A novel synthetic method for backbone-cyclized polypeptide POL7080 with the help of hydrophobic-support materials†
Xiaotong
Gu‡
,
Weijin
Chen‡
,
Ting
Guo
,
Xiaohong
Chang
,
Shenyan
Zhang
,
Bingfang
Bai
and
Shutao
Ma
 *
*
Department of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University. 44 West Wenhua Road, Jinan 250012, China. E-mail: mashutao@sdu.edu.cn
First published on 4th December 2023
Abstract
Murepavadin (POL7080) in phase III clinical trials, a backbone-cyclized polypeptide composed of 14 amino acids, has a novel mode of action and shows a specific and efficient bactericidal effect against multidrug-resistant Pseudomonas aeruginosa. It is a potential candidate to treat severe P. aeruginosa infections in the future and still has significant commercial value for further research and development. In this paper, we report a liquid-phase peptide synthetic route for this valuable candidate polypeptide assisted by hydrophobic-support materials (tags), which overcomes the difficulties of high cost and poor yield in the traditional solid-phase synthesis of macrocyclic peptides. Through the careful optimization of reaction conditions and the innovative strategy of synthetic post-treatment, we established a simple and efficient liquid-phase synthetic route suitable for POL7080 and other similar structures, with satisfactory yield, high purity and a production process not being controlled by scale.
Introduction
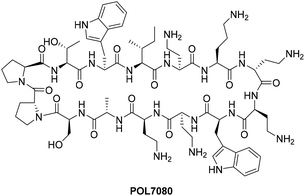
Nowadays, the resistance of bacterial pathogens to traditional antibiotics has attracted worldwide attention.1,2 More seriously, the development speed of new antibiotics is much lower than the current speed of development of antibiotic resistance. Therefore, it is urgent to develop new antibiotics against drug-resistant bacteria to address the severe emergence and spread of drug-resistant pathogens.3In terms of the crisis caused by drug-resistant bacteria, Pseudomonas aeruginosa poses the most serious challenge due to the lack of effective antibiotics.4,5 The World Health Organization has evaluated the antibacterial agents in clinical development with activity toward carbapenem-resistant P. aeruginosa (CRPA). Among them, the antibacterial cyclic peptide POL7080 (Fig. 1, murepavadin) shows an efficient antibacterial effect on P. aeruginosa because of its unique mechanism.6–8 We have observed excellent characteristics such as a high clinical cure rate, low mortality rate, and low drug resistance tendency in previous studies on treating ventilator-associated bacterial pneumonia (NCT02096328) with POL7080. Although it has presented unexpected problems in phase III clinical trials, researchers are trying to solve them by changing the mode of administration. In general, it is a potential candidate to treat severe P. aeruginosa infections in the future and still has significant commercial value for further research and development. At present, the synthetic routes for POL7080 are still based on solid-phase peptide synthesis (SPPS), but the disadvantages of high cost and poor yield brought by SPPS cannot be ignored.
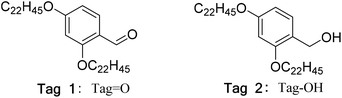
In recent years, the liquid-phase peptide synthesis (LPPS) method has increasingly attracted the attention of researchers. LPPS is more suitable for synthesizing oligopeptides and peptide fragments, and all organic synthesis methods can be used, which makes it easy to scale up the reaction.9,10 Kazuhiro Chiba reported synthetic routes for polypeptide drugs assisted by a kind of hydrophobic support (tag) (Fig. 2), the function of which was similar to that of resin materials in SPPS.11–14 With its help, the synthetic reaction can be carried out in a homogeneous liquid phase environment and monitored by common analytical techniques, such as thin layer chromatography (TLC). After the reaction is completed, a polar solvent can be added into the reaction liquid to precipitate the product in solid form by taking advantage of the fact that the hydrophobic supports are difficult to dissolve in polar solvents, while other components such as excess raw materials and by-products can be retained in polar solvents. This means that a relatively pure precipitated product can be obtained through a simple separation operation.
