Molecular clips with spatially proximal urea residues for efficient transmembrane co-transport of H+/Cl− ions†
Nyaya Prakash
Pradhan
 ,
Kavthekar Rupesh
Namdev
and
Aasheesh
Srivastava
,
Kavthekar Rupesh
Namdev
and
Aasheesh
Srivastava
 *
*
Department of Chemistry, Indian Institute of Science Education and Research (IISER) Bhopal, Bhopal Bypass Road, Bhauri, Bhopal 462 066, Madhya Pradesh, India. E-mail: asrivastava@iiserb.ac.in
First published on 29th November 2023
Abstract
We report the design of bis(urea) functionalized amphiphilic molecular clips viz. 1a–1e to achieve efficient transmembrane co-transport of H+/Cl− ions. The most promising molecule 1a demonstrated a low nanomolar EC50 value (6.96 nM) to co-transport H+/Cl− ions via a carrier-mediated pathway and showed selective toxicity against cancerous HeLa cells as a result.
Controlled transport of ions across cell membranes is pivotal for maintaining cell tonicity and potential across the membranes. It is also crucial in ion homeostasis, regulation of intracellular pH, and signal transduction inside cells. Specialized ion channels and carrier proteins therefore exist to manage this critical task.1 However, dysregulation or malfunctioning of these proteins can result in various channelopathies, including cystic fibrosis (CF), epilepsy, Bartter syndrome, and certain types of cancers.2–4 Recent studies revealed that synthetic transmembrane H+/Cl− transporters can be used to treat certain channelopathies and act as anticancer agents since they induce cell apoptosis by disrupting the pH gradient across cell membranes.5–7 Hence, the design and evaluation of synthetic ion transporters that mimic the function of natural ion channels have attracted much attention in recent times.8 The shuttling of H+/Cl− ions across the cell membrane with the help of such synthetic ion transporters can disrupt the cell's electrochemical balance and potentially trigger the apoptosis of cancer cells. This mechanism is employed by prodigiosin, a natural ion transporter that induces apoptosis in cancer cells.9,10 Synthetic pyrrole-based analogs of prodigiosin such as cycloprodigiosin reported by Inoue et al.,11 obatoclax12 and tambjamine derivatives13 reported by Quesada et al., and perenosins reported by the Gale group,14,15 show prodigious H+/Cl− co-transport across cell membranes and induce apoptosis in cancer cells. Recently, Talukdar and co-workers have reported pyridyl-linked benzimidazolyl hydrazones as H+/Cl− co-transporters that show superior transport activity than prodigiosin, leading to apoptosis in cancer cells.16
Due to their high pharmacological potential, designing new H+/Cl− co-transporting motifs has gained prominence in recent times. A careful tuning of the acidity of anion-interacting protons and modulating the hydrophilic–lipophilic balance of the molecule are needed to achieve efficient ion transport.17,18 Many synthetic H+/Cl− co-transporters containing urea and thiourea residues that offer two acidic N–H units as active chloride binding sites have also been reported, which suggests potential biomedical application in anticancer treatment.19–21 For instance, Gale and co-workers have recently reported a coumarin-based fluorescent transporter consisting of a bis(urea) moiety that allows for the co-transport of H+/Cl− across lipid bilayer membranes, resulting in organelle deacidification and apoptosis in cancer cells.22 Amongst the series of macrocyclic chloride ion channels developed by Madhavan and co-workers,23 the one with optimal lipophilicity (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 11.7) showed the highest Cl− transport efficiency in lipid vesicles.
P = 11.7) showed the highest Cl− transport efficiency in lipid vesicles.
