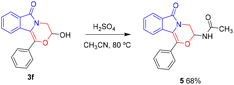Synthesis of fungicidal morpholines and isochromenopyridinones via acid-catalyzed intramolecular reactions of isoindolinones†
Xiaoyu
Liu
 a,
Yaru
Sun
a,
Shuang
Hong
a,
Xia
Ji
a,
Wei
Gao
a,
Haolin
Yuan
a,
Yaru
Sun
a,
Shuang
Hong
a,
Xia
Ji
a,
Wei
Gao
a,
Haolin
Yuan
 a,
Yue
Zhang
a,
Bin
Lei
b,
Liangfu
Tang
*ac and
Zhijin
Fan
*ac
a,
Yue
Zhang
a,
Bin
Lei
b,
Liangfu
Tang
*ac and
Zhijin
Fan
*ac
aState Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, People's Republic of China
bPesticide Production and Experiment Center, Xinjiang Academy of Agricultural Sciences, Urumqi 830091, China
cFrontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin 300071, People's Republic of China
First published on 28th November 2023
Abstract
Acid-catalyzed intramolecular cyclization or rearrangement of isoindolinone derivatives is described. 3-Hydroxy/ethoxy-3,4-dihydro-6H-[1,4]-oxazino-[3,4-a]-isoindol-6-ones are obtained in moderate to good yields. Further acid-catalyzed intramolecular rearrangement reactions give 6H-isochromeno-[4,3-b]-pyridin-6-ones. The mild reaction conditions with convenient starting materials show broad substrate scope and provide the target compounds as novel pesticide leads with good fungicidal or systemical acquired resistance activities.
Introduction
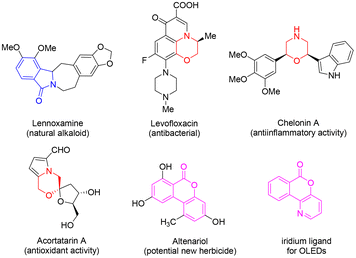
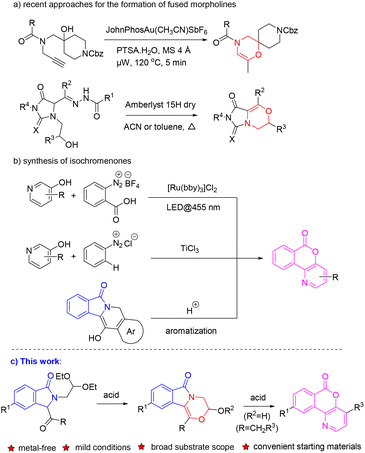
Nitrogen- and oxygen-containing heterocyclic compounds are widely found in natural products, drugs and agrochemicals. As important heterocyclic molecules, morpholines, isoindolinones and isochromenones show a wide range of applications in the fields of medicine, agriculture and materials science. For instance, the natural alkaloid lennoxamine contains isoindolinone.1 Antibacterial drug levofloxacin contains a morpholine structure.2 Chelonin A with anti-inflammatory activity3 and acotatarin A with antioxidant activity are also morpholine derivatives.4 As biological isochromenone chemicals, altenariol is a new lead compound with herbicidal activity for weed control,5 and isochromenopyridinone is used as the iridium ligand for OLEDs (Fig. 1).6Due to the wide applications of morpholines, construction of their core skeleton has attracted considerable attention.7 The most common approach for the synthesis of the morpholine ring is the intramolecular cyclization of a substituted 1,2-amino alcohol. Compared to the base-mediated SN2 reaction,8 transition metal-catalyzed synthesis draws more and more attention;9 for example, Soklou et al. reported a gold-catalyzed method for the cyclization of tertiary alcohols with terminal alkyne to yield morpholine-containing aza-spirocycles;9a Borah and co-workers reported palladium-catalyzed intramolecular cyclization of nitrogen-tethered alkenols to substituted morpholines;9b the Morandi group reported that an iron salt-catalyzed aliphatic ether metathesis reaction could be utilized to furnish the morpholine core (Scheme 1a).9c On the other hand, isochromenopyridinones could be prepared in moderate yield by treating the diazonium salt of anthranilic acid in the presence of TiCl3 with 3-hydroxypyridines in aqueous hydrochloric acid.10 Among the preparation methods for isochromenopyridinones, rearrangement of isoindolinones showed the highest yield; however, the starting material of isoindolinone is relatively complex with the substrate scope limited only to three fused aromatic heterocycles (Scheme 1b).11 Therefore, the development of synthetic methods for isochromenopyridinones with improved efficiency is necessary. There are various methods for the synthesis of isoindolinones.12 Among them, our former studies on the convenient synthesis of 3-position functionalized isoindolinone derivatives provided a basis for the efficient transformation of fused morpholines and isochromenopyridinones.13 Herein, we report an efficient synthesis method for 3-ethoxy/hydroxy-3,4-dihydro-6H-[1,4]-oxazino-[3,4-a]-isoindol-6-ones and 6H-isochromeno-[4,3-b]-pyridin-6-ones via acid-catalyzed intramolecular reactions of isoindolinones under mild conditions.