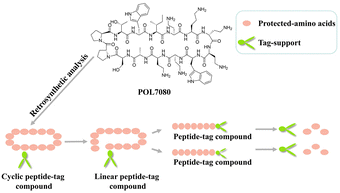
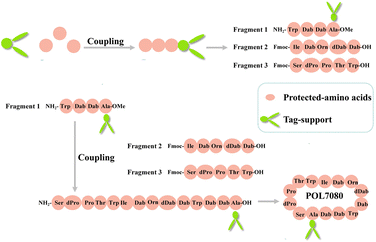
Based on this hydrophobic support-assisted synthesis method, we made a bold attempt to apply these hydrophobic supports to the synthesis of POL7080. First, we conducted a retrosynthetic analysis of POL7080 according to its structural characteristics (Fig. 3). A specific amino acid needs to be connected to a hydrophobic support to make the auxiliary material play its role in synthesis.
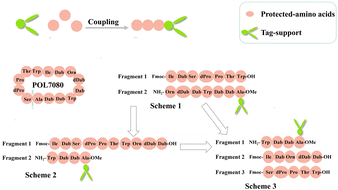
In the synthesis of POL7080, we first synthesized a linear peptide precursor assisted by hydrophobic materials and then closed the ring to obtain a cyclic peptide. Thus, we defined the carboxy-terminal amino acid of the ring opening site as a bridgehead amino acid and connected it to a hydrophobic support. In order to make the reaction efficient, we further divided the linear peptide precursor with fourteen amino acids into several intermediate fragments, which can be synthesized by using hydrophobic supports and the corresponding amino acids at the same time (Table 1).
| Scheme | Fragment | Amino acid sequence |
|---|---|---|
| Scheme 1 | Fragment 1 | Fmoc–Ile–Dab(Boc)–Ser(tBu)–D-Pro–Pro–Thr(tBu)–Trp(Boc)–OH |
| Fragment 2 | NH2–Orn(Boc)–D-Dab(Boc)–Dab(Boc)–Trp(Boc)–Dab(Boc)–Dab(Boc)–Ala(Tag)–OMe | |
| Scheme 2 | Fragment 1 | Fmoc–Ile–Dab(Boc)–Ser(tBu)–D-Pro–Pro–Thr(tBu)–Trp(Boc)–Orn(Boc)–D-Dab(Boc)–Dab(Boc)–OH |
| Fragment 2 | NH2–Trp(Boc)–Dab(Boc)–Dab(Boc)–Ala(Tag)–OMe | |
| Scheme 3 | Fragment 1 | NH2–Trp(Boc)–Dab(Boc)–Dab(Boc)–Ala(Tag)–OMe |
| Fragment 2 | Fmoc–Ile–Dab(Boc)–Orn(Boc)–D-Dab(Boc)–Dab(Boc)–OH | |
| Fragment 3 | Fmoc–Ser(tBu)–D-Pro–Pro–Thr(tBu)–Trp(Boc)–OH |
After carefully analyzing the structure of POL7080, we selected isoleucine and alanine with the lowest steric hindrance as the bridgehead amino acid candidates. Unfortunately, we found that the yield of coupling synthesis of the isoleucine-tag with the corresponding amino acid fragments was very low, while the alanine-tag was relatively easy to participate in a similar reaction. However, there was still a poor yield for the above types of reactions, which may be closely related to the steric hindrance of the amino acid-tags and their corresponding amino acid fragments. Thus, in the follow-up study, we made improvements.
After determining alanine as the bridgehead amino acid, POL7080 was divided equally into two fragments for the synthesis (Scheme 1 in Fig. 4), so as to improve the synthesis efficiency. During the synthesis of fragment 2, however, the solubility of the polypeptide-tag significantly decreased as the number of amino acids increased, which was very unfavorable for the subsequent reactions. Therefore, we redesigned a new scheme (Scheme 2 in Fig. 4). Although the synthesis problem of fragment 2 had been solved, a similar problem was also encountered in the synthesis of fragment 1. On the basis of comprehensively considering these two schemes, we designed a final scheme (Scheme 3 in Fig. 4) and confirmed the feasibility of this scheme in subsequent practice.