Nonetheless, an important attribute to be kept in mind when designing synthetic ion transporters is their physiological stability, since a physiologically unstable molecule may break down and lose its activity rapidly, even before reaching its target, or it may interact with unintended targets, leading to undesirable side effects. Hydrolytically sensitive linkages like imines or esters should therefore be avoided in the ion transporting scaffolds to enhance their potential for translation. Keeping these aspects in mind, herein we designed a series of pyridine-2,6-dicarboxamide (PDC) based molecular clips 1a–1e, each with two spatially proximal urea residues for potential bipodal interaction with the anion. The PDC unit present in these molecules enforces a hairpin-bend type conformation due to the strong bifurcated intramolecular hydrogen bonding present in it.24 This brings the urea residues present in the molecules into spatial proximity during anion binding (Fig. 2). Previously, we reported anion binding-induced white light emission from PDC-based scaffolds25 and also demonstrated their high solid-state proton conduction abilities.26 Herein, we exploited the unique structural attributes of transporters 1a–1e to achieve efficient co-transport of H+/Cl− ions across lipid membranes.
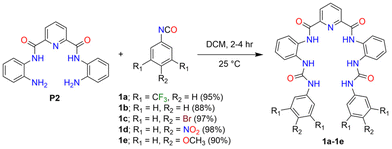
The target molecules were readily synthesized in excellent yields by the reaction of precursor P226 with different commercially available phenyl isocyanate derivatives (Scheme 1). Compounds 1a and 1d have electron-withdrawing residues attached to the phenyl rings, while electron-donating residues are appended in molecules 1c and 1e. Thus, 1a and 1d were expected to interact more strongly with the added anions than the other molecules investigated here. For screening of the anion-binding potential of these molecules, a series of anions (Cl−, Br−, I−, NO3−, ClO4− and AcO−) were added as tetrabutylammonium (TBA) salts to the DMSO-d6/0.5% H2O solution of 1a and changes in the 1H NMR spectrum of 1a were studied (Fig. 1A and S1†).27 It was observed that, after adding 5 eq. of TBA salts, the urea N–Hd and N–He protons underwent deshielding in the cases of Cl− and AcO−, whereas no such changes were observed with other anions. This shows the selective binding of 1a with Cl− and AcO− ions through hydrogen bonding interaction. The stronger binding of 1a towards AcO− over the Cl− ion was attributed to the affinity of the pre-organised intramolecular bifurcated H-bonded network in the PDC framework to selectively bind charge-dense AcO− anions.28 Furthermore, to determine the binding constant values, the binding stoichiometry between 1a and the Cl− ion was first obtained from Job's plot using the continuous variation method, which showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry in the solution phase (Fig. S2†). This was further confirmed by mass spectrometric analysis, in which a strong peak corresponding to the [M + Cl−] species was observed (Fig. S3†). The binding constants of 1a with different TBA salts were then calculated by fitting the titration data of N–Hd and N–He protons employing an inbuilt 1
1 binding stoichiometry in the solution phase (Fig. S2†). This was further confirmed by mass spectrometric analysis, in which a strong peak corresponding to the [M + Cl−] species was observed (Fig. S3†). The binding constants of 1a with different TBA salts were then calculated by fitting the titration data of N–Hd and N–He protons employing an inbuilt 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model of the BindFit v0.5 program,29 which provided the sequence of binding as follows: Cl− (71.65 M−1 ± 1.90%) > Br− (8.49 M−1 ± 3.01%) > NO3− (7.03 M−1 ± 1.14%) (Fig. 1B and S4–S6†). The binding constants for the AcO− ion were calculated using a 1
1 binding model of the BindFit v0.5 program,29 which provided the sequence of binding as follows: Cl− (71.65 M−1 ± 1.90%) > Br− (8.49 M−1 ± 3.01%) > NO3− (7.03 M−1 ± 1.14%) (Fig. 1B and S4–S6†). The binding constants for the AcO− ion were calculated using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding model to account for the binding of a second AcO− anion. The resulting values were K11 = 3127 M−1 ± 27.44% and K12 = 17.53 M−1 ± 4.95% (Fig. S7†). Binding of 1a with I− and ClO4− was too weak to be determined due to the absence of any changes in chemical shifts (Fig. S8 and S9†). The interaction between 1a and Cl− could be readily overcome through the addition of a slight excess of Ag(SbF6), resulting in the return of the chemical shift of the N–Hd and N–He protons of 1a to their original positions (as was the case in the absence of Cl− ions, Fig. 1C).