Results and discussion
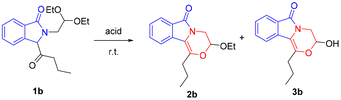
According to the previous methods developed by our group,13 the starting materials N-acetal-substituted-3-acyl isoindolinones (1) are readily available from a one-pot reaction of o-lithiated aromatic imines with carbon monoxide followed by the use of acyl chlorides as the electrophiles under mild reaction conditions on a gram scale (see the ESI†). With compounds 1 in hand, we were able to explore their reactivity under acid-catalyzed conditions (Table 1). When 1b was treated with trifluoromethanesulfonic acid (TfOH) in dichloromethane, dichloroethane or toluene (entries 1–3), 2b was obtained as the main product, and a trace amount of 3b could be detected by TLC. Using dichloromethane as the solvent, we selectively synthesized compound 2b with the highest yield (74%, entry 1). However, in solvent THF, 3b became the main product, no 2b was detected (entry 4), and the above results show a direct influence of the solvent on the formation of final products. In addition, when the acid was changed to concentrated sulfuric acid and THF was used as the solvent, the yield of 3b increased to 60% along with the extension of the reaction time (entry 8), which provides a method for the selective synthesis of 3b. Acetic acid could not catalyze this reaction (entry 11). p-Toluenesulfonic acid (pTSA) and AlCl3 could catalyze this reaction, but the selectivity of 2b and 3b was poor (entries 12 and 13).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Acid | Solvent | Time |
2b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f (%) f (%) |
3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f (%) f (%) |
|---|---|---|---|---|---|
| a Reaction conditions: all reactions were performed with 1b (0.5 mmol, 1.0 equiv.), acid (1.0 or 8.0 equiv.) and solvent (25 mL) in a round bottom flask at room temperature under air conditions. b 1.0 equiv. c 8.0 equiv. d Detected by TLC. e Isolated unconverted 1b (21%). f Isolated yield based on 1b. | |||||
| 1 | TfOH | CH 2 Cl 2 | 1 min | 74 | Trace |
| 2 | TfOHb | ClCH2CH2Cl | 1 min | 48 | Traced |
| 3 | TfOHb | Toluene | 1 min | 50 | Traced |
| 4 | TfOHb | THF | 1 min | 0 | 43 |
| 5 | TfOHb | CH2Cl2 | 12 h | 50 | Traced |
| 6 | TfOHb | Toluene | 4 h | 48 | Traced |
| 7 | H2SO4c | CH2Cl2 | 12 h | Traced | Traced |
| 8 | H 2 SO 4 | THF | 12 h | 0 | 60 |
| 9 | H2SO4b | THF | 12 h | 0 | 38e |
| 10 | CF3COOHb | CH2Cl2 | 12 h | 26 | 45 |
| 11 | CH3COOHb | CH2Cl2 | 12 h | 0 | 0 |
| 12 | pTSAb | CH2Cl2 | 12 h | 49 | 42 |
| 13 | AlCl3b | CH2Cl2 | 12 h | 43 | 33 |
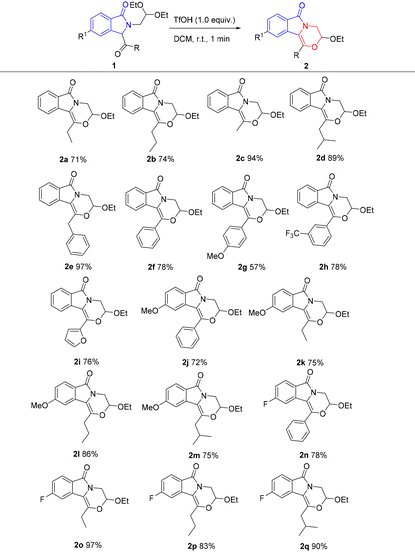
With the established optimal conditions, compounds 2 were selectively obtained by treating compounds 1 with TfOH in dichloromethane. This rapid and mild reaction shows broad substrate scope. As shown in Table 2, alkyl (2a–2d), benzyl (2e), phenyl (2f), electron-donating aryl (2g), electron-withdrawing aryl (2h), and furanyl (2i) are all compatible, generating the fused morpholine derivatives in yields of 57–97%. Electron-donating methoxy (2j–2m) and electron-withdrawing fluorine (2n–2q) on the isoindolinone ring are tolerable with yields of 72–86% and 78–97%, respectively.