We first synthesized three target fragments based on a strategy for the total synthesis of POL7080 (Fig. 5), and then using fragment 1 as the starting material for synthesis, fragment 2 and fragment 3 were coupled in sequence to obtain a linear fully protected target fragment. Next, we removed the protective groups at the amino and carboxyl terminals to expose the cyclization reaction sites. Subsequently, a cyclization reaction was carried out to obtain the fully protected POL7080. Finally, we removed the hydrophobic support and protective groups from the pyrolysis solution to obtain the crude product of POL7080, followed by purification using preparative HPLC to give the high-purity product of POL7080.
Results and discussion
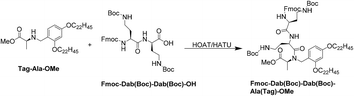
In general, with the help of hydrophobic-support materials (Tag![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Tag–OH), we successfully established a liquid-phase synthetic route for POL7080. In addition, Fmoc chemistry was applied in the total synthesis of POL7080 to supplement the hydrophobic-support materials. The poor yield of coupling synthesis of the alanine-tag with the corresponding amino acid fragment, as we have discussed before, is the crucial aspect of this synthesis route. Through careful optimization of the reaction conditions, the yield of this coupling synthesis was satisfactorily improved (Fig. 6).
O and Tag–OH), we successfully established a liquid-phase synthetic route for POL7080. In addition, Fmoc chemistry was applied in the total synthesis of POL7080 to supplement the hydrophobic-support materials. The poor yield of coupling synthesis of the alanine-tag with the corresponding amino acid fragment, as we have discussed before, is the crucial aspect of this synthesis route. Through careful optimization of the reaction conditions, the yield of this coupling synthesis was satisfactorily improved (Fig. 6).
The influence of conventional factors, such as temperature and feed ratio, on the coupling synthesis was investigated in detail (Table 2). Considering that the alanine-tag was relatively cheap and recyclable, we mainly increased its feed equivalent. It was observed that when the feed ratio of alanine-tag to the corresponding amino acid fragment was 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the reaction temperature was 40 °C, the highest yield of this coupling synthesis was achieved, which was 75.1%.
1 and the reaction temperature was 40 °C, the highest yield of this coupling synthesis was achieved, which was 75.1%.
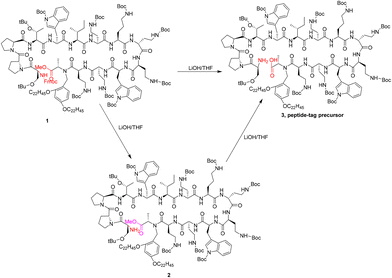
In addition, there are some noteworthy reaction details. For example, after obtaining a linear protected peptide-tag precursor 3, we needed to remove the protective groups at the amino and carboxyl terminals to expose the cyclization reaction sites (Fig. 7). Compared with the slow removal of the methoxy group, the Fmoc group in compound 1 could be removed very quickly. As a result, the rapid production of intermediate 2 was likely to lead to the misjudgment of the reaction endpoint.
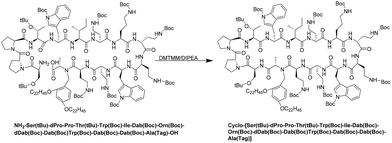
Furthermore, the cyclization reaction is also a critical step of this synthetic route (Fig. 8). There are two main ways for amide bond formation in macrocyclic peptides, namely head-to-tail cyclization and side chain to side chain cyclization. The former is usually carried out under liquid-phase conditions, while the latter is carried out under solid-phase conditions. POL7080 is prepared using the head-to-tail cyclization way. Kazuhiro Chiba has successfully cyclized a seven-amino acid polypeptide under similar reaction conditions. However, it is challenging to obtain the cyclic peptide-tag precursor of POL7080 using the previous coupling conditions due to its large steric hindrance. The substrate is more likely to undergo intermolecular reactions between adjacent molecules to form dimers, rather than intramolecular reactions at low concentrations because of the influence of the high activity of exposed active groups and large steric hindrance.