2 binding model to account for the binding of a second AcO− anion. The resulting values were K11 = 3127 M−1 ± 27.44% and K12 = 17.53 M−1 ± 4.95% (Fig. S7†). Binding of 1a with I− and ClO4− was too weak to be determined due to the absence of any changes in chemical shifts (Fig. S8 and S9†). The interaction between 1a and Cl− could be readily overcome through the addition of a slight excess of Ag(SbF6), resulting in the return of the chemical shift of the N–Hd and N–He protons of 1a to their original positions (as was the case in the absence of Cl− ions, Fig. 1C).
Furthermore, the binding constants of all the bis(urea) derivatives viz. 1a–1e towards Cl− anions were determined. As hypothesized, compounds 1a and 1d with electron-deficient substituents showed the most potent binding and the other molecules followed the sequence: 1a (Ka = 71.65 M−1 ± 1.90%) > 1d (57.67 M−1 ± 2.31%) > 1c (51.25 M−1 ± 1.25%) > 1e (38.15 M−1 ± 0.58%) > 1b (32.45 M−1 ± 1.07%) (Fig. S4, S10–S13† and Table 1). Hence the binding affinity of these bis(urea) functionalized molecular clips towards Cl− ions was correlated with the acidity of urea N–H protons. Furthermore, a significant increase in the binding between 1a and Cl− ions (Ka = 324.75 M−1 ± 13.67%) was noticed in acetone-d6, a solvent of comparatively less polarity (Fig. S14†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), binding constants Ka (M−1), effective concentrations needed for half-maximal activity (EC50), and the binding stoichiometry (n) of transporters 1a–1e with Cl−
P), binding constants Ka (M−1), effective concentrations needed for half-maximal activity (EC50), and the binding stoichiometry (n) of transporters 1a–1e with Cl−
| Transporter | R1 | R2 | clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa Pa |
K
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (M−1)
(M−1) |
EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (nM) c (nM) |
n |
|---|---|---|---|---|---|---|
a clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of the transporters were calculated using the MarvinSketch program.
b Binding constants (Ka) were calculated using the 1 P values of the transporters were calculated using the MarvinSketch program.
b Binding constants (Ka) were calculated using the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model with the BindFit program through 1H NMR experiments.
c EC50 values were calculated using the HPTS assay, n = Hill coefficient. 1 binding model with the BindFit program through 1H NMR experiments.
c EC50 values were calculated using the HPTS assay, n = Hill coefficient.
|
||||||
| 1a | CF3 | H | 10.09 | 71.65 ± 1.90% | 6.96 ± 1.13 | 0.73 |
| 1b | H | H | 6.56 | 32.45 ± 1.07% | 1061.6 ± 54.93 | 1.37 |
| 1c | H | Br | 8.15 | 51.25 ± 1.25% | 414.4 ± 40.32 | 1.04 |
| 1d | H | NO2 | 6.47 | 57.67 ± 2.31% | 29.1 ± 4.46 | 1.10 |
| 1e | H | OCH3 | 5.45 | 38.15 ± 0.58% | 1634.5 ± 70.06 | 2.54 |
Single crystals of 1a suitable for X-ray diffraction studies were obtained by slow infusion of diethyl ether vapours into the acetone solution of 1a at 25 °C. The crystal structure of 1a shows that one urea moiety of the molecule is pointing inward, whereas the other is pointing outward, as illustrated in Fig. 2A. The chloride-bound structure of 1a was optimized with the help of Gaussian 09 software, taking 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry using the B3LYP/6-311G(d,p) basis set (Fig. 2B). The optimized structure of [1a + Cl−] was a tetra-coordinated chloride complex with the N–H residues of both the urea moieties present in 1a that interact strongly with the chloride ions having N–H⋯Cl bond distances in the range of 2.243–2.347 Å. Importantly, the amide N–Hc protons of the PDC residue showed no such interaction with the Cl− ions since they were already engaged in the bifurcated intramolecular H-bonding.
1 binding stoichiometry using the B3LYP/6-311G(d,p) basis set (Fig. 2B). The optimized structure of [1a + Cl−] was a tetra-coordinated chloride complex with the N–H residues of both the urea moieties present in 1a that interact strongly with the chloride ions having N–H⋯Cl bond distances in the range of 2.243–2.347 Å. Importantly, the amide N–Hc protons of the PDC residue showed no such interaction with the Cl− ions since they were already engaged in the bifurcated intramolecular H-bonding.
 | ||
| Fig. 2 (A) Single crystal structure of 1a. (B) DFT optimized structure of [1a + Cl−] showing hydrogen bonding interactions with Cl−, using the Gaussian 09, B3LYP/6-311G(d,p) basis set. | ||
The Cl− binding affinity of 1a–1e studied through 1H NMR, mass spectroscopy, DFT optimized structures, and the optimal lipophilicity values (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 5.45–10.09) encouraged us to investigate the transmembrane Cl− ion transport activity of these molecules across the synthetic lipid bilayer membranes by fluorescence assays based on a pH-sensitive dye 8-hydroxypyrene-1,3,6-trisulfonate (HPTS).30 For that, large unilamellar vesicles (LUVs) entrapping HPTS were prepared using egg yolk phosphatidylcholine (EYPC) to get EYPC-LUVs⊃HPTS (see the ESI†). Fluorescence kinetics experiments were performed to study the ion-transport activities of 1a–1e across EYPC-LUVs⊃HPTS by adding these compounds to the vesicular solution and increasing the external pH (Fig. S16†).31 Compounds 1a and 1d showed maximum ion transport activity even at a nanomolar concentration (10 nM) (Fig. 3A, Table 1). The optimum clog
P = 5.45–10.09) encouraged us to investigate the transmembrane Cl− ion transport activity of these molecules across the synthetic lipid bilayer membranes by fluorescence assays based on a pH-sensitive dye 8-hydroxypyrene-1,3,6-trisulfonate (HPTS).30 For that, large unilamellar vesicles (LUVs) entrapping HPTS were prepared using egg yolk phosphatidylcholine (EYPC) to get EYPC-LUVs⊃HPTS (see the ESI†). Fluorescence kinetics experiments were performed to study the ion-transport activities of 1a–1e across EYPC-LUVs⊃HPTS by adding these compounds to the vesicular solution and increasing the external pH (Fig. S16†).31 Compounds 1a and 1d showed maximum ion transport activity even at a nanomolar concentration (10 nM) (Fig. 3A, Table 1). The optimum clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value and the presence of electron-withdrawing 3,5-bis(trifluoromethyl)phenyl residue in 1a contribute to the highest ion transport activity of it amongst molecules 1a–1e. A dose–response ion transport study was performed using HPTS assay experiments followed by Hill analysis to obtain the concentration needed for half-maximum activity (EC50) and the Hill coefficient (n) (Fig. 3B and S17–S21†). The EC50 value refers to the concentration of the transporter required to obtain 50% transport of ions, and the Hill coefficient indicates the binding stoichiometry. Compounds 1a and 1d were found to be the most efficient transporters in the series with EC50 values of 6.96 nM and 29.1 nM, respectively, with the sequence of EC50 values being 1a > 1d > 1c > 1b > 1e (Table 1). These data correlate well with the binding constant (Ka) and lipophilicity (clog
P value and the presence of electron-withdrawing 3,5-bis(trifluoromethyl)phenyl residue in 1a contribute to the highest ion transport activity of it amongst molecules 1a–1e. A dose–response ion transport study was performed using HPTS assay experiments followed by Hill analysis to obtain the concentration needed for half-maximum activity (EC50) and the Hill coefficient (n) (Fig. 3B and S17–S21†). The EC50 value refers to the concentration of the transporter required to obtain 50% transport of ions, and the Hill coefficient indicates the binding stoichiometry. Compounds 1a and 1d were found to be the most efficient transporters in the series with EC50 values of 6.96 nM and 29.1 nM, respectively, with the sequence of EC50 values being 1a > 1d > 1c > 1b > 1e (Table 1). These data correlate well with the binding constant (Ka) and lipophilicity (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) of the transporters. Furthermore, the Hill coefficient values n ≈ 1 confirmed the 1
P) of the transporters. Furthermore, the Hill coefficient values n ≈ 1 confirmed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of Cl− ions.
1 binding of Cl− ions.
The ion selectivity and transport mechanism studies were conducted across EYPC-LUVs⊃HPTS using 1a as the active ion transporter. Initially, anion selectivity studies were conducted using a modified HPTS assay based on the anion gradient and without any base pulse.18,32 The EYPC-LUVs⊃HPTS vesicles were suspended in various external buffer solutions containing NaX (X− = Cl−, Br−, I−, NO3−, ClO4−, and AcO−) salts at pH 7.0, followed by the addition of the transporter to initiate the transport activity (Fig. S23†). The data showed the higher transport of ClO4− and NO3− ions due to their larger size and higher hydrophobicity, followed by I−, Br−, Cl−, and AcO− anions exhibiting an inverse Hofmeister pattern (Fig. S24†). The lower selectivity of smaller-size anions like Cl− and Br− ions may arise due to the structural flexibility present in the anion binding residues.18,33 Previously, Gale and co-workers have also observed similar trends in the anion selectivity sequence of urea-based ion transporters.34,35 Our anion transporter 1a was unaffected by the variation of counter cations and the change of the extravesicular cations to MCl (M+ = Li+, Na+, K+, Rb+, and Cs+) using a dual gradient assay, which resulted in no significant change in the Cl− ion transport activity (Fig. S25 and S26†). This indicates that the cations have no role in the anion transport process by 1a.
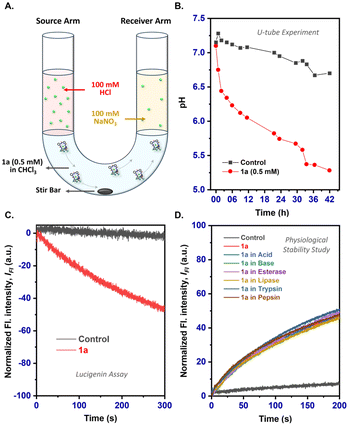
Subsequently, the mechanism of anion transport by 1a was investigated across EYPC-LUVs⊃HPTS. Different pathways such as M+/H+ antiport, OH−/X− antiport, H+/X− symport, and M+/OH− symport are possible for the transport process. However, the comparable ion transport rate in the cation selectivity study excludes the possibility of M+/H+ antiport and M+/OH− symport processes (Fig. S26†). To gain further insights into the transport mechanism, we studied the ion transport in the presence and absence of carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP; an H+ ionophore)36 and valinomycin (Val; a K+ ionophore).37 If the transport process of 1a occurs through the OH−/X− antiport process, the presence of FCCP would increase the transport activity due to the cooperative effect of both transporter 1a and FCCP. However, the presence of FCCP produced no substantial enhancement in the ion transport activity of 1a (Fig. 3C and S27†), which suggests that the H+/X− symport pathway is operating for the equilibration of the pH gradient. Similarly, the valinomycin-coupled HPTS assay conducted across EYPC-LUVs⊃HPTS in KCl buffer showed similar ion transport activity with or without valinomycin. This suggested the preferential transport of Cl− over OH− ions (Fig. 3D and S27†), which further confirms the co-transport of H+/Cl− by the transporters.31,38,39 To get direct evidence of the H+/Cl− co-transport by 1a, we conducted the conventional U-tube experiment40 by keeping isomolar HCl and NaNO3 solutions in the source (left) and the receiver (right) arms, respectively, separated by an organic layer (CHCl3 containing 0.5 mM 1a) (Fig. 4A). A significant reduction in the pH at the receiver arm was observed with time (Fig. 4B). This confirmed the co-transport of H+/Cl− ions by the bis(urea) derivative 1a. The Cl− ion transport of 1a was further established with the help of a halide-sensitive lucigenin dye across EYPC/CHOL-LUVs⊃lucigenin vesicles following a previously reported protocol (Fig. S28†).41 The influx of the Cl− ion was evident from the quenching of fluorescence of the lucigenin dye with time when a Cl−/NO3− gradient was created across the LUVs (Fig. 4C).
Keeping the physiological aspect of the compounds in mind, we investigated the chemical stability and transport activity of 1a under different conditions as well as in the presence of enzymes. Films of compound 1a were incubated in acid, base, and enzymes such as esterase from porcine liver, lipase, trypsin, and pepsin solutions separately for 6 h, and then 1H NMR and HPTS assays were performed (Fig. S29†). Notably, no change in the NMR spectra as well as the transport activity of the compound was observed in all cases, which shows the applicability of the compound under different physiological conditions even where pH extremes may be encountered (Fig. 4D and S30†).
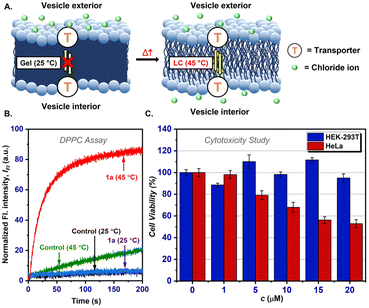
To investigate whether carrier-mediated or channel-mediated transport is occurring with 1a, a temperature-dependent HPTS assay was performed using vesicles prepared from DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) lipid that has a gel-to-liquid crystal transition temperature of 41 °C. The assay revealed a negligible ion transport activity of 1a (25 nM) at 25 °C (Fig. 5A and B) that increased drastically when the experiment was performed at 45 °C. This suggests that a carrier mediated ion transport mechanism is in operation.
The impressive H+/Cl− co-transport ability across synthetic lipid bilayer membranes and the physiological stability of 1a inspired us to investigate its effect on different cell lines.16 The cytotoxicity of 1a was studied using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay in cancerous (HeLa, human breast cancer) and non-cancerous (HEK-293T, human embryonic kidney) cell lines. The cells were incubated with different concentrations of 1a (0–20 μM) for 24 h, and the results showed that 1a was selectively toxic to HeLa cells (IC50 = 19.84 μM) and not to HEK-293T cells (Fig. 5C and ESI†), pointing towards the potential of 1a in anticancer applications that would require more elaborate investigations to establish unequivocally.
Taken together, this work presents PDC-based molecular clips 1a–1e containing spatially proximal bis(urea) units for bipodal interaction with anions as effective and efficient anion transporters. Compound 1a with the most acidic urea protons facilitated the co-transport of H+/Cl− ions across synthetic lipid bilayer membranes. Job's plot from the 1H NMR experiment, mass analysis, and the Hill coefficient values from the HPTS assay collectively indicate a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry. The most effective transport activity of 1a (EC50 = 6.96 nM) through a carrier mediated H+/Cl− symport pathway was established through various assays. The cytotoxicity study indicates the preliminary anticancer potential of the transporter. This study presents the successful design of potent, physiologically stable anion transporting scaffolds.
1 binding stoichiometry. The most effective transport activity of 1a (EC50 = 6.96 nM) through a carrier mediated H+/Cl− symport pathway was established through various assays. The cytotoxicity study indicates the preliminary anticancer potential of the transporter. This study presents the successful design of potent, physiologically stable anion transporting scaffolds.
Author contributions
NPP and AS conceived and designed the project and wrote the manuscript. NPP conducted all the experiments and analysis. KRN performed the cytotoxicity studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank SERB India for funding this work through project no. CRG/2021/007029. We thank Central Instrumentation Facility (CIF) IISER Bhopal for spectroscopic characterization of the samples. We also gratefully acknowledge Prof. Himanshu Kumar and Dr Sanjeev Shukla (both from Dept. of Biological Sciences, IISER Bhopal) for providing the cell lines. N. P. P. and K. R. N. thank the CSIR and UGC, respectively, for Senior Research Fellowships.References
- B. Hille, Ion Channels of Excitable Membranes, Sinauer Associates Inc., 3rd edn, 2001 Search PubMed.
- B. Dworakowska and K. Dołowy, Acta Biochim. Pol., 2000, 47, 685–703 CrossRef CAS PubMed.
- T. J. Jentsch, C. A. Hübner and J. C. Fuhrmann, Nat. Cell Biol., 2004, 6, 1039–1047 CrossRef CAS PubMed.
- H. Li, H. Valkenier, L. W. Judd, P. R. Brotherhood, S. Hussain, J. A. Cooper, O. Jurček, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed.
- B. A. Webb, M. Chimenti, M. P. Jacobson and D. L. Barber, Nat. Rev. Cancer, 2011, 11, 671–677 CrossRef CAS PubMed.
- J. Yang, G. Yu, J. L. Sessler, I. Shin, P. A. Gale and F. Huang, Chem, 2021, 7, 3256–3291 CAS.
- S.-H. Park, S.-H. Park, E. N. W. Howe, J. Y. Hyun, L.-J. Chen, I. Hwang, G. Vargas-Zuñiga, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Chem, 2019, 5, 2079–2098 CAS.
- A. Mondal, M. Ahmad, D. Mondal and P. Talukdar, Chem. Commun., 2023, 59, 1917–1938 RSC.
- T. Sato, H. Konno, Y. Tanaka, T. Kataoka, K. Nagai, H. H. Wasserman and S. Ohkuma, J. Biol. Chem., 1998, 273, 21455–21462 CrossRef CAS PubMed.
- J. L. Sessler, L. R. Eller, W.-S. Cho, S. Nicolaou, A. Aguilar, J. T. Lee, V. M. Lynch and D. J. Magda, Angew. Chem., Int. Ed., 2005, 44, 5989–5992 CrossRef CAS PubMed.
- C. Yamamoto, H. Takemoto, K. Kuno, D. Yamamoto, K. Nakai, T. Baden, K. Kamata, H. Hirata, T. Watanabe and K. Inoue, Oncol. Rep., 2001, 8, 821–824 CAS.
- B. Díaz de Greñu, P. I. Hernández, M. Espona, D. Quiñonero, M. E. Light, T. Torroba, R. Pérez-Tomás and R. Quesada, Chem. – Eur. J., 2011, 17, 14074–14083 CrossRef PubMed.
- V. Soto-Cerrato, P. Manuel-Manresa, E. Hernando, S. Calabuig-Fariñas, A. Martínez-Romero, V. Fernández-Dueñas, K. Sahlholm, T. Knöpfel, M. García-Valverde, A. M. Rodilla, E. Jantus-Lewintre, R. Farràs, F. Ciruela, R. Pérez-Tomás and R. Quesada, J. Am. Chem. Soc., 2015, 137, 15892–15898 CrossRef CAS PubMed.
- W. Van Rossom, D. J. Asby, A. Tavassoli and P. A. Gale, Org. Biomol. Chem., 2016, 14, 2645–2650 RSC.
- L. A. Jowett, E. N. W. Howe, V. Soto-Cerrato, W. Van Rossom, R. Pérez-Tomás and P. A. Gale, Sci. Rep., 2017, 7, 9397 CrossRef PubMed.
- A. Mondal, J. A. Malla, H. Paithankar, S. Sharma, J. Chugh and P. Talukdar, Org. Lett., 2021, 23, 6131–6136 CrossRef CAS PubMed.
- H. Valkenier, C. J. E. Haynes, J. Herniman, P. A. Gale and A. P. Davis, Chem. Sci., 2014, 5, 1128–1134 RSC.
- X. Wu and P. A. Gale, Chem. Commun., 2021, 57, 3979–3982 RSC.
- X. Wu, J. R. Small, A. Cataldo, A. M. Withecombe, P. Turner and P. A. Gale, Angew. Chem., Int. Ed., 2019, 58, 15142–15147 CrossRef CAS PubMed.
- O. Biswas, N. Akhtar, Y. Vashi, A. Saha, V. Kumar, S. Pal, S. Kumar and D. Manna, ACS Appl. Bio Mater., 2020, 3, 935–944 CrossRef CAS.
- P. Vieira, M. Q. Miranda, I. Marques, S. Carvalho, L. Chen, E. N. W. Howe, C. Zhen, C. Y. Leung, M. J. Spooner, B. Morgado, O. A. B. da Cruz e Silva, C. Moiteiro, P. A. Gale and V. Félix, Chem. – Eur. J., 2020, 26, 888–899 CrossRef CAS PubMed.
- M. Fares, X. Wu, D. A. McNaughton, A. M. Gilchrist, W. Lewis, P. A. Keller, A. Arias-Betancur, P. Fontova, R. Pérez-Tomás and P. A. Gale, Org. Biomol. Chem., 2023, 21, 2509–2515 RSC.
- P. Saha and N. Madhavan, Org. Lett., 2020, 22, 5104–5108 CrossRef CAS PubMed.
- R. Kumar, H. Aggarwal, R. Bhowal, D. Chopra and A. Srivastava, Chem. – Eur. J., 2019, 25, 10756–10762 CrossRef CAS PubMed.
- R. Kumar and A. Srivastava, Chem. – Eur. J., 2016, 22, 3224–3229 CrossRef CAS PubMed.
- H. Aggarwal, P. A. Gaikwad, A. Dahat, S. N. Ghosh, P. Mehra, A. Paul, S. Talukder and A. Srivastava, Chem. – Eur. J., 2023, 29, e202300019 CrossRef CAS PubMed.
- A. Kerckhoffs and M. J. Langton, Chem. Sci., 2020, 11, 6325–6331 RSC.
- S. J. Brooks, S. E. García-Garrido, M. E. Light, P. A. Cole and P. A. Gale, Chem. – Eur. J., 2007, 13, 3320–3329 CrossRef CAS PubMed.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC.
- K. Kano and J. H. Fendler, Biochim. Biophys. Acta, Biomembr., 1978, 509, 289–299 CrossRef CAS PubMed.
- S. V. Shinde and P. Talukdar, Org. Biomol. Chem., 2019, 17, 4483–4490 RSC.
- A. M. Gilchrist, P. Wang, I. Carreira-Barral, D. Alonso-Carrillo, X. Wu, R. Quesada and P. A. Gale, Supramol. Chem., 2021, 33, 325–344 CrossRef CAS.
- X. Wu, P. Wang, W. Lewis, Y.-B. Jiang and P. A. Gale, Nat. Commun., 2022, 13, 4623 CrossRef CAS PubMed.
- A. M. Gilchrist, X. Wu, B. A. Hawkins, D. E. Hibbs and P. A. Gale, iScience, 2023, 26, 105988 CrossRef CAS PubMed.
- P. Wang, X. Wu and P. A. Gale, Supramol. Chem., 2021, 33, 143–149 CrossRef CAS.
- R. Benz and S. McLaughlin, Biophys. J., 1983, 41, 381–398 CrossRef CAS PubMed.
- P. Bhattacharyya, W. Epstein and S. Silver, Proc. Natl. Acad. Sci., 1971, 68, 1488–1492 CrossRef CAS PubMed.
- S. V. Shinde and P. Talukdar, Chem. Commun., 2018, 54, 10351–10354 RSC.
- S. V. Shinde and P. Talukdar, Angew. Chem., Int. Ed., 2017, 56, 4238–4242 CrossRef CAS PubMed.
- A. Saha, N. Akhtar, V. Kumar, S. Kumar, H. K. Srivastava, S. Kumar and D. Manna, Org. Biomol. Chem., 2019, 17, 5779–5788 RSC.
- T. Saha, M. S. Hossain, D. Saha, M. Lahiri and P. Talukdar, J. Am. Chem. Soc., 2016, 138, 7558–7567 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2258898. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ob01431b |
| This journal is © The Royal Society of Chemistry 2024 |