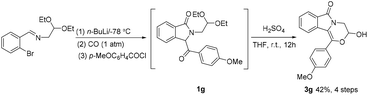
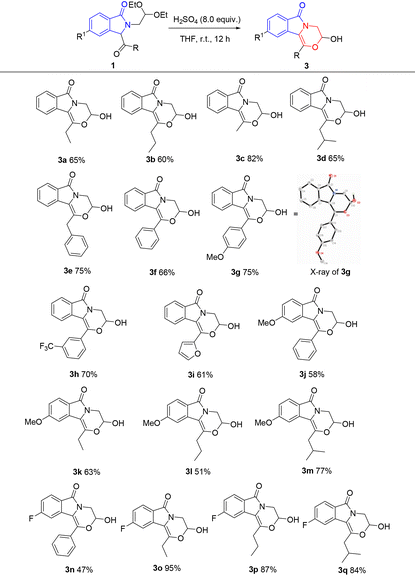
Compounds 3 are obtained under the optimal conditions by treating 1 with concentrated sulfuric acid in THF. This reaction also shows broad substrate scope. As shown in Table 3, alkyl (3a–3d), benzyl (3e), phenyl (3f), electron-donating aryl (3g), electron-withdrawing aryl (3h), and heterocyclic furanyl (3i) are all compatible, leading to the synthesis of fused morpholines with a hemiacetal structure in yields of 60%–82%. Electron-donating methoxy on the isoindolinone ring is tolerable with yields of 51%–77% (3j–3m), and electron-withdrawing fluorine is also compatible with yields of 47–95% (3n–3q). Moreover, compound 3g can be obtained through a “one-pot” method starting from o-bromobenzaldehyde imine with a yield of 42%, namely the isolation of the starting material 1g is not necessary (Scheme 2).
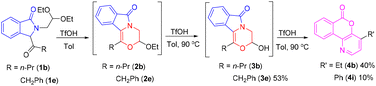
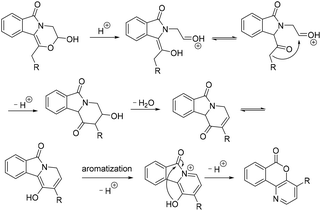
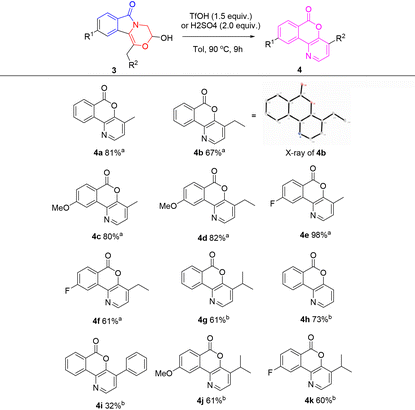
Surprisingly, 2b cannot be transformed into 3b irrespective of whether water is added or not under the conditions listed in Table 3, and the results show that the acetal structure of 2b is considerably stable under these reaction conditions. Although compounds 1 are potential precursors for the intramolecular aldol reaction,14 a rearrangement reaction is observed instead of only the aldol reaction. When TfOH was added to a toluene (Tol) solution of 1b, rapid formation of 2b was observed (entry 3, Table 1). With the increase of reaction temperature, 2b was transformed into 3b, which subsequently underwent an intramolecular rearrangement to produce compound 4b in 40% overall yield (Scheme 3). However, a similar reaction of 1e gave 3e as the major product in 53% yield, along with the desired rearrangement product 4i in only 10% yield. We also found that 3e was considerably stable, and the conversion of 3e into 4i hardly occurred under a heated TfOH/toluene system. However, the direct conversion of 3b into 4b was successful under such conditions, which gave 4b in 67% yield (Table 4). At the same time, the rearrangement products 4a and 4c–4f were also obtained from the corresponding hemiacetals 3 under a heated TfOH/toluene system in good to excellent yields. To our surprise, the rearrangement reaction of 3e to 4i occurred in 32% yield when concentrated sulfuric acid was used instead of TfOH. Compounds 4g, 4h, 4j and 4k were also obtained in good yields under a heated concentrated H2SO4/toluene system. Different acids possibly affect the equilibrium of hemiacetal to aldehyde, which subsequently undergoes a tandem aldol/rearrangement reaction to generate isochromenopyridinones 4 (Scheme 4).11
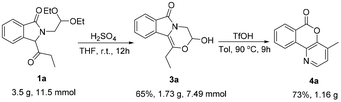
To prove the practical application value of this method, amplification experiments were done where 1a was used as the starting material (Scheme 5). Compounds 3a (65%) and 4a (73%) were obtained on a gram scale.
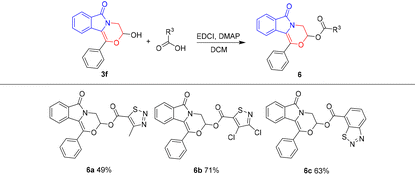
It is known that hemiacetal hydroxyl is an active functional group, so compounds 3 are anticipated to show great potential in other organic transformations. For example, the Ritter reaction of 3f with acetonitrile was achieved, which afforded compound 5 in 68% yield (Scheme 6). In addition, the esterification reaction of 3f was also successful (Table 5), which gave potential biologically active compounds 6.15
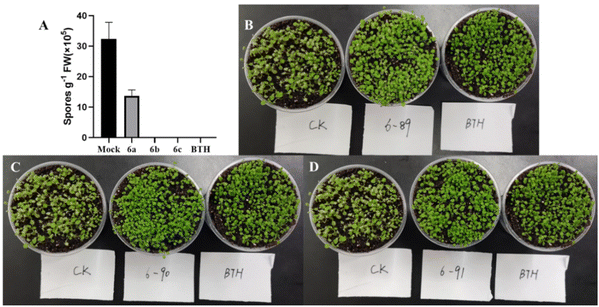
To evaluate the biological activities of compounds, the fungicidal activities of compounds against Alternaria solani (A. s), B. cinerea (B. c), Cercospora arachidicola (C. a), F. graminearum (F. g), Physalospora piricola (P. p), R. solani (R. s), and Sclerotinia sclerotiorum (S. s) were preliminarily evaluated in vitro (Table 6).16 The bioassay results indicated that compounds 2g, 2n, 4g, 4h and 4k exhibited good activities against the above-mentioned phytopathogens in inhibiting mycelial growth at a concentration of 50 μg mL−1. The activities of 6b and 6c against Hyaloperonospora arabidopsidis on Arabidopsis thaliana showed good systemical acquired resistance activities in vivo (Fig. 2),17 which highlights the important value of this investigation.
| Compound | A. s | B. c | C. a | F. g | P. p | R. s | S. s |
|---|---|---|---|---|---|---|---|
| 2a | 22 ± 1 | 8 ± 1 | 19 ± 2 | 29 ± 2 | 12 ± 1 | 30 ± 1 | 13 ± 1 |
| 2b | 16 ± 1 | 0 | 13 ± 2 | 24 ± 2 | 2 ± 1 | 25 ± 1 | 14 ± 3 |
| 2c | 0 ± 3 | 0 | 7 ± 1 | 9 ± 1 | 0 | 12 ± 3 | 0 ± 2 |
| 2d | 12 ± 1 | 0 | 5 ± 1 | 34 ± 1 | 7 ± 1 | 27 ± 1 | 15 ± 3 |
| 2e | 29 ± 2 | 0 | 23 ± 1 | 37 ± 1 | 16 ± 3 | 24 ± 2 | 36 ± 2 |
| 2f | 25 ± 3 | 20 ± 3 | 25 ± 1 | 47 ± 2 | 22 ± 0 | 27 ± 3 | 33 ± 3 |
| 2g | 57 ± 1 | 42 ± 1 | 55 ± 1 | 74 ± 0 | 48 ± 1 | 52 ± 1 | 66 ± 1 |
| 2h | 48 ± 1 | 55 ± 1 | 34 ± 1 | 63 ± 1 | 35 ± 2 | 39 ± 1 | 35 ± 2 |
| 2i | 13 ± 2 | 0 | 10 ± 1 | 22 ± 0 | 0 | 22 ± 2 | 20 ± 1 |
| 2j | 22 ± 1 | 5 ± 1 | 17 ± 1 | 20 ± 2 | 11 ± 2 | 18 ± 1 | 21 ± 0 |
| 2k | 27 ± 1 | 31 ± 1 | 53 ± 0 | 72 ± 1 | 52 ± 1 | 57 ± 1 | 65 ± 1 |
| 2l | 26 ± 1 | 27 ± 1 | 25 ± 1 | 31 ± 1 | 13 ± 2 | 24 ± 1 | 27 ± 1 |
| 2m | 17 ± 1 | 26 ± 1 | 27 ± 1 | 33 ± 2 | 14 ± 2 | 26 ± 1 | 18 ± 3 |
| 2n | 67 ± 2 | 40 ± 2 | 57 ± 2 | 72 ± 1 | 45 ± 1 | 65 ± 2 | 70 ± 1 |
| 2o | 15 ± 1 | 0 | 27 ± 1 | 29 ± 1 | 24 ± 1 | 20 ± 1 | 26 ± 2 |
| 2p | 7 ± 1 | 0 | 20 ± 2 | 21 ± 2 | 16 ± 3 | 17 ± 1 | 27 ± 1 |
| 2q | 11 ± 1 | 6 ± 1 | 15 ± 4 | 17 ± 2 | 10 ± 2 | 27 ± 1 | 14 ± 2 |
| 3a | 2 ± 3 | 0 | 17 ± 2 | 33 ± 1 | 4 ± 3 | 29 ± 3 | 23 ± 3 |
| 3b | 10 ± 1 | 0 | 19 ± 3 | 30 ± 1 | 5 ± 3 | 28 ± 1 | 18 ± 1 |
| 3c | 12 ± 0 | 27 ± 0 | 16 ± 4 | 16 ± 1 | 8 ± 1 | 28 ± 0 | 14 ± 2 |
| 3d | 10 ± 1 | 19 ± 1 | 9 ± 1 | 11 ± 3 | 13 ± 5 | 22 ± 1 | 14 ± 2 |
| 3e | 49 ± 1 | 0 | 28 ± 2 | 38 ± 1 | 9 ± 1 | 28 ± 1 | 27 ± 1 |
| 3f | 25 ± 1 | 0 | 23 ± 1 | 70 ± 6 | 24 ± 0 | 29 ± 1 | 23 ± 1 |
| 3g | 18 ± 1 | 0 | 11 ± 1 | 63 ± 1 | 14 ± 1 | 15 ± 1 | 15 ± 3 |
| 3h | 36 ± 1 | 1 ± 1 | 20 ± 2 | 70 ± 1 | 30 ± 1 | 30 ± 1 | 31 ± 1 |
| 3i | 27 ± 1 | 0 | 22 ± 3 | 29 ± 1 | 2 ± 2 | 19 ± 1 | 15 ± 1 |
| 3j | 27 ± 1 | 31 ± 1 | 18 ± 1 | 32 ± 2 | 10 ± 2 | 33 ± 1 | 15 ± 1 |
| 3k | 23 ± 1 | 0 | 13 ± 2 | 13 ± 2 | 1 ± 2 | 18 ± 1 | 18 ± 1 |
| 3l | 31 ± 1 | 26 ± 1 | 21 ± 1 | 21 ± 1 | 9 ± 2 | 22 ± 1 | 25 ± 1 |
| 3m | 31 ± 2 | 0 | 10 ± 1 | 8 ± 2 | 15 ± 2 | 9 ± 2 | 18 ± 1 |
| 3n | 34 ± 1 | 0 | 19 ± 2 | 42 ± 1 | 12 ± 3 | 27 ± 1 | 23 ± 1 |
| 3o | 26 ± 1 | 0 | 13 ± 1 | 27 ± 0 | 4 ± 3 | 15 ± 1 | 22 ± 2 |
| 3p | 33 ± 1 | 49 ± 1 | 13 ± 1 | 26 ± 3 | 16 ± 2 | 19 ± 1 | 19 ± 2 |
| 3q | 30 ± 1 | 38 ± 1 | 8 ± 2 | 22 ± 2 | 11 ± 1 | 18 ± 1 | 19 ± 2 |
| 4a | 48 ± 1 | 42 ± 1 | 33 ± 0 | 41 ± 3 | 36 ± 0 | 61 ± 0 | 25 ± 0 |
| 4b | 51 ± 0 | 56 ± 0 | 30 ± 0 | 49 ± 2 | 43 ± 0 | 64 ± 0 | 33 ± 0 |
| 4c | 30 ± 0 | 20 ± 9 | 17 ± 1 | 33 ± 2 | 35 ± 1 | 34 ± 0 | 23 ± 1 |
| 4d | 52 ± 0 | 33 ± 2 | 36 ± 0 | 64 ± 4 | 69 ± 1 | 64 ± 0 | 55 ± 1 |
| 4e | 53 ± 2 | 51 ± 2 | 73 ± 2 | 0 | 55 ± 2 | 73 ± 1 | 46 ± 2 |
| 4f | 57 ± 0 | 17 ± 2 | 58 ± 1 | 21 ± 2 | 48 ± 0 | 69 ± 0 | 53 ± 2 |
| 4g | 66 ± 0 | 73 ± 2 | 43 ± 2 | 66 ± 0 | 53 ± 0 | 71 ± 0 | 50 ± 2 |
| 4h | 52 ± 1 | 89 ± 1 | 78 ± 1 | 30 ± 1 | 66 ± 1 | 76 ± 1 | 51 ± 1 |
| 4i | 13 ± 0 | 39 ± 0 | 15 ± 0 | 31 ± 2 | 22 ± 1 | 28 ± 1 | 3 ± 0 |
| 4j | 13 ± 0 | 29 ± 2 | 21 ± 0 | 33 ± 2 | 3 ± 3 | 24 ± 4 | 3 ± 0 |
| 4k | 53 ± 2 | 51 ± 2 | 75 ± 1 | 0 | 62 ± 2 | 70 ± 1 | 46 ± 2 |
| 6a | 25 ± 5 | 82 ± 2 | 15 ± 1 | 17 ± 0 | 16 ± 1 | 25 ± 1 | 30 ± 1 |
| 6b | 23 ± 9 | 60 ± 3 | 18 ± 1 | 13 ± 0 | 15 ± 1 | 23 ± 5 | 16 ± 1 |
| 6c | 19 ± 4 | 80 ± 1 | 20 ± 1 | 24 ± 0 | 22 ± 2 | 35 ± 1 | 25 ± 0 |
| BTH | 13 ± 2 | 14 ± 8 | 18 ± 0 | 12 ± 1 | 19 ± 1 | 74 ± 1 | 5 ± 1 |
Conclusions
In summary, we developed new approaches for the synthesis of bioactive leads with systemical acquired resistance by fusing morpholines and isochromenopyridinones via acid-catalyzed intramolecular tandem reactions of isoindolinone derivatives. The methods are simple and efficient with broad substrate scope and good to excellent yields. The protocol is easy to apply at the gram level and is helpful for further lead modification of these bioactive derivatives in the areas of pharmacy and agrochemistry.Author contributions
Xiaoyu Liu: investigation, methodology, bioactivity test, and writing – original draft and editing; Yaru Sun: methodology; Shuang Hong, Xia Ji, Wei Gao, Haolin Yuan, Yue Zhang and Bin Lei: bioactivity test; Liangfu Tang and Zhijin Fan: project administration, supervision, funding acquisition and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by the National Key Research and Development Program of China (no. 2022YFD1700400 and 2022YFD1700402); the Tianjin Natural Science Foundation (no. 21JCZXJC00110); the Frontiers Science Center for New Organic Matter, Nankai University (no. 63181206); and the Key Research and Development Program of Xinjiang Uygur Autonomous Region (no. 2022B02001).References
- (a) E. Valencia, I. Weiss, S. Firdous, A. J. Freyer, M. Shamma, A. Urzús and V. Fajardo, Tetrahedron, 1984, 40, 3957 CrossRef CAS; (b) D. L. Comins, S. Schilling and Y. Zhang, Org. Lett., 2005, 7, 95 CrossRef CAS.
- (a) S. Atarashi, S. Yokohama, K. Yamazaki, K. Sakano, M. Imamura and I. Hayakawa, Chem. Pharm. Bull., 1987, 35, 1896 CrossRef CAS PubMed; (b) V. R. Anderson and C. M. Perry, Drugs, 2008, 68, 535 CrossRef CAS PubMed.
- (a) S. C. Bobzin and D. J. Faulkner, J. Org. Chem., 1991, 56, 4403 CrossRef CAS; (b) Y. Wang, W. Y. Zhang and S. L. You, J. Am. Chem. Soc., 2019, 141, 2228 CrossRef CAS; (c) S. M. S. Atta, D. S. Farrag, A. M. K. Sweed and A. H. Abdel-Rahman, Eur. J. Med. Chem., 2010, 45, 4920 CrossRef CAS PubMed; (d) L. A. Baltina, H. C. Lai, Y. C. Liu, S. H. Huang, M. J. Hour, L. A. Baltina, T. R. Nugumanov, S. S. Borisevich, L. M. Khalilov, S. F. Petrova, S. L. Khursan and C. W. Lin, Bioorg. Med. Chem., 2021, 41, 116204 CrossRef CAS.
- X.-G. Tong, L.-L. Zhou, Y.-H. Wang, C. Xia, Y. Wang, M. Liang, F.-F. Hou and Y.-X. Cheng, Org. Lett., 2010, 12, 1844 CrossRef CAS PubMed.
- (a) Z. Mao, W. Sun, L. Fu, H. Luo, D. Lai and L. Zhou, Molecules, 2014, 19, 5088 CrossRef PubMed; (b) H. Wang, Y. Guo, Z. Luo, L. Gao, R. Li, Y. Zhang, H. M. Kalaji, S. Qiang and S. Chen, J. Fungi, 2022, 8, 168 CrossRef CAS.
- (a) X. Ren, M. E. Kondakova, D. J. Giesen, M. Rajswaran, M. Madaras and W. C. Lenhart, Inorg. Chem., 2010, 49, 1301 CrossRef CAS PubMed; (b) M. J. Jurow, C. Mayr, T. D. Schmidt, T. Lampe, P. I. Djurovich, W. Brütting and M. E. Thompson, Nat. Mater., 2016, 15, 85 CrossRef CAS PubMed.
- (a) R. Wijtmans, M. K. S. Vink, H. E. Schoemaker, F. L. van Delft, R. H. Blaauw and F. P. J. T. Rutjes, Synthesis, 2004, 641 CAS; (b) J. Ilaš, P. Š. Anderluh, M. S. Dolenc and D. Kikelj, Tetrahedron, 2005, 61, 7325 CrossRef; (c) V. A. Pal'chikov, Russ. J. Org. Chem., 2013, 49, 787 CrossRef; (d) A. Tzara, D. Xanthopoulos and A. P. Kourounakis, ChemMedChem, 2020, 15, 392 CrossRef CAS.
- (a) F. Foschi, D. Albanese, I. Pecnikaj, A. Tagliabue and M. Penso, Org. Lett., 2017, 19, 70 CrossRef CAS PubMed; (b) J. V. Matlock, T. D. Svejstrup, P. Songara, S. Overington, E. M. McGarrigle and V. K. Aggarwal, Org. Lett., 2015, 17, 5044 CrossRef CAS PubMed; (c) J. Zhou and Y.-Y. Yeung, J. Org. Chem., 2014, 79, 4644 CrossRef CAS PubMed; (d) E. Lenci, A. Rossi, G. Menchi and A. Trabocchi, Org. Biomol. Chem., 2017, 15, 9710 RSC.
- (a) K. E. Soklou, H. Marzag, B. Vallée, S. Routier and K. Plé, Adv. Synth. Catal., 2022, 364, 218 CrossRef CAS; (b) M. Borah, U. Borthakur and A. K. Saikia, J. Org. Chem., 2017, 82, 1330 CrossRef CAS PubMed; (c) T. Biberger, S. Makai, Z. Lian and B. Morandi, Angew. Chem., Int. Ed., 2018, 57, 6940 CrossRef CAS PubMed; (d) M. U. Luescher, C.-V. T. Vo and J. W. Bode, Org. Lett., 2014, 16, 1236 CrossRef CAS; (e) C. Jindakun, S.-Y. Hsieh and J. W. Bode, Org. Lett., 2018, 20, 2071 CrossRef CAS; (f) Y. Y. Lau, H. Zhai and L. L. Schafer, J. Org. Chem., 2016, 81, 8696 CrossRef CAS; (g) S. A. Ruider, S. Müller and E. M. Carreira, Angew. Chem., Int. Ed., 2013, 52, 11908 CrossRef CAS.
- (a) M. C. D. Fürst, L. R. Bock and M. R. Heinrich, J. Org. Chem., 2016, 81, 5752 CrossRef PubMed; (b) S. Crespi, S. Jäger, B. König and M. Fagnoni, Eur. J. Org. Chem., 2017, 2147 CrossRef CAS.
- M. Othman, P. Pigeon and B. Decroix, Tetrahedron, 1998, 54, 8737 CrossRef CAS.
- (a) R. Savela and C. Méndez-Gálvez, Chem. – Eur. J., 2021, 27, 5344 CrossRef CAS PubMed; (b) S. Samanta, S. A. Ali, A. Bera, S. Giri and K. Samanta, New J. Chem., 2022, 46, 7780 RSC; (c) Z. Xie, L. Jin, H. Li, J. Meng and Z. Le, Synthesis, 2023, 3172 CrossRef CAS; (d) M. K. Gupta, A. Panda, S. Panda and N. K. Sharma, Org. Biomol. Chem., 2023, 21, 5104 RSC; (e) C. Wiethan, I. B. Braga, V. H. Menezes da Silva, J. M. Batista Jr. and C. R. D. Correia, ChemCatChem, 2023, 15, e2023004 CrossRef; (f) T. Ma, J. Hua, M. Bian, H. Qin, X. Lin, X. Yang, C. Liu, Z. Yang, Z. Fang and K. Guo, Org. Chem. Front., 2022, 9, 25 RSC; (g) X. Du, Y. Hu, D. Yang, D. Huang, W. Yang, H. Wu and H. Zhao, Org. Biomol. Chem., 2022, 20, 783 RSC.
- (a) H.-J. Li, Y.-Q. Zhang and L.-F. Tang, Tetrahedron, 2015, 71, 7681 CrossRef CAS; (b) Y. Xu, X.-Y. Liu, Z.-H. Wang and L.-F. Tang, Tetrahedron, 2017, 73, 7245 CrossRef CAS; (c) X.-Y. Liu, X.-M. Luo and L.-F. Tang, Tetrahedron, 2020, 76, 131341 CrossRef CAS.
- Y. Quevedo-Acosta, I. D. Jurberg and D. Gamba-Sánchez, Org. Lett., 2020, 22, 239 CrossRef CAS PubMed.
- (a) M. Oostendorp, W. Kunz, B. Dietrich and T. Staub, Eur. J. Plant Pathol., 2001, 107, 19 CrossRef CAS; (b) K. Tsubata, K. Kuroda, Y. Yamamoto and N. Yasokawa, J. Pestic. Sci., 2006, 31, 161 CrossRef CAS; (c) Z. J. Fan, Z. G. Shi, H. K. Zhang, X. F. Liu, L. L. Bao, L. Ma, X. Zhou, Q. X. Zheng and N. Mi, J. Agric. Food Chem., 2009, 57, 4279 CrossRef CAS; (d) Y. Bektas and T. Eulgem, Front. Plant Sci., 2015, 5, 804 Search PubMed; (e) M. Markiewicz, P. Lewandowski, M. Spychalski, R. Kukawka, J. Feder-Kubis, S. Beil, M. Smiglak and S. Stolte, Green Chem., 2021, 23, 5138 RSC; (f) Y. Zhang, K. Li, W. Gao, X. Liu, H. Yuan, L. Tang and Z. Fan, Org. Lett., 2022, 24, 6599 CrossRef CAS PubMed.
- Z. Hao, W. Wang, B. Yu, X. Qi, Y. Lv, X. Liu, H. Chen, T. A. Kalinina, T. V. Glukhareva and Z. Fan, Chin. J. Chem., 2021, 39, 1531 CrossRef CAS.
- (a) K. A. Lawton, L. Friedrich, M. Hunt, K. Weymann, T. Delaney, H. Kessmann, T. Staub and J. Ryals, Plant J., 1996, 10, 71 CrossRef CAS; (b) X. Qi, K. Li, L. Chen, Y. Zhang, N. Zhang, W. Gao, Y. Li, X. Liu and Z. Fan, Int. J. Mol. Sci., 2022, 23, 5348 CrossRef CAS; (c) X. Qi, L. Chen, Y. Zhang, W. Gao, L. Chen, D. Wang, L. Tang, Z. Wang, N. N. Wang and Z. Fan, J. Agric. Food Chem., 2022, 70, 3142 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2298345 and 2298279. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ob01717f |
| This journal is © The Royal Society of Chemistry 2024 |