Therefore, we mainly optimized the coupling agents and their concentrations for the POL7080 intramolecular cyclization reaction, referring to other macrocyclic peptide synthesis protocols. To avoid side reactions during the intermolecular reaction, we controlled the concentration of the linear protected peptide-tag precursor between 0.15 and 0.6 mmol L−1. And it was observed that when the feed ratio of peptide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMTMM
DMTMM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIPEA was 1
DIPEA was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and the reaction temperature was 40 °C, the highest yield of this intramolecular cyclization reaction was 89.1%. Interestingly, when monitoring the reaction endpoint through TLC, we noticed that the cyclic protected peptide-tag product showed characteristic fluorescence at 365 nm. Prior to this, our raw materials and target products only exhibited fluorescence at 254 nm. Therefore, this feature can also be used to judge whether the cyclization reaction has occurred (Table 3).
20 and the reaction temperature was 40 °C, the highest yield of this intramolecular cyclization reaction was 89.1%. Interestingly, when monitoring the reaction endpoint through TLC, we noticed that the cyclic protected peptide-tag product showed characteristic fluorescence at 365 nm. Prior to this, our raw materials and target products only exhibited fluorescence at 254 nm. Therefore, this feature can also be used to judge whether the cyclization reaction has occurred (Table 3).
| Entry | Feed ratioa | Concb (mmol L−1) | Time (min) | Yield (%) |
|---|---|---|---|---|
a Feed ratio, method A: peptide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HATU HATU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HOAT HOAT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIPEA = 1 DIPEA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; method B: peptide 20; method B: peptide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMTMM DMTMM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIPEA = 1 DIPEA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; method C: peptide 20; method C: peptide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMTMM DMTMM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIPEA = 1 DIPEA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. HATU: O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate; HOAT: 1-hydroxy-7-azabenzotriazole; DMTMM: 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride.
b Substrate concentration. 20. HATU: O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate; HOAT: 1-hydroxy-7-azabenzotriazole; DMTMM: 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride.
b Substrate concentration.
|
||||
| 1 | A | 0.6 | 30 | 63.2 |
| 2 | B | 0.6 | 30 | 89.1 |
| 3 | C | 0.6 | 120 | 54.5 |
| 4 | B | 1.6 | >120 | <10 |
| 5 | B | 3.1 | >120 | <10 |
In addition, after the coupling reaction or removal of Fmoc groups, adverse solvents were added to precipitate the product in a solid state. However, the precipitated product may be very sticky, which can be ignored in small-scale reactions but can increase the difficulty of post-treatment in large-scale reactions. Our laboratory has creatively proposed a “capture and release” strategy using diatomite to solve the above problem. During post-treatment, an appropriate amount of diatomite was added and mixed with the precipitated product. The powdered polypeptide-tag products with diatomite can be quickly obtained by vacuum filtration and washing. Afterward, we added the powdered polypeptide-tag products with diatomite to a weakly polar solvent. We subjected them to ultrasonic treatment and filtration to give the polypeptide-tag products, which were directly used in the subsequent coupling or deprotection reaction. We also found that the reaction impurities were removed more thoroughly in the post-treatment using the “capture and release” strategy.
The fully protected POL7080 was added to a solid acid solution to remove all protective groups and the hydrophobic support. Then, an appropriate amount of isopropyl ether was added to precipitate the crude POL7080. After separation and purification by preparative HPLC, the pure product of POL7080 was obtained with a purity of 98%.
The antibacterial activity of POL7080 from our group was tested through the broth micro-dilution method. The minimum inhibitory concentration (MIC) data of the test compounds against two Gram-positive strains (Bacillus subtilis ATCC9372 and Staphylococcus aureus ATCC25923) and three Gram-negative strains (Escherichia coli ATCC25922, P. aeruginosa ATCC27853 and P. aeruginosa PAO1) are listed in Table S1 in the ESI.† Ciprofloxacin (Adamas life, China) and vancomycin (Meryer, China) were tested as positive controls. Our group's sample of POL7080 showed similar activity against P. aeruginosa (MIC = 0.25 μg mL−1) to the POL7080 (MIC = 0.125–0.25 μg mL−1) reported in the literature.7
Conclusions
In summary, after meticulous optimization of reaction conditions and innovation of post-synthetic treatment methods, we have successfully established a simple, efficient, and low-cost synthetic route for POL7080 with the help of hydrophobic supports (tags). This synthetic route has laid a good foundation for the large-scale production of POL7080. This successful and feasible practice of synthesizing 14 amino acid cyclic peptides provides a new idea for synthesizing other similar backbone-cyclized polypeptides.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (81973179).References
- R. P. Pandey, R. Mukherjee and C. M. Chang, Antibiotics, 2022, 11, 476–495 CrossRef CAS.
- T. Pulingam, T. Parumasivam, A. M. Gazzali, A. M. Sulaimana, J. Y. Chee, M. Lakshmanan, C. F. Chin and K. Sudesh, Eur. J. Pharm. Sci., 2022, 170, 106103–106120 CrossRef CAS PubMed.
- A. Rai, R. Ferrao, P. Palma, T. Patricio, P. Parreira, E. Anes, C. Tonda-Turo, M. C. L. Martins, N. Alves and L. Ferreira, J. Mater. Chem. B, 2022, 10, 2384–2429 RSC.
- M. Assefa, Pneumonia, 2022, 14, 1–12 CrossRef.
- F. C. Tenover, D. P. Nicolau and C. M. Gill, Emerging Microbes Infect., 2022, 11, 811–814 CrossRef CAS PubMed.
- M. Rijsbergen, R. Rijneveld, M. Todd, G. L. Feiss, S. T. P. Kouwenhoven, K. D. Quint, D. van Alewijk, M. N. C. de Koning, E. S. Klaassen, J. Burggraaf, R. Rissmann and M. I. E. van Poelgeest, Br. J. Clin. Pharmacol., 2020, 86, 2133–2143 CrossRef CAS.
- I. Martin-Loeches, G. E. Dale and A. Torres, Expert Rev. Anti-Infect. Ther., 2018, 16, 259–268 CrossRef CAS.
- J. A. Robinson, S. C. Shankaramma, P. Jettera, U. Kienzl, R. A. Schwendener, J. W. Vrijbloed and D. Obrecht, Bioorg. Med. Chem., 2005, 13, 2055–2064 CrossRef CAS.
- X. B. Liu, N. Zhang, X. T. Gu, Y. H. Qin, D. Song, L. Zhang and S. T. Ma, ACS Comb. Sci., 2020, 22, 821–825 CrossRef CAS PubMed.
- N. Zhang, X. T. Gu, D. Song, P. P. Zhang, N. Zhang, L. Zhang and S. T. Ma, Tetrahedron Lett., 2021, 79, 153299–153303 CrossRef CAS.
- G. Tana, S. Kitada, S. Fujita, Y. Okada, S. Kim and K. Chiba, Chem. Commun., 2010, 46, 8219–8221 RSC.
- S. Kitada, S. Fujita, Y. Okada, S. Kim and K. Chiba, Bioorg. Med. Chem. Lett., 2011, 21, 4476–4479 CrossRef CAS PubMed.
- S. Kitada, S. Fujita, Y. Okada, S. Kim and K. Chiba, Tetrahedron, 2013, 69, 2555–2559 CrossRef CAS.
- Y. Okada, H. Suzuki, T. Nakae, S. Fujita, H. Abe, K. Nagano, T. Yamada, N. Ebata, S. Kim and K. Chiba, J. Org. Chem., 2013, 78, 320–327 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob01670f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |