluoromethylated indole-3-carbinols, bisindolylmethanes and indole-3-methanamines by a Friedel–Crafts reaction. The reaction involves the in situ generation of difluoroacetaldehyde from difluoroacetaldehyde ethyl hemiacetal in the presence of a base or an acid. This protocol is distinguished by its good to excellent yields, broad substrate compatibility, good functional group tolerance and scalability.
Introduction
Indoles represent a class of privileged nitrogen-containing heterocyclic frameworks present in many natural products, diverse pharmaceutical agents, fine chemicals and agrochemicals.1 Among the indole family, indole-3-carbinol (I3C) emerges as an important and versatile pro-electrophile for constructing indole-containing molecules and their related dimeric products, namely substituted bisindolylmethanes (BIMs) that exhibit diverse therapeutic activities such as anticancer, antimicrobial, antioxidant and anti-inflammatory effects (Fig. 1).2,3 Meanwhile, indole-3-methanamine derivatives exhibit diverse therapeutic activities (Fig. 1).4 Due to the importance of I3C, BIMs and indole-3-methanamine derivatives, the development of facile and sustainable synthetic procedures to obtain these indole derivatives has emerged as an important topic for synthetic organic chemists.5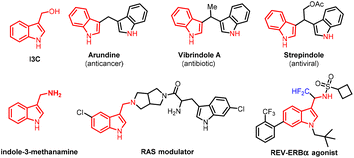 | ||
| Fig. 1 Indole-3-carbinol, representative 3,3′-BIMs and indole-3-methanamine derivatives with relevant bioactivity. | ||
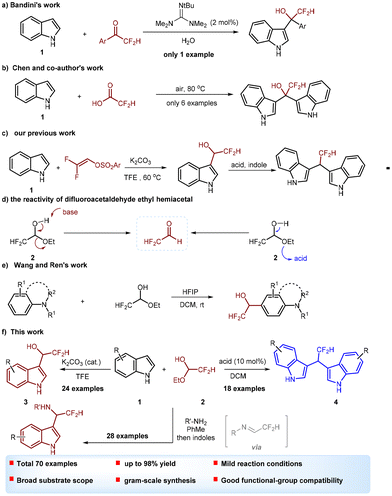
On the other hand, the difluoromethyl (CHF2) group as a good hydrogen bond donor and bioisostere for hydroxy, thiol and amide groups has attracted significant attention in drug discovery and development.6 Meanwhile, incorporation of CHF2 into molecules has a profound effect on the acidity, stability and lipophilicity of parent molecules. Thus, difluoromethyl-containing compounds have significant potential in pharmaceuticals, agrochemicals and advanced materials.7 However, compared with the fruitful development of non-fluorinated I3C, BIMs and indole-3-methanamine derivatives, the synthesis of difluoromethylated I3C, BIMs and indole-3-methanamine derivatives is highly challenging and remains to be extensively explored. In general, when carbonyl compounds are used as electrophilic reagents, the dimeric products BIMs are formed via a double Friedel–Crafts reaction in the presence of acids or bases.5 The methods used for introducing a fluoromethyl group into a BIM scaffold generally involve step-by-step Friedel–Crafts-type alkylations,8,9 probably because the electron-withdrawing fluoromethyl group can stabilize the fluorinated indole-3-methanol. The known method for the synthesis of difluoromethylated I3C, BIMs and indole-3-methanamines is the Friedel–Crafts reaction of indole with difluoromethylketones (Scheme 1a),10 difluoroacetic acid (Scheme 1b)11 or difluoroacetamide.12 Recently, we reported a new method for the synthesis of difluoromethylated I3C from the reaction of indole with difluorovinyl arysulfonates (Scheme 1c). Mechanistic studies revealed that it probably involves a difluoroacetaldehyde intermediate.13 Obviously, difluoromethyl carbonyls are the most common reagents used in the Friedel–Crafts reaction to construct difluoromethylated I3C and BIMs. Among the relative difluoromethylated carbonyls, α,α-difluoroacetaldehyde is a high-value synthon. It is worth noting that difluoroacetaldehyde ethyl hemiacetal is used as a convenient α,α-difluoroacetaldehyde equivalent as it can transform into difluoroacetaldehyde under basic or acidic conditions (Scheme 1d).14 Recently, Wang and Ren's group reported the elegant work on HFIP-promoted hydroxydifluoromethylation of aniline, indole, and pyrrole derivatives with difluoroacetaldehyde ethyl hemiacetal for the synthesis of difluoromethylated carbinols (Scheme 1e).15 We reasoned that by taking advantage of the reactivity of difluoroacetaldehyde ethyl hemiacetal, difluoromethylated I3C, BIMs and indole-3-methanamines would be successfully constructed via a Friedel–Crafts reaction of indole with difluoroacetaldehyde ethyl hemiacetal. With this thought in mind and as part of our continuous research on the synthesis of fluoroalkylated molecules,13,16,17 herein we report a catalyst-controlled approach for accessing difluoromethylated I3C, BIMs and indole-3-methanamines via Friedel–Crafts alkylation.
Results and discussion
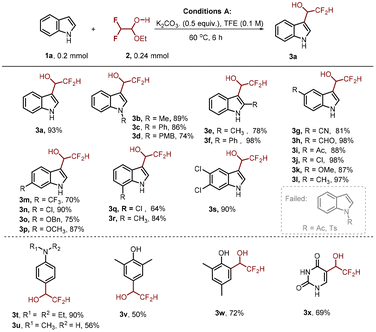
To examine the feasibility of our proposed approach, we commenced our investigation with model substrates indole 1a and difluoroacetaldehyde ethyl hemiacetal 2 (Table 1). We found that the reaction proceeds well in the presence of 0.5 equivalents of K2CO3 at 60 °C in TFE (trifluoroethanol), affording the desired product 3a in 93% yield (entry 1). Switching the solvent to DCM and other alcohol solvents (MeOH and EtOH) resulted in the decrease of yield (entries 2–4). The yield of product 3a was dramatically decreased without the addition of K2CO3 (entry 5) or lowering the temperature to room temperature (entry 7). Similarly, increasing the loading of K2CO3 to 1 equivalent (entry 6) or raising the temperature to 90 °C (entry 8) had a negative effect on the yield of 3a, probably because these conditions accelerate the decomposition of difluoroacetaldehyde ethyl hemiacetal.With the optimized reaction conditions, the robustness and compatibility of this transformation were then explored. As shown in Scheme 2, for a wide range of indoles bearing different substituents, regardless of their electronic properties and substitution positions, the reaction proceeded smoothly, giving the corresponding difluoromethyl indole-3-carbinols in good to excellent yields (64–98%). It should be noted that, regardless of the substituent position, indoles bearing either electron-donating groups (3b–f, 3k, 3l, 3o and 3p) or electron-withdrawing groups (3g–j, 3m and 3n) could be obtained in good yields. Some commonly encountered functional groups such as halogen (3j, 3n, and 3q), trifluoromethyl (3m), cyano (3g), acetyl (3i), and even aldehyde (3h) were tolerated, providing good opportunities for further functionalizations. –OMe (3k and 3p) and OBn (3o) groups were well tolerated, which allowed further simple derivatization. Although substitutions at the C2 position of indole may cause steric effects at the C3 position, this reaction proceeded well to give the corresponding products 3e and 3f in 78% and 98% yields, respectively. It is worth mentioning that 5,6-dichloroindole is also compatible under the reaction conditions, giving the corresponding product 3s in a good yield.
Meanwhile, the reaction of anilines, phenols and uracil with difluoroacetaldehyde ethyl hemiacetal 2 was studied (Scheme 2). Under the standard conditions, these reactions proceeded well to give the corresponding compounds (3t–3x) in 50–90% yields.
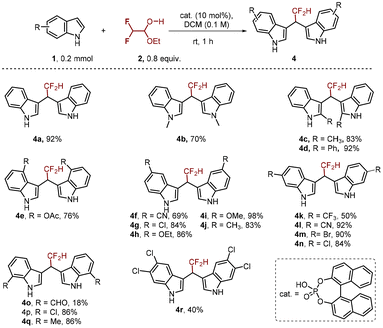
Next, we focused on Friedel–Crafts alkylation to construct difluoromethylated BIMs (Scheme 3). We first tested the reaction of indole 1 with difluoroacetaldehyde ethyl hemiacetal 2, catalyzed by 10 mol% 1,1-binaphthyl-2,2-diyl hydrogen phosphate, in DCM at room temperature. To our delight, this reaction afforded the corresponding product 4a in an excellent yield (92%). Then the reaction scope was investigated with diverse indoles, as summarized in Scheme 3. Interestingly, regardless of the electronic properties of the substituents on the indole ring, the reaction was compatible to give the desired products (4b–r) in moderate to excellent yields. In addition, the substituents at the C2 position of indole had no obvious steric effect on the reaction outcome since both Me (4c) and Ph (4d) substituents were all compatible to give the desired products in 83% and 92% yields, respectively. Of note, the use of 5,6-dichloroindole was feasible to give 4r in 40% yield.
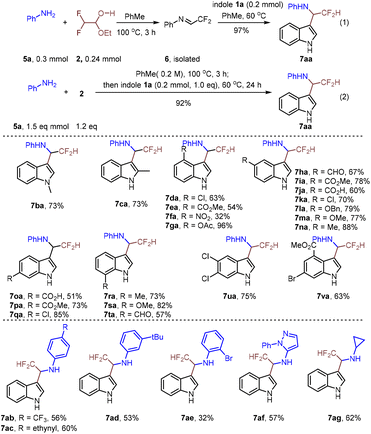
These satisfactory results encouraged us to investigate the construction of difluoromethylated indole-3-methanamines via Friedel–Crafts alkylation (Scheme 4). Difluoroacetaldehyde ethyl hemiacetal 2 was smoothly transformed into difluoromethylated imine 6, which was isolated to react with indole 1a to give N-(2,2-difluoro-1-(1H-indol-3-yl)ethyl)aniline 7aa in 97% yield (eqn (1)). To enhance the efficiency of the Friedel–Crafts alkylation, a telescoping synthesis of 7aa without the isolation of intermediate 6 was realized with a good yield of 92% (eqn (2)). The generality of the synthesis of difluoromethylated indole-3-methanamines 7 was then investigated. As shown in Scheme 4, differently substituted indoles, regardless of the electronic properties and the positions of the substituents, underwent a smooth reaction to give the corresponding products (7aa–7sa) in moderate to excellent yields. Various substituents were tolerated and the functionalization occurred at the less hindered position of anilines (7ab–7ad). It is noteworthy that ortho-substituents of aniline were also tolerated in this reaction, albeit with lower yield (7ae). This is due to the relatively low yield for the formation of imine 5e. Gratifyingly, 1-phenyl-1H-pyrazol-5-amine (5h) and cyclopropanamine (5i) were compatible to give the desired products (7ag–7ai) in moderate yields.
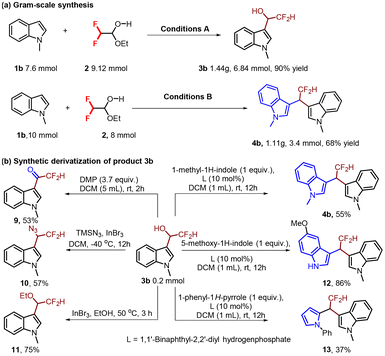
To demonstrate the scalability of the reaction, we executed a scale-up experiment of 1b with difluoroacetaldehyde ethyl hemiacetal 2 under the optimized conditions, giving the products 3b and 4b in 90% and 68% yields, respectively, indicative of the same efficiency as in a 0.2 mmol scale (Scheme 5a). To document the potential utility of 3b, its derivatization was conducted (Scheme 5b).12 First, oxidation of the secondary alcohol 3b to ketone 5 was conducted in the presence of Dess–Martin periodinane reagent. Second, direct azidation of 3b was catalyzed by InBr3 to achieve 10 in 57% yield. Next, etherification of 3b gave 11 in 75% yield. Finally, the Friedel–Crafts reaction was carried out with rac-phosphoric acid catalysis to give compounds 4b, 12 and 13 in 55%, 86% and 37% yields, respectively.
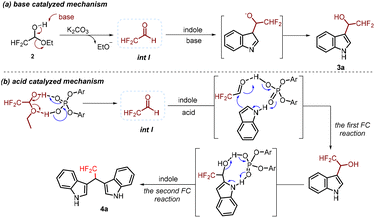
According to the experimental results and previous studies,7–12 a plausible mechanism of this transformation was proposed (Scheme 6). In the presence of a catalytic amount of base, the difluoroacetaldehyde ethyl hemiacetal 2 has a tendency to remove ethoxide that can serve as a base catalyzing the next reaction cycle, resulting in the in situ generation of difluoroacetaldehyde. Then, the nucleophilic addition of indoles occurs to give difluoromethylated I3C 3a (Scheme 6a). In the presence of a catalytic amount of 1,1-binaphthyl-2,2-diyl hydrogen phosphate, by taking advantage of intermolecular hydrogen bonding interaction between difluoroacetaldehyde ethyl hemiacetal 2 and the phosphoric acid motif of the catalyst, a molecule of ethanol was removed to form difluoroacetaldehyde (int I). Subsequently, a two-step Friedel–Crafts alkylation of indole would proceed to give the desired product difluoromethylated BIM 4avia hydrogen bonding interaction with 1,1-binaphthyl-2,2-diyl hydrogen phosphate (Scheme 6b).
Conclusions
In conclusion, we reported a facile and straightforward method for the successful synthesis of difluoromethylated indole-3-carbinols, bisindolylmethanes and indole-3-methanamines by a Friedel–Crafts reaction. The reaction involves the in situ generation of difluoroacetaldehyde from difluoroacetaldehyde ethyl hemiacetal in the presence of a base or an acid. This protocol is distinguished by its good to excellent yields, broad substrate compatibility, good functional group tolerance and scalability. The present method would allow the facile synthesis of difluoromethylated indole-3-carbinols, bisindolylmethanes and indole-3-methanamines with potential bioactivity.Author contributions
Y. Q., X. C. and Y. G. contributed equally to this work. J. H., W.-H. C., and J.-Q. W. conceived the idea and designed the research. Y. Q., X. C., Y. G., M. L., Y. H. and J. T. performed the research. X. C. analyzed the data. J.-Q. W. wrote the original manuscript. W.-H. C. and J. H. reviewed the manuscript and suggested improvements.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the support of this work from the Natural Science Foundation of Guangdong Province (2023A1515030144), the National Natural Science Foundation of China (21901188), the High-level Talent Research Start-up Project (2018AL001), the Science Foundation for Young Teachers (2019td04), and the Innovation and Entrepreneurship Project (2020CX03) of Wuyi University.References
- A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Comprehensive Heterocyclic Chemistry III, Elsevier, Oxford, 2008 Search PubMed.
- For selected reviews, see: (a) M. N. Karimabad, M. Mohamadi, S. A. Torabizadeh and G. Hassanshahi, Mini-Rev. Med. Chem., 2023, 23, 150–158 CrossRef CAS PubMed; (b) M. N. Karimabad, M. Mohamadi, A. Jafarzadeh, A. Darekordi, M. R. Hajizadeh and G. Hassanshahi, Mini-Rev. Med. Chem., 2019, 19, 540–554 CrossRef CAS PubMed.
- For selected reviews and articles, see: (a) M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250–2293 CrossRef CAS PubMed; (b) N. Ichite, M. B. Chougule, T. Jackson, S. V. Fulzele, S. Safe and M. Singh, Clin. Cancer Res., 2009, 15, 543–552 CrossRef CAS; (c) S. Sarva, J. S. Harinath, S. P. Sthanikam, S. Ethiraj, M. Vaithiyalingam and S. R. Cirandur, Chin. Chem. Lett., 2016, 27, 16–20 CrossRef CAS.
- (a) J. R. Abbott, T. R. Hodges, R. N. Daniels, P. A. Patel, J. P. Kennedy, J. E. Howes, D. T. Akan, M. C. Burns, J. Sai, T. Sobolik, Y. Beesetty, T. Lee, O. W. Rossanese, J. Phan, A. G. Waterson and S. W. Fesik, J. Med. Chem., 2018, 61, 6002–6017 CrossRef CAS PubMed; (b) T. M. Kamenecka, K. Greenman, L. Solt, Y. He, M. Lizarzaburu and B. C. Bookser, WO2021263278A1, 2021.
- For selected reviews and articles, see: (a) Z. Zhang, X. Liu, X. Zong, Y. Yuan, S. Liu, T. Zhang, Z. Wu, J. Yang and Z. Jia, Chin. J. Org. Chem., 2021, 41, 52–64 CrossRef CAS; (b) D. Bag and S. D. Sawant, Org. Lett., 2022, 24, 4930–4934 CrossRef CAS; (c) P. K. Jat, K. K. Dabaria, R. Bai, L. Yadav and S. S. Badsara, J. Org. Chem., 2022, 87, 12975–12985 CrossRef CAS PubMed; (d) X. Liu, S. Ma and P. H. Toy, Org. Lett., 2019, 21, 9212–9216 CrossRef CAS; (e) K. A. Chavan, M. Shukla, A. N. S. Chauhan, S. Maji, G. Mali, S. Bhattacharyya and R. D. Erande, ACS Omega, 2022, 7, 10438–10446 CrossRef CAS; (f) W.-J. Zhu, J.-F. Gong and M.-P. Song, J. Org. Chem., 2020, 85, 9525–9537 CrossRef CAS.
- For selected articles, see: (a) Y. Zafrani, G. Parvari, D. Amir, L. Ghindes-Azaria, S. Elias, A. Pevzner, G. Fridkin, A. Berliner, E. Gershonov, Y. Eichen, S. Saphier and S. Katalan, J. Med. Chem., 2021, 64, 4516–4531 CrossRef CAS PubMed; (b) Y. Zafrani, G. Sod-Moriah, D. Yeffet, A. Berliner, D. Amir, D. Marciano, S. Elias, S. Katalan, N. Ashkenazi, M. Madmon, E. Gershonov and S. Saphier, J. Med. Chem., 2019, 62, 5628–5637 CrossRef CAS PubMed; (c) Y. Zafrani, D. Yeffet, G. Sod-Moriah, A. Berliner, D. Amir, D. Marciano, E. Gershonov and S. Saphier, J. Med. Chem., 2017, 60, 797–804 CrossRef CAS PubMed; (d) J. Li, W. Xi, S. Liu, Y. Yang, J. Yang, H. Ding and Z. Wang, Chin. Chem. Lett., 2022, 33, 3007–3011 CrossRef CAS; (e) J. Li, W. Xi, S. Liu, C. Ruan, X. Zheng, J. Yang, L. Wang and Z. Wang, Org. Lett., 2021, 23, 7264–7269 CrossRef CAS PubMed.
- For selected reviews, see: (a) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2014 Search PubMed; (b) I. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, John Wiley & Sons, Ltd, Chichester, UK, 2009 CrossRef; (c) T. Nakajima and H. Groult, Fluorinated Materials for Energy Conversion, Elsevier, London, 2005 Search PubMed.
- For selected examples, see: (a) J. Nie, H.-C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455–529 CrossRef CAS PubMed; (b) W.-R. Zhu, Q. Su, X.-Y. Deng, J.-S. Liu, T. Zhong, S.-S. Meng, J. T. Yi, J. Weng and G. Lu, Chem. Sci., 2022, 13, 170–177 RSC; (c) D. S. Nipate, S. Jaspal, V. N. Shinde, K. Rangan and A. Kumar, Org. Lett., 2021, 23, 1373–1377 CrossRef CAS PubMed; (d) L. Cai, Y. Zhao, T. Huang, S. Meng, X. Jia, A. S. C. Chan and J. Zhao, Org. Lett., 2019, 21, 3538–3542 CrossRef CAS PubMed; V. K. Pandey and P. Anbarasan, J. Org. Chem., 2017, 82, 12328–12336 Search PubMed.
- (a) Y. Ling, D. An, Y. Zhou and W. Rao, Org. Lett., 2019, 21, 3396–3401 CrossRef CAS; (b) X. X. Yuan, L. L. Wu, C. L. Xu, Z. L. Pan, L. J. Shi, G. Y. Yang, C. X. Wang and S. F. Fan, Tetrahedron Lett., 2019, 60, 151329 CrossRef CAS; (c) S. Sasaki, Y. Ikekame, M. Tanayama, T. Yamauchi and K. Higashiyama, Synlett, 2012, 23, 2699–2703 CrossRef CAS.
- M. Bandini and R. Sinisi, Org. Lett., 2009, 11, 2093–2096 CrossRef.
- X. T. Wu, F. Ma, E.-K. Xiao, J. Yin, F. Sun, Q. Wang, Y.-J. Jiang and P. Chen, Org. Biomol. Chem., 2022, 20, 7491–7498 RSC.
- (a) P. J. Czerwiński, B. Grzeszczyk and B. Furman, Org. Lett., 2022, 24, 9269–9274 CrossRef PubMed; (b) P. J. Czerwiński and B. Furman, Chem. Commun., 2019, 55, 9436–9439 RSC; (c) G.-W. Zhang, L. Wang, J. Nie and J.-A. Ma, Adv. Synth. Catal., 2008, 350, 1457–1463 CrossRef CAS.
- X. Cai, J. Xu, X. Cui, J. Qu, W. Sun, J. Hu, S. Zhao, W.-H. Chen, H. Li and J.-Q. Wu, Org. Chem. Front., 2022, 9, 6273–6280 RSC.
- For selected examples of using difluoroacetaldehyde ethyl hemiacetal as a difluoromethylated aldehyde equivalent, see: (a) J. M. Cabrera, J. Tauber, W. Zhang, M. Xiang and M. J. Krische, J. Am. Chem. Soc., 2018, 140, 9392–9395 CrossRef CAS; (b) Y. Ning, X. Zhang, Y. Gai, Y. Dong, P. Sivaguru, Y. Wang, B. R. P. Reddy, G. Zanoni and X. Bi, Angew. Chem., Int. Ed., 2020, 59, 6473–6481 CrossRef CAS PubMed; (c) Y. Ren, R. Ma, Y. Feng, K.-H. Wang, J. Wang, D. Huang, X. Lv and Y. Hu, Asian J. Org. Chem., 2022, 11, e202200438 CrossRef CAS; (d) K. Mikami, T. Takasaki, S. Matsukawa and M. Maruta, Synlett, 1995, 1057–1058 CrossRef CAS; (e) S. Kaneko, T. Yamazaki and T. Kitazume, J. Org. Chem., 1993, 58, 2302–2312 CrossRef CAS.
- J. Yang, J. Cui, M. Mu, S. Liu, J. Li, J. Ren and Z. Wang, J. Org. Chem., 2023, 88, 4790–4798 CrossRef CAS PubMed.
- X. Cui, J. Qu, J. Yi, W. Sun, J. Hu, S. Guo, J.-W. Jin, W.-H. Chen, W.-L. Wong and J.-Q. Wu, Chem. Commun., 2023, 59, 3747–3750 RSC.
- B. Shu, S.-Y. Chen, N.-X. Deng, T. Zheng, H. Xie, X.-L. Xie, J.-Q. Wu, H. Cao and S.-S. Zhang, Org. Chem. Front., 2021, 8, 4445–4451 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob01735d